Abstract
Effective and safe delivery of anticancer agents is among the major challenges in cancer therapy. The majority of anticancer agents are toxic to normal cells, have poor bioavailability, and lack in vivo stability. Recent advancements in nanotechnology provide safe and efficient drug delivery systems for successful delivery of anticancer agents via nanoparticles. The physicochemical and functional properties of the nanoparticle vary for each of these anticancer agents, including chemotherapeutics, nucleic acid-based therapeutics, small molecule inhibitors, and photodynamic agents. The characteristics of the anticancer agents influence the design and development of nanoparticle carriers. This review focuses on strategies of nanoparticle-based drug delivery for various anticancer agents. Recent advancements in the field are also highlighted, with suitable examples from our own research efforts and from the literature.
1. INTRODUCTION
Despite advancements in diagnostic and treatment strategies, cancer is still the second most common cause of disease-related death in the United States, according to the American Cancer Society. An estimated 1.6 million new cases of cancer and 0.6 million cancer-related deaths are predicted for the year 2017 (Cancer Facts & Figures, 2017, American Cancer Society). Cancers that spread to many parts of the body require more rigorous and comprehensive treatment regimens that usually involve chemotherapy as the first-line approach (Morabito et al., 2007; Telli & Carlson, 2009; Younes, Pereira, Fares, & Gross, 2011). Most chemotherapy drugs are difficult to administer directly, due to hydrophobicity. In addition, many are toxic to healthy tissues and produce undesirable side effects. As an emerging treatment modality, gene therapy utilizes nucleic acid materials, such as small interfering (si)RNA, DNA, and oligonucleotides, to silence cancer-causing genes, correct mutated genes, or enhance the expression of beneficial proteins that can prevent cancer cell growth and metastasis (Cross & Burmester, 2006; Song, 2007).
Recently, the combined effects of chemotherapy and gene therapy have been realized, especially in overcoming multidrug resistance (MDR). Delivery of gene therapy agents, alone or in combination with chemotherapy, is a hurdle because of their poor stability, lack of tumor selectivity, and rapid clearance from the body (Cross & Burmester, 2006; Wang, Lu, Wientjes, & Au, 2010). Other treatment modalities that use chemical or natural products as anticancer agents or small molecule inhibitors are hampered due to comparable delivery issues. For example, photodynamic therapy (PDT) uses photosensitizers (PSs), which are mostly hydrophobic, lack tumor selectivity, and require soluble formulation for in vivo administration (Babu, Jeyasubramanian, Gunasekaran, & Murugesan, 2012). Therefore, many anticancer agents share similar issues affecting safe and effective delivery to the tumor site via the systemic route.
To overcome these limitations, various delivery vehicles have been developed to achieve high therapeutic efficiency of anticancer agents by providing protection in the circulation and enhancing their bioavailability (Haley & Frenkel, 2008). Nanoparticles are attractive vehicles for anticancer agents, because oftheir controlled drug release and tumor-selective properties (Prabhu, Uzzaman, & Guruvayoorappan, 2011). Nanoparticles have been developed based on liposomes/lipids, polymers ofsynthetic and natural origin, and inorganic particles (Panyam & Labhasetwar, 2003; Wang, Li, Cheng, & Yuan, 2016; Zhao & Huang, 2014). This review describes the strategies using nanoparticles to deliver anticancer agents, such as chemotherapy drugs, PDT agents, small molecule inhibitors, and therapeutic genes. Nanoparticle designs for stimuli-responsive drug release are also emphasized. In addition, this review outlines some of the major cancer-specific cell surface receptors that have been explored for ligand-based targeted drug delivery using nanoparticles.
2. TYPES OF NANOPARTICLES FOR THERAPEUTIC DELIVERY
Liposome-based nanoparticle systems are widely used for drug and gene delivery. Since the 1995 US Food and Drug Administration (FDA) approval of the liposomal formulation for anticancer chemotherapeutic doxorubicin, Doxil®, many new liposomal formulations have been developed, entered into clinical trials, and FDA approved for clinical use (Table 1).
Table 1.
Clinically Approved Liposomal Chemotherapeutic Formulations for Cancer Therapy
| Brand Name | Drug (Active Agent) | Liposome Composition | Target Cancer | Manufacturer |
|---|---|---|---|---|
| Doxil | Doxorubicin | HSPC:Cholesterol:PEG 2000-DSPE | Ovarian, Breast, AIDS-Related Kaposi’s Sarcoma | Ben Venue Laboratories |
| Lipodox | Doxorubicin | HSPC:Cholesterol:PEG 2000-DSPE | Ovarian, Breast, AIDS-Related Kaposi’s Sarcoma, Multiple Myeloma | Sun Pharmaceuticals |
| Myocet | Doxorubicin | EPC:Cholesterol | Breast | Teva Pharma B.V. |
| DaunoXome | Daunorubicin | DSPC:Cholesterol | AIDS-Related Kaposi’s Sarcoma | NeXtar Pharmaceuticals |
| Mepact | Mifamurtide | DOPS:POPC | Osteosarcoma | Takeda Pharmaceuticals Limited |
| Onivyde | Irinotecan | DSPC:MPEG-2000:DSPE | Pancreatic Adenocarcinoma | Merrimack Pharmaceuticals Limited |
| Marqibo | Vincristine | Sphingomyelin and Cholesterol | Philadelphia Chromosome-Negative (Ph–) Acute Lymphoblastic Leukemia | Talon Therapeutics, Inc. |
| Lipoplatin/Nanoplatin | Cisplatin | Soy Phosphatidyl Choline (SPC-3): Cholesterol mPEG-DSPE:DPPG | Pancreatic, Lung | Regulon, Inc. |
Liposomes are small nanosized vesicles formed by lipid bilayers with an aqueous core. Hydrophilic drugs are encapsulated inside the core, whereas hydrophobic drugs are entrapped in the lipid bilayer. If the active agent is nucleic acid, liposomes are cationic in nature (Simoes et al., 2005). These cationic liposomes form a complex with negatively charged nucleic acid therapeutics through electrostatic interaction. If the liposomes are anionic, the nucleic acid materials are either complexed in the presence of calcium ions or encapsulated inside the core (Patil, Rhodes, & Burgess, 2004). Neutral lipids are used as helper lipids for efficient gene delivery (Balazs & Godbey, 2011). The major limitation of liposomes is their poor stability and burst release of drug.
Solid lipid nanoparticles (SLNs) offer unique properties, including small size, large surface area, high drug loading, the interaction of phases at the interfaces, and improved therapeutic efficacy of the loaded drug. SLNs are also more stable than liposomes and offer controlled release of their cargo (Muller, Mader, & Gohla, 2000). SLNs consist of solid-phase lipids at room temperature and surfactants for emulsification.
Polymer nanoparticles form a significant category of drug delivery vehicles, in which both synthetic and natural polymers are used in the preparation of nanoparticles (Amreddy, Babu, Muralidharan, Munshi, & Ramesh, 2017). Polymer nanoparticles are versatile in delivering numerous materials, including chemotherapy drugs, small molecules, genes, and proteins. Numerous polymer nanoparticles, such as polylactic acid (PLA), polylactic acid-co-glycolic acid (PLGA), poly(alkyl cyanoacrylate) (PACA), poly-caprolactone (PCL), polyanhydrides, polyethyleneimine (PEI), chitosan, and gelatin, are being tested in the laboratory (Zhang & Saltzman, 2013). The aforementioned polymers are biodegradable. Among these polymers, PLGA has been approved by the FDA for drug delivery purposes. Lupron Depot® is an FDA-approved drug for prostate cancer therapy that is based on a PLGA nanoparticle platform (Zhang & Saltzman, 2013). PCL nanoparticles are another controlled release platform for chemotherapeutics and other anticancer agents. They are commonly used as a long-term controlled delivery system because of their slow degradation rate (Danafar & Schumacher, 2016). In addition, polyanhydride-controlled release systems can be manipulated to release drugs during a large time range by adjusting the ratios of the copolymers’ aliphatic and aromatic anhydrides (Fu & Kao, 2010). Such a polyanhydride system, GLIADEL®, is used in the treatment of recurrent malignant glioma (Perry, Chambers, Spithoff, & Laperriere, 2007). Furthermore, PEI is a cationic linear or branched polymer commonly used for the delivery of siRNA or DNA for gene therapy (Lungwitz, Breunig, Blunk, & Gopferich, 2005). PEI has numerous free cationic groups that can electrostatically interact with nucleic acids and thereby condense them to form nanosized particles. However, PEI has a major limitation in that it is toxic to cells. Modification of PEI with neutral polymer PEG (Wu et al., 2010) or hybridization with other biocompatible polymer could reduce its toxicity and enhance its in vivo stability (Zheng et al., 2012).
Chitosan and gelatin are natural polymers that are commonly used for drug or gene delivery. Chitosan is a cationic polysaccharide, a modified form of chitin that is known for its biocompatibility and biodegradability. The presence of numerous functional groups allows for easy modification for use in drug delivery applications. Chitosan nanoparticles can encapsulate chemotherapeutics, genes, peptides, or small molecules, and efficiently deliver the cargo for tumor therapy (Babu & Ramesh, 2017; Layek, Lipp, & Singh, 2015; Patel, Patel, & Patel, 2010). The cationic nature of chitosan is harnessed for gene delivery, as negatively charged nucleic acids readily form complexes with chitosan to form nanoparticles. Gelatin-based nanocarriers are also highly biocompatible and biodegradable and can be easily functionalized due to their numerous free chemical groups. Gelatin is often crosslinked to form stable nanoparticles and to improve its performance as a drug delivery system. Gelatin nanocarriers can deliver a variety of drugs, including chemotherapeutics, protein and peptides, and siRNA (Kulsharova et al., 2013; Lee, Yhee, Kim, Kwon, & Kim, 2013; Xu, Singh, & Amiji, 2014).
Other commonly used nanocarriers include dendrimers, metal-based nanoparticles, such as gold and iron oxide nanoparticles, quantum dots, cyclodextrin, and silica nanoparticles. Dendrimers form unique nanocarriers and are uniformly branched structures originating from a core molecule. Numerous multivalent surface functional groups offer wide potential for multiple interactions and modifications, and can link therapeutic molecules and nucleic acids onto the surface functional groups and in the cavities formed by the branches radiating from the core molecule (Dufes, Uchegbu, & Schatzlein, 2005; Madaan, Kumar, Poonia, Lather, & Pandita, 2014).
The most commonly used dendrimer for drug and gene delivery applications is the polyamidoamine (PAMAM) dendrimer. PAMAM dendrimers of different generations can have drugs loaded into their cavities or attached to the periphery using chemical methods, and controlled and specified drug delivery can occur. Moreover, PAMAM dendrimers can also carry nucleic acid therapeutics, aided by electrostatic interaction-based complex formation (Abbasi et al., 2014). Most of these characteristics make dendrimers a good choice for drug and gene delivery for cancer therapy.
Gold nanoparticles exhibit a combination of unique physicochemical and optical properties that permit their use in various biomedical applications, such as diagnostic imaging and drug and gene delivery (Cai, Gao, Hong, & Sun, 2008). Gold nanoparticles are typically very small (<10nm), can easily penetrate into cells, and have the capability to deliver drugs, genes, and imaging agents with low solubility and poor pharmacokinetics. In general, gold nanoparticles exist in different shapes and structures, such as spheres, rods, stars, and clusters. All of these shapes have been explored for drug delivery. Gold nanoparticles are routinely surface functionalized with ligands to achieve increased selectivity in tumors and to specifically deliver their payload. These nanoparticles are generally modified with polymers or PEG-containing linkers to conjugate or complex with drug or siRNA/DNA. Hence, they are increasingly being used for drug or gene delivery purposes by exploiting the advantages of increased drug/gene loading, low toxicity due to the inert nature of gold, efficiency in cell uptake, fast endosomal escape, and stability in the circulation (Mendes, Fernandes, & Baptista, 2017).
Iron oxide nanoparticles, otherwise known as magnetic nanoparticles, have been used in biomedical applications for the last 3 decades. However, the potential of magnetic nanoparticles in drug and gene delivery for cancer therapy has only recently been realized. Therapeutic agents are either attached to or encapsulated within the nanoparticle. These particles have either magnetic cores with a polymer or metal coating that can be modified, or a porous polymer matrix that has magnetic particles precipitated within the pores (McBain, Yiu, & Dobson, 2008). Under the influence of an external magnetic field, magnetic nanoparticles are attracted to the tumor site and deliver the drugs. This is an important advantage, because this strategy can prevent accumulation of drug in healthy tissue. Superparamagnetic nanoparticles behave like magnets only when this external magnetic field is applied, and cause no toxicity on their own.
Mesoporous silica nanoparticles (MSNs) are a special class of nanoparticles that are small, rigid, and nonbiodegradable nontoxic nanocarriers for drugs, peptides, and genes. The characteristics of MSNs include a mesoporous nature, tunable pore size, high drug loading efficiency, and surface properties that can be altered to favor efficient drug or gene delivery, depending on additives used in the preparation of MSNs (Bharti, Nagaich, Pal, & Gulati, 2015). To enhance the surface properties, PEG molecules can be used to modify the MSNs surface by covalent conjugation or by adsorption or entrapment of PEG molecules in the MSNs surface. PEG functionalization can be utilized for covalent conjugation of drug molecules and targeting ligands for selective drug delivery. For gene delivery, a cationic polymer, such as PEI, is useful in modifying the MSNs surface to electrostatically attract nuclei acid molecules for delivery. The presence of PEI also enhances the endolysosomal escape capability of MSNs carrying nucleic acids (Hom et al., 2010).
Recently, researchers have explored the possibilities of using extracellular vesicles as drug/gene delivery vehicles (Jiang, Vader, & Schiffelers, 2017). Exosomes are the most prominently studied (Jiang et al., 2017; Srivastava, Amreddy, et al., 2016; Srivastava, Babu, et al., 2016). Exosomes are nanosized (30–100nm) lipid bilayer cellular vesicles that are involved in transportation of cellular cargo. Because of their small size, cellular origin, flexibility to incorporate macromolecules, such as DNA, RNA, and micro-RNAs (miRNA), into their lumen, and ability to cross-stringent biological barriers, such as the blood–brain barrier, exosomes are considered ideal candidates for drug/gene delivery vehicles (Shahabipour et al., 2017). Studies have shown that therapeutic siRNA and miRNA can be inserted into exosomes either by transforming the cell producing exosomes or by directly manipulating their lumen by adding molecules using techniques such as incubation, sonication, or electroporation (Johnsen et al., 2014). Although the field of exosome biology and application is relatively new, it is predicted that exosomes will have an important role in personalized and precision medicine, as patients’ own cells can be used to produce exosomes that will be used as therapeutic carriers.
Thus, a multitude of nanoparticle formulations are currently under investigation in the laboratory and clinical settings to enhance drug delivery efficacy for cancer therapy. Therefore, it is expected that the use of nanoparticle drug delivery systems will spread rapidly across different human ailments, especially cancer. The following sections discuss the different types of therapeutic molecules and delivery strategies using specifically designed nanoparticles, citing recent examples.
3. ANTICANCER THERAPEUTICS AND NANOPARTICLE-BASED DELIVERY AGENTS
Anticancer agents such as chemotherapeutics, photodynamic agents, small molecule inhibitors, and nucleic acid-based therapeutic agents often requires safe and efficient delivery systems for stability enhancement, delaying fast bioclearance, high accumulation in tumor site, and enhanced therapeutic potential upon systemic administration. In addition, nanoparticle-based drug delivery systems offer minimized exposure of drug toward normal tissues in the body. This section discusses different kinds of anticancer agents and nanoparticle-based drug delivery strategies for cancer therapy with suitable examples.
3.1. Chemotherapeutic Drugs
In cancer treatment, chemotherapy has been practiced for more than a century. Numerous antitumor agents that inhibit and reduce tumor growth are available (Isoldi, Visconti, & Castrucci, 2005; Monks et al., 1991). Chemotherapeutic agents include alkylating agents, plant alkaloids, antitumor antibiotics, and antimetabolics. Some agents are specifically used against certain cancers, while others are used in the treatment of multiple cancers. For example, hydrophilic drugs, such as doxorubicin (DOX), platinum compounds (cisplatin, carboplatin, and oxaliplatin), 5-fluorouracil, gefitinib, epirubicin, paclitaxel, camptothecin, and curcumin are used to treat various cancers. Most chemotherapeutic agents target cell division, eventually inhibiting cell growth via mechanisms such as inhibiting microtubule function, protein function, or DNA synthesis (Dumontet & Sikic, 1999; Palchaudhuri & Hergenrother, 2007). The direct administration of drugs into the body can cause severe side effects and toxicity to normal healthy tissues (Celikoglu, Karayel, Demirci, Celikoglu, & Cagatay, 1997). Hence, chemotherapy requires a withdrawal period to allow patients to recover from the effects of toxicity. Therefore, researchers are focused on the development of systems to deliver antitumor drugs to the targeted location. The specific delivery of drugs can be achieved safely by optimizing drug dose and monitoring drug levels in the body. These methods must allow for a controlled rate of delivery, sustained release, and targeted delivery to improve therapeutic efficacy while simultaneously reducing toxicity to normal tissues.
