Abstract
Per- and polyfluoroalkyl substances (PFAS) are incorporated into an ever-increasing number of modern products and inevitably enter the environment and ultimately human bodies. Herein, we show that chemical ionization mass spectrometry with iodide reagent ion chemistry is a useful technique for the detection of fluorotelomer alcohols (FTOHs) and other oxygenated PFAS, including per- and polyfluoro carboxylic acids such as hexafluoropropylene oxide dimer acid. This technique offers direct, high-time resolution measurement capability with parts per trillion by volume (nanograms per cubic meter) gas-phase detection limits. Measurements were taken by direct volatilization of samples without prior processing, allowing for fast measurements and reduced sample treatment compared to established PFAS methods. We demonstrate the utility of this technique by sampling volatile and semivolatile PFAS from fluoro additives and fluoro products to quantify levels of FTOHs and identify additional fluorinated compounds for which standards were unavailable.
Graphical Abstract
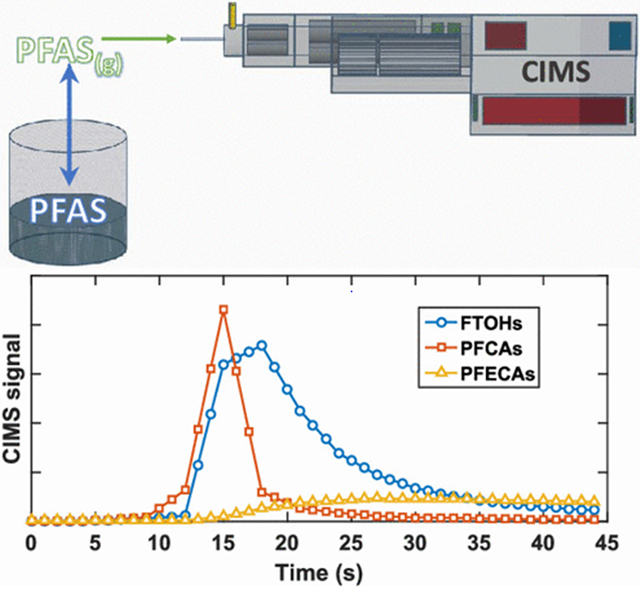
Introduction
Per- and polyfluoroalkyl substances (PFAS) are used in a wide variety of industrial applications, including textiles, materials, and chemical manufacturing, due to the beneficial mechanical properties and relative inertness they can lend to consumer and industrial products.(1) Upon being released into the environment, these compounds are subject to slow or negligible environmental degradation and are thus considered as an emerging class of pollutants capable of persistent global buildup and increasing human exposure.(2,3)
PFAS are believed to number in the thousands,(4) without considering the likely complex array of related environmental degradates. The rate at which these chemicals are produced and released to environments often vastly outpaces the time required for the development of traditional measurement approaches that typically focus on a small subset of chemicals that occurs over multiple years. As a consequence, human populations incur exposure and risk potentially for years before the presence of contamination is recognized. Advances in high-resolution mass spectrometry and data analytics provide a means of broadening the window of PFAS identification to evaluate current occurrence rather than the multiyear lag of traditional methods.(5)
Fluorotelomer alcohols (FTOHs), perfluorinated carboxylic acids (PFCAs), and perfluoro ether carboxylic acids (PFECAs) are three environmentally relevant and related classes of PFAS. In this study, we focus on four representative FTOHs, two PFCAs, and one PFECA: 4:2, 6:2, 8:2, and 10:2 FTOH; perfluorooctanoic acid (PFOA); perfluorobutanoic acid (PFBA); and hexafluoropropylene oxide dimer acid (HFPO-DA). FTOHs have been shown to be the dominant PFAS measured in landfill gas, but measurements are limited to the fraction that can be collected on impregnated foam disks.(6) FTOHs can degrade to form certain PFCAs, which are extremely stable compounds, and have been implicated to play a major role in the global dissemination of PFCAs.(7-9) PFOA has been linked to adverse health effects.(10,11) It is often termed a “legacy” PFAS because it has largely been phased out of industrial use over the past decade in the United States and Europe but remains environmentally relevant due to persistent levels in ground and surface waters and landfills. HFPO-DA (perfluoro-2-propoxypropanoic acid) is often referred to by the product name “GenX” and has received attention in states such as North Carolina where PFAS manufacturing processes take place.(12)
Considering the long-chain fluorinated “tails” present on most PFAS, these compounds have a vapor pressure considerably higher than those of equivalent hydrocarbon-based ones. For example, dodecan-1-ol (C12H26O) has an equilibrium vapor pressure that is almost 500 times lower than that of 10:2 FTOH (C12F21H5O), an analogous FTOH.(13,14) Thus, a significant fraction of PFAS can reasonably be expected to be present in the gas phase, which is therefore a likely exposure pathway.
