Abstract
PURPOSE
The selective cyclin-dependent kinase 4/6 inhibitor palbociclib was approved in Argentina in 2015 for postmenopausal women with hormone receptor (HR)–positive, human epidermal growth factor receptor 2 (HER2)–negative advanced breast cancer (ABC) or metastatic breast cancer (MBC) based on phase III study results. The Ibrance Real World Insights (IRIS) study aims to evaluate palbociclib in patients with HR-positive/HER2-negative ABC or MBC in the real-world setting in multiple countries globally. Here we report results from patients enrolled in the IRIS study in Argentina.
PATIENTS AND METHODS
This retrospective medical chart review study included postmenopausal women with confirmed HR-positive/HER2-negative ABC or MBC who were treated with palbociclib plus letrozole as first-line endocrine-based therapy or with palbociclib plus fulvestrant in women with disease progression after endocrine therapy. Participating physicians reviewed medical records of up to six patients each, collecting demographic and clinical data. Outcomes included progression-free and overall survival rates.
RESULTS
Records were extracted for 162 patients in Argentina (palbociclib plus letrozole, n = 105 [65%]; palbociclib plus fulvestrant, n = 57 [35%]). The 6-month progression-free survival rate was 94% for patients treated with palbociclib plus letrozole and 95% for patients treated with palbociclib plus fulvestrant; 85% and 80% of patients treated with palbociclib plus letrozole were progression free at 12 and 18 months, respectively. Six-month survival rates were 98% for palbociclib plus letrozole and 98% for palbociclib plus fulvestrant; 93% and 89% of patients treated with palbociclib plus letrozole were alive at 12 and 18 months, respectively.
CONCLUSION
Results from this first real-world evaluation of clinical outcomes in Argentina suggest that palbociclib plus letrozole or fulvestrant delivers favorable effectiveness, as measured by progression-free and overall survival rates.
INTRODUCTION
Breast cancer is a common concern in women in Argentina, with an estimated 71 breast cancers per 100,000 women.1 Hormone receptor (HR)–positive, human epidermal growth factor receptor 2 (HER2)–negative breast cancer is the most common breast cancer subtype in Argentina, affecting 70% of patients.2 Even though mortality rates from breast cancer have been in decline in Argentina during recent years, the mortality rate is higher in Argentina (17.56 deaths per 100,000 women in 2012) than in most other Latin American countries with the exception of Uruguay (17.78 deaths per 100,000 women in 2012).3
Endocrine agents have been the mainstay of treatment of HR-positive/HER2-negative breast cancer; however, recognition of the complexity of breast cancer subtypes and resistance mechanisms has led to the development of targeted treatments designed to overcome endocrine resistance and improve patient outcomes. One such agent is palbociclib, a selective cyclin-dependent kinase 4/6 inhibitor with proven efficacy for HR-positive/HER2-negative advanced breast cancer (ABC) or metastatic breast cancer (MBC) in the phase III placebo-controlled PALOMA-2 (ClinicalTrials.gov identifier: NCT01740427) and PALOMA-3 (Clinicaltrials.gov identifier: NCT01942135) studies.4-8 Palbociclib is approved in Argentina for the treatment of women with HR-positive/HER2-negative ABC or MBC, in combination with letrozole as initial endocrine-based therapy for postmenopausal women and in combination with fulvestrant for women with disease progression after endocrine therapy.9
CONTEXT
Key Objective
The Ibrance Real World Insights (IRIS) study is, to our knowledge, the first multicountry real-world study to evaluate palbociclib in patients with hormone receptor–positive, human epidermal growth factor receptor 2–negative advanced or metastatic breast cancer outside of clinical trials. This report presents data from the Argentina cohort.
Knowledge Generated
Between August and November 2017, 162 patients in Argentina received palbociclib plus letrozole (n = 105; 65%) or palbociclib plus fulvestrant (n = 57; 35%). Most patients (85%) initiated palbociclib at the recommended starting dose of 125 mg/d, whereas 15% of patients received a starting dose of 100 mg/d. Dose reductions were uncommon. Patients had favorable progression-free and overall survival rates that were comparable with the results of clinical trials.
