Abstract
Dominant mutations of Gata4, an essential cardiogenic transcription factor (TF), were known to cause outflow tract (OFT) defects in both human and mouse, but the underlying molecular mechanism was not clear. In this study, Gata4 haploinsufficiency in mice was found to result in OFT defects including double outlet right ventricle (DORV) and ventricular septum defects (VSDs). Gata4 was shown to be required for Hedgehog (Hh)-receiving progenitors within the second heart field (SHF) for normal OFT alignment. Restored cell proliferation in the SHF by knocking-down Pten failed to rescue OFT defects, suggesting that additional cell events under Gata4 regulation is important. SHF Hh-receiving cells failed to migrate properly into the proximal OFT cushion, which is associated with abnormal EMT and cell proliferation in Gata4 haploinsufficiency. The genetic interaction of Hh signaling and Gata4 is further demonstrated to be important for OFT development. Gata4 and Smo double heterozygotes displayed more severe OFT abnormalities including persistent truncus arteriosus (PTA). Restoration of Hedgehog signaling renormalized SHF cell proliferation and migration, and rescued OFT defects in Gata4 haploinsufficiency. In addition, there was enhanced Gata6 expression in the SHF of the Gata4 heterozygotes. The Gata4-responsive repressive sites were identified within 1kbp upstream of the transcription start site of Gata6 by both ChIP-qPCR and luciferase reporter assay. These results suggested a SHF regulatory network comprising of Gata4, Gata6 and Hh-signaling for OFT development.
Author summary
Gata4 is an important transcription factor that regulates the development of the heart. Human possessing a single copy of Gata4 mutation display congenital heart defects (CHD), including double outlet right ventricle (DORV). DORV is an alignment problem in which both the Aorta and Pulmonary Artery originate from the right ventricle, instead of originating from the left and the right ventricles, respectively. In this study, a Gata4 mutant mouse model was used to study how Gata4 mutations cause DORV. We showed that Gata4 is required in the cardiac precursor cells for the normal alignment of the great arteries. Although Gata4 mutations inhibit the rapid increase in the cardiac precursor cell numbers, resolving this problem does not recover the normal alignment of the great arteries. It indicates that there is a migratory issue of the cardiac precursor cells as they navigate to the great arteries during development. The study further showed that a specific molecular signaling, Hh-signaling and Gata6 are responsible to the Gata4 action in the cardiac precursor cells. Importantly, over-activation of the Hh-signaling pathways rescues the DORV in the Gata4 mutant embryos. This study provides a molecular model to explain the ontogeny of a subtype of CHD.
Introduction
Congenital Heart Defects (CHDs) occur in approximately 1% of live births [1] and are the most common serious birth defects in humans [2, 3]. Approximately one third of the CHDs involve malformations of the outflow tract (OFT), which leads to significant morbidity and mortality of children and adults [4]. Multiple OFT abnormalities involve the defective relationship between the Aorta and Pulmonary Artery to the underlying left and right ventricles. For example, double-outlet right ventricle (DORV) is an anomaly in which the Aorta and Pulmonary Artery originate from the right ventricle [4]. A key characteristic of DORV that distinguishes it from other OFT defects is that the aorta and pulmonary trunk are well separated but are improperly aligned over the right ventricle. The molecular basis of OFT misalignment has remained unclear.
SHF-derived cells migrate into the developing poles of the heart tube, to direct the morphogenesis of the cardiac inflow and outflow. The anterior SHF (aSHF) is essential for OFT and great artery development [5–9]. The failure of the aSHF-derived myocardial and endocardial contributions to the arterial pole of the heart causes a shortened OFT and arterial pole misalignment, resulting in inappropriate connections of the great arteries to the ventricular mass [10–12]. Deletion of genes responsible for SHF morphogenesis, such as Isl1, Mef2c, and Jagged1, leads to abnormal OFT formation including DORV [5, 6, 12–19]. These observations lay the groundwork for investigating the molecular pathways required for OFT development in SHF cardiac progenitor cells.
Gata4, a member of the GATA family of zinc finger transcription factors, is an essential cardiogenic transcriptional regulator implicated in many aspects of cardiac development and function [13–27]. Human genetic studies have implicated haploinsufficiency of GATA4 in human CHDs, such as atrial septal defects (ASD), ventral septal defects (VSD), and tetralogy of Fallot (TOF) [17, 28–32]. In mouse models, decreased expression of Gata4 results in the development of common atrioventricular canal (CAVC), DORV, and hypoplastic ventricular myocardium in a large proportion of mouse embryos [20, 33]. Multiple studies have demonstrated the molecular requirement of Gata4 in the endocardium for normal cardiac valve formation [15, 23, 34]. Furthermore, our previous study demonstrated that Gata4 is required in the posterior SHF (pSHF) for atrial septation. Both Hedgehog (Hh) signaling and Pten-mediated cell-cycle progression were shown to be downstream of Gata4 in atrial septation [35]. However, the mechanistic requirement for Gata4 in OFT development was not clear and seemed different as in atrial septation. For example, from the multiple Gata4 transcriptional targets that have been identified in the context of heart development, including Nppa, α-MHC, α-CA, B-type natriuretic peptide (BNP), Ccnd2, and Cyclin D2, Gli1, and Mef2c [13, 15, 16, 18, 36–38], only Mef2c has a known functional role in OFT development [12].
In this study, the mechanistic requirement for Gata4 in OFT development was investigated. Gata4-dependent pathways were revealed to be contributors to OFT development in Gata4 heterozygous mouse embryos.
Results
Gata4 is required for OFT alignment
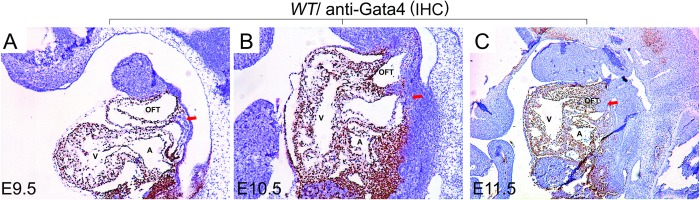
Gata4 is strongly expressed in the heart, pSHF and OFT at E9.5 [20, 35, 39]. There is a gap in expression between the OFT and the pSHF at embryonic day 9.5 (Fig 1A, indicated by a “↓”). IHC staining for Gata4 at later stages during OFT development showed strong Gata4 expression in the heart, the developing OFT and the pSHF, but only in a limited subset of aSHF cells at E10.5 (Fig 1B, indicated by a “↓”). At E11.5, both the chamber myocardium and the developing OFT had strong Gata4 expression. However, Gata4 expression was absent from the cardiac neural crest (CNC)-derived distal OFT (Fig 1C, indicated by a “↓”).
Fig 1. Gata4 is strongly expressed in the developing heart, the OFT and the pSHF.
Gata4 expression was detected in wildtype mouse embryos by IHC at (A) E9.5, (B) E10.5 and (C) E11.5. Red arrows indicate anterior second heart field at E9.5 or E10.5 (A and B), and distal outflow tract at E11.5 (C). OFT: out flow tract; A: atrium; V: ventricle. Magnification: A, B and C: 100X.
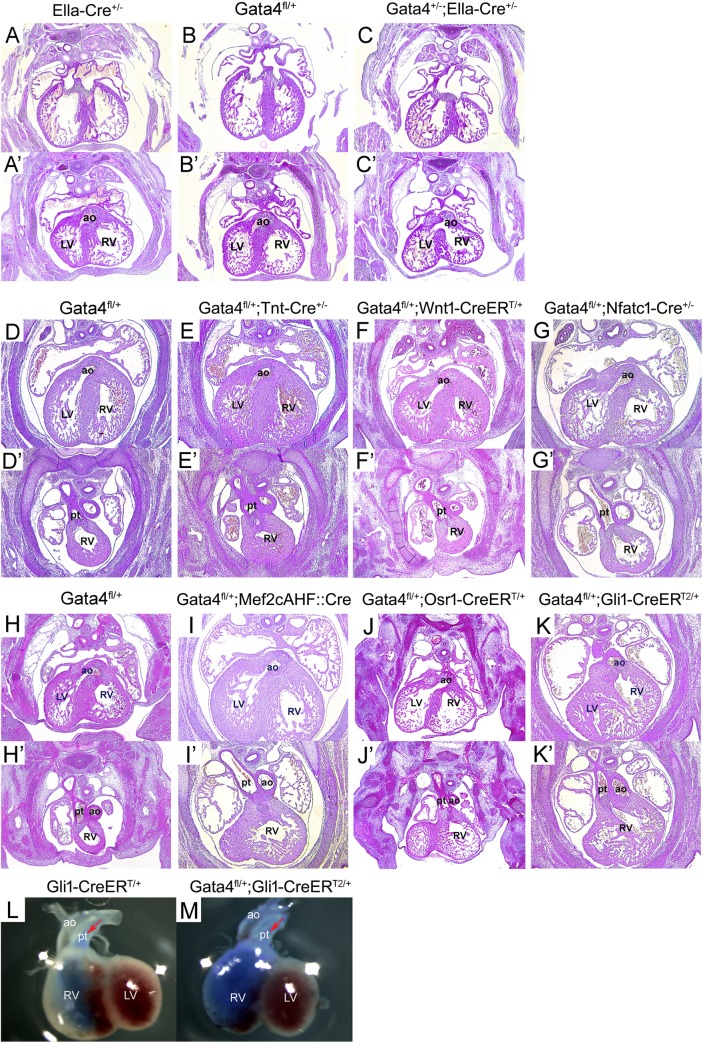
Gata4 was previously reported to be required for OFT alignment [20]. To study the role of Gata4 in OFT development, Gata4 heterozygotes were examined for OFT defects. As described previously [35], Gata4 heterozygotes were generated by crossing Gata4fl/+ with Ella-Cre+/-, which drives Cre expression in the germline [40] to produce the germline Gata4 deletion. The Gata4 germline deletion was ensured by genotyping using the embryonic tail DNA (Fig 1S). Whereas Gata4fl/+ (n = 13) and EllaCre/+ (n = 12) embryos demonstrated normal heart at E14.5 (Fig 2A and 2A’, 2B and 2B’), 61.1% of Gata4+/-; Ella-Cre+/-embryos demonstrated VSD and DORV (Fig 2C’, 11/18, P = 0.0004). Consistent with prior work, primum ASDs with an absence of the DMP observed in 8 out of 18 Gata4+/; Ella-Cre+/-embryos [35] (Fig 2C). In addition, 37.5% of these embryos displayed A-V cushion defects (3 out of 8, Fig 2C vs. 2A) and 62.5% expressed right ventricle hypoplasia (possibly right ventricle non-compaction) (5 out of 8, Fig 2C vs. 2A).To determine the lineage requirement for Gata4 in AV septation, we analyzed mouse embryos haploinsufficient for Gata4 in the myocardium, CNC, endocardium or SHF (Fig 1S). We combined Tnt: Cre [41] with Gata4fl/+ to create Gata4 haploinsufficiency in the myocardium. Normal OFT alignment was observed in all Tnt-Cre+/-; Gata4fl/+ (12/12) and littermate control Gata4fl/+ embryos (9/9) at E14.5 (P = 1) (Fig 2E and 2E’ vs. 2D and 2D’, P = 1). Wnt1: CreERT/+ and Gata4fl/+ was combined to create Gata4 haploinsufficiency in the CNC induced by tamoxifen (TMX) administration at E8.5 and E9.5 [42, 43]. Normal OFT alignment was observed in all Wnt1-CreERT/+; Gata4fl/+ mutant embryos (24/24) and littermate control Gata4fl/+ embryos (16/16) at E14.5 (Fig 2F and 2F’ vs. 2D and 2D’, P = 1). Nfat1c: Cre [42, 43] and Gata4fl/+ were combined to create Gata4 haploinsufficiency in the endocardium. Normal OFT alignment was observed in 93.3% of the Nfatc1-Cre+/-; Gata4fl/+ mutant embryos (14/15) and all of littermate control Gata4fl/+ embryos (10/10) at E14.5 (Fig 2G and 2G’ vs. 2D and 2D’, P = 1). All embryos are viable at E14.5 and no other heart defects such as the atrial septal defects (ASDs), ventricle septal defects (VSDs), or malformations of the ventricular wall, were observed at this stage. These results demonstrated that Gata4 haploinsufficiency in the myocardium, CNC or endocardium did not contribute to abnormal OFT alignment.
