Abstract
We investigated the impact of diabetes on US life expectancy by sex and race/ethnicity using a prospective cohort study design. Cohorts were drawn from 1997–2009 waves of the National Health Interview Survey and linked to death records through December 31, 2011. We combined data on the prevalence of diabetes among decedents with estimates of the hazard ratios of individuals diagnosed with diabetes to calculate population attributable fractions (PAFs) by age, sex, and race/ethnicity at ages 30 and above. These estimates were then applied to deaths in the official US life table for 2010 to estimate effects of diabetes on life expectancy.
Diabetes was responsible for a reduction of 0.83 years of life expectancy for men at age 30 and 0.89 years for 30-year-old women. The impact was greatest among Black women at 1.05 years. Estimates based on traditional demographic and actuarial methods using the frequency with which a disease appears as an underlying cause of death on death certificates produced a reduction in life expectancy at age 30 of only 0.33 years.
We conclude that diabetes is substantially reducing US longevity and that its effect is seriously underestimated when using data on underlying causes of death.
Keywords: Diabetes, Race/ethnicity, Life Expectancy, Mortality, Population Attributable Fraction
Diabetes is a growing problem in the United States. In 2011–12, 9.1% of the population aged 20 years and above reported that they had been diagnosed with diabetes.1 The prevalence of diabetes is much higher among deaths. Among those aged 30–84 in the National Health Interview Surveys (NHIS) from 1997 to 2006, 23.7% of those who died over the next five years had been diagnosed with diabetes at baseline.2 The prevalence of diabetes varies by race and ethnicity. When measured by HbA1c, fasting plasma glucose or 2-hour plasma glucose level, the age-adjusted prevalence was significantly higher among non-Hispanic blacks (21.8%) and Hispanics (22.6%) than among non-Hispanic whites (11.3%) in 2011–2012.1
This paper is aimed at estimating the effect of diabetes on life expectancy in the United States. The conventional approach used by demographers and actuaries to address such questions is to identify the fraction of deaths at a particular age that have been attributed to a particular disease as the underlying cause of death. These authors then hypothetically eliminate those deaths and recalculate life expectancy.3,4 One recent calculation of this nature by the National Center for Health Statistics found that diabetes reduced life expectancy at age 30 in the United States in 1999–2001 by 0.34 years.5
A disadvantage of this approach in the case of diabetes is that it may be seriously underreported as an underlying cause of death.6,7 A prior study by Stokes and Preston used population-attributable fractions (PAF) to estimate that about 12% of deaths in the United States at ages 30–84 were attributable to diabetes over the period 1997–2011. This percentage was substantially greater than the value of 3.3% estimated using underlying cause of death data.2 People who die with diabetes typically have other conditions, especially cardiovascular diseases, that may also contribute to death. When both diabetes and cardiovascular disease are mentioned on a death certificate, whether or not diabetes is listed as the underlying cause is highly variable.8
In this paper, we show that Population Attributable Fractions can also be used to estimate the impact of a disease on life expectancy. PAF is defined as the proportion of disease cases or deaths in a defined population that would be eliminated if a particular exposure were eliminated.9 It is calculated based on estimates of the prevalence of the exposure combined with information on the risks of death associated with that exposure. To extend the PAF concept to life expectancy requires using age-specific values of risk and prevalence and applying them to the age distribution of years lived in the life cycle of individuals. The study by Stokes and Preston (2017) estimated the fraction of deaths at all ages combined attributed to diabetes, without differentiating among age groups. That approach is not tenable when estimating the effect of diabetes on life expectancy because large differences in attributable fractions by age, which we will demonstrate, combine with an age distribution of years lived in the period life table that is very different from the age distribution of the population.
It is useful to recognize that we are not comparing life expectancies of those with diabetes to those without.10–13 Rather, we are applying population-attributable fractions age-by-age to the entire population to estimate the effect of this disease on national life expectancy. As far as we are aware, this is the first study to use PAF values in this fashion.
Because the prevalence of diabetes and mortality vary by age, sex, and race/ethnicity, we will estimate the effect of diabetes on life expectancy for men and women and for three racial/ethnic groups. As noted above, Blacks and Hispanics have a much higher incidence and prevalence of diabetes than Non-Hispanic Whites.14 Blacks have also higher mortality than either Non-Hispanic Whites or Hispanics, whereas mortality of Hispanics is much closer to that of Non-Hispanic Whites.15 The results from the application of the age-specific PAFs to the age distribution in the life table will then be compared to estimates of the impact of diabetes on life expectancy when using diabetes as an underlying cause of death.
Methods
The estimates of the contribution of diabetes to life expectancy were based on two data sources. To estimate the relative risk of death for those with diabetes and the prevalence of diabetes among deaths, we used the public use 1997–2009 National Health Interview Survey and Linked Mortality Files (NHIS-LMF). The NHIS is a nationally representative, cross-sectional survey of the non-institutionalized population in the United States. The National Center for Health Statistics (NCHS) has linked respondents in NHIS to death certificate records from the National Death Index (NDI) through December 31, 2011.16
Diabetes status in the NHIS was collected for sample adults ages 18 and above using the question: “Other than during pregnancy, have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” While less precise than clinical measures, self-reports of diabetes have been shown to have high sensitivity and specificity.6 One advantage of the NHIS data relative to other data sources with clinical measures of diabetes is its large sample size, which allows us to examine how the contribution of diabetes to life expectancy varies by age, race/ethnicity and gender. We limited our sample to individuals who were eligible for mortality follow-up at ages 30 and above in 1997–2009 waves of the NHIS. Individuals with missing information on diabetes, race/ethnicity, educational attainment, smoking or drinking status and hypertension are excluded (n=7,856) along with those who died in the same quarter year in which they were interviewed (n=260). The final sample size is 286,810. There were 18,199 deaths from all causes within five years after the year in which respondents were surveyed. This data source was also used to provide estimates of the proportion of deaths at a particular age that are attributed to diabetes as an underlying cause of death.
