ABSTRACT
Cell–cell communication is essential for tissue homeostasis, but its contribution to disease prevention remains to be understood. We demonstrate the involvement of connexin 43 (Cx43, also known as GJA1) and related gap junction in epithelial homeostasis, illustrated by polarity-mediated cell cycle entry and mitotic spindle orientation (MSO). Cx43 localization is restricted to the apicolateral membrane of phenotypically normal breast luminal epithelial cells in 3D culture and in vivo. Chemically induced blockade of gap junction intercellular communication (GJIC), as well as the absence of Cx43, disrupt the apicolateral distribution of polarity determinant tight junction marker ZO-1 (also known as TJP1) and lead to random MSO and cell multilayering. Induced expression of Cx43 in cells that normally lack this protein reestablishes polarity and proper MSO in 3D culture. Cx43-directed MSO implicates PI3K–aPKC signaling, and Cx43 co-precipitates with signaling node proteins β-catenin (CTNNB1) and ZO-2 (also known as TJP2) in the polarized epithelium. The distribution of Cx43 is altered by pro-inflammatory breast cancer risk factors such as leptin and high-fat diet, as shown in cell culture and on tissue biopsy sections. The control of polarity-mediated quiescence and MSO may contribute to the tumor-suppressive role of Cx43.
KEY WORDS: Gap junction, Apical polarity, Epithelial differentiation, PI3K, Cancer risk, Quiescence
Summary: Gap junction protein connexin 43 (GJA1) contributes to breast epithelial polarity by modulating mitotic spindle orientation to prevent multilayering of the epithelium typically associated with tumor onset.
INTRODUCTION
The formation of multilayers of cells during cancer onset reflects the loss of epithelial architecture that results from disruption of the polarity axis (Lesko et al., 2015), and particularly, its apical portion established by tight junctions. The implication of tight junction proteins in proliferation control and tumor suppression (Bazzoun et al., 2013), and of tight junction structures in the maintenance of cell quiescence (Chandramouly et al., 2007; Yue et al., 2012), has established apical polarity as essential for epithelial homeostasis. The contribution of apical polarity to the control of cancer onset makes this architectural feature worthy of consideration for cancer prevention research (Eccles et al., 2013; Lelièvre, 2010).
Another tissue structure involved in epithelial homeostasis is the gap junction (GJ) built from connexins. Lack of cell coupling via GJs and/or downregulation of connexins have been initially reported in liver, thyroid, stomach, lung, prostate, cervical and intestinal tumors (Kanno and Matsui, 1968; Ruch et al., 1998; Yamasaki et al., 1995). The ectopic reintroduction of connexin 43 (Cx43), Cx26 or Cx40 (also known as GJA1, GJB2 and GJA5, respectively) into tumor cells has been shown to reduce tumor growth and partially redifferentiate transformed cells (Hirschi et al., 1996; McLachlan et al., 2006). In the mammary gland GJs have been implicated in normal development and their dysregulation has been correlated with tumorigenesis (Kanczuga-Koda et al., 2003). Specifically, the downregulation of Cx43 and Cx26 expression in primary breast tumors (Laird, 2006; Lee et al., 1992), and the transfection of breast cancer cells with either of these connexins leading to mesenchymal to epithelial transition and partial reversion of the tumor phenotype, indicate tumor suppressor function in these proteins (McLachlan et al., 2007; Qin et al., 2002; Talhouk et al., 2013).
Connections between GJs and tight junctions exist. Indeed, Cx30 (also known as GJB4), Cx40, Cx43 or Cx26 may regulate the expression of ZO-1 (also known as TJP1), claudins and occludins in epithelia and sometime interact with these proteins (Go et al., 2006; Li et al., 2010; Nagasawa et al., 2006; Nusrat et al., 2000). Interestingly, in light of the overlap of Cx43-mediated GJs, but not Cx32 (also known as GJB1)-mediated GJs, and tight junctions in the subapical region of the thyroid follicular cells, it has been suggested that the location of Cx43 might be linked to apical polarity (Guerrier et al., 1995). Moreover, Cx43 assembly into GJs and its association with ZO-2 (also known as TJP2) – and also adherens junction-related α- and β-catenins – has been related to mammary epithelial differentiation (Talhouk et al., 2008). Whether GJs and tight junctions work together or separately to prevent tumor development remains to be investigated.
Originally, Cx43 had been documented to mediate GJ intercellular communication (GJIC) in mammary myoepithelial cells that are notoriously lacking in tight junctions (El-Sabban et al., 2003; Locke et al., 2004; Mroue et al., 2015; Plante et al., 2010; Talhouk et al., 2005). Yet, induced expression of Cx43 in human breast cancer cells could drive mesenchymal to epithelial transition (Talhouk et al., 2013). The expression of Cx43 in luminal epithelial cells has been indicated in human breast in the past (Monaghan et al., 1996) and was shown to be present in mice (Dianati et al., 2016). Homomeric Cx32 and heteromeric Cx26–Cx32, Cx26–Cx30 and Cx30–Cx32 form channels in the murine luminal epithelium. Cx26 is variably expressed through murine development, with a peak during pregnancy and lactation (Locke et al., 2004; Talhouk et al., 2005), and appears to have a low impact on mammary function (Stewart et al., 2014). Cx26 has been detected in low amounts and localized intracellularly when human luminal cells are separated from the myoepithelium and cultured in vitro, but only rare displays of dye transfer and no formation of GJ plaques were detected (Monaghan et al., 1996). Cx30 and Cx32 are transiently expressed as well; in contrast, Cx43 is continuously present, at similar levels, throughout development stages of the murine mammary gland (Dianati et al., 2016). Although suspected to act as a major contributor to the normal behavior of cells, the function of Cx43 remains poorly appreciated in polarized epithelia.
Here, we report the presence of Cx43 specifically at the apical cell–cell contacts of the luminal breast epithelium in 3D cell culture and in resting human breast. Cx43-mediated GJs are required for the establishment and maintenance of apical polarity, and obesity, a risk factor for mammary cancer, is associated with loss of the apical distribution of Cx43. We further demonstrate that Cx43-dependent polarity controls glandular morphogenesis, not only by influencing cell cycle entry, but also by directing mitotic spindle orientation (MSO) through the PI3K–aPKC–NuMA pathway. Our findings establish a preventive function of Cx43 in major aspects of cancer onset that are polarity loss, random MSO and cell multilayering.
RESULTS
Cx43 is distributed apically in differentiated breast luminal epithelial cells
The non-neoplastic human mammary epithelial HMT-3522 S1 cell line (Briand et al., 1987) forms well-differentiated glandular structures or acini, characterized by cell cycle exit and a polarity axis with apicolateral tight junctions (Plachot and Lelièvre, 2004) when cultured in 3D, in the presence of basement membrane components. Such a level of differentiation renders the S1 cells suitable for studying mechanisms of phenotypically normal homeostasis associated with connexins. Analyses of mRNAs from GJA1, GJB6, GJB1 and GJB2 genes coding for Cx43, Cx30, Cx32 and Cx26, respectively, detected only expression of GJA1 in acini formed by S1 cells. Western blotting confirmed that Cx43, but not Cx26, Cx30 or Cx32, was present in these cells (Fig. 1A; Fig. S1). Immunofluorescence staining revealed the expected dotted pattern correlated with cell–cell junction localization of Cx43 in S1 cells cultured as 2D monolayer, whereas Cx43 was mainly concentrated into foci towards the center of the acini, and thus within the apical cellular poles, in 3D cell culture (Fig. 1B). Immunohistochemistry performed on archival biopsy sections of normal-appearing breast tissue reaffirmed the presence of Cx43 in myoepithelial cells (Laird et al., 1999), but it also showed an apicolateral concentration of the protein in the luminal epithelium, similar to the pattern observed in acini in 3D cell culture (Fig. 1C). In vivo basal Cx43 colocalized with α-smooth muscle actin (α-SMA, also known as ACTA2) protein, a marker of myoepithelial cells; however, apicolateral Cx43 appeared strictly confined to luminal cells since it did not overlap with α-SMA, ruling out the possibility that myoepithelial cytoplasmic extensions brought Cx43 toward the apical pole of acini (Fig. 1D).
Fig. 1.
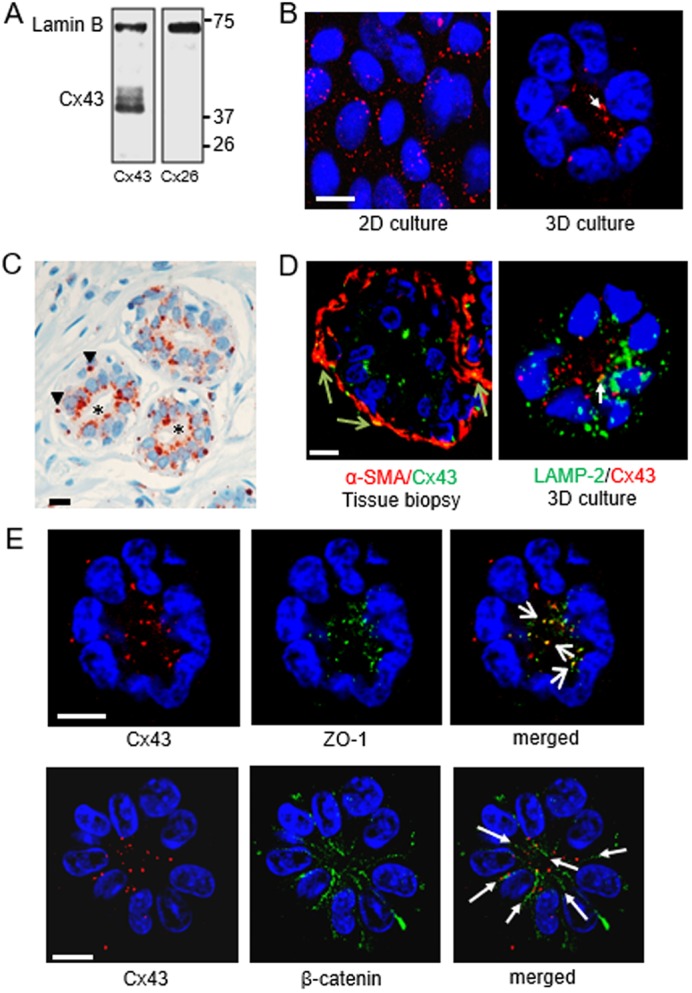
Cx43 is located apically in the breast luminal epithelium. S1 non-neoplastic mammary epithelial cells were cultured in 2D (A,B) or in 3D (B,D,E), as indicated, for 10 days. A thin section from breast tissue biopsy was used in C. (A) Western blot shows that Cx43, but not Cx26, is expressed in S1 cells; lamin B is used as loading control. (B) Immunostaining for Cx43 (red), with apical localization indicated by the arrow. (C) Immunohistochemistry for Cx43 (reddish-brown) in normal-appearing breast glandular tissue, with display of basal localization in myoepithelial cells (arrowheads) and apical localization in luminal cells (asterisks). Nuclei are counterstained with hematoxylin (blue). (D) Left: dual fluorescence staining for Cx43 (green) and a myoepithelial cell marker (α-smooth muscle actin protein, α-SMA; red) in normal-appearing breast glandular tissue. Cx43 staining overlap with α-SMA staining in myoepithelial cells appears in yellow (arrows). Right: dual immunostaining for Cx43 (red) and a lysosomal marker (lysosomal-associated membrane protein 2, LAMP-2) (green) in an acinus formed by S1 cells; the arrow points to a rare spot with colocalization (yellow). (E) Dual staining for Cx43 (red) and ZO-1 (green) or β-catenin (green). Colocalization of Cx43 and ZO-1 staining appears yellow (short arrows); cell–cell contacts with Cx43 aligned with β-catenin are indicated (long arrows). Nuclei are counterstained with DAPI (blue). Scale bars: 10 µm. Single immunofluorescence staining was done on multiple (>5) biological replicates (cell cultures and tissue samples); dual immunostaining was done on 2–3 biological replicates.
In cells defective for connexin trafficking and GJ assembly, connexins are found in lysosomes owing to their lysosomal degradation (Qin et al., 2001). The distribution pattern of Cx43 in acini seen in 3D cell culture was not linked to lysosomal degradation of the protein since dual immunostaining for Cx43 and lysosomal marker LAMP-2 did not reveal striking colocalization (Fig. 1D). In contrast, dual immunostaining for Cx43 and ZO-1 revealed extensive colocalization at the apical side of luminal cells (Fig. 1E), suggesting a close association of Cx43 with tight junction proteins. Moreover, Cx43 was primarily localized along lines marked by cell–cell adhesion marker β-catenin (also known as CTNNB1), indicating its presence at cell–cell junctions and consequently, its possible involvement in GJIC (Fig. 1E).
GJIC controls epithelial homeostasis
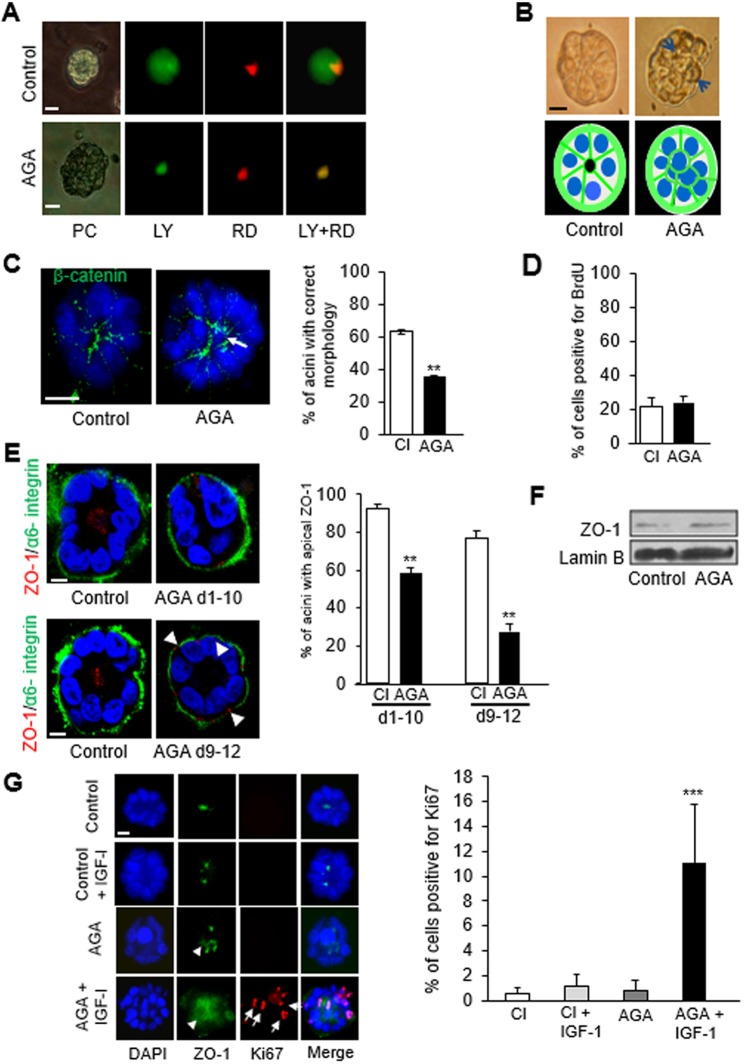
Communication among S1 cells via GJ was initially determined by scrape loading of a mixture of Lucifer yellow (LY) and rhodamine-B isothiocyanate–dextran (RD) in 2D culture. The GJ-permeable LY diffused over a longer distance inside the cell layer compared to RD, a dye too large to diffuse through GJ and that remained at the wound site (Fig. S2A). For the assessment of GJIC in the differentiated glandular epithelium, microinjection of a mixture of LY and RD was performed into a single cell, in at least 10 acini. The localization of RD confirmed that only one cell had received the injection, whereas LY diffused throughout each of the acini, indicating the presence of functional GJs (Fig. 2A). A concentration of 18α-glycerrhitinic acid (AGA) that effectively blocked GJs without toxicity, based on TUNEL and Trypan Blue exclusion assays, was first determined in 2D culture (Fig. S2B). The treatment of cells with AGA in 3D culture at day 4, during the proliferation stage of acinar morphogenesis (Fig. S2C), or at day 10, upon completion of acinar morphogenesis, confirmed the blockade of GJ communication, as shown by the strict localization of both RD and LY to the injected cells (Fig. 2A).
