Abstract
Background and objectives:
Vaccination with Human Papillomavirus (HPV) vaccine is recommended for 11–12 years-old, but uptake is suboptimal. Current messaging focuses on HPV infection transmission and prevention. Parents and providers are often uncomfortable discussing sexual practices of adolescents, contributing to the delay/refusal of vaccine. We created a cervical cancer-salient message encouraging HPV vaccination, emphasizing disease salience and disease threat, while promoting self-efficacy. We hypothesized this message would have greater effects on vaccine confidence and intent to vaccinate compared to Centers for Disease Control and Prevention (CDC) and non-vaccine control messages.
Methods:
A 3-arm randomized trial was conducted. Parents of girls aged 9–17 were eligible for the study. We measured participants’ vaccine confidence and intent to vaccinate at baseline and post intervention message. Recruitment and surveys were administered online through Amazon Mechanical Turk.
Results:
762 participants completed both surveys. We saw modest increases in vaccine confidence when comparing cervical cancer arm and control arm, and CDC arm and control arm; estimates were not statistically significant. The odds of reporting intent to vaccinate among the cervical cancer message arm were 1.13 times the odds of reporting intent to vaccinate in the control arm (95% CI: 0.30. 4.29). Intent to vaccinate was also not statistically significantly different between CDC message arm and control arm (OR = 1.25, 95%CI: 0.66, 2.37).
Conclusion:
Neither message had effect on intent to vaccinate, highlighting need for research to identify successful messaging strategies for HPV. Exploratory analyses suggest among parents with ‘Low’ vaccine confidence at baseline, the cervical cancer framed message may be more effective in changing intention than the CDC message or non-vaccine control. Future work should target groups with ‘Low’ or ‘Medium’ vaccine confidence at baseline - they may be more amenable to change, and more receptive to disease salient messaging.
Clinical Trial Registration:
ClinicalTrials.gov, Reference #: NCT03002324.
1. Introduction
Human Papillomavirus (HPV) is the most common sexually transmitted infection in the United States [1]. Persistent HPV infection can lead to cancer, with cervical cancer being the most common cancer caused by HPV among women [2]. Across genders, incidence and prevalence of HPV-related oropharyngeal cancer (OPC) have increased, making the oropharynx most common site of HPV-related cancers [2,3]. There is no cure for HPV, but HPV infections are preventable through vaccination. Three HPV vaccines are currently licensed for use in the United States, and are given in 2 or 3 dose series depending on age at vaccination [4]. Despite being recommended for more than a decade, HPV vaccine uptake remains suboptimal [5,6]. Among adolescents aged 13–17 years old, uptake of at least 1 dose of HPV vaccine was 60.4%, while uptake of 3 doses was only 37.1%. For parents, an important factor for HPV vaccine uptake is provider recommendation [7–9]; however, provider discomfort when discussing child sexuality with parents is a significant barrier for providers in recommending HPV vaccination [10–12]. Additionally, parents may deem the vaccine unnecessary as their child is not sexually active [7]. However, this reasoning highlights the lack of parental appreciation of the HPV vaccine, as they miss the fundamental goal of vaccination as a prevention strategy provided prior to exposure [7,13].
Parental decisions to vaccinate their children, specifically with the HPV vaccine, can be tied to multiple behavioral constructs. These include perceived susceptibility of HPV infection (is my child at risk of infection), perceived benefit, and perceived severity (of disease, and of vaccine related adverse events) [14,15]. This would suggest that messaging surrounding the HPV vaccine should be highly salient within these constructs. However, messages currently being used - including messages from the CDC - are not focused on perceived susceptibility of cervical cancer, or perceived severity of cervical cancer; even though these factors have been identified as predictive to vaccination status among adolescents [16]. For example, the CDC’s Vaccine Information Statement (VIS) has direct reference to HPV as a sexually transmitted disease, despite literature suggesting sexual reference is a factor contributing to vaccine refusal and delay [7,11,12]. Additionally, current CDC and other messages are not disease salient in regard to cervical cancer.
We sought to create a message to promote HPV vaccination, framing HPV vaccination as protection against cervical cancer. We designed a message to emphasize disease salience and disease threat, and promote self-efficacy. We hypothesized that a cervical cancer targeted message would have an equal or stronger effect on intent to vaccinate than currently available messages from the CDC, compared to a control message.
2. Methods
We conducted a 3-arm randomized trial, comparing three messages - a CDC HPV message, a cervical cancer-salient message, and a non-vaccine control message - on attitudes towards adolescent vaccines and intent to vaccinate adolescents with the HPV vaccine. This study was conducted among parents of females 9–17 years old. Study participants were recruited online through Amazon Mechanical Turk, and followed for 2 weeks to assess attitudes toward vaccination, vaccine confidence and intent to vaccinate before and after message delivery. The Emory University Institutional Review Board approved all study activities (Study #00087211). This trial is registered on ClinicalTrials.gov, under reference number NCT03002324.