The second data source, used to estimate the age-distribution of years lived and deaths in the life cycle, is the 2010 official U.S. life table produced by the National Center for Health Statistics.17 Such tables are available by sex for the whole population and for non-Hispanic whites (hereafter Whites), non-Hispanic blacks (hereafter Blacks), and Hispanics.
Using data from the 1997–2009 NHIS-LMF, we estimated Cox proportional hazard regressions with duration since baseline as exposure time. The outcome was all-cause mortality. Individuals were followed for five years unless their observations were truncated by death or by reaching January 1, 2012. We limited follow-up to a maximum of 5 years to add precision by reducing the possibility that someone was diagnosed with diabetes after they were surveyed. We confirmed the proportional hazards assumption by testing the slope of the Schoenfeld residuals for the model used to generate estimates of the effect of diabetes on life expectancy.
Explanatory variables in the hazard model based on NHIS data were self-reported diabetes, age, gender, race/ethnicity, educational attainment, diagnosis of hypertension, alcohol consumption and smoking history. Educational attainment was categorized into five groups: less than 9th grade, 9–11 years, high school graduate, some college, and college degree. Smoking was categorized as never (reference category), former smoker and current smoker. Respondents were asked whether they had ever smoked 100 cigarettes in their entire lifetime. If they reported “no” to the question, we assigned them as never smoker. If they said “yes” to the question, but were currently not smoking, they were considered former smokers. Current smokers are those who reported that they are currently smoking. Alcohol consumption categories are current drinker (one plus drinks in the past year), former drinker, and never drinker. Hypertension status was based on a question asking sample adults whether they had ever been told by a doctor or other health professional that they had “hypertension, also called high blood pressure.” We introduced age at baseline/diabetes interactions in the model as well as interactions between diabetes and sex and between diabetes and race/ethnicity. These interactions were significant and were retained in the model. Interactions between sex and age and between race/ethnicity and age were also introduced but were insignificant and were dropped in the final model. The hazard ratio used for the age interval x to x+5 was that predicted for age x+2.5.
The prevalence of diabetes among deaths in each age-sex-race/ethnicity group is obtained from the 5-year follow-up in NHIS. The prevalence for age interval x to x+5 was based on the 5-year follow up for cohorts aged x-5 to x+ 5 at baseline, a group whose exposure over the next five years was centered on the age interval x to x+5.
The Population Attributable Fraction, the proportion of deaths attributable to diabetes, is a function of the hazard ratio of death associated with diabetes and the prevalence of diabetes among those who died during the follow-up period:
where pd is the proportion of deaths occurring to those with diabetes and HR is the model-based hazard ratio of death for an individual with diabetes relative to those without. The PAF values used in this paper are estimated separately in 5-year wide age intervals 30–34, 35–39…80–84, with a terminal category of 85+.
To calculate life expectancy without deaths attributable to diabetes, we constructed an associated single decrement life table for all causes other than diabetes.3 We first employed age-sex-race/ethnicity specific rates of death from 2010 U.S. life tables.17 At each age, we then eliminated the proportion of deaths attributable to diabetes that was indicated by our estimated age-specific PAF values and recalculated the life table, including the calculation of life expectancy. We used U.S. life tables that include detail on single-years of age up to age 100.17 We assumed that the prevalence of diabetes among deaths for age intervals above 85 was constant and equal to the prevalence for the age category 85+. Our estimates of years of life lost to diabetes are based on a comparison of official US life tables to life tables after the removal of deaths attributable to diabetes.
We also calculated the probability of dying from diabetes using multiple decrement life tables.3 To do so, we applied age-specific PAF values to deaths at a particular age in the life table. The sum of deaths attributable to diabetes above age x, divided by the sum of all deaths above age x, is the estimated probability of eventually dying from diabetes at age x.
For calculations of both life expectancy and the probability of dying, we compared results obtained using PAF values to those obtained using the age-specific proportion of deaths assigned to diabetes as an underlying cause of death. Data analyses were performed using Stata version 15 (StataCorp, Texas, USA). All analyses incorporated sample weights and accounted for the complex design of the survey. Standard errors were estimated using the SVY routine.
Results
Table 1 provides a description of the sample. A total of 286,810 individuals are included, contributing a total of 1,311,397 person-years. As expected, Blacks and Hispanics had a much higher prevalence of reported diagnosed diabetes than Whites. The difference in prevalence between men and women was relatively small and inconsistent across racial/ethnic groups. Compared to Whites, Blacks had higher mortality while Hispanics had lower mortality. This pattern is consistent with national vital statistics.15 The same racial/ethnic pattern of mortality differentials was present among individuals both with and without diagnosed diabetes.
Table 1.
