Abstract
OBJECTIVES
The aim of the current investigation is to examine whether use of high-frequency jet ventilation (HFJV) during pulmonary vein isolation (PVI) performed with force-sensing catheters is associated with improved outcomes.
BACKGROUND
Catheter ablation is well established as therapy for symptomatic atrial fibrillation (AF). Reconnection following PVI is commonly observed during repeat ablation procedures. Technologies that may optimize catheter stability and lesion delivery include both force-sensing ablation catheters and HFJV.
METHODS
Patients undergoing PVI at Johns Hopkins Hospital were prospectively enrolled in a registry. The study compared procedural characteristics, adverse event rates, and 1-year procedural outcomes in patients undergoing PVI supported either by standard ventilation or HFJV. Patient and procedural aspects were otherwise constant.
RESULTS
Eighty-four HFJV patients and 84 matched control patients with 1-year outcome data were identified. Atrial arrhythmia recurrence occurred in 26 of 84 HFJV patients (31%) and 42 of 84 control patients (50%; p = 0.019). In patients with paroxysmal AF, arrhythmia recurrence in HFJV and control patients was 27.3% and 47.3%, respectively (p = 0.045). In patients with persistent AF, arrhythmia recurrence rates were not significantly different (37.9% in HFJV patients, 55.2% in control patients; p = 0.184). On multivariate analysis, HFJV was independently associated with improved freedom from arrhythmia recurrence. Vasopressor use during HFJV cases was significantly higher than during standard ventilation (79.7% vs. 22.4%; p = 0.001). Indices of catheter stability and contact force adequacy were significantly higher in the HFJV patients than in control patients. Complication rates in the 2 groups were similarly low.
CONCLUSIONS
Use of HFJV in patients undergoing PVI with radiofrequency force-sensing catheters is associated with improved outcomes, without appreciable increase in adverse procedural events.
Keywords: atrial fibrillation ablation, high frequency jet ventilation, pulmonary vein isolation
Catheter ablation is well established for the treatment of symptomatic atrial fibrillation (AF) (1). Despite improvements in AF ablation techniques, single-procedure success rates remain <80%, even in optimal procedural candidates (1). In patients undergoing repeat ablation for recurrent AF, pulmonary vein (PV) reconnection is commonly observed.
Optimizing the catheter-tissue interface and attendant lesion delivery may improve procedural outcomes. Force-sensing radiofrequency (RF) ablation catheters have become widely adopted, with mixed results in terms of both outcomes and safety. An additional measure to assist in stable catheter-tissue contact is the use of high-frequency jet ventilation (HFJV) (2). This strategy employs low-volume, high-frequency ventilation via endotracheal tube, maintaining adequate gas exchange while minimizing respirophasic cardiac excursion (2). Initial studies have suggested that HFJV for PV isolation (PVI) is safe However, limited data exist concerning the impact of HFJV on ablation outcomes (3,4), and no studies have been performed examining the additive value of HFJV in patients undergoing PVI with RF force-sensing catheters.
Based on the findings of early reports on the utility of HFJV, we incorporated that approach in our AF ablation program in November 2015. In the present paper, we report on the safety and 1-year outcomes in patients undergoing PVI with HFJV support, compared with appropriately matched patients with standard ventilation.
METHODS
STUDY COHORT.
All patients undergoing AF ablation at Johns Hopkins Hospital from 2015 to 2017 were approached for enrollment in a prospective electronic database, with demographics, clinical history, procedural data, complications, and outcomes recorded for each case. Those who agreed to participate were enrolled in an Institutional Review Board–approved AF ablation registry. The study population included in this report were those patients who underwent AF ablation using a force-sensing RF ablation catheter and in whom HFJV was used. Control patients consisted of database-enrolled patients undergoing PVI with RF force-sensing catheters and supported by standard ventilation, with frequency matching for paroxysmal versus persistent AF. Control patients were selected from consecutive patients undergoing PVI with RF force-sensing catheters, with standard ventilator support, contemporaneous with the case population, and with 1-year outcome data available. Demographic and procedural characteristics, adverse events, and outcomes were compared between the 2 groups.
CLINICAL FOLLOW-UP.