Fig. 2.
Loss of GJIC disrupts acinar differentiation and primes luminal epithelial cells for cell cycle entry. S1 cells were cultured in 3D for 10 days and treated with AGA (50 μM) or vehicle (A–F). (A) One cell per acinus was ionophoretically microinjected with a mixture of Lucifer Yellow (LY, 2.5% w/v) and rhodamine B isothiocyanate-dextran (RD, 2% w/v) in 0.1 mM LiCl. Cells were observed with epifluorescence microscopy 15 min following injection. Representative acini are shown (n=10 acini in different cell cultures). The gap junction impermeable dye, RD (red), marks the injected cells; LY diffuses throughout the acinus when gap junctions are functional. (B) Phase contrast images with accompanying drawings of a normal-looking acinus (left panel) and an acinus with cells positioned abnormally (right panel, arrows). (C) Immunostaining for β-catenin (green) and DAPI (blue) to better visualize the arrangement of cells. The correct acinar morphology is defined as one layer of cells; the arrow indicates an aberrantly localized (central) cell observed on the optical section through the middle of an acinus. Graph shows the mean±s.e.m. percentages of acini with correct morphology; at least 100 acini analyzed per condition; n=3 (biological replicates corresponding to distinct cell cultures usually of different passages). (D) Mean±s.e.m. percentages of BrdU-positive cells at day 4 of 3D culture; a minimum of 500 cells analyzed per condition; n=3. (E) Dual immunostaining for apical polarity marker ZO-1 (red) and basal polarity marker α6 integrin (green); arrowheads indicate aberrant basal localization of ZO-1. Graph shows mean±s.e.m. percentages of S1 acini with apical location of ZO-1 during (days 1–10) or following (days 9–12) acinar differentiation; at least 100 acini analyzed per condition; n=3. (F) Western blot for ZO-1 shows no effect of AGA treatment on expression levels; lamin B is used as loading control. (G) S1 cells were cultured for nine days in 3D with or without AGA (50 µM) followed by incubation with 100 ng/ml of insulin-like growth factor I (IGF-1) or vehicle for 36 h. Graph shows mean±s.e.m. percentages of cells positive for cell proliferation marker Ki67. In representative dual fluorescence immunostaining for ZO-1 (green) and Ki67 (red) under different treatment conditions, nuclei are counterstained with DAPI (blue). Diffuse ZO-1 distribution is indicated by arrowheads; positive intranuclear Ki67 localization is shown by arrows in some of the cells. At least 100 cells analyzed for each condition, n=3. **P<0.01, ***P<0.001; unpaired t-test (C,D,E) and one-way ANOVA with Dunn's comparison (G). Scale bar: 10 µm.
The effect of blocking GJIC was examined by assessing proliferation, acinar morphology, and basoapical polarity following treatment of S1 cells with AGA during the 10-day process of acinar morphogenesis. A marked defect in morphology was illustrated by acini that appeared bumpy and lacked the usual organization of cells like ‘slices in a pie’ (Fig. 2B). Noticeably, immunostaining for β-catenin revealed the aberrant presence of cells in the center of the acini of the AGA-treated group (Fig. 2C). The alteration of the arrangement of cells was not accompanied by a change in proliferation rate as measured by BrdU labeling on day 4 of 3D culture (Fig. 2D). Furthermore, the distribution of polarity marker ZO-1, typically apicolateral in differentiated breast acini in vivo (Martin et al., 2004) and in vitro (Plachot and Lelièvre, 2004), was significantly altered by AGA treatment during (days 1–10) or following (days 9–12) acinar differentiation, and displayed a diffused and/or basal staining pattern (Fig. 2E). There was no apparent alteration in the localization of α6-integrin (ITGA6) compared to untreated acini (Fig. 2E), ruling out the possibility that loss of apical polarity was a consequence of gross perturbations in basal polarity. The alteration of ZO-1 distribution was not accompanied by a noticeable change in the expression levels of this protein (Fig. 2F).
These results suggest that GJIC is required for the proper organization of tight junctions, and hence for the establishment of apical polarity in acini. We have shown previously that acini lacking apical polarity can be pushed into the cell cycle (Chandramouly et al., 2007). Initially, this demonstration was done by altering the distribution of mitotic spindle and chromatin organizer NuMA (also known as NUMA1), a protein involved in mammary acinar differentiation (Abad et al., 2007), with an antibody (B1C11) targeted against this protein and introduced in live acinar cells. To determine whether apical polarity loss induced by GJ blockage also primed acinar cells for cell cycle entry, we treated S1 acini, in which GJs were blocked by AGA treatment, with the B1C11 antibody against NuMA. There was a significant increase in the percentage of cells positive for cell proliferation marker Ki67 (also known as MKI67) in acini with combined AGA and B1C11 treatments compared to those treated either with a nonspecific immunoglobulin (IgG), regardless of AGA treatment, or with B1C11 alone (Fig. S3). The impact of GJIC blockage on fostering cell cycle entry was confirmed by exposing AGA-treated S1 acini to insulin-like growth factor 1 (IGF-1), a potent mitogenic stimulus implicated in normal and cancerous mammary development (Fig. 2G). Thus, GJs influence apical polarity and cell quiescence, two features of normal homeostasis that need to be disrupted for tumor onset.
Cx43 directs the establishment of apical polarity
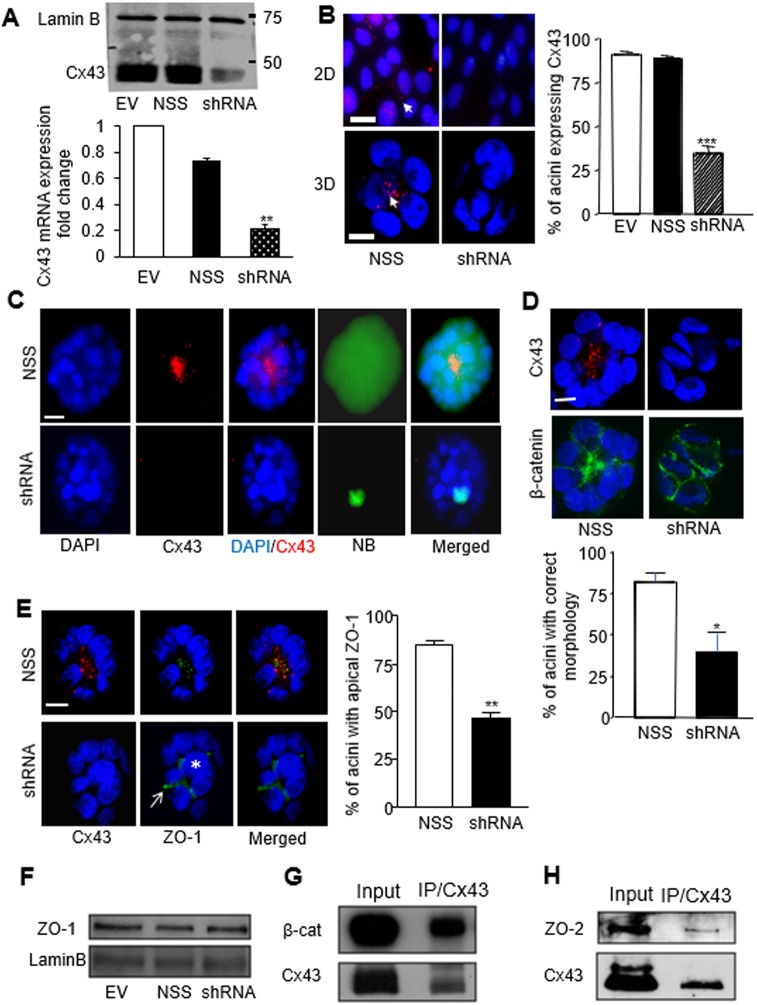
The most abundantly expressed gap junction protein in several epithelial tissues, including breast, is Cx43, and it has been shown to be involved in the maintenance of epithelial homeostasis in organs other than the breast (Ey et al., 2009; Li et al., 2010; Marquez-Rosado et al., 2012). To confirm its direct implication in the regulation of mammary acinar differentiation, Cx43 was downregulated in S1 breast luminal cells through retroviral delivery of shRNA (Shao et al., 2005). The expression of Cx43 was markedly reduced at mRNA and protein levels in Cx43 shRNA-treated cells compared to cells infected with a non-specific sequence (NSS) or the empty vector (EV), without exhibiting compensatory expression of Cx30, Cx32 and Cx26 (Fig. 3A; Fig. S4A). As evaluated using immunofluorescence staining, at least 65% of the acini in the shRNA-treated group did not express Cx43, whereas almost 90% of the acini in the control groups exhibited Cx43 expression (Fig. 3B). Upon GJA1 silencing GJ-mediated communication was inhibited in the majority (at least 75%) of the acini analyzed, as determined by the lack of transfer of microinjected neurobiotin (NB), a fixable GJ permeable dye. In contrast, acinar cells in the control group had an effective dye coupling, as evidenced by the distribution of NB throughout the multicellular structures (Fig. 3C). All the acini that lacked GJIC were deficient in Cx43, as shown by immunostaining in the acini injected with NB.
Fig. 3.
Downregulation of Cx43 expression disrupts GJIC and apical polarity in S1 cells. (A) Western blot (upper image) and quantitative real-time PCR (bottom graph) for Cx43 expression in S1 cells following retroviral delivery of empty vector (EV) or non-specific sequence shRNA (NSS) used as negative controls, and Cx43-specific shRNA (shRNA). Lamin B serves as loading control; n=3. Cx43 mRNA expression normalized to EV control; data represented as mean±s.e.m. (B) Fluorescence immunostaining for Cx43 (red) in S1 cells treated as indicated and cultured for 10 days in 2D or 3D conditions. Arrows indicate Cx43 foci. Graph shows the mean±s.e.m. percentages of acini that express Cx43 in each treatment condition; at least 200 acini were analyzed per condition; n=3. (C) S1 cells infected with NSS (upper panel) or Cx43 shRNA (lower panel) were cultured in 3D for 10 days and microinjected with 3% NB in 0.15 M LiCl, followed by dual fluorescence staining with streptavidin–FITC and an antibody against Cx43 (red). Merged images show the extent of NB spread within the acini; n=10 acini. (D) Dual immunostaining of acini for Cx43 (red) and β-catenin (green; indicating cell–cell limits) in S1 cells treated as indicated. The peripheral organization of cells around a hollow center (left lower panel) is considered morphologically correct. Graph shows mean±s.e.m. percentages of acini with correct morphology; at least 100 acini analyzed per condition; n=3. (E) Dual immunostaining for Cx43 (red) and ZO-1 (green) in acini formed by S1 cells treated as indicated. The arrow points to the peripheral location of ZO-1 and the asterisk indicates the central location of a cell (optical section through the middle of the acinus), illustrating abnormal morphogenesis. Graph shows mean±s.e.m. percentages of acini with apical ZO-1 staining; at least 100 acini analyzed per condition; n=3. (F) Western blots show unchanged levels of total ZO-1 expression in 10-day-old S1 acini treated as indicated. Lamin B was used as loading control. (G,H) Western blots for Cx43 and co-immunoprecipitated β-catenin (G) or ZO-2 (H) following immunoprecipitation with Cx43 antibody in S1 acini. *P<0.05, **P<0.01, ***P<0.001; one way ANOVA, with Dunn's comparison (A,B), nonpaired t-test (D,E). Nuclei are counterstained with DAPI (blue). Scale bar: 10 µm.
Based on β-catenin immunostaining, the typical arrangement of luminal epithelial cells, like slices in a pie, was lost in the Cx43-shRNA acini population, with >75% of acini properly organized in the control group compared to <40% in the Cx43-shRNA group (Fig. 3D). There was no observable effect on basal polarity, as shown by α6-integrin distribution (Fig. S4B), yet apical polarity was compromised. Indeed, apical localization of ZO-1 was evident in <50% of the acini in the Cx43-shRNA group compared to >80% of the acini in the NSS group (Fig. 3E). ZO-1 mislocalization was not accompanied with a detectable change in the expression level of this protein (Fig. 3F). These data resemble those obtained upon blockage of GJs, confirming that Cx43-mediated GJIC is essential for the morphological differentiation of S1 cells.
The interaction of connexins with proteins that control polarity is selective and depends on stages of murine mammary development, which supports the idea that connexins participate in signaling nodes and hubs involved in epithelial homeostasis (Dianati et al., 2016). In particular, Cx43 interacts with β-catenin in mammary luminal cells as we showed in a murine 3D culture model (Talhouk et al., 2008). We have repeatedly observed the accumulation of β-catenin at the apical portion of polarized human acini in 3D culture (Yue et al., 2012), with some overlap of Cx43 and β-catenin staining patterns (Fig. 1E). Indeed, β-catenin was present in Cx43 precipitates in human mammary epithelial cells differentiated into acini (Fig. 3G). We have also observed that ZO-2, a partner of Cx43 in murine mammary epithelial tissue (Talhouk et al., 2008), has a location and behavior similar to ZO-1 in mammary acini (Yue et al., 2012). Here, ZO-2 coimmunoprecipitated with Cx43 in polarized acini (Fig. 3H). Thus, Cx43 connects with proteins essential for polarity organization and signaling in differentiated mammary epithelium.
We reasoned that if Cx43 were essential to mediate apical polarity formation in the mammary epithelium, its induced expression in non-neoplastic mammary epithelial MCF10A cells, which normally harbor negligible endogenous Cx43, might rectify their demonstrated inability to polarize apically (Plachot et al., 2009). Following infection with a lentiviral construct coding for Cx43, the expression of this connexin was increased in MCF10A/HuCx43 cells compared to uninfected cells and cells infected with the empty vector (EV) control in both 2D and 3D culture conditions (Fig. 4A). Accordingly, the percentage of MCF10A/HuCx43 acini with immunostaining signals for Cx43 was drastically higher than in the EV group (Fig. 4B,C), and expression of Cx43 was accompanied by apical localization of the protein in a majority of the acini population. As much as 50% of MCF10A/HuCx43 acini displayed apicolateral ZO-1 when Cx43 was at the apical pole of cells, which was significantly higher than in acini with non-apical Cx43. In contrast, <15% of the Cx43-positive MCF10A/EV multicellular structures displayed ZO-1 apically localized, which represents a mere 2% of the whole population of structures made by MCF10A/EV cells (Fig. 4C). It is to be noted that Cx43-positive MCF10A/EV acini usually do not display Cx43 apically. The change in ZO-1 distribution associated with de novo expression of Cx43 was not accompanied by striking alterations in ZO-1 expression level (Fig. 4A). In contrast, plasmid-induced de novo expression of Cx32 was observed throughout the cytoplasm and not at cell–cell junctions (with rare exceptions upon transfection of S1 cells) and appeared to be associated with a mesenchymal shape of MCF10A cells (Fig. S4C,D). We conclude that Cx43 is capable of driving apical polarity in the mammary epithelium.
Fig. 4.
