ABSTRACT
Apical polarity of cystic fibrosis transmembrane conductance regulator (CFTR) is essential for solute and water transport in secretory epithelia and can be impaired in human diseases. Maintenance of apical polarity in the face of CFTR non-polarized delivery and inefficient apical retention of mutant CFTRs lacking PDZ-domain protein (NHERF1, also known as SLC9A3R1) interaction, remains enigmatic. Here, we show that basolateral CFTR delivery originates from biosynthetic (∼35%) and endocytic (∼65%) recycling missorting. Basolateral channels are retrieved via basolateral-to-apical transcytosis (hereafter denoted apical transcytosis), enhancing CFTR apical expression by two-fold and suppressing its degradation. In airway epithelia, CFTR transcytosis is microtubule-dependent but independent of Myo5B, Rab11 proteins and NHERF1 binding to its C-terminal DTRL motif. Increased basolateral delivery due to compromised apical recycling and accelerated internalization upon impaired NHERF1–CFTR association is largely counterbalanced by efficient CFTR basolateral internalization and apical transcytosis. Thus, transcytosis represents a previously unrecognized, but indispensable, mechanism for maintaining CFTR apical polarity that acts by attenuating its constitutive and mutation-induced basolateral missorting.
KEY WORDS: CFTR, Airway epithelium, Polarity, Trafficking
Highlighted Article: Apical transcytosis is indispensable for the maintenance of highly polarized CFTR expression by counteracting its basolateral missorting from the secretory and apical endocytic pathways in human bronchial epithelial cells.
INTRODUCTION
The anion selective channel CFTR, a member (ABCC7) of the ABC transporter superfamily, is predominantly expressed at the apical plasma membrane (PM) of secretory and resorptive epithelia (Riordan, 2008). CFTR maintains the ionic, composition and volume homeostasis of the airway surface liquid, which are prerequisites for the physiological regulation of mucocilliary clearance and innate immune response of airway epithelia, and to prevent uncontrolled infection and inflammation of the lung, the primary causes of mortality in cystic fibrosis (CF) (Cutting, 2015; Rowe et al., 2005). An inherited defect in functional expression of CFTR at the apical PM of epithelia causes CF, while acquired loss of CFTR expression in airway epithelia and pancreatic duct is associated with chronic obstructive pulmonary disease (COPD) and asthma, and pancreatitis, respectively (Schnúr et al., 2016).
Four vesicular transport routes are recognized as important determinants of steady-state CFTR PM expression: biosynthetic secretion, slow constitutive internalization, and recycling, as well as endo-lysosomal/autophagosomal degradation (Ameen et al., 2007; Fu and Sztul, 2009; Gentzsch et al., 2004; Sharma et al., 2004). Given the long cellular and PM half-life of CFTR in epithelia (Okiyoneda et al., 2018; Sharma et al., 2004; Varga et al., 2008), a slow internalization rate with a high fidelity of apical recycling is essential for the maintenance of Cl− transport capacity by minimizing premature channel degradation and basolateral missorting.
In polarized epithelia, apical and basolateral PM proteins partition between apical sorting endosomes (ASEs), apical recycling endosomes (AREs), basolateral sorting endosomes (BSEs), and common recycling endosomes (CREs), respectively (Stoops and Caplan, 2014). Previous studies have shown that apically internalized CFTR is confined to EEA1+ ASEs and recycles via Rab11+ AREs in airway cells (Cholon et al., 2009; Swiatecka-Urban et al., 2007), in a PKA-dependent manner (Holleran et al., 2013), but the molecular basis of rapid degradation of basolaterally delivered and natively folded CFTR is not known. Nevertheless, forced missorting of CFTR to the basolateral PM suppressed the transepithelial anion secretion efficiency (Farmen et al., 2005).
Apical confinement of PM proteins can be achieved either by direct apical targeting from the trans-Golgi network (TGN) (Takeda et al., 2003), indirectly by transcytosis from the basolateral PM (Anderson et al., 2005) or by non-polarized delivery with selective retention at the apical PM and rapid degradation from the basolateral PM, as described for CFTR (Swiatecka-Urban et al., 2002). The association of postsynaptic density/disc large/ZO-1 (PDZ) domain adaptor proteins with PM proteins exposing a PDZ-binding motif can serve as polarized targeting and/or retention signal for both the apical and basolateral PM (Brône and Eggermont, 2005). NHERF1 and NHERF2 (also known as SLC9A3R1 and EBP50, and SLC9A3R2, respectively) binding to the CFTR C-terminal PDZ-binding motif (DTRL1480) has been implicated in the apical channel targeting, retention, confined lateral diffusion and endocytic recycling (Haggie et al., 2004; Moyer et al., 1999; Swiatecka-Urban et al., 2002) by tethering it, via ezrin, to the subapical actin network (Short et al., 1998; Sun et al., 2000). Deletion of the DTRL motif caused by the S1455X premature termination codon, however, manifests in the isolated elevation of sweat Cl− concentration in the absence of a lung and pancreatic phenotype in the background of an in-frame deletion of exon 14a, which encodes a non-functional CFTR (Mickle et al., 1998). These observations, together with extensive mutagenesis studies (Benharouga et al., 2003; Milewski et al., 2005, 2001; Ostedgaard et al., 2003) suggest that CFTR tethering to the subapical cytoskeleton via NHERF proteins is not essential for the channel apical polarity. To identify additional mechanism(s) that preserve CFTR polarity in the absence of the NHERF tethering function, we investigated candidate trafficking pathways, sorting signals and molecular players that may be involved in CFTR polarity development.
Here, we show that both the limited fidelity of apical CFTR biosynthetic secretion and recycling from apical endosomes account for the basolateral missorting of the channel. Basolateral CFTR accumulation is effectively counteracted by its rapid internalization rate and basolateral-to-apical transcytosis (hereafter denoted apical transcytosis), contributing to the high apical levels of the channel and its metabolic stability. The microtubule-dependent, but Myo5B-independent, CFTR transcytosis in airway cells avoids both apical and basolateral endocytic recycling compartments but traverses EEA1+ sorting endosomes. Furthermore, apical transcytosis offsets the augmented basolateral missorting caused by C-terminal truncations of the PDZ protein-binding sites in CFTR, providing a plausible explanation for the isolated resorbtive, but not secretory, defects in the sweat gland and respiratory epithelia, respectively, of individuals harboring the S1445X–CFTR mutant.
RESULTS
Human airway epithelial models for studying CFTR polarized sorting
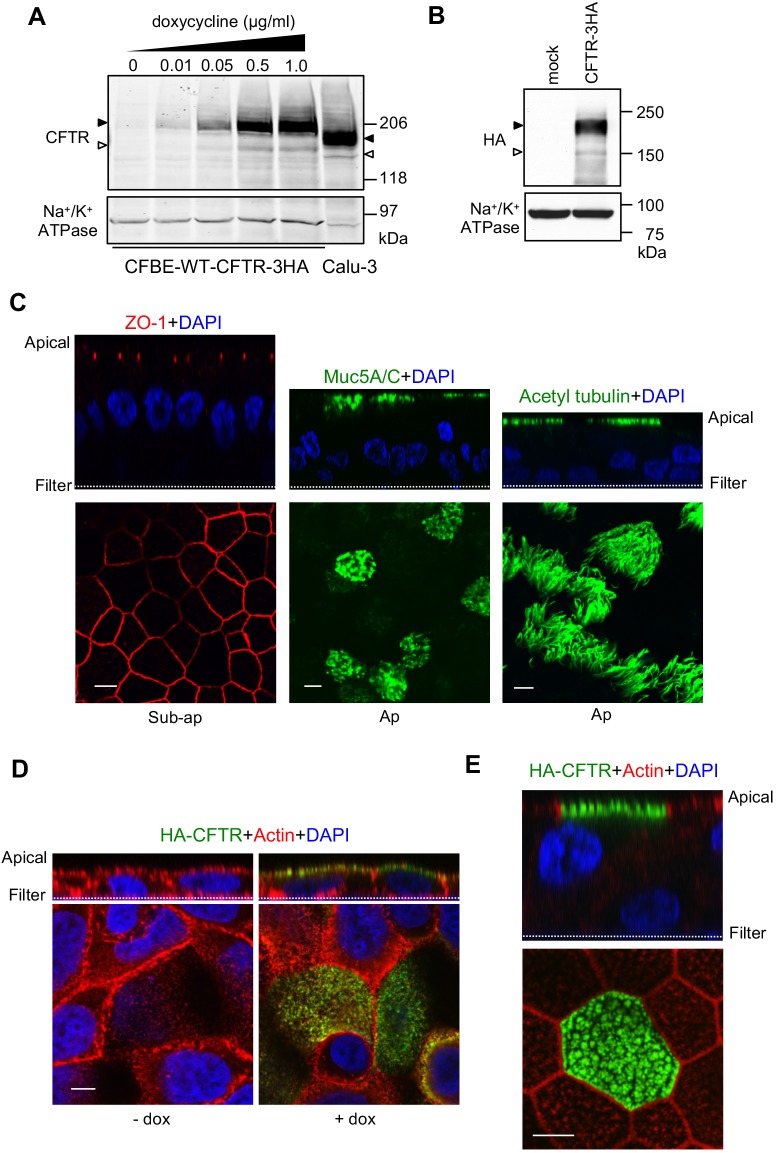
To investigate polarized trafficking, CFTR containing a 3HA tag in its fourth extracellular loop was expressed in CF bronchial epithelial cells (CFBE41o– or CFBE) under the control of the tetracycline transactivator (Ehrhardt et al., 2006; Veit et al., 2012). Filter-grown CFBE cells were cultured for 4–5 days post-confluence and CFTR expression was induced with doxycycline (+dox) to reach similar or lower expression to that in Calu-3 cells, a lung adenocarcinoma epithelia cell line endogenously expressing CFTR (Fig. 1A). The mass difference between the endogenous and tagged CFTR can be attributed to 3HA tag and altered complex glycosylation, demonstrated by the mobility shift observed after glycosidase digestion (Fig. S1A), but had no significant impact on the channel folding, trafficking, stability and function (Peters et al., 2011). As a second model, CFTR–3HA was expressed in conditionally reprogrammed human primary bronchial epithelial cells (CR-HBE) differentiated under an air–liquid interface for ≥4 weeks after lentiviral transduction (Fig. 1B). Hallmarks of airway epithelia differentiation were assessed: tight-junctional localization of ZO-1 (also known as TJP1), mucin 5 accumulation in goblet cells and accumulation of acetylated tubulin in cilia, as well as development of transepithelial electrical resistance (>500 Ω/cm2), airway surface liquid (ASL, ∼10 µm) and polarized H+/K+ ATPase and transferrin receptor (TfR) expression (Fig. 1C; Fig. S1B–D and see Materials and Methods). Apical localization and function of exogenously expressed CFTR–3HA was demonstrated by immunostaining and the presence of dox-inducible and CFTR inhibitor (Inh172)-sensitive PKA-activated short circuit current (Isc) (Fig. 1D,E, see also Fig. 6I).
Fig. 1.
Characterization of human CF bronchial epithelial models to study polarized CFTR sorting. (A,B) Immunoblot analysis of CFTR–3HA expression (A) in CFBE cells after 3 days induction with the indicated amount of doxycycline and (B) following lentivirus transduction of CR-HBE cells. For comparison, an equal amount of Calu-3 cell lysate was loaded (A). Black and white arrowheads indicate complex- and core-glycosylated CFTR, respectively. Na+/K+ ATPase was probed as a loading control. (C) CR-HBE cells were differentiated at an air–liquid interface (ALI) and stained for tight junctions (ZO-1, red), mucin 5 (muc5A/C, green), cilia (acetylated-tubulin, green) and nuclei (DAPI, blue). Cells were visualized by laser confocal fluorescence microscopy. The upper and lower panels are vertical and horizontal optical sections, respectively. Ap, apical surface. (D,E) WT-CFTR–3HA is apically expressed in CFBE (D) and CR-HBE (E) cells. Cells were stained for WT-CFTR–3HA (green), actin (red) and DAPI (blue). Scale bars: 5 µm. Representative of at least three independent experiments.
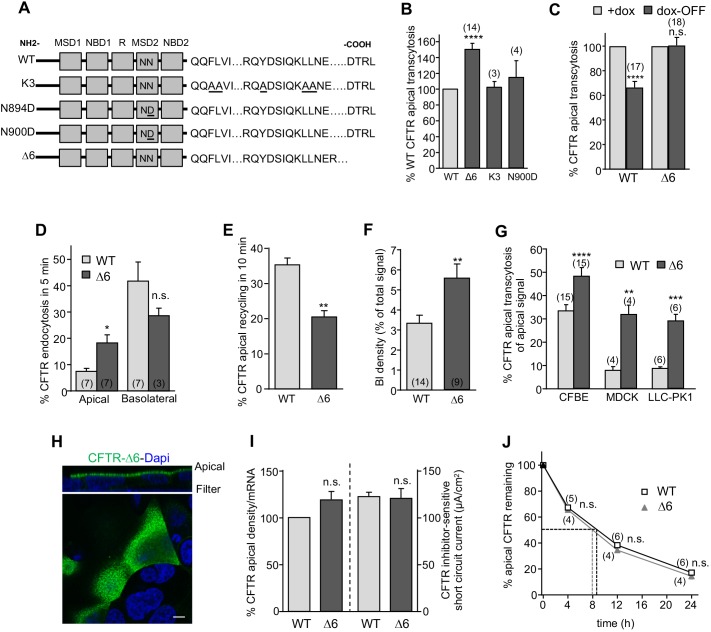
Fig. 6.
