Abstract
Climbing fiber-driven long-term depression (LTD) of parallel fiber synapses onto cerebellar Purkinje cells has long been investigated as a putative mechanism of motor learning. We recently discovered that the rules governing the induction of LTD at these synapses vary across different regions of the cerebellum. Here, we discuss the design of LTD induction protocols in light of this heterogeneity in plasticity rules. The analytical advantages of the cerebellum provide an opportunity to develop a deeper understanding of how the specific plasticity rules at synapses support the implementation of learning.
Main text
Nearly half a century ago, Marr, Albus, and Ito proposed a model of cerebellum-dependent learning, wherein Climbing fibers carry instructive signals that guide plasticity at the parallel fiber synapses onto Purkinje cells [1–3]. A decade later, Ito and colleagues demonstrated that Climbing fiber activation could trigger long-term depression (LTD) at conjunctively activated parallel-fiber synapses [4,5]. Since then, the idea that the cerebellum learns by sculpting away synapses that cause errors through this anti-Hebbian form of synaptic plasticity has had a powerful influence on the cerebellar field. LTD and its links to cerebellum-dependent learning have been extensively investigated, yet there is still much disagreement about whether and how LTD at parallel fiber-to-Purkinje cell synapses contributes to learning [6,7,16–25,8,26–30,9–15]. Here, we review findings that suggest a path toward a Clearer and more sophisticated understanding of the contribution of LTD to cerebellum-dependent learning.
A variety of recording, stimulation and perturbation approaches have yielded considerable, convergent evidence for a role of LTD in learning [10,11,37–46,15,18,31–36] but also yielded results that call into question the necessity of parallel fiber-to-Purkinje cell LTD for learning [13,40,41,47–49]. One of the most widely used experimental approaches has been to employ pharmacological or molecular-genetic techniques to perturb LTD, and then test the effects on one or more cerebellum-dependent learning tasks. Results obtained with this approach have been mixed in their support for a role of LTD in learning. There are a number of potential reasons for the lack of consistent findings on the link between LTD and learning, including potential off-target effects of the manipulations used to perturb LTD (see discussion in Schonewille et al., 2011), compensation for LTD deficits by other forms of plasticity [8], and selective contribution of LTD to certain cerebellar leaning tasks and not others [7,40,41,50]. Here, we focus on one specific challenge in connecting in vitro LTD results and in vivo learning results, which was highlighted by two recent studies [51,52], namely the lack of a single, definitive protocol for studying LTD in vitro, and our rudimentary understanding of the sensitivity of LTD to variations in the protocols used to induce LTD.
Over the years, LTD at parallel fiber-to-Purkinje cell synapses has been studied using a wide range of induction protocols (see Table 1 for a representative sample of LTD protocols), which have generally been treated as equivalent. However, a recent study [51, see also 53] demonstrated that the measurement of LTD impairments can be highly sensitive to the parameters used for LTD induction. Four different LTD induction protocols, which all induced significant LTD in wild type mice, were used to study two lines of mice that had previously been reported as having normal cerebellum-dependent learning despite a lack of LTD [13]. When tested with different LTD induction protocols, the same line of mice could exhibit everything from considerable sparing of LTD to near-total perturbation of LTD. The demonstration that one can get different answers about whether and how much LTD is impaired, depending on the protocol used to study LTD makes it tempting to ask what is the “correct”, “physiological”, or “behaviorally relevant” protocol for testing LTD. However, another recent study illustrates that this question is unlikely to have a single or straightforward answer.
Table 1.
Representative stimulation protocols which have been used to induce long term depression at parallel fiber-to-Purkinje cell synapses. The pairing of parallel fibers (PF, black) and Climbing fibers (CF, red) is schematized in the first column, with each stimulus shown as a line. The time between stimuli is not drawn to scale. The following columns describe the parameters of the parallel fiber and Climbing fiber stimuli and the pairings between them, the region of the cerebellum where the investigation was conducted, and the presence or absence of blockers of inhibition [5,13,6,1,62,68,82–8,5,102,138,139,31,140–149,34,150,151,51,52,57–60].
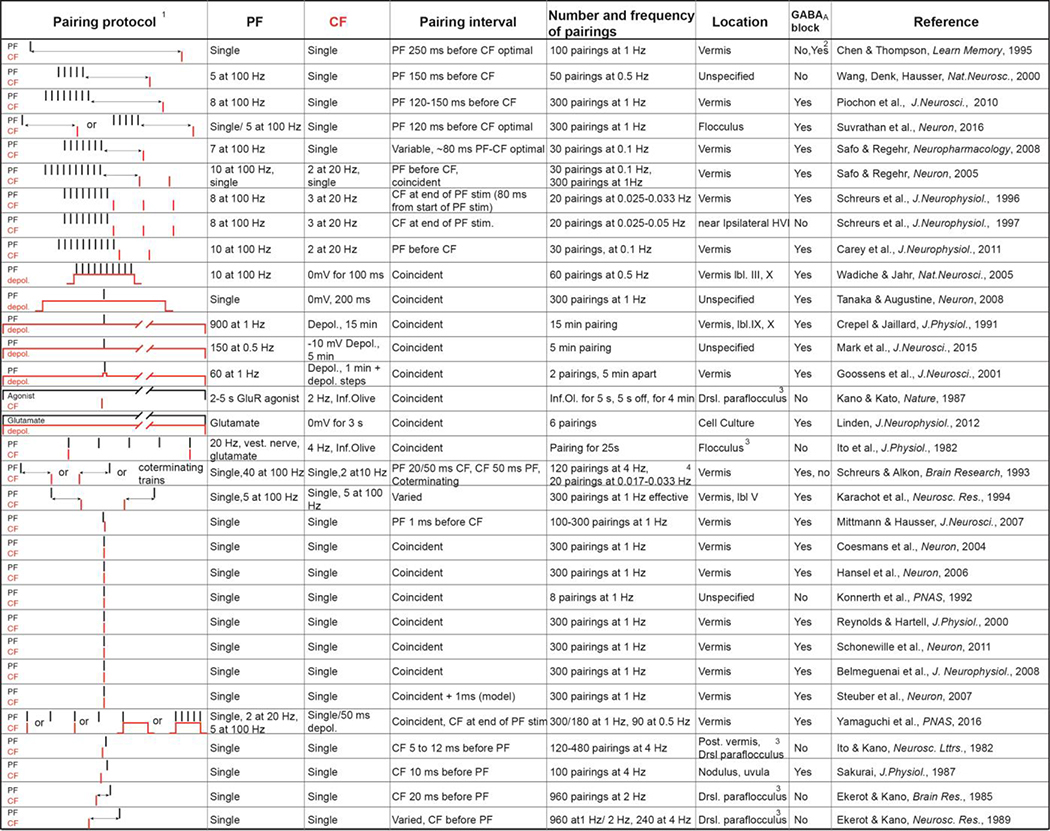 |
In order of increasing PF-CF interval.
100 coincident pairings only effective with GABA block, 600 pairings effective at all tested intervals including CF before PF.
