Abstract
Pigment production is an important biological process throughout the tree of life. Some pigments function for collecting light energy, or for visual identification, while others have dramatic antimicrobial functions, or camouflage capabilities. The functions of these pigments and their biosynthesis is of great interest if only because of their diversity. The biochemistry of echinoderm pigmentation has been intensively studied for many years and with more recent technologies, the origin and functions of these pigments are being exposed. Here we summarize the major pigment types in biology and emphasize the status of the field in echinoderms, taking full advantage of the new genomic and technologic resources for studying these important animals, and their beautiful pigmentation.
Keywords: melanin, carotenoids, porphyrins, quinones, polyketides, polyketide synthase, CRISPR/Cas9, echinoderm, sea urchin
1. Introduction
Color is everywhere on our planet. Whether it is the sky, the mountains, the forest, or the caterpillar, color provides the signals for us to recognize and discriminate objects in our world. Color in biology is especially prevalent, and perhaps a key feature for many organisms. The green, then red, then brown for the leaf of a maple tree, or the diverse skin tones of humans, color is everywhere in our biological world and the regulation of pigment biosynthesis is key for this ubiquitous quality of organisms. For many organisms, pigments convey information for mating, camouflage, socialization, molecular protection, and many other functions.
Biological pigments have broad ranges of colors, and a vast repertoire of pigment types with which to generate colors. In animals, the biosynthetic machinery for some pigments is inherently encoded within the genome of the organism e.g. melanin in humans, whereas in other organisms the color may come from a symbiont e.g. corals and their algae colleagues. Some pigments in animals are derived even from the diet, such as the dependence of the rich pink plumage in flamingos on carotenoids from their diet.
Here we consider pigments in echinoderms. These animals have been a rich source of pigment research for over 125 years (MacMunn, 1883), and for many good reasons. One, echinoderms are enormously diverse in color with rich differences even within a species. Second, many echinoderm species are easy to harvest, and highly abundant, making deep biochemical analysis feasible. Third, echinoderms are basal branching deuterostomes whose sister taxa include chordates. Thus, many of the genes involved in development, in regeneration, and in pigment formation are more closely related to mammals for example, than those of any other invertebrate. Thus, due to a rich literature in pigment research in echinoderms (see e.g. (Fox and Hopkins, 1966)), now enhanced by tools of modern molecular biology, one can begin to parse out the regulatory mechanism and biochemical steps to elucidate pigment biosynthesis, and even function, in these animals.
2. Pigments for consideration
2. 1. Melanins
are widely present in plants, animals, fungi and bacteria. Although its name is from the Greek meaning dark, or black, the many variations in melanin formation and modification result in a wide range of colors and properties. In echinoderms, melanin formation and distribution has been studied in several taxa, especially in sea urchins, sea cucumbers, and brittle stars (Fox and Hopkins, 1966). Often associated with the melanin granules in echinoderms are fluorescent pigments, which are perhaps involved in the biosynthesis of melanin and/or melanin-containing granules (Fontaine, 1962). Biosynthesis of the melanin pigment is initiated by oxidation of tyrosine, which is then polymerized into larger molecular structures that will be variably modified, depending on the cell type and organism studied. Melanin variants have been studied biochemically in echinoderm for many years e.g. see review by (Fox and Hopkins, 1966). In many animals, melanin is thought to be a block to UV radiation, and the appearance and distribution of melanin in human evolution is support for such a contention. Melanin is also involved in the innate immune system of many invertebrates in which invading microbes are coated with melanin, the so-called melanization reaction, which neutralizes the invader and aids in its destruction. The function of melanins in echinoderms is not clear, but being rich in the integument of e.g. sea cucumbers, suggests it may also function in blocking UV radiation. However, melanin has also been found in the amoebocytes of sea urchins, cells active in combating microbes located within the body cavity of the animal, suggesting a role instead (or in addition to) for protecting against pathogen infection (Jacobson and Millott, 1953). Understanding the biosynthesis of this pigment is, however, complicated since the major enzyme responsible for converting tyrosine to DOPA, tyrosinase – a copper-containing phenol oxidase, is not detectable yet in any of the echinoderm transcriptomes/genomes listed (echinobase.org; Dec 2017). Perhaps this enzyme activity is accomplished by various different genes, or is highly variable in sequence in certain taxa.
