Abstract
Objective:
Studies of depressed psychiatric patients have suggested that antidepressant efficacy can be increased by adding eicosapentaenoic acid (EPA), one of the omega-3 fatty acids found in fish oils. The purpose of this study was to determine whether the addition of EPA improves the response to sertraline in depressed patients with or at high risk for coronary heart disease (CHD).
Methods:
Between May 2014 and June 2018, 144 patients with DSM-V major depressive disorder seen at the Washington University School of Medicine with or at high risk for coronary heart disease were randomized to receive either 50mg/day of sertraline and 2g/day of EPA or 50mg/day sertraline and corn oil placebo capsules for 10 weeks. The Beck Depression Inventory II (BDI-II) was the primary outcome measure.
Results:
After ten weeks of treatment there were no differences between the arms on the mean baseline-adjusted BDI-II(Placebo, 10.3; EPA, 12.1; p=.22), the Hamilton Rating Scale for Depression (HAM-D-17)(Placebo, 7.2; EPA, 8.0; p=.40), or the 10-week remission rate (BDI-II ≤ 8)(Placebo, 50.6%; EPA, 46.7%; OR, 0.85; 95% C.I., 0.43,1.68; p=.63).
Conclusions:
Augmentation of sertraline with 2g/day of EPA for ten weeks did not result in greater improvement in depression symptoms compared to sertraline and corn oil placebo in patients with major depression and CHD or CHD risk factors. Identifying the characteristics of cardiac patients whose depression may benefit from omega-3, and clarifying the pathways linking omega-3 to improvement in depression symptoms are important directions for future research.
Keywords: Depression, Coronary Heart Disease, Omega-3 fatty acid, Clinical trial
Depression increases the risk for cardiac morbidity and mortality in patients with coronary heart disease (CHD).1–3 There is evidence that depression can be successfully treated in these patients, although the effect sizes in clinical trials have been modest.1 Nevertheless, there is considerable interest in whether treating depression improves cardiac event-free survival in CHD. Unfortunately, few randomized clinical trials (RCTs) have addressed this question4–7, and the interventions tested in these trials have had relatively small effects on depression. A depression treatment that is both safe and effective is needed to adequately answer this important question.
Marine-derived omega-3 fatty acids supplements have been found to be safe and may offer cardiovascular benefits in patients with or at risk for CHD.8,9 Recent studies have also shown that the long-chain omega-3 fatty acid eicosapentaenoic (EPA) may improve the efficacy of antidepressants in depressed psychiatric patients.10–14 In contrast, supplements containing docosahexaenoic (DHA) or a predominance of DHA relative to EPA, generally have shown little or no effect on depression. EPA may be more effective in patients with major depression than in patients with subclinical depression symptoms10,15,16, and combining omega-3 supplements with antidepressants may be more effective than omega-3 monotherapy.12,16
In a previous trial, we found that 50 mg/day of sertraline combined with an omega-3 supplement (930 mg/day EPA and 750 mg/day DHA) compared to sertraline and placebo for 10 weeks improved a cardiovascular risk factor17, but it was not efficacious for depression.18 Based on the findings of the reviews and meta-analyses that were published following our trial 10–13,15,16,19, we conducted a second, randomized, double-blind, placebo-controlled superiority trial. This trial was designed to determine whether 50mg/day of sertraline combined with 2g/day of EPA is superior to 50mg/day sertraline plus corn oil placebo for major depression in patients with or at high risk for CHD.
METHODS
Recruitment and Eligibility Screening
Patients were recruited between May 2014 and June 2018 from the practices of cardiologists affiliated with Washington University School of Medicine in St. Louis. Patients with >50% documented stenosis in ≥1 major coronary artery determined by cardiac catheterization and angiography, a history of revascularization, or hospitalization >3 months earlier for an acute coronary syndrome (ACS) were asked to participate in the study by their physicians or study staff. In order to achieve our recruitment goal in the allotted time, starting in June 2016 we expanded the medical eligibility criteria to include patients with two or more major cardiac risk factors who had not undergone diagnostic cardiac catheterization but who were being followed by a university cardiologist. Patients who expressed an interest in participating were provided a complete description of the study. Those who continued to express an interest were asked to provide written informed consent, and were then administered the Patient Health Questionnaire (PHQ-9).20 Those who scored >8 on the PHQ-9 were administered a structured clinical interview (the Depression Interview and Structured Hamilton [DISH])21 to document depression symptoms and history.
Patients were excluded if they 1) had moderate to severe cognitive impairment; 2) had another major Axis I diagnosis other than an anxiety disorder, or a high risk of suicide; 3) were not expected to survive one year; 4) had a known sensitivity to sertraline or omega-3, or an allergy to fish oil or shellfish; or 5) were currently taking an antidepressant, lithium, or omega-3 supplements. Those patients without exclusions who met the DSM-5 criteria for a current major depressive episode and scored ≥17 on the BDI-II22, were enrolled and randomized following a blood draw. The study was approved by the Human Research Protection Office at Washington University School of Medicine in St. Louis and registered at ClinicalTrials.gov (NCT02021669).
Cardiovascular and Medical History
Patients’ medical history, cardiac risk factors including history of smoking, diabetes, hypertension, hyperlipidemia, family history of heart disease, body mass index [kg/m2]), history of coronary revascularization, cardiac events including acute coronary syndrome (ACS), and arrhythmias or other ECG abnormalities, as well as current medications, were obtained from electronic medical records.
