ABSTRACT
Mutations in the cardiac ryanodine receptor Ca2+ release channel (RyR2) can cause deadly ventricular arrhythmias and atrial fibrillation (AF). The RyR2-P2328S mutation produces catecholaminergic polymorphic ventricular tachycardia (CPVT) and AF in hearts from homozygous RyR2P2328S/P2328S (denoted RyR2S/S) mice. We have now examined P2328S RyR2 channels from RyR2S/S hearts. The activity of wild-type (WT) and P2328S RyR2 channels was similar at a cytoplasmic [Ca2+] of 1 mM, but P2328S RyR2 was significantly more active than WT at a cytoplasmic [Ca2+] of 1 µM. This was associated with a >10-fold shift in the half maximal activation concentration (AC50) for Ca2+ activation, from ∼3.5 µM Ca2+ in WT RyR2 to ∼320 nM in P2328S channels and an unexpected >1000-fold shift in the half maximal inhibitory concentration (IC50) for inactivation from ∼50 mM in WT channels to ≤7 μM in P2328S channels, which is into systolic [Ca2+] levels. Unexpectedly, the shift in Ca2+ activation was not associated with changes in sub-conductance activity, S2806 or S2814 phosphorylation or the level of FKBP12 (also known as FKBP1A) bound to the channels. The changes in channel activity seen with the P2328S mutation correlate with altered Ca2+ homeostasis in myocytes from RyR2S/S mice and the CPVT and AF phenotypes.
This article has an associated First Person interview with the first author of the paper.
KEY WORDS: RyR2 P2328S ion channel, P2328S-RyR2 mouse, Atrial fibrillation, Catecholaminergic polymorphic ventricular tachycardia, Cytoplasmic Ca2+ activation, Cytoplasmic Ca2+ inactivation, FKBP
Summary: The RyR2–P2328S mutation, precipitating potentially fatal arrhythmia in humans, can be attributed to leftward shifts in cytoplasmic Ca2+–dependent RyR2 activation and inactivation in the absence of adrenergic stimulation.
INTRODUCTION
Mammalian heart rhythm depends on the activity of ion channels, ion transporters and Ca2+-binding proteins in the surface membrane, cytoplasm and intracellular sarcoplasmic reticulum (SR) Ca2+ store of cardiac myocytes. The intracellular Ca2+-handling proteins control Ca2+ release and contraction during systole, as well as diastolic Ca2+ re-uptake and storage in the SR. Mutations or acquired changes in these proteins lead to arrhythmia and heart failure (Bers, 2001). Mutations in the cardiac ryanodine receptor (RyR2), the ligand-gated SR Ca2+ release channel, can increase channel open probability during diastole, resulting in excess diastolic SR Ca2+ release, and lead to catecholaminergic polymorphic ventricular tachycardia (CPVT) (Laitinen et al., 2001; Lehnart et al., 2004; Priori et al., 2001; Swan et al., 1999), ventricular fibrillation (VF) (Cerrone et al., 2005; Jiang et al., 2007; Paech et al., 2014) or atrial fibrillation (AF) (Pizzale et al., 2008; Sumitomo et al., 2007). CPVT is an inherited channelopathy, and is characterised by often-fatal ventricular tachycardia exacerbated by physical or emotional adrenergic stress. To date, more than 150 different mutations have been found to disturb RyR2 ion channel function, and these account for 70–80% of CPVT cases. The mutations lead to abnormal SR Ca2+ handling and subsequent pro-arrhythmic SR Ca2+ ‘leak’ (Priori and Chen, 2011; Ronen and Lili, 2016; Sumitomo, 2016). In contrast to the typically monogenic nature of CPVT, VF and AF are multifactorial, but can also involve disrupted Ca2+ homeostasis. AF is the most common sustained arrhythmia, resulting in significant clinical morbidity and mortality (Benjamin et al., 1998; Davis et al., 2012; Kourliouros et al., 2009). Abnormally high diastolic cytoplasmic [Ca2+] triggers the surface membrane sodium calcium exchanger (NCX; herein referring to the isoform encoded by SCL8A1) to extrude Ca2+, generating a delayed after-depolarisation (DAD) as NCX imports three Na+ ions for every Ca2+ extruded. Triggered arrhythmogenic action potentials are generated when the DAD reaches the action potential threshold.
The molecular properties of RyR2 carrying CPVT and/or AF mutations that lead to diastolic Ca2+ leak are frequently studied using RyR2 channels expressed in HEK 293 cells (Jiang et al., 2005; Liu et al., 2013; Meli et al., 2011; Paavola et al., 2007). Mammalian models of RyR2 mutations are particularly useful because the ion channel is subjected to many post-translational modifications that also occur in patients, but only a few have been developed (Cerrone et al., 2005; Goddard et al., 2008; Lehnart et al., 2008; Shan et al., 2010; Shan et al., 2012). An advantage of animal models is that the ion channel properties can be directly correlated with changes in heart function and susceptibility to arrhythmia (Huang, 2017).
The RyR2-P2328S mutation is one of a few CPVT-related mutations that has been reported to also be associated with AF (Glukhov et al., 2015; Goddard et al., 2008; King et al., 2013; Laitinen et al., 2001; Lehnart et al., 2004; Salvage et al., 2015; Xiao et al., 2016; Zhang et al., 2011). We have generated a mouse model of the P2328S mutation; RyR2P2328S/P2328S (RyR2S/S) (Goddard et al., 2008). The mutation lies within an RyR2 mutation ‘hot-spot’. This hot-spot, known as domain II or the central region, encompassing amino acids 2246–2534, is conserved across species and isoforms (Yano et al., 2005) and is included in the HD1 domain of RyR2 (Dhindwal et al., 2017). The RyR2S/S mouse demonstrates the atrial and ventricular arrhythmic phenotype seen in patients (Goddard et al., 2008; Sabir et al., 2010; Zhang et al., 2011). The arrhythmia is associated with reduced action potential conduction velocity, reduced expression of NaV1.5 (also known as SCN5A) and reduced Na+ channel function (King et al., 2013; Ning et al., 2016; Salvage et al., 2015; Zhang et al., 2013). It is also associated with DAD phenomena (King et al., 2013), suggesting that RyR2 may be hyperactive. Indeed increased RyR2 sensitivity to cytosolic Ca2+ was indicated in a study of cellular Ca2+ handling (Goddard et al., 2008). P2328S RyR2 channels expressed in HEK 293 cells show increased activity at 150 nM cytoplasmic Ca2+, but only after protein kinase A (PKA) activation led to increased phosphorylation and reduced FKBP binding (Lehnart et al., 2004). Thus, in addition to its clinical phenotype, the P2328S mutation is of interest because of this reported loss of regulatory 12.6 kDa FK506-binding protein (FKBP12.6, also known as FKBP1B). In this context, the mutation is in the general proximity of a putative, albeit controversial, FKBP12- (also known as FKBP1A) and FKBP12.6-binding site (amino acids 2361–2496) within the central HD1 domain of RyR2 (Dhindwal et al., 2017; Laitinen et al., 2001; Lehnart et al., 2004; Marx et al., 2000; Xiao et al., 2016; Zissimopoulos and Lai, 2005). FKBP12 and FKBP12.6 are RyR2-associated proteins, reported to stabilise channel opening to the maximum conductance (Ahern et al., 1994; Brillantes et al., 1994; Lehnart et al., 2004; Marx et al., 2000; Wehrens et al., 2003). We find that FKBP12 and FKBP12.6 (hereafter FKBP12/12.6) removal from sheep RyR2 is correlated with increased sub-conductance activity in one RyR2 channelopathy linked to a mutation in the RyR2-associated regulatory protein CLIC-2 (Richardson et al., 2017). While the RyR2–FKBP12/12.6 association is widely documented, its role in modulating RyR2 channel activity is disputed (Meli et al., 2011; Shan et al., 2010; Xiao et al., 2007; Zissimopoulos and Lai, 2005).
Taking these considerations together, the ion channel properties of P2328S RyR2 from the RyR2S/S mouse model have wide-reaching implications in cardiac dysfunction and physiology. Here, we examined the conductance and single-channel parameters of RyR2 from WT and RyR2S/S mice, their cytoplasmic Ca2+ sensitivity, their FKBP12 association and S2814 and S2808 phosphorylation. [Note, that we refer to FKBP12 rather than FKBP12.6 as we found here that there was only one FKBP band associated with RyR2 in mouse heart, consistent with a previous report there is a 100-fold excess of FKBP12 compared to FKBP12.6 that is associated with RyR2 (Zissimopoulos et al., 2012).] Overall, we find that neither the full conductance of the channel, nor its sub-conductance properties are altered by the mutation. Ca2+ activation and, surprisingly, Ca2+ inactivation of mutant channels, are shifted to a lower cytoplasmic [Ca2+] so that the channel is maximally activated between 100 nM and 1 µM Ca2+. In contrast to channels expressed in HEK 293 cells, these changes are apparent without adrenergic stimulation and without loss of RyR2-associated FKBP12 or change in RyR2 S2814 or S2808 phosphorylation. The differences between the properties of channels expressed in mouse and in HEK 293 cells emphasise the importance of examining channels expressed in mature mammals.
RESULTS
Conductance of WT and P2328S RyR2 channels at 1 mM and 1 µM cytoplasmic [Ca2+]
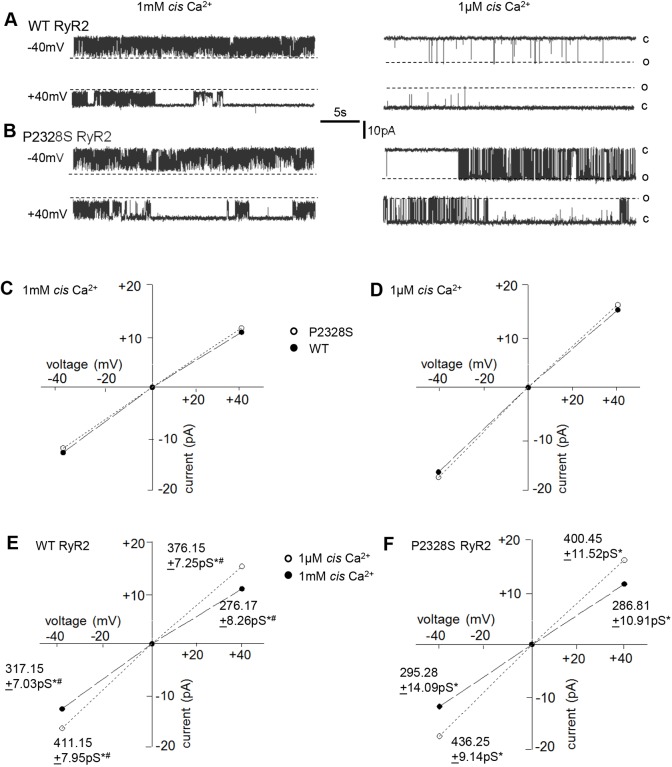
In initial experiments (experiment #1), WT and P2328S RyR2 activity was recorded continuously following channel incorporation into bilayers, starting with solutions on either side of the bilayer containing 1 mM Ca2+. The cytoplasmic [Ca2+] was lowered after 3 to 5 min to 1 μM (see Materials and Methods), a concentration that might exist in myocytes towards the end of diastole. There were no significant differences between the average maximum conductance of WT and P2328S channels recorded at the same bilayer voltage and cytoplasmic [Ca2+] (Fig. 1). However, currents in both channel types were greater with 1 μM cytoplasmic Ca2+ than with 1 mM Ca2+, likely due to removal of the partial pore block caused by Ca2+ binding (Hanna et al., 2014; Friel and Tsien, 1989; Gillespie et al., 2005). There was a small but significantly greater conductance in WT channels at −40 mV (with current flow from lumen to cytoplasm), which is also observed in sheep RyR2 (A.F.D., unpublished observations), and a similar trend in P2328S channels.
Fig. 1.