3.2. PDT Drugs
PDT is an emerging approach for improved and effective cancer treatment. Compared with other cancer treatment approaches, PDT has several advantages. PDT is cost effective and reduces the need for repeated and prolonged treatment. Most PSs exhibit auto fluorescence, which makes them easy to track and observe uptake inside the body (Usuda et al., 2007). Moreover, PDT can be controlled with near-infrared (NIR) light irradiation; once in the tumor region of interest, the PS can be irradiated and the drug released, thus reducing toxicity to normal tissues (Wang, Tao, Cheng, & Liu, 2011). PDT is mainly recommended for oral, head and neck, and ocular cancers, although researchers are currently exploring the use of PDT in the treatment of other cancers (Yoshida et al., 2008). PDT occurs through the dynamic nature of the three essential components: NIR light, PS (e.g., porphyrins, phthalocyanines, and chlorines), and molecular oxygen. The PS generates reactive oxygen species (ROS) in the presence of light, and causes oxidative damage to DNA and proteins that are involved in cell growth and proliferation of the tumor and its surrounding vasculature, as illustrated in Fig. 1 (Castano, Demidova, & Hamblin, 2004). The physical and chemical properties of the materials that are used in PDT will influence the effectiveness of the therapy. Injecting the PS directly into the body results in nonspecific biodistribution and poor tumor accumulation. Most PSs are hydrophobic in nature. Thus, the hydrophobic PS exhibits poor solubility under physiological conditions that reduce the tumor-targeting ability. The specific delivery of PS into tumor tissue is a challenge and requires a carrier that specifically delivers the PS into the targeted tissue region. Nanoparticles are appropriate carriers for delivering PS molecules. By conjugating or encapsulating the PS in nanoparticles, the above-mentioned limitations could be addressed (Chatterjee, Fong, & Zhang, 2008). In this section, we discuss recent advances in PDT with nanoparticles.
Fig. 1.
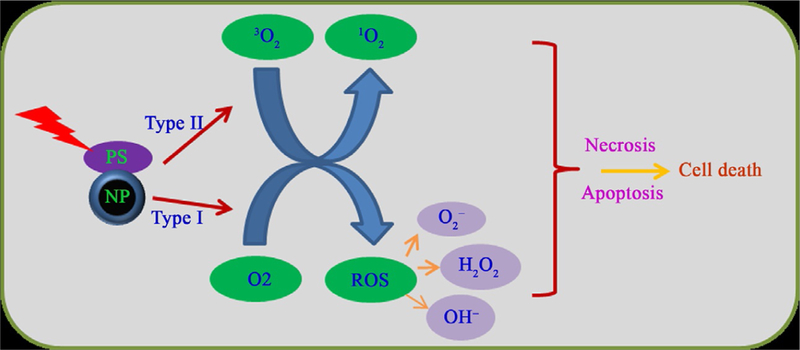
Schematic representation of the types I and II mechanisms of PDT upon light irradiation to generate ROS and singlet oxygen that cause necrosis and apoptosis, respectively.
Flak et al. demonstrated that ZnPc@TiO2 hybrid nanostructures, in the form of nanoparticles and nanotubes, could be used for dual therapy by loading PDT agent zinc phthalocyanine (ZnPc) and anticancer drug doxorubicin with folic acid targeting. They observed that these hybrid nanoparticles selectively targeted and displayed more uptake in human cervical cancer cells (HeLa) than in normal fibroblasts (MSU-1.1). Enhanced in vitro cytotoxicity and photocytotoxic activity was demonstrated with hybrid nanostructures selectively targeting to cancer cells (Flak, Yate, Nowaczyk, &Jurga, 2017).
Recently, Wang et al. used cold atmospheric plasma (CAP) as a light source, instead of NIR light. The researchers used Protoporphyrin IX (PpIX), which is the most popular PDT PS, using polymerosomes as carrier. They demonstrated that cancer cells can be effectively killed by using a CAP light source with a wide range of wavelengths, compared with UV light sources. After CAP irradiation, melanoma cell killing efficiency significantly increased to 80% when compared with no light source (45%) or a UV light source (65%) (Wang, Geilich, Keidar, & Webster, 2017).
Gold nanoparticles can be used as photothermal therapy (PTT) agents that show a synergistic therapeutic effect when combined with PDT. Li and colleagues reported the use of gold nanoclusters for dual PTT/PDT therapy for pancreatic ductal adenocarcinoma (PDAC). Gold nanoclusters were used as the PTT agent and Cathepsin E (CTSE) was used as the PDT prodrug. In this system, they observed that CTSE-sensitive nanoclusters with targeting U11 peptide can significantly increase the uptake and apoptosis of pancreatic cancer cells, compared with that of the nontargeted nanocluster AuS-PEG and the insensitive nanocluster AuC-PEG. They reported that enzyme-triggered drug release of 5-ALA with tumor targeting in nanoclusters AuS-U11 could achieve optimal therapeutic efficacy with endomicroscopy-guided PTT/PDT, with reduced side effects (Li, Wang, et al., 2017).
Gold nanorods are used as PTT agents with high laser doses, usually 808 nm, 1–48W/cm2 irradiations that kill the cancer cells. Vankayala et al. reported that gold nanorods themselves act as PDT and PTT therapeutic agents by optimizing the irradiation wavelengths. The researchers demonstrated that gold nanorods alone can sensitize the formation of singlet oxygen (1O2) as PDT affects inhibition of tumors in mice under very low LED/laser doses of single photon NIR (915nm, <130mW/cm2) light excitation. By changing the NIR light excitation wavelengths, Au NRs-mediated phototherapeutic effects can be switched from PDT to PTT or a combination (Vankayala, Huang, Kalluru, Chiang, & Hwang, 2014).
Magnetic nanoparticles are well known as magnetic resonance (MR) imaging agents. When a PDT agent is delivered with magnetic nanoparticles, the magnetic nanoparticles are used for image-guided PDT cancer therapy, which integrates diagnostic and therapeutic functionalities into a single system. Zhou et al. conjugated the IR820 onto the surface of iron oxide nanoparticles with 6-amino hexanoic acid to form IR820-CSQ-Fe conjugates. The IR820-conjugated iron oxide nanoparticles showed an enhanced ability to produce singlet oxygen almost double that of free dyes, which improved its efficiency for PDT, and showed increased T1 and T2 relaxation values (Zhou et al., 2016). In another approach, magnetic and fluorescent lanthanide-doped gadolinium oxide (Gd2O3) UCNs with bright upconversion luminescence (UCL) and high longitudinal relaxivity (r1) were used for simultaneous magnetic resonance imaging (MRI)/UCL dual-modal imaging and PDT. The results showed that these UCN/UCL/MRI nanoparticles act as good MRI contrast agents and fluorescence nanoprobes for live cell imaging. The researchers utilized the luminescence-emission capability of the UCNs for the activation of a PS to achieve significant PDT results and image-guided delivery (Liu, Huang, et al., 2017).
3.3. Small Molecule Inhibitors
Small molecule inhibitors are organic compounds with different chemical structures and molecular weights ranging from 500 to 900 Da; these inhibitors can easily diffuse across cell membranes to reach intracellular sites (Veber et al., 2002). Once the inhibitor enters the cells, it affects various other molecules, such as proteins, and ultimately kills cancer cells (Arkin & Wells, 2004). Small molecule inhibitors can be classified as natural, such as secondary metabolites, or artificial, such as antiviral drugs. These agents are structurally similar to chemotherapy drugs, but function as RNAi to knockdown specific proteins. Small molecule inhibitors can be used to study aspects of cell biology, such as cell cycle control, mitosis, oncogenic signaling pathways, gene expression, apoptosis, and autophagy (Weiss, Taylor, & Shokat, 2007). Inhibitors are used either alone or encapsulated with nanoparticles to treat various cancers.
Small molecule inhibitors have advantages over larger molecules, such as monoclonal antibodies. These advantages include easy diffusion across the cell membrane, which facilitates large-scale experiments in which there might be difficulties in transfection or knockdown using RNAi (Gowda, Jones, Banerjee, & Robertson, 2013; Madani, Naderi, Dissanayake, Tan, & Seifalian, 2011; Parhi, Mohanty, & Sahoo, 2012; Perumal, Banerjee, Das, Sen, & Mandal, 2011). Small molecule inhibitors can be administered orally, whereas biologics generally require injection or another parenteral administration. These inhibitors can be easily combined with other treatments. Titration of doses is relatively easy, and the compounds can be used for different cell lines and even from different species.
MiaPaCa-2 tumor cells treated with PH-427, a small molecule inhibitor of AKT/PDK1, showed rapid internalization. However, PH-427 encapsulated with PNP (PH-427-PNP) treatment improved the therapeutic effect in in vitro and in vivo studies in pancreatic cancer cells (Lucero-Acuna et al., 2014). Encapsulation of Mcl-1 small molecule inhibitors with lipid nanoparticles showed better cell killing efficiency in monocytes infected with human cytomegalovirus (HCMV) (Burrer et al., 2017).
Prostate-specific membrane antigen (PSMA) is a diagnostic and therapeutic marker for prostate cancer. Targeted delivery of gold nanoparticles with small molecule inhibitor phosphoramidate peptidomimetic to PSMA-expressing prostate cancer cells showed significantly higher and selective binding to LNCap cells than did nontargeted gold nanoparticles (Kasten, Liu, Nedrow-Byers, Benny, & Berkman, 2013). The small molecule inhibitor for Niemann–Pick type C1 (NPC1), NP3.47, formulated with lipid nanoparticles containing siRNA, enhanced the therapeutic efficacy of LNP–siRNA by increasing endosomal escape (Wang, Tam, et al., 2016).
Polymeric biodegradable nanoparticle poly-L-glutamic acid (PGA) is used for the conjugation of small molecules. Xyotax (PGA-paclitaxel) and CT-2106 (PGA-camptothecin) are now in clinical trials (Bhatt et al., 2003; Sabbatini et al., 2004). Hh pathway inhibitor (HPI)-1 is an antagonist of Gli1. It has been shown that encapsulation of HPI-1 using PLGA conjugated with PEG improved the systemic bioavailability compared with the parent compound in pancreatic cancer (Chenna et al., 2012). Aurora B is involved in cytokinesis, and inhibition of this kinase has shown mitotic catastrophe. Encapsulation of AZD2811 and AZD1152 using polymeric and lipid nanoparticles has resulted in increased drug accumulation in tumors (Ashton et al., 2016).
3.4. Nucleic Acid Therapeutic Agents
Targeted regulation of the expression of genes that are known to be involved in cancer-related pathways is the ultimate goal of various experimental approaches for cancer treatment. Efforts are being made to achieve complete knockdown of the targeted gene to stop the downstream network of genes/proteins, preventing disease progression. The initial efforts to knockdown genes involved the antisense method, ribozymes, and chimeric oligonucleotides. However, only a very weak suppression of gene expression was achieved (Watts & Corey, 2012).
In the 1990s, a novel method of controlling gene expression was discovered in a worm model organism (Caenorhabditis elegans). A short double-stranded RNA (dsRNA) was used to specifically and selectively suppress the expression of the target gene (Fire et al., 1998). This phenomenon is called RNAi or RNA interference. The dsRNA was termed small interfering RNA, or siRNA. RNAi begins when an enzyme, DICER, encounters dsRNA and chops it into pieces called small interfering RNAs or siRNAs (Bernstein et al., 2003). This enzyme belongs to the RNase III nuclease family. A complex of proteins gathers up these RNA remains and uses their code as a guide to search out and destroy any RNAs with a matching sequence, such as target mRNA, in the cell. The mechanism of RNAi-mediated gene silencing is commonly called posttranscription gene silencing, or PTGS (Zamore, Tuschl, Sharp, & Bartel, 2000).
In cancer treatment, RNAi technology has opened a new arena of opportunities to develop innovative gene therapy strategies. The targeted approach and the ability to design and custom synthesize siRNA with the desired sequence can be exploited to selectively knockdown the expression of specific genes that are critical for pathophysiology of cancer, such as different signaling pathways involved in cancer cell growth, metastasis, angiogenesis, or drug resistance. In the following sections, we discuss the advancements and methods for using siRNA, DNA, shRNA, and miRNA with different types of nanocarriers.
3.4.1. siRNA Delivery
Although the phenomenon of siRNA-based cancer gene therapy is promising, direct administration of siRNA to cells did not yield the expected results, largely because naked siRNA has a short half-life in vivo, as it is rapidly degraded by nucleases in biological fluids (Choung, Kim, Kim, Park, & Choi, 2006). Furthermore, with an average size of 15kDa, the naked siRNA cannot penetrate endothelial cells and tissues, and the extra-cellular matrix (ECM) further prevents siRNA from diffusing efficiently into the cellular membrane. In addition to these biological barriers, the negative charge present on siRNA prevents it from penetrating through negatively charged cell membranes. Inside the cell, the siRNA encounters harsh environment produced by the endosomes, which often results in clearing of siRNA through endosomal pathways. Finally, siRNA is cleared by the site of disease and bioaccumulates in the kidneys and liver. To circumvent these limitations and exploit the benefits of PTGS of targeted cancer-related genes by siRNA, researchers have developed novel nanoparticle-based delivery vehicles to transport siRNA into cells at the site of action in cancer cells. This method of delivery has additional benefits, such as that target moieties can be added to the nanoparticles that cause targeted delivery of complex to the desired cells. However, the physicochemical properties must be considered before selecting a delivery vehicle for siRNAs, as described in Tatiparti, Sau, Kashaw, and Iyer (2017).
Several nanoparticle formulations combining lipids and polymers have been developed as hybrid delivery systems that harness the properties of lipids, which facilitate the penetration and efficient delivery of siRNA to the cell, and properties of polymers, which provide stability and biocompatibility. Some of the siRNA delivery systems using liposome and polymer nanoparticles are in clinical trials, as represented in Table 2. A similar hybrid delivery system delivery of p53 siRNA was successfully tested by Kundu et al. in a mouse osteocarcinoma cell line (Kundu et al., 2017). Neutral lipids are biocompatible, but have fewer interactions with anionic polynucleotides, resulting in their poor bioavailability. The siRNA can be loaded inside the core of nanoparticles or can be coated into the surface of nanoparticles. Organic in nature, these nanoparticles show low immunogenic response and are biodegradable. Furthermore, siRNA can be complexed with lipids for additional stability of the siRNA in complexes, which promotes endocytosis of siRNA into the cells, as shown by Kim et al. (2007) in their work combining siRNA with DOTAP/cholesterol. In ovarian cancer liposomes, zwitterion 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC) was added to provide optimum stability and was shown to efficiently target oncoprotein EphA2 (Landen et al., 2005).
Table 2.
Current Clinical Trials With Different siRNA Delivery Platforms
| NCT Number | Title | Recruitment | Conditions | Interventions | Gender | Phases | Mode of Delivery |
|---|---|---|---|---|---|---|---|
| NCT00938574 | Study With Atu027 in Advanced Solid Cancer | Completed | Advanced Solid Tumors | Drug: Atu027 | All | Phase 1 | Lipid nanoparticle |
| NCT00689065 | Safety Study of CALAA-01 in Solid Tumor Cancers | Terminated | Cancer Solid Tumor | Drug: CALAA-01 | All | Phase 1 | Polymer nanoparticle |
| NCT02166255 | APN401 in Treating Cancer That Are Metastatic or Cannot Be Removed By Surgery | Active, not recruiting | Recurrent tumors at different stages | Biological: siRNA | All | Phase 1 | siRNA transfection |
| NCT01591356 | EphA2 Gene Targeting | Recruiting | Advanced Cancers | Drug: siRNA-EphA2-DOPC | All | Phase 1 | Lipid nanoparticle |
| NCT01437007 | TKM 080301 for Primary or Secondary Liver Cancer | Completed | Cancers With Hepatic Metastases | Drug: TKM-080301 | All | Phase 1 | Lipid nanoparticle |
| NCT02110563 | Study of DCR–MYC in Solid Tumors, Multiple Myeloma, or Lymphoma | Active, not recruiting | Solid Tumors | Drug: DCR-MYC | All | Phase 1 | Lipid nanoparticle |
| NCT01808638 | Atu027 Plus Gemcitabine in Advanced or Metastatic Pancreatic Cancer | Completed | Carcinoma, Pancreatic Ductal | Drug: Atu027 and gemcitabine | All | Phase 1 | Phase 2 | Lipid nanoparticle |
| NCT00672542 | Immunotherapy of Melanoma With siRNA | Completed | Metastatic Melanoma | Absence of CNS Metastases | siRNA | All | Phase 1 | Transfection |
| NCT01676259 | Study of siG12D LODER in Combination With Chemotherapy | Not yet recruiting | Pancreatic Cancer | Drug: siG12D- LODER | Drug: Gemcitabine + nab-Paclitaxel | All | Phase 2 | Polymer nanoparticle |
| NCT02314052 | DCR-MYC in Hepatocellular Carcinoma | Active, not recruiting | Hepatocellular Carcinoma | Drug: DCR-MYC | All | Phase 1 | Phase 2 |
Data retrieved from www.clinicaltrial.gov on June 28, 2017.
Chitosan is another negatively charged biopolymer that has been explored for drug/gene delivery formulations because of physical compatibility. Anderson et al. reported delivery of siRNA in lung adenocarcinoma H1299 cells via cationic chitosan (Andersen, Howard, Paludan, Besenbacher, & Kjems, 2008). The formulations of chitosan/siRNA nanoparticles vary, especially in the ratio of molecular weight to charge, which affects its stability and efficiency (Hsu et al., 2013).
