Abstract
A major breakthrough in cancer treatment occurred with the development of strategies that overcome T-cell tolerance toward tumor cells. These approaches enhance antitumor immunity by overcoming mechanisms that are normally in place to prevent autoimmunity but simultaneously prevent rejection of tumor cells. Although tolerance mechanisms that restrict antitumor immunity take place both in the thymus and periphery, only immunotherapies that target peripheral tolerance mechanisms occurring outside of the thymus are currently available. We review here recent gains in our understanding of how thymic tolerance mediated by the autoimmune regulator (Aire) impedes antitumor immunity. It is now clear that transient depletion of Aire-expressing cells in the thymus can be achieved with RANKL blockade. Finally, we discuss key findings that support the repurposing of anti-RANKL as a cancer immunotherapy with a unique mechanism of action.
Keywords: immunotherapy, RANK, RANKL, Aire, thymus central tolerance
Introduction
Immune checkpoint inhibitors that target CTLA-4 and PD1-PDL1 have revolutionized the treatment of advanced melanoma and other cancers. Checkpoint inhibitors prolong overall survival in melanoma patients, and have resulted in durable responses and even cure (1,2). However, clinical benefit is currently limited to a subgroup of patients and cancer types. Efforts are, therefore, underway to expand the therapeutic benefit of checkpoint inhibition (3,4). Checkpoint inhibitors rely on modulating peripheral (i.e. extrathymic) immune tolerance to activate tumor-specific T cells after they have left the thymus. To date, much less is known about how central (i.e. thymic) tolerance inhibits antitumor immunity, and a pharmacologic means to inhibit central tolerance is not yet available in the clinic. Such a therapy has the potential to function in a synergistic fashion with checkpoint inhibitors, given their distinct mechanisms of action.
We present here recent advances in identifying key thymic pathways that restrict antitumor immunity. In particular, it is now known that the autoimmune regulator (Aire) gene plays a critical role in preventing robust antitumor immunity. Early evidence points to synergistic antitumor effects of simultaneously blocking central Aire-mediated tolerance and peripheral checkpoint proteins, and have led to growing interest in repurposing an FDA-approved osteoporosis therapy, anti-RANKL (denosumab), to enhance peripherally acting checkpoint inhibition.
Aire prevents tissue-specific autoimmune diseases
Aire is a master transcriptional regulator with a well-defined role in enforcing self-tolerance (5). The importance of Aire in maintaining self-tolerance is clearly demonstrated by the development of multi-organ autoimmunity in Aire-deficient mice (6,7) and humans (8). Within medullary thymic epithelial cells (mTECs) of the thymus, a primary role for Aire is to upregulate expression of thousands of tissue-specific self-antigens (TSAs), so that developing T cells in the thymus that recognize these TSAs with high affinity/avidity undergo negative selection or are diverted into the regulatory T-cell (Treg) lineage. Thus, Aire enforces self-tolerance through both recessive (deletional) as well as dominant (suppressive) tolerance mechanisms.
Aire’s role in the regulation of recessive tolerance was initially demonstrated using double transgenic systems, in which T-cell receptor (TCR) transgenic T cells undergo negative selection upon recognizing transgenic neo–self-antigens expressed by mTECs (9,10). Aire deficiency in these mice disrupts the negative selection of TCR transgenic T cells, suggesting that Aire is required for their thymic deletion. Given the potential caveats of using these transgenic systems (e.g., early transgenic TCR expression), whether these findings reflect normal physiology is uncertain. However, these findings were subsequently extended to naturally occurring CD4+ T-cell specificities, using tetramers to detect rare autoreactive T cells specific for the Aire-dependent, tissue-specific antigen interphotoreceptor retinoid-binding protein (IRBP) (11). Increased frequencies of IRBP-specific T cells are seen in the thymus and periphery of Aire knockout mice, suggesting that thymic deletion of IRBP-specific T cells within the polyclonal repertoire are dependent on Aire. Defective negative selection of IRBP-specific T cells has important implications for development of autoimmunity. In the C57BL/6 background, only one-third of 10–20 week-old Aire knockout mice show histologic evidence of autoimmune uveitis, and development of uveitis was correlated with higher peripheral tetramer frequencies. Together, these findings demonstrate that Aire’s removal of autoreactive T cells from the polyclonal repertoire protects against tissue-specific autoimmunity.