The effect of sorting signal and PDZ-domain-binding motif mutation on CFTR polarized sorting and function in epithelia. (A) Mutagenesis of sorting signals that may influence CFTR transcytosis. Endocytic consensus sites Tyr1424 (Y) and Leu1430-1431 (L) were mutated to Ala (A). NN represents Asn895 and Asn900, two N-linked glycosylation sites in the MSD2. DTRL, PDZ-domain-binding motif at the C-terminus; MSD, membrane spanning domain; NBD, nucleotide-binding domain; R, regulatory domain. (B) Deletion of the DTRL motif (Δ6) increases CFTR apical transcytosis relative to WT-CFTR, but the K3 and N900D mutations do not. (C) Biosynthetic basolateral missorting of CFTR depends on the binding of PDZ-domain protein(s). CFTR apical transcytosis was measured in control (+dox) or dox-OFF (24 h after washout) cells. (D) Apical but not basolateral CFTR internalization is accelerated upon deletion of the PDZ-binding motif (two-tailed unpaired t-test). (E) The Δ6 mutation impairs CFTR apical recycling (n=6), as measured by PM-ELISA. (F) WT- and Δ6-CFTR basolateral cell-surface density was measured by PM-ELISA (two-tailed unpaired t-test). (G) The effect of the Δ6 mutation on CFTR apical transcytosis in the indicated cell line. (H) Δ6-CFTR is predominantly detected at the apical PM in CFBE by indirect immunostaining, performed as in Fig. 1D. Scale bar: 5 µm. (I) The apical PM density and channel function of WT- and Δ6-CFTR were measured by PM-ELISA and by determining the 10 µM forskolin-stimulated short-circuit current (Isc), respectively, and corrected for their relative mRNA level (n=3). The CFTR-mediated Isc was quantified after inhibition with CFTRinh 172. (J) Apical stability of WT- and Δ6-CFTR is similar. CFTR was labeled with anti-HA (1 h, ice) and chased for the indicated time before PM-ELISA (two-tailed unpaired t-test). Data are mean±s.e.m. on each panel, parentheses indicate the number of independent experiments. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001; n.s. not significant.
CFTR is constitutively transcytosed in human airway epithelial cells
Newly synthesized CFTR is targeted randomly to both apical and basolateral PM in MDCK cells (Swiatecka-Urban et al., 2002). To study CFTR biosynthetic targeting in CFBE cells, we determined the kinetics of domain-specific appearance of a cohort of newly translated CFTR molecules, containing a horseradish peroxidase (HRP) tag in its fourth extracellular loop (Veit et al., 2014). HRP activity, as a surrogate measure of CFTR PM appearance, was monitored by measuring the amount of Amplex Red oxidation to the fluorescent resorufin after dox-induction of CFTR–HRP transcription in CFBE and MDCK cells. We could not detect a significant difference in the delivery kinetics of CFTR–HRP activity at the apical and basolateral PM (236 min versus 242 min in CFBE; 164 min versus 156 min in MDCK, Fig. S1E–G), which confirms the non-polarized biosynthetic secretion of CFTR in both epithelia (Swiatecka-Urban et al., 2002). Considering that basolateral sorting of CFTR takes place at the TGN, we hypothesized that natively folded channels are targeted for apical transcytosis instead of lysosomal degradation.
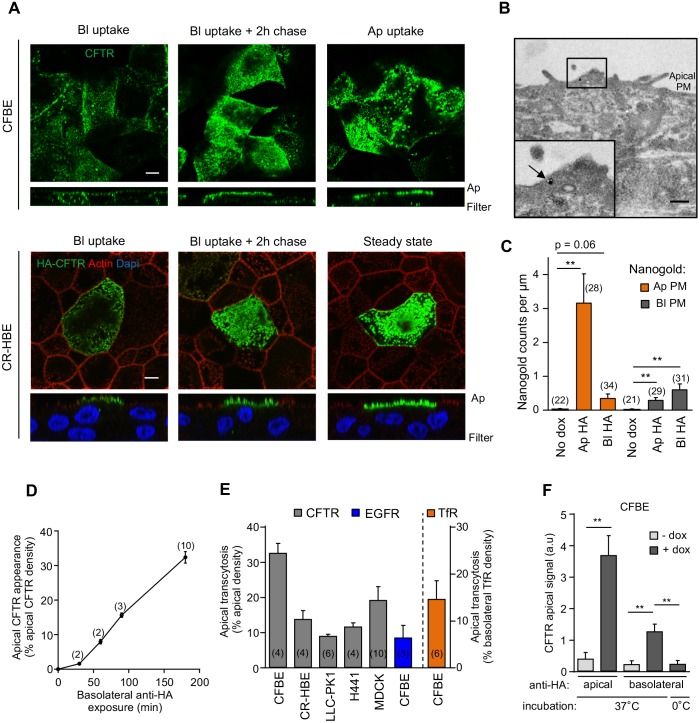
To assess apical transcytosis, we monitored the postendocytic fate of basolateral CFTR–3HA after labeling the channel with anti-HA antibody (Ab) capture from the basolateral compartment of filter-grown CFBE and CR-HBE cells at 37°C for 3 h. CFTR–anti-HA-Ab complexes were accumulated at the apical PM as well as intracellularly (Fig. 2A, left panel), visualized by Alexa Fluor 488 (AF488)-conjugated secondary Ab and laser confocal fluorescence microscopy (LCFM). Following a 2 h chase, the intracellular CFTR staining was diminished (Fig. 2A, middle panel). No staining was detected in the absence of CFTR (Fig. S1H), indicating that the primary Ab staining cannot be attributed to non-specific binding or CFTR-independent Ab transcytosis. We used similar assays to confirm CFTR transcytosis by electron microscopy (EM) with post-embedding secondary Ab-decorated nanogold labeling. Anti-HA–CFTR complex accumulation at the apical or basolateral PM was detected following anti-HA labeling from contralateral compartments only in CFTR-expressing CFBE cells (+dox, 3 h, 37°C) (Fig. 2B,C; Fig. S2A), suggesting that CFTR undergoes bidirectional transcytosis.
Fig. 2.
CFTR is apically transcytosed in human airway epithelial cells. (A) CFTR–3HA was labeled by anti-HA Ab capture (3 h, 37°C) from the basolateral (Bl) or the apical (Ap) compartment in filter-grown CFBE or CR-HBE cells. Cells were chased for 2 h (37°C) in the absence of anti-HA Ab. CFTR–anti-HA Ab complexes were visualized with AF488-anti-mouse Ab and laser confocal fluorescence microscopy. Lower panels represent z-sections. Scale bars: 5 µm. (B,C) Monitoring CFTR transcytosis with immuno-EM. (B) Immuno-EM detection of CFTR-3HA at the apical PM after basolateral labeling with anti-HA (3 h, 37°C) in CFBE. CFTR-anti-HA complexes labeled with secondary Ab conjugated to nanogold (arrow). The inset represents a higher magnification of the indicated area. Scale bar: 0.5 µm. (C) CFTR-expressing or non-expressing (no dox) CFBE cells were labeled either by apical (Ap) or basolateral (Bl) anti-HA Ab capture for 3 h (37°C). CFTR–anti-HA antibody complexes were visualized with anti-mouse Ab conjugated to nanogold particles as in B. The number of nanogold particles per micrometer was counted along the apical and basolateral PM segments (n=2–3 independent experiments, Mann–Whitney U-test). (D) CFTR apical transcytosis was measured over time as in Fig. S2B. (E) Apical transcytosis of CFTR–3HA, and endogenous EGFR and TfR were measured as described in the Materials and Methods in the indicated cells. (F) Apical transcytosis of CFTR is abolished when the channel is basolaterally labeled by anti-HA Ab for 3 h on ice or when CFTR transcription is not induced (- dox) (n=4). Data are means±s.e.m. on each panel. **P<0.01, parentheses indicate the number of independent experiments (D,E) or regions of interest (C).
Determination of CFTR apical transcytotic flux
To estimate the CFTR transcytotic flux, we labeled basolateral channels by anti-HA Ab capture and detected the transcytosed CFTR–Ab complexes by PM-ELISA at the apical PM (Fig. S2B, right). The amount of transcytosed CFTR amount was expressed as the percentage of the steady-state apical CFTR density (Fig. S2B, left). CFTR accumulation at the apical PM increased with increasing basolateral Ab-labeling time (0.5–3 h) at 37°C (Fig. 2D). After 3 h of Ab capture, 32±3% and 14±3% (means±s.e.m.) of the apical PM pool was labeled, indicating it had undergone transcytosis, in CFBE and CR-HBE cells, respectively (Fig. 2E). These values, owing to constitutive internalization of transcytosed CFTR–anti-HA Ab complexes, are likely underestimates. The channel transcytosis flux remained unaltered at ∼50% reduced CFTR PM density, achieved by reduced dox induction (0.05 µg · ml−1), which adjusted the mature CFTR expression to ∼25% of that in Calu-3 cells (Fig. 1A; Fig. S2C).
Several lines of evidence validate the specificity of the ELISA-based transcytosis assay. First, transepithelial or paracellular Ab transport was ruled out based on the lack of HRP-conjugated Ab translocation from the basolateral into the apical compartment at 37°C (Fig. S2D,E). Second, neither non-specific mouse nor rabbit Ab was apically transcytosed, indicating that FcRn- or pIgR-mediated Ab transport cannot contribute to the transcytotic signal (Fig. S2F). Likewise, the contribution of fluid-phase transcytosis of anti-HA Ab was negligible (Fig. S2F; Ap HA versus Ap+Bl HA). Third, CFTR transcytosis was completely abolished on ice or in the absence of CFTR expression (Fig. 2F). Fourth, we showed previously that binding of primary anti-HA and secondary Abs do not alter CFTR turnover (Sharma et al., 2004). Finally, we confirmed that endogenous transferrin and EGF receptors (TfR and EGFR) also undergo apical transcytosis in CFBE cells (Fig. 2E) despite the fact that they display highly polarized expression at the basolateral PM. Jointly, these results suggest that CFTR belongs to a handful of PM proteins [e.g. TfR, aquaporin 2 (AQP2) and the Cu-ATPase] whose polarized missorting is ‘corrected’ by transcytosis.
Contribution of biosynthetic and endocytic missorting to CFTR basolateral delivery
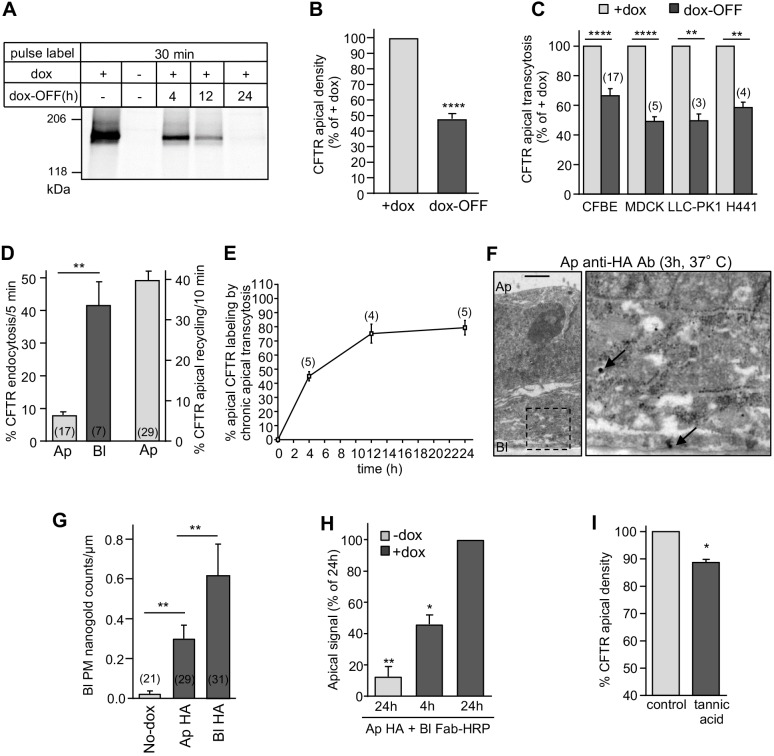
Basolateral delivery of newly synthesized CFTR may occur from the endoplasmic reticulum by non-conventional trafficking (Gee et al., 2011; Yoo et al., 2002), the TGN (Swiatecka-Urban et al., 2002) and/or, in principle, from the apical PM via basolateral (apical-to-basolateral) transcytosis. To evaluate the biosynthetic pathway contribution to CFTR basolateral delivery, apical transcytosis was measured following termination of CFTR transcription/translation. We confirmed by metabolic pulse-labeling that after 24 h dox washout (dox-OFF), CFTR translation was completely inhibited (Fig. 3A). This resulted in CFTR clearance of the biosynthetic pathways and a 52±3.3% reduction in channel apical density (Fig. 3B). Considering that the CFTR transcytosis rate (expressed as percentage of its PM density) is independent of the apical expression of the channel (Fig. S2C), the 33.6±4.8% attenuation of CFTR transcytosis in dox-OFF CFBE (Fig. 3C) can be attributed to the lack of basolateral CFTR delivery from the TGN. CFTR apical transcytosis was similarly reduced (∼40–50%) after translational arrest in polarized kidney (MDCK, LLC-PK1) and airway (H441) epithelial cells (Fig. 3C), underscoring cell-type-independent biosynthetic polarized missorting of the channel.
Fig. 3.