In comparison to condensed-phase measurements of PFAS, gas-phase assessments are rare, largely due to the lack of established methods for reliable sample collection and quantification, but such measurements are needed considering recent increases in awareness that gas-phase releases may contribute significantly to overall PFAS from landfills and industrial sources. For example, attempts to constrain HFPO-DA in industrial stack emissions at one facility resulted in vastly different estimates of annual emissions: early estimates of 66.6 lb year−1 were adjusted to 2758 lb year−1, a 40-fold difference, when alternative methods were used.(15)
Previous publications regarding gas-phase PFAS field sampling techniques have largely been limited to offline analyses of liquid-phase extracts [i.e., capture of PFAS from ambient air on sorbent-impregnated disks, followed by extraction and concentration for detection by gas chromatography–mass spectrometry (GC–MS) and/or liquid chromatography–mass spectrometry (LC–MS)].(6,16) Given the collection times, these approaches are rarely able to capture valuable temporal information such as diurnal variations or emission plume intercepts that inevitably are averaged and/or diluted over the entire collection period. Clearly, a real-time continuous measurement technique for the detection of these compounds would be valuable.
In addition to field sampling, there is a need to accurately and directly quantify PFAS in products like aqueous firefighting foams, industrial dispersions, and cleaners to better constrain the contributions of such materials to the environmental PFAS burden from product manufacturing and landfills. Previous publications that reported the PFAS content of products used extraction and cleanup steps where volatile or semivolatile PFAS could potentially be lost. For example, Kotthoff et al.(1) reported 6:2 FTOH concentrations obtained using extraction procedures that included evaporation to dryness followed by reconstitution prior to analysis.
Our objective here is to demonstrate that an iodide-adduct chemical ionization mass spectrometer shows potential as a reliable method for direct detection of FTOHs and other PFAS that have previously been difficult to detect in the gas phase at appropriate time resolution. We attempt to illustrate that utility through instrument calibrations of FTOHs, PFCAs, and a PFECA and by directly sampling semivolatile PFAS emitted from four commercially available fluoro products: three fluoro surfactants and an aqueous film-forming firefighting foam. PFAS present in those products for which corresponding standards were available were quantified, and additional PFAS signals were identified by probable molecular compositions.
Materials and Methods
Chemical Ionization Mass Spectrometry
A high-resolution time-of-flight chemical ionization mass spectrometer (ToF-CIMS, Aerodyne Research Inc./TOFWERK AG) operated in negative ion mode with iodide reagent ion chemistry was used for this study (see Table S1 and Figure S1 for instrument details). This technique has been used previously to quantify inorganic and polar organic atmospheric trace gases and offers online, direct, and fast measurements without the need for lengthy collection, extraction, and/or derivatization prior to detection.(17-19) Sample air is drawn directly into the inlet where analyte species are ionized by forming molecular adducts with the iodide reagent ion, a soft ionization process that typically preserves parent ions with little fragmentation. Analyte ion detection occurs at the parent + iodide mass-to-charge ratio (m/Q), and all signals were normalized to the total reagent ion signal [I− + I(H2O)−] and reported per million counts per second (cps) of total reagent ion. As in other studies utilizing an iodide-adduct CIMS, the ion optics were tuned to maximize both the total ion current and the ratio of the iodide-water cluster to iodide [I(H2O)−/I−].(17) The latter was used as an indication of the collision-induced molecular ion declustering potential, which we hoped to minimize thereby preserving parent ions.
