Abstract
PURPOSE
In PALOMA-2, palbociclib plus letrozole significantly improved progression-free survival (PFS) as initial treatment of estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer. We assessed the benefit of palbociclib plus letrozole in Asians.
PATIENTS AND METHODS
Of 666 enrolled postmenopausal women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (no prior treatment of advanced disease), 95 were Asian. Patients were randomly assigned 2:1 to receive palbociclib plus letrozole or placebo plus letrozole. The primary end point was investigator-assessed PFS. Secondary end points were overall survival, objective response, patient-reported outcomes, pharmacokinetics, and safety.
RESULTS
Median PFS was significantly longer in Asian patients who received palbociclib plus letrozole versus placebo plus letrozole (25.7 months [95% CI, 19.2 months to not estimable] v 13.9 months [95% CI, 7.4 to 22.0 months]; hazard ratio, 0.49; 95% CI, 0.27 to 0.87; P = .007). The most common toxicities with palbociclib were hematologic and more frequent among Asians versus non-Asians: neutropenia (any grade, 95.4% v 76.8%; grade 3/4, 89.2% v 62.5%), leukopenia (43.1% v 38.3%; 32.3% v 23.5%), and thrombocytopenia (27.7% v 13.5%; 4.6% v 1.1%). No Asians had febrile neutropenia. Discontinuation rates as a result of adverse events were similar among Asian and non-Asian patients who received palbociclib plus letrozole (10.8% and 9.5%). In Asians, quality of life (QOL) was maintained with no significant differences observed between treatments from baseline in breast cancer–specific QOL and general health status scores. Change from baseline in EuroQol five dimensions index scores was significantly higher with palbociclib plus letrozole (0.013 v –0.069; P = .0132). Geometric mean palbociclib trough concentration values were higher in Asians versus non-Asians (93.8 v 61.7 ng/mL).
CONCLUSION
Consistent with the overall study population, the addition of palbociclib to letrozole significantly improved PFS in Asians. Hematologic toxicities were more frequent in Asians versus non-Asians but manageable with early dose modifications while maintaining QOL.
INTRODUCTION
Breast cancer accounts for 25% of all cancers and 15% of all cancer deaths among women worldwide, with incidence rates generally higher in North America than in Asia.1 Although the incidence of breast cancer has been declining in some regions of the world,2 it remains a leading cause of cancer death among Asian women.3 The age-standardized incidence rates of advanced breast cancer in recent generations of Asian women have risen and, in some countries, surpassed the historically high rates in the United States. Globally, the majority of breast malignancies are hormone receptor positive/human epidermal growth factor receptor 2 (HER2) negative for which endocrine therapy has been a mainstay of treatment.4,5 However, de novo and acquired resistance to endocrine therapy can develop, which often results in a therapeutic switch to chemotherapy that is associated with clinically significant toxicity.5 Treatment options that improve outcomes with endocrine-based therapy and delay the use of chemotherapy are needed.
Palbociclib is a first-in-class oral small-molecule inhibitor of cyclin-dependent kinases 4 and 6 indicated for the treatment of hormone receptor‒positive/HER2-negative advanced or metastatic breast cancer in combination with fulvestrant in women with disease progression after endocrine therapy and in combination with an aromatase inhibitor as initial endocrine-based therapy in postmenopausal women.6 PALOMA-1 was an open-label, phase II study that evaluated palbociclib plus letrozole versus letrozole alone as frontline treatment in postmenopausal women with advanced estrogen receptor (ER)–positive/HER2-negative breast cancer.4 This study was based on preclinical data that demonstrated that palbociclib preferentially inhibited the growth of ER-positive breast cancer cell lines and was synergistic with anti-estrogens.7 In PALOMA-1, the addition of palbociclib significantly improved progression-free survival (PFS), which supported the accelerated approval of the combination in the United States.4 Although relatively few Asians were enrolled in PALOMA-1, the phase III PALOMA-3 study included 102 pre- and postmenopausal Asian patients with hormone receptor‒positive/HER2-negative advanced breast cancer and prior endocrine resistance whose median PFS was significantly improved with palbociclib plus fulvestrant versus fulvestrant monotherapy.8 The combination also was well tolerated, and global quality of life (QOL) was maintained.8 To date, there are limited data on Asian patients in the first-line setting in which the duration of treatment generally is prolonged.
PALOMA-2 was designed to confirm the results of PALOMA-1 and further evaluate the safety and efficacy of the combination in a larger patient population. Published results from this study have reported significant improvement in median PFS with palbociclib plus letrozole versus placebo plus letrozole (24.8 v 14.5 months; hazard ratio [HR], 0.58; 95% CI, 0.46 to 0.72; P < .001).9 Because ethnic differences may play a role in the efficacy and safety of anticancer drugs,10-12 it is also important to analyze results from clinical studies that test new drugs in subgroups with different ethnicities. Because more Asian patients were enrolled in the PALOMA-2 study than in PALOMA-1, we examined the efficacy and safety results in Asian and non-Asian patients enrolled in PALOMA-2.
PATIENTS AND METHODS
Study Design and Patients
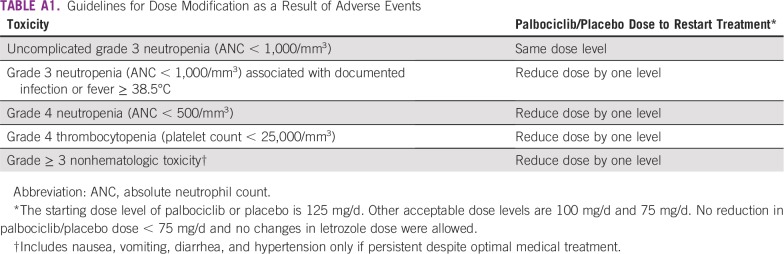
In the double-blind, international, phase III PALOMA-2 study, women with advanced ER-positive/HER2-negative breast cancer were randomly assigned 2:1 to receive palbociclib (125 mg/d orally for 3 weeks followed by 1 week off of a 4-week cycle) or matching placebo. Both treatment arms received letrozole 2.5 mg/d orally continuously. Palbociclib or placebo dose reductions as a result of adverse events (AEs) were allowed, but dose reduction of letrozole was not permitted (Appendix Table A1). Women were required to be postmenopausal with no prior systemic therapy for advanced disease, adequate organ function, and an Eastern Cooperative Oncology Group performance status of 0 to 2. They also were required to have measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.113 or bone-only disease. Additional eligibility criteria and study design details were described previously.9
The PALOMA-2 study was approved by an institutional review board/independent ethics committee and was conducted in accordance with Good Clinical Practice principles and the Declaration of Helsinki. All patients provided informed consent before the study.
End Points and Assessments
The primary study end point was investigator-assessed PFS. Subgroup analyses by baseline characteristics, including race, were preplanned in the study analysis plan; the current analysis examines results in patients of Asian ethnicity, regardless of their geographic region. Additional information on end points, assessments, procedures, and statistical analyses are described in the Appendix.
