Abstract
Background
This is an updated version of the original Cochrane Review published in Issue 10, 2013.
Extramammary Paget's disease is a rare form of superficial skin cancer. The most common site of involvement is the vulva. It is seen mainly in postmenopausal white women. Paget's disease of the vulva often spreads in an occult fashion, with margins extending beyond the apparent edges of the lesion. There is a range of interventions from surgical to non‐invasive techniques or treatments. The challenges of interventions are to remove or treat disease that may not be visible, without overtreatment and with minimisation of morbidity from radical surgery. There is little consensus regarding treatment. Surgery, by default, is the most common treatment, but it is challenging to excise the disease adequately, and recurrence is common, leading to repeated operations, and destruction of anatomy. Alternative treatments of photodynamic therapy, laser therapy, radiotherapy, topical treatments or even chemotherapy have been mooted, and it is important to evaluate the available evidence. It is essential to assess whether newer cell‐specific treatments, such as photodynamic therapy and imiquimod, can reduce the need for radical surgery.
Objectives
To evaluate the benefits and harms of different treatment modalities for the management of Paget's disease of the vulva.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (via Ovid) and Embase (via Ovid) up to 8 May 2018. We also searched registers of clinical trials, abstracts of scientific meetings and reference lists of review articles.
Selection criteria
We searched for randomised controlled trials (RCTs) and well‐designed non‐randomised studies that compared different interventions in women with Paget's disease of the vulva,
Data collection and analysis
Two review authors independently assessed whether potentially relevant studies met the inclusion criteria. We found no trials and, therefore, analysed no data.
Main results
The search for the original version of the review identified 635 unique references. We found 31 references (which reported on 30 studies) in full text after inspection of titles and abstracts, but we excluded them all as they did not meet the inclusion criteria. However, we have included a comprehensive narrative account of studies where we identified an analysis of more than 10 women, as this forms the only evidence base in this rare disease. Surgery continues to be the mainstay of treatment in the current literature, with other treatments limited to case reports or treatment of inoperable or recurrent disease.
This update between September 2013 and May 2018 identified 35 new studies. None of these met the inclusion criteria. There was only one prospective study of 5% imiquimod in recurrent Paget's disease of the vulva, which although of good quality only included eight women.
Authors' conclusions
Since the last version of the review was published there are many more cases in the literature reporting a clinical response to 5% imiquimod cream. There is one prospective study of eight women treated with 5% imiquimod for recurrent Paget's disease of the vulva, and one prospective trial of 20 women was due to be reported. This increasing evidence for the safety and efficacy of 5% imiquimod will be helpful for women and clinicians alike. Ideally, a multicentre RCT of reasonable size is needed, but ongoing publications of high‐quality non‐randomised prospective studies will enhance the current available literature.
Plain language summary
The comparison of different treatments for women with Paget's disease of the vulva
Background Extramammary Paget’s disease of the vulva is a rare type of superficial skin cancer. It is most common in postmenopausal white women. It is an intraepithelial (layer of cells that forms the surface or lining of an organ) condition that can present as white and red scaly areas on the vulva that may be itchy and painful. The diagnosis is made by examination and tissue sampling. Abnormal cells often extend outside the clinically abnormal area, so some studies suggest frozen section at time of surgery, where a pathologist can give a rapid report of small biopsies to say whether the skin is involved with Paget's or not. Other treatments include: topical medication, such as imiquimod (self‐applied cream); radiotherapy; chemotherapy; photodynamic therapy (form of phototherapy using light‐sensitive compounds that are exposed selectively to light, whereupon they become toxic to targeted cancerous and other diseased cells); laser therapy; or a combination of these approaches. The challenges of interventions are to remove or treat disease that may not be visible, without overtreatment. Avoiding the long‐term complications of radical surgery, such as pain and scarring, a feeling of mutilation and loss of femininity, is very important to women. Surgery is still the most common treatment, but it is challenging to remove the disease completely, and recurrence is common, leading to repeated operations and mutilation of the vulva. The aim of this review was to evaluate the benefits and harms of different treatments for Paget's disease of the vulva.
Study characteristics We searched for randomised controlled trials (trials where treatment is allocated to women in a random manner) and well‐designed non‐randomised studies that compared different treatments in women aged 18 years or older with biopsy‐confirmed Paget's disease of the vulva.
Key results and quality of evidence We searched scientific databases and contacted experts and identified and checked the titles and abstracts of 635 possibly relevant articles and retrieved 31 of these references in full text. However, we found no studies that met our inclusion criteria. We identified several non‐randomised studies and drafted a detailed narrative of their results, but these studies were of poor quality and were at high risk of bias. Therefore, there is currently no evidence to determine whether any form of treatment is better or worse in terms of prolonging survival, delaying progression or recurrence, improving QoL or minimising toxicity. The review highlights the need for good‐quality studies comparing different interventions for the management of Paget's disease of the vulva. Women and clinicians would value more evidence for guiding surgical and non‐surgical management of this disease. In particular, non‐invasive medical management would spare women from the side effects and consequences of surgery.
Background
Description of the condition
Extramammary Paget's disease is a rare form of intraepithelial skin cancer (adenocarcinoma). The most common site of involvement is the vulva. It is seen mainly in postmenopausal white women. Paget's disease of the vulva often spreads in an occult fashion, with margins extending beyond the apparent edges of the lesion. It is characterised by infiltration of the squamous mucosa or adenexa by vacuolated Paget cells. It is an intraepithelial adenocarcinoma that presents as slowly expanding, asymmetrical white and red scaly plaques on the vulva, which may be itchy and painful. The diagnosis is made by finding the characteristic changes on skin biopsy. Immunohistochemistry is required to exclude the differential diagnoses of melanoma and vulval intraepithelial neoplasia. Paget's disease may be primary, arising as an intraepithelial adenocarcinoma, or secondary due to Pagetoid spread of an adjacent or contiguous in situ or invasive tumour. There have been reports of an association with distant tumours, particularly breast cancer, although the strength of this association is unknown. Any screening for distant tumours may involve lengthy and invasive investigations, and also delay the appropriate treatment of the Paget's disease itself (Heymann 1993; Kanitakis 2007). Immunohistochemical stains can guide further investigations. Tissue staining negative for cytokeratin 20 favours a cutaneous origin for Paget's (primary or secondary due to an underlying adnexal adenocarcinoma) and positive staining favours an endodermal origin, where a visceral malignancy should be more keenly sought. The presence of Paget’s disease around the perianal area or around the urethra should prompt a search for urothelial or rectal tumours. One clinicopathological study in 1977 reported 13 cases of Paget's disease of the vulva (Lee 1977): four (31%) women had underlying invasive carcinoma of the adnexal structures and three (23%) had adnexal carcinoma in situ. In four (31%) women, there was a second malignancy (Lee 1977). A more recent case series reviewed 10 women with vulval Paget's disease (Fanning 1999). About 34% of women had recurrent disease, at a median time of three years, with 12% having invasive Paget's disease of the vulva and 4% had a vulval adenocarcinoma. The association between Paget's disease and other malignancies is variable across the literature, with one literature review showing the association to be between 0% and 50%, and with no significant difference between women with Paget's disease and the rate of cancer in that demographic group (Preti 2003).
Wilkinson and Brown subclassified Paget's disease of the vulva into primary or secondary disease (Wilkinson 2002). Primary Paget's disease is of vulval cutaneous origin, and secondary vulval Paget's disease is due to a non‐cutaneous neoplasm, often of adjacent sites.
Description of the intervention
Surgery can involve local excision, radical excision or vulvectomy. Margins often extend outside the clinically abnormal area, so some studies suggest frozen section or preoperative mapping biopsies to delineate the margins of excision. Other interventions include topical imiquimod (an immune response modifier), radiotherapy, chemotherapy, photodynamic therapy (topical and systemic), laser therapy or a combination of these approaches. The challenges of interventions are to remove or treat disease that may not be visible, without overtreatment and with minimisation of morbidity from radical surgery.
How the intervention might work
Surgery removes the abnormal area. Disease tends to be multifocal, and complete eradication is not guaranteed. Surgical excision can also cause significant vulval mutilation, with consequent psychological morbidity (Tsutsumida 2003). Photodynamic therapy works by the exposure of sensitised cells to a specific wavelength of light, which activates a cascade of photochemical and photobiological events, causing irreversible damage to tumour tissue (Shieh 2002). Radiotherapy destroys tissues by damaging deoxyribonucleic acid (DNA), affecting normal as well as abnormal tissues. Chemotherapy has been used alone or in conjunction with radiotherapy. Topical imiquimod, usually a 5% cream, is an immune response modifier that induces high levels of interferon, although the complete mechanism of action is complex and not fully understood. It causes inflammation, which in some cases can be poorly tolerated (Woodmansee 2006). Carbon dioxide laser has been used in other vulval conditions, such as vulval intraepithelial neoplasia (Maclean 1995), since the 1970s and was initially used for treatment of disease of the cervix. The depth of destruction can be controlled and planned, but needs to be deep enough to decrease the likelihood of recurrence.