Relevance
These results highlight the pattern of palbociclib use in the real-world setting, providing the clinical community with the data needed to demonstrate that palbociclib is effective and well tolerated in the real-world setting in patients with advanced or metastatic breast cancer.
Although palbociclib has been widely studied in the clinical trial setting, evidence for its use in real-world patients with ABC or MBC is scarce. The Ibrance Real World Insights (IRIS) study is a retrospective chart review initiated to assess the use of palbociclib-based regimens according to approved indications in real-world clinical practice across multiple countries in North America, South America, and Europe. Here we describe the results from patients in Argentina, which was among the first countries in which palbociclib was approved.
PATIENTS AND METHODS
Study Design
IRIS is a retrospective medical chart review study of patients who received palbociclib with either letrozole or fulvestrant based on labeled indications across multiple countries in North America, South America, and Europe. Results from the United States have been previously reported.10 The study protocol was approved by the Western Institutional Review Board.
Study Population
Oncologists and gynecologists from both private and public practices who treated two or more patients with ABC or MBC were included in the study. Eligible patients were age 18 years or older and had confirmed HR-positive/HER2-negative ABC or MBC treated with palbociclib as licensed in Argentina (ie, in combination with letrozole as initial endocrine-based therapy in postmenopausal women [approved December 14, 2015] or in combination with fulvestrant in women with disease progression after endocrine therapy [approved August 16, 2016]).9 In addition, eligible patients had to have follow-up data for 6 months or more since the initiation of palbociclib plus letrozole or for 3 months or more since the initiation of palbociclib plus fulvestrant. No prior or current enrollment in interventional clinical trials for ABC or MBC was permitted.
Data Source and Extraction
Physicians extracted data from medical records for up to six sequential patients meeting the inclusion criteria. The index date was defined as 60 days after the physician first prescribed palbociclib plus letrozole or fulvestrant after regulatory approval in Argentina. Data were extracted from the index date until the last available medical record, death, or date of record abstraction, whichever was earliest. To ensure sufficient follow-up after palbociclib initiation, a period of 3 months or longer for palbociclib plus fulvestrant or 6 months or longer for palbociclib plus letrozole was required before the date of chart review.
Data extracted included demographic and clinical characteristics, palbociclib treatment patterns, response to treatment, and time to response, discontinuation, or death. Derived from these data were objective response rate (ORR), clinical benefit rate (CBR; the proportion of patients with a complete response [CR], partial response [PR], or stable disease lasting 24 weeks or longer), and progression-free and overall survival rates at 6, 12, and 18 months. Insufficient follow-up data were available to derive 12- and 18-month progression-free and overall survival rates for patients who received palbociclib plus fulvestrant at the time of the analysis.
Statistical Analysis
Analyses were descriptive, and no formal hypothesis was tested. Time-to-event outcomes were calculated using Kaplan-Meier estimates. CBR data were censored for patients with less than 24 weeks of data who were still receiving palbociclib with no evidence of CR, PR, or disease progression. Missing data were not imputed. Analyses were conducted using STATA statistical software version 15.1 (StataCorp, College Station, TX).
RESULTS
Physicians and Patients
Between August and November 2017, 41 physicians (40 oncologists and one gynecologist) extracted data for 162 patients; 105 patients (65%) were treated with palbociclib plus letrozole, and 57 patients (35%) were treated with palbociclib plus fulvestrant. The mean time since first prescription of palbociclib was 17 months (standard deviation [SD], 5 months) for patients treated with palbociclib plus letrozole and 11 months (SD, 3 months) for patients treated with palbociclib plus fulvestrant. Patient characteristics are listed in Table 1.
TABLE 1.
Patient Demographic and Clinical Characteristics
A total of 99 patients (61%) had received treatment of early breast cancer, including adjuvant chemotherapy (n = 68; 69%) and endocrine therapy (n = 76; 77%; Table 1). The mean time from breast cancer diagnosis to palbociclib therapy was 38 months (SD, 52 months). At the time of data extraction, palbociclib treatment was ongoing in 138 patients (85%), including 88 patients (84%) in the palbociclib plus letrozole group and 50 patients (88%) in the palbociclib plus fulvestrant group.
