ABSTRACT
Intellectual disability (ID) and autism spectrum disorders (ASD) are frequently co-occurring neurodevelopmental disorders and affect 2-3% of the population. Rapid advances in exome and genome sequencing have increased the number of known implicated genes by threefold, to more than a thousand. The main challenges in the field are now to understand the various pathomechanisms associated with this bewildering number of genetic disorders, to identify new genes and to establish causality of variants in still-undiagnosed cases, and to work towards causal treatment options that so far are available only for a few metabolic conditions. To meet these challenges, the research community needs highly efficient model systems. With an increasing number of relevant assays and rapidly developing novel methodologies, the fruit fly Drosophila melanogaster is ideally positioned to change gear in ID and ASD research. The aim of this Review is to summarize some of the exciting work that already has drawn attention to Drosophila as a model for these disorders. We highlight well-established ID- and ASD-relevant fly phenotypes at the (sub)cellular, brain and behavioral levels, and discuss strategies of how this extraordinarily efficient and versatile model can contribute to ‘next generation’ medical genomics and to a better understanding of these disorders.
KEY WORDS: Neurodevelopment, ASD, ID, Drosophila, Fruit fly, Brain
Summary: This Review summarizes some of the exciting work that has used Drosophila as a model for the ever-expanding field of intellectual disability and autism spectrum disorders. It highlights disease-relevant assays, emerging themes and future challenges.
Introduction
Intellectual disability (ID) and autism spectrum disorders (ASD) are major neurodevelopmental disorders with a frequency of 2-3% in western countries (Bourke et al., 2016). ID is defined by significant limitations in both intellectual functioning and adaptive behavior before the age of 18 years, and is usually reflected by an IQ below 70 (Ropers, 2010). ASD is a collective term for a spectrum of behavioral phenotypes including deficits in communication and social interaction, and restricted and repetitive behaviors, interests and activities. ID and ASD often co-occur, with an estimated 10% of children with ID having autistic symptoms and with 70% of individuals with autism also having ID (Oeseburg et al., 2011; Schwartz and Neri, 2012).
Because of their frequency and lifelong nature, ID and ASD are an immense socioeconomic burden for the affected families and for healthcare systems. They represent a large unsolved problem in modern medicine due to limited treatability, partially caused by their poorly understood biology. Most ID cases are monogenic, meaning that mutations in a single gene are sufficient to lead to the disorder. Inheritance patterns, such as sporadic de novo mutations or homozygosity in consanguineous families (Deciphering Developmental Disorders Study, 2017; Najmabadi et al., 2011), facilitate disease gene and variant identification (Vissers et al., 2016). So far, little is known about oligogenic inheritance (see Box 1 for a glossary of terms) in ID and the identity of modifiers contributing to a large clinical variability and incomplete penetrance in some cases. In contrast, ASD often represent a genetically complex disorder with oligogenic or polygenic causes, including a combination of both rare de novo variants and more common inherited variants (Chaste et al., 2017). This complex genetic architecture hampers the identification of high-confidence risk-conferring ASD genes. However, this is mainly true for the subset of ‘high-functioning’ ASD cases, who have normal cognitive function. ASD in combination with ID is often monogenic (Arnett et al., 2018). Owing to this large clinical and molecular overlap, monogenic causes of ID also provide us with an unique molecular window into the biology and (patho)mechanisms of ASD.
Box 1. Glossary.
Angelman syndrome (OMIM #105830): neurodevelopmental disorder characterized by intellectual disability (ID), typical abnormal behaviors, movement or balance problems, and severe speech and language impairments. Around 75% of cases are caused by de novo deletions in 15q11.2-q13 on the maternal chromosome 15. The remaining cases are because of paternal uniparental disomy 15, point mutations in the UBE3A gene or rare imprinting defects (Buiting et al., 2016).
Arborization pattern: tree-like morphological arrangement of dendritic branches.
Basal ganglia: group of subcortical nuclei (neuronal population) in the vertebrate brain that play a critical role in motor control and cognition (e.g. in reward-based learning).
Boutons: round-shaped varicosities of the neuromuscular junction (NMJ) presynaptic terminal that house active zones (the neurotransmitter release machinery).
Central complex: a set of neuropil-rich structures (protocerebral bridge, fan-shaped body and ellipsoid body) that integrate complex sensorial (environmental) information with the fly's internal state and previous experience into an appropriate behavioral response (shaped as a motor output) (Wolff and Rubin, 2018).
Dendritic arborization (da) sensory neurons: nociceptive dopaminergic neurons present in the larval body wall.
Dendritic spine: postsynaptic compartment protruding from dendrites, receiving input from a single synapse (axon terminal).
Electroretinogram: Drosophila eye voltage recording reflecting retinal electrical activity upon light stimulation (Ugur et al., 2016).
Fragile X syndrome (OMIM # 300624): most common monogenic cause of ID and ASD, caused by CGG-repeat expansion (>200) in the 5′ untranslated region (5′-UTR) of the FMR1 gene.
Giant-fiber system (recordings): neural circuit controlling escape-response behavior in adult Drosophila. Electrophysiological recordings can be performed through the direct stimulation of the giant fiber neurons and recording from their output muscles (Allen and Godenschwege, 2010).
Inborn errors of metabolism: genetic disorders causing specific metabolic defects due to mutations in genes encoding metabolic enzymes or transporters.
Light-off jump habituation: paradigm used to assess non-associative learning habituation. Repeated light-off stimuli generate an initial jump (startle reflex) response that gradually diminishes due to a learned adaptation to the stimuli, not due to sensory desensitization or motor fatigue.
Non-declarative memory: implicit memory acquired and used without conscious awareness. A classic example is motor memory.
Oligogenic inheritance: trait modulated by a small number of genes or loci (Badano and Katsanis, 2002).
Purkinje cell: large GABAergic neurons in the cerebellar cortex that regulate and coordinate motor function.
Non-REM and REM sleep: the two main components of sleep. REM stands for and is characterized by rapid eye movement, and by low-amplitude and mixed-frequency waves on electroencephalogram (EEG). In contrast, non-REM sleep shows mainly slow wave activity on EEG.
Rett syndrome (OMIM #312750): neurodevelopmental disorder characterized by an arrest in development before the second year of life and a regression of all acquired skills; patients present with ID, loss of speech, stereotypic hand movements, microcephaly and seizures. Rett syndrome occurs almost exclusively in females, and is caused by mutations in the MECP2 gene (Amir et al., 1999).
Suprachiasmatic nucleus: principal circadian pacemaker of the mammalian brain located in the hippocampus.
T2A-Gal4: a cassette that disrupts the gene into which it is integrated and at the same time permits Gal4-mediated induction of UAS alleles under the gene's endogenous regulatory elements (Diao et al., 2015).
Whole-exome sequencing: genomic technique to investigate all protein-coding regions of the genome (exome).
The development of new tools, such as next-generation sequencing, has brought substantial progress in ID/ASD gene and variant identification (Sanders, 2018; Vissers et al., 2016). Genetically, both ID and ASD are extremely heterogeneous, with more than 1150 confirmed disease-associated genes (Kochinke et al., 2016; SysID database, updated on October 2018, https://sysid.cmbi.umcn.nl/). Within this large group, molecular pathways and networks emerge, linking variants with overlapping phenotypes (Kochinke et al., 2016). However, as chromosomal microarray analysis currently identifies ca. 20% (Miller et al., 2010) and (trio) whole-exome sequencing (Box 1) ca. 40% (Deciphering Developmental Disorders Study, 2017) of causative aberrations, a significant fraction of ID and the majority of ASD patients remain without a genetic diagnosis.
Although current treatment options are limited to a small number of ID/ASD disorders deriving from metabolic deficits [inborn errors of metabolism (Box 1)] (van Karnebeek and Stockler, 2012), this does not necessarily mean that opportunities to improve cognitive impairments and associated behavioral problems are non-existent. Generalizations, such as deeming ID and ASD as barely reversible based on their early onset and classification as neurodevelopmental disorders, might hinder efforts to identify effective treatment for specific conditions. In fact, still very little is known about the degree of developmental versus postnatal (acute lack of a required gene/protein function) contribution to brain dysfunction in most ID and ASD disorders, i.e. it is unclear to what extent the brain is not functioning because it has wrongly ‘hardwired' during development and to what extent because an important component for postnatal functioning is acutely missing. In the past years, several studies have provided impressive examples of how impaired gene/protein function can be restored in adult animals (Guo et al., 2000; Guy et al., 2007; Kramer et al., 2011; Lee et al., 2014; McBride et al., 2005). These findings raise hope that cognitive impairment in several forms of ID and ASD can be reversed or mitigated.
In summary, ID and ASD are dynamic fields of research with a number of big challenges ahead, including the identification of additional disease genes to allow better diagnostics, the characterization of candidate genes to better understand the neurobiology of the associated disorders, and the development of successful treatment approaches. Model organisms are widely used in the endeavor to overcome these bottlenecks. Drosophila melanogaster, the fruit fly, is a well-established genetic model, and highly suited to study the nervous system from genes to behavior (Ugur et al., 2016). In general, Drosophila is a cheap, genetically highly accessible, and, compared to vertebrates, a rather simple organism with high potential for both in-depth and high-throughput research.
The aim of this Review is to summarize some of the exciting work that has already drawn attention to Drosophila as a model for ID and ASD. We highlight disease-relevant fly phenotypes at the morphological, functional and behavioral levels, and discuss the future challenges in medical genomics that could be met by this extraordinarily efficient and versatile model.
Using Drosophila to overcome bottlenecks in ID and ASD research: relevant features and paradigms
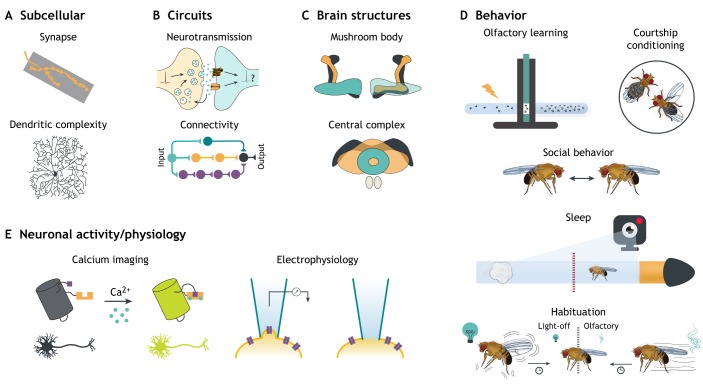
With the advent of exome sequencing, the major bottleneck in ID changed from gene identification to understanding gene function, interpreting the effect of the variants found in patients, and understanding various pathomechanisms. About three-quarters of all ID genes identified are conserved in Drosophila (Oortveld et al., 2013; Vissers et al., 2016). Despite the low conservation of the central nervous system (CNS) anatomy between flies and humans, ID-relevant biological processes are highly conserved at the molecular, cellular and synaptic level (Tian et al., 2017). While Drosophila research has so far focused on modeling ID rather than ASD, their genetic and clinical overlap makes the potential of such studies obvious. In Fig. 1, we have summarized the most widely used assays and systems to study the hallmarks and underlying mechanisms of ID and ASD in Drosophila.
Fig. 1.
Modeling ID and ASD in Drosophila – from (sub)cellular defects to aberrant behavior. This figure summarizes the commonly utilized ID- and ASD-relevant phenotype assays at various levels of complexity: from subcellular and circuit-level to brain structures, neuronal activity and behavior. (A) At the subcellular level, an NMJ and a type-IV da neuron with its complex dendritic tree serve as models to assess synapse morphology and dendritic complexity, respectively. (B) Circuits can be studied at the functional or connectivity level. Top: a synaptic cartoon with ongoing neurotransmission, with neurotransmitter release from the presynaptic terminal into the synaptic cleft and subsequent binding to receptors present in the postsynaptic terminal. Bottom: a hypothetical circuit, which is a parallel after-discharge circuit: an input neuron discharges to different chains of neurons, each one with a different number of synapses, and eventually all converge onto a single output neuron. (C) Many neuroanatomical entities can be studied in Drosophila, and the mushroom body (MB) and the central complex (CC) are of particular interest for ID and ASD modeling (see text). (D) Many behavioral assays can be used to assess ID- and ASD-relevant readouts. At the top of the panel, the two most widely used assays to assess associative learning and memory are depicted: olfactory learning, as conducted with a T-maze in which an electric shock is used as a negative stimulus, and courtship conditioning, with a naïve male courting a pre-mated female. Social behavior in Drosophila can be assessed, for instance, through the study of intra-fly distance. Sleep has been classically studied in the fly with single-beam activity monitors (red dashed line), but video tracking is increasingly being used. Lastly, non-associative learning is studied in Drosophila in light-off or olfactory habituation learning paradigms. Initial responses to these cues gradually wane. (E) Neuronal activity/physiology levels can be assessed by Ca2+ imaging (left) using genetically encoded Ca2+ indicators and by electrophysiological recordings, such as patch-clamp (right).
Neuromuscular junction as a model synapse
A significant number of ID/ASD genes are required for synaptic transmission (Srivastava and Schwartz, 2014) and/or synaptic organization, which may directly contribute to the synaptic morphology defects found in postmortem studies and various animal models (reviewed in Varghese et al., 2017). The Drosophila larval neuromuscular junction (NMJ) has been used for decades to investigate synapse morphology, development and neurotransmission in fundamental and disease model studies (Fig. 1A). The structural characteristics of NMJs make them an ideal model: they are relatively large and readily accessible, and thus suitable for electrophysiological and morphological investigation (Frank, 2014; Nijhof et al., 2016). However, the NMJ is peripheral and connects to a muscle instead of a postsynaptic neuron; therefore, some processes that operate at NMJs can differ from those at CNS synapses. Despite this, Drosophila NMJs share many features with vertebrate CNS synapses. For instance, they are glutamatergic, like the majority of excitatory synapses in the mammalian brain. The presynaptic component is composed of boutons (Box 1). The opposing postsynaptic membrane contains ionotropic glutamate receptors as well as postsynaptic signaling complexes, assembled in the postsynaptic density (Harris and Littleton, 2015). Pre- and postsynaptic molecular machineries include many highly conserved key regulatory proteins involved in ID and ASD, such as neurexins, synapsin I, synaptotagmins, ionotropic glutamate receptors (e.g. GRIN2A, GRIN2B and GRIK2) and PSD-95 (Dlg in Drosophila) (Han et al., 2015; Harris and Littleton, 2015). Similarities also extend to conserved processes regulating fundamental synaptic features, including synaptic plasticity, homeostasis, development and neurotransmitter recycling (Menon et al., 2013). Recent work in Drosophila has unraveled novel synaptic functions of classic ID/ASD genes. For instance, the fly NMJ was key in identifying presynaptic roles of proteins traditionally thought of as being only postsynaptic. These include Shank, the unique ortholog of human SHANK1-SHANK3, implicated in ASD and other neuropsychiatric conditions (Harris et al., 2016; Wu et al., 2017), and Dnlg4 (NLGN4 ortholog), a member of the neuroligin family, several of which are implicated in ID/ASD (Zhang et al., 2017).
Multidendritic neurons as a model for dendrites
Changes in dendritic architecture have long been reported in various neurodevelopmental conditions (Kaufmann and Moser, 2000; Kulkarni and Firestein, 2012). The first histological studies of ID patients' brains back in the 1970s showed a reduced complexity of the arborization pattern (Box 1) of their dendrites, and an increased number of immature dendritic spines (Box 1) (Purpura, 1975). Similar findings have been reported in Rett syndrome (Box 1) and other forms of ID/ASD, e.g. ID/ASD associated with mutations in CAMK2A, SHANK3 or IL1RAPL1 (Pardo and Eberhart, 2007; Stephenson et al., 2017).
A well-established model to study dendritic tree morphology in Drosophila are the dendritic arborization (da) sensory neurons (Box 1) of the peripheral nervous system. Depending on their morphology and function, four different classes of da neurons can be defined (I-IV). Type-IV da neurons display the most complex arborization, and tile the complete body wall with minimum overlap between neighboring neurons (Corty et al., 2009; Jan and Jan, 2010; Fig. 1A). Owing to this, as well as their location in the larval body wall and their planar nature, they are easy to identify, access, trace and quantify. Moreover, like NMJs, they can also be imaged in vivo over time (Jan and Jan, 2010; Satoh et al., 2012). The da neurons have a well-characterized and stereotyped architecture, which is achieved through a strict regulation of genetic programs and molecular pathways (Corty et al., 2009; Gao et al., 1999; Jan and Jan, 2010; Tassetto and Gao, 2006). One limitation of using da neurons as a dendritic model is that these, and most other Drosophila neurons, lack dendritic spines.