Fig 2. Gata4 is required in Hh-receiving cells for OFT development.
(A-K’) Histology of Gata4 transgenic mouse embryonic heart at E14.5. The lower panels (A’-C’) and upper panels (D-K) showed that the opening of the AO connected to the right ventricle in the Gata4 haploinsufficency, while in control embryos the AO opened to the left ventricle. LV, left ventricle; RV, right ventricle; ao, aorta artery; pt, pulmonary trunk. Magnification: 40X (L-M) Blue ink stained embryo heart at E14.5. LV, left ventricle; RV, right ventricle; ao, aorta artery, pt, pulmonary trunk. Red arrow indicates the ink injected direction.
Gata4 is required in the SHF Hedgehog (Hh) signal-receiving progenitors for OFT alignment
We hypothesized that Gata4 is required in the aSHF for OFT alignment in aSHF-specific Gata4 heterozygous mice. This hypothesis was tested by combining Mef2cAHF: Cre with Gata4fl/+. Surprisingly, OFT misalignment with DORV was only observed in 1 out of 22 embryos and none of the littermate controls (Fig 2I and 2I’ vs. 2H and 2H’, P = 1). We next tested if Gata4 is required in the pSHF for OFT alignment in in pSHF-specific Gata4 heterozygous mice by crossing Osr1-CreERT/+ with Gata4fl/+ [44, 45]. CreERT was activated by TMX administration at E7.5 and E8.5 in Osr1-CreERT/+; Gata4fl/+ embryos to results in pSHF Gata4 happloinsufficiency [44]. Similarly, neither Gata4fl/+;Osr1-CreERT/+ embryos (0/5) nor littermate control Gata4fl/+ embryos (0/6) demonstrated OFT misalignments at E14.5 (Fig 2J and 2J’ vs. 2H and 2H’, P = 1). However, right ventricular hypoplasia was observed in 5 out of 8 embryos (62.5%, Fig 2J and 2J’ vs. 2H and 2H’). Nonetheless, these results demonstrated that Gata4 haploinsufficiency in either aSHF or pSHF supported normal OFT alignment.
Previous studies have shown that Hh signal-receiving progenitors localized in both the aSHF and the pSHF, and regulate the migration of SHF toward the OFT and inflow tract (IFT) to form the pulmonary artery and the atrial septum respectively [46–48]. We combined Gli1-CreERT2/+ with Gata4fl/+ to create Gata4 haploinsufficiency in SHF Hh signal-receiving progenitors. CreERT2 was activated by TMX administration at E7.5 and E8.5 in Gli1-CreERT2/+; Gata4fl/+ embryos. The reduced expression of Gata4 by the deletion of Gli1-CreERT2 recombination was confirmed by realtime-PCR using the SHF tissue of E9.5 embryos (Fig 1S–1G). With TMX administration at E7.5 and E8.5, 66.7% of Gli1-CreERT2/+; Gata4fl/+ embryos displayed DORV, while the littermate control Gata4fl/+ embryos displayed normal OFT alignment (Fig 2K and 2K’ vs. 2H, 2H’, 8/12 vs. 0/15, P = 0.0002). Blue ink was injected in the pulmonary artery of the Gata4fl/+ E14.5 embryos, resulting in the staining of the pulmonary artery and the right ventricle. However, the Gli1-CreERT2/+; Gata4fl/+ embryos showed staining in not only the right ventricle and the pulmonary artery, but also the aortic artery, which confirms the phenotype of DORV in these embryos (Fig 2L and 2M). In addition, when the embryos were given TMX at E8.5 and E9.5, normal OFT alignment was observed in all Gli1-CreERT2/+; Gata4fl/+ embryos (Table 1). To exclude the possibility that the phenotype might be due to the double heterozygosity for Gata4 and Gli1, the phenotype of the Gli1-CreERT2/+; Gata4fl/+ embryos without TMX treatment was examined. There were no heart defects observed in the embryos at E14.5 (Table 1). Considering that TMX activates expression 12 h after injection and that the action lasts for 36 hours [49, 50], we concluded that Gata4 is required in the SHF Hedgehog (Hh) signal-receiving progenitors from E8 to E10.5 for proper OFT alignment.
Table 1. Incidence of OFT defect in Gata4 mutant embryos.
| Genotype | OFT defect | Total | Type | vs. control | p value |
|---|---|---|---|---|---|
| Conditional Gata4 mutant embryos | |||||
| Gata4+/-;EIIa-Cre+/- | 11 | 18 | DORV, OA | Gata4fl/+ (0/13) | 0.0004 |
| Gata4fl/+;Tnt-Cre+/- | 0 | 12 | —— | Gata4fl/+ (0/9) | 1 |
| Gata4fl/+; Mef2cAHF::Cre | 1 | 22 | —— | Gata4fl/+ (0/15) | 1 |
| 1Gata4fl/+; Wnt1-CreERT/+ | 0 | 24 | —— | Gata4fl/+ (0/16) | 1 |
| 1Gata4fl/+;Osr1GCE/+ | 0 | 5 | —— | Gata4fl/+ (0/6) | 1 |
| Gata4fl/+;Nfatc1-Cre+/- | 1 | 15 | DORV | Gata4fl/+ (0/10) | 1 |
| 1Gata4fl/+;Gli1-CreERT2/+ | 8 | 12 | DORV, OA | Gata4fl/+ (0/15) | 0.0002 |
| 2Gata4fl/+;Gli1-CreERT2/+ | 0 | 9 | —— | Gata4fl/+ (0/9) | 1 |
| 3Gata4fl/+;Gli1-CreERT2/+ | 0 | 7 | —— | Gata4fl/+ (0/9) | 1 |
| 1Ptenfl/+;Gli1-CreERT2/+ | 1 | 20 | DORV | Ptenfl/+ (0/7) | 1 |
| 1SmoM2fl/+;Gli1-CreERT2/+ | 2 | 7 | DORV | SmoM2fl/+ (0/7) | 0.5677 |
| Pten—Gata4 compound mutant embryos | |||||
| 1Gata4fl/+;Ptenfl/+; Gli1-CreERT2/+ | 6 | 20 | DORV |
Ptenfl/+;Gli1-CreERT2/+(1/20) Gata4fl/+;Gli1-CreERT2/+(12/29) |
0.0915 0.5495 |
| Smo—Gata4 compound mutant embryos | |||||
| 1Gata4fl/+;Smofl/+; Gli1-CreERT2/+ | 5 | 9 | DORV, OA, PTA |
Smofl/+;Gli1cre/+ (0/7) Gata4fl/+;Gli1-CreERT2/+(4/6) |
0.0337 1 |
| 1Gata4fl/+;SmoM2fl/+;Gli1-CreERT2/+ | 0 | 9 | —— |
SmoM2fl/+;Gli1-CreERT2/+(2/7) Gata4fl/+;Gli1-CreERT2/+(7/12) |
0.1750 0.0071 |
| Gata4+/-;Smo+/- | 5 | 7 | DORV, OA |
Gata4+/- (1/5) Smo+/- (0/4) |
0.2424 0.0606 |
NOTE
1 TMX E7.5+8.5
2 TMX E8.5+9.5
3 No treatment
Rescue of SHF proliferation by disruption of Pten does not rescue DORV in Gata4 mutant embryos
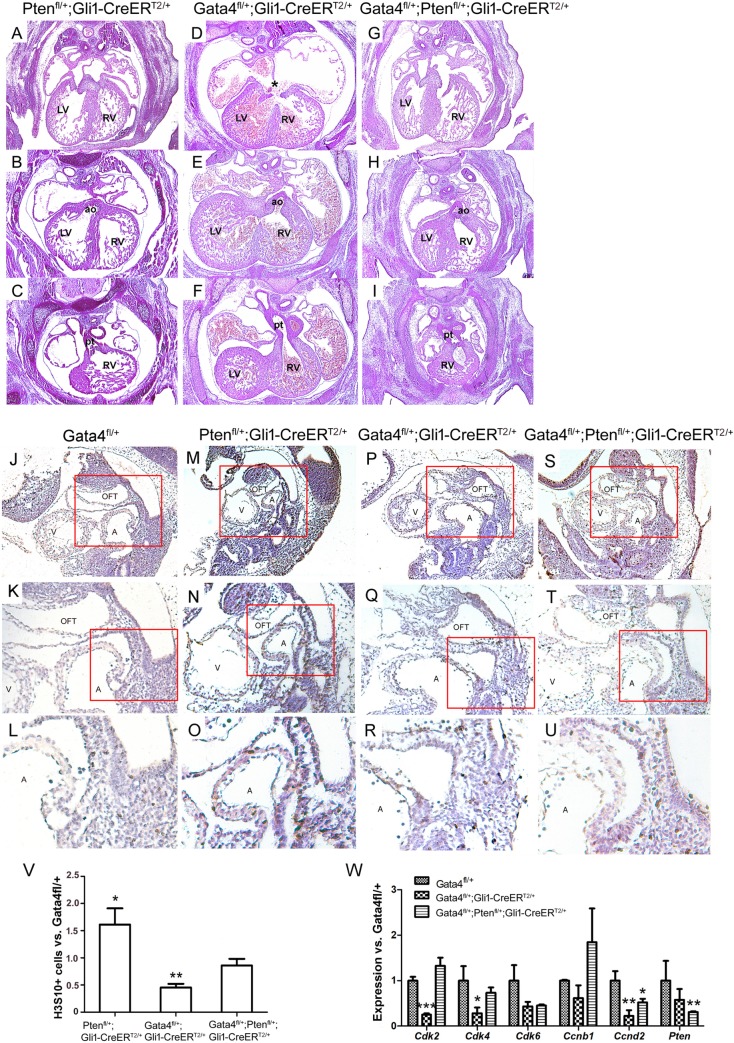
Our previous study demonstrated that Gata4 mutants disrupted cell cycle progression in the pSHF cardiac precursors resulting in atrial septal defects and genetically targeted downregulation of Pten rescued the proliferation defects in SHF of the Gata4 heterozygotes [35]. Would the defected cell cycle by Gata4 mutants lead to OFT alignment defects? In order to answer this question, the analysis was conducted to qualify if Pten downregulation (TMX at E7.5 and E8.5), could also rescue DORV in Hh-receiving cell-specific Gata4 heterozygotes. Decreased dosage of Pten caused DORV in only 1 of the 20 embryos, and none with ASD (Fig 3G, 3H and 3I). Consistent with the previous report, ASD in Gli1-CreERT2/+; Gata4fl/+ embryos was rescued by Pten downregulation (Fig 3G vs. 3D, 1/20 in Gli1-CreERT2/+; Gata4fl/+;Ptenfl/+ vs. 14/29 in Gli1Cre-ERT2/+;Gata4fl/+, P = 0.0013), but the Gli1-CreERT2/+; Gata4fl/+;Ptenfl/+ embryos displayed DORV, consistent with the incidence rate from Gli1-CreERT2/+; Gata4fl/+ embryos (Fig 3H vs. 3E, 12/29 vs. 6/20, Table 1, P = 0.5495). We next performed immunohistochemical (IHC) staining for H3S10 phosphorylation to assess the cell proliferation in the SHF at E10.5. This showed a significantly less percentile of H3S10+ cells in the SHF of the Gli1-CreERT2/+; Gata4fl/+ embryos versus the Gata4fl/+ (Fig 3R vs. 3L and Fig 3V, P = 0.013), suggesting a proliferation defect. However, this proliferation defect was restored in the Gli1-CreERT2/+; Gata4fl/+; Ptenfl/+ embryos (Fig 3U vs. 3L and Fig 3V, P = 0.500 vs. Gata4fl/+; P = 0.062 vs. Gli1-CreERT2/+; Ptenfl/+). Consistently, expression of the cell proliferation genes including Cdk2, Cdk4 and Ccnd2 was lower in the Gli1-CreERT2/+; Gata4fl/+ embryos but was restored to normal levels with a Pten knockdown (Fig 3W). This data suggested that correction of the SHF proliferation defects failed to rescue the OFT misalignment of the Gata4 mutant embryos, and thus different mechanisms were involved in the regulations of Gata4 for atrial septal and OFT.