Air containing the gas-phase analytes was sampled directly into the instrument at a rate of 2.1 L min−1. Though the CIMS is capable of faster data acquisition (>1 Hz), full mass spectra (3–1132 Da) measured at 0.33 Hz were sufficient for the investigations presented here. Preliminary proof-of-concept tests in which the CIMS sampled the headspace above neat FTOH and per- and polyfluorocarboxylic acid standards indicated that the instrument effectively detected these classes of compounds and that the volatility was such that they were present in significant gas-phase quantities. Instrument calibrations were then performed on 4:2, 6:2, 8:2, and 10:2 FTOH, PFOA, PFBA, and HFPO-DA. During calibrations, dilute solutions of the standards were prepared in ethyl acetate at concentrations ranging from 1 to 18 ng μL−1, depending on the standard. During calibrations, 1–10 μL of the standard solution was injected onto a 47 mm diameter, 2.0 μm pore size polytetrafluoroethylene (PTFE) filter (Pall Corp.) within a perfluoroalkoxy (PFA) polymer filter holder (Savillex) placed immediately upstream of the CIMS inlet. Humidified clean air [50% relative humidity (RH)] was used as the dilution/carrier gas. Upon contact with the filter, analytes were continuously volatilized into the CIMS, and signals were integrated until <1% of the maximum value remained, typically ∼5 min, to determine the total signal. The instrument response or sensitivity was determined from a linear fit of the integrated signals generated by varying the volumes of standard injections.
Volatile and semivolatile compounds within condensed-phase commercial samples were transferred through a short section of PTFE tubing (15 cm length, 3.175 mm inner diameter) to the inlet of the CIMS by passing nitrogen gas through the headspace of a sealed vessel containing a small aliquot of the sample (20–500 μL) at a rate of 0.5 L min−1. Humidified clean air (50% RH) made up the remainder of the inlet draw. Transfer distances were kept to a minimum (<25 cm) to minimize potential line losses during sampling, though there was no indication that analyte species were lost to the PTFE or PFA surfaces. During the measurements and calibrations described above, the PTFE and PFA surfaces of the filters, filter holder, and transfer lines did not produce signals above the detection limits for any of the compounds or chemical compositions reported (see Figure S2 for a PFOA example).
Commercial Fluoro Products
Four commercial fluoro products were sampled for the presence of PFAS, with special emphasis on FTOH detection: Masurf FS-115 Fluorosurfactant, Zonyl FSA Fluorosurfactant, Capstone FS-35 Fluorosurfactant, and Arctic U.S. Type 3 (3%) MIL-SPEC AFFF Foam Concentrate, an aqueous film-forming foam (AFFF) used for firefighting applications. Products were stored in sealed containers at room temperature prior to sampling. We acknowledge the potential that some fraction could have undergone some degree of degradation during storage. However, the focus of this investigation was demonstration of the CIMS measurement of volatile PFAS present in complex mixtures, and the fluoro products, regardless of any degradation, were representative of such mixtures.
Results and Discussion
CIMS Detection of FTOHs, PFCAs, and PFECAs
CIMS calibrations for 4:2, 6:2, 8:2, and 10:2 FTOH are shown in Figure 1. PFOA, PFBA, and HFPO-DA calibration curves are provided in Figure S3. Following the approach outlined by Bertram et al.(20) and assuming a signal-to-noise ratio of 3, we also calculated gas-phase detection limits for these compounds. These limits could be lowered significantly for longer signal averaging times at the expense of time resolution. For example, averaging over 1 min versus the 3 s used here reduces the detection limit of 6:2 FTOH by ∼80% while still providing measurements fast enough for most applications. The results from all calibrations are summarized in Table 1, where the errors are calculated as the standard deviation of the calibration factors obtained from each individual injection. These detection limits correspond broadly to larger values reported by Ahrens et al.(16) for ambient air samples taken at wastewater treatment plants and landfills. It is important to keep in mind that those values likely have been diluted to some extent over the course of collection and that collection efficiencies are <100%. Therefore, instantaneous mixing ratios should be expected to be larger, and industrial sources such as incinerator stack, process, and fugitive emissions have the potential for much higher concentrations.
Figure 1.