All patients were observed in hospital for a minimum of 1 night post-ablation. Routine follow-up (history, exam, and electrocardiography or Holter) was performed at the outpatient clinic or by a local cardiologist at 3, 6, and 12 months, and additionally if prompted by symptoms. Holter monitoring was not performed routinely on all patients. Antiarrhythmic drug therapy, if present at the time of ablation, was discontinued at the 3-month follow-up visit. Arrhythmia recurrence was defined on the basis of the 2017 Heart Rhythm Society consensus document as any AF or atrial tachycardia sustained for >30 s recorded by a surface electrocardiogram or rhythm monitoring device after a 90-day blanking period (1). One-year outcomes were assessed in all patients at clinic follow-up, electronic health record review, or phone interview.
DEFINITIONS.
Paroxysmal AF was defined as AF that terminates spontaneously or with intervention within 7 days of onset, whereas persistent AF was defined as AF sustained for >7 days (1). Major procedure-related complications were defined based on the recommendations of the 2017 AF Ablation Consensus Document as those complications resulting in permanent injury or death, requiring intervention for treatment, or prolonging or requiring hospitalization for more than 48 h (1).
VENTILATION STRATEGY.
All patients had ablation performed under general anesthesia. For patients undergoing standard ventilation, anesthesia was maintained with volatile anesthetics at 0.5 to 1 minimal alveolar concentration using a GE Aisys anesthesia machine (GE Healthcare, Madison, Wisconsin). Volume control ventilation was utilized with tidal volumes between 6 and 10 ml/kg, positive end-expiratory pressure between 0 and 5 mm Hg, FiO2 between 60% and 100%, and respiratory rate at 8 to 24 breaths/min to achieve end-tidal CO2 levels of 35 to 40 mm Hg. For patients undergoing HFJV (Monsoon III Jet Ventilators, Acutronic Medical Systems AG, Hirzel, Switzerland), anesthesia was maintained with total intravenous anesthesia (TIVA) using propofol 30- to 200-µg/kg/min and remifentanil 0.05- and 0.40-µg/kg/min infusions. HFJV was delivered with a driving pressure (DP) of 20 mm Hg, inspiratory time 40%, frequency (analog of respiratory rate) of 120 cycles/min, FiO2 of 60% to 100%, and 40% humidity. Target ventilation parameters included arterial CO2 level of 40 mm Hg with accepted range of 35 to 45 mm Hg. In the event of hyperventilation (with attendant respiratory alkalosis), DP pressure was reduced by 2 mm Hg first to a minimum of 16 mm Hg, followed by reduction in inspiratory time from 40% to 35%. In the event of hypoventilation and respiratory acidosis, DP was increased by 2 mm Hg, with arterial blood gas in 20 to 30 min after each change. Bispectral index monitoring (electroencephalographic monitoring to determine depth of sedation) was used in all cases to monitor the depth of anesthesia at the lowest possible doses of TIVA agents (5–7).
ABLATION STRATEGY.
Pre-procedure transesophageal echocardiography was performed at the discretion of the operator. Femoral site access was obtained and intravenous heparin administered to maintain activated clotting times >350 s. After performing a double transseptal puncture, a Lasso circular mapping catheter (Biosense-Webster, Diamond Bar, California) was positioned in the left atrium. An electroanatomic map of the left atrium was obtained using the CARTO System (Biosense-Webster), and superimposed on pre-acquired CAT scan or magnetic resonance images. A 4-mm open-tip irrigated RF catheter with contact force sensor (Thermocool SmartTouch, Biosense-Webster) was then positioned in the left atrium: PVI was performed using real-time automated display of RF application points (Visitag, Biosense-Webster) with predefined catheter stability settings. Starting energy delivery parameters were 25 to 35 W on the posterior wall and 35 to 40 W at other sites. Target contact force was between 5 and 20 g at all sites. Esophageal temperature was monitored, and RF delivery paused if esophageal temperature increased by 0.5°C. Electric isolation of PVs was confirmed by entrance block to individual PVs, assessed by Lasso catheter positioned at the PV antrum.
Ablation point parameters were collected from CARTO software and analyzed for force variability (force measure sampling of 20 Hz), mean force amplitude, and duration of contact above 5 g. Force variability index was defined as (Fmax – Fmin)/Fmax at each ablation point.
STATISTICAL ANALYSIS.