Fig 3. Genetically targeted ablation of Pten rescues atrioventricular septal defect.
(A-I) Histology of Gata4 transgenic mouse embryonic hearts at E14.5. Two panel images of each embryonic heart were used to show the DORV phenotypes. The panels B-H showed that the opening of AO connected to the right ventricle in the Gata4 haploinsufficency (E and H), while in control embryos the AO opened to the left ventricle (B). The lower panel C-I showed the opening of PT connecting to the right ventricle. LV, left ventricle; RV, right ventricle; ao, aorta artery; pt, pulmonary trunk. Magnification: 40X. (J-U) H3S10 expression was detected in Gata4 transgenic mouse embryos by IHC at E9.5. Red rectangle area was amplified below. Magnification for panels J, M, P and S is 100X. Magnification for panels K, N, Q and T is 200X. Magnification for panels L, O, R and U is 400X. (V) Quantification of H3S10 labelled cells. Data is expressed as ratio to Gata4fl/+. Data is presented as Mean±SEM, **p<0.05, ***p<0.01, n = 4, compared with the Gata4fl/+ embryos. (W) Gene expression in the aSHF of Gata4fl/+, Gata4fl/+;Gli1-CreERT2/+,or Gata4fl/+;Ptenfl/+;Gli1-CreERT2/+ embryos was measured by real-time PCR. Data is presented as Mean±SEM, n = 4, *P < 0.1, **P < 0.05 and ***P < 0.01 compared with the expression in Gata4fl/+ embryos.
Gata4 acts upstream of Hh signaling in OFT development
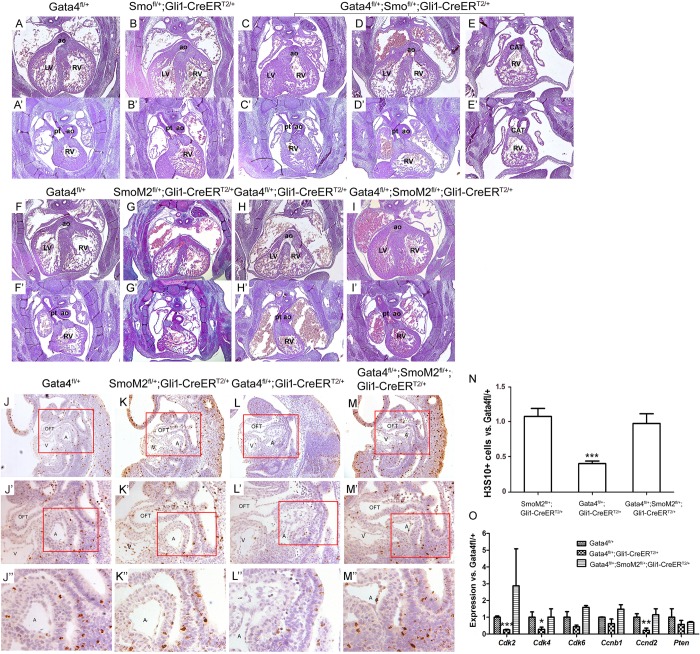
We have previously reported that Gata4 acts upstream of Hh-signaling for atrial septation [35]. The requirement of Gata4 in Hh-receiving cells for OFT alignment suggested that Gata4 and Hh signaling might interact genetically in the SHF for OFT development. This hypothesis was tested in the Gata4 and Smo compound heterozygotes (Gli1-CreERT2/+; Gata4fl/+; Smofl/+) versus the littermate controls (Gli1-CreERT2/+; Gata4fl/+; or Gli1-CreERT2/+; Smofl/+) both induced by TMX administration at E7.5 and E8.5. Consistent OFT defects were observed in compound Gata4; Smo haploinsufficient embryos (Gli1-CreERT2/+; Gata4fl/+; Smofl/+) (5/9, Fig 4C–4E) whereas no OFT defects were observed in Gli1-CreERT2/+; Smofl/+ embryos (0/7, Fig 4B and 4B’; P = 0.0337). The total incidence of OFT defects occurred in the Gli1-CreERT2/+; Gata4fl/+; Smofl/+was not different from in the Gli1-CreERT2/+; Gata4fl/+ embryos (Fig 4C–4E, 5/9 vs. 4/6, P = 0.7326). However, a larger range of OFT defects was observed in Gli1-CreERT2/+; Gata4fl/+; Smofl/+ embryos, including DORV (3 out of 5, Fig 4C and 4C’), OA (1 out of 5, Fig 4D and 4D’) and persistent truncus arteriosus (PTA) (1 out of 5, Fig 4E and 4E’). PTA, caused by a combined defect of alignment and separation, was only observed in Gli1-CreERT2/+; Gata4fl/+; Smofl/+. This result suggested an interaction between Gata4 and Hh-signaling in OFT development.
Fig 4. Gata4 acts upstream of Hh signaling pathway.
(A-I’) Histology of Gata4 transgenic mouse embryo heart at E14.5. Two panel images of each embryonic heart were used to show the DORV phenotypes. The upper panels showed that the opening of AO connected to the right ventricle (C, DORV), or override on the ventricular septum (D and H, OA), or shared a common trunk with the PT which opens to the right ventricle (E, CAT or PTA) in the Gata4 haploinsufficency, while in control embryos the AO opened to the left ventricle (A, B, F, G and I). The lower panel showed the opening of PT connecting to the right ventricle (A’-I’). LV, left ventricle; RV, right ventricle; ao, aorta artery, pt, pulmonary trunk; CAT, common artery trunk. Magnification: 40X. (J-M’) H3S10 expression was detected in Gata4 transgenic mouse embryos by IHC at E9.5. Red rectangle area was amplified below. (J-M) Magnification: 100X. (J’-M’) Magnification: 200X. (J”-M”) Magnification: 400X. (N) Quantification of H3S10 labelled cells. Data is expressed as ratio to Gata4fl/+. Data is presented as Mean±SEM, ***p<0.01, n = 3, compared with the Gata4fl/+ embryos. (O) Gene expression in the aSHF of Gata4fl/+, Gata4fl/+;Gli1-CreERT2/+ or Gata4fl/+;SmoM2fl/+;Gli1-CreERT2/+ embryos was measured by real-time PCR. Data is presented as Mean±SEM, n = 3, *P < 0.1, **P < 0.05, ***P < 0.01, compared with the expression in Gata4fl/+ embryos.
We tested the hypothesis that Gata4 acts upstream of Hh-signaling for OFT development using a genetic epistasis study. The purpose was to understand if increased Hh-signaling via a constitutively activated Smo mutant, SmoM2 [35], induced by TMX administration at E7.5 and E8.5, could rescue the OFT misalignment in Gata4-heterozygotes. DORV was observed in 28.6% of littermate control Gli1-CreERT2/+; SmoM2fl/+embryos (2/7) (Fig 4G and 4G’) and 58.3% of littermate control Gli1-CreERT2/+; Gata4fl/+ embryos at E14.5 (7/12) (Fig 4H and 4H’). In contrast, none of Gli1-CreERT2/+; Gata4fl/+; SmoM2fl/+ embryos showed DORV (Fig 4I and 4I’), indicating significant rescue by Gli1-CreERT2/+; SmoM2fl/+(Fig 4I vs Fig 4H, P = 0.0071, Table 1). This results demonstrated rescue of DORV in Gata4-mutant embryos by constitutive Hh signaling.
Next, IHC staining for H3S10 phosphorylation in the SHF was performed to determine if the cell proliferation defects observed in the Gli1-CreERT2/+; Gata4fl/+ were rescued by overactivation of Hh-signaling. Clearly, the cell proliferation defects, indicated by less percentile of H3S10+ cells, observed in the SHF of the Gli1-CreERT2/+; Gata4fl/+ (Fig 4L” vs. 4J” and Fig 4N, P<0.01 vs. Gata4fl/+) were recovered by Gli1-CreERT2/+; SmoM2fl/+ (Fig 4M” vs. 4J” and Fig 4N, P>0.05 vs. Gata4fl/+). Consistently, gene expression was downregulated for multiple cell proliferation genes including the Cdk2, Cdk4, Cdk6 and Ccnd2 in the SHF of Gli1-CreERT2/+; Gata4fl/+ comparing to the Gata4fl/+ embryos, which was recovered in the Gli1-CreERT2/+; Gata4fl/+; SmoM2fl/+ embryos (Fig 4O). These results suggested that overactivating the Hh-signaling rescued proliferation defects in the SHF of Gata4 haploinsufficiency.