Taking advantage of this approach, researchers have uncovered the role of multiple ID/ASD genes and pathways in dendrite development. These include the gene DYRK1A (minibrain in Drosophila), gain of which is associated with Down syndrome (Altafaj et al., 2001; Guimera et al., 1996), whereas heterozygous disruption of the gene causes ID, ASD and microcephaly (Møller et al., 2008; O'Roak et al., 2012; van Bon et al., 2011). Using da neurons as a model, Ori-McKenney et al. found that altering Minibrain levels disrupts dendrite morphology and neuronal physiology due to abnormal phosphorylation of β-tubulin, a direct Minibrain substrate, which results in inhibited tubulin polymerization (Ori-McKenney et al., 2016). Additionally, several upstream (e.g. Wnt5) and downstream (e.g. Trio and Rho1) effectors of the Wnt pathway, implicated in the etiology and pathophysiology of many ID and ASD disorders (Kwan et al., 2016; Vorstman et al., 2017), were also recently uncovered to be critical in dendrite termination and delimitation of dendritic boundaries in Drosophila (Yasunaga et al., 2015).
Neuronal activity assessed by electrophysiology and calcium imaging
It appears likely that the above-mentioned morphological anomalies in ID and ASD correlate with anomalies in neuronal activity. Indeed, altered neuronal activity, measurable by non-invasive methods, has been reported in patients (Carter Leno et al., 2018; Guy et al., 2007; Knoth et al., 2018), as well as in some in vitro models, such as cortical neuron cultures and induced pluripotent stem cells (Griesi-Oliveira et al., 2015; Martens et al., 2016). The manipulable nature and reduced complexity of the Drosophila brain allows in-depth assessment of neuronal function, from a single cell to the whole network (Fig. 1E). In this context, electrophysiological assays from patch-clamp (Murthy and Turner, 2013) to whole-brain (van Swinderen and Greenspan, 2003) recordings, as well as electroretinograms (Box 1), NMJ electrophysiology and giant-fiber-system recordings (Box 1), have proven to be informative tools to assess neuronal activity (Ugur et al., 2016).
Several of these electrophysiological measurements can be combined with live imaging of protein or organelle trafficking and calcium (Ca2+) imaging, as facilitated by ever-improving genetically encoded calcium indicators (Simpson and Looger, 2018; Yang et al., 2018), to provide insights into the molecular control of neurotransmission. Furthermore, Ca2+ imaging can be performed ex vivo (Tong et al., 2016) and in vivo to simultaneously measure activity and behavior in the context of various circuits and developmental stages (Macleod, 2012; Seelig et al., 2010).
Mushroom body
Deficits in learning and memory are one of the main hallmarks of ID (Detterman, 1987; Vicari, 2004). Moreover, children with ASD also show impaired memory for complex information and poor working memory for spatial information (Williams et al., 2006). Drosophila has been widely used to investigate learning and memory. Before discussing behavioral paradigms used for learning and memory assessment in the next section, we will briefly describe the brain areas important for learning, and memory formation and consolidation. One of the key mammalian brain centers involved in several forms of learning and memory is the hippocampus (Moser et al., 2008; Squire, 1992; Winocur, 1990). Numerous ID and ASD genes have been shown to be important for hippocampal development and function, including genes involved in epigenetic remodeling (Lagali et al., 2010), neuronal migration and differentiation (Kepa et al., 2017; Wegiel et al., 2010), or synaptic circuitry maturation (Lanore et al., 2012; Roussignol et al., 2005).
Although structurally very different from the mammalian brain, some Drosophila brain centers have been argued to have analogy with human brain structures in terms of neuronal connectivity and behavioral output. The mushroom body (MB) is often referred to as the brain structure analogous to the mammalian hippocampus, as it has been widely implicated in insect learning and memory (Campbell and Turner, 2010; Heisenberg et al., 1985). It has also been proposed as an analog to both the cerebellum and the cortex due to a similar architecture and gene expression, respectively (Farris, 2011; Tomer et al., 2010). Interestingly, although the cerebellum has classically been associated with motor function, there is increasing evidence for its role in cognition (Leiner et al., 1993; Vandervert, 2016) and as a key region in ASD susceptibility (Chen et al., 2017; Peter et al., 2016; Wang et al., 2014). This association has, however, been attributed to dysfunction of Purkinje cells (Box 1) (Clifford et al., 2019; Tsai et al., 2012), for which no correlate has been identified in Drosophila, thus limiting studies into this interesting topic.
The MB is a neuropil-rich structure composed of ∼2500 Kenyon cell axons. These neurons receive and integrate inputs from several sensory pathways, including olfactory, gustatory, visual and auditory (Masek and Scott, 2010; Vogt et al., 2014) information that can be modified by reward or punishment via dopaminergic input (Liu et al., 2012; Riemensperger et al., 2005; Fig. 1C). MB output is glutamatergic, GABAergic or cholinergic (Aso et al., 2014) and is carried to convergent brain areas, ultimately resulting in modified behavior. MBs have been studied mainly for their role in associative learning. However, they are also involved in other behaviors, such as olfactory learning (Heisenberg et al., 1985), habituation (Acevedo et al., 2007; Glanzman, 2011), sleep (Joiner et al., 2006; Sitaraman et al., 2015), context generalization (Liu et al., 1999), habit formation (Brembs, 2009), temperature preference (Bang et al., 2011; Hong et al., 2008) and, recently, perceptual decision-making (DasGupta et al., 2014; Groschner et al., 2018). Some of these behaviors are highly relevant for ID and ASD, as will be discussed further in this Review. The MB is thus a very attractive system to link disease genes to their cellular function and disease-relevant behavior, and thus to a better understanding of disease pathology.
Associative learning and memory
The most commonly used assay to investigate learning and memory in Drosophila is olfactory classical conditioning (Fig. 1D). In this paradigm, odors (the conditional stimulus) are coupled to either a positive (e.g. sugar reward) or negative (e.g. electric shock) stimulus (the unconditioned stimulus). Upon successful learning, the flies will either avoid or prefer the associated odor even in the absence of the unconditional stimulus (Busto et al., 2010; Quinn et al., 1974). Another widely used approach to assess associative learning is courtship conditioning. This paradigm is based on the reduction of male courtship behavior in response to sexual rejection of a non-receptive pre-mated female (Siegel and Hall, 1979). Changes in courtship behavior can be easily scored by assessing the stereotyped pattern of behavior in males (summarized in Spieth, 1974). Learning, and short- and long-term memory can be assessed with both olfactory and courtship conditioning paradigms (Busto et al., 2010; Quinn et al., 1974), and both behaviors depend on the MB (de Belle and Heisenberg, 1994; McBride et al., 1999). In Drosophila, short-term memory is referred to as the memory present immediately after training. It rapidly decays, within an hour, whereas long-term memory can persist for days (Kahsai and Zars, 2011). An obvious limitation of Drosophila is that the established short/long-term memory paradigms probe analogs of non-declarative memory (Box 1; Brem et al., 2013) only.
The groundbreaking contribution of Drosophila to our molecular understanding of learning and memory is undebatable. Seymor Benzer and colleagues identified the first learning and memory genes, dunce and rutabaga, in Drosophila (Byers et al., 1981; Dudai et al., 1976; Livingstone et al., 1984). Both genes act in the cyclic AMP (cAMP) pathway, a second messenger activated by G protein-coupled receptor activation and Ca2+/Calmodulin. This pathway converges on the cAMP response element-binding protein (CREB) transcription factor to regulate a transcriptional program driving long-term but not short-term memory (Androschuk et al., 2015). Several ID genes have been linked to cAMP signaling, including CREBBP (encoding CBP, a CREB co-factor) (Petrif et al., 1995), FMR1 (Akshoomoff et al., 2015) and NF1 (Guo et al., 1997). Numerous additional ID/ASD genes converge onto CREB, which also integrates other learning- and memory-related pathways. This includes the Ras-MAPK signaling pathway (Guo et al., 2000; Pagani et al., 2009), which is mutated in a group of ID/ASD disorders referred to as rasopathies (Krab et al., 2008). Recent research into ID/ASD-associated genes highlights the complexity of regulating short- and long-term memory. Unexpectedly, ID genes encoding different subunits of the same protein complex, SWI/SNF, differentially affect MB-encoded short- versus long-term memory (Chubak et al., 2019).
Some ID/ASD gene orthologs have been unbiasedly identified as genes regulating Drosophila learning and/or memory, independent of their disease implication, e.g. the Drosophila ortholog of FLNA (Battaglia et al., 1997), cheerio (Dubnau et al., 2003).
Circadian rhythm and sleep
Many individuals with ID and/or ASD suffer from sleep disturbances (Ballester et al., 2019; Geoffray et al., 2016; van de Wouw et al., 2013; Veatch et al., 2017). A study from 2013 reported 72% of ID patients to have sleep disturbances (van de Wouw et al., 2013), while a more recent study characterized various qualitative components of sleep in ASD patients, and found an increased number of awakenings during the night, sleep onset latency and reduced sleep efficiency (Ballester et al., 2019). Disturbed sleep does not only negatively affect the emotional status and social behavior of patients, but also their cognitive functioning (Geoffray et al., 2016; Veatch et al., 2017).
Some sleep problems can be attributed to defects in the circadian rhythm driven by dysregulation of a highly conserved molecular pacemaker/clock that oscillates in a ∼24 h rhythm and synchronizes physiology and behavior to the time of the day (Dubruille and Emery, 2008). Some ID/ASD patients have a shift in their circadian clock (Ballester et al., 2019; Maaskant et al., 2013). Drosophila is an excellent model organism to study the circadian clock and circuit, as supported by the 2017 Nobel Prize in Physiology or Medicine for the discoveries of molecular mechanisms controlling circadian rhythm. In the fly brain, the expression of the pacemaker is restricted to a small set of neurons and glia cells (Zhang et al., 2018), resembling the function of the mammalian suprachiasmatic nucleus (Box 1) (Dubowy and Sehgal, 2017).
Drosophila has also delivered fundamental insights into the regulation and function of sleep (Dubowy and Sehgal, 2017; Emery and Reppert, 2004). Sleep in Drosophila is defined as five or more minutes of inactivity in which flies show an increased arousal threshold. Circadian behavior and sleep can be measured by assessing locomotor activity (Greenspan et al., 2001), as classically done in the Drosophila Activity Monitor (DAM) system (TriKinetics, Waltham, MA, USA). Increasingly used video-tracking-based methods may be more accurate (Garbe et al., 2015) and allow assessment of additional parameters, such as arousal, sleep pressure and feeding [e.g. DART (Drosophila ARousal Tracking) system (Faville et al., 2015), ethoscope (Geissmann et al., 2017), ARC (Activity Recording Capillary Feeder) or CAFE (Murphy et al., 2017)]. Moreover, sleep can be modified by stimulants and hypnotics, and is regulated by both the circadian clock and a homeostatic system that determines sleep need, which shows the conserved nature of sleep properties (Shaw et al., 2000). Although there is increasing evidence for dynamic changes in the sleep intensity of Drosophila (van Alphen et al., 2013), flies do not display the typical sleep stages described in humans, e.g. non-REM/REM sleep (Box 1). Many brain centers and neuronal clusters have been involved in sleep promotion or inhibition (reviewed in Dubowy and Sehgal, 2017).
When mutated, many Drosophila orthologs of human ID and ASD genes have been reported to cause sleep disturbances. Neurexins and neuroligins are key adhesion molecules required for proper synapse formation, homeostasis and function (Dean et al., 2003; Missler et al., 2003). Neurexin 1 in flies regulates nighttime sleep due to its role in mediating synaptic transmission of a subset of MB neurons (Tong et al., 2016), and its loss leads to sleep fragmentation and circadian defects (Larkin et al., 2015). Neurexin receptors, the neuroligins (Nlg proteins), have also been implicated in sleep. Nlg4 mutant flies display abnormal nighttime sleep due to impaired GABA neurotransmission in clock neurons (Li et al., 2013). This effect on sleep is not exclusive of Dnlg4, as has recently been reported for Dnlg2 (Corthals et al., 2017). Patients with mutations in these genes suffer from sleep disturbances (Harrison et al., 2011; Vaags et al., 2012).
High potential: central complex, social behavior and habituation learning
The assays discussed above are providing more insights into the pathology of ID and ASD disorders than we are able to acknowledge in this Review. Nevertheless, an increasing amount of novel paradigms have been barely tapped into to investigate ID/ASD but have, we believe, high potential to make significant contributions to the field in the future. In this section, we draw attention to some of these: the Drosophila central complex (CC; Box 1), to social behaviors and habituation learning (Fig. 1D).
Increasingly, the literature has pointed to dysfunction in the basal ganglia (Box 1) in ASD and other neuropsychiatric conditions (Riva et al., 2018; Subramanian et al., 2017). This subcortical structure shows homology with the insect CC regarding genetic developmental programs, microarchitecture and regulated behaviors (Lin et al., 2013; Strausfeld and Hirth, 2013). It serves as the integration center for sensory inputs, particularly for space representation and spatial control of motor behavior, and is also involved in various types of memory (Liu et al., 2006; Neuser et al., 2008; Ofstad et al., 2011), arousal and sleep (Donlea et al., 2018, 2011). So far, reports of ID/ASD gene function in the CC are scarce [e.g. RSK2 (Kuntz et al., 2012; Thran et al., 2013); SIM2 (Pielage et al., 2002)]. However, given its key role in memory, arousal and sleep, processes highly relevant to ID/ASD (van Alphen and van Swinderen, 2013), it is likely to emerge as a pertinent system to be investigated in Drosophila ID/ASD models.
One of the main criteria for diagnosing ASD as stated in the latest Diagnostic and Statistical Manual of Mental Disorders (DSM-5) are ‘persistent deficits in social communication and social interaction across multiple contexts’. These can manifest as a wide variety of deficits: from social-emotional reciprocity, to verbal and nonverbal communicative behaviors needed for social interactions, as well as deficits in establishing and understanding relationships (American Psychiatric Association, 2013). Similar deficits are also observed in children and adults with ID (Sigafoos et al., 2017). Although Drosophila is a simple model, complex social interactions exist. Classically, fly sociability has been studied in the context of mating and aggression, by studying courtship behavior (Dockendorff et al., 2002; Villella and Hall, 2008) and male social dominance (Zwarts et al., 2012), respectively. Whereas the concept of sociability in these contexts substantially differs from human behaviors in this domain, new paradigms explore other, potentially more translatable, types of social behaviors, mostly based on inter-fly distance, and some have begun to be applied to ID/ASD genes.
One of the first approaches to characterize social interactions of Drosophila ID/ASD models was in Fragile X syndrome (FXS; Box 1), which showed that dFMR1 mutant flies spend less time interacting with another fly in a neighboring chamber (divided by a mesh) (Bolduc et al., 2010). In a novel assay evaluating group formation, Dnlg-2-deficient flies showed decreased social interaction, whereas, in Dnlg-4-deficient flies, group formation was enhanced, implicating different members of the ID/ASD-associated Neuroligin family into opposite regulation of this social behavior (Corthals et al., 2017). Dnlg-2 mutants also showed courtship and aggression deficits, implicating this gene in further aspects of social behavior (Hahn et al., 2013). Another assay with emerging relevance to ID/ASD assesses social space, the average distance in which flies position themselves relative to each other (Simon et al., 2012). Social space was increased in rg mutants, the ortholog of human NBEA, supporting it as an ASD-candidate gene (Wise et al., 2015). Social space was also affected in FoxP-null and pan-neuronal knockdown flies (Castells-Nobau et al., 2019). Interestingly, social space positively correlates with paternal and maternal age (Brenman-Suttner et al., 2018). As advanced paternal age at conception has been strongly linked with increased risk to ASD and other neuropsychiatric conditions due to increased rates of de novo mutations (Janecka et al., 2017; Sandin et al., 2016), it will be interesting to determine whether similar mechanisms underlie the Drosophila phenomenon.