All continuous variables are reported as mean ± SD, and categorical variables are reported as percentages. Univariate analysis for comparison of the groups was done using 2 sample t test for continuous variables and chi-square or Fisher’s exact test for categorical variables. Kaplan-Meier analysis and Cox regression were used for survival analysis. A multivariate analysis model was created with age, sex, body mass index, CHA2DS2-VASc (congestive heart failure, hypertension, age, diabetes mellitus, stroke/transient ischemic attack/ thromboembolism, vascular disease, sex category female) score, AF type, AF duration, and ventilation strategy. Baseline significance was set at p ≤0.05. All analysis was done using SPSS Statistics Software for Windows version 24.0 (IBM Corporation, Armonk, New York).
RESULTS
At our institution, HFJV support during AF ablation procedures was introduced in November 2015 and has since become standard practice (Figure 1). In that interval, 285 registry patients underwent PVI using RF force sensing. Of these 285 patients, HFJV was used in 249 (87.4%), while standard ventilation was used in 35 (12%). Of the 249 HFJV patients, 84 had complete demographic, procedural, and follow-up data for at least 1 year. Our matched control population (n = 84) included 35 patients undergoing ablation from November 2015 to November 2016, supported by standard ventilation, as well as 49 frequency-matched patients who underwent AF ablation with force-sensing ablation catheters from January 2015 to November 2015.
FIGURE 1. Utilization of HFJV Over Time.
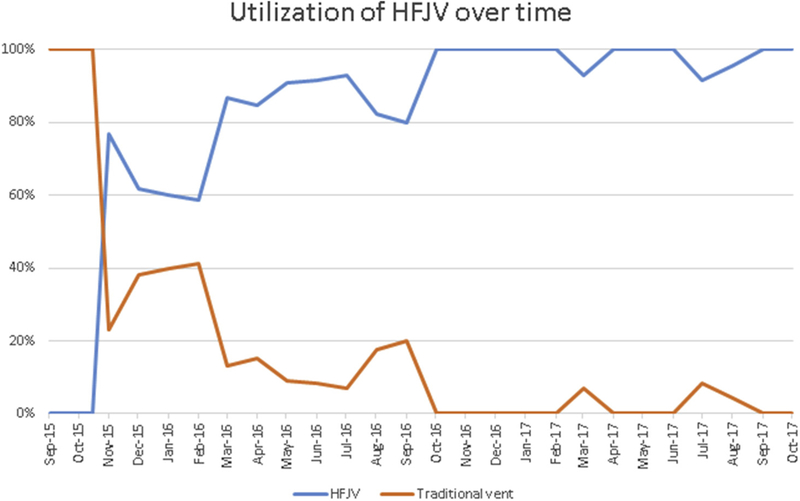
The utilization of traditional ventilation and HFJV, expressed as % of all PVI cases performed from September 2015 through October 2017. HFJV = high-frequency jet ventilation; PVI = pulmonary vein isolation.
PATIENT DEMOGRAPHICS.
Patients undergoing PVI with HFJV or standard ventilation were similar in age (63.1 years vs. 61.1 years; p = 0.237), gender (34.5% women vs. 26.2% women; p = 0.24), and body mass index (30.3 kg/m2 vs. 31.1 kg/m2; p = 0.303). Cohorts were matched for prevalence of paroxysmal AF (65.5%) and persistent AF (35.5%). Comorbidities including rates of diabetes, hypertension, prior cerebrovascular accident, obstructive sleep apnea, and coronary artery disease were similar between the 2 groups (Table 1). CHA DS -VASc score in the HFJV cohort was 2.12 T 1.64 and in the standard ventilation group was 1.98 T 1.57 (p = 0.564). Both groups had similar time since AF diagnosis (4.6 T 4.7 years vs. 4.5 T 4.4 years; p = 0.923). There was no difference in left atrial size between the HFJV and control cohorts (4.40 ± 0.57 cm vs. 4.41 ± 0.54 cm; p = 0.899); however, left ventricular ejection fraction on pre-procedure transthoracic echocardiography was higher in the HFJV group in the control group (57.3 ± 8.1% vs. 53.1 ± 10.9%; p = 0.017).
TABLE 1.