Aire also promotes dominant (active) tolerance (12–14) by promoting generation of immunosuppresive Tregs in the thymus (14), particularly early in life (13). Aire-deficient mice have decreased Treg numbers in the first 10 days of life and decreased Treg frequencies in the first 35 days of life (13). Aire deficiency is also associated with loss of Treg immunosuppressive function in mice and humans. Adoptive transfer of perinatally tagged Tregs from Aire-wildtype mice protected Aire-deficient recipients from autoimmune manifestations, whereas Tregs from Aire-deficient mice did not (13). Tregs isolated from Aire-deficient patients similarly have impaired function in in vitro suppression assays (15).
In addition to decreased Treg numbers and function, Aire deficiency is also associated with Treg TCR repertoire alterations, both in mice (16,17) and humans (15). In depth analysis of the TCR repertoire of Tregs from the spleen and lymph nodes of Aire-deficient mice revealed an absence of the most prevalent Treg TCRs found in Aire-wildtype mice (17).The specificity of dominant conventional T (Tconv)-cell clonotypes within infiltrated organs of Aire-deficient mice are preferentially expressed by Tregs in Aire-wildtype mice. Thus, Aire deficiency allows for the development of “Trogue” cells, named because these Tconv cells share the same TCR specificity as Tregs and may, therefore, represent Tregs that have undergone conversion in the absence of Aire. Thus, Aire may shape the Treg repertoire by directing self-reactive T cells away from a conventional T-cell lineage and into a Treg lineage.
Thymic Aire restricts antitumor immunity
Aire-regulated TSAs include antigens that are shared by normal tissues and their malignant counterpart. TRP-1, tyrosinase, and gp100 (18,19) are Aire-regulated self/tumor antigens expressed by normal melanocyte cells and melanoma cells. Loss of these melanocyte/melanoma antigens with Aire deficiency may be predicted to have dual effects: Aire deficiency may not only disrupt tolerance toward normal melanocytes, but also enhance immunity against melanoma cells. Aire deficiency leads to a predisposition to vitiligo, an autoimmune condition in which T cells destroy melanocytes. Rare patients with autoimmune polyendocrinopathy syndrome (APS Type 1) due to homozygous loss-of-function mutations in Aire are >10-times more likely to develop vitiligo than the general population (20). Aire polymorphisms that occur at higher frequencies in the general population are associated with increased risk of vitiligo in humans (21).
At the same time, multiple lines of evidence now show that Aire deficiency enhances immune rejection of melanoma cells. We and others independently reported that mice with genetic Aire deficiency have decreased expression of melanocyte/melanoma antigens in the thymus (18,19). Aire-deficient mice demonstrate slower growth of transplanted B16-F10 melanoma tumors and improved survival compared to wildtype controls. In Aire-deficient mice, increased T-cell numbers are found within melanoma tumors, as well as higher frequencies of tumor-infiltrating lymphocytes expressing pro-inflammatory cytokines, which suggests a more effective T-cell response.
Aire is expressed within thymic mTECs but is also expressed at lower levels in peripheral lymphoid organs (22). Because of this, it is possible that Aire expression in either the thymus or peripheral lymphoid organs may be important in mediating tumor tolerance. Extrathymic Aire regulates a distinct array of self-antigens, which also include antigens expressed by tumors. Ladinin 1 (Lad1) is expressed in extrathymic Aire-expressing cells (eTACs) under the control of Aire. Lad1 is an autoantigen in linear IgA dermatosis and is also expressed by breast tumor cells (23). Our data suggest that loss of thymic Aire is sufficient to protect against melanoma because decreased tumor growth and increased survival was seen with transplantation of Aire-deficient thymi into nude mice (18). Neverthless, it remains possible that the loss of peripheral Aire also contributes.
Aire restricts antitumor immunity not only in mice, but also in humans. The autoantibody repertoire found in AIRE-deficient APS1 patients targets many (~20) cancer testes antigens (24), including MAGE-A and MAGE-B family members that are expressed by melanoma and other tumor types and otherwise expressed by testes in non-tumor tissues. This provides solid evidence for a break in tolerance toward tumor/self-antigens in APS1 patients and are consistent with a previous report that human mTECs promiscuously express multiple tumor-associated antigens, including MAGE-A1, MAGE-A3, MAGE-A4, NY-ESO, MART1, tyrosinase, MUC1, and CEA (25,26). An AIRE polymorphism (rs1800520 SNP) that destabilizes AIRE mRNA is associated with protection from melanoma development (27). Together, these findings suggest a critical role for Aire in limiting immune rejection of tumors in humans.