RESULTS
Patients
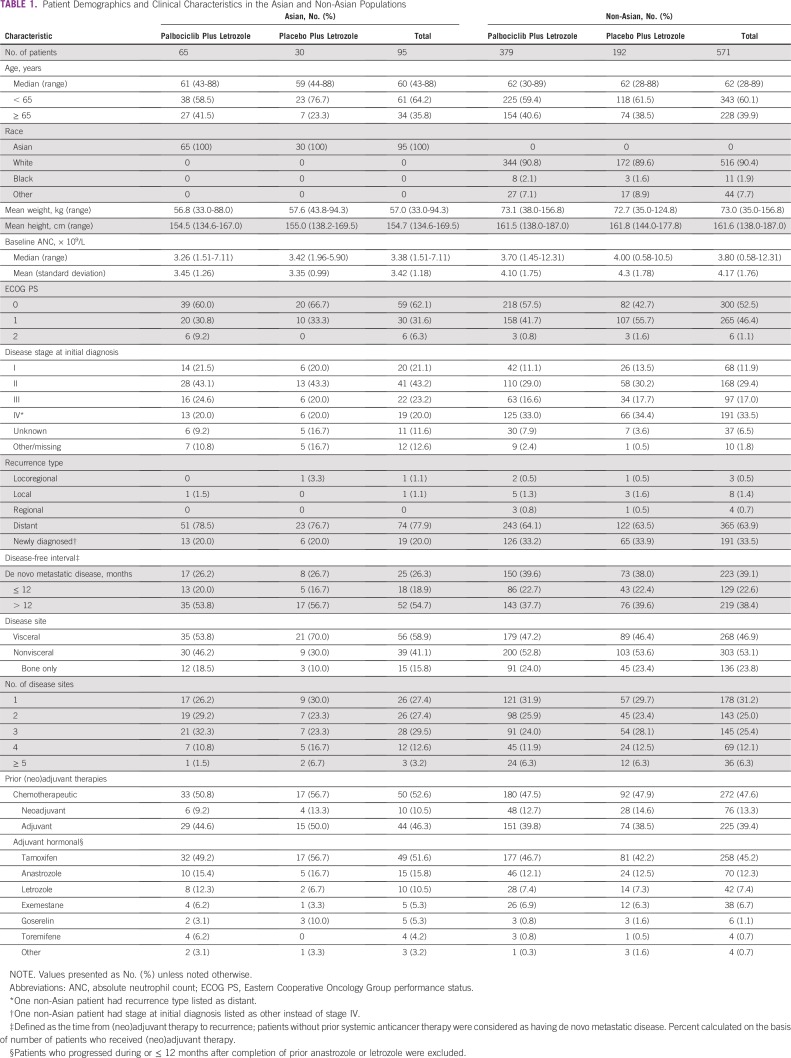
Between February 2013 and July 2014, 95 patients of Asian ethnicity were enrolled in the study, with 65 in the palbociclib plus letrozole group and 30 in the placebo plus letrozole group (Appendix Fig A1). Demographic and baseline disease characteristics were generally similar between the Asian and non-Asian populations except for weight (mean, 57.0 v 73.0 kg, respectively), the proportion of patients with bone-only disease (15.8% v 23.8%), the proportion who were newly diagnosed (20.0% v 33.5%), and baseline mean absolute neutrophil count (ANC; 3.42 v 4.17 × 109/L; Table 1). The median age of Asian patients was 60 years. Most (58.9%) had visceral disease at baseline, and 66.3% had received prior adjuvant hormonal therapy; more than one half (52.6%) had received prior chemotherapy in the early breast cancer setting. Among Asian patients, demographic and baseline disease characteristics were generally balanced between the treatment arms.
TABLE 1.
Patient Demographics and Clinical Characteristics in the Asian and Non-Asian Populations
Efficacy
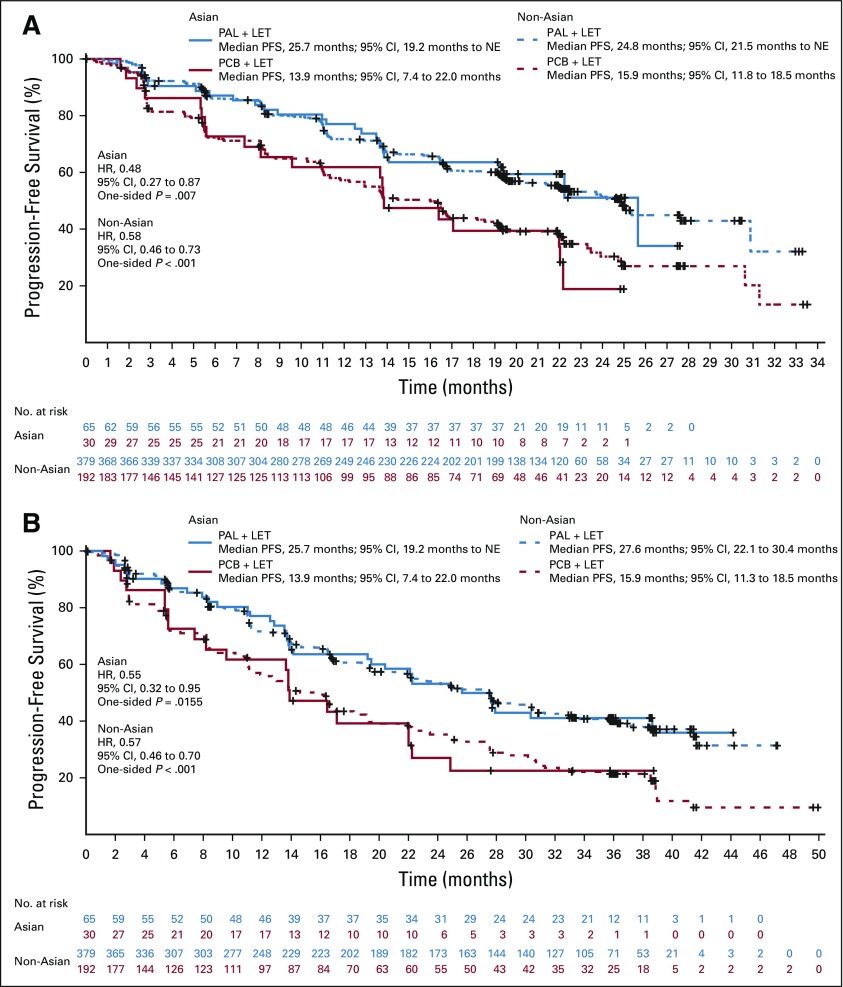
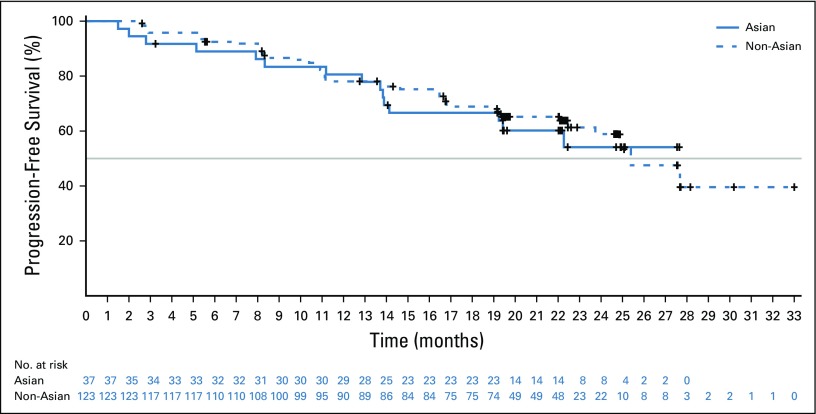
In both Asian and non-Asian patients, the addition of palbociclib resulted in improved PFS (Figs 1A and 1B). Data assessed at the February 26, 2016, cutoff date demonstrated that median PFS in Asians was significantly longer in the palbociclib arm than in the placebo arm (25.7 months [95% CI, 19.2 months to not estimable (NE)] v 13.9 months [95% CI, 7.4 to 22.0 months]; HR, 0.49; 95% CI, 0.27 to 0.87; one-sided P = .007; Fig 1A). Among non-Asians, the median PFS was 24.8 months (95% CI, 21.5 months to NE) in the palbociclib arm versus 15.9 months (95% CI, 11.8 to 18.5 months) in the placebo arm (HR, 0.58; 95% CI, 0.46 to 0.73; one-sided P < .001). Data assessed at the cutoff date of May 31, 2017, showed that PFS in Asians remained significantly longer with palbociclib than with placebo (25.7 months [95% CI, 19.2 months to NE] v 13.9 months [95% CI, 7.4 to 22.0 months]; HR, 0.55; 95% CI, 0.32 to 0.95]; one-sided P = .0155; Fig 1B). Among non-Asians, the median PFS was 27.6 months (95% CI, 22.1 to 30.4 months) in the palbociclib arm versus 15.9 months (95% CI, 11.3 to 18.5 months) in the placebo arm (HR, 0.57; 95% CI, 0.46 to 0.70; one-sided P < .001). In addition, dose reductions did not have a negative impact on the PFS benefit observed with palbociclib in both groups (Fig 2).
