Abstract
Background.
An increasing number of studies have linked air pollution to decreased fertility. Whether this is due to an effect on ovarian reserve is unknown.
Method.
Our study included 632 women attending the Massachusetts General Hospital Fertility Center (2004–2015) who had a measured antral follicle count. Validated spatiotemporal models estimated daily particulate matter <2.5 μg/m3 (PM2.5) (based on residential address) for the 3 months prior to the antral follicle count. We analyzed associations with Poisson regression.
Results.
Every 2 μg/m3 increase in estimated PM2.5 exposure was associated with a −7.2% (95% CI −10.4%, −3.8%) lower antral follicle count adjusting for age, BMI, smoking status, and year and season of the count. The association of PM2.5 with antral follicle count was stronger among women with female factor infertility (−16.3% per 2 μg/m3).
Conclusion.
Among women from an infertility clinic, higher PM2.5 exposure was associated with lower ovarian reserve, raising concern that air pollution may accelerate reproductive aging.
Keywords: air pollution, ovarian reserve, fertility, fecundity
Introduction
Air pollution, specifically exposure to fine particulate matter (PM2.5), is a substantial global health concern,1 responsible for a growing number of adverse health effects such as cardiovascular disease,2 stroke,3 and cancer.4 In the past decade, there have also been an increasing number of reports linking air pollution to diminished fertility.5,6 Specifically, live birth rates were lower among women residing in census tracts with higher exposure to PM2.5 and other traffic-related air pollutants7 and women with higher PM2.5 exposure had slightly longer time to pregnancy.8 Adverse effects of PM2.5 on fertility may be due to perturbations in semen quality9 and reproductive hormones10 among men; however, mechanisms underlying the potential effects of PM2.5 on markers of female fertility have been understudied. Animal studies suggest that increased exposure to PM2.5 may compromise female reproductive potential by accelerating reproductive aging.11–13 Therefore, to address this question in women, we investigated the association between estimated residential exposure to PM2.5 and antral follicle counts, the gold standard measure of ovarian reserve,14 among women presenting to an infertility clinic.
Methods
Study participants were women (18 to 45 years) presenting to the Massachusetts General Hospital (MGH) Fertility Center for infertility treatment who enrolled in the Environment and Reproductive Health (EARTH) Study.15 Approximately 60% of eligible women contacted by the research nurses enrolled. Of the initial 806 women (954 antral follicle scans) available for analysis, we excluded incomplete scans, those done while the woman was on Lupron, those done on women with polycystic ovaries, repeated scans, and scans lacking complete exposure data (eFigure 1). This resulted in a final sample size of 632 women who contributed one unstimulated antral follicle count between 2004 and 2015. The EARTH study was approved by the Human Studies Institutional Review Boards of MGH and the Harvard T.H. Chan School of Public Health.
Upon enrollment, all participants provided their residential address and this was geocoded using ArcGIS. We estimated daily residential PM2.5 exposures starting from three months prior to the antral follicle count date to correspond with the proposed window of antral follicle development (~2–4 months).14 As a negative control window, we also estimated daily PM2.5 exposures in the 3 months after the antral follicle count. PM2.5 exposures were estimated with a validated hybrid model of satellite–derived aerosol optical depth measurements and land-use terms.16 These daily 1 km2 spatial resolution values were then averaged to obtain the woman’s 3-month average PM2.5 exposure preceding the scan. Ovarian antral follicle count was measured using transvaginal ultrasonography by one of the MGH reproductive endocrinologists on the 3rd day of an unstimulated menstrual cycle or progesterone withdrawal bleed. All follicles above 2 mm were counted. No fertility medications were used in the cycle prior to the count. To reduce the influence of very high counts, we truncated the measure at 30 (15 women, 2% of population). Date of birth was collected at entry, trained study staff measured weight and height to calculate body mass index (BMI) (kg/m2). A detailed take-home questionnaire contained questions on lifestyle factors, reproductive health, and medical history. We defined regular menstrual cycles as being predictable within 10 days, and assessed time spent in leisure time physical and sedentary activities using a validated questionnaire.17 We abstracted infertility diagnosis from electronic medical records.