Aire mediates clonal deletion of T cells capable of tumor rejection
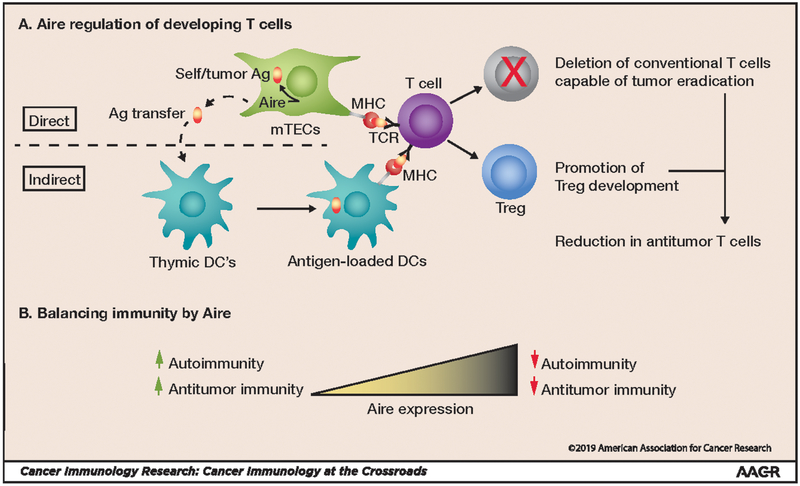
As discussed above, Aire prevents tissue-specific autoimmunity through multiple cellular mechanisms. Aire regulates TSA expression in mTECs, and these TSAs are either processed and presented by mTECs themselves or transferred to thymic dendritic cells (DCs) (Fig. 1A). Developing T cells in the thymus recognizing Aire-regulated TSAs either undergo clonal deletion or are diverted into the Treg lineage. Because Aire regulates TSAs shared between normal tissues and tumors, it would seem logical that these same mechanisms may also restrict antitumor immunity (Fig. 1A). Increased Aire expression protects against autoimmunity but prevents tumor rejection, whereas decreased Aire expression predisposes to autoimmunity but enhances antitumor immunity (Fig. 1B). Substantial evidence now exists that Aire mediates the clonal deletion of T cells capable of rejecting tumor cells. TRP-1 is a melanocyte/melanoma antigen that is also expressed by mTECs. As a consequence, TRP-1-reactive CD4+ T cells normally undergo clonal deletion (28,29). Using TRP-1 antigen–negative mice, Muranski et al. cloned and expressed a TRP-1–specific T-cell receptor (TCR) transgene into C57BL/6 mice to establish the TRP-1 TCR transgenic (Tg) mouse line (28). TRP-1 TCR Tg CD4+ T cells from these mice possess melanoma-specific antitumor activity (29) and are capable of mediating destruction of B16 melanoma cells in an IFNγ-dependent manner (28). We have reported that TRP-1 expression in mTECs is Aire-dependent. In Aire-deficient mice, decreased thymic TRP-1 expression by mTECs is associated with rescue of TRP-1 TCR Tg CD4+ T cells from thymic deletion (18). Aire deficiency in TRP-1 TCR Tg mice prevents growth of transplanted B16 melanoma cells and significantly prolongs survival. Depletion of Aire+ mTECs after anti-RANKL administration similarly rescued TRP-1-specific T cells from clonal deletion and increased survival in melanoma-challenged TRP-1 TCR Tg mice (30). Together these findings suggest that Aire restricts anti-melanoma immunity through clonal deletion of T cells with antitumor activity.
Figure 1: Thymic Aire restricts antitumor immunity.