Not in slice preparation.
with trains unpaired stimulation causes LTD
Suvrathan and colleagues discovered that the requirements for the induction of plasticity at the parallel fiber-to-Purkinje cell synapses can vary across regions of cerebellum, and even between nearby Purkinje cells within a region [52]. More specifically, there is heterogeneity in the optimal parallel fiber – Climbing fiber pairing interval for inducing LTD at parallel fiber synapses onto Purkinje cells in different regions of the cerebellum. The timing requirements for inducing LTD in the flocculus, the region of the cerebellum that supports oculomotor learning, are strikingly different from the timing requirements in the well-studied vermis. In the flocculus, LTD is only induced if the delay between parallel fiber and Climbing fiber activation is matched to the feedback delay for Climbing fibers to signal errors in vivo during oculomotor learning, which is approximately 120 ms (Fig. 1) [52,54,55]. Moreover, this delay must be precise to within a few tens of milliseconds to be effective in driving LTD. The 120-ms feedback delay in the flocculus is relatively long, because the error signals are visual, and visual processing is slow compared to other sensory modalities such as somatosensation. Crucially, in the vermis, which supports different behaviors and thus receives error signals from the Climbing fibers of different modalities and different delays, the associative synaptic depression has different timing requirements. An important direction for future research will be to test the match between the feedback delays and the timing requirements for LTD in different Purkinje cells of the vermis. Mechanistically, there are known molecular differences between Purkinje cells [56], which could potentially support the tuning of plasticity to the appropriate Climbing fiber delay, however the molecular mechanism for the temporal tuning of LTD has not yet been identified. These findings demonstrate that the rules governing the induction of LTD are not uniform across all parallel fiber-to-Purkinje cell synapses, but rather appear to be locally tuned to the functional requirements of the circuit for a specific behavior. Conversely, a single LTD induction protocol can vary in its effectiveness for inducing LTD in different cerebellar regions [57].
Figure 1.
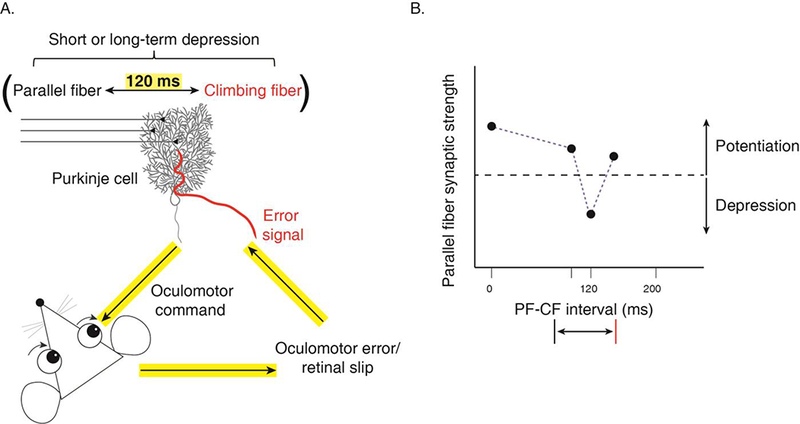
A. The optimal parallel fiber – Climbing fiber (PF-CF) pairing interval for induction of plasticity at parallel fiber-to-Purkinje cell synapses in the cerebellar flocculus is 120 ms, which is the feedback delay for Climbing fibers to signal an error during oculomotor learning. B. Schematic illustrating the tuning of LTD at parallel fiber-to-Purkinje cell synapses in the flocculus to a Climbing fiber delay of 120 ms (Schematic adapted from Suvrathan et al., Neuron, 2016).
The demonstration of heterogeneity in the optimal pairing interval for LTD of parallel fiber-to-Purkinje cell synapses in different regions of the cerebellum opens up the possibility that there may be heterogeneity in other aspects of the LTD learning rule as well. Might there also be regional tuning of the LTD rule to the typical patterns of parallel fiber activation, Climbing fiber activation, or the inhibitory or neuromodulatory tone in different cerebellar microcircuits? In a “standard” LTD experiment, the slice physiologist must make choices related to all of these factors (Table 1). Below, we consider some of the findings that can inform these decisions about experimental design (Fig. 2).
Figure 2.
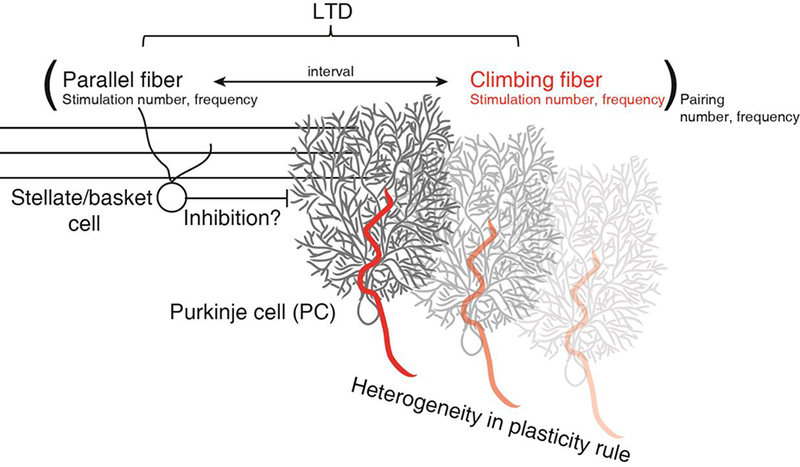
LTD is a function of the frequency and number of parallel fiber and Climbing fiber stimuli, the parallel fiber-Climbing fiber pairing interval, and the frequency and number of pairings: LTD(PF-to-PC) = f([PF(n)(ν) CF(n)(ν)] (n)(ν)), where n = number of stimuli or pairings, ν = frequency, t = PF-to-CF time nterval. The role played by inhibition and neuromodulators remains unclear. There is also heterogeneity in the plasticity rules implemented at the parallel fiber synapses onto different Purkinje cells.
Biologically plausible variations that may influence the induction of LTD
Parallel fiber activation
In vitro, LTD can be induced by pairing Climbing fiber stimulation with either a single stimulus to the parallel fibers, or a brief, high frequency train of parallel fiber activation (Table 1). Many influential studies have used single parallel fiber stimulation during LTD induction [13,31,58–62]. Yet, there is evidence that metabotropic glutamate receptors (mGluRs) contribute to motor learning and to LTD [46,63,64], and mGluR activation requires multiple parallel fiber stimuli at high frequency [65,66]. Also, stimulation of parallel fibers either in larger numbers or at a higher frequency can overcome the necessity for Climbing fiber co-activation [67,68].