Habituation, a form of non-associative learning, represents a selective filter through which an organism learns to ignore (and stops to react to) a familiar irrelevant stimulus. This mechanism, highly conserved throughout the entire animal kingdom, is thought to prevent information overload and to allow focusing on the available cognitive resources on relevant matters (McDiarmid et al., 2017; Ramaswami, 2014). Habituation is a proxy for synaptic plasticity (Castellucci et al., 1970; Larkin et al., 2010; Weber et al., 2002) and represents an important prerequisite for higher cognitive functions (Colombo and Mitchell, 2009; Kavšek, 2004; McDiarmid et al., 2017; Ramaswami, 2014). ASD is characterized by defective cortical filtering of sensory stimuli and information overload, which manifests in hypersensitivities, an ‘intense world’ perception (Ramaswami, 2014; Sinha et al., 2014), and probably also contributes to social deficits and other hallmark features (Barron et al., 2017; Kleinhans et al., 2009). A number of studies reported defective habituation in idiopathic ASD (Dinstein et al., 2012; Ewbank et al., 2015; Kleinhans et al., 2009; Pellicano et al., 2013). Habituation deficits have also been demonstrated in patients with FXS and in its mouse model (Restivo et al., 2005), as well as in a number of other ID/ASD mouse, zebrafish and fly models (Bariselli et al., 2018; Stessman et al., 2017; Wolman et al., 2014). Different types of habituation have been described in Drosophila, and a variety of assays are available for their assessment (Asztalos et al., 2007; Das et al., 2011; Kuntz et al., 2012; Paranjpe et al., 2012). Recently, Drosophila knockdown models of ∼300 ID genes were investigated in the light-off jump habituation paradigm (Box 1), revealing habituation deficits in more than 100 models (Fenckova et al., 2018). Interestingly, among the habituation-defective ID models, those with comorbid ASD were particularly enriched, suggesting that habituation could be a widely applicable readout for Drosophila studies of both disorders. Although habituation appears to exhibit strong face- and construct-validity, important prerequisites for accurate disease-modeling (Hmeljak and Justice, 2019), the predictive value of fly models for human habituation levels, and for ID and ASD clinical features, remains to be further characterized.
The above-discussed and other available assays and systems provide a rich repertoire to study the disease mechanisms of ID and ASD; they already made important contributions that significantly improve our understanding of the genetics and biology underlying specific aspects of neuronal morphology, function and behavior. In addition to the examples highlighted above, others have been previously featured in other reviews (Androschuk et al., 2015; Bolduc and Tully, 2009; van der Voet et al., 2014). With this large repertoire, Drosophila is a very powerful model that allows researchers to work across these different levels to accelerate fundamental and translational research for ID and ASD disorders.
From fundamental gene function insights towards molecular networks and translational application
Fragile X syndrome: from molecular mechanisms and novel functions to clinical trials
FXS is the most frequent and best-studied cause of monogenic ID and ASD (de Vries et al., 1997). It arises from a CGG-trinucleotide expansion and subsequent transcriptional silencing of the FMR1 gene (Verkerk et al., 1991). The characteristic low IQ is highly comorbid with ASD traits, with a prevalence as high as 50% (Abbeduto et al., 2014). FXS has always been the forerunner in research for both disorders, in humans and other systems, including Drosophila. This is reflected by numerous discoveries in Drosophila, from abnormal synaptic architecture to learning and memory deficits (Bolduc et al., 2008; McBride et al., 2005; Sudhakaran et al., 2014; Zhang et al., 2001). The pathophysiological mechanisms underlying FXS and the contribution of Drosophila to this knowledge have been extensively discussed in dedicated reviews (De Rubeis et al., 2012; Drozd et al., 2018; McBride et al., 2013; Specchia et al., 2019). As illustrated by past work on FXS, Drosophila can be a useful tool to reveal changes in certain neurotransmitter systems, as now widely implicated in ID/ASD (Bear et al., 2004; Mariani et al., 2015; Muller et al., 2016). Drosophila provided the first pharmacological rescue of FXS-associated phenotypes, with mGluR antagonists that have been tested in clinical trials, unfortunately without success, as described and reviewed in detail elsewhere (Braat and Kooy, 2015; Chang et al., 2008; Duy and Budimirovic, 2017; McBride et al., 2005; Youssef et al., 2018). Decrease of the inhibitory neurotransmitter γ-aminobutyric acid (GABA) has also been intensively investigated in FXS Drosophila and other animal models (Gatto et al., 2014; Lozano et al., 2014). Importantly, the first and so far only unbiased large-scale in vivo drug screen for FXS, conducted in Drosophila, identified small molecules that interfere with both glutamatergic (excitatory) and GABAergic (inhibitory) signaling (Chang et al., 2008). Whereas for most ID/ASD genes it is still unknown in which neurons they act, it is obvious that this question can be efficiently addressed in Drosophila with its versatile genetic tools. Such knowledge is relevant to the development of treatment strategies for FXS and other ID/ASD disorders.
Also for FXS research, Drosophila continues to reveal aspects that may hint at treatment options, including those that could be relevant more widely to ID/ASD disorders. One aspect of FXS that has classically been rather overlooked is metabolic dysfunction (Bailey et al., 2010; Berry-Kravis et al., 2015; de Vries et al., 1993). A key regulator of metabolism in mammals and invertebrates, insulin signaling, was increased in the FXS Drosophila model (Monyak et al., 2017), along with deregulation of both carbohydrate and lipid metabolism (Weisz et al., 2018). This increase in insulin signaling in dfmr1 mutants was shown to underlie the circadian defect of these flies, which could be rescued by either restoring dfmr1 expression in the insulin-producing cells of the fly brain or by reducing the signaling pathway. Moreover, the enhanced insulin signaling also led to memory deficits (Monyak et al., 2017). Interestingly, pharmacological downregulation of insulin signaling with metformin also rescued the memory defects in dfmr1 mutants. Similar findings have subsequently been reported in the FXS mouse model (Dy et al., 2018), and a controlled clinical trial has been recommended. Of note, the influence of metabolic state on cognition has been shown in both in flies and mammals (Chambers et al., 2015; Dou et al., 2005; Hirano et al., 2013; Placais and Preat, 2013). Since metabolic homeostasis is affected in a number of ID patients and models (Blanchet et al., 2017; Dunkley et al., 2017; Hsieh et al., 2014; Lin et al., 2010; Zheng et al., 2017), these findings provide avenues for developing innovative therapeutic approaches.
ID/ASD genes cooperate in molecular networks: the EHMT1 module example
Another disorder for which Drosophila contributed much of our current knowledge is Kleefstra syndrome. The disorder, caused by haploinsufficiency of the eukaryotic histone methyltransferase 1 gene (EHMT1) (Kleefstra et al., 2010; Vermeulen et al., 2017), is characterized by ID, comorbid ASD in all patients reported so far (Vermeulen et al., 2017), behavioral problems and other clinical features, including recurrent infections and obesity (Kleefstra et al., 2012). Loss of Drosophila G9a, the ortholog of EHMT1, only resulted in subtle anomalies of da neurons and did not show other detectable nervous system architecture anomalies (Kramer et al., 2011). Nevertheless, G9a deficiency resulted in dramatic defects in courtship memory and light-off jump habituation caused by epigenetic changes in a set of target genes that featured the majority of known learning and memory genes. Interestingly, courtship memory could be restored by G9a re-expression in adulthood (Kramer et al., 2011), adding Kleefstra syndrome to a growing list of potentially reversible ID/ASD disorders.
Apart from learning and memory genes, G9a ChIP-seq data also revealed marked enrichment of genes implicated in immune defense and stress responses. Subsequent studies confirmed these results: G9a mutants were susceptible to virus infection (Merkling et al., 2015) and oxidative stress, the latter being caused by metabolic dysregulation (An et al., 2017; Riahi et al., 2019). This work identified energy availability as a generally limiting factor for oxidative stress resistance and further adds to metabolic dysregulation as a wider theme in ID/ASD.
G9a-related Drosophila work also makes a compelling case for the utility of this model organism in diagnostics, for what could be referred to as a ‘bedside-to-bench-and-back’ approach. In a cohort of patients with Kleefstra-syndrome-like appearance but no EHMT1 mutations, next-generation sequencing approaches revealed single de novo mutations in five novel candidate genes (MBD5, SMARCB1, KMT2C, NR1I3 and MTMR9) in four patients (Kleefstra et al., 2012). Testing pairwise genetic interactions with G9a, Kleefstra et al. showed that KMT2C, MBD5, SMARCB1 and NR1I3 genetically interact with EHMT1, uncovering an EHMT1-associated chromatin remodeling module of both synergistic and antagonistic interactions (Kleefstra et al., 2012). Notably, the fifth candidate gene, MTMR9, which was co-mutated in the patient with an NR1I3 mutation, did not show any genetic interaction. This work strengthened NR1I3 as the gene underlying the Kleefstra-syndrome-like phenotype in this specific patient, and enabled the genetic diagnosis of all four investigated patients.
Another study that further investigated the molecular pathology/transcriptional dysregulation common to EHMT1 and KMT2C mutations found significant overlap in misregulated downstream target genes of the Drosophila EHMT1 and KMT2C orthologs (G9a and trr) (Koemans et al., 2017a). One of the few direct target genes, dysregulated in both mutants, was the Drosophila ortholog of Arc (Arc1) (Koemans et al., 2017a), which also emerged as a relevant EHMT1 target in recent mouse studies into Kleefstra syndrome (Benevento et al., 2016). Arc is an important neuron-specific regulator orchestrating multiple aspects of synaptic plasticity (reviewed in Shepherd and Bear, 2011), learning and memory (Whitlock et al., 2006). Interestingly, Arc had been previously linked to ID/ASD, both in the context of FXS (Krueger et al., 2011; Park et al., 2008; Yan et al., 2018) and Angelman syndrome (Box 1) (Greer et al., 2010; Kuhnle et al., 2013), suggesting convergent mechanisms between multiple ID/ASD disorders. Excitingly, Drosophila was key in groundbreaking work on the Arc mode of action (Ashley et al., 2018). The Drosophila Arc1 protein was shown to bind its own RNA in vivo and assemble into retrovirus-like capsids that are transferred in extracellular vesicles from the presynaptic NMJ terminal to its postsynaptic compartment. Abrogation of this process disrupted synaptic plasticity, uncovering a fundamentally new mechanism of synaptic communication (Ashley et al., 2018). A parallel study reported similar results in mice (Pastuzyn et al., 2018). Together, these examples highlight the relevance of findings in Drosophila for both fundamental and translational ID/ASD research.
Future outlook
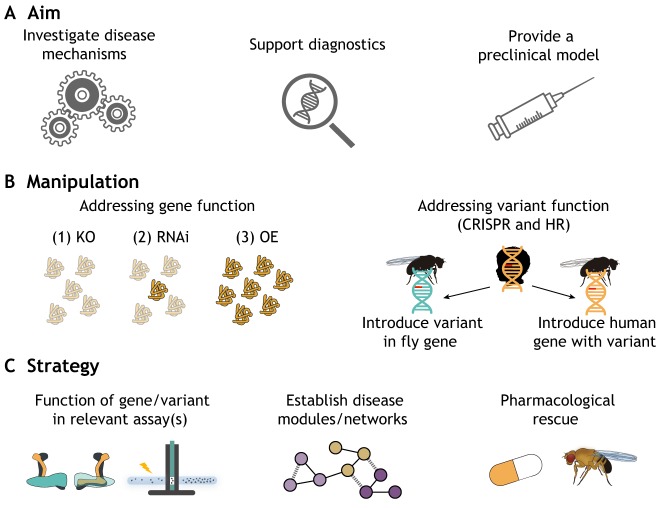
As the above examples illustrate, Drosophila has made important contributions to our understanding of molecular mechanisms underlying ID/ASD disorders in the past decade. With the available resources and technologies, Drosophila is set to continue to contribute fundamental insights to this important field, and serve the great need for efficient and effective model organisms in translational research. Complementary to recent progress in uncovering ID and ASD genetics, Drosophila bears potential to push the boundaries of this field's main challenges by: (1) generating a better conceptual understanding of the pathophysiology of these disorders, (2) facilitating diagnostics, and (3) serving as a preclinical model for testing drugs and other treatment strategies (Fig. 2A). This final section further discusses how Drosophila can be exploited on all these fronts, and the important milestones and limitations of this endeavor.
Fig. 2.
Main challenges and applications of Drosophila as a model in future medical genomics for ID/ASD disorders. (A) Drosophila research into ID and ASD can facilitate various aims, from dissection of disease mechanisms to shedding light onto pathogenicity of variants/mutations identified in the clinic, to providing preclinical models to assess the potential of treatment strategies. (B) Different genetic manipulations can be performed to target an ID/ASD gene of interest. Left: the most widely used manipulations to address gene function are: (1) complete ablation of proteins by gene knockout (KO), (2) decreased protein levels via RNA interference (RNAi)-mediated knockdown, or (3) increased protein levels via overexpression (OE) of the gene of interest. Right: the function of genetic variants can be addressed by either introducing the human variant [at the corresponding residue(s)] into the fly gene or by introducing the whole human gene with its variant in the fly genome. Both approaches can be realized using CRISPR/Cas9 (CRISPR) or homologous recombination (HR). (C) Several strategies can be followed to achieve the aims stated above, from assessing gene/variant function in ID/ASD-relevant assays (Fig. 1), establishing disease networks, to generating preclinical models, e.g. for pharmacological rescue.
Strategies and opportunities for Drosophila disease modeling to overcome current bottlenecks
Unquestionably, future Drosophila work on ID/ASD-associated genes will also be based on manipulating the expression of their Drosophila orthologs through classical approaches. This includes the generation of knockout animals by various techniques, transgenic knockdown and/or overexpression (Fig. 2B), depending on the established or presumptive effect of the human disease alleles and on the further approach to be taken. Beyond addressing gene function, different studies have also investigated the effect of specific gene mutations by expressing these either in wild-type (Wan et al., 2000) or null/mutant backgrounds (Wu et al., 2015; Zamurrad et al., 2018), and comparing them to the effect of the non-mutated proteins. For such attempts, either transgenes expressing the human mutant proteins, or transgenes expressing the Drosophila genes with engineered, analogous mutations, can be used. Alternatively, gene replacement by homologous recombination and CRISPR/Cas9 genome-editing approaches now allow manipulation of the fly gene at its endogenous locus (de Brouwer et al., 2018; Mariappa et al., 2018) (Fig. 2B).
To evaluate the effect of specific mutations is not only of fundamental interest; it may well be that patients carrying different mutations also require different interventions, as most obvious for loss- versus gain-of-function mutations that likely require opposite manipulation. Furthermore, in the era of diagnostic exome sequencing in ID and ASD, the interpretation of genetic variants of unknown significance has become the major challenge in diagnostics (Di Resta et al., 2018). We can safely assume that the resulting need for functional investigation will further increase, at least in cases where human genetics/genomics fail to detect the same mutation in additional patients with similar phenotypes (van der Voet et al., 2014).
Need for speed!
Extraordinarily efficient models are required to meet the current challenges, particularly in diagnostics, where the generation of relevant information is required in a rather short time and on demand. Drosophila already is in a pole position in this respect. Furthermore, we expect that Drosophila disease modeling will continue to benefit from the ever-increasing pool of readily usable resources of mutants, and from increasingly efficient phenotyping approaches. To date, large-scale resources for genetic manipulation, such as gene-disrupting P-element collections and libraries to induce conditional RNA interference or overexpression, exist. These allow researchers to manipulate the majority of genes in the Drosophila genome (Bellen et al., 2011; Bischof et al., 2013; Dietzl et al., 2007; Perkins et al., 2015), and thus also any evolutionarily conserved, established or newly identified, ID/ASD gene. A recent achievement that accelerates testing variants by rescue approaches is gene targeting with CRISPR-mediated integration cassettes (CRIMICs), which can be converted to T2A-Gal4 (or Trojan Gal4; Box 1) lines (Diao et al., 2015; Lee et al., 2018). A library of >1000 mutant T2A lines is already available (Lee et al., 2018), and genes can be nominated for CRIMIC generation via the webpage http://flypush.imgen.bcm.tmc.edu/pscreen/crimic/crimic-technique.html. The technology has been applied in a first study to demonstrate that de novo variants in the EBF3 gene found in three individuals with ID are deleterious (Chao et al., 2017a).
Phenotypic characterization, particularly large-scale, remains laborious and often limited by data analysis and quantification processes. We discussed specific setups that facilitate data acquisition in the above-discussed disease-relevant paradigms. Other recent examples include the Fiji/ImageJ macro NMJ morphometrics to quantify morphological parameters in high throughput (Castells-Nobau et al., 2017; Nijhof et al., 2016). In behavioral research, several tools have been developed to assess and quantify learning and memory through courtship conditioning behavior, although their implementation appears to require programming or other skills to get operational (Dankert, 2009; Reza, 2013; Schneider, 2014). However, the assay can be efficiently conducted (Koemans et al., 2017b). Liu and colleagues developed a novel tracking and analysis pipeline that allows a large number of flies to be followed, and their social network quantified (Liu et al., 2018). One step further, the Janelia Automatic Animal Behavior Annotator (JAABA) is a machine-learning-based system to automatically track and quantify a wide variety of pre-defined behaviors (e.g. walking, touching, righting, etc.), and provides the computational framework for the quantification of additional behaviors of interest (Kabra et al., 2013). Further development of open-source setups and software for (semi)automated assessment and analyses of quantitative biological data can greatly contribute to the future success of Drosophila as a versatile disease model.