We calculated descriptive statistics and compared them across quartiles of estimated average PM2.5 exposure. Differences in demographic and reproductive characteristics were evaluated using Kruskal–Wallis tests for continuous variables and chi-squared tests for categorical variables (or Fisher’s exact test where appropriate). We used Poisson regression models to estimate the association of estimated average PM2.5 exposure with antral follicle count. Non-linearity was assessed non-parametrically with restricted cubic splines, which used the likelihood ratio test comparing the model with the linear term to the model with the linear and the cubic spline terms.18 Results are presented as either adjusted % change in antral follicle count per 2 μg/m3 increase in estimated PM2.5 exposure (the interquartile range) or population marginal means. We assessed confounding based on biological relevance and descriptive statistics from our study population. Final models were adjusted for age, BMI, smoking status, year of antral follicle count (as a quadratic function), and season of count. We tested effect modification of the relationship between estimated average PM2.5 exposure and AFC by age, BMI, smoking status, infertility diagnosis, and menstrual cycle characteristics, all well-known predictors of ovarian reserve, by adding a cross-product term to the final multivariable model.
Results
The 632 women had a mean (standard deviation) age of 35.3 (4.2) years and BMI of 24.4 (4.7) kg/m2. The majority of women were never smokers (73%) and of Caucasian race (84%) with a college degree or higher (93%). The most common infertility diagnosis at enrollment was unexplained (41%). Women in our cohort resided in Massachusetts (96%), New Hampshire (2%), and Rhode Island (1%), as well as Maine and a few states outside of New England (<1%). The median antral follicle count in our study was 12 (range=1 to 30) (eFigure 2). During the study period (2005–2015), the average estimated PM2.5 concentration in our population was 9.0 μg/m3 (range=5.4 to 16.4 μg/m3), which was similar to the average PM2.5 concentration across the US (mean=10.2 μg/m3) (eFigure 2).19 Our PM2.5 concentrations also tended to decrease over time, mirroring national trends.19 Women in the highest quartile of estimated exposure to PM2.5 were, on average, heavier and their antral follicle counts tended to be measured during the earlier years of the study and during the summer months compared to women in the lowest quartile (Table 1). All other characteristics were similar.
Table 1.
Baseline demographic and reproductive characteristics by quartiles of ambient exposure to particulate matter <2.5 μm in diameter (PM2.5) among 632 women in the EARTH Study.