Metal-based nanoparticles, such as gold or iron oxide and carbon materials, are also used to deliver siRNA. Wang et al. used an iron oxide-based nanoparticle vector for tumor-targeted siRNA delivery in an orthotropic hepatocellular carcinoma xenograft mouse model (Wang, Kievit, et al., 2016). Hybrid nanoparticles are the latest approach to explore improvements in gene delivery efficiency by overcoming the limitations associated with individual methods. Sardo et al. reported that transfection efficiency and low bioavailability can be considerably improved by using polymer-modified gold nanostars. They coated the gold nanostar with layers of hydrophilic and amphiphilic polymers that contained –SH and –SS linker groups to anchor the siRNA layers. The results indicated improved transfection efficiency and enhanced colloidal stability (Sardo et al., 2017). In another example, Lee et al. fabricated a hybrid delivery system by combining chitosan–deoxycholic acid with perflouropentane and iron oxide. To this hybrid system, siRNA was electrostatistically loaded and delivered to lung and breast cancer cell lines. The results demonstrated enhanced delivery and effect of siRNA in the recipient cells upon ultrasound exposure (Lee et al., 2017).
Since cancer is a multifaceted disease, the pooling of multiple siRNA has been considered. Chen et al. described a strategy for using a metallic nanoparticle consisting of selenium and ruthenium to deliver pools of siRNA for MDR and to interfere with microtubule dynamics. They showed enhanced uptake of siRNA in breast cancer cells and induction of cytotoxic and downstream signaling pathways, leading to improved therapeutic effects (Chen, Xu, et al., 2017).
Iron oxide nanoparticles can be used during MRI. Hence, delivery of therapeutic siRNA using these nanoparticles can be used for the dual purposes of noninvasive cancer imaging and treatment (Medarova, Balcioglu, & Yigit, 2016). Many recent studies have demonstrated the flexibility of gold-, iron-, and silver-based metallic nanoparticles when developing hybrid nanoparticles. This flexibility offers increasing possibilities to further develop metallic nanoparticles that could be used for delivery of siRNA in clinics in the near future.
siRNA efficacy would be further improved if the cells’ own vesicles could be used as delivery vehicles. This approach will overcome the body’s immunogenic response. Exosomes, the 30–100-nm-sized nanovesicles produced from almost all cells, have attracted recent attention as promising drug delivery vehicles. Previously considered the trash bag of the cell, with the major function of removing toxic materials from the cell, exosomes are now recognized for transporting cellular molecules, such as nucleic acids, proteins, and lipids, from one cell to another and/or to intercellular spaces. Alvarez-Ervit et al. used exosomes derived from immature dendritic cells to deliver siRNA for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and BACE1 into the brains of C57BL/6 mice, demonstrating for the first time that siRNA can be delivered via exosomes. Exosome-based vehicles are reported to overcome cytotoxic and immunogenic responses produced by synthetic nanoparticles. Since exosomes can pass through the stringent blood–brain barrier, exosome-based siRNA delivery for genes involved in brain cancer could be successful in the future (Alvarez-Erviti et al., 2011).
3.4.2. Plasmid DNA Delivery
DNAs are high molecular-weight molecules with double strands that contain transgenes for specific proteins. In therapeutics, DNA inhibits the generation of specific protein through the release of transgenes into cells. The design and construction of plasmids with transgene(s) of interest is an important step in developing DNA therapeutics. Nanoparticle-based carriers play an important role in improving transfection efficiency and therapeutic effects. Cationic DOTAP liposome formulations are used for the conjugation of CXCR4 siRNA, HMGA1 DNA, IL-24 DNA, and FUS1 DNA in lung adenocarcinoma cells (Ito et al., 2004; Panneerselvam et al., 2015, 2016). Codelivery of p53-encoding plasmid and DOX using double-walled PLGA microspheres reportedly improved drug activity (Xu, Xia, Wang, & Pack, 2012). A nonviral dendrimer-based delivery system was used to deliver a combination of TRAIL gene therapy with DOX chemotherapy in human glioblastoma (Liu et al., 2012). Furthermore, SLNs composed of 3β-[N-(N’,N’-dimethylaminoethane)-carbamoyl]cholesterol hydrochloride (DC-Chol), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), and Tween 80 were used to complex with p53-EGFP plasmid DNA in NSCLC cells (Choi et al., 2008). Lecithin-based SNLs were used for the combination therapy of EGFP plasmid DNA and doxorubicin in mice bearing A549 tumors (Han, Zhang, Chen, Sun, & Kong, 2014). Cationic poly-amino acid conjugated magnetic nanoparticles were complexed with NM230HA-GFP plasmid DNA for gene transfection in mice bearing B16F10 melanoma tumors (Jiang, Eltoukhy, Love, Langer, & Anderson, 2013). Nonviral DNA delivery using biodegradable poly(β-amino ester)s (PBAEs) has been shown to be more effective than naked DNA in targeting malignant glioma (Guerrero-Cazares et al., 2014).
3.4.3. shRNA Delivery
shRNA, or short hairpin or small hairpin RNA, is another mode of inducing RNA interference-mediated posttranscriptional gene silencing for target genes. The shRNA consists of an RNA molecule with a hairpin-like structure; the molecule is slightly larger than siRNA molecules and, unlike siRNA, is produced inside the cell in the nucleus. From a therapeutics perspective, shRNA can be used to treat different diseases, including cancers. Clinical trials using shRNA are listed in Table 3. Several shRNA delivery methods have been studied. In prostate cancer, a folate-targeted nanoparticle complexed with AR-shRNA enhanced radiosensitivity (Zhang, Liu, et al.,2017). In addition, WT-shRNA was delivered using a transferrin-targeted PEG liposome in melanoma (Saavedra-Alonso et al., 2016).
Table 3.
Current Clinical Trials With Different shRNA Delivery Platforms
| Title | Recruitment | Conditions | Interventions |
|---|---|---|---|
| Bi-shRNA-furin and Granulocyte Macrophage Colony Stimulating Factor Tumor Cell Vaccine for Advanced Cancer | Active, not recruiting | Ewing’s Sarcoma, Nonsmall Cell Lung Cancer, Liver Cancer | Biological: Vigil™|Biological: Vigil™|Biological: Vigil™ |
| Pbi-shRNA STMN1 LP in Advanced and/or Metastatic Cancer | Completed | Advanced Cancer Metastatic Cancer Solid Tumors | Biological: pbi- shRNA STMN1 LP |
| Pbi-shRNA™ EWS/ FLI1 Type 1 LPX in Advanced Ewing’s Sarcoma | Recruiting | Ewing’s Sarcoma | Biological: pbi- shRNA™ EWS/ FLI1 Type 1 LPX |
| Treatment of Chronic Lymphocytic Leukemia | Not yet recruiting | Leukemia, Lymphocytic, Chronic, B-cell | Genetic: shRNA |
| Human Immunodeficiency Virus-Related Lymphoma Receiving Stem Cell Transplant | Recruiting | HIV Infection, Mature T cell and NK cell, and cancer | Lentivirus Vector CCR5 shRNA and other interventions |
Data retrieved from www.clinicaltrial.gov on June 28, 2017.
3.4.4. miRNA Delivery
miRNAs are another class of regulatory RNA epigenetically controlling various cellular processes under normal and pathological conditions. These miRNAs are conventionally classified as a small, noncoding class of RNA with a size of 18–22 nucleotides. The mechanism of action of miRNA overlaps with that of siRNA in certain aspects, but miRNA can regulate more than one mRNA and, thus, more than one gene. Furthermore, the mode of synthesis of miRNA and siRNA is different. miRNA is synthesized endogenously from cell DNA in a two-step process ending in the nucleus and cytoplasm, while siRNA is added exogenously to the cell. Since miRNA can regulate the expression of multiple mRNAs and miRNAs are involved in cancer physiology, they have been recently explored as both therapeutics and targets for cancer treatment. Studies involving miRNA therapeutics are increasing, as there are currently approximately 160 active clinical trials (www.clinicaltrial.gov).
Various nanoparticle formulations have been developed for in vitro and in vivo delivery of miRNAs and anti-miRNAs (antimirs) for therapeutic purposes. However, the challenge of miRNA delivery is the lack of a specific, nontoxic, and efficient method of delivery. Since miRNA can also act as an oncoMiR, an antimir is often used to suppress the expression of such oncogenic miRNA. miR-21 is an oncoMir in many cancers, including triple-negative breast cancer (TNBC). In a recent study by Shu et al., an RNA nanoparticle conjugated with epidermal growth factor receptor (EGFR) aptamer was used to target delivery of antimirs for miR-21 in TNBC, in vitro and in vivo (Shu et al., 2015). Ultrasound-induced microbubble cavitation has been widely recognized as a safer way of delivering therapeutics. Wang et al. used ultrasound cavitation to deliver miR-122 loaded with PLGA–PEG nanoparticles into human colon cancer xenografts in mice. The findings showed that this novel approach can be used for noninvasive delivery of therapeutic miRNA in cancer treatment (Wang et al., 2015).
Multiple miRNAs often work together as clusters that are produced from a single pri-miRNA. In a study by Subramanian et al., an aptamer for the miT17 ~ 92 cluster was used in retinoblastoma cell lines and resulted in downregulation of miRNA of the cluster (Subramanian, Kanwar, Kanwar, & Krishnakumar, 2015). In addition, various conventional metallic nanoparticles, such as galadonium, gold, and iron, have been found to functionalize with miRNA and have been extensively explored (Hsu et al., 2013; Yoo et al., 2014). Recently, the presence of miRNAs was reported in the lumen of cellular vesicle, such as exosomes, which are already known for carrying and delivering cellular cargo across the cellular microenvironment. In recent years, the miRNA present in exosomes has been exploited as a therapeutic drug delivery vehicle (Srivastava, Babu, et al., 2016; Srivastava et al., 2015). In addition, loading exosomes with miRNA of therapeutic importance has been reported. Researchers have also adopted the use of biomimetics, which are mimics of natural vectors and have similar properties. Although the concept of miRNA as gene therapy molecule is relatively new, this multitargeted approach is expected to become a popular tool for cancer treatment and a novel approach for efficient drug delivery.
4. NANOPARTICLE-BASED CODELIVERY OF DRUGS AND GENES
Individual cancer therapeutic agents and approaches may not sufficient to cure cancers. Using high doses of drug to increase the therapeutic effect may lead to drug resistance and undesirable side effects (Liu, 2009). Therefore, combined therapy may be more effective than the corresponding individual treatments alone. The rationale to use combination therapies is that the therapeutics work by different mechanisms of action, which leads to reduced resistance in cancer cells (Herman et al., 1988). In combination therapy, each therapeutic agent can be used at the optimal dose that is tolerable to healthy tissues to reduce toxicity. Based on the type and stage of cancer, the appropriate combination therapy will be used. Combination therapies are more suitable for advanced cancers, such as nonsmall cell lung cancer, esophageal cancer, or bladder cancer.
Since cancer is a complex disease involving multiple targets of therapy, the codelivery of a chemotherapeutics with genes has been explored. Chemotherapy is one of most used approaches in cancer treatment; however, it has some drawbacks, such as drug resistance and toxicity to healthy tissues (Liu, 2009). RNAi has been incorporated into nucleic acid medicines for cancer treatment. The codelivery of nucleic acids with chemotherapy drugs can have additive or synergistic effects that maximize therapeutic efficacy. Codelivery overcomes many obstacles of individual treatments, such as drug resistance and toxicity to normal tissues (Wang, Zhao, et al., 2010). However, a safe and efficient delivery system is needed for the codelivery of nucleic acids and chemotherapeutic drug molecules. Nanoparticle-based delivery systems are excellent carriers and have been extensively studied in this context. This section discusses the codelivery of RNAi molecules with chemotherapy drugs via different types of nanoparticles (Khan, Ong, Wiradharma, Attia, & Yang, 2012).
Zhu et al. developed binary polymer low-density lipoprotein-N-succinyl chitosan–cystamine–urocanic acid (LDL-NSC-SS-UA) micelles with dual pH/redox sensitivity and targeting for the codelivery of breast cancer resistance protein, siRNA, and paclitaxel (PTX). These siRNA–PTX-loaded micelles exhibit stability under physiological conditions. In the tumor microenvironment (pH/redox), the micelles showed fast release of gene and drug molecules. These micelles showed enhanced antitumor activity and downregulated the protein and mRNA expression levels ofbreast cancer resistance protein in MCF-7/Taxol cells. The in vivo study results revealed that the siRNA-PTX-loaded micelles showed prolonged circulation time with a remarkable tumor-targeting effect, and effectively inhibited tumor growth (Zhu et al., 2017). Another group reported a redox-sensitive micellar system that was synthesized with hyaluronic acid-based amphiphilic conjugate HA-ss-(OA-g-bPEI), HSOP, and was loaded with paclitaxel (PTX) and AURKA-specific siRNA (si-AURKA) with tumor-targeting molecules. In vitro and in vivo, the HSOP micelles simultaneously delivered the PTX and siRNA, producing synergistic effects between the drugs that led to greater antitumor efficacy than that observed with single drug-loaded micelles and nonsensitive coloaded micelles (Yin, Wang, Yin, Zhou, & Huo, 2015).
IL17RB siRNA and DOX were codelivered using a chitosan-based nanoparticle and showed enhanced anticancer efficacy. Similarly, VEGF and Bcl-2 dual-targeted siRNA was used to study prostate cancer, and the results revealed enhanced treatment efficacy when the dual siRNA was administered (Lee et al., 2015). In theory, such an approach can be adopted in which the drug resistance gene is silenced, followed by the delivery of drug. Similarly, a lipid–polymer nanoparticle system was used to deliver gefitinib and HIF1α gene treatment for pancreatic cancer (Zhao et al., 2015). The use of chitosan increases the functionality of codelivery nanoparticle systems. For instance, Babu et al. (2014) prepared a nanoparticle system based on chitosan and PLA for codelivery of P62 siRNA, proteasome β5 plasmid, and cisplatin. The nanoparticle was designed such that the PLA nanoparticle encapsulated the drug cisplatin and was coated with chitosan, onto which sip62 and pβ5 were adsorbed by maintaining a siRNA:pDNA:CS ratio of 1:0.2:53 (w/w). This codelivery strategy using a chitosan–PLA nanoparticle system resulted in P62 knockdown, β5 overexpression, and enhanced cisplatin sensitivity in drug-resistant ovarian cancer cells. Fig. 2 shows the effect of P62 and β5 protein expression levels in cisplatin-resistant 2008/C13 cells that received various treatments using the chitosan–PLA codelivery system (Babu et al., 2014).
Fig. 2.
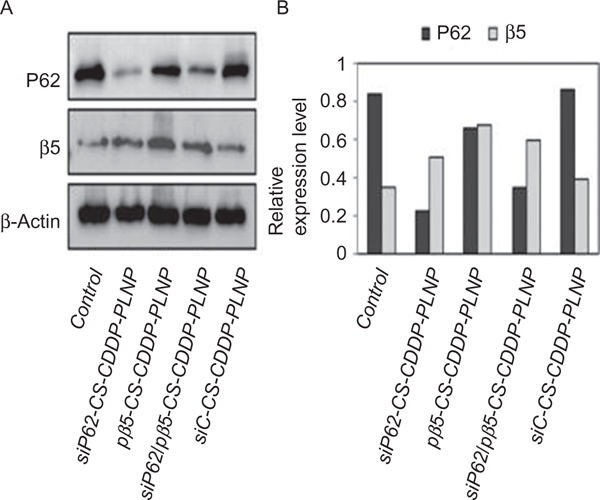
(A) The effect of codelivery of siP62 and/or pβ5 using cisplatin-encapsulated, chitosan-coated polylactic acid nanoparticles (CS-CDDP-PLNP) on P62 and β5 protein expression levels in cisplatin-resistant 2008/C13 cells. (B) The corresponding diagram shows relative expression levels of the two proteins normalized to beta actin. Figure reproduced from Babu, A, Wang, Q., Muralidharan, R., Shanker, M., Munshi, A, & Ramesh, R. (2014). Chitosan coated polylactic acid nanoparticle-mediated combinatorial delivery of cisplatin and siRNA/plasmid DNA chemosensitizes cisplatin-resistant human ovarian cancer cells. Molecular Pharmaceutics, 11(8), 2720–2733. doi:10.1021/mp500259e. Copyright © 2014, American Chemical Society.
Different nanoparticles with drug combinations have gained popularity recently. In a solid tumor model, shRNA against thymidylate synthase (TS shRNA) complexed with cationic liposome (TS shRNA-lipoplex) was codelivered with oxaliplatin and showed enhanced effects and accumulation of the TS shRNA lipoplex. In breast cancer, a combination treatment using doxorubicin functionalized to graphene oxide (GO-PAMAM) with shRNA against MMPS showed an enhanced therapeutic response (Gu et al., 2017). Zhang et al. used a combined approach to deliver Akt-shRNA with drug demethylcantharate. The results in three cancer cell lines confirmed the enhanced therapeutic response ofthis combination molecule (Zhang et al., 2016).