The in vivo data currently available do not provide strong guidance for the in vitro physiologist’s choice of parallel fiber stimulation parameters for studying LTD. Our knowledge about the natural patterns of activity in the parallel fibers in vivo during learning is extremely limited, because of technical difficulties in recording from the small, densely packed granule cells, whose axons form the parallel fibers. In anesthetized animals, granule cells were reported to be largely silent, and emit brief, high-frequency bursts of spikes in response to a punctate whisker stimulus [69,70], whereas a study in decerebrate animals found more sustained granule cell spiking in response to a more sustained somatosensory stimulus (cutaneous forepaw stimulus; [71]). In the vestibulocerebellum, granule cells exhibit a range of responses to vestibular stimuli, including high-frequency bursts of spikes, sustained firing with smooth firing rate modulation in response to the vestibular stimulus, or, in some granule cells, a combination of both response types [72]. Likewise, calcium imaging studies suggest that many granule cells have high levels of sustained activity in awake behaving animals [73–75], although this approach does not provide spike-level resolution of granule cell activity.
Another variable in studies of LTD that is not well constrained by in vivo data is the number of co-activated parallel fiber inputs to a given Purkinje cell. A prominent and compelling theory proposes sparse coding of information by granule cells [76–79]. However, recent imaging studies challenge the hypothesis that activation of the granule cell population is sparse in behaving animals [73,74,80,81]. In vitro, the number of co-activated parallel fibers, which can be controlled by the strength of parallel fiber stimulation, can influence whether LTD is induced or not [67,68], suggesting that cooperativity at parallel fiber synapses is an important consideration. However, the mechanisms underlying LTD induced by stimulation of multiple parallel fiber inputs vs. a few inputs may be different [82]. Distant parallel fiber synapses that are not activated during LTD induction, do not undergo modification [4,83– 86]. LTD does seem to spread to nearby synapses [61,67,87], however, LTD at synapses that were not paired with Climbing fiber stimulation appears to involve different mechanisms [88,89].
Thus, in vivo, during learning, there may be considerable heterogeneity in the patterning and overall rate of firing in individual granule cells, from mostly silent with an occasional burst of spikes, to tonically firing [75,90–92]. Likewise, there may be heterogeneity in the level of parallel fiber co-activation. This heterogeneity of parallel fiber activity patterns could influence the likelihood of LTD induction at parallel fiber synapses in different cerebellar regions. Alternatively, the parallel fiber activation requirements for LTD in different cerebellar regions may be tuned to the relevant patterns of parallel fiber activity, as observed for the parallel fiber-Climbing fiber pairing interval [52].
Climbing fiber activation
A spike in a Climbing fiber triggers a characteristic “complex spike” in its Purkinje cell targets, which is associated with a prolonged calcium transient [93–98]. Historically, the Climbing fiber-triggered calcium transient in the Purkinje cell was considered a binary, all-or-none error signal, and many of the seminal studies of LTD used depolarization of the Purkinje cell and the resulting calcium entry through voltage-gated calcium channels as a substitute for Climbing fiber-induced calcium entry (Table 1). However, more recent studies have suggested that the calcium transient produced by the Climbing fiber and its efficacy at inducing LTD is graded. The efficacy of LTD induction is strongly correlated with the amount of calcium influx during induction [11,82,99–102]. In slice preparations, the number of spikes in a Climbing fiber burst affects the likelihood of LTD induction [103]. Moreover, in vivo, the duration of the Climbing fiber-triggered complex spike in a Purkinje cell is correlated with the amount of single-trial plasticity [104]. Thus, the choice of Climbing fiber stimulation parameters for studying LTD is a critical one, and, like the parameters of parallel fiber activity, can vary in vivo, even from trial to trial in the same cell.
Number and frequency of parallel fiber-Climbing fiber pairings
The number and frequency of parallel fiber-Climbing fiber pairings have varied across studies of cerebellar LTD, but a common choice is 300 pairings at 1 Hz (Table 1). This choice has similarities and differences with the number and frequency of behavioral training trials typically used to induce cerebellum-dependent learning, which develops over hundreds of training trials. For example, a typical oculomotor learning experiment might pair 1 Hz sinusoidal vestibular and visual stimuli, with learning developing over 30–60 min of training, for a total of approximately 2000–3000 stimulus cycles, or “trials” [7,13,35,37,105,106]. An eyeblink conditioning procedure may include 100 trials per session, with learning developing over 5–10 sessions, delivered over multiple days, for a total of about 1000 trials [107,108]. Thus, the number of pairings used in a typical LTD experiment is on the low end of the number of behavioral pairings used to induce cerebellum-dependent learning, and, not surprisingly, a greater number of pairings is more effective at inducing LTD [85]. In terms of the spacing between pairings, the 1 Hz frequency typical in LTD experiments is in the range used in oculomotor learning, but the inter-trial interval used in eyeblink conditioning is typically much longer, in the range of tens of seconds between trials within a session and 24 hours between sessions.
Notably, plasticity at parallel fiber-to-Purkinje cell synapses can be triggered not only by repeated parallel fiber-Climbing fiber pairings, as discussed above, but also by the extreme case of a single parallel fiber-Climbing fiber pairing [52], and similar single-trial plasticity has been observed in vivo [20,41,104,109,110]. In the flocculus, the properties of short-term, single-trial associative synaptic depression has striking parallels with those of LTD, including tight tuning for the functionally relevant Climbing fiber delay [52]. The molecular mechanisms of short-term associative depression induced by one or a few pairings are not well understood, and may be different from those of LTD [111].
Inhibition and other heterosynaptic modulatory influences
In order to isolate plasticity at the parallel fiber-to-Purkinje cell synapses from plasticity occurring at the synapses of inhibitory interneurons, most investigations of LTD are performed with GABAergic transmission blocked. However, there are some notable exceptions (see Table 1, seventh column). Whereas some studies [83,88,89] suggest that LTD can only be induced in the absence of GABAergic inhibition, successful LTD induction has been reported without a block of GABAergic inhibition (Table 1, [4,60,82,112]). Some investigators have suggested that the study of LTD with inhibition blocked is “unphysiological”, however, another interpretation is that in vivo, the induction of LTD requires a transient reduction in the level of inhibitory input to the Purkinje cell from the molecular layer interneurons. Moreover, there could be additional factors gating the induction of LTD by paired activation of parallel fibers and Climbing fibers [41,113]. Neuromodulators, such as acetylcholine and norepinephrine, which are known to influence associative synaptic plasticity in other brain areas [114–117], are also present in the cerebellum
Implications for future research
For years, the study of synaptic plasticity in vitro, in the cerebellum and other brain areas, focused on uncovering the cellular-molecular mechanisms. Driven by this goal, the protocols for inducing plasticity were selected mainly to optimize their reliability at inducing synaptic changes, and thereby facilitate the analysis of underlying cellular-molecular processes. More recently, there has been renewed effort to establish more direct, causal links between specific synaptic plasticity mechanisms and learning. In the cerebellar field, this has prompted consideration of how to design studies of LTD in vitro to facilitate this goal. The synaptic physiologist must make many choices, as outlined above. There have been attempts to make choices that recreate some of the specific conditions that are present in vivo [52,68,112]. However, our lack of knowledge about what exactly these conditions are during learning remains a challenge. Moreover, the conditions in vitro will always, by definition, differ in certain respects from those in vivo (let’s not forget that we are removing a piece of a neural circuit from the brain, and that the strength of reduced preparations is the ability to create simplified and more controlled conditions). The vexed question of which protocol, within the intimidatingly large parameter space, to use for induction of LTD is further complicated by heterogeneity in the plasticity rules operating at different parallel fiber-to-Purkinje cell synapses [51], and the possibility that there could be multiple heterosynaptic influences that govern the induction of LTD, in addition to the patterns of parallel fiber and Climbing fiber activation. Hence, there is no single, straightforward answer to the question of the most appropriate way to conduct LTD experiments in slice—there is no one-size-fits-all “standard” protocol. Nor is it possible for every study to explore the relevant parameter space. This represents both a significant challenge and a major opportunity.