FIG 1.
Investigator-assessed progression-free survival in Asian and non-Asian patients (intention-to-treat population) at the data cutoff dates of (A) February 26, 2016, and (B) May 31, 2017. HR, hazard ratio; LET, letrozole; NE, not estimable; PAL, palbociclib; PCB, placebo.
FIG 2.
Investigator-assessed progression-free survival in Asian and non-Asian patients with palbociclib dose reductions (intention-to-treat population). Gray line indicates the median progression-free-survival.
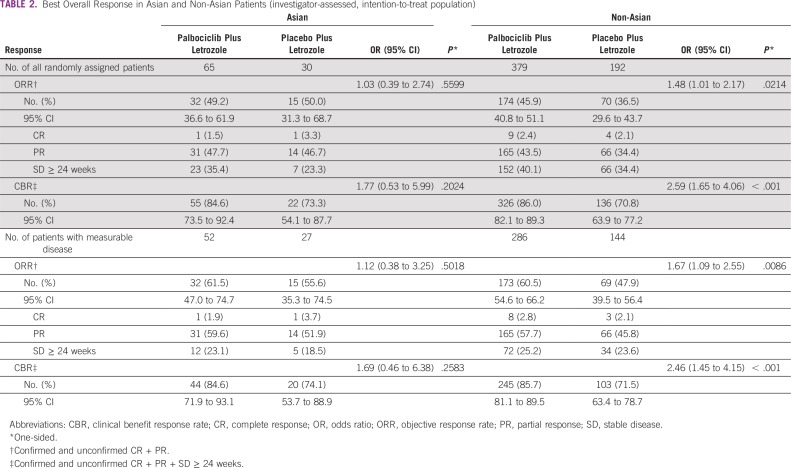
The objective response rate among Asians in the intention-to-treat (ITT) population and in those with measurable disease was 49.2% (95% CI, 36.6% to 61.9%) and 61.5% (47.0% to 74.7%), respectively, in the palbociclib arm versus 50.0% (95% CI, 31.3% to 68.7%) and 55.6% (35.3% to 74.5%) in the placebo arm (Table 2). The rate of clinical benefit response was numerically higher with palbociclib versus placebo in the ITT population (84.6% [95% CI, 73.5% to 92.4%] v 73.3% [95% CI, 54.1% to 87.7%]) and in patients with measurable disease (84.6% [95% CI, 71.9% to 93.1%] v 74.1% [95% CI, 53.7% to 88.9%]). The amount of improvement in clinical benefit response with palbociclib was similar in Asians and non-Asians and in the ITT population (Table 2).9 At the time of this analysis, survival data were not yet mature. Double blinding has been maintained to allow for ongoing follow-up for overall survival.
TABLE 2.
Best Overall Response in Asian and Non-Asian Patients (investigator-assessed, intention-to-treat population)
Study Treatment Exposure
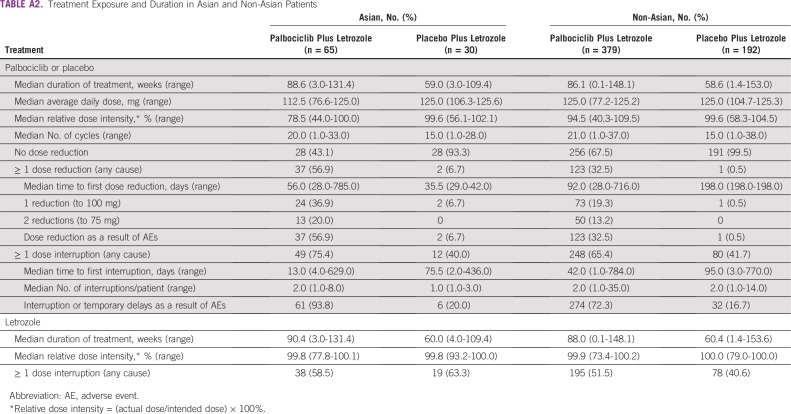
Consistent with the overall study population, median treatment duration was longer in the palbociclib versus placebo arm for both Asians (88.6 weeks [range, 3.0 to 131.4 weeks] v 59.0 weeks [range, 3.0 to 109.4 weeks]) and non-Asians (86.1 weeks [range, 0.1 to 148.1 weeks] v 58.6 weeks [range, 1.4 to 153.0 weeks]; Appendix Table A2). Palbociclib dose reductions (all causality) were more common among Asians versus non-Asians (56.9% v 32.5%), and median time to first palbociclib dose reduction was shorter in Asians versus non-Asians (56 days [range, 28 to 785 days] v 92 days [range, 28 to 716 days]). Dose reductions and dose interruptions/delays as a result of AEs in the palbociclib arm were also more common among Asians versus non-Asians (56.9% and 93.8%, respectively, v 32.5% and 72.3%), with neutropenia (38.5%) and neutrophil count decrease (16.9%) being the main reasons for palbociclib dose reductions in Asian patients. Median relative palbociclib dose intensity was lower in Asians versus non-Asians (78.5% v 94.5%).