The P2328S mutation enhances RyR2 channel activity when cytoplasmic [Ca2+] is 1 µM, without altering maximum channel conductance. (A,B) Representative 25 s recordings from one WT RyR2 channel (A) and one P2328S RyR2 channel (B), with 1 mM Ca2+ (left panels) or 1 µM cytoplasmic Ca2+ (right panels), at −40 mV (upper record) or +40 mV (lower record). Broken lines (o) indicate maximum open currents, and solid lines (c) indicate the closed current levels. (C,D) Average maximum single channel currents plotted against voltage with 1 mM (C) or 1 μM (D) Ca2+. Dashed lines through filled circles connect WT data and dotted lines through open circles connect P2328S data. (E,F) Data from C and D replotted to emphasise the conductance difference between 1 mM with 1 μM Ca2+ in WT (E) or P2328S (F) RyR2. Dashed lines through filled circles connect 1 mM Ca2+ data, and dotted lines through open circles connect 1 µM Ca2+ data. Data points include mean±s.e.m. Error bars are within the symbol dimensions in all cases. Mean conductance (±s.e.m.) values are given beside their respective symbols. n=20 channels, WT, 1 mM Ca2+, −40 mV; n=24 channels, WT, 1 μM, −40 mV; n=22 channels, WT 1 mM Ca2+, +40 mV; n=23 channels, WT, 1 μM Ca2+, +40 mV. n=17 channels P2328S, 1 mM Ca2+, −40 mV; n=21 channels, P2328S, 1 μM Ca2+, −40 mV; n=18 channels, P2328S 1 mM Ca2+, +40 mV; n=22 channels, P2328S, 1 μM, +40 mV. #P<0.05 between average data at −40 and +40 mV; *P<0.05 between conductances with 1 mM and 1 µM Ca2+. There was no significant difference between average WT and P2328S RyR2 conductances under any condition.
The activity of WT and P2328S RyR2 channels at 1 mM and 1 µM cytoplasmic Ca2+ and ATP sensitivity
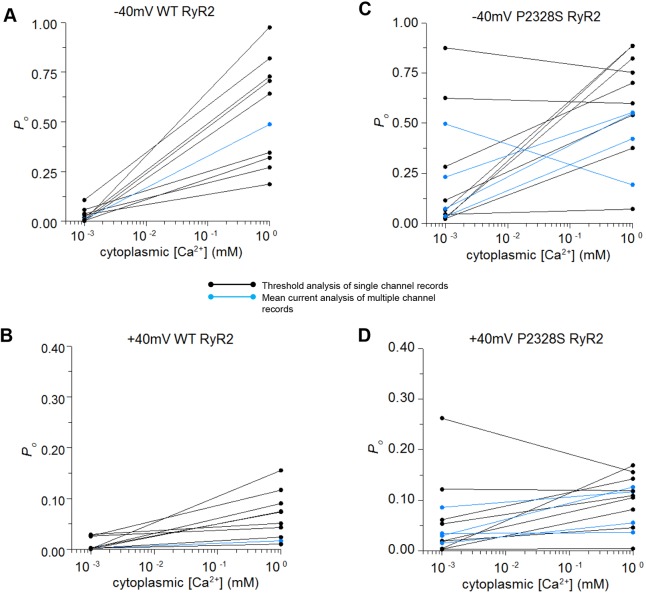
The biphasic cytoplasmic [Ca2+]–dependence of sheep and canine RyR2 channels shows a strong increase in activity between 1 μM and 10 μM Ca2+, a plateau between 10 µM and 1 mM and inactivation at concentrations ≫1 mM (Laver et al., 1995; Xu and Meissner, 1998). Thus, the decline in the activity of the mouse WT channel as shown in Fig. 1A when cytoplasmic Ca2+ was lowered from 1 mM to 1 μM was expected. The activity of the P2328S channel was similar to that of the WT channel with 1 mM cytoplasmic Ca2+, but was markedly higher when the [Ca2+] was lowered to 1 μM. This difference is reflected in the open probability (Po) of most individual channels (Fig. 2; Figs S1, S3 and S4: where lines connect data for individual channels at 1 mM and 1 µM Ca2+). Despite the usual (>10-fold) variability between the Po of individual channels (Copello et al., 1997) at both 1 mM and 1 µM cytoplasmic Ca2+, the open probability of each WT channel was substantially lower with 1 µM than 1 mM Ca2+ (Fig 2A,B). The P2328S channels were more variable, with activity being lower at 1 µM than at 1 mM in 9 of 14 channels, changing very little in two channels and being higher for three channels (Fig 2C,D). As in Fig. 1 above, a robust voltage-dependence of Po in these mouse RyR2 channels is notable in Fig. 2 and Figs S1, S3 and S4, with higher Po at −40 than +40 mV in both WT and P2328S channels. The higher Po at −40 mV suggests that the mutation would have the greatest impact when Ca2+ moves from the lumen of the SR into the cytoplasm, during systole and during diastolic Ca2+ leak. This voltage-dependence of Po has been reported in sheep RyR2 (Dulhunty et al., 1999; Laver and Lamb, 1998; Sigalas et al., 2009), but is not apparent under all conditions (Dulhunty et al., 1999; Hewawasam et al., 2010; Laver and Lamb, 1998; Sigalas et al., 2009).
Fig. 2.
The Po of individual channels at 1 µM and 1 mM cytoplasmic [Ca2+] show substantial variability between P2328S channels with 1 µM Ca2+. (A,B) WT channels. (C,D) P2328S channels. Po at −40 mV plotted in A and C; Po at +40 mV plotted in B and D. Black lines link individual channel data from bilayers containing one active channel (Po determined with a threshold discriminator). Blue lines link measurements from bilayers with more than one channel opening (Po estimated from I′F, the mean current normalised to maximum current, see Materials and Methods). Notably, Po values derived from I′F fall within the range of values obtained from direct measurement of Po.
The Po data in Fig. 2 is replotted on a logarithmic scale in Fig. S1 to show the spread of lower Po values over an ∼100-fold range with 1 µM cytoplasmic Ca2+ at +40 mV. This in most cases exceeds the ∼10-fold range with 1 mM cytoplasmic Ca2+. The tighter range of values with 1 mM cytoplasmic Ca2+ may reflect more cohesive gating of Po at maximally activated levels or a clustering due to the limiting Po value of 1.00.
Parameter values from single and multiple RyR2 channels are shown as the average Po in Fig. 3A (assuming that I′F for multiple channels is equal to the average Po of the individual channels, see Materials and Methods). The mean open time (To), mean closed time (Tc) and event frequency (Fo) could be measured only in single-channel recordings (Fig. 3B–D). There were no significant differences between WT and P2328S in any of the average parameters with 1 mM Ca2+. In marked contrast, at 1 μM Ca2+ there was a significant difference between WT and P2328S channels in average Po To and Tc, with P2328S channels having a higher Po, with longer openings and briefer closures. The higher WT Po at 1 mM compared to 1 µM Ca2+ was due to a significantly longer To, briefer Tc and higher Fo. The average P2328S Po was also higher with 1 mM than with 1 µM Ca2+ due to a significantly higher Fo and a trend towards longer open durations. The difference between Po at 1 µM and 1 mM Ca2+ was substantially less in P2328S than in WT channels. Given the voltage-dependence of channel activity (Figs 1 and 2), the gating parameters are plotted separately for −40 and +40 mV in Fig. S2. The difference between the WT and P2328S Po were significant at both potentials, but the other parameters mainly showed trends in the same directions as the significant changes in the combined data (Fig. 3A–D).
Fig. 3.
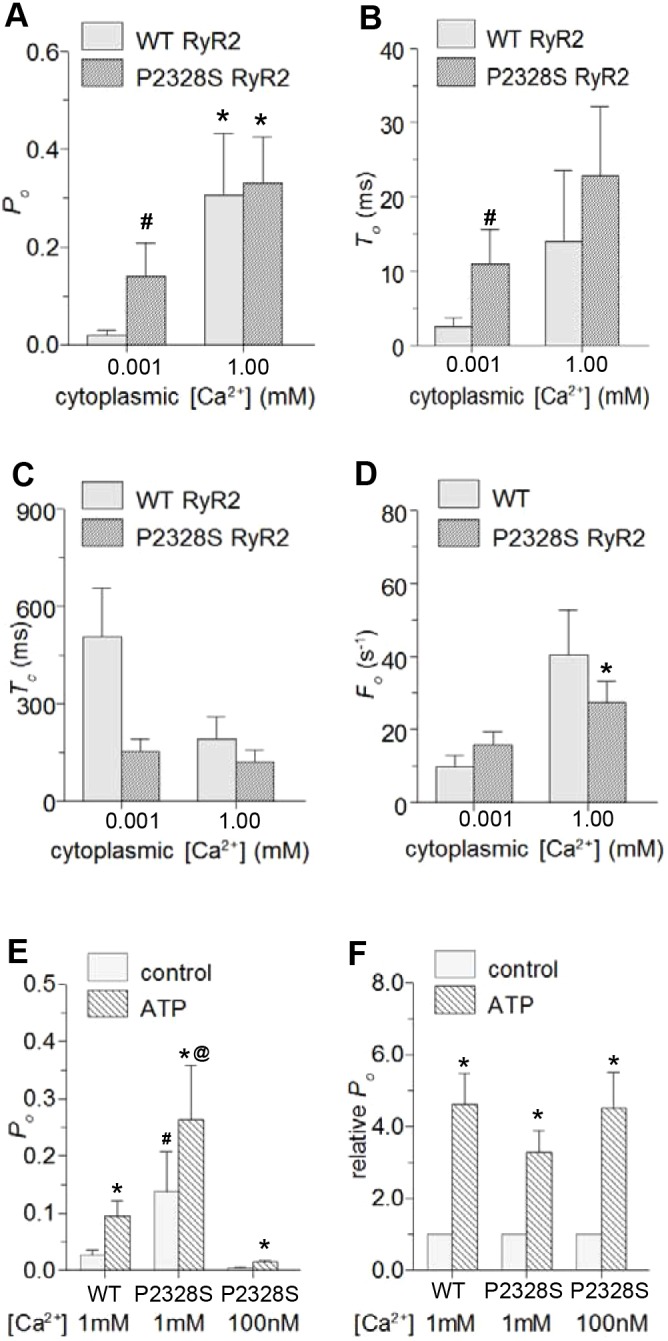
Average gating parameters for WT and P2328S RyR2 channels exposed to 1 μM or 1 mM cytoplasmic Ca2+ and effects of ATP reveal significantly higher activity in P2328S with 1 μM Ca2+. Average data (−40 and +40 mV pooled) for WT (light grey) and P2328S RyR2 (dark grey). (A–D) Mean±s.e.m. values for Po (WT n=20; P2328S n=26), To (WT n=16; P2328S n=18), Tc (WT n=18; P2328S n=20) and Fo (WT n=18; P2328S n=20). *P<0.05 between 1 µM and 1 mM Ca2+. # P<0.05 between WT and P2328S. (E,F) Mean±s.e.m. data for WT (n=7) and P2328S (n=5) channels before (solid grey bars) and after (cross-hatched bars) exposure to 2 mM ATP, with cytoplasmic Ca2+ of 1 μM (n=7 WT; n=5 P2328S) or 100 nM (n=2 P2328S) as indicated. Absolute Po (E); relative Po (F). *P<0.05 for ATP being greater than control, #P<0.05 for P2328S control with 1 μM Ca2+ being greater than WT or P2328S control at 100 nM Ca2+; @P<0.05 for P2328S with 1 µM Ca2+ plus ATP being greater WT with 1 μM Ca2+ plus ATP or P2328S with 100 nM Ca2+ plus ATP.
Overall the results in Fig. 3A–D and Fig. S2 show that increases in WT channel Po between 1 µM and 1 mM Ca2+ are due to cytoplasmic Ca2+ increasing the duration and frequency of channel openings, while reducing the closed times. There were smaller differences between 1 µM and 1 mM Ca2+ in P2328S RyR2, with the significant increases in Po and Fo, and trends towards a longer To and shorter Tc. The reduced sensitivity to this change in [Ca2+] in P2328S RyR2 could be due to either a reduced cytoplasmic Ca2+ sensitivity or a shift in the cytoplasmic Ca2+ dependence. These possibilities are explored in the following section.
Since the P2328 residue is contained within an RyR2 sequence that also binds ATP (Blayney et al., 2013), we examined the effect of the P2328S mutation on ATP activation. Cytoplasmic addition of 2 mM Na2ATP caused an increase in Po in all WT and P2328S channels examined and a significant increase in the average Po of WT RyR2 in the presence of 1 µM Ca2+ and of P2328S channels in the presence of 1 µM and 100 nM cytoplasmic Ca2+ (Fig. 3E). In the presence of ATP, the average Po was significantly greater in P2328S with 1 μM Ca2+ than in WT channels or P2328S with 100 nM Ca2+. Prior to ATP addition, the Po with 1 μM Ca2+ was significantly greater in P2328S than WT channels or P2328S channels with 100 nM Ca2+. However, relative increases in Po were the same in all cases (Fig. 3F), indicating that ATP-activation per se is unaffected by the P2328S mutation.