The complexity and heterogeneity of learning rules identified at the parallel fiber-to-Purkinje cell synapses in the cerebellum may reflect a broader principle of how learning is implemented throughout the brain. The cerebellum is described as having the most uniform, “crystalline” cytoarchitecture in the mammalian brain. If there is heterogeneity of synaptic learning rules in this context, it seems likely that this is a more widespread property of synaptic plasticity, and there are already suggestions of this in other brain areas [118–121]. Exploration of the large parameter space and potential interactions between parameters governing the induction of plasticity at any type of synapse in the brain represents a major undertaking that will take years of effort by multiple labs. The cerebellum, with its relatively simple circuit architecture and its support of a number of simple and analytically tractable behaviors, offers unique opportunities to make sense of this newly recognized complexity of synaptic plasticity rules [51, 52], and to establish how the brain utilizes this complexity in the service of learning. The discovery of the tuning of LTD for different parallel fiber-Climbing fiber intervals illustrates how existing knowledge of the broader circuits supporting simple cerebellum-dependent forms of learning can be leveraged to inform experimental manipulations in vitro that can uncover new features of synaptic learning rules. Moreover, we can draw on a vast literature on the molecular mechanisms that support parallel fiber-to-Purkinje cell LTD [10,11,38,39,44,106,122,18,31–37], and on the heterogeneity of Purkinje cells [123,124,133–137,125–132]. Thus, studies of plasticity at the cerebellar Purkinje cells’ synapses offers unique insights and opportunities to move beyond broad concepts of Hebbian and anti-Hebbian plasticity to a more sophisticated understanding of the complex rules governing plasticity at synapses in a behaving animal undergoing learning.
In summary, recent findings on novel features of the rules governing synaptic plasticity in the cerebellum force us to consider the role of heterogeneity in the properties of LTD at parallel fiber-to-Purkinje cell synapses. This heterogeneity has been observed in the sensitivity to one key parameter of the protocols that induce LTD, the pairing interval; however, there are other relevant variables that may also show heterogeneous properties. Rather than considering this explosion of parameter space as a problem, we suggest that it may provide an opportunity, given the unique properties of the cerebellum and cerebellum-dependent behavior, to better understand the logic of how the recruitment of synaptic plasticity is precisely controlled during learning.
Acknowledgements.
We are grateful for Jaydev Bhateja for his comments and suggestions.
Funding. AS was supported by the Research Institute of the McGill University Health Centre and McGill University as well as by funding from the Canada First Research Excellence Fund, awarded to McGill University for the Healthy Brains for Healthy Lives initiative. JR was supported by NIH R01NS072406, R01DC004154 and the Simons Foundation Collaboration on the Global Brain #54031.
Footnotes
The authors declare that they have no conflict of interest
References
- 1.Marr D A theory of cerebellar cortex. J Physiol. 1969;202:437–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61. [Google Scholar]
- 3.Ito M Neural design of the cerebellar motor control system. Brain Res. 1972;40:81–4. [DOI] [PubMed] [Google Scholar]
- 4.Ito M, Sakurai M, Tongroach P. Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar purkinje cells. J Physiol. 1982;324:113–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito M, Kano M. Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and Climbing fibers in the cerebellar cortex. Neurosci Lett. 1982;33:253–8. [DOI] [PubMed] [Google Scholar]
- 6.Boyden ES, Katoh A, Raymond JL. Cerebellum-dependent learning: The Role of Multiple Plasticity Mechanisms. Annu Rev Neurosci. 2004;27:581–609. [DOI] [PubMed] [Google Scholar]
- 7.Boyden ES, Katoh A, Pyle JL, Chatila TA, Tsien RW, Raymond JL. Selective engagement of plasticity mechanisms for motor memory storage. Neuron. 2006;51:823–34. [DOI] [PubMed] [Google Scholar]
- 8.Gao Z, van Beugen BJ, De Zeeuw CI. Distributed synergistic plasticity and cerebellar learning. Nat Rev Neurosci. 2012;13:619–35. [DOI] [PubMed] [Google Scholar]
- 9.Galliano E, De Zeeuw CI. Questioning the Cerebellar Doctrine. Prog. Brain Res 2014;210:59–77 [DOI] [PubMed] [Google Scholar]
- 10.Ito M Cerebellar Long-Term Depression: Characterization, signal transduction, and functional roles. Physiol Rev. 2001;81:1143–95. [DOI] [PubMed] [Google Scholar]
- 11.Ito M, Yamaguchi K, Nagao S, Yamazaki T. Long-Term Depression as a Model of Cerebellar Plasticity. Prog Brain Res. 2014;210:1–30. [DOI] [PubMed] [Google Scholar]
- 12.Hirano T Around LTD Hypothesis in Motor Learning. Cerebellum. 2014;13:645–50. [DOI] [PubMed] [Google Scholar]
- 13.Schonewille M, Gao Z, Boele H-J, Veloz MFV, Amerika WE, Simek AAM, et al. Reevaluating the role of LTD in cerebellar motor learning. Neuron. 2011;70:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galliano E, Schonewille M, Peter S, Rutteman M, Houtman S, Jaarsma D, et al. Impact of NMDA Receptor Overexpression on Cerebellar Purkinje Cell Activity and Motor Learning. Eneuro. 2018;5:ENEURO.0270-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoshita T, Hirano T. Occurrence of long-term depression in the cerebellar flocculus during adaptation of optokinetic response. Elife. 2018; 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aizenman CD, Linden DJ. Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nat Neurosci. 2000;3:109–11. [DOI] [PubMed] [Google Scholar]
- 17.D’Angelo E The Organization of Plasticity in the Cerebellar Cortex: From Synapses to Control. Prog. Brain Res 2014;210:31–38. [DOI] [PubMed] [Google Scholar]
- 18.Ito M Mechanisms of motor learning in the cerebellum. Brain Res. 2000;886:237–45. [DOI] [PubMed] [Google Scholar]
- 19.Jörntell H Cerebellar physiology: links between microcircuitry properties and sensorimotor functions. J Physiol. 2017;595:11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khilkevich A, Halverson HE, Canton-Josh JE, Mauk MD. Links Between Single-Trial Changes and Learning Rate in Eyelid Conditioning. Cerebellum. 2016;15:112–21. [DOI] [PubMed] [Google Scholar]