Gata4 is required for the contribution of Hh-receiving cells to the OFT
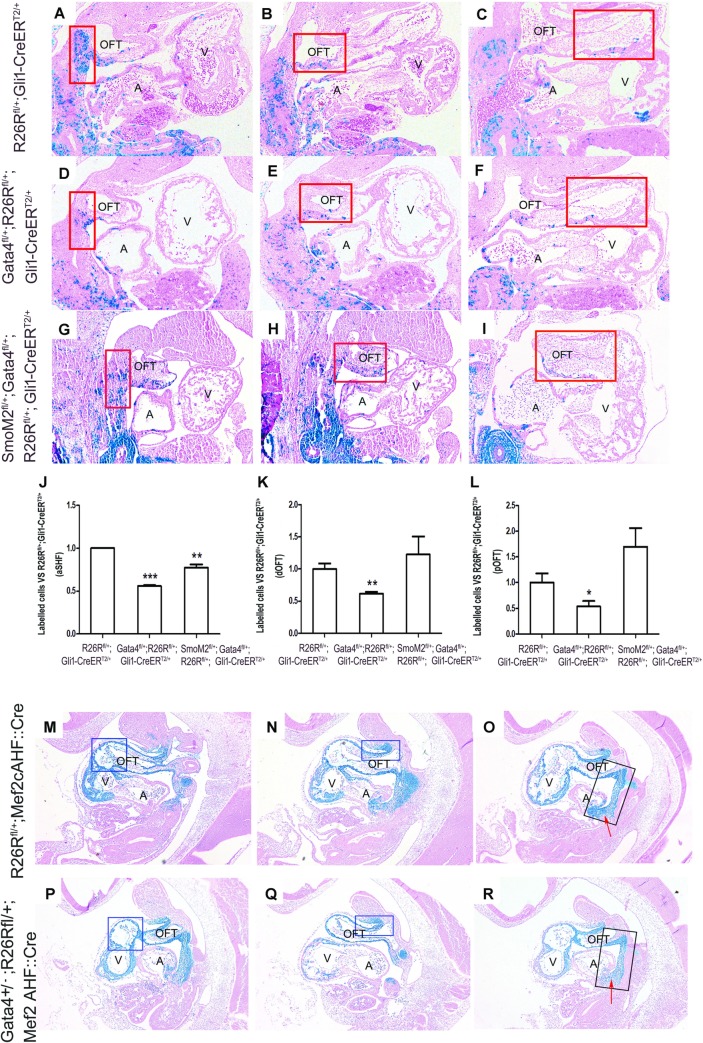
Hh signaling has been reported to regulate the migration of SHF Hh-receiving cells toward the arterial pole of the heart [46]. We therefore hypothesized that Gata4 drives SHF Hh-receiving cells migration toward the developing OFT. This hypothesis was tested by using genetic inducible fate mapping (GIFM) [51]. The Hh-receiving lineage cells were marked by TMX administration at E7.5 and E8.5 (Gli1-CreERT2/+; R26Rfl/+) and β-gal expression was evaluated at E11.5. We assessed if there was less migrating Hh-receiving SHF cells migrating through the distal OFT (dOFT) towards the proximal OFT (pOFT) in the Gata4 haploinsufficient embryos (Gli1-CreERT2/+; Gata4fl/+; R26Rfl/+) than the control embryos (Gli1-CreERT2/+; R26Rfl/+), and if this defects rescued in Gli1-CreERT2/+; R26Rfl/+; Gata4fl/+; SmoM2fl/+ embryos. Previous reports indicate a decreased number of Hh-receiving cells in the pSHF at E9.5 associated with developing defects of DMP in the Gata4fl/+; R26Rfl/+; Gli1Cre-ERT2/+embryos [35]. In concurrence, significantly less Hh-receiving cells within the aSHF region (Fig 5A vs. 5D and Fig 5J, 334.0 ± 1.4 vs. 186.7 ± 4.9, P = 0.001) of the Gli1-CreERT2/+; Gata4fl/+; R26Rfl/+ embryos was also observed. The cells of Hh-receiving lineage were analyzed in the developing OFT at this stage. By counting the number of β-galactosidase-expressing cells in the proximal half and the distal half of the OFT myocardium of the Gata4fl/+; R26Rfl/+ embryos, both of the regions of the Gli1-CreERT2/+; Gata4fl/+; R26Rfl/+ had less β-galactosidase-expressing cells than the littermate controls (Fig 5B vs. 5E and 5K, 91.7 ± 9.2 vs. 56.7 ± 1.4, P = 0.013 for dOFT; Fig 5C vs. 5F and 5L, 49.7 ± 10.6 vs. 26.7 ± 6.4, P = 0.091 for pOFT). Importantly, the lower number of Hh-receiving cells in the Gli1-CreERT2/+; Gata4fl/+ was partially recovered by overactivating the Hh-signaling in the aSHF (Fig 5A vs. 5G and 5J, Gli1-CreERT2/+; R26Rfl/+ vs. Gli1-CreERT2/+; R26Rfl/+; Gata4fl/+; SmoM2fl/+: 334.0 ± 1.4 vs. 258 ± 18.4, P = 0.028; Fig 5G vs. 5D and 5J, Gli1-CreERT2/+; R26Rfl/+; Gata4fl/+; SmoM2fl/+ vs. Gli1-CreERT2/+; R26Rfl/+: 258 ± 18.4 vs. 186.7 ± 4.9, P = 0.034). There was also complete restoration of the amount of β-galactosidase-expressing cells in the dOFT (Fig 5B vs. 5H and 5K, Gli1-CreERT2/+; R26Rfl/+ vs. Gli1-CreERT2/+; R26Rfl/+; Gata4fl/+; SmoM2fl/+: 91.7 ± 9.2 vs. 109.3 ± 19.8, P = 0.505) and the pOFT (Fig 5C vs. 5I and 5L, Gli1-CreERT2/+; R26Rfl/+ vs. Gli1-CreERT2/+; R26Rfl/+; Gata4fl/+; SmoM2fl/+: 49.7 ± 10.6 vs. 84.3 ± 15.7, P = 0.161).
Fig 5. Gata4 is required for the contribution of Hh-receiving cells to the OFT.
The embryos were given TMX at E7.5 and E8.5 and the β-galactosidase expression was evaluated at E11.5. (A-I) LacZ staining of Gli1-expressing cells in Gata4 transgenic mouse embryos at E11.5 focusing on aSHF (A, D and G), dOFT (B, E and H) and pOFT (C,F and I). Magnification: 100X (J-L) Quantification of stained cells within selected regions. Data is expressed as ratio to R26Rfl/+; Gli1-CreERT2/+. Data is presented as Mean±SE, *p<0.1, ** p<0.05 and *** p<0.01 vs. Gata4fl/+, n = 3–5, One-way ANOVA. (M-R) LacZ staining of cells with Mef2cAHF::Cre expression in Gata4 transgenic mouse embryos at E10.5. The red arrow indicated a developing DMP region. Magnification: 100X.
To examine if a Gata4 heterozygous influenced the SHF cell recruitment within the proximal OFT, we analyzed the fate map of SHF lineage cells in the OFT of the Gata4 heterozygotes. Defined by Mef2cAHF::Cre driven β-galactosidase-expressing cells, the total number of the SHF lineage cells within the proximal and distal half of the OFT were compared between the Mef2cAHF::Cre; R26Rfl/+;Gata4+/-; and the Mef2cAHF::Cre;R24Rfl/+ embryos at E10.5. The number of SHF lineage cells populating the pOFT of the Mef2cAHF::Cre;Gata4+/-; R26Rfl/+ embryos was significantly less than those in the control Mef2cAHF::Cre; R26Rfl/+ embryos (Fig 5M vs. 5P); however, this decrement was not observed in the distal OFT (Fig 5N vs. 5Q). The distribution pattern of the SHF lineage was not different in the Mef2cAHF::Cre;Gata4+/-; R26Rfl/+ and the Mef2cAHF::Cre;R26Rfl/+embryos (Fig 5O vs. 5R). Fewer cells were observed to populate the developing dorsal mesocardium protrusion (DMP) in Mef2cAHF::Cre; Gata4+/-; R26Rfl/+(red arrow, Fig 5O vs. 5R). This was consistent with the previous report that Gata4 is required in the SHF for the DMP [35]. These results demonstrated the requirement of Gata4 for the SHF lineage cells populating in the developing OFT.
Gata4 is involved in the endothelial-to-mesenchymal transformation (EMT) and mesenchymal cell proliferation for OFT cushion development
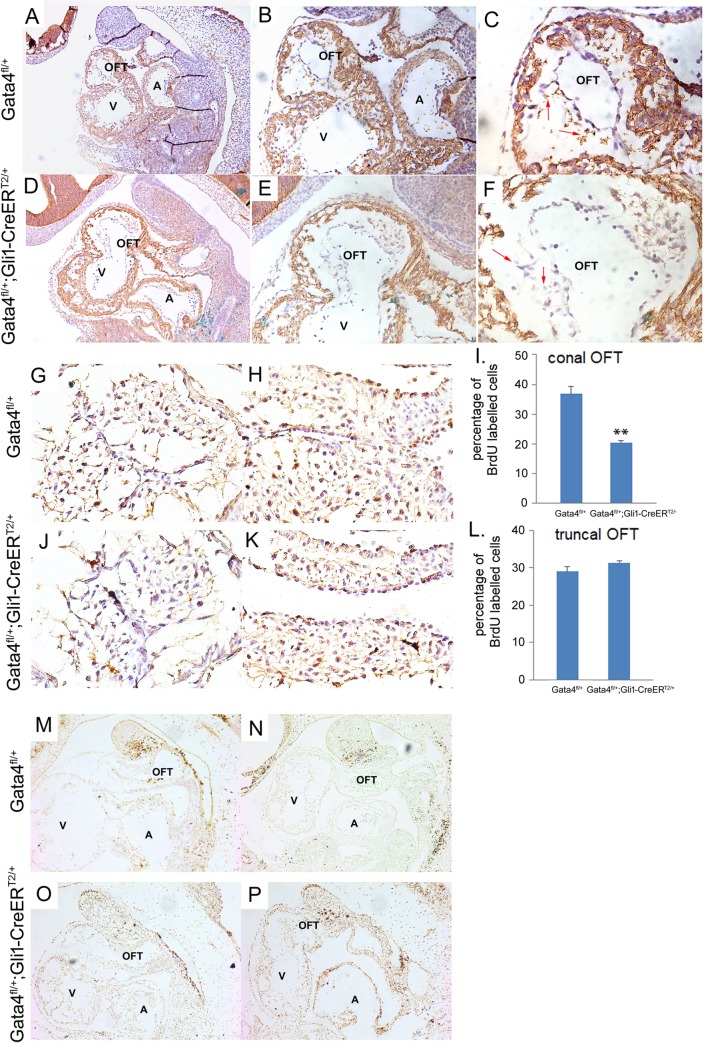
The role of Gata4 in the EMT for the endocardial cushion development has been well described previously [20, 23, 52]. Since less SHF lineage cells populating in the developing OFT myocardium were observed, we asked if this defect would affect the sequential events such as EMT, cell proliferation or cell survival in the developing OFT via a non-cell autonomous manner. Expression of mesenchymal marker N-cadherin was used to label the cushion cells undergoing EMT. LacZ staining was performed before the IHC staining, which indicated the active Cre recombination for specifically Gata4 knocking-down. The Hh-receiving cells were shown to populate at the conal OFT as early as E14.5. Significantly less N-cadherin staining of the conal OFT cushion cells in the Gli1-CreERT2/+; Gata4fl/+; R26Rfl/+ embryos versus the Gata4fl/+; R26Rfl/+ littermate control embryos at E10.5 (Fig 6A–6C vs. 6D–6E) was observed, suggesting that the EMT process of the OFT cushion was inhibited by the lower Gata4 expression in Hh-receiving cells. Cell proliferation was examined by BrdU incorporation at E11.5. Gli1-CreERT2/+; Gata4fl/+ embryos demonstrated 17% fewer BrdU-positive SHF cells in the OFT conal cushion (Fig 6G vs. 6J and 6I; P = 0.0134), but not the OFT truncal cushion (Fig 6H vs. 6K and 6L; P = 0.1998), when compared to the littermate Gata4fl/+embryos at E11.5. Cell death were assessed by TUNEL staining and no differences in either the conal or truncal cushion between Gli1-CreERT2/+; Gata4fl/+ and the Gata4fl/+embryos was observed (Fig 6M–6P). Together, these results demonstrated that Gata4 is required for normal cell EMT and proliferation in OFT conal cushion development, possibly through a non-cell autonomous manner.
Fig 6. Gata4 regulates cell proliferation in conal OFT.
(A-F) IHC staining for N-Cadherin in R26Rfl/+; Gata4fl/+ and R26Rfl/+; Gata4fl/+; Gli1-CreERT2/+ embryos at E9.5. LacZ staining was performed before the IHC staining, which indicated the active Cre recombination for specifically Gata4 knocking-down. The Hh-receving cells were shown to populate at the conal OFT as early as E14.5. Magnification: A and D, 100X; B and E, 200X, C and F, 400X. (G-L) BrdU staining in conal OFT and truncal OFT in Gata4fl/+; Gli1-CreERT2/+ embryos and control embryos at E11.5. Magnification: 400X. (I and L) Quantification of BrdU labelled cells. Data is expressed as percentile of Gata4fl/+. Read arrow indicate the cells undergoing EMT. Data is presented as Mean±SE, **p<0.05, n = 3–5, compared with the Gata4fl/+ embryos. (M-P) TUNEL staining in both Gata4fl/+; Gli1Cre-ERT2/+ embryos and control embryos at E11.5. OFT: out flow tract; A: atrium; V: ventricle. Magnification: 100X.