Blood Specimens and Assays
During the baseline evaluation, the patient rested supine on an examination table for ten minutes. Blood was drawn and centrifuged by a research nurse who was blinded to group assignment, and the serum was divided into aliquots and frozen at −80oc. At the end of the study, the samples were assayed in a single batch. The red blood cell (RBC) membrane fatty acid composition was assessed by capillary gas chromatography after extraction and conversion to fatty acid methyl esters. EPA and DHA were expressed as percent of total RBC fatty acids.23 C-reactive protein (CRP) was also measured at baseline and after treatment by an enhanced immunonephelometric assay on a BN-II analyser (Dade Behring; Newark, NJ).
Randomization
Patients were randomly assigned to receive 50mg/day of sertraline plus 2g/day of EPA (4 capsules of EPA, Atrium Innovations, Inc.), or 50mg/day of sertraline plus identical corn oil capsules. Randomization was stratified by whether the patient had been taking an antidepressant at any time during the previous 3 months. Group allocation was determined by a SAS (SAS Institute, Cary, NC) permuted block random allocation program. The assignments were coded and concealed to ensure that the double-blind was maintained.
Treatment and Follow-Up
Patients were given a five-week supply of the assigned medications at randomization, and an additional five-week supply at the end of week 4. Only the pharmacist, who had no direct contact with the patients or study staff, was unblinded to group assignment during the trial. Patients were maintained on 50mg/day of sertraline for the entire 10 weeks of the trial. During weekly telephone contacts, the study nurse identified new potential side-effects, changes in medical status and adverse events, administered the PHQ-9 to assess changes in depression severity and new or worsening depression symptoms including suicidal ideation, and assessed and encouraged medication adherence.
The DISH interview was re-administered ten weeks after randomization. At that time, the patient again provided a blood sample and completed the same assessments that were administered at baseline. Patients were compensated $100.00 for completion of the baseline and post-treatment assessments.
Treatment Adherence
Remaining medications were counted at weeks 5 and 10 and subtracted from the number provided to determine adherence. RBC membrane EPA and DHA levels were obtained at baseline and post-treatment to confirm adherence to the EPA capsules in the intervention arm and to confirm that no participants were taking a non-study omega-3 dietary supplement.
Primary and Secondary Outcomes
The post-treatment BDI-II score adjusted for baseline score was chosen à priori as the primary outcome measure.22 Secondary outcomes included baseline adjusted post-treatment scores on The Hamilton Rating Scale for Depression (HAM-D-17)24, response (>50% reduction from the baseline BDI-II score) and remission rates (BDI-II<8) from dichotomized BDI-II scores, and post-treatment Beck Anxiety Inventory (BAI) scores.25
Data and Safety Monitoring Board (DSMB)
The external DSMB received quarterly reports on enrollment, side-effects, and non-serious adverse events, and were immediately informed if a serious adverse event occurred. The committee advised the investigators as to whether to continue the study, based on the latest adverse event and recruitment data.
Statistical Analysis
Chi-square tests, Fisher’s exact tests, and analysis of variance models were used to compare demographic, psychiatric, and medical characteristics, EPA RBC levels, adverse events, and possible drug side-effects. Model diagnostics, including residual, influence, and outlier analyses, were performed for each statistical model. Efficacy analyses were conducted according to the intention-to-treat (ITT) principle.26 Data that were plausibly missing at random were imputed from 15 datasets, and the analysis models were fitted to each imputed dataset and then aggregated. Analysis-of-covariance (ANCOVA) models were fitted to the week 10 BDI-II (primary) and HAM-D-17 (secondary) scores to determine depression outcomes and to the BAI scores to determine the effect of the intervention on anxiety. The scores were regressed on the treatment group, recent use of antidepressant strata and baseline scores.
In other secondary analyses, a mixed-effects linear regression model was fitted to weekly PHQ-9 score to determine whether the course of depression differed between arms. The proportions of patients in each arm who achieved remission and who responded to treatment were also compared at 10 weeks. These were regressed on the treatment group parameter in logistic regression models. Potential moderators of the primary outcome including antidepressant use during the previous 3 months, age, gender, minority status, cardiac status (established vs. at high risk for CHD), CRP, and anxiety level27 were tested by adding interaction terms to the model. Per protocol analyses were also conducted for those patients who completed baseline and post-treatment depression assessments and remained on the medication regimen for the ten weeks. All hypothesis tests were two-tailed with p<0.05 denoting statistical significance. SAS© version 9.1 was used for all statistical analyses.
Power Analysis
We defined the minimal clinically important difference for the primary outcome as >3 points on the BDI-II and a within-group standard deviation of 5.0 and an alpha (two-sided) of .05 per comparison. This was exceeded by most previous studies in EPA trials of depressed psychiatric patients.28,29 We planned to randomize 75 patients per group to provide .85 power to detect a 3-point differences on the BDI-II and HAM-D-17.