| PM2.5 Exposure in the 3 Months Prior to AFC | ||||
|---|---|---|---|---|
| Quartile (Range, μg/m3) |
Q1 (5.4–7.6) |
Q2 (7.7–8.6) |
Q3 (8.7–9.9) |
Q4 (10.0–16.4) |
| Number of Women | 158 | 158 | 158 | 158 |
| Demographic characteristicsb | ||||
| Age (years), mean (SD) | 35.0 (4.4) | 35.1 (4.1) | 35.5 (4.3) | 35.4 (4.3) |
| Body Mass Index (kg/m2), mean (SD) | 23.9 (4.6) | 24.0 (4.3) | 25.2 (5.0) | 24.7 (4.8) |
| Total physical activity (hr/week), mean (SD) | 8.0 (11.2) | 6.9 (9.7) | 5.8 (7.0) | 6.0 (6.8) |
| Race/Ethnic group, n (%) | ||||
| White/Caucasian | 130 (82.3) | 134 (84.8) | 131 (82.9) | 133 (84.2) |
| Black | 6 (3.8) | 3 (1.9) | 8 (5.1) | 2 (1.3) |
| Asian | 13 (8.2) | 15 (9.5) | 13 (8.2) | 14 (8.9) |
| Other | 9 (5.7) | 6 (3.8) | 6 (3.8) | 9 (5.7) |
| Smoking status, n (%) | ||||
| Never smoked | 112 (70.9) | 115 (72.8) | 117 (74.1) | 118 (74.7) |
| Ever smoked | 46 (29.1) | 43 (27.2) | 41 (26.0) | 40 (25.3) |
| Education, n (%) | ||||
| < College | 11 (7.0) | 9 (5.7) | 12 (7.6) | 14 (8.9) |
| College graduate | 73 (46.2) | 72 (45.6) | 64 (40.5) | 59 (37.3) |
| Graduate degree | 74 (46.8) | 77 (48.7) | 82 (51.9) | 85 (53.8) |
| Reproductive characteristics | ||||
| Usual menstrual cycle length (days), mean (SD) | 30.5 (9.3) | 30.9 (12.8) | 31.6 (13.7) | 30.7 (13.7) |
| Year of AFC, mean (SD) | 2012.6 (2.3) | 2011.1 (2.6) | 2010.0 (2.3) | 2008.1 (1.9) |
| Regular menstrual cycles, n (%) | 135 (85.4) | 135 (85.4) | 144 (91.1) | 144 (91.1) |
| History of being pregnant, n (%) | 74 (46.8) | 75 (47.5) | 67 (42.4) | 69 (43.7) |
| Previous infertility exam, n (%) | 132 (83.5) | 130 (82.3) | 121 (76.6) | 128 (81.0) |
| Previous infertility treatment, n (%) | 80 (50.6) | 86 (54.4) | 72 (45.6) | 82 (51.9) |
| Initial infertility diagnosisa, n (%) | ||||
| Male factor | 32 (20.3) | 44 (27.9) | 43 (27.2) | 44 (27.9) |
| Female factor | 53 (33.5) | 46 (29.1) | 60 (38.0) | 50 (31.7) |
| DOR | 19 (12.0) | 19 (12.0) | 21 (13.3) | 15 (9.5) |
| Endometriosis | 5 (3.2) | 9 (5.7) | 10 (6.3) | 11 (7.0) |
| Ovulation Disorders | 18 (11.4) | 9 (5.7) | 16 (10.1) | 14 (8.9) |
| Tubal | 8 (5.1) | 6 (3.8) | 11 (7.0) | 9 (5.7) |
| Uterine | 3 (1.9) | 3 (1.9) | 2 (1.3) | 1 (0.6) |
| Unexplained | 73 (46.2) | 66 (41.8) | 54 (34.2) | 64 (40.5) |
| Season of AFC, n (%) | ||||
| Jan-Mar | 22 (13.9) | 55 (34.8) | 46 (29.1) | 51 (32.3) |
| Apr-Jun | 54 (34.2) | 47 (29.8) | 30 (19.0) | 19 (12.0) |
| Jul-Sept | 15 (9.5) | 19 (12.0) | 28 (17.7) | 63 (39.9) |
| Oct-Dec | 67 (42.4) | 37 (23.4) | 54 (34.2) | 25 (15.8) |
Abbreviations: AFC, antral follicle count; DOR, diminished ovarian reserve;
Numbers may not add up to the total due to missing values (i.e. 3 women missing infertility diagnosis).