Comparison of Baseline Characteristics
| HFJV Group (n = 84) |
Control Group (n = 84) |
p Value | |
|---|---|---|---|
| Age, yrs | 63.1 ± 10.9 | 61.1 ± 11.1 | 0.237 |
| Female | 29 (34.5) | 22 (26.2) | 0.240 |
| BMI, kg/m2 | 30.3 ± 5.4 | 31.1 ± 6.1 | 0.303 |
| CHF | 6 (7.1) | 14 (16.7) | 0.057 |
| DM | 15 (17.9) | 15 (17.9) | 1.000 |
| CVA | 10 (11.9) | 6 (7.1) | 0.293 |
| HTN | 48 (57.1) | 53 (63.1) | 0.431 |
| OSA | 14 (16.7) | 20 (23.8) | 0.249 |
| CHA2DS2-VASc score | 2.12 ± 1.64 | 1.98 ± 1.57 | 0.564 |
| Paroxysmal AF | 55 (65.5) | 55 (65.5) | 1.000 |
| Persistent AF | 29 (34.5) | 29 (34.5) | 1.000 |
| Time since AF diagnosis, yrs | 4.6 ± 4.7 | 4.5 ± 4.4 | 0.923 |
| LVEF, % | 57.3 ± 8.1 | 53.1 ± 10.9 | 0.017 |
| LA size, cm | 4.40 ± 0.57 | 4.41 ± 0.54 | 0.899 |
Values are mean T SD or n (%).
AF = atrial fibrillation; BMI = body mass index; CHA2DS2-VASc = congestive heart failure, hypertension, age, diabetes mellitus, stroke/transient ischemic attack/thromboembolism, vascular disease, sex category female; CHF = congestive heart failure; CVA = cerebrovascular accident; DM = diabetes mellitus; HFJV = high-frequency jet ventilation; HTN = hypertension; LA = left atrium; LVEF = left ventricular ejection fraction; OSA = obstructive sleep apnea.
ANESTHESIA OUTCOMES.
Patients undergoing HFJV were monitored closely for adequacy of ventilation with serial arterial blood gas monitoring. Hypoventilation with concurrent respiratory acidosis was observed in 19% of the patients (n = 16), and hyper-ventilation with concurrent respiratory alkalosis was observed in 20.2% (n = 17), requiring adjustment of ventilator settings. None of the patients were converted to standard ventilation due to abnormal gas exchange.
The need for vasopressors during the procedure was significantly higher in HFJV compared with standard ventilation (79.7% vs. 22.4%; p = 0.001). However mean vasopressor (phenylephrine) dose in the HFJV population was similar to standard ventilation patients (4.35 mg vs. 4.6 mg; p = 0.82). Only 1 patient undergoing HFJV required conversion to standard ventilation due to persistent hypotension despite vasopressor support. The use of vasopressors did not increase the incidence of any adverse events or prolong the hospital stay compared with patients who did not require vasopressor support.
Total room time in the HFJV and standard ventilation groups was similar (265 ± 72 min vs. 253 ± 72 min; p = 0.295) as was total RF time (44.2 ± 13.4 min vs. 43.9 ± 15.4 min; p = 0.867).
ABLATION OUTCOMES.
PVI was achieved acutely in 100% of patients. Recurrence of atrial arrhythmia within 1 year was seen in 26 of 84 (31%) patients undergoing PVI supported with HFJV, compared with 42 of 84 (50%) matched control patients (p = 0.019) (Figure 2). In patients undergoing PVI for paroxysmal AF, the atrial arrhythmia recurrence rates were 27.3% in the HFJV group and 47.3% in the control group (p = 0.045) (Table 2). Persistent AF patients were found to have recurrent atrial arrhythmia rates of 37.9% (HFJV) and 55.2% (standard ventilation; p = 0.184) (Table 2).
FIGURE 2. Kaplan-Meier Curve for Atrial Arrhythmia–Free Survival.