Palbociclib Plus Letrozole
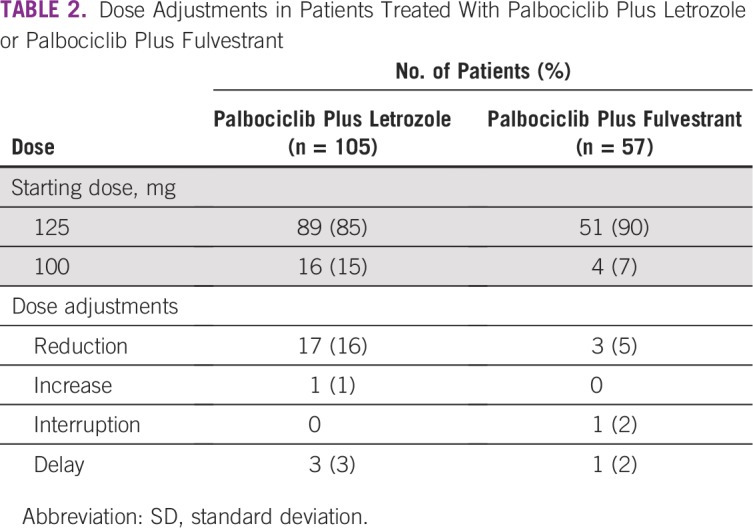
At the time of data extraction, palbociclib treatment was ongoing in 88 patients (84%) in the palbociclib plus letrozole group. The mean time from diagnosis of ABC or MBC to palbociclib plus letrozole initiation was 5 months (SD, 12 months); 97 patients (92%) initiated palbociclib treatment within 12 months of ABC or MBC diagnosis. Most patients receiving palbociclib plus letrozole (n = 100; 95%) did so as an initial endocrine-based therapy, per the labeled indication. The most common starting dose of palbociclib was 125 mg/d (n = 89; 85%; Table 2). Most patients (n = 20; 95%) required only one dose adjustment. Among the 17 patients (16%) who discontinued treatment, eight patients (47%) did so because of disease progression after initial response.
TABLE 2.
Dose Adjustments in Patients Treated With Palbociclib Plus Letrozole or Palbociclib Plus Fulvestrant
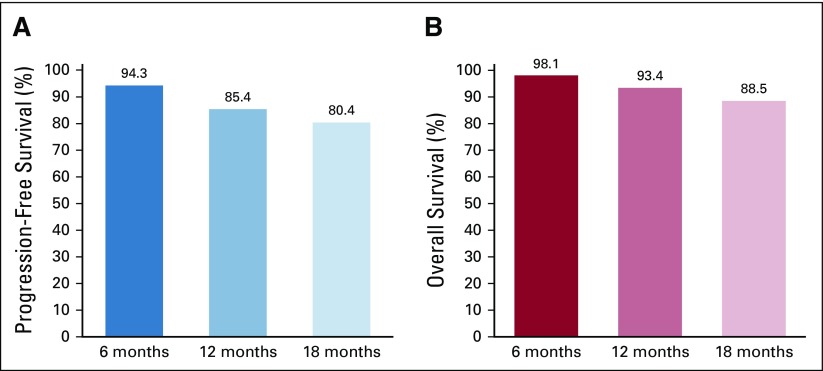
At the time of data collection, after a mean follow-up time of 10 months (SD, 4 months), 12 patients receiving palbociclib plus letrozole (11%) had disease progression. The 12-month progression-free survival rate was 85%; at 18 months, 85% of patients remained progression free (Fig 1A). At the time of data collection, six patients (6%) had died; 12- and 18-month survival rates were 93% and 89%, respectively (Fig 1B).
FIG 1.
(A) Progression-free survival rate and (B) overall survival rate in patients treated with palbociclib plus letrozole. Rates were estimated using Kaplan-Meier analyses.
Objective responses, calculated based on the physicians’ assessments recorded in patients’ charts, were achieved in 68 patients (65%; Table 3). The mean time to CR after palbociclib initiation was 4 months (SD, 2 months), and the mean time to PR was 3 months (SD, 2 months). The CBR was 93%; no patients were censored for this analysis.