Eligibility criteria for our study included: men and women who were over 18 years at the time of recruitment, who had at least one daughter aged 9–17 years, who currently lived in the United States, and had heard of HPV. Recruitment was conducted through Amazon Mechanical Turk web services, and screening and survey administration were conducted using SurveyMonkey. Participants, regardless of eligibility, were given $0.05 for successfully completing the screening questions. Participants eligible to enroll in the study who finished the baseline survey were rewarded $0.95, to a total of $1.00. Participants who returned 2 weeks later to complete the follow-up survey were rewarded an additional $2.00, for a total $3.00 in compensation for all counted in the final sample.
Participants randomized to the non-vaccine related control arm read a passage about bird feeding, which was used as a control in similar trials [17,18]. Participants randomized to the CDC message arm read a message taken almost directly from the CDC VIS on HPV [19] that was minimally altered for length and clarity. Participants randomized to the cervical cancer-salient messaging arm read a message developed by the study team. All messages fell between 8.7 and 9.1, inclusively, on the Flesh-Kincaid grade level reading scale. This range was used to keep our messages’ reading level consistent and comparable to the CDC message, which has a reading level of 9.1.
There were two co-primary outcome measures in this study: (1) vaccine confidence, quantified by change in score on the Vaccine Confidence Scale (VCS) [20,21], and (2) intent to vaccinate daughters with the HPV vaccine, measured through questions constructed by the study team. The VCS is an 8-point questionnaire built on constructs of ‘benefits’, ‘harms’ and ‘trust’. Four questions on the scale contribute to the ‘benefits’ factor, and are related to safety and advantages of vaccinating your teenager. Two questions on the scale correspond to the ‘harms’ factor, and touch on perceived negative effects of vaccinating your teenager, including adverse events. The final two questions of the scale focus on the parent and healthcare provider relationship, which correspond to the ‘trust’ factor [20]. The response to each of these eight statements is a scaled response from 0 to 10, with higher score relating to positive attitudes towards vaccines [20]. Overall VCS scores were calculated by averaging the numeric answers to the eight questions, while reverse coding the responses for the two ‘harms’ related questions. We assessed participant’s scores on the VCS prior to message delivery, with comparison to scores after they received one of the randomized messages.
The baseline survey assessed participants’ attitudes towards vaccines, quantified hesitancy toward vaccines, knowledge of HPV, and sociodemographic characteristics. Key sociodemographic data collected included the eligible child’s age, parent age, race/ethnicity of parent, gender of parent, number of children in household, average income of household, marital status of participant and participants’ education level. Questions used to assess participants’ attitudes, knowledge, and beliefs during this study were adapted directly from the Vaccine Confidence Scale and the Parent Attitudes about Childhood Vaccines (PACV) short scales [20–23]. Participants were asked if their child has received at least one dose of HPV vaccine (yes/no/I don’t know), and if they intend to complete the series (if yes) or their intent to vaccinate their child (if no or I don’t know). In analysis, these questions were combined to create one value for intention. The post intervention questionnaire included Vaccine Confidence Scale and PACV short scale questions, as well as six questions about overall engagement in the messages. Participants were again asked about their intent to complete the vaccine series or intent to vaccinate their child with the HPV vaccine, dependent on their child’s vaccine status.
Sample size calculations were completed using PASS (version 11, NCSS LLC, Kaysvile, Utah) using the one-way ANOVA procedure. A mean score of 8.19 (standard deviation = 3.0) on the Vaccine Confidence Scale was used as the baseline, based on the work of Gilkey et al. on the Vaccine Confidence Scale [20]. Given these parameters, the proposed number of study participants was 699, with 233 participants in each study arm, to yield 90% power to detect a 0.5-point change in VCS. We assumed 50% of participants would return for the second survey, and thus aimed to enroll 1450 participants at baseline.
Descriptive statistics were used to summarize participant sociodemographic characteristics. Bivariate analyses were conducted to assess randomization of sociodemographic characteristics by intervention arm using chi-square and t tests. Differences between post-intervention and baseline VCS scores were computed and used as a primary outcome. Mean differences in VCS scores were compared between each intervention arm and the control arm using unpaired t tests with unequal variance assumptions.