Redox buffering was addressed in a subset of three WT and three P2328S ATP-activated channels. The GSH:GSSG ratio was set to an oxidising potential (see Materials and Methods) in cytoplasmic and luminal solutions to mimic cardiac oxidative stress (Oda et al., 2015). There was a trend towards the expected increase in Po in WT channels (Pessah et al., 2002). The average WT Po (data at +40 and −40 mV pooled) was 0.086±0.045 with ATP before, and 0.162±0.108 after, redox buffering. P2328S Po was 0.191±0.078 before, and 0.163±0.051 after redox buffering (mean±s.e.m.). Although incomplete, these results suggest that the mutant channels may be less sensitive to oxidising cytoplasmic conditions than WT channels.
Cytoplasmic Ca2+ sensitivity of WT and P2328S RyR2 channels
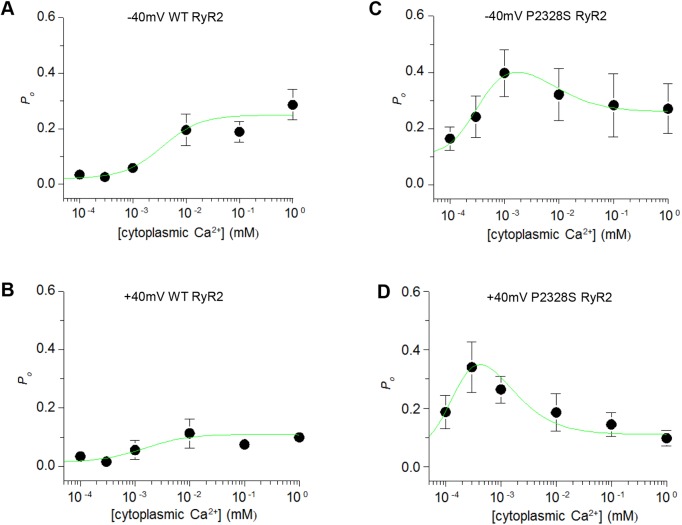
In experiment #2, the cytoplasmic incorporation solution containing 1 mM Ca2+ was replaced immediately after incorporation by perfusion with solutions containing 100 nM or 300 nM Ca2+ (see Materials and Methods). The [Ca2+] was then increased stepwise to 1 mM by addition of appropriate aliquots of CaCl2. The Po for WT channels at 1 mM and 1 µM (Fig. 4) were not significantly different from those obtained in the first experiment. However, in contrast to experiment #1, the activity of most P2328S channels in experiment #2 declined at the higher Ca2+ concentrations (see individual channels in Figs S3 and S4). The average P2328S Po was significantly less with 1 mM Ca2+ than with lower [Ca2+] at +40 mV (Fig. 4D), while at −40 mV there was a trend towards a lower Po at 1 mM than 1 µM Ca2 (Fig. 4C).
Fig. 4.
The Ca2+-concentration dependence of Po reveals substantial effects of the P2328S mutation on Ca2+-activation and Ca2+-inactivation. (A,B) Average Po from experiment #2 for WT RyR2 at −40 and +40 mV respectively. (C,D) Average Po for P2328S RyR2 at −40 and +40 mV, respectively. Data points show mean±s.e.m. (s.e.m. markers are not visible when contained within the dimensions of the symbols). The numbers of observations were the same at −40 and +40 mV for each [Ca2+], but varied between each [Ca2+], depending on whether Ca2+ was initially reduced to 100 nM or 300 nM before subsequent concentration increases. For WT channels, n=x ([Ca2+]): 4 (100 nM); 3 (300 nM); 12 (1 µM); 8 (10 µM); 12 (100 μM); 8 (1 mM). For P2328S RyR2 channels, n=([Ca2+]): 10 (100 nM); 11 (300 nM); 10 (1 µM); 7 (10 µM); 12 (100 μM; 8 (1 mM). The green line Hill curves were fitted to the data using parameters in Table 1.
Although the difference between the two experiments may reflect the different order in which the solutions were changed, it is more likely that it reflects differences between individual P2328S channels. Po was lower with 1 µM Ca2+ than 1 mM Ca2+ in all WT channels. By contrast, P2328S Po in experiment #1 was greater or no different with 1 µM than 1 mM Ca2+ in three of 11 channels, while in experiment #2 Po was greater or no different in seven of eight channels. Taking the two experiments together, the activity of ten of 19 P2328S channels was lower with 1 mM Ca2+ than with 1 µM Ca2+.
The average Po values for WT channels described classical Ca2+ activation curves for RyR2 with Po increasing steeply between 1 µM and 10 µM Ca2+ and reaching a plateau between 10 µM and 1 mM Ca2+ (Fig. 4A,B). Inactivation is not apparent in the WT data as it occurs when the cytoplasmic [Ca2+] is increased to non-physiological levels >1 mM (Laver et al., 1995). The Ca2+ dependence of Po was substantially altered by the RyR2-P2328S mutation (Fig. 4C,D). The increase in P2328S RyR2 Po with Ca2+ activation was shifted to a lower [Ca2+], with a maximum at 1 μM Ca2+ (−40 mV) or ∼300 nM (+40 mV). The average Po declined with further increases in [Ca2+], likely reflecting Ca2+–dependent inactivation, and suggesting an unexpected shift in the inactivation curve into the physiological range of cytoplasmic [Ca2+].
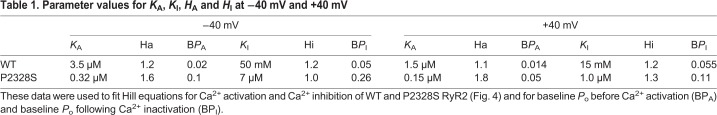
Hill equations for Ca2+-dependent activation and inactivation (see Materials and Methods) were fitted to the data in Fig. 4. Affinity constants for activation and inactivation (KA and KI, respectively) and Hill coefficients for activation and inhibition (HA and HI, respectively) for the fitted curves are listed in Table 1. The P2328S mutation caused an ∼10-fold shift in Ca2+ activation. The affinity constant decreased from WT values of 3.5 μM to 0.32 μM Ca2+ in mutant channels at −40 mV, and from 1.5 μM in WT to 0.15 μM Ca2+ in mutant channels at +40 mV. Inactivation for P2328S RyR2 channels was shifted ∼1000-fold from millimolar range levels in WT channels to 7 µM at +40 mV or 1 μM at −40 mV. Additionally, the P2328S RyR2 inactivation curve at +40 mV (KI=1.0 μM) overlapped the activation curve (KA=0.15 μM). Therefore, the maximum Po achieved of 0.35 was less than the maximum of 0.52 that would occur if activation had proceeded in the absence of inactivation. This is the first report of a RyR2 mutation causing such a dramatic shift in the Ca2+ dependence of inactivation. In contrast to the marked changes in KI and KA, there was no change in the Hill coefficients, indicating that, as expected, the number of binding sites does not change.
Table 1.
Parameter values for KA, KI, HA and HI at −40 mV and +40 mV
The best fit of the Hill equations to the data required two assumptions. First, that there is a baseline Po (BPA) when [Ca2+] is lower than the activating level, of 0.02 for WT RyR2 at −40 mV or 0.014 at +40 mV, or for P2328S of 0.1 at −40 mV or 0.05 at +40 mV. Second, that inactivation in P2328S RyR2 reduced Po to a baseline level (BPI) of 0.26 at −40 mV or 0.11 at +40 mV, which is higher than for the WT channel BPI of 0.05 at −40 mV and 0.055 at +40 mV.
Sub-conductance activity in WT and P2328S RyR2 channels
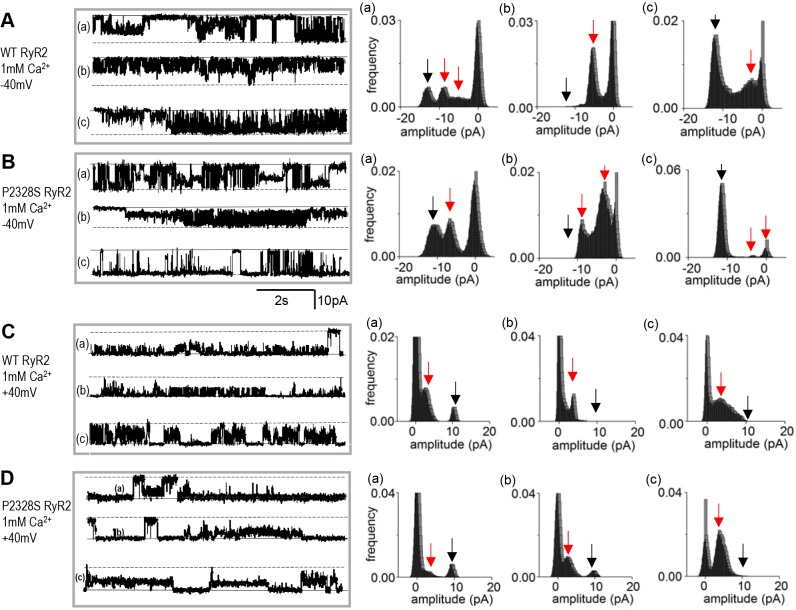
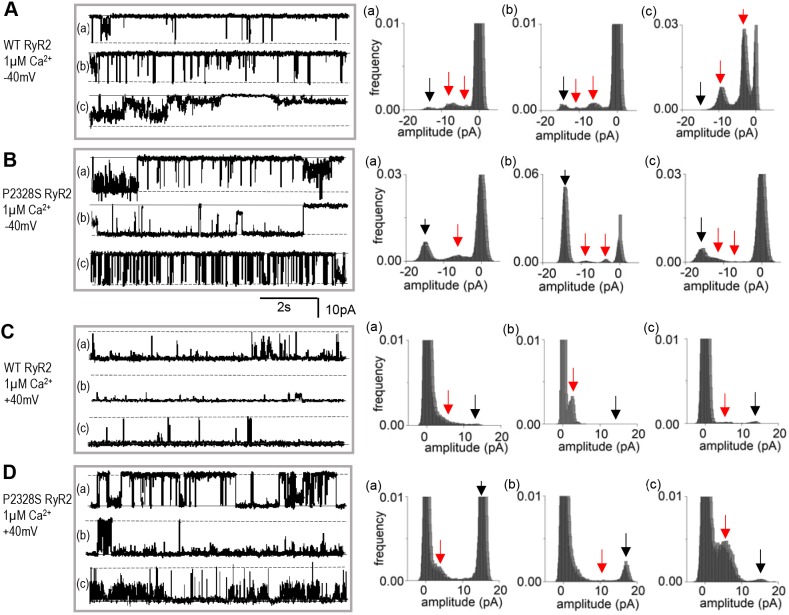
Given the reported associations between arrhythmogenic mutations in RyR2, sub-conductance activity, phosphorylation and amounts of FKBP12 associated with RyR2 (see Introduction), we examined sub-conductance levels, FKBP12 binding to, and phosphorylation of, WT and P2328S RyR2 channels. Strong sub-conductance opening to current levels less than the maximum single-channel current was apparent in both channel types and is apparent in selected segments of activity from 24 different channels in Fig. 5 (with 1 mM cytoplasmic Ca2+) and Fig. 6 (with 1 µM cytoplasmic Ca2+). There were no consistent differences between the WT and P2328S RyR2 channels in sub-conductance levels or in amounts of sub-conductance activity. In each case, there are brief and very long openings to levels between 25% and 75% of the maximum current, with multiple levels, as well as one or two dominant levels. The same lack of difference is apparent in the longer recordings from 18 WT and 18 P2328S channels shown in Figs S3 and S4. Sub-conductance levels were generally scaled to the maximum current, so that the intervals between levels were least with 1 mM cytoplasmic Ca2+ and at +40 mV. Channel openings in WT and P2328S RyR2 are generally more clearly defined at −40 mV with 1 µM Ca2+ (Fig. 6) than with 1 mM cytoplasmic Ca2+ (Fig. 5). The lack of differences between the sub-conductance activity was not due to selection of the current segments in Figs 5 and 6 as they are clearly seen in the longer records of continuous activity from a larger number of channels, as shown in Figs S3 and S4. No quantitative evaluation of sub-conductance levels was attempted as we concluded that the activity was not substantially altered by the P2328S mutation.
Fig. 5.
Sub-conductance openings apparent in both WT and P2328S RyR2 channels with 1 mM cytoplasmic Ca2+. Representative 8.5 s current recordings from three different bilayers (a, b and c) shown on the left, with corresponding amplitude histograms on the right. WT at −40 mV (A); P2328S at −40 mV (B); WT at +40 mV (C); P2328S at +40 mV (D). In current records, solid lines indicate the zero current and broken lines indicate maximum open current. In histograms, the black arrow points to the maximum single channel current and the red arrows indicate prominent sub-conductance levels.
Fig. 6.