Codelivery of miRNA (or antimiRs) and drugs is expected to produce an enhanced effect, as miRNA can affect the expression of several genes involved in the signaling pathways, which can enhance the sensitivity of cell for a given drug. Similarly, tumor suppressor miR can be delivered to curtail the effect of metastatic relapse and rapid cell proliferation, shielding the cells from the effects of the drug. A combined formulation of DOX and miR 34a in breast cancer produced enhanced antitumor effects in breast cancer in vivo and in vitro. The therapeutic effect of DOX is further complemented with miR-34a, which inhibits cancer cell migration by targeting the Notch-1 signaling pathway. A hyaluronic acid–chitosan nanoparticle was formulated for delivery of this combination (Deng et al., 2014). Another approach to achieve synergistic therapeutic response is to use multiple miRNAs involved in a critical pathway (Pencheva et al., 2012).
5. STIMULI-RESPONSIVE DRUG DELIVERY
Recent progress in materials science and drug delivery allows spatial-, temporal-, and dosage-controlled mechanisms to be introduced in nanoparticle drug delivery systems. Such a modification to nanoparticles can be achieved by using stimuli-responsive materials in their synthesis. The stimuli-response delivery systems address the issues of controlled dose release of drug in response to various stimuli signals specifically produced in the tumor microenvironment, as represented in Fig. 3. Therefore, there will in theory be almost zero drug release until stimuli are applied. Major stimuli signals in the tumor microenvironment include pH, redox state and concentration, and types and concentrations of proteins and enzymes (Lopes, Santos, Barata, Oliveira, & Lopes, 2013). The response of nanoparticle drug delivery systems to these internal stimuli allows tumor-specific release of the payload. External stimuli can also be applied for additional specificity in tumor-targeted drug delivery (Puoci, Iemma, & Picci, 2008). In the following subsections, we focus on recent developments in the design of nanoparticle-based stimuli-responsive systems that are able to control drug release in response to external (temperature, magnetic field, ultrasound, light, or electric pulses) or internal (pH, enzyme concentration, or redox gradients) stimuli.
Fig. 3.
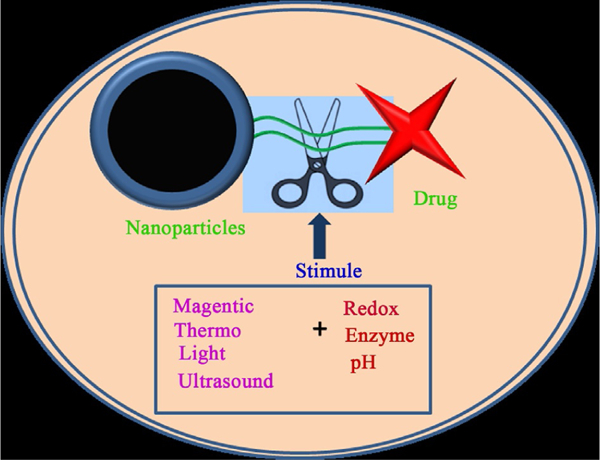
Schematic representation illustrating stimuli-responsive, tumor-targeted drug delivery under extracellular and intracellular gradients in nanoparticle–drug conjugates.
5.1. Thermostimuli
Thermoresponsive drug delivery systems are more stable at body temperature, 37°C. However, with a slight increase in temperature to 40–42°C, the carrier exhibits stimuli responsiveness and released the payload (Bikram & West, 2008). Usually, liposomes and polymers respond to changes in the temperature of the external environment, which then changes their conformation, solubility, and hydrophilic/hydrophobic balance (Kneidl, Peller, Winter, Lindner, & Hossann, 2014). The most commonly used thermosensitive polymer is poly(N-isopropyl acrylamide), commonly called PNIPAM. PNIPAM exhibits a lower critical solution temperature (LCST) parameter (Gong et al., 2013).
Tagami et al. reported the development of thermoresponsive liposomes (HaT liposome) for image-guided drug delivery in which they codelivered Gd-DTPA for T1 MRI and chemotherapy drug doxorubicin (DOX). They observed that 100% of Dox was released at 40–42°C in 3min with a correlation of MR T1 values that indicated a 60% reduction in MR T1 relaxation values. In the in vivo study, researchers observed that DOX showed more antitumor efficacy with enhancement of T1 signal in a heated tumor environment (Tagami et al., 2011). Another group utilized the dissociative properties of thermosensitive liposomes, which combine with unfolding advantages of temperature-sensitive peptide to improve DOX accumulation in tumors with leucine zipper peptide–liposome hybrid nanocarriers (Al-Ahmady et al., 2012). Another hydrophobic chemotherapy drug, paclitaxel (PTX), also showed enhanced antitumor activity in vitro and in vivo when delivered using thermosensitive liposomes. Wang and coworkers reported more PTX release at 42°C than at 37°C. An in vivo study of a xenograft lung tumor model showed enhanced drug release in tumor tissues when hyperthermia occurred, resulting in increased tumor growth suppression, compared with nontemperature-sensitive liposome and free drug treatment (Wang, Zhang, et al., 2016).
Polymers are another important class of thermosensitive materials. Among these, poly(N-isopropylacrylamide-co-acrylamide), commonly called P(NIPA-co-AAm), is extensively utilized. Zhang et al. studied P(NIPA-co-AAm) as a hydrogel in delivering multiple hydrophobic drugs, 5-fluorouracil (5-FU), fluorescein, docetaxel (DTX), and near-infrared dye-12 (NIRD-12), and performed in vitro release studies at different temperatures. DTX-loaded thermoresponsive hydrogels enhanced the tumor inhibition to 78.15% with hyperthermia in Kunming mice bearing S180 sarcoma compared with the same mice without hyperthermia (48.78%), and reduced toxicity to normal tissues (Zhang, Qian, & Gu, 2009). Another polymer, poly(γ−2-(2-(2-methoxyethoxy)-ethoxy) ethoxy-ε-caprolactone)-b-poly(γ-octyloxy-ε-caprolactone), PMEEECL-b-POCTCL, exhibits LCST at 38°C and demonstrated thermoresponsive properties. Dox-loaded thermoresponsive micelles of PMEEECL-b-POCTCL showed increased cytotoxicity at the above LCST in the MCF7 breast cancer cell line (Cheng et al., 2012).
5.2. Magnetic Stimuli
Magnetic nanoparticles convert an applied magnetic field into heat. Because of the transformation of magnetic energy into heat by the dynamic response of a dipole with their magnetic moments, that heat acts as a stimulus in delivering the drugs (Giri, Trewyn, Stellmaker, & Lin, 2005). Magnetic-responsive drug delivery has great potential for the development of safe and dual therapy agents (Kumar & Mohammad, 2011). The external magnetic field plays an important role in the controlled release of drugs from magnetic drug delivery carriers.
Magnetic nanoparticles must be modified with other materials to load drugs for stimuli-responsive delivery. Zhang et al. developed a fluorescent-magnetic biotargeting system with PEI-modified fluorescent Fe3O4@mSiO2 yolk-shell nanobioprobes as a model for simultaneous fluorescence imaging and magnetically guided drug delivery in liver cancer cells (Zhang et al., 2012). Another group developed poly[aniline-co-N-(1-one-butyric acid) aniline] (SPAnH) coated on Fe3O4 cores, which enhanced the therapeutic capacity and thermal stability of 1,3-bis (2-chloroethyl)-1-nitrosourea (BCNU), a compound used to treat brain tumors. Both in vitro and in vivo studies showed that, after applying an external magnetic field, nanoparticles bound-BCNU-3 accumulated at targeted tissues and increased the therapeutic efficacy, while reducing toxicity (Hua et al., 2011).
In another approach, Harries and coworkers demonstrated stimuli-responsive controlled delivery of vancomycin antibiotic with Fe3O4 nanoparticles. Both the antibiotic and Fe3O4 nanoparticles were loaded onto chitosan microbeads with polyethylene glycol dimethacrylate as the crosslinker. Researchers studied the elution properties with successive magnetic stimulations at multiple field strengths and frequencies. They observed that 30 min of magnetic stimulation induced a temporary increase in the daily elution rate of up to 45%. This increase was influenced by field strength, field frequency, and crosslinker length. After 3 days, 70% of the beads were degraded, but continued to elute antibiotic for up to 8 days. No cytotoxic effects were observed in vitro, compared with controls (Harris et al., 2017). Recently, another group reported using the MnFe2O4 core and NIR/pH-coupling sensitive mesoporous silica shell nanocarriers for enhanced antitumor efficacy and T1/T2-weighted dual-mode MRI applications in vivo. This nanocomposite showed the photothermal/chemo dual-modal synergistic therapies triggered by NIR/pH. They observed that, under 808 nm irradiation, MnFe2O4 can transform light into heat, which ablates tumor cells directly and also promotes the release of DOX from the mesoporous layer (_S1_Reference30_S1_Reference30Chen, Deng, et al., 2017). Deng and colleagues reported the development of a dual, external triggered, and magnetic stimuli-responsive delivery system with hybrid microcapsule (h-MC) by an electrostatic layer-by-layer assembly of polysaccharides (sodium alginate, chitosan, and hyaluronic acid), iron oxide, and graphene oxide (GO), in which the drug is loaded through pH control. The alternative irradiation of magnetic field and NIR laser applied on triggerable Fe3O4@GO component for dual high-energy and high-penetration hyperthermia therapy (Deng et al., 2016).
5.3. Light Stimuli
Drug release from light-responsive drug delivery systems can be controlled temporally and spatially using specific light irradiation of different wavelengths. Delivery systems have been developed that respond to light of ultra-violet (UV), visible, or NIR wavelengths (Alvarez-Lorenzo, Bromberg, & Concheiro, 2009). Light-induced delivery systems can be achieved through different mechanisms, such as light-induced isomerization, bond cleavage, and disaggregation of materials (Fomina, Sankaranarayanan, & Almutairi, 2012). Drugs can be conjugated with nanoparticles through photocleavable ligands. Upon light irradiation, ligands cleave or activate and release the drugs.
Zhang et al. reported a titanium dioxide-based visible light-sensitive nanoplatform (HA–TiO2–IONPs/ART) for codelivery of artemisinin as a type of Fe2+-dependent drug and Fe2+ cotransport system. This system showed improved antitumor effects. The researchers observed that the HA–TiO2–IONPs/ART system synchronized the codelivery of Fe2+ and artemisinin tumor-responsive release, and generated ROS under visual light irradiation. Both in vitro and in vivo results indicated that the HA–TiO2–IONPs/ART system exhibits enhanced antitumor activity when combined with laser irradiation (Zhang, Zhang, et al., 2017). This switchable dual light- and temperature-responsive drug carrier system was reported to use a gold nanoparticles (Au NPs)-grafted poly(dimethylacrylamide-co-acrylamide)/poly(acrylic) acid [P(DMA-co-AAm)/PAAc] hydrogel. Then, the researchers studied the swelling, thermal sensitivity, and thermal and optical switching properties of the prepared hydrogels in acidic (pH 1.2) and neutral (pH 7.4) buffered solutions to simulate stomach and intestinal conditions. During ofloxacin antibiotic release studies, it was observed that the “on” state occurred at higher temperatures and the “off” state occurred at lower temperatures (Amoli-Diva, Sadighi-Bonabi, & Pourghazi, 2017) under thermal and optical switching conditions.
Li et al. demonstrated a CDDP drug delivery system with NIR light stimuli-responsive drug release properties based on the coordination chemistry of CDDP and tellurium-containing block polymer (PEG–PUTe–PEG). This nanocarrier was loaded with indocyanine green (ICG) as an NIR-sensitive dye. Under NIR laser irradiation, ROS was generated from ICG. The ROS oxidizes the tellurium. The oxidation of tellurium weakened the coordination bond between CDDP and tellurium and led to the rapid release of CDDP. In addition, the researchers observed that the encapsulated ICG had a synergistic antitumor effect through photothermal effects after mild laser irradiation (Li, Li, Cao, Wang, & Xu, 2017). Another group reported the use of arylboronic ester and cholesterol-modified hyaluronic acid (PPE–Chol1–HA), denoted as the PCH–DI nanosystem, for light-triggered DOX release, along with ICG PTT. The ICG produced ROS that cleaved arylboronic ester to realize controllable drug release. The results were confirmed by NIR laser irradiation. The in vitro results indicated that DOX in the PCH–DI/laser group showed the most efficient nuclear binding toward HCT-116 colon cells; the results of the in vivo studies indicated that this system also enhanced the cytotoxicity and tumor suppression effect of PCH–DI on a nude mouse model of HCT-116 tumor xenografts (Chen, Deng, et al., 2017).
5.4. Ultrasound Stimuli
Ultrasound-sensitive drug delivery systems can improve targeted delivery of drugs into specified tissues, while reducing the systemic dose and toxicity (Paliwal & Mitragotri, 2006). Sonoporation is an important mechanism for stimuli-enhanced delivery of therapeutics in this system through vasculature openings, induced by ultrasound-triggered oscillations and destruction of microbubbles (Ferrara, 2008).
Ultrasound interaction with nanoparticles induces enhanced drug delivery and can also be used in image-guided delivery. Chen et al. developed an ultrasound imaging contrast and ultrasound-triggered DOX using polymer microcapsules composed of hydrogen-bonded multilayers of tannic acid and poly(N-vinylpyrrolidone). These capsules work as diagnostic agents upon low-power (~ 100mW/cm2) ultrasound irradiation and act as therapeutics upon high-power (>10W/cm2) ultrasound irradiation. The researchers observed that DOX release was gradual at lower power and swift at higher power. In vitro results indicated that 50% of DOX release induced 97% cytotoxicity in MCF-7 breast cancer cells, whereas no cytotoxicity was found in the absence of ultrasound irradiation (Chen, Ratnayaka, et al., 2017).
Another group developed multifunctional smart curcumin-loaded chitosan/perfluorohexane nanodroplets for ultrasound imaging mediated, on-demand drug delivery systems. The researchers observed that 63.5% of curcumin was released from the nanoformulation upon sonication at the frequency of 1 MHz, 2W/cm2 for 4min. Cell growth inhibition was significantly increased in curcumin-loaded nanodroplets of 4T1 human breast cancer cells upon ultrasound exposure (Baghbani, Chegeni, Moztarzadeh, Hadian-Ghazvini, & Raz, 2017). Another report from same group demonstrated that codelivery of doxorubicin and curcumin using multifunctional smart alginate/perfluorohexane nanodroplets (Dox-Cur-NDs) had a synergistic therapeutic effect in adriamycin-resistant A2780 ovarian cancer cells for Dox-Cur-NDs with ultrasound irradiation compared to Dox-NDs; in vivo results also demonstrated efficient tumor suppression (Baghbani & Moztarzadeh, 2017).
5.5. Redox Stimuli
Biologically reduced substances vitamin C (ascorbic acid), vitamin E, and glutathione (GSH) are widely distributed in all body tissues (Sun, Meng, Cheng, Deng, & Zhong, 2014). Reduced state GSH plays an important role in human metabolism. In intratumoral conditions, GSH expression is almost a 1000-fold greater than in normal tissues (Wen et al., 2011). Hence, this property has motivated researchers to fabricate redox-sensitive drug delivery systems using disulfide crosslinkers.
The redox-controlled release of drugs can revert MDR in tumors, with fewer side effects. Qiao et al. created a D-α-Tocopherol polyethylene 1000 succinate (TPGS)-based drug delivery system that contains both TPGS and mitoxantrone (MTO) via a disulfide bond and assembles into micelles (TSMm). The disulfide bonds are more sensitive at high concentrations of intracellular glutathione (GSH), which lead to rapid release of MTO. These redox-sensitive TSMm showed significantly increased therapeutic effect in treatment-resistant MDA–MB-231/MDR breast tumor cells, compared with either free MTO or disulfide-free prodrug micelle (TCMm). Furthermore, TSMm has demonstrated significantly stronger antitumor activity in xenograft nude mice, without causing toxicity (Qiao et al., 2017).
PTX is highly effective in many cancer types, but its highly hydrophobic nature limits the usage in current formulations. Redox-sensitive PTX delivery overcomes this obstacle. Li and coworkers developed a nanoformulation with α-amylase- and redox-responsive nanoparticles based on hydroxyethyl starch (HES) for the redox-sensitive delivery of PTX. These HES-SS-PTX nanoparticles showed a prolonged half-life compared with that of the commercial PTX formulation (Taxol), resulting in more tumor accumulation. In reductive conditions, HES-SS-PTX NPs showed better therapeutic efficiency than did Taxol in in vitro and in vivo models in 4T1 tumor-bearing mice (Li, Hu, et al., 2017). In another approach, a mixed micelle system was developed with redox-sensitive mPEG-SS-PTX and mPEG-SS-DOX conjugates to reduce the side effects. The in vitro release profile and in vitro anticancer activity of mixed micelles showed redox-controlled release and significant cytotoxicity in A549 and B16 cells. The in vivo results demonstrated that the mixed micelles had fewer side effects than free PTX/DOX in mice (Zhao et al., 2017).
Pluronic F127 (F127), an amphiphilic triblock copolymer, improved the drug delivery efficiency by introducing a redox-sensitive disulfide linker into micelles. Liu et al. synthesized α-tocopherol (TOC) conjugated with F127 polymer (F127-SS-TOC) through a redox-sensitive disulfide bond between F127 and TOC. These F127-SS-TOC micelles showed high hemocompatibility and low cytotoxicity in Bel 7402 and L02 cells (Liu, Fu, et al., 2017).