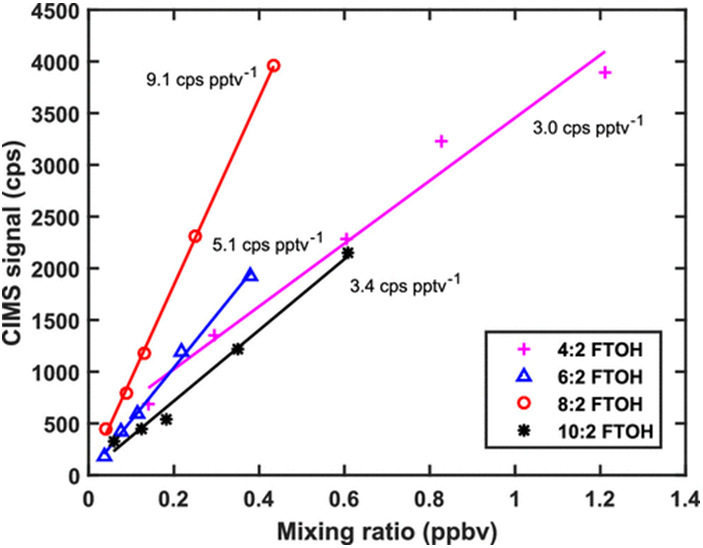
CIMS calibration curves for 4:2, 6:2, 8:2, and 10:2 FTOH with the corresponding calibration factors.
Table 1.
CIMS PFAS Calibration Summary
| compound | structural formula | calibration factor (cps pptv−1) |
detection limit (pptv) |
|---|---|---|---|
| 4:2 fluorotelomer alcohol | F(CF2)4(CH2)2OH | 3.0 ± 0.7 | 7.9 |
| 6:2 fluorotelomer alcohol | F(CF2)6(CH2)2OH | 5.1 ± 0.3 | 2.1 |
| 8:2 fluorotelomer alcohol | F(CF2)8(CH2)2OH | 9.1 ± 0.8 | 1.8 |
| 10:2 fluorotelomer alcohol | F(CF2)10(CH2)2OH | 3.4 ± 1.1 | 4.8 |
| perfluorooctanoic acid (PFOA) | F(CF2)7COOH | 2.9 ± 0.6 | 5.9 |
| perfluorobutanoic acid (PFBA) | F(CF2)3COOH | 24.8 ± 3.9 | 1.4 |
| hexafluoropropylene oxide dimer acid (HFPO-DA) | F(CF2)3OC(COOH)FCF3 | 3.7 ± 1.1 | 7.6 |
Additional PFAS such as fluorosulfonic acids [e.g., perfluorooctanesulfonic acid (PFOS)] and polyfluoroalkyl phosphate diesters (diPAPs) were also screened for detection with the CIMS but were not detected. This is likely because they were not sufficiently volatile or did not form an iodide-adduct ion, but these species are routinely quantified in the condensed phase by LC–MS methods.(21,22)
FTOH Levels in Commercial Fluoro Products
Masurf FS-115, Zonyl FSA, Capstone FS-35, and Arctic 3 AFFF were sampled over ∼1 h; thereafter, all products started to form highly viscous gels or waxes. These waxes still produced substantial signals with only marginal decay and thus precluded complete volatilization of all analyte species. For this reason, any estimates of PFAS presented here are considered lower limits to those present in the commercial samples, given these matrix effects. Gas-phase mixing ratios were converted to the total mass of analyte per volume of sample (nanograms per microliter), a more commonly used unit for condensed-phase samples.
6:2 FTOH was the only FTOH detected in all four samples. 4:2 FTOH was detected Masurf FS-115, Zonyl FSA, and Capstone FS-35. 8:2 FTOH was detected in Masurf FS-115, Zonyl FSA, and Artic 3 AFFF, and 10:2 FTOH was detected in only Masurf FS-115 and Zonyl FSA. PFOA, PFBA, and HFPO-DA were not detected in any of the products. This was confirmed by LC–MS. All concentrations are reported in Table 2.
Table 2.