RESULTS
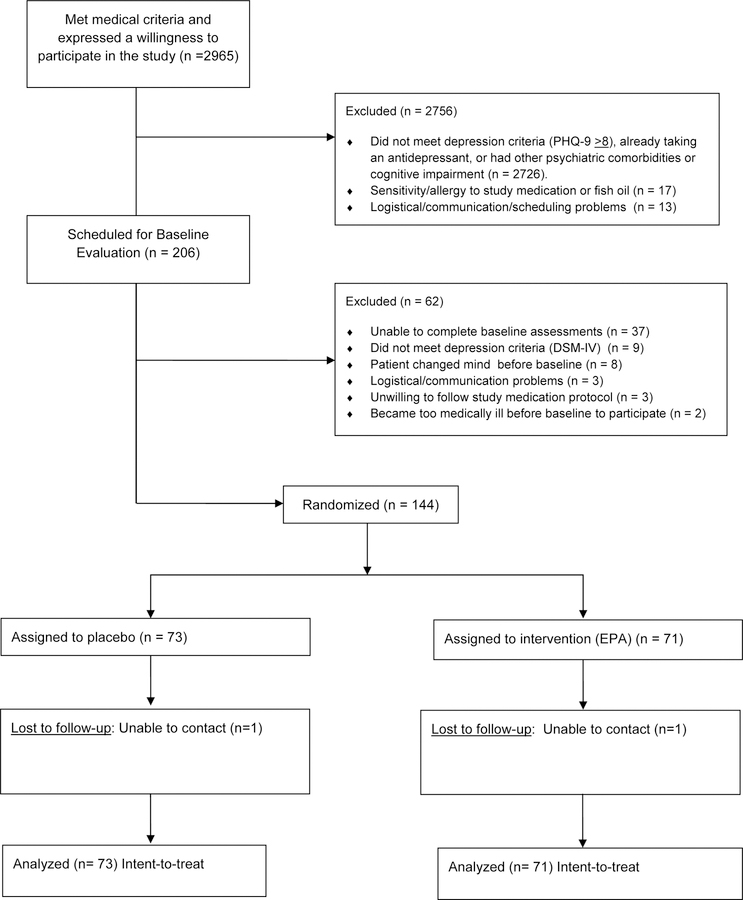
Two thousand nine hundred and sixty-five outpatients agreed to be considered for enrollment in the study (Figure 1). Two hundred six met all medical inclusion and no known exclusion criteria, scored > 8 on the PHQ-9, and agreed to further evaluation including a depression diagnostic interview. One hundred forty-four of these patients met the DSM-5 criteria for major depression, met no exclusion criteria, and agreed to participate in the trial. Seventy-three of these patients were randomly assigned to the placebo arm and 71 to the EPA arm. Seventy-one (97%) of the patients assigned to the placebo arm and 65 (92%) of those assigned to the EPA arm completed all phases of the study.
Figure 1:
CONSORT Diagram
Baseline Characteristics
Baseline medical, demographic, and depression history data are presented in Table 1. There were no significant differences between arms on any demographic or baseline medical variable. Baseline EPA levels were in the expected range for patients not taking supplements or eating more than the average number of servings of foods high in omega-3.30 The baseline mean BDI-II (p=.59) and HAM-D-17 scores (p=.67) did not differ between arms at baseline (Table 2).
Table 1:
Baseline Demographic, Medical, and Depression Characteristics (N=144)
| Characteristic a | Group Assignment |
P-value b | |
|---|---|---|---|
| Placebo (n=73) | Omega-3 (n=71) | ||
| Age (years) | 60.5 ± 9.3 | 58.5 ± 9.6 | .21 |
| Female gender | 30 (41.1) | 26 (36.6) | .58 |
| Caucasian race | 45 (61.6) | 45 (63.4) | .83 |
| Education > 12 years | 51 (69.9) | 48 (67.6) | .77 |
| BMI (kg/m2) | 35.4 ± 7.7 | 35.2 ± 8.7 | .89 |
| Cigarette Smoker (current) | 13 (17.8) | 11 (15.5) | .71 |
| Hypertension | 67 (91.8) | 66 (93.0) | .79 |
| Diabetes | 37 (50.7) | 30 (42.3) | .31 |
| History of MI | 30 (41.1) | 36 (50.7) | .25 |
| History of CABG | 12 (16.4) | 17 (23.9) | .26 |
| History of PTCA | 41 (56.2) | 44 (62.0) | .48 |
| Canadian Cardiovascular Society Angina Class | .94 | ||
| Asymptomatic | 20 (64.5) | 27 (65.9) | |
| I | 1 (3.2) | 3 (7.3) | |
| II | 4 (12.9) | 4 (9.8) | |
| III | 2 (6.5) | 3 (7.3) | |
| IV | 4 (12.9) | 4 (9.8) | |
| Medications | |||
| Aspirin | 61 (83.6) | 62 (87.3) | .52 |
| Ace Inhibitors | 29 (39.7) | 34 (47.9) | .32 |
| Beta Blockers | 55 (75.3) | 55 (77.5) | .76 |
| Statins | 65 (89.0) | 63 (88.7) | .95 |
| Calcium Channel Blockers | 28 (38.4) | 20 (28.2) | .19 |
| Biomarkers | |||
| EPA, % in RBC | 0.48 ± 0.19 | 0.46 ± 0.18 | .48 |
| DHA, % in RBC | 4.06 ± 1.05 | 3.83 ± 0.93 | .18 |
| High Sensitivity C-Reactive Protein (mg/dL) | 1.39 ± 3.41 | 1.59 ± 3.19 | .73 |
| Total Cholesterol (mg/dL) | 169.4 ± 55.3 | 167.3 ± 45.8 | .81 |
| HDL Cholesterol (mg/dL) | 45.7 ± 12.2 | 48.5 ± 15.7 | .26 |
| Triglycerides, fasting (mg/dL) | 224.5 ± 468.0 | 162.7 ± 155.2 | .29 |
| Depression Treatment/History | |||
| Recent (3 month) antidepressant medication use | 17 (23.3) | 16 (22.5) | .91 |
| Previous episode(s) of depression | 53 (72.6) | 47 (66.2) | .40 |
| History of depression treatment | 32 (43.8) | 30 (42.3) | .85 |
Continuous variables are reported as mean (SD); categorical variables are reported as number and (percentage).
