Abstract
Introduction
Marked elevation in serum ferritin levels may be seen in disseminated infection or severe organ failure states, but it is also present in hemophagocytic lymphohistiocytosis (HLH). Herpes simplex virus (HSV) hepatitis has a high mortality rate, even in immunocompetent individuals, in whom it is rarely reported. We present a case of hyperferritinemia with features initially suggestive of a diagnosis of HLH but that ultimately proved to be fulminant HSV hepatitis.
Case Presentation
A 56-year-old man with an indolent undiagnosed brain mass presented with progressive neurologic deficits and was found to have fevers, cytopenias, transaminitis, and hyperferritinemia. Initially, HLH was suspected; however, the ultimate diagnosis was HSV hepatitis with dissemination. Although the patient was treated with intravenous acyclovir, multiorgan failure developed, and he died.
Discussion
This case highlights the importance of considering alternative causes for a rise in ferritin levels when HLH is on the differential. Additionally, we discuss the diagnostic and therapeutic implications of HSV hepatitis, and we review the literature for cases presenting in immunocompetent hosts.
Keywords: herpes simplex virus hepatitis, HSV hepatitis, hemophagocytic lymphohistiocytosis, HLH, hyperferritinemia
INTRODUCTION
Marked elevation in serum ferritin levels may be seen in states of substantial inflammation such as hemophagocytic lymphohistiocytosis (HLH) or adult-onset Still disease, but it can also be present in disseminated infection or severe organ failure. We present a case of hyperferritinemia with features initially suggestive of a diagnosis of HLH but that ultimately proved to be fulminant herpes simplex virus (HSV) hepatitis. This rare1 case of HSV hepatitis highlights the importance of considering alternative causes for a rise in ferritin levels.
CASE PRESENTATION
Presenting Concerns
A 56-year-old man with a 12-year history of an indolent, undiagnosed brain mass, presented with fevers and subacute progressive left-sided weakness. Six weeks before admission, left-sided numbness and paresthesias had developed, and he was evaluated as an outpatient by a neurosurgeon. Results of magnetic resonance imaging of the brain demonstrated an abnormal white-matter signal in the anterior right cerebral hemisphere suggestive of primary glioma of the central nervous system. There was minimal interval progression in this mass from prior imaging. He was treated with dexamethasone, 24 mg daily in divided doses for 2 weeks, with minimal improvement in his symptoms. A week before admission, the left-sided weakness developed.
On presentation to the hospital, he additionally reported sinus congestion and pharyngitis, but otherwise his review of symptoms yielded normal findings. He had a history of hypertension, diabetes mellitus, fibromyalgia, and peripheral neuropathy. The brain mass was suspected to represent a low-grade glioma or meningioma but had not undergone a biopsy. He reported occasional consumption of alcohol but denied use of tobacco or illicit drugs. He had not recently traveled outside the country. His family history included an unspecified brain tumor in his mother.
On examination, his temperature was 38.4°C; otherwise, his vital signs were within normal limits. Although he appeared drowsy, he was alert and oriented to person, place, time, and situation. He had 5 of 5 strength proximally and distally on the right side, but he had 4+ of 5 proximally and distally on the left side in the upper and lower extremities. Laboratory evaluations disclosed the following values: White blood cells, 11.76 × 103/mL; hemoglobin, 17 g/dL; hematocrit, 48.7%; and platelets, 82 × 103/mL. A complete metabolic panel revealed these values: Serum urea nitrogen, 33 mg/dL; creatinine, 1.15 mg/dL; aspartate aminotransferase, 6030 U/L; alanine aminotransferase, 7042 U/L; alkaline phosphatase, 77 IU/L; and total bilirubin, 2.1 mg/dL (Table 1). Results of a computed tomography scan of the head did not show acute intracranial abnormalities. The patient was admitted to the intensive care unit for neurologic and laboratory monitoring.
Table 1.