Sample Characteristics by Race/Ethnicity, Ages 30 and Above, NHIS-LMF 1997–2009
| All Racesa | Non- Hispanic White | Non- Hispanic Black | Hispanic | |||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | Men | Women | |
| Death rateb | 19.2 | 12.2 | 18.8 | 11.9 | 23.6 | 15.6 | 17.4 | 10.4 |
| Diabetesb | 29.8 | 21.3 | 31.2 | 22.4 | 31.3 | 22.1 | 25.2 | 17.8 |
| No diabetesb | 17.6 | 11.0 | 17.3 | 10.8 | 22.4 | 13.8 | 15.5 | 8.7 |
| Diabetes prevalence (%) | 8.9 | 8.4 | 8.3 | 7.4 | 11.9 | 13.5 | 9.9 | 10.4 |
| Mean age (years) | 50.7 | 52.2 | 51.9 | 53.4 | 48.6 | 49.8 | 45.9 | 47.5 |
| Age categories (%) | ||||||||
| 30–44 | 39.7 | 37.0 | 36.4 | 33.8 | 45.3 | 42.9 | 55.1 | 50.3 |
| 45–64 | 41.6 | 40.3 | 42.9 | 41.1 | 40.6 | 39.7 | 34.3 | 35.7 |
| 65 and over | 18.7 | 22.7 | 20.8 | 25.1 | 14.1 | 17.4 | 10.6 | 14.0 |
| Education | ||||||||
| Less than 9th grade | 7.0 | 6.7 | 4.1 | 3.9 | 7.3 | 6.3 | 27.5 | 27.2 |
| 9th – 11th grade | 7.6 | 8.0 | 6.5 | 6.7 | 11.5 | 13.0 | 12.9 | 12.9 |
| High school graduate | 30.8 | 32.8 | 31.2 | 33.8 | 36.4 | 33.6 | 27.3 | 27.5 |
| Some college | 25.7 | 28.0 | 26.5 | 29.0 | 27.8 | 30.2 | 19.6 | 20.3 |
| 4 Yrs college + | 29.0 | 24.5 | 31.7 | 26.5 | 17.0 | 16.9 | 12.7 | 12.1 |
| Smoking | ||||||||
| Never | 45.6 | 59.4 | 43.1 | 55.2 | 50.0 | 65.0 | 55.0 | 76.2 |
| Former smoker | 31.0 | 21.6 | 33.7 | 24.6 | 21.9 | 14.5 | 23.7 | 12.2 |
| Current smoker | 23.4 | 19.0 | 23.2 | 20.2 | 28.2 | 20.5 | 21.3 | 11.7 |
| Alcohol status | ||||||||
| Never | 13.1 | 27.7 | 11.0 | 22.3 | 19.8 | 38.6 | 16.6 | 45.8 |
| Former drinker | 18.0 | 16.7 | 18.2 | 17.0 | 21.0 | 19.6 | 15.8 | 13.1 |
| Current drinker | 68.8 | 55.6 | 70.8 | 60.7 | 59.2 | 41.8 | 67.5 | 41.1 |
| Hypertension (% Yes) | 30.7 | 31.7 | 31.4 | 31.0 | 37.1 | 43.8 | 21.1 | 26.0 |
| Number of deathsc | 8,759 | 9,440 | 6,501 | 7,080 | 1,237 | 1,458 | 839 | 726 |
| Person-yearsc | 565,675 | 745,722 | 389,327 | 496,118 | 68,274 | 110,793 | 85,425 | 112,159 |
| Unweighted N | 124,272 | 162,538 | 85,367 | 107,956 | 15,215 | 24,351 | 18,617 | 24,258 |
All races include other racial/ethnic groups that are not separately identified.
Age-standardized rates per 1,000 person-years using 2010 US population age distribution as the standard for both sexes. Source: 2010 US Census.28
Individuals in the sample are followed up to five years following the interview.
Table 2 presents results from Cox proportional hazard models. Results are expressed as hazard ratios. Model 1 is a baseline model with interactions between diabetes and age and between diabetes and sex. As expected, better educated people, those who have never smoked, and those without hypertension had significantly lower death rates. Men had a significantly lower risk associated with diabetes than women. The interaction term between diabetes status and age was significant and implied that the risk associated with diabetes decreased by a factor of 0.981 for each year of age beyond age 30. Thus a 30-year-old female had a hazard ratio of 3.807 and an 80-year-old woman a hazard ratio of 3.807(0.981)50 = 1.459.
Table 2.
Hazard Ratios from the Cox Proportional Hazard Model Predicting All-Cause Mortality, Ages 30 and Above, NHIS-LMF 1997–2011 (N=286,810)
| Model 1 | 95% CI | Model 2 | 95% CI | |
|---|---|---|---|---|
| Race/Ethnicitya | ||||
| Non-Hispanic White (ref) | 1.000 | |||
| Non-Hispanic Black | 1.217 | [1.146–1.291] | ||
| Hispanic | 0.848 | [0.785–0.916] | ||
| Diabetes | 3.807 | [3.170–4.574] | 4.205 | [3.483–5.076] |
| Age (in years since age 30) | 1.097 | [1.095–1.099] | 1.097 | [1.095–1.099] |
| Male | 1.519 | [1.458–1.582] | 1.523 | [1.462–1.587] |
| Education | ||||
| Less than 9th grade | 1.200 | [1.142–1.263] | 1.235 | [1.172–1.300] |
| 9th – 11th grade | 1.215 | [1.152–1.282] | 1.210 | [1.147–1.278] |
| High school graduate (ref) | 1.000 | 1.000 | ||
| Some college | 0.931 | [0.886–0.979] | 0.932 | [0.887–0.980] |
| 4 yrs college + | 0.782 | [0.738–0.829] | 0.788 | [0.743–0.835] |
| Smoking | ||||
| Never (ref) | 1.000 | 1.000 | ||
| Former smoker | 1.428 | [1.370–1.488] | 1.419 | [1.361–1.479] |
| Current smoker | 2.525 | [2.340–2.661] | 2.492 | [2.365–2.623] |
| Drinking status | ||||
| Never (ref) | 1.000 | 1.000 | ||
| Former drinker | 1.046 | [0.998–1.010] | 1.037 | [0.989–1.087] |
| Current drinker | 0.716 | [0.684–0.750] | 0.715 | [0.683–0.749] |
| Hypertension | 1.189 | [1.145–1.234] | 1.177 | [1.133–1.223] |
| Interactions with diabetesa | ||||
| Diabetes X Age (in years since age 30) | 0.981 | [0.977–0.985] | 0.980 | [0.976–0.983] |
| Diabetes X Male | 0.889 | [0.816–0.969] | 0.889 | [0.815–0.969] |
| Diabetes X Non-Hispanic Black | 0.793 | [0.697–0.902] | ||
| Diabetes X Hispanic | 0.828 | [0.711–0.963] |
ref: Reference group.