TABLE 3.
Outcomes in Patients Treated With Palbociclib Combination Therapy
Palbociclib Plus Fulvestrant
At the time of data extraction, palbociclib treatment was ongoing in 50 patients (88%) in the palbociclib plus fulvestrant group. Palbociclib-based therapy was the second-line treatment of 43 patients (75%) and the third-line treatment of six patients (11%). Prior therapies for ABC or MBC included endocrine therapy in 40 patients (82%) and chemotherapy in seven patients (14%; Table 1). The most common prior regimens were anastrozole monotherapy (n = 16; 28%) and letrozole monotherapy (n = 12; 21%).
The mean time from diagnosis of ABC or MBC to initiation of palbociclib plus fulvestrant was 18 months (SD, 19 months); 26 patients (47%) initiated palbociclib plus fulvestrant treatment within 12 months of ABC or MBC diagnosis. The most common starting dose of palbociclib was 125 mg/d (n = 51; 89%; Table 2). Seven patients (14%) had their treatment discontinued, most commonly because of disease progression after initial response (n = 2; 29%). The mean duration of therapy among patients who discontinued treatment was 4 months (SD, 2 months); among those in whom treatment was ongoing, the median duration of treatment was 6 months (SD, 2 months).
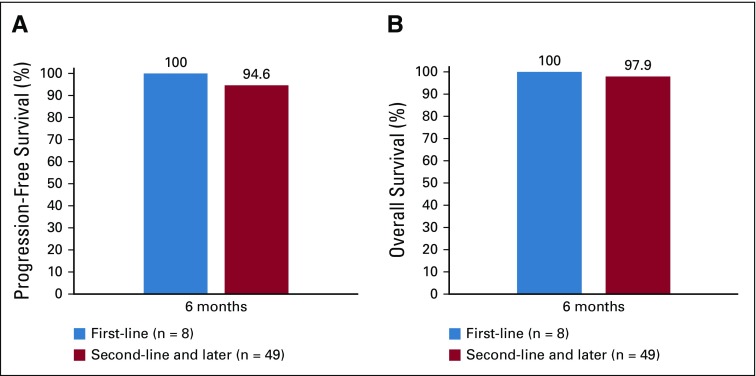
At the time of data collection, after a mean follow-up time of 6 months (SD, 2 months), three patients (5%) in the palbociclib plus fulvestrant group had disease progression. The 6-month progression-free survival rate was 95% overall; corresponding rates were 100% in patients receiving first-line treatment and 95% in patients receiving second- or later-line treatment (Fig 2A). At the time of data collection, two patients (4%) had died. The 6-month survival rate was 98% overall; corresponding survival rates were 100% and 98% in the first- and second- or later-line settings, respectively (Fig 2B). Progression-free and overall survival rates were not available after 6 months for this group because of the limited time on treatment.
FIG 2.
(A) Progression-free survival rate and (B) overall survival rate in patients treated with palbociclib plus fulvestrant. Rates were estimated using Kaplan-Meier analyses.
Objective responses were reported in 37 patients (70%) in the palbociclib plus fulvestrant group (Table 3). The mean time to CR after palbociclib initiation was 6 months (SD, not estimable), and the mean time to PR was 3 months (SD, 1 month). The CBR, including patients censored for having stable disease, was 94%; when censored patients were excluded, the CBR was 76%.