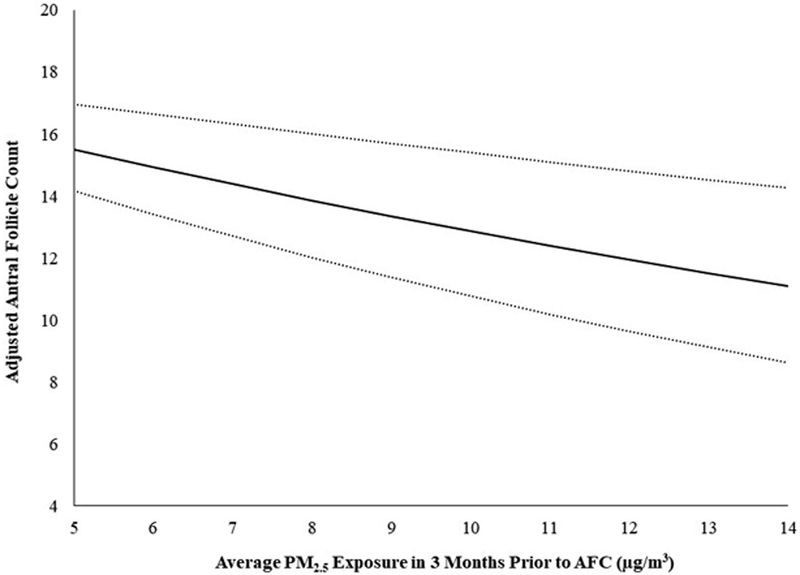
A 2 μg/m3 increase in estimated average residential exposure to PM2.5 in the 3 months prior to the antral follicle count was associated with a −7.2% (95% CI −10.4%, −3.8%) lower count adjusting for age, BMI, smoking status, and year and season of count (Table 2). This translated into approximately 1.1 fewer antral follicles for every 2 μg/m3 increase in estimated average exposure to PM2.5 (Figure. 1). There was no evidence of a departure from linearity. For comparison, a 2-year increase in age was associated with a −8.0% (95% CI −8.9%, −7.0%) lower antral follicle count. In contrast, a 2 μg/m3 increase in estimated average residential exposure to PM2.5 in the 3 months after antral follicle count was associated with an imprecisely measured −1.4% (95% CI −5.0%, 2.2%) lower count after multivariable adjustment.
Table 2.
Association between ambient exposure to particulate matter <2.5 μm in diameter (PM2.5) and antral follicle counts (AFC) among 632 women in the EARTH Study.
| Unadjusted % Change in AFC | Adjusted % Change in AFCa | |
|---|---|---|
| 3 Months Prior to AFC | ||
| Per 2 μg/m3 increase in PM2.5 | −7.2 (−9.4, −5.0) | −7.2 (−10.4, −3.8) |
| Quartiles of PM2.5 | ||
| Q1 (5.4–7.6 μg/m3) | REF | REF |
| Q2 (7.7–8.6 μg/m3) | −4.0 (−9.5, 1.8) | −2.2 (−8.1, 4.2) |
| Q3 (8.7–9.9 μg/m3) | −8.8 (−14.1, −3.2) | −5.5 (−11.8, 1.1) |
| Q4 (10.0–16.4 μg/m3) | −13.6 (−18.7, −8.2) | −9.0 (−16.3, −1.1) |
| 3 Months After the AFCb | ||
| Per 2 μg/m3 increase in PM2.5 | −4.8 (−7.1, −2.4) | −1.4 (−5.0, 2.2) |
| Quartiles of PM2.5 | ||
| Q1 (3.9–7.6 μg/m3) | REF | REF |
| Q2 (7.7–8.5 μg/m3) | −9.2 (−14.6, −3.5) | −5.5 (−11.4, 0.9) |
| Q3 (8.6–9.9 μg/m3) | −4.6 (−10.2, 1.3) | −0.7 (−7.3, 6.3) |
| Q4 (10.0–16.8 μg/m3) | −12.3 (−17.5, −6.7) | −4.4 (−12.2, 4.0) |
| Per 2 year increase in age | −8.1 (−9.1, −7.2) | −8.0 (−8.9, −7.0) |
We obtained effect estimates using Poisson regression.
Adjusted models account for age (continuous), BMI (continuous), smoking status (ever, never), year of AFC (quadratic), season of AFC (Jan-Mar, Apr-Jun, Jul-Sept, Oct-Dec).
615 women were included in this analysis (17 women who had their AFC performed in late 2015 were excluded because they were missing more than 7 days of PM data).
Figure. 1. Association between ambient exposure to particulate matter <2.5 μm in diameter (PM2.5) and antral follicle counts (AFC) among 632 women in the EARTH Study.
The solid line shows the predicted mean antral follicle counts for the average woman in our cohort (mean age 35 years, BMI of 23.3 kg/m2, never smoker, and AFC scan performed in 2010 during Oct to Dec) from the 1st to 99th percentile of PM2.5 exposure levels. The dotted lines are the 95% confidence intervals.