Laboratory values during hospital course
| Laboratory value | HD 1 | HD 2 | HD 3 | HD 4 | HD 5 |
|---|---|---|---|---|---|
| White blood cells, × 106/μL | 11.76 | 10.3 | 10.8 | 12.9 | 17.6 |
| Hemoglobin, g/dL | 17.0 | 15.3 | 14.7 | 11.8 | 11.5 |
| Hematocrit, % | 48.7 | 45.5 | 43.4 | 33.9 | 32.4 |
| Platelets, × 103/μL | 82 | 41 | 37 | 37 | 73 |
| Sodium, mmol/L | 138 | 138 | 140 | 140 | 141 |
| Potassium, mmol/L | 4.9 | 4.9 | 5.0 | 5.3 | 5.3 |
| Chloride, mmol/L | 103 | 105 | 106 | 107 | 104 |
| Bicarbonate, mmol/L | 26 | 21 | 21 | 20 | 23 |
| Serum urea nitrogen, mg/dL | 33 | 79 | 105 | 143 | 116 |
| Serum creatinine, mg/dL | 1.15 | 3.61 | 4.20 | 6.42 | 6.00 |
| AST, U/L | 6030 | 16,490 | 10,613 | 6242 | 5365 |
| ALT, U/L | 7042 | 11,315 | 8946 | 5747 | 4904 |
| ALP, IU/L | 77 | 104 | 148 | 157 | 221 |
| Total bilirubin, mg/dL | 2.1 | 3.1 | 3.0 | 3.7 | 6.5 |
| Ferritin, ng/dLa | > 30,000 | ||||
| Triglyceride, mg/dLa | 154 | ||||
| Fibrinogen, mg/dLa | 206 |
Blank cell indicates laboratory study was not performed.
ALP = alkaline phosphatase; ALT = alanine aminotransferase; AST = aspartate aminotransferase;.HD = hospital day.
During the next 24 hours, the platelet count declined to 41 × 103/mL, the serum creatinine level rose to 3.61 mg/dL, and aspartate and alanine aminotransferase levels surpassed 10,000 U/L. The ferritin level exceeded 30,000 ng/mL. Fibrinogen and triglycerides were within the normal range, at 206 mg/dL and 154 mg/dL, respectively (Table 1). Given his markedly elevated transaminase levels, thrombocytopenia, hyperferritinemia, and concurrent fever, there was substantial concern and debate regarding a diagnosis of HLH. Positron emission tomography scan results did not demonstrate evidence of lymphoma, although there was increased fluorodeoxyglucose avidity in the brain lesion.
Therapeutic Intervention and Treatment
A bone marrow biopsy was performed on the second hospital day. Dexamethasone therapy (10 mg every 12 hours) was initiated on hospital day 3.
A broad infectious workup was undertaken, with negative serologic findings for HIV, cytomegalovirus, and hepatitis A, B, C, D, and E viruses. Epstein-Barr virus serostatus was consistent with prior infection without evidence of reactivation. On hospital day 3, a serum polymerase chain reaction for herpes simplex virus 2 (HSV-2) returned positive. Concurrently, a new facial rash was noted, consisting of innumerable 1- to 2-mm erythematous and hemorrhagic papules, pustules, and vesicles (Figure 1). Given the suspicion for disseminated HSV, intravenous acyclovir therapy, 10 mg/kg every 8 hours, was started. Results of the bone marrow biopsy showed no evidence of hemophagocytosis. A transjugular liver biopsy specimen revealed pathologic findings consistent with HSV hepatitis, with multifocal hepatocyte necrosis with minimal inflammation. An immunohistochemical stain was positive for HSV but negative for varicella zoster virus, cytomegalovirus, and Epstein-Barr virus.
Figure 1.