2.2. Carotenoids
are organic pigments responsible for the bright red, yellow, and orange colors that make many fruits and vegetables so recognizable. They are present in a wide variety of plants, algae, fungi, and bacteria and help the cell both absorb light energy as well as protect the chlorophyll pigment from photodamage. Several hundred carotenoids are known, all derivatives of tetraterpenes, which contain 40 carbon atoms derived from 8 polymerized isoprene units. This carotenoid building block is modified into hundreds of variants, which all have an absorption ranging from 400 nm – 550 nms (violet to green) and not in the yellow, orange and red wavelengths. Animals benefit from carotenoids as vitamin precursors (Vitamin A activity) and serve as an important antioxidant, yet these molecules must come from the diet of the animal since carotenoids are not generally produced in animals. The few exceptions to this rule are the spider mite and aphids, which appear to have acquired the genetic machinery to synthesize their own carotenoids as a result of horizontal gene transfer from a fungus (Moran and Jarvik, 2010; Novakova and Moran, 2012). Echinoderms do not have carotenoid biosynthetic genes, but do have many varied types of carotenoid pigments in their adult body. These pigments are diet derived, and then likley modified by the adult echinoderm in varied ways.
2. 3. Porphyrins
are heterocyclic organic molecules. Heme, from mammalian red blood cells, is the posterchild of porphyrins. In animals, fungi, and protozoa the commitment step of porphyrin biosynthesis is through aminolevulinic acid (ALA) synthase, which produce 5-amino levulinic acid from glycine and succinyl-CoA. The ALA building blocks are then used by the cell to make the macrocyclic ring that becomes modified in many ways to make varied porphyrin pigments (and other organic compounds). Although many echinoderms have substantial levels of porphyrins in their integument, and animals are capable of biosynthesizing porphyrin pigments, it is less clear in echinoderms which species biosynthesize their own tetrapyrrole rings and which species acquire these structures from their diet and only modify the parent molecule. Further, some echinoderms (Holothurians) appear to use tetrapyrroles as a prosthetic for their respiratory molecules. The genomic resources now available in echinoderms and the well-known pathways for porphyrin biosynthesis should enable at least a cursory test of biosynthesis of this important pigment precursor. Indeed, a gene (SPU_024016) was found in the sea urchin Strongylocentrotus purpuratus with strong identity (62% amino acid identity; e-164 significance) to the mouse gene encoding aminolevulinic acid synthase 2 (EDL07517) supporting a synthetic pathway at least in this sea urchin for the porphyrin biosynthesis (echinobase.org; (Cameron et al., 2009).
2. 4. Quinones
are a large family of reactive aromatic ring structures, of which naphthoquinones are a subgroup. These compounds are also a major family of pigments in echinoderms. Originally thought to be present naturally only in plants, the discovery of echinochrome in the coelomic fluid of sea urchins expanded that vocabulary. It is now known that naphthoquinones are prevalent in echinoderms with several major members having been identified and characterized from the larval pigment cells, the test, spines and tube feet in addition to the coelomocytes of sea urchins (Fox and Hopkins, 1966; Figure 1 and 2).
Figure 1.

Pigmentation differences in echinoderms. A) The sea urchin Strongylocenrotus purpuratus (purple) adjacent to the sea star Patiria miniata (batstar) and to B) the sea urchin Lytechnus variegatus. Note the stripped banding of pigment on the tube feet (arrow in A). (Photo in (A) complements of Adrian Reich.
Figure 2.