The association between estimated PM2.5 exposure and antral follicle count was similar across age (<35 vs. ≥35 yrs), BMI (<25 vs. ≥25 kg/m2), and smoking status (never vs. ever) groups; however, the estimated effect of PM2.5 exposure on antral follicle count was stronger among women whose primary infertility diagnosis was attributable to a female cause (% change= −16.3% 95% CI −21.5, −10.7%) compared to women with an unexplained or male factor diagnosis (% change= −2.8% 95% CI −6.9, 1.6%) (p-for-interaction=<0.001) (Table 3). Moreover, this effect was consistently negative across all diagnostic categories of female factor infertility (e.g. diminished ovarian reserve, endometriosis, ovulation disorders, tubal, and uterine). The association between estimated PM2.5 exposure and antral follicle count was also more pronounced among women with non-regular menstrual cycles (p-for-interaction=<0.001) and those with short (<24 days) or long (>38 days) cycles (p-for-interaction=0.01) compared to women with regular and normal length menstrual cycles, respectively. While the association between estimated PM2.5 and antral follicle count was slightly stronger between years 2005–2009 (% change= −7.6% 95% CI −12.7%, −2.4%) versus 2010–2015 (% change= −5.0% 95% CI −9.8%, 0.1%); the difference was imprecise. Results for the main analysis were also consistent after excluding women who resided outside of Massachusetts (% change= −6.8% 95% CI −10.2, −3.3%).
Table 3.
Effect modification of the association between ambient exposure to particulate matter <2.5 μm in diameter (PM2.5) and antral follicle counts (AFC) among 632 women in the EARTH Study.
| n | Adjusted % Change in AFC per 2 μg/m3 increase in PM2.5a |
|
|---|---|---|
| Age | ||
| <35 years | 330 | −7.5 (−11.8, −3.0) |
| ≥35 years | 302 | −6.8 (−11.9, −1.4) |
| P for interactionb | 0.79 | |
| BMI | ||
| <25 kg/m2 | 413 | −8.7 (−12.6, −4.7) |
| ≥25 kg/m2 | 219 | −4.1 (−10.1, 2.3) |
| P for interaction | 0.35 | |
| Smoking Status | ||
| Never smoker | 462 | −7.8 (−11.5, −3.9) |
| Ever smoker | 170 | −8.1 (−14.8, −0.9) |
| P for interaction | 0.33 | |
| Infertility Diagnosis | ||
| Female | 209 | −16.3 (−21.5, −10.7) |
| Male & Unexplained | 423 | −2.8 (−6.9, 1.6) |
| P for interaction | <0.001 | |
| Year of AFC | ||
| 2005–2009 | 263 | −7.6 (−12.7, −2.4) |
| 2010–2015 | 369 | −5.0 (−9.8, 0.1) |
| P for interaction | 0.06 | |
| Regular Menstrual Cycles | ||
| Yes | 558 | −3.0 (−6.8, 0.9) |
| No | 74 | −19.0 (−26.3, −10.9) |
| P for interaction | <0.001 | |
| Menstrual Cycle Length | ||
| 24–38 days | 571 | −5.0 (−8.5, −1.2) |
| <24 or >38 days | 61 | −17.8 (−26.8, −7.7) |
| P for interaction | 0.01 |
Effect estimates were obtained using Poisson regression adjusted for age (continuous), BMI (continuous), smoking status (ever, never), year of AFC (quadratic), season of AFC (Jan-Mar, Apr-Jun, Jul-Sept, Oct-Dec).
P for interaction was calculated by adding a cross product term to the final multivariable model.
Discussion
In our prospective study of women seeking infertility treatment, we found that higher residential exposure to PM2.5 was inversely associated with antral follicle count, a well-accepted marker of ovarian reserve. Moreover, the magnitude of this association was approximately equivalent to a 2-year increase in female age. Our results also suggest that the effects of PM2.5 on antral follicle count may be more pronounced among women with a female factor infertility diagnosis and abnormal menstrual cycles, whose counts are generally lower than those of women presenting with other diagnoses or regular menstrual cycles. To our knowledge, this is the first study in humans to investigate a link between air pollution exposure and a biomarker of ovarian aging.