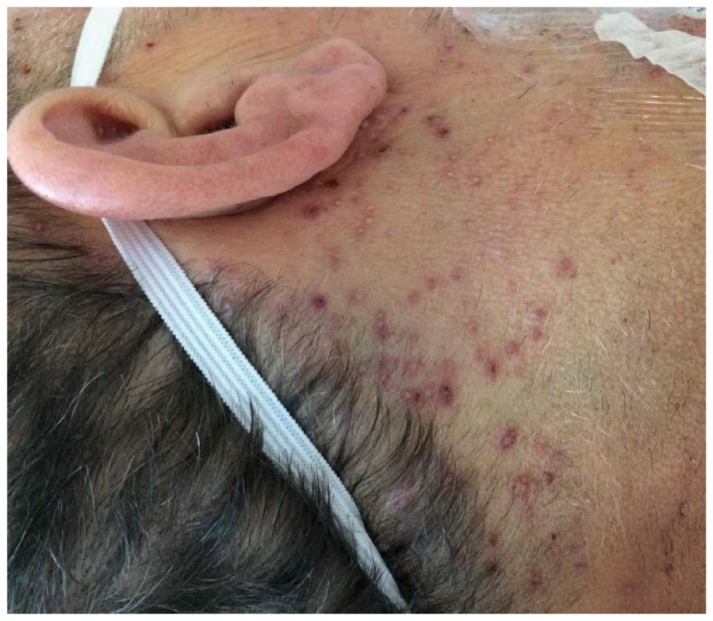
Skin lesions consisting of small erythematous and hemorrhagic papules, pustules, and vesicles present on the face, consistent with a diagnosis of herpes simplex virus infection.
Follow-up and Outcomes
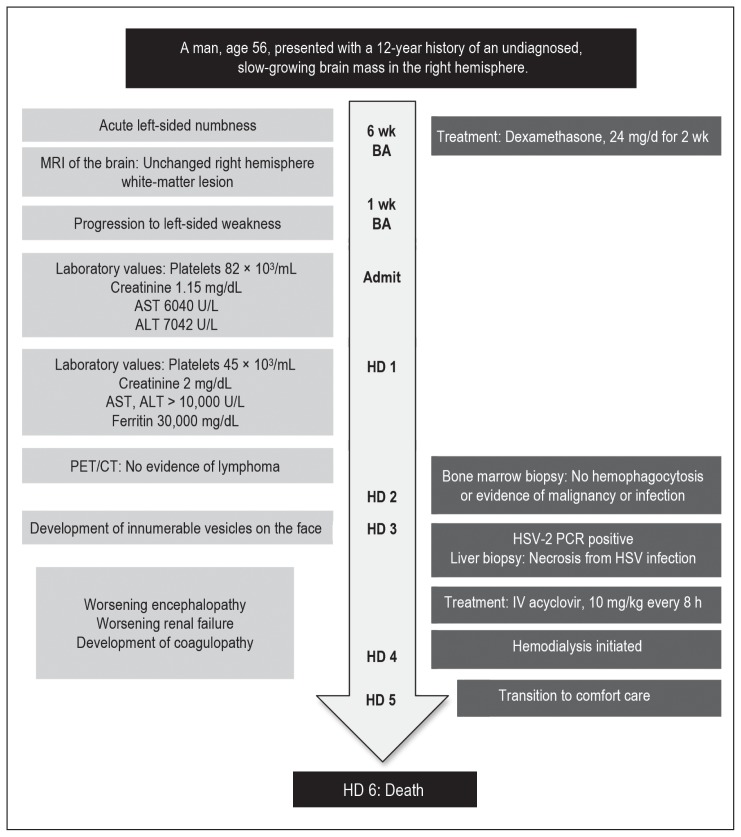
The patient experienced progressive encephalopathy, acute renal failure, and worsening coagulopathy. He was initially given hemodialysis. However, with ongoing encephalopathy and clinical decline, after a discussion with his family, he was transitioned to comfort care measures on hospital day 5. He died 24 hours later. Figure 2 shows a timeline of the case.
Figure 2.
Timeline of the case.
ALT = alanine aminotransferase; AST = aspartate aminotransferase; BA = before admission; HD = hospital day; HSV = herpes simplex virus; IV = intravenous; MRI = magnetic resonance imaging; PCR = polymerase chain reaction; PET/CT = positron emission tomography/computed tomography.
His autopsy confirmed the hepatic findings of disseminated HSV infection. There was also evidence of HSV involvement in the lung and the adrenal glands. There was focal corticomedullary necrosis of the adrenal glands, with positive immunohistochemical staining for HSV. Neuropathology revealed a high-grade infiltrating astrocytoma of the cerebral white matter on the right side. Consent to publish was obtained from the patient’s family after his death. Institutional review board approval was not required.