“Other racial groups” are separately identified in Model 2: results are not shown.
Model 2 introduces variables representing race and ethnicity and includes interaction terms between race/ethnicity and diabetes status. Blacks have significantly higher mortality than Whites while Hispanics enjoy a significant advantage. Relative to Whites with diabetes, both Blacks and Hispanics with diabetes had significantly lower hazard ratios. Age-specific hazard ratios produced by these models are presented in Table 3.
Table 3.
Age-specific Hazard Ratios Associated with Diabetes, Ages 30 and Above, NHIS-LMF 1997–2011
| Age Interval | All Racesa | Non-Hispanic White | Non-Hispanic Black | Hispanic | ||||
|---|---|---|---|---|---|---|---|---|
| Menb | Womenb | Menc | Womenc | Menc | Womenc | Menc | Womenc | |
| 30 – 34 | 3.224 | 3.625 | 3.549 | 3.993 | 2.815 | 3.167 | 2.938 | 3.306 |
| 35 – 39 | 2.923 | 3.287 | 3.201 | 3.601 | 2.538 | 2.856 | 2.650 | 2.981 |
| 40 – 44 | 2.650 | 2.980 | 2.886 | 3.248 | 2.289 | 2.576 | 2.390 | 2.689 |
| 45 – 49 | 2.403 | 2.702 | 2.603 | 2.929 | 2.064 | 2.323 | 2.155 | 2.425 |
| 50 – 54 | 2.179 | 2.450 | 2.348 | 2.641 | 1.862 | 2.095 | 1.943 | 2.187 |
| 55 – 59 | 1.975 | 2.221 | 2.117 | 2.382 | 1.679 | 1.889 | 1.753 | 1.972 |
| 60 – 64 | 1.791 | 2.014 | 1.909 | 2.148 | 1.514 | 1.704 | 1.581 | 1.778 |
| 65 – 69 | 1.624 | 1.826 | 1.722 | 1.937 | 1.366 | 1.536 | 1.425 | 1.604 |
| 70 – 74 | 1.472 | 1.655 | 1.553 | 1.747 | 1.231 | 1.386 | 1.285 | 1.446 |
| 75 – 79 | 1.335 | 1.501 | 1.400 | 1.576 | 1.111 | 1.250 | 1.159 | 1.304 |
| 80 – 84 | 1.210 | 1.361 | 1.263 | 1.421 | 1.002 | 1.127 | 1.045 | 1.176 |
| 85 + | 1.097 | 1.234 | 1.139 | 1.281 | 1.000 | 1.016 | 1.000 | 1.061 |
All races include other racial/ethnic groups that are not separately identified.
Hazard ratios are from Model 1 in Table 2.
Hazard ratios are from Model 2 in Table 2.
Age-specific hazard ratio is set to 1.000 if the value is lower than 1.000.
In calculating age-specific hazard ratios, we used more decimal places than shown in Table 2.
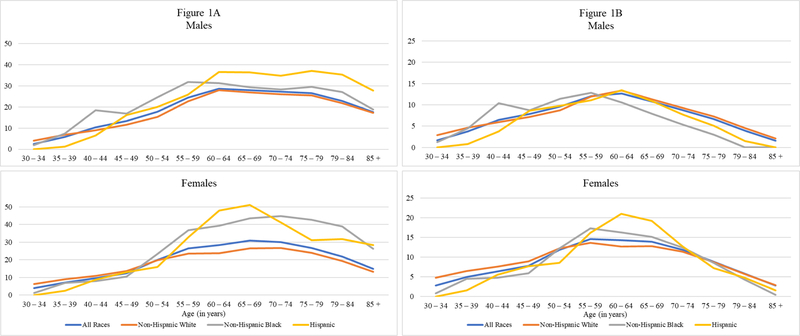
In addition to hazard ratios, the calculation of PAF values requires data on the prevalence of diabetes among decedents. These values, pertaining to deaths in a five-year period following survey enumeration, are presented graphically in Figure 1A and numerically in Table 4. The 22.8% prevalence of diabetes among the full sample of male decedents in Table 4 is in sharp contrast to the prevalence of diabetes at baseline of 8.9% (Table 1). Equivalent figures for women are 22.5% and 8.4%. The disparity between these prevalence values is a product of the very high death rate of people with diabetes.
Figure 1.
Prevalence of Diagnosed Diabetes among Deaths (Figure 1A) and Proportion of Deaths Attributable to Diabetes (PAF) (Figure 1B) by Sex and Race/Ethnicity at Ages 30 and Above, 1997–2011 NHIS-LMF.
Table 4.