Challenge 1: towards a conceptual understanding of the pathophysiology of these disorders
Reaching a higher throughput in the characterization of ID/ASD genes does not only increase data quantity, but also its quality. Based on shared phenotypes, gene modules that operate together can be recognized, with implications for fundamental (i.e. recognition of key pathways) and translational (i.e. the potential to target multiple ID/ASD models/disorders with the same treatment) research. So far, only a few large-scale studies into monogenic ID/ASD disorders have been conducted. These studies have implicated dozens of novel genes in neurotransmission and/or learning, and revealed neuronal substrates underlying the latter. Moreover, they uncovered functional modules that can predict additional phenotypes and demonstrated that ID genes associated with similar phenotypes in Drosophila are also associated with significant phenotypic similarity in humans (Fenckova et al., 2018 preprint; Kochinke et al., 2016; Oortveld et al., 2013).
Increasing the throughput of assays will also allow the transition from identifying monogenic to genetically more complex causes of ID/ASD. Two studies dissected phenotypes and genetic interactions among the Drosophila orthologs of genes co-affected by ID/ASD-associated copy number variations (CNVs). They tested pairwise interactions between conserved genes in both CNVs, and used readouts from cellular to behavioral systems (Grice et al., 2015; Iyer et al., 2018). Both studies identified extensive genetic interactions among the genes located in a single CNV locus and beyond, and proposed that variants in multiple genes contribute to the respective disease phenotypes.
To our knowledge, no studies systematically mined public genome-wide Drosophila data to identify characteristic phenotypes or patterns associated with Drosophila ID/ASD orthologs. Drosophila can further contribute to the identification of common phenotypes and mechanisms underlying ID/ASD in the future.
Challenge 2: towards Drosophila as a tool in diagnostics
As discussed, the need for systems that can inform medical genomics about the causal relationship between a mutation and a clinical phenotype is enormous. For ID/ASD, Drosophila researchers have so far taken two approaches. First, they investigated whether manipulating the expression of a candidate gene can cause an ID/ASD-relevant phenotype in flies, providing support for such a causal relationship. Second, they addressed whether an identified mutation affects gene function, even if this does not (or not obviously) relate to the clinical phenotype. Both approaches have value; ideally, future studies will combine testing patient-specific mutations with an assay tailored to the clinical phenotype (Fig. 2B,C). In addition, the genetic interaction/network approaches with known disease genes can be exploited where one or more genes have already been implicated in a specific syndrome (Fig. 2C).
To facilitate the use of Drosophila in diagnostics, it is not only important to generate disease-relevant data in this organism, but also to organize them in a way that they can be accessible across disciplines. However, major barriers in the communication between clinicians and fundamental Drosophila researchers often hinder the development of effective interdisciplinary collaborations (Chao et al., 2017b). These pitfalls, as well as the initiatives, resources and tools for clinicians and researchers to facilitate effective bi-directional dialogues, have been discussed in detail elsewhere (Chao et al., 2017b; Şentürk and Bellen, 2018; Yamamoto et al., 2014). Open-access databases – such as MARRVEL, which integrates data from human disease research to biochemical data and that from multiple model organisms (Wang et al., 2017); FlyBase [http://flybase.org (Gramates et al., 2017)], with its implemented Human Disease Model section (Millburn et al., 2016); and the Monarch Initiative, connecting genotypes to phenotypes across species (Mungall et al., 2017) – are at least a start to increasing interspecies research collaborations. A series of recent papers in the ID/ASD field that combine clinical and Drosophila data with the identification of genetic defects in patients argue that clinicians, and human and Drosophila geneticists nowadays find each other more efficiently (de Brouwer et al., 2018; Fattahi et al., 2018; Gonçalves et al., 2018; Koemans et al., 2017a; Nixon et al., 2019; Straub et al., 2018).
A persistent limitation to the implementation of Drosophila in diagnostics is its evolutionary distance from humans. A quarter of all human genes do not have a Drosophila counterpart, and a significant amount of human coding variants will not affect conserved residues. The former will, in many cases, also limit the fly's value to point to causal variants or genes among multiple ones affected in a patient (i.e. by a CNV or by multiple de novo mutations); if some variants cannot be modeled, the outcome of such experiments will remain incomplete.
Challenge 3: towards successful treatment strategies
Research in one or even across different animal models has demonstrated that the cognitive defects in some ID/ASD disorders, such as FXS, neurofibromatosis type 1 and Kleefstra syndrome, may be reversible in adulthood (Kramer et al., 2011; Lee et al., 2014; McBride et al., 2005). Drosophila could readily be used to assert reversibility for dozens to hundreds of uncharacterized ID/ASD genes with the same approach. Such disorders could then be prioritized for intervention.
While FXS appeared as a success story in translational medicine for some years, so far clinical trials have failed. Despite the progress, our treatment options for ID/ASD remain limited. How can we improve in the future? Intervention strategies that were successful in Drosophila will need confirmation in other systems and, if positive, to be tested in clinical trials. One still unexplored, conceptually novel approach in ID/ASD drug identification would be to use high-throughput amenable cognitive readouts (i.e. learning or memory paradigms) for large-scale drug screening in ID/ASD. The identified compounds would eventually need to be tested in higher organisms and prove their utility in patients.
Conclusions
A number of major challenges in ID and ASD research lie ahead. Drosophila, with its unique resources and advantages, may be one of the organisms that is best equipped to meet many of the current bottlenecks limiting the translation of successful preclinical research to clinical application. Importantly, the community needs not only this model, but also research funding and training to raise the next generation of creative interdisciplinary scientist who will take up this translational endeavor.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was supported by a Radboudumc personal PhD fellowship to M.C.-T., a TOP grant (912-12-109) from the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) to A.S., and by a Horizon 2020 Marie Sklodowska-Curie European Training Network grant (MiND, 643051) to A.S.
References
- Abbeduto L., McDuffie A. and Thurman A. J. (2014). The fragile X syndrome-autism comorbidity: what do we really know? Front. Genet. 5, 355 10.3389/fgene.2014.00355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo S. F., Froudarakis E. I., Kanellopoulos A. and Skoulakis E. M. C. (2007). Protection from premature habituation requires functional mushroom bodies in Drosophila. Learn. Mem. 14, 376-384. 10.1101/lm.566007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akshoomoff N., Mattson S. N. and Grossfeld P. D. (2015). Evidence for autism spectrum disorder in Jacobsen syndrome: identification of a candidate gene in distal 11q. Genet. Med. 17, 143-148. 10.1038/gim.2014.86 [DOI] [PubMed] [Google Scholar]
- Allen M. J. and Godenschwege T. A. (2010). Electrophysiological recordings from the Drosophila giant fiber system (GFS). Cold Spring Harb. Protoc. 2010, pdb.prot5453 10.1101/pdb.prot5453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altafaj X., Dierssen M., Baamonde C., Marti E., Visa J., Guimera J., Oset M., Gonzalez J. R., Florez J., Fillat C. et al. (2001). Neurodevelopmental delay, motor abnormalities and cognitive deficits in transgenic mice overexpressing Dyrk1A (minibrain), a murine model of Down's syndrome. Hum. Mol. Genet. 10, 1915-1923. 10.1093/hmg/10.18.1915 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub. [Google Scholar]
- Amir R. E., Van den Veyver I. B., Wan M., Tran C. Q., Francke U. and Zoghbi H. Y. (1999). Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 23, 185-188. 10.1038/13810 [DOI] [PubMed] [Google Scholar]
- An P. N. T., Shimaji K., Tanaka R., Yoshida H., Kimura H., Fukusaki E. and Yamaguchi M. (2017). Epigenetic regulation of starvation-induced autophagy in Drosophila by histone methyltransferase G9a. Sci. Rep. 7, 7343 10.1038/s41598-017-07566-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androschuk A., Al-Jabri B. and Bolduc F. V. (2015). From learning to memory: what flies can tell us about intellectual disability treatment. Front. Psychiatry 6, 85 10.3389/fpsyt.2015.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett A. B., Trinh S. and Bernier R. A. (2018). The state of research on the genetics of autism spectrum disorder: methodological, clinical and conceptual progress. Curr. Opin. Psychol. 27, 1-5. 10.1016/j.copsyc.2018.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley J., Cordy B., Lucia D., Fradkin L. G., Budnik V. and Thomson T. (2018). Retrovirus-like Gag protein Arc1 binds RNA and traffics across synaptic boutons. Cell 172, 262-274.e11. 10.1016/j.cell.2017.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y., Hattori D., Yu Y., Johnston R. M., Iyer N. A., Ngo T.-T., Dionne H., Abbott L. F., Axel R., Tanimoto H. et al. (2014). The neuronal architecture of the mushroom body provides a logic for associative learning. eLife 3, e04577 10.7554/eLife.04577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asztalos Z., Arora N. and Tully T. (2007). Olfactory jump reflex habituation in Drosophila and effects of classical conditioning mutations. J. Neurogenet. 21, 1-18. 10.1080/01677060701247508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badano J. L. and Katsanis N. (2002). Beyond Mendel: an evolving view of human genetic disease transmission. Nat. Rev. Genet. 3, 779-789. [DOI] [PubMed] [Google Scholar]
- Bailey D. B., Raspa M. and Olmsted M. G. (2010). Using a parent survey to advance knowledge about the nature and consequences of fragile X syndrome. Am. J. Intellect. Dev. Disabil. 115, 447-460. 10.1352/1944-7558-115.6.447 [DOI] [PubMed] [Google Scholar]
- Ballester P., Martinez M. J., Javaloyes A., Inda M. M., Fernandez N., Gazquez P., Aguilar V., Perez A., Hernandez L., Richdale A. L. et al. (2019). Sleep problems in adults with autism spectrum disorder and intellectual disability. Autism Res. 12, 66.- . 10.1002/aur.2000 [DOI] [PubMed] [Google Scholar]
- Bang S., Hyun S., Hong S.-T., Kang J., Jeong K., Park J.-J., Choe J. and Chung J. (2011). Dopamine signalling in mushroom bodies regulates temperature-preference behaviour in Drosophila. PLoS Genet. 7, e1001346 10.1371/journal.pgen.1001346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bariselli S., Contestabile A., Tzanoulinou S., Musardo S. and Bellone C. (2018). SHANK3 downregulation in the ventral tegmental area accelerates the extinction of contextual associations induced by juvenile non-familiar conspecific interaction. Front. Mol. Neurosci. 11, 360 10.3389/fnmol.2018.00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron H. C., Vogels T. P., Behrens T. E. and Ramaswami M. (2017). Inhibitory engrams in perception and memory. Proc. Natl. Acad. Sci. USA 114, 6666-6674. 10.1073/pnas.1701812114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia G., Granata T., Farina L., D'Incerti L., Franceschetti S. and Avanzini G. (1997). Periventricular nodular heterotopia: epileptogenic findings. Epilepsia 38, 1173-1182. 10.1111/j.1528-1157.1997.tb01213.x [DOI] [PubMed] [Google Scholar]
- Bear M. F., Huber K. M. and Warren S. T. (2004). The mGluR theory of fragile X mental retardation. Trends Neurosci. 27, 370-377. 10.1016/j.tins.2004.04.009 [DOI] [PubMed] [Google Scholar]
- Bellen H. J., Levis R. W., He Y., Carlson J. W., Evans-Holm M., Bae E., Kim J., Metaxakis A., Savakis C., Schulze K. L. et al. (2011). The Drosophila gene disruption project: progress using transposons with distinctive site specificities. Genetics 188, 731-743. 10.1534/genetics.111.126995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevento M., Iacono G., Selten M., Ba W., Oudakker A., Frega M., Keller J., Mancini R., Lewerissa E., Kleefstra T. et al. (2016). Histone methylation by the Kleefstra syndrome protein EHMT1 mediates homeostatic synaptic scaling. Neuron 91, 341-355. 10.1016/j.neuron.2016.06.003 [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E., Levin R., Shah H., Mathur S., Darnell J. C. and Ouyang B. (2015). Cholesterol levels in fragile X syndrome. Am. J. Med. Genet. A 167a, 379-384. 10.1002/ajmg.a.36850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof J., Bjorklund M., Furger E., Schertel C., Taipale J. and Basler K. (2013). A versatile platform for creating a comprehensive UAS-ORFeome library in Drosophila. Development 140, 2434-2442. 10.1242/dev.088757 [DOI] [PubMed] [Google Scholar]
- Blanchet P., Bebin M., Bruet S., Cooper G. M., Thompson M. L., Duban-Bedu B., Gerard B., Piton A., Suckno S., Deshpande C. et al. (2017). MYT1L mutations cause intellectual disability and variable obesity by dysregulating gene expression and development of the neuroendocrine hypothalamus. PLoS Genet. 13, e1006957 10.1371/journal.pgen.1006957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc F. V. and Tully T. (2009). Fruit flies and intellectual disability. Fly (Austin) 3, 91-104. 10.4161/fly.3.1.7812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc F. V., Bell K., Cox H., Broadie K. S. and Tully T. (2008). Excess protein synthesis in Drosophila fragile X mutants impairs long-term memory. Nat. Neurosci. 11, 1143-1145. 10.1038/nn.2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc F. V., Valente D., Nguyen A. T., Mitra P. P. and Tully T. (2010). An assay for social interaction in Drosophila fragile X mutants. Fly (Austin) 4, 216-225. 10.4161/fly.4.3.12280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke J., de Klerk N., Smith T. and Leonard H. (2016). Population-based prevalence of intellectual disability and autism spectrum disorders in Western Australia: a comparison with previous estimates. Medicine (Baltimore) 95, e3737 10.1097/MD.0000000000003737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braat S. and Kooy R. F. (2015). Insights into GABAAergic system deficits in fragile X syndrome lead to clinical trials. Neuropharmacology 88, 48-54. 10.1016/j.neuropharm.2014.06.028 [DOI] [PubMed] [Google Scholar]
- Brem A.-K., Ran K. and Pascual-Leone A. (2013). Learning and memory. Handb. Clin. Neurol. 116, 693-737. 10.1016/B978-0-444-53497-2.00055-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brembs B. (2009). Mushroom bodies regulate habit formation in Drosophila. Curr. Biol. 19, 1351-1355. 10.1016/j.cub.2009.06.014 [DOI] [PubMed] [Google Scholar]
- Brenman-Suttner D. B., Long S. Q., Kamesan V., de Belle J. N., Yost R. T., Kanippayoor R. L. and Simon A. F. (2018). Progeny of old parents have increased social space in Drosophila melanogaster. Sci. Rep. 8, 3673 10.1038/s41598-018-21731-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buiting K., Williams C. and Horsthemke B. (2016). Angelman syndrome - insights into a rare neurogenetic disorder. Nat. Rev. Neurol. 12, 584-593. 10.1038/nrneurol.2016.133 [DOI] [PubMed] [Google Scholar]
- Busto G. U., Cervantes-Sandoval I. and Davis R. L. (2010). Olfactory learning in Drosophila. Physiology (Bethesda) 25, 338-346. 10.1152/physiol.00026.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers D., Davis R. L. and Kiger J. A. Jr. (1981). Defect in cyclic AMP phosphodiesterase due to the dunce mutation of learning in Drosophila melanogaster. Nature 289, 79-81. 10.1038/289079a0 [DOI] [PubMed] [Google Scholar]