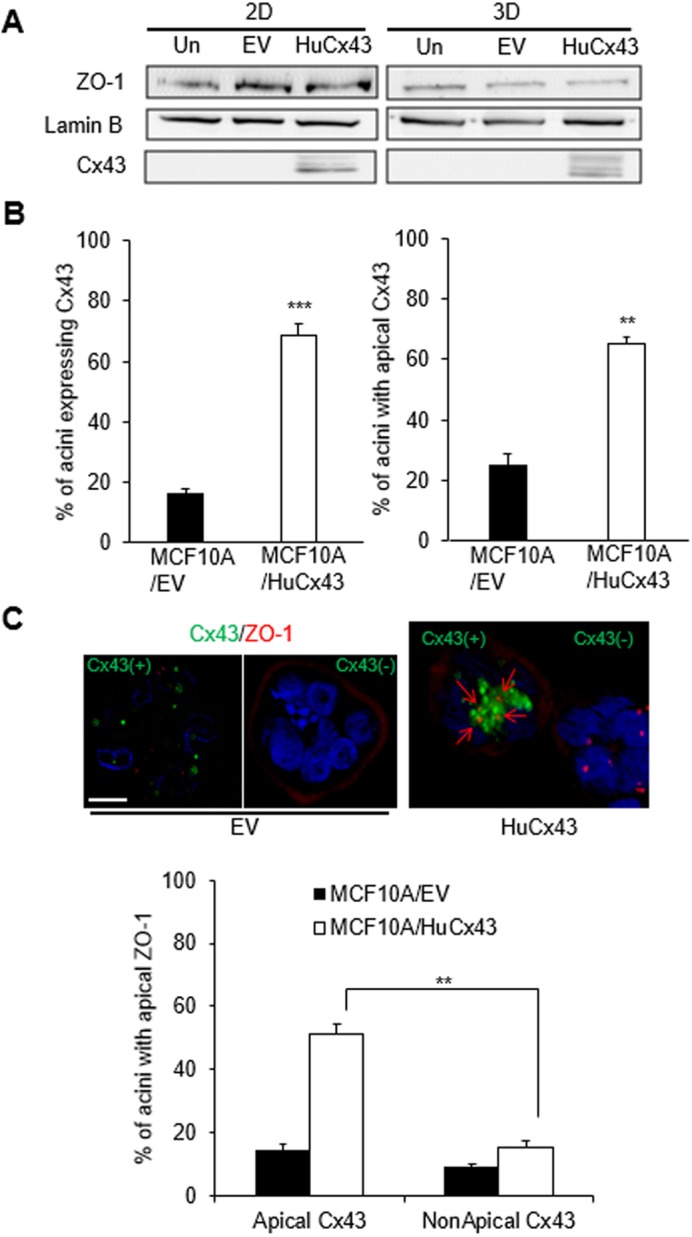
Cx43 expression induces apical polarity in MCF10A acini. (A) Western blots for Cx43 and ZO-1 expression from 10-day 2D and 3D cultures of MCF10A cells that were uninfected (Un) or infected with empty vector (EV) for controls, or infected with HuCx43 vector (HuCx43). Lamin B is used as loading control. (B) MCF10A cells stably infected with EV (MCF10A/EV) or with HuCx43 (MCF10A/HuCx43) were cultured in 3D for 10 days to induce acinar differentiation. Graphs show mean±s.e.m. percentages of acinar structures expressing Cx43 (left) and of acinar structures with Cx43 apically localized among those expressing Cx43 (right). (C) Representative images of dual immunostaining for Cx43 (green) and ZO-1 (red) in acini formed by MCF10A/HuCx43 and by MCF10A/EV with (Cx43+) and without (Cx43−) Cx43 expression. Red arrows on merged image indicate the apical location of ZO-1. Nuclei are counterstained with DAPI (blue). Graph shows mean±s.e.m. percentages of acini with apically localized ZO-1 among those with apical Cx43 and non-apical Cx43. A minimum of 100 acini were analyzed in each condition although the number of acini analyzed was less in EV population due to the paucity of structures expressing ZO-1 in C; n=3; unpaired-test (B) and one-way ANOVA with Dunn's comparison (C), **P<0.01, ***P<0.001 (significance only shown for comparison of interest in C). Scale bar: 10 µm.
Cx43-mediated gap junction intercellular communication influences acinar morphology via the control of mitotic spindle orientation
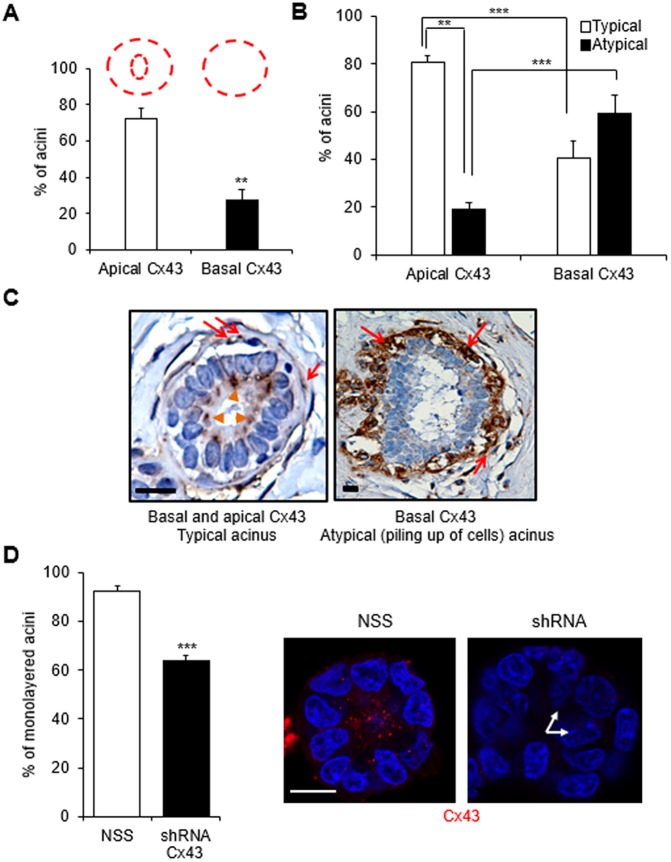
The loss of epithelial tissue polarity has been linked to multilayering of cells within the ductal lumen in pathological specimens (Jones and Young, 1994; Fitzgibbons et al., 1998; Tobi, 1999). A recurrent observation following blockage of GJIC and silencing of GJA1 coding for Cx43 in the experiments presented above is the disruption of acinar morphogenesis shown by the aberrant presence of cells in the center of the acinus (see Fig. 2B,C,G, Fig. 3B–D). We further investigated the link between Cx43 localization and epithelial multilayering. Immunohistochemical analysis of Cx43 in archival biopsy sections from breast cancer-free women revealed that most of the acini displayed apically localized Cx43 in the luminal epithelium compared to only basally localized Cx43 (i.e., Cx43 localized in the myoepithelial cells of the acini) in myoepithelial cells (Fig. 5A). Furthermore, the apical localization of Cx43 significantly correlated with a typical epithelial organization (i.e. only one layer of luminal cells lining the inner side of the layer of myoepithelial cells) (Fig. 5B,C). An atypical epithelium (i.e. with cells piling up in places, suggesting additional layering compared to the layer of myoepithelial cells and the layer of luminal cells) was more frequently associated with exclusively basal Cx43 localization than with normally distributed Cx43 (i.e. both at the apical pole of the luminal epithelium and basally in the myoepithelial layer). Similarly, GJA1 silencing-mediated loss of Cx43 in luminal S1 cells was accompanied by a significant increase in the number of acini displaying abnormal multilayering of cells (Fig. 5D).
Fig. 5.
Absence of luminal expression of Cx43 is associated with cell multilayering. (A–C) Immunohistochemical staining for Cx43 was performed on archival breast tissue biopsy sections from 22 women with no history of breast cancer. Graphs show mean±s.e.m. percentages of acini with apical localization of Cx43 in the luminal epithelium (in addition to basal myoepithelial localization; see drawing in red) and basal localization of Cx43 only (A); percentages of acini displaying typical (one layer of luminal cells at the inner side of the layer of myoepithelial cells) or atypical (piling up of cells) organization depending on the location of Cx43 (B), n=22 acini; paired t-test. (C) Representative images of acini with basal (red arrows) and apical (orange arrowheads) Cx43 localization in a typical (normal-appearing) epithelial structure and only basal Cx43 (red arrows) in an atypical structure. Nuclei are stained with hematoxylin (blue). (D) S1 cells stably silenced for Cx43 expression were cultured in 3D for 10 days and immunolabeled for Cx43. Graph shows mean±s.e.m. percentages of acini with a monolayer of cells in acini of cells transduced with non-specific sequence (NSS) control and Cx43 shRNA. Shown are representative images of a monolayered acinus with Cx43 (red) apically localized and a multilayered (arrows) acinus lacking Cx43 staining. Nuclei are stained with DAPI. At least 100 acini analyzed; n=3, unpaired t-test. **P<0.01, ***P<0.001. Scale bars: 10 µm.
Factors such as cell–cell adhesion, polarity and the orientation of cell divisions contribute to the formation of monolayered epithelia (Martin-Belmonte and Perez-Moreno, 2011; Akhtar and Streuli, 2013); however, the mechanisms underlying abnormal cell multilayering remain poorly understood. Compromised adhesion linked to impaired interaction of epithelial cells with their ECM (Myllymaki et al., 2011; Daley et al., 2012) or mutations in adhesion receptor E-cadherin (CDH1) (Jia et al., 2011) can give rise to aberrantly polarized epithelial structures along with the loss of monolayered epithelium. Cx43 might participate in cellular organization by influencing adhesion, since a significant correlation between its expression and that of E-cadherin has been reported (Jin et al., 2010; Tang et al., 2011). We found no difference in the percentage of acini displaying E-cadherin at cell–cell junctions (versus a diffused cytoplasmic distribution, for instance) upon AGA treatment (leading to loss of apical Cx43) (Fig. S5A,B), thus ruling out the possibility that disruption of cell–cell adhesion underlies acinus malformation.
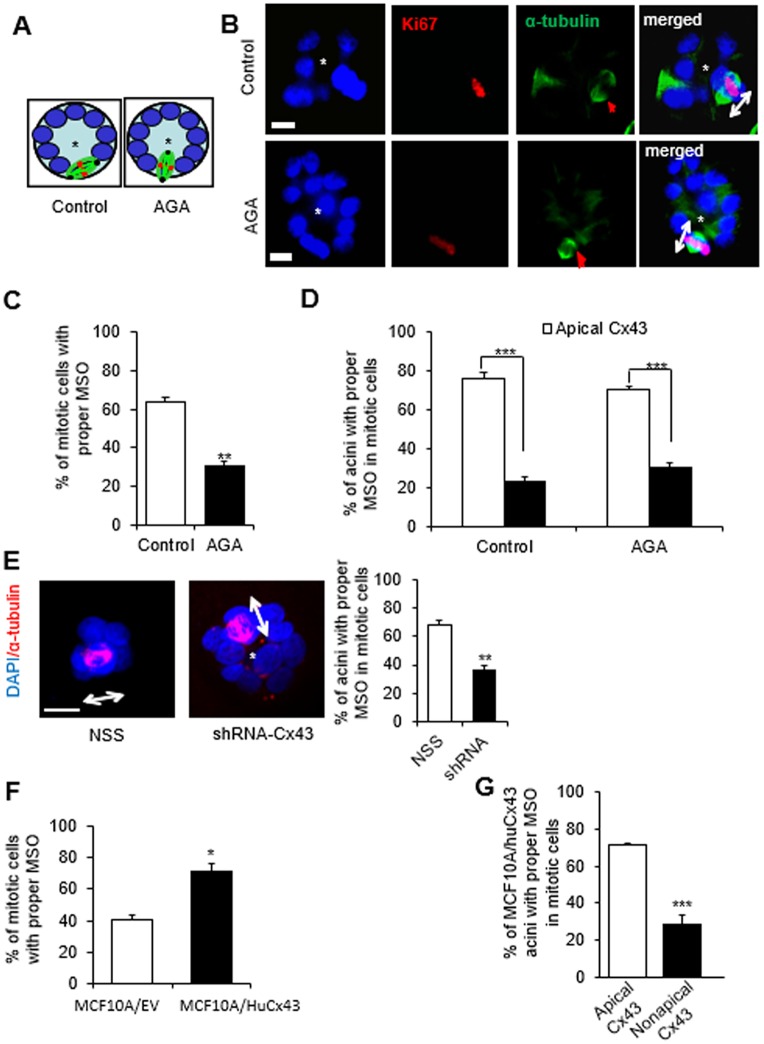
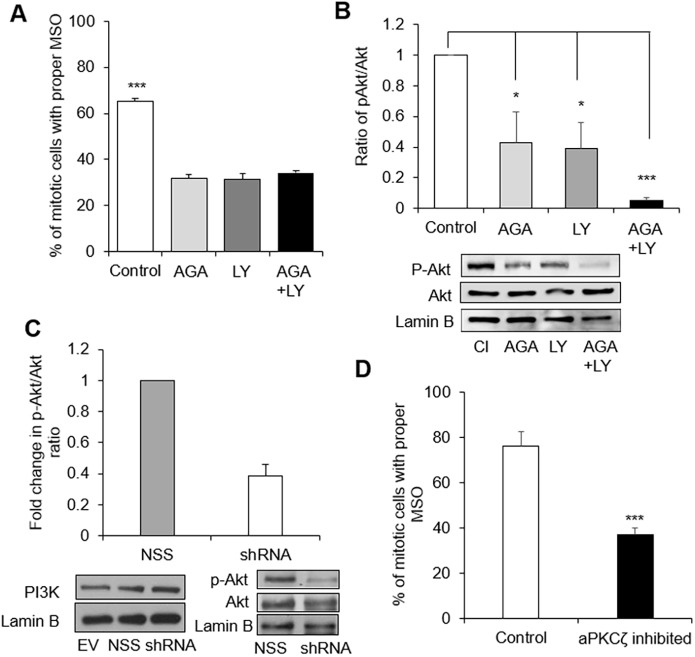
Cell multilayering might result from aberrant directional division, a known phenomenon for altered morphogenesis and disorganization of the neuroepithelium (Zheng et al., 2010; Peyre et al., 2011). In the mammary gland an aberrant MSO would be any direction whereby the spindle poles are not tangential to the circumference of the acinus, in other words, not ‘parallel to the basement membrane or substratum’. In such a situation, one or more of the daughter cells would remain in the center of the acinar structure upon completion of cytokinesis (Fig. 6A). The orientation of the mitotic spindle was assessed in cells treated with AGA and with vehicle until day 4 of 3D culture by immunostaining for α-tubulin. A significantly reduced number of mitotic cells in the AGA-treated cultures displayed proper MSO (i.e. tangential to the acinar circumference) compared to the control group (Fig. 6B,C). Dual immunostaining for α-tubulin and Cx43 revealed that at least 70% of acini containing mitotic cells with proper MSO (there is usually at most one mitotic cell per acinus) displayed apical Cx43 (Fig. 6D). Furthermore, S1 acini in the Cx43 shRNA-treated group exhibited proper MSO at a significantly lower rate than the control group (Fig. 6E). To confirm that expression of Cx43 is required for proper MSO, we analyzed MCF10A/EV cells that barely express Cx43, and MCF10A/HuCx43 cells in which Cx43 expression is mainly apical (see Fig. 4B). Proper MSO was significantly less frequently observed in MCF10A/EV group than in the group of MCF10A/HuCx43 cells (Fig. 6F). A higher percentage of MCF10A/HuCx43 acini displayed proper MSO when Cx43 was apically localized compared to structures with nonapical Cx43 (Fig. 6G).
Fig. 6.