Apical transcytosis retrieves basolaterally missorted CFTR from the biosynthetic and apical recycling pathways. (A) Onset of CFTR translational arrest in dox-OFF CFBE. Translational inhibition was measured by metabolic pulse labeling with 35S-metionine/35S-cysteine (30 min) after 0–24 h of dox-OFF (i.e. after washout) by fluorography. (B,C) CFTR apical density in CFBE cells (n=12) (B) and apical transcytosis after 24 h of dox-OFF in the indicated epithelial cells (C). (D) CFTR endocytosis and recycling in CFBE cells monitored by PM-ELISA (two-tailed unpaired t-test). (E) Apical CFTR labeling after basolateral-to-apical transcytosis. CFTR was labeled by continuous basolateral anti-HA capture at 37°C. Apical CFTR-Anti-HA Ab complexes were measured by PM-ELISA. (F,G) CFTR basolateral transcytosis detection by immuno-EM in CFBE cells after apical capture of anti-HA Ab (3 h, 37°C). The micrograph on the right is a higher magnification of the selected area. Arrows, CFTR-anti-HA-nanogold-anti-mouse complex. Scale bar: 1 µm. (G) Quantification of the number of nanogold particles at the basolateral PM after apical or basolateral anti-HA Ab uptake (data extracted from Fig. 2C, n=2–3 independent experiments, Mann–Withney U-test). (H) ‘Round-trip’ CFTR transcytosis was monitored by simultaneous exposure of CFBE to anti-HA and anti-mouse Fab–HRP in the apical and basolateral compartment, respectively, for 4 h or 24 h. Transcytosed CFTR was quantified by PM-ELISA (n=3). (I) Inhibition of transcytosis accelerates apical CFTR turnover. After blocking newly synthesized CFTR arrival (dox-OFF, 24 h), CFBE cells were exposed basolaterally to 0.5% tannic acid (15 min, 37°C) or mock treatment. Apical CFTR density was measured after 1 h incubation at 37°C by PM-ELISA (n=3) and expressed as a percentage of control. Ap, apical; Bl, basolateral. Data are means±s.e.m. on each panel, parentheses indicate the number of independent experiments (C–E) or regions of interest (G). *P<0.05, **P<0.01, ****P<0.0001.
Under steady-state conditions, we detected only a small fraction of the total CFTR–HRP (2.4±0.6%) or CFTR–3HA (3.3±0.4% in CFBE, 0.5±0.2% in CR-HBE and 2.7±1.1% in LLC-PK1) expression at the basolateral PM by assessing HRP activity and PM ELISA, respectively (Fig. S3A). The modest steady-state basolateral density of CFTR can be attributed to the concerted result of the surprisingly rapid basolateral (41.8±7.3%/5 min) and the >5-fold slower apical (8.0±1.0%/5 min) internalization rate, in concert with efficient apical endocytic recycling (40±2.2%/10 min) of CFTR (Fig. 3D). These processes are complemented by CFTR constitutive apical transcytosis to preserve the channel polarity, as substantiated by the observation that at least 75% of the apical CFTR pool could be labeled with anti-HA Ab from the basolateral compartment after 12 h incubation (Fig. 3E).
Based on our previous results, we inferred that, of the basolaterally delivered and then apically transcytosed CFTR, ∼33% originates from the TGN (Fig. 3B) and ∼66% comes from the apical PM/endosomes (Figs 1D and 2A) through apical-to-basolateral transcytosis. To visualize CFTR apical-to-basolateral transcytosis, the channel was apically labeled by anti-HA Ab capture (3 h, 37°C), and followed by immunoelectron microscopy (immuno-EM). Apically endocytosed CFTR–Ab complex was detectable at the basolateral PM (Fig. 3F,G).
If CFTR is basolaterally missorted from apical endosomes, a ‘round-trip’ transcytosis of apically labeled CFTRs should be observed. To this end, we simultaneously exposed CFTR-expressing CFBE cells to apical anti-HA Ab and basolateral HRP-conjugated secondary Fab at 37°C. We monitored the time-dependent appearance of the HRP activity at the apical PM (Fig. 3H). The results confirmed that apical CFTR-anti-HA Ab complexes successively underwent apical-to-basolateral targeting, association with HRP–Fab, and then basolateral-to-apical transcytosis.
Jointly, these results suggest that constitutive apical transcytosis preserves the CFTR apical polarity, which would be compromised in the absence of trancytosis. To test this conjecture, we inhibited cargo delivery to and retrieval from the basolateral PM by short tannic acid (TA) crosslinking (15 min, 37°C) (Polishchuk et al., 2004). Basolateral TA exposure accelerated the loss of apical CFTR to 11.3±1.2%/h as compared to control (∼5.5%/h) in dox-OFF CFBE cells (Fig. 3I). This translates to a reduction of CFTR half-life from ∼12.4 h to ∼5.8 h, assuming an exponential decay kinetic in the absence of apical transcytosis, and underscores the significance of transcytosis in CFTR apical PM stability.
The CFTR transcytotic route in airway epithelia cells
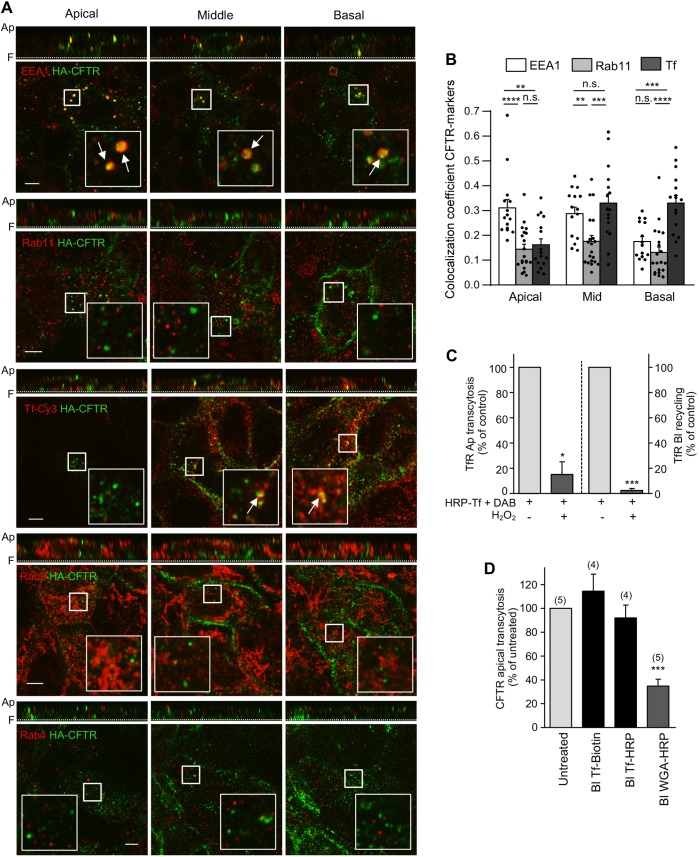
To delineate the transcytotic route of CFTR in CFBE cells, the channel was basolaterally labeled with anti-HA Ab (45 min, 37°C), while HA detection at the cell surface was prevented with anti-mouse Fab. CFTR localization was then assessed by examining colocalization with organelle markers for ASEs (EEA1) and AREs (Rab11, herein referring to both Rab11a and Rab11b), as well as CREs and BSEs (Tf), by indirect immunostaining. Quantification of the colocalization between transcytotic CFTR and these organelle markers in the 3D-volume of z-stacks was evaluated in three volume clusters, representing the basal, middle and apical third volume of epithelia. The results show that basolaterally labeled CFTR colocalized with Tf in basal and middle layers, and with EEA1 in middle and apical layers of the cells, while it marginally colocalized with Rab11 throughout the cells (Fig. 4A,B).
Fig. 4.
Apical transcytotic route of CFTR in polarized CFBE. (A) Immunocolocalization of transcytotic CFTR. CFTR was labeled by basolateral anti-HA Ab capture (45 min, 37°C), while staining of apically delivered CFTR-anti-HA complexes were blocked with goat anti-mouse Fab. Then, the colocalization of transcytotic CFTR–anti-HA (green) with EEA1, Rab11, Rab4 or Rab8 (red) was determined by indirect immunostaining and laser confocal fluorescence microscopy at the apical, middle, and basal planes of filter-grown CFBE cells (lower panels). Vertical optical sections are shown as top panels. TfR was labeled by means of Cy3–Tf uptake (45 min). Inserts represent a ∼3.5-fold magnification of the selected area. Ap, apical PM; F, filter. Arrows indicate colocalization. (B) Quantitative colocalization of transcytosed CFTR with organellar markers. The Manders' colocalization coefficient was calculated on each slice of 3D-volumes [n=15 (EEA1), 20 (Rab11) and 16 (Tf) z-stacks from three independent experiments] that were divided into three approximately equal sections (apical, middle and basal). Two-tailed unpaired t-test. (C) Functional inactivation of TfR-containing endosomes. Tf–HRP was basolaterally loaded (30 min, 37°C) and incubated with H2O2 and DAB or DAB alone (control). Apical (Ap) transcytosis or basolateral (Bl) recycling of Tf–HRP was measured as described in the Materials and Methods and expressed as percentage of control (n=3). (D) CFTR apical transcytosis is largely independent of the TfR trafficking route but intersects with BSEs. Following functional ablation of Tf–HRP- or HRP–WGA-loaded endosomes, CFTR apical transcytosis was measured for 3 h at 37°C. Tf–biotin was used as control. Parentheses indicate the number of independent experiments. Data are means±s.e.m. on each panel. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001; n.s., not significant.
After 1 h of apical labeling, CFTR preferentially accumulated in EEA1+ endosomes and, after a 3 h chase, showed modest colocalization with Rab11. This is consistent with the longer residency time for internalized CFTR in sorting rather than recycling endosomal compartments (Fig. S3B–E). We could not resolve significant colocalization of CFTR with the fast recycling endosome marker Rab4a or Rab8a (Fig. 4A). This is compatible with the model that basolaterally retrieved CFTR enters a TfR-containing compartment but segregates from this compartment and primarily accumulates in EEA1+ endosomes en route to the apical PM. To verify functionally whether transcytotic CFTR traverses TfR-containing endocytic compartments, basolateral endosomes were loaded with HRP–Tf (30 min) to target the CREs (and to a much lesser extent the BSEs) and these compartments were functionally inactivated by HRP-mediated cross-linking (Cresawn et al., 2007; Henry and Sheff, 2008). This previously validated protocol efficiently abrogated basolateral recycling and apical transcytosis of TfR but failed to alter CFTR transcytosis (Fig. 4C,D). In contrast, ablation of BSEs by using HRP-conjugated wheat germ agglutinin (HRP–WGA) profoundly decreased CFTR transcytosis (Fig. 4D). Overall, these results indicate that basolaterally internalized CFTRs predominantly accumulate in EEA1+ ASEs and segregate from TfR+ CREs and Rab11+ AREs for apical transcytosis.
Missorting of internalized CFTR for basolateral transcytosis
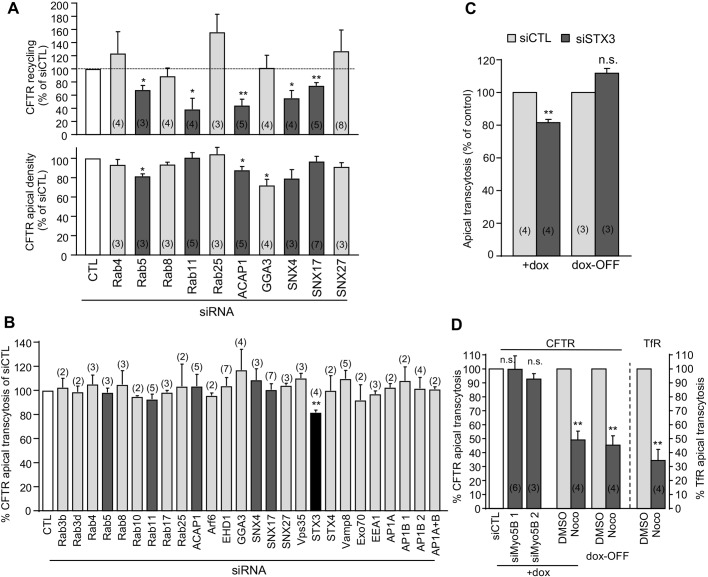
To confirm CFTR transcytotic itinerary in CFBE cells and assess the origin of CFTR-loaded apical-to-basolateral transcytotic vesicles, we knocked down putative or proven regulators of CFTR endocytic trafficking by means of siRNA (Fig. S4A,B). Although ablation of Rab5a and Rab11 affected CFTR recycling (Gentzsch et al., 2004) (Fig. 5A, top; Fig. S4C), they failed to influence CFTR transcytosis (Fig. 5B). Similar results were observed after ablation of SNX4, a Rab11-interacting protein, ACAP1, a coat protein associated with recycling endosomes, and SNX17, which have been implicated in E-cadherin, Glut4 and integrin, but not in CFTR recycling (Li et al., 2007; Solis et al., 2013; Steinberg et al., 2012) (Fig. 5A,B). CFTR transcytosis also remained unaltered upon Rab4a and Rab8a depletion, confirming our immunostaining results (Figs 4A and 5B). These results suggest that basolateral missorting of internalized CFTR occurs before its Rab5-dependent entry into the ASEs and confirm that CFTR apical transcytosis is independent of the Tf+ CREs and Rab11+ AREs in CFBE, in contrast to other transcytosed cargoes (e.g. pIgR), which transit the CREs and AREs prior to exocytosis (Jerdeva et al., 2010).
Fig. 5.