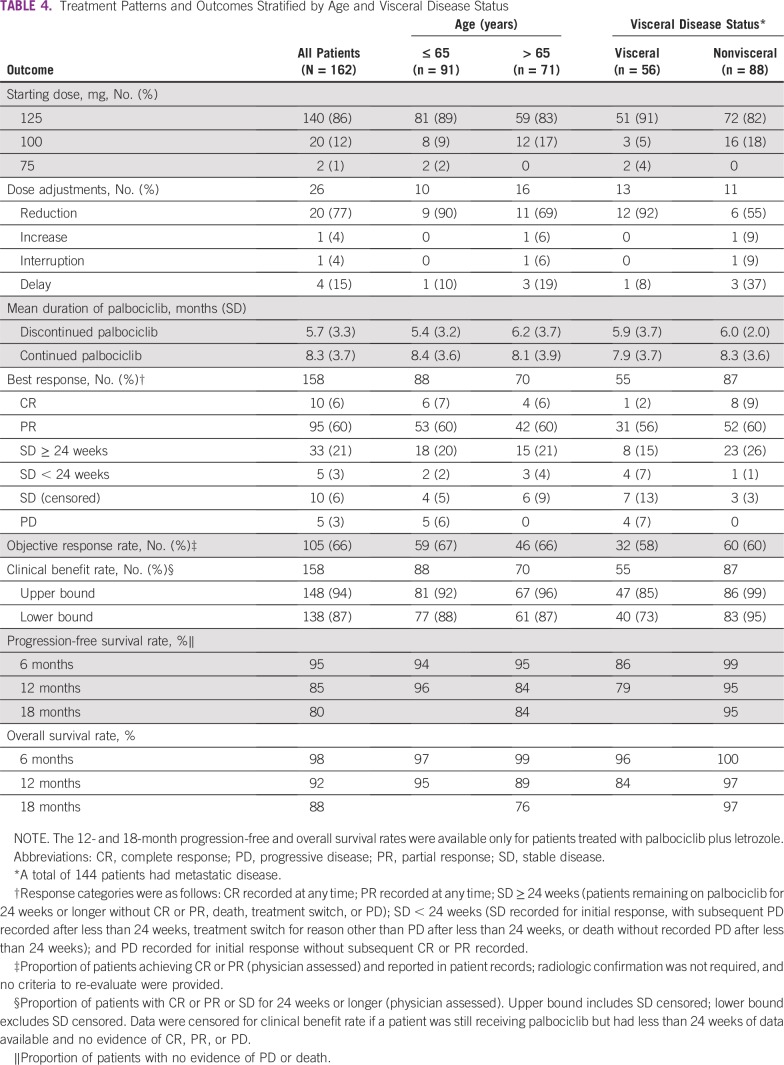
Effect of Age and Visceral Disease Status on Treatment With Palbociclib
The most common palbociclib starting dose in patients older than age 65 years and patients age 65 years and younger was 125 mg/d (Table 4). Doses were adjusted in 23% of older patients and 11% of younger patients, with dose reductions in 15% of older patients and 10% of younger patients. Discontinuation rates were 13% in older patients and 17% in younger patients.
TABLE 4.
Treatment Patterns and Outcomes Stratified by Age and Visceral Disease Status
Dose adjustments were made in 23% of patients with visceral disease and 13% of patients with nonvisceral disease, with dose reductions in 21% of patients with visceral disease and 7% of patients with nonvisceral disease. Patients with visceral disease had a discontinuation rate of 27%, whereas those with nonvisceral disease had a discontinuation rate of 6%.
ORRs and CBRs according to age and presence or absence of visceral disease are listed in Table 4. ORRs of 67% and 66% were reported for patients age 65 years or younger and patients older than age 65 years, respectively. Twelve-month progression-free survival rates, which were calculated based on data for patients treated with palbociclib plus letrozole only, were 86% in patients age 65 years or younger and 84% patients older than age 65 years. In patients age 65 years or younger and patients older than age 65 years, 12-month overall survival rates, also for patients treated with palbociclib plus letrozole only, were 95% and 89%, respectively.
ORRs of 58% and 69% were reported for patients with visceral and nonvisceral disease, respectively. In patients with visceral and nonvisceral disease, 12-month progression-free survival rates were 79% and 95%, respectively, and 12-month survival rates were 84% and 97%, respectively (for patients treated with palbociclib plus letrozole only; Table 4).
DISCUSSION
Extrapolation of clinical trial data to the real-world clinical practice setting can be challenging because clinical trials are conducted in a tightly controlled environment with protocol-driven assessment schedules that may not be applicable in the real world. Therefore, physicians in the real-world setting can be faced with a lack of data to inform their decision making in the day-to-day treatment of the broad range of patients encountered in clinical practice. To address the lack of real-world evidence for palbociclib, the IRIS study investigated treatment patterns and outcomes in patients with HR-positive/HER2-negative ABC or MBC who were treated with palbociclib in multiple countries. Patients receiving palbociclib in Argentina in IRIS had favorable response rates and progression-free and overall survival rates, with low discontinuation and dose-adjustment rates.