Why it is important to do this review
Paget's disease is a rare condition, and there is little consensus regarding treatment. Surgery, by default, is the most common treatment, but it is challenging to excise the disease adequately, and recurrence is common, leading to repeated operations and mutilation of the vulva. Alternative treatments of photodynamic therapy, laser therapy, radiotherapy, topical treatments or even chemotherapy have been mooted, and we considered that it was important to evaluate the available evidence. Paget's disease most commonly occurs in elderly women, and having evidence‐based alternative treatments to surgery would be of benefit to these women.
Objectives
To evaluate the benefits and harms of different treatment modalities for the management of Paget's disease of the vulva.
Methods
Criteria for considering studies for this review
Types of studies
We wanted to include randomised controlled trials (RCTs). However, since RCTs were unlikely, we also searched for non‐randomised studies with concurrent comparison groups:
quasi‐randomised trials;
non‐randomised trials;
prospective and retrospective cohort studies;
case series of 10 or more women.
We excluded case‐controlled studies, uncontrolled observational studies and case series of fewer than 10 women.
To minimise selection bias if no RCTs were identified, we only included studies that used statistical adjustment for baseline case mix using multivariable analyses (e.g. disease severity, age, comorbidity, previous treatment).
Types of participants
Women (aged 18 years or older) with biopsy‐confirmed Paget's disease. We applied no exclusion criteria.
Types of interventions
We searched for all interventions used in Paget's disease. The mainstay of treatment is surgery, which is either conservative or radical.
Other interventions that are used include: radiotherapy; topical treatments, including steroids; photodynamic therapy; imiquimod; systemic treatments, including chemotherapeutic agents and any treatment combinations. We considered comparisons of any two treatment modalities.
Types of outcome measures
We did not use outcome measures as part of the inclusion criteria.
Primary outcomes
Overall survival, assessed from the time when women were enrolled in the study.
Disease‐free survival, defined as the documented time between treatment and confirmed recurrence.
Secondary outcomes
Quality of life (QoL), measured using a validated scale.
-
Adverse events classified according to CTCAE 2006:
direct surgical morbidity;
surgically related systemic morbidity;
delayed (hospital) discharge and recovery;
toxicity of photodynamic therapy;
radiotherapy toxicity;
chemotherapy toxicity.
-
Toxicity was grouped as:
haematological;
gastrointestinal;
genitourinary;
skin;
neurological;
pulmonary.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases on 8 May 2018:
the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 5), in the Cochrane Library (Appendix 1);
MEDLINE via Ovid (September 2013 to May 2018; Appendix 2);
Embase via Ovid (September 2013 to May 2018; Appendix 3).
We identified all relevant articles on PubMed and, using the 'related articles' feature, we carried out a further search for newly published articles.
Searching other resources
We searched the following:
the online bibliography of Paget's disease (www.tiny.cc/PagetsOnline) set up by the Oxford University library service;
metaRegister, Physicians Data Query, www.controlled‐trials.com/rct, www.clinicaltrials.gov, and www.cancer.gov/clinicaltrials for ongoing studies;
conference proceedings and abstracts through ZETOC (zetoc.mimas.ac.uk) and WorldCat Dissertations.
Handsearching
We handsearched and checked the citation lists of included studies, key textbooks and previous systematic reviews. We handsearched reports of the following conferences:
Annual Meeting of the American Society of Gynecologic Oncologist;
Annual Meeting of the International Gynecologic Cancer Society;
British Cancer Research Meeting;
Annual Meeting of European Society of Medical Oncology;
Annual Meeting if the American Society of Clinical Oncology;
British Society for the study of Vulval Disease;
International Society for the Study of Vulvo‐Vaginal Disease;
European College for the Study of Vulval Disease.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to the reference management database Endnote. We removed duplicates and two review authors (KE, JM) independently examined the remaining references. We excluded studies that clearly did not meet the inclusion criteria and obtained copies of the full text of potentially relevant references. Two review authors (KE, EA) independently assessed the eligibility of the retrieved papers and resolved any disagreements by discussion. When necessary, a third review author (SC or AB) participated in discussions. We documented reasons for exclusions. We excluded all articles that we retrieved, as they did not meet the inclusion criteria. We did not identify any studies that met our inclusion criteria from our searches of the grey literature. However, we included a comprehensive narrative account of studies where we identified an analysis of more than 10 women, as this forms the only evidence base in this rare disease (See Effects of interventions for studies, which included at least 10 women and Agreements and disagreements with other studies or reviews for a general discussion of a wider range of studies and reviews).
Results
Description of studies
Results of the search
The search strategy for the original review identified 635 separate references by title and abstracts (Figure 1; Edey 2013). The updated search identified 35 new studies between September 2013 and May 2018.
1.
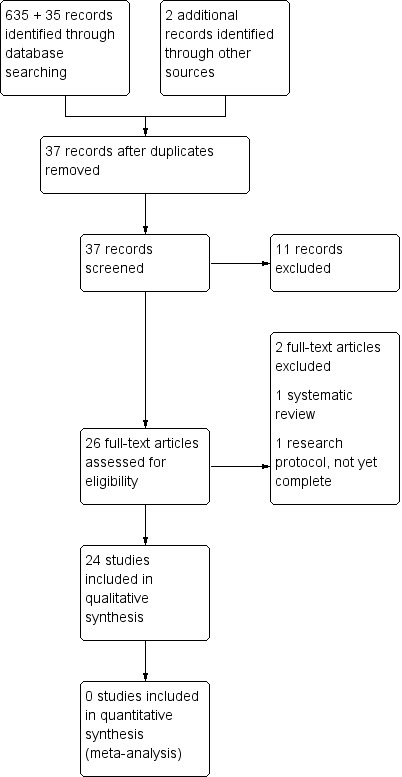
Study flow diagram.
For the original review, two review authors (KE, JM) sifted through these and excluded abstracts that did not include at least 10 women with Paget's disease of the vulva and then applied the other inclusion criteria. Where the contents of the paper were not clear from the abstract, we obtained the full‐text papers in order to be rigorous in the searching. We identified 31 references that reported on 30 studies that potentially met the inclusion criteria. We excluded all studies after inspection of the full papers.
For this update, none of the 35 new studies identified met the inclusion criteria; four papers contained more than 10 women treated for Paget's disease of the vulva and these were reviewed in full, but excluded after inspection. The outcomes for these four papers have been added to the Characteristics of excluded studies table.
Two review authors (KE, JM) independently searched the grey literature and identified no relevant studies. All studies were at a high risk of bias and retrospective in nature.
Included studies
None of the studies met our inclusion criteria and we excluded them all from the review. However, 20 studies were initially included in a narrative discussion in Effects of interventions, with a further four added in the update. All the studies were retrospective data analyses; the earliest woman included was diagnosed in 1939. Across the studies, the age range was 35 to 100 years, but there were minimal other demographic data available due to the retrospective nature of the data collection. There were 581 women included with some data for analysis. The most common surgery was a wide local excision (202 (35%) women) and radical vulvectomy (157 (27%) women). In total, 135 women had a simple vulvectomy, 48 women had a hemivulvectomy and one woman had a skinning vulvectomy. Ultracision was used to treat one woman, primary chemotherapy was used in seven cases, primary radiotherapy in 14 cases and laser therapy was in 23 cases. Two women undergoing surgery had been treated with neoadjuvant radiotherapy. Treatment regimens for radiotherapy and chemotherapy were not clearly documented in any of the papers, and, therefore, further discussion will focus on the surgical management.
Although some women received treatments in addition to surgery, all 20 studies discussed contained women having a variety of surgical interventions, ranging from wide excisions to radical vulvectomies. Case series ranged from 10 to 100 women. Data were variable between the papers; some contained limited clinical data because the main focus was discussion of pathology. Forty of the 306 women across all the studies had a cancer at another site, although 10 of these were definitely metachronous, and for many it was unclear. They are described in greater detail in Table 1.