Modulation of CFTR transcytosis. (A,B) CFTR recycling, apical density and transcytosis were measured in CFBE cells silenced for the indicated protein. Results are expressed as a percentage of that seen with the control siRNA (CTL). (C) STX3 may contribute to the basolateral missorting of newly synthesized CFTR. CFTR apical transcytosis was measured after 24 h dox-OFF (i.e. after washout) in CFBE cells transfected with control (CTL) or STX3 siRNAs (siSTX3). (D) CFTR apical transcytosis is MT dependent. CFTR and TfR transcytosis was determined in CFBE cells transfected with control (CTL) or Myo5B-specific siRNAs (siMyo5B 1 or 2) or pretreated for 30 min and during transcytosis with 33 µM nocodazole (Noco) or DMSO. Data are means±s.e.m. on each panel, parentheses indicate the number of independent experiments. *P<0.05; **P<0.01; n.s., not significant.
Only syntaxin 3 (STX3) knockdown decreased CFTR apical transcytosis significantly (Fig. 5B; Fig. S4A,B). Depletion or mutation of the apical targeting signal of this t-SNARE enhances basolateral mistargeting of NHE3 and GFP-tagged p75 (neurotrophin receptor) (Sharma et al., 2006; Vogel et al., 2015) but did not induce a global inversion of epithelial (Vogel et al., 2015) or CFTR (Fig. S4D) polarity. Interestingly, a fraction of STX3 was localized at the basolateral PM and its retrieval through an ubiquitin-dependent mechanism facilitates the recruitment of cargoes into apical exosomes (Giovannone et al., 2017). Considering that biosynthesis arrest abrogated the effect of STX3 siRNA on CFTR transcytosis (Fig. 5C, dox-OFF), we suggest that STX3, besides controlling CFTR apical exocytosis (Collaco et al., 2010), might be involved in the delivery and/or retrieval of basolaterally targeted channels from the biosynthetic pathway.
CFTR transcytosis requires microtubules
To test the contribution of myosin VB (Myo5B) and microtubules (MTs), which are important proteins for transport of transcytotic cargoes (Jerdeva et al., 2010; Tzaban et al., 2009), MTs were disrupted with nocodazole and Myo5B expression was silenced by means of siRNA. While Myo5B knockdown modestly decreased CFTR apical expression as a consequence of reduced entry of apically endocytosed channel into AREs (Swiatecka-Urban et al., 2007), it failed to interfere with apical transcytosis (Fig. 5D; Fig. S4A,E). Nocodazole, however, profoundly decreased CFTR transcytosis, similar to what was observed for TfR (Fig. 5D; Fig. S4F). This cannot be attributed to the impeded biosynthetic basolateral transport of CFTR, as comparable inhibition of transcytosis (54.7±6.7% versus 50.9±6.3%) was observed in dox-OFF cells (Fig. 5D; Fig. S4G). Considering that the channel endocytosis and recycling rates remained unaltered upon MT disruption (Fig. S4H), the decreased CFTR apical expression could be attributed to its impeded transcytosis (13.4±4% in 3 h) (Fig. S4I and Fig. 5D).
Transcytosis counteracts CFTR missorting upon disruption of the interaction with PDZ proteins
N- and O-glycans, glycosylphosphatidylinositol (GPI) anchors, and cytosolic and transmembrane segments have been identified as apical sorting determinants (Cholon et al., 2009; Kundu et al., 1996; Lisanti et al., 1989; Potter et al., 2006; Sharma et al., 2006). Basolateral targeting signals may partly overlap with internalization motifs of transmembrane cargoes, represented by tyrosine- (YxxΦ, NPxY, where Φ represents a hydrophobic amino acid) and di-leucine-based (D/ExxxLL) motifs (Stoops and Caplan, 2014).
To identify targeting motifs that may influence CFTR transcytosis efficacy, we inactivated three potential polarized sorting signals (Fig. 6A): first, three di-leucine and tyrosine-based endocytic motifs (Hu et al., 2001) were mutated to alanine residues (K3-CFTR); second, two N-linked glycosylation sites (N894D- and N900D-CFTR) were inactivated in the fourth extracellular loop; and, finally, considering the role of PDZ proteins in apical targeting, tethering and recycling of CFTR, we truncated the PDZ-binding motif (DTRL) by eliminating the last six residues (Δ6-CFTR) at the C-terminus. All of these CFTR variants were stably expressed in CFBE cells (Fig. S5A).
Surprisingly, apical transcytosis of Δ6-CFTR, but not K3- or N900D-CFTR, was increased by 49.5±7.8%, suggesting that the Δ6 mutation compromised the fidelity of CFTR apical biosynthetic targeting, endocytic recycling or both processes (Fig. 6B). The comparable expression level of Δ6- and wild-type (WT)-CFTR–3HA and the inability of Δ6-, but not WT-CFTR to associate with the GST–NHERF1 fusion protein were confirmed (Fig. S5B,C). If the amplified basolateral arrival Δ6-CFTR was caused by increased missorting at the TGN, inhibition of biosynthetic secretion would affect its apical transcytosis by a greater extent than for WT-CFTR. However, we found that transcytotic flux of Δ6-CFTR, in contrast to that of WT-CFTR, was insensitive to translation inhibition (Fig. 6C, dox-OFF), which suggests that the amplified basolateral arrival of Δ6-CFTR likely emanates from augmented missorting at apical endosomes and not from the biosynthetic secretion. This inference is supported by the increased apical internalization (18.3±3.1 versus 7.4±1.2%/5 min) and impeded recycling (20.5±1.8 versus 35.3±2.0%/10 min) of Δ6-CFTR relative to WT-CFTR (Fig. 6D,E), which both increase the intracellular pool of the mutant channel. These data are also in line with CFTR tethering to the subapical actin cytoskeleton and enhanced channel recycling by the PDZ domain-containing NHERF proteins (Haggie et al., 2004; Swiatecka-Urban et al., 2002), as well as with the ∼1.5-fold increased basolateral density of Δ6-CFTR (Fig. 6F). The accelerated basolateral transcytosis of Δ6-CFTR mirrors the stimulated apical transcytosis of TfR upon inhibition of its basolateral recycling after AP-1B ablation (Perez Bay et al., 2013). The apical transcytosis of Δ6-CFTR was also augmented in MDCK and LLC-PK1 cells (Fig. 6G).
Remarkably, Δ6-CFTR displayed similar biochemical and functional apical PM expression to WT-CFTR despite its accelerated internalization and impeded apical recycling, as measured through immunostaining, PM ELISA and short-circuit current (Isc) determination, respectively (Fig. 6H,I). Finally, we could not resolve a detectable difference in the PM turnover of Δ6- and WT-CFTR by PM-ELISA or a biotinylation assay (Fig. 6J; Fig. S5D), suggesting that 2-fold augmented round-trip transcytosis maintains the WT-like apical PM stability of Δ6-CFTR and highlights the sorting capacity and significance of transcytotic pathway to preserve CFTR polarity.
NHERF1 association attenuates CFTR basolateral missorting and transcytosis
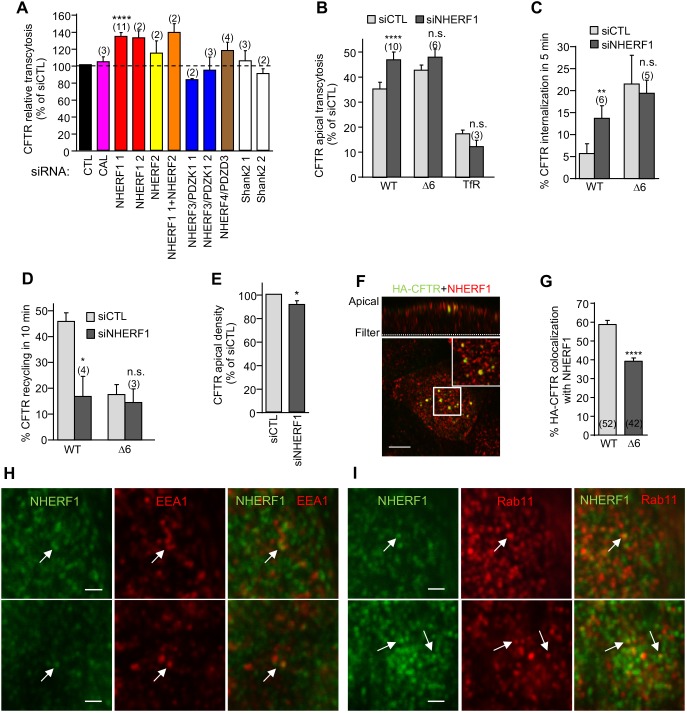
To search for additional proteins that may contribute to the recycling fidelity of apically internalized CFTR, the consequence of siRNA-mediated depletion of CFTR-interacting PDZ domain proteins was measured (Figs S4B and S5F). The apical transcytosis of WT- but not Δ6-CFTR or TfR was increased only by NHERF1 knockdown (Fig. 7A,B). NHERF1 siRNA accelerated the internalization and inhibited the recycling of WT-, but not Δ6-CFTR, at a moderately altered CFTR apical density (Fig. 7C–E), consistent with previous reports (Benharouga et al., 2003; Ostedgaard et al., 2003). Finally, we showed that NHERF1 was partly colocalized with internalized CFTR in subapical vesicular compartments (Fig. 7F,G; Fig. S5G,H), as well as with the EEA1+ ASEs and, to a lesser extent with the Rab11+ AREs (Fig. 7H–I). These results are consistent with the physiological relevance of the NHERF1–CFTR interaction in the channel apical retention and endosomal recycling via the ASEs/AREs compartments in CFBE cells, and provide a plausible explanation for the augmented basolateral missorting seen for CFTR upon preventing NHERF1–CFTR association, either by CFTR C-terminal truncation or NHERF1 ablation.
Fig. 7.
NHERF1 ablation phenocopies the Δ6-CFTR cellular phenotype. (A) CFTR transcytosis was assayed after siRNA-mediated knockdown of the indicated PDZ proteins in CFBE and expressed as the percentage of that seen with the control siRNA (CTL). (B–E) NHERF1 silencing mirrors the effect of the PDZ-binding motif truncation of CFTR. CFBE cells were transfected with control siCTL or NHERF1 1 siRNA and then assayed for (B) CFTR and TfR transcytosis following 3 h basolateral anti-HA or Tf-HRP capture, (C) CFTR internalization, (D) CFTR recycling, (E) CFTR apical PM density (n=10), measured as described in the Materials and Methods. (F) NHERF1 colocalizes with the apical endocytosed CFTR. CFTR was labeled by anti-HA capture (1 h, 37°C) from the apical compartment. Residual CFTR apical PM was blocked with goat anti-mouse Fab on ice. NHERF1 (red) and CFTR (green) were visualized by indirect immunostaining and laser confocal fluorescence microscopy in permeabilized cells. The upper panel represents a z-section. The insert is a 4-fold magnification of the indicated area. Scale bar: 5 µm. (G) Quantification of colocalization between NHERF1 and subapical endocytosed WT- and Δ6-CFTR in CFBE cells (two-tailed unpaired t-test). (H,I) NHERF1 can localize to both ASEs and AREs. Fixed and permeabilized filter-grown CFBE cells were stained for NHERF1 (green) and EEA1 (red, H) or Rab11 (red, I). Arrows indicate colocalization. Scale bars: 1 µm. Data are mean±s.e.m. on each panel, parentheses indicate the number of independent experiments (A–D) or number of regions of interest from two independent experiments (G). *P<0.05; **P<0.01; ****P<0.0001; n.s. not significant.
DISCUSSION
Here, we report that apical and basolateral transcytosis represent previously unrecognized membrane trafficking pathways that significantly contribute to the development and maintenance of CFTR apical polarity, as well as the phenotypic suppression of CFTR C-terminal truncations in multiple epithelial cells, including immortalized and primary human airway cells. We show that CFTR apical transcytosis and highly efficient basolateral internalization, jointly, play a role in counteracting the constitutive basolateral missorting of the channel and maintaining a ∼30-fold higher amount of CFTR at the apical than at the basolateral PM in CFBE cells (Figs S5E and S6). The physiological significance of CFTR apical transcytosis is illustrated by the >2.1-fold reduction of its half-life in conditions with preserved apical internalization but inhibition of basolateral retrieval. CFTR basolateral accumulation in the absence of apical transcytosis would decrease apical Cl− secretion by reducing basolateral Cl− entry, causing attenuated coupled water secretion and mucociliary clearance of airway epithelia (Ballard et al., 2002; Farmen et al., 2005).
By taking advantage of a Tet-ON-inducible CFTR expression system and the relatively rapid termination of CFTR biosynthesis, while maintaining ∼50% of the mature CFTR pool, we show that ∼65% of the basolaterally delivered CFTR originates from apical endocytic vesicles via reversed transcytosis. A similar phenomenon prevails for endosomal missorting of TfR and EGFR at basolateral endosomes (Cotton et al., 2013; Gravotta et al., 2007). In addition, missorting of newly synthesized CFTR from the TGN fuels ∼35% of the basolateral channel delivery, likely in a STX3-dependent manner. Interestingly, the contribution of apical transcytosis to CFTR polarity and the basolateral channel expression varies between epithelia, suggesting cell-specific alterations in the fidelity of CFTR polarity maintenance. Notably, the reduced rate of CFTR transcytosis in MDCK cells and the limited efficiency of CFTR biotinylation relative to an ELISA-based assay may offer an explanation for the previously undetected transcytosis of CFTR or EGFR (Cotton et al., 2013; Swiatecka-Urban et al., 2002).