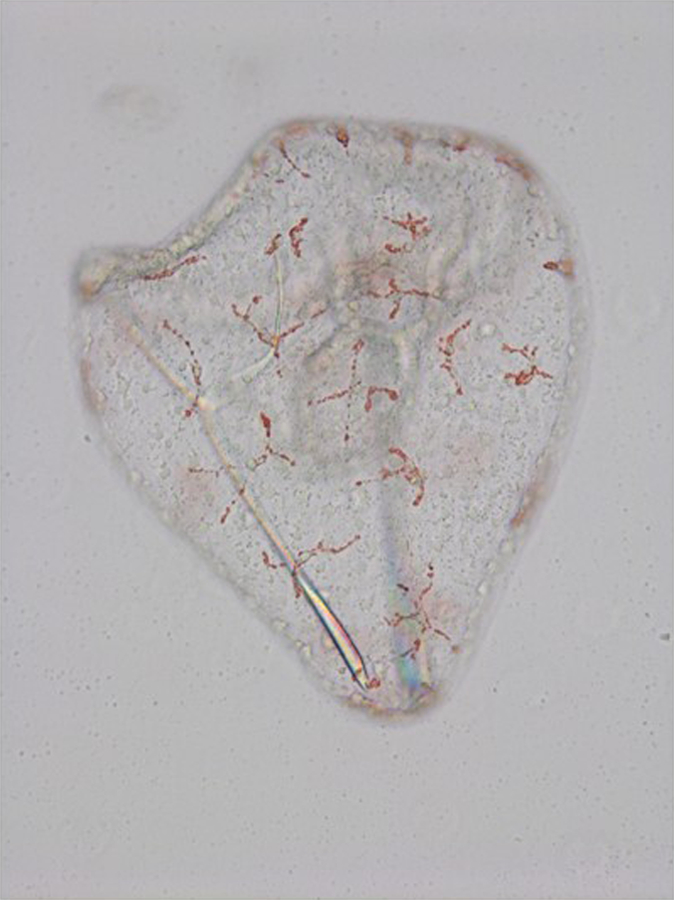
Sea urchin (S. purpuratus) pluteus larvae showing the reddish pigment cells embedded in the ectoderm. Pigment cells have a stellar shape, and they can extend and re-tract their pseudopods and move within the ectoderm.
Within the family of quinones are the naphthoquinones, a group of heterocyclic polyketides that are highly modified into different pigment types. The sea urchin has several closely related naphthoquinones, including Echinochrome A, and Spinochromes A (Figure 3). This review will now focus on the enzyme presumably responsible for the production of these polyketides, polyketide synthase (PKS).
Figure 3.

The chemical structure of two of the many polyketide pigments in echinoderms. From Fox and Hopkins, 1966.
3. What is the major enzymatic activity of polyketide synthases?
Polyketide synthases (PKSs) catalyze the synthesis of a wide variety of compounds ranging from antibiotics to plant pigments (Hopwood, 1997; Staunton and Weissman, 2001; Hopwood 2004; Weissman, 2015a; Weissman, 2015b). PKSs have been mainly studied in bacteria, fungi and plants and produce compounds with antibiotic, mycotoxic, anti-inflammatory and anti-cancer properties (Hopwood, 2004). Plants polyketides have very diverse roles including flower pigmentation, UV and visible light responses, and symbiotic plant–pathogen interactions (Schroder et al., 1998; Winkel-Shirley, 2002). Due to the vast array of biological activities of polyketide compounds and especially to their medicinal properties, research on the structural biology of PKSs has been growing. These studies led to the new field of genetic engineering and synthetic biology of PKSs to produce new compounds (e.g. Weissman 2015b; Weissman 2016).
The sea urchin SpPks1 belongs to the type I class of PKSs (Castoe et al., 2007) and it is required for the larval echinochrome biosynthesis (Calestani et al., 2003). The major pigments of many echinoderms are echinochromes and spinochromes (Figure 3) and these are napthoquinone polyketides. The PKS encoded by XM__788471.4 (Sp-Pks1) is a multifunctional enzyme that contains KS-AT-DH-MT-ER-KR-ACP domains most typical of a branched (methylated) saturated fatty acid synthase, although there is no TE-like domains in this protein. This PKS therefore most likely belongs to the class known as iterative PKSs, in which the product of one round of chain elongation and modification serves as the substrate for repeated rounds of the same set of elongation/modification reactions. There are no good algorithms to predict the length of the final chain or any deviations from the canonical methylation-ketoreduction-dehydration-enoyl reduction for any given cycle of elongation/modification.
3.1. How is echinochrome synthesized?
Echinochrome seems to be produced from acetic acid molecules (Salaque et al., 1967), which are condensed and modified by SpPKS1. In addition to Sp-PKS1 a flavin monooxygenase (Sp-FMO1) appears to be involved in the oxygenation of the precursor of echinochrome since Sp-FMO1knock-downs produced albino larvae (Calestani et al., 2003). A similar involvement in the oxygenation of phenols to quinones compounds has been observed in the biosynthesis of the antibiotic actinorhodin by Streptomyces coelicolor (Kendrew et al., 1997). Since polyketides are cytotoxic, most likely the echinochrome in the sea urchin larval pigment cells is either conjugated with some protein or other compound(s) to neutralize the toxicity and/or sequestered in vesicles, to protect the cell synthesizing the toxic compound.