1. Details of excluded studies.
| Study | Methods | Participants | Intervention | Outcomes | Notes |
| Bakalianou 2008 | Retrospective review of records 1996 – 2005 |
11 women with Paget's disease of the vulva from 1 centre in Athens Median age 64 years; range 53 – 75 years |
Ultracision, SV, RV with groin node dissection | All had topical steroid use before diagnosis. 1/11 had ultracision, 7/11 had SV, 3/11 had radical surgery. All had FSs (10/11 margins negative at FS, 9/11 negative at final pathology). 3/11 had a recurrence, 1/1 who had ultracision, 1/7 with SV, 1/3 with RV. 2/2 with positive margins. |
1 had concurrent breast cancer. |
| Black 2007 | Retrospective review from pathology database | 28 women with Paget's disease of the vulva Median age 68 years; range 48 – 86 years |
RV, SV, WLE | 3/28 had RV, 18/28 had SV, 7/28 had WLE. 14/20 with positive margins recurred, 3/8 with negative margins recurred. 14/17 further surgery, 1/17 treated with tretinoin. |
11 women had a secondary malignancy. |
| Cowan 2016 | Prospective Pilot study | 8 women with biopsy‐proven recurrent extramammary Paget's | Topical application 5% imiquimod tiw for 12 wks. Punch biopsy and photography at baseline and 12 wk time point | 6/8 had complete clinical and histologic response at 12 wks. 1/8 had complete clinical but no histologic response. | 1/8 withdrew due to intolerable irritation. |
| Crawford 1999 | Retrospective data collection Time period not defined |
21 women with diagnosis of Paget's disease of the vulva | WLE, hemivulvectomy, RV | WLE 13, hemivulvectomy 2, RV 5. 1 woman had biopsies only. Margins not documented by type of surgery. 13/20 had positive margins, 1/7 recurrence with negative margins, 7/13 recurrence with positive margins. |
2 women died of other malignancies, 1 died from CVA after hip surgery, 1 died of metastatic Paget's disease. |
| Curtin 1990 | Retrospective data 1939–1987 |
31 women with a histological diagnosis of Paget's disease of the vulva Aged 49 – 84 years |
WLE, vulvectomy or skinning vulvectomy, RV | 4 had WLE, 22 had vulvectomy and 3 had an RV. 1 woman had a skinning vulvectomy. Out of 28 women, 14 had negative margins, 8 had positive margins, 6 were not documented. 3/14 with positive margins recurred, 2/8 with negative margins recurred. |
No mortality. 5 adenocarcinomas excluded. |
| Fan 2016 | Retrospective data 2001–2012 |
18 women | WLE, vulvectomy, WLE with reconstruction | Overall recurrence rate 11%. | Recurrence rate low due to loss of data (3/18 participants lost to follow‐up). |
| Fanning 1999 | Retrospective data 1962–1996 Multiple institutions |
100 women with Paget's disease of the vulva Aged 35–100 years |
RV with or without node dissection, radical hemivulvectomy, WLE | 58 radical vulvectomies, 10 radical hemivulvectomies, 32 WLE. Recurrence 18/58 radical, 2/10 hemivulvectomy, 14/32 WLE. Median time to recurrence 3 years. Not able to assess margins in this study as retrospective. 4 women had an adenocarcinoma at resection. |
1 woman DoD. |
| Feuer 1990 | Retrospective review 1969–1987 |
19 women with Paget's disease of the vulva Mean age 65.2 years; range 44 – 81 years |
WLE, SV, hemivulvectomy, RV | 4/19 WLE, 9/19 SV, 2/19 hemivulvectomy, 4/19 RV. 9/19 recurred, 1 DoD. 8/9 recurrences treated surgically 1 treated with laser and 5 with 5‐FU. 2/4 WLE recurred, 5/9 SV recurred, 1/2 hemivulvectomy recurred, 1/4 RV recurred. |
5 had an associated malignancy. No comment on margin status. |
| Fishman 1995 | Retrospective review and re‐review of all histology 1982–1993 |
14 women from 1 centre with known Paget's disease of the vulva Mean age 70.5 years; range 57 – 83 years |
WLE, SV, modified RV | 8/14 WLE, 3/14 SV, 3/14 modified RV. 2/5 with positive margins recurred, 3/9 with negative margins recurred. After clinical judgement of negative margins, 6/17 were positive at final pathology. After FS, 3/8 that were negative at FS were positive at final pathology. No difference between FS and inspection (P = 1). 2/8 having a WLE recurred, 1/3 having a SV recurred, 2/3 having a modified RV recurred. |
7 women had a metachronous cancer. |
| Goldblum 1997 | Retrospective review from 1 centre | 19 women with Paget's disease of the vulva Median age 65 years; range 56–86 years |
SV, RV, RV + groin node dissection | 13/19 SV, 4/19 RV, 2/19 RV + groin node dissection. 4/13 recurrences in SV group, 1/4 recurrences in RV. Minimal invasion had no effect on recurrence or prognosis; 2/7 minimally invasive recurred compared with 3/7 intraepithelial (5 were initially invasive). |
No comment on margin status. |
| Kim 2017 | Retrospective review from 1 centre 1961–2012 |
94 women with Paget's disease of the vulva | WLE, Moh's micrographic surgery | Paper compared local excision to Moh's micrographic surgery, but none of the women with vulva Paget's disease had Moh's surgery. | Vulval outcomes not reported separately. |
| Lee 1977 | Retrospective data 1940–1976 |
13 women with confirmed Paget's disease of the vulva Median age 65 years; range 38–86 years |
SV, RV, radiotherapy | 8/13 SV, 4/13 RV. 2 had presurgical radiotherapy. 1/8 recurrence after SV, 1/4 re‐excision for positive margins after RV, 2/4 recurrences with RV but all free of disease after re‐excision. |
4 had other cancers, 3 deaths of other causes. |
| Lee 2010 | Retrospective data 1990–2009 |
14 women with diagnosis of Paget's disease of the vulva Mean age 54.3 years; range 29 – 72 years |
Hemivulvectomy, WLE, RV | 2/14 hemivulvectomy, 5/14 WLE, 7/14 RV. Positive margins in 8/14. 3/8 recurrences with positive margins, 2/6 recurrences with negative margins. |
Recurrences treated with radiotherapy. |
| Long 2017 | Retrospective review 1992–2015 |
90 women with Paget's disease of the vulva | WLE, AP resection | 87/90 WLE, 3/90 AP resection. 48/79 negative margin, 31/79 positive margin. |
— |
| Louis‐Sylvestre 2001 | Retrospective review | 52 women with biopsy‐confirmed Paget's disease of the vulva Mean age 67 years; range 39–94 years |
Surgery, surgery + laser therapy, laser therapy alone | 31 had surgery alone, 7 recurrences at 1 year (23%); 15 had surgery + laser therapy with 5 recurring within 1 year (33%); 6 had laser therapy alone with 4 recurrences at 1 year (67%). | Treatment was chosen by size of lesion so the largest lesions had laser therapy, so the survival rate may be related to disease rather than treatment. |
| Machida 2015 | Systematic review | 68 women with vulva Paget's disease | Topical 5% imiquimod therapy used between 3 ‐ 7 tiw for a median duration of 4 months and median follow‐up of 12 months. | In 46 (73.0%) cases, a complete remission (CR) to imiquimod therapy was reported, with 2, 4, and 6‐month cumulative CR rates being 9.8%, 31.1%, and 71.6%, respectively. | Frequency‐reduction due to adverse effects was seen in 9.5%, with the initial 5 ‐ 7 times/week regimen being associated with the highest reduction rate (1‐2, 3‐4, and 5‐7times/week: 0%, 2.3%, and 81.8%). |
| Molinie 1993 | Retrospective data 1976–1990 |
36 women with a histological diagnosis of Paget's disease of the vulva Aged 45 – 91 years |
Local excision, partial vulvectomy or RV | 29 women followed up. 11 vulvectomies; 4 involved margins, 2/4 recurred. 4 clear margins, 3/4 recurred. 14 partial vulvectomies; 12 involved margins, 3/12 recurred. 1 clear margin, 1/1 recurred. 4 local excisions, all clear margins 2/4 recurred. Others margins not known. Altogether 11/29 recurrences, 5/16 with positive margins, 5/9 with negative margins. |
3 died of associated malignancy. |
| Niikura 2006 | Retrospective data 1986–2005 |
22 women with Paget's disease of the vulva Mean age 71.5 years; range 51–85 years |
SV, RV + groin node dissection, chemotherapy (cisplatin and 5‐FU) | 18 SV, 3 RV, 1 primary chemotherapy (stage 4 disease) DoD after 12/12. 3/18 SV had positive margins; 2 treated with etoposide, 1 with radiotherapy. 2/3 RV positive margins; 1 treated with radiotherapy, 1 with MEP. Only 1 recurrence in whole series (RV + MEP). | 2 associated malignancies. |
| Parashurama 2017 | Retrospective data 1988–2016 |
18 women with Paget's disease of the vulva | WLE ± graft reconstruction | 69% of women with positive margins had a recurrence, 60% women with negative margins had a recurrence. | — |
| Parker 2000 | Retrospective data 1944–1997 |
76 women with a diagnosis of Paget's disease of the vulva Mean age 67.5 years |
6 women had chemotherapy, 12 had radiotherapy, 20 had WLE, 26 had SV, 20 had RV, 2 had no treatment | 53% having surgery had positive margins, 23% negative, 26% unknown. Recurrence was 31% in those with positive margins, 33% in those with negative margins. |
16% DoD. Exact chemotherapy used not documented. |
| Petkovic 2006 | Retrospective review 1995–2000 |
10 women in 1 institution with Paget's disease of the vulva Mean age 58 years; range 46 – 84 years |
WLE, RV and bilateral groin node dissection | 8/10 WLE, 2/10 RV; 2/8 recurrences with WLE, 1/2 recurrences with radical surgery. No comment on margins. | 2 women previously treated before treatment in the centre. |
| Pierie 2003 | Retrospective data review of all participants with extramammary Paget's disease | 25/33 women had Paget's disease of the vulva Median age 70 years |
Local excision, hemivulvectomy, radiotherapy | 5/25 local excision, 18/25 hemivulvectomy, 2/25 radiotherapy. 10/25 recurred; 2/5 local excisions, 8/18 hemivulvectomy, 0/2 radiotherapy. Margin status not broken down for tumour type. No radical surgery. |
No perioperative mortality or disease‐related mortality. |
| Roh 2010 | Retrospective review 1996–2008 |
11 women with histologically verified Paget's disease of the vulva Mean age 66.6 years; range 52–77 years |
WLE, RV, SV | 7/11 WLE, 2/11 SV, 2/11 RV. 6/11 recurred; 4/8 with positive margins, 2/3 with negative margins. 4/7 with WLE, 0/2 with SV, 2/2 with RV. 4/6 recurrences had further excisions with no further disease, 1 had photodynamic therapy and was alive with disease, 1 declined treatment and DoD. |
No surgical mortality/morbidity. 1 DoD. 3 had previous cancers 3–15 years before diagnosis of Paget's disease. |
| Shaco‐Levy 2010 | Retrospective | 56 women with known diagnosis of Paget's disease of the vulva Mean age 69 years; range 42 – 89 years |