5.6. Enzyme Stimuli
Biochemists have explored the role of biomolecules in biochemical reactions and their distribution within the body. It is well established that enzymes are involved in most of the biocatalytic reactions in the body (de la Rica, Aili, & Stevens, 2012). Enzymes exhibit properties that catalyze the biodegradation of biomacromolecules; however, some enzymes are overexpressed in cancer cells (Hu, Katti, & Gu, 2014). Enzyme-degradable linker molecules can be used to make enzyme-responsive drug delivery systems. For example, Zhang et al. developed an enzyme-responsive PEGylated lysine peptide dendrimer–gemcitabine conjugate (dendrimer–GEM)-based nanoparticle through a click reaction. Glycyl phenylalanyl leucyl glycine tetra-peptide (GFLG) was used as an enzyme-cleavable linker. The nanoparticle showed significantly rapid GEM release in the secreted Cathepsin B environment, compared with conditions without Cathepsin B. This dendrimer–GEM nanoparticle displayed enhanced antitumor efficacy in a 4T1 murine breast cancer model, with minimal toxicity; twofold higher tumor growth inhibition was induced by the dendrimer–GEM nanoparticle than by free GEM (Zhang, Pan, et al., 2017).
In addition, there was a recent report of an enzyme-responsive drug delivery system based on enzymatic biodegradable amphiphilic poly(ester-urethane)s from L-tyrosine amino acid resources. The self-assembled nanoparticles were used to deliver anticancer drugs DOX and camptothecin (CPT). These nanoparticles showed more intracellular drug release than extracellular drug release, due to enzymatic biodegradation. The cell uptake and in vitro cytotoxicity studies demonstrated that the L-tyrosine nanoparticles showed more cell internalization and cell killing efficiency in HeLa cervical cancer cells, with reduced toxicity to normal cells (Aluri &Jayakannan, 2017).
Kumar and colleagues used guar gum, a natural carbohydrate polymer, as a capping layer to encapsulate chemotherapy drug 5-flurouracil (5FU) within the channels of MSNs for oral delivery of a colon-specific drug. They observed that the specific release of 5FU from guar gum-capped MSN (GG-MSN) occurs via enzymatic biodegradation of guar gum by enzymes in the colonic microenvironment. However, there was no release in the gastrointestinal tract. The released drug showed anticancer activity in colon cancer cell lines (Kumar et al., 2017).
5.7. pH Stimuli
Owing to enhanced glycolysis and accumulation of lactic acid, the tumor microenvironment (endo/lysosomes) has a lower pH gradient (4–6.5) than that (7.4) of the corresponding normal tissues (Estrella et al., 2013). pH-sensitive drug delivery has been shown to overcome the MDR and reduce toxicity to normal cells. Furthermore, pH-responsive drug delivery systems are mostly created with pH-sensitive linkers (hydrazone and ester linkages) that are conjugated at one end with chemotherapy drug and at the other end with nanocarriers (Prabaharan, Grailer, Pilla, Steeber, & Gong, 2009).
Ir (III) drugs have potent anticancer activity. Cai and colleagues examined the pH- and redox-sensitive delivery of these Ir (III) drugs. The researchers developed CD44-targetable conjugates of HA-cystamin-pyrenyl (HA-ss-Py) containing disulfide bonds, and HA-pyrenyl (HA-Py), which forms micelle particles (Cai et al., 2017). Almeida et al. developed a method of pH- and thermoresponsive curcumin (CUR) delivery of magnetic microgels using pectin maleate, N-isopropyl acrylamide, and Fe3O4 nanoparticles. CUR was loaded into the microgels, and slow and sustainable CUR release was achieved under the influence of an external magnetic field (Almeida et al., 2017). Qiu et al. reported novel tumor-targeted hyaluronic acid-2-(octadecyloxy)-1,3-dioxan-5-amine (HOD) conjugates. The HOD micelles were sensitive to degradation in the acidic tumor microenvironment. Then, DOX was loaded on HOD micelles (DOX/HOD), which showed enhanced release at low pH. The DOX/HOD micelles exhibited enhanced cell killing efficiency, compared with pH-insensitive micelles and the free DOX control (Qiu et al., 2017).
Our group also extensively studied pH-sensitive DOX release using nanosomes as carriers. The nanosomes were made up of gold nanoparticles and exosomes. We conjugated DOX to gold nanoparticles through a hydrazone-based, pH-sensitive drug delivery linker. Then, the nanoparticles were loaded on exosomes that were isolated from normal MRC9 lung fibroblast cells. Nanosome formation was confirmed by transmission electron microscopy (TEM) and zeta potential, as represented in Fig. 4. The highest DOX release was observed in conditions that mimicked the tumor microenvironment. Increased cell uptake and cytotoxicity were observed in lung cancer cell lines, while toxicity to normal fibroblast cells and DOX-sensitive human coronary artery smooth muscle cells were reduced (Srivastava, Amreddy, et al., 2016).
Fig. 4.
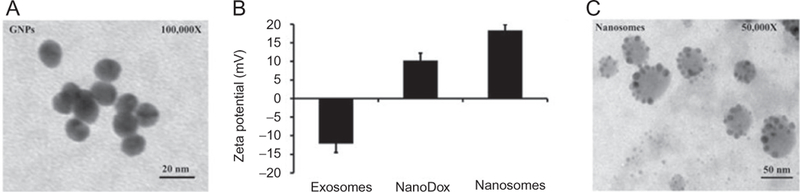
(A) TEM image of gold nanoparticles, (B) surface zeta potential values of exosomes that were isolated from MRC9 cells, NanoDox (GNP–DOX), nanosomes (exosomes+GNP–DOX), and (C) TEM image of nanosomes. Figure reproduced from Srivastava, A., Amreddy, N., Babu, A., Panneerselvam, J., Mehta, M., Muralidharan, R., ..., Ramesh, R. (2016). Nanosomes carrying doxorubicin exhibit potent anticancer activity against human lung cancer cells. Scientific Reports, 6, 38541. doi:10.1038/srep38541. Copyright © 2016, Rights Managed by Nature Publishing Group.
The above examples of stimuli-responsive nanoparticle systems demonstrate the growing importance for these systems in drug delivery applications. However, most are still in the early stages of development. Thorough optimization of the synthesis procedures is needed before these systems can transition into clinical settings. Many of the above-discussed systems failed to surpass in vitro levels, but a few have entered in vivo trials. Simplified systems with strong stimuli-responsive characteristics may drastically influence the chances for clinical applications. Despite these hurdles, stimuli-responsive drug delivery systems have the potential to replace current drug delivery approaches soon.
6. NANOPARTICLE-BASED RECEPTOR-TARGETED DELIVERY
Translation of cancer therapeutics and gene therapy into the clinic depends on the successful development of targeted delivery systems. Conventional chemotherapeutic drugs cause several side effects, including nonspecific killing of both cancerous and noncancerous cells (Morrow, 1985). These findings led to advancements in the development of molecularly targeted nanocarriers for selective uptake of the therapeutic molecules in the tumor site (Brannon-Peppas & Blanchette, 2004). These targeted nanocarriers protect the therapeutics from serum proteins, increase their systemic circulation, and enhance their efficacy, while minimizing specific toxicity. An efficient targeted delivery system should remain in the systemic circulation until it reaches the tumor site, while retaining its characteristics and releasing the therapeutics at the tumor site. Once the nanocarriers are injected into the systemic circulation, they will reach the tumor site through active or passive targeting mechanisms. In passive targeting, the nanocarriers reach the tumor site as a result of enhanced permeability and retention phenomena, known as the EPR effect; which depends on the size of the nanocarriers, and leaky vasculature and impaired lymphatics. Passive targeting is influenced by several independent properties that affect the delivery system, such as size, shape, surface charge, surface chemistry, composition, and other physicochemical properties (Yu et al., 2016). In active targeting, the nanocarriers reach the tumor site based on the affinity of the ligand toward the antigens or receptors that are highly expressed on the tumors.
The approach of targeting biologically active pathways to produce synergistic effects without causing any toxic effects leads to successful translation (Wang, Yu, Han, Sha, & Fang, 2007). Therefore, identifying several targeted molecules and designing nanocarriers became desirable in the field of targeted delivery systems. Multifunctionality can be improved if combined with receptor-mediated targeted delivery by conjugating targeting ligands on the surface of nanocarriers, as illustrated in Fig. 5. Here, we discuss the recent examples of EGFR-, transferrin-, luteinizing hormone-releasing hormone (LHRH) receptor-, folate receptor- (FR), and Arg-Gly-Asp (RGD) receptor-targeted therapeutic delivery with nanocarriers, and their anticancer activity.
Fig. 5.
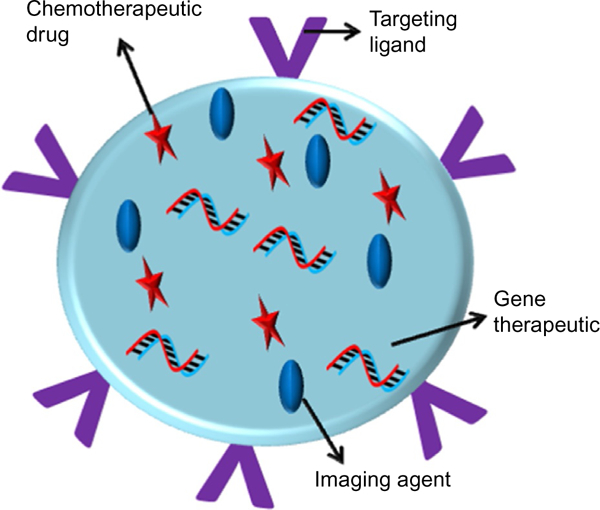
Schematic illustration of multifunctional nanoparticles for tumor-targeted delivery of therapeutics and imaging agents using targeted ligands functionalized on the surface of nanocarriers.
6.1. EGFR Receptor
EGFR is a transmembrane tyrosine kinase receptor that is overexpressed in many types of cancer, including lung, breast, ovarian, and pancreatic cancers (Inicholson, WGee, & Harper, 2001). EGFR plays a major role in regulating cell proliferation and survival through three different pathways: RAS–RAF mitogen-activated protein, PI3K/AKT, and JAK/STAT (Okayama et al., 2012).
A polymer-based unimolecular NP formulation with pH/redox dual stimuli release of siRNA for EGFR-targeted delivery was recently reported (Chen, Wang, Xie, & Gong, 2017). The researchers utilized GE11 peptide for EGFR targeting, and it overcame the limitations associated with endosomal and lysosomal escape of siRNA. In this study, siRNA was complexed at the multiarm cationic core polymer through electrostatic interaction. An imidazole group in the cationic polymer absorbed the protons from acidic endosomes, leading to osmotic swelling and facilitating the endosomal escape of the siRNA complex. These pH/redox dual-sensitive nanoparticles were functionalized using EGFR-targeting GE11 peptide to enhance the active targeting and efficient gene silencing in GFP-MDA-MB-468 TNBC cells, without any significant cytotoxicity (Chen, Wang, et al., 2017). Researchers developed superparamagnetic iron oxide (SPIO) nanoparticles for MRI sensitivity and energy deposition efficiency using a clinical MRgFUS system. The SPIO surface was decorated with EGFR monoclonal antibody (mAb) to target EGFR overexpressed in lung cancer cells. They demonstrated that the anti-EGFR, mAb-targeted SPIO nanoparticles significantly improved the targeting efficiency in H460 lung tumor cells, and this MRI contrast imaging facilitated the monitoring of therapeutic efficiency in a nude rat model (Wang, Qiao, et al., 2017).
Later, the same group reported Cetuximab-conjugated micelles loaded with IR-780 (radiolabeled with Indium-111) for targeted imaging and PTT. These micelles specifically bind to EGFR at the tumor site and showed enhanced therapeutic effect. Micro-SPECT/CT imaging and biodistribution studies showed the accumulation of Cetuximab micelles at the tumor. The radiolabeled radioisotopes provided real-time monitoring of the micelles in the circulation. This approach can be used for optical and nuclear imaging, and to deliver therapeutics simultaneously (Shih et al., 2017). Studies from our lab demonstrated the conjugation of anti-EGF antibody into plasmonic resonance magnetic nanoparticles, which were selectively taken up by EGFR-overexpressing lung tumor cells. This EGFR-targeted nanoparticle showed enhanced antitumor activity by inducing apoptosis and autophagy in NSCLC cells (Yokoyama et al., 2011).
6.2. Transferrin Receptor
Transferrin receptor (TfR1), also known as CD71, is ubiquitously expressed at low levels in most human tissues. It is expressed on the outer cell membrane and transports iron into cells through a clathrin/dynamin-dependent endocytotic mechanism (Daniels et al., 2012). Higher expression ofTfR1 in malignant cells is correlated with tumor progression, and make this receptor an attractive target for delivery of nanotherapeutics. Direct conjugation of chemotherapeutic drugs to transferrin showed enhanced and selective delivery in malignant cells. Covalent linkage of transferrin to doxorubicin showed cytotoxicity in multiple cancer cell lines (Szwed, Kania, & Jozwiak, 2014). Surface functionalization of nanoparticles using transferrin peptide, antibody, or protein provided promising results. Conjugation of transferrin as a targeting moiety on the surface of the nanoparticle showed enhanced delivery due to the availability of the receptors on malignant cells. Sahoo et al. developed PLGA nanoparticles loaded with paclitaxel and conjugated to transferrin, and tested the nanoparticles in PC3 human prostate cancer cells. Systemic administration of these nanoparticles resulted in complete regression of the tumor and increased survival in nude mice bearing PC3 cells (Sahoo, Ma, & Labhasetwar, 2004). A pulmonary siRNA delivery system using Tf-PEI for asthma therapy showed selective delivery of siRNA to activate T cells in the lung. In vitro studies demonstrated the enhanced uptake of these polyplexes in human primary activated T cells and JURKAT cells. Biodistribution studies in murine asthma model showed selective delivery of siRNA to activated T cells (Xie, Kim, et al., 2016).
Researchers have shown that the cationic polymer branched polyethyleneamine (bPEI) coupled with TfR targeting peptides (HAIYPRH peptide) efficiently delivered siRNA into TfR-overexpressing lung cancer cells. The targeted delivery resulted in GAPDH gene knockdown that was more efficient than that observed with bPEI alone (Xie, Killinger, Moszczynska, & Merkel, 2016).
Studies from our lab and others have shown that the RNA-binding protein HuR regulates several target mRNAs that are involved in cancer progression; thus, HuR has emerged as a promising target for cancer treatment. The target genes of HuR are key regulators of the cell cycle, angiogenesis, and cell migration and invasion. Hence, we investigated whether siRNA-mediated knockdown of HuR would induce cell death, leading to better survival. To test this approach, we developed an efficient lipid nanoparticle delivery system targeting transferrin receptors (TfNP) that are overexpressed on lung tumor cells. Our in vitro studies demonstrated the selective uptake of TfNP, which led to downregulation of HuR and HuR-regulated oncogenes. Biodistribution studies revealed the selective accumulation of TfNPs at the tumor site. In vivo efficacy studies showed significant reductions in xenograft tumor volumes and in the number of lung met tumor nodules upon intravenous administration of TfNP into nude mice (Muralidharan et al., 2017).
Liposomal nanocarriers have gained popularity for applications as drug carriers. We attempted to develop a liposomal nanocarrier to enhance the delivery of tyrosine kinase inhibitor gefitinib to a lung tumor model. We successfully loaded the poorly soluble drug gefitinib into the lipid bilayer of a liposomal structure. These gefitinib-loaded liposomes delivered a combination of gefitinib and HuR siRNA. This system demonstrated the combined inhibitory activity of gefitinib and HuR siRNA in the tested lung tumor cells, which were analyzed by Western blot, as represented in Fig. 6. The transferrin-targeted liposomal nanoparticles showed enhanced pEGFR and HuR protein downregulation upon treatment with the combination of gefitinib and HuR siRNA, compared with individual treatments.
Fig. 6.
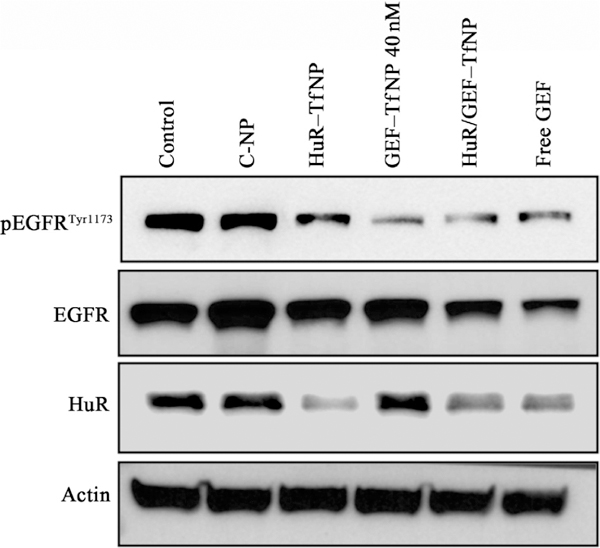
Western blot analysis of Tf receptor-targeted codelivery of HuR siRNA and gefitinib (40nM) with DOTAP-chol liposome nanoparticles to HCC827 lung cancer cells. The results showed the reduction of HuR and pEGFR expression.