Cx43-mediated GJs regulate mitotic spindle orientation (MSO). (A–D) S1 cells were cultured in 3D and treated, pre-lumen assembly, with vehicle or with AGA until day 4. Resulting multicellular structures were dual-immunostained for Ki67 (red) and α-tubulin (green). MSO was analyzed based on the directionality of the α-tubulin poles, either parallel to the basement membrane (or tangential to the circumference of the growing acini), which is the proper MSO to maintain a monolayered epithelium, or non-tangential to the circumference (conducive to cell multilayering) as drawn in A, and shown on representative structures in B. Red arrows in α-tubulin panels point to mitotic spindles and double-headed arrows in merge panels indicate MSO; the asterisk indicates the center of the acinar structure; nuclei are counterstained with DAPI (blue). (C) Graph shows mean±s.e.m. percentages of S1 cells that demonstrate proper MSO; at least 60 mitotic cells analyzed per group; n=3; non-paired t-test. (D) Mean±s.e.m. percentages of acinar structures with mitotic cells that show ‘correct’ MSO depending on the location of Cx43 (usually only one mitotic cell seen per acinus). At least 60 cells in each treatment group analyzed; n=3. (E) Representative acinar structures of S1 cells transduced with non-specific sequence (NSS) shRNA or with Cx43 shRNA (shRNA-Cx43) and immunostained for α-tubulin (red); double-headed arrows indicate MSO; the asterisk indicates the center of the acinar structure; nuclei are counterstained with DAPI (blue). Graph shows mean±s.e.m. percentages of S1 cells that show proper MSO; at least 60 mitotic cells analyzed per group; n=3; unpaired t-test. (F,G) MCF10A/HuCx43 and MCF10A/EV cells were cultured in 3D for four days and dual immunostained for Cx43 and α-tubulin. Graphs show mean±s.e.m. percentages of cells that display ‘correct’ MSO; n=3 (F) and percentages of MCF10A/HuCx43 acini with mitotic cells that that show ‘correct’ MSO depending on the location of Cx43 (G). At least 60 mitotic cells analyzed per group; n=3. *P<0.05, **P<0.01, ***P<0.001; unpaired t-test (C,F,G), one-way ANOVA with Dunn's comparison (D). Scale bars: 10 µm.
Cx43-mediated control of MSO involves the PI3K–AKT pathway
It has been established that β1-integrin regulates MSO in many cell types including epithelial cells (Théry et al., 2005; Reverte et al., 2006; Toyoshima and Nishida, 2007). Since β1-integrin participates in mammary acinar development (Berdichevsky et al., 1994), we investigated the potential role of this adhesion molecule in regulating MSO in the breast epithelium. Upon incubation of S1 cells with 15 µg/ml of AIIB2, a function-blocking antibody against β1-integrin (Damsky et al., 1992), between day 4 and day 6 of 3D culture, there was a slight, but significant, increase in the percentage of cells with proper MSO compared to mitotic cells in acini treated with 15 µg/ml of nonspecific IgG, suggesting that β1-integrin can influence MSO to be perpendicular to the lining of the epithelium; thus, an effect opposite to the impact of Cx43. Inhibition of both GJs and β1-integrin (AGA+AIIB2 treatment) also resulted in a significant increase in the number of acini with proper MSO compared to acini with GJ blockage only (Fig. S6A); however, blocking β1-integrin was insufficient to bring the percentage of cells with proper MSO back to the level measured in the control.
The protein kinase PI3K pathway has been involved in the regulation of MSO; notably its orientation parallel to the cell layer (Tuncay and Ebnet, 2016). Inhibition of PI3K in S1 cells up to day 4 of 3D culture, during the proliferation stage (pre-lumen assembly), with 1 µM of LY294002 induced a significant decrease in the percentage of cells with MSO ‘tangential to the circumference’ of the growing acini, as shown with α-tubulin immunostaining (Fig. 7A). The low percentage of cells with proper MSO in the group treated with both LY294002 and AGA was comparable to the groups treated either with AGA or with LY294002. Thus, PI3K proteins (PI3K hereafter) are also important players in Cx43-mediated control of MSO in the mammary epithelium. Indeed, phosphorylated (p)-AKT1 (p-AKT hereafter), a downstream target of PI3K, was significantly downregulated in the AGA-treated group and in the LY294002-treated group compared to control (Fig. 7B). A twofold decrease in p-AKT activity was also measured in acini formed by S1 cells treated with Cx43 shRNA compared to controls, without a decrease in PI3K levels (Fig. 7C). We conclude that blocking GJs inhibits PI3K, consequently disrupting the PI3K-dependent pathway that normally directs proper MSO.
Fig. 7.
Regulation of MSO by Cx43 involves PI3K–pAKT. (A,B) S1 cells were treated with vehicle (control) or with AGA, or with 1 μM of PI3K inhibitor LY294002 (LY), or with AGA+LY294002 pre-lumen assembly and until day 4 in 3D culture. Graphs show mean±s.e.m. percentages of cells with proper MSO (i.e. tangential to the acinus circumference) based on immunostaining for α-tubulin and analysis of at least 60 mitotic cells per group (A), and mean±s.e.m. ratios of expression levels of downstream effector of PI3K, p-AKT, over AKT, normalized to the control group based on quantification of western blot bands (B); n=3, one-way ANOVA with Dunn's comparison. A representative image of western blots for one of the replicates is also shown in B; lamin B is used as loading control. (C) Representative images of western blots for PI3K, AKT and p-AKT expression from 3D cultures of S1 cells transduced with Cx43 shRNA (shRNA), non-specific sequence shRNA (NSS) or empty vector (EV). Graph shows mean±s.e.m. relative levels of p-AKT expression compared to total AKT upon Cx43 silencing, normalized to expression in NSS control cells. (D) S1 cells were treated with 5 μM aPKC pseudosubstrate inhibitor until day 4 of 3D culture. Graph shows mean±s.e.m. percentages of cells with MSO tangential to the acini circumference based on α-tubulin immunostaining and analysis of a minimum of 60 cells; n=3; *P<0.05, ***P<0.001; unpaired t-test.
Among the top candidates that might act as effector proteins downstream of PI3K to control MSO is aPKCζ (also known as PRKCZ); this kinase is part of the Par6–aPKC polarity complex. Once activated it excludes NuMA–LGN, a major protein complex controlling MSO, from the apical domain of epithelial cells during mitosis (Zheng et al., 2010; Tuncay and Ebnet, 2016), ultimately allowing NuMA to interact at the lateral cortex with dynein, a member of the motor complex that orients the spindle in the equatorial cell membrane area (Johnston et al., 2009). As with α-tubulin staining, GJ inhibition in acini following AGA treatment resulted in a significant decrease in the number of cells with proper MSO when measured by the presence of NuMA at the mitotic spindle pole; inhibiting β1-integrin with function-blocking AIIB2 antibody increased the percentage of cells with proper MSO in control and AGA-treated acini, confirming results obtained with tubulin staining (Fig. S6B). NuMA has been considered a major anchor to orient the mitotic spindle via an interaction with proteins linked to the cell membrane (di Pietro et al., 2016; Tuncay and Ebnet, 2016). In an attempt to visualize a potential spatial relationship between Cx43 and NuMA for MSO control, we cultured S1 cells as a monolayer on a flat surface with induction of polarity by coating the culture surface with laminin 111 (Grafton et al., 2011). Proliferation rate is typically very low in the non-neoplastic epithelium. Among eight images of spindle poles recorded with confocal microscopy, we saw colocalization of Cx43 and NuMA at a mitotic spindle pole located next to a neighboring cell with MSO in the equatorial portion of the cell membrane only in one instance; thus, we could not correlate a specific location of Cx43 to MSO (Fig. S6C). Nevertheless, we could confirm the involvement of aPKCζ in MSO in S1 acini since treatment between day 1 and day 4 with 5 μM aPKC pseudosubstrate inhibitor (to halt aPKCζ activity) resulted in a significantly decreased occurrence of proper MSO compared to the control group (Fig. 7D).
The apical localization of Cx43 is compromised under conditions associated with mammary cancer risk
We have shown previously with the S1 model of acini that treatment with pro-inflammatory ω6 polyunsaturated fatty acid, arachidonic acid, leads to the disruption of tight junctions (Yue et al., 2012). Poor diet leading to a pro-inflammatory environment and overweight or obesity condition have been associated with increased risk for breast cancer (Calle and Kaaks, 2004; Deng et al., 2016). Here, we found that S1 acini co-cultured with adipose tissue explants derived from reduction mammoplasties performed on overweight women had a markedly reduced proportion of multicellular structures with strictly apical Cx43 (Fig. 8A,B). Similar results were obtained for growth-arrested acini in culture treated on day 10 (post-lumen formation) with fat-conditioned medium, consistent with a paracrine mechanism. Fat-tissue-derived adipokine leptin is a key mediator of the oncogenic effects of obesity (Andò and Catalano, 2012; Saxena and Sharma, 2013). Leptin treatment of S1 acini upon differentiation (day 10 of 3D culture) significantly altered Cx43 distribution, and this effect was abrogated by a leptin receptor antagonist, as well as with LY294002-mediated inhibition of PI3K, a downstream effector of leptin signaling (Fig. 8C,D). Since we could not include live breast adipose tissue from women who were not overweight in our conditioned medium experiments, we obtained mammary tissue sections from mice fed a normal or an obesity-inducing diet. The proportion of mammary acini displaying Cx43 at the apical side of the luminal cells was significantly reduced in animals fed the obesity-inducing diet compared to controls (Fig. 8E).
Fig. 8.
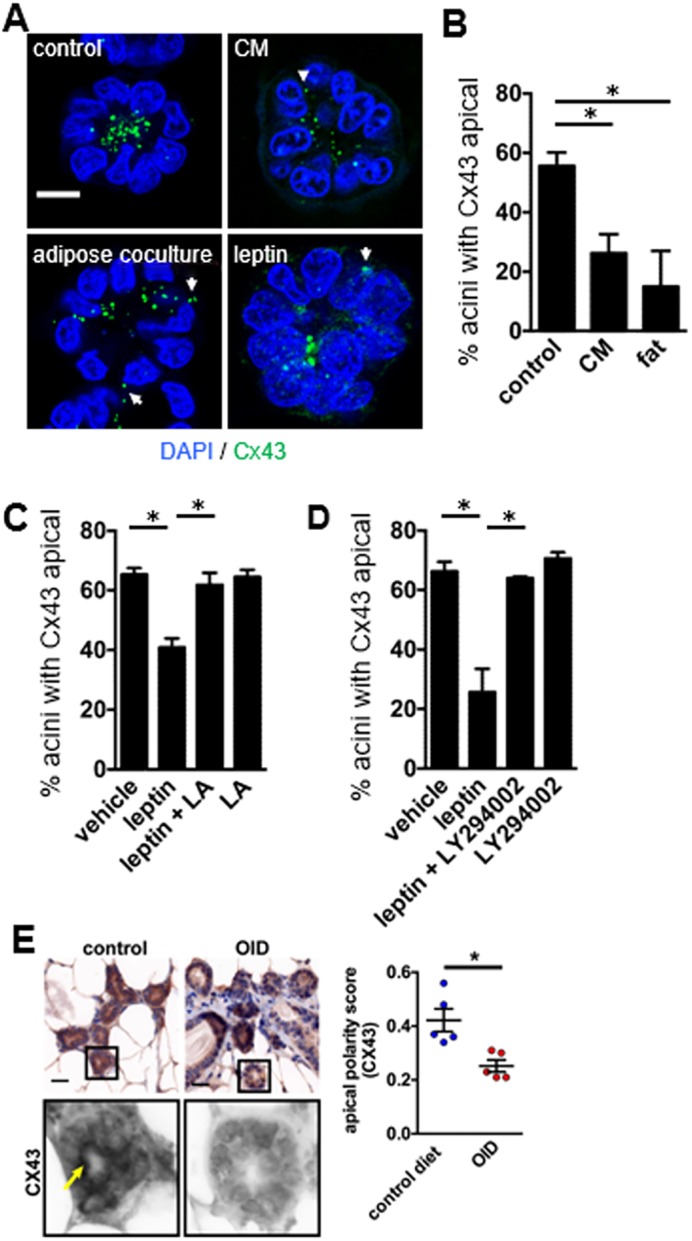
Cx43 localization is altered in models of overweight condition. (A) Representative images of Cx43 localization in S1 acini, treated for 72 h on day 10 of culture (post-lumen assembly) with mammary adipose tissue explants (adipose coculture), with conditioned medium (CM) from adipose tissue, or with recombinant leptin (100 ng/ml). Nuclei were stained with DAPI. Arrows and arrowhead indicate non-apical distribution of Cx43. Scale bar: 10 µm. (B–D) Mean±s.e.m. quantification of apical Cx43 localization in acini cultured in the presence of adipose tissue explants or CM (B), and in acini treated post-lumen assembly with leptin, a leptin receptor antagonist (LA) (C), or leptin and PI3K inhibitor LY294002 (D). *P<0.05, one-way ANOVA with Tukey test. (E) Immunohistochemical analysis of Cx43 distribution in whole-mount mammary glands from mice fed control or obesity-inducing diets (OID). Cx43 signals were extracted from immunohistochemistry images using spectral imaging (bottom images). The arrow points to apical Cx43 signals. Cx43 localization was evaluated with a three-grade scale (0=no apical signal; 1=partial, discontinuous apical signal lining the lumen; 3=continuous apical signal) by two investigators blind to the treatment conditions (≥25 acini or mammary ducts analyzed for each animal). Cx43 localization is quantified in the graph. Data represented as mean±s.e.m.; *P<0.01; unpaired t-test. Scale bar: 25 µm.
DISCUSSION
We demonstrate the implication of Cx43 and its connexon in epithelial homeostasis. In cell culture, Cx43 controls the establishment and maintenance of apical polarity and, thus, cell cycle entry. Moreover, it directs MSO, contributing to the maintenance of epithelial organization. The absence of Cx43 from the luminal epithelium is associated with an atypical structure (with cells piling up) as observed in breast tissue sections.
We show that both leptin and adiposity activate the loss of apicolateral distribution of Cx43. These results substantiate our recent publication that these risk factors disrupt apical polarity (as measured by the distribution of tight junctions) and alter MSO in the breast epithelium (Tenvooren et al., 2019). Ultimately, as shown in mammary tissue sections related to obesity, Cx43 is lost from the luminal epithelium. Possible routes for Cx43 gene silencing include disruption of GATA-3 binding or expression of oncogenic microRNAs (Hao et al., 2012; Liu et al., 2015). Further work will determine the place of Cx43 in obesity-mediated risk of tumor development. Interestingly, the response to Cx43 alterations in cell culture involved only a portion of the acini. The epigenetic status of the mammary epithelium might condition the response to polarity disruptors, since we have shown previously that apical polarity is under epigenetic control (Plachot and Lelièvre, 2004).
Relationship between the location of Cx43 and epithelial polarity
The presence of Cx26, Cx30, Cx32 and Cx43 has been reported in the mammary gland, but in humans Cx43 was considered a feature of myoepithelial cells. Yet, only Cx43 was associated with better prognosis of breast cancer (Teleki et al., 2014), stressing its importance for normal breast homeostasis. Our immunohistological investigation of archival breast tissue sections revealed the punctate apicolateral location of Cx43 in the luminal epithelium, as we initially observed in breast acini in vitro. We wish to point out that apical staining for Cx43 is also observable on some of the epithelial structures in Fig. 3 of the report from Teleki et al. (2014), although not indicated in their manuscript. Apical polarity is a labile feature that is modulated by many factors, including aging; thus, certain polarity components can be easily missed from tissue sections. Cx43 is present at luminal cell–cell interactions in the murine mammary gland and is expressed in these cells throughout developmental stages (Dianati et al., 2016). Our observation of Cx43 at the apical side of the breast epithelium was essential to pursue the functional study of the role of this connexin in luminal epithelial differentiation. Cx43 was previously reported to be apical in the intestine and the lens epithelia where it forms functional GJs, but it was not studied in the context of epithelial polarity (Donaldson et al., 1994; White et al., 2001).