WLE, SV, partial vulvectomy, RV, laser ablation | Surgical extent did not affect recurrence rate. 11/37 recurrences in those with conservative surgery, 5/16 recurrences with radical surgery, 2/2 recurrences with laser ablation. 6 women received adjuvant radiotherapy; 1 DoD, 5 had no further recurrences. 32% recurrence rate. 20/30 with positive margins recurred, 3/17 with negative margins recurred. |
Currently 43% no evidence of disease. |
| Stacy 1986 | Retrospective data 1975–1984 |
13 women with biopsy‐ confirmed Paget's disease of the vulva or anus. Women with anal disease excluded for this review; data from 10 women analysed Aged 45 – 81 years |
SV, RV | 9 simple vulvectomies, 1 RV. FS showed positive margins in 4/8. 2 had positive margins on final specimens with no FS, 1 had re‐excision, 1 no re‐excision and no recurrence. All free of disease at follow‐up. |
— |
| Sawada 2018 | Prospective study | 9 women with extramammary Paget's disease | 5% imiquimod cream 3 times a week for 16 weeks; one case only 6 weeks. | The response rate was 100% including five complete remissions. | Local irritation was observed in three patients, which was controlled by a provisional withdrawal of the treatment. |
| Tebes 2002 | Retrospective data 1988–2000 |
23 women with Paget's disease of the vulva Aged 46 – 84 years |
WLE, and if adenocarcinoma present proceed to RV and node dissection. | 17 WLE, 6 radical resections. 13 women with negative FS margins, 6 of these had positive margins in final resection. 2/17 with negative margins recurred, 6/16 with positive margins recurred. Mean time to recurrence 30 months. 2/6 with radical resections recurred, 6/17 with conservative surgery recurred. All those with invasive disease had a radical resection. |
5 (22%) women had other primary cancers all treated previously. |
5‐FU: fluorouracil; CVA: cerebrovascular accident; DoD: dead of disease; FS: frozen section; MEP: mitomycin + etoposide + cisplatin; RV: radical vulvectomy; tiw: three times a week SV: simple vulvectomy; WLE: wide local excision.
All studies were from large hospitals in Europe and Northern America, reporting from retrospective reviews of women's and histopathological records. Few information were available in the studies regarding participant demographics although all reported age.
Excluded studies
For the original review, we excluded 30 references after obtaining the full‐text paper (Edey 2013). The updated search identified four references including more than 10 women, but these were all excluded. Reasons for exclusion are explained below.
Twenty‐four references reported studies that did not use statistical adjustment to reduce the risk of selection bias (Bakalianou 2008; Black 2007; Crawford 1999; Curtin 1990; Fan 2016; Fanning 1999; Feuer 1990; Fishman 1995; Goldblum 1997; Kim 2017; Lee 1977; Lee 2010; Long 2017; Louis‐Sylvestre 2001; Molinie 1993; Niikura 2006; Parashurama 2017; Parker 2000; Petkovic 2006; Pierie 2003; Roh 2010; Shaco‐Levy 2010; Stacy 1986; Tebes 2002). We discussed all of these studies further.
One study only included eight women with Paget's disease (two with melanoma) (Koss 1968), and two studies included just seven women with full information (Parmley 1975; Tanaka 2009). Another study contained three cases of Paget's diseases only (Strempel 1958).
Three references reported case reports only (Martorell‐Calatayud 2009; Wang 2003; Zawislak 2004), and one contained only one women with Paget's disease in a study on carcinoma of the vulva (Shingleton 1970).
One prospective study was included looking at 5% imiquimod for the treatment of Paget's disease of the vulva in eight women (Cowan 2016) and another looking at 5% imiquimod for the treatment extramammary Paget's disease in nine participants.
One systematic review (Machida 2015).
Two references were not available through any UK source, including the British Library and we excluded them from the review (Lu 1999; Shrestha 2010). We will continue search for full‐text copies for completeness of the review and to reduce possibility of biases.
For further details of all the excluded studies, see Table 1 and the Characteristics of excluded studies table.
Risk of bias in included studies
No studies met our inclusion criteria and we excluded them all from the review. However, we did report some results in a narrative discussion in the Effects of interventions section. These studies were retrospective case series and were at a high risk of bias, although we did not objectively and subjectively assess this since they did not meet the inclusion criteria.
Effects of interventions
None of the studies used any statistical adjustment for baseline case mix using multivariable analyses. The number of included women in most of the studies was low, which may account for the lack of statistical adjustment. We presented the main results of the studies but made no inferences due to the problem of selection bias and fact that all studies were at a high risk of bias.
The 20 studies discussed here met all the inclusion criteria, except having any statistical adjustment to decrease the risk of bias. Therefore, any meta‐analysis would be invalid, but their results warrant discussion.
Eight studies provided data on survival (Crawford 1999; Curtin 1990; Fanning 1999; Lee 1977; Molinie 1993; Parker 2000; Pierie 2003; Roh 2010). These included information on 306 women and reported 23 deaths. There were 14 deaths related to disease, five deaths from other malignancies and four deaths related to other causes. Since nearly all treatment in the studies was surgical, we could not comment on mortality to treatment modality. Two series used chemotherapy as first‐line treatment (Niikura 2006; Parker 2000). One woman in the Niikura 2006 study received chemotherapy using cisplatin plus fluorouracil for stage IV disease, and she died of the disease. Six women out of 76 (8%) in the Parker 2000 study had chemotherapy as the primary treatment. However, there was no documentation regarding what chemotherapy regimens were used, or how women were selected for chemotherapy. In this series of women, chemotherapy was associated with poor prognosis with regards to survival, compared with other treatment, but this is likely to be due to selection bias, and this difference could not be expanded any further due to the small series and lack of detailed information. In this same study, 12 women were also treated with primary radiotherapy (16%), although again there was no documentation of treatment regimens, and whether doses used were of palliative or radical levels (Parker 2000). Survival was again shown to be worse after radiotherapy, but the same caveats applied as to their comments on chemotherapy.
The largest series of laser therapy included six women having laser alone and 15 women having a combination of laser and surgery (Louis‐Sylvestre 2001). The recurrence rates for the small number of women having laser alone were very high (67% at one year), but laser alone was only used in women with large, likely inoperable lesions, so it is not possible to draw conclusions regarding the efficacy of the treatment.
Radiotherapy was used as a first‐line treatment in only four women across all the other papers. With these small numbers, it was not possible to form any conclusions as to its effectiveness as a treatment. With the data available in the studies, it was also not possible to draw any conclusions with regards to disease‐free survival, as, due to the nature of retrospective series, there was no adequate information with which to categorise recurrences.
The studies contained variable length of follow‐up, varying from nine months to 38 years. Accurate survival data were not possible given the number of women followed up for fewer than five years. Of the 40 women across all the studies who had a confirmed malignancy at another site (13%), at least 10 of these were metachronous and treated before the presentation of Paget's disease of the vulva. It was not possible from the data in the studies to define clearly the number of secondary Paget's disease included.
For the review update, we identified four further studies, which were all retrospective data series of between 18 and 94 women (Fan 2016; Kim 2017; Long 2017; Parashurama 2017). The data, by nature of the retrospective collection, was of poor quality and included surgery only as a treatment modality. We also identified two prospective studies with very small numbers of participants, eight and nine respectively (Cowan 2016; Sawada 2018) and one systematic review (Machida 2015).
Margin status
Thirteen of the 19 studies clearly documented the margin status of the initial specimens. Due to the small numbers in each series, most papers favoured the margin status making no difference to chance of recurrence. However, with the small numbers involved, inferences should not be made as to the risk of recurrence based on margin status in these women. Out of the 529 women in the 19 studies, margin status and recurrence data were known in 307, although follow‐up length was variable, from months to more than 20 years. This highlights the difficulties of extracting meaningful information from data collected retrospectively, when in these studies, margin status was only known in 57% of women.
Radical versus conservative surgery
Different studies described the exact surgery performed in different ways. The most common were wide local excision, hemivulvectomy, simple vulvectomy and radical vulvectomy sometimes with groin node dissection. It could be assumed that the more radical the surgery, the larger the lesion, but it was not possible to extract these data from the papers. None of the study authors described in detail the surgery undertaken for women in their study. Tebes 2002 stated that all women with invasive disease had radical surgery, but this did not apply to other studies. In all of the studies, almost twice as many women had conservative surgery rather than radical. It seems reasonable that surgery should be tailored to the size of the lesion, and radical surgery to be used only when necessary to obtain clear margins.