For all regression analyses, the sample was restricted to allow for comparisons between one intervention arm and the control arm, with independent comparisons between the CDC message and control arms, and the cervical cancer-salient message and the control arms. Logistic regression models were used to compare respondents who reported an intent to vaccinate to those who did not report intent to vaccinate by intervention message. We stratified by baseline intention to vaccinate to account for prior intentions. Generalized linear regression models were run to examine the relationship between mean difference in VCS scores between each intervention arm and the control arm. Sociodemographic variables identified in bivariate analyses as significantly different between arms were included in the model as control variables.
Exploratory analyses were conducted to identify potentially sensitive subgroups of future interest, and also to understand overall engagement of participants with the intervention. Frequency statistics were calculated for a set of six engagement questions by intervention arm, which were all answered on a 7-point Likert-type scale of ‘‘Strongly Disagree” to ‘‘Strongly Agree” with the statement. Baseline and post intervention mean VCS scores were grouped into three classes, ‘‘Low Confidence” for VCS scores <6, ‘‘Medium Confidence” for VCS scores 6–8, and ‘‘High Confidence” for VCS scores >8. Differences in classification were examined by each intervention message. Additionally, VCS scores were calculated by the three constructs of the scale, ‘Benefits’, ‘Harms’, and ‘Trust’. Mean VCS scores for each construct were calculated at baseline and follow-up, and compared by message to identify if messages differentially affected populations by scale factor. When stratified by baseline confidence, change in intention to vaccinate was compared to assess if particular subgroups had a greater increase in intention to vaccinate by intervention message. All analyses were conducted in SAS 9.4 (Cary, NC) at an alpha level of 0.05.
3. Results
A total of 16,474 participants were assessed for eligibility using Amazon Mechanical Turk. Of those, 1479 participants were eligible for entry in the study, and 1320 completed the baseline survey and were invited to participate in the second survey. Nine hundred and two participants were randomized to an intervention arm: 323 to the CDC message arm, 296 to the cervical cancer message arm, and 283 to the control message arm. The final study sample included 762 participants, with 57.7% of eligible sample accounted for in the analysis (Fig. 1).
Fig. 1.
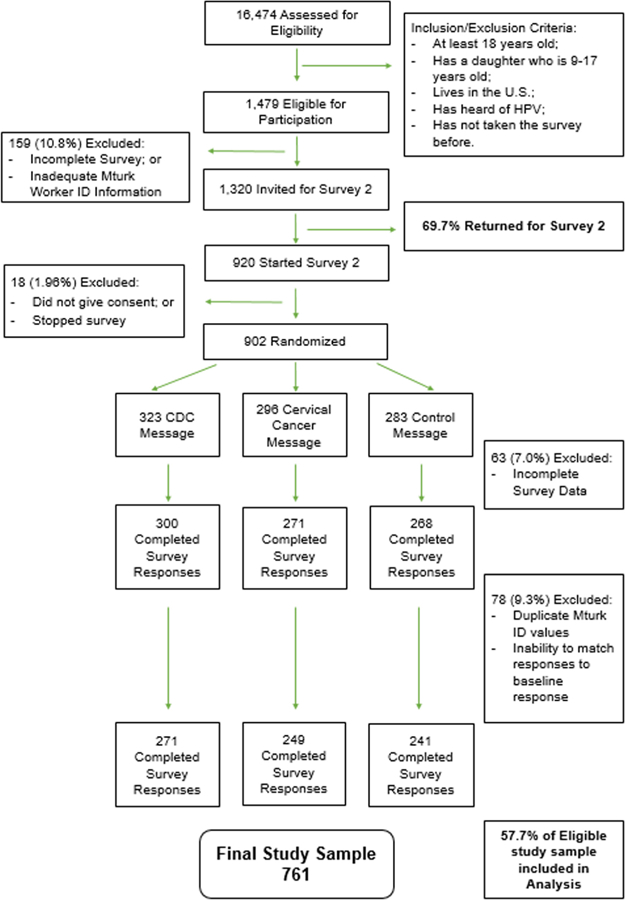
Study CONSORT Diagram: Selection, Inclusion and Exclusion Criteria, and Randomization.
Most respondents were female (70.5%), white (74.7%) and married (76.2%) (Table 1). Total household income was evenly distributed between six categories, with 12.5% of the sample reporting earning less than $25,000 per year, and 20.1% of the sample reporting earning over $100,000 per year. The sample was evenly distributed by daughters’ age in three categories: 9–11 years old, 12–14 years old, and 15–17 years old. A large proportion of the sample reported having some college education or college degree (67.5%). Bivariate analyses indicated a successful randomization, and identified baseline differences between daughters’ age by intervention message received (p < .05) (Table 1).
Table 1.