Polarity is critical for epithelial homeostasis (McCaffrey and Macara, 2011). The plethora of different connexins enables selectivity in channel permeability and selective participation in homeostasis via interaction with other proteins depending on the tissue type and development stage (Dianati et al., 2016). We show that breast luminal differentiation is influenced by the apical location of Cx43, where this connexin forms gap junctions and interacts with homeostasis signaling protein β-catenin. Apical polarity (illustrated by core protein ZO-1) is restored in MCF10A acini when Cx43 is apical upon do novo expression (Fig. 4). Moreover, Cx43 and ZO-2 interact in differentiated mammary epithelial cells (Fig. 3H), which reinforces the proposed existence of a cross-talk between GJs and tight junctions (Kojima et al., 2002; Morita et al., 2004; Zemljic-Harpf et al., 2014). The literature supports the assembly of junctional nexuses with overlapping and common regulatory pathways between GJ, tight and adherens junctions (Bazzoun et al., 2013; Dianati et al., 2016), which suggests that components of these junctions might influence each other during polarity development and maintenance; thus, we cannot rule out that Cx43 might help recruit specific elements of the tight junctions.
Cx43 controls the direction of cell division
Normally, luminal cells divide within the plane of the epithelium (Villegas et al., 2014). A random MSO upon blocking gap junctions or via the silencing of Cx43 in the luminal epithelium revealed the involvement of connexins in the control of cell multilayering. Interestingly, stratification of skin has been shown to be positively influenced by β1-integrin (Lechler and Fuchs, 2005), whereas in other cell types, this integrin has been reported to direct MSO tangential to the epithelium (LaFlamme et al., 2008). Indeed, a lack of functional β1-integrin has been shown to induce mammary myoepithelial cell division perpendicularly to the basement membrane (Taddei et al., 2008). The mammary gland is part of the integumentary system and develops as an ectodermal skin specialization; yet, we show that Cx43 is paramount for MSO instead of β1-integrin. We speculate that the interaction between Cx43 junctional complex and tight junction ZO proteins, or with molecules like Par3 that are involved in the PI3K–AKT pathway controlling MSO (Tenvooren et al., 2019), might underlie Cx43-mediated MSO. It is unlikely that Cx43 exerts an impact on MSO via its known interaction with tubulin (Sorgen et al., 2018; Vanderpuye et al., 2016), since we rarely observed Cx43 localized near the mitotic spindle (Fig. S6C).
An altered MSO might contribute to cancer progression by increasing aneuploidy, expanding the cancer stem cell pool and facilitating tissue disorganization (Caldwell et al., 2007; Qin et al., 2010; Quyn et al., 2010). Our immunohistochemical analysis of normal human breast tissues supports a relation between Cx43 and maintenance of an organized or typical luminal epithelium (without cells piling up towards the lumen) in vivo. Piling up of epithelial cells is already seen in premalignant mammary lesions such as ductal hyperplasia (Godde et al., 2014). Even if misoriented spindles have been observed in certain tumor models, not all tumors display striking spindle misorientation, and not all spindle misorientation events are tumorigenic (Fischer et al., 2006; Fleming et al., 2009; Patel et al., 2008). Nevertheless, altered MSO might play a synergistic role with other changes during tumorigenesis.
We have reported a dominant involvement of Cx43-mediated GJs in the control of apical polarity and MSO. Specifically, the loss of activity of PI3K (an activator of Cdc42) due to Cx43 silencing and loss of GJIC reduced aPKC activity (usually triggered by Cdc42–GTP) that regulates MSO (Jaffe et al., 2008; Bray et al., 2011). Moreover, the fact that Par3 (also known as PARD3), typically localized to tight junctions, complexes with Cdc42–GTP and aPKC (Joberty et al., 2000; Etienne-Manneville and Hall, 2003) integrates the control of polarity and MSO in a single pathway. Indeed, altered MSO has been linked with loss of controllers of apical polarity, such as Par3 and aPKC, that resulted in the formation of mammary ducts with multilayers of cells (Kojima et al., 2008; McCaffrey et al., 2012; Xue et al., 2013). Interestingly, adherens junction cadherins control MSO in a NuMA–LGN-dependent manner and require β-catenin (Gloerich et al., 2017). As apicolateral Cx43 also interacts with β-catenin in acini, it suggests a powerful control of Cx43 over MSO via an impact on two distinct regulatory pathways (Fig. S7).
Somehow, the apicolateral localization of Cx43 may contribute to the structure necessary to establish polarity in tissues, notably since polarity is organized as part of a morphogenesis process, like acinus formation, in which cell–cell interaction is paramount. The GJs might mediate this role early on when cells make contact via E-cadherins, in a channel-dependent manner, by enabling the exchange of signals involved in polarity initiation. For instance, in Drosophila, polarity is mediated across the tissue by the passage of yet-to-be-determined polarity signals through direct cell–cell interactions; in zebrafish, communication through GJ has been shown to control important aspects of polarity related to morphogenesis (Park et al., 1994; Mathews and Levin, 2017). The GJs might also be involved in later stages of basoapical axis formation, possibly in a channel-independent manner by regulating core polarity and signaling molecules via an interaction with Cx43 (Fig. S7). The question remains whether channel-dependent GJ communication or Cx43 per se (i.e. channel-independent mechanism) is a key element in polarity control. Possibly, both might be involved with loss of Cx43 leading to a more profound impact on homeostasis if connections with control proteins for major channel-independent signaling pathways, such as β-catenin, are lost. Earlier studies showed that transfecting breast cancer cells with full-length Cx43 downregulated replication and invasion in a GJIC-independent manner, due to the association of Cx43 (via its carboxy terminus) with β-catenin and ZO-2 at the cell membrane. Carboxy-terminus truncated Cx43 restored GJIC but did not reduce proliferation or invasion (Talhouk et al., 2013). New information suggests that Cx43 loss induces cell cycle entry (Fostok et al., 2019), whereas AGA treatment only primed the cells for cycle entry (Fig. 2G).
Finally, the concentration of gap junctions to apicolateral tight junctions might provide local signaling to maintain the integration of polarity and MSO homeostasis. Interestingly, ZO-1, localized with ZO-2 in tight junctions in the mammary gland, plays a role in MSO specifically in the context of an acinar geometry (Odenwald et al., 2017). Mechanical tension linked to the curvature of the acinus or duct might exert a specific impact on homeostasis, possibly favored by signaling in its vicinity. Deciphering the role of Cx43 in the mechanotransduction of acinar morphogenesis would be interesting, considering the importance of this type of signaling to complement biochemical pathways.
MATERIALS AND METHODS
Cell culture
Non-neoplastic S1 HMT-3522 human mammary epithelial cells (Briand et al., 1987), between passages 52 and 60, were routinely maintained as a monolayer (2D culture) in chemically defined serum-free H14 medium (Blaschke et al., 1994; Plachot and Lelièvre, 2004). MCF10A cells (ATCC, Manassas, VA) were cultured in DMEM/F12 supplemented with 5% donor horse serum, 20 ng/ml epidermal growth factor (EGF), 10 µg/ml insulin, 0.5 µg/ml hydrocortisone and 100 ng/ml cholera toxin. The drip method of three-dimensional (3D) cell culture was used to induce the formation of acini. Briefly, S1 and MCF10A cells were plated on Matrigel (50 µl/cm2, BD Biosciences, Bedford, MA) at a density of 4.2×104 cells/cm2 in the presence of culture medium containing 5% Matrigel (Plachot and Lelièvre, 2004). The EGF was omitted from the culture medium after day 7 to allow completion of acinar differentiation (usually observed on day 8 or 9) (Plachot and Lelièvre, 2004). To induce basoapical polarity formation in S1 cells cultured as a flat monolayer, cells were seeded on coverslips coated with 2.07 μg/cm2 laminin (entactin-free) (Corning, Corning, NY) (Plachot et al., 2009). S1 cells were obtained in 2000 from Mina J. Bissell’s laboratory (Lawrence Berkeley National Laboratory, Berkeley, CA); the most recent genetic test on their characteristics was performed a few years ago by the Lelièvre laboratory during experiments done for the present manuscript (Jayaraman et al., 2017). These cells were shipped to the Talhouk laboratory. Assessment of contamination with mycoplasma is done regularly. In addition, the phenotype of S1 and MCF10A cells is frequently assessed in 3D cell culture by the Lelièvre and Talhouk laboratories to confirm proper differentiation and identity since the differentiation status of each of these cell lines is typical and easily recognizable with polarity markers (Plachot et al., 2009). Finally, to avoid cross-contamination of cell lines, each cell line is split for passage on an individual basis.
Treatments encompassed 18α-glycerrhitinic acid (AGA; 10–100 μM) (Sigma-Aldrich, St Louis, MO), 5 μM of aPKCζ pseudosubstrate (myristoylated) inhibitor (Enzo LifeSciences, Farmingdale, NY), 1 or 8 µM LY294002 (Cell Signaling Technology, Danvers, MA), 15 µg/ml rat IgG anti-human β1 integrin (AIIB2) antibody (a kind gift from Caroline Damsky, University of California San Francisco, CA) and 15 µg/ml nonspecific IgG (Jackson ImmunoResearch, West Grove, PA). The cell culture medium was changed every 48 h.
Co-culture with fat tissue explants or conditioned medium
Breast adipose tissue from reduction mammoplasties were obtained with the patients' consent. The Purdue BioMedical Institutional Review Board (IRB) (IRB registration number: 0004580) can affirm that S.A.L. and colleagues obtained approval of the document (Purdue IRB protocol 1206012467, entitled Interplay Between Tissue Architecture and Nuclear Organization in the DNA Damage) used to obtain written informed consent from the subjects. The IRB conducted an ethical review of the study protocol in accordance with recognized ethical guidelines (e.g. the Belmont Report and U.S. Common Rule).
Within 30 min following surgery, tissues were resected to trim ≥1 cm of the surgical margins, minced to ∼0.5 cm fragments, and then immediately rinsed with RPMI and transferred into RPMI-containing vials for transport. Adipose tissue explants were co-cultured for 72 h with differentiated acini in 3D culture (day 10 of culture, post-lumen assembly). Histological assessment of the adipose tissue explants revealed minimal content of epithelium and confirmed viability. To generate conditioned medium (CM), adipose tissue explants were transferred into H14 culture medium (10 ml CM were made per tissue collection, corresponding to 2.1±0.2 g of tissue per ml of medium) supplemented with penicillin (50 U/ml), streptomycin (50 µg/ml), and amphotericin B (0.5 µg/ml). After 48 h incubation at 37°C in a humidified cell culture incubator, CM was collected, centrifuged, sterile-filtered, and flash-frozen.
Transfection and infection protocols
The recombinant shRNA retroviral constructs against Cx43 have been described previously (Shao et al., 2005). Retroviral vectors (2 µg) containing Cx43 shRNA, non-specific sequence shRNA (NSS), and empty vector control (EV) were transfected into Phoenix packaging cells using calcium phosphate (Stratagene, La Jolla, CA) according to the supplier's protocol. For infection, filtered retroviral supernatants were applied to monolayers of S1 cells at day 3. Cells were incubated with hexadimethrine bromide (polybreen) (6 µg/ml; Sigma-Aldrich) for 8 h. The infection medium was removed, and cells were incubated in regular H14 medium for 24 h. Infection was repeated two additional times and selection with hygromycin-B (150 µg/ml; Calbiochem, San Diego, CA) was started 72 h after the last infection.
The pCDF-MCS1 plasmid vectors containing HuCx43 and EV control were transfected into 293TN cells according to the supplier's protocol. MCF10A cells were plated in a 60 mm dish 72 h prior to viral infection in 3.8 ml of H14+EGF. Viral supernatant (2 ml) was added directly to MCF10A cells and incubated with polybreen (5 μg/ml) at 37°C overnight. The medium was replaced with a polybreen-free medium the next day and left overnight. Infection was repeated two additional times and selection with puromycin (50 µg/ml; Sigma-Aldrich) in H14+EGF medium was started 48 h after the last infection.
mEmerald-Cx32-7 used for transient transfection was deposited by Michael Davidson (Addgene plasmid 54054). MCF10A cells and S1 cells cultured on laminin 111 (133 μg/ml final concentration corresponding to 2.07 μg/cm2 of culture surface) were transfected, at 30% confluence, with 1 μg of plasmid DNA (Fort et al., 2011) using Lipofectamine 3000 (Thermo Fisher Scientific). Transfection efficiency was determined based on the percentage of cells positive with mEmerald staining (green). Control cells were transfected with empty vector.
GJIC assay by scrape-loading and microinjection
The scrape-loading method (El-Fouly et al., 1987) was used to determine the minimum concentration of AGA that blocks GJ in S1 cells. Cells were plated as a monolayer in 35 mm dishes and cultured for 10 days. Cells were then treated for 1 h with AGA (10, 20, 50, 75 and 100 µM) or corresponding concentrations of vehicle (DMSO). After rinsing the cells with warm phosphate-buffered saline (PBS) twice, a dye mixture of 2.5% Lucifer Yellow CH (LY) (Sigma-Aldrich) and 2% rhodamine-B isothiocyanate-dextran (RD) (Sigma-Aldrich) in PBS was added. Scrapes were made through the monolayer with a surgical scalpel blade #20 (Swann-Morton, UK) and culture dishes were incubated at 37°C for 5 min. The dye mixture was removed and cultures were rinsed three times with warm PBS, and fixed with 4% buffered paraformaldehyde (Sigma-Aldrich). Dye spread was observed using epifluorescence microscopy and images were recorded.
GJ assay in 3D culture was performed by microinjection of a dye mixture of LY (2.5%) and RD (2%) dissolved in 0.15 M LiCl in single S1 cells of acini formed in 3D culture, in 35 mm dishes. Some of the acini populations were treated with AGA as described above. Penetration of the cell membrane was determined by monitoring the membrane potential, which ranged between −19 mV and −35 mV (AxoClamp 2B amplifier, Axon Instruments, Foster City, CA). Cells were injected ionophoretically with micropipettes using hyperpolarizing pulses, 4–5.5 nA/500 ms at a frequency of 0.75 Hz for 1 min, produced by a Grass S88 stimulator (Grass Instruments, West Warwick, RI). The micropipettes, when filled with 3 M KCl, had tip resistance of 15–20 MΩ and were pulled using a P-97 microelectrode puller (Sutter Instruments, Novato, CA). Single cells in 9–12 acini in each experiment group were microinjected. During microinjections, acini were kept in culture medium at room temperature (RT). Intercellular dye diffusion was allowed to proceed for 15 min and acini were observed using epifluorescence microscopy. Dye coupling was determined by assessing the spread of LY from the injected cell (marked by RD) to the other cells in the acinus.
GJ assay also encompassed microinjection of neurobiotin (NB) (Invitrogen Molecular Probes, Eugene, OR). Cells were plated in 3D on Matrigel-coated 13 mm diameter cell culture Thermanox coverslips (Nunc, Rochester, NY). The coverslips were then transferred into 35 mm cell culture dishes and maintained in H14 medium. On day 10, 10–12 acini were microinjected with 3% NB in 0.15 M LiCl. A single cell in an acinus was injected ionophoretically with depolarizing pulses of 2 nA/600 ms at a frequency of 1 Hz for 2 min. Intercellular dye diffusion was allowed to proceed for 15 min. NB was revealed with streptavidin–FITC (1:40 dilution, Vector Laboratories, Burlingame, CA) according to the protocol described in the immunostaining section.