Discussion
Summary of main results
We identified no studies that met our inclusion criteria. Consequently, there was no evidence from this review as to which treatment modality was most effective and safe in Paget's disease of the vulva. We were able to review the results of 20 studies in detail that reported on at least 10 women with biopsy‐confirmed Paget's disease of the vulva. We reviewed four subsequent studies in the update in 2019, but these did not add any further information. We were unable to make any comparisons between treatment modalities, due to minimal numbers of women having non‐surgical treatment. Due the variation in the radicality of the surgery itself, we could not make any inferences about disease recurrence.
Overall completeness and applicability of evidence
At the outset of this review, we were aware, due to the rare nature of Paget's disease of the vulva, that accumulation of helpful outcome data would be difficult. We set a cut‐off of series with at least 10 women, in order to try to include as much of the published data as possible, while excluding case reports and very small case series. The accumulation of meaningful data on rare diseases remains challenging, but is a vital part of extending our knowledge base on rare conditions.
It was noticeable that the majority of published data were now relatively old (10/20 studies were pre‐2000) and there was no published prospective data collection. This meant that it was not possible to gain any evidence regarding morbidity from these papers, as morbidity data need to be collected prospectively. Other flaws in retrospective data collection include a lack of clarity in decision making regarding choices of surgery and whether any other treatment options had been offered or discussed. The update identified one prospective study looking at 5% imiquimod for the treatment of Paget's disease of the vulva. This is the best‐quality study in the literature to date, but only included eight women (Cowan 2016). Sawada 2018 is another small prospective study of nine patients of which five were women with Paget's disease of the vulva.
We have specifically reviewed only women with Paget's disease of the vulva. There is evidence that may be available for perianal disease or scrotal disease in men that may be appropriate to guide treatment.
Quality of the evidence
There is no available evidence of adequate quality; selected retrospective studies formed the only available evidence base and were the best that was available currently in the literature, but these study designs are significantly flawed. While taking into account the inherent problems with these data, the way forward is to collect prospective data on all treatments for Paget's disease of the vulva, ideally as a national database, in order that clear outcome and morbidity data, as well as patient satisfaction data, can be collected.
Potential biases in the review process
Due to the retrospective nature of all the studies, there were no morbidity data available, or patient satisfaction analysis, and, therefore, no comment can be made on QoL following treatments. This is significant when considering radical surgery, and re‐excision after previous surgery. Selection bias is challenging when dealing with retrospective studies, when radicality of treatment would be dependent on surgeon choice. Most of the women in the studies had treatment before the advent of the multidisciplinary team, and, therefore, alternative treatments to surgery may not have been available, or not considered. It was not possible to extract this information from the studies.
Agreements and disagreements with other studies or reviews
There is one published systematic review of the effects of imiquimod on vulva Paget's disease (Machida 2015). This systematic review contained 63 cases of women treated with imiquimod for Paget's disease of the vulva. They reported a 73% complete remission rate with 5% imiquimod, which was demonstrated in later published case series (our initial search strategy included several case reports of successful treatment with 5% imiquimod in eight women (Bertozzi 2009; Hatch 2008; Sendagorta 2010; Tonguc 2011; Wang 2003). Other treatments that have been used and published as case reports included laser ablation (Valentine 1992), primary curative intent radiotherapy (Luk NM 2003; Moreno‐Arias 2003), and photodynamic therapy (Raspagliesi 2006, which was a pilot study of six women, but with no control group). Nardelli and colleagues carried out a review into photodynamic therapy for mammary and extramammary Paget's disease (Nardelli 2011). It contained 23 studies, but nine were single case reports only. The largest study included only two women with Paget's disease of the vulva (Li 2010). These data were collected prospectively, but neither woman had a complete response. Across all Paget's disease, this non‐systematic review concluded that evidence was limited, and most women across all studies and Paget's sites had follow‐up of one year or less.
The published literature regarding treatment of Paget's disease of the vulva is dominated by surgical treatment. This is likely to be due to the nature of the published studies, which are retrospective series of women who have undergone surgery, rather than a proper cohort. However, there is also no evidence for surgery as a treatment of Paget's disease of the vulva, and currently any treatment is not based on evidence, but on empirical reasoning.
Authors' conclusions
Implications for practice.
There is no evidence to support the use of surgery over any other treatment modality, yet the published research implies that surgery is the mainstay of treatment of Paget's disease of the vulva, although there is a wide variation in the radicality of surgery carried out. Therefore, no recommendations regarding treatment modality can be made from the current available literature and women need to be made aware that any treatment including surgery does not have a clear evidence base. Women should be able to discuss different treatment modalities with their clinician and be referred to other centres for alternatives to surgery, if appropriate. Ideally, treatment should be offered as part of a trial. However, the highest quality data currently in the literature is the only prospective trial of 5% imiquimod in eight women, which has shown a 75% response rate.
Implications for research.
In rare diseases, it is accepted that prospective randomised controlled trials are difficult but not impossible, so in order to provide further evidence regarding management every attempt should be made to set one up. Practically, the way forwards would be to design a large international multicentre trial, as well as carefully planned prospective data collection with consideration to bias. The collection of cases centrally with a key dataset would enable data to be extracted retrospectively, which will aid development of a more robust literature. The flaws in the excluded studies examined in this review were that data were collected retrospectively over a long time period, and important information was not available including morbidity data, reasons for treatment selection and robust survival data. Publication of any significant case series of treatments other than surgery would enhance the available literature regarding the management of Paget's disease of the vulva. Prospective, collated data on imiquimod use would help improve the available literature, and give clinicians and women more robust evidence, when considering treatment modalities. We await the publication of The Paget Trial, which is a prospective multicentre study looking at outcomes of 5% imiquimod which was expected to report in 2020 (van der Linden 2017).
What's new
| Date | Event | Description |
|---|---|---|
| 24 June 2019 | Amended | PLs title amended. |
History
Protocol first published: Issue 7, 2011 Review first published: Issue 10, 2013
| Date | Event | Description |
|---|---|---|
| 23 May 2019 | New citation required but conclusions have not changed | Thirty‐five new studies identified but none of these met the inclusion criteria. One trial added to ongoing studies. |
| 23 May 2019 | New search has been performed | Search updated May 2018 |
| 11 February 2015 | Amended | Contact details updated. |
| 27 March 2014 | Amended | Contact details updated. |
Acknowledgements
We thank Jo Morrison for clinical expertise, Jo Platt for updating and running searches, and Gail Quinn, Clare Jess amd Tracey Harrison for their contribution to the editorial process. We are also very grateful for the helpful and detailed feedback given by the peer referees. We are also very grateful to George Smith for his many useful comments throughout the editorial process.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
MeSH descriptor Paget Disease, Extramammary, this term only
paget*
(#1 OR #2)
MeSH descriptor Vulva explode all trees
vulva*
(#4 OR #5)
(#3 AND #6)
Appendix 2. MEDLINE search strategy
Paget Disease Extramammary/
paget*.mp.
1 or 2
exp Vulva/
vulva*.mp.
4 or 5
3 and 6
key: mp=title, original title, abstract, name of substance word, subject heading word, unique identifier
Appendix 3. Embase search strategy
Paget skin disease/
paget*.mp.
1 or 2
vulva/
vulva*.mp.
4 or 5
3 and 6
key: mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bakalianou 2008 | No statistical adjustment to reduce the threat of selection bias. |
| Black 2007 | No statistical adjustment to reduce the threat of selection bias. |
| Cowan 2016 | Numbers too small for inclusion. |
| Crawford 1999 | No statistical adjustment to reduce the threat of selection bias. |
| Curtin 1990 | No statistical adjustment to reduce the threat of selection bias. |
| Fan 2016 | No statistical adjustment to reduce the threat of selection bias. |
| Fanning 1999 | No statistical adjustment to reduce the threat of selection bias. |
| Feuer 1990 | No statistical adjustment to reduce the threat of selection bias. |
| Fishman 1995 | No statistical adjustment to reduce the threat of selection bias. |
| Goldblum 1997 | No statistical adjustment to reduce the threat of selection bias. |
| Kim 2017 | No statistical adjustment to reduce the threat of selection bias. |
| Koss 1968 | A report of 10 cases, but on review of the paper, 2 participants had melanoma, therefore only 8 participants had Paget's disease. |
| Lee 1977 | No statistical adjustment to reduce the threat of selection bias. |
| Lee 2010 | No statistical adjustment to reduce the threat of selection bias. |
| Long 2017 | No statistical adjustment to reduce the threat of selection bias. |
| Louis‐Sylvestre 2001 | No statistical adjustment to reduce the threat of selection bias. |
| Machida 2015 | Systematic review |
| Martorell‐Calatayud 2009 | Was only a case report. |
| Molinie 1993 | No statistical adjustment to reduce the threat of selection bias. |
| Niikura 2006 | No statistical adjustment to reduce the threat of selection bias. |
| Parashurama 2017 | No statistical adjustment to reduce the threat of selection bias. |
| Parker 2000 | No statistical adjustment to reduce the threat of selection bias. |
| Parmley 1975 | Only comments on 7 participants with invasive Paget's disease, no data on the 10 non‐invasive so cohort too small. |
| Petkovic 2006 | No statistical adjustment to reduce the threat of selection bias. |
| Pierie 2003 | No statistical adjustment to reduce the threat of selection bias. |
| Roh 2010 | No statistical adjustment to reduce the threat of selection bias. |
| Sawada 2018 | No statistical adjustment to reduce the threat of selection bias. Numbers too small for inclusion. |
| Shaco‐Levy 2010 | No statistical adjustment to reduce the threat of selection bias. |
| Shingleton 1970 | Contained only 1 participant with biopsy‐confirmed Paget's disease. |
| Stacy 1986 | No statistical adjustment to reduce the threat of selection bias. |
| Strempel 1958 | Contained only 3 participants with biopsy‐confirmed Paget's disease. |
| Tanaka 2009 | Contained only 7 participants with biopsy‐confirmed Paget's disease |
| Tebes 2002 | No statistical adjustment to reduce the threat of selection bias. |
| Wang 2003 | Case report only of use of imiquimod 5%. |
| Zawislak 2004 | Case report only of photodynamic therapy. |
Characteristics of studies awaiting assessment [ordered by study ID]
Fontanelli 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Paper not available from any UK source. |
Lu 1999.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Paper not available from any UK source. |
Shrestha 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Paper not available from any UK source. |
Characteristics of ongoing studies [ordered by study ID]
van der Linden 2017.