In the laboratory setting, three previous studies have examined the association between air pollution exposure and ovarian reserve in mice. In the first study, mice exposed to nonfiltered ambient air sampled close to a highly trafficked street (average PM2.5 of 27.5 μg/m3 per day) prior to and during pregnancy had reduced numbers of antral follicles compared to mice who only received filtered air.13 In the second study, there was a reduction in the proportional area occupied by primordial follicles in mice exposed to diesel exhaust (average PM2.5 of 21.5 μg/m3 per day) during pregnancy, postnatally, or both periods compared to mice exposed to only clean air.12 In the third study, mice treated with a PM2.5 suspension (10 mg/kg), every 2 days, for 22 days had serum anti-Müllerian hormone levels that were decreased by more than half compared to the control group.11 Furthermore, IL-6 and TNF-α concentrations and the number of apoptotic cells were increased in ovarian tissue and ovarian histologic structures showed evidence of hemorrhage and vascular congestion in mice exposed to PM2.5 compared to the control group.11 Taken together with our findings, these data suggest that exposure to PM2.5 throughout adult life may enhance follicular atresia through effects on ovarian inflammation, oxidative stress, and apoptosis, even at exposure levels within the World Health Organization guidelines (e.g. <25 μg/m3 24-hour mean).
Limitations of our study are worth noting. Due to the sole inclusion of women undergoing infertility treatment, it may not be possible to generalize our findings to all women of reproductive age. However, previous work has shown that infertile women <40 years have similar antral follicle counts compared with women of the same age with no history of infertility.20 We also used residence-based PM2.5 exposure as a proxy for personal exposure, potentially leading to exposure misclassification particularly due to lack of information on the women’s work addresses or their time–activity patterns. However, the spatio-temporal models we used are validated and the use of outdoor ambient exposures can be valuable because regulation typically focuses on these concentrations. We also lacked information on other spatial variables such as noise or light pollution, which tend to correlate with PM2.5 exposure and may still confound our association between PM2.5 and antral follicle counts. Strengths of our study include its prospective design, large sample size, gold-standard assessment of ovarian reserve,14 and our comprehensive adjustment for other reproductive and lifestyle factors that enhanced our ability to adjust for confounding. The null results we observed in our sensitivity analysis using the average estimated PM2.5 concentrations in the 3 months after antral follicle assessment (our negative control window) also further strengthen our argument for a causal association between PM2.5 in the 3 months prior to antral follicle count and assessment of ovarian reserve.
In conclusion, our study’s findings are consistent with the hypothesis that exposure to relatively low concentrations of PM2.5 may decrease human fertility by accelerating ovarian aging. Moreover, this association may be particularly pronounced among women with an existing female-specific cause of infertility and women with abnormal menstrual cycles.
Supplementary Material
Acknowledgments:
We would like to thank all members of the EARTH study team, specifically our research nurses Myra G. Keller and Jennifer B. Ford, senior research staff Ramace Dadd, the physicians and staff at Massachusetts General Hospital Fertility Center and all the EARTH study participants.
Sources of Financial Support: This work was supported by grants ES009718, ES022955, ES000002, and K99ES026648 from the National Institute of Environmental Health Sciences (NIEHS). This publication was also made possible by U.S. Environmental Protection Agency (U.S. EPA): RD-834798 and RD-83587201. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the U.S. EPA. Further, U.S. EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Footnotes
Competing Financial Interests: The authors declare they have no actual or potential competing financial interests.
Data are not publicly available to protect human subjects' confidentiality. Code for the statistical analysis is available from the authors by request.
References.