6.3. LHRH Receptor
LHRH is a hormonal decapeptide that specifically targets LHRH receptors expressed on the cell membrane. Expression of LHRH is high in several cancers, including ovarian, endometrial, prostate, and breast cancer, whereas LHRH expression levels are very low in healthy tissues. Studies have shown that LHRH receptor-targeted dendrimers and liposomes produced enhanced accumulation of the drug at the tumor site (Minko et al., 2010; Saad et al., 2008).
Nukolva et al. developed targeted nanogels for delivery of chemotherapeutic agent cisplatin targeting luteinizing hormone receptors. They synthesized soft polymeric nanogels that can serve as carriers for hydrophilic drugs, such as cisplatin. These nanogels were less toxic and showed enhanced antitumor activity (Nukolova et al., 2013). Multifunctional lipid nanocarriers can deliver both chemotherapeutic drug and siRNA to the lungs through inhalation. These lipid nanocarriers were used for the delivery of anticancer drug PTX or DOX, and BCL2 siRNA. Inhalation resulted in the specific accumulation of these nanocarriers in the lung compared with other organs, whereas intravenous injection resulted in more accumulation in the liver, kidney, and spleen, and significantly less accumulation in the lung (Taratula, Kuzmov, Shah, Garbuzenko, & Minko, 2013). Iron oxide nanoparticles were coated with gold to form SPIONS-Au conjugated to the LHRH protein to target LHRH-overexpressing cancer cells. This study demonstrated that the SPIONs can generate a therapeutic amount of heat under magnetic fluid conditions, with controlled drug release (Mohammad & Al-Lohedan, 2017).
6.4. Folate Receptor
FR is membrane-bound protein on the cell surface, which strongly binds with folic acid molecules in neutral pH and transports them into cells. Folate mainly plays a role in DNA synthesis, methylation, and repair (Crider, Yang, Berry, & Bailey, 2012). It is widely overexpressed in many types of cancer tissues (Parker et al., 2005). Thus, FR can be used as a delivery target in cancer treatment by incorporating folic acid conjugation on the nanocarrier surfaces.
Fernandes et al. labeled chitosan with folic acid and used it as a carrier for siRNA delivery in HeLa and OV-3 cells, which express high levels of FRs. The researchers reported excellent siRNA transfection efficiency and low toxicity of the folate–chitosan nanoparticle in FR-positive cells (Fernandes et al., 2012). Dendrimer-based PAMAM polymer nanoparticles were covalently conjugated and used for hydrophobic baicalin (BAI) drug delivery. Administration of these PAMAM–FA/BAI complexes resulted in more cellular uptake in FR-positive HeLa cancer cells than in FR-negative A549 cells. Enhanced cell killing efficiency was observed with PAMAM–FA/BAI complexes compared with non-FA-modified PAMAM/BAI complexes (Lv et al., 2017). Recently, albumin coated on gold nanoparticles modified with FA- for FR-targeted drug delivery was combined with the chemotherapeutic DOX and computed tomography (CT) imaging. The Au-BSA-DOX-FA nanocomposites exhibited antitumor activity specifically on FR-overexpressing tumors, and no toxicity was observed in normal healthy tissues (Huang et al., 2017).
Our group recently published regarding FA-targeted HuR siRNA delivery in nonsmall cell lung cancer using DOTAP:cholesterol liposomes (HuR-FNP). FA was covalently conjugated on the surface of liposomes, and HuR siRNA was encapsulated on the liposomes through electrostatic interaction. HuR-FNP showed enhanced cell uptake and therapeutic effects in FRA-overexpressing H1299 cancer cells compared with normal CCD16 lung fibroblast cells with no FRA expression. HuR-FNP enhanced inhibition ofcell migration in H1299 cells compared with C-FNP and control at both 24 and 48 h (Fig. 7), thus HuR-FNP exhibited activity on FR overexpressing tumors (Muralidharan et al., 2016).
Fig. 7.
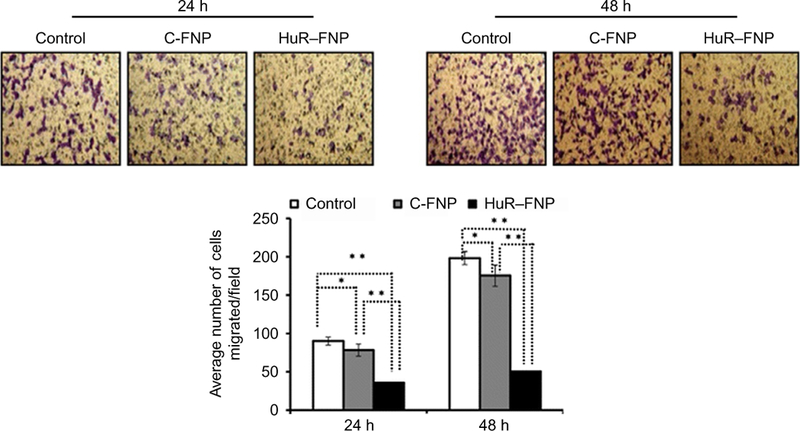
Folic acid-conjugated DOTAP:cholesterol-based liposomes (HuR-FNP) greatly inhibit tumor cell migration compared with control siRNA (C-FNP) and untreated control at 24 and 48h. Figure reproduced from Muralidharan, R., Babu, A., Amreddy, N., Basalingappa, K., Mehta, M., Chen, A., ..., Ramesh, R. (2016). Folate receptor-targeted nanoparticle delivery of HuR-RNAi suppresses lung cancer cell proliferation and migration. Journal of Nanobiotechnology, 14(1), 47. doi:10.1186/s12951–016-0201–1. Creativecommons.org license 2016.
6.5. Integrins
Integrins are cell adhesion molecules that comprise a large family of receptors involving alpha and beta subunits expressed on the cell surface. A peptide sequence with an RGD (argmine–glycone–aspartic acid) motif has an affinity toward integrins, such as αvβ3, αvβ5, and α5β1, that are broadly expressed on the surface of tumor cells and tumor vasculature, but are largely absent from normal cells. Thus, RGD peptide is an attractive tumor-targeting protein for drug delivery using nanoparticles (Wang, Chui, & Ho, 2010). Two types of RGD peptides are commonly used as ligands to functionalize the nanoparticle surface: linear and cyclic. Cyclic RGD peptides [c(RGDfK)] showed high affinity and selectivity toward integrins compared with linear peptides, and thus can be used for tumor-targeted nanoparticle delivery of cancer therapeutics. Recently, Miura et al. utilized a micellar delivery system conjugated with cyclic RGD peptides for platinum drugs toward glioblastoma. Compared with nontargeted control peptide-conjugated micelles, cyclic RGD-conjugated micelle systems accumulated rapidly and had high permeability to the tumor parenchyma from vessels. The cyclic RGD-conjugated micelle delivery of platinum drug also resulted in significant tumor regression in an orthotopic mouse model of U87MG human glioblastoma (Miura et al., 2013).
Another derivative of RGD peptide, iRGD (CRGDKGPDC) peptide, has affinity toward integrin αvβ3 and neuropilin (NRP) receptors overexpressed in cancer cells (Yin et al., 2017). In addition to targeting integrins, the iRGD peptide has tumor-homing properties that have been harnessed for tumor therapy and targeted drug delivery using nanoparticles (Nie et al., 2014). In a typical study, iRGD peptide conjugated to paclitaxel albumin nanoparticles increased paclitaxel accumulation in tumor up to 4-folds when compared with unmodified nanoparticles (Sugahara et al., 2009). Another study utilized iRGD in conjunction with doxorubicin-conjugated gold nanoparticles-gelatin nanoparticle system (DOX–AuNPs–GNPs) to achieve deep tumor penetration and drug delivery (Cun et al., 2015). The use of iRGD increased the vascular and tumor permeability, resulting in enhanced tumor accumulation of nanoparticles and apoptosis induced by DOX.
7. CONCLUSION AND PROSPECTS
The main advantages of using delivery vehicles for cancer therapeutic agents are their enhanced bioavailability, controlled release, ability to prevent degradation, and preferential delivery to the tumor site. Therapeutic agents, such as chemotherapy drugs, nucleic acid therapeutics, small molecule inhibitors, and photodynamic agents can be loaded onto nanoparticles for cancer treatment. Each of these therapeutic agents differ in their physicochemical characteristics and their mechanisms of action, and require a specific dose to be released in the tumor site. However, many biological barriers must be overcome before drug reaches the target cancer cells.
Various nanoparticle formulations are currently being developed and explored as delivery vehicles for cancer therapeutics. These include a wide range of lipids, polymers of natural and synthetic origin, inorganic nanoparticles, and cellular vesicles that have been used for targeted delivery of biomolecules and therapeutic agents with promising in vitro and in vivo efficacy. Advanced delivery systems have also been designed to deliver drugs specifically to the tumor site, based on their stimuli-responsive properties. Targeted nanoparticles utilize ligands conjugated onto their surfaces to deliver therapeutic agents to specific receptors that are overexpressed in tumor cells, thereby enhancing their therapeutic efficiency. However, translating these nanoparticle systems to clinical settings is challenging. Obstacles, such as maintaining the stability of therapeutic agents, controlling their pharmacokinetics, reducing toxicity, and achieving targeted delivery, have been overcome to some extent by designing nanoparticles that can delay bioclearance, use of stimuli-responsive delivery strategy, and through a proper understanding of ligand-receptor interaction specific to tumor cells.
However, the unprecedented behavior of materials used for nanoparticle formulations, such as off-target effects or nonspecific toxicity, maintaining consistency in particle synthesis, and controlling penetration of biological barriers, are major hurdles to FDA approval. Therefore, many of the nanoparticle systems that appear promising in vitro may not be successful in vivo. Proper standards should be established for the examination of safety and efficacy issues before expanding the newly developed nanoparticle carriers into preclinical and clinical testing. Implementing proper regulatory measures, a deep understanding of tumor biology, and thoughtful use of technology advancements will speed the possible use of these nanoparticle systems in mainstream cancer treatment.
ACKNOWLEDGMENTS
The study was supported in part by a grant received from the National Institutes of Health (NIH), R01 CA167516 (R.R.), an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences (P20 GM103639) of the National Institutes of Health (A.M. and R.R.), and by funds received from the Stephenson Cancer Center Seed Grant (R.R.), Presbyterian Health Foundation Seed Grant (R.R.), Presbyterian Health Foundation Bridge Grant (R.R.), and Jim and Christy Everest Endowed Chair in Cancer Developmental Therapeutics (R.R.) at the University of Oklahoma Health Sciences Center. The authors thank Ms. Kathy Kyler at the office of the Vice President of Research, OUHSC, for editorial assistance. R.R. is an Oklahoma TSET Research Scholar and holds the Jim and Christy Everest Endowed Chair in Cancer Developmental Therapeutics.
REFERENCES
- Abbasi E, Aval SF, Akbarzadeh A, Milani M, Nasrabadi HT, Joo SW, et al. (2014). Dendrimers: Synthesis, applications, and properties. Nanoscale Research Letters, 9(1), 247 10.1186/1556-276X-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ahmady ZS, Al-Jamal WT, Bossche JV, Bui TT, Drake AF, Mason AJ, et al. (2012). Lipid–peptide vesicle nanoscale hybrids for triggered drug release by mild hyperthermia in vitro and in vivo. ACS Nano, 6(10), 9335–9346. 10.1021/nn302148p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida E, Bellettini IC, Garcia FP, Farinacio MT, Nakamura CV, Rubira AF, et al. (2017). Curcumin-loaded dual pH- and thermo-responsive magnetic microcarriers based on pectin maleate for drug delivery. Carbohydrate Polymers, 171, 259–266. 10.1016/j.carbpol.2017.05.034. [DOI] [PubMed] [Google Scholar]
- Aluri R, & Jayakannan M (2017). Development of l-tyrosine-based enzyme-responsive amphiphilic poly(ester-urethane) nanocarriers for multiple drug delivery to cancer cells. Biomacromolecules, 18(1), 189–200. 10.1021/acs.biomac.6b01476. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, & Wood MJ (2011). Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotechnology, 29(4), 341–345. 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- Alvarez-Lorenzo C, Bromberg L, & Concheiro A (2009). Light-sensitive intelligent drug delivery systems. Photochemistry and Photobiology, 85(4), 848–860. 10.1111/j.1751-1097.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- Amoli-Diva M, Sadighi-Bonabi R, & Pourghazi K (2017). Switchable on/off drug release from gold nanoparticles-grafted dual light- and temperature-responsive hydrogel for controlled drug delivery. Materials Science &Engineering C, Materials for Biological Applications, 76, 242–248. https://doi.org/10.10167j.msec.2017.03.038. [DOI] [PubMed] [Google Scholar]
- Amreddy N, Babu A, Muralidharan R, Munshi A, & Ramesh R (2017). Polymeric nanoparticle-mediated gene delivery for lung cancer treatment. Topics in Current Chemistry, 375(2), 35 10.1007/s41061-017-0128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen MO, Howard KA, Paludan SR, Besenbacher F, & Kjems J (2008). Delivery of siRNA from lyophilized polymeric surfaces. Biomaterials, 29(4), 506–512. 10.1016/j.biomaterials.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Arkin MR, & Wells JA (2004). Small-molecule inhibitors of protein–protein interactions: Progressing towards the dream. Nature Reviews. Drug Discovery, 3(4), 301–317. 10.1038/nrd1343. [DOI] [PubMed] [Google Scholar]
- Ashton S, Song YH, Nolan J, Cadogan E, Murray J, Odedra R, et al. (2016). Aurora kinase inhibitor nanoparticles target tumors with favorable therapeutic index in vivo. Science Translational Medicine, 8(325), 325–317. 10.1126/scitranslmed.aad2355. [DOI] [PubMed] [Google Scholar]
- Babu A, Jeyasubramanian K, Gunasekaran P, & Murugesan R (2012). Gelatin nanocarrier enables efficient delivery and phototoxicity of hypocrellin B against a mice tumour model. Journal of Biomedical Nanotechnology, 8(1), 43–56. [DOI] [PubMed] [Google Scholar]
- Babu A, & Ramesh R (2017). Multifaceted applications of chitosan in cancer drug delivery and therapy. Marine Drugs, 15(4), pii: E96. 10.3390/md15040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu A, Wang Q, Muralidharan R, Shanker M, Munshi A, & Ramesh R (2014). Chitosan coated polylactic acid nanoparticle-mediated combinatorial delivery of cisplatin and siRNA/plasmid DNA chemosensitizes cisplatin-resistant human ovarian cancer cells. Molecular Pharmaceutics, 11(8), 2720–2733. 10.1021/mp500259e. [DOI] [PubMed] [Google Scholar]
- Baghbani F, Chegeni M, Moztarzadeh F, Hadian-Ghazvini S, & Raz M (2017). Novel ultrasound-responsive chitosan/perfluorohexane nanodroplets for image-guided smart delivery of an anticancer agent: Curcumin. Materials Science & Engineering C: Materials for Biological Applications, 74, 186–193. 10.1016/j.msec.2016.11.107. [DOI] [PubMed] [Google Scholar]
- Baghbani F, & Moztarzadeh F (2017). Bypassing multidrug resistant ovarian cancer using ultrasound responsive doxorubicin/curcumin co-deliver alginate nanodroplets. Colloids and Surfaces B: Biointerfaces, 153, 132–140. 10.1016/jxolsurfb.2017.01.051. [DOI] [PubMed] [Google Scholar]
- Balazs DA, & Godbey W (2011). Liposomes for use in gene delivery. Journal of Drug Delivery, 2011, 326497. 10.1155/2011/326497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, et al. 2003). Dicer is essential for mouse development. Nature Genetics, 35(3), 215–217. 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- Bharti C, Nagaich U, Pal AK, & Gulati N (2015). Mesoporous silica nanoparticles in target drug delivery system: A review. International Journal of Pharmaceutical Investigation, 5(3), 124–133. 10.4103/2230-973X.160844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt R, de Vries P, Tulinsky J, Bellamy G, Baker B, Singer JW, et al. (2003). Synthesis and in vivo antitumor activity of poly(l-glutamic acid) conjugates of 20S-camptothecin. Journal of Medicinal Chemistry, 46(1), 190–193. 10.1021/jm020022r. [DOI] [PubMed] [Google Scholar]
- Bikram M, & West JL (2008). Thermo-responsive systems for controlled drug delivery. Expert Opinion on Drug Delivery, 5(10), 1077–1091. 10.1517/17425247.5.10.1077. [DOI] [PubMed] [Google Scholar]
- Brannon-Peppas L, &Blanchette JO (2004). Nanoparticle and targeted systems for cancer therapy. Advanced Drug Delivery Reviews, 56(11), 1649–1659. 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Burrer CM, Auburn H, Wang X, Luo J, Abulwerdi FA, Nikolovska-Coleska Z, et al. (2017). Mcl-1 small-molecule inhibitors encapsulated into nanoparticles exhibit increased killing efficacy towards HCMV-infected monocytes. Antiviral Research, 138, 40–46. https://doi.org/10.10167j.antiviral.2016.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Gao T, Hong H, & Sun J (2008). Applications of gold nanoparticles in cancer nanotechnology. Nanotechnology, Science and Applications, 1, 17–32. 10.2147/NSA.S3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Zhang H, Wei Y, Wei Y, Xie Y, & Cong F (2017). Reduction- and pH-sensitive hyaluronan nanoparticles for delivery of iridium(III) anticancer drugs. Biomacromolecules, 18, 2102–2117. 10.1021/acs.biomac.7b00445. [DOI] [PubMed] [Google Scholar]
- Cancer Facts & Figures (2017). American Chemical Society, Retrieved from URL: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html. on June 17, 2017.