Product FTOH Concentrations and Nontargeted Chemical Compositions
| targeted (ng μL−1) | nontargeted | ||||||
|---|---|---|---|---|---|---|---|
| product | 4:2 FTOH |
6:2 FTOH |
8:2 FTOH |
10:2 FTOH |
composition | peak centerm/Q |
mass error (ppm) |
| Masurf FS-115 | 26.1 | 578.5 | 106.8 | 43.0 | C6F10H2O | 279.996 | 5.0 |
| C7F13H3O | 349.994 | 10.4 | |||||
| C16F26H8O | 710.009 | 9.9 | |||||
| C18F30H8O | 810.002 | 9.4 | |||||
| C20F34H8O | 909.988 | 16.7 | |||||
| Zonyl FSA | 9.4 | 948.1 | 130.7 | 17.9 | C14F23H5O | 626.002 | 7.5 |
| C16F27H5O | 725.999 | 11.1 | |||||
| C18F31H5O | 825.997 | 14.8 | |||||
| Capstone FS-35 | 6.7 | 644.6 | – | – | C4F5H5O | 164.025 | 6.4 |
| C5F7H5O | 214.023 | 0.6 | |||||
| C7F11H5O | 314.015 | 4.7 | |||||
| C14F24H6O | 645.996 | 11.7 | |||||
| Arctic 3 AFFF | – | 1.6 | 0.3 | – | C7F11H5O | 314.018 | 4.9 |
| C16F27H5O | 726.002 | 15.3 | |||||
| C18F31H5O | 825.986 | 1.8 | |||||
Analysis of Unknown Peaks
In addition to the FTOHs, there are several peaks at m/Q >300 Da in the mass spectra that did not correspond to any of the PFAS standards available to us. Examples of these peaks as well as the FTOH peaks are shown in Figure S4. In the absence of heating, peaks at these large m/Q values are relatively rare given the typically low vapor pressure for high-molecular weight organic compounds. Several of the peaks were separated by multiples of ∼50 Da, corresponding to a CF2group within the perfluorinated tail of the molecule, similar to the case for the FTOHs.(23)Considering these factors, we concluded that these peaks were likely representative of PFAS and have identified the most probable chemical compositions for each using the Tofware data analysis package (version 2.5.13, TOFWERK AG),(24-26) which has been used previously.(27-29) Most probable compositions were selected by the smallest mass error compared to the observed peak centers (also termed the center of mass). Comparison to theoretical isotopic signatures was also coarsely considered for each composition assignment, but minor isotopes were occasionally near the signal noise, especially at lower m/Q values, or had mass interferences preventing such comparisons from providing much value beyond a cursory check (Figures S5-S8). These compositions along with the peak center locations (with iodide removed) and mass errors are listed in Table 2 for each of the commercial products. Note that with iodide removed some of the peak centers still show a characteristic negative mass defect expected for certain PFAS,(30) and some of the predicted compositions differ by one or more CF2 groups. These compositions that contain one oxygen atom are indicative of PFAS containing alcohol functionalities. Because structural information beyond the presence of an alcohol (or carboxylic acid) group is not available from this measurement technique, we have refrained from speculating about the structural identities of the compositions but note that accurate mass and composition are valuable in narrowing the list of possible candidates.
Potential Extension to Environmental Measurements
The CIMS was designed as a lab and field capable instrument,(17,31) and considering the results presented here, a logical next step would be the extension of this technique to gas-phase field measurements of PFAS. As stated above, previous measurements of gas-phase FTOHs and other PFAS used techniques that require lengthy collection with a heightened likelihood of analyte losses. The fast and direct measurements offered by the CIMS are an improvement on these limitations while also showing potential to perform analyses on volatilized condensed-phase environmental samples in a manner similar to that of the commercial product measurements described above. Coupled with well-established LC–MS condensed-phase PFAS measurements, utilization of the CIMS would capture a large spectrum of PFAS that encompasses the liquid and gas phases.
It is also worth noting that the measurements performed here are representative of PFAS volatility at room temperature. At higher temperatures, such as those used during industrial applications, volatility would be increased, driving up gas-phase concentrations and potentially the number of detectable gas-phase compounds. Continuous emission monitoring of such sources and ambient measurements in regions influenced by these sources would be beneficial for assessing gas-phase deposition, reaction, and atmospheric transport of PFAS, all of which are important factors in environmental loadings. Such assessments would benefit greatly from measurements on fast time scales (minutes to seconds). These measurements are arguably necessary to properly characterize certain exposure pathways in areas with existing and emerging PFAS issues.