Chi-square tests and analyses of variance were used to determine significance
Abbreviations: EPA = eicosapentaenoic acid; DHA = docosahexaenoic acid; RBC = red blood cells
Table 2:
Depression, Anxiety, and Adherence at Baseline and 10-Weeks
| Measure a | Group Assignment |
P | |
|---|---|---|---|
| Placebo (n = 73) | Omega-3 (n = 71) | ||
| Beck Depression Inventory II (BDI-II) | |||
| Baseline b | 29.1 (8.8) | 29.9 (9.0) | .59 |
| Post Treatment (10 weeks) c | 9.1 (7.7) | 11.0 (9.9) | .20 |
| Hamilton Rating Scale for Depression (HAM-D) | |||
| Baseline b | 17.0 (5.0) | 17.4 (5.4) | .67 |
| Post Treatment c | 6.2 (5.5) | 7.1 (7.0) | .38 |
| Patient Health Questionnaire (PHQ-9) | |||
| Baseline b | 15.4 (4.1) | 15.9 (4.6) | .54 |
| Post Treatment c | 4.9 (4.7) | 5.4 (5.2) | .60 |
| Beck Anxiety Inventory (BAI) | |||
| Baseline b | 12.4 (9.7) | 12.7 (10.0) | .83 |
| Post Treatment c | 6.4 (7.2) | 7.6 (9.0) | .34 |
| Cumulative mean treatment adherence (% days pill removed) | |||
| Omega-3/Placebo | 95.9 (5.8) | 95.3 (5.8) | .53 |
| Sertraline | 97.5 (3.8) | 97.6 (3.2) | .87 |
| Omega-3 % RBC | |||
| EPA | |||
| Baseline a | 0.48 (0.19) | 0.46 (0.18) | .48 |
| Post Treatment b | 0.51 (0.40) | 1.74 (0.77) | <.0001 |
| DHA | |||
| Baseline a | 4.06 (1.05) | 3.83 (0.93) | .18 |
| Post Treatment 10 weeks (n = 124) b | 4.06 (0.89) | 3.86 (1.09) | .23 |
Continuous outcomes are reported as mean (SD)
All baseline means were imputed due to limited missing data on two participants assigned to the placebo arm.
Post treatment means are not adjusted for the baseline score.
Abbreviations: EPA = eicosapentaenoic acid; DHA = docosahexaenoic acid; RBC = red blood cells
Adherence to the Treatment Regimen
Based on pill counts, adherence to the trial medication regimen was >95% in both arms (Table 2). EPA levels were not available for nine of the patients and the missing data were imputed. Mean EPA RBC levels were nearly identical for the two arms at baseline (p=.48). At 10 weeks, the EPA level in the placebo arm was unchanged from baseline whereas it increased more than four fold in the EPA arm (Table 2). This is within range of the expected level for the dose of EPA and the duration of the trial.30 There was no difference in the mean number of weekly servings of fatty fish (e.g., mackerel, salmon) reportedly consumed by the participants in the placebo (0.76 ± 0.76) and EPA (0.63±0.72) arms during the ten weeks of the trial (p=0.30).
Post–Treatment (10-week) Outcomes
Primary Outcome:
The depression scores at baseline and post-treatment are presented in Table 2. There was no difference between the placebo and EPA arms on the BDI-II at the post-treatment evaluation after adjusting for baseline scores (p=0.22) (Table 3). Two-way interaction terms added to the primary outcome model produced no evidence for treatment moderation by gender (est. β=−1.66 [95%CI:−7.42, 4.09]; t123 = −0.57; p=.57), minority status (est. β = −4.33 [95%CI:−10.16,1.50]; t125 = −1.47; p=.14), age (est. β=.12 [95%CI:−0.18,0.41]; t134= 0.79; p=.43); recent antidepressant treatment (est. β = 3.39 [95%CI −3.28, 10.05]; t126=1.01; p=.32), cardiac status (est. β=0.03 [95%CI −6.60, 6.65]; t123 = 0.01; p=.99), CRP (est. β=0.02 [95%CI −0.84, 0.89]; t122 = 0.05; p=0.96), or by anxiety (est. β = −0.09[95%CI −0.38, 0.19]; t128= −0.65; p=.51).
Table 3:
Primary and Secondary Depression and Anxiety Outcomes
| Outcome | ITT Parameter Estimate a, (95% CI) | Cohen’s D (95% CI) | Test Statistic b | P-Value |
|---|---|---|---|---|
| Primary | ||||
| Baseline-Post Treatment BDI-II Scores | 1.74 (−1.04, 4.52) |
0.19 (−0.14, 0.52) |
t131 = 1.24 | .22 |
| Secondary | ||||
| Baseline-Post Treatment HAM-D Scores | 0.81 (−1.11, 2.73) |
0.13 (−0.20, 0.46) |
t118 = 0.84 | .40 |
| Baseline-Post Treatment PHQ-9 Scores | 0.33 (−1.19, 1.85) |
0.06 (−0.26, 0.39) |
t133 = 0.43 | .67 |
| Baseline-Post Treatment BAI Scores | 1.11 (−1.07, 3.30) |
0.15 (−0.18, 0.48) |
t122 = 1.01 | .32 |
| Remission | ||||
| (BDI-II score ≤ 8 at 10 weeks) | 0.85 (0.43, 1.68) |
t3605 = −0.48 | .63 | |
| Response | ||||
| (>50% reduction in BDI-II score from baseline) | 0.64 (0.28, 1.47) |
t2661 = −1.05 | .29 | |
The ITT parameter estimate represents the treatment effect, that is, the difference between placebo and omega-3 group means at the post-treatment evaluation. The pre-post scores were adjusted for the baseline outcome measure in each ANCOVA model.