Gata6 was overexpressed in the SHF of the Gata4 transgenic mouse embryos
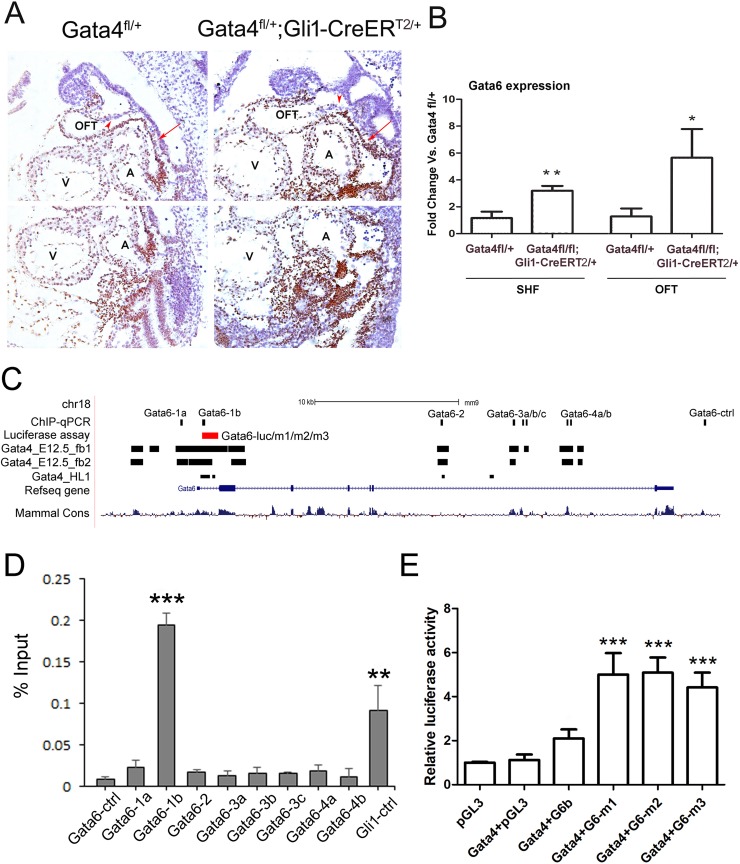
Because Gata4 and Gata6 double mutant embryos display PTA [33], Gata6 expression in Gata4 mutants was examined. Gata6 was expressed in the heart, the OFT and strongly in the splanchnic mesoderm (Fig 7A, arrow), but not neural crest cell derivatives (Fig 7A, arrowhead) of the Gata4fl/+ embryo at E9.5. In Gata4 knockdown embryos specifically in the Hh-receiving cells, the Gata6 expression domain was strongly enhanced in the OFT and the splanchnic mesoderm. Consistently, enhanced expression of Gata6 in the OFT and the SHF of the Gata4fl/fl; Gli1-CreERT2/+ was further confirmed by the real-time PCR at the mRNA level (Fig 7B). The Gata6 expression in the SHF of Gata4fl/fl; Gli1-CreERT2/+ mouse embryo was increased by 1.7-fold comparing to the control Gata4fl/+ embryos (P<0.05). Gata6 expression in the OFT of the Gata4fl/fl; Gli1-CreERT2/+ mouse embryo was increased by 3.4-fold comparing to the littermate control (P<0.01). These results suggested a negative association between the expression of Gata4 and Gata6 in the SHF and developing OFT.
Fig 7. Gata6 is a repressing target of Gata4 in the SHF.
(A) IHC of the Gata6 in Gata4fl/+ and Gata4fl/+; Gli1-CreERT2/+ embryos at E9.5. The arrowhead indicated the NCCs-derived cells and the arrow indicates the splanchnic mesoderm. Magnification: 200X. (B) Gata6 was measured by realtime-PCR in the micro-dissected SHF and the OFT of the Gata4fl/+ and Gata4fl/fl; Gli1-CreERT2/+ embryos at E9.5. *p<0.1 vs. Gata4fl/+, **p<0.05, n = 3–5, compared with the expression in Gata4fl/+ embryos. OFT: out flow tract; A: atrium; V: ventricle. (C) Schematic of the mouse Gata6 genomic locus including Gata4-binding regions and the cloned genomic fragments used for Gata4 regulation assays (luciferase reporter assay and ChIP-qPCR). (D) Enrichment of Gata4-responsive Gata6 genomic fragments in the SHF by Gata4 ChIP-qPCR. Results are presented as mean ± SEM; n = 4; **P < 0.05 vs. Gata6-ctrl, ***P < 0.01, compared with Gata6-ctrl. (E) Gata4-stimulated firefly luciferase activity in wild-type Gata6 and Gata6 mutant fragments. Results are presented as mean ± SEM; n = 4; ***P < 0.01, compared with Gata4+pGL3.
We tested the possibility of Gata4 regulating the expression of Gata6 in the SHF as a repressor by ChIP-qPCR using the microdissected SHF from the E9.5 wildtype mouse embryos. The Gata6 loci was bioinformatically interrogated for potential Gata4-responsive elements using the overlap of evolutionary conservation and Gata4 occupancy in HL-1 cells or embryonic mouse hearts [53, 54] (Fig 7C). Eight potential Gata4-binding regions for Gata6 were screened and our previously identified Gata4 responsive Gli1 loci [35] was used as the positive control (Gli1-ctrl). The results showed enrichments of the region Gli1-ctrl and the region Gata6-1b, but not the others (Fig 7D). This suggested that the region Gata6-1b, within 1 Kbp upstream of Gata6 start codon, was responsive to Gata4 binding. Luciferase reporter assay showed no changes of firefly luciferase activity under Gata6-1b expression in HEK293 cells (Fig 7E, Gata4+G6-luc vs. Gata4+pGL3, P>0.05). However, three mutant constructs for Gata6-1b, each ablating one Gata4-binding site, significantly enhanced the luciferase activity (Fig 7E, P<0.001 for all three mutants versus the Gata4+pGL3). Together, these results place Gata4 upstream of Gata6 as a repressor in the SHF.
Discussion
The requirement of Gata4 for OFT development has been reported in mice and humans, and Gata4 mutations causing DORV have been reported in mice [19, 20, 33]. Here Gata4 is demonstrated required in the SHF Hh-receiving cells for OFT alignment. Our previous study has demonstrated that Gata4 is required for Hh signaling in the SHF for cell proliferation. However, the current study suggested that the cell proliferation defects in the SHF caused by Gata4 mutation may not be the only mechanism underlying the OFT misalignment. Another important contributing factor is the migration defect of the SHF cells, which were associated with disrupted Hh-signaling, as proved by the rescue of over-activating Hh-signaling. As subsequent events, Gata4 haploinsufficency in the Hh-receiving cells disrupted EMT process and cell proliferation in the conal OFT cushion, suggesting that both the cell-autonomous and non-cell autonomous effects of Gata4 drive OFT development. In addition, we demonstrated Gata4 as a repressor of Gata6 in the SHF by identifying Gata4-responsive binding sites in its promoter regions. This result provides a molecular explanation for the severity of OFT defects observed in Gata4 and Gata6 double mutant embryos. These data suggested that breaking down the threshold of GATA including Gata4 and Gata6, and Hh signaling tone might be associated with the severity of OFT defects.
The SHF was initially described as a progenitor field for the cardiac OFT and a rich literature has established the requirement of aSHF contributions for OFT development [5, 12, 35, 55–65]. More recently, the contribution of pSHF cardiac progenitors to the OFT and the future subpulmonary myocardium has been reported; however, the mechanistic requirement for this contribution is not well understood [46, 66–68]. The cell lineage in which Gata4 is required for OFT development has not been reported. Gata4 is expressed in both the aSHF and pSHF, although its expression is much stronger in the pSHF [35]. The decreased number of Mef2C-AHF::Cre positive cells in the proximal OFT cushion of E10.5 Gata4−/+ embryos demonstrated that Gata4 plays a role in adding the SHF progenitor cells to the developing OFT. Surprisingly, OFT defects were not observed in either aSHF-specific (Mef2c-AHF::Cre) or pSHF-specific (Osr1-CreERT) Gata4 haploinsufficiency. Instead, it was found that the severity of the OFT defects and incidence rate in embryos with Gata4 haploinsufficiency in Hh-receiving cells were identical to those in Gata4−/+ embryos. Because Hh-receiving cells are located throughout the SHF, these observations suggested that Gata4 is required in both pSHF and aSHF progenitor cells for OFT alignment.
Evidence was provided that Gata4 acts upstream of Hh-signaling in the SHF for OFT development. The Gata4−/+ embryos have combined phenotypes of ASD and DORV [35]. This study, adding new knowledge to the previous [35], have disclosure that Gata4-Hh-signaling plays active, but different, roles at the venous and atrial pole of the developing heart. In the SHF, Gata4-Hh-signaling controls cell cycle progression and thereby the proliferation of the cardiac progenitors. At the venous pole, diminished Gata4-Hh signaling for cell cycle regulation is balanced by Pten through transcriptional inhibition of Cyclin D4 and Cdk4 [13, 35], as DMP hypoplasia and SHF cell cycle defects are rescued by Pten knockdown [13, 35]. At the atrial pole, the Pten knockdown was able to rescue the cell cycle defects in the SHF, but failed to rescue DORV or OA defects in Gata4 heterozygous mutants. This observation suggests that correction of SHF cell proliferation is not sufficient to support a normal OFT development in Gata4 mutants. In this study, increased apoptosis was not observed in the SHF of Gata4 heterozygote mutant embryos [35]. Indeed, cell migration under the regulation of Gata4-Hh signaling is important for OFT development. However, fate mapping of the SHF using either Mef2c-AHF::Cre or the Gli1-CreERT2 disclosed less SHF-derived cells in the distal OFT in Gata4 mutant embryos. Specifically, there was a decreased number of SHF Hh-receiving cells throughout the migration route from the SHF into the OFT, which traveled from the dorsal mesocardium and continued through the rostral splanchnic mesoderm, past the distal OFT and reached the proximal OFT. Hh-receiving progenitors have been found to migrate from the aSHF to populate the pulmonary trunk between E9.5 to E11.5 [46], suggesting that Hh-signaling is required for SHF cell migration. The observation that DORV in Gata4 mutant embryos can be rescued by constitutive Hh-signaling is correlated with restored cell migration and cell proliferation in the SHF. Therefore, this data suggested that the normal Gata4 regulation of both the proliferation and the migration of the SHF cardiac progenitors are required for OFT development.
During development, the ventricular outlets are aligned to the ventricles by the fusion of conal cushions with the interventricular septum [69], and improper lengthening of the conal cushion may cause rotation problems resulting in misalignment. We demonstrated that Gata4 plays a non-cell autonomous role in the EMT process to give rise to the conal cushions mesenchyme. It is still unknown what specific signals the SHF-derived OFT Gli1+ myocardial cells provide for EMT. Future studies should aim to identify the ligands secreted by Hh-receiving cells. Moreover, a smaller percentage of BrdU+ cells in the conal cushion of the OFT was found at E11.5 of the Gata4fl/+; Gli1Cre-ERT2/+ embryos, suggesting Gata4 plays a role in regulating the OFT cushion cell proliferation. Inactivating N-Cadherin in the SHF resulted in hypoplastic OFT and right ventricle, associated with decreased proliferation [70, 71]. Thus, the lower number of proliferating cells might be associated with the lower expression of N-Cadherin. Overall, cellular, molecular and genetic evidence proved that Gata4-Hh signaling is required in OFT alignment via both the cell-autonomous and non-cell autonomous manners.