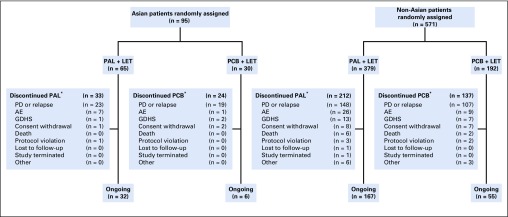
As of the data cutoff (February 26, 2016), 57 Asian patients (60.0%) had permanently discontinued study treatment, including 33 in the palbociclib arm (50.8%) and 24 in the placebo arm (80.0%). Of the 571 non-Asians, 349 (61.1%) discontinued study treatment, including 212 in the palbociclib arm (55.9%) and 137 in the placebo arm (71.4%). Disease progression was the most common reason for permanent discontinuation overall; however, fewer patients in the palbociclib arm (Asians, 23, 35.4%; non-Asians, 148, 39.1%) versus the placebo arm (Asians, 19, 63.3%; non-Asians, 107, 55.7%) discontinued for this reason. Discontinuation as a result of AEs occurred in a similar proportion of Asian (nine, 9.5%) and non-Asian patients (47, 8.2%), among whom seven (10.8%) and 36 (9.5%), respectively, were in the palbociclib arm.
Safety
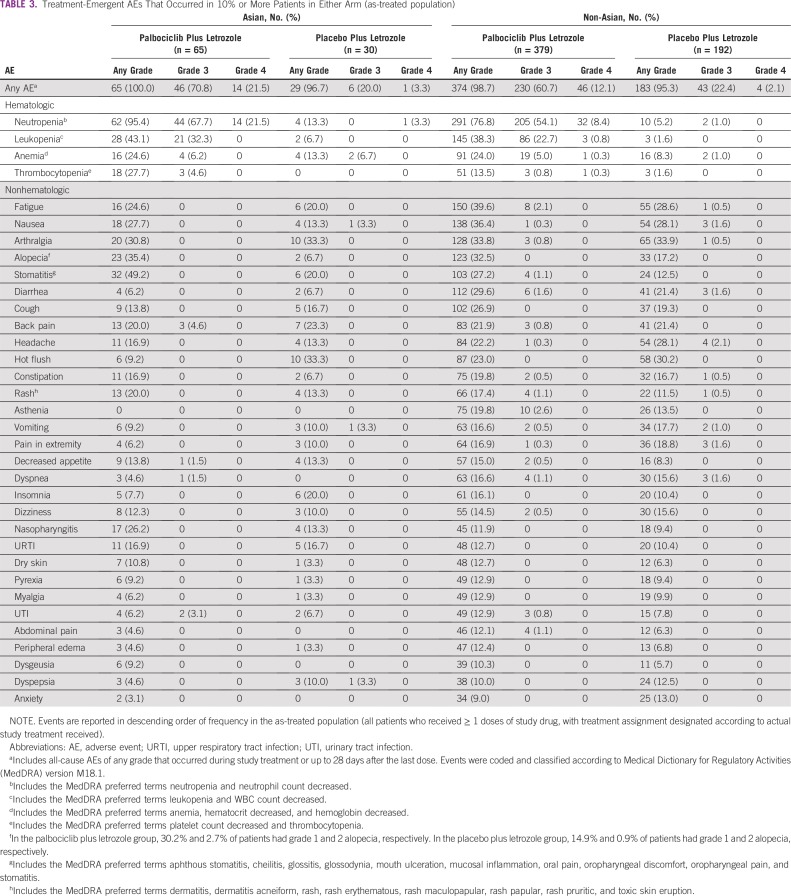
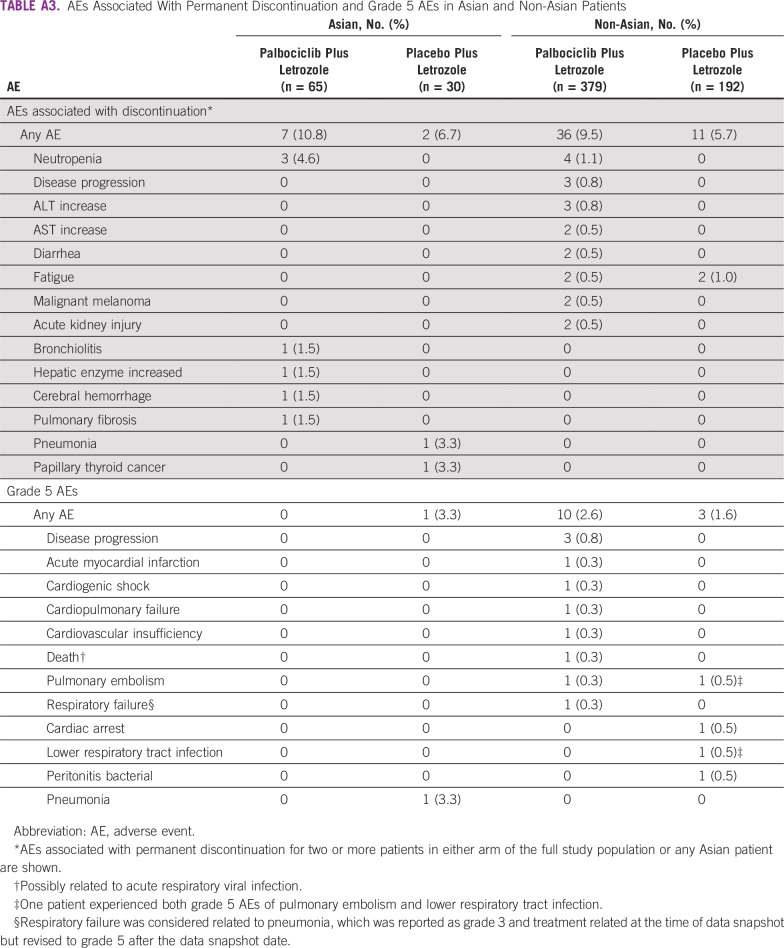
The most common AEs among both Asian and non-Asian patients in the palbociclib arm were hematologic (Table 3). Hematologic AEs were generally more frequent with palbociclib versus placebo. In the palbociclib arm, incidence of any-grade neutropenia and thrombocytopenia was higher among Asians versus non-Asians (95.4% v 76.8% and 27.7% v 13.5%, respectively). With the exception of neutropenia and leukopenia, AEs were typically of grade 1 or 2 severity. The most common grade 3/4 hematologic AEs among Asians and non-Asians were neutropenia (89.2% v 62.5%), leukopenia (32.3% v 23.5%), anemia (6.2% v 5.3%), and thrombocytopenia (4.6% v 1.1%); neutropenia was the only grade 4 AE experienced by Asian patients in the palbociclib plus letrozole arm (21.5%). Although the incidence of neutropenia was high, only three Asians (4.6%) and four non-Asians (1.1%) permanently discontinued palbociclib treatment because of this AE (Appendix Table A3). Febrile neutropenia occurred in eight patients (1.8%) in the palbociclib arm in the overall population, none of whom were of Asian ethnicity. Nonhematologic AEs are described in the Appendix.
TABLE 3.