DISCUSSION
This case of fatal, disseminated HSV in the face of a known brain lesion challenged the physicians’ diagnostic and therapeutic decision making, given that the strategies for treating HLH and disseminated HSV are diametrically opposed. Empiric immunosuppressive treatment of HLH would have worsened the HSV infection, and treatment of HSV would have had no impact on HLH. Yet, at presentation, both disorders could have presented similarly, with hyperferritinemia, cytopenias, and progressive multiorgan dysfunction.
HLH is a syndrome of immune dysregulation with cytokine storm and the proliferation and activation of macrophages, and should be suspected in cases of major liver dysfunction, cytopenias, coagulopathy, fevers, and evidence of systemic inflammation. The 2004 diagnostic criteria for HLH (see Sidebar: 2004 Diagnostic Criteria for Hemphagocytic Lymphohistiocytois) include 5 of the following 8 features: Fever, splenomegaly, cytopenia in at least 2 cell lines, hypertriglyceridemia or hypofibrinogenemia, hyperferritinemia, elevated soluble interleukin-2 receptor, decreased or absent natural killer cell activity, and evidence of hemophagocytosis in bone marrow, cerebrospinal fluid, or lymph nodes.2,3 Our patient met only 3 of these criteria; we were unable to obtain testing for soluble interleukin-2 receptor or natural killer cell activity before his death. Additionally, there was no evidence of hemophagocytosis on either bone marrow or liver biopsy; however, this finding is also neither sensitive nor specific for HLH in adults.2–13 Primary HLH is caused by genetic and hereditary defects, whereas secondary HLH may be related to malignancy, autoimmune disorders, or infections.2–4 Viral-associated HLH is most commonly caused by Epstein-Barr virus, which carries a high mortality,5,6 but has rarely been associated with HSV. There are 5 previously reported cases of HSV hepatitis complicated by HLH (Table 2). In 1 case, HSV-2 was implicated6; in another, the patient was found to have reactivation of both HSV-1 and HSV-2.8 In these cases, ferritin values ranged from 7000 to 200,000 ng/mL. The overall mortality was 40% although only 2 of the patients described were treated for HLH with chemotherapeutic agents.7–11
Table 2.
Herpes simplex virus (HSV) hepatitis complicated by hemophagocytic lymphohistiocytosis (HLH) reported in the literature
| Source | HSV serotype | HLH criteria6 met | Ferritin (ng/dL) | Bone marrow obtained? | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Fenaux et al,7 1986 | 1 | Fever Splenomegaly Pancytopenia Hypertriglyceridemia/hypofibrinogenemia Hemophagocytosis in bone marrow |
NA | Yes | Acyclovir, IVIG, vinblastine | Died |
| Mihalcea-Danciu et al,8 2014 | 1+2 | Fever Bicytopenia Hypertriglyceridemia Hyperferritinemia |
201,000 | No | Acyclovir | Survived |
| Lasserre et al,9 1993 | 2 | Fever Bicytopenia Hemophagocytosis in bone marrow |
NA | Yes | Acyclovir, IVIG | Survived |
| Alidjinou et al,10 2015 | 1 | Fever Bicytopenia Hypertriglyceridemia/hypofibrinogenemia Hyperferritinemia Hemophagocytosis in bone marrow |
62,427 | Yes | Acyclovir, etoposide | Survived |
| Honsig et al,11 2017 | 1 | Fever Splenomegaly Pancytopenia Hyperferritinemia Increased soluble IL-2 receptor Hemophagocytosis on liver biopsy |
7058 | No | Acyclovir | Died |
IL = interleukin; IVIG = intravenous immunoglobulin; NA = not available.