- 21.Otis TS, Mathews PJ, Lee KH, Maiz J. How do Climbing fibers teach? Front Neural Circuits. 2012;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popa LS, Hewitt a L, Ebner TJ. Predictive and Feedback Performance Errors Are Signaled in the Simple Spike Discharge of Individual Purkinje Cells. J Neurosci. 2012;32:15345–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen A, Jirenhed D-A, Zucca R, Johansson F, Svensson P, Hesslow G. Number of spikes in Climbing fibers determines the direction of cerebellar learning. J Neurosci. 2013;33:13436–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Titley HK, Heskin-Sweezie R, Broussard DM. The bidirectionality of motor learning in the vestibulo-ocular reflex is a function of cerebellar mGluR1 receptors. J Neurophysiol. 2010;104:3657–66. [DOI] [PubMed] [Google Scholar]
- 25.Wetmore DZ, Jirenhed D-a., Rasmussen a., Johansson F, Schnitzer MJ, Hesslow G Bidirectional Plasticity of Purkinje Cells Matches Temporal Features of Learning. J Neurosci. 2014;34:1731–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, Lisberger SG. Interaction of plasticity and circuit organization during the acquisition of cerebellum-dependent motor learning. Elife. 2013;2:e01574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miles F, Lisberger SG. Plasticity in the vestibulo-ocular reflex: a new hypothesis. Annu Rev Neurosci. 1981; [DOI] [PubMed] [Google Scholar]
- 28.Dean P, Porrill J, Ekerot CF, Jörntell H. The cerebellar microcircuit as an adaptive filter: experimental and computational evidence. Nat Rev Neurosci. Nature Publishing Group; 2009; 11:30–43. [DOI] [PubMed] [Google Scholar]
- 29.Broussard DM, Titley HK, Antflick J, Hampson DR. Motor learning in the VOR: the cerebellar component. Exp Brain Res. 2011;210:451–63. [DOI] [PubMed] [Google Scholar]
- 30.du Lac S, Raymond JL, Sejnowski TJ. Learning and memory in the vestibulo-ocular reflex. Annu Rev Neurosci. 1995;409:409–41. [DOI] [PubMed] [Google Scholar]
- 31.Hansel C, de Jeu M, Belmeguenai A, Houtman SH, Buitendijk GHS, Andreev D, et al. alphaCaMKII Is essential for cerebellar LTD and motor learning. Neuron. 2006;51:835–43. [DOI] [PubMed] [Google Scholar]
- 32.De Zeeuw CI, Hansel C, Bian F, Koekkoek SK, van Alphen a M, Linden DJ, et al. Expression of a protein kinase C inhibitor in Purkinje cells blocks cerebellar LTD and adaptation of the vestibulo-ocular reflex. Neuron. 1998;20:495–508. [DOI] [PubMed] [Google Scholar]
- 33.Goossens HHLM, Alphen AM, Steen J Van Der, Stahl JS, Zeeuw CI De, Frens MA. Simple spike and complex spike activity of floccular Purkinje cells during the optokinetic reflex in mice lacking cerebellar long-term depression. Neuroscience. 2004;19:687–97. [DOI] [PubMed] [Google Scholar]
- 34.Goossens J, Daniel H, Rancillac A, van Der Steen J, Oberdick J, Crépel F, et al. Expression of protein kinase C inhibitor blocks cerebellar long-term depression without affecting Purkinje cell excitability in alert mice. J Neurosci. 2001;21:5813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anzai M, Nagao S. Motor learning in common marmosets: Vestibulo-ocular reflex adaptation and its sensitivity to inhibitors of Purkinje cell long-term depression. Neurosci Res. 2014;83:33–42. [DOI] [PubMed] [Google Scholar]
- 36.Le TD, Shirai Y, Okamoto T, Tatsukawa T, Nagao S, Shimizu T, et al. Lipid signaling in cytosolic phospholipase A2 -cyclooxygenase-2 cascade mediates cerebellar long-term depression and motor learning. Proc Natl Acad Sci. 2010;107:3198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katoh a, Kitazawa H, Itohara S, Nagao S. Inhibition of nitric oxide synthesis and gene knockout of neuronal nitric oxide synthase impaired adaptation of mouse optokinetic response eye movements. Learn Mem. 2000;7:220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katoh A, Yoshida T, Himeshima Y, Mishina M, Hirano T. Defective control and adaptation of reflex eye movements in mutant mice deficient in either the glutamate receptor δ2 subunit or Purkinje cells. Eur J Neurosci. 2005;21:1315–26. [DOI] [PubMed] [Google Scholar]
- 39.Kawaguchi S, Hirano T. Gating of long-term depression by Ca 2+ /calmodulin-dependent protein kinase II through enhanced cGMP signalling in cerebellar Purkinje cells. J Physiol. 2013;591:1707–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen-Vu TDB, Kimpo RR, Rinaldi JM, Kohli A, Zeng H, Deisseroth K, et al. Cerebellar Purkinje cell activity drives motor learning. Nat Neurosci. 2013;16:1734–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kimpo RR, Rinaldi JM, Kim CK, Payne HL, Raymond JL. Gating of neural error signals during motor learning. Elife. 2014;3:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilbert PFC, Thach WT. Purkinje cell activity during motor learning. Brain Res. 1977;128:309–28. [DOI] [PubMed] [Google Scholar]
- 43.Nagao S Behavior of floccular Purkinje cells correlated with adaptation of horizontal optokinetic eye movement response in pigmented rabbits. Exp Brain Res. 1988;73:489–97. [DOI] [PubMed] [Google Scholar]
- 44.Wang W, Nakadate K, Masugi-tokita M, Shutoh F, Aziz W, Etsuko T, et al. Distinct cerebellar engrams in short-term and long-term motor learning. Proc Natl Acad Sci. 2014;111:E188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aziz W, Wang W, Kesaf S, Mohamed AA, Fukazawa Y, Shigemoto R. Distinct kinetics of synaptic structural plasticity, memory formation, and memory decay in massed and spaced learning. Proc Natl Acad Sci. 2014;111:E194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alba A Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–88. [PubMed] [Google Scholar]
- 47.Welsh JP, Yamaguchi H, Zeng X-H, Kojo M, Nakada Y, Takagi A, et al. Normal motor learning during pharmacological prevention of Purkinje cell long-term depression. Proc Natl Acad Sci. 2005;102:17166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lisberger SG. Neural Basis for Motor Learning in the Vestibuloocular Reflex of Primates .3. Computational and Behavioral-Analysis of the Sites of Learning. J Neurophysiol. 1994;72:974–98. [DOI] [PubMed] [Google Scholar]