Treatment-Emergent AEs That Occurred in 10% or More Patients in Either Arm (as-treated population)
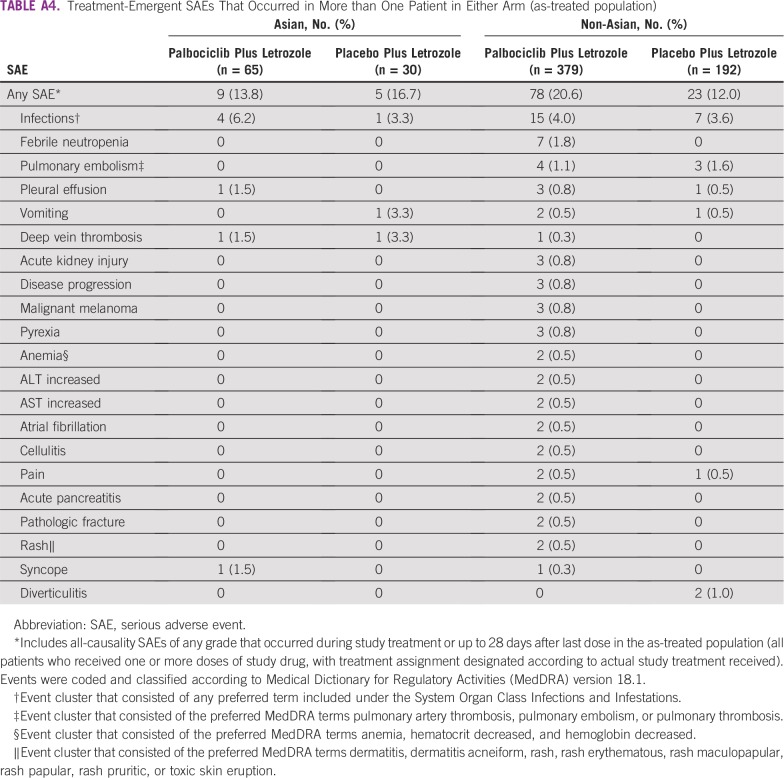
In the palbociclib versus placebo arms, respectively, serious AEs (SAEs) of any cause occurred in nine (13.8%) versus five (16.7%) Asian patients and 78 (20.6%) versus 23 (12.0%) non-Asian patients (Appendix Table A4). Febrile neutropenia was reported as serious in seven patients (1.1%) overall, all of whom were non-Asians in the palbociclib arm. No other individual SAE occurred at an incidence rate of 2% or more in either Asians or non-Asians in the palbociclib arm. Infections (any preferred term under the System Organ Class Infections and Infestations) were the most common SAEs among both Asians and non-Asians; however, no individual SAE classified as an infection occurred in more than one Asian patient. Ten on-study deaths (2.3%) occurred in the palbociclib arm and four (1.8%) occurred in the placebo arm (Appendix Table A3). One on-study death occurred among Asian patients (as a result of disease progression in a patient who received placebo plus letrozole). One death was considered treatment related (as a result of pulmonary embolism and lower respiratory tract infection in a non-Asian patient who received placebo plus letrozole).
Pharmacokinetics
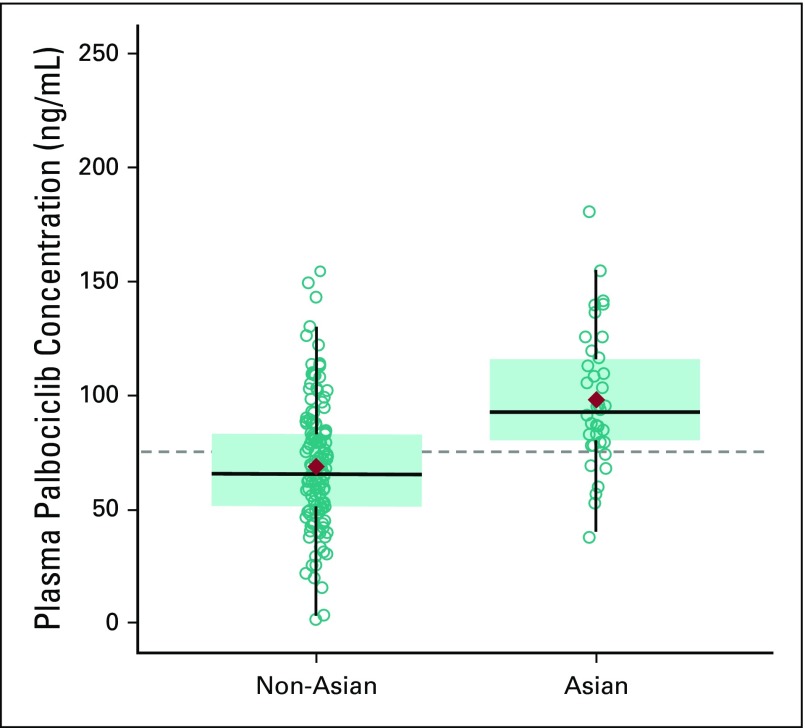
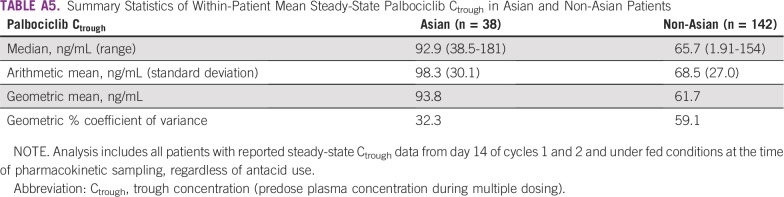
Within-patient mean steady-state trough concentration (Ctrough) of palbociclib was examined in 38 Asian and 142 non-Asian patients. Geometric mean palbociclib Ctrough values were higher in Asians relative to non-Asians (93.8 v 61.7 ng/mL), which indicated greater palbociclib exposure in Asians (Appendix Table A5). However, variability (percent coefficient of variance) was substantially higher in non-Asians (59.1%) than in Asians (32.3%), and the distribution of Ctrough values in Asian patients was generally within range of those observed in non-Asian patients (Appendix Fig A2). There was no apparent relationship between Ctrough and body dimensions in Asian and non-Asian populations (data not shown).
Patient-Reported Outcomes
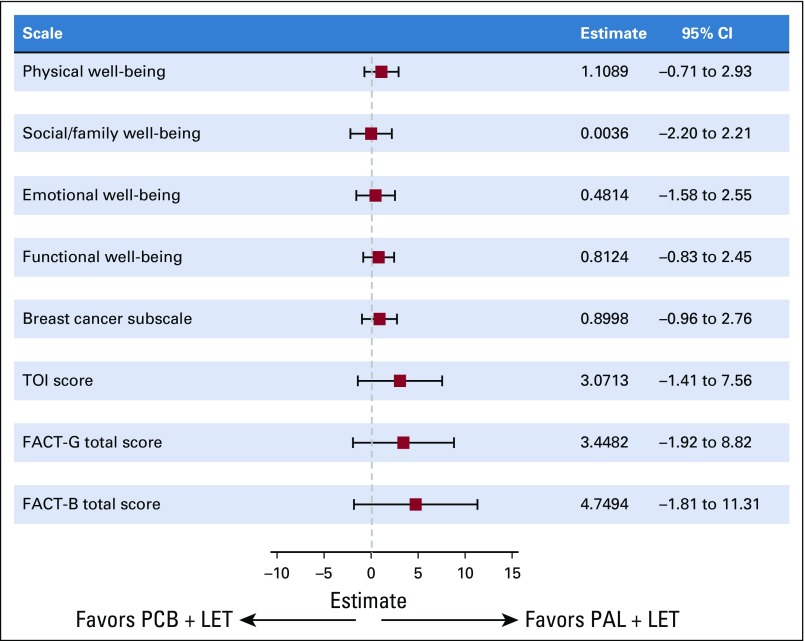
Across all cycles, 90% to 100% of all Asian patients in each treatment arm completed at least one question. No statistically significant between-arm differences were observed in overall change from baseline scores among Asian patients for the Functional Assessment of Cancer Therapy-Breast (FACT-B) or FACT-General total scores, the Breast Cancer Subscale, or the Trial Outcome Index score as well as for the FACT-B scales that describe physical, social/family, emotional, and functional well-being (Fig 3). Among Asians, overall change from baseline in EuroQol five dimensions (EQ-5D) index scores was significantly higher in the palbociclib versus placebo arm (0.013 v –0.069; P = .0132). No significant differences were observed between treatment arms in general health status scores assessed by EQ-5D.