Demographic Characteristics of study sample by randomized message.
| CDC Message (n = 271) |
Cervical Cancer Message (n = 249) |
Control Message (n = 242) |
p-value | ||||
|---|---|---|---|---|---|---|---|
| n or mean | % or SD | n or mean | % or SD | n or mean | % or SD | ||
| Parent Gender | .52 | ||||||
| Male | 73 | 26.9 | 70 | 28.1 | 75 | 31.0 | |
| Female | 197 | 72.7 | 176 | 70.7 | 164 | 67.8 | |
| Parent Age, Years | 39.5 | 7.6 | 39.2 | 6.6 | 40.2 | 7.3 | .83 |
| Parent Race/Ethnicity | .59 | ||||||
| White | 201 | 74.2 | 188 | 75.5 | 180 | 74.4 | |
| African American | 19 | 7.0 | 21 | 8.5 | 9 | 3.7 | |
| Asian | 18 | 6.6 | 8 | 3.2 | 16 | 6.6 | |
| Hispanic | 12 | 4.4 | 10 | 4.0 | 11 | 4.6 | |
| American Indian/Alaska Native Hawaiian/Pacific Islander | 14 | 5.2 | 11 | 4.4 | 18 | 7.4 | |
| Other/Multi-race | 7 | 2.6 | 11 | 4.4 | 8 | 3.3 | |
| Parent Marital Status | .66 | ||||||
| Single, Never Married | 24 | 8.9 | 23 | 9.2 | 19 | 7.9 | |
| Married | 208 | 76.7 | 183 | 73.5 | 190 | 78.5 | |
| Widowed/Divorced/Separated | 39 | 14.4 | 43 | 17.3 | 33 | 13.6 | |
| Household Income | .71 | ||||||
| Less than $25,000 | 44 | 16.2 | 25 | 10.0 | 24 | 9.9 | |
| $25,000–$34,999 | 34 | 12.6 | 34 | 13.6 | 26 | 10.7 | |
| $35,000–$49,999 | 29 | 10.7 | 37 | 14.9 | 42 | 17.4 | |
| $50,000–$74,999 | 57 | 21.0 | 63 | 25.3 | 52 | 21.5 | |
| $75,000–$99,999 | 46 | 17.0 | 48 | 19.3 | 48 | 19.8 | |
| Over $100,000 | 61 | 22.5 | 42 | 16.9 | 50 | 20.7 | |
| Number of children in household | .31 | ||||||
| 1 child | 54 | 19.9 | 32 | 12.8 | 40 | 16.5 | |
| 2 children | 92 | 34.0 | 102 | 41.0 | 99 | 40.9 | |
| 3 children | 68 | 25.1 | 54 | 21.7 | 58 | 24.0 | |
| 4 or more children | 57 | 21.0 | 61 | 24.5 | 45 | 18.6 | |
| Parent Education Level | .68 | ||||||
| High School or GED | 34 | 12.6 | 24 | 9.6 | 25 | 10.3 | |
| College Degree | 176 | 64.9 | 175 | 70.3 | 163 | 67.4 | |
| Graduate or Professional Degree | 61 | 22.5 | 50 | 20.1 | 54 | 22.3 | |
| Daughter’s Age | .05 | ||||||
| 9–11 years old | 99 | 36.5 | 83 | 33.3 | 75 | 31.0 | |
| 12–14 years old | 97 | 35.8 | 68 | 27.3 | 83 | 34.3 | |
| 15–17 years old | 75 | 27.7 | 98 | 39.4 | 84 | 34.7 | |
| Vaccine Confidence Scale Score | .31 | ||||||
| Baseline | 7.4 | 2.0 | 7.2 | 2.0 | 7 | 2.0 | |
| Vaccine Intention | .18 | ||||||
| Baseline Intent = Yes | 166 | 61.3 | 134 | 53.8 | 148 | 61.2 | |
| HPV Vaccine Uptake at Baseline | |||||||
| At least 1 dose of HPV vaccine | 90 | 33.2 | 73 | 29.3 | 89 | 36.8 | .21 |
| Vaccine Confidence Scale Classifications at Baseline | .24 | ||||||
| Low Confidence | 56 | 20.6 | 65 | 24 | 57 | 23.6 | |
| Medium Confidence | 95 | 35.1 | 78 | 28.8 | 71 | 29.3 | |
| High Confidence | 120 | 44.3 | 128 | 47.2 | 114 | 47.1 | |
We saw modest increases in VCS score when controlling for daughters age when comparing cervical cancer-salient message arm and control arm, and CDC arm and control arm, however estimates were not statistically significant (Table 2). When examining differences in VCS scores by the scale sub factors ‘Benefits’, ‘Harms’, and ‘Trust’, we saw the largest increase in benefits score in the CDC message arm (Table 3). When examining those who reported no intent to vaccinate at baseline, the odds of reporting intent to vaccinate among participants randomized to the cervical cancer-salient message arm was 1.13 times the odds of reporting intent to vaccinate in the control group (95% CI: 0.30, 4.29), however, these results were not statistically significant. Similarly, within the group reporting no intent to vaccinate at baseline, the odds of reporting intent to vaccinate among participants randomized to the CDC message was 1.25 times the odds of reporting intent to vaccinate in participants randomized to the control arm, these results were not statistically significant (95% CI: 0.66, 2.37) (Table 4).