- 49.Ke MC, Guo CC, Raymond JL. Elimination of Climbing fiber instructive signals during motor learning. Nat Neurosci. Nature Publishing Group; 2009;12:1171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen-Vu TDB, Zhao GQ, Lahiri S, Kimpo RR, Lee H, Ganguli S, et al. A saturation hypothesis to explain both enhanced and impaired learning with enhanced plasticity. Elife. 2017;6:1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamaguchi K, Itohara S, Ito M. Reassessment of long-term depression in cerebellar Purkinje cells in mice carrying mutated GluA2 C terminus. Proc Natl Acad Sci. 2016;113:10192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suvrathan A, Payne HL, Raymond JL. Timing rules for synaptic plasticity matched to behavioral function. Neuron. 2016;92:959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McConnell MJ, Huang YH, Datwani A, Shatz CJ. H2-K(b) and H2-D(b) regulate cerebellar long-term depression and limit motor learning. Proc Natl Acad Sci USA. 2009;106:6784–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raymond JL, Lisberger SG. Neural learning rules for the vestibulo-ocular reflex. J Neurosci. 1998;18:9112–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stone LS, Lisberger SG. Visual responses of Purkinje cells in the cerebellar flocculus during smooth-pursuit eye movements in monkeys. II. Complex spikes. J Neurophysiol. 1990;63:1262–75. [DOI] [PubMed] [Google Scholar]
- 56.Cerminara NL, Lang EJ, Sillitoe R V., Apps R. Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits. Nat Rev Neurosci. Nature Publishing Group; 2015;16:79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jl Wadiche, Jahr CE. Patterned expression of Purkinje cell glutamate transporters controls synaptic plasticity. Nat Neurosci. 2005;8:1329–34. [DOI] [PubMed] [Google Scholar]
- 58.Mittmann W, Häusser M. Linking synaptic plasticity and spike output at excitatory and inhibitory synapses onto cerebellar Purkinje cells. J Neurosci. 2007;27:5559–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coesmans M, Weber JT, De Zeeuw CI, Hansel C. Bidirectional parallel fiber plasticity in the cerebellum under Climbing fiber control. Neuron. 2004;44:691–700. [DOI] [PubMed] [Google Scholar]
- 60.Konnerth A, Dreessen J, Augustine GJ. Brief dendritic calcium signals initiate long-lasting synaptic depression in cerebellar Purkinje cells. Proc Natl Acad Sci USA. 1992;89:7051–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reynolds T, Harteil NA. An evaluation of the synapse specificity of long-term depression induced in rat cerebellar slices. J Physiol. 2000;5273:563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Belmeguenai A, Botta P, Weber JT, Carta M, De Ruiter M, De Zeeuw CI, et al. Alcohol impairs long-term depression at the cerebellar parallel fiber-Purkinje cell synapse. J Neurophysiol. 2008;100:3167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kano M, Hashimoto K, Tabata T. Type-1 metabotropic glutamate receptor in cerebellar Purkinje cells: A key molecule responsible for long-term depression, endocannabinoid signalling and synapse elimination. Philos Trans R Soc B Biol Sci. 2008;363:2173–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jin Y, Kim SJ, Kim J, Worley PF, Linden DJ. Long-term depression of mGluR1 signaling. Neuron. 2007;55:277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brasnjo G, Otis TS. Neuronal glutamate transporters control activation of postsynaptic metabotropic glutamate receptors and influence cerebellar long-term depression. Neuron. 2001;31:607–16. [DOI] [PubMed] [Google Scholar]
- 66.Meera P, Pulst S, Otis T. A positive feedback loop linking enhanced mGluR function and basal calcium in spinocerebellar ataxia type 2. Elife. 2017;6:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hartell ΝΑ. Strong activation of parallel fibers produces localized calcium transients and a form of LTD that spreads to distant synapses. Neuron. 1996;16:601–10. [DOI] [PubMed] [Google Scholar]
- 68.Schreurs BG, Alkon DL. Rabbit cerebellar slice analysis of long-term depression and its role in classical conditioning. Brain Res. 1993;631:235–40. [DOI] [PubMed] [Google Scholar]
- 69.Chadderton P, Margrie TW, Häusser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428:856–60. [DOI] [PubMed] [Google Scholar]
- 70.Rancz EA, Ishikawa T, Duguid I, Chadderton P, Mahon S, Häusser M. High-fidelity transmission of sensory information by single cerebellar mossy fibre boutons. Nature. 2007;450:1245–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jorntell H, Ekerot C-F. Properties of Somatosensory Synaptic Integration in Cerebellar Granule Cells In Vivo. J Neurosci. 2006;26:11786–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barmack NH, Yakhnitsa V. Functions of interneurons in mouse cerebellum. J Neurosci. 2008;28:1140–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Giovannucci A, Badura A, Deverett B, Najafi F, Pereira TD, Gao Z, et al. Cerebellar granule cells acquire a widespread predictive feedback signal during motor learning. Nat Neurosci. 2017;20:727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wagner MJ, Hyun Kim T, Savall J, Schnitzer MJ, Luo L. Cerebellar granule cells encode the expectation of reward. Nat Lett. Nature Publishing Group; 2017;1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ozden I, Dombeck D a, Hoogland TM, Tank DW, Wang SS-H. Widespread state-dependent shifts in cerebellar activity in locomoting mice. PLoS One. 2012;7:e42650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Billings G, Piasini E, Lorincz A, Nusser Z, Silver RA. Network Structure within the Cerebellar Input Layer Enables Lossless Sparse Encoding. Neuron. 2014;83:960–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sawtell NB. Multimodal integration in granule cells as a basis for associative plasticity and sensory prediction in a cerebellum-like circuit. Neuron. 2010;66:573–84. [DOI] [PubMed] [Google Scholar]
- 78.Chabrol FP, Arenz A, Wiechert MT, Margrie TW, DiGregorio DA. Synaptic diversity enables temporal coding of coincident multisensory inputs in single neurons. Nat Neurosci. 2015;18:718–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ishikawa T, Shimuta M, Häusser M. Multimodal sensory integration in single cerebellar granule cells in vivo. Elife. 2015;4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Badura A, De Zeeuw CI. Cerebellar Granule Cells: Dense, Rich and Evolving Representations. Curr Biol. 2017;27:R415–8. [DOI] [PubMed] [Google Scholar]
- 81.Knogler LD, Markov DA, Dragomir El, Štih V, Portugues R. Sensorimotor Representations in Cerebellar Granule Cells in Larval Zebrafish Are Dense, Spatially Organized, and Non-temporally Patterned. Curr Biol. 2017;27:1288–302. [DOI] [PubMed] [Google Scholar]
- 82.Wang SS, Denk W, Häusser M. Coincidence detection in single dendritic spines mediated by calcium release. Nat Neurosci. 2000;3:1266–73. [DOI] [PubMed] [Google Scholar]
- 83.Ekerot CF, Kano M. Long-term depression of parallel fibre synapses following stimulation of Climbing fibres. Brain Res. 1985;342:357–60. [DOI] [PubMed] [Google Scholar]
- 84.Ekerot C-F, Kano M. Stimulation parameters influencing Climbing fibre induced long-term depression of parallel fibre synapses. 1989;6:264–8. [DOI] [PubMed] [Google Scholar]