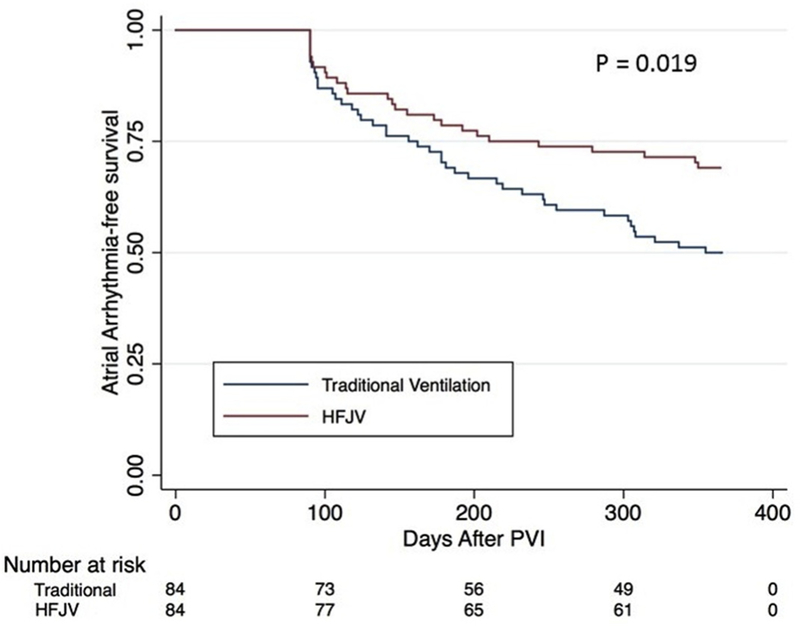
Arrhythmia-free survival during post-ablation follow-up for patients supported during PVI by traditional or HFJV. Abbreviations as in Figure 1.
TABLE 2.
Outcomes at 1 Year
| HFJV Group (n = 84) |
Control Group (n = 84) |
p Value | |
|---|---|---|---|
| AF recurrence at 1 yr | 26 (31.0) | 42 (50.0) | 0.019 |
| AF recurrence at 1 yr, paroxysmal AF | 15 (27.3) | 26 (47.3) | 0.045 |
| AF recurrence at 1 yr, persistent AF | 11 (37.9) | 16 (55.2) | 0.184 |
Values are n (%).
Abbreviations as in Table 1.
Multivariate analysis incorporating age, sex, body mass index, CHA2DS2-VASc score, AF years, AF type, and ventilation strategy found that HFJV was associated with reduced arrhythmia recurrence at 1 year (hazard ratio: 0.41; p = 0.009) (Table 3).
TABLE 3.
Multivariate Analysis of Factors Associated With Arrhythmia Recurrence
| HR | p Value | |
|---|---|---|
| Age per yr | 1.000 | 0.982 |
| Sex | 0.496 | 0.101 |
| BMI per kg/m2 | 1.000 | 0.998 |
| CHA2DS2-VASc score | 1.086 | 0.592 |
| Duration of AF per yr | 1.070 | 0.073 |
| Persistent AF | 1.197 | 0.624 |
| Jet ventilation used | 0.410 | 0.009 |
HR = hazard ratio; other abbreviations as in Table 1.
LESION ANALYSIS.
To investigate potential differences in catheter stability and lesion delivery in HFJV versus standard ventilation patients, post-procedural offline analysis of force, power, and time characteristics was performed at a total of 29,647 ablation points (HFJV 14,653, standard ventilation 14,814). Mean force variability index, defined as (Fmax — Fmin)/Fmax, was significantly lower in the HFJV group than in standard ventilation patients (0.84 ± 0.18 vs. 0.90 ± 0.15; p ≤ 0.001). Maintenance of target contact force >5 g during lesion delivery was significantly higher in patients supported by HFJV compared with standard ventilation (79.8% vs 76.7%; p < 0.001).
ADVERSE EVENTS.
One patient in the HFJV group experienced transient ischemic attack. This occurred 18 days after the index ablation; anticoagulation had been discontinued for 5 days for pacemaker insertion. In the control cohort, 2 patients experienced femoral hematoma, and were both managed conservatively (observation). No other adverse events occurred in either group.
DISCUSSION
We investigated the effects of instituting HFJV on patients undergoing PVI with RF force-sensing catheters, comparing procedural characteristics, adverse events, and 1-year outcomes in matched cohorts of patients (standard vs. HFJV ventilation). Our principal findings include: 1) institution of HFJV by our anesthesia service was not associated with increase procedural duration or adverse events; 2) anticipatory vasopressor use was increased in HFJV patients, without any appreciable adverse events; and 3) 1-year freedom from recurrent atrial arrhythmias was significantly higher in patients supported with HFJV compared with standard ventilation.
STRATEGIES TO IMPROVE LESION DELIVERY AND ABLATION OUTCOMES.
Recovery of PV conduction is a likely cause of recurrent AF following acutely successful PVI (1,8). Strategies to improve RF-mediated lesion delivery and to thereby minimize the likelihood of electrical reconnection of PVs are varied. One group of strategies focuses on feedback to the operator of surrogate markers for adequate catheter-tissue interface, including change in impedance during ablation, contact force between catheter and tissue (9,10), and calculated indices incorporating such variables as force, time, and power (11,12). A different approach to ensuring optimal lesion delivery is to stabilize catheter-tissue interface by minimizing respirophasic cardiac motion with HFJV (3,4).