3. 2. PKS diversity in sea urchins
Several PKSs have been identified in different sea urchin species and they are highly conserved. Lytechinus variegatus PKS (NCBI sequence ID DAA05847.1) has 92% amino acid sequence identity with Sp-Pks1; a partial sequence has been isolated in Hemicentrotus pulcherrimus (NCBI sequence ID BAQ21222.1 with a 99% amino acid sequence identity, from Strongylocentrotus intermedius (NCBI sequence ID AEL14404.1) with 99% amino acid sequence identity, from Paracentrotus lividus (NCBI sequence ID SMC15637.1) with a 90% amino acid sequence identity. A second PKS has been identified in the sea urchin genome, transcriptome and by phylogenetic analysis, Sp-PKS2 (NCBI sequence ID NP_001239013.1). Sp-PKS2 however, has only 32% amino acid sequence identity with Sp-PKS1 (Castoe et al., 2007; Tu et al., 2014). Interestingly, Sp-Pks2 is not expressed in pigment cells but in the skeletogenic cells of the sea urchin embryo and larva (Beeble and Calestani, 2012). Sp-PKS2 is phylogenetically close to several bacterial PKSs and is composed by the conserved domains KS-AT-DH-ER-KR-PP-TE (Castoe et al., 2007). Recently it was discovered that SpPKS2 is required for skeletogenic development, specifically for the calcium carbonate mineralization process of the spicules (Hojo et al., 2015). A PKS homologous to Sp-PKS2 is also required for the initial step of biomineralization in the ear otolith of medaka (Hojo et al., 2015).
3. 3. Phylogeny of PKS.
The phylogenetic relationships of PKSs are quite complex. PKSs are present in bacteria, fungi, plants and more animals than previously thought (Hojo et al., 2015). PKSs are related to fatty acid synthases (FAS) but they have a separate evolutionary origin (Castoe et al., 2007; Hojo et al., 2015). Sp-PKS1 and Sp-PKS2 are most closely related to PKSs from the lancelet Branchiostoma floridae and more distantly related to another animal PKS clade, which includes corals, hemichordates, reptile, birds, fish and marsupials (Hojo et al., 2015). A PKS, belonging to a separate animal clade, has been also identified in the protostome Caenorhabditis elegans but not in Drosophila melanogaster (Hojo et al., 2015; O’Brien et al., 2014). No PKS orthologs have been identified in humans or other mammals (Hojo et al., 2015). Therefore PKSs seems to have been lost from several animal lineages.
3. 4. The Gene Regulatory Network of PKS.
The Sp-Pks1 gene is first activated in pigment cell precursors at the blastula stage (between 15 and 18 hours post-fertilization in S. purpuratus) and its expression is maintained throughout larval development (Calestani et al., 2003). The specification process of pigment cells is regulated by a DELTA (DL)–NOTCH (N) signaling (Sherwood and McClay, 1999; Sweet et al., 1999, 2002; McClay et al., 2000; Oliveri et al., 2002). Previous studies demonstrated that the pigment cell gene regulatory network (GRN) including Sp-Pks1 is quite shallow. In fact, Sp-Pks1 is directly activated by the transcription factors Glial Cell Missing (Sp-GCM), Sp-GATAE and Kruppel-like (Sp-KRL; Calestani and Rogers, 2010). Upstream, Sp-Gcm and Sp-GataE are directly activated by the Notch intracellular domain (Ransick et al., 2002; Ransick and Davidson, 2006; Ransick and Davidson, 2012). Sp-Krl is DL-N independent, therefore it is possible that PKS is activated by additional positive regulatory inputs. Such a GRN architecture including parallel positive regulatory inputs into differentiation genes has been also observed for the skeletogenic precursors in S. purpuratus (http://www.spbase.org/endomes/#; Sun and Ettensohn, 2014). Sp-Pks1 expression is maintained by a positive feedback loop of Sp-Gcm throughout development (Ransick et al., 2002; Ransick and Davidson, 2006), while Sp-GataE and Sp-Krl are most likely required only up to mesenchyme blastula–early gastrula stages (Calestani and Rogers, 2010).
3. 5. What is the function of the PKS-derived pigment in sea urchin?