| Trial name or title | Topical 5% imiquimod cream for vulva Paget's disease: clinical efficacy, safety and immunological response |
| Methods | Interventional open‐label Phase 3 trial |
| Participants | Women aged ≥ 18 years with non‐invasive vulval Paget's disease, primary or recurrence after earlier surgery. |
| Interventions | Imiquimod topical 5% imiquimod cream (Aldara) 3 times a week for 16 weeks Paracetamol Lidocaine in Vaseline ointment |
| Outcomes | Clinical response will be assessed by vulval examination and measurement and defined as complete remission, partial remission (decrease by ≥ 50% of total lesion size) or no remission. |
| Starting date | May 2015 |
| Contact information | Joanne A de Hullu, MD, PhD; University Medical Center Nijmegen, the Netherlands |
| Notes |
Differences between protocol and review
Results of retrospective studies and case reports are discussed in the Effects of interventions and Agreements and disagreements with other studies or reviews sections.
In updates of the review, we will employ the following methods.
Data extraction and management
We will use a specifically designed data extraction form to document data on characteristics of participants (inclusion criteria, age, comorbidity, previous treatment, number enrolled) and interventions (surgery, radiotherapy, photodynamic therapy, chemotherapy), risk of bias, duration of follow‐up and outcomes. Two review authors (KE, EA) will extract and document author, year of publication, journal, language and data.
For time to event data, we will extract the log of the hazard ratio (HR) and its standard error from trial reports; if these are not reported, we will attempt to estimate the log of the HR and its standard error using the methods of Parmar 1998.
For dichotomous outcomes (e.g. adverse events or deaths if it is not possible to use an HR), we will extract the number of women in each treatment arm who experienced the outcome of interest and the number of women assessed at endpoint, in order to estimate a risk ratio.
For continuous outcomes (e.g. QoL), we will extract the final value and standard deviation of the outcome of interest and the number of women assessed at endpoint in each treatment arm at the end of follow‐up, in order to estimate the mean difference between treatment arms and its standard error.
For non‐randomised studies (if applicable), we will extract adjusted statistics.
All data will be extracted using an intention‐to‐treat analysis, where possible and we will note the time points at which outcomes are collected and reported.
We will resolve differences between review authors by discussion or by appeal to a third review author (SC) if necessary.
Assessment of risk of bias in included studies
We will assess the risk of bias in included studies using Cochrane's 'Risk of bias' tool and the criteria specified in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a): this will include assessment of:
sequence generation;
allocation concealment;
-
blinding (of participants, healthcare providers and outcome assessors). Surgical assessment is unlikely to be blinded.
-
We will record the proportion of participants whose outcomes are not reported at the end of the study. We will code the satisfactory level of loss to follow‐up for each outcome as:
low risk of bias, if less than 20% of women were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms;
high risk of bias, if more than 20% of women were lost to follow‐up or reasons for loss to follow‐up differed between treatment arms;
unclear risk of bias, if loss to follow‐up was not reported;
-
selective reporting of outcomes;
other possible sources of bias.
Potential biases are likely to be greater for non‐randomised studies compared with randomised trials. We will assess the risk of bias in non‐randomised controlled trials in accordance with four additional questions and criteria.
Cohort selection
-
Were relevant details of criteria for assignment of women to treatments provided?
Yes (low risk of bias)
No (high risk of bias)
Unclear
-
Was the group of women who received the intervention (surgery, radiotherapy, photodynamic therapy, chemotherapy) representative?
Low risk of bias, if they were representative of women with Paget's disease of the vulva.
High risk of bias, if group of women was selected.
Unclear risk of bias, if selection of group was not described.
-
Was the group of women who received the comparison intervention (surgery, radiotherapy, photodynamic therapy, chemotherapy) representative?
Low risk of bias, if drawn from the same population as the intervention group.
High risk of bias, if drawn from a different source.
Unclear risk of bias, if selection of group not described.
Comparability of treatment groups
-
Were there no differences between the two groups or differences controlled for, in particular with reference to age, histological grade, performance status, grade of operating surgeon (if direct comparison of surgical interventions)?
Yes, if at least two of these characteristics were reported and any reported differences were controlled for.
No, if the two groups differed and differences were not controlled for.
Unclear, if fewer than two of these characteristics were reported even if there were no other differences between the groups, and other characteristics had been controlled for.
Two review authors (KE, JM) will independently apply the 'Risk of bias' tool and resolve differences by discussion or by appeal to a third review author (SC or EA). We will present results in both a 'Risk of bias' graph and a 'Risk of bias' summary and interpret results of meta‐analyses in light of the findings with respect to risk of bias.
Measures of treatment effect
We will use the following measures of the effect of treatment.
For time to event data, such as disease‐free survival, we will use the HR, with 95% confidence interval (CI), if possible.
For dichotomous outcomes, we will use the risk ratio, with 95% CI.
For continuous outcomes, such as QoL scores, we will use the mean difference between treatment arms if all trials measured the outcome on the same scale, otherwise we will use the standardised mean differences, with 95% CI.
If adjusted results are available in RCTs, they will be preferred; otherwise, we will use unadjusted results. If we identify no RCTs, we will use adjusted results or exclude the study (see above).
Dealing with missing data
We will not impute missing outcome data for the primary outcome. If data are missing or only imputed data are reported, we will contact trial authors to request data on the outcomes only among participants who were assessed.
Assessment of heterogeneity
We will assess heterogeneity between studies by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials that could not be ascribed to sampling variation (Higgins 2003), and by a formal statistical test of the significance of the heterogeneity (Deeks 2001). If there is evidence of substantial heterogeneity, we will investigate and report the possible reasons for this.
Assessment of reporting biases
We will examine funnel plots corresponding to meta‐analysis of the primary outcome to assess the potential for small‐study effects such as publication bias. If these plots suggest that treatment effects may not be sampled from a symmetric distribution, as assumed by the random‐effects model, we will perform further meta‐analyses using fixed‐effect models.
Data synthesis
If sufficient, clinically similar studies are available, we will pool their results in meta‐analyses.
For time‐to‐event data, we will pool HRs using the generic inverse variance facility of Review Manager 5 (Review Manager 2012).
For any dichotomous outcomes, we will calculate the risk ratio for each study and then pool them.
For continuous outcomes, we will pool the mean differences between the treatment arms at the end of follow‐up if all trials measured the outcome on the same scale, otherwise we will pool standardised mean differences.
If any trials have multiple treatment groups, we will divide the 'shared' comparison group into the number of treatment groups and treat the comparisons between each treatment group and the split comparison group as independent comparisons.
We will use random‐effects models with inverse variance weighting for all meta‐analyses (DerSimonian 1986). If possible, we will synthesise studies making different comparisons using the methods of Bucher 1997.
Subgroup analysis and investigation of heterogeneity
In interpretation of any heterogeneity, we will consider factors such as age, cancer stage, type of intervention, length of follow‐up and adjusted/unadjusted analysis. We will not carry out any subgroup analyses a priori.
Sensitivity analysis
We will perform sensitivity analyses excluding studies at high risk of bias.
Summary of findings for assessing the certainty of the evidence
We will present the overall certainty of the evidence for each outcome according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which takes into account issues not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity such as directness of results (Langendam 2013; Schünemann 2011). We will create a 'Summary of findings' table based on the methods described the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and using GRADEPro GDT 2014. We will use the GRADE checklist and GRADE Working Group certainty of evidence definitions (Meader 2014). We will downgrade the evidence from 'high' certainty by one level for serious (or by two for very serious) concerns for each limitation:
Contributions of authors
KE: writing of protocol and review; assessment of literature for inclusion; analysis of papers; updated review February 2019. EA: paper analysis. JM: assessment of literature for inclusion. SC: review of protocol and review. AB: statistical support and assistance in writing of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Department of Health, UK.