Based on colocalization and functional endosomal ablation studies, we propose that basolaterally internalized CFTR enters transcytotic endosomes (TEs), which are EEA1+ and show reduced TfR content, that fuel the transcellular migration of CFTR in a MT-dependent, but Myo5B-independent, pathway (Fig. S6). Several observations suggest that CFTR apical transcytosis has some unique characteristics in CFBE cells. First, unlike basolateral sorting of TfR, which is thought to proceed at CREs (Gravotta et al., 2012), commitment of CFTR to apical recycling likely takes place before its Rab5-mediated entry into ASEs. This inference is supported by the observation that accelerated endocytosis of CFTR lacking its PDZ-binding motif diplays enhanced basolateral targeting and subsequent apical transcytosis, whereas silencing of proteins along CFTR endocytic sorting (Rab5, EEA1 or SNX17) or recycling (Rab11, ACAP1, or SNX4) pathways failed to do so. Second, the results of CFTR mutagenesis and siRNA screens showed that transcytosis of CFTR in CFBE cells is independent of N-glycans, AP-1B, Rab3b, Rab17, Rab25 and the exocyst complex, which are essential for the apical transcytosis of pIgR, TfR and FcRn (Hunziker and Peters, 1998; Nelms et al., 2017; Perez Bay et al., 2013, 2014; Tzaban et al., 2009; van IJzendoorn et al., 2002). Finally, while we cannot rule out a small contribution of the AREs (Fig. 4A,B), transcytotic CFTR seems to mostly avoid the CRE and ARE compartments that are commonly used by other transcytotic cargoes (Jerdeva et al., 2010; Lalioti et al., 2016).
Interestingly, constitutive TfR transcytosis appears to preferentially bypass the Rab11+ AREs in CFBE cells, which express AP-1B (Figs S7A–C and S4J), similar to that of the neonatal Fc receptor in MDCK cells (Tzaban et al., 2009). Hence, it is plausible that both the transcytotic and endocytic/recycling pathways for CFTR and other cargoes interconnect at a common compartment with sorting capacity for direct or indirect cargo targeting through the AREs prior to exocytosis in certain epithelia, as proposed for the TfR in MDCK cells (Perez Bay et al., 2013). These alternative, but parallel, pathways may be able to compensate for each other activity upon loss of function, which would prevail for the EEA1-independent CFTR transcytosis (Fig. 5B). The activation of an alternative slow recycling pathway may also explain the surprising inability of Rab11 silencing to reduce the apical density of CFTR, in contrast to what is seen with Rab5a or ACAP1 depletion, despite inhibition of the channel endocytic recycling (Fig. 5A), which is consistent with the operation of alternative recycling pathways described for CFTR in other epithelia (Saxena et al., 2006; Silvis et al., 2009; Swiatecka-Urban et al., 2005, 2007). In addition, the combined effect of the lysosomal and basolateral targeting of some internalized CFTRs and the short duration of the recycling assay (10 min), to minimize re-internalization of exocytosed channels, are jointly responsible for the underestimation of CFTR recycling efficiency in our assay. This inference is in line with the observation that Rab11 silencing only enhances CFTR intracellular accumulation by 10% after 30 min (Fig. S4C and Materials and Methods). Improved assay sensitivity, permitting shorter recycling time and correction for apical CFTR re-internalization, will be necessary to achieve a more-precise assessment of the role of Rab11 in CFTR trafficking in CFBE cells.
Comparison of the exocytotic machinery of apical CFTR in relation to other transcytotic cargoes derived from biosynthetic, recycling and transcytotic pathways of airway epithelia remains to be investigated. Intriguingly, we were unable to detect a significant reduction of CFTR apical transcytosis and PM density upon ablation of Myo5B, which has been implicated in maintaining CFTR apical expression along recycling and secretory pathways in CFBE and CaCo2 cells (Swiatecka-Urban et al., 2007; Vogel et al., 2015). Recent studies were also unable to detect profound changes in functional CFTR expression in intestinal epithelia cells expressing an inactive Myo5B or following Myo5B knockdown (Kravtsov et al., 2016). Furthermore, Myo5B-independent apical polarization of CFTR is in line with the augmented Cl− secretion seen in microvillus inclusion disease, which is caused by loss of function of Myo5B (Kravtsov et al., 2016).
Our results expand the established role of NHERF1 in CFTR traffic at multiple cellular locations. It is known that by tethering CFTR to the subapical cytoskeleton, NHERF1 enhances the channel apical retention (Haggie et al., 2004; Swiatecka-Urban et al., 2002). Importantly, CFTR retention at basolateral PM is compromised as a consequence of modest expression level of NHERFs, ezrin and cortical actin (Reczek et al., 1997; Short et al., 1998), accounting for the unprecendently fast CFTR internalization, ensuring its low basolateral PM density. Here, we provide evidence for enhanced colocalization of NHERF1 with the endocytic pool of WT-CFTR relative to that of Δ6-CFTR (Fig. 7F,G), supporting the paradigm that NHERF1-mediated sorting directs the channel towards recycling at subapical endosomes (ASEs and/or AREs) (Cardone et al., 2015; Cushing et al., 2008). Finally, we demonstrate that PDZ motif elimination has a negligible influence on CFTR apical expression, transport activity and stability. Increased CFTR basolateral internalization and apical transcytosis flux largely offset the accelerated removal of the channel and decreased recycling at the apical PM. These results expand on previous observations obtained on CFTR variants with compromised binding for PDZ proteins (Benharouga et al., 2003; Ostedgaard et al., 2003) and offer a plausible explanation for the isolated elevation of sweat Cl− concentration in the absence of pancreatic and lung phenotype in individuals harboring a single copy of CFTR lacking the C-terminal 26 residues (S1455X-CFTR) (Mickle et al., 1998). Finally, increased apical transcytosis of CFTR may also explain the mild CF cellular phenotype (Ameen et al., 2007) of the naturally occurring N287Y-CFTR mutant, exhibiting accelerated apical internalization (Silvis et al., 2003).
Whether transcytotic CFTR routes are affected by CF-causing mutations and can influence a non-native channel degradation via ubiquitin-dependent lysosomal targeting from the PM, such as ΔF508 (Okiyoneda et al., 2018), remains to be explored. Interestingly, the CF-causing folding mutant P67L-CFTR (Bagdany et al., 2017; Sabusap et al., 2016) displays considerably reduced transcytotic flux that can be restored to that of WT upon folding correction with VX-809 (data not shown). Intriguingly, overexpression of NHERF1 or impeding lysosomal CFTR by ablation of CFTR-associated ligand (CAL, also known as GOPC), partly rescues the PM expression of ΔF508 (Guerra et al., 2005; Wolde et al., 2007). Considering that deletion of the PDZ motif decreased CFTR biosynthetic delivery to the basolateral PM (Fig. 6C, dox-OFF), we speculate that NHERF1 overexpression may rescue ΔF508 not only by increasing its retention/recycling at the apical PM but also by promoting its biosynthetic over endocytic basolateral missorting, which may further reduce the lysosomal degradation propensity of the channel upon reducing its basolateral transcytosis.
MATERIALS AND METHODS
Antibodies and reagents
Antibodies used in this study are listed in Table S1. Nocodazole, sodium 2-mercaptoethanesulfonate (MESNA), 3,3′-diaminobenzidine tetrahydrochloride (DAB), horseradish peroxidase (HRP) and tannic acid were purchased from Sigma-Aldrich. Y-27632 was from Tocris. HRP–Tf and Ultroser-G were purchased at Jackson Immunoresearch and Pall Corporation, respectively.
Cell culture and medium
CFBE, Calu-3 and 3T3-J2 cells are generous gifts from Dieter Gruenert (University of California-San Francisco, USA), John W. Hanrahan (McGill University, Canada) and Bob Scholte (Erasmus MC, Rotterdam, The Netherlands), respectively. LLC-PK1 and NCI-H441 were purchased from the ATCC. MDCKII was described before (Benharouga et al., 2003; Veit et al., 2012). CFBE cells were propagated in minimal essential medium (MEM) supplemented with fetal bovine serum (FBS), L-glutamine and HEPES (Invitrogen) on coated plastic flasks as described previously (Veit et al., 2012) and were seeded and differentiated for ≥3 days on coated plastic wells or polyester permeable supports (Transwell filters, Corning). Both MDCKII and LLC-PK1 cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 10% FBS, and NCI-H441 cells were cultured in RPMI-1640 (Gibco) supplemented with 10% FBS and 10 mM Hepes, and were differentiated on polyester permeable supports for >3 days post-confluence. In these cell types, CFTR displayed a similar polarized expression at the apical PM (Benharouga et al., 2003; Veit et al., 2012) (Fig. S3A). Human lung adenocarcinoma Calu-3 cells were cultured in DMEM/F12 (Invitrogen) supplemented with 10% FBS. All cells were maintained in a 37°C incubator under 5% CO2. CFBE, MDCK, NCI-H441 and LLC-PK1 cell lines expressing inducible WT- and Δ6- CFTR with a 3HA or HRP tag were generated using the ClonTech pLVX-Tight-Puro lentivirus technology, as described previously (Veit et al., 2014, 2012, 2018) and induced with 250–500 ng/ml dox. MDCKII cells, stably expressing WT- or Δ6-CFTR-3HA, were generated by transduction with retroviral particles (Benharouga et al., 2003).
CR-HBE characterization and expression of CFTR-3HA
Primary human bronchial epithelial cells (HBE) cells were isolated in Walter E. Finkbeiner's laboratory under approval from the University of California, San Francisco Committee on Human Research as well as purchased from the CF Canada Primary Airway Cell Biobank at McGill University. For both primary HBE sources, informed consent was obtained for all lung tissue donors and all clinical investigation was conducted according to the principles expressed in the Declaration of Helsinki. HBE were conditionally reprogrammed and differentiated according to a modified protocol of Liu et al. (Liu et al., 2012). Briefly, HBE cells were cultured on irradiated 3T3-J2 fibroblasts in proliferation F-Medium (Liu et al., 2012) with 10 µM of ROCK inhibitor Y-27632. After expansion, cells were plated on collagen IV-coated transwell filters and differentiated in Ultroser-G medium (Neuberger et al., 2011) at the air–liquid interface for at least 4 weeks with the basolateral medium being changed every 2-3 days. CR-HBE cells constitutively expressing WT-CFTR–3HA were developed by transducing the cells with lentiviral particles encoding the WT-CFTR–3HA in pTZV4-CMV-IRES-puro (Open Biosystems) as described previously (Veit et al., 2012) during proliferation, followed by 2 days of puromycin selection before seeding on filter supports. The Air Surface Liquid (ASL) height was determined after staining the ASL with 2 mg/ml Tetramethylrhodamine-conjugated dextran (D1819, 70,000 MW, Neutral, Invitrogen), dispersed in a low boiling point perfluorocarbon (Fluorinert FC-72, boiling point 56°C, 3 M Company) and the epithelia with Cell tracker (Invitrogen). The ASL height was determined in 3–10 areas of two filters using a Zeiss700 upright fluorescence laser confocal microscope equipped with an environmental chamber at 37°C and 5% CO2.
CFTR cell surface ELISA and transcytosis assay
CFTR apical density and apical transcytosis were measured by performing PM-ELISA. Cells were incubated with anti-HA antibody (1:1000) in the apical (apical density) or basolateral (transcytosis) compartment, respectively, at 37°C for 0.5–4 h. After washing of the apical PM with ice-cold PBS (Gibco) supplemented with 1 mM MgCl2 and 0.1 mM CaCl2 (PBSCM), all the cells were incubated apically with HRP-conjugated anti-mouse-IgG or Fab (1:1000) in PBSCM with 0.5% bovine serum albumin (PBSCM-BSA) (1 h, on ice). Cells were washed with PBSCM and the HRP activity in the apical compartment was determined in the presence of Amplex Red fluorogenic substrate (Invitrogen) at 544 nm excitation and 590 nm emission wavelengths, using a POLARstar OPTIMA (BMG Labtech) or a Tecan Infinite M1000 (Tecan Group) fluorescence plate reader. CFTR apical transcytosis was expressed relative to its apical density, measured in parallel [percentage Ap transcytosis=(Ap signaltranscytosis/Ap signalapical density)×100]. Non-specific Ab binding was determined by using either non-expressing CFBE cells or replacing the anti-HA with non-specific mouse IgG under the same experimental conditions. EGFR transcytosis was measured by using the same PM-ELISA assay, with anti-EGFR instead of anti-HA antibody. To monitor TfR apical transcytosis, endogenous Tf was first depleted in serum-free medium (1 h), before incubation with HRP–Tf or HRP (both 5 µg/ml) in the apical or basolateral compartment. After 3 h, the peroxidase activity was measured on the apical PM and in the medium. TfR transcytosis was expressed as the signal intensity in the apical compartment relative to that of the steady-state TfR density at the basolateral PM. ‘Round-trip’ transcytosis was measured by incubating the cells simultaneously with mouse anti-HA antibody (1:1000) in the apical chamber and with HRP-conjugated anti-mouse-Fab (1:1000) (Jackson Immunoresearch) in the basolateral chamber for 4 to 24 h at 37°C. Cells were then extensively washed with ice-cold PBSCM and assessed for HRP activity in the apical compartment.