A) Within thymic mTECs, Aire drives the expression of antigens (Ag) shared between tumors and normal (self) tissues. These antigens are then presented by mTECs, or dendritic cells (DCs) by antigen transfer, to developing thymocytes in the thymus. Recognition of self/tumor antigens by thymocytes results in 2 potential fates: clonal deletion (red “X”) of conventional T cells capable of tumor eradication or diversion into the immunosuppressive Treg lineage, reducing antitumor immunity. TCR: T-cell receptor; MHC: major histocompatibility complex. B) Decreased Aire expression predisposes to autoimmunity but also enhances antitumor immunity, whereas increased Aire expression prevents autoimmunity but restricts antitumor immunity.
Aire has also been linked to thymic expression of the self/melanoma antigen gp100. The precursor frequency of gp100 tetramer-positive CD8+ T cells is estimated to be low (1 in a million) (31), and Aire deficiency in melanoma-bearing mice is associated with a trend toward increased gp100-tetramer-positive T-cell clones (19), consistent with the idea that the protective effects of Aire are mediated through the escape of self/melanoma-reactive T cells from thymic negative selection. This study also reported that Treg depletion with anti-CD25 further enhanced melanoma rejection in Aire-deficient mice (19). This observation would suggest that enhanced tumor rejection seen with Aire-deficiency is not solely due to lack of Treg numbers or activity.
Finally, in support of an important role for deletional tolerance in Aire-mediated restriction of antitumor immunity, patients bearing more than one melanoma-protective AIRE SNP have an increased frequency of T-cell clonotypes specific for the self/melanoma antigen MAGE-1 (27). Although these findings need to be validated in a larger patient cohort, one of these protective SNPs (rs1800522) occurs naturally in the C57BL/6 mouse strain and also protects against B16 melanoma outgrowth in these mice (32). mTECs from mice harboring the protective Aire allele, cocultured with MAGEB2-specific T cells, induces apoptosis in T cells, suggesting that deletional tolerance is the mechanism by which protection from melanoma outgrowth occurs.
Aire promotes thymic development of tumor-associated Tregs
Along with clonal deletion of T cells capable of tumor rejection, evidence also exists that Aire promotes thymic selection of tumor-associated Tregs. Immunosuppressive Tregs are present in tumor infiltrates, and Treg density is correlated with clinical outcome, depending on tumor context (33). As a consequence, depleting Tregs to enhance antitumor immunity has garnered interest as a therapeutic approach. An important question toward this end is the developmental origins of tumor-infiltrating Tregs. In a seminal paper, Malchow et al. demonstrate that a Treg clone derived from the thymus is found in prostate tumors and show that Aire plays a critical role in the thymic development of this prostate cancer-associated Treg clone (MJ23) (34). MJ23 Treg clones are enriched in the tumor of Aire-wildtype mice with oncogene-driven prostate cancer, and in Aire-deficient mice, these Treg clones are absent in the thymus, but their TCRs are expressed by Tconv cells within prostate gland infiltrates. The antigen targeted by MJ23 clones is likely an unmutated, prostate-specific self-antigen because male TCR Tg mice expressing this TCR (MJ23Tg) exhibit prostatic infiltration by activated CD4+ T cells in the absence of prostate tumor. Together these findings suggest that Aire-deficiency may enhance antitumor immunity by preventing development of Tregs with self/tumor-reactive specificities and promoting their diversion into the Tconv-cell compartment.
Nevertheless, not all tumor-associated, thymus-derived Treg clones are Aire-dependent. The absolute numbers of TRP-1 TCR Tg Tregs are unchanged in the thymus of Aire-deficient and Aire-wildtype mice, suggesting that Aire is not required for the thymic development of this particular Treg specificity (18). It remains unclear what factors determine whether a particular self-reactive clone undergoes Aire-dependent negative selection or is diverted into the Treg lineage and is an area that will require further study.
mTEC-depleting anti-RANKL is a potential cancer immunotherapy
Given the key role that Aire-expressing mTEC’s play in restraining T-cell responses against tumors, this cell population could be an attractive target to improve antitumor responses. Our group and others have shown that targeting RANK-RANKL interactions can selectively target Aire+ mTEC’s in the thymus (30). mTECs have a high rate of turnover, with an estimated 2-week half-life, and the inductive signal to induce Aire-expression comes mainly from RANK-RANKL signaling in these cells. Work in the neonatal setting has shown that when immature mTEC’s are administered RANKL, they can acquire Aire expression, and conversely, if RANK/RANKL are knocked out in mice, there is a paucity of Aire+ mTECs (35–37). In the adult setting, our group determined that treatment of young mice with anti-RANKL for 2 weeks led to >80% depletion of mTECs (30). Although other agents deplete multiple thymic cell subtypes, anti-RANKL is unique in its selective depletion of Aire+ mTECs within the thymic stromal cell population. Thus, disrupting RANK-RANKL interactions induces a transient “Aire-deficient” state. Importantly, anti-RANKL administration is associated with improved survival of melanoma-challenged mice, suggesting that pharmacologic depletion of Aire+ mTECs improves antitumor immunity.