Prevalence of Diabetes (Percent) among Those Who Died, Ages 30 and Above, NHIS-LMF 1997–2011
| Age Interval | All Racesa | Non-Hispanic White | Non-Hispanic Black | Hispanic | ||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | Men | Women | |
| 30 – 34 | 2.5 | 3.9 | 4.0 | 6.4 | 1.9 | 1.3 | 0.0 | 0.0 |
| 35 – 39 | 5.7 | 7.2 | 6.8 | 9.0 | 7.4 | 7.0 | 1.3 | 2.4 |
| 40 – 44 | 10.3 | 9.6 | 9.0 | 11.0 | 18.5 | 7.9 | 6.4 | 8.8 |
| 45 – 49 | 13.4 | 12.4 | 11.7 | 13.6 | 17.0 | 10.5 | 16.1 | 13.1 |
| 50 – 54 | 17.8 | 20.0 | 15.2 | 19.7 | 24.6 | 23.4 | 20.1 | 15.9 |
| 55 – 59 | 24.4 | 26.5 | 22.6 | 23.5 | 31.7 | 36.8 | 25.9 | 32.8 |
| 60 – 64 | 28.7 | 28.4 | 28.0 | 23.8 | 31.2 | 39.4 | 36.6 | 47.9 |
| 65 – 69 | 27.9 | 30.8 | 27.0 | 26.5 | 29.4 | 43.5 | 36.4 | 51.0 |
| 70 – 74 | 27.2 | 30.1 | 26.0 | 26.7 | 28.3 | 44.8 | 34.8 | 41.3 |
| 75 – 79 | 26.5 | 26.6 | 25.4 | 23.9 | 29.5 | 42.8 | 37.1 | 31.1 |
| 80 – 84 | 22.8 | 22.0 | 21.8 | 19.4 | 27.1 | 38.8 | 35.3 | 31.8 |
| 85 + | 17.6 | 14.8 | 17.2 | 13.2 | 18.8 | 26.3 | 27.7 | 28.4 |
| Ages 30+ | 22.8 | 22.5 | 22.0 | 20.1 | 26.0 | 33.7 | 27.2 | 31.0 |
All races include other racial/ethnic groups that are not separately identified.
The complex NHIS survey design is taken into account.
The prevalence of diabetes among decedents at survey in the full sample peaked at 27–30% in the age interval 60–74. The hill-shaped age-pattern was generally shared between the sexes and among racial/ethnic groups (Figure 1A). The peak in prevalence was most dramatic among Hispanic women, among whom 51% of decedents aged 65–69 had reported at survey being diagnosed with diabetes. Sex differences in the full sample were minor (a prevalence of 22.8% of deaths among men and 22.5% among women) but that similarity hides some racial/ethnic distinctions. At all ages above 55, the prevalence of diabetes among Black female decedents exceeded that of Black males. Female prevalence also exceeded male prevalence among Hispanic decedents between ages 55 and 74 (Table 4). A related pattern is that the difference in prevalence across race/ethnic groups is generally greater for women than for men.
Prevalence values and hazard ratios combine to produce PAF values in the manner shown in the earlier PAF equation. Table 5 presents PAF values by age, sex and race/ethnicity. The values are graphed in Figure 1B. The age pattern is a combination of hazard ratios that decline systematically with age and hill-shaped prevalence values that peak in the age interval 60–74. The result is a slightly earlier peak in PAF’s in the age interval 55–69 and a sharp fall-off in PAF values as age advances beyond the peak.
Table 5.
Percentage of Deaths Attributable to Diabetes (PAF), Ages 30 and Above, NHIS-LMF 1997–2011
| Age Interval | All Racesa | Non-Hispanic White | Non-Hispanic Black | Hispanic | ||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | Men | Women | |
| 30 – 34 | 1.7 | 2.8 | 2.9 | 4.8 | 1.2 | 0.9 | 0.0 | 0.0 |
| 35 – 39 | 3.8 | 5.0 | 4.7 | 6.5 | 4.5 | 4.6 | 0.8 | 1.6 |
| 40 – 44 | 6.4 | 6.4 | 5.9 | 7.6 | 10.4 | 4.8 | 3.7 | 5.5 |
| 45 – 49 | 7.8 | 7.8 | 7.2 | 8.9 | 8.7 | 6.0 | 8.6 | 7.7 |
| 50 – 54 | 9.6 | 11.8 | 8.8 | 12.2 | 11.4 | 12.2 | 9.8 | 8.6 |
| 55 – 59 | 12.0 | 14.6 | 11.9 | 13.6 | 12.8 | 17.3 | 11.1 | 16.2 |
| 60 – 64 | 12.7 | 14.3 | 13.3 | 12.7 | 10.6 | 16.3 | 13.4 | 21.0 |
| 65 – 69 | 10.7 | 13.9 | 11.3 | 12.8 | 7.9 | 15.2 | 10.9 | 19.2 |
| 70 – 74 | 8.7 | 11.9 | 9.3 | 11.4 | 5.3 | 12.5 | 7.7 | 12.8 |
| 75 – 79 | 6.7 | 8.9 | 7.3 | 8.7 | 2.9 | 8.5 | 5.1 | 7.3 |
| 80 – 84 | 4.0 | 5.8 | 4.5 | 5.8 | 0.0 | 4.4 | 1.5 | 4.8 |
| 85 + | 1.6 | 2.8 | 2.1 | 2.9 | 0.0 | 0.4 | 0.0 | 1.6 |
| Ages 30+ | 7.2 | 8.4 | 7.4 | 8.0 | 6.6 | 9.4 | 6.7 | 9.5 |
PAF values by race and ethnicity reflect the fact that the higher prevalence of diabetes among Black and Hispanic decedents was substantially offset by the lower hazard ratios associated with diabetes for these two groups. For example, the Black/White ratio of prevalence for women at all ages combined was 33.7/20.1 = 1.68, whereas the Black/White ratio of PAF’s for women was only 9.4/8.0 = 1.18. Among Blacks and Hispanics, women had much higher PAF values above age 55 than men, whereas the sex differences were much smaller among Whites. At the peak value of PAF, 21% of deaths among Hispanic women aged 60–64 were attributable to diabetes (Table 5).