- Castano AP, Demidova TN, & Hamblin MR (2004). Mechanisms in photodynamic therapy: Part one-photosensitizers, photochemistry and cellular localization. Photodiagnosis and Photodynamic Therapy, 1(4), 279–293. 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celikoglu SI, Karayel T, Demirci S, Celikoglu F, & Cagatay T (1997). Direct injection of anti-cancer drugs into endobronchial tumours for palliation of major airway obstruction. Postgraduate Medical Journal, 73(857), 159–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee DK, Fong LS, & Zhang Y (2008). Nanoparticles in photodynamic therapy: An emerging paradigm. Advanced Drug Delivery Reviews, 60(15), 1627–1637. 10.1016/j.addr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Chen Y, Deng X, Li C, He F, Liu B, Hou Z, et al. (2017). Stimuli-responsive nanocomposites for magnetic targeting synergistic multimodal therapy and T1/T2-weighted dual-mode imaging. Nanomedicine, 13(3), 875–883. 10.1016/j.nano.2016.12.004. [DOI] [PubMed] [Google Scholar]
- Chen J, Ratnayaka S, Alford A, Kozlovskaya V, Liu F, Xue B, et al. (2017). Theranostic multilayer capsules for ultrasound imaging and guided drug delivery. ACS Nano, 11(3), 3135–3146. 10.1021/acsnano.7b00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Wang Y, Xie R, & Gong S (2017). Tumor-targeted pH/redox dual-sensitive unimolecular nanoparticles for efficient siRNA delivery. Journal of Controlled Release, 259, 105–114. 10.1016/j-jconrel.2017.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Xu M, Zheng W, Xu T, Deng H, & Liu J (2017). Se/Ru-decorated porous metal-organic framework nanoparticles for the delivery of pooled siRNAs to reversing multidrug resistance in Taxol-resistant breast cancer cells. ACS Applied Materials & Interfaces, 9(8), 6712–6724. 10.1021/acsami.6b12792. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Hao J, Lee LA, Biewer MC, Wang Q, & Stefan MC (2012). Thermally controlled release of anticancer drug from self-assembled gamma-substituted amphiphilic poly(epsilon-caprolactone) micellar nanoparticles. Biomacromolecules, 13(7), 2163–2173. 10.1021/bm300823y. [DOI] [PubMed] [Google Scholar]
- Chenna V, Hu C, Pramanik D, Aftab BT, Karikari C, Campbell NR, et al. (2012). A polymeric nanoparticle encapsulated small-molecule inhibitor of hedgehog signaling (NanoHHI) bypasses secondary mutational resistance to smoothened antagonists. Molecular Cancer Therapeutics, 11(1), 165–173. 10.1158/1535-7163.MCT-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Jin SE, Lee MK, Lim SJ, Park JS, Kim BG, et al. (2008). Novel cationic solid lipid nanoparticles enhanced p53 gene transfer to lung cancer cells. European Journal of Pharmaceutics and Biopharmaceutics, 68(3), 545–554. 10.1016/j.ejpb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Choung S, Kim YJ, Kim S, Park HO, & Choi YC (2006). Chemical modification of siRNAs to improve serum stability without loss of efficacy. Biochemical and Biophysical Research Communications, 342(3), 919–927. [DOI] [PubMed] [Google Scholar]
- Crider KS, Yang TP, Berry RJ, &Bailey LB (2012). Folate and DNA methylation: A review of molecular mechanisms and the evidence for folate’s role. Advances in Nutrition, 3(1), 21–38. 10.3945/an.111.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross D, & Burmester JK (2006). Gene therapy for cancer treatment: Past, present and future. Clinical Medicine & Research, 4(3), 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cun X, Chen J, Ruan S, Zhang L, Wan J, He Q, et al. (2015). A novel strategy through combining iRGD peptide with tumor-microenvironment-responsive and multistage nanoparticles for deep tumor penetration. ACS Applied Materials & Interfaces, 7(49), 27458–27466. 10.1021/acsami.5b09391. [DOI] [PubMed] [Google Scholar]
- Danafar H, & Schumacher U (2016). MPEG–PCL copolymeric nanoparticles in drug delivery systems. Cogent Medicine, 3, Article No. 1142411. [DOI] [PubMed] [Google Scholar]
- Daniels TR, Bernabeu E, Rodriguez JA, Patel S, Kozman M, Chiappetta DA, et al. (2012). The transferrin receptor and the targeted delivery of therapeutic agents against cancer. Biochimica et Biophysica Acta, 1820(3), 291–317. 10.1016/j.bbagen.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Rica R, Aili D, & Stevens MM (2012). Enzyme-responsive nanoparticles for drug release and diagnostics. Advanced Drug Delivery Reviews, 64(11), 967–978. 10.1016/j.addr.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Deng X, Cao M, Zhang J, Hu K, Yin Z, Zhou Z, et al. (2014). Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials, 35(14), 4333–4344. 10.1016/j.biomaterials.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Deng L, Li Q, Al-Rehili S, Omar H, Almalik A, Alshamsan A, et al. (2016). Hybrid iron oxide-graphene oxide-polysaccharides microcapsule: A micro-Matryoshka for on-demand drug release and antitumor therapy in vivo. ACS Applied Materials & Interfaces, 8(11), 6859–6868. 10.1021/acsami.6b00322. [DOI] [PubMed] [Google Scholar]
- Dufes C, Uchegbu IF, & Schatzlein AG (2005). Dendrimers in gene delivery. Advanced Drug Delivery Reviews, 57(15), 2177–2202. 10.1016/j.addr.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Dumontet C, & Sikic BI (1999). Mechanisms of action of and resistance to antitubulin agents: Microtubule dynamics, drug transport, and cell death. Journal of Clinical Oncology, 17(3), 1061–1070. 10.1200/JCO.1999.17.3.1061. [DOI] [PubMed] [Google Scholar]
- Estrella V, Chen T, Lloyd M, Wojtkowiak J, Cornnell HH, Ibrahim-Hashim A, et al. (2013). Acidity generated by the tumor microenvironment drives local invasion. Cancer Research, 73(5), 1524–1535. 10.1158/0008-5472.CAN-12-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes JC, Qiu X, Winnik FM, Benderdour M, Zhang X, Dai K, et al. (2012). Low molecular weight chitosan conjugated with folate for siRNA delivery in vitro: Optimization studies. International Journal of Nanomedicine, 7, 5833–5845. 10.2147/IJN.S35567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara KW (2008). Driving delivery vehicles with ultrasound. Advanced Drug Delivery Reviews, 60(10), 1097–1102. 10.1016/j.addr.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, & Mello CC (1998). Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature, 391(6669), 806–811. 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Flak D, Yate L, Nowaczyk G, & Jurga S (2017). Hybrid ZnPc@TiO2 nanostructures for targeted photodynamic therapy, bioimaging and doxorubicin delivery. Materials Science & Engineering. C, Materials for Biological Applications, 78, 1072–1085. 10.1016/j.msec.2017.04.107. [DOI] [PubMed] [Google Scholar]
- Fomina N, Sankaranarayanan J, & Almutairi A (2012). Photochemical mechanisms of light-triggered release from nanocarriers. Advanced Drug Delivery Reviews, 64(11), 1005–1020. 10.1016/j.addr.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, & Kao WJ (2010). Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opinion on Drug Delivery, 7(4), 429–444. 10.1517/17425241003602259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri S, Trewyn BG, Stellmaker MP, & Lin VS (2005). Stimuli-responsive controlled-release delivery system based on mesoporous silica nanorods capped with magnetic nanoparticles. Angewandte Chemie (International Ed. in English), 44(32), 5038–5044. 10.1002/anie.200501819. [DOI] [PubMed] [Google Scholar]
- Gong C, Qi T, Wei X, Qu Y, Wu Q, Luo F, et al. (2013). Thermosensitive polymeric hydrogels as drug delivery systems. Current Medicinal Chemistry, 20(1), 79–94. [PubMed] [Google Scholar]
- Gowda R, Jones NR, Banerjee S, & Robertson GP (2013). Use of nanotechnology to develop multi-drug inhibitors for cancer therapy. Journal of Nanomedicine & Nanotechnology, 4(6), 184 10.4172/2157-7439.1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Guo Y, Wang C, Xu J, Wu J, Kirk TB, et al. (2017). A polyamidoamne dendrimer functionalized graphene oxide for DOX and MMP-9 shRNA plasmid co-delivery. Materials Science & Engineering C : Materials for Biological Applications, 70(Pt. 1), 572–585. https://doi.org/10.1016Zj.msec.2016.09.035. [DOI] [PubMed] [Google Scholar]
- Guerrero-Cazares H, Tzeng SY, Young NP,Abutaleb AO, Quinones-Hinojosa A, & Green JJ (2014). Biodegradable polymeric nanoparticles show high efficacy and specificity at DNA delivery to human glioblastoma in vitro and in vivo. ACS Nano, 8(5), 5141–5153. 10.1021/nn501197v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley B, & Frenkel E (2008). Nanoparticles for drug delivery in cancer treatment. Urologic Oncology, 26(1), 57–64. 10.1016/j.urolonc.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Han Y, Zhang P, Chen Y, Sun J, & Kong F (2014). Co-delivery of plasmid DNA and doxorubicin by solid lipid nanoparticles for lung cancer therapy. International Journal of Molecular Medicine, 34(1), 191–196. 10.3892/ijmm.2014.1770. [DOI] [PubMed] [Google Scholar]
- Harris M, Ahmed H, Barr B, LeVine D, Pace L, Mohapatra A, et al. (2017). Magnetic stimuli-responsive chitosan-based drug delivery biocomposite for multiple triggered release. International Journal of Biological Macromolecules, 104(Pt. B), 1407–1414. 10.1016/j.ijbiomac.2017.03.141. [DOI] [PubMed] [Google Scholar]
- Herman TS, Teicher BA, Jochelson M, Clark J, Svensson G, & Coleman CN (1988). Rationale for use of local hyperthermia with radiation therapy and selected anticancer drugs in locally advanced human malignancies. International Journal of Hyperthermia, 4(2), 143–158. [DOI] [PubMed] [Google Scholar]
- Hom C, Lu J, Liong M, Luo H, Li Z, Zink JI, et al. (2010). Mesoporous silica nanoparticles facilitate delivery of siRNA to shutdown signaling pathways in mammalian cells. Small, 6(11), 1185–1190. 10.1002/smll.200901966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SH, Yu B, Wang X, Lu Y, Schmidt CR, Lee RJ, et al. (2013). Cationic lipid nanoparticles for therapeutic delivery of siRNA and miRNA to murine liver tumor. Nanomedicine, 9(8), 1169–1180. 10.1016/j.nano.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Katti PS, & Gu Z (2014). Enzyme-responsive nanomaterials for controlled drug delivery. Nanoscale, 6(21), 12273–12286. 10.1039/c4nr04249b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua MY, Liu HL, Yang HW, Chen PY, Tsai RY, Huang CY, et al. (2011). The effectiveness of a magnetic nanoparticle-based delivery system for BCNU in the treatment of gliomas. Biomaterials, 32(2), 516–527. 10.1016/j.biomaterials.2010.09.065. [DOI] [PubMed] [Google Scholar]
- Huang H, Yang DP, Liu M, Wang X, Zhang Z, Zhou G,et al. (2017). pH-sensitive Au-BSA-DOX-FA nanocomposites for combined CT imaging and targeted drug delivery. International Journal of Nanomedicine, 12, 2829–2843. 10.2147/IJN.S128270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inicholson R, WGee JM, & Harper M (2001). EGFR and cancer prognosis. European Journal of Cancer, 37, 9–15. [DOI] [PubMed] [Google Scholar]
- Isoldi MC, Visconti MA, & Castrucci AM (2005). Anti-cancer drugs: Molecular mechanisms of action. Mini Reviews in Medicinal Chemistry, 5(7), 685–695. [DOI] [PubMed] [Google Scholar]
- Ito I, Ji L, Tanaka F, Saito Y, Gopalan B, Branch CD, et al. (2004). Liposomal vector mediated delivery of the 3p FUS1 gene demonstrates potent antitumor activity against human lung cancer in vivo. Cancer Gene Therapy, 11(11), 733–739. 10.1038/sj.cgt.7700756. [DOI] [PubMed] [Google Scholar]
- Jiang S, Eltoukhy AA, Love KT, Langer R, & Anderson DG (2013). Lipidoid-coated iron oxide nanoparticles for efficient DNA and siRNA delivery. Nano Letters, 13(3), 1059–1064. 10.1021/nl304287a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Vader P, & Schiffelers RM (2017). Extracellular vesicles for nucleic acid delivery: Progress and prospects for safe RNA-based gene therapy. Gene Therapy, 24(3), 157–166. [DOI] [PubMed] [Google Scholar]
- Johnsen KB, Gudbergsson JM, Skov MN, Pilgaard L, Moos T, & Duroux M (2014). A comprehensive overview of exosomes as drug delivery vehicles—Endogenous nanocarriers for targeted cancer therapy. Biochimica et Biophysica Acta, 1846(1), 75–87. [DOI] [PubMed] [Google Scholar]
- Kasten BB, Liu T, Nedrow-Byers JR, Benny PD, & Berkman CE (2013). Targeting prostate cancer cells with PSMA inhibitor-guided gold nanoparticles. Bioorganic & Medicinal Chemistry Letters, 23(2), 565–568. 10.1016/j.bmcl.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Ong ZY, Wiradharma N, Attia AB, & Yang YY (2012). Advanced materials for co-delivery of drugs and genes in cancer therapy. Advanced Healthcare Materials, 1(4), 373–392. 10.1002/adhm.201200109. [DOI] [PubMed] [Google Scholar]
- Kim SI, Shin D, Choi TH, Lee JC, Cheon GJ, Kim KY, et al. (2007). Systemic and specific delivery of small interfering RNAs to the liver mediated by apolipoprotein A–I. Molecular Therapy, 15(6), 1145–1152. 10.1038/sj.mt.6300168. [DOI] [PubMed] [Google Scholar]
- Kneidl B, Peller M, Winter G, Lindner LH, & Hossann M (2014). Thermosensitive liposomal drug delivery systems: State of the art review. International Journal of Nanomedicine, 9, 4387–4398. 10.2147/IJN.S49297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulsharova GK, Lee MB, Cheng F, Haque M, Choi H, Kim K, et al. (2013). In vitro and in vivo imaging ofpeptide-encapsulated polymer nanoparticles for cancer biomarker activated drug delivery. IEEE Transactions on Nanobioscience, 12(4), 304–310. 10.1109/TNB.2013.2274781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B, Kulanthaivel S, Mondal A, Mishra S, Banerjee B, Bhaumik A, et al. (2017). Mesoporous silica nanoparticle based enzyme responsive system for colon specific drug delivery through guar gum capping. Colloids and Surfaces B: Biointerfaces, 150, 352–361. 10.1016/jxolsurfb.2016.10.049. [DOI] [PubMed] [Google Scholar]
- Kumar CS, & Mohammad F (2011). Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Advanced Drug Delivery Reviews, 63(9), 789–808. https://doi.org/10.1016Zj.addr.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu AK, Iyer SV, Chandra S, Adhikari AS, Iwakuma T, & Mandal TK (2017). Novel siRNA formulation to effectively knockdown mutant p53 in osteosarcoma. PLoS One, 12(6), e0179168. 10.1371/journal.pone.0179168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landen CN Jr., Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, et al. (2005). Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Research, 65(15), 6910–6918. [DOI] [PubMed] [Google Scholar]
- Layek B, Lipp L, & Singh J (2015). Cell penetrating peptide conjugated chitosan for enhanced delivery of nucleic acid. International Journal of Molecular Sciences, 16(12), 28912–28930. 10.3390/ijms161226142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Crake C, Teo B, Carugo D, de Saint Victor M, Seth A, et al. (2017). Ultrasound-enhanced siRNA delivery using magnetic nanoparticle-loaded chitosan-deoxycholic acid nanodroplets. Advanced Healthcare Materials, 6(8). 10.1002/adhm.201601246. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Yhee JY, Kim SH, Kwon IC, & Kim K (2013). Biocompatible gelatin nanoparticles for tumor-targeted delivery of polymerized siRNA in tumor-bearing mice. Journal of Controlled Release, 172(1), 358–366. 10.1016/j.jconrel.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Yook S, Yhee JY, Yoon HY, Kim MG, Ku SH, et al. (2015). Codelivery of VEGF and Bcl-2 dual-targeted siRNA polymer using a single nanoparticle for synergistic anti-cancer effects in vivo. Journal of Controlled Release, 220(Pt. B), 631–641. https://doi.Org/10.1016/j.jconrel.2015.08.032. [DOI] [PubMed] [Google Scholar]
- Li Y, Hu H, Zhou Q, Ao Y, Xiao C, Wan J, et al. (2017). Alpha-amylase- and redox-responsive nanoparticles for tumor-targeted drug delivery. ACS Applied Materials & Interfaces, 9(22), 19215–19230. 10.1021/acsami.7b04066. [DOI] [PubMed] [Google Scholar]