Table 2.
Estimated Increase in Vaccine Confidence Scale Score with 95% Confidence Intervals, by Intervention Message, when Compared to a Control Message, from Linear Risk Regression Models.
| Estimate | 95% CI | ||
|---|---|---|---|
| Cervical Cancer Message | 0.1 | −0.08 | 0.27 |
| CDC Message | 0.13 | −0.04 | 0.29 |
Table 3.
Mean Vaccine Confidence Scale (VCS) Scores for each message at baseline and follow-up, separated by scale factors.
| Scale Score (8 items) |
Benefits (4 items) |
Harms (2 items) |
Trust (2 items) |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Difference (SD) | Mean (SD) | Difference (SD) | Mean (SD) | Difference (SD) | Mean (SD) | Difference (SD) | |
| Control Message | ||||||||
| Baseline | 7.35 (2.04) | −0.04 (0.93) | 7.77 (2.20) | −0.05 (1.03) | 4.01 (2.76) | −0.2 (2.05) | 7.87 (2.20) | −0.11 (1.49) |
| Post Message | 7.29 (1.98) | 7.72 (2.19) | 3.99 (2.53) | 7.75 (2.22) | ||||
| CDC Message | ||||||||
| Baseline | 7.42 (2.04) | 0.08 (0.97) | 7.73 (2.28) | 0.12 (1.16) | 3.69 (2.64) | −0.4 (1.75) | 7.90 (2.09) | 0.05 (1.52) |
| Post Message | 7.51 (1.93) | 7.85 (2.15) | 3.65 (2.49) | 7.94 (2.04) | ||||
| Cervical Cancer Message | ||||||||
| Baseline | 7.12 (1.97) | 0.06 (1.01) | 7.43 (2.23) | 0.10 (1.14) | 3.99 (2.61) | 0.07 (2.02) | 7.60 (2.00) | 0.07 (1.41) |
| Post Message | 7.19 (1.89) | 7.54 (2.12) | 4.06 (2.45) | 7.68 (2.00) | ||||
Table 4.
Estimated Odds Ratios and 95% Confidence Intervals for the Effect of Intervention Message on Intent to Vaccinate, Compared to Non-Vaccine Control.
| Estimate | 95% CI | ||
|---|---|---|---|
| Intent to Vaccinate at Baseline | |||
| Cervical Cancer Message | 0.90 | 0.58 | 2.06 |
| CDC Message | 0.93 | 0.28 | 3.12 |
| No Intent to Vaccinate at Baseline | |||
| Cervical Cancer Message | 1.13 | 0.3 | 4.29 |
| CDC Message | 1.25 | 0.66 | 2.37 |
In secondary analysis, we found that 52% of the CDC message arm and 48% of the cervical cancer-salient message arm agree or strongly agree with the statement, ‘‘I learned something from this passage about vaccines.” When asked about the statement, ‘‘I could really relate to this passage.” 51% of the CDC message arm and 50% of the cervical cancer-salient message arm reported they agree or strongly agree with the statement (Fig. 2). When looking at participants that reported low vaccine confidence at baseline, 12.3% changed intent to vaccinate from ‘No’ to ‘Yes’ in the cervical cancer-salient message arm, compared to 5.3% and 5.4% in the control and CDC message arm, however these results were not statistically significant (Fig. 3).
Fig. 2.
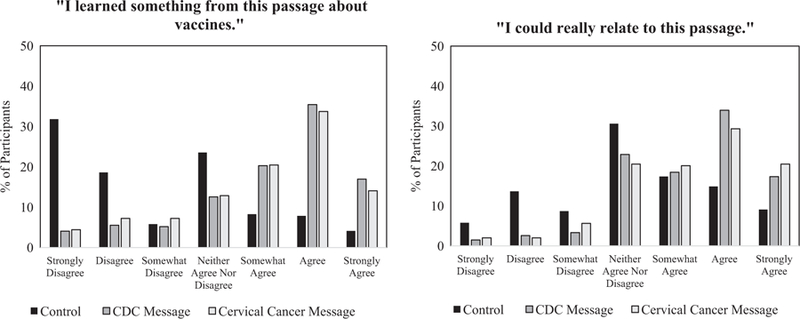
Participant responses to 7-point Likert-Scale engagement questions, by intervention message.