The most commonly prescribed palbociclib dose was 125 mg/d, in line with the prescribing information.11 This dose seemed to be well tolerated, with only 16% of patients requiring a dose adjustment over the study period; dose adjustments were more common in patients age 65 years or older and those with visceral disease. In previous phase III studies, the palbociclib dose was reduced in 36% of patients in PALOMA-24 and in 34% of patients in PALOMA-36; thus, the IRIS data suggest that dose reduction rates may be lower in routine clinical practice than observed in clinical studies. This may be driven by the fact that patients could be started on lower doses in clinical practice (75 or 100 mg/d) rather than the protocol-mandated 125 mg/d dose used in PALOMA-2 and PALOMA-3, with physicians tailoring the dose according to patient characteristics. The use of lower initial palbociclib doses may be responsible for the low dose-reduction rates, although the number of patients beginning treatment on the 100-mg dose was low, limiting the conclusions that can be drawn from the data.
A total of 85% of patients in the palbociclib plus letrozole group were progression free and 93% were alive after 12 months. Patients treated with palbociclib plus fulvestrant had 6-month progression-free and overall survival rates of 95% and 98%, respectively. This group had a shorter follow-up period than patients in the palbociclib plus letrozole group, and no progression or survival data were available after 6 months because the combination of palbociclib plus fulvestrant was approved more recently in Argentina than the combination of palbociclib plus letrozole. These outcomes were broadly comparable to those reported for US patients participating in IRIS,10 indicating similar benefits for palbociclib in the treatment of patients across different countries and health care systems.
Response and survival data from IRIS support the efficacy data for palbociclib in combination with letrozole or fulvestrant reported in the PALOMA studies,4,6 despite a higher proportion of patients in our patient population receiving a lower starting dose of palbociclib. Although not directly comparable because of different study designs and patient populations, some differences between the real world and clinical studies may have affected the efficacy findings. Palbociclib treatment seemed to be initiated earlier in this real-world study, with a higher percentage of patients in the current cohort receiving palbociclib after the failure of first-line treatment compared with PALOMA-3. Physicians in IRIS had more flexibility in selecting patients for whom palbociclib was appropriate, including those with less advanced disease, compared with the clinical trial setting. Our findings suggest that earlier initiation of palbociclib in the treatment pathway is occurring, rather than its use in later lines of therapy; further studies are needed to confirm why this is the case.
The effectiveness of palbociclib plus letrozole or fulvestrant did not seem to depend on age, visceral disease status, or line of therapy in this study, and favorable outcomes were observed for all subgroups analyzed, including older patients, those with visceral disease, and heavily pretreated patients. Patients in the palbociclib plus fulvestrant group included those who had experienced progression on prior endocrine therapy; nonetheless, second- and third-line palbociclib plus fulvestrant yielded excellent response and CBR rates and favorable 6-month progression-free and overall survival rates. Although these data are based on a limited follow-up period for palbociclib plus fulvestrant, they represent, to the best of our knowledge, the first real-world evidence for the effectiveness of palbociclib plus fulvestrant outside of clinical trials and the first evidence from Latin American patients.
The results of studies such as IRIS can provide valuable data that complement the findings of randomized clinical trials, particularly with the inclusion of patients not well represented in clinical trials. IRIS included patients with poorer Eastern Cooperative Oncology Group performance status, who compose a considerable proportion of the population seen in clinical practice but are under-represented in clinical trials. Overall, 12% of patients in IRIS had an Eastern Cooperative Oncology Group performance status of 2, with a higher proportion of these patients in the palbociclib plus fulvestrant group (16% v 10% for palbociclib plus letrozole). This is in line with the more advanced disease typically seen in patients treated with palbociclib plus fulvestrant.