Using two different approaches to reduce and/or eliminate PKS function, the resulting larvae are albino (Calestani et al., 2003; Oulhen and Wessel, 2016). They appear to still have pigment cells, but these cells are no longer pigmented. The albino animals still develop, swim, and feed - aside from color, the developing albino larvae are otherwise identical to the wild-type larvae. Thus, PKS and the pigments resulting from its activity, appear to not be involved in developmental signaling. The mechanisms of eliminating PKS activity has been demonstrated by both morpholino (phosphorodiamidate morpholino oligomer; MO) knock-down (Calestani et al., 2003), in which the MO is designed to block translation by sequence - specifically blocking ribosome scanning along the 5’UTR, and by CRISPR-Cas9 targeting the PKS upstream coding exons (Oulhen and Wessel, 2016). Both approaches give the same result of albinism in the larvae. With such an easy visual indicator of pigment in larvae as a read out – the CRISPR/Cas9 system appears to be over 90% efficient for gene disruption. Thus, neither the pigment, nor the PKS enzyme has any noticeable developmental role at least several days into formation of the larvae. Echinochrome and related pigments may have roles in immuno-defense (Perry and Epel, 1981; Service and Wardlaw, 1984; Hibino et al., 2006; Kiselev et al., 2013). In fact, echinochrome isolated from the coelomic fluid of the adult sea urchin Echinus esculentus showed antibiotic properties against six gram-positive and gram-negative marine bacteria (Service and Wardlaw, 1984). In eggs echinochrome A is oxydated and released, especially after fertilization, producing H2O2 potentially at sufficient levels for anti-microbial function (Perry and Epel, 1981). Moreover Hibino et al. (2006) showed that pigment cells can phagocyte bacteria. More recently Kiselev et al. (2013) tested the effects of sea urchin larvae exposure to several pathogenic marine bacteria and found that pigment cell number and Sp-Pks1 expression were both increased as compared to untreated larvae. Thus, an immune function for these pigments might be worthy for additional testing, especially using albinos as test subjects for pigment effect.
4. Concluding Statements
With the new technologies and genomic resources available, pigment function can now be effectively tested in echinoderms both in vivo and in vitro. With genetic knockouts feasible for PKS, one can test the developmental role, the ecological role, and use these pigment-null animals in lineage analyses for rapid and easy visual identification of cellular fates and morphogenesis. While most efforts have been focused on the PKS gene activity and the polyketides produced, the presence, biosynthesis, and functions of other pigments, especially the porphyrins, for which defined genes are present in echinoderms, can now be pursued. The future looks colorful for these remarkable and brightly pigmented animals!
Figure 4.

SpPKS1 – knock down of function by CRISPR/Cas9 (Oulhen and Wessel, 2016) resulted in normal larvae except that they lacked pigment. Tests of the CRISPR/Cas-directed inactivation of genes involved in the synthesis of pigment: Larvae contain over 100 pigment cells in their ectoderm (arrows in A; inset is a magnification of the pigment (red) from control-injected embryos). (B) CRISPR/Cas9-targeted gene inactivation of the enzyme responsible for the biosynthesis of pigment, polyketide synthase (Sp-PKS1) (Calestani et al., 2003; inset shows lack of pigment). (C) Knock-down of Glial cells missing (Sp-GCM), the main transcription factor responsible for Sp-Pks1 transcription (Ransick and Davidson,2012). Elimination of either target genes resulted in albinism in otherwise healthy, developing embryos. (D) Brightfield of E and F showing Cas9 immunolabeling enrichment in the nuclei of early embryos (E), highlighted by DAPI staining (F). Scale bar, 100mm (From Oulhen and Wessel, 2016; with permission)
References
- Beeble A, Calestani C, 2012. Expression pattern of polyketide synthase-2 during sea urchin development. Gene Expr Patterns 12, 7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calestani C, Rast JP, Davidson EH, 2003. Isolation of pigment cell specific genes in the sea urchin embryo by differential macroarray screening. Development 130, 4587–4596. [DOI] [PubMed] [Google Scholar]