Sub-conductance openings apparent in WT and P2328S RyR2 channel activity with 1 µM cytoplasmic Ca2+. Representative 8.5 s current recordings from three different bilayers (a, b and c) shown on the left, with corresponding amplitude histograms on the right. WT at −40 mV (A); P2328S at −40 mV (B); WT at +40 mV (C); P2328S at +40 mV (D). In current records, solid lines indicate the zero current and the broken lines indicate maximum open current. In histograms, the black arrow points to the maximum single channel current and the red arrows indicate prominent sub-conductance levels.
FKBP12 and FKBP12.6 association with WT and P2328S RyR2 channels
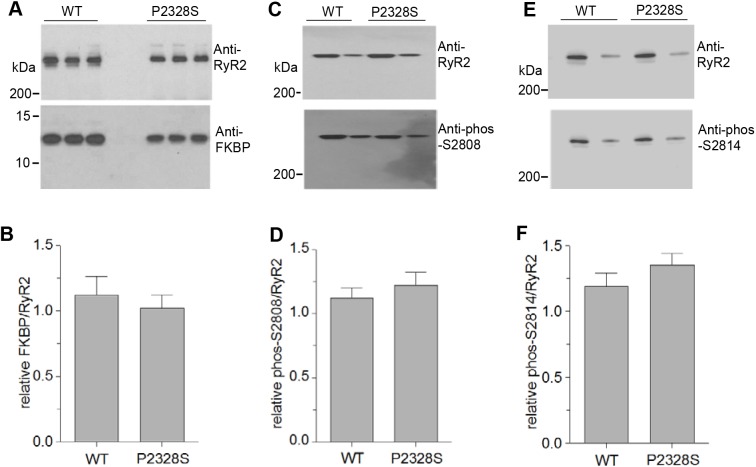
FKBPs associated with the RyR2 was examined in the context of two controversial questions. First, regarding whether there is a correlation between sub-conductance activity and the amount of FKBP bound to RyR2 (Galfré et al., 2012; Lam et al., 1995) and, second, whether the binding of FKBPs to RyR2 is generally altered as a result of disease-associated mutations in RyR2 (Lehnart et al., 2004; Meli et al., 2011) or heart failure (Marx and Marks, 2002). Consistent with reported amounts of FKBP12.6 associated with RyR2 in mouse heart being ∼100-fold lower than FKBP12 (Zissimopoulos et al., 2012), we failed to see any convincing band corresponding to FKBP12.6 in western blots of WT or P2328S RyR2 mouse hearts. We routinely see bands corresponding to both isoforms in western blots of sheep and human heart (Richardson et al., 2017; Walweel et al., 2017). Blots of FKBP12 associated with RyR2 in SR vesicles suggest that there is no difference in its levels between WT or P2328S RyR2 (Fig. 7A) and there was no significant difference between the average relative amounts (Fig. 7B).
Fig. 7.
Neither FKBP associated with RyR2 channels nor S2804 or S2814 phosphorylation are altered by the P2328S mutation. (A) Representative blots of RyR2 and associated FKBP12 from WT and RyR2S/S mouse hearts following co-IP of the FKBP–RyR2 complex with anti-RyR2 antibody, following by SDS-PAGE and immunoblotting (see Materials and Methods). (B) Average relative levels of FKBP12 bound to RyR2. FKBP12 band densities were normalised to the RyR2 band density for each lane, then expressed relative to the WT ratio. n=21 for WT and n=18 for P2328S data. (C–F) SR proteins separated via SDS-PAGE, subjected to western blotting and probed with antibodies to phosphorylated (phos) S2808 (C) or S2814 (E), then stripped and re-probed with anti-RyR2 as a loading control. Average data for RyR2 phosphorylation at S2808 (D) or S2814 (F). Band densities were normalised to total RyR2, then expressed relative to the WT phos-S2808/RyR2 or phos-S2814/RyR ratio. n=9 for S2808, n=6 for S2814. Data bars show mean±s.e.m.
Phosphorylation of WT and P2328S RyR2 channels
Hyperphosphorylation of RyR2 at S2808 and S2814 is associated with ventricular arrhythmia and FKBP dissociation from RyR2 (Dobrev and Wehrens, 2014). S2808 is hyperphosphorylated in humans and dogs with chronic AF (Vest et al., 2005). Human and rat S2804 and/or S2814 are basally phosphorylated and hyperphosporylated after β-adrenergic stimulation (Denniss et al., 2018; Li et al., 2013; Walweel et al., 2017). Phosphorylation of RyR2 S2804 and S2814 in SR vesicles from WT and RyR2S/S mouse hearts was immuno–detected in western blots using antibodies that specifically recognise those residues only when they are phosphorylated. Both residues are basally phosphorylated in the absence of any experimental adrenergic treatment and there was no difference between the basal phosphorylation in WT or P2328S RyR2 in the individual blots (Fig. 7C,E) or in the average normalised data (Fig. 7D,F).
DISCUSSION
Our findings demonstrate that mouse RyR2-P2328S channels are more active than WT RyR2 channels over the lower spectrum of physiologically relevant cytoplasmic Ca2+ concentrations (0.1–1 µM), with both Ca2+ activation and inactivation shifted, respectively, to nanomolar or micromolar Ca2+ levels. Notably this change in Ca2+ sensitivity occurred in the absence of adrenergic challenge. The channel properties indicate that P2328S RyR2 activity may be close to a balance between controlled and uncontrolled Ca2+ leak prior to adrenoreceptor activation and that such a challenge could tip the balance and trigger the cascade of events leading to potentiation of aberrant diastolic Ca2+ release. The greatly altered channel characteristics occurred without alterations in maximum conductance or sub-conductance activity and without change of FKBP12 binding or RyR2 S2808/S2814 hyperphosphorylation, again in the absence of adrenergic challenge. This suggests a mechanism for enhanced channel activity in P2328S-associated CPVT that does not depend on hyperphosphorylation or loss of FKBP12/12.6-mediated stabilisation of RyR2 channels. Notably our findings parallel cellular evidence showing that cardiomyocytes from RyR2S/S hearts have higher incidences of spontaneous diastolic events than WT hearts without adrenergic challenge (Goddard et al., 2008; Zhang et al., 2011).
Channel activity and its Ca2+ dependence in murine WT and P2328S RyR2 channels
The significantly higher Po at 1 mM compared with 1 μM cytoplasmic Ca2+ in mouse WT RyR2 was similar to that for sheep and canine RyR2 (Sitsapesan and Williams, 1994; Xu and Meissner, 1998). The P2328S mutation reduced this difference between Po at 1 µM and 1 mM Ca2+ (experiment #1) so that P2328S RyR2 channels were more active than WT RyR2 at 1 µM cytoplasmic Ca2+ (Figs 1–3). Activity in both channel types was significantly greater with current flow from lumen to cytosol at −40 mV.
The higher activity in P2328S RyR2 channels at 1 µM Ca2+ was due to a strong leftward shift to activation at lower [Ca2+], such that the channels were between 30% and 50% activated with 100 nM Ca2+ and fully activated with 1 µM Ca2+. This would lead to pronounced diastolic Ca2+ leak through P2328S channels that would be expected to be strongly pro-arrhythmic. A leftward shift in the Ca2+ activation curve is common with CPVT mutations (Meli et al., 2011; Xiao et al., 2016) and not surprising. However, the stronger ∼1000-fold shift in Ca2+ inactivation is a novel finding. This inactivation is within the normal range of systolic [Ca2+] and, hence, could truncate systolic Ca2+ release, thus reducing contraction. However, we predict that systolic Ca2+ release may not be affected, because the weaker shift in inactivation at −40 mV (current flow from lumen to cytoplasm) meant that Po at −40 mV was similar in WT and P2328S channels at peak Ca2+ transient levels of ∼10–15 µM Ca2+. Consistent with this prediction, there are no reports suggesting reduced contraction with RyR2-P2328S mutations in humans or in the mouse model, while systolic Ca2+ transient amplitudes are similar in cardiomyocytes from WT and RyR2S/S mice (Goddard et al., 2008; Zhang et al., 2011).
To fit Hill equations to the data, we assumed finite baseline activity levels. The baseline Po at [Ca2+] below activation was higher in P2328S than WT channels at −40 mV and with Ca2+ inactivation, P2328S Po decayed to a baseline level greater than the level before Ca2+ activation (Table 1), suggesting incomplete inactivation. The magnitude of the baseline Po reported here would lead to massive diastolic Ca2+ leak. This would be less in myocytes where other factors including Mg2+ modulate channel activity. The effect of Mg2+ was not addressed in bilayer experiments because, in the absence of ATP and with cytoplasmic Ca2+ ≤1 μM, Mg2+ effectively reduces WT channel activity to such low values that quantification of channel activity is unreliable (Laver et al., 1997a; Xu et al., 1996). Nevertheless, we predict that even a smaller increase in baseline activity with the P2328S mutation would contribute significantly to increased diastolic Ca2+ leak.
The impact of ATP activation and potential Mg2+ inhibition on P2328S channels
The ATP-binding site involves RyR1 residues M4954 and F4959 at the C-terminus of transmembrane S6 helix (des Georges et al., 2016). Equivalent RyR2 residues, M4884 and F4889, are presumably also adjacent to S6. P2328 is situated in the HD1 helical domain, which interacts extensively with regions near the ATP-binding site (Dhindwal et al., 2017). Indeed, Blayney et al. (2013) found high affinity ATP binding to the G2236–G2491 fragment (encompassing HD1, HD2 and P2328) (Blayney et al., 2013). That we did not see an effect of P2328S on ATP activation suggests that the P2328 residue is not involved in ATP binding. Similar ATP scaling of WT and P2328S activity suggests that the Ca2+ dependence of activation and inactivation might not be altered by ATP, although Po would be higher.
The effects of the P2328S mutation on cytoplasmic Mg2+ and luminal Ca2+ and Mg2+ sensitivity, or other regulatory factors like H+, calmodulin, oxidation and nitrosylation were beyond the scope of this study. However it is interesting that Lehnart et al. (2004) found a right shift in cytoplasmic ‘Mg2+ inhibition’ of P2328S channels, which might appear inconsistent with the left shift we see in Ca2+ inactivation because cytoplasmic Mg2+ of ∼1 mM can occupy the Ca2+ inactivation site and inhibit RyR2. However, Mg2+ can bind to two independent cytoplasmic Ca2+-binding sites on RyR2, with effects that depend on cytoplasmic [Ca2+]. The low-affinity inhibitory (I1) site does not discriminate between Ca2+ and Mg2+, and Mg2+ binding to this site leads to Mg2+ inhibition with ≥100 μM cytoplasmic [Ca2+] (Laver, 2010; Walweel et al., 2014). This would indeed parallel the Ca2+ inactivation that we describe here. Mg2+ also binds to the higher affinity Ca2+ activation (A-) site, which selects Ca2+ over Mg2+ by ∼50–fold (Laver, 2010). 1 mM Mg2+ binds to the A-site and prevents Ca2+ in low micromolar range from binding and activating RyR2 (Laver, 2010), that is, it prevents Ca2+ activation rather than inhibiting the channel. The ‘Mg2+ inhibition’ described by Lehnart et al. (2004) using 150 nM cytoplasmic Ca2+ likely reflects Mg2+ binding to the A-site. A right shift in Mg2+ inhibition at the A-site in P2328S RyR2 is consistent with reduced Mg2+ binding as a result of enhanced Ca2+ affinity, allowing activation by 150 nM Ca2+. The relief of A-site Mg2+ binding would further enhance Ca2+ leak during diastole.
Lack of alterations in FKBP association or phosphorylation in mouse P2328S RyR2 channels
The significant changes in the Ca2+ activation curve in mouse P2328S channels did not depend on adrenergic challenge, which is unlike what is seen for P2328S channels expressed in HEK 293 cells where increased activity with 150 nM Ca2+ was seen only after PKA phosphorylation (Lehnart et al., 2004). Our observations are more consistent with those of Zhang et al. (Zhang et al., 2011) who found significantly higher incidences of arrhythmia in isolated perfused RyR2S/S hearts, compared to WT, prior to adding isoproterenol. In addition, diastolic Ca2+ release events are seen in isolated RyR2S/S, but not RyR2+/S or WT atrial myocytes in the absence of isoproterenol (Goddard et al., 2008; Zhang et al., 2011). The difference between results with RyR2 P2328S channels expressed in HEK 293 cells and those with adult mice may not be surprising given that native channels from mouse are associated with regulatory proteins including triadin, junction and calsequestrin, which are lacking in the recombinant system. These contrasts underline the importance of examining the effects of mutations on RyR2 channels expressed in adult mammalian tissue.