Immunohistochemistry
Archival formalin-fixed normal adult breast tissues were obtained from the Department of Surgical Pathology at the Indiana School of Medicine, Indianapolis, IN. Tissue samples were used according to IRB approval 0502000712. For immunohistochemistry, tissue sections (4 µm) were sequentially processed for deparaffinization, rehydrated and antigens were revealed by boiling in Antigen Unmasking Solution (Vector). Following washes with Tris-buffered saline (TBS) (10 mM Tris base, 150 mM NaCl), endogenous peroxidase was blocked by incubating in 0.03% hydrogen peroxide in TBS for 15 min. Samples were then washed with TBS and TBST (TBS with 0.05% Tween-20) and incubated for 1 h at RT with blocking reagents (Avidin/Biotin Blocking Kit, Vector) followed by overnight incubation at 4°C with rabbit anti-Cx43 primary antibody (0.5 mg/ml; C6219, Sigma-Aldrich). Samples were washed with TBS and TBST and incubated with biotynyl-conjugated anti-rabbit polyclonal antibody (1:1000; E043, DakoCytomation, Carpinteria, CA) for 1 h at RT. Following washes with TBS and TBST, specimens were incubated for 30 min at RT in streptavidin–horse radish peroxidase (SA–HRP) (PerkinElmer Life Sciences, Waltham, MA) diluted to 1:100 in blocking reagent. Following washes with TBS and TBST, specimens were incubated for 15 min at RT with biotinyl-tyramide signal amplification solution (PerkinElmer Life Sciences). Specimens were then washed with TBS and TBST and incubated with SA–HRP for 30 min at RT. After washes with TBS and TBST, specimens were incubated with 3, 3'-diaminobenzidine (DAB) (Peroxidase Substrate Kit, Vector) for 5 min. Nuclear counterstaining was performed for 45 s with Gill III Hematoxylin (Leica Biosystems, Buffalo Grove, IL), and coverslips were fixed to the tissue sections on slides with Permount mounting reagent (Fisher Scientific, Fair Lawn, NJ).
For the study with animal tissues, we obtained sections of mammary glands from mice fed a normal diet (TD.08806) or an obesity-inducing diet (60% kcal from fat; TD.06414), both from Teklad diets (Envigo, Indianapolis, IN, USA) (Tenvooren et al., 2019). Animal experimentation was compliant with ethical standards and was approved by the Animal Care and Use Committee of the Wake Forest School of Medicine (IACUC protocol A16-010). The animals were not euthanized as part of this work. Instead, control groups from another study on obesity and carcinogenesis were repurposed for the present study. Mammary glands had been collected at 20 weeks of age, fixed, and embedded in paraffin. Immunohistochemistry was performed using an antibody against CX43 (0.5 µg/ml; C6219, Sigma-Aldrich). Tissue sections were stained with hematoxylin, and a Mantra system (Perkin Elmer, Waltham, MA) was used for spectral imaging and channel separation of diaminobenzidine (DAB) and hematoxylin signals.
Immunofluorescence labeling
Fresh 3D cultures or cryosections from frozen embedded cultures were stained for markers of differentiation as described previously (Plachot and Lelièvre, 2004). Briefly, cells were either permeabilized with 0.5% peroxide and carbonyl-free Triton X-100 (Sigma-Aldrich) in cytoskeleton buffer (100 mM NaCl, 300 mM sucrose, 10 mM PIPES pH 6.8, 5 mM MgCl2, 1 mM pefabloc, 10 µg/ml aprotinin, 250 µM NaF) prior to fixation in 4% paraformaldehyde (Sigma-Aldrich), or directly fixed with 4% paraformaldehyde. Antibodies used were rabbit polyclonal against Cx43 (0.5 mg/ml; C6219, Sigma-Aldrich; Tong et al., 2009), Ki67 (100 μg/ml; VP-K451, Vector; Knezevic et al., 2015), mouse monoclonal against NuMA (1:2; B1C11, a gift from Dr Jeffrey Nickerson, University of Massachusetts, Worcester, MA; Vidi et al., 2011), ZO-1 (2.5 μg/ml; 33-9100, Life Technologies; Grand Island, NY; Mao et al., 2017), α-tubulin (1:500; T5168, Sigma-Aldrich; Piao et al., 2014), β-catenin (2.5 μg/ml; C19220, BD Biosciences; Gomes et al., 2013), E-cadherin (1:50; 610181, BD Biosciences; Elisha et al., 2018), and rat anti-α6 integrin (5 μg/ml; clone NKI-GoH3, EMD Millipore, Billerica, MA; Villa-Diaz et al., 2016). For immunostaining for lysosomal associated membrane 2 (LAMP-2) (1:100; mouse, A15464, Life Technologies), cells were fixed with methanol acetone solution (1:1 ratio) without permeabilization. Secondary antibodies conjugated with Alexa Fluor 488 (green), Alexa Fluor 594 (red) and Alexa Fluor 568 (red) (Invitrogen Molecular Probes) were used at the manufacturer's proposed dilutions. Nuclei were counterstained with 0.5 µg/ml 4′,6-diamidino-2-phenylindole (DAPI) and specimens were mounted in ProLong Gold antifade reagent (Invitrogen Molecular Probes).
BrdU labeling
Active cell proliferation was determined by immunofluorescence detection of 5-bromo-2′-deoxy-uridine (BrdU) incorporated into DNA during the S phase of the cell cycle. The BrdU assay was performed on day 4 of cell culture using a BrdU labeling kit according to the manufacturer's instructions (Roche Diagnostics Corporation, Indianapolis, IN).
Preparation of whole cell protein extracts and western blot analysis
Cells were harvested from 3D cultures as described earlier (Plachot and Lelièvre, 2004). Briefly, acini were released from the Matrigel by incubation with Dispase (BD Biosciences), and whole cell extracts were prepared in lysis buffer (2% SDS in PBS with 10 mg/ml aprotinin, 100 mM Pefabloc, 250 mM NaF) (Lelièvre et al., 1998), except for p-AKT that was prepared in RIPA buffer (25 mM Tris HCl, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS). For western blot analysis equal amounts of proteins were separated and immunoblotted with rabbit polyclonal antibodies against Cx43 (0.16 μg/ml; C6219 and 5 μg/ml; SAB4501175, Sigma-Aldrich; Ni et al., 2017), p-Akt (1:100; 9271, Cell Signaling Technology; Zhang et al., 2018), Akt (9272, 1:1000, Cell Signaling Technology; Geyer et al., 2018), β-catenin (0.04 μg/ml; sc7199, Santa Cruz Biotechnology; Nedvetsky et al., 2012); goat polyclonal antibody against ZO-2 (2 μg/ml, sc-8148, Santa Cruz Biotechnology; Talhouk et al., 2008); and monoclonal antibodies against Cx26 (0.5 μg/ml; 13-8100, Zymed Laboratories, San Francisco, CA), Cx43 (2 μg/ml; sc-271837, Santa Cruz Biotechnology; Lai et al., 2018) and ZO-1 (1 μg/ml; 33-9100, Life Technologies). Equal protein loading was verified by immunoblotting for lamin B (0.6 μg/ml; rabbit, Ab16048, Abcam; Jayaraman et al., 2017) and β-actin (1:1000; rabbit, A8227, Abcam; Chen et al., 2018). Protein levels were quantified using Scion NIH Image software (Scion Image, Scion Corporation, NIH) or ImageJ (http://imagej.nih.gov/ij/) and normalized to lamin B or β-actin expression levels.
Immunoprecipitation
Acini from 3D culture were collected after dispase treatment as published (Plachot and Lelièvre, 2004) and immunoprecipitation followed a previously described protocol (Talhouk et al., 2008) with the following modifications: samples were sheared using a 27 gauge needle and protein G sepharose beads were used for this experiment with 0.25 μg rabbit polyclonal Cx43 antibody (710700, Invitrogen; Mroue et al., 2015). At the end, supernatants were loaded into a 12% polyacrylamide gel for electrophoresis.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from cells using RNeasy Minikit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Total RNA (1 µg) was reverse transcribed to cDNA using Revertaid first strand cDNA synthesis kit (Fermentas, Grand Island, NY). qRT-PCR was performed using iQSYBR Green Supermix in a CFX96 system (Bio-Rad Laboratories, Hercules, CA). Products were amplified using primers for: Cx43, 5′ CAA TCA CTT GGC GTG ACT TC 3′ (forward) and 5′ GTT TGG GCA ACC TTG AGT TC 3′ (reverse); Cx30, 5′ TCA ACA AAC ACT CCA CCA GC 3′ (forward) and 5′ CAA TCC CAC ATT TCA ACA CC 3′ (reverse); Cx32, 5′ GAC AGG TTT GTA CAC CTT GC 3′ (forward) and 5′ CGT CGC ACT TGA CCA GCC GC 3′ (reverse); Cx26, 5′ TCT TTT CCA GAG CAA ACC GC 3′ (forward) and 5′ GAC ACG AAG ATC AGC TGC AG 3′ (reverse); Cx45, 5′ GGA GCT TTC TGA CTC GCC TG 3′ (forward) and 5′ CGG CCA TCA TGC TTA GGT TT 3′ (reverse); GAPDH, 5′ AAG GTG AAG GTC GGA GTC AAC 3′ (forward) and 5′ GGG GTC ATT GAT GGC AAC AAT A 3′ (reverse). To quantify changes in gene expression, the ΔCt method was used to calculate the relative fold-changes normalized to GAPDH gene expression levels.
Image processing and analysis
Images of immunofluorescence labeling were recorded using an inverted IX70 fluorescence microscope (Olympus) equipped with a Retiga 1300 camera (QImaging, Surrey, BC, Canada) and 40×, NA 1.4 fluor lens, and a multiphoton laser scanning confocal Radiance 2100 MP (Bio-Rad Laboratories, Hemel Hampstead, UK) linked to a TE2000 (Nikon, Tokyo, Japan) inverted microscope, and oil immersion 60×, numerical aperture (NA) 1.4 apochromatic. Brightfield images of H&E-stained slides were acquired on a Nikon Diaphot 300 equipped with a QImaging color Retiga 1300i FAST. Images were processed using Confocal Assistant 4.02 (Bio-Rad Laboratories) and assembled using Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA).
Statistical analysis
Data are presented as mean±s.e.m. Statistical comparisons were done using GraphPad Prism 3.0 software (GraphPad Software Inc., San Diego, CA). Unpaired t-test was used for comparison of two groups in general; paired t-test was used for comparison of two conditions within a same culture or archival tissue sample (e.g. multilayering depending on Cx43 polarity on archival tissue sections; ZO-1 location depending on Cx43 in acini formed by MCF10A); whereas one-way ANOVA with Dunn's multiple comparison test or with Tukey test was employed for three or more groups of treatments. P<0.05 was considered significant. For immunofluorescence, when quantification was used, two investigators analyzed the same slides or experiments; if only one investigator was analyzing, whenever possible s/he was blinded to the group allocation (code used on slides). Unless otherwise specified, ‘n’ in the figure legends corresponds to the number of biological replicates (i.e. independent cell cultures usually of different passages).
Supplementary Material
Acknowledgements
P.-A.V., R.S.T. and S.A.L. are members of International Breast Cancer and Nutrition (IBCN).
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: H.A.A., P.-A.V., S.A.L.; Methodology: A.U., J.S., S.A.L.; Validation: D.B., K.H.; Formal analysis: H.A.A., D.B., L.W., A.U., S.C., J.S., G.C., P.-A.V., S.A.L.; Investigation: H.A.A., D.B., L.W., A.U., I.T., S.F.F., S.C., G.C., P.-A.V.; Resources: K.H.; Writing - original draft: H.A.A., D.B., S.A.L.; Writing - review & editing: H.A.A., R.S.T., S.A.L.; Visualization: H.A.A., D.B., L.W., S.C., J.S., G.C., P.-A.V., S.A.L.; Supervision: P.-A.V., R.S.T., S.A.L.; Project administration: S.A.L.; Funding acquisition: S.A.L., P.-A.V., R.S.T.