FIG 3.
Between-treatment comparison of changes from baseline scores among Asian patients (patient-reported outcome analysis set). FACT-B, Functional Assessment of Cancer Therapy-Breast; FACT-G, Functional Assessment of Cancer Therapy-General; LET, letrozole; PAL, palbociclib; PCB, placebo; TOI, Trial Outcome Index.
DISCUSSION
There have been considerable efforts to identify therapies that improve outcomes in patients with advanced breast cancer. Results from PALOMA-2 confirmed those observed in PALOMA-1, with both studies demonstrating that the addition of palbociclib to a standard endocrine therapy (letrozole) as first-line treatment of ER-positive/HER2-negative breast cancer significantly improves outcomes, including investigator-assessed PFS, irrespective of age, performance status, disease site, prior chemotherapy, prior endocrine therapy, disease-free interval after adjuvant treatment, or histologic subtype.4,9 However, outcomes by ethnicity have not previously been reported from this study.
Palbociclib has been approved in the United States since February 2015,14 and substantial clinical data are now available; however, most data are in white patients.4,15 Although few Asian patients were enrolled in PALOMA-1,4 95 (14.3%) in PALOMA-2 were Asian, which allowed for the assessment of palbociclib efficacy, safety, pharmacokinetics, and patient-reported outcomes (PROs) in patients of this ethnicity. In addition, to our knowledge, this report is the first to provide prospective data with regard to clinical outcomes with letrozole treatment in Asian patients with breast cancer, despite its wide use. Because disparities in the safety and efficacy of anticancer therapies in patients of different ethnicities exist,10-12 it is important to explore whether distinct safety profiles and/or anticancer activity of therapies are observed in different patient populations.
In this study, stomatitis was more common among Asians versus non-Asians in the palbociclib plus letrozole arm. However, all but four patients (all non-Asian) experienced grade 1 or 2 AEs and the incidence of grade 2 stomatitis was similar among Asians and non-Asians. The incidence of any-grade neutropenia (95.4% v 76.8%) and thrombocytopenia (27.7% v 13.5%) was also higher among Asians. These results could be due to higher exposure in Asian patients relative to non-Asian patients, as assessed by within-patient mean steady-state Ctrough values. However, recent results have shown that patients with a lower baseline ANC are significantly more likely to experience grade 3/4 neutropenia while receiving palbociclib plus letrozole.16 Of note, in the PALOMA-3 study population, Asian patients had a baseline ANC that was 20% lower on average compared with non-Asians.8 In the current analysis, Asian patients had a mean baseline ANC value approximately 18% lower than non-Asians. Although the incidence of hematologic toxicities was high, the toxicities were effectively managed with dose adjustments, and few patients permanently discontinued palbociclib because of these AEs. The US prescribing information for palbociclib currently recommends that all patients have a CBC before and during palbociclib therapy.6 Dosing interruption (including cycle delay) or dose reduction is recommended for patients who develop grade 3 or 4 neutropenia.6
Despite the higher incidence of hematologic toxicity and a lower proportion of patients with either bone-only or de novo stage IV disease in the Asian group, median treatment duration in both arms was similar among Asians and non-Asians and in the full study population and consistently longer in the palbociclib treatment arm. A difference in palbociclib dose intensity was observed between Asians and non-Asians in the palbociclib arm, with Asian patients having a lower mean relative dose intensity than non-Asian patients. This is reflected by a greater percentage of Asian patients who experienced dose reductions and dose interruptions compared with non-Asian patients. Dose reductions occurred early during treatment, yet even with a higher frequency of dose reductions and dose interruptions, the palbociclib treatment effect was comparable in Asian and non-Asian patients, with a similar median PFS. Furthermore, palbociclib exposure was higher in Asian patients versus non-Asian patients, although, within-patient mean steady-state palbociclib Ctrough values in Asians were generally within the range of those observed for non-Asians.
The efficacy and safety findings presented herein are comparable to preliminary results of other cyclin-dependent kinase 4/6 inhibitors in Asians with breast cancer. In subgroup analyses of MONALESSA-2 and MONARCH-2, the addition of ribociclib or abemaciclib to endocrine therapy significantly prolonged median PFS in Asians, with safety profiles consistent with each study’s respective overall population.17,18 In the phase Ib study of ribociclib in Asian, non-Japanese patients with hormone receptor–positive, HER2-negative advanced breast cancer (MONALESSASIA), neutropenia was the most common AE.19 Similar to the results presented with palbociclib, the incidence of grade 3/4 neutropenia with ribociclib plus letrozole in MONALEESA-2 was higher in Asians than in non-Asians (71% and 58%, respectively).17 In subgroup analyses of PALOMA-3, all-grade neutropenia in the palbociclib arm was higher in Asians versus non-Asians (92% v 78%, respectively) and was also increased in Japanese patients versus the overall PALOMA-3 population (93% v 79%, respectively).8,20 Moreover, in a Japanese phase II study with palbociclib plus letrozole, neutropenia was the most common AE (100%).21
In addition to efficacy and safety, assessment of PROs is crucial to fully understand risk-benefit profiles and to optimize treatment outcomes. Analyses of PROs among Asian patients in the study revealed significantly higher EQ-5D index scores in the palbociclib arm versus placebo arm. Scores for all scales indicated maintenance of quality of life even with the addition of palbociclib to letrozole. Together, these results suggest that the addition of palbociclib to endocrine therapy is well tolerated, which allows Asian patients to maintain quality of life while benefiting from the same degree of significant disease control as non-Asians.
As observed in the full PALOMA-2 study population, the addition of palbociclib to letrozole resulted in significantly improved PFS compared with letrozole alone in Asian patients with advanced ER-positive/HER2-negative breast cancer. The safety profile of palbociclib plus letrozole was consistent with those previously reported and was similar in Asians and non-Asians with the exception of hematologic AEs, which were more frequent among Asian patients. Although relatively common, hematologic toxicities were manageable with early dose modifications, with no deterioration in quality of life and few permanent discontinuations as a result of these events. Furthermore, febrile neutropenia was not observed in any Asian patients in this study. Overall, these findings confirm the efficacy and safety results observed in the full study population and indicate that palbociclib plus letrozole is a reasonable treatment option in Asian patients with ER-positive/HER2-negative advanced breast cancer.
ACKNOWLEDGMENT
Editorial/medical writing support was provided by Alan Klopp, PhD, and Jill Shults, PhD, of Complete Healthcare Communications (North Wales, PA), a CHC Group company, and was funded by Pfizer. We thank the patients who participated in the PALOMA-2 study and their families as well as the study investigators, nurses, and site staff for their support. We also thank Hiroko Godai of Pfizer Japan for data analysis in Asian and non-Asian patients.