The correlation between leaky RyR2 channels carrying CPVT mutations and FKBP binding to the channels is controversial. Reduced FKBP12.6 binding to recombinant RyR2 channels has been reported with P2328S, S2226L, R2474S and R4497C CPVT mutations (Lehnart et al., 2008; Wehrens et al., 2003), and increasing FKBP12.6 association with RyR2 can prevent aberrant SR Ca2+ release with AF and CPVT (Meli et al., 2011; Shan et al., 2012; Wehrens et al., 2003). Conversely, the CPVT R2474S RyR2 demonstrated increased FKBP12.6 affinity (Tiso et al., 2002), while enhanced FKBP12.6 binding did not alter arrhythmias in R4496C mice (Liu et al., 2006).
A complication with evaluating reported FKBP interactions with RyR2 was the assumption that RyR2 associates with FKBP12.6 alone, as FKBP12.6 was discovered in heart (Timerman et al., 1996). Therefore, many studies assumed that any FKBP bound to RyR2 was FKBP12.6, and only FKBP12.6 has been added to RyR2 in numerous functional studies. However many mammalian species (e.g. mouse, pig and rabbit), have more FKBP12 associated with RyR2 than FKBP12.6 (Zissimopoulos et al., 2012). A further complication is that FKBP12 and FKBP12.6 have different actions on RyR2; FKBP12 is a high-affinity sheep RyR2 activator, whereas FKBP12.6 has low efficacy, but antagonises the effects of FKBP12 (Galfré et al., 2012). There are no similar comparative reports for human or mouse RyR2. However we find substantial amounts of FKBP12 bound to healthy human (Walweel et al., 2017) and mouse RyR2 (Fig. 7). It may be more relevant to examine FKBP12 rather than, or combined with, FKBP12.6, association with RyR2 channels.
Altered sub-conductance opening has been attributed to altered FKBP12/12.6 binding to RyR2 (Lehnart et al., 2004; Marx et al., 2000; Richardson et al., 2017; Wehrens et al., 2003) or FKBP12 binding to RyR1 (Ahern et al., 1994; Brillantes et al., 1994). Our observations showing that neither the amount of FKBP12 bound to RyR2 nor sub-conductance activity are altered by the P2328S mutation again indicate a correlation between these parameters. That FKBP12 was not dissociated from P2328S RyR2 is consistent with a mutation in the HD1 domain (Dhindwal et al., 2017) which is distant from FKBP12 binding sites within RyR2 305–784 and 1815–1855 (Meli et al., 2011; Xiao et al., 2016) in both the linear sequence and 3D structure. Although FKBP12.6 stabilises HD2 (2982–3528), it does not alter HD1 (2110–2679) structure (Dhindwal et al., 2017).
As neither FKBP12 nor phosphorylation are important in the RyR2S/S model, ‘intramolecular domain unzipping’ may lead to CPVT in RyR2 P2328S carriers. Interactions between N-terminal and central domains stabilise the channel closed state (Liu and Priori, 2007; Marks, 2002). As P2328S lies within the leucine-rich HD1 region (Dhindwal et al., 2017), the mutation could cause unzipping, hence decreasing the ability of the channel to remain closed and rendering it more sensitive to changes in cytosolic Ca2+ (Priori and Chen, 2011; Sumitomo, 2016), independently of FKBP12/12.6 or phosphorylation (Oda et al., 2015). Consistent with this, the closed times found here were significantly shorter in P2328S channels. Since our findings are in the absence of adrenergic activation, the changes could be more extreme during adrenergic stimulation, which increases channel activity at diastolic cytoplasmic [Ca2+] primarily through effects on luminal Ca2+ and Mg2+ regulation without altering A-site Ca2+ or Mg2+ regulation (Li et al., 2013). Provided the mutation did not alter the influence of phosphorylation on these luminal sites, we predict that adrenergic stimulation would further enhance channel activity at diastolic [Ca2+].
The impact of changes in P2328S channel activity on Ca2+ efflux from the SR during diastole, DAD generation and arrhythmia
It is significant that, with diastolic cytoplasmic Ca2+ levels between 300 nM and 1 µM, Po is three to five times greater in P2328S than WT channels. At 1 µM cytoplasmic Ca2+, the P2328S channel Po is similar to the maximum Ca2+-activated Po of WT channels with >10 µM cytoplasmic Ca2+. Therefore, robust local Ca2+ release from the RyR2S/S mouse SR during diastole could lead to Ca2+ waves. Indeed, we previously reported ectopic Ca2+ transients in regularly stimulated RyR2S/S murine hearts without adrenergic challenge, but not in their WT counterparts (Goddard et al., 2008). It is estimated that release of 30 to 40 µM of Ca2+ would be required to depolarise myocyte membranes to action potential threshold (Schlotthauer and Bers, 2000). Several factors may contribute to preventing this release from triggering an action potential in normal myocytes, providing a 3- to 4-fold safety margin (Schlotthauer and Bers, 2000). The three to five times greater activity of P2328S RyR2 channels during diastole could overcome this safety margin, allowing a greater local increase in cytoplasmic [Ca2+] and stronger depolarisation, triggering action potentials. It is likely that subclinical arrhythmic events, such as ectopic action potentials or non-sustained VT, occur without harming individuals and therefore remain undetected. Indeed, this may occur more frequently than anticipated as more-severe arrhythmic events, including polymorphic VT and sudden cardiac death, occur in CPVT patients at rest and during sleep (Allouis et al., 2005; D'Amati et al., 2005; Goddard et al., 2008; Postma et al., 2005). The incidence of severe arrhythmic events may be further increased by even relatively mild adrenergic challenge.
General characteristics of single mouse WT and P2328S RyR2 channels
Single-channel analysis revealed some fundamental properties common to WT and P2328S RyR2 channels, which have not been reported, or which have been relatively overlooked in recent literature. First, channel conductance is significantly greater in WT channels when current flow is from lumen to cytoplasm (−40 mV), as during systole or diastolic ‘leak’, with a similar trend seen in P2328S channels. Second, a millimolar cytoplasmic [Ca2+] lowers channel conductance, due to Ca2+ blocking the pore (Hanna et al., 2014; Friel and Tsien, 1989; Gillespie et al., 2005). Third, Po is highest when current flow is from the lumen to the cytoplasm. This has been attributed to ‘feed-through activation’ whereby luminal Ca2+ flows through the channel and binds to cytoplasmic Ca2+ activation sites (Sitsapesan and Williams, 1994; Tripathy and Meissner, 1996), or to Ca2+ binding to luminal Ca2+ activation sites (Laver, 2007). We see a greater Po at −40 mV when both cytoplasmic and luminal solutions contain 1 mM Ca2+, so that both cytoplasmic and luminal Ca2+ activation sites would be occupied. Therefore, our results suggest that there is indeed a voltage, or direction of current flow sensor, within the transmembrane domain of the RyR2 that regulates channel gating and is not influenced by the P2328S mutation. The overall significance of this sensor would be to amplify channel activation through Ca2+-induced Ca2+ release during systole and during diastolic leak, particularly with the excess Ca2+ release imposed on the channel by the P2328S mutation.
Finally, our results demonstrate the well–documented (Copello et al., 1997) spread in parameter values in individual WT channels, which is exacerbated in P2328S channels as it is in human heart failure (Walweel et al., 2017). In experiment #1 (1 μM cytoplasmic Ca2+ at −40 mV), the range of WT Po was 0.0016 to 0.11, compared to 0.0027 to 0.875 in P2328S channels. The WT variability is likely a consequence of the size of the RyR2 protein and number of regulatory sites, which may be vacated or occupied on each of the four subunits to different extents in each functional ion channel. Added to this, the increased P2328S variability was partly due to Po remaining unchanged or increasing in many P2328S channels when cytoplasmic Ca2+ was reduced from 1 mM to 1 μM, in contrast to WT channels where Po fell to lower values in all cases. A consequence of the variability is that, although changes in average gating parameters are often not significant, trends are indicative of underlying gating changes.
Concluding comments
P2328S channels show increased sensitivity to cytosolic Ca2+ in the absence of adrenergic challenge. This could contribute significantly to the potentiation of aberrant SR Ca2+ leak under resting conditions, thus triggering cardiac arrhythmias and sudden cardiac death. The increase in open probability was observed in the absence of altered levels of FKBP12 binding or phosphorylation. This is in contrast to findings with FKBP12.6 and phosphorylation with the same mutation, and also some other mutations in RyR2 channels expressed in a HEK cell system, albeit following PKA stimulation (Wehrens et al., 2003; Lehnart et al., 2004; Meli et al., 2011). We uncovered a novel leftward shift in Ca2+-dependent inactivation towards the range of [Ca2+] achieved during systole, with incomplete inactivation. While this might not impact on the peak systolic Ca2+ transient, incomplete inactivation would contribute to maintaining cytoplasmic Ca2+ at a higher than normal level during diastole and add to the potential for DADs and arrhythmia. Overall, our results are consistent with the increased sensitivity to cytosolic Ca2+ suggested in RyR2S/S mouse cells (Goddard et al., 2008; Zhang et al., 2011). It is worth considering that the location of P2328 and its proximity to Ca2+ activation and inactivation sites in the high-resolution structure of the RyR. The cytoplasmic Ca2+ and Mg2+ A-site and ATP-binding sites are located near the transmembrane channel pore region (des Georges et al., 2016; Jones et al., 2017) that interacts with HD1 residues containing P2328 (Dhindwal et al., 2017). A predicted Ca2+ and Mg2+ I1-binding site in residues 1873–1903 is located in the handle domain of RyR1 (Laver, 2018; Laver et al., 1997b), close in space to the HD domains (Yan et al., 2015). Therefore, structural changes in the HD1 domain caused by the P2328S mutation could sterically influence both the A and I1 Ca2+-binding sites producing the changes in channel activity reported here.
MATERIALS AND METHODS
Harvesting of mouse hearts
WT and homozygous RyR2-P2328S inbred 129/Sv mice, age matched across 3–7 months (in order to obtain a range that would represent a similar magnitude of age distribution in the human population), were killed by cervical dislocation in licensed institutional premises under the UK Animals (Scientific Procedures) Act 1986. Homozygous mice were used to ensure that all RyR2 channels were P2328S homotetramers and to reveal the full extent of the effect of the mutation. The hearts were rapidly excised and transferred to ice cold Krebs–Henseleit buffer (in mM: NaCl 119, NaHCO3 25, KCl4, KH2PO4 1.2, MgCl2 1, CaCl2 1.8, glucose 10 and Na-pyruvate 2; pH 7.4, 95% O2/5% CO2) to rinse and remove excess tissue and blood. The whole heart was then snap frozen in liquid N2. Hearts were couriered to Australia on dry ice and then stored at −80°C.
Isolation of the RyR2 SR vesicle preparation
All steps of the SR vesicle preparation were performed on ice and/or at 4°C. For lipid bilayer experiments, five to seven hearts were homogenised in cardiac homogenising buffer (CHB, containing, in mM: sucrose 290, imidazole 10 and NaN3 3, pH 6.9). The homogenate was then centrifuged at 12,000 g for 20 min, then the pellet discarded and the supernatant centrifuged at 43,000 g for 2 h. The pellet was resuspended in Buffer A (CHB plus 649 mM KCl) and centrifuged at 46,000 g for 1.5 h. This pellet was re-suspended in 125 µl per g of mouse heart (∼5 hearts) of buffer A plus protease inhibitor mixture and stored in 8 μl aliquots at −80°C for use in lipid bilayer experiments. All individual protease inhibitors were obtained from Sigma-Aldrich and were added to the final suspension at the following final concentrations: benzamidine hydrochloride hydrate (catalogue #B6506), 1.0 mM; pepstatin A (catalogue #P4265), 2.1 µM; leupeptin (catalogue #L2884), 1 µM; AEBSF/Pefabloc SC (catalogue #76307), 0.5 mM; calpain inhibitor I –(catalogue #A6185), 3 µM; calpain inhibitor II (catalogue no. A6060), 3 µM.