- Campbell R. A. A. and Turner G. C. (2010). The mushroom body. Curr. Biol. 20, R11-R12. 10.1016/j.cub.2009.10.031 [DOI] [PubMed] [Google Scholar]
- Carter Leno V., Chandler S., White P., Yorke I., Charman T., Pickles A. and Simonoff E. (2018). Alterations in electrophysiological indices of perceptual processing and discrimination are associated with co-occurring emotional and behavioural problems in adolescents with autism spectrum disorder. Mol Autism 9, 50 10.1186/s13229-018-0236-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castells-Nobau A., Nijhof B., Eidhof I., Wolf L., Scheffer-de Gooyert J. M., Monedero I., Torroja L., van der Laak J. A. W. M. and Schenck A. (2017). Two algorithms for high-throughput and multi-parametric quantification of Drosophila neuromuscular junction morphology. J. Vis. Exp. 123, e55395 10.3791/55395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castells-Nobau A., Eidhof I., Fenckova M., Brenman-Suttner D. B., Scheffer-de Gooyert J. M., Christine S., Schellevis R. L., van der Laan K., Quentin C., van Ninhuijs L. et al. (2019). Conserved regulation of neurodevelopmental processes and behavior by FoxP in Drosophila. PLoS ONE 14, e0211652 10.1371/journal.pone.0211652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V., Pinsker H., Kupfermann I. and Kandel E. R. (1970). Neuronal mechanisms of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science 167, 1745-1748. 10.1126/science.167.3926.1745 [DOI] [PubMed] [Google Scholar]
- Chambers D. B., Androschuk A., Rosenfelt C., Langer S., Harding M. and Bolduc F. V. (2015). Insulin signaling is acutely required for long-term memory in Drosophila. Front. Neural Circuits 9, 8 10.3389/fncir.2015.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Bray S. M., Li Z., Zarnescu D. C., He C., Jin P. and Warren S. T. (2008). Identification of small molecules rescuing fragile X syndrome phenotypes in Drosophila. Nat. Chem. Biol. 4, 256-263. 10.1038/nchembio.78 [DOI] [PubMed] [Google Scholar]
- Chao H.-T., Davids M., Burke E., Pappas J. G., Rosenfeld J. A., McCarty A. J., Davis T., Wolfe L., Toro C., Tifft C. et al. (2017a). A syndromic neurodevelopmental disorder caused by de novo variants in EBF3. Am. J. Hum. Genet. 100, 128-137. 10.1016/j.ajhg.2016.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao H.-T., Liu L. and Bellen H. J. (2017b). Building dialogues between clinical and biomedical research through cross-species collaborations. Semin. Cell Dev. Biol. 70, 49-57. 10.1016/j.semcdb.2017.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaste P., Roeder K. and Devlin B. (2017). The Yin and Yang of autism genetics: how rare de novo and common variations affect liability. Annu. Rev. Genomics Hum. Genet. 18, 167-187. 10.1146/annurev-genom-083115-022647 [DOI] [PubMed] [Google Scholar]
- Chen L. Y., Jiang M., Zhang B., Gokce O. and Südhof T. C. (2017). Conditional deletion of all neurexins defines diversity of essential synaptic organizer functions for neurexins. Neuron 94, 611-625.e4. 10.1016/j.neuron.2017.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chubak M. C., Nixon K. C. J., Stone M. H., Raun N., Rice S. L., Sarikahya M., Jones S. G., Lyons T. A., Jakub T. E., Mainland R. L. M. et al. (2019). Individual components of the SWI/SNF chromatin remodelling complex have distinct roles in memory neurons of the Drosophila mushroom body. Dis. Model. Mech. 12, dmm037325 10.1242/dmm.037325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford H., Dulneva A., Ponting C. P., Haerty W. and Becker E. B. E. (2019). A gene expression signature in developing Purkinje cells predicts autism and intellectual disability co-morbidity status. Sci. Rep. 9, 485 10.1038/s41598-018-37284-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo J. and Mitchell D. W. (2009). Infant visual habituation. Neurobiol. Learn. Mem. 92, 225-234. 10.1016/j.nlm.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthals K., Heukamp A. S., Kossen R., Grosshennig I., Hahn N., Gras H., Göpfert M. C., Heinrich R. and Geurten B. R. H. (2017). Neuroligins Nlg2 and Nlg4 affect social behavior in Drosophila melanogaster. Front. Psychiatry 8, 113 10.3389/fpsyt.2017.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corty M. M., Matthews B. J. and Grueber W. B. (2009). Molecules and mechanisms of dendrite development in Drosophila. Development 136, 1049-1061. 10.1242/dev.014423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankert H., Wang L., Hoopfer E. D., Anderson D. J. and Perona P. (2009). Automated monitoring and analysis of social behavior in Drosophila. Nat. Methods 6, 297-303. 10.1038/nmeth.1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Sadanandappa M. K., Dervan A., Larkin A., Lee J. A., Sudhakaran I. P., Priya R., Heidari R., Holohan E. E., Pimentel A. et al. (2011). Plasticity of local GABAergic interneurons drives olfactory habituation. Proc. Natl. Acad. Sci. USA 108, E646-E654. 10.1073/pnas.1106411108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta S., Ferreira C. H. and Miesenbock G. (2014). FoxP influences the speed and accuracy of a perceptual decision in Drosophila. Science 344, 901-904. 10.1126/science.1252114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Belle J. S. and Heisenberg M. (1994). Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science 263, 692-695. 10.1126/science.8303280 [DOI] [PubMed] [Google Scholar]
- de Brouwer A. P. M., Abou Jamra R., Körtel N., Soyris C., Polla D. L., Safra M., Zisso A., Powell C. A., Rebelo-Guiomar P., Dinges N. et al. (2018). Variants in PUS7 cause intellectual disability with speech delay, microcephaly, short stature, and aggressive behavior. Am. J. Hum. Genet. 103, 1045-1052. 10.1016/j.ajhg.2018.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rubeis S., Fernández E., Buzzi A., Di Marino D. and Bagni C. (2012). Molecular and cellular aspects of mental retardation in the Fragile X syndrome: from gene mutation/s to spine dysmorphogenesis. Adv. Exp. Med. Biol. 970, 517-551. 10.1007/978-3-7091-0932-8_23 [DOI] [PubMed] [Google Scholar]
- de Vries B. B., Fryns J. P., Butler M. G., Canziani F., Wesby-van Swaay E., van Hemel J. O., Oostra B. A., Halley D. J. and Niermeijer M. F. (1993). Clinical and molecular studies in fragile X patients with a Prader-Willi-like phenotype. J. Med. Genet. 30, 761-766. 10.1136/jmg.30.9.761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries B. B. A., van den Ouweland A. M. W., Mohkamsing S., Duivenvoorden H. J., Mol E., Gelsema K., van Rijn M., Halley D. J. J., Sandkuijl L. A., Oostra B. A. et al. (1997). Screening and diagnosis for the fragile X syndrome among the mentally retarded: an epidemiological and psychological survey. Collaborative Fragile X Study Group. Am. J. Hum. Genet. 61, 660-667. 10.1086/515496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Scholl F. G., Choih J., DeMaria S., Berger J., Isacoff E. and Scheiffele P. (2003). Neurexin mediates the assembly of presynaptic terminals. Nat. Neurosci. 6, 708-716. 10.1038/nn1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deciphering Developmental Disorders Study. (2017). Prevalence and architecture of de novo mutations in developmental disorders. Nature 542, 433-438. 10.1038/nature21062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detterman D. K. (1987). Theoretical notions of intelligence and mental retardation. Am. J. Ment. Defic. 92, 2-11. [PubMed] [Google Scholar]
- Di Resta C., Galbiati S., Carrera P. and Ferrari M. (2018). Next-generation sequencing approach for the diagnosis of human diseases: open challenges and new opportunities. Ejifcc 29, 4-14. [PMC free article] [PubMed] [Google Scholar]
- Diao F., Ironfield H., Luan H., Diao F., Shropshire W. C., Ewer J., Marr E., Potter C. J., Landgraf M. and White B. H. (2015). Plug-and-play genetic access to drosophila cell types using exchangeable exon cassettes. Cell Rep. 10, 1410-1421. 10.1016/j.celrep.2015.01.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K.-C., Barinova Y., Fellner M., Gasser B., Kinsey K., Oppel S., Scheiblauer S. et al. (2007). A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151-156. 10.1038/nature05954 [DOI] [PubMed] [Google Scholar]
- Dinstein I., Heeger D. J., Lorenzi L., Minshew N. J., Malach R. and Behrmann M. (2012). Unreliable evoked responses in autism. Neuron 75, 981-991. 10.1016/j.neuron.2012.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockendorff T. C., Su H. S., McBride S. M. J., Yang Z., Choi C. H., Siwicki K. K., Sehgal A. and Jongens T. A. (2002). Drosophila lacking dfmr1 activity show defects in circadian output and fail to maintain courtship interest. Neuron 34, 973-984. 10.1016/S0896-6273(02)00724-9 [DOI] [PubMed] [Google Scholar]
- Donlea J. M., Thimgan M. S., Suzuki Y., Gottschalk L. and Shaw P. J. (2011). Inducing sleep by remote control facilitates memory consolidation in Drosophila. Science 332, 1571-1576. 10.1126/science.1202249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlea J. M., Pimentel D., Talbot C. B., Kempf A., Omoto J. J., Hartenstein V. and Miesenböck G. (2018). Recurrent circuitry for balancing sleep need and sleep. Neuron 97, 378-389.e4. 10.1016/j.neuron.2017.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou J.-T., Chen M., Dufour F., Alkon D. L. and Zhao W. Q. (2005). Insulin receptor signaling in long-term memory consolidation following spatial learning. Learn. Mem. 12, 646-655. 10.1101/lm.88005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drozd M., Bardoni B. and Capovilla M. (2018). Modeling fragile X syndrome in Drosophila. Front. Mol. Neurosci. 11, 124 10.3389/fnmol.2018.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau J., Chiang A.-S., Grady L., Barditch J., Gossweiler S., McNeil J., Smith P., Buldoc F., Scott R., Certa U. et al. (2003). The staufen/pumilio pathway is involved in Drosophila long-term memory. Curr. Biol. 13, 286-296. 10.1016/S0960-9822(03)00064-2 [DOI] [PubMed] [Google Scholar]
- Dubowy C. and Sehgal A. (2017). Circadian rhythms and sleep in Drosophila melanogaster. Genetics 205, 1373-1397. 10.1534/genetics.115.185157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubruille R. and Emery P. (2008). A plastic clock: how circadian rhythms respond to environmental cues in Drosophila. Mol. Neurobiol. 38, 129-145. 10.1007/s12035-008-8035-y [DOI] [PubMed] [Google Scholar]
- Dudai Y., Jan Y. N., Byers D., Quinn W. G. and Benzer S. (1976). dunce, a mutant of Drosophila deficient in learning. Proc. Natl. Acad. Sci. USA 73, 1684-1688. 10.1073/pnas.73.5.1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley A. J., Tyrer F., Gray L. J., Bhaumik S., Spong R., Chudasama Y., Cooper S.-A., Ganghadaran S., Davies M. and Khunti K. (2017). Type 2 diabetes and glucose intolerance in a population with intellectual disabilities: the STOP diabetes cross-sectional screening study. J. Intellect. Disabil. Res. 61, 668-681. 10.1111/jir.12380 [DOI] [PubMed] [Google Scholar]
- Duy P. Q. and Budimirovic D. B. (2017). Fragile X syndrome: lessons learned from the most translated neurodevelopmental disorder in clinical trials. Transl. Neurosci. 8, 7-8. 10.1515/tnsci-2017-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dy A. B. C., Tassone F., Eldeeb M., Salcedo-Arellano M. J., Tartaglia N. and Hagerman R. (2018). Metformin as targeted treatment in fragile X syndrome. Clin. Genet. 93, 216-222. 10.1111/cge.13039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery P. and Reppert S. M. (2004). A rhythmic Ror. Neuron 43, 443-446. 10.1016/j.neuron.2004.08.009 [DOI] [PubMed] [Google Scholar]
- Ewbank M. P., Rhodes G., von dem Hagen E. A. H., Powell T. E., Bright N., Stoyanova R. S., Baron-Cohen S. and Calder A. J. (2015). Repetition suppression in ventral visual cortex is diminished as a function of increasing autistic traits. Cereb. Cortex 25, 3381-3393. 10.1093/cercor/bhu149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris S. M. (2011). Are mushroom bodies cerebellum-like structures? Arthropod. Struct. Dev. 40, 368-379. 10.1016/j.asd.2011.02.004 [DOI] [PubMed] [Google Scholar]
- Fattahi Z., Sheikh T. I., Musante L., Rasheed M., Taskiran I. I., Harripaul R., Hu H., Kazeminasab S., Alam M. R., Hosseini M. et al. (2018). Biallelic missense variants in ZBTB11 can cause intellectual disability in humans. Hum. Mol. Genet. 27, 3177-3188. 10.1093/hmg/ddy220 [DOI] [PubMed] [Google Scholar]
- Faville R., Kottler B., Goodhill G. J., Shaw P. J. and van Swinderen B. (2015). How deeply does your mutant sleep? Probing arousal to better understand sleep defects in Drosophila. Sci. Rep. 5, 8454 10.1038/srep08454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenckova M., Blok L. E. R, Asztalos L., Goodman D. P., Cizek P., Singgih E. L., Glennon J. C., IntHout J., Zweier C., Eichler E. E. et al. (2019). A hundred genes implicated in intellectual disability and autism regulate habituation learning and reveal an opposing role for Ras-MAPK signaling in inhibitory and excitatory neurons. BioRxiv. 10.1101/285981 [DOI] [Google Scholar]
- Frank C. A. (2014). Homeostatic plasticity at the Drosophila neuromuscular junction. Neuropharmacology 78, 63-74. 10.1016/j.neuropharm.2013.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F.-B., Brenman J. E., Jan L. Y. and Jan Y. N. (1999). Genes regulating dendritic outgrowth, branching, and routing in Drosophila. Genes Dev. 13, 2549-2561. 10.1101/gad.13.19.2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe D. S., Bollinger W. L., Vigderman A., Masek P., Gertowski J., Sehgal A. and Keene A. C. (2015). Context-specific comparison of sleep acquisition systems in Drosophila. Biol. Open 4, 1558-1568. 10.1242/bio.013011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto C. L., Pereira D. and Broadie K. (2014). GABAergic circuit dysfunction in the Drosophila Fragile X syndrome model. Neurobiol. Dis. 65, 142-159. 10.1016/j.nbd.2014.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann Q., Garcia Rodriguez L., Beckwith E. J., French A. S., Jamasb A. R. and Gilestro G. F. (2017). Ethoscopes: an open platform for high-throughput ethomics. PLoS Biol. 15, e2003026 10.1371/journal.pbio.2003026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffray M.-M., Nicolas A., Speranza M. and Georgieff N. (2016). Are circadian rhythms new pathways to understand Autism Spectrum Disorder? J. Physiol. Paris 110, 434-438. 10.1016/j.jphysparis.2017.06.002 [DOI] [PubMed] [Google Scholar]
- Glanzman D. L. (2011). Olfactory habituation: fresh insights from flies. Proc. Natl. Acad. Sci. USA 108, 14711-14712. 10.1073/pnas.1111230108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves S., Patat J., Guida M. C., Lachaussée N., Arrondel C., Helmstädter M., Boyer O., Gribouval O., Gubler M.-C., Mollet G. et al. (2018). A homozygous KAT2B variant modulates the clinical phenotype of ADD3 deficiency in humans and flies. PLoS Genet. 14, e1007386 10.1371/journal.pgen.1007386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramates L. S., Marygold S. J., Santos G. D., Urbano J.-M., Antonazzo G., Matthews B. B., Rey A. J., Tabone C. J., Crosby M. A., Emmert D. B. et al. (2017). FlyBase at 25: looking to the future. Nucleic Acids Res. 45, D663-D671. 10.1093/nar/gkw1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan R. J., Tononi G., Cirelli C. and Shaw P. J. (2001). Sleep and the fruit fly. Trends Neurosci. 24, 142-145. 10.1016/S0166-2236(00)01719-7 [DOI] [PubMed] [Google Scholar]