NHS Cochrane Collaboration Programme Grant Scheme CPG‐10/4001/12
Declarations of interest
KE: none known. EA: none known. JM: none known. SC: none known. AB: none known.
Edited (no change to conclusions)
References
References to studies excluded from this review
Bakalianou 2008 {published data only}
- Bakalianou K, Salakos N, Iavazzo C, Paltoglou G, Papadias K, Gregoriou O, et al. Paget's disease of the vulva. A ten‐year experience. European Journal of Gynecologic Oncology 2008;29(4):368‐70. [PubMed] [Google Scholar]
Black 2007 {published data only}
- Black D, Tornos C, Soslow RA, Awtrey CS, Barakat RR, Chi DS. The outcomes of patients with positive margins after excision for intraepithelial Paget's disease of the vulva. Gynaecologic Oncology 2007;104:547‐50. [DOI] [PubMed] [Google Scholar]
Cowan 2016 {published data only}
- Cowan RA, Black DR, Hoang LN, Park KJ, Soslow RA, Backes FJ, et al. A pilot study of topical imiquimod therapy for the treatment of recurrent extramammary Paget's disease. Gynaecologic Oncology 2016;142(1):139‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crawford 1999 {published data only}
- Crawford D, Nimmo M, Clement PB, Thomson T, Benedet JL, Miller D, et al. Prognostic factors in Paget's disease of the vulva: a study of 21 cases. International Journal of Gynaecological Pathology 1999;18(4):351‐9. [DOI] [PubMed] [Google Scholar]
Curtin 1990 {published data only}
- Curtin JP, Rubin SC, Jones WB, Hoskins WJ, Lewis JL. Paget's disease of the vulva. Gynecologic Oncology 1990;39:374‐77. [DOI] [PubMed] [Google Scholar]
Fan 2016 {published data only}
- Fan L, Zhu J, Tao X, Xu C. Intraepithelial extramammary Paget's disease of the vulva; the clinicopathological characteristics, Management, and outcome in a study of 18 female patients. Dermatologic Surgery 2016;42(10):1142‐6. [DOI] [PubMed] [Google Scholar]
Fanning 1999 {published data only}
- Fanning JD, Lambert HC, Hale TM, Morris PC, Schuerch C. Paget's disease of the vulva: prevalence of associated vulvar adenocarcinoma, invasive Paget's disease and recurrence after surgical excision. American Journal of Obstetrics and Gynecology 1999;180:24‐7. [DOI] [PubMed] [Google Scholar]
Feuer 1990 {published data only}
- Feuer GA, Shevchuk M, Calanog A. Vulvar Paget's disease: the need to exclude an invasive lesion. Gynecologic Oncology 1990;38:81‐9. [DOI] [PubMed] [Google Scholar]
Fishman 1995 {published data only}
- Fishman DA, Chambers SK, Schwartz PE, Kohorn EI, Chambers JT. Extramammary Paget's disease of the vulva. Gynaecologic Oncology 1995;56:266‐70. [DOI] [PubMed] [Google Scholar]
Goldblum 1997 {published data only}
- Goldblum JR, Hart WR. Vulvar Paget's disease: a clinicopathologic and immunohistochemical study of 19 cases. American Journal of Surgical Pathology 1997;21(10):1178‐87. [DOI] [PubMed] [Google Scholar]
Kim 2017 {published data only}
- Kim SJ, Thompson AK, Zubair AS, Otley CC, Arpey CJ, Baum CL, et al. Surgical treatment and outcomes of patients with extramammary Paget's disease: a cohort study. Dermatologic Surgery 2017;43(5):708‐14. [DOI] [PubMed] [Google Scholar]
Koss 1968 {published data only}
- Koss LG, Ladinsky S, Brockunier A. Paget's disease of the vulva. Obstetrics and Gynaecology 1968;31(4):513‐25. [PubMed] [Google Scholar]
Lee 1977 {published data only}
- Lee SC, Roth LM, Ehrlich C, Hall JA. Extramammary Paget's disease of the vulva. Cancer 1977;39:2540‐9. [DOI] [PubMed] [Google Scholar]
Lee 2010 {published data only}
- Lee CW, Song MJ, Lee SH, Kim JH, Lee SJ, Lee KH, et al. Clinicopathologic analysis of extramammary Paget's disease. European Journal of Gynecological Oncology 2010;32(1):34‐6. [PubMed] [Google Scholar]
Long 2017 {published data only}
- Long B, Schmitt AR, Weaver AL, McGree M, Bakkum‐Gamez JN, Brewer J, et al. A matter of margins: surgical and pathologic risk factors for recurrence in extramammary Paget's disease. Gynecologic Oncology 2017;2:358‐63. [DOI] [PubMed] [Google Scholar]
Louis‐Sylvestre 2001 {published data only}
- Louis‐Sylvestre C, Haddad B, Paniel BJ. Paget's disease of the vulva‐results of different conservative treatments. European Journal of Obstetrics, Gynaecology and Reproductive Biology 2001;99:253‐5. [DOI] [PubMed] [Google Scholar]
Machida 2015 {published data only}
- Machida H, Moeini A, Roman LD, Matsuo K. Effects of imiquimod on vulva Paget's disease: a systematic review of literature. Gynecologic Oncology 2015;139(1):165‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martorell‐Calatayud 2009 {published data only}
- Martorell‐Calatayud A, Sanmartín‐Jiménez O, Escutia‐Muñoz B, Guillén‐Barona C. Imiquimod topical at 5% as therapeutic alternative in primary extramammary Paget's disease [Imiquimod tópico al 5% como alternativa terapéutica en la enfermedad de Paget extramamaria primaria]. Piel 2009;10:568‐9. [Google Scholar]
Molinie 1993 {published data only}
- Molinie V, Paniel BJ, Lessana‐Leibowitch M, Moyal‐Barracco M, Pelisse M, Escande JP. Paget disease of the vulva: 36 cases [Maladie de Paget vulvaire: 36 cas]. Annales de Dermatologie et de Vénéréologie 1993;120:522‐7. [PubMed] [Google Scholar]
Niikura 2006 {published data only}
- Niikura H, Yoshida H, Ito K, Takano T, Watanabe H, Aiba S, et al. Paget's disease of the vulva: clinicopathologic study of type 1 cases treated at a single institution. International Journal of Gynecological Cancer 2006;16:1212‐5. [DOI] [PubMed] [Google Scholar]
Parashurama 2017 {published data only}
- Parashurama R, Nama V, Hutson R. Paget's disease of the vulva: a review of 20 years' experience. International Journal of Gynecological Cancer 2017;4:791‐3. [DOI] [PubMed] [Google Scholar]
Parker 2000 {published data only}
- Parker LP, Parker JR, Bodurka‐Bevers D, Deavers M, Bevers MW, Shen‐Gunther J, et al. Paget's disease of the vulva: pathology, pattern of involvement, and prognosis. Gynaecologic Oncology 2000;77:183‐9. [DOI] [PubMed] [Google Scholar]
Parmley 1975 {published data only}
- Parmley TH, Woodruff JD, Julian CG. Invasive vulvar Paget's disease. Obstetrics and Gynaecology 1975;46(3):341‐6. [PubMed] [Google Scholar]
Petkovic 2006 {published data only}
- Petkovic S, Jeremic K, Vidakovic S, Jeremic J, Lazovic G. Paget's disease of the vulva – a review of our experience. European Journal of Gynaecological Oncology 2006;27(6):611‐2. [PubMed] [Google Scholar]
Pierie 2003 {published data only}
- Pierie JE, Choudry U, Muzikansky A, Finkelstein DM, Ott MJ. Prognosis and management of extramammary Paget's disease and the association with secondary malignancies. Journal of American College of Surgeons 2003;196:45‐50. [DOI] [PubMed] [Google Scholar]
Roh 2010 {published data only}
- Roh H‐J, Kim D‐Y, Kim J‐H, Kim Y‐M, Kim Y‐K, Nam J‐H. Paget's disease of the vulva: evaluation of recurrence relative to symptom duration, volumetric excision of lesion, and surgical margin status. Acta Obstetricia et Gynaecologica 2010;89:962‐5. [DOI] [PubMed] [Google Scholar]
Sawada 2018 {published data only}
- Sawada M, Kato J, Yamashita T, Yoneta A, Hida T, Horimoto K, et al. Imiquimod 5% cream as a therapeutic option for extramammary Paget's disease. Journal of Dermatology 2018;45(2):216‐9. [DOI] [PubMed] [Google Scholar]
Shaco‐Levy 2010 {published data only}
- Shaco‐Levy R, Bean SM, Vollmer RT, Jewell E, Jones EL, Valdes CL, et al. Paget disease of the vulva: a study of 56 cases. European Journal of Obstetrics & Gynecology and Reproductive Biology 2010;149:86‐91. [DOI] [PubMed] [Google Scholar]
- Shaco‐Levy R, Bean SM, Vollmer RT, Papalas JA, Bentley RC, Selim MA, et al. Paget disease of the vulva: a histologic study of 56 cases corelating pathologic features and disease course. International Journal of Gynecological Pathology 2010;29(1):69‐78. [DOI] [PubMed] [Google Scholar]
Shingleton 1970 {published data only}
- Shingleton HM, Fowler WC, Palumbo L, Koch GG. Carcinoma of the vulva, influence of radical operation on cure. Obstetrics and Gynaecology 1970;35(1):1‐6. [PubMed] [Google Scholar]
Stacy 1986 {published data only}