- 1.Burnett R, Chen H, Szyszkowicz M, et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci U S A 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An Z, Jin Y, Li J, Li W, Wu W. Impact of Particulate Air Pollution on Cardiovascular Health. Curr Allergy Asthma Rep 2018;18(3):15. [DOI] [PubMed] [Google Scholar]
- 3.Lin H, Guo Y, Di Q, et al. Ambient PM2.5 and Stroke: Effect Modifiers and Population Attributable Risk in Six Low- and Middle-Income Countries. Stroke 2017;48(5):1191–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer (IARC). Air Pollution and Cancer. IARC Scientific Publication No. 161 Straif K, Cohen A, Samet J, eds. World Health Organization Press, 2013. [Google Scholar]
- 5.Checa Vizcaino MA, Gonzalez-Comadran M, Jacquemin B. Outdoor air pollution and human infertility: a systematic review. Fertil Steril 2016;106(4):897–904 e1. [DOI] [PubMed] [Google Scholar]
- 6.Carre J, Gatimel N, Moreau J, Parinaud J, Leandri R. Does air pollution play a role in infertility?: a systematic review. Environ Health 2017;16(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nieuwenhuijsen MJ, Basagana X, Dadvand P, et al. Air pollution and human fertility rates. Environ Int 2014;70:9–14. [DOI] [PubMed] [Google Scholar]
- 8.Slama R, Bottagisi S, Solansky I, et al. Short-term impact of atmospheric pollution on fecundability. Epidemiology 2013;24(6):871–9. [DOI] [PubMed] [Google Scholar]
- 9.Wu L, Jin L, Shi T, et al. Association between ambient particulate matter exposure and semen quality in Wuhan, China. Environ Int 2017;98:219–228. [DOI] [PubMed] [Google Scholar]
- 10.Radwan M, Jurewicz J, Polanska K, et al. Exposure to ambient air pollution--does it affect semen quality and the level of reproductive hormones? Ann Hum Biol 2016;43(1):50–6. [DOI] [PubMed] [Google Scholar]
- 11.Gai HF, An JX, Qian XY, et al. Ovarian Damages Produced by Aerosolized Fine Particulate Matter (PM2.5) Pollution in Mice: Possible Protective Medications and Mechanisms. Chin Med J (Engl) 2017;130(12):1400–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogliari KS, Lichtenfels AJ, de Marchi MR, et al. Intrauterine exposure to diesel exhaust diminishes adult ovarian reserve. Fertil Steril 2013;99(6):1681–8. [DOI] [PubMed] [Google Scholar]
- 13.Veras MM, Damaceno-Rodrigues NR, Guimaraes Silva RM, et al. Chronic exposure to fine particulate matter emitted by traffic affects reproductive and fetal outcomes in mice. Environ Res 2009;109(5):536–43. [DOI] [PubMed] [Google Scholar]
- 14.Broekmans FJ, de Ziegler D, Howles CM, et al. The antral follicle count: practical recommendations for better standardization. Fertil Steril 2010;94(3):1044–51. [DOI] [PubMed] [Google Scholar]
- 15.Messerlian C, Williams PL, Ford JB, et al. The Environment and Reproductive Health (EARTH) Study: A Prospective Preconception Cohort. Hum Reprod Open 2018;2018(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kloog I, Chudnovsky AA, Just AC, et al. A New Hybrid Spatio-Temporal Model For Estimating Daily Multi-Year PM2.5 Concentrations Across Northeastern USA Using High Resolution Aerosol Optical Depth Data. Atmos Environ (1994) 2014;95:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 1994;23(5):991–9. [DOI] [PubMed] [Google Scholar]
- 18.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8(5):551–61. [DOI] [PubMed] [Google Scholar]
- 19.United States Environmental Protection Agency. Particulate Matter (PM2.5) Trends 2000–2017 Available: https://www.epa.gov/air-trends/particulate-matter-pm25-trends Accessed September 17, 2018 Last Updated July 31, 2018.
- 20.Hvidman HW, Bentzen JG, Thuesen LL, et al. Infertile women below the age of 40 have similar anti-Mullerian hormone levels and antral follicle count compared with women of the same age with no history of infertility. Hum Reprod 2016;31(5):1034–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.