Single-channel lipid bilayer recordings
Lipid bilayers were formed as previously described (Laver et al., 1995), by spreading a lipid mixture (phosphatidylethanolamine, phosphatidylserine and phosphatidylcholine in n-decane) across a 100 µm aperture in a partition separating the cis chamber from the trans chamber. SR vesicles were added to the cis solution so that, following incorporation, the cytoplasmic surface of SR and RyR2 faced that solution, which was then equivalent to the cytoplasmic solution. SR vesicles were incorporated using a cytoplasmic (cis) incorporation solution containing 230 mM caesium methanesulfonate (CsMS), 20 mM CsCl, 1 mM CaCl2 and 10 mM tetraethylsulfamide (TES) pH 7.4, and a luminal (trans) solution containing 30 mM CsMS, 20 mM CsCl, 1 mM CaCl2 and 10 mM TES pH 7.4. Following channel incorporation, CsMS was added to the trans side to equalise the concentration of the charge carrier [Cs+] in both solutions. Continuous current recording began at this point and continued for the duration of the experiment. A cis solution containing physiological cytoplasmic Ca2+ concentrations of 100 nM, 300 nM or 1 μM (with all other components identical to 1 mM Ca2+ cis solution) was introduced by a back-to-back 10 ml syringe aspiration-perfusion system designed to effectively replace the entire cis bathing solution. The Ca2+ concentration in the cis solution was later increased by adding appropriate amounts of CaCl2, which were again determined using a Ca2+ electrode. In addition, in many channels, after increasing [Ca2+] stepwise from 100 or 300 nM to 1 μM, 10 μM, 100 μM and 1 mM, we then re-perfused the cis chambers with the 100 or 300 nM Ca2+ solution and increased Ca2+ again to 1 μM or 10 μM. This allowed us to bracket at least some of the measurement and have more confidence in the results obtained for individual channels at lower [Ca2+]. All experiments were performed at a room temperature of 19±1oC.
Note that the trans (luminal) [Ca2+] was maintained at its physiological level of 1 mM throughout. Note also that the initial cytoplasmic [Ca2+] of 1 mM used for vesicle incorporation was higher than the cellular range of 100 nM to 10 µM, but was required to facilitate channel incorporation. Measurement of channel activity with 1 mM cytoplasmic Ca2+ was nevertheless of considerable interest because it provided an indication of whether the plateau of RyR2 Ca2+ activation was maintained up to that concentration or whether activity declined due to the lower affinity Ca2+ inactivation process.
In some channels the redox potential of the cytoplasmic and luminal solutions was clamped to an oxidising level of −180 mV by adding GSH:GSSG in the ratio of 0.95 mM:0.1 mM to each solution (Feng et al., 2000; Pessah et al., 2002), in the presence of cytoplasmic 1 μM Ca2+ and 2 mM ATP2−.
Single-channel lipid bilayer electrophysiology and analysis
Electrodes in the solutions on either side of the bilayer were used to voltage clamp the bilayer potential to −40 or +40 mV (Vcis – Vtrans) and to detect current flow through the channel. Bilayer potential was switched between −40 mV and +40 mV every 30 s. The open probability (Po), mean open time (To) and mean closed time (Tc) were measured, and sub-conductance activity analysed over 60 to 90 s of recordings in which only one channel opened in the bilayer, using the programs Channel 2 (now depreciated; developed by P. W. Gage and M. Smith, John Curtin School of Medical Research) or Channel 3 (developed by N. W. Laver, University of Newcastle, UK). Channel 3 software can be obtained from Professor D. R. Laver (University of Newcastle, Newcastle, NSW, Australia). Threshold levels for channel opening were set to exclude baseline noise at ∼20% of the maximum single-channel conductance. Po alone was evaluated in recordings containing more than one channel from the mean current divided by the maximum open current to obtain a value for the fractional mean current (I′F), which reflects Po and is equal to Po under ideal conditions.
A curve describing the changes in Po with Ca2+ concentration was constructed by multiplying Hill equations for activation and inhibition (Eqns 1 and 2, respectively) which were modified from Laver et al. (1995) to include non-zero Po values as indicated before Ca2+ activation (BPA) and after Ca2+-dependent inhibition BPI, as required to best fit the equations to the data.
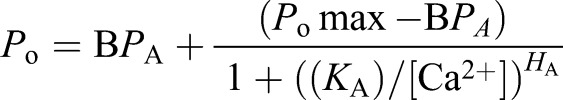 |
(1) |
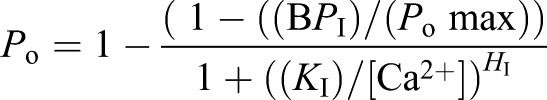 |
(2) |
Po max is the Po of the achieved with maximal Ca2+ activation of the channel under the conditions of our experiment, KA and KI are the Ca2+ affinities of the activation and inhibition sites.
Co-immunoprecipitation
Anti-RyR2 co-immunoprecipitation (Co-IP) of RyR2 complexes was performed to assess levels of FKBP12 and FKBP12.6 bound to RyR2 using the Pierce Co-IP kit and anti-RyR2 C3-33 antibody, following the manufacturer's instructions (Walweel et al., 2017). In brief, 100 μg of SR were diluted to 1 μg /μl in IP buffer (20 mM MOPS, 150 mM NaCl, 1 mM CaCl2, 1× cOmplete, EDTA-free protease inhibitor cocktail ; pH 7.4) with 5% glycerol and 0.1%Triton X-100 for 15 min on ice. Diluted SR samples were precleared with non-activated resin for 30 min at room temperature, with rotation. Precleared SR vesicles were separated from non-activated resin by centrifugation (1 min, 1000 g), and incubated for 15 h at 4°C (with rotation) with anti-RyR2-antibody-bound resin in IP buffer. Unbound protein was removed by five washes of the complex with 200 μl of IP buffer, prior to elution of the bound RyR2 complexes from the anti-RyR2 antibody resin using 35 μl of 1×LDL sample buffer with reducing agent at 60°C for 10 min. Samples were then subject to SDS-PAGE and western blotting (below).
SDS-PAGE and western blotting
SR vesicles (1–4 μg, for phosphorylated protein detection) or co-immunoprecipitates (for detection of FKBPs and RyR2), were subject to SDS-PAGE and western blotting as previously described (Laemmli, 1970; Towbin et al., 1979). Briefly, proteins were denatured in 1× Bolt LSL sample buffer with reducing agent (Life Technologies) at 60°C for 10 min. Samples and standards were loaded onto a 4–15% BOLT SDS-polyacrylamide gel (Thermo Fisher Scientific, Scorsby, Australia) and separated via electrophoresis using a Bolt Mini Gel system (Thermo Fisher Scientific) at 165 V until the dye from reached the bottom on the gel. The proteins were transferred onto PVDF membrane within a Bio-Rad Mini-Protean Tetra cell (Bio-Rad, Gladesville, Australia) in cold (4°C) transfer buffer (37 mM Tris, 140 mM glycine, 20% ethanol, no pH adjustment). To maximise transfer of large molecular mass proteins (such as RyR2), the transfer was carried out 1 h at 100 mV, and then for an additional 30 min at 200 mV. The PVDF membrane was blocked for 1–2 h at room temperature in blocking solution (3% BSA in PBS), and incubated with primary antibody (in PBS with 0.05% Tween 20 buffer) overnight at 4°C. Membranes were washed in PBS with 0.05% Tween 20 (5×), and incubated with appropriate secondary HRP-conjugated antibodies (IgG; in PBS with 0.05% Tween 20 for 1.5–2 h at room temperature). Blots were washed 5×in PBS+0.05% tween, once in PBS, prior to chemiluminescence detection.
Primary antibodies used were: anti-RyR2 C3-33 [MA3-916 ryanodine receptor monoclonal antibody (C3-33), used at a concertation of 1 μg/ml (Thermo Fisher Scientific)], anti-FKBP12 H5 [sc-133067 FKBP12 (H-5) mouse monoclonal antibody, used at a 1:200 dilution (Santa Cruz Biotechnology); this detects both 12.0 and 12.6 isoforms of FKBP] and RyR2 pSer28084 [A010-30AP RyR2 (pSer2808) rabbit polyclonal antibody, used at a 1:2000 dilution] and RyR2 pSer2814 [A010-31AP RyR2 (pSer2814) rabbit polyclonal antibody, used at a 1:5000 dilution], which detect the phosphorylated form of RyR2 residues S2808 or S2814, respectively (Badrilla, Leeds, UK). The specificity of the pSer2814 and pSer2814 antibodies for phosphorylated S2808 and S2814 (respectively) was validated by maximally phosphorylating (with PKA and CamKII) and dephosphorylating (with PP1) RyR2, as previously described (Walweel et al., 2017). Specificity of anti-RyR2 C3-33 for RyR2 was validated by probing native RyR2 (in SR vesicles) and purified mouse RyR2.
Statistics
Average data is presented as mean±s.e.m. The significance of difference between various channel parameters for all channel data was assessed with one- or two-sided Student's t-tests as appropriate. The significance of differences in the co-IP and phosphorylation data were evaluated by performing one-way ANOVA with Tukey post hoc testing. Differences were considered significant with P<0.05.
Supplementary Material
Acknowledgements
The authors are grateful to Ms Suzy Pace and Ms Joan Stivala for their assistance with preparation of SR vesicles and general laboratory duties and to Dr Hermia Willemse for assistance with some channel and co-IP work.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: S.C.S., N.A.B., S.A., J.A.F., C.L.-H.H., A.F.D.; Methodology: S.C.S., E.M.G., N.A.B., H.V., J.A.F., C.L.-H.H., A.F.D.; Validation: C.L.-H.H., A.F.D.; Formal analysis: S.C.S., N.A.B., A.F.D.; Investigation: E.M.G., N.A.B., H.V., A.F.D.; Resources: S.C.S., S.A., C.L.-H.H., A.F.D.; Data curation: S.C.S., E.M.G., N.A.B., J.A.F., A.F.D.; Writing - original draft: S.C.S., N.A.B., C.L.-H.H., A.F.D.; Writing - review & editing: S.C.S., N.A.B., S.A., J.A.F., C.L.-H.H., A.F.D.; Supervision: S.C.S., C.L.-H.H., A.F.D.; Project administration: S.C.S., N.A.B., S.A., C.L.-H.H., A.F.D.; Funding acquisition: S.A., C.L.-H.H., A.F.D.