Supplementary Material
Acknowledgments
The authors thank James McCord (ORISE, U.S. EPA) for valuable discussions. The U.S. EPA through its Office of Research and Development funded and managed the research described here. This paper has been subjected to the Agency’s administrative review and approved for publication. The U.S. EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Footnotes
Supporting Information
CIMS schematic and details; PFOA, PFBA, and HFPO-DA calibration curves; Masurf FS-115 unit mass resolution difference mass spectrum; and molecular composition assignment isotopic distribution
References
- 1.Kotthoff M; Müller J; Jürling H; Schlummer M; Fiedler D Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Environ. Sci. Pollut. Res. 2015, 22 (19), 14546–14559, DOI: 10.1007/s11356-015-4202-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwok KY; Yamazaki E; Yamashita N; Taniyasu S; Murphy MB; Horii Y; Petrick G; Kallerborn R;Kannan K; Murano K, et al. Transport of Perfluoroalkyl substances (PFAS) from an arctic glacier to downstream locations: Implications for sources. Sci. Total Environ. 2013, 447, 46–55, DOI: 10.1016/j.scitotenv.2012.10.091 [DOI] [PubMed] [Google Scholar]
- 3.Pickard HM; Criscitiello AS; Spencer C; Sharp MJ; Muir DCG; De Silva AO; Young CJ Continuous non-marine inputs of per- and polyfluoroalkyl substances to the High Arctic: a multi-decadal temporal record. Atmos. Chem. Phys. 2018, 18 (7), 5045–5058, DOI: 10.5194/acp-18-5045-2018 [DOI] [Google Scholar]
- 4.Buck RC; Franklin J; Berger U; Conder JM; Cousins IT; de Voogt P; Jensen AA; Kannan K;Mabury SA; van Leeuwen SP Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manage. 2011, 7 (4), 513–541, DOI: 10.1002/ieam.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newton S; McMahen R; Stoeckel JA; Chislock M; Lindstrom A; Strynar M Novel Polyfluorinated Compounds Identified Using High Resolution Mass Spectrometry Downstream of Manufacturing Facilities near Decatur, Alabama. Environ. Sci. Technol. 2017, 51 (3), 1544–1552, DOI: 10.1021/acs.est.6b05330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian Y; Yao Y; Chang S; Zhao Z; Zhao Y; Yuan X; Wu F; Sun H Occurrence and Phase Distribution of Neutral and Ionizable Per- and Polyfluoroalkyl Substances (PFASs) in the Atmosphere and Plant Leaves around Landfills: A Case Study in Tianjin, China. Environ. Sci. Technol. 2018, 52 (3), 1301–1310, DOI: 10.1021/acs.est.7b05385 [DOI] [PubMed] [Google Scholar]
- 7.Zhao L; Folsom PW; Wolstenholme BW; Sun H; Wang N; Buck RC 6:2 Fluorotelomer alcohol biotransformation in an aerobic river sediment system. Chemosphere 2013, 90 (2), 203–209, DOI: 10.1016/j.chemosphere.2012.06.035 [DOI] [PubMed] [Google Scholar]
- 8.Dinglasan MJA; Ye Y; Edwards EA; Mabury SA Fluorotelomer Alcohol Biodegradation Yields Poly- and Perfluorinated Acids. Environ. Sci. Technol. 2004, 38 (10), 2857–2864, DOI: 10.1021/es0350177 [DOI] [PubMed] [Google Scholar]
- 9.Ellis DA; Martin JW; De Silva AO; Mabury SA; Hurley MD; Sulbaek Andersen MP;Wallington TJ Degradation of Fluorotelomer Alcohols: A Likely Atmospheric Source of Perfluorinated Carboxylic Acids. Environ. Sci. Technol. 2004, 38 (12), 3316–3321, DOI: 10.1021/es049860w [DOI] [PubMed] [Google Scholar]