Fig. 3.
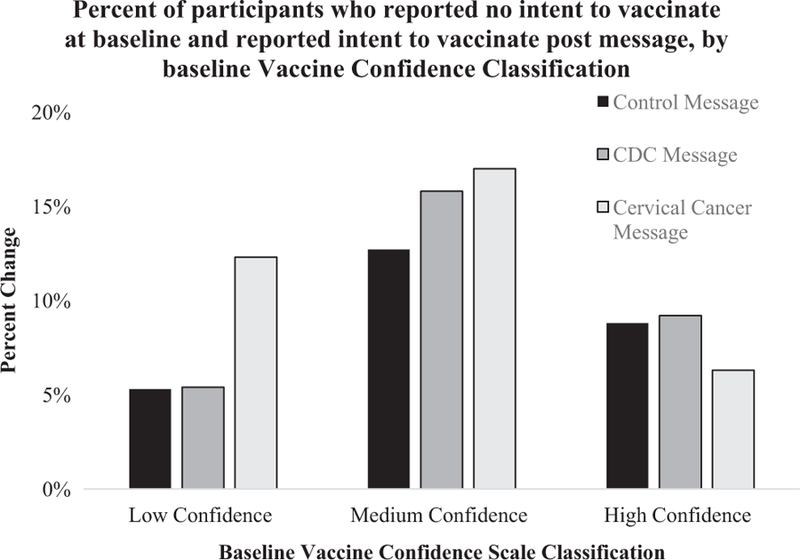
Participants who identified no intent to vaccinate at baseline, and shifted to reporting intent to vaccinate after intervention. 12.3% of participants with low vaccine confidence at baseline in cancer-salient arm changed intentions, compared to 5.4% and 5.3% of participants with low vaccine confidence at baseline in CDC and control arms.
4. Discussion
By incorporating current CDC messages into this study, we were able to provide an important and relevant comparison between a cancer-focused message and currently available CDC materials. Our results suggest that neither message affected intention to vaccinate or overall vaccine confidence in the study population. Other studies have examined the effects of the HPV VIS on parental HPV vaccine acceptability. In a randomized study conducted by Dempsey et al., researchers found that parents given information about HPV were no more likely to be accepting of the HPV vaccine and no more likely to get their child vaccinated compared to parents given no information [24]. Our study findings are in line with this work, and add that written information about HPV also does not increase parental intent to vaccinate their children, or overall confidence in vaccines.
The cervical cancer passage constructed by the study team was designed to induce participants to vaccinate against HPV. We focused on disease severity, disease threat, as and overall self-efficacy to get the vaccine because these constructs were identified in prior literature as predictive to vaccination.[12,14–16,25] The Vaccine Confidence Scale, used in the study as a primary outcome, was developed to measure overall confidence in adolescent vaccines using three factors: benefits, harms and trust [20,21]. While the message developed by the study team does highlight the benefits of vaccination and the harms of cervical cancer it was not developed to align directly with the constructs of the Vaccine Confidence Scale. With this in mind, it is not entirely surprising that our message and the CDC message did not have drastic effects on overall confidence by VCS standards. Additionally, our sample was heavily skewed at baseline to participants with ‘high’ confidence (45% of the sample), which was highly correlated with intent to vaccinate. The skewness of the sample is a possible explanation for the null result as well, because such a large portion of the sample intended to get their daughter vaccinated at baseline. However, it is reassuring to see that this new message did not backfire among parents with high intention to vaccinate [18,26]. Subgroup analyses suggest that within participants who had scores of ‘low’ confidence at baseline, there was more potential for change in intention by intervention message. Within this subgroup we saw the most movement in the cervical cancer-salient message intervention. These findings have potentially identified a sensitive subgroup, those initially having ‘low’ confidence at baseline, as those most receptive to a cancer-salient message. In light of these findings, it might be more appropriate for future studies to create a tailored intervention, using VCS as a means to identify the target population and assess vaccine intention as a primary endpoint.
Participants who saw the CDC and cervical cancer-salient message were equally engaged in their respective message compared to the bird-feeding control. Roughly 50% of the sample reported they learned something from the passage, and roughly 50% of the sample reported they could relate to the message (Fig. 2). Levels of engagement were consistent between our message and the CDC message, providing further evidence that what is available could be improved. We believe that employing different modes of delivery in future studies could increase overall engagement in our messages. A randomized trial of hospital patients showed that patients who received digital video disc (DVD) messages were more confident in knowledge of falls prevention than patients who received a written pamphlet [27]. Providing an audio-visual message, such as a video clip or infographic, could prove to be more successful than text paragraphs in getting our message across to parents and providers [27,28].