Imputation inference (ITT analysis) is based on a t reference distribution with adjusted degrees of freedom (tADF), computed from the SAS© MIANALYZE procedure42 For the dichotomous remission and improvement outcomes, the estimated ITT parameters are reported as odds ratios; a Wald test is used to test the null hypothesis of no treatment effect on the probability of remission/improvement. The placebo arm is the reference group.
Abbreviations: BDI-II = Beck Depression Inventory II; HAM-D = Hamilton Rating Scale for Depression; BAI = Beck Anxiety Inventory; ITT = Intention-to-Treat.
Secondary Outcomes:
There was no difference on the HAM-D-17 between the placebo and the EPA arm at the post-treatment evaluation after adjusting for baseline scores (p=.40)(Table 3). A profile plot of weekly PHQ-9 scores is presented in Figure 2. Depressive symptoms improved over time at comparable rates in both groups (treatment x time est. β=0.03 [95%CI:−0.14, 0.20]; t138=0.32; p=.75). No major violations of model assumptions and no influential observations were identified in any of the analyses.
Figure 2:
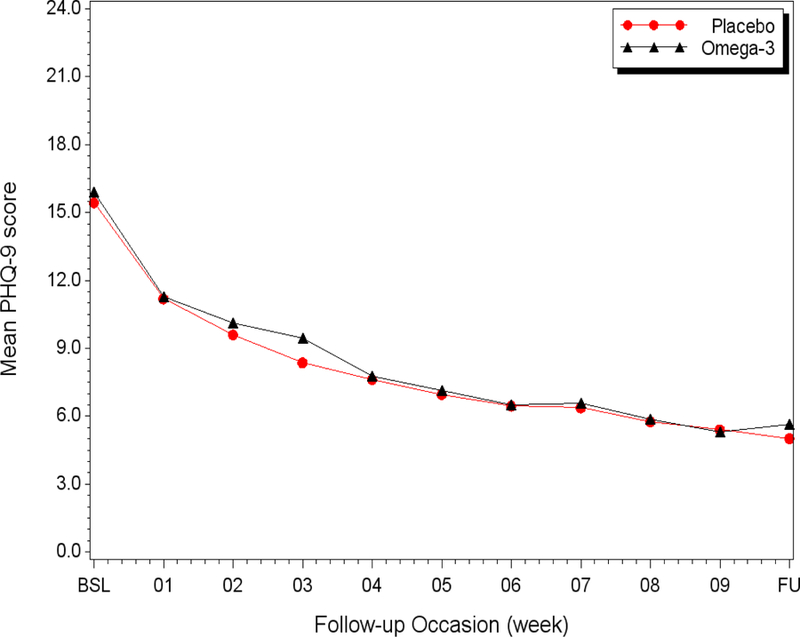
Profile plot of the weekly course of depression symptoms (PHQ-9) by treatment group adjusted for baseline score
As reported in Table 3, there were no between-group differences in the remission rate (BDI-II ≤ 8)(50.6% placebo vs. 46.7% EPA; OR=0.85 [95%CI 0.43,1.68]; p=.63), treatment response rate (>50% reduction in BDI-II scores from baseline; 80.5% placebo vs. 73.1% EPA (OR=0.64, 95%CI:0.28,1.47; p=.29), or on baseline-adjusted post-treatment anxiety levels (BAI between-groups difference=1.11,95%CI:−1.07,3.30; p=.32)(Table 3).
The preceding analyses were repeated for the subgroup of participants who completed all phases of the study (n=136), defined as completing both baseline and follow up assessments and having taken the prescribed medication regimen for the ten weeks of the study. These results were very similar to the ITT analyses, with no significant differences between arms for any analysis (data not shown).
Patients were asked at 10 weeks to guess the group to which they had been assigned. The difference between actual and suspected group assignment was not significant (estimated kappa [κ] = 0.16; 95%CI −0.03, 0.36).
Side Effects and Symptoms
New or worsened medical symptoms were recorded weekly during the 10 weeks of the study. Gastrointestinal complaints (diarrhea, indigestion, constipation, flatulence, nausea) were the most common, reported by 33 (45.2%) of the placebo and 35 (49.3%) of patients in the EPA arm (χ2=0.24; df=1; p=.62). Neurological symptoms (tremors, dizziness, headache) were the next most common, reported by 14 (19.2%) of the placebo and 11 (15.5%) of the patients in the EPA arm (χ2=0.34; df=1; p=.56). Other symptoms (e.g. dry eyes, bitter taste, dry skin, muscle aches) were rarely reported, and the total number of reported symptoms was similar between arms (p=0.84). Nearly all symptoms were described as mild to moderate, and resolved or greatly improved within a few weeks with three exceptions, all in the EPA arm. One patient reported severe indigestion, another reported unremitting diarrhea, and the third complained of frequent bowel movements. All three discontinued the drugs and dropped out of the study after less than three weeks.
Adverse Events
Emergency department visits that resulted in hospitalization were not counted as separate adverse events. Fourteen patients (19.7%) in the EPA arm and ten (13.7%) in the placebo arm were seen in an emergency department or urgent care center and/or were hospitalized during the 10 weeks of the study (χ2 = 0.94; df=1; p=.33). Twelve of the hospitalizations and emergency visits were cardiac-related. Of these, one patient in the placebo arm was admitted for ACS, two in the EPA arm and one in the placebo arm were hospitalized for cardiac catheterization and percutaneous intervention, one EPA patient was hospitalized for a hypertensive crisis, and another received an automatic implantable cardioverter-defibrillator. One patient in the placebo arm was hospitalized for symptoms of acute heart failure. All of the cardiac-related emergency room visits that did not lead to hospitalization were for angina or shortness of breath (n=2 EPA; n=1 placebo). All but two of the non-cardiac hospitalizations (pneumonia [placebo], blood clot [EPA]) were for non-life-threatening conditions (e.g. fractures, falls, pain). None of the adverse events were deemed by the investigators, the IRB, or the DSMB to be study-related.