- 10.Lau C; Anitole K; Hodes C; Lai D; Pfahles-Hutchens A; Seed J Perfluoroalkyl Acids: A Review of Monitoring and Toxicological Findings. Toxicol. Sci. 2007, 99 (2), 366–394, DOI: 10.1093/toxsci/kfm128 [DOI] [PubMed] [Google Scholar]
- 11.IARC Some Chemicals Used as Solvents and in Polymer Manufacture. IARC Monogr. Eval. Carcinog. Risks Hum. 2017, 110, 1–276 [Google Scholar]
- 12.Hogue C What’s GenX still doing in the water downstream of a Chemours Plant? Chem. Eng. News, 2018https://cen.acs.org/articles/96/i7/whats-genx-still-doing-in-the-water-downstream-of-a-chemours-plant.html. [Google Scholar]
- 13.Daubert TE; Danner RP Physical and Thermodynamic Properties of Pure Chemicals: Data Compilation;Taylor and Francis: Washington, DC, 1989. [Google Scholar]
- 14.Lei YD; Wania F; Mathers D; Mabury SA Determination of Vapor Pressures, Octanol–Air, and Water–Air Partition Coefficients for Polyfluorinated Sulfonamide, Sulfonamidoethanols, and Telomer Alcohols. J. Chem. Eng. Data 2004, 49 (4), 1013–1022, DOI: 10.1021/je049949h [DOI] [Google Scholar]
- 15.Abraczinskas M Emerging Compounds Update: Division of Air Quality. North Carolina Department of Environmental Quality, 2018. https://files.nc.gov/ncdeq/GenX/epa-comm-mtg/Abraczinskas-EPA-PFAS-Stakeholder-mtg-Aug14-2018.pdf. [Google Scholar]
- 16.Ahrens L; Shoeib M; Harner T; Lee SC; Guo R; Reiner EJ Wastewater Treatment Plant and Landfills as Sources of Polyfluoroalkyl Compounds to the Atmosphere. Environ. Sci. Technol. 2011, 45 (19),8098–8105, DOI: 10.1021/es1036173 [DOI] [PubMed] [Google Scholar]
- 17.Lee BH; Lopez-Hilfiker FD; Mohr C; Kurtén T; Worsnop DR; Thornton JA An Iodide-Adduct High-Resolution Time-of-Flight Chemical-Ionization Mass Spectrometer: Application to Atmospheric Inorganic and Organic Compounds. Environ. Sci. Technol. 2014, 48 (11), 6309–6317, DOI: 10.1021/es500362a [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Hilfiker FD; Iyer S; Mohr C; Lee BH; D’Ambro EL; Kurtén T; Thornton JA Constraining the sensitivity of iodide adduct chemical ionization mass spectrometry to multifunctional organic molecules using the collision limit and thermodynamic stability of iodide ion adducts. Atmos. Meas. Tech. 2016, 9 (4),1505–1512, DOI: 10.5194/amt-9-1505-2016 [DOI] [Google Scholar]
- 19.Riedel TP; Bertram TH; Crisp TA; Williams EJ; Lerner BM; Vlasenko A; Li S-M; Gilman J;de Gouw J; Bon DM, et al. Nitryl Chloride and Molecular Chlorine in the Coastal Marine Boundary Layer.Environ. Sci. Technol. 2012, 46 (19), 10463–10470, DOI: 10.1021/es204632r [DOI] [PubMed] [Google Scholar]
- 20.Bertram TH; Kimmel JR; Crisp TA; Ryder OS; Yatavelli RLN; Thornton JA; Cubison MJ;Gonin M; Worsnop DR A field-deployable, chemical ionization time-of-flight mass spectrometer. Atmos. Meas. Tech. 2011, 4 (7), 1471–1479, DOI: 10.5194/amt-4-1471-2011 [DOI] [Google Scholar]
- 21.Yoo H; Washington JW; Jenkins TM; Ellington JJ Quantitative Determination of Perfluorochemicals and Fluorotelomer Alcohols in Plants from Biosolid-Amended Fields using LC/MS/MS and GC/MS. Environ. Sci. Technol. 2011, 45 (19), 7985–7990, DOI: 10.1021/es102972m [DOI] [PubMed] [Google Scholar]