Although important Gata4 transcriptional targets in the heart have been identified [13, 18, 37], Gata4-dependent molecular pathways required for OFT development remain unknown. Gli1 was previously identified as a downstream target of Gata4 in the pSHF for atrial septation [35]. In the current study, it was further demonstrated that Gata4 controls Hh-signaling though Gli1 transcriptional regulation for cell migration and OFT alignment. In addition, Gata4 was demonstrated to be a transcriptional repressor of Gata6 in the SHF, and the Gata4-responsive sites in the Gata6 promoter region were identified. Previously, several downstream targets of Gata4 including the Mef2c and Gli1 have been recognized [13, 15, 16, 18, 36, 37], while none of them respond to the inhibitory effects. Gata6 is the first identified repressing target of Gata4, providing direct evidence that Gata4, as a transcription factor, is not only an activator but also a repressor. Enhanced Gata6 expression in Gata4 mutants might illustrate a compensatory feedback loop, given that Gata6 and Gata4 are redundant for cardiac myocyte differentiation [72, 73]. Gata4/Gata6 compound heterozygotes displayed persistent truncus arteriosus (PTA), a severe OFT defect caused by combined alignment and OFT septation defects [33]. This study shows that Gata4/Smo compound heterozygotes show a similar phenotype. Gata4 heterozygote alone does not display PTA, which might be due to the partial recovery of GATA function from enhanced Gata6 expression. Together with previous study [33], these data suggest a threshold of Gata4, Gata6, and Hh signaling and that is required for OFT development. This implies that GATA TFs may be essential for the quantitative regulation of Hh signaling, and diminished GATA function or reduced GATA and Hh signaling together may cause more severe OFT defects. Future studies will focus on the quantitative relationship between GATA tone and Hh signaling tone, as well as the Gata4 dependent gene regulatory network (GRN) [74] for OFT development.
Materials and methods
Mouse lines
All mouse experiments were performed in a mixed B6/129/SvEv background. Gata4fl/+, Gli1-CreERT2/+, Mef2cAHF::Cre, Smofl/+ mouse lines were kind gifts from Dr. Ivan Moskowitz lab (University of Chicago, Chicago). TnT-Cre+/- mouse line was from Dr. Yiping Chen lab (Tulane University, New Orleans). Nfat1c-Cre+/- mouse line was from Dr. Bin Zhou lab (Albert Einstein College of Medicine, Bronx, NY). The SmoM2fl/+, Osr1-CreERT/+ and EIIa-Cre+/- mouse lines were purchased from the Jackson Laboratory.
Ethics statement
Mouse experiments were completed according to a protocol reviewed and approved by the Institutional Animal Care and Use Committee of Texas A&M University (#2015–0398), in compliance with the USA Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Tamoxifen administration and X-gal staining
TMX-induced activation of CreERT2 was accomplished by oral gavage with two doses of 75 mg/kg TMX at E7.5 and E8.5 [44, 46]. X-gal staining of embryos was performed as described [46]. The total number of β-gal positive cells was obtained by counting those on each individual sections and adding up all through the SHF and the OFT.
BrdU incorporation and Immunohistochemistry Staining (IHC)
Standard procedures were used for histology and IHC. For BrdU incorporation, pregnant mice were given 100mg BrdU per kg bodyweight at 10mg/mL concentration solutions at E11.25 with two doses, 3 hours and 6 hours before sacrifice, respectively. The BrdU staining was performed using a BrdU In-Situ detection kit (EMD Millipore). For TUNNEL staining, an ApopTag plus peroxidase In-Situ apoptosis detection kit was used (EMD Millipore). IHC was performed using the following antibodies: anti-Gata4 (Abcam, #ab84593), anti-Gata6 (Abcam, #ab175349), and anti-N-cadherin (Abcam, #ab18203). After incubating with the first antibody, a VECTASTAIN ABC HRP Kit (LifeSpan BioSciences, Inc) was used for detecting the protein expression signal. For counting the ratio of proliferating cells, a total of 100 random cells within the SHF and the specific OFT regions per each section were counted using the Particle Analysis tool of ImageJ and the ratio of positively stained cells was recorded. For each sample, five equivalent serial sections were counted and the averages were taken for statistical analysis.
Micro-dissection of pSHF and RNA extraction
To obtain the pSHF splanchnic mesoderm for use in quantitative realtime-PCR, E9.5 embryos were dissected as described before [45, 75]. The heart, aSHF, and pSHF were collected separately in RNA-later, and then stored at −20°C until genotyping was completed.
Realtime-PCR
Total RNA was extracted from the PSHF regions of mouse embryos hearts using RNeasy Mini Kit (QIAGEN), according to the manufacturer’s instructions. Two hundred ng of total RNA was reverse transcribed using a SuperScriptTM III Reverse Transcriptase kit from Invitrogen. qPCR was performed using a POWER SYBER Green PCR mater mix from Applied Biosystems. Results were analyzed using the delta-delta Ct method with GAPDH as a normalization control [76].
Chromatin immunoprecipitation
Chromatin Immunoprecipitation was performed as described previously [35]. The ChIP assay was performed using a Gata4 antibody (Santa Cruz, #sc-1237 X). Genomic regions with potential Gata4-binding sites and negative control sites are listed in Table 2. Primers used for evaluating the enrichment of the Gata4 pull-down fragments via realtime-PCR are listed in Table 3.
Table 2. Genomic regions with Gata4-binding sites assessed by luciferase reporter assay and ChIP-qPCR.
| Luciferase assay | ChIP-qPCR | ||||||
|---|---|---|---|---|---|---|---|
| Gene name | Genomic fragment | Locus | Luciferase results | Gata4-binding sites in subcloned fragments | Genomic fragment | Locus | ChIP results |
| Gata6 | Gata6-luc | chr18: 11052898–11054024 | 1.51±0.97 P = 0.0945 |
chr18: 11052933–11053086 | Gata6-1a | chr18: 11051387–11069602 | 0.02±0.01 P = 0.1128 |
| Gata6-luc/m1 | 0.90±0.03 P = 0.1198 |
chr18: 11052933–11052964 | Gata6-1b | chr18: 11052900–11053090 | 0.19±0.01 P = 0.0141 |
||
| Gata6-luc/m2 | 0.93±0.03 P = 0.3169 |
chr18: 11053019–11053024 | Gata6-2 | chr18: 11069461–11069602 | 0.02±0.00 P = 0.1530 |
||
| Gata6-luc/m3 | 0.81±0.05 P = 0.3552 |
chr18: 11053062–11053066 | Gata6-3a | chr18: 11074459–11074606 | 0.01±.01 P = 0.4378 |
||
| Gata6-3b | chr18: 11075106–11075234 | 0.02±0.01 P = 0.3491 |
|||||
| Gata6-3c | chr18: 11075344–11075487 | 0.02±0.00 P = 0.1789 |
|||||
| Gata6-4a | chr18: 11078180–11078329 | 0.02±0.01 P = 0.1773 |
|||||
| Gata6-4b | chr18: 11078433–11078559 | 0.02±0.01 P = 0.8491 |
|||||
| Gata6-ctrl | chr18: 11087740–11087888 | 0.00±0.00 | |||||
| Gli1-ctrl | chr10: 126771190–126771322 | 0.09±0.03 | |||||
All genomic coordinates are shown in mouse genome build mm9.
Table 3. Primers for ChIP-qPCR.
| Fragment | Forward 5’ → 3’ | Reverse 5’ → 3’ |
|---|---|---|
| Gata6-1a | ATATCACTGCTGCTGCCTGG | CACACACCCCTTAGTCGCTC |
| Gata6-1b | ATACTCCGGACCAGCCTCC | CAAATCGCTTAGGCTCATCGG |
| Gata6-2 | CGAGGAACATATTTTGCCTGCC | ACACCCAGCAAAGCAGAAGTG |
| Gata6-3a | CACTTGCCACATGCTGCAAAC | ACATTCTCTGCTACGGTGAC |
| Gata6-3b | CTGCCAAAAGGGTTACCAGC | TCCGGTGACACCTGTCTTTG |
| Gata6-3c | CTGAGGCTAGCCAGGAACTCC | CAGGCCATGGAGTTGGTCCTC |
| Gata6-4a | ATGCTATGCTAAGCCCAGGC | GTGCATTGAGGGCAGAGTAGA |
| Gata6-4b | TCTCTGCTTTCTCCTAGGGAC | TCCATGAGTTAACATTTTCCCAC |
| Gata6-ctrl | CCCCTGGGAGGTAACACAGAC | CCTCAGTTTCCCTGTACCCAC |
| Gli1-ctrl | GAGGGATACTTAGGCGGC | GTTGCAGCAAGGCCTTTAGC |
Dual Luciferase Reporter Assay
Dual Luciferase Reporter Assay was performed as described previously [35, 44, 45]. Genomic regions with potential Gata4-binding sites tested are listed in Table 2. Primers used for site-specific mutation and subclone are listed in Table 4.
Table 4. Primers for luciferase reporter assay.
| Fragment | Forward 5’ → 3’ | Reverse 5’ → 3’ |
|---|---|---|
| Gata6-luc | GTCACCCGGGATACTCCGGACCAGCCTC | AGTCAAGCTTGAGTGAGGAACAAGACGG |
| Gata6-luc/m1 | CCCCCAGTGCAAAGCCCACAGCCCGAGTTTCAGCGCCAAG | CTTGGCGCTGAAACTCGGGCTGTGGGCTTTGCACTGGGGG |
| Gata6-luc/m2 | GAGAAACTTCTTTCTTGTGCCTGGGTCTGTGTGTGG | CCACACACAGACCCAGGCACAAGAAAGAAGTTTCTC |
| Gata6-luc/m3 | GGGCATTAATTTTAGTGTGGCCGATGAGCCTAAGCG | CGCTTAGGCTCATCGGCCACACTAAAATTAATGCCC |
Supporting information
A) DNA was extracted from SHF, heart and tail tissues of Gata4fl/+;Gli1-CreERT2/+ mice and was tested for Cre-mediated knockdown of Gata4. B) DNA was extracted from heart and tail tissues of Gata4fl/+;Nfatc1-Cre+/- mice and was tested for Cre-mediated knockdown of Gata4. C) DNA was extracted fromheart and tail tissues of Gata4fl/+;Wnt1-CreERT/+ mice and was tested for Cre-mediated knockdown of Gata4. D) DNA was extracted from SHF, heart and tail tissues of Gata4fl/+;Osr1-CreERT/+ mice and was tested for Cre-mediated knockdown of Gata4. E) DNA was extracted from SHF, heart and tail tissues of Gata4fl/+;Mef2cAHF::Cre mice and was tested for Cre-mediated knockdown of Gata4. F) DNA was extracted from heart and tail tissues of Gata4fl/+;Tnt-Cre+/- mice and was tested for Cre-mediated knockdown of Gata4. The following primers were used: Cre forward: 5′-TCGACCAGGTTCGTTC ACTCATGG-3′; Cre reverse: 5′-CAGGCTAAGTGCCTTCTCTACACC-3′; Gata4-WT/flox forward: 5′-ACCCTGGAAGAC ACCCCAATCTCGG-3′; Gata4-del forward: 5′-TGTCATTCTTCGCTGGAGCCGC-3′; Gata4 reverse: 5′-TCCATGAGAC CCCAGAGTGTGCCTGA-3′. The size of Cre product is ∼220 bp. The Gata4 wild type and Gata4-flox products are ∼510 bp and 530 bp in size, respectively. The size of the Gata4-del product is ∼300 bp. G) Realtime-PCR results for Gata4 expression in the SHF of the Gata4fl/+;Gli1-CreERT2/+ versus the Gata4fl/+ embryos at E9.5 (TMX at E7.5 and E8.5). Data is presented as Mean±SEM, n = 6, ***P < 0.001 compared with the expression in Gata4fl/+ embryos. The forward primer used is 5’-GAAGAGATGCGCCCCATCAA-3’ and the reverse primer used is 5’- GCAGACAGCACTGGATGGAT-3’.