Considering these findings, RANKL blockade represents a potential immunotherapy with a unique mechanism of action. Fortuitously, anti-RANKL (denosumab) is an FDA-approved drug for osteoporosis and multiple other bone-related indications in people. As a consequence, anti-RANKL has a well-known safety profile, which makes it an attractive candidate for repurposing as a cancer immunotherapy. In support of its use as a cancer immunotherapy, anti-RANKL treatment of patients with lung cancer and bone metastases is associated with improved overall survival (38). This survival benefit seems to occur outside of the bone-protective effects of the antibody because overall survival is also seen in patients with visceral metastases, which dictates timing to disease progression and death. In support anti-RANKL’s possible function to augment antitumor responses against tumors, administration of anti-RANKL every 6 months to women with osteoporosis has been associated with increased peripheral T-cell numbers (39). A caveat is that this increase appears to be transient, as it is present at 3 and 6 months after treatment, but not at 12 months. A transient increase in T-cell numbers may be sufficient to enhance antitumor immunity, but this will require additional study. Of note, the mean age in this study was 75 years, suggesting that anti-RANKL treatment may have immune effects even in late adulthood. This is of interest, given that age-related immune changes (e.g., thymic involution) have been described (40), which may modify the effects of anti-RANKL. Thus, it remains to be seen whether anti-RANKL may have differential effects throughout the lifespan. In any case, these observations support additional studies to determine its potential antitumor effects in patients.
In addition to its status as an FDA-approved drug, anti-RANKL has also garnered much attention as a potential cancer immunotherapy due its potential efficacy in combination with checkpoint inhibitors. Distinct from anti-RANKL, immune checkpoint inhibitors exert their effects on peripheral tolerance mechanisms. As such, anti-RANKL and checkpoint inhibitors in combination may have potentially additive effects. We have reported that the checkpoint inhibitor anti–CTLA-4 inhibits melanoma growth and prolongs host survival to a greater degree in Aire-deficient mice than in wild-type littermates (41). In anti–CTLA-4–treated Aire-deficient mice, slower melanoma growth is accompanied by increased frequency of CD4+ tumor-infiltrating lymphocytes expressing the proliferation marker Ki67 and cytolytic activity markers KLRG1 and granzyme B. These findings in mouse models may be directly translatable to humans, as early evidence indicates that a human AIRE polymorphism (rs1055311) is associated with progression-free survival in advanced melanoma patients treated with anti–CTLA-4 (41).
In line with these findings, we and others have reported that anti-RANKL and anti–CTLA-4 in combination have additive effects on immune rejection of B16F10 melanoma in mice (41,42). These additive antitumor effects of anti-RANKL and anti–CTLA-4 are not restricted to melanoma tumor growth and have now been demonstrated in multiple tumor types (CT26 colon carcinoma, MCA1956 fibrosarcoma, Tramp-C1 prostate cancer) and metastatic models (B16F10 melanoma, RM1 prostate cancer, LWT1 melanoma) in mice (42). In patients, this additive effect is suggested by case reports in which unexpected and near-complete responses to anti–CTLA-4 are seen in metastatic melanoma patients also receiving anti-RANKL (43,44).
In addition to anti-CTLA-4, anti-RANKL also has additive anti-cancer effects when combined with the checkpoint inhibitors anti–PD-1 or anti–PDL-1 (45). Anti-RANKL also expands the therapeutic efficacy of checkpoint inhibition when anti–CTLA-4/PD-1 are used in combination (45). Together, these findings suggest anti-RANKL expands the therapeutic benefit of checkpoint inhibitors and have provided impetus for multiple clinical trials to evaluate the anti-cancer efficacy of anti-RANKL in combination with checkpoint inhibitors (46). These include two phase 2 clinical trials (NCT03620019 and NCT03161756) currently underway in advanced melanoma patients to test the efficacy of combining anti-RANKL and checkpoint inhibitors.