- Cameron RA, Samanta M, Yuan A, He D, Davidson E, 2009. SpBase: the sea urchin genome database and web site. Nucleic acids research 37, D750–D754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine AR, 1962. Colours of Ophiocomina Nigra (Abildgaard) .2. Occurrence of Melanin and Fluorescent Pigments. J Mar Biol Assoc Uk 42, 9-&. [Google Scholar]
- Fox DL, Hopkins TS, 1966. The Comparative Biochemistry of Pigments in: Boolootian RA (Ed.), Physiology of Echinodermata Interscience Publishers, New York, pp. 277–300. [Google Scholar]
- Jacobson FW, Millott N, 1953. Phenolases and Melanogenesis in the Coelomic Fluid of the Echinoid Diadema-Antillarum Phillippi. Proc R Soc Ser B-Bio 141, 231–247. [PubMed] [Google Scholar]
- MacMunn CA, 1883. Studies in animal chromatology. Proc. Bgham Philos. Soc 3, 351–407. . [Google Scholar]
- Moran NA, Jarvik T, 2010. Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science 328, 624–627. [DOI] [PubMed] [Google Scholar]
- Novakova E, Moran NA, 2012. Diversification of genes for carotenoid biosynthesis in aphids following an ancient transfer from a fungus. Mol Biol Evol 29, 313–323. [DOI] [PubMed] [Google Scholar]
- Oulhen N, Wessel GM, 2016. Albinism as a visual, in vivo guide for CRISPR/Cas9 functionality in the sea urchin embryo. Mol Reprod Dev 83, 1046–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G, Epel D, 1981. Ca2+ -stimulated production of H2O2 from naphthoquinone oxidation in Arbacia eggs. Exp Cell Res 134, 65–72. [DOI] [PubMed] [Google Scholar]
- Ransick A, Davidson EH, 2006. Cis-regulatory processing of Notch signaling input to the sea urchin glial cells missing gene during mesoderm specification. Dev Biol 297, 587–602. [DOI] [PubMed] [Google Scholar]
- Ransick A, Rast JP, Minokawa T, Calestani C, Davidson EH, 2002. New early zygotic regulators expressed in endomesoderm of sea urchin embryos discovered by differential array hybridization. Dev Biol 246, 132–147. [DOI] [PubMed] [Google Scholar]
- Ransick A, Davidson EH, 2012. Cis-regulatory logic driving glial cells missing: self-sustaining circuitry in later embryogenesis. Dev Biol 364, 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaque A, Barbier M, Lederer E, 1967. Sur la biosynthèse de l’échinochrome A par l’oursin Arbacia pustulosa. Bull Soc Chim Biol 49, 841–848. [PubMed] [Google Scholar]
- Schroder J, Raiber S, Berger T, Schmidt A, Schmidt J, Soares-Sello AM, Bardshiri E, Strack D, Simpson TJ, Veit M et al. , 1998. Plant polyketide synthases: a chalcone synthase-type enzyme which performs a condensation reaction with methylmalonyl-CoA in the biosynthesis of C-methylated chalcones. Biochemistry 37, 8417–8425. [DOI] [PubMed] [Google Scholar]
- Service M, Wardlaw AC, 1984. Echinochrome-A as a bactericidal substance in the coelomic fluid of Echinus esculentus (L.). Comp Biochem 79B, 161–165. [Google Scholar]
- Sherwood DR, McClay DR, 1999. LvNotch signaling mediates secondary mesenchyme specification in the sea urchin embryo. Development 126, 1703–1713. [DOI] [PubMed] [Google Scholar]
- Staunton J,Weissman KJ, 2001. Polyketide biosynthesis: a millenium review. Nat Prod Rep 18, 380–416. [DOI] [PubMed] [Google Scholar]
- Sun Z, Ettensohn CA, 2014. Signal-dependent regulation of the sea urchin skeletogenic gene regulatory network. Gene Expr Patterns 16, 93–103. [DOI] [PubMed] [Google Scholar]
- Sweet HC, Hodor PG, Ettensohn CA, 1999. The role of micromere signaling in Notch activation and mesoderm specification during sea urchin embryogenesis. Development 126, 5255–5265. [DOI] [PubMed] [Google Scholar]
- Sweet HC, Gehring M, Ettensohn CA, 2002. LvDelta is a mesoderm-inducing signal in the sea urchin embryo and can endow blastomeres with organizer-like properties. Development 129, 1945–1955. [DOI] [PubMed] [Google Scholar]
- Tu Q, Cameron RA, Davidson EH, 2014. Quantitative developmental transcriptomes of the sea urchin Strongylocentrotus purpuratus. Dev Biol 385, 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman K, 2015a. Uncovering the structures of modular polyketide synthases. Nat Prod Rep 32, 436–453. [DOI] [PubMed] [Google Scholar]
- Weissman K, 2015b. The structural biology of biosynthetic megaenzymes. Nat Chem Biol 11, 660–670. [DOI] [PubMed] [Google Scholar]
- Weissman K, 2016. Genetic engineering of modular PKSs: from combinatorial biosynthesis to synthetic biology. Nat Prod Rep 33, 203–230. [DOI] [PubMed] [Google Scholar]
- Winkel-Shirley B, 2002. Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol 5, 218–223. [DOI] [PubMed] [Google Scholar]