CFTR internalization and recycling assays
The CFTR internalization rate was measured by determining the rate at which CFTR–Ab complexes disappeared from the apical PM by ELISA, as described previously (Glozman et al., 2009). Apical CFTR was labeled with anti-HA (1:1000) for 1 h on ice. After washing with ice-cold PBSCM, one plate was incubated for 5 min at 37°C (denoted P5′), while the control plate was kept on ice (denoted P0). The amount of CFTR remaining at the PM was determined using HRP-conjugated secondary Ab and Amplex Red in both plates. The internalization rate was expressed as the percentage of the initial amount of CFTR removed in 5 min [percentage CFTR internalization=(P0−P5′)×100/P0].
The CFTR recycling rate was assessed by monitoring the exocytosis of internalized CFTR–Ab complexes at the apical PM. To this end, CFTR-expressing CFBE cells were seeded on four plates (P1–P4). After anti-HA binding to apical CFTR (1 h on ice, P1–P4), the CFTR–Ab complex was allowed to internalize for 30 min at 37°C (P2, P3 and P4) while a control plate was kept in cold medium (P1). The anti-HA antibody remaining on the PM was blocked with anti-mouse monovalent Fab (Jackson ImmunoResearch, 1:75, 1 h, on ice, P3 and P4). Recycling of endocytosed CFTR was elicited at 37°C for 10 min (P4), while a blocking efficiency plate was kept in cold medium (P3). All plates were apically incubated with HRP-conjugated anti-mouse-IgG or Fab (1:1000) for 1 h on ice, washed and peroxidase activity was measured. The recycling efficiency in 10 min was expressed as a percentage of the endocytosed pool of CFTR, taking into consideration the blocking efficiency of the anti-mouse Fab [percentage apical recycling=(P4−P3)×100/(P1−P2)]. In addition, we also took into consideration that the recyclable endosomal CFTR pool was slightly reduced due to missorting of internalized CFTR to the basolateral PM (∼5.6%) and lysosomes (∼5.6%) (Fig. 3D).
Time course of polarized delivery of newly synthesized CFTR–HRP
To measure the kinetics for delivery of newly translated CFTR to the apical and basolateral PM, CFTR–HRP expression was induced for ∼3.5 h at 37°C. Then cells were washed with buffer H (154 mM NaCl, 3 mM KCl, 10 mM Hepes pH 7.8, 1 mM MgCl2, 0.1 mM CaCl2 and 10 mM glucose) and incubated further in buffer H, supplemented with Amplex Red at 37°C. Apical and basolateral media were sampled for Amplex Red fluorescence every 5 min for ∼20 min. Non-induced cells were used to determine the background fluorescence.
Basolateral PM turnover inhibition by tannic acid
The basolateral membrane turnover was inhibited by tannic acid, using a modified protocol of Polishchuk et al. (2004). CFBE cells were treated for 15 min with 0.5% tannic acid in serum-free medium, then washed two times in serum-free medium and once in full medium followed by PM-ELISA. Reversibility of the tannic acid treatment was ascertained by measurement of CFTR–anti-HA complex basolateral uptake after an 8 h chase (data not shown).
Inactivation of endosomal compartments by HRP-mediated ablation
Tf-containing endosomes were functionally ablated as described previously (Cresawn et al., 2007) with the following modifications. Tf-depleted CFBE cells were loaded with HRP–Tf (5 µg/ml) for 30 min (37°C) from the basolateral chamber. Cells were then washed with ice-cold PBSCM and incubated twice for 5 min with 150 mM NaCl and 20 mM citric acid, pH 5.0 to remove cell surface-bound HRP–Tf. WGA-labeled endosomes were functionally inactivated after 20 min internalization of WGA–HRP (10 µg/ml, Sigma) from the basolateral compartment. WGA–HRP remaining at the cell surface was removed with 3×10 min incubation with 100 mM N-acetyl-D-glucosamine. Endosomal functional ablation was induced by initiating crosslinking upon the addition of 0.1 mg/ml DAB and 0.025% H2O2 for 1 h on ice in the dark. The reaction was quenched with PBSCM containing 1% BSA. As controls, DAB or H2O2 were omitted, or Tf–biotin and WGA–Alexa Fluor 647 were used instead of Tf–HRP and WGA–HRP, respectively.
siRNA-mediated inhibition of gene expression
Stealth siRNAs and siRNAs were purchased from Invitrogen, Origene and Qiagen, respectively (Table S2). To limit off-target effects, the siRNAs were used at a final concentration of 25 nM and phenotypic screens were carried out using pools of two to four siRNAs. For filter-based experiments, CFBE cells were electroporated using the Neon Transfection system (Invitrogen) with three 10 ms 1500 V pulses using 100 µl Neon pipette tips according to the manufacturer's protocol. Cells on coated plastic were transfected with Lipofectamine RNAiMax (Invitrogen) according to the manufacturer's protocol and then were polarized for 4 days in presence of dox-induced CFTR expression.
Immunofluorescence microscopy and colocalization
Differentiated filter-grown CFBE cells were fixed in 4% paraformaldehyde and permeabilized with 0.2% Triton X-100. After blocking in PBSCM-BSA, cells were incubated with primary Ab, washed with PBSCM and incubated with Alexa Fluor-conjugated secondary Abs. Extensively washed and cut-out pieces of filters were mounted between a glass slide and coverslip. For monitoring intracellular CFTR colocalization, CFTR was labeled by anti-HA capture at 37°C for the indicated time. When indicated, PM CFTR staining was blocked with anti-mouse Fab prior to fixation (1 h, on ice). TfR was visualized by exposing the cells to Cy3–Tf (20 µg/ml, Jackson Immunoresearch, 45 min–1 h at 37°C) in the basolateral compartment after depletion of the endogenous Tf in serum-free medium (1 h, 37°C). Nuclei were stained with DAPI. Horizontal optical sections (20–30) were acquired using a LSM-710 or LSM-780 LFCM equipped with a Plan-Apochromat 63×/1.40 oil objective (Carl Zeiss), reconstituted using the Zen 2012 software package and representative vertical sections are shown. For colocalization study, the Manders’ colocalization coefficient was calculated using the JACOP plugin of Fiji software (Bolte and Cordelieres, 2006) after background subtraction and thresholding from 3D volumes of z-stacks. Then, the colocalization coefficient of all the slices of a z-stack was sorted in three groups to evaluate the colocalization at the apical, middle and basal volume of the cells.
Monitoring CFTR apical stability by cell surface biotinylation and immunoblotting
Apical surface proteins of filter-grown CFBE cells were biotinylated for 15 min on ice with 1 mg/ml EZ Link sulfo-NHS-SS-biotin (Thermo Fisher Scientific) in buffer H (as above). Excess biotin reagent was quenched with 100 mM glycine in PBSCM. Cells were then shifted to 37°C for 0, 4, 12 or 24 h before being lysed in RIPA buffer (150 mM NaCl, 20 mM Tris-HCl, 1% Triton X-100, 0.1% SDS and 0.5% sodium deoxycholate, pH 8.0) containing protease inhibitor (5 µg · ml–1 leupeptin, 5 µg · ml–1 pepstatin, 500 µM PMSF, all from Sigma-Aldrich). Biotinylated proteins were isolated from postnuclear lysates with streptavidin–agarose beads (Invitrogen) at 4°C with end-over-end rotation. Proteins were eluted with 5× Laemmli sample buffer and visualized by immunoblotting with anti-HA antibody or a mixture of L12B4 and M3A7 mouse monoclonal anti-CFTR Abs followed by either IRDye 800-conjugated anti-mouse-IgG Abs (Licor Biosciences, Lincoln, NE) with the Odyssey Infrared Imaging System (Licor Biosciences) or using enhanced chemiluminescence as described (Benharouga et al., 2001).
Electron microscopy
Filter-grown CFBE cells were incubated with anti-HA antibody (1:500) in the basolateral or apical compartment for 3 h at 37°C. Cells were washed, fixed, permeabilized and incubated with 1.4 nm nanogold-conjugated anti-mouse Fab fragment (1:50) (Nanoprobes, Yaphank, NY) for 1 h on ice. Cell monolayers were then post-fixed with 1% osmium tetroxide in 0.1 M phosphate buffer for 1 h at 4°C. Samples were dehydrated through a series of graded ethanol baths and embedded in epon resin. Semi-thin sections of ∼1 µm were obtained with a diamond knife on an ultramicrotome (Ultracut E, Reichert-Jung) and stained with 1% Toluidine Blue. Then, 60-nm-thick sections were cut and counterstained with 4% uranyl acetate and Reynold's lead citrate. Sections were observed under a Philips CM120 electron microscope equipped with a Gatan digital camera. The number of nanogold particles per micrometer of PM was calculated using the ImageJ software. Post-labeling steps and imaging were performed at the EM facility of the Department of Pharmacology, McGill University.
Short-circuit current measurement
The Isc of CFTR-expressing CFBE cells was measured on cells differentiated on 12 mm Snapwell filters (Corning) mounted in Ussing chambers and bathed in Krebs-bicarbonate buffer (140 mM Na+, 120 mM Cl−, 5.2 mM K+, 25 mM HCO3−, 2.4 mM HPO4, 0.4 mM H2PO4, 1.2 mM Ca2+, 1.2 mM Mg2+ and 5 mM glucose, pH 7.4) at 37°C in the presence of an apical-to-basolateral chloride gradient. To functionally isolate apical PM, the contralateral domain was permeabilized with 100 μM amphotericin B. In each experiment, 100 µM amiloride and 20 µM forskolin were added sequentially to the apical and basolateral side to inhibit the epithelial Na+ channel and activate CFTR, respectively. CFTR activity was inhibited by 20 µM of channel blocker CFTRinh 172 (Tocris Bioscience, Ellisville, MO).
Recombinant NHERF1 purification and pulldown
GST or GST–NHERF1 was expressed in the BL21 Escherichia coli by means of a pGEX-4T plasmid. Bacterial pellets were resuspended in 50 mM Tris-HCl pH 8, 50 mM NaCl, 5 mM EDTA, 0.5% NP-40, 5% glycerol (50 µl/ml) and sonicated. The lysate was centrifuged at 23,700 g (30 min, 4°C) and passed through a Dowex 50X2-400 ion-exchange resin (Acros Organics). The flow through was incubated with glutathione–Sepharose 4B beads (GE Healthcare) for 2 h at 4°C. After three washes, beads were incubated with the post-nuclear supernatant of RIPA lysate (obtained as above) from CFBE cells expressing WT- or Δ6-CFTR for 2 h at 4°C under rotation. After three washes with RIPA medium, co-isolated CFTR was immunoblotted.
Metabolic pulse labeling
Post-confluent filter-grown CFBE cells were incubated with methionine- and cysteine-free α-MEM medium for 45 min at 37°C. Cells were then pulse labeled in the presence of [35S]-methionine and [35S]-cysteine (0.1 mCi/ml; Perkin Elmer, Waltham, MA) from the basolateral compartment for 30 min at 37°C in a humid chamber. After washing with ice-cold PBSCM, CFTR was immunoprecipitated with a mixture of M3A7 and L12B4 anti-CFTR Abs. Following autoradiography, radioactivity incorporated into CFTR was quantified by phosphorimage analysis, using a Typhoon workstation (GE Healthcare).
EndoH and PNGase F digestion of CFTR
CFBE and Calu-3 cells expressing CFTR–3HA and endogenous CFTR were grown on 6-cm coated plastic dishes for 4–5 days post confluency. CFTR expression in CFBE cells was induced by treatment with 250 ng/ml dox for 4 days. Cells were lysed (0.3% Triton X100, 150 mM NaCl, 20 mM Tris-HCl pH 8.0) and after centrifugation (at 4°C, 12,000×rpm for 10 min), the supernatants were digested with EndoH or PNGase F enzyme according to the manufacturer's protocol. Samples were immunoblotted with anti-CFTR antibodies (L12B4, M3A7).
RT-qPCR
For WT- and Δ6-CFTR mRNA expression, total RNA was extracted from CFBE lysed in Qiazol and analyzed using the one-step QuantiFast SYBR Green RT-PCR kit (Qiagen, 204154) as recommended by the manufacturer. Briefly, total RNA was extracted from polarized CFBE grown on coated plastic in 24-well plates using the miRNeasy Mini Kit (Qiagen, 217004). Reverse transcription and PCR amplification was performed sequentially in a Stratagene Mx3005P real-time thermocycler (Agilent, 401513) during the same thermocycler protocol on 50–100 ng total RNA, as determined by measuring its Nanodrop UV-Vis light absorbance. The abundance of transcripts was determined using a SYBR Green fluorescence amplification curve and its intersection with a preset threshold, yielding a Ct value. Data were analyzed by efficiency-corrected comparative quantification with MxPro QPCR software (Agilent) and the variations in initial RNA loading amount was normalized by using GAPDH as a reference gene. mRNA expression differences between samples were reported as the percentage abundance relative to a reference sample (e.g. WT-CFTR). PDZ protein downregulation was evaluated with a Quanti-Tect reverse transcription kit (Qiagen) as previously described (Veit et al., 2012). Primers are given in Table S3.
Statistical analysis
Results are presented as mean±s.e.m. of the number of independent experiments indicated in the figure legends, as biological replicates. Unless specified, P-values were calculated with the means of at least three independent experiments by two-tailed paired Student's t-test and P<0.05 was considered significant. Normal distribution of data and homogeneity of variance were validated by calculating the skew factor (−2> skew <2) and performing the F-test, respectively. For non-normal data, a Mann-Withney U-test was used for calculating the P-values, as indicated in the figure legends. For normal distributions with non-homogenous variances, the Welch correction was applied to two-tailed unpaired t-test to calculate the P-values, as indicated in the figure legends. All ELISA-based assays were performed using two to four technical replicates, except for CFTR–HRP polarized delivery (one or two wells assayed per timepoint). Short-circuit current measurement and quantitative PCR (qPCR) were performed with two technical replicates.