For this study, we chose to target parents of adolescent girls. As an initial evaluation of these messages, we felt targeting one group and one cancer specifically could potentially provide the best argument and framework for future interventions. In future studies, we plan to expand this reasoning to other cancers and include other populations as the target. For example, we might seek to target parents of adolescent males, with messages focused on HPV-related OPC.
There were a number of strengths to this study, the largest being study design. Social desirability bias of our study participants was limited compared to other randomized messaging studies due to the online mode of data collection. Furthermore, given the online mode of collection we had a very high percent of our sample return for follow-up (57.7%), which limits bias in the sample due to loss-to-follow-up compared to other messaging studies. The generalizability of our sample to the United States population is comparable to other studies conducted through Amazon Mechanical Turk – and studies have found that populations accessed through Amazon Mechanical Turk are at least as representative to the U.S. population as traditional subject pools [29–32]. With the strengths of the study come a few limitations. Mainly, the self-report nature of vaccine intentions and vaccine status provide a potential source of bias in our sample. However, given data were collected online, we have no reason to believe the participants intentionally provided misinformation [33]. Additionally, participants reported vaccine intention, which may or may not be correlated with vaccine uptake. Lastly, we could not confirm that participants read the intervention. This is a limitation of conducting a trial online. However, we have no evidence to suggest that participants randomized to one particular message are more or less likely to read the message. Thus, this limitation would be non-differentially biasing the estimate toward the null.
5. Conclusion
Providing parents with a cervical cancer-salient message or a HPV infection salient message had no effect on intention to vaccinate with the HPV vaccine or on overall vaccine confidence. While overall effects were null, subgroup analysis suggests that participants with a baseline ‘low’ confidence could be more amenable to intervention, and suitable as a target population for study. These conclusions highlight the need for additional research in this field to identify the best communication strategy to reach target immunization levels for the HPV vaccine.
Acknowledgments
Funding source
Dr. Bednarczyk was supported in part by NIH grant K01AI106961. The funder had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. Dr. Bednarczyk has received speaking fees and travel support from the Three Rivers Area Health Education Center for HPV-related educational talks.
Abbreviations:
- HPV
Human Papillomavirus
- CDC
Centers for Disease Control
- VIS
Vaccine Information Statement
- VCS
Vaccine Confidence Scale
- PACV
Parent Attitudes about Childhood Vaccines
- CI
Confidence Interval
Footnotes
Financial disclosure
All authors have indicated they have no financial relationships relevant to this article to disclose.
Conflict of interest
All authors have indicated they have no potential conflicts of interest to disclose.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.vaccine.2018.01.040.
References
- [1].Jemal A, Simard EP, Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst 2013;105(3):175–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Veins LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers - United States, 2008–2012. MMWR Morb Mortal Wkly Rep 2016;65 (26):661–6. [DOI] [PubMed] [Google Scholar]
- [3].Berman TA, Schiller JT. Human papillomavirus in cervical cancer and oropharyngeal cancer: one cause, two diseases. Cancer 2017;123 (12):2219–29. [DOI] [PubMed] [Google Scholar]
- [4].Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination - updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 2016;65(49):1405–8. [DOI] [PubMed] [Google Scholar]
- [5].Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years-United States, 2014. MMWR Morb Mortal Wkly Rep 2015;64(29):784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years - United States, 2016. MMWR Morb Mortal Wkly Rep 2017;66(33):874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr 2014;168(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Daley MF, Crane LA, Markowitz LE, et al. Human papillomavirus vaccination practices: a survey of US physicians 18 months after licensure. Pediatrics 2010;126(3):425–33. [DOI] [PubMed] [Google Scholar]