Table 6 presents values of the gain in life expectancy resulting from the removal of deaths attributable to diabetes. Life expectancy in 2010 at age 30 was 47.8 years for men and 52.0 years for women based on the official U.S. life table. After eliminating deaths from diabetes estimated via the age-specific PAF function, life expectancy at age 30 rose by 0.83 years for men and by 0.89 years for women. The variation in gains across sexes and race/ethnicity groups was relatively small, ranging from a gain of 0.65 years among Hispanic males to 1.05 years among Black females at age 30. This narrow range was a product of the offsetting effects of higher prevalence of diabetes among Black and Hispanic decedents and their lower hazard ratios. Similar patterns of change are apparent in life expectancy at ages 50 and 70.
Table 6.
Life Expectancy at Ages 30, 50 and 70 in the 2010 U.S. Life Table and Gains in Life Expectancy from Eliminating Deaths Attributable to Diabetes
| 2010 U.S. Life Tablea | Gain in Life Expectancy from Eliminating Diabetes | |||||||
|---|---|---|---|---|---|---|---|---|
| All | NHW | NHB | Hispanic | All | NHW | NHB | Hispanic | |
| Men | Men | |||||||
| 30 | 47.8 | 48.0 | 44.3 | 50.0 | 0.83 | 0.85 | 0.81 | 0.65 |
| 50 | 29.6 | 29.7 | 26.6 | 31.4 | 0.77 | 0.80 | 0.70 | 0.61 |
| 70 | 14.2 | 14.2 | 12.9 | 15.4 | 0.36 | 0.39 | 0.17 | 0.23 |
| Women | Women | |||||||
| 30 | 52.0 | 52.2 | 49.5 | 54.6 | 0.89 | 0.85 | 1.05 | 0.80 |
| 50 | 33.2 | 33.3 | 31.2 | 35.4 | 0.84 | 0.79 | 1.02 | 0.77 |
| 70 | 16.4 | 16.5 | 15.8 | 18.0 | 0.47 | 0.46 | 0.44 | 0.37 |
Source: National Center for Health Statistics (2014).
All: All racial/ethnic groups, NHW: non-Hispanic White, NHB: non-Hispanic Black.
A more conventional method of estimating the effect of diabetes on life expectancy is to employ cause-deleted life tables to eliminate the deaths assigned to diabetes as an underlying cause of death. The latest period for which such calculations have been made in the US is 1999–2001.5 In that period, life expectancy for men at age 30 was 45.89 years before the elimination of deaths from diabetes and 46.20 years after their elimination. The gain of 0.31 years is only about one-third of the gain from eliminating deaths attributable to diabetes that we have estimated using PAF’s based on the 2010 US life table. The gain in life expectancy at age 30 for women was only 0.36 years using diabetes as an underlying cause of death.
We were able to repeat the underlying-cause analysis for the population studied in this paper because the deaths linked to NHIS surveys included information on underlying cause of death. Results are shown in Table 7. At age 30, the gain in life expectancy of 0.33 years for each sex from eliminating diabetes as an underlying cause of death was highly consistent with those calculated for 1999–2001 by the National Center for Health Statistics (NCHS). The gains were only about 37–40% of those obtained using PAF. This result primarily reflects conditions in the White population.
Table 7.
Comparison of Gains in Life Expectancy from Eliminating Diabetes and Probability of Dying from Diabetes at Age 30: Population Attributable Fraction versus Underlying Cause of Death Calculations
| Life Years Lost to Diabetes Using PAF | Life Years Lost to Diabetes Using Underlying Cause of Death | |||||||
|---|---|---|---|---|---|---|---|---|
| All | NHW | NHB | Hispanic | All | NHW | NHB | Hispanic | |
| Males | 0.83 | 0.85 | 0.81 | 0.65 | 0.33 | 0.30 | 0.44 | 0.58 |
| Females | 0.89 | 0.85 | 1.05 | 0.80 | 0.33 | 0.23 | 0.68 | 0.80 |
| Probability of Dying from Diabetes using PAF | Probability of Dying from Diabetes Using Underlying Cause of Death | |||||||
| Males | 0.055 | 0.059 | 0.044 | 0.036 | 0.027 | 0.025 | 0.033 | 0.050 |
| Females | 0.062 | 0.061 | 0.060 | 0.050 | 0.028 | 0.022 | 0.053 | 0.070 |
All: all racial/ethnic groups, NHW: non-Hispanic White, NHB: non-Hispanic Black
Both the NCHS estimates and our estimates based on PAF values use identical methods based on associated single-decrement life tables. The difference in results is entirely attributable to the much lower volume of deaths assigned to diabetes as an underlying cause of death than to deaths from diabetes identified using the PAF approach. This difference is consistent with the results of Stokes and Preston, who examined the volume of deaths at all ages combined rather than life expectancy.2
A closely-related measure is the probability that an individual will die from diabetes. Table 7 shows that, using PAF values, the probability of dying from diabetes for a 30-year old male was 5.5%. Using underlying cause of death criteria, the probability is only 2.7%. Equivalent values for women are 6.2% and 2.8%. Once again, the disparity between these two probabilities is greatest in the White population and underlying cause calculations are actually somewhat higher for Hispanics.
Discussion
We estimate that diabetes was responsible for the loss of 0.89 years of life expectancy at age 30 for women in 2010 and 0.83 years for men. Using PAF values has elevated its importance as a source of mortality relative to its much less frequent citation as an underlying cause of death (0.33 years). At the male/female average of 0.86 years, diabetes is responsible for the loss of approximately as many years of life above age 30 as widely recognized major killers: malignant neoplasms of the trachea, bronchus and lung (0.87), acute myocardial infarction (0.88), and cerebrovascular diseases (0.66). 5 The loss of 0.86 years of life to diabetes gains significance in view of the recent stubbornness of levels of life expectancy in the United States: life expectancy at birth grew by only 0.1 years between 2011 and 2015.18 Our results clearly underscore the importance of diabetes as a major disease process affecting American longevity.