Supplementary Material
Acknowledgements
We are grateful to J. W. Hanrahan, B. Scholte and to the late D. Gruenert for cell lines and W. E. Finkbeiner for primary HBE. We thank G. Bertolin and M. Tramier for helpful discussions on colocalization quantification, R. Robert and R.G. Avramescu for help with initial Isc and qPCR measurements, respectively, and D. da Fonte for TEER measurement. Post-labeling steps and EM imaging were performed at the EM facility of the Department of Pharmacology, McGill University. The core facility of W. E. Finkbeiner (Department of Pathology, University of California, San Francisco) was supported by grants from National Institutes of Health [DK072517] and Cystic Fibrosis Foundation Research and Translational Core Center [VERKMA15R0].
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: A.B.-M., F.B., G.L.L.; Methodology: A.B.-M., F.B., G.L.L.; Validation: A.B.-M., G.L.L.; Formal analysis: A.B.-M., F.B., A.S., R.F., G.V., H.X.; Investigation: A.B.-M., F.B., A.S., R.F., G.V., H.X.; Writing - original draft preparation: A.B.-M., G.L.L.; Writing-review and editing: A.B.-M., G.L.L.; Visualizatioin: A.B.-M., G.L.L.; Supervision: G.L.L.; Project administration: G.L.L.; Funding acquisition: G.L.L.
Funding
This work was supported by the the Canadian Institutes of Health Research (MOP-142221), National Institute of Diabetes and Digestive and Kidney Diseases (5R01DK075302) and the Cystic Fibrosis Canada. A.B.-M. was supported by a travel grant from Cystic Fibrosis Canada. F.B. was a recipient of a Richard & Edith Strauss Fellowship, McGill Univeristy. G.L.L. is a Canada Research Chair. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.226886.supplemental
References
- Ameen N., Silvis M. and Bradbury N. A. (2007). Endocytic trafficking of CFTR in health and disease. J. Cyst. Fibros. 6, 1-14. 10.1016/j.jcf.2006.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E., Maday S., Sfakianos J., Hull M., Winckler B., Sheff D., Fölsch H. and Mellman I. (2005). Transcytosis of NgCAM in epithelial cells reflects differential signal recognition on the endocytic and secretory pathways. J. Cell Biol. 170, 595-605. 10.1083/jcb.200506051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdany M., Veit G., Fukuda R., Avramescu R. G., Okiyoneda T., Baaklini I., Singh J., Sovak G., Xu H., Apaja P. M. et al. (2017). Chaperones rescue the energetic landscape of mutant CFTR at single molecule and in cell. Nat. Commun. 8, 398 10.1038/s41467-017-00444-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard S. T., Trout L., Mehta A. and Inglis S. K. (2002). Liquid secretion inhibitors reduce mucociliary transport in glandular airways. Am. J. Physiol. Lung Cell. Mol. Physiol. 283, L329-L335. 10.1152/ajplung.00277.2001 [DOI] [PubMed] [Google Scholar]
- Benharouga M., Haardt M., Kartner N. and Lukacs G. L. (2001). COOH-terminal truncations promote proteasome-dependent degradation of mature cystic fibrosis transmembrane conductance regulator from post-golgi compartments. J. Cell Biol. 153, 957-970. 10.1083/jcb.153.5.957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benharouga M., Sharma M., So J., Haardt M., Drzymala L., Popov M., Schwapach B., Grinstein S., Du K. and Lukacs G. L. (2003). The role of the C terminus and Na+/H+ exchanger regulatory factor in the functional expression of cystic fibrosis transmembrane conductance regulator in nonpolarized cells and epithelia. J. Biol. Chem. 278, 22079-22089. 10.1074/jbc.M301030200 [DOI] [PubMed] [Google Scholar]
- Bolte S. and Cordelieres F. P. (2006). A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213-232. 10.1111/j.1365-2818.2006.01706.x [DOI] [PubMed] [Google Scholar]
- Brône B. and Eggermont J. (2005). PDZ proteins retain and regulate membrane transporters in polarized epithelial cell membranes. Am. J. Physiol. 288, C20-C29. 10.1152/ajpcell.00368.2004 [DOI] [PubMed] [Google Scholar]
- Cardone R. A., Greco M. R., Zeeberg K., Zaccagnino A., Saccomano M., Bellizzi A., Bruns P., Menga M., Pilarsky C., Schwab A. et al. (2015). A novel NHE1-centered signaling cassette drives epidermal growth factor receptor-dependent pancreatic tumor metastasis and is a target for combination therapy. Neoplasia 17, 155-166. 10.1016/j.neo.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholon D. M., O'Neal W. K., Randell S. H., Riordan J. R. and Gentzsch M. (2009). Modulation of endocytic trafficking and apical stability of CFTR in primary human airway epithelial cultures. Am. J. Physiol. Lung Cell. Mol. Physiol. 298, L304-L314. 10.1152/ajplung.00016.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaco A., Marathe J., Kohnke H., Kravstov D. and Ameen N. (2010). Syntaxin 3 is necessary for cAMP- and cGMP-regulated exocytosis of CFTR: implications for enterotoxigenic diarrhea. Am. J. Physiol. 299, C1450-C1460. 10.1152/ajpcell.00029.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton C. U., Hobert M. E., Ryan S. and Carlin C. R. (2013). Basolateral EGF receptor sorting regulated by functionally distinct mechanisms in renal epithelial cells. Traffic 14, 337-354. 10.1111/tra.12032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresawn K. O., Potter B. A., Oztan A., Guerriero C. J., Ihrke G., Goldenring J. R., Apodaca G. and Weisz O. A. (2007). Differential involvement of endocytic compartments in the biosynthetic traffic of apical proteins. EMBO J. 26, 3737-3748. 10.1038/sj.emboj.7601813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing P. R., Fellows A., Villone D., Boisguérin P. and Madden D. R. (2008). The relative binding affinities of PDZ partners for CFTR: a biochemical basis for efficient endocytic recycling. Biochemistry 47, 10084-10098. 10.1021/bi8003928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting G. R. (2015). Cystic fibrosis genetics: from molecular understanding to clinical application. Nat. Rev. Genet. 16, 45-56. 10.1038/nrg3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt C., Collnot E.-M., Baldes C., Becker U., Laue M., Kim K.-J. and Lehr C.-M. (2006). Towards an in vitro model of cystic fibrosis small airway epithelium: characterisation of the human bronchial epithelial cell line CFBE41o. Cell Tissue Res. 323, 405-415. 10.1007/s00441-005-0062-7 [DOI] [PubMed] [Google Scholar]
- Farmen S. L., Karp P. H., Ng P., Palmer D. J., Koehler D. R., Hu J., Beaudet A. L., Zabner J. and Welsh M. J. (2005). Gene transfer of CFTR to airway epithelia: low levels of expression are sufficient to correct Cl− transport and overexpression can generate basolateral CFTR. Am. J. Physiol. Lung Cell. Mol. Physiol. 289, L1123-L1130. 10.1152/ajplung.00049.2005 [DOI] [PubMed] [Google Scholar]
- Fu L. and Sztul E. (2009). ER-associated complexes (ERACs) containing aggregated cystic fibrosis transmembrane conductance regulator (CFTR) are degraded by autophagy. Eur. J. Cell Biol. 88, 215-226. 10.1016/j.ejcb.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee H. Y., Noh S. H., Tang B. L., Kim K. H. and Lee M. G. (2011). Rescue of ΔF508-CFTR trafficking via a GRASP-dependent unconventional secretion pathway. Cell 146, 746-760. 10.1016/j.cell.2011.07.021 [DOI] [PubMed] [Google Scholar]
- Gentzsch M., Chang X.-B., Cui L., Wu Y., Ozols V. V., Choudhury A., Pagano R. E. and Riordan J. R. (2004). Endocytic trafficking routes of wild type and ΔF508 cystic fibrosis transmembrane conductance regulator. Mol. Biol. Cell 15, 2684-2696. 10.1091/mbc.e04-03-0176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannone A. J., Reales E., Bhattaram P., Fraile-Ramos A. and Weimbs T. (2017). Monoubiquitination of syntaxin 3 leads to retrieval from the basolateral plasma membrane and facilitates cargo recruitment to exosomes. Mol. Biol. Cell 28, 2843-2853. 10.1091/mbc.e17-07-0461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glozman R., Okiyoneda T., Mulvihill C. M., Rini J. M., Barriere H. and Lukacs G. L. (2009). N-glycans are direct determinants of CFTR folding and stability in secretory and endocytic membrane traffic. J. Cell Biol. 184, 847-862. 10.1083/jcb.200808124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravotta D., Deora A., Perret E., Oyanadel C., Soza A., Schreiner R., Gonzalez A. and Rodriguez-Boulan E. (2007). AP1B sorts basolateral proteins in recycling and biosynthetic routes of MDCK cells. Proc. Natl. Acad. Sci. USA 104, 1564-1569. 10.1073/pnas.0610700104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravotta D., Carvajal-Gonzalez J. M., Mattera R., Deborde S., Banfelder J. R., Bonifacino J. S. and Rodriguez-Boulan E. (2012). The clathrin adaptor AP-1A mediates basolateral polarity. Dev. Cell 22, 811-823. 10.1016/j.devcel.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra L., Fanelli T., Favia M., Riccardi S. M., Busco G., Cardone R. A., Carrabino S., Weinman E. J., Reshkin S. J., Conese M. et al. (2005). Na+/H+ exchanger regulatory factor isoform 1 overexpression modulates cystic fibrosis transmembrane conductance regulator (CFTR) expression and activity in human airway 16HBE14o- cells and rescues ΔF508 CFTR functional expression in cystic fibrosis cells. J. Biol. Chem. 280, 40925-40933. 10.1074/jbc.M505103200 [DOI] [PubMed] [Google Scholar]
- Haggie P. M., Stanton B. A. and Verkman A. S. (2004). Increased diffusional mobility of CFTR at the plasma membrane after deletion of Its C-terminal PDZ binding Motif. J. Biol. Chem. 279, 5494-5500. 10.1074/jbc.M312445200 [DOI] [PubMed] [Google Scholar]
- Henry L. and Sheff D. R. (2008). Rab8 regulates basolateral secretory, but not recycling, traffic at the recycling endosome. Mol. Biol. Cell 19, 2059-2068. 10.1091/mbc.e07-09-0902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran J. P., Zeng J., Frizzell R. A. and Watkins S. C. (2013). Regulated recycling of mutant CFTR is partially restored by pharmacological treatment. J. Cell Sci. 126, 2692-2703. 10.1242/jcs.120196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., Howard M. and Lukacs G. L. (2001). Multiple endocytic signals in the C-terminal tail of the cystic fibrosis transmembrane conductance regulator. Biochem. J. 354, 561-572. 10.1042/bj3540561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W. and Peters P. J. (1998). Rab17 localizes to recycling endosomes and regulates receptor-mediated transcytosis in epithelial cells. J. Biol. Chem. 273, 15734-15741. 10.1074/jbc.273.25.15734 [DOI] [PubMed] [Google Scholar]
- Jerdeva G. V., Tesar D. B., Huey-Tubman K. E., Ladinsky M. S., Fraser S. E. and Bjorkman P. J. (2010). Comparison of FcRn- and pIgR-mediated transport in MDCK cells by fluorescence confocal microscopy. Traffic 11, 1205-1220. 10.1111/j.1600-0854.2010.01083.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravtsov D. V., Ahsan M. K., Kumari V., van Ijzendoorn S. C. D., Reyes-Mugica M., Kumar A., Gujral T., Dudeja P. K. and Ameen N. A. (2016). Identification of intestinal ion transport defects in microvillus inclusion disease. Am. J. Physiol. Gastrointest. Liver Physiol. 311, G142-G155. 10.1152/ajpgi.00041.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu A., Avalos R. T., Sanderson C. M. and Nayak D. P. (1996). Transmembrane domain of influenza virus neuraminidase, a type II protein, possesses an apical sorting signal in polarized MDCK cells. J. Virol. 70, 6508-6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalioti V., Peiro R., Perez-Berlanga M., Tsuchiya Y., Munoz A., Villalba T., Sanchez C. and Sandoval I. V. (2016). Basolateral sorting and transcytosis define the Cu+-regulated translocation of ATP7B to the bile canaliculus. J. Cell Sci. 129, 2190-2201. 10.1242/jcs.184663 [DOI] [PubMed] [Google Scholar]
- Li J., Peters P. J., Bai M., Dai J., Bos E., Kirchhausen T., Kandror K. V. and Hsu V. W. (2007). An ACAP1-containing clathrin coat complex for endocytic recycling. J. Cell Biol. 178, 453-464. 10.1083/jcb.200608033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M. P., Caras I. W., Davitz M. A. and Rodriguez-Boulan E. (1989). A glycophospholipid membrane anchor acts as an apical targeting signal in polarized epithelial cells. J. Cell Biol. 109, 2145-2156. 10.1083/jcb.109.5.2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Ory V., Chapman S., Yuan H., Albanese C., Kallakury B., Timofeeva O. A., Nealon C., Dakic A., Simic V. et al. (2012). ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am. J. Pathol. 180, 599-607. 10.1016/j.ajpath.2011.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickle J. E., Macek M. Jr, Fulmer-Smentek S. B., Egan M. M., Schwiebert E., Guggino W., Moss R. and Cutting G. R. (1998). A mutation in the cystic fibrosis transmembrane conductance regulator gene associated with elevated sweat chloride concentrations in the absence of cystic fibrosis. Hum. Mol. Genet. 7, 729-735. 10.1093/hmg/7.4.729 [DOI] [PubMed] [Google Scholar]