This HFJV approach has been investigated previously by Hutchinson et al. (3) in a cohort of patients undergoing AF ablation. Combinations of electro-anatomic mapping, steerable introducer sheaths, and HFJV were used, with the conclusion that use of those 3 elements in conjunction improved 1-year freedom from AF compared with patients in which all 3 elements were not used. This study is the first (and only) to suggest that use of HFJV contributes to improved ablation outcomes; of note, it was performed before the advent of force-sensing catheters, which have become widely used to monitor and optimize tissue-catheter contact.
Our findings support and extend the findings of Hutchinson et al. (3), providing rationale for the use of HFJV even in the era of force-sensing catheter use. It seems reasonable to conclude that feedback mechanisms alone (including force sensing) are important but not sufficient to ensure catheter stability and that employment of strategies to minimize cardiac motion provides significant improvement in lesion delivery. This idea is supported by our observation that, even when compared with patients undergoing PVI with RF force-sensing catheters, the addition of a HFJV strategy led to reductions in force variability and improvements in maintaining force adequacy during ablation. These improved metrics are a plausible explanation for the improved out-comes seen in the HFJV cohort.
SAFETY OF HFJV.
Our anesthesia service was able to institute nearly universal use of HFJV in patients undergoing RF-mediated PVI without appreciable increase in adverse periprocedural events. Several trends appear to be noteworthy, including both the universal use of TIVA with propofol and remifentanil infusions and the significantly increased use of vasopressors (phenylephrine) during HFJV-supported PVI than during standard ventilation. TIVA use without surgical stimulation with HFJV leads to systemic hypotension. To avoid high doses of vasopressor agents to counteract hypotension, relatively lower doses of propofol and remifentanil were routinely utilized during ablation (13). However, anticipatory vasopressor use was still employed to maintain adequate perfusion. To avoid the possibility inadequate depth of anesthesia, bispectral index monitoring was used in all cases to ensure adequate depth of sedation at the lowest possible doses of TIVA agents (7).
A unique feature of HFJV is that it does not allow monitoring of end-tidal CO2 in expiratory gases to assess adequacy of ventilation; hypo-or hyperventilation may occur with development of respiratory acidosis or alkalosis, respectively. To maintain normocapnia, we utilized intermittent blood gas samples to monitor arterial pH and CO2 levels. No patients in our series required cessation of the HFJV approach secondary to compromised gas exchange. These practice changes (universal TIVA, vasopressor agent use, bispectral index monitoring, arterial blood gas monitoring to assess for ventilation adequacy) allowed for HFJV support with a minimum of adverse clinical events.
STUDY LIMITATIONS.
We report findings from a single-center, matched-cohort study. Intrinsic limitations include the study design (frequency matched cohort study, controlling for AF subtype); we have endeavored to carefully match cohorts while blinded to outcomes and feel that the comparison reported here is informative. Certainly, though, the results here are hypothesis-generating and would be well tested by a prospective, randomized clinical trial.
Patients undergoing standard ventilation were predominantly ablated between January 2015 and November 2015, while HFJV patients were ablated following November 2015. These different epochs introduce the specter of confounding increased operator experience in the HFJV cohort. All operators in the study, however, were high-volume, experienced operators before the enrollment period. Our limited sample size likely resulted in a study under-powered to detect outcome differences in patients undergoing ablation for persistent AF. A larger sample size may have shown a statistically significant difference. Strategies for HFJV may vary among institutions, and this may affect procedural safety and outcomes.
CONCLUSIONS
HFJV during PVI using RF force-sensing catheters is independently associated with improved procedural outcomes. Adverse events attributable to use of HFJV were not observed. Use of this ventilatory strategy should be considered by AF ablation programs performing RF-mediated PVI.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
Providing care to AF patients that is effective and safe is at the core of 2 basic clinical competencies: medical knowledge as well as patient care and procedural skills. We feel that our investigation allows readers to supplement these 2 competencies by providing data about whether a particular ventilator strategy (HFJV) during AF ablation improves effective lesion delivery, improves outcomes, and whether there are safety concerns associated with jet ventilation.