- 85.Chen C, Thompson RF. Temporal specificity of long-term depression in parallel fiber-Purkinje synapses in rat cerebellar slice. Learn Mem. 1995;2:185–98. [DOI] [PubMed] [Google Scholar]
- 86.Linden DJ. Input-specific induction of cerebellar long-term depression does not require presynaptic alteration. Learn Mem. 1994;1:121–8. [PubMed] [Google Scholar]
- 87.Wang SS, Khiroug L, Augustine GJ. Quantification of spread of cerebellar long-term depression with chemical two-photon uncaging of glutamate. Proc Natl Acad Sci USA. 2000;97:8635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hartell ΝΑ. Parallel fiber plasticity. The Cerebellum. 2002;3–18. [DOI] [PubMed] [Google Scholar]
- 89.Hartell ΝΑ. Receptors, second messengers and protein kinases required for heterosynaptic cerebellar long-term depression. Neuropharmacology. 2000;40:148–61. [DOI] [PubMed] [Google Scholar]
- 90.Powell K, Mathy A, Duguid I, Häusser M. Synaptic representation of locomotion in single cerebellar granule cells. Elife. 2015;4:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hartmann MJ, Bower JM. Tactile responses in the granule cell layer of cerebellar folium crus lla of freely behaving rats. J Neurosci. 2001;21:3549–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Beugen BJ, Gao Z, Boele H-J, Hoebeek F, De Zeeuw CI. High Frequency Burst Firing of Granule Cells Ensures Transmission at the Parallel Fiber to Purkinje Cell Synapse at the Cost of Temporal Coding. Front Neural Circuits. 2013;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Llinas R, Nicholson C, Llinas R. Reversal properties of Climbing fiber potential in cat Purkinje cells: an example of a distributed synapse. J Neurophysiol. 1976;39:311–23. [DOI] [PubMed] [Google Scholar]
- 94.Eccles J, Llinás RR, Sasaki K. Excitation of cerebellar Purkinje cells by Climbing fibers. Nature. 1964;201:1212–3. [DOI] [PubMed] [Google Scholar]
- 95.De Zeeuw CI, Simpson JI, Hoogenraad CC, Galjart N, Koekkoek SKE, Ruigrok TJH. Microcircuitry and function of the inferior olive. 1998;2236:391–400. [DOI] [PubMed] [Google Scholar]
- 96.Perkel DJ, Hestrin S, Sah P, Nicoll RA. Excitatory synaptic currents in Purkinje cells. Proc. R. Soc. Lond. B 1990;100:116–21. [DOI] [PubMed] [Google Scholar]
- 97.Konnerth A, Llanot I, Armstrongt CM. Synaptic currents in cerebellar Purkinje cells. Neurobiology. 1990;87:2662–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schmolesky MT, Weber JT, De Zeeuw CI, Hansel C. The Making of a Complex Spike: Ionic Composition and Plasticity. Ann N Y Acad Sci. 2002;978:359–90. [DOI] [PubMed] [Google Scholar]
- 99.Piochon C, Titley HK, Simmons DH, Grasselli G, Elgersma Y, Hansel C. Calcium threshold shift enables frequency-independent control of plasticity by an instructive signal. Proc Natl Acad Sci. 2016;113:13221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Eilers J, Takechi H, Fich EA, Augustine GJ, Konnerth A. Local dendritic Ca2 + signaling induces cerebellar Long-Term Depression. Learn Mem. 1997;3:159–68. [DOI] [PubMed] [Google Scholar]
- 101.Finch EA, Augustine GJ. Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature. 1998;396:753–6. [DOI] [PubMed] [Google Scholar]
- 102.Tanaka K, Augustine GJ. A positive feedback signal transduction loop determines timing of cerebellar long-term depression. Neuron. 2008;59:608–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mathy A, Ho SSN, Davie JT, Duguid IC, Clark BA, Häusser M. Encoding of Oscillations by Axonal Bursts in Inferior Olive Neurons. Neuron. 2009;62:388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang Y, Lisberger SG. Purkinje-cell plasticity and cerebellar motor learning are graded by complex-spike duration. Nature. Nature Publishing Group; 2014;510:529–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kimpo RR, Boyden ES, Katoh A, Ke MC, Raymond JL. Distinct patterns of stimulus generalization of increases and decreases in VOR gain. J Neurophysiol. 2005;94:3092–100. [DOI] [PubMed] [Google Scholar]
- 106.Schonewille M, Belmeguenai a, Koekkoek SK, Houtman SH, Boele HJ, van Beugen BJ, et al. Purkinje cell-specific knockout of the protein phosphatase PP2B impairs potentiation and cerebellar motor learning. Neuron. 2010;67:618–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Perrett SP, Ruiz BP, Mauk MD. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. J Neurosci. 1993;13:1708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kalmbach BE, Davis T, Ohyama T, Riusech F, Nores WL, Mauk MD. Cerebellar Cortex Contributions to the Expression and Timing of Conditioned Eyelid Responses. J Neurophysiol. 2010;103:2039–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Medina JF, Lisberger SG. Links from complex spikes to local plasticity and motor learning in the cerebellum of awake-behaving monkeys. Nat Neurosci. 2008;11:1185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang Y, Lisberger SG. Learning on multiple timescales in smooth pursuit eye movements. J Neurophysiol. 2010;104:2850–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brenowitz SD, Regehr WG. Associative short-term synaptic plasticity mediated by endocannabinoids. Neuron. 2005;45:419–31. [DOI] [PubMed] [Google Scholar]
- 112.Bouvier G, Clopath C, Bimbard C, Nadal J-P, Brunei N, Hakim V, et al. Cerebellar learning using perturbations. bioRxiv. 2016;053785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nisimaru N, Mittal C, Shirai Y, Sooksawate T, Anandaraj P, Hashikawa T, et al. Orexin- neuromodulated cerebellar circuit controls redistribution of arterial blood flows for defense behavior in rabbits. Proc Natl Acad Sci. 2013;110:14124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marder E Neuromodulation of Neuronal Circuits: Back to the Future. Neuron. 2012;76:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gu O Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience. 2002;111:815–35. [DOI] [PubMed] [Google Scholar]
- 116.Nadim F, Bucher D. Neuromodulation of neurons and synapses. Curr Opin Neurobiol. 2014;29:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rasmusson DD. The role of acetylcholine in cortical synaptic plasticity. Behav Brain Res. 2000;115:205–18. [DOI] [PubMed] [Google Scholar]
- 118.Schaefer AT. Coincidence Detection in Pyramidal Neurons Is Tuned by Their Dendritic Branching Pattern. J Neurophysiol. 2003;89:3143–54. [DOI] [PubMed] [Google Scholar]
- 119.Giocomo LM, Stensola T, Bonnevie T, Van Cauter T, Moser MB, Moser EI. Topography of head direction cells in medial entorhinal cortex. Curr Biol.; 2014;24:252–62. [DOI] [PubMed] [Google Scholar]