- Milewski M. I., Mickle J. E., Forrest J. K., Stafford D. M., Moyer B. D., Cheng J., Guggino W. B., Stanton B. A. and Cutting G. R. (2001). A PDZ-binding motif is essential but not sufficient to localize the C terminus of CFTR to the apical membrane. J. Cell Sci. 114, 719-726. [DOI] [PubMed] [Google Scholar]
- Milewski M. I., Lopez A., Jurkowska M., Larusch J. and Cutting G. R. (2005). PDZ-binding motifs are unable to ensure correct polarized protein distribution in the absence of additional localization signals. FEBS Lett. 579, 483-487. 10.1016/j.febslet.2004.11.106 [DOI] [PubMed] [Google Scholar]
- Moyer B. D., Denton J., Karlson K. H., Reynolds D., Wang S., Mickle J. E., Milewski M., Cutting G. R., Guggino W. B., Li M. et al. (1999). A PDZ-interacting domain in CFTR is an apical membrane polarization signal. J. Clin. Invest. 104, 1353-1361. 10.1172/JCI7453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelms B., Dalomba N. F. and Lencer W. (2017). A targeted RNAi screen identifies factors affecting diverse stages of receptor-mediated transcytosis. J. Cell Biol. 216, 511-525. 10.1083/jcb.201609035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger T., Burton B., Clark H. and Van Goor F. (2011). Use of primary cultures of human bronchial epithelial cells isolated from cystic fibrosis patients for the pre-clinical testing of CFTR modulators. Methods Mol. Biol. 741, 39-54. 10.1007/978-1-61779-117-8_4 [DOI] [PubMed] [Google Scholar]
- Okiyoneda T., Veit G., Sakai R., Aki M., Fujihara T., Higashi M., Susuki-Miyata S., Miyata M., Fukuda N., Yoshida A. et al. (2018). Chaperone-independent peripheral quality control of CFTR by RFFL E3 ligase. Dev. Cell 44, 694-708 e697. 10.1016/j.devcel.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostedgaard L. S., Randak C., Rokhlina T., Karp P., Vermeer D., Ashbourne Excoffon K. J. and Welsh M. J. (2003). Effects of C-terminal deletions on cystic fibrosis transmembrane conductance regulator function in cystic fibrosis airway epithelia. Proc. Natl. Acad. Sci. USA 100, 1937-1942. 10.1073/pnas.2627982100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Bay A. E., Schreiner R., Mazzoni F., Carvajal-Gonzalez J. M., Gravotta D., Perret E., Lehmann Mantaras G., Zhu Y. S. and Rodriguez-Boulan E. J. (2013). The kinesin KIF16B mediates apical transcytosis of transferrin receptor in AP-1B-deficient epithelia. EMBO J. 32, 2125-2139. 10.1038/emboj.2013.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Bay A. E., Schreiner R., Benedicto I. and Rodriguez-Boulan E. J. (2014). Galectin-4-mediated transcytosis of transferrin receptor. J. Cell Sci. 127, 4457-4469. 10.1242/jcs.153437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters K. W., Okiyoneda T., Balch W. E., Braakman I., Brodsky J. L., Guggino W. B., Penland C. M., Pollard H. B., Sorscher E. J., Skach W. R. et al. (2011). CFTR Folding consortium: methods available for studies of CFTR folding and correction. Methods Mol. Biol. 742, 335-353. 10.1007/978-1-61779-120-8_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polishchuk R., Di Pentima A. and Lippincott-Schwartz J. (2004). Delivery of raft-associated, GPI-anchored proteins to the apical surface of polarized MDCK cells by a transcytotic pathway. Nat. Cell Biol. 6, 297-307. 10.1038/ncb1109 [DOI] [PubMed] [Google Scholar]
- Potter B. A., Hughey R. P. and Weisz O. A. (2006). Role of N- and O-glycans in polarized biosynthetic sorting. Am. J. Physiol. Cell Physiol. 290, C1-C10. 10.1152/ajpcell.00333.2005 [DOI] [PubMed] [Google Scholar]
- Reczek D., Berryman M. and Bretscher A. (1997). Identification of EBP50: A PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J. Cell Biol. 139, 169-179. 10.1083/jcb.139.1.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J. R. (2008). CFTR Function and prospects for therapy. Annu. Rev. Biochem. 77, 701-726. 10.1146/annurev.biochem.75.103004.142532 [DOI] [PubMed] [Google Scholar]
- Rowe S. M., Miller S. and Sorscher E. J. (2005). Cystic fibrosis. N. Engl. J. Med. 352, 1992-2001. 10.1056/NEJMra043184 [DOI] [PubMed] [Google Scholar]
- Sabusap C. M., Wang W., McNicholas C. M., Chung W. J., Fu L., Wen H., Mazur M., Kirk K. L., Collawn J. F., Hong J. S. et al. (2016). Analysis of cystic fibrosis-associated P67L CFTR illustrates barriers to personalized therapeutics for orphan diseases. JCI Insight 1, e86581 10.1172/jci.insight.86581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S. K., Kaur S. and George C. (2006). Rab4GTPase modulates CFTR function by impairing channel expression at plasma membrane. Biochem. Biophys. Res. Commun. 341, 184-191. 10.1016/j.bbrc.2005.12.170 [DOI] [PubMed] [Google Scholar]
- Schnúr A., Hegyi P., Rousseau S., Lukacs G. L. and Veit G. (2016). Epithelial anion transport as modulator of chemokine signaling. Mediators Inflamm. 2016, 7596531 10.1155/2016/7596531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M., Pampinella F., Nemes C., Benharouga M., So J., Du K., Bache K. G., Papsin B., Zerangue N., Stenmark H. et al. (2004). Misfolding diverts CFTR from recycling to degradation: quality control at early endosomes. J. Cell Biol. 164, 923-933. 10.1083/jcb.200312018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N., Low S. H., Misra S., Pallavi B. and Weimbs T. (2006). Apical targeting of syntaxin 3 is essential for epithelial cell polarity. J. Cell Biol. 173, 937-948. 10.1083/jcb.200603132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short D. B., Trotter K. W., Reczek D., Kreda S. M., Bretscher A., Boucher R. C., Stutts M. J. and Milgram S. L. (1998). An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J. Biol. Chem. 273, 19797-19801. 10.1074/jbc.273.31.19797 [DOI] [PubMed] [Google Scholar]
- Silvis M. R., Picciano J. A., Bertrand C., Weixel K., Bridges R. J. and Bradbury N. A. (2003). A mutation in the cystic fibrosis transmembrane conductance regulator generates a novel internalization sequence and enhances endocytic rates. J. Biol. Chem. 278, 11554-11560. 10.1074/jbc.M212843200 [DOI] [PubMed] [Google Scholar]
- Silvis M. R., Bertrand C. A., Ameen N., Golin-Bisello F., Butterworth M. B., Frizzell R. A. and Bradbury N. A. (2009). Rab11b regulates the apical recycling of the cystic fibrosis transmembrane conductance regulator in polarized intestinal epithelial cells. Mol. Biol. Cell 20, 2337-2350. 10.1091/mbc.e08-01-0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis G. P., Hülsbusch N., Radon Y., Katanaev V. L., Plattner H. and Stuermer C. A. O. (2013). Reggies/flotillins interact with Rab11a and SNX4 at the tubulovesicular recycling compartment and function in transferrin receptor and E-cadherin trafficking. Mol. Biol. Cell 24, 2689-2702. 10.1091/mbc.e12-12-0854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg F., Heesom K. J., Bass M. D. and Cullen P. J. (2012). SNX17 protects integrins from degradation by sorting between lysosomal and recycling pathways. J. Cell Biol. 197, 219-230. 10.1083/jcb.201111121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops E. H. and Caplan M. J. (2014). Trafficking to the apical and basolateral membranes in polarized epithelial cells. J. Am. Soc. Nephrol. 25, 1375-1386. 10.1681/ASN.2013080883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F., Hug M. J., Lewarchik C. M., Yun C.-H. C., Bradbury N. A. and Frizzell R. A. (2000). E3KARP mediates the association of ezrin and protein kinase A with the cystic fibrosis transmembrane conductance regulator in airway cells. J. Biol. Chem. 275, 29539-29546. 10.1074/jbc.M004961200 [DOI] [PubMed] [Google Scholar]
- Swiatecka-Urban A., Duhaime M., Coutermarsh B., Karlson K. H., Collawn J., Milewski M., Cutting G. R., Guggino W. B., Langford G. and Stanton B. A. (2002). PDZ Domain interaction controls the endocytic recycling of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 277, 40099-40105. 10.1074/jbc.M206964200 [DOI] [PubMed] [Google Scholar]
- Swiatecka-Urban A., Brown A., Moreau-Marquis S., Renuka J., Coutermarsh B., Barnaby R., Karlson K. H., Flotte T. R., Fukuda M., Langford G. M. et al. (2005). The short apical membrane half-life of rescued {Delta}F508-cystic fibrosis transmembrane conductance regulator (CFTR) results from accelerated endocytosis of {Delta}F508-CFTR in polarized human airway epithelial cells. J. Biol. Chem. 280, 36762-36772. 10.1074/jbc.M508944200 [DOI] [PubMed] [Google Scholar]
- Swiatecka-Urban A., Talebian L., Kanno E., Moreau-Marquis S., Coutermarsh B., Hansen K., Karlson K. H., Barnaby R., Cheney R. E., Langford G. M. et al. (2007). Myosin Vb is required for trafficking of the cystic fibrosis transmembrane conductance regulator in Rab11a-specific apical recycling endosomes in polarized human airway epithelial cells. J. Biol. Chem. 282, 23725-23736. 10.1074/jbc.M608531200 [DOI] [PubMed] [Google Scholar]
- Takeda T., Yamazaki H. and Farquhar M. G. (2003). Identification of an apical sorting determinant in the cytoplasmic tail of megalin. Am. J. Physiol. 284, C1105-C1113. 10.1152/ajpcell.00514.2002 [DOI] [PubMed] [Google Scholar]
- Tzaban S., Massol R. H., Yen E., Hamman W., Frank S. R., Lapierre L. A., Hansen S. H., Goldenring J. R., Blumberg R. S. and Lencer W. I. (2009). The recycling and transcytotic pathways for IgG transport by FcRn are distinct and display an inherent polarity. J. Cell Biol. 185, 673-684. 10.1083/jcb.200809122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van IJzendoorn S. C., Tuvim M. J., Weimbs T., Dickey B. F. and Mostov K. E. (2002). Direct interaction between Rab3b and the polymeric immunoglobulin receptor controls ligand-stimulated transcytosis in epithelial cells. Dev. Cell 2, 219-228. 10.1016/S1534-5807(02)00115-6 [DOI] [PubMed] [Google Scholar]
- Varga K., Goldstein R. F., Jurkuvenaite A., Chen L., Matalon S., Sorscher E. J., Bebok Z. and Collawn J. F. (2008). Enhanced cell-surface stability of rescued DeltaF508 cystic fibrosis transmembrane conductance regulator (CFTR) by pharmacological chaperones. Biochem. J. 410, 555-564. 10.1042/BJ20071420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit G., Bossard F., Goepp J., Verkman A. S., Galietta L. J. V., Hanrahan J. W. and Lukacs G. L. (2012). Proinflammatory cytokine secretion is suppressed by TMEM16A or CFTR channel activity in human cystic fibrosis bronchial epithelia. Mol. Biol. Cell 23, 4188-4202. 10.1091/mbc.e12-06-0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit G., Avramescu R. G., Perdomo D., Phuan P. W., Bagdany M., Apaja P. M., Borot F., Szollosi D., Wu Y. S., Finkbeiner W. E. et al. (2014). Some gating potentiators, including VX-770, diminish DeltaF508-CFTR functional expression. Sci. Transl. Med. 6, 246ra297 10.1126/scitranslmed.3008889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit G., Xu H., Dreano E., Avramescu R. G., Bagdany M., Beitel L. K., Roldan A., Hancock M. A., Lay C., Li W. et al. (2018). Structure-guided allosteric corrector combination for ΔF508 and rare cystic fibrosis folding mutants. Nat. Med. 24, 1732-1742. 10.1038/s41591-018-0200-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel G. F., Klee K. M. C., Janecke A. R., Müller T., Hess M. W. and Huber L. A. (2015). Cargo-selective apical exocytosis in epithelial cells is conducted by Myo5B, Slp4a, Vamp7, and Syntaxin 3. J. Cell Biol. 211, 587-604. 10.1083/jcb.201506112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolde M., Fellows A., Cheng J., Kivenson A., Coutermarsh B., Talebian L., Karlson K., Piserchio A., Mierke D. F., Stanton B. A. et al. (2007). Targeting CAL as a negative regulator of DeltaF508-CFTR cell-surface expression: an RNA interference and structure-based mutagenetic approach. J. Biol. Chem. 282, 8099-8109. 10.1074/jbc.M611049200 [DOI] [PubMed] [Google Scholar]
- Yoo J.-S., Moyer B. D., Bannykh S., Yoo H.-M., Riordan J. R. and Balch W. E. (2002). Non-conventional trafficking of the cystic fibrosis transmembrane conductance regulator through the early secretory pathway. J. Biol. Chem. 277, 11401-11409. 10.1074/jbc.M110263200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.