- [9].Bynum SA, Staras SAS, Malo TL, Giuliano AR, Shenkman E, Vadaparampil ST. Factors associated with medicaid providers’ recommendation of the HPV vaccine to low-income adolescent girls. J Adolescent Health 54(2):190–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gargano LM, Herbert NL, Painter JE, et al. Impact of a physician recommendation and parental immunization attitudes on receipt or intention to receive adolescent vaccines. Hum Vaccin Immunother 2013;9 (12):2627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hansen CE, Credle M, Shapiro ED, Niccolai LM. ‘‘It All Depends”: a qualitative study of parents’ views of human papillomavirus vaccine for their adolescents at ages 11–12 years. J Cancer Educ: Off J Am Assoc Cancer Educ 2016;31 (1):147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Krawczyk A, Perez S, King L, Vivion M, Dube E, Rosberger Z. Parents’ decision-making about the human papillomavirus vaccine for their daughters: II. Qualitative results. Hum Vaccin Immunother 2015;11(2):330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dorell C, Yankey D, Strasser S. Parent-reported reasons for nonreceipt of recommended adolescent vaccinations, national immunization survey: teen, 2009. Clin Pediatrics 2011;50(12):1116–24. [DOI] [PubMed] [Google Scholar]
- [14].Krawczyk A, Knauper B, Gilca V, et al. Parents’ decision-making about the human papillomavirus vaccine for their daughters: I. Quantitative results. Hum Vaccin Immunother 2015;11(2):322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mays RM, Sturm LA, Zimet GD. Parental perspectives on vaccinating children against sexually transmitted infections. Soc Sci Med 2004;58(7):1405–13. [DOI] [PubMed] [Google Scholar]
- [16].Reiter PL, Brewer NT, Gottlieb SL, McRee AL, Smith JS. Parents’ health beliefs and HPV vaccination of their adolescent daughters. Soc Sci Med 2009;69 (3):475–80. [DOI] [PubMed] [Google Scholar]
- [17].Horne Z, Powell D, Hummel JE, Holyoak KJ. Countering antivaccination attitudes. Proc Natl Acad Sci USA 2015;112(33):10321–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nyhan B, Reifler J, Richey S, Freed GL. Effective messages in vaccine promotion: a randomized trial. Pediatrics 2014;133(4):e835–842. [DOI] [PubMed] [Google Scholar]
- [19]. HPV (Human Papillomavirus) Vaccine - Gardasil-9: What You Need to Know. In: Prevention CfDCa, ed2016.
- [20].Gilkey MB, Magnus BE, Reiter PL, McRee AL, Dempsey AF, Brewer NT. The Vaccination Confidence Scale: a brief measure of parents’ vaccination beliefs. Vaccine 2014;32(47):6259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gilkey MB, Reiter PL, Magnus BE, McRee AL, Dempsey AF, Brewer NT. Validation of the vaccination confidence scale: a brief measure to identify parents at risk for refusing adolescent vaccines. Acad Pediatr 2016;16(1):42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Opel DJ, Taylor JA, Zhou C, Catz S, Myaing M, Mangione-Smith R. The relationship between parent attitudes about childhood vaccines survey scores and future child immunization status: a validation study. JAMA Pediatr 2013;167(11):1065–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Opel DJ, Mangione-Smith R, Taylor JA, et al. Development of a survey to identify vaccine-hesitant parents. Human Vaccines 2014;7(4):419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dempsey AF, Zimet GD, Davis RL, Koutsky L. Factors that are associated with parental acceptance of human papillomavirus vaccines: a randomized intervention study of written information about HPV. Pediatrics 2006;117 (5):1486–93. [DOI] [PubMed] [Google Scholar]
- [25].Brewer NT, Fazekas KI. Predictors of HPV vaccine acceptability: a theory-informed, systematic review. Prev Med 2007;45(2–3):107–14. [DOI] [PubMed] [Google Scholar]
- [26].Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine 2015;33(3):459–64. [DOI] [PubMed] [Google Scholar]
- [27].Hill AM, McPhail S, Hoffmann T, et al. A randomized trial comparing digital video disc with written delivery of falls prevention education for older patients in hospital. J Am Geriatrics Soc 2009;57(8):1458–63. [DOI] [PubMed] [Google Scholar]
- [28].Lee NJ, Chae SM, Kim H, Lee JH, Min HJ, Park DE. Mobile-based video learning outcomes in clinical nursing skill education: a randomized controlled trial. Comput Inform Nursing: CIN 2016;34(1):8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Goodman JK, Cryder CE, Cheema A. Data collection in a flat world: the strengths and weaknesses of Mechanical Turk samples. J Behav Decision Making 2012.
- [30].Buhrmester M, Kwang T, Gosling SD. Amazon’s Mechanical Turk: a new source of inexpensive, yet high-quality, data? Perspect Psychol Sci: J Assoc Psychol Sci 2011;6(1):3–5. [DOI] [PubMed] [Google Scholar]
- [31].Huff C, Tingley D. ‘‘Who are these people?” Evaluating the demographic characteristics and political preferences of MTurk survey respondents. Res Politics 2015;2(3). 2053168015604648. [Google Scholar]
- [32].Berinsky AJ, Huber GA, Lenz GS. Evaluating On-Line Labor Markets for Experimental Research: Amazon.com’s Mechanical Turk Vol. 202012. [Google Scholar]
- [33].Paolacci G, Chandler J, Ipeirotis PG. Running experiments on Amazon Mechanical Turk. Judgem Decision Making 2010;5(5):411–9. [Google Scholar]