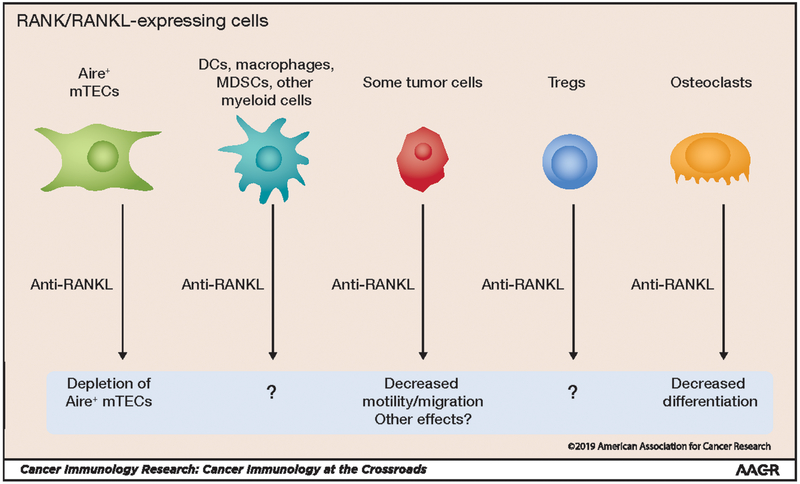
As discussed above, RANK is expressed by tolerogenic mTECs in the thymus and is required for mTEC development (Fig. 2). However, RANK is not just expressed by mTECs in the thymus, but also by DCs, macrophages, and other immune cell types. Thus, it is possible that blocking RANK/RANKL signaling in one of these additional cell types also contributes to enhanced antitumor immunity (46). Because anti-RANKL enhances antitumor immunity, RANK or RANKL expression would be predicted to occur in a tolerogenic cell type. It is likely that anti-RANKL also has direct effects on tumor cells that express RANK and/or RANKL. RANK expression on breast cancer cells has been reported to regulate its potential for metastases (47). Interestingly, RANKL in the breast cancer microenvironment is expressed by Tregs, suggesting that RANK signaling in breast cancer cells may be mediated by Tregs. Further investigation is needed to delineate the nature of these additional RANK/RANKL signals and how their disruption may enhance antitumor immunity (Fig. 2).
Figure 2: Effects of anti-RANKL antibody administration on multiple cell types.
RANK/RANKL is expressed by a wide range of cell types, and blockade of this signaling pathway with anti-RANKL has pleiotropic effects. However, for some cell types, the effects of anti-RANKL are not yet known (indicated by “?”).
The study of Aire and its function in the thymus has opened potential new avenues for manipulating immune tolerance. It has become clear that central tolerance in the thymus is a barrier to effective antitumor responses and targeting this process with RANKL blockade may offer a method to transiently overcome this barrier. Similar to immune checkpoint blockade, this approach will also likely increase the risk for autoimmune side effects, and it will be important to determine how extensive these risks are as clinical trials testing this approach ensue. It is also widely appreciated that the thymus involutes with age causing an age-related decline in T-cell ouput (40), and thus, the most efficacious setting for using this approach may be in younger individuals. Finally, another attractive feature of this approach is that it can be transient, in terms of its effects on the thymus, as we have demonstrated that proper development of Aire+ mTECs can be re-established after treatment is halted. Overall, this complementary approach to enhancing T-cell responses against tumors may lead to improving responses in more patients and in more cancer disease settings.
Acknowledgements:
M.S.A. is supported by the NIAID, NIDDK, and The Helmsley Charitable Trust. M.A.S. is supported by NINDS, NIAID, Parker Center for Cancer Immunotherapy, and APS Type 1 Foundation.