DISCUSSION
The results of this trial do not support the hypothesis that co-administration of 2g/day of EPA improves the efficacy of 50mg/day of sertraline in patients with major depression and CHD or major CHD risk factors. This is inconsistent with most previous studies of depressed psychiatric patients in which EPA supplements substantially augmented the efficacy of standard antidepressants.
To our knowledge, only two previous clinical trials have evaluated the effects of omega-3 supplements on depression in patients with CHD. The first was our initial ten-week trial which found no effect for the combination of 50mg/day of sertraline plus a supplement containing 930mg/day EPA and 750mg/day DHA, compared to sertraline plus placebo in patients with CHD and major depression. The second tested a 12-week regimen of omega-3 (1.2g/day EPA and 0.6g/day DHA) monotherapy vs. placebo on depression symptoms in 37 depressed and 55 nondepressed patients with CHD.31 Twelve of these patients had been taking antidepressants for at least 3 months before the trial began. There were no differences between the arms on post-treatment HAM-D or BDI-II scores. The null findings of both trials are consistent with trials of psychiatric patient samples that tested supplements with < 2:1 ratios of EPA to DHA.13
Three studies have explored the antidepressant effects of omega-3 supplements in secondary analyses of trials that were designed primarily to determine the effects of omega-3 supplements on cardiac outcomes in patients with CHD32–34, and only one reported any effect on depression. In a secondary analysis of the OMEGA trial, Zimmer et al. evaluated 2,081 of the 3,851 post-MI patients enrolled in the study who received either an omega-3 supplement (480 mg/day EPA and 380 mg/day DHA) or a placebo for 12 months.34 Depression was not assessed at baseline, so they reported unadjusted post-trial depression scores only. There was no difference on the BDI-II between arms in the total sample, but among patients who were receiving antidepressants and had elevated BDI-II scores (>14 BDI-II) at 12 months, those who received the omega-3 supplement scored lower on the BDI-II than did those who received a placebo. In all three trials, the dosages and ratios of EPA to DHA were lower and the duration of treatment was considerably longer than in most of the successful omega-3 treatment trials of depressed psychiatric patients.
Several limitations should be considered in interpreting the results of this trial. First, the treatment may have been too brief to have a measurable effect on depression. However, trials in psychiatric patients have found EPA treatment effects in 10 weeks or less.13, 28 Furthermore, there is no indication from our study that longer treatment would have favored the omega-3 group. The weekly PHQ-9 scores (Figure 2) showed that most of the improvement in both arms in our trial occurred during the first few weeks with marginal improvement during the last few weeks, consistent with findings of most depression treatment trials.35,36 Finally, we found more than a four-fold increase in RBC EPA in the EPA arm, suggesting that a substantial amount of EPA was biologically available within the 10 weeks of the trial.
It is also possible that an effect of EPA augmentation would have been detectable had we chosen another antidepressant or administered a higher dose of sertraline. However, positive trials have employed similar SSRIs, and some evaluated the effects of omega-3 supplements in patients who were receiving a variety of different antidepressants at the time of randomization. Previous studies showed that higher doses of sertraline (100–200 mg/day) produce no better response rates than 50mg/day, but higher rates of side-effects.37–39
Finally, a different dose of EPA might have produced a different outcome. The choice of the omega-3 dosage for the present trial was based on previous studies of psychiatric patients. The meta-analysis by Lin and Su found that high doses of EPA did not add much to its efficacy40, and Peet and Horrobin29 reported that doses of EPA over one gram per day yielded more side effects without much additional improvement in depression. The meta-analysis by Sublette and colleagues reported a nonlinear relationship between EPA dose and antidepressant efficacy13, with diminishing returns somewhere above 2.5–3.0g/day. The optimal dose has not been established, but the dose we used was within the range of that used in most of the positive studies.
Although most of the trials of EPA for depression have been positive, there have been others besides the present study that did not find an effect 41,42, mirroring the findings of trials of omega-3 supplements for reducing cardiac morbidity and mortality.43 Consistent with earlier studies that have found no reliable moderators of the antidepressant effect of any omega-3 formulation40, we found no evidence of treatment moderation by age, gender, minority status, recent antidepressant treatment, baseline CRP level, or CHD status. Nevertheless, more effort should be made to identify the characteristics of depressed patients who may benefit from omega-3 monotherapy or augmentation of standard antidepressants. For example, consistent with the suggestion that EPA may improve depression by decreasing inflammation, Mazereeuw and colleagues identified lipid peroxidation with high oxidative stress as a predictor of improvement in depression symptoms following administration of omega-3 supplements.44 Although CRP levels did not moderate the effect of EPA on depression in this study, other markers of inflammation including lipid peroxidation with high oxidative stress, may do so. Research on variations in the genes involved in the metabolism, synthesis, uptake, and utilization of omega-3 may also help to identify patients who will respond to omega-3 supplements as monotherapy or as augmentation of standard antidepressants.45–48
In conclusion, this randomized, double-blind, placebo-controlled trial found no evidence that adding 2 grams/day of EPA to 50mg/day of sertraline is superior to 50 mg/day of sertraline plus placebo for the treatment of depression in patients with or at high risk for CHD and with major depression. The relatively high remission rates in both arms following 10 weeks of treatment suggest that a standard antidepressant medication combined with supportive clinical management may have a clinically significant effect on major depression in these patients. Identifying the characteristics of cardiac patients whose depression may benefit from omega-3, and clarifying the pathways linking omega-3 to improvement in depression symptoms, are important directions for future research.