Funding
The work was supported by grants to A.F.D. and N.A.B. from the Australian National Health and Medical Research Council (APP108477 to A.F.D., APP1021342 to N.A.B and A.F.D.), to C.L-H.H. from the Medical Research Council (MR/M001288/1), the Wellcome Trust (105727/Z/14/Z) and British Heart Foundation (PG/14/79/31102 and PG/15/12/31280), and the Isaac Newton Trust/Wellcome Trust ISSF/University of Cambridge Joint Research Grants Scheme (to J.A.F.). Deposited in PMC for immediate release.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.229039.supplemental
References
- Ahern G. P., Junankar P. R. and Dulhunty A. F. (1994). Single channel activity of the ryanodine receptor calcium release channel is modulated by FK-506. FEBS Lett. 352, 369-374. 10.1016/0014-5793(94)01001-3 [DOI] [PubMed] [Google Scholar]
- Allouis M., Probst V., Jaafar P., Schott J.-J. and Le Marec H. (2005). Unusual clinical presentation in a family with catecholaminergic polymorphic ventricular tachycardia due to a G14876A ryanodine receptor gene mutation. Am. J. Cardiol. 95, 700-702. 10.1016/j.amjcard.2004.10.057 [DOI] [PubMed] [Google Scholar]
- Benjamin E. J., Wolf P. A., D'Agostino R. B., Silbershatz H., Kannel W. B. and Levy D. (1998). Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 98, 946-952. 10.1161/01.CIR.98.10.946 [DOI] [PubMed] [Google Scholar]
- Bers D. M. (2001). Excitation-Contraction Coupling and Cardiac Contractile Force. Netherlands: Springer [Google Scholar]
- Blayney L., Beck K., MacDonald E., D'Cruz L., Nomikos M., Griffiths J., Thanassoulas A., Nounesis G. and Lai F. A. (2013). ATP interacts with the CPVT mutation-associated central domain of the cardiac ryanodine receptor. Biochim. Biophys. Acta - Gen. Subj. 1830, 4426-4432. 10.1016/j.bbagen.2013.05.038 [DOI] [PubMed] [Google Scholar]
- Brillantes A. M. B., Ondrias K., Scott A., Kobrinsky E., Ondriašová E., Moschella M. C., Jayaraman T., Landers M., Ehrlich B. E. and Marks A. R. (1994). Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell 77, 513-523. 10.1016/0092-8674(94)90214-3 [DOI] [PubMed] [Google Scholar]
- Cerrone M., Colombi B., Santoro M., di Barletta M. R., Scelsi M., Villani L., Napolitano C. and Priori S. G. (2005). Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Circ. Res. 96, e77-e82. 10.1161/01.RES.0000169067.51055.72 [DOI] [PubMed] [Google Scholar]
- Copello J. A., Barg S., Onoue H. and Fleischer S. (1997). Heterogeneity of Ca2+ gating of skeletal muscle and cardiac ryanodine receptors. Biophys. J. 73, 141-156. 10.1016/S0006-3495(97)78055-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amati G., Bagattin A., Bauce B., Rampazzo A., Autore C., Basso C., King K., Romeo M. D., Gallo P., Thiene G. et al. (2005). Juvenile sudden death in a family with polymorphic ventricular arrhythmias caused by a novel RyR2 gene mutation: Evidence of specific morphological substrates. Hum. Pathol. 36, 761-767. 10.1016/j.humpath.2005.04.019 [DOI] [PubMed] [Google Scholar]
- Davis R. C., Hobbs F. D. R., Kenkre J. E., Roalfe A. K., Iles R., Lip G. Y. H. and Davies M. K. (2012). Prevalence of atrial fibrillation in the general population and in high-risk groups: the ECHOES study. Europace 14, 1553-1559. 10.1093/europace/eus087 [DOI] [PubMed] [Google Scholar]
- Denniss A., Dulhunty A. F. and Beard N. A. (2018). Ryanodine receptor Ca2+ release channel post-translational modification: Central player in cardiac and skeletal muscle disease. Int. J. Biochem. Cell Biol. 101, 49-53. 10.1016/j.biocel.2018.05.004 [DOI] [PubMed] [Google Scholar]
- des Georges A., Clarke O. B., Zalk R., Yuan Q., Condon K. J., Grassucci R. A., Hendrickson W. A., Marks A. R. and Frank J. (2016). Structural basis for gating and activation of RyR1. Cell 167, 145-157.e17. 10.1016/j.cell.2016.08.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhindwal S., Lobo J., Cabra V., Santiago D. J., Nayak A. R., Dryden K. and Samsó M. (2017). A cryo-EM-based model of phosphorylation- and FKBP12.6-mediated allosterism of the cardiac ryanodine receptor. Sci. Signal. 10, eaai8842 10.1126/scisignal.aai8842 [DOI] [PubMed] [Google Scholar]
- Dobrev D. and Wehrens X. H. T. (2014). Role of RyR2 Phosphorylation in Heart Failure and Arrhythmias: controversies around ryanodine receptor phosphorylation in cardiac disease. Circ. Res. 114, 1311-1319. 10.1161/CIRCRESAHA.114.300568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty A. F., Laver D. R., Gallant E. M., Casarotto M. G., Pace S. M. and Curtis S. (1999). Activation and inhibition of skeletal RyR channels by a part of the skeletal DHPR II-III loop: effects of DHPR Ser 687 and FKBP12. Biophys. J. 77, 189-203. 10.1016/S0006-3495(99)76881-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W., Liu G., Allen P. D. and Pessah I. N. (2000). Transmembrane redox sensor of ryanodine receptor complex. J. Biol. Chem. 275, 35902-35907. 10.1074/jbc.C000523200 [DOI] [PubMed] [Google Scholar]
- Friel D. D. and Tsien R. W. (1989). Voltage-gated calcium channels: direct observation of the anomalous mole fraction effect at the single-channel level. Proc. Natl. Acad. Sci. 86, 5207-5211. 10.1073/pnas.86.13.5207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfré E., Pitt S. J., Venturi E., Sitsapesan M., Zaccai N. R., Tsaneva-Atanasova K., O'Neill S. and Sitsapesan R. (2012). FKBP12 activates the cardiac ryanodine receptor Ca2+-release channel and is antagonised by FKBP12.6. PLoS ONE 7, e31956 10.1371/journal.pone.0031956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Xu L., Wang Y. and Meissner G. (2005). (De)constructing the ryanodine receptor: modeling ion permeation and selectivity of the calcium release channel. J. Phys. Chem. B 109, 15598-15610. 10.1021/jp052471j [DOI] [PubMed] [Google Scholar]
- Glukhov A. V., Kalyanasundaram A., Lou Q., Hage L. T., Hansen B. J., Belevych A. E., Mohler P. J., Knollmann B. C., Periasamy M., Györke S. et al. (2015). Calsequestrin 2 deletion causes sinoatrial node dysfunction and atrial arrhythmias associated with altered sarcoplasmic reticulum calcium cycling and degenerative fibrosis within the mouse atrial pacemaker complex1. Eur. Heart J. 36, 686-697. 10.1093/eurheartj/eht452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard C. A., Ghais N. S., Zhang Y., Williams A. J., Colledge W. H., Grace A. A. and Huang C. L.-H. (2008). Physiological consequences of the P2328S mutation in the ryanodine receptor (RyR2) gene in genetically modified murine hearts. Acta Physiol. 194, 123-140. 10.1111/j.1748-1716.2008.01865.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna A. D., Lam A., Thekkedam C., Gallant E. M., Beard N. A. and Dulhunty A. F. (2014). Cardiac ryanodine receptor activation by a high Ca2+ store load is reversed in a reducing cytoplasmic redox environment. J. Cell Sci. 127, 4531-4541. 10.1242/jcs.156760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewawasam R., Liu D., Casarotto M. G., Dulhunty A. F. and Board P. G. (2010). The structure of the C-terminal helical bundle in glutathione transferase M2-2 determines its ability to inhibit the cardiac ryanodine receptor. Biochem. Pharmacol. 80, 381-388. 10.1016/j.bcp.2010.04.019 [DOI] [PubMed] [Google Scholar]
- Huang C. L.-H. (2017). Murine electrophysiological models of cardiac arrhythmogenesis. Physiol. Rev. 97, 283-409. 10.1152/physrev.00007.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Wang R., Xiao B., Kong H., Hunt D. J., Choi P., Zhang L. and Chen S. R. W. (2005). Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circ. Res. 97, 1173-1181. 10.1161/01.RES.0000192146.85173.4b [DOI] [PubMed] [Google Scholar]
- Jiang D., Chen W., Wang R., Zhang L. and Chen S. R. W. (2007). Loss of luminal Ca2+ activation in the cardiac ryanodine receptor is associated with ventricular fibrillation and sudden death. Proc. Natl. Acad. Sci. 104, 18309-18314. 10.1073/pnas.0706573104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. P., Guo W. and Chen S. R. W. (2017). Control of cardiac ryanodine receptor by sarcoplasmic reticulum luminal Ca2+. J. Gen. Physiol. 149, 867-875. 10.1085/jgp.201711805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J. H., Zhang Y., Lei M., Grace A. A., Huang C. L.-H. and Fraser J. A. (2013). Atrial arrhythmia, triggering events and conduction abnormalities in isolated murine RyR2-P2328S hearts. Acta Physiol. 207, 308-323. 10.1111/apha.12006 [DOI] [PubMed] [Google Scholar]
- Kourliouros A., Savelieva I., Kiotsekoglou A., Jahangiri M. and Camm J. (2009). Current concepts in the pathogenesis of atrial fibrillation. Am. Heart J. 157, 243-252. 10.1016/j.ahj.2008.10.009 [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680-685. 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- Laitinen P. J., Brown K. M., Piippo K., Swan H., Devaney J. M., Brahmbhatt B., Donarum E. A., Marino M., Tiso N., Viitasalo M. et al. (2001). Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation 103, 485-490. 10.1161/01.CIR.103.4.485 [DOI] [PubMed] [Google Scholar]
- Lam E., Martin M. M., Timerman A. P., Sabers C., Fleischer S., Lukas T., Abraham R. T., O'Keefe S. J., O'Neill E. A. and Wiederrecht G. J. (1995). A novel FK506 binding protein can mediate the immunosuppressive effects of FK506 and is associated with the cardiac ryanodine receptor. J. Biol. Chem. 270, 26511-26522. 10.1074/jbc.270.44.26511 [DOI] [PubMed] [Google Scholar]
- Laver D. R. (2007). Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites. Biophys. J. 92, 3541-3555. 10.1529/biophysj.106.099028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver D. R. (2010). Regulation of RyR channel gating by Ca2+, Mg2+ and ATP. Curr. Top. Membr. 66, 69-89. 10.1016/S1063-5823(10)66004-8 [DOI] [PubMed] [Google Scholar]
- Laver D. R. (2018). Regulation of the RyR channel gating by Ca2+ and Mg2+. Biophys. Rev. 10, 1087-1095. 10.1007/s12551-018-0433-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver D. R. and Lamb G. D. (1998). Inactivation of Ca2+ release channels (ryanodine receptors RyR1 and RyR2) with rapid steps in [Ca2+] and voltage. Biophys. J. 74, 2352-2364. 10.1016/S0006-3495(98)77944-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver D. R., Roden L. D., Ahern G. P., Eager K. R., Junankar P. R. and Dulhunty A. F. (1995). Cytoplasmic Ca2+ inhibits the ryanodine receptor from cardiac muscle. J. Membr. Biol. 147, 7-22. 10.1007/BF00235394 [DOI] [PubMed] [Google Scholar]
- Laver D. R., Baynes T. M. and Dulhunty A. F. (1997a). Magnesium inhibition of ryanodine-receptor calcium channels: evidence for two independent mechanisms. J. Membr. Biol. 156, 213-229. 10.1007/s002329900202 [DOI] [PubMed] [Google Scholar]
- Laver D. R., Owen V. J., Junankar P. R., Taske N. L., Dulhunty A. F. and Lamb G. D. (1997b). Reduced inhibitory effect of Mg2+ on ryanodine receptor-Ca2+ release channels in malignant hyperthermia. Biophys. J. 73, 1913-1924. 10.1016/S0006-3495(97)78222-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnart S. E., Wehrens X. H. T., Laitinen P. J., Reiken S. R., Deng S.-X., Cheng Z., Landry D. W., Kontula K., Swan H. and Marks A. R. (2004). Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation 109, 3208-3214. 10.1161/01.CIR.0000132472.98675.EC [DOI] [PubMed] [Google Scholar]
- Lehnart S. E., Mongillo M., Bellinger A., Lindegger N., Chen B. X., Hsueh W., Reiken S., Wronska A., Drew L. J., Ward C. W. et al. (2008). Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J. Clin. Invest. 118, 2230-2245. 10.1172/JCI35346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Imtiaz M. S., Beard N. A., Dulhunty A. F., Thorne R., VanHelden D. F. and Laver D. R. (2013). ß-Adrenergic stimulation increases RyR2 activity via intracellular Ca2+ and Mg2+ regulation. PLoS ONE 8, e58334 10.1371/journal.pone.0058334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N. and Priori S. G. (2007). Disruption of calcium homeostasis and arrhythmogenesis induced by mutations in the cardiac ryanodine receptor and calsequestrin. Cardiovasc. Res. 77, 293-301. 10.1093/cvr/cvm004 [DOI] [PubMed] [Google Scholar]
- Liu N., Colombi B., Memmi M., Zissimopoulos S., Rizzi N., Negri S., Imbriani M., Napolitano C., Lai F. A. and Priori S. G. (2006). Arrhythmogenesis in catecholaminergic polymorphic ventricular tachycardia: insights From a RyR2 R4496C knock-in mouse model. Circ. Res. 99, 292-298. 10.1161/01.RES.0000235869.50747.e1 [DOI] [PubMed] [Google Scholar]
- Liu Y., Kimlicka L., Hiess F., Tian X., Wang R., Zhang L., Jones P. P., Van Petegem F. and Chen S. R. W. (2013). The CPVT-associated RyR2 mutation G230C enhances store overloadinduced Ca2+ release and destabilizes the N-terminal domains. Biochem. J. 454, 123-131. 10.1042/BJ20130594 [DOI] [PubMed] [Google Scholar]