(TIF)
Acknowledgments
We would specifically like to acknowledge the support of Dr. Boon Chew for the study.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This project was supported by grants from the National Institutes of Health to LX (NIH-1R15HL117238 and NIH-1R56HL138479-01) and LX and the AHA to LX (13SDG14650009), KZ (15GRNT25700195). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jain R, Rentschler S, Epstein JA. Notch and cardiac outflow tract development. Ann N Y Acad Sci. 2010;1188:184–90. Epub 2010/03/06. 10.1111/j.1749-6632.2009.05099.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58(21):2241–7. Epub 2011/11/15. 10.1016/j.jacc.2011.08.025 . [DOI] [PubMed] [Google Scholar]
- 3.Dolk H, Loane MA, Abramsky L, de Walle H, Garne E. Birth prevalence of congenital heart disease. Epidemiology. 2010;21(2):275–7; author reply 7. Epub 2010/02/18. 10.1097/EDE.0b013e3181c2979b . [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. Epub 2013/12/20. 10.1161/01.cir.0000441139.02102.80 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roux M, Laforest B, Capecchi M, Bertrand N, Zaffran S. Hoxb1 regulates proliferation and differentiation of second heart field progenitors in pharyngeal mesoderm and genetically interacts with Hoxa1 during cardiac outflow tract development. Dev Biol. 2015;406(2):247–58. Epub 2015/08/19. 10.1016/j.ydbio.2015.08.015 . [DOI] [PubMed] [Google Scholar]
- 6.High FA, Jain R, Stoller JZ, Antonucci NB, Lu MM, Loomes KM, et al. Murine Jagged1/Notch signaling in the second heart field orchestrates Fgf8 expression and tissue-tissue interactions during outflow tract development. J Clin Invest. 2009;119(7):1986–96. Epub 2009/06/11. 10.1172/JCI38922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang S, Li HC, Wang YX, Wu SS, Cai YJ, Cui HL, et al. Pulmonary endoderm, second heart field and the morphogenesis of distal outflow tract in mouse embryonic heart. Dev Growth Differ. 2014;56(4):276–92. Epub 2014/04/05. 10.1111/dgd.12129 . [DOI] [PubMed] [Google Scholar]
- 8.Rochais F, Dandonneau M, Mesbah K, Jarry T, Mattei MG, Kelly RG. Hes1 is expressed in the second heart field and is required for outflow tract development. PLoS One. 2009;4(7):e6267 Epub 2009/07/18. 10.1371/journal.pone.0006267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang YP, Li HR, Cao XM, Wang QX, Qiao CJ, Ya J. Second heart field and the development of the outflow tract in human embryonic heart. Dev Growth Differ. 2013;55(3):359–67. Epub 2013/03/16. 10.1111/dgd.12050 . [DOI] [PubMed] [Google Scholar]
- 10.Neeb Z, Lajiness JD, Bolanis E, Conway SJ. Cardiac outflow tract anomalies. Wiley Interdiscip Rev Dev Biol. 2013;2(4):499–530. Epub 2013/09/10. 10.1002/wdev.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keyte A, Hutson MR. The neural crest in cardiac congenital anomalies. Differentiation. 2012;84(1):25–40. Epub 2012/05/19. 10.1016/j.diff.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnes RM, Harris IS, Jaehnig EJ, Sauls K, Sinha T, Rojas A, et al. MEF2C regulates outflow tract alignment and transcriptional control of Tdgf1. Development. 2016;143(5):774–9. Epub 2016/01/27. 10.1242/dev.126383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rojas A, Kong SW, Agarwal P, Gilliss B, Pu WT, Black BL. GATA4 is a direct transcriptional activator of cyclin D2 and Cdk4 and is required for cardiomyocyte proliferation in anterior heart field-derived myocardium. Mol Cell Biol. 2008;28(17):5420–31. Epub 2008/07/02. 10.1128/MCB.00717-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajagopal SK, Ma Q, Obler D, Shen J, Manichaikul A, Tomita-Mitchell A, et al. Spectrum of heart disease associated with murine and human GATA4 mutation. J Mol Cell Cardiol. 2007;43(6):677–85. Epub 2007/07/24. 10.1016/j.yjmcc.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Misra C, Sachan N, McNally CR, Koenig SN, Nichols HA, Guggilam A, et al. Congenital heart disease-causing Gata4 mutation displays functional deficits in vivo. PLoS Genet. 2012;8(5):e1002690 Epub 2012/05/17. 10.1371/journal.pgen.1002690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Misra C, Chang SW, Basu M, Huang N, Garg V. Disruption of myocardial Gata4 and Tbx5 results in defects in cardiomyocyte proliferation and atrioventricular septation. Hum Mol Genet. 2014. Epub 2014/05/27. 10.1093/hmg/ddu215 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garg V, Kathiriya IS, Barnes R, Schluterman MK, King IN, Butler CA, et al. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature. 2003;424(6947):443–7. Epub 2003/07/08. 10.1038/nature01827 . [DOI] [PubMed] [Google Scholar]
- 18.Dodou E, Verzi MP, Anderson JP, Xu SM, Black BL. Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development. 2004;131(16):3931–42. Epub 2004/07/16. 10.1242/dev.01256 . [DOI] [PubMed] [Google Scholar]
- 19.Maitra M, Schluterman MK, Nichols HA, Richardson JA, Lo CW, Srivastava D, et al. Interaction of Gata4 and Gata6 with Tbx5 is critical for normal cardiac development. Dev Biol. 2009;326(2):368–77. Epub 2008/12/17. 10.1016/j.ydbio.2008.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pu WT, Ishiwata T, Juraszek AL, Ma Q, Izumo S. GATA4 is a dosage-sensitive regulator of cardiac morphogenesis. Dev Biol. 2004;275(1):235–44. Epub 2004/10/07. 10.1016/j.ydbio.2004.08.008 . [DOI] [PubMed] [Google Scholar]
- 21.Zeisberg EM, Ma Q, Juraszek AL, Moses K, Schwartz RJ, Izumo S, et al. Morphogenesis of the right ventricle requires myocardial expression of Gata4. J Clin Invest. 2005;115(6):1522–31. Epub 2005/05/20. 10.1172/JCI23769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bisping E, Ikeda S, Kong SW, Tarnavski O, Bodyak N, McMullen JR, et al. Gata4 is required for maintenance of postnatal cardiac function and protection from pressure overload-induced heart failure. Proc Natl Acad Sci U S A. 2006;103(39):14471–6. Epub 2006/09/20. 10.1073/pnas.0602543103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivera-Feliciano J, Lee KH, Kong SW, Rajagopal S, Ma Q, Springer Z, et al. Development of heart valves requires Gata4 expression in endothelial-derived cells. Development. 2006;133(18):3607–18. Epub 2006/08/18. 10.1242/dev.02519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi S, Lackey T, Huang Y, Bisping E, Pu WT, Boxer LM, et al. Transcription factor gata4 regulates cardiac BCL2 gene expression in vitro and in vivo. Faseb J. 2006;20(6):800–2. Epub 2006/02/14. 10.1096/fj.05-5426fje . [DOI] [PubMed] [Google Scholar]
- 25.Kuo CT, Morrisey EE, Anandappa R, Sigrist K, Lu MM, Parmacek MS, et al. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11(8):1048–60. Epub 1997/04/15. 10.1101/gad.11.8.1048 . [DOI] [PubMed] [Google Scholar]
- 26.Ip HS, Wilson DB, Heikinheimo M, Leiden JM, Parmacek MS. The GATA-4 transcription factor transactivates the cardiac-specific troponin C promoter-enhancer in non-muscle cells. Adv Exp Med Biol. 1995;382:117–24. Epub 1995/01/01. . [DOI] [PubMed] [Google Scholar]
- 27.Ip HS, Wilson DB, Heikinheimo M, Tang Z, Ting CN, Simon MC, et al. The GATA-4 transcription factor transactivates the cardiac muscle-specific troponin C promoter-enhancer in nonmuscle cells. Mol Cell Biol. 1994;14(11):7517–26. Epub 1994/11/01. 10.1128/mcb.14.11.7517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajagopal SK, Ma Q, Obler D, Shen J, Manichaikul A, Tomita-Mitchell A, et al. Spectrum of heart disease associated with murine and human GATA4 mutation. J Mol Cell Cardiol. 2007;43(6):677–85. Epub 2007/07/24. 10.1016/j.yjmcc.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reamon-Buettner SM, Borlak J. GATA4 zinc finger mutations as a molecular rationale for septation defects of the human heart. J Med Genet. 2005;42(5):e32 Epub 2005/05/03. 10.1136/jmg.2004.025395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nemer G, Fadlalah F, Usta J, Nemer M, Dbaibo G, Obeid M, et al. A novel mutation in the GATA4 gene in patients with Tetralogy of Fallot. Hum Mutat. 2006;27(3):293–4. Epub 2006/02/14. 10.1002/humu.9410 . [DOI] [PubMed] [Google Scholar]
- 31.Yang YQ, Gharibeh L, Li RG, Xin YF, Wang J, Liu ZM, et al. GATA4 loss-of-function mutations underlie familial tetralogy of fallot. Hum Mutat. 2013;34(12):1662–71. Epub 2013/09/04. 10.1002/humu.22434 . [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Li X, Shen A, Jiao W, Guan X, Li Z. GATA4 mutations in 486 Chinese patients with congenital heart disease. Eur J Med Genet. 2008;51(6):527–35. Epub 2008/08/02. 10.1016/j.ejmg.2008.06.005 . [DOI] [PubMed] [Google Scholar]
- 33.Xin M, Davis CA, Molkentin JD, Lien CL, Duncan SA, Richardson JA, et al. A threshold of GATA4 and GATA6 expression is required for cardiovascular development. Proc Natl Acad Sci U S A. 2006;103(30):11189–94. Epub 2006/07/19. 10.1073/pnas.0604604103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moskowitz IP, Wang J, Peterson MA, Pu WT, Mackinnon AC, Oxburgh L, et al. Transcription factor genes Smad4 and Gata4 cooperatively regulate cardiac valve development. [corrected]. Proc Natl Acad Sci U S A. 2011;108(10):4006–11. Epub 2011/02/19. 10.1073/pnas.1019025108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou L, Liu J, Xiang M, Olson P, Guzzetta A, Zhang K, et al. Gata4 potentiates second heart field proliferation and Hedgehog signaling for cardiac septation. Proc Natl Acad Sci U S A. 2017;114(8):E1422–E31. Epub 2017/02/09. 10.1073/pnas.1605137114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamak A, Latinkic BV, Dali R, Temsah R, Nemer M. Cyclin D2 is a GATA4 cofactor in cardiogenesis. Proc Natl Acad Sci U S A. 2014;111(4):1415–20. Epub 2014/01/30. 10.1073/pnas.1312993111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morin S, Charron F, Robitaille L, Nemer M. GATA-dependent recruitment of MEF2 proteins to target promoters. Embo J. 2000;19(9):2046–55. Epub 2000/05/03. 10.1093/emboj/19.9.2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–7. Epub 2001/12/14. 10.1038/414782a . [DOI] [PubMed] [Google Scholar]
- 39.Molkentin JD, Lin Q, Duncan SA, Olson EN. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11(8):1061–72. Epub 1997/04/15. 10.1101/gad.11.8.1061 . [DOI] [PubMed] [Google Scholar]
- 40.Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, et al. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci U S A. 1996;93(12):5860–5. Epub 1996/06/11. 10.1073/pnas.93.12.5860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiao K, Kulessa H, Tompkins K, Zhou Y, Batts L, Baldwin HS, et al. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003;17(19):2362–7. Epub 2003/09/17. 10.1101/gad.1124803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230(2):230–42. Epub 2001/02/13. 10.1006/dbio.2000.0106 S0012160600901064 [pii]. . [DOI] [PubMed] [Google Scholar]