Clinical Points.
Depression is associated with increased mortality in persons with heart disease.
In an effort to identify safe, effective treatments for depression in these patients we tested whether sertraline is more effective when administered with eicosapentaenoic acid, an omega-3 fatty acid.
Contrary to our hypothesis, the results show that EPA plus sertraline is not superior to placebo plus sertraline.
Acknowledgments
Funding and Support: This study was funded by Grant Number RO1 HL117805 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland. Atrium Innovations Inc. provided the EPA and matching placebo capsules for the trial. Neither the National Institutes of Health nor Atrium Innovations, Inc. had a role in the design, analysis, interpretation or publication of this study.
Trial Registration: NCT02021669 www.clinicaltrials.gov.
Footnotes
Disclosures: Dr. Carney or a member of his family is a stockholder in Pfizer Inc. Drs. Freedland, Rich, and Rubin, and Mr. Steinmeyer have no potential conflicts of interest to report. Dr. Harris is the President of OmegaQuant Analytics, LLC, Dr. Harris is the President of OmegaQuant Analytics, LLC.
REFERENCES
- 1.Carney RM and Freedland KE. Depression and coronary heart disease. Nat Rev Cardiol 2017;14:145–155. [DOI] [PubMed] [Google Scholar]
- 2.Lichtman JH, Froelicher ES, Blumenthal JA, et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation 2014;129:1350–69. [DOI] [PubMed] [Google Scholar]
- 3.Meijer A, Conradi HJ, Bos EH, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis of 25 years of research. Gen Hosp Psychiatry 2011;33:203–16. [DOI] [PubMed] [Google Scholar]
- 4.Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003;289:3106–16. [DOI] [PubMed] [Google Scholar]
- 5.Glassman AH, O’Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002;288:701–9. [DOI] [PubMed] [Google Scholar]
- 6.Kim JM, Stewart R, Lee YS, et al. Effect of Escitalopram vs Placebo Treatment for Depression on Long-term Cardiac Outcomes in Patients With Acute Coronary Syndrome: A Randomized Clinical Trial. JAMA 2018;320:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Melle JP, de Jonge P, Honig A, et al. Effects of antidepressant treatment following myocardial infarction. Br J Psychiatry 2007;190:460–6. [DOI] [PubMed] [Google Scholar]
- 8.Lai HT, de Oliveira Otto MC, Lemaitre RN, et al. Serial circulating omega 3 polyunsaturated fatty acids and healthy ageing among older adults in the Cardiovascular Health Study: prospective cohort study. BMJ 2018;363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rimm EB, Appel LJ, Chiuve SE, et al. Seafood Long-Chain n-3 Polyunsaturated Fatty Acids and Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation 2018;138:e35–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallahan B, Ryan T, Hibbeln JR, et al. Efficacy of omega-3 highly unsaturated fatty acids in the treatment of depression. Br J Psychiatry 2016;209:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martins JG, Bentsen H and Puri BK. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: a critique of Bloch and Hannestad and updated meta-analysis. Mol Psychiatry 2012;17:1144–9; discussion 1163–7. [DOI] [PubMed] [Google Scholar]
- 12.Mocking RJ, Harmsen I, Assies J, et al. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl Psychiatry 2016;6:e756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sublette ME, Ellis SP, Geant AL, et al. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J Clin Psychiatry 2011;72:1577–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mischoulon D and Freeman MP. Omega-3 fatty acids in psychiatry. Psychiatr Clin North Am 2013;36:15–23. [DOI] [PubMed] [Google Scholar]
- 15.Appleton KM, Rogers PJ and Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr 2010;91:757–70. [DOI] [PubMed] [Google Scholar]
- 16.Grosso G, Pajak A, Marventano S, et al. Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PLoS One 2014;9:e96905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carney RM, Freedland KE, Stein PK, et al. Effect of omega-3 fatty acids on heart rate variability in depressed patients with coronary heart disease. Psychosom Med 2010;72:748–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carney RM, Freedland KE, Rubin EH, et al. Omega-3 augmentation of sertraline in treatment of depression in patients with coronary heart disease: a randomized controlled trial. JAMA 2009;302:1651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martins JG. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J Am Coll Nutr 2009;28:525–42. [DOI] [PubMed] [Google Scholar]
- 20.Spitzer RL, Kroenke K and Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. JAMA 1999;282:1737–44. [DOI] [PubMed] [Google Scholar]
- 21.Freedland KE, Skala JA, Carney RM, et al. The Depression Interview and Structured Hamilton (DISH): rationale, development, characteristics, and clinical validity. Psychosom Med 2002;64:897–905. [DOI] [PubMed] [Google Scholar]
- 22.Beck AT, Steer RA and Brown GK. BDI-II Manual Second ed. San Antonio: Harcourt Brace & Company; 1996. [Google Scholar]
- 23.Harris WS and Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med 2004;39:212–20. [DOI] [PubMed] [Google Scholar]
- 24.Hedlund JL and Viewig BW. The Hamilton rating scale for depression: A comprehensive review. Journal of Operational Psychiatry 1979;10:149–165. [Google Scholar]