- Stacy D, Burrell MO, Franklin EW. Extramammary Paget's disease of the vulva and anus: use of intraoperative frozen‐section margins. American Journal of Obstetrics and Gynecology 1986;155:519‐23. [DOI] [PubMed] [Google Scholar]
Strempel 1958 {published data only}
- Strempel R. Study on Paget's disease of the vulva [Beitrag zum morbus paget der vulva]. Dermatologische Wochenschrif 1958;138(46):1241‐52. [PubMed] [Google Scholar]
Tanaka 2009 {published data only}
- Tanaka VD, Sanches JA, Torezan L, Niwa AB, Neto CF. Mammary and extramammary Paget's disease: a study of 14 cases and therapeutic difficulties. Clinics (Sao Paolo) 2009;64(6):599‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tebes 2002 {published data only}
- Tebes S, Cardosi R, Hoffman M. Paget's disease of the vulva. American Journal of Obstetrics and Gynecology 2002;1887:281‐4. [DOI] [PubMed] [Google Scholar]
Wang 2003 {published data only}
- Wang LC, Blanchard A, Judge DE, Lorincz AA, Medenica MM, Busbey S. Successful treatment of recurrent extramammary Paget's disease of the vulva with topical imiquimod 5% cream. Journal of the American Academy of Dermatology 2003;49(4):769‐72. [DOI] [PubMed] [Google Scholar]
Zawislak 2004 {published data only}
- Zawislak AA, McCarron PA, McCluggae WG, Price JH, Donnelly RF, McClelland HR, et al. Successful photodynamic therapy of vulval Paget's disease using a novel patch‐based delivery system containing 5‐aminolevulinic acid. British Journal of Obstetrics and Gynaecology 2004;111:1143‐5. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Fontanelli 2013 {published data only}
- Fontanelli R, Papadia A, Martinelli F, Lorusso D, Grijuela B, Merola M, et al. Photodynamic therapy with M‐ALA as non surgical treatment option in patients with primary extramammary Paget's disease. Journal of Clinical Oncology 2013;130:90‐4. [DOI] [PubMed] [Google Scholar]
Lu 1999 {published data only}
- Lu H, Zhu G. Clinicopathologic and immunohistochemical features of vulvar Paget diseases. Chinese Journal of Obstetrics and Gynaecology 1999;34(3):156‐8. [PubMed] [Google Scholar]
Shrestha 2010 {published data only}
- Shrestha E. Clinical analysis of 13 cases of vulvar Paget's disease. Journal of Chinese Clinical Medicine 2010;5(6):322‐5. [Google Scholar]
References to ongoing studies
van der Linden 2017 {published data only}
- Linden M, Meeuwis K, Hees C, Dorst E, Bulten J, Bosse T, et al. The Paget trial: a multicenter, observational cohort intervention study for clinical efficacy, safety, and immunological response of topical 5% imiquimod cream for vulvar Paget disease. JMIR Research Protocols 2017; Vol. 6, issue 9:e178. [DOI] [PMC free article] [PubMed]
Additional references
Bertozzi 2009
- Bertozzi S, Londero AP, Fruscalzo A, Marchesoni D, Lellé RJ. Paget disease of the vulva: resolution after local treatment with imiquimod – report of a case and review of the literature. Gynakol Geburtshilfliche Rundsch 2009;49(4):326‐30. [DOI] [PubMed] [Google Scholar]
Bucher 1997
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. [DOI] [PubMed] [Google Scholar]
CTCAE 2006
- CTCAE v3.0. Common Terminology Criteria for Adverse Events. ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf (29 September 2013).
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. Systematic Reviews in Health Care: Meta‐analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
GRADEPro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University, Hamilton (ON). GRADEpro Guideline Development Tool (GDT)[www.gradepro.org]. Version [insert date of use]. GRADE Working Group, McMaster University, Hamilton (ON), 2014.
Hatch 2008
- Hatch KD, Davis JR. Complete resolution of Paget disease of the vulva with imiquimod cream. Journal of Lower Genital Tract Disease 2008;12(2):90‐4. [DOI] [PubMed] [Google Scholar]
Heymann 1993
- Heymann WR. Extramammary Paget's disease. Clinical Dermatology 1993;11:83‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011a
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kanitakis 2007
- Kanitakis J. Mammary and extramammary Paget's disease. Journal of the European Academy of Dermatology and Venereology 2007;21(5):581‐90. [DOI] [PubMed] [Google Scholar]
Langendam 2013
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schunemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;23(2):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2010
- Li Q, Gao T, Jiao B, Qi X, Long H, Qiao H, et al. Long‐term follow‐up of in‐situ extramammary Paget's disease in Asian skin types IIII/V treated with photodynamic therapy. Acta Dermato‐Venereologica 2010;90:159‐64. [DOI] [PubMed] [Google Scholar]
Luk NM 2003
- Luk NM, Yu KH, Yeung WK, Choi CL, Teo ML. Extramammary Paget's disease: outcome of radiotherapy with curative intent. Clinical and Experimental Dermatology 2003;28(4):360‐3. [DOI] [PubMed] [Google Scholar]
Maclean 1995
- Maclean AB, Reid WM. Benign and premalignant disease of the vulva. British Journal of Obstetrics and Gynaecology 1995;102:359‐63. [DOI] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moreno‐Arias 2003
- Moreno‐Arias GA, Conill C, Sola‐Casas MA, Mascaro‐Galy JM, Grimalt R. Radiotherapy for in situ extramammary Paget disease of the vulva. Journal of Dermatological Treatment 2003;14(2):119‐23. [DOI] [PubMed] [Google Scholar]
Nardelli 2011
- Nardelli AA, Stafinski T, Menon D. Effectiveness of photodynamic therapy for mammary and extra‐mammary Paget's disease: a state of the science review. BMC Dermatology 2011;11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Preti 2003
- Preti M, Micheletti L, Massobrio M, Ansai S, Wilkinson EJ. Vulvar Paget disease: one century after first reported. Journal of Lower Genital Tract Disease 2003;7(2):122‐35. [DOI] [PubMed] [Google Scholar]
Raspagliesi 2006
- Raspagliesi F, Fontanelli R, Rossi G, Ditto A, Solima E, Hanozet F, et al. Photodynamic therapy using a methyl ester of 5‐aminolevulinic acid in recurrent Paget's disease of the vulva: a pilot study. Gynecologic Oncology 2006;103(2):581‐6. [DOI] [PubMed] [Google Scholar]
Review Manager 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Sendagorta 2010
- Sendagorta E, Herranz P, Feito M, Ramírez P, Floristán U, Feltes R, et al. Successful treatment of three cases of primary extramammary Paget's disease of the vulva with Imiquimod – proposal of a therapeutic schedule. Journal of the European Academy of Dermatology and Venereology 2010;24(4):490‐2. [DOI] [PubMed] [Google Scholar]
Shieh 2002
- Shieh S, Dee AS, Cheyney RT, Frawley NP, Zeitouni NC, Oseroff AR. Photodynamic therapy for the treatment of extramammary Paget's disease. British Journal of Dermatology 2002;146:1000‐5. [DOI] [PubMed] [Google Scholar]
Tonguc 2011
- Tonguc E, Gungor T, Var T, Ozat M, Sahin I, Sirvan L. Treatment of recurrent vulvar Paget disease with imiquimod cream: a case report and review of the literature. Archives of Gynecology and Obstetrics 2011;283(1):97‐101. [DOI] [PubMed] [Google Scholar]
Tsutsumida 2003
- Tsutsumida A, Yamamoto Y, Minakawa H, Yosida T, Kokubu I, Sugihara T. Indications for lymph node dissection in the treatment of extramammary Paget's disease. Dermatological Surgery 2003;29:21‐4. [DOI] [PubMed] [Google Scholar]
Valentine 1992
- Valentine BH, Arena B, Green E. Laser ablation of recurrent Paget's disease of vulva and perineum. Journal of Gynecologic Surgery 1992;8(1):21‐4. [DOI] [PubMed] [Google Scholar]
Wilkinson 2002
- Wilkinson EJ, Brown HM. Vulvar Paget disease of urothelial origin: a report of three cases and a proposed classification of vulvar Paget disease. Human Pathology 2002;33:549‐54. [DOI] [PubMed] [Google Scholar]
Woodmansee 2006
- Woodmansee C, Pillow J, Skinner RB Jr. The role of topical immune response modifiers in skin cancer. Drugs 2006;66(13):1657‐64. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Edey 2013
- Edey KA, Allan E, Murdoch JB, Cooper S, Bryant A. Interventions for the treatment of Paget's disease of the vulva. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD009245.pub2] [DOI] [PubMed] [Google Scholar]