Appendix
Methods
End points, assessments, and procedures.
Secondary end points were overall survival, objective response (partial or complete response according to Response Evaluation Criteria in Solid Tumors [RECIST] version 1.1), clinical benefit response (objective response or stable disease lasting 24 or more weeks according to RECIST version 1.1), patient-reported outcomes (PROs), pharmacokinetics, and safety assessments. Patient-reported health-related quality of life and general health status were assessed using the Functional Assessment of Cancer Therapy-Breast and EuroQol five dimensions questionnaires.1,2
Computed tomography scan and/or magnetic resonance imaging were performed at screening and every 12 ± 1 weeks. Bone scans were performed within 12 weeks before random assignment and every 24 ± 1 weeks after assignment. Both imaging and bone scans continued until disease progression, initiation of new anticancer therapy, or permanent discontinuation of study treatment. Laboratory analyses were performed on days 1 and 14 of the first two cycles and on day 1 of subsequent cycles. Adverse events (AEs) were determined by the investigator and classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). Assessment of PROs was based on health-related quality-of-life scores on the Functional Assessment of Cancer Therapy-Breast and EuroQol five dimensions questionnaires (Brady et al: J Clin Oncol 15:974-986, 1997; EuroQol Group: Health Policy 16:199-208, 1990).
Statistical analyses.
The Kaplan-Meier method was used to estimate median progression-free survival (PFS) and corresponding 95% CIs. Between-arm comparison of PFS was done using a predefined log-rank test. Hazard ratios were calculated from a Cox proportional hazards regression model. Between-arm comparisons of objective response rate and clinical benefit response were done using Fisher’s exact test. AEs were summarized using descriptive statistics in Asians and non-Asians who received one or more doses of study treatment. Repeated-measures mixed-effects models were used to assess the impact on overall change from baseline on PROs. No adjustments for multiple comparisons were made for the analyses of secondary end points and subgroup analyses of the primary end point (PFS). The nominal P values are presented in this report.
Results
Nonhematologic AEs.
The most common (greater than 20% incidence) nonhematologic AEs among Asians who received palbociclib plus letrozole were stomatitis (49.2% v 27.2% among non-Asians), alopecia (35.4% v 32.5%), arthralgia (30.8% v 33.8%), nausea (27.7% v 36.4%), nasopharyngitis (26.2% v 11.9%), and fatigue (24.6% v 39.6%), all of which were grade 1 or 2 (Table 3). Of all AEs reported in the palbociclib arm, only stomatitis and nasopharyngitis occurred with a more than 10% higher incidence among Asians versus non-Asians. In Asian patients, these two AEs were more common with palbociclib plus letrozole than with placebo plus letrozole (stomatitis, 49.2% v 20.0%; nasopharyngitis, 26.2% v 13.3%). Incidence of grade 2 stomatitis was similar among Asians (9.2%) and non-Asians (6.1%) treated with palbociclib plus letrozole; there were no grade 3 or higher events among Asians, whereas four non-Asians experienced grade 3 stomatitis (no grade 4). Alopecia was more common in both Asians and non-Asians in the palbociclib arm (35.4% and 32.5%, respectively) versus the placebo arm (6.7% and 17.2%) with most events (greater than 90%) being grade 1. Incidence of grade 3 or higher infections (any preferred term under the System Organ Class Infections and Infestations) among Asian patients was similar between the two arms (4.6% v 3.3%). No grade 3/4 nonhematologic AEs occurred in more than three Asian patients who received palbociclib plus letrozole, and there were no grade 5 events among Asians in this arm.
FIG A1.
Patient disposition. (*) Patients who discontinued palbociclib (PAL) or placebo (PCB) could continue to receive letrozole (LET) alone. AE, adverse event; GDHS, global deterioration of health status; PD, progressive disease.
FIG A2.
Individual and geometric mean steady-state palbociclib trough concentration (Ctrough) in Asian and non-Asian patients. Analysis included all patients with reported steady-state Ctrough data from day 14 of cycles 1 and 2 and under fed conditions at the time of pharmacokinetic sampling, regardless of antacid use. The diamonds represent the geometric mean of within-patient mean values of steady-state Ctrough, the circles represent individual of within-patient mean values of steady-state Ctrough, and the dashed line represents the arithmetic mean of data from all patients. Box plots provide median and 25%/75% quartiles with whiskers to the last point within 1.5 times the interquartile range.
TABLE A1.
Guidelines for Dose Modification as a Result of Adverse Events
TABLE A2.
Treatment Exposure and Duration in Asian and Non-Asian Patients
TABLE A3.
AEs Associated With Permanent Discontinuation and Grade 5 AEs in Asian and Non-Asian Patients
TABLE A4.
Treatment-Emergent SAEs That Occurred in More than One Patient in Either Arm (as-treated population)
TABLE A5.
Summary Statistics of Within-Patient Mean Steady-State Palbociclib Ctrough in Asian and Non-Asian Patients
Footnotes
Supported by Pfizer. The Korean Cancer Study Group received support for the PALOMA-2 study from Translational Research in Oncology. Presented at the Second European Society for Medical Oncology Asia Congress, Singapore, December 16-19, 2016.
Clinical trial information: NCT01740427.
AUTHOR CONTRIBUTIONS
Conception and design: Seock-Ah Im, Cynthia Huang Bartlett, Yuko Mori, Ave Mori, Eric Gauthier, Richard S. Finn, Masakazu Toi
Provision of study material or patients: Seock-Ah Im, Hirofumi Mukai, In Hae Park, Sung-Bae Kim, Young-Hyuck Im, Shoichiro Ohtani, Richard S. Finn, Masakazu Toi
Collection and assembly of data: Seock-Ah Im, Hirofumi Mukai, In Hae Park, Norikazu Masuda, Chikako Shimizu, Sung-Bae Kim, Shoichiro Ohtani, Cynthia Huang Bartlett, Yuko Mori, Eric Gauthier, Richard S. Finn, Masakazu Toi
Data analysis and interpretation: Seock-Ah Im, Hirofumi Mukai, In Hae Park, Norikazu Masuda, Chikako Shimizu, Sung-Bae Kim, Young-Hyuck Im, Cynthia Huang Bartlett, Dongrui R. Lu, Shrividya Iyer, Yuko Mori, Ave Mori, Eric Gauthier, Richard S. Finn, Masakazu Toi
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jgo/site/misc/authors.html.