- 22.De Silva AO; Allard CN; Spencer C; Webster GM; Shoeib M Phosphorus-Containing Fluorinated Organics: Polyfluoroalkyl Phosphoric Acid Diesters (diPAPs), Perfluorophosphonates (PFPAs), and Perfluorophosphinates (PFPIAs) in Residential Indoor Dust. Environ. Sci. Technol. 2012, 46 (22), 12575–12582, DOI: 10.1021/es303172p [DOI] [PubMed] [Google Scholar]
- 23.Trier X; Granby K; Christensen JH Tools to discover anionic and nonionic polyfluorinated alkyl surfactants by liquid chromatography electrospray ionisation mass spectrometry. J. Chromatogr. A 2011,1218 (40), 7094–7104, DOI: 10.1016/j.chroma.2011.07.057 [DOI] [PubMed] [Google Scholar]
- 24.Cubison MJ; Jimenez JL Statistical precision of the intensities retrieved from constrained fitting of overlapping peaks in high-resolution mass spectra. Atmos. Meas. Tech. 2015, 8 (6), 2333–2345, DOI: 10.5194/amt-8-2333-2015 [DOI] [Google Scholar]
- 25.Timonen H; Cubison M; Aurela M; Brus D; Lihavainen H; Hillamo R; Canagaratna M; Nekat B;Weller R; Worsnop D, et al. Applications and limitations of constrained high-resolution peak fitting on low resolving power mass spectra from the ToF-ACSM. Atmos. Meas. Tech. 2016, 9 (7), 3263–3281, DOI: 10.5194/amt-9-3263-2016 [DOI] [Google Scholar]
- 26.Stark H; Yatavelli RLN; Thompson SL; Kimmel JR; Cubison MJ; Chhabra PS;Canagaratna MR; Jayne JT; Worsnop DR; Jimenez JL Methods to extract molecular and bulk chemical information from series of complex mass spectra with limited mass resolution. Int. J. Mass Spectrom. 2015, 389, 26–38, DOI: 10.1016/j.ijms.2015.08.011 [DOI] [Google Scholar]
- 27.Yuan B; Koss A; Warneke C; Gilman JB; Lerner BM; Stark H; de Gouw JA A high-resolution time-of-flight chemical ionization mass spectrometer utilizing hydronium ions (H3O+ ToF-CIMS) for measurements of volatile organic compounds in the atmosphere. Atmos. Meas. Tech. 2016, 9 (6), 2735–2752, DOI: 10.5194/amt-9-2735-2016 [DOI] [Google Scholar]
- 28.Thompson SL; Yatavelli RLN; Stark H; Kimmel JR; Krechmer JE; Day DA; Hu W;Isaacman-VanWertz G; Yee L; Goldstein AH, et al. Field intercomparison of the gas/particle partitioning of oxygenated organics during the Southern Oxidant and Aerosol Study (SOAS) in 2013. Aerosol Sci. Technol. 2017, 51 (1), 30–56, DOI: 10.1080/02786826.2016.1254719 [DOI] [Google Scholar]
- 29.Aljawhary D; Lee AKY; Abbatt JPD High-resolution chemical ionization mass spectrometry (ToF-CIMS): application to study SOA composition and processing. Atmos. Meas. Tech. 2013, 6 (11), 3211–3224, DOI: 10.5194/amt-6-3211-2013 [DOI] [Google Scholar]
- 30.Strynar M; Dagnino S; McMahen R; Liang S; Lindstrom A; Andersen E; McMillan L; Thurman M;Ferrer I; Ball C Identification of Novel Perfluoroalkyl Ether Carboxylic Acids (PFECAs) and Sulfonic Acids (PFESAs) in Natural Waters Using Accurate Mass Time-of-Flight Mass Spectrometry (TOFMS). Environ. Sci. Technol. 2015, 49 (19), 11622–11630, DOI: 10.1021/acs.est.5b01215 [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Hilfiker FD; Mohr C; D’Ambro EL; Lutz A; Riedel TP; Gaston CJ; Iyer S; Zhang Z;Gold A; Surratt JD, et al. Molecular Composition and Volatility of Organic Aerosol in the Southeastern U.S.: Implications for IEPOX Derived SOA. Environ. Sci. Technol. 2016, 50 (5), 2200–2209, DOI: 10.1021/acs.est.5b04769 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