- Greer P. L., Hanayama R., Bloodgood B. L., Mardinly A. R., Lipton D. M., Flavell S. W., Kim T. K., Griffith E. C., Waldon Z., Maehr R. et al. (2010). The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell 140, 704-716. 10.1016/j.cell.2010.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice S. J., Liu J. L. and Webber C. (2015). Synergistic interactions between Drosophila orthologues of genes spanned by de novo human CNVs support multiple-hit models of autism. PLoS Genet. 11, e1004998 10.1371/journal.pgen.1004998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesi-Oliveira K., Acab A., Gupta A. R., Sunaga D. Y., Chailangkarn T., Nicol X., Nunez Y., Walker M. F., Murdoch J. D., Sanders S. J. et al. (2015). Modeling non-syndromic autism and the impact of TRPC6 disruption in human neurons. Mol. Psychiatry 20, 1350-1365. 10.1038/mp.2014.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groschner L. N., Chan Wah Hak L., Bogacz R., DasGupta S. and Miesenböck G. (2018). Dendritic integration of sensory evidence in perceptual decision-making. Cell 173, 894-905.e13. 10.1016/j.cell.2018.03.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimera J., Casas C., Pucharcos C., Solans A., Domenech A., Planas A. M., Ashley J., Lovett M., Estivill X. and Pritchard M. A. (1996). A human homologue of Drosophila minibrain (MNB) is expressed in the neuronal regions affected in Down syndrome and maps to the critical region. Hum. Mol. Genet. 5, 1305-1310. 10.1093/hmg/5.9.1305 [DOI] [PubMed] [Google Scholar]
- Guo H. F., The I., Hannan F., Bernards A. and Zhong Y. (1997). Requirement of Drosophila NF1 for activation of adenylyl cyclase by PACAP38-like neuropeptides. Science 276, 795-798. 10.1126/science.276.5313.795 [DOI] [PubMed] [Google Scholar]
- Guo H.-F., Tong J., Hannan F., Luo L. and Zhong Y. (2000). A neurofibromatosis-1-regulated pathway is required for learning in Drosophila. Nature 403, 895-898. 10.1038/35002593 [DOI] [PubMed] [Google Scholar]
- Guy J., Gan J., Selfridge J., Cobb S. and Bird A. (2007). Reversal of neurological defects in a mouse model of Rett syndrome. Science 315, 1143-1147. 10.1126/science.1138389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn N., Geurten B., Gurvich A., Piepenbrock D., Kastner A., Zanini D., Xing G., Xie W., Göpfert M. C., Ehrenreich H. et al. (2013). Monogenic heritable autism gene neuroligin impacts Drosophila social behaviour. Behav. Brain Res. 252, 450-457. 10.1016/j.bbr.2013.06.020 [DOI] [PubMed] [Google Scholar]
- Han T. H., Dharkar P., Mayer M. L. and Serpe M. (2015). Functional reconstitution of Drosophila melanogaster NMJ glutamate receptors. Proc. Natl. Acad. Sci. USA 112, 6182-6187. 10.1073/pnas.1500458112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K. P. and Littleton J. T. (2015). Transmission, development, and plasticity of synapses. Genetics 201, 345-375. 10.1534/genetics.115.176529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K. P., Akbergenova Y., Cho R. W., Baas-Thomas M. S. and Littleton J. T. (2016). Shank modulates postsynaptic Wnt signaling to regulate synaptic development. J. Neurosci. 36, 5820-5832. 10.1523/JNEUROSCI.4279-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison V., Connell L., Hayesmoore J., McParland J., Pike M. G. and Blair E. (2011). Compound heterozygous deletion of NRXN1 causing severe developmental delay with early onset epilepsy in two sisters. Am. J. Med. Genet. A 155a, 2826-2831. 10.1002/ajmg.a.34255 [DOI] [PubMed] [Google Scholar]
- Heisenberg M., Borst A., Wagner S. and Byers D. (1985). Drosophila mushroom body mutants are deficient in olfactory learning. J. Neurogenet. 2, 1-30. 10.3109/01677068509100140 [DOI] [PubMed] [Google Scholar]
- Hirano Y., Masuda T., Naganos S., Matsuno M., Ueno K., Miyashita T., Horiuchi J. and Saitoe M. (2013). Fasting launches CRTC to facilitate long-term memory formation in Drosophila. Science 339, 443-446. 10.1126/science.1227170 [DOI] [PubMed] [Google Scholar]
- Hmeljak J. and Justice M. J. (2019). From gene to treatment: supporting rare disease translational research through model systems. Dis. Model. Mech. 12, dmm039271 10.1242/dmm.039271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S.-T., Bang S., Hyun S., Kang J., Jeong K., Paik D., Chung J. and Kim J. (2008). cAMP signalling in mushroom bodies modulates temperature preference behaviour in Drosophila. Nature 454, 771-775. 10.1038/nature07090 [DOI] [PubMed] [Google Scholar]
- Hsieh K., Rimmer J. H. and Heller T. (2014). Obesity and associated factors in adults with intellectual disability. J. Intellect. Disabil. Res. 58, 851-863. 10.1111/jir.12100 [DOI] [PubMed] [Google Scholar]
- Iyer J., Singh M. D., Jensen M., Patel P., Pizzo L., Huber E., Koerselman H., Weiner A. T., Lepanto P., Vadodaria K. et al. (2018). Pervasive genetic interactions modulate neurodevelopmental defects of the autism-associated 16p11.2 deletion in Drosophila melanogaster. Nat. Commun. 9, 2548 10.1038/s41467-018-04882-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan Y.-N. and Jan L. Y. (2010). Branching out: mechanisms of dendritic arborization. Nat. Rev. Neurosci. 11, 316-328. 10.1038/nrn2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janecka M., Mill J., Basson M. A., Goriely A., Spiers H., Reichenberg A., Schalkwyk L. and Fernandes C. (2017). Advanced paternal age effects in neurodevelopmental disorders-review of potential underlying mechanisms. Transl Psychiatry 7, e1019 10.1038/tp.2016.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner W. J., Crocker A., White B. H. and Sehgal A. (2006). Sleep in Drosophila is regulated by adult mushroom bodies. Nature 441, 757-760. 10.1038/nature04811 [DOI] [PubMed] [Google Scholar]
- Kabra M., Robie A. A., Rivera-Alba M., Branson S. and Branson K. (2013). JAABA: interactive machine learning for automatic annotation of animal behavior. Nat. Methods 10, 64-67. 10.1038/nmeth.2281 [DOI] [PubMed] [Google Scholar]
- Kahsai L. and Zars T. (2011). Learning and memory in Drosophila: behavior, genetics, and neural systems. Int. Rev. Neurobiol. 99, 139-167. 10.1016/B978-0-12-387003-2.00006-9 [DOI] [PubMed] [Google Scholar]
- Kaufmann W. E. and Moser H. W. (2000). Dendritic anomalies in disorders associated with mental retardation. Cereb. Cortex 10, 981-991. 10.1093/cercor/10.10.981 [DOI] [PubMed] [Google Scholar]
- Kavšek M. (2004). Predicting later IQ from infant visual habituation and dishabituation: A meta-analysis. J Appl Dev Psychol. 25, 369-393. 10.1016/j.appdev.2004.04.006 [DOI] [Google Scholar]
- Kepa A., Martinez Medina L., Erk S., Srivastava D. P., Fernandes A., Toro R., Levi S., Ruggeri B., Fernandes C., Degenhardt F. et al. (2017). Associations of the intellectual disability gene MYT1L with helix-loop-helix gene expression, hippocampus volume and hippocampus activation during memory retrieval. Neuropsychopharmacology 42, 2516-2526. 10.1038/npp.2017.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleefstra T., de Leeuw N., Wolf R., Nillesen W. M., Schobers G., Mieloo H., Willemsen M., Perrotta C. S., Poddighe P. J., Feenstra I. et al. (2010). Phenotypic spectrum of 20 novel patients with molecularly defined supernumerary marker chromosomes 15 and a review of the literature. Am. J. Med. Genet. A 152a, 2221-2229. 10.1002/ajmg.a.33529 [DOI] [PubMed] [Google Scholar]
- Kleefstra T., Kramer J. M., Neveling K., Willemsen M. H., Koemans T. S., Vissers L. E. L. M., Wissink-Lindhout W., Fenckova M., van den Akker W. M. R., Kasri N. N. et al. (2012). Disruption of an EHMT1-associated chromatin-modification module causes intellectual disability. Am. J. Hum. Genet. 91, 73-82. 10.1016/j.ajhg.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans N. M., Johnson L. C., Richards T., Mahurin R., Greenson J., Dawson G. and Aylward E. (2009). Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. Am. J. Psychiatry 166, 467-475. 10.1176/appi.ajp.2008.07101681 [DOI] [PubMed] [Google Scholar]
- Knoth I. S., Lajnef T., Rigoulot S., Lacourse K., Vannasing P., Michaud J. L., Jacquemont S., Major P., Jerbi K. and Lippé S. (2018). Auditory repetition suppression alterations in relation to cognitive functioning in fragile X syndrome: a combined EEG and machine learning approach. J. Neurodev. Disord. 10, 4 10.1186/s11689-018-9223-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochinke K., Zweier C., Nijhof B., Fenckova M., Cizek P., Honti F., Keerthikumar S., Oortveld M. A. W., Kleefstra T., Kramer J. M. et al. (2016). Systematic phenomics analysis deconvolutes genes mutated in intellectual disability into biologically coherent modules. Am. J. Hum. Genet. 98, 149-164. 10.1016/j.ajhg.2015.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koemans T. S., Kleefstra T., Chubak M. C., Stone M. H., Reijnders M. R. F., de Munnik S., Willemsen M. H., Fenckova M., Stumpel C. T. R. M., Bok L. A. et al. (2017a). Functional convergence of histone methyltransferases EHMT1 and KMT2C involved in intellectual disability and autism spectrum disorder. PLoS Genet. 13, e1006864 10.1371/journal.pgen.1006864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koemans T. S., Oppitz C., Donders R. A. T., van Bokhoven H., Schenck A., Keleman K. and Kramer J. M. (2017b). Drosophila courtship conditioning as a measure of learning and memory. J. Vis. Exp. 124, e55808 10.3791/55808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krab L. C., Goorden S. M. I. and Elgersma Y. (2008). Oncogenes on my mind: ERK and MTOR signaling in cognitive diseases. Trends Genet. 24, 498-510. 10.1016/j.tig.2008.07.005 [DOI] [PubMed] [Google Scholar]
- Kramer J. M., Kochinke K., Oortveld M. A. W., Marks H., Kramer D., de Jong E. K., Asztalos Z., Westwood J. T., Stunnenberg H. G., Sokolowski M. B. et al. (2011). Epigenetic regulation of learning and memory by Drosophila EHMT/G9a. PLoS Biol. 9, e1000569 10.1371/journal.pbio.1000569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger D. D., Osterweil E. K., Chen S. P., Tye L. D. and Bear M. F. (2011). Cognitive dysfunction and prefrontal synaptic abnormalities in a mouse model of fragile X syndrome. Proc. Natl. Acad. Sci. USA 108, 2587-2592. 10.1073/pnas.1013855108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnle S., Mothes B., Matentzoglu K. and Scheffner M. (2013). Role of the ubiquitin ligase E6AP/UBE3A in controlling levels of the synaptic protein Arc. Proc. Natl. Acad. Sci. USA 110, 8888-8893. 10.1073/pnas.1302792110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni V. A. and Firestein B. L. (2012). The dendritic tree and brain disorders. Mol. Cell. Neurosci. 50, 10-20. 10.1016/j.mcn.2012.03.005 [DOI] [PubMed] [Google Scholar]
- Kuntz S., Poeck B., Sokolowski M. B. and Strauss R. (2012). The visual orientation memory of Drosophila requires Foraging (PKG) upstream of Ignorant (RSK2) in ring neurons of the central complex. Learn. Mem. 19, 337-340. 10.1101/lm.026369.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan V., Unda B. K. and Singh K. K. (2016). Wnt signaling networks in autism spectrum disorder and intellectual disability. J. Neurodev. Disord. 8, 45 10.1186/s11689-016-9176-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagali P. S., Corcoran C. P. and Picketts D. J. (2010). Hippocampus development and function: role of epigenetic factors and implications for cognitive disease. Clin. Genet. 78, 321-333. 10.1111/j.1399-0004.2010.01503.x [DOI] [PubMed] [Google Scholar]
- Lanore F., Labrousse V. F., Szabo Z., Normand E., Blanchet C. and Mulle C. (2012). Deficits in morphofunctional maturation of hippocampal mossy fiber synapses in a mouse model of intellectual disability. J. Neurosci. 32, 17882-17893. 10.1523/JNEUROSCI.2049-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin A., Karak S., Priya R., Das A., Ayyub C., Ito K., Rodrigues V. and Ramaswami M. (2010). Central synaptic mechanisms underlie short-term olfactory habituation in Drosophila larvae. Learn. Mem. 17, 645-653. 10.1101/lm.1839010 [DOI] [PubMed] [Google Scholar]
- Larkin A., Chen M.-Y., Kirszenblat L., Reinhard J., van Swinderen B. and Claudianos C. (2015). Neurexin-1 regulates sleep and synaptic plasticity in Drosophila melanogaster. Eur. J. Neurosci. 42, 2455-2466. 10.1111/ejn.13023 [DOI] [PubMed] [Google Scholar]
- Lee Y.-S., Ehninger D., Zhou M., Oh J.-Y., Kang M., Kwak C., Ryu H.-H., Butz D., Araki T., Cai Y. et al. (2014). Mechanism and treatment for learning and memory deficits in mouse models of Noonan syndrome. Nat. Neurosci. 17, 1736-1743. 10.1038/nn.3863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P.-T., Zirin J., Kanca O., Lin W.-W., Schulze K. L., Li-Kroeger D., Tao R., Devereaux C., Hu Y., Chung V. et al. (2018). A gene-specific T2A-GAL4 library for Drosophila. eLife 7, e35574 10.7554/eLife.35574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiner H. C., Leiner A. L. and Dow R. S. (1993). Cognitive and language functions of the human cerebellum. Trends Neurosci. 16, 444-447. 10.1016/0166-2236(93)90072-T [DOI] [PubMed] [Google Scholar]
- Li Y., Zhou Z., Zhang X., Tong H., Li P., Zhang Z. C., Jia Z., Xie W. and Han J. (2013). Drosophila neuroligin 4 regulates sleep through modulating GABA transmission. J. Neurosci. 33, 15545-15554. 10.1523/JNEUROSCI.0819-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P.-Y., Lin L.-P. and Lin J.-D. (2010). Hypertension, hyperglycemia, and hyperlipemia among adolescents with intellectual disabilities. Res. Dev. Disabil. 31, 545-550. 10.1016/j.ridd.2009.12.002 [DOI] [PubMed] [Google Scholar]
- Lin C.-Y., Chuang C.-C., Hua T.-E., Chen C.-C., Dickson B. J., Greenspan R. J. and Chiang A.-S. (2013). A comprehensive wiring diagram of the protocerebral bridge for visual information processing in the Drosophila brain. Cell Rep. 3, 1739-1753. 10.1016/j.celrep.2013.04.022 [DOI] [PubMed] [Google Scholar]
- Liu L., Wolf R., Ernst R. and Heisenberg M. (1999). Context generalization in Drosophila visual learning requires the mushroom bodies. Nature 400, 753-756. 10.1038/23456 [DOI] [PubMed] [Google Scholar]
- Liu G., Seiler H., Wen A., Zars T., Ito K., Wolf R., Heisenberg M. and Liu L. (2006). Distinct memory traces for two visual features in the Drosophila brain. Nature 439, 551-556. 10.1038/nature04381 [DOI] [PubMed] [Google Scholar]
- Liu C., Plaçais P.-Y., Yamagata N., Pfeiffer B. D., Aso Y., Friedrich A. B., Siwanowicz I., Rubin G. M., Preat T. and Tanimoto H. (2012). A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature 488, 512-516. 10.1038/nature11304 [DOI] [PubMed] [Google Scholar]
- Liu G., Nath T., Linneweber G. A., Claeys A., Guo Z., Li J., Bengochea M., De Backer S., Weyn B., Sneyders M. et al. (2018). A simple computer vision pipeline reveals the effects of isolation on social interaction dynamics in Drosophila. PLoS Comput. Biol. 14, e1006410 10.1371/journal.pcbi.1006410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M. S., Sziber P. P. and Quinn W. G. (1984). Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga, a Drosophila learning mutant. Cell 37, 205-215. 10.1016/0092-8674(84)90316-7 [DOI] [PubMed] [Google Scholar]
- Lozano R., Hare E. B. and Hagerman R. J. (2014). Modulation of the GABAergic pathway for the treatment of fragile X syndrome. Neuropsychiatr. Dis. Treat. 10, 1769-1779. 10.2147/ndt.s42919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaskant M., van de Wouw E., van Wijck R., Evenhuis H. M. and Echteld M. A. (2013). Circadian sleep-wake rhythm of older adults with intellectual disabilities. Res. Dev. Disabil. 34, 1144-1151. 10.1016/j.ridd.2012.12.009 [DOI] [PubMed] [Google Scholar]
- Macleod G. T. (2012). Calcium imaging at the Drosophila larval neuromuscular junction. Cold Spring Harb. Protoc. 2012, 758-766. 10.1101/pdb.top070078 [DOI] [PubMed] [Google Scholar]
- Mariani J., Coppola G., Zhang P., Abyzov A., Provini L., Tomasini L., Amenduni M., Szekely A., Palejev D., Wilson M. et al. (2015). FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell 162, 375-390. 10.1016/j.cell.2015.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariappa D., Ferenbach A. T. and van Aalten D. M. F. (2018). Effects of hypo-O-GlcNAcylation on Drosophila development. J. Biol. Chem. 293, 7209-7221. 10.1074/jbc.RA118.002580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens M. B., Frega M., Classen J., Epping L., Bijvank E., Benevento M., van Bokhoven H., Tiesinga P., Schubert D. and Nadif Kasri N. (2016). Euchromatin histone methyltransferase 1 regulates cortical neuronal network development. Sci. Rep. 6, 35756 10.1038/srep35756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masek P. and Scott K. (2010). Limited taste discrimination in Drosophila. Proc. Natl. Acad. Sci. USA 107, 14833-14838. 10.1073/pnas.1009318107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride S. M. J., Giuliani G., Choi C., Krause P., Correale D., Watson K., Baker G. and Siwicki K. K. (1999). Mushroom body ablation impairs short-term memory and long-term memory of courtship conditioning in Drosophila melanogaster. Neuron 24, 967-977. 10.1016/S0896-6273(00)81043-0 [DOI] [PubMed] [Google Scholar]