References
- 1.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010;363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014. [DOI] [PubMed] [Google Scholar]
- 3.Hodi FS, Lawrence D, Lezcano C, Wu X, Zhou J, Sasada T, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res. 2014;2:632–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N Engl J Med. 2013;369:122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson MS, Su MA. Aire and T cell development. Curr Opin Immunol. 2011;23:198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–401. [DOI] [PubMed] [Google Scholar]
- 7.Su MA, Giang K, umer K, Jiang H, Oven I, Rinn JL, et al. Mechanisms of an autoimmunity syndrome in mice caused by a dominant mutation in Aire. J Clin Invest. 2008;118:1712–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–8. [DOI] [PubMed] [Google Scholar]
- 9.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The Cellular Mechanism of Aire Control of T Cell Tolerance. Immunity. 2005;23:227–39. [DOI] [PubMed] [Google Scholar]
- 10.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nature Immunology. Nature Publishing Group; 2003;4:350–4. [DOI] [PubMed] [Google Scholar]
- 11.Taniguchi RT, DeVoss JJ, Moon JJ, Sidney J, Sette A, Jenkins MK, et al. Detection of an autoreactive T-cell population within the polyclonal repertoire that undergoes distinct autoimmune regulator (Aire)-mediated selection. Proceedings of the National Academy of Sciences. 2012;109:7847–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nature Immunology. 2007;8:351–8. [DOI] [PubMed] [Google Scholar]
- 13.Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lei Y, Ripen AM, Ishimaru N, Ohigashi I, Nagasawa T, Jeker LT, et al. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. Journal of Experimental Medicine. 2011 ed. 2011;208:383–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kekäläinen E, Tuovinen H, Joensuu J, Gylling M, Franssila R, Pöntynen N, et al. A defect of regulatory T cells in patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Immunol. 2007;178:1208–15. [DOI] [PubMed] [Google Scholar]
- 16.Perry JSA, Lio C-WJ, Kau AL, Nutsch K, Yang Z, Gordon JI, et al. Distinct contributions of aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity. 2014;41:414–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malchow S, Leventhal DS, Lee V, Nishi S, Socci ND, Savage PA. Aire Enforces Immune Tolerance by Directing Autoreactive T Cells into the Regulatory T Cell Lineage. Immunity. 2016;44:1102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu M-L, Nagavalli A, Su MA. Aire deficiency promotes TRP-1-specific immune rejection of melanoma. Cancer Research. 2013;73:2104–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Träger U, Sierro S, Djordjevic G, Bouzo B, Khandwala S, Meloni A, et al. The immune response to melanoma is limited by thymic selection of self-antigens. PLoS ONE. 2012;7:e35005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahonen P, Myllärniemi S, Sipilä I, Perheentupa J. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med. 1990;322:1829–36. [DOI] [PubMed] [Google Scholar]
- 21.Tazi-Ahnini R, McDonagh AJG, Wengraf DA, Lovewell TRJ, Vasilopoulos Y, Messenger AG, et al. The autoimmune regulator gene (AIRE) is strongly associated with vitiligo. British Journal of Dermatology. 2008;:???–??? [DOI] [PubMed] [Google Scholar]
- 22.Gardner JM, DeVoss JJ, Friedman RS, Wong DJ, Tan YX, Zhou X, et al. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roth L, Srivastava S, Lindzen M, Sas-Chen A, Sheffer M, Lauriola M, et al. SILAC identifies LAD1 as a filamin-binding regulator of actin dynamics in response to EGF and a marker of aggressive breast tumors. Sci Signal. 2018;11. [DOI] [PubMed] [Google Scholar]
- 24.Fishman D, Kisand K, Hertel C, Rothe M, Remm A, Pihlap M, et al. Autoantibody Repertoire in APECED Patients Targets Two Distinct Subgroups of Proteins. Front Immunol. 2017;8:976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gotter J, Brors B, Hergenhahn M, Kyewski B. Medullary epithelial cells of the human thymus express a highly diverse selection of tissue-specific genes colocalized in chromosomal clusters. 2004;199:155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cloosen S, Arnold J, Thio M, Bos GMJ, Kyewski B, Germeraad WTV. Expression of Tumor-Associated Differentiation Antigens, MUC1 Glycoforms and CEA, in Human Thymic Epithelial Cells: Implications for Self-Tolerance and Tumor Therapy. Cancer Research. 2007;67:3919–26. [DOI] [PubMed] [Google Scholar]