TRANSLATIONAL OUTLOOK:
Making ablation for AF patients a more safe and effective procedure is the subject of active investigation worldwide. This investigation raises important questions that are central in the delivery of effective and safe lesions during AF ablation— questions that clearly demand further exploration. PVI remains the cornerstone of ablation for both PAF and persistent AF and demands effective linear ablation for long distances around complex structures. Force-sensing catheters, jet ventilation, high-power or low-time lesion delivery, and other novel aspects of PVI all focus on the same end—effective lesion delivery. Strategies incorporating some or all of these approaches can and should be investigated with prospective, randomized exploration.
Acknowledgments
Funding for this research was provided in part by the Edward St. John Fund for AF Research, the Roz and Marvin H. Weiner and Family Foundation, the Dr. Francis P. Chiaramonte Foundation, the Marilyn and Christian Poindexter Arrhythmia Research Fund, Norbert and Louise Grunwald Cardiac Arrhythmia Research Fund, and the Mr. & Mrs. Larry Small AF Research Fund. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND A CRONYMS
- AF
atrial fibrillation
- DP
driving pressure
- HFJV
high-frequency jet ventilation
- PV
pulmonary vein
- PVI
pulmonary vein isolation
- RF
radiofrequency
- TIVA
total Intravenous anesthesia
Footnotes
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Clinical Electrophysiology author instructions page.
REFERENCES
- 1.Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017; 14:e275–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klain M, Keszler H. High-frequency jet ventilation. Surg Clin North Am 1985;65:917–30. [DOI] [PubMed] [Google Scholar]
- 3.Hutchinson MD, Garcia FC, Mandel JE, et al. Efforts to enhance catheter stability improve atrial fibrillation ablation outcome. Heart Rhythm 2013; 10:347–53. [DOI] [PubMed] [Google Scholar]
- 4.Goode JS Jr., Taylor RL, Buffington CW, Klain MM, Schwartzman D. High-frequency jet ventilation: utility in posterior left atrial catheter ablation. Heart Rhythm 2006;3:13–9. [DOI] [PubMed] [Google Scholar]
- 5.Sigl JC, Chamoun NG. An introduction to bispectral analysis for the electroencephalogram. J Clin Monit 1994;10:392–404. [DOI] [PubMed] [Google Scholar]
- 6.Myles PS, Leslie K, McNeil J, Forbes A, Chan MT. Bispectral index monitoring to prevent awareness during anaesthesia: the B-Aware randomised controlled trial. Lancet 2004;363:1757–63. [DOI] [PubMed] [Google Scholar]
- 7.Mashour GA, Shanks A, Tremper KK, et al. Prevention of intraoperative awareness with explicit recall in an unselected surgical population: a randomized comparative effectiveness trial. Anesthesiology 2012;117:717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ouyang F, Antz M, Ernst S, et al. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation 2005;111:127–35. [DOI] [PubMed] [Google Scholar]
- 9.Natale A, Reddy VY, Monir G, et al. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J Am Coll Cardiol 2014;64: 647–56. [DOI] [PubMed] [Google Scholar]
- 10.Reddy VY, Dukkipati SR, Neuzil P, et al. Randomized, Controlled Trial of the Safety and Effectiveness of a Contact Force-Sensing Irrigated Catheter for Ablation of Paroxysmal Atrial Fibrillation: Results of the TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation (TOCCASTAR) Study. Circulation 2015;132:907–15. [DOI] [PubMed] [Google Scholar]
- 11.Phlips T, Taghji P, El Haddad M, et al. Improving procedural and one-year outcome after contact force-guided pulmonary vein isolation: the role of interlesion distance, ablation index, and contact force variability in the ‘CLOSE’-protocol. Europace 2018. January 4 [E-pub ahead of print]. [DOI] [PubMed]
- 12.Wolf M, El Haddad M, Fedida J, et al. Evaluation of left atrial linear ablation using contiguous and optimized radiofrequency lesions: the ALINE study. Europace 2018;20:f401–9. [DOI] [PubMed] [Google Scholar]
- 13.Short TG, Hannam JA, Laurent S, et al. Refining target-controlled infusion: an assessment of pharmacodynamic target-controlled infusion of propofol and remifentanil using a response surface model of their combined effects on bispectral index. Anesth Analg 2016; 122:90–7. [DOI] [PubMed] [Google Scholar]