- 120.Mallory CS, Giocomo LM. Heterogeneity in hippocampal place coding. Curr Opin Neurobiol. 2018;49:158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cembrowski MS, Bachman JL, Wang L, Sugino K, Shields BC, Spruston N. Spatial Gene-Expression Gradients Underlie Prominent Heterogeneity of CA1 Pyramidal Neurons. Neuron. 2016;89:351–68. [DOI] [PubMed] [Google Scholar]
- 122.Lev-Ram V, Nebyelul Z, Ellisman M, Huang P, Tsien R. Absence of cerebellar long-term depression in mice lacking neuronal nitric oxide synthase. Learn Mem. 1997;4:169–77. [DOI] [PubMed] [Google Scholar]
- 123.Fujita H, Aoki H, Ajioka I, Yamazaki M, Abe M, Oh-Nishi A, et al. Detailed expression pattern of aldolase C (aldoc) in the cerebellum, retina and other areas of the CNS studied in aldoc-venus knock-in mice. PLoS One. 2014;9:e86679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fujita H, Morita N, Furuichi T, Sugihara I. Clustered fine compartmentalization of the mouse embryonic cerebellar cortex and its rearrangement into the postnatal striped configuration. J Neurosci. 2012;32:15688–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Apps R, Hawkes R. Cerebellar cortical organization: a one-map hypothesis. Nat Rev Neurosci. Nature Publishing Group; 2009;10:670–81. [DOI] [PubMed] [Google Scholar]
- 126.Schonewille M, Luo C, Ruigrok TJH, Voogd J, Schmolesky MT, Rutteman M, et al. Zonal organization of the mouse flocculus: physiology, input, and output. J Comp Neurol. 2006;497:670–82. [DOI] [PubMed] [Google Scholar]
- 127.Sillitoe R V, Gopal N, Joyner AL. Embryonic origins of Zebrinll parasagittal stripes and establishment of topographic Purkinje cell projections. Neuroscience. 2009;162:574–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sugihara I, Quy PN. Identification of aldolase C compartments in the mouse cerebellar cortex by olivocerebellar labeling. J Comp Neurol. 2007;500:1076–92. [DOI] [PubMed] [Google Scholar]
- 129.Sugihara I, Shinoda Y. Molecular, topographic, and functional organization of the cerebellar cortex: a study with combined aldolase C and olivocerebellar labeling. J Neurosci. 2004;24:8771–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang X, Chen G, Gao W, Ebner TJ. Parasagittally aligned, mGluR1-dependent patches are evoked at long latencies by parallel fiber stimulation in the mouse cerebellar cortex in vivo. J Neurophysiol. 2011;105:1732–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cerminara NL, Aoki H, Loft M, Sugihara I, Apps R. Structural basis of cerebellar microcircuits in the rat. J Neurosci. 2013;33:16427–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhou H, Lin Z, Voges K, Ju C, Gao Z, Bosman LWJ, et al. Cerebellar modules operate at different frequencies. Elife. 2014;2014:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhou H, Voges K, Lin Z, Ju C, Schonewille M. Differential Purkinje cell simple spike activity and pausing behavior related to cerebellar modules. J Neurophysiol. 2015;113:2524–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim C-H, Oh S-H, Lee JH, Chang SO, Kim J, Kim SJ. Lobule-specific membrane excitability of cerebellar Purkinje cells. J Physiol. 2012;590:273–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xiao J, Cerminara NL, Kotsurovskyy Y, Aoki H, Burroughs A, Wise AK, et al. Systematic regional variations in purkinje cell spiking patterns. PLoS One. 2014;9:e105633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Witter L, De Zeeuw CI. In Vivo Differences in Inputs and Spiking Between Neurons in Lobules VI/VII of Neocerebellum and Lobule X of Archaeocerebellum. The Cerebellum. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Witter L, De Zeeuw CI. Regional functionality of the cerebellum. Curr Opin Neurobiol. 2015;33:150–5. [DOI] [PubMed] [Google Scholar]
- 138.Piochon C, Levenes C, Ohtsuki G, Hansel C. Purkinje cell NMDA receptors assume a key role in synaptic gain control in the mature cerebellum. J Neurosci. 2010;30:15330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Safo P, Regehr WG. Timing dependence of the induction of cerebellar LTD. Neuropharmacology. 2008;54:213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Safo PK, Regehr WG. Endocannabinoids control the induction of cerebellar LTD. Neuron. 2005;48:647–59. [DOI] [PubMed] [Google Scholar]
- 141.Schreurs BG, Oh MM, Alkon DL. Pairing-specific long-term depression of Purkinje cell excitatory postsynaptic potentials results from a classical conditioning procedure in the rabbit cerebellar slice. J Neurophysiol. 1996;75:1051–60. [DOI] [PubMed] [Google Scholar]
- 142.Schreurs BG, Tomsic D, Gusev PA, Alkon DL. Dendritic excitability microzones and occIuded long-term depression after classical conditioning of the rabbit’s nictitating membrane response. J Neurophysiol. 1997;77:86–92. [DOI] [PubMed] [Google Scholar]
- 143.Carey MR, Myoga MH, McDaniels KR, Marsicano G, Lutz B, Mackie K, et al. Presynaptic CB1 Receptors Regulate Synaptic Plasticity at Cerebellar Parallel Fiber Synapses. J Neurophysiol. 2011;105:958–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jörnteil H, Ekerot CF. Reciprocal bidirectional plasticity of parallel fiber receptive fields in cerebellar Purkinje cells and their afferent interneurons. Neuron. 2002;34:797–806. [DOI] [PubMed] [Google Scholar]
- 145.Crepel F and, Jaillard D, Paris-sud U. Pairing of pre- and postsynaptic activities in cerebellar Purkinje cells induces long-term changes in synaptic efficacy in vitro. J Physiol. 1991; 123–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mark MD, Krause M, Boele HJ, Kruse W, Pollok S, Kuner T, et al. Spinocerebellar ataxia type 6 protein aggregates cause deficits in motor learning and cerebellar plasticity. J Neurosci. 2015;35:8882–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kano M, Kato M. Quisqualate receptors are specifically involved in cerebellar synaptic plasticity. Nature. 1987;325:276–9. [DOI] [PubMed] [Google Scholar]
- 148.Linden DJ. A late phase of LTD in cultured cerebellar Purkinje cells requires persistent dynamin-mediated endocytosis. J Neurophysiol. 2012;107:448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Karachot L, Kado RT, Ito M. Stimulus parameters for induction of long-term depression in in vitro rat Purkinje cells. Neurosci Res. 1994;21:161–8. [DOI] [PubMed] [Google Scholar]
- 150.Steuber V, Mittmann W, Hoebeek FE, Silver RA, De Zeeuw CI, Häusser M, et al. Cerebellar LTD and pattern recognition by Purkinje cells. Neuron. 2007;54:121–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sakurai M Synaptic modification of parallel fibre-Purkinje cell transmission in in vitro guinea-pig cerebellar slices. J Physiol. 1987;394:463–80. [DOI] [PMC free article] [PubMed] [Google Scholar]