2004 Diagnostic Criteria for Hemophagocytic Lymphohistiocytosis2,3.
Fulfilment of either 1) or 2):
A molecular diagnosis consistent with hemophagocytic lymphohistiocytosis is made
-
5 of the 8 diagnostic criteria are fulfilled:
Fever
Splenomegaly
-
Cytopenias affecting ≥ 2 cell lineages in the peripheral blood
○ Hemoglobin < 9 g/dL
○ Platelets < 100 × 103/mL
○ Neutrophils < 1 × 103/mL
-
Hypertriglyceridemia and/or hypofibrinogenemia
○ Fasting triglyceride > 265 mg/dL
○ Fibrinogen < 150 mg/dL
Hyperferritinemia ≥ 500 ng/L
Hemophagocytosis in bone marrow, spleen, or lymph nodes
Low or absent natural killer cell activity
Soluble CD25 (interleukin-2 receptor)
The differential diagnosis of marked hyperferritinemia is often considered limited to HLH and adult-onset Still disease, an autoinflammatory disorder whose diagnosis requires the exclusion of infectious, malignant, and autoimmune conditions. However, a retrospective analysis in adults identified that a ferritin greater than 50,000 ng/mL may also be found in individuals with renal failure, marked hepatocellular damage, infection, as well as HLH.12 Consequently, an extremely elevated ferritin level is not specific for HLH in adults. In the end, our patient’s elevated ferritin level was likely related to his disseminated HSV and resultant hepatic necrosis, with contribution from his renal failure.
Hepatitis caused by HSV infection is rare, accounting for about 1% of cases of acute liver failure in adults.1 It may be part of disseminated disease and can be caused by a primary infection or reactivation of either HSV-1 or HSV-2.14 Fulminant liver failure is associated with high mortality. HSV hepatitis and dissemination are more common in immunocompromised individuals, such as transplant recipients, patients receiving long-term corticosteroid therapy, and individuals with burns. Pregnant women and neonates also account for a large number of cases. Immunocompetent, nonpregnant adults account for 21% to 24% of all patients in prior case reports.1,14,15 In our patient, who was otherwise immunocompetent, the course of glucocorticoid therapy for his brain lesion probably was the inciting factor for his HSV infection. Common clinical manifestations and laboratory findings include fever (82%–98%), leukopenia (71%), thrombocytopenia (94%), coagulopathy (35%–90%), and elevated transaminase levels without cholestasis (anicteric hepatitis).1,14,16 There may also be concomitant acute renal failure in 65%.1 Mucocutaneous findings of herpetic infection range from 44% to 80% in literature reviews1,14–16; they are not a sensitive finding. Diagnosis is best accomplished with polymerase chain reaction for HSV DNA.1,15 Liver biopsy is the gold standard, with findings of confluent hemorrhagic necrosis of liver parenchyma, and ground-glass intranuclear inclusions consistent with viral cytopathic effect.14–17
The mortality from HSV hepatitis, even in immunocompetent individuals, is high. In a 1986 literature review, the mortality was greater than 80%,13 which was again demonstrated in a 1997 review.15 Norvell et al,1 in 2007, found an overall 74% mortality for all cases of HSV hepatitis reported since 1969. Additionally, it is sobering to note that mortality remains approximately 50% even for those treated with acyclovir.1 A 1997 review of patients with HSV hepatitis noted that only 28% of patients received antiviral agents,15 yet by 2007, this rate had increased to only 37%.1
CONCLUSION
The clinical spectrum of an acute inflammatory febrile syndrome associated with marked hepatic dysfunction, cytopenias, coagulopathy, and hyperferritinemia should prompt diagnostic consideration of not only HLH but other causes as well. Hyperferritinemia in the setting of multiorgan dysfunction or failure is a known association with HLH, but it may be seen in other inflammatory and infectious processes. Such diagnoses must be excluded before the treatment of HLH, because this treatment may have deleterious effects if the diagnosis is incorrect. In our patient, the ultimately diagnosed disseminated HSV, likely triggered by high-dose corticosteroid treatment for mass-associated brain edema, was the cause of death. HSV hepatitis has a high mortality rate, even in immunocompetent individuals, in whom it is rarely reported. One must have a high index of suspicion for this diagnosis because cutaneous lesions are not always present. The early use of acyclovir may improve outcomes, although it did not alter the course in our patient.