Funding
This work was supported by a grant from the Purdue University Center for Cancer Research to S.A.L., the National Institutes of Health (NIH) (CA171704 to S.A.L. and CA163957 to P.-A.V.), the National Council for Scientific Research–Lebanon (CNRS-L) and the American University of Beirut (AUB) University Research Board (URB) to R.S.T., and a United Nations Educational, Scientific and Cultural Organization (UNESCO)–Fondation L'Oréal International Fellowship for Women in Science to D.B. Some of the experiments were performed thanks to support from the Purdue University Center for Cancer Research, under National Institutes of Health grant P30 CA023168. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.223313.supplemental
References
- Abad P. C., Lewis J., Mian I. S., Knowles D. W., Sturgis J., Badve S., Xie J. and Lelièvre S. A. (2007). NuMA influences higher order chromatin organization in human mammary epithelium. Mol. Biol. Cell 18, 348-361. 10.1091/mbc.e06-06-0551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar N. and Streuli C. H. (2013). An integrin–ILK–microtubule network orients cell polarity and lumen formation in glandular epithelium. Nat. Cell Biol. 15, 17-27. 10.1038/ncb2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andò S. and Catalano S. (2012). The multifactorial role of leptin in driving the breast cancer microenvironment. Nat. Rev. Endocrinol. 8, 263-275. 10.1038/nrendo.2011.184 [DOI] [PubMed] [Google Scholar]
- Bazzoun D., Lelièvre S. and Talhouk R. (2013). Polarity proteins as regulators of cell junction complexes: Implications for breast cancer. Pharmacol. Ther. 138, 418-427. 10.1016/j.pharmthera.2013.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdichevsky F., Alford D., D'Souza B. and Taylor-Papadimitriou J. (1994). Branching morphogenesis of human mammary epithelial cells in collagen gels. J. Cell Sci. 107, 3557-3568. [DOI] [PubMed] [Google Scholar]
- Blaschke R. J., Howlett A. R., Desprez P.-Y., Petersen O. W. and Bissell M. J. (1994). Cell differentiation by extracellular matrix components. Methods Enzymol. 245, 535-556. 10.1016/0076-6879(94)45027-7 [DOI] [PubMed] [Google Scholar]
- Bray K., Brakebusch C. and Vargo-Gogola T. (2011). The Rho GTPase Cdc42 is required for primary mammary epithelial cell morphogenesis in vitro. Small GTPases 2, 247-258. 10.4161/sgtp.2.5.18163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand P., Petersen O. and Van Deurs B. (1987). A new diploid nontumorigenic human breast epithelial cell line isolated and propagated in chemically defined medium. In Vitro Cell Dev. Biol. 23, 181-188. 10.1007/BF02623578 [DOI] [PubMed] [Google Scholar]
- Caldwell C. M., Green R. A. and Kaplan K. B. (2007). APC mutations lead to cytokinetic failures in vitro and tetraploid genotypes in Min mice. J. Cell Biol. 178, 1109-1120. 10.1083/jcb.200703186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calle E. E. and Kaaks R. (2004). Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 4, 579-591. 10.1038/nrc1408 [DOI] [PubMed] [Google Scholar]
- Chandramouly G., Abad P. C., Knowles D. W. and Lelièvre S. A. (2007). The control of tissue architecture over nuclear organization is crucial for epithelial cell fate. J. Cell Sci. 120, 1596-1606. 10.1242/jcs.03439 [DOI] [PubMed] [Google Scholar]
- Chen Y., Wei H., Liu Y. and Zheng S. (2018). Promotional effect of microRNA-194 on breast cancer cells via targeting F-box/WD repeat-containing protein 7. Oncol. Lett. 15, 4439-4444. 10.3892/ol.2018.7842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley W. P., Gervais E. M., Centanni S. W., Gulfo K. M., Nelson D. A. and Larsen M. (2012). ROCK1-directed basement membrane positioning coordinates epithelial tissue polarity. Development 139, 411-422. 10.1242/dev.075366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky C., Tremble P. and Werb Z. (1992). Signal transduction via the fibronectin receptor: do integrins regulate matrix remodeling? Matrix Suppl. 1, 184-191. [PubMed] [Google Scholar]
- Deng T., Lyon C. J., Bergin S., Caligiuri M. A. and Hsueh W. A. (2016). Obesity, inflammation, and cancer. Annu. Rev. Pathol. 11, 421-449. 10.1146/annurev-pathol-012615-044359 [DOI] [PubMed] [Google Scholar]
- Dianati E., Poiraud J., Weber-Ouellette A. and Plante I. (2016). Connexins, E-cadherin, Claudin-7 and β-catenin transiently form junctional nexuses during the post-natal mammary gland development. Dev. Biol. 416, 52-68. 10.1016/j.ydbio.2016.06.011 [DOI] [PubMed] [Google Scholar]
- di Pietro F., Echard A. and Morin X. (2016). Regulation of mitotic spindle orientation: an integrated view. EMBO Rep. 17, 1106-1130. 10.15252/embr.201642292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson P. J., Roos M., Evans C., Beyer E. and Kistler J. (1994). Electrical properties of mammalian lens epithelial gap junction channels. Invest. Ophthalmol. Vis. Sci. 35, 3422-3428. [PubMed] [Google Scholar]
- Eccles S. A., Aboagye E. O., Ali S., Anderson A. S., Armes J., Berditchevski F., Blaydes J. P., Brennan K., Brown N. J. and Bryant H. E. (2013). Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer. Breast Cancer Res. 15, R92 10.1186/bcr3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Fouly M. H., Trosko J. E. and Chang C.-C. (1987). Scrape-loading and dye transfer: a rapid and simple technique to study gap junctional intercellular communication. Exp. Cell Res. 168, 422-430. 10.1016/0014-4827(87)90014-0 [DOI] [PubMed] [Google Scholar]
- Elisha Y., Kalchenko V., Kuznetsov Y. and Geiger B. (2018). Dual role of E-cadherin in the regulation of invasive collective migration of mammary carcinoma cells. Sci Rep. 8, 4986 10.1038/s41598-018-22940-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sabban M. E., Sfeir A. J., Daher M. H., Kalaany N. Y., Bassam R. A. and Talhouk R. S. (2003). ECM-induced gap junctional communication enhances mammary epithelial cell differentiation. J. Cell Sci. 116, 3531-3541. 10.1242/jcs.00656 [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S. and Hall A. (2003). Cell polarity: Par6, aPKC and cytoskeletal crosstalk. Curr. Opin. Cell Biol. 15, 67-72. 10.1016/S0955-0674(02)00005-4 [DOI] [PubMed] [Google Scholar]
- Ey B., Eyking A., Gerken G., Podolsky D. and Cario E. (2009). TLR2-mediates gap junctional intercellular communication through connexin-43 in intestinal epithelila barrier injury. J. Biol. Chem. 284, 22332-22343. 10.1074/jbc.M901619200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E., Legue E., Doyen A., Nato F., Nicolas J.-F., Torres V., Yaniv M. and Pontoglio M. (2006). Defective planar cell polarity in polycystic kidney disease. Nat. Genet. 38, 21-23. 10.1038/ng1701 [DOI] [PubMed] [Google Scholar]
- Fitzgibbons P. L., Henson D. E. and Hutter R. V. (1998). Benign breast changes and the risk for subsequent breast cancer: an update of the 1985 consensus statement. Arch. Pathol. Lab. Med. 122, 1053-1055. [PubMed] [Google Scholar]
- Fleming E. S., Temchin M., Wu Q., Maggio-Price L. and Tirnauer J. S. (2009). Spindle misorientation in tumors from APCmin/+ mice. Mol. Carcinog. 48, 592-598. 10.1002/mc.20506 [DOI] [PubMed] [Google Scholar]
- Fort A. G., Murray J. W., Dandachi N., Davidson M. W., Dermietzel R., Wolkoff A. W. and Spray D. C. (2011). In vitro motility of liver connexin vesicles along microtubules utilizes kinesin motors. J. Biol. Chem. 286, 22875-22885. 10.1074/jbc.M111.219709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fostok S., El-Sabai M., Bazzoun D., Lelièvre S. and Talhouk R. (2019). Connexin 43 loss triggers cell cycle entry and invation in non-neoplastic breast epithelium: a role for noncanonical Wnt signaling. Cancers 11, 339 10.3390/cancers11030339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer F. C., Li A., Papanastasiou A. D., Smith A., Selenica P., Burke K. A., Edelweiss M., Wen H. C., Piscuoglio S., Schultheis A. M. et al. (2018). Recurrent hotspot mutations in HRAS Q61 and PI3K-AKT pathway genes as drivers of breast adenomyoepitheliomas. Nat. Commun. 9, 1816 10.1038/s41467-018-04128-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloerich M., Bianchini J. M., Siemers K. A., Cohen D. J. and Nelson W. J. (2017). Cell division orientation is coupled to cell–cell adhesion by the E-cadherin/LGN complex. Nat. Commun. 8, 13996 10.1038/ncomms13996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go M., Kojima T., Takano K.-I., Murata M., Koizumi J., Kurose M., Kamekura R., Osanai M., Chiba H. and Spray D. C. (2006). Connexin 26 expression prevents down-regulation of barrier and fence functions of tight junctions by Na+/K+-ATPase inhibitor ouabain in human airway epithelial cell line Calu-3. Exp. Cell Res. 312, 3847-3856. 10.1016/j.yexcr.2006.08.014 [DOI] [PubMed] [Google Scholar]
- Godde N. J., Sheridan J. M., Smith L. K., Pearson H. B., Britt K. L., Galea R. C., Yates L. L., Visvader J. E. and Humbert P. O. (2014). Scribble modulates the MAPK/Fra1 pathway to disrupt luminal and ductal integrity and suppress tumour formation in the mammary gland. PLoS Genet. 10, e1004323 10.1371/journal.pgen.1004323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes D. S., Porto S. S., Rocha R. M. and Gobbi H. (2013). Usefulness and limitations of E-cadherin and β-catenin in the classification of breast carcinomas in situ with mixed pattern. Diagn Pathol. 8, 114 10.1186/1746-1596-8-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton M. M., Wang L., Vidi P.-A., Leary J. and Lelièvre S. A. (2011). Breast on-a-chip: mimicry of the channeling system of the breast for development of theranostics. Integr. Biol. 3, 451-459. 10.1039/c0ib00132e [DOI] [PubMed] [Google Scholar]
- Guerrier A., Fonlupt P., Morand I., Rabilloud R., Audebet C., Krutovskikh V., Gros D., Rousset B. and Munari-Silem Y. (1995). Gap junctions and cell polarity: connexin32 and connexin43 expressed in polarized thyroid epithelial cells assemble into separate gap junctions, which are located in distinct regions of the lateral plasma membrane domain. J. Cell Sci. 108, 2609-2617. 10.1016/0248-4900(91)90115-4 [DOI] [PubMed] [Google Scholar]
- Hao J., Zhang C., Zhang A., Wang K., Jia Z., Wang G., Han L., Kang C. and Pu P. (2012). miR-221/222 is the regulator of Cx43 expression in human glioblastoma cells. Oncol. Rep. 27, 1504-1510. 10.3892/or.2012.1652 [DOI] [PubMed] [Google Scholar]
- Hirschi K. K., Xu C., Tsukamoto T. and Sager R. (1996). Gap junction genes Cx26 and Cx43 individually suppress the cancer phenotype of human mammary carcinoma cells and restore differentiation potential. Cell Growth Differ. 7, 861-870. [PubMed] [Google Scholar]
- Jaffe A. B., Kaji N., Durgan J. and Hall A. (2008). Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J. Cell Biol. 183, 625-633. 10.1083/jcb.200807121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman S., Doucet M. and Kominsky S. L. (2017). Down-regulation of CITED2 attenuates breast tumor growth, vessel formation and TGF-β-induced expression of VEGFA. Oncotarget 8, 6169-6178. 10.18632/oncotarget.14048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L., Liu F., Hansen S. H., ter Beest M. B. A. and Zegers M. M. (2011). Distinct roles of cadherin-6 and E-cadherin in tubulogenesis and lumen formation. Mol. Biol. Cell 22, 2031-2041. 10.1091/mbc.e11-01-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin E.-J., Park K. S., Kim D., Lee Y.-S., Sonn J. K., Jung J. C., Bang O.-S. and Kang S.-S. (2010). TGF-β3 inhibits chondrogenesis by suppressing precartilage condensation through stimulation of N-cadherin shedding and reduction of cRREB-1 expression. Mol. Cells 29, 425-432. 10.1007/s10059-010-0078-z [DOI] [PubMed] [Google Scholar]
- Joberty G., Petersen C., Gao L. and Macara I. G. (2000). The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat. Cell Biol. 2, 531-539. 10.1038/35019573 [DOI] [PubMed] [Google Scholar]
- Johnston C. A., Hirono K., Prehoda K. E. and Doe C. Q. (2009). Identification of an Aurora-A/PinsLINKER/Dlg spindle orientation pathway using induced cell polarity in S2 cells. Cell 138, 1150-1163. 10.1016/j.cell.2009.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. C. and Young R. H. (1994). The differential diagnosis of prostatic carcinoma. Its distinction from premalignant and pseudocarcinomatous lesions of the prostate gland. Am. J. Clin. Pathol. 101, 48-64. 10.1093/ajcp/101.1.48 [DOI] [PubMed] [Google Scholar]
- Kanczuga-Koda L., Sulkowska M., Koda M., Reszec J., Famulski W., Baltaziak M. and Sulkowski S. (2003). Expression of connexin 43 in breast cancer in comparison with mammary dysplasia and the normal mammary gland. Folia. Morphol. (Warsz) 62, 439-442. 10.2478/v10042-008-0026-3 [DOI] [PubMed] [Google Scholar]
- Kanno Y. and Matsui Y. (1968). Cellular uncoupling in cancerous stomach epithelium. Nature 218, 775-776. 10.1038/218775b0 [DOI] [PubMed] [Google Scholar]
- Knezevic J., Pfefferle A. D., Petrovic I., Greene S. B., Perou C. M. and Rosen J. M. (2015). Expression of miR-200c in claudin-low breast cancer alters stem cell functionality, enhances chemosensitivity and reduces metastatic potential. Oncogene 34, 5997 10.1038/onc.2015.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T., Spray D. C., Kokai Y., Chiba H., Mochizuki Y. and Sawada N. (2002). Cx32 formation and/or Cx32-mediated intercellular communication induces expression and function of tight junctions in hepatocytic cell line. Exp. Cell Res. 276, 40-51. 10.1006/excr.2002.5511 [DOI] [PubMed] [Google Scholar]
- Kojima Y., Akimoto K., Nagashima Y., Ishiguro H., Shirai S., Chishima T., Ichikawa Y., Ishikawa T., Sasaki T., Kubota Y. et al. (2008). The overexpression and altered localization of the atypical protein kinase C lambda/iota in breast cancer correlates with the pathologic type of these tumors. Hum. Pathol. 39, 824-831. 10.1016/j.humpath.2007.11.001 [DOI] [PubMed] [Google Scholar]
- LaFlamme S. E., Nieves B., Colello D. and Reverte C. G. (2008). Integrins as regulators of the mitotic machinery. Curr. Opin. Cell Biol. 20, 576-582. 10.1016/j.ceb.2008.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S. W., Huang B. R., Liu Y. S., Lin H. Y., Chen C. C., Tsai C. F., Lu D. Y. and Lin C. (2018). Differential characterization of temozolomide-resistant human glioma cells. Int. J. Mol. Sci. 19, E127 10.3390/ijms19010127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird D. W. (2006). Life cycle of connexins in health and disease. Biochem. J. 394, 527-543. 10.1042/BJ20051922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird D. W., Fistouris P., Batist G., Alpert L., Huynh H. T., Carystinos G. D. and Alaoui-Jamali M. A. (1999). Deficiency of connexin43 gap junctions is an independent marker for breast tumors. Cancer Res. 59, 4104-4110. [PubMed] [Google Scholar]
- Lechler T. and Fuchs E. (2005). Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437, 275-280. 10.1038/nature03922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. W., Tomasetto C., Paul D., Keyomarsi K. and Sager R. (1992). Transcriptional downregulation of gap-junction proteins blocks junctional communication in human mammary tumor cell lines. J. Cell Biol. 118, 1213-1221. 10.1083/jcb.118.5.1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelièvre S. A. (2010). Tissue polarity-dependent control of mammary epithelial homeostasis and cancer development: an epigenetic perspective. J. Mammary Gland Biol. Neoplasia 15, 49-63. 10.1007/s10911-010-9168-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelièvre S. A., Weaver V. M., Nickerson J. A., Larabell C. A., Bhaumik A., Petersen O. W. and Bissell M. J. (1998). Tissue phenotype depends on reciprocal interactions between the extracellular matrix and the structural organization of the nucleus. Proc. Natl. Acad. Sci. USA 95, 14711-14716. 10.1073/pnas.95.25.14711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesko A. C., Goss K. H., Yang F. F., Schwertner A., Hulur I., Onel K. and Prosperi J. R. (2015). The APC tumor suppressor is required for epithelial cell polarization and three-dimensional morphogenesis. Biochim. Biophys. Acta 1853, 711-723. 10.1016/j.bbamcr.2014.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. W., Mruk D. D., Lee W. M. and Cheng C. Y. (2010). Connexin 43 is critical to maintain the homeostasis of the blood-testis barrier via its effects on tight junction reassembly. Proc. Natl. Acad. Sci. USA 107, 17998-18003. 10.1073/pnas.1007047107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Cao K., Xum C., Hu T., Zhou L., Cao D., Xiao J., Luo L., Guo Y. and Qi Y. (2015). GATA-3 augmentation down-regulates Connexin43 in Helicobacter pylori associated gastric carcinogenesis. Cancer Biol. Ther. 16, 987-996. 10.1080/15384047.2015.1030552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke D., Stein T., Davies C., Morris J., Harris A. L., Evans W. H., Monaghan P. and Gusterson B. (2004). Altered permeability and modulatory character of connexin channels during mammary gland development. Exp. Cell Res. 298, 643-660. 10.1016/j.yexcr.2004.05.003 [DOI] [PubMed] [Google Scholar]