The estimates in this paper are based on values of prevalence and hazards observed over the period 1997–2011, while the US life table to which the resulting PAF values are applied pertains to 2010, near the end of the period. We chose 2010 because we wanted a timely estimate. To investigate the effect of choosing a life table in the middle of the period, we repeated the analysis using the same set of prevalence and hazard values but using the official U.S. life table for 2005.19 This substitution produced an increase in estimated years lost to diabetes at age 30 of 0.02 years for both men and women. Thus, results were not sensitive to the year selected for the life table. A wider range between the life tables may produce greater sensitivity in other applications.
On the basis of the higher prevalence of diabetes among Blacks and Hispanics, we anticipated that Blacks and Hispanics would suffer a greater loss of life from diabetes than Whites. Apart from results for Black women, the losses were relatively similar because lower hazard ratios associated with diabetes for Blacks and Hispanics offset most or all of the higher prevalence among these groups.
Several explanations may account for the finding that hazard ratios associated with diabetes are lower for Blacks and Hispanics than for Whites. One possible explanation for the lower hazard ratio for Blacks is that the risks associated with diabetes and with membership in a particular racial/ethnic group are additive rather than multiplicative. Such a claim has been made about race and obesity.20 If so, the same absolute risk associated with diabetes for Whites and Blacks would constitute a lower relative risk among Blacks, who have higher mortality from other causes of death. Table 1 suggests that this is not a promising explanation; Black men and women show lower absolute increases in mortality associated with diabetes than do Whites.
Misclassification bias associated with use of self-reported data on diabetes status may provide another potential explanation for the lower hazard ratios observed for Blacks and Hispanics. It is well understood that misclassification of a dichotomous predictor variable is likely to bias coefficients towards the null.21 Prior studies indicate that racial/ethnic minorities with diabetes are less likely to receive a diagnosis compared to non-Hispanic whites22,23, suggesting use of self-reported data may lead to greater downward bias in risk estimates among these groups.
A third potential explanation for the lower hazard ratios may be related to the finding that different racial and ethnic groups present different joint distributions of levels of Hemoglobin A1c (HbA1c) and blood glucose levels. At a given HbA1c level, blood glucose levels are typically lower among racial/ethnic minorities than non-Hispanic Whites.24,25 These different combinations present opportunities for systematic racial and ethnic differences in the conditions of individuals diagnosed with diabetes. Future research on the sources of variation in relative risks across racial/ethnic groups would be valuable for clinical care, risks stratification, and for identifying disease mechanisms. Such research would need to go beyond comparisons of the medical conditions of those with diabetes across racial/ethnic groups26,27 and include racial/ethnic contrasts between those without diabetes.
In addition to the reliance on self-reported data on diabetes status, an additional limitation of the study was that diabetes status was assessed at the time of the survey and could change during the follow-up period. Transitions from not having to having diabetes are not rare. To reduce this potential misclassification error, we limited our mortality follow-up to 5 years.
We emphasize that the estimated hazards associated with diabetes depend on the model used to estimate those hazards. In this paper, the mortality hazards associated with diabetes were estimated with a model that included variables for smoking, educational attainment, hypertension, alcohol consumption, race/ethnicity, sex, and age. In one sensitivity analysis, we dropped the variables representing hypertension and alcohol consumption. These omissions raised the estimated years lost to diabetes at age 30 from 0.83 years to 0.87 years for men and from 0.89 to 0.94 years for women. In a second sensitivity analysis, we added a four-category obesity variable to the basic model. This addition raised the estimated years lost to diabetes at age 30 to 0.88 years for men and 0.98 years for women. These changes show mild sensitivity of our results to model specification. It is certainly possible that other changes in the model would produce larger effects. If we have omitted variables not on the causal chain between diabetes and death but that are positively correlated with having diabetes and that also raise mortality, it is likely that we will have overestimated the impact of diabetes on life expectancy.
One methodological point deserves to be emphasized. Individuals have a lower probability of dying from diabetes (5.5% for males and 6.2% for females from Table 7) than the estimated PAF value for the population (7.2% and 8.4% from Table 5), despite the fact that both figures are based on the same set of age-specific PAF values. The reason for the disparity is that, because the US population has been growing, the age distribution of deaths in the population is younger than the age distribution of deaths in the life table.3 As a result, the declining age-specific PAF values after age 60–64 receive heavier weight in the life table than in the population. This example illustrates that characteristics of populations and of life cycles do not map directly onto implications for individuals without the intervention of a life table.
Conclusion
In 2010, diabetes was responsible for a reduction of 0.89 years of life expectancy at age 30 for US women and 0.83 years for US men. There is relatively little variation in this reduction among Whites, Blacks and Hispanics and between males and females. The small range among racial/ethnic groups reflects the offsetting effects of higher prevalence among Blacks and Hispanics and the lower hazard ratios among these groups. Our estimates suggest that the importance of diabetes in US longevity is seriously underestimated when data on underlying cause of death are used to estimate its impact. The results demonstrate that diabetes is a major feature on the landscape of American mortality and reinforce the need for robust population-level interventions aimed at diabetes prevention and care. The long-term rise in the prevalence of diabetes clearly poses a major threat to the health and longevity of Americans.
Acknowledgments
Funding: This work was supported by the National Institute of Aging under Grants R03AG 055724–01, R24AG045061 and P30AG012836.