Acknowledgments
Kathleen Louden, ELS, of Louden Health Communications performed a primary copy edit.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
References
- 1.Norvell JP, Blei AT, Jovanovic BD, Levitsky J. Herpes simplex virus hepatitis: An analysis of the published literature and institutional cases. Liver Transpl. 2007 Oct;13(10):1428–34. doi: 10.1002/lt.21250. [DOI] [PubMed] [Google Scholar]
- 2.Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118(15):4041–52. doi: 10.1182/blood-2011-03-278127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henter JI, Horne A, Arico M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007 Feb;48(2):124–31. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 4.Schram AM, Berliner N. How I treat hemophagocytic lymphohistiocytosis in the adult patient. Blood. 2015 May 7;125(19):2908–14. doi: 10.1182/blood-2015-01-551622. [DOI] [PubMed] [Google Scholar]
- 5.Rouphael NG, Talati NJ, Vaughan C, Cunningham K, Moreira R, Gould C. Infections associated with haemophagocytic syndrome. Lancet Infect Dis. 2007 Dec;7(12):814–22. doi: 10.1016/S1473-3099(07)70290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maakaroun NR, Moanna A, Jacob JT, Albrecht H. Viral infections associated with haemophagocytic syndrome. Rev Med Virol. 2010 Mar;20(2):93–105. doi: 10.1002/rmv.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenaux P, Jouet JP, Zandecki M, et al. Hemophagocytic syndrome associated with herpes simplex virus. Apropos of a case with a fatal outcome [article in French] Nouv Rev Fr Hematol. 1986;28(5):303–7. [PubMed] [Google Scholar]
- 8.Mihalcea-Danciu M, Ellero B, Gandoin M, Harlay ML, Schneider F, Bilbault P. Herpes simplex hepatitis with macrophage activation syndrome in an immunocompetent patient [article in French] Rev Med Interne. 2014 Dec;35(12):823–6. doi: 10.1016/j.revmed.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Lasserre M, Huguet C, Terno O. Acute severe herpes simplex hepatitis with virus-associated hemophagocytic syndrome in an immunocompetent adult. J Hepatol. 1993 Jun;18(2):256–7. doi: 10.1016/s0168-8278(05)80255-7. [DOI] [PubMed] [Google Scholar]
- 10.Alidjinou EK, Dewilde A, Terriou L, Lazrek M, Engelmann I, Hober D. Persistent viral DNA detection in blood after primary herpes simplex 1 infection revealed by hepatitis with hemophagocytic syndrome. J Clin Virol. 2015 Aug;69:101–3. doi: 10.1016/j.jcv.2015.06.087. [DOI] [PubMed] [Google Scholar]
- 11.Honsig C, Beinhardt S, Tomasits J, Dienes HP. Haemophagocytic lymphohistiocytosis associated with fulminant hepatitis and multiorgan failure following primary Epstein-Barr virus and herpes simplex virus type 1 infection. BMJ Case Rep. 2017 Mar 29;2017 doi: 10.1136/bcr-2016-218310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schram AM, Campigotto F, Mullally A, et al. Marked hyperferritinemia does not predict for HLH in the adult population. Blood. 2015 Mar 5;125(10):1548–52. doi: 10.1182/blood-2014-10-602607. [DOI] [PubMed] [Google Scholar]
- 13.Schram AM, Comstock P, Campo M, et al. Haemophagocytic lymphohistiocytosis in adults: A multicentre case series over 7 years. Br J Haematol. 2016 Feb;172(3):412–9. doi: 10.1111/bjh.13837. [DOI] [PubMed] [Google Scholar]
- 14.Chase RA, Pottage JC, Jr, Haber MH, Kistler G, Jensen D, Levin S. Herpes simplex viral hepatitis in adults: Two case reports and review of the literature. Rev Infect Dis. 1987 Mar-Apr;9(2):329–33. doi: 10.1093/clinids/9.2.329. [DOI] [PubMed] [Google Scholar]
- 15.Farr RW, Short S, Weissman D. Fulminant hepatitis during herpes simplex virus infection in apparently immunocompetent adults: Report of two cases and review of the literature. Clin Infect Dis. 1997 Jun;24(6):1191–4. doi: 10.1086/513646. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman B, Gandhi SA, Louie E, Rizzi R, Illei P. Herpes simplex virus hepatitis: Case report and review. Clin Infect Dis. 1997 Mar;24(3):334–8. doi: 10.1093/clinids/24.3.334. [DOI] [PubMed] [Google Scholar]
- 17.Goodman ZD, Ishak KG, Sesterhenn IA. Herpes simplex hepatitis in apparently immunocompetent adults. Am J Clin Pathol. 1986 Jun;85(6):694–9. doi: 10.1093/ajcp/85.6.694. [DOI] [PubMed] [Google Scholar]