- 25.Beck AT and Steer RA. BAI, Beck Anxiety Inventory San Antonio: Harcourt Brace Jovanovich; 1990. [Google Scholar]
- 26.Mallinckrodt CH, Sanger TM, Dube S, et al. Assessing and interpreting treatment effects in longitudinal clinical trials with missing data. Biol Psychiatry 2003;53:754–60. [DOI] [PubMed] [Google Scholar]
- 27.Lesperance F, Frasure-Smith N, St-Andre E, et al. The efficacy of omega-3 supplementation for major depression: a randomized controlled trial. J Clin Psychiatry 2011;72:1054–62. [DOI] [PubMed] [Google Scholar]
- 28.Nemets B, Stahl Z and Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 2002;159:477–9. [DOI] [PubMed] [Google Scholar]
- 29.Peet M and Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry 2002;59:913–9. [DOI] [PubMed] [Google Scholar]
- 30.Krul ES, Lemke SL, Mukherjea R, et al. Effects of duration of treatment and dosage of eicosapentaenoic acid and stearidonic acid on red blood cell eicosapentaenoic acid content. Prostaglandins Leukot Essent Fatty Acids 2012;86:51–9. [DOI] [PubMed] [Google Scholar]
- 31.Mazereeuw G, Herrmann N, Oh PI, et al. Omega-3 Fatty Acids, Depressive Symptoms, and Cognitive Performance in Patients With Coronary Artery Disease: Analyses From a Randomized, Double-Blind, Placebo-Controlled Trial. J Clin Psychopharmacol 2016;36:436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andreeva VA, Galan P, Torres M, et al. Supplementation with B vitamins or n-3 fatty acids and depressive symptoms in cardiovascular disease survivors: ancillary findings from the SUpplementation with FOLate, vitamins B-6 and B-12 and/or OMega-3 fatty acids (SU.FOL.OM3) randomized trial. Am J Clin Nutr 2012;96:208–14. [DOI] [PubMed] [Google Scholar]
- 33.Giltay EJ, Geleijnse JM and Kromhout D. Effects of n-3 fatty acids on depressive symptoms and dispositional optimism after myocardial infarction. Am J Clin Nutr 2011;94:1442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimmer R, Riemer T, Rauch B, et al. Effects of 1-year treatment with highly purified omega-3 fatty acids on depression after myocardial infarction: results from the OMEGA trial. J Clin Psychiatry 2013;74:e1037–45. [DOI] [PubMed] [Google Scholar]
- 35.Sakurai H, Uchida H, Abe T, et al. Trajectories of individual symptoms in remitters versus non-remitters with depression. J Affect Disord 2013;151:506–13. [DOI] [PubMed] [Google Scholar]
- 36.Taylor MJ, Freemantle N, Geddes JR, et al. Early onset of selective serotonin reuptake inhibitor antidepressant action: systematic review and meta-analysis. Arch Gen Psychiatry 2006;63:1217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fabre LF, Abuzzahab FS, Amin M, et al. Sertraline safety and efficacy in major depression: a double-blind fixed-dose comparison with placebo. Biol Psychiatry 1995;38:592–602. [DOI] [PubMed] [Google Scholar]
- 38.Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic Review and Meta-Analysis: Dose-Response Relationship of Selective Serotonin Reuptake Inhibitors in Major Depressive Disorder. Am J Psychiatry 2016;173:174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schweizer E, Rynn M, Mandos LA, et al. The antidepressant effect of sertraline is not enhanced by dose titration: results from an outpatient clinical trial. Int Clin Psychopharmacol 2001;16:137–43. [DOI] [PubMed] [Google Scholar]
- 40.Lin PY and Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry 2007;68:1056–61. [DOI] [PubMed] [Google Scholar]
- 41.Bot M, Pouwer F, Assies J, et al. Eicosapentaenoic acid as an add-on to antidepressant medication for co-morbid major depression in patients with diabetes mellitus: a randomized, double-blind placebo-controlled study. J Affect Disord 2010;126:282–6. [DOI] [PubMed] [Google Scholar]
- 42.Mischoulon D, Nierenberg AA, Schettler PJ, et al. A double-blind, randomized controlled clinical trial comparing eicosapentaenoic acid versus docosahexaenoic acid for depression. J Clin Psychiatry 2015;76:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.London B, Albert C, Anderson ME, et al. Omega-3 fatty acids and cardiac arrhythmias: prior studies and recommendations for future research: a report from the National Heart, Lung, and Blood Institute and Office Of Dietary Supplements Omega-3 Fatty Acids and their Role in Cardiac Arrhythmogenesis Workshop. Circulation 2007;116:e320–35. [DOI] [PubMed] [Google Scholar]
- 44.Mazereeuw G, Herrmann N, Andreazza AC, et al. Oxidative stress predicts depressive symptom changes with omega-3 fatty acid treatment in coronary artery disease patients. Brain Behav Immun 2017;60:136–141. [DOI] [PubMed] [Google Scholar]
- 45.Alsaleh A, Maniou Z, Lewis FJ, et al. ELOVL2 gene polymorphisms are associated with increases in plasma eicosapentaenoic and docosahexaenoic acid proportions after fish oil supplement. Genes Nutr 2014;9:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simopoulos AP. Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: their role in the determination of nutritional requirements and chronic disease risk. Exp Biol Med (Maywood) 2010;235:785–95. [DOI] [PubMed] [Google Scholar]
- 47.Superko HR, Superko SM, Nasir K, et al. Omega-3 fatty acid blood levels: clinical significance and controversy. Circulation 2013;128:2154–61. [DOI] [PubMed] [Google Scholar]
- 48.Zeman M, Vecka M, Jachymova M, et al. Fatty acid CoA ligase-4 gene polymorphism influences fatty acid metabolism in metabolic syndrome, but not in depression. Tohoku J Exp Med 2009;217:287–93. [DOI] [PubMed] [Google Scholar]