References
- 1.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. JAMA [Internet] 2015;314(10):1021–9. Available from: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2015.10029 [DOI] [PubMed] [Google Scholar]
- 2.Stokes A, Preston S. Deaths Attributable to Diabetes in the United States: Comparison of Data Sources and Estimation Approaches. PLoS One [Internet] 2017;12(1):e0170219 Available from: 10.1371/journal.pone.0170219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Preston SH, Heuveline P, Guillot M. Demography: Measuring and Modeling Population Processes Wiley-Blackwell; 2001. [Google Scholar]
- 4.Bowers N, Gerber H, Hickman J, Jones D, Nesbitt C. Actuarial Mathematics 2nd ed. Society of Actuaries; 1997. [Google Scholar]
- 5.Arias E, Heron M, Teleda-Vera B. United States life tables eliminating certain causes of death, 1999–2001. Natl Vital Stat Reports 2013;61(9):1–128. [PubMed] [Google Scholar]
- 6.Saydah SH, Geiss LS, Tierney E, Benjamin SM, Engelgau M, Brancati F. Review of the performance of methods to identify diabetes cases among vital statistics, administrative, and survey data. Ann Epidemiol 2004;14(7):507–16. [DOI] [PubMed] [Google Scholar]
- 7.Diabetes Saydah S. and Mortality. In: Narayan KMV, Williams DE, Gregg EW, Cowie CC, editors. Diabetes Public Health 2011. p. 267–84.21524316
- 8.Murray CJL, Dias RH, Kulkarni SC, Lozano R, Stevens GA, Ezzati M. Improving the comparability of diabetes mortality statistics in the U.S. and Mexico. Diabetes Care 2008;31(3):451–8. [DOI] [PubMed] [Google Scholar]
- 9.Benichou J. A review of adjusted estimators of attributable risk. Stat Methods Med Res [Internet] 2001;10:195–216. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11446148 [DOI] [PubMed] [Google Scholar]
- 10.Andrade FCD. Measuring the impact of diabetes on life expectancy and disability-free life expectancy among older adults in Mexico. J Gerontol B Psychol Sci Soc Sci 2010;65 B(3):381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang SH, Yu YC, Carlsson NP, Liu X, Colditz GA. Racial disparity in life expectancies and life years lost associated with multiple obesity-related chronic conditions. Obesity 2017;25(5):950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franco OH, Steyerberg EW, Hu FB, Mackenbach J, Nusselder W. Associations of Diabetes Mellitus With Total Life Expectancy and Life Expectancy With and Without Cardiovascular Disease. Arch Intern Med [Internet] 2007;167(11):1145–51. Available from: http://archinte.jamanetwork.com/article.aspx?doi=10.1001/archinte.167.11.1145 [DOI] [PubMed] [Google Scholar]
- 13.Laditka SB, Laditka JN. Active life expectancy of Americans with diabetes: Risks of heart disease, obesity, and inactivity. Diabetes Res Clin Pract [Internet] 2015;107(1):37–45. Available from: 10.1016/j.diabres.2014.10.008 [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of diabetes and its burden in the United States. Natl Diabetes Stat Rep 2014;
- 15.Arias E. United States Life Tables, 2011. Natl Vital Stat Reports 2015;64(11):1–63. [PubMed] [Google Scholar]
- 16.National Center for Health Statistics Office of Analysis and Epidemiology. Public-use Linked Mortality File, 2015 [Internet] Hyattsville, Maryland: 2016. Available from: http://www.cdc.gov/nchs/data_access [Google Scholar]
- 17.Arias E. United States Life Tables, 2010. Natl Vital Stat Reports [Internet] 2014;63(7):1–63. Available from: http://www.cdc.gov/nchs/data/nvsr/nvsr58/nvsr58_10.pdf [PubMed] [Google Scholar]
- 18.Xu J, Murphy SL, Kochanek KD, Arias E. Mortality in the United States, 2015. NCHS Data Brief [Internet] 2016;267:1–7. Available from: https://www.cdc.gov/nchs/products/databriefs/db267.htm [PubMed] [Google Scholar]
- 19.Arias E, Rostron BL, Tejada-Vera B. National Vital Statistics Reports: United States Life Tables , 2009. Natl Vital Stat Reports 2010;58(10). [PubMed] [Google Scholar]
- 20.Mehta N, Preston S. Are major behavioral and sociodemographic risk factors for mortality additive or multiplicative in their effects? Soc Sci Med [Internet] 2016;154:93–9. Available from: 10.1016/j.socscimed.2016.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jurek AM, Greenland S, Maldonado G. Brief Report: How far from non-differential does exposure or disease misclassification have to be to bias measures of association away from the null? Int J Epidemiol 2008;37(2):382–5. [DOI] [PubMed] [Google Scholar]
- 22.Menke A, Casagrande S, És-Santa MLA, Cowie CC. Factors associated with being unaware of having diabetes. Diabetes Care 2017;40(5):e55–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010;362(9):800–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolfenbuttel BH, Herman WH, Gross JL, Dharmalingam M, Honghua HJ, Hardin DS. Ethnic Differences in Glycemic Markers in Patients With Type 2 Diabetes. Diabetes Care 2013;36(October):2931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ziemer DC, Kolm P, Weintraub WS, et al. Glucose-Independent, Black-White Differences in Hemoglobin A1C Levels: A Cross-sectional analysis of 2 Studies. Ann Intern Med 2010;152:770–7. [DOI] [PubMed] [Google Scholar]
- 26.Kirk JK, Bell RA, Bertoni AG, et al. Ethnic disparities: Control of glycemia, blood pressure, and LDL cholesterol among US adults with type 2 diabetes. Ann Pharmacother 2005;39(9):1489–501. [DOI] [PubMed] [Google Scholar]
- 27.Lanting LC, Joung IM., Mackenbach JP, Lamberts SWJ, Bootsma AH. Ethnic Differences in Mortality , End- Stage Complications , and Quality of Care. Diabetes Care 2005;28(9). [DOI] [PubMed] [Google Scholar]
- 28.US Census Bureau. Census Summary File 1 United States 2011.