Seock-Ah Im
Consulting or Advisory Role: AstraZeneca, Novartis, Roche, Genentech, Eisai, Pfizer
Research Funding: AstraZeneca, Pfizer
Travel, Accommodations, Expenses: Novartis, Roche, Genentech, MSD Oncology
Other Relationship: Roche
Hirofumi Mukai
Honoraria: Astra Zeneca, Pfizer, Daiichi Sankyo, Taiho, Takeda, Novartis
Research Funding: Japanese government, Pfizer, Eisai, Daiichi Sankyo, Nippon Kayaku
Norikazu Masuda
Leadership: Japan Breast Cancer Research Group Association
Honoraria: Chugai Pharma, AstraZeneca, Pfizer, Eisai, Eli Lilly Japan, Takeda Pharmaceuticals
Research Funding: Chugai Pharma (Inst), AstraZeneca (Inst), Kyowa Hakko Kirin (Inst), MSD (Inst), Novartis (Inst), Pfizer (Inst), Eli Lilly (Inst), Daiichi Sankyo (Inst)
Chikako Shimizu
Honoraria: AstraZeneca, Kyowa Hakko Kirin, Chugai Pharma, Asuka Seiyaku, Taiho Pharmaceutical, Mochida Pharmaceutical
Consulting or Advisory Role: Eli Lilly Japan, Pfizer, Eisai, AstraZeneca
Research Funding: Eli Lilly, MSD, Chugai Pharma, Pfizer
Sung-Bae Kim
Consulting or Advisory Role: Novartis (Inst), Odonate Therapeutics (Inst), Enzychem Lifesciences (Inst), Eli Lilly (Inst)
Research Funding: Novartis (Inst), Dongkook Pharma (Inst), Genzyme (Inst)
Shoichiro Ohtani
Honoraria: Chugai Pharma, AstraZeneca, Pfizer, Eisai
Cynthia Huang Bartlett
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Dongrui R. Lu
Employment: Pfizer, Pfizer (I)
Stock and Other Ownership Interests: Pfizer, Pfizer (I)
Shrividya Iyer
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Research Funding: Pfizer
Travel, Accommodations, Expenses: Pfizer
Yuko Mori
Employment: Pfizer R&D Japan
Stock and Other Ownership Interests: Pfizer
Ave Mori
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Eric Gauthier
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Travel, Accommodations, Expenses: Pfizer
Richard S. Finn
Consulting or Advisory Role: Pfizer, Bayer AG, Novartis, Bristol-Myers Squibb, Merck, Eisai, Eli Lilly, Genentech, Roche, AstraZeneca, Exelixis
Research Funding: Pfizer (Inst), Bayer AG (Inst), Novartis (Inst), Eisai (Inst), Eli Lilly (Inst), Merck (Inst), Bristol-Myers Squibb (Inst), Roche (Inst), Genentech (Inst)
Expert Testimony: Novartis
Masakazu Toi
Honoraria: Novartis, MSD, Takeda Pharmaceuticals, AstraZeneca, Eisai, Genomic Health, Chugai Pharma, Taiho Pharmaceutical, Bayer AG, Eli Lilly, Daiichi Sankyo, Kyowa Hakko Kirin, C&C Research Laboratories, Yakult, Sanofi, Shimadzu, Pfizer, Konica Minolta
Consulting or Advisory Role: Daiichi Sankyo, Kyowa Hakko Kirin, Taiho Pharmaceutical
Speakers’ Bureau: Pfizer, AstraZeneca, Eli Lilly
Research Funding: Taiho Pharmaceutical, Chugai Pharma, Shimadzu (Inst), Astellas Pharma (Inst), AFI Technology, C&C Research Laboratories, Japan Breast Cancer Research Group (Inst), Pfizer, Eisai, Daiichi Sankyo (Inst), AstraZeneca, Ono Pharmaceutical
Patents, Royalties, Other Intellectual Property: JP 2017-143763WO2017/131162A1 (Inst), PCT/JP2016/004374 (Inst)
Travel, Accommodations, Expenses: Genomic Health, Eli Lilly
Other Relationship: Japan Breast Cancer Research Group, Kyoto Breast Cancer Research Network, Organization for Oncology and Translational Research
No other potential conflicts of interest were reported.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 3.Sung H, Rosenberg PS, Chen WQ, et al. Female breast cancer incidence among Asian and Western populations: More similar than expected. J Natl Cancer Inst. 2015;107:djv107. doi: 10.1093/jnci/djv107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2) Ann Oncol. 2014;25:1871–1888. doi: 10.1093/annonc/mdu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Comprehensive Cancer Network: Clinical practice guidelines in oncology (NCCN guidelines). Breast cancer. Version 2.2017. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/breast_blocks.pdf.
- 7.Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwata H, Im SA, Masuda N, et al. PALOMA-3: Phase III trial of fulvestrant with or without palbociclib in premenopausal and postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer that progressed on prior endocrine therapy—Safety and efficacy in Asian patients. J Glob Oncol. 2017;3:289–303. doi: 10.1200/JGO.2016.008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 10.Swain SM, Im YH, Im SA, et al. Safety profile of pertuzumab with trastuzumab and docetaxel in patients from Asia with human epidermal growth factor receptor 2-positive metastatic breast cancer: Results from the phase III trial CLEOPATRA. Oncologist. 2014;19:693–701. doi: 10.1634/theoncologist.2014-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu B, Zhang XQ, Chi HD, et al. Consistent efficacy and safety of gemcitabine-paclitaxel in patients with metastatic breast cancer: A retrospective comparison of East Asian and global studies. Asia Pac J Clin Oncol. 2014;10:330–339. doi: 10.1111/ajco.12116. [DOI] [PubMed] [Google Scholar]
- 12.Verma S, Bartlett CH, Schnell P, et al. Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: Detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3) Oncologist. 2016;21:1165–1175. doi: 10.1634/theoncologist.2016-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14. Pfizer: 2015 Annual Review: Advances in Oncology. https://www.pfizer.com/files/investors/financial_reports/annual_reports/2015/assets/pdfs/pfi2015ar-advances-in-oncology.pdf.
- 15.Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 16. Diéras V, Harbeck N, Joy AA, et al: PALOMA-2: Neutropenia (NP) patterns in patients (Pts) with estrogen receptor–positive (ER+)/human epidermal growth factor receptor 2–negative (HER2–) first-line advanced breast cancer (ABC) receiving palbociclib + letrozole (P+L). Ann Oncol 28:v74-v-108, 2017. [Google Scholar]
- 17. Yap YS, Tseng LM, Blackwell KL, et al: First-line ribociclib + letrozole in postmenopausal Asian women with hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2–) advanced breast cancer (ABC): A subgroup analysis from MONALEESA-2. Ann Oncol 27, 2016 (suppl; abstr LBA1) [Google Scholar]
- 18. doi: 10.1200/JCO.2017.73.7585. Toi M, Huang C, Im YH, et al: MONARCH 2: Abemaciclib in combination with fulvestrant in Asian women with HR+, HER2- advanced breast cancer who progressed on endocrine therapy. Ann Oncol 28:x26-x34, 2017. [DOI] [PubMed] [Google Scholar]
- 19. Yap YS, Suarez-Vizcarra J, Liu X, et al: Phase Ib study of ribociclib + letrozole in Asian non-Japanese patients with hormone receptor-positive, HER2-negative advanced breast cancer: MONALEESASIA. Cancer Res 78, 2018 (suppl; abstr P5-21-28) [Google Scholar]
- 20. doi: 10.1007/s10147-018-1359-3. Masuda N, Inoue K, Nakamura R, et al: Palbociclib in combination with fulvestrant in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: PALOMA-3 subgroup analysis of Japanese patients. Int J Clin Oncol, 24:262-273, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masuda N, Nishimura R, Takahashi M, et al. Palbociclib in combination with letrozole as first-line treatment for advanced breast cancer: A Japanese phase II study. Cancer Sci. 2018;109:803–813. doi: 10.1111/cas.13507. [DOI] [PMC free article] [PubMed] [Google Scholar]