- Li F, Li T, Cao W, Wang L, & Xu H (2017). Near-infrared light stimuli-responsive synergistic therapy nanoplatforms based on the coordination of tellurium-containing block polymer and cisplatin for cancer treatment. Biomaterials, 133, 208–218. 10.1016/j.biomaterials.2017.04.032. [DOI] [PubMed] [Google Scholar]
- Li H, Wang P, Deng Y, Zeng M, Tang Y, Zhu WH, et al. (2017). Combination of active targeting, enzyme-triggered release and fluorescent dye into gold nanoclusters for endomicroscopy-guided photothermal/photodynamic therapy to pancreatic ductal adenocarcinoma. Biomaterials, 139, 30–38. 10.1016/j.biomaterials.2017.05.030. [DOI] [PubMed] [Google Scholar]
- Liu FS (2009). Mechanisms of chemotherapeutic drug resistance in cancer therapy—A quick review. Taiwanese Journal of Obstetrics & Gynecology, 48(3), 239–244. 10.1016/S1028-4559(09)60296-5. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fu S, Lin L, Cao Y, Xie X, Yu H, et al. (2017). Redox-sensitive pluronic F127-tocopherol micelles: Synthesis, characterization, and cytotoxicity evaluation. International Journal of Nanomedicine, 12, 2635–2644. 10.2147/IJN.S122746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Guo Y, Huang R, Li J, Huang S, Kuang Y, et al. (2012). Gene and doxorubicin co-delivery system for targeting therapy of glioma. Biomaterials, 33(19), 4907–4916. 10.1016/j.biomaterials.2012.03.031. [DOI] [PubMed] [Google Scholar]
- Liu J, Huang L, Tian X, Chen X, Shao Y, Xie F, et al. (2017). Magnetic and fluorescent Gd2O3:Yb3+/Ln3+ nanoparticles for simultaneous upconversion luminescence/MR dual modal imaging and NIR-induced photodynamic therapy. International Journal of Nanomedicine, 12, 1–14. 10.2147/IJN.S118938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes JR, Santos G, Barata P, Oliveira R, & Lopes CM (2013). Physical and chemical stimuli-responsive drug delivery systems: Targeted delivery and main routes of administration. Current Pharmaceutical Design, 19(41), 7169–7184. [DOI] [PubMed] [Google Scholar]
- Lucero-Acuna A, Jeffery JJ, Abril ER, Nagle RB, Guzman R, Pagel MD, et al. (2014). Nanoparticle delivery of an AKT/PDK1 inhibitor improves the therapeutic effect in pancreatic cancer. International Journal of Nanomedicine, 9, 5653–5665. 10.2147/IJN.S68511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungwitz U, Breunig M, Blunk T, & Gopferich A (2005). Polyethylenimine-based non-viral gene delivery systems. European Journal of Pharmaceutics and Biopharmaceutics, 60(2), 247–266. https://doi.org/10.1016Zj.ejpb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Lv T,Yu T, Fang Y, Zhang S, Jiang M, Zhang H, et al. (2017). Role of generation on folic acid-modified poly(amidoamine) dendrimers for targeted delivery of baicalin to cancer cells. Materials Science & Engineering C: Materials for Biological Applications, 75, 182–190. 10.1016/j.msec.2016.12.134. [DOI] [PubMed] [Google Scholar]
- Madaan K, Kumar S, Poonia N, Lather V, & Pandita D (2014). Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. Journal of Pharmacy & Bioallied Sciences, 6(3), 139–150. 10.4103/0975-7406.130965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madani SY, Naderi N, Dissanayake O, Tan A,& Seifalian AM (2011). A new era of cancer treatment: Carbon nanotubes as drug delivery tools. International Journal of Nanomedicine, 6, 2963–2979. 10.2147/IJN.S16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain SC, Yiu HH, & Dobson J (2008). Magnetic nanoparticles for gene and drug delivery. International Journal of Nanomedicine, 3(2), 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medarova Z, Balcioglu M, & Yigit MV (2016). Controlling RNA expression in cancer using iron oxide nanoparticles detectable by MRI and in vivo optical imaging. Methods in Molecular Biology, 1372, 163–179. 10.1007/978-1-4939-3148-4_13. [DOI] [PubMed] [Google Scholar]
- Mendes R, Fernandes AR, & Baptista PV (2017). Gold nanoparticle approach to the selective delivery of gene silencing in cancer-the case for combined delivery? Genes (Basel), 8(3), pii: E94. 10.3390/genes8030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minko T, Patil ML, Zhang M, Khandare JJ, Saad M, Chandna P, et al. (2010). LHRH-targeted nanoparticles for cancer therapeutics. Methods in Molecular Biology, 624, 281–294. 10.1007/978-1-60761-609-2_19. [DOI] [PubMed] [Google Scholar]
- Miura Y, Takenaka T, Toh K, Wu S, Nishihara H, Kano MR, et al. (2013). Cyclic RGD-linked polymeric micelles for targeted delivery of platinum anticancer drugs to glioblastoma through the blood—brain tumor barrier. ACS Nano, 7(10), 8583–8592. 10.1021/nn402662d. [DOI] [PubMed] [Google Scholar]
- Mohammad F, & Al-Lohedan HA (2017). Luteinizing hormone-releasing hormone targeted superparamagnetic gold nanoshells for a combination therapy of hyperthermia and controlled drug delivery. Materials Science & Engineering C: Materials for Biological Applications, 76, 692–700. 10.1016/j.msec.2017.03.162. [DOI] [PubMed] [Google Scholar]
- Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, et al. (1991). Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. Journal of the National Cancer Institute, 83(11), 757–766. [DOI] [PubMed] [Google Scholar]
- Morabito A, Piccirillo MC, Monaco K, Pacilio C, Nuzzo F, Chiodini P, et al. (2007). First-line chemotherapy for HER-2 negative metastatic breast cancer patients who received anthracyclines as adjuvant treatment. The Oncologist, 12(11), 1288–1298. 10.1634/theoncologist.12-11-1288. [DOI] [PubMed] [Google Scholar]
- Morrow GR (1985). The effect of a susceptibility to motion sickness on the side effects of cancer chemotherapy. Cancer, 55(12), 2766–2770. [DOI] [PubMed] [Google Scholar]
- Muller RH, Mader K, & Gohla S (2000). Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. European Journal of Pharmaceutics and Biopharmaceutics, 50(1), 161–177. [DOI] [PubMed] [Google Scholar]
- Muralidharan R, Babu A, Amreddy N, Basalingappa K, Mehta M, Chen A, et al. (2016). Folate receptor-targeted nanoparticle delivery of HuR-RNAi suppresses lung cancer cell proliferation and migration. Journal of Nanobiotechnology, 14(1), 47 10.1186/s12951-016-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan R, Babu A, Amreddy N, Srivastava A, Chen A, Zhao YD, et al. (2017). Tumor-targeted nanoparticle delivery of HuR siRNA inhibits lung tumor growth in vitro and in vivo by disrupting the oncogenic activity of the RNA-binding protein HuR. Molecular Cancer Therapeutics, 16(8), 1470–1486. 10.1158/1535-7163.MCT-17-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie X, Zhang J, Xu Q, Liu X, Li Y, Wu Y, et al. (2014). Targeting peptide iRGD-conjugated amphiphilic chitosan-co-PLA/DPPE drug delivery system for enhanced tumor therapy. Journal of Materials Chemistry B, 2, 3232–3242. [DOI] [PubMed] [Google Scholar]
- Nukolova NV, Oberoi HS, Zhao Y, Chekhonin VP, Kabanov AV, & Bronich TK (2013). LHRH-targeted nanogels as a delivery system for cisplatin to ovarian cancer. Molecular Pharmaceutics, 10(10), 3913–3921. 10.1021/mp4003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H, Kohno T, Ishii Y, Shimada Y, Shiraishi K, Iwakawa R, et al. (2012). Identification of genes upregulated in ALK-positive and EGFR/KRAS/ALK-negative lung adenocarcinomas. Cancer Research, 72(1), 100–111. 10.1158/0008-5472.CAN-11-1403. [DOI] [PubMed] [Google Scholar]
- Palchaudhuri R, & Hergenrother PJ (2007). DNA as a target for anticancer compounds: Methods to determine the mode of binding and the mechanism of action. Current Opinion in Biotechnology, 18(6), 497–503. 10.1016/j.copbio.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Paliwal S, &Mitragotri S (2006). Ultrasound-induced cavitation: Applications in drug and gene delivery. Expert Opinion on Drug Delivery, 3(6), 713–726. 10.1517/17425247.3.6.713. [DOI] [PubMed] [Google Scholar]
- Panneerselvam J, Jin J, Shanker M, Lauderdale J, Bates J, Wang Q, et al. (2015). IL-24 inhibits lung cancer cell migration and invasion by disrupting the SDF-1/ CXCR4 signaling axis. PLoS One, 10(3), e0122439. 10.1371/journal.pone.0122439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneerselvam J, Srivastava A, Muralidharan R, Wang Q, Zheng W, Zhao L, et al. (2016). IL-24 modulates the high mobility group (HMG) A1/miR222/AKT signaling in lung cancer cells. Onco Target, 7(43), 70247–70263. 10.18632/oncotarget.11838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyam J, & Labhasetwar V (2003). Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Advanced Drug Delivery Reviews, 55(3), 329–347. [DOI] [PubMed] [Google Scholar]
- Parhi P, Mohanty C, & Sahoo SK (2012). Nanotechnology-based combinational drug delivery: An emerging approach for cancer therapy. Drug Discovery Today, 17(17–18), 1044–1052. 10.1016/j.drudis.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, & Leamon CP (2005). Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Analytical Biochemistry, 338(2), 284–293. 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Patel MP, Patel RR, & Patel JK (2010). Chitosan mediated targeted drug delivery system: A review. Journal of Pharmacy & Pharmaceutical Sciences, 13(4), 536–557. [DOI] [PubMed] [Google Scholar]
- Patil SD, Rhodes DG, & Burgess DJ (2004). Anionic liposomal delivery system for DNA transfection. The AAPS Journal, 6(4), e29 10.1208/aapsj060429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pencheva N, Tran H, Buss C, Huh D, Drobnjak M, Busam K, et al. (2012). Convergent multi-miRNA targeting of ApoE drives LRP1/LRP8-dependent melanoma metastasis and angiogenesis. Cell, 151(5), 1068–1082. 10.1016/j.cell.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J, Chambers A, Spithoff K, & Laperriere N (2007). Gliadel wafers in the treatment of malignant glioma: A systematic review. Current Oncology, 14(5), 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumal V, Banerjee S, Das S, Sen RK, & Mandal M (2011). Effect of liposomal celecoxib on proliferation of colon cancer cell and inhibition of DMBA-induced tumor in rat model. Cancer Nanotechnology, 2(1–6), 67–79. 10.1007/s12645-011-0017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabaharan M, Grailer JJ, Pilla S, Steeber DA,& Gong S (2009). Amphiphilic multiarm-block copolymer conjugated with doxorubicin via pH-sensitive hydrazone bond for tumor-targeted drug delivery. Biomaterials, 30(29), 5757–5766. 10.1016/j.biomaterials.2009.07.020. [DOI] [PubMed] [Google Scholar]
- Prabhu S, Uzzaman V, & Guruvayoorappan GC (2011). Nanoparticles in drug delivery and cancer therapy: The Giant rats tail. Journal of Cancer Therapy, 2, 325–334. [Google Scholar]
- Puoci F, Iemma F, &Picci N (2008). Stimuli-responsive molecularly imprinted polymers for drug delivery: A review. Current Drug Delivery, 5(2), 85–96. [DOI] [PubMed] [Google Scholar]
- Qiao H, Zhu Z, Fang D, Sun Y, Kang C, Di L, et al. (2017). Redox-triggered mitoxantrone prodrug micelles for overcoming multidrug-resistant breast cancer. Journal of Drug Targeting, 18, 1–11. 10.1080/1061186X.2017.1339195. [DOI] [PubMed] [Google Scholar]
- Qiu L, Zhu M, Gong K, Peng H, Ge L, Zhao L, et al. (2017). pH-triggered degradable polymeric micelles for targeted anti-tumor drug delivery. Materials Science & Engineering C : Materials for Biological Applications, 78, 912–922. 10.1016/j.msec.2017.04.137. [DOI] [PubMed] [Google Scholar]
- Saad M, Garbuzenko OB, Ber E, Chandna P, Khandare JJ, Pozharov VP, et al. (2008). Receptor targeted polymers, dendrimers, liposomes: Which nanocarrier is the most efficient for tumor-specific treatment and imaging? Journal of Controlled Release, 130(2), 107–114. 10.1016/j.jconrel.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra-Alonso S, Zapata-Benavides P, Chavez-Escamilla AK, Manilla-Munoz E, Zamora-Avila DE, Franco-Molina MA, et al. (2016). WT1 shRNA delivery using transferrin-conjugated PEG liposomes in an in vivo model of melanoma. Experimental and Therapeutic Medicine, 12(6), 3778–3784. 10.3892/etm.2016.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbatini P, Aghajanian C, Dizon D, Anderson S, Dupont J, Brown JV, et al. (2004). Phase II study of CT-2103 in patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal carcinoma. Journal of Clinical Oncology, 22(22), 4523–4531. 10.1200/JCO.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Sahoo SK, Ma W, & Labhasetwar V (2004). Efficacy of transferrin-conjugated paclitaxel-loaded nanoparticles in a murine model of prostate cancer. International Journal of Cancer, 112(2), 335–340. 10.1002/ijc.20405. [DOI] [PubMed] [Google Scholar]
- Sardo C, Bassi B, Craparo EF, Scialabba C, Cabrini E, Dacarro G, et al. (2017). Gold nanostar-polymer hybrids for siRNA delivery: Polymer design towards colloidal stability and in vitro studies on breast cancer cells. International Journal of Pharmaceutics, 519(1–2), 113–124. 10.1016/j.ijpharm.2017.01.022. [DOI] [PubMed] [Google Scholar]
- Shahabipour F, Barati N, Johnston TP, Derosa G, Maffioli P,& Sahebkar A (2017). Exosomes: Nanoparticulate tools for RNA interference and drug delivery. Journal of Cellular Physiology, 232(7), 1660–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih YH, Luo TY, Chiang PF, Yao CJ, Lin WJ, Peng CL, et al. (2017). EGFR-targeted micelles containing near-infrared dye for enhanced photothermal therapy in colorectal cancer. Journal of Controlled Release, 258, 196–207. 10.1016/j.jconrel.2017.04.031. [DOI] [PubMed] [Google Scholar]
- Shu D, Li H, Shu Y, Xiong G, Carson WE 3rd, Haque F, et al. (2015). Systemic delivery of anti-miRNA for suppression of triple negative breast cancer utilizing RNA nanotechnology. ACS Nano, 9(10), 9731–9740. 10.1021/acsnano.5b02471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes S, Filipe A, Faneca H, Mano M, Penacho N, Duzgunes N, et al. (2005). Cationic liposomes for gene delivery. Expert Opinion on Drug Delivery, 2(2), 237–254. 10.1517/17425247.2.2.237. [DOI] [PubMed] [Google Scholar]
- Song CZ (2007). Gene silencing therapy against cancer In Hunt KK, Vorburger SA, & Swisher SG (Eds.), Gene therapy for cancer (pp. 185–196). Human Press. [Google Scholar]
- Srivastava A, Amreddy N, Babu A, Panneerselvam J, Mehta M, Muralidharan R, et al. (2016). Nanosomes carrying doxorubicin exhibit potent anticancer activity against human lung cancer cells. Scientific Reports, 6, 38541 10.1038/srep38541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Babu A, Filant J, Moxley KM, Ruskin R, Dhanasekaran D, et al. (2016). Exploitation of exosomes as nanocarriers for gene-, chemo-, and immune- therapy of cancer. Journal of Biomedical Nanotechnology, 12(6), 1159–1173. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Filant J, Moxley KM, Sood A, McMeekin S, & Ramesh R (2015). Exosomes: A role for naturally occurring nanovesicles in cancer growth, diagnosis and treatment. Current Gene Therapy, 15(2), 182–192. [DOI] [PubMed] [Google Scholar]
- Subramanian N, Kanwar JR, Kanwar RK, & Krishnakumar S (2015). Blocking the maturation of OncomiRNAs using pri-miRNA-17 approximately 92 aptamer in retinoblastoma. Nucleic Acid Therapeutics, 25(1), 47–52. 10.1089/nat.2014.0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Girard OM, et al. (2009). Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell, 16(6), 510–520. 10.1016/j.ccr.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Meng F, Cheng R, Deng C, & Zhong Z (2014). Reduction-responsive polymeric micelles and vesicles for triggered intracellular drug release. Antioxidants & Redox Signaling, 21(5), 755–767. 10.1089/ars.2013.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szwed M, Kania KD, & Jozwiak Z (2014). Relationship between