- McBride S. M. J., Choi C. H., Wang Y., Liebelt D., Braunstein E., Ferreiro D., Sehgal A., Siwicki K. K., Dockendorff T. C., Nguyen H. T. et al. (2005). Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron 45, 753-764. 10.1016/j.neuron.2005.01.038 [DOI] [PubMed] [Google Scholar]
- McBride S. M. J., Holloway S. L. and Jongens T. A. (2013). Using Drosophila as a tool to identify pharmacological therapies for fragile X syndrome. Drug Discov. Today Technol. 10, e129-e136. 10.1016/j.ddtec.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDiarmid T. A., Bernardos A. C. and Rankin C. H. (2017). Habituation is altered in neuropsychiatric disorders-a comprehensive review with recommendations for experimental design and analysis. Neurosci. Biobehav. Rev. 80, 286-305. 10.1016/j.neubiorev.2017.05.028 [DOI] [PubMed] [Google Scholar]
- Menon K. P., Carrillo R. A. and Zinn K. (2013). Development and plasticity of the Drosophila larval neuromuscular junction. Wiley Interdiscip. Rev. Dev. Biol. 2, 647-670. 10.1002/wdev.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkling S. H., Bronkhorst A. W., Kramer J. M., Overheul G. J., Schenck A. and Van Rij R. P. (2015). The epigenetic regulator G9a mediates tolerance to RNA virus infection in Drosophila. PLoS Pathog. 11, e1004692 10.1371/journal.ppat.1004692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millburn G. H., Crosby M. A., Gramates L. S. and Tweedie S. (2016). FlyBase portals to human disease research using Drosophila models. Dis. Model. Mech. 9, 245-252. 10.1242/dmm.023317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. T., Adam M. P., Aradhya S., Biesecker L. G., Brothman A. R., Carter N. P., Church D. M., Crolla J. A., Eichler E. E., Epstein C. J. et al. (2010). Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am. J. Hum. Genet. 86, 749-764. 10.1016/j.ajhg.2010.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missler M., Zhang W., Rohlmann A., Kattenstroth G., Hammer R. E., Gottmann K. and Südhof T. C. (2003). Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature 423, 939-948. 10.1038/nature01755 [DOI] [PubMed] [Google Scholar]
- Møller R. S., Kübart S., Hoeltzenbein M., Heye B., Vogel I., Hansen C. P., Menzel C., Ullmann R., Tommerup N., Ropers H.-H. et al. (2008). Truncation of the Down syndrome candidate gene DYRK1A in two unrelated patients with microcephaly. Am. J. Hum. Genet. 82, 1165-1170. 10.1016/j.ajhg.2008.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyak R. E., Emerson D., Schoenfeld B. P., Zheng X., Chambers D. B., Rosenfelt C., Langer S., Hinchey P., Choi C. H., McDonald T. V. et al. (2017). Insulin signaling misregulation underlies circadian and cognitive deficits in a Drosophila fragile X model. Mol. Psychiatry 22, 1140-1148. 10.1038/mp.2016.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser E. I., Kropff E. and Moser M.-B. (2008). Place cells, grid cells, and the brain's spatial representation system. Annu. Rev. Neurosci. 31, 69-89. 10.1146/annurev.neuro.31.061307.090723 [DOI] [PubMed] [Google Scholar]
- Muller C. L., Anacker A. M. J. and Veenstra-VanderWeele J. (2016). The serotonin system in autism spectrum disorder: From biomarker to animal models. Neuroscience 321, 24-41. 10.1016/j.neuroscience.2015.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungall C. J., McMurry J. A., Köhler S., Balhoff J. P., Borromeo C., Brush M., Carbon S., Conlin T., Dunn N., Engelstad M. et al. (2017). The Monarch Initiative: an integrative data and analytic platform connecting phenotypes to genotypes across species. Nucleic Acids Res. 45, D712-D722. 10.1093/nar/gkw1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K. R., Park J. H., Huber R. and Ja W. W. (2017). Simultaneous measurement of sleep and feeding in individual Drosophila. Nat. Protoc. 12, 2355-2366. 10.1038/nprot.2017.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy M. and Turner G. (2013). Whole-cell in vivo patch-clamp recordings in the Drosophila brain. Cold Spring Harb. Protoc. 2013, 140-148. 10.1101/pdb.prot071704 [DOI] [PubMed] [Google Scholar]
- Najmabadi H., Hu H., Garshasbi M., Zemojtel T., Abedini S. S., Chen W., Hosseini M., Behjati F., Haas S., Jamali P. et al. (2011). Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature 478, 57-63. 10.1038/nature10423 [DOI] [PubMed] [Google Scholar]
- Neuser K., Triphan T., Mronz M., Poeck B. and Strauss R. (2008). Analysis of a spatial orientation memory in Drosophila. Nature 453, 1244-1247. 10.1038/nature07003 [DOI] [PubMed] [Google Scholar]
- Nijhof B., Castells-Nobau A., Wolf L., Scheffer-de Gooyert J. M., Monedero I., Torroja L., Coromina L., van der Laak J. A. W. M. and Schenck A. (2016). A new Fiji-based algorithm that systematically quantifies nine synaptic parameters provides insights into Drosophila NMJ morphometry. PLoS Comput. Biol. 12, e1004823 10.1371/journal.pcbi.1004823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K. C. J., Rousseau J., Stone M. H., Sarikahya M., Ehresmann S., Mizuno S., Matsumoto N., Miyake N., Baralle D., McKee S. et al. (2019). A syndromic neurodevelopmental disorder caused by mutations in SMARCD1, a core SWI/SNF subunit needed for context-dependent neuronal gene regulation in flies. Am. J. Hum. Genet. 104, 596-610. 10.1016/j.ajhg.2019.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeseburg B., Dijkstra G. J., Groothoff J. W., Reijneveld S. A. and Jansen D. E. M. C. (2011). Prevalence of chronic health conditions in children with intellectual disability: a systematic literature review. Intellect. Dev. Disabil. 49, 59-85. 10.1352/1934-9556-49.2.59 [DOI] [PubMed] [Google Scholar]
- Ofstad T. A., Zuker C. S. and Reiser M. B. (2011). Visual place learning in Drosophila melanogaster. Nature 474, 204-207. 10.1038/nature10131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oortveld M. A. W., Keerthikumar S., Oti M., Nijhof B., Fernandes A. C., Kochinke K., Castells-Nobau A., van Engelen E., Ellenkamp T., Eshuis L. et al. (2013). Human intellectual disability genes form conserved functional modules in Drosophila. PLoS Genet. 9, e1003911 10.1371/journal.pgen.1003911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori-McKenney K. M., McKenney R. J., Huang H. H., Li T., Meltzer S., Jan L. Y., Vale R. D., Wiita A. P. and Jan Y. N. (2016). Phosphorylation of beta-tubulin by the Down syndrome kinase, Minibrain/DYRK1a, regulates microtubule dynamics and dendrite morphogenesis. Neuron 90, 551-563. 10.1016/j.neuron.2016.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Roak B. J., Vives L., Fu W., Egertson J. D., Stanaway I. B., Phelps I. G., Carvill G., Kumar A., Lee C., Ankenman K. et al. (2012). Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science 338, 1619-1622. 10.1126/science.1227764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani M. R., Oishi K., Gelb B. D. and Zhong Y. (2009). The phosphatase SHP2 regulates the spacing effect for long-term memory induction. Cell 139, 186-198. 10.1016/j.cell.2009.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjpe P., Rodrigues V., VijayRaghavan K. and Ramaswami M. (2012). Gustatory habituation in Drosophila relies on rutabaga (adenylate cyclase)-dependent plasticity of GABAergic inhibitory neurons. Learn. Mem. 19, 627-635. 10.1101/lm.026641.112 [DOI] [PubMed] [Google Scholar]
- Pardo C. A. and Eberhart C. G. (2007). The neurobiology of autism. Brain Pathol. 17, 434-447. 10.1111/j.1750-3639.2007.00102.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Park J. M., Kim S., Kim J.-A., Shepherd J. D., Smith-Hicks C. L., Chowdhury S., Kaufmann W., Kuhl D., Ryazanov A. G. et al. (2008). Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron 59, 70-83. 10.1016/j.neuron.2008.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastuzyn E. D., Day C. E., Kearns R. B., Kyrke-Smith M., Taibi A. V., McCormick J., Yoder N., Belnap D. M., Erlendsson S., Morado D. R. et al. (2018). The Neuronal Gene Arc Encodes a Repurposed Retrotransposon Gag Protein that Mediates Intercellular RNA Transfer. Cell 172, 275-288.e18. 10.1016/j.cell.2017.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicano E., Rhodes G. and Calder A. J. (2013). Reduced gaze aftereffects are related to difficulties categorising gaze direction in children with autism. Neuropsychologia 51, 1504-1509. 10.1016/j.neuropsychologia.2013.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins L. A., Holderbaum L., Tao R., Hu Y., Sopko R., McCall K., Yang-Zhou D., Flockhart I., Binari R., Shim H.-S. et al. (2015). The transgenic RNAi project at harvard medical school: resources and validation. Genetics 201, 843-852. 10.1534/genetics.115.180208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter S., Ten Brinke M. M., Stedehouder J., Reinelt C. M., Wu B., Zhou H., Zhou K., Boele H.-J., Kushner S. A., Lee M. G. et al. (2016). Dysfunctional cerebellar Purkinje cells contribute to autism-like behaviour in Shank2-deficient mice. Nat. Commun. 7, 12627 10.1038/ncomms12627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrif F., Giles R. H., Dauwerse H. G., Saris J. J., Hennekam R. C. M., Masuno M., Tommerup N., van Ommen G.-J. B., Goodman R. H., Peters D. J. et al. (1995). Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature 376, 348-351. 10.1038/376348a0 [DOI] [PubMed] [Google Scholar]
- Pielage J., Steffes G., Lau D. C., Parente B. A., Crews S. T., Strauss R. and Klämbt C. (2002). Novel behavioral and developmental defects associated with Drosophila single-minded. Dev. Biol. 249, 283-299. 10.1006/dbio.2002.0770 [DOI] [PubMed] [Google Scholar]
- Placais P.-Y. and Preat T. (2013). To favor survival under food shortage, the brain disables costly memory. Science 339, 440-442. 10.1126/science.1226018 [DOI] [PubMed] [Google Scholar]
- Purpura D. P. (1975). Dendritic differentiation in human cerebral cortex: normal and aberrant developmental patterns. Adv. Neurol. 12, 91-134. [PubMed] [Google Scholar]
- Quinn W. G., Harris W. A. and Benzer S. (1974). Conditioned behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 71, 708-712. 10.1073/pnas.71.3.708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami M. (2014). Network plasticity in adaptive filtering and behavioral habituation. Neuron 82, 1216-1229. 10.1016/j.neuron.2014.04.035 [DOI] [PubMed] [Google Scholar]
- Restivo L., Ferrari F., Passino E., Sgobio C., Bock J., Oostra B. A., Bagni C. and Ammassari-Teule M. (2005). Enriched environment promotes behavioral and morphological recovery in a mouse model for the fragile X syndrome. Proc. Natl. Acad. Sci. USA 102, 11557-11562. 10.1073/pnas.0504984102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reza M. A., Mhatre S. D., Morrison J. C., Utreja S., Saunders A. J., Breen D. E. and Marenda D. R. (2013). Automated analysis of courtship suppression learning and memory in Drosophila melanogaster. Fly (Austin) 7, 105-111. 10.4161/fly.24110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riahi H., Brekelmans C., Foriel S., Merkling S. H., Lyons T. A., Itskov P. M., Kleefstra T., Ribeiro C., van Rij R. P., Kramer J. M. et al. (2019). The histone methyltransferase G9a regulates tolerance to oxidative stress-induced energy consumption. PLoS Biol. 17, e2006146 10.1371/journal.pbio.2006146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemensperger T., Völler T., Stock P., Buchner E. and Fiala A. (2005). Punishment prediction by dopaminergic neurons in Drosophila. Curr. Biol. 15, 1953-1960. 10.1016/j.cub.2005.09.042 [DOI] [PubMed] [Google Scholar]
- Riva D., Taddei M. and Bulgheroni S. (2018). The neuropsychology of basal ganglia. Eur. J. Paediatr. Neurol. 22, 321-326. 10.1016/j.ejpn.2018.01.009 [DOI] [PubMed] [Google Scholar]
- Ropers H. H. (2010). Genetics of early onset cognitive impairment. Annu. Rev. Genomics Hum. Genet. 11, 161-187. 10.1146/annurev-genom-082509-141640 [DOI] [PubMed] [Google Scholar]
- Roussignol G., Ango F., Romorini S., Tu J. C., Sala C., Worley P. F., Bockaert J. and Fagni L. (2005). Shank expression is sufficient to induce functional dendritic spine synapses in aspiny neurons. J. Neurosci. 25, 3560-3570. 10.1523/JNEUROSCI.4354-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders S. J. (2018). Next-generation sequencing in autism spectrum disorder. Cold Spring Harb. Perspect. Med. 10.1101/cshperspect.a026872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin S., Schendel D., Magnusson P., Hultman C., Surén P., Susser E., Grønborg T., Gissler M., Gunnes N., Gross R. et al. (2016). Autism risk associated with parental age and with increasing difference in age between the parents. Mol. Psychiatry 21, 693-700. 10.1038/mp.2015.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh D., Suyama R., Kimura K.-I. and Uemura T. (2012). High-resolution in vivo imaging of regenerating dendrites of Drosophila sensory neurons during metamorphosis: local filopodial degeneration and heterotypic dendrite-dendrite contacts. Genes Cells 17, 939-951. 10.1111/gtc.12008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J. and Levine J. D. (2014). Automated identification of social interaction criteria in Drosophila melanogaster. Biol. Lett. 10, 20140749 10.1098/rsbl.2014.0749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C. E. and Neri G. (2012). Autism and intellectual disability: two sides of the same coin. Am. J. Med. Genet. C Semin. Med. Genet. 160C, 89-90. 10.1002/ajmg.c.31329 [DOI] [PubMed] [Google Scholar]
- Seelig J. D., Chiappe M. E., Lott G. K., Dutta A., Osborne J. E., Reiser M. B. and Jayaraman V. (2010). Two-photon calcium imaging from head-fixed Drosophila during optomotor walking behavior. Nat. Methods 7, 535-540. 10.1038/nmeth.1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şentürk M. and Bellen H. J. (2018). Genetic strategies to tackle neurological diseases in fruit flies. Curr. Opin. Neurobiol. 50, 24-32. 10.1016/j.conb.2017.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P. J., Cirelli C., Greenspan R. J. and Tononi G. (2000). Correlates of sleep and waking in Drosophila melanogaster. Science 287, 1834-1837. 10.1126/science.287.5459.1834 [DOI] [PubMed] [Google Scholar]
- Shepherd J. D. and Bear M. F. (2011). New views of Arc, a master regulator of synaptic plasticity. Nat. Neurosci. 14, 279-284. 10.1038/nn.2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. W. and Hall J. C. (1979). Conditioned responses in courtship behavior of normal and mutant Drosophila. Proc. Natl. Acad. Sci. USA 76, 3430-3434. 10.1073/pnas.76.7.3430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigafoos J., Lancioni G. E., Singh N. N. and O'Reilly M. F. (2017). Intellectual disability and social skills. In Handbook of Social Behavior and Skills in Children (ed. Matson J. L.), pp. 249-271. Cham: Springer International Publishing. [Google Scholar]
- Simon A. F., Chou M.-T., Salazar E. D., Nicholson T., Saini N., Metchev S. and Krantz D. E. (2012). A simple assay to study social behavior in Drosophila: measurement of social space within a group. Genes Brain Behav. 11, 243-252. 10.1111/j.1601-183X.2011.00740.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J. H. and Looger L. L. (2018). Functional Imaging and Optogenetics in Drosophila. Genetics 208, 1291-1309. 10.1534/genetics.117.300228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha P., Kjelgaard M. M., Gandhi T. K., Tsourides K., Cardinaux A. L., Pantazis D., Diamond S. P. and Held R. M. (2014). Autism as a disorder of prediction. Proc. Natl. Acad. Sci. USA 111, 15220-15225. 10.1073/pnas.1416797111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaraman D., Aso Y., Jin X., Chen N., Felix M., Rubin G. M. and Nitabach M. N. (2015). Propagation of homeostatic sleep signals by segregated synaptic microcircuits of the Drosophila mushroom body. Curr. Biol. 25, 2915-2927. 10.1016/j.cub.2015.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specchia V., Puricella A., D'Attis S., Massari S., Giangrande A. and Bozzetti M. P. (2019). Drosophila melanogaster as a model to study the multiple phenotypes, related to genome stability of the fragile-X syndrome. Front. Genet. 10, 10 10.3389/fgene.2019.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieth H. T. (1974). Courtship behavior in Drosophila. Annu. Rev. Entomol. 19, 385-405. 10.1146/annurev.en.19.010174.002125 [DOI] [PubMed] [Google Scholar]