- Mao X., Li P., Wang Y., Liang Z., Liu J., Li J., Jiang Y., Bao G., Li L., Zhu B. et al. (2017). CRB3 regulates contact inhibition by activating the Hippo pathway in mammary epithelial cells. Cell Death Dis. 8, e2546 10.1038/cddis.2016.478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez-Rosado L., Solan J. L., Dunn C. A., Norris R. P. and Lampe P. D. (2012). Connexin43 phosphorylation in brain, cardiac, endothelial and epithelial tissues. Biochim. Biophys. Acta 1818, 1985-1992. 10.1016/j.bbamem.2011.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Belmonte F. and Perez-Moreno M. (2011). Epithelial cell polarity, stem cells and cancer. Nat. Rev. Cancer 12, 23-38. 10.1038/nrc3169 [DOI] [PubMed] [Google Scholar]
- Martin T. A., Watkins G., Mansel R. E. and Jiang W. G. (2004). Loss of tight junction plaque molecules in breast cancer tissues is associated with a poor prognosis in patients with breast cancer. Eur. J. Cancer 40, 2717-2725. 10.1016/j.ejca.2004.08.008 [DOI] [PubMed] [Google Scholar]
- Mathews J. and Levin M. (2017). Gap junctional signaling in pattern regulation: Physiological network connectivity instructs growth and form. Dev. Neurobiol. 77, 643-673. 10.1002/dneu.22405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey L. M. and Macara I. G. (2011). Epithelial organization, cell polarity and tumorigenesis. Trends Cell Biol. 21, 727-735. 10.1016/j.tcb.2011.06.005 [DOI] [PubMed] [Google Scholar]
- McCaffrey L. M., Montalbano J. A., Mihai C. and Macara I. G. (2012). Loss of the Par3 polarity protein promotes breast tumorigenesis and metastasis. Cancer Cell 22, 601-614. 10.1016/j.ccr.2012.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E., Shao Q. and Laird D. W. (2007). Connexins and gap junctions in mammary gland development and breast cancer progression. J. Membr. Biol. 218, 107-121. 10.1007/s00232-007-9052-x [DOI] [PubMed] [Google Scholar]
- McLachlan E., Shao Q., Wang H.-L., Langlois S. and Laird D. W. (2006). Connexins act as tumor suppressors in three-dimensional mammary cell organoids by regulating differentiation and angiogenesis. Cancer Res. 66, 9886-9894. 10.1158/0008-5472.CAN-05-4302 [DOI] [PubMed] [Google Scholar]
- Monaghan P., Clarke C., Perusinghe N. P., Moss D. W., Chen X.-Y. and Evans W. H. (1996). Gap junction distribution and connexin expression in human breast. Exp. Cell Res. 223, 29-38. 10.1006/excr.1996.0055 [DOI] [PubMed] [Google Scholar]
- Morita H., Katsuno T., Hoshimoto A., Hirano N., Saito Y. and Suzuki Y. (2004). Connexin 26-mediated gap junctional intercellular communication suppresses paracellular permeability of human intestinal epithelial cell monolayers. Exp. Cell Res. 298, 1-8. 10.1016/j.yexcr.2004.03.046 [DOI] [PubMed] [Google Scholar]
- Mroue R., Inman J., Mott J., Budunova I. and Bissell M. J. (2015). Asymmetric expression of connexins between luminal epithelial- and myoepithelial- cells is essential for contractile function of the mammary gland. Dev. Biol. 399, 15-26. 10.1016/j.ydbio.2014.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllymäki S. M., Teräväinen T. P. and Manninen A. (2011). Two distinct integrin-mediated mechanisms contribute to apical lumen formation in epithelial cells. PLoS ONE 6, e19453 10.1371/journal.pone.0019453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa K., Chiba H., Fujita H., Kojima T., Saito T., Endo T. and Sawada N. (2006). Possible involvement of gap junctions in the barrier function of tight junctions of brain and lung endothelial cells. J. Cell. Physiol. 208, 123-132. 10.1002/jcp.20647 [DOI] [PubMed] [Google Scholar]
- Nedvetsky P. I., Kwon S.-H., Debnath J. and Mostov K. E. (2012). Cyclic AMP regulates formation of mammary epithelial acini in vitro. Mol. Biol. Cell 23, 2973-2981. 10.1091/mbc.e12-02-0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni X., Wang A., Zhang L., Shan L. Y., Zhang H. C., Li L., Si J. Q., Luo J., Li X. Z. and Ma K. T. (2017). Up-regulation of gap junction in peripheral blood T lymphocytes contributes to the inflammatory response in essential hypertension. PLoS ONE 12, e0184773 10.1371/journal.pone.0184773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusrat A., Chen J. A., Foley C. S., Liang T. W., Tom J., Cromwell M., Quan C. and Mrsny R. J. (2000). The coiled-coil domain of occludin can act to organize structural and functional elements of the epithelial tight junction. J. Biol. Chem. 275, 29816-29822. 10.1074/jbc.M002450200 [DOI] [PubMed] [Google Scholar]
- Odenwald M. A., Choi W., Buckley A., Shashikanth N., Joseph N. E., Wang Y., Warren M. H., Buschmann M. M., Pavlyuk R., Hildebrand J. et al. (2017). ZO-1 interactions with F-actin and occludin direct epithelial polarization and single lumen specification in 3D culture. J. Cell Sci. 130, 243-259. 10.1242/jcs.188185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park W.-J., Liu J. and Adler P. N. (1994). The frizzled gene of Drosophila encodes a membrane protein with an odd number of transmembrane domains. Mech. Dev. 45, 127-137. 10.1016/0925-4773(94)90026-4 [DOI] [PubMed] [Google Scholar]
- Patel V., Li L., Cobo-Stark P., Shao X., Somlo S., Lin F. and Igarashi P. (2008). Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum. Mol. Genet. 17, 1578-1590. 10.1093/hmg/ddn045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyre M., Goutagny S., Imbeaud S., Bozorg-Grayeli A., Felce M., Sterkers O. and Kalamarides M. (2011). Increased growth rate of vestibular schwannoma after resection of contralateral tumor in neurofibromatosis type 2. Neuro Oncol. 13, 1125-1132. 10.1093/neuonc/nor101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao H. L., Yuan Y., Wang M., Sun Y., Liang H. and Ma L. (2014). α-catenin acts as a tumour suppressor in E-cadherin-negative basal-like breast cancer by inhibiting NF-κB signalling. Nature Cell Biol. 16, 245 10.1038/ncb2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plachot C. and Lelièvre S. A. (2004). DNA methylation control of tissue polarity and cellular differentiation in the mammary epithelium. Exp. Cell Res. 298, 122-132. 10.1016/j.yexcr.2004.04.024 [DOI] [PubMed] [Google Scholar]
- Plachot C., Chaboub L. S., Adissu H. A., Wang L., Urazaev A., Sturgis J., Asem E. K. and Lelièvre S. A. (2009). Factors necessary to produce basoapical polarity in human glandular epithelium formed in conventional and high-throughput three-dimensional culture: example of the breast epithelium. BMC Biol. 7, 77 10.1186/1741-7007-7-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante I., Wallis A., Shao Q. and Laird D. W. (2010). Milk secretion and ejection are impaired in the mammary gland of mice harboring a Cx43 mutant while expression and localization of tight and adherens junction proteins remain unchanged. Biol. Reprod. 82, 837-847. 10.1095/biolreprod.109.081406 [DOI] [PubMed] [Google Scholar]
- Qin H., Shao Q., Belliveau D. J. and Laird D. W. (2001). Aggregated DsRed-tagged Cx43 and over-expressed Cx43 are targeted to lysosomes in human breast cancer cells. Cell Commun. Adhes. 8, 433-439. 10.3109/15419060109080766 [DOI] [PubMed] [Google Scholar]
- Qin H., Shao Q., Curtis H., Galipeau J., Belliveau D. J., Wang T., Alaoui-Jamali M. A. and Laird D. W. (2002). Retroviral delivery of connexin genes to human breast tumor cells inhibits in vivo tumor growth by a mechanism that is independent of significant gap junctional intercellular communication. J. Biol. Chem. 277, 29132-29138. 10.1074/jbc.M200797200 [DOI] [PubMed] [Google Scholar]
- Qin Y., Meisen W. H., Hao Y. and Macara I. G. (2010). Tuba, a Cdc42 GEF, is required for polarized spindle orientation during epithelial cyst formation. J. Cell Biol. 189, 661-669. 10.1083/jcb.201002097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quyn A. J., Appleton P. L., Carey F. A., Steele R. J. C., Barker N., Clevers H., Ridgway R. A., Sansom O. J. and Näthke I. S. (2010). Spindle orientation bias in gut epithelial stem cell compartments is lost in precancerous tissue. Cell Stem Cell 6, 175-181. 10.1016/j.stem.2009.12.007 [DOI] [PubMed] [Google Scholar]
- Reverte C. G., Benware A., Jones C. W. and LaFlamme S. E. (2006). Perturbing integrin function inhibits microtubule growth from centrosomes, spindle assembly, and cytokinesis. J. Cell Biol. 174, 491-497. 10.1083/jcb.200603069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch R. J., Cesen-Cummings K. and Malkinson A. M. (1998). Role of gap junctions in lung neoplasia. Exp. Lung Res. 24, 523-539. 10.3109/01902149809087384 [DOI] [PubMed] [Google Scholar]
- Saxena N. K. and Sharma D. (2013). Multifaceted leptin network: the molecular connection between obesity and breast cancer. J. Mammary Gland Biol. Neoplasia 18, 309-320. 10.1007/s10911-013-9308-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Q., Wang H., McLachlan E., Veitch G. I. L. and Laird D. W. (2005). Down-regulation of Cx43 by retroviral delivery of small interfering RNA promotes an aggressive breast cancer cell phenotype. Cancer Res. 65, 2705-2711. 10.1158/0008-5472.CAN-04-2367 [DOI] [PubMed] [Google Scholar]
- Sorgen P. L., Trease A. J., Spagnol G., Delmar M. and Nielsen M. S. (2018). Protein–protein interactions with connexin 43: regulation and function. Int. J. Mol. Sci. 19, 1428-1449. 10.3390/ijms19051428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M. K., Plante I., Bechberger J. F., Naus C. C. and Laird D. W. (2014). Mammary gland specific knockdown of the physiological surge in Cx26 during lactation retains normal mammary gland development and function. PLoS ONE 9, e101546 10.1371/journal.pone.0101546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei I., Deugnier M.-A., Faraldo M. M., Petit V., Bouvard D., Medina D., Fässler R., Thiery J. P. and Glukhova M. A. (2008). Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat. Cell Biol. 10, 716-722. 10.1038/ncb1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk R. S., Elble R. C., Bassam R., Daher M., Sfeir A., Mosleh L. A., El-Khoury H., Hamoui S., Pauli B. U. and El-Sabban M. E. (2005). Developmental expression patterns and regulation of connexins in the mouse mammary gland: expression of connexin30 in lactogenesis. Cell Tissue Res. 319, 49-59. 10.1007/s00441-004-0915-5 [DOI] [PubMed] [Google Scholar]
- Talhouk R. S., Mroue R., Mokalled M., Abi-Mosleh L., Nehme R., Ismail A., Khalil A., Zaatari M. and El-Sabban M. E. (2008). Heterocellular interaction enhances recruitment of alpha and beta-catenins and ZO-2 into functional gap-junction complexes and induces gap junction-dependant differentiation of mammary epithelial cells. Exp. Cell Res. 314, 3275-3291. 10.1016/j.yexcr.2008.07.030 [DOI] [PubMed] [Google Scholar]
- Talhouk R. S., Fares M.-B., Rahme G. J., Hariri H. H., Rayess T., Dbouk H. A., Bazzoun D., Al-Labban D. and El-Sabban M. E. (2013). Context dependent reversion of tumor phenotype by connexin-43 expression in MDA-MB231 cells and MCF-7 cells: role of beta-catenin/connexin43 association. Exp. Cell Res. 319, 3065-3080. 10.1016/j.yexcr.2013.10.002 [DOI] [PubMed] [Google Scholar]
- Tang B., Peng Z.-H., Yu P.-W., Yu G. and Qian F. (2011). Expression and significance of Cx43 and E-cadherin in gastric cancer and metastatic lymph nodes. Med. Oncol. 28, 502-508. 10.1007/s12032-010-9492-5 [DOI] [PubMed] [Google Scholar]
- Teleki I., Szasz A. M., Maros M. E., Gyorffy B., Kulka J., Meggyeshazi N., Kiszner G., Balla P., Samu A. and Krenacs T. (2014). Correlations of differentially expressed gap junction connexins Cx26, Cx30, Cx32, Cx43 and Cx46 with breast cancer progression and prognosis. PLoS ONE 9, e112541 10.1371/journal.pone.0112541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenvooren I., Jenks M. Z., Rashi H., Cook K. L., Muhlemann J. K., Sistrunk C., Holmes J., Wang K., Bonin K., Hodges K., (2019). Elevated leptin disrupts epithelial polarity and promotes premalignant alterations in the mammary gland. Oncogene. 10.1038/s41388-019-0687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry M., Racine V., Pépin A., Piel M., Chen Y., Sibarita J.-B. and Bornens M. (2005). The extracellular matrix guides the orientation of the cell division axis. Nat. Cell Biol. 7, 947-953. 10.1038/ncb1307 [DOI] [PubMed] [Google Scholar]
- Tobi M. (1999). Polyps as biomarkers for colorectal neoplasia. Front. Biosci. 4, D329-D338. 10.2741/A431 [DOI] [PubMed] [Google Scholar]
- Tong D., Lu X., Wang H. X., Plante I., Lui E., Laird D. W., Bai D. and Kidder G. M. (2009). A dominant loss-of-function GJA1 (Cx43) mutant impairs parturition in the mouse. Biol. Reprod. 80, 1099-1106. 10.1095/biolreprod.108.071969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima F. and Nishida E. (2007). Spindle orientation in animal cell mitosis: roles of integrin in the control of spindle axis. J. Cell. Physiol. 213, 407-411. 10.1002/jcp.21227 [DOI] [PubMed] [Google Scholar]
- Tuncay H. and Ebnet K. (2016). Cell adhesion molecule control of planar spindle orientation. Cell. Mol. Life Sci. 73, 1195-1207. 10.1007/s00018-015-2116-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderpuye O. A., Bell C. L. and Murray S. A. (2016). Redistribution of connexin 43 during cell division. Cell Biol. Int. 40, 387-396. 10.1002/cbin.10576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidi P.-A., Chandramouly G., Gray M., Wang L., Liu E., Kim J. J., Roukos V., Bissell M. J., Moghe P. V. and Lelièvre S. A. (2011). Interconnected contribution of tissue morphogenesis and the nuclear protein NuMA to the DNA damage response. J. Cell Sci. 125, 350-361. 10.1242/jcs.089177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Diaz L. G., Kim J. K., Laperle A., Palecek S. P. and Krebsbach P. H. (2016). Inhibition of focal adhesion kinase signaling by integrin α6β1 supports human pluripotent stem cell self-renewal. Stem Cells 34, 1753-1764. 10.1002/stem.2349 [DOI] [PubMed] [Google Scholar]
- Villegas E., Kabotyanski E. B., Shore A. N., Creighton C. J., Westbrook T. F. and Rosen J. M. (2014). Plk2 regulates mitotic spindle orientation and mammary gland development. Development 141, 1562-1571. 10.1242/dev.108258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. W., Sellitto C., Paul D. L. and Goodenough D. A. (2001). Prenatal lens development in connexin43 and connexin50 double knockout mice. Invest. Ophthalmol. Vis. Sci. 42, 2916-2923. [PubMed] [Google Scholar]
- Xue B., Krishnamurthy K., Allred D. C. and Muthuswamy S. K. (2013). Loss of Par3 promotes breast cancer metastasis by compromising cell-cell cohesion. Nat. Cell Biol. 15, 189-200. 10.1038/ncb2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H., Mesnil M., Omori Y., Mironov N. and Krutovskikh V. (1995). Intercellular communication and carcinogenesis. Mutat. Res. 333, 181-188. 10.1016/0027-5107(95)00144-1 [DOI] [PubMed] [Google Scholar]
- Yue S., Cárdenas-Mora J. M., Chaboub L. S., Lelievre S. A. and Cheng J.-X. (2012). Label-free analysis of breast tissue polarity by Raman imaging of lipid phase. Biophys. J. 102, 1215-1223. 10.1016/j.bpj.2012.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemljic-Harpf A. E., Godoy J. C., Platoshyn O., Asfaw E. K., Busija A. R., Domenighetti A. A. and Ross R. S. (2014). Vinculin directly binds zonula occludens-1 and is essential for stabilizing connexin-43-containing gap junctions in cardiac myocytes. J. Cell Sci. 127, 1104-1116. 10.1242/jcs.143743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Li G., Zhang Q., Tang Q., Huang J., Hu C., Liu Y., Wang Q., Liu W., Gao N. et al. (2018). Hirsutine induces mPTP-dependent apoptosis through ROCK1/PTEN/PI3K/GSK3β pathway in human lung cancer cells. Cell Death Dis. 9, 598 10.1038/s41419-018-0641-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Zhu H., Wan Q., Liu J., Xiao Z., Siderovski D. P. and Du Q. (2010). LGN regulates mitotic spindle orientation during epithelial morphogenesis. J. Cell Biol. 189, 275-288. 10.1083/jcb.200910021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.