- 43.Goddeeris MM, Rho S, Petiet A, Davenport CL, Johnson GA, Meyers EN, et al. Intracardiac septation requires hedgehog-dependent cellular contributions from outside the heart. Development. 2008;135(10):1887–95. Epub 2008/04/29. 10.1242/dev.016147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou L, Liu J, Olson P, Zhang K, Wynne J, Xie L. Tbx5 and Osr1 interact to regulate posterior second heart field cell cycle progression for cardiac septation. J Mol Cell Cardiol. 2015;85:1–12. 10.1016/j.yjmcc.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie L, Hoffmann AD, Burnicka-Turek O, Friedland-Little JM, Zhang K, Moskowitz IP. Tbx5-hedgehog molecular networks are essential in the second heart field for atrial septation. Dev Cell. 2012;23(2):280–91. Epub 2012/08/18. 10.1016/j.devcel.2012.06.006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoffmann AD, Peterson MA, Friedland-Little JM, Anderson SA, Moskowitz IP. sonic hedgehog is required in pulmonary endoderm for atrial septation. Development. 2009;136(10):1761–70. Epub 2009/04/17. 10.1242/dev.034157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goddeeris MM, Schwartz R, Klingensmith J, Meyers EN. Independent requirements for Hedgehog signaling by both the anterior heart field and neural crest cells for outflow tract development. Development. 2007;134(8):1593–604. Epub 2007/03/09. 10.1242/dev.02824 . [DOI] [PubMed] [Google Scholar]
- 48.Dyer LA, Kirby ML. Sonic hedgehog maintains proliferation in secondary heart field progenitors and is required for normal arterial pole formation. Dev Biol. 2009;330(2):305–17. 10.1016/j.ydbio.2009.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mugford JW, Sipila P, McMahon JA, McMahon AP. Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Dev Biol. 2008;324(1):88–98. 10.1016/j.ydbio.2008.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mugford JW, Sipila P, Kobayashi A, Behringer RR, McMahon AP. Hoxd11 specifies a program of metanephric kidney development within the intermediate mesoderm of the mouse embryo. Dev Biol. 2008;319(2):396–405. 10.1016/j.ydbio.2008.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joyner AL, Zervas M. Genetic inducible fate mapping in mouse: establishing genetic lineages and defining genetic neuroanatomy in the nervous system. Dev Dyn. 2006;235(9):2376–85. 10.1002/dvdy.20884 . [DOI] [PubMed] [Google Scholar]
- 52.Flagg AE, Earley JU, Svensson EC. FOG-2 attenuates endothelial-to-mesenchymal transformation in the endocardial cushions of the developing heart. Dev Biol. 2007;304(1):308–16. Epub 2007/02/06. 10.1016/j.ydbio.2006.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He A, Kong SW, Ma Q, Pu WT. Co-occupancy by multiple cardiac transcription factors identifies transcriptional enhancers active in heart. Proc Natl Acad Sci U S A. 2011;108(14):5632–7. 10.1073/pnas.1016959108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He A, Gu F, Hu Y, Ma Q, Ye LY, Akiyama JA, et al. Dynamic GATA4 enhancers shape the chromatin landscape central to heart development and disease. Nat Commun. 2014;5:4907 Epub 2014/09/25. 10.1038/ncomms5907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.von Both I, Silvestri C, Erdemir T, Lickert H, Walls JR, Henkelman RM, et al. Foxh1 is essential for development of the anterior heart field. Dev Cell. 2004;7(3):331–45. Epub 2004/09/15. 10.1016/j.devcel.2004.07.023 . [DOI] [PubMed] [Google Scholar]
- 56.Sinha T, Li D, Theveniau-Ruissy M, Hutson MR, Kelly RG, Wang J. Loss of Wnt5a disrupts second heart field cell deployment and may contribute to OFT malformations in DiGeorge syndrome. Hum Mol Genet. 2015;24(6):1704–16. Epub 2014/11/21. 10.1093/hmg/ddu584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seo S, Kume T. Forkhead transcription factors, Foxc1 and Foxc2, are required for the morphogenesis of the cardiac outflow tract. Dev Biol. 2006;296(2):421–36. Epub 2006/07/15. 10.1016/j.ydbio.2006.06.012 . [DOI] [PubMed] [Google Scholar]
- 58.Neeb Z, Lajiness JD, Bolanis E, Conway SJ. Cardiac outflow tract anomalies. Wiley Interdiscip Rev Dev Biol. 2013;2(4):499–530. Epub 2013/09/10. 10.1002/wdev.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Milgrom-Hoffman M, Harrelson Z, Ferrara N, Zelzer E, Evans SM, Tzahor E. The heart endocardium is derived from vascular endothelial progenitors. Development. 2011;138(21):4777–87. Epub 2011/10/13. 10.1242/dev.061192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin Q, Lu J, Yanagisawa H, Webb R, Lyons GE, Richardson JA, et al. Requirement of the MADS-box transcription factor MEF2C for vascular development. Development. 1998;125(22):4565–74. Epub 1998/10/21. . [DOI] [PubMed] [Google Scholar]
- 61.Li P, Pashmforoush M, Sucov HM. Retinoic acid regulates differentiation of the secondary heart field and TGFbeta-mediated outflow tract septation. Dev Cell. 2010;18(3):480–5. Epub 2010/03/17. 10.1016/j.devcel.2009.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keyte A, Hutson MR. The neural crest in cardiac congenital anomalies. Differentiation. 2012;84(1):25–40. Epub 2012/05/19. 10.1016/j.diff.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen L, Fulcoli FG, Ferrentino R, Martucciello S, Illingworth EA, Baldini A. Transcriptional control in cardiac progenitors: Tbx1 interacts with the BAF chromatin remodeling complex and regulates Wnt5a. PLoS Genet. 2012;8(3):e1002571 Epub 2012/03/23. 10.1371/journal.pgen.1002571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, et al. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5(6):877–89. Epub 2003/12/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bi W, Drake CJ, Schwarz JJ. The transcription factor MEF2C-null mouse exhibits complex vascular malformations and reduced cardiac expression of angiopoietin 1 and VEGF. Dev Biol. 1999;211(2):255–67. Epub 1999/07/09. 10.1006/dbio.1999.9307 . [DOI] [PubMed] [Google Scholar]
- 66.Bertrand N, Roux M, Ryckebusch L, Niederreither K, Dolle P, Moon A, et al. Hox genes define distinct progenitor sub-domains within the second heart field. Dev Biol. 2011;353(2):266–74. Epub 2011/03/10. 10.1016/j.ydbio.2011.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dominguez JN, Meilhac SM, Bland YS, Buckingham ME, Brown NA. Asymmetric fate of the posterior part of the second heart field results in unexpected left/right contributions to both poles of the heart. Circ Res. 2012;111(10):1323–35. Epub 2012/09/08. 10.1161/CIRCRESAHA.112.271247 . [DOI] [PubMed] [Google Scholar]
- 68.Lescroart F, Mohun T, Meilhac SM, Bennett M, Buckingham M. Lineage tree for the venous pole of the heart: clonal analysis clarifies controversial genealogy based on genetic tracing. Circ Res. 2012;111(10):1313–22. Epub 2012/08/03. 10.1161/CIRCRESAHA.112.271064 . [DOI] [PubMed] [Google Scholar]
- 69.Lin CJ, Lin CY, Chen CH, Zhou B, Chang CP. Partitioning the heart: mechanisms of cardiac septation and valve development. Development. 2012;139(18):3277–99. Epub 2012/08/23. 10.1242/dev.063495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soh BS, Buac K, Xu H, Li E, Ng SY, Wu H, et al. N-cadherin prevents the premature differentiation of anterior heart field progenitors in the pharyngeal mesodermal microenvironment. Cell Res. 2014;24(12):1420–32. Epub 2014/11/05. 10.1038/cr.2014.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M, Hynes RO. Developmental defects in mouse embryos lacking N-cadherin. Dev Biol. 1997;181(1):64–78. Epub 1997/01/01. 10.1006/dbio.1996.8443 . [DOI] [PubMed] [Google Scholar]
- 72.Borok MJ, Papaioannou VE, Sussel L. Unique functions of Gata4 in mouse liver induction and heart development. Dev Biol. 2016;410(2):213–22. Epub 2015/12/22. 10.1016/j.ydbio.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao R, Watt AJ, Battle MA, Li J, Bondow BJ, Duncan SA. Loss of both GATA4 and GATA6 blocks cardiac myocyte differentiation and results in acardia in mice. Dev Biol. 2008;317(2):614–9. Epub 2008/04/11. 10.1016/j.ydbio.2008.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Davidson EH, Erwin DH. Gene regulatory networks and the evolution of animal body plans. Science. 2006;311(5762):796–800. Epub 2006/02/14. 10.1126/science.1113832 . [DOI] [PubMed] [Google Scholar]
- 75.Zhang KK, Xiang M, Zhou L, Liu J, Curry N, Heine Suner D, et al. Gene network and familial analyses uncover a gene network involving Tbx5/Osr1/Pcsk6 interaction in the second heart field for atrial septation. Hum Mol Genet. 2016;25(6):1140–51. 10.1093/hmg/ddv636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–8. Epub 2008/06/13. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) DNA was extracted from SHF, heart and tail tissues of Gata4fl/+;Gli1-CreERT2/+ mice and was tested for Cre-mediated knockdown of Gata4. B) DNA was extracted from heart and tail tissues of Gata4fl/+;Nfatc1-Cre+/- mice and was tested for Cre-mediated knockdown of Gata4. C) DNA was extracted fromheart and tail tissues of Gata4fl/+;Wnt1-CreERT/+ mice and was tested for Cre-mediated knockdown of Gata4. D) DNA was extracted from SHF, heart and tail tissues of Gata4fl/+;Osr1-CreERT/+ mice and was tested for Cre-mediated knockdown of Gata4. E) DNA was extracted from SHF, heart and tail tissues of Gata4fl/+;Mef2cAHF::Cre mice and was tested for Cre-mediated knockdown of Gata4. F) DNA was extracted from heart and tail tissues of Gata4fl/+;Tnt-Cre+/- mice and was tested for Cre-mediated knockdown of Gata4. The following primers were used: Cre forward: 5′-TCGACCAGGTTCGTTC ACTCATGG-3′; Cre reverse: 5′-CAGGCTAAGTGCCTTCTCTACACC-3′; Gata4-WT/flox forward: 5′-ACCCTGGAAGAC ACCCCAATCTCGG-3′; Gata4-del forward: 5′-TGTCATTCTTCGCTGGAGCCGC-3′; Gata4 reverse: 5′-TCCATGAGAC CCCAGAGTGTGCCTGA-3′. The size of Cre product is ∼220 bp. The Gata4 wild type and Gata4-flox products are ∼510 bp and 530 bp in size, respectively. The size of the Gata4-del product is ∼300 bp. G) Realtime-PCR results for Gata4 expression in the SHF of the Gata4fl/+;Gli1-CreERT2/+ versus the Gata4fl/+ embryos at E9.5 (TMX at E7.5 and E8.5). Data is presented as Mean±SEM, n = 6, ***P < 0.001 compared with the expression in Gata4fl/+ embryos. The forward primer used is 5’-GAAGAGATGCGCCCCATCAA-3’ and the reverse primer used is 5’- GCAGACAGCACTGGATGGAT-3’.
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.