- 27.Conteduca G, Ferrera F, Pastorino L, Fenoglio D, Negrini S, Sormani MP, et al. The role of AIRE polymorphisms in melanoma. Clinical Immunology. Elsevier Inc; 2010;136:96–104. [DOI] [PubMed] [Google Scholar]
- 28.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. Journal of Experimental Medicine. 2010;207:637–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan IS, Mouchess ML, Zhu M-L, Conley B, Fasano KJ, Hou Y, et al. Enhancement of an anti-tumor immune response by transient blockade of central T cell tolerance. Journal of Experimental Medicine. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rizzuto GA, Merghoub T, Hirschhorn-Cymerman D, Liu C, Lesokhin AM, Sahawneh D, et al. Self-antigen-specific CD8+ T cell precursor frequency determines the quality of the antitumor immune response. Journal of Experimental Medicine. 2009;206:849–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conteduca G, Fenoglio D, Parodi A, Battaglia F, Kalli F, Negrini S, et al. AIRE polymorphism, melanoma antigen-specific T cell immunity, and susceptibility to melanoma. Oncotarget. 2016;7:60872–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.deLeeuw RJ, Kost SE, Kakal JA, Nelson BH. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res. 2012;18:3022–9. [DOI] [PubMed] [Google Scholar]
- 34.Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339:1219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hikosaka Y, Nitta T, Ohigashi I, Yano K, Ishimaru N, Hayashi Y, et al. The Cytokine RANKL Produced by Positively Selected Thymocytes Fosters Medullary Thymic Epithelial Cells that Express Autoimmune Regulator. Immunity. 2008;29:438–50. [DOI] [PubMed] [Google Scholar]
- 36.Rossi SW, Kim MY, Leibbrandt A, Parnell SM, Jenkinson WE, Glanville SH, et al. RANK signals from CD4+3 inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. Journal of Experimental Medicine. 2007;204:1267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, et al. The Tumor Necrosis Factor Family Receptors RANK and CD40 Cooperatively Establish the Thymic Medullary Microenvironment and Self-Tolerance. Immunity [Internet]. 2008;29:423–37. Available from: http://www.sciencedirect.com/science/article/pii/S1074761308003683 [DOI] [PubMed] [Google Scholar]
- 38.Scagliotti GV, Hirsh V, Siena S, Henry DH, Woll PJ, Manegold C, et al. Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: subgroup analysis from a randomized phase 3 study. J Thorac Oncol. 2012;7:1823–9. [DOI] [PubMed] [Google Scholar]
- 39.Rossini M, Viapiana O, Adami S, Idolazzi L, Ghellere F, Tripi G, et al. Effects of denosumab on peripheral lymphocyte subpopulations. Endocrine. 2016;53:857–9. [DOI] [PubMed] [Google Scholar]
- 40.Bakhru P, Zhu M-L, Wang H-H, Hong LK, Khan I, Mouchess M, et al. Combination central tolerance and peripheral checkpoint blockade unleashes antimelanoma immunity. JCI Insight. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahern E, Harjunpää H, Barkauskas D, Allen S, Takeda K, Yagita H, et al. Co-administration of RANKL and CTLA4 Antibodies Enhances Lymphocyte-Mediated Antitumor Immunity in Mice. Clin Cancer Res. 2017;23:5789–801. [DOI] [PubMed] [Google Scholar]
- 42.Smyth MJ, Yagita H, McArthur GA. Combination Anti-CTLA-4 and Anti-RANKL in Metastatic Melanoma. Journal of Clinical Oncology. 2016;34:e104–6. [DOI] [PubMed] [Google Scholar]
- 43.Bostwick AD, Salama AK, Hanks BA. Rapid complete response of metastatic melanoma in a patient undergoing ipilimumab immunotherapy in the setting of active ulcerative colitis. J Immunother Cancer. 2015;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahern E, Harjunpää H, O’Donnell JS, Allen S, Dougall WC, Teng MWL, et al. RANKL blockade improves efficacy of PD1-PD-L1 blockade or dual PD1-PD-L1 and CTLA4 blockade in mouse models of cancer. Oncoimmunology. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahern E, Smyth MJ, Dougall WC, Teng MWL. Roles of the RANKL-RANK axis in antitumour immunity - implications for therapy. Nat Rev Clin Oncol. 2018;15:676–93. [DOI] [PubMed] [Google Scholar]
- 46.Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470:548–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palmer DB. The Effect of Age on Thymic Function. Front Immunol. 2013;4:316. [DOI] [PMC free article] [PubMed] [Google Scholar]