- Squire L. R. (1992). Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 99, 195-231. 10.1037/0033-295X.99.2.195 [DOI] [PubMed] [Google Scholar]
- Srivastava A. K. and Schwartz C. E. (2014). Intellectual disability and autism spectrum disorders: causal genes and molecular mechanisms. Neurosci. Biobehav. Rev. 46, 161-174. 10.1016/j.neubiorev.2014.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Wang X., Perfitt T. L., Parrish W. P., Shonesy B. C., Marks C. R., Mortlock D. P., Nakagawa T., Sutcliffe J. S. and Colbran R. J. (2017). A novel human CAMK2A mutation disrupts dendritic morphology and synaptic transmission, and causes ASD-related behaviors. J. Neurosci. 37, 2216-2233. 10.1523/JNEUROSCI.2068-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stessman H. A. F., Xiong B., Coe B. P., Wang T., Hoekzema K., Fenckova M., Kvarnung M., Gerdts J., Trinh S., Cosemans N. et al. (2017). Targeted sequencing identifies 91 neurodevelopmental-disorder risk genes with autism and developmental-disability biases. Nat. Genet. 49, 515-526. 10.1038/ng.3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub J., Konrad E. D. H., Grüner J., Toutain A., Bok L. A., Cho M. T., Crawford H. P., Dubbs H., Douglas G., Jobling R. et al. (2018). Missense variants in RHOBTB2 cause a developmental and epileptic encephalopathy in humans, and altered levels cause neurological defects in Drosophila. Am. J. Hum. Genet. 102, 44-57. 10.1016/j.ajhg.2017.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausfeld N. J. and Hirth F. (2013). Deep homology of arthropod central complex and vertebrate basal ganglia. Science 340, 157-161. 10.1126/science.1231828 [DOI] [PubMed] [Google Scholar]
- Subramanian K., Brandenburg C., Orsati F., Soghomonian J.-J., Hussman J. P. and Blatt G. J. (2017). Basal ganglia and autism - a translational perspective. Autism Res. 10, 1751-1775. 10.1002/aur.1837 [DOI] [PubMed] [Google Scholar]
- Sudhakaran I. P., Hillebrand J., Dervan A., Das S., Holohan E. E., Hulsmeier J., Sarov M., Parker R., VijayRaghavan K. and Ramaswami M. (2014). FMRP and Ataxin-2 function together in long-term olfactory habituation and neuronal translational control. Proc. Natl. Acad. Sci. USA 111, E99-E108. 10.1073/pnas.1309543111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassetto M. and Gao F.-B. (2006). Transcriptional control of dendritic patterning in Drosophila neurons. Genome Biol. 7, 225 10.1186/gb-2006-7-7-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thran J., Poeck B. and Strauss R. (2013). Serum response factor-mediated gene regulation in a Drosophila visual working memory. Curr. Biol. 23, 1756-1763. 10.1016/j.cub.2013.07.034 [DOI] [PubMed] [Google Scholar]
- Tian Y., Zhang Z. C. and Han J. (2017). Drosophila Studies on Autism Spectrum Disorders. Neurosci. Bull. 33, 737-746. 10.1007/s12264-017-0166-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomer R., Denes A. S., Tessmar-Raible K. and Arendt D. (2010). Profiling by image registration reveals common origin of annelid mushroom bodies and vertebrate pallium. Cell 142, 800-809. 10.1016/j.cell.2010.07.043 [DOI] [PubMed] [Google Scholar]
- Tong H., Li Q., Zhang Z. C., Li Y. and Han J. (2016). Neurexin regulates nighttime sleep by modulating synaptic transmission. Sci. Rep. 6, 38246 10.1038/srep38246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai P. T., Hull C., Chu Y. X., Greene-Colozzi E., Sadowski A. R., Leech J. M., Steinberg J., Crawley J. N., Regehr W. G. and Sahin M. (2012). Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature 488, 647-651. 10.1038/nature11310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugur B., Chen K. and Bellen H. J. (2016). Drosophila tools and assays for the study of human diseases. Dis Model Mech 9, 235-244. 10.1242/dmm.023762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaags A. K., Lionel A. C., Sato D., Goodenberger M. K., Stein Q. P., Curran S., Ogilvie C., Ahn J. W., Drmic I., Senman L. et al. (2012). Rare deletions at the neurexin 3 locus in autism spectrum disorder. Am. J. Hum. Genet. 90, 133-141. 10.1016/j.ajhg.2011.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alphen B. and van Swinderen B. (2013). Drosophila strategies to study psychiatric disorders. Brain Res. Bull. 92, 1-11. 10.1016/j.brainresbull.2011.09.007 [DOI] [PubMed] [Google Scholar]
- van Alphen B., Yap M. H. W., Kirszenblat L., Kottler B. and van Swinderen B. (2013). A dynamic deep sleep stage in Drosophila. J. Neurosci. 33, 6917-6927. 10.1523/JNEUROSCI.0061-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bon B. W. M., Hoischen A., Hehir-Kwa J., de Brouwer A. P. M., Ruivenkamp C., Gijsbers A. C. J., Marcelis C. L., de Leeuw N., Veltman J. A., Brunner H. G. et al. (2011). Intragenic deletion in DYRK1A leads to mental retardation and primary microcephaly. Clin. Genet. 79, 296-299. 10.1111/j.1399-0004.2010.01544.x [DOI] [PubMed] [Google Scholar]
- van de Wouw E., Evenhuis H. M. and Echteld M. A. (2013). Objective assessment of sleep and sleep problems in older adults with intellectual disabilities. Res. Dev. Disabil. 34, 2291-2303. 10.1016/j.ridd.2013.04.012 [DOI] [PubMed] [Google Scholar]
- van der Voet M., Nijhof B., Oortveld M. A. W. and Schenck A. (2014). Drosophila models of early onset cognitive disorders and their clinical applications. Neurosci. Biobehav. Rev. 46, 326-342. 10.1016/j.neubiorev.2014.01.013 [DOI] [PubMed] [Google Scholar]
- van Karnebeek C. D. M. and Stockler S. (2012). Treatable inborn errors of metabolism causing intellectual disability: a systematic literature review. Mol. Genet. Metab. 105, 368-381. 10.1016/j.ymgme.2011.11.191 [DOI] [PubMed] [Google Scholar]
- van Swinderen B. and Greenspan R. J. (2003). Salience modulates 20-30 Hz brain activity in Drosophila. Nat. Neurosci. 6, 579-586. 10.1038/nn1054 [DOI] [PubMed] [Google Scholar]
- Vandervert L. (2016). The prominent role of the cerebellum in the learning, origin and advancement of culture. Cerebellum Ataxias 3, 10 10.1186/s40673-016-0049-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese M., Keshav N., Jacot-Descombes S., Warda T., Wicinski B., Dickstein D. L., Harony-Nicolas H., De Rubeis S., Drapeau E., Buxbaum J. D. et al. (2017). Autism spectrum disorder: neuropathology and animal models. Acta Neuropathol. 134, 537-566. 10.1007/s00401-017-1736-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch O. J., Sutcliffe J. S., Warren Z. E., Keenan B. T., Potter M. H. and Malow B. A. (2017). Shorter sleep duration is associated with social impairment and comorbidities in ASD. Autism Res. 10, 1221-1238. 10.1002/aur.1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerk A. J. M. H., Pieretti M., Sutcliffe J. S., Fu Y.-H., Kuhl D. P. A., Pizzuti A., Reiner O., Richards S., Victoria M. F., Zhang F. P. et al. (1991). Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65, 905-914. 10.1016/0092-8674(91)90397-H [DOI] [PubMed] [Google Scholar]
- Vermeulen K., de Boer A., Janzing J. G. E., Koolen D. A., Ockeloen C. W., Willemsen M. H., Verhoef F. M., van Deurzen P. A. M., van Dongen L., van Bokhoven H. et al. (2017). Adaptive and maladaptive functioning in Kleefstra syndrome compared to other rare genetic disorders with intellectual disabilities. Am. J. Med. Genet. A 173, 1821-1830. 10.1002/ajmg.a.38280 [DOI] [PubMed] [Google Scholar]
- Vicari S. (2004). Memory development and intellectual disabilities. Acta Paediatr. Suppl. 93, 60-63; discussion 63-4 10.1111/j.1651-2227.2004.tb03059.x [DOI] [PubMed] [Google Scholar]
- Villella A. and Hall J. C. (2008). Neurogenetics of courtship and mating in Drosophila. Adv. Genet. 62, 67-184. 10.1016/S0065-2660(08)00603-2 [DOI] [PubMed] [Google Scholar]
- Vissers L. E. L. M., Gilissen C. and Veltman J. A. (2016). Genetic studies in intellectual disability and related disorders. Nat. Rev. Genet. 17, 9-18. 10.1038/nrg3999 [DOI] [PubMed] [Google Scholar]
- Vogt K., Schnaitmann C., Dylla K. V., Knapek S., Aso Y., Rubin G. M. and Tanimoto H. (2014). Shared mushroom body circuits underlie visual and olfactory memories in Drosophila. eLife 3, e02395 10.7554/eLife.02395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorstman J. A. S., Parr J. R., Moreno-De-Luca D., Anney R. J. L., Nurnberger J. I. Jr and Hallmayer J. F. (2017). Autism genetics: opportunities and challenges for clinical translation. Nat. Rev. Genet. 18, 362-376. 10.1038/nrg.2017.4 [DOI] [PubMed] [Google Scholar]
- Wan L., Dockendorff T. C., Jongens T. A. and Dreyfuss G. (2000). Characterization of dFMR1, a Drosophila melanogaster homolog of the fragile X mental retardation protein. Mol. Cell. Biol. 20, 8536-8547. 10.1128/MCB.20.22.8536-8547.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. S.-H., Kloth A. D. and Badura A. (2014). The cerebellum, sensitive periods, and autism. Neuron 83, 518-532. 10.1016/j.neuron.2014.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Al-Ouran R., Hu Y., Kim S.-Y., Wan Y.-W., Wangler M. F., Yamamoto S., Chao H.-T., Comjean A., Mohr S. E. et al. (2017). MARRVEL: integration of human and model organism genetic resources to facilitate functional annotation of the human genome. Am. J. Hum. Genet. 100, 843-853. 10.1016/j.ajhg.2017.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M., Schnitzler H.-U. and Schmid S. (2002). Synaptic plasticity in the acoustic startle pathway: the neuronal basis for short-term habituation? Eur. J. Neurosci. 16, 1325-1332. 10.1046/j.1460-9568.2002.02194.x [DOI] [PubMed] [Google Scholar]
- Wegiel J., Kuchna I., Nowicki K., Imaki H., Wegiel J., Marchi E., Ma S. Y., Chauhan A., Chauhan V., Bobrowicz T. W. et al. (2010). The neuropathology of autism: defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuropathol. 119, 755-770. 10.1007/s00401-010-0655-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz E. D., Towheed A., Monyak R. E., Toth M. S., Wallace D. C. and Jongens T. A. (2018). Loss of Drosophila FMRP leads to alterations in energy metabolism and mitochondrial function. Hum. Mol. Genet. 27, 95-106. 10.1093/hmg/ddx387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock J. R., Heynen A. J., Shuler M. G. and Bear M. F. (2006). Learning induces long-term potentiation in the hippocampus. Science 313, 1093-1097. 10.1126/science.1128134 [DOI] [PubMed] [Google Scholar]
- Williams D. L., Goldstein G. and Minshew N. J. (2006). The profile of memory function in children with autism. Neuropsychology 20, 21-29. 10.1037/0894-4105.20.1.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G. (1990). Anterograde and retrograde amnesia in rats with dorsal hippocampal or dorsomedial thalamic lesions. Behav. Brain Res. 38, 145-154. 10.1016/0166-4328(90)90012-4 [DOI] [PubMed] [Google Scholar]
- Wise A., Tenezaca L., Fernandez R. W., Schatoff E., Flores J., Ueda A., Zhong X., Wu C.-F., Simon A. F. and Venkatesh T. (2015). Drosophila mutants of the autism candidate gene neurobeachin (rugose) exhibit neuro-developmental disorders, aberrant synaptic properties, altered locomotion, and impaired adult social behavior and activity patterns. J. Neurogenet. 29, 135-143. 10.3109/01677063.2015.1064916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff T. and Rubin G. M. (2018). Neuroarchitecture of the Drosophila central complex: A catalog of nodulus and asymmetrical body neurons and a revision of the protocerebral bridge catalog. J. Comp. Neurol. 526, 2585-2611. 10.1002/cne.24512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolman M. A., de Groh E. D., McBride S. M., Jongens T. A., Granato M. and Epstein J. A. (2014). Modulation of cAMP and ras signaling pathways improves distinct behavioral deficits in a zebrafish model of neurofibromatosis type 1. Cell Rep 8, 1265-1270. 10.1016/j.celrep.2014.07.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Gause M., Xu D., Misulovin Z., Schaaf C. A., Mosarla R. C., Mannino E., Shannon M., Jones E., Shi M. et al. (2015). Drosophila nipped-B mutants model Cornelia de Lange syndrome in growth and behavior. PLoS Genet. 11, e1005655 10.1371/journal.pgen.1005655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Gan G., Zhang Z., Sun J., Wang Q., Gao Z., Li M., Jin S., Huang J., Thomas U. et al. (2017). A presynaptic function of shank protein in Drosophila. J. Neurosci. 37, 11592-11604. 10.1523/JNEUROSCI.0893-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Jaiswal M., Charng W.-L., Gambin T., Karaca E., Mirzaa G., Wiszniewski W., Sandoval H., Haelterman N. A., Xiong B. et al. (2014). A drosophila genetic resource of mutants to study mechanisms underlying human genetic diseases. Cell 159, 200-214. 10.1016/j.cell.2014.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Porch M. W., Court-Vazquez B., Bennett M. V. L. and Zukin R. S. (2018). Activation of autophagy rescues synaptic and cognitive deficits in fragile X mice. Proc. Natl. Acad. Sci. USA 115, E9707-E9716. 10.1073/pnas.1808247115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Liu N., He Y., Liu Y., Ge L., Zou L., Song S., Xiong W. and Liu X. (2018). Improved calcium sensor GCaMP-X overcomes the calcium channel perturbations induced by the calmodulin in GCaMP. Nat. Commun. 9, 1504 10.1038/s41467-018-03719-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasunaga K., Tezuka A., Ishikawa N., Dairyo Y., Togashi K., Koizumi H. and Emoto K. (2015). Adult Drosophila sensory neurons specify dendritic territories independently of dendritic contacts through the Wnt5-Drl signaling pathway. Genes Dev. 29, 1763-1775. 10.1101/gad.262592.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef E. A., Berry-Kravis E., Czech C., Hagerman R. J., Hessl D., Wong C. Y., Rabbia M., Deptula D., John A., Kinch R. et al. (2018). Effect of the mGluR5-NAM Basimglurant on behavior in adolescents and adults with fragile X syndrome in a randomized, double-blind, placebo-controlled trial: FragXis Phase 2 results. Neuropsychopharmacology 43, 503-512. 10.1038/npp.2017.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamurrad S., Hatch H. A. M., Drelon C., Belalcazar H. M. and Secombe J. (2018). A Drosophila model of intellectual disability caused by mutations in the histone demethylase KDM5. Cell Rep. 22, 2359-2369. 10.1016/j.celrep.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Q., Bailey A. M., Matthies H. J. G., Renden R. B., Smith M. A., Speese S. D., Rubin G. M. and Broadie K. (2001). Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell 107, 591-603. 10.1016/S0092-8674(01)00589-X [DOI] [PubMed] [Google Scholar]
- Zhang X., Rui M., Gan G., Huang C., Yi J., Lv H. and Xie W. (2017). Neuroligin 4 regulates synaptic growth via the bone morphogenetic protein (BMP) signaling pathway at the Drosophila neuromuscular junction. J. Biol. Chem. 292, 17991-18005. 10.1074/jbc.M117.810242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. L., Yue Z., Arnold D. M., Artiushin G. and Sehgal A. (2018). A circadian clock in the blood-brain barrier regulates xenobiotic efflux. Cell 173, 130-139.e10. 10.1016/j.cell.2018.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Zhang L., Li S., Zhao F., Wang Y., Huang L., Huang J., Zou R., Qu Y. and Mu D. (2017). Association among obesity, overweight and autism spectrum disorder: a systematic review and meta-analysis. Sci. Rep. 7, 11697 10.1038/s41598-017-12003-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwarts L., Versteven M. and Callaerts P. (2012). Genetics and neurobiology of aggression in Drosophila. Fly (Austin) 6, 35-48. 10.4161/fly.19249 [DOI] [PMC free article] [PubMed] [Google Scholar]