Some limitations of this study should be acknowledged. Data were only collected by physicians willing to participate in the study, introducing a potential selection bias, although physicians were asked to select consecutive patients in line with the index date to mitigate this. Data were only collected for patients initiating treatment 6 months or more before chart abstraction for palbociclib plus letrozole and 3 months or more before chart abstraction for palbociclib plus fulvestrant because the latter combination was not approved in Argentina until August 2016. Consequently, progression and survival data at 12 months and beyond were not available for patients undergoing treatment with palbociclib plus fulvestrant. Despite these limitations, the current study provides much needed insight into the real-world use of palbociclib and related outcomes in patients with MBC or ABC in Argentina, supporting the evidence from the US IRIS population10 and adding to the body of real-world evidence for palbociclib. In conclusion, the IRIS study provides novel information regarding real-world treatment patterns and clinical outcomes associated with palbociclib in combination with letrozole or fulvestrant in Argentina, complementing the data reported previously from clinical studies.
ACKNOWLEDGMENT
We thank Suarez Luis Alberto for his support during the study. Medical writing and editorial assistance were provided by Deirdre Carman, PhD, of Alispera Communications (Cheshire, United States) and funded by Adelphi.
Footnotes
Supported by Pfizer.
Presented in part at the 54th Annual Meeting of the American Society for Clinical Oncology, Chicago, IL, June 1-5, 2018.
Clinical trial information: NCT03159195.
AUTHOR CONTRIBUTIONS
Conception and design: Debanjali Mitra, Katie Mycock, Gavin Taylor-Stokes, Lin Zhan, Shrividya Iyer
Collection and assembly of data: John Waller, Debanjali Mitra, Katie Mycock, Gavin Taylor-Stokes, Lin Zhan
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jgo/site/misc/authors.html.
John Waller
Employment: Adelphi
Debanjali Mitra
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Katie Mycock
Employment: Adelphi
Gavin Taylor-Stokes
Research Funding: Pfizer (Inst)
Gary Milligan
Employment: Adelphi
Lin Zhan
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Travel, Accommodations, Expenses: Pfizer
Shrividya Iyer
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Research Funding: Pfizer
Travel, Accommodations, Expenses: Pfizer
No other potential conflicts of interest were reported.
REFERENCES
- 1.Huñis AP. A current view of oncology in Argentina. Ecancermedicalscience. 2016;10:622. doi: 10.3332/ecancer.2016.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meiss Kress RP, Novelli JE, Gago FE, et al. Breast cancer in Argentina: Feasibility for the implementation of the new TNM staging system in 2018 in a middle-income country. J Cancer Epidemiol Treat. 2018;2:4–12. [Google Scholar]
- 3.Carioli G, Malvezzi M, Rodriguez T, et al. Trends and predictions to 2020 in breast cancer mortality: Americas and Australasia. Breast. 2018;37:163–169. doi: 10.1016/j.breast.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 5.Rugo HS, Finn RS, Diéras V, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2–negative advanced breast cancer: efficacy and safety updates with longer follow-up across patient subgroups. Cancer Res. 2018;78(suppl 4; abstr P5-21-03) [Google Scholar]
- 6.Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 7.Verma S, Bartlett CH, Schnell P, et al. Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: Detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3) Oncologist. 2016;21:1165–1175. doi: 10.1634/theoncologist.2016-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner NC, Finn RS, Martin M, et al. Clinical considerations of the role of palbociclib in the management of advanced breast cancer patients with and without visceral metastases. Ann Oncol. 2018;29:669–680. doi: 10.1093/annonc/mdx797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miniterio de Salud Disposicion No. 9253. http://www.anmat.gov.ar/boletin_anmat/agosto_2016/Dispo_9253-16.pdf
- 10.Mitra D, Taylor-Stokes G, Waller J, et al. Real world treatment clinical outcomes associated with palbociclib combination therapy in the United States: Results from the IRIS study; 35th Annual Miami Breast Cancer Conference; Miami Beach, FL. March 8-11, 2018. Presented at the. [Google Scholar]
- 11.US Food and Drug Administration Highlights of prescribing information: IBRANCE® (palbociclib) capsules, for oral use, March 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/207103s007lbl.pdf