- Marks A. R. (2002). Ryanodine receptors, FKBP12, and heart failure. Front. Biosci. 7, d970-d977. 10.2741/a822 [DOI] [PubMed] [Google Scholar]
- Marx S. O. and Marks A. R. (2002). Regulation of the ryanodine receptor in heart failure. Basic Res. Cardiol. 97 Suppl. 1, I49-I51. [DOI] [PubMed] [Google Scholar]
- Marx S. O., Reiken S., Hisamatsu Y., Jayaraman T., Burkhoff D., Rosemblit N. and Marks A. R. (2000). PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell 101, 365-376. 10.1016/S0092-8674(00)80847-8 [DOI] [PubMed] [Google Scholar]
- Meli A. C., Refaat M. M., Dura M., Reiken S., Wronska A., Wojciak J., Carroll J., Scheinman M. M. and Marks A. R. (2011). A novel ryanodine receptor mutation linked to sudden death increases sensitivity to cytosolic calcium. Circ. Res. 109, 281-290. 10.1161/CIRCRESAHA.111.244970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning F., Luo L., Ahmad S., Valli H., Jeevaratnam K., Wang T., Guzadhur L., Yang D., Fraser J. A., Huang C. L. H. et al. (2016). The RyR2-P2328S mutation downregulates Nav1.5 producing arrhythmic substrate in murine ventricles. Pflugers Arch. Eur. J. Physiol. 468, 655-665. 10.1007/s00424-015-1750-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda T., Yang Y., Uchinoumi H., Thomas D. D., Chen-Izu Y., Kato T., Yamamoto T., Yano M., Cornea R. L. and Bers D. M. (2015). Oxidation of ryanodine receptor (RyR) and calmodulin enhance Ca release and pathologically alter, RyR structure and calmodulin affinity. J. Mol. Cell. Cardiol. 85, 240-248. 10.1016/j.yjmcc.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavola J., Viitasalo M., Laitinen-Forsblom P. J., Pasternack M., Swan H., Tikkanen I., Toivonen L., Kontula K. and Laine M. (2007). Mutant ryanodine receptors in catecholaminergic polymorphic ventricular tachycardia generate delayed afterdepolarizations due to increased propensity to Ca2+ waves. Eur. Heart J. 28, 1135-1142. 10.1093/eurheartj/ehl543 [DOI] [PubMed] [Google Scholar]
- Paech C., Gebauer R. A., Karstedt J., Marschall C., Bollmann A. and Husser D. (2014). Ryanodine receptor mutations presenting as idiopathic ventricular fibrillation: a report on two novel familial compound mutations, c.6224T>C and c.13781A>G, with the clinical presentation of idiopathic ventricular fibrillation. Pediatr. Cardiol. 35, 1437-1441. 10.1007/s00246-014-0950-2 [DOI] [PubMed] [Google Scholar]
- Pessah I. N., Kim K. H. and Feng W. (2002). Redox sensing properties of the ryanodine receptor complex. Front. Biosci. 7, a72-a79. 10.2741/A741 [DOI] [PubMed] [Google Scholar]
- Pizzale S., Gollob M. H., Gow R. and Birnie D. H. (2008). Sudden death in a young man with catecholaminergic polymorphic ventricular tachycardia and paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 19, 1319-1321. 10.1111/j.1540-8167.2008.01211.x [DOI] [PubMed] [Google Scholar]
- Postma A. V., Denjoy I., Kamblock J., Alders M., Lupoglazoff J. M., Vaksmann G., Dubosq-Bidot L., Sebillon P., Mannens M. M. A. M., Guicheney P. et al. (2005). Catecholaminergic polymorphic ventricular tachycardia: RYR2 mutations, bradycardia, and follow up of the patients. J. Med. Genet. 42, 863-870. 10.1136/jmg.2004.028993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori S. G. and Chen S. R. W. (2011). Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ. Res. 108, 871-883. 10.1161/CIRCRESAHA.110.226845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori S. G., Napolitano C., Tiso N., Memmi M., Vignati G., Bloise R., Sorrentino V. and Danieli G. A. (2001). Mutataions in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation 103, 196-200. 10.1161/01.CIR.103.2.196 [DOI] [PubMed] [Google Scholar]
- Richardson S. J., Steele G. A., Gallant E. M., Lam A., Schwartz C. E., Board P. G., Casarotto M. G., Beard N. A. and Dulhunty A. F. (2017). Association of FK506 binding proteins with RyR channels – effect of CLIC2 binding on sub-conductance opening and FKBP binding. J. Cell Sci. 130, 3588-3600. 10.1242/jcs.204461 [DOI] [PubMed] [Google Scholar]
- Ronen B. J. and Lili B. (2016). Patient specific induced pluripotent stem cell-derived cardiomyocytes for drug development and screening in catecholaminergic polymorphic ventricular Tachycardia. J. Atr. Fibrillation 9, 91-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabir I. N., Ma N., Jones V. J., Goddard C. a, Zhang Y., Kalin A., Grace A. a and Huang C. L. H. (2010). Alternans in genetically modified langendorff-perfused murine hearts modeling catecholaminergic polymorphic ventricular tachycardia. Front. Physiol. 1, 126 10.3389/fphys.2010.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvage S. C., King J. H., Chandrasekharan K. H., Jafferji D. I. G., Guzadhur L., Matthews H. R., Huang C. L. H. and Fraser J. A. (2015). Flecainide exerts paradoxical effects on sodium currents and atrial arrhythmia in murine RyR2-P2328S hearts. Acta Physiol. 214, 361-375. 10.1111/apha.12505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlotthauer K. and Bers D. M. (2000). Sarcoplasmic reticulmn Ca2+ release causes myocyte depolarization: underlying mechanism and threshold for triggered action potentials. Circ. Res. 87, 774-780. 10.1161/01.RES.87.9.774 [DOI] [PubMed] [Google Scholar]
- Shan J., Betzenhauser M. J., Kushnir A., Reiken S., Meli A. C., Wronska A., Dura M., Chen B. X. and Marks A. R. (2010). Role of chronic ryanodine receptor phosphorylation in heart failure and β-adrenergic receptor blockade in mice. J. Clin. Invest. 120, 4375-4387. 10.1172/JCI37649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan J., Xie W., Betzenhauser M., Reiken S., Chen B.-X., Wronska A. and Marks A. R. (2012). Calcium leak through ryanodine receptors leads to atrial fibrillation in 3 mouse models of catecholaminergic polymorphic ventricular tachycardia. Circ. Res. 111, 708-717. 10.1161/CIRCRESAHA.112.273342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigalas C., Mayo-Martin M. B., Jane D. E. and Sitsapesan R. (2009). Ca2+ -Calmodulin increases RyR2 open probability yet reduces ryanoid association with RyR2. Biophys. J. 97, 1907-1916. 10.1016/j.bpj.2009.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan R. and Williams A. J. (1994). Regulation of the gating of the sheep cardiac sarcoplasmic reticulum Ca2+ -release channel by luminal Ca2+. J. Membr. Biol. 137, 215-226. 10.1007/BF00232590 [DOI] [PubMed] [Google Scholar]
- Sumitomo N. (2016). Current topics in catecholaminergic polymorphic ventricular tachycardia. J. Arrhythmia 32, 344-351. 10.1016/j.joa.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumitomo N., Sakurada H., Taniguchi K., Matsumura M., Abe O., Miyashita M., Kanamaru H., Karasawa K., Ayusawa M., Fukamizu S. et al. (2007). Association of atrial arrhythmia and sinus node dysfunction in patients with catecholaminergic polymorphic ventricular tachycardia. Circ. J. 71, 1606-1609. 10.1253/circj.71.1606 [DOI] [PubMed] [Google Scholar]
- Swan H., Piippo K., Viitasalo M., Heikkilä P., Paavonen T., Kainulainen K., Kere J., Keto P., Kontula K. and Toivonen L. (1999). Arrhythmic disorder mapped to chromosome 1q42–q43 causes malignant polymorphic ventricular tachycardia in structurally normal hearts. J. Am. Coll. Cardiol. 34, 2035-2042. 10.1016/S0735-1097(99)00461-1 [DOI] [PubMed] [Google Scholar]
- Timerman A. P., Onoue H., Xin H.-B., Barg S., Copello J., Wiederrecht G. and Fleischer S. (1996). Selective binding of FKBP12.6 by the cardiac ryanodine receptor. J. Biol. Chem. 271, 20385-20391. 10.1074/jbc.271.34.20385 [DOI] [PubMed] [Google Scholar]
- Tiso N., Salamon M., Bagattin A., Danieli G. A., Argenton F. and Bortolussi M. (2002). The binding of the RyR2 calcium channel to its gating protein FKBP12.6 is oppositely affected by ARVD2 and VTSIP mutations. Biochem. Biophys. Res. Commun. 299, 594-598. 10.1016/S0006-291X(02)02689-X [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T. and Gordon J. (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. 76, 4350-4354. 10.1073/pnas.76.9.4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy A. and Meissner G. (1996). Sarcoplasmic reticulum lumenal Ca2+ has access to cytosolic activation and inactivation sites of skeletal muscle Ca2+ release channel. Biophys. J. 70, 2600-2615. 10.1016/S0006-3495(96)79831-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vest J. A., Wehrens X. H. T., Reiken S. R., Lehnart S. E., Dobrev D., Chandra P., Danilo P., Ravens U., Rosen M. R. and Marks A. R. (2005). Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation 111, 2025-2032. 10.1161/01.CIR.0000162461.67140.4C [DOI] [PubMed] [Google Scholar]
- Walweel K., Li J., Molenaar P., Imtiaz M. S., Quail A., dos Remedios C. G., Beard N. A., Dulhunty A. F., van Helden D. F. and Laver D. R. (2014). Differences in the regulation of RyR2 from human, sheep, and rat by Ca2+ and Mg2+ in the cytoplasm and in the lumen of the sarcoplasmic reticulum. J. Gen. Physiol. 144, 263-271. 10.1085/jgp.201311157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walweel K., Molenaar P., Imtiaz M. S., Denniss A., dos Remedios C., van Helden D. F., Dulhunty A. F., Laver D. R. and Beard N. A. (2017). Ryanodine receptor modification and regulation by intracellular Ca2+ and Mg2+ in healthy and failing human hearts. J. Mol. Cell. Cardiol. 104, 53-62. 10.1016/j.yjmcc.2017.01.016 [DOI] [PubMed] [Google Scholar]
- Wehrens X. H. T., Lehnart S. E., Huang F., Vest J. A., Reiken S. R., Mohler P. J., Sun J., Guatimosim S., Song L.-S., Rosemblit N. et al. (2003). FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell 113, 829-840. 10.1016/S0092-8674(03)00434-3 [DOI] [PubMed] [Google Scholar]
- Xiao J., Tian X., Jones P. P., Bolstad J., Kong H., Wang R., Zhang L., Duff H. J., Gillis A. M., Fleischer S. et al. (2007). Removal of FKBP12.6 does not alter the conductance and activation of the cardiac ryanodine receptor or the susceptibility to stress-induced ventricular arrhythmias. J. Biol. Chem. 282, 34828-34838. 10.1074/jbc.M707423200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z., Guo W., Sun B., Hunt D. J., Wei J., Liu Y., Wang Y., Wang R., Jones P. P., Back T. G. et al. (2016). Enhanced cytosolic Ca2+ activation underlies a common defect of central domain cardiac ryanodine receptor mutations linked to arrhythmias. J. Biol. Chem. 291, 24528-24537. 10.1074/jbc.M116.756528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L. and Meissner G. (1998). Regulation of cardiac muscle Ca2+ release channel by sarcoplasmic reticulum lumenal Ca2+. Biophys. J. 75, 2302-2312. 10.1016/S0006-3495(98)77674-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Mann G. and Meissner G. (1996). Regulation of cardiac Ca2+ release channel (ryanodine receptor) by Ca2+, H+, Mg2+, and adenine nucleotides under normal and simulated ischemic conditions. Circ. Res. 79, 1100-1109. 10.1161/01.RES.79.6.1100 [DOI] [PubMed] [Google Scholar]
- Yan Z., Bai X., Yan C., Wu J., Li Z., Xie T., Peng W., Yin C., Li X., Scheres S. H. W. et al. (2015). Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature 517, 50-55. 10.1038/nature14063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M., Yamamoto T., Ikemoto N. and Matsuzaki M. (2005). Abnormal ryanodine receptor function in heart failure. Pharmacol. Ther. 107, 377-391. 10.1016/j.pharmthera.2005.04.003 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Fraser J. A., Jeevaratnam K., Hao X., Hothi S. S., Grace A. A., Lei M. and Huang C. L.-H. (2011). Acute atrial arrhythmogenicity and altered Ca2+ homeostasis in murine RyR2-P2328S hearts. Cardiovasc. Res. 89, 794-804. 10.1093/cvr/cvq229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wu J., Jeevaratnam K., King J. H., Guzadhur L., Ren X., Grace A. A., Lei M., Huang C. L.-H. and Fraser J. A. (2013). Conduction slowing contributes to spontaneous ventricular arrhythmias in intrinsically active murine RyR2-P2328S hearts. J. Cardiovasc. Electrophysiol. 24, 210-218. 10.1111/jce.12015 [DOI] [PubMed] [Google Scholar]
- Zissimopoulos S. and Lai F. A. (2005). Central domain of the human cardiac muscle ryanodine receptor does not mediate interaction with FKBP12.6. Cell Biochem. Biophys. 43, 203-220. 10.1385/CBB:43:2:203 [DOI] [PubMed] [Google Scholar]
- Zissimopoulos S., Seifan S., Maxwell C., Williams A. J. and Lai F. A. (2012). Disparities in the association of the ryanodine receptor and the FK506-binding proteins in mammalian heart. J. Cell Sci. 125, 1759-1769. 10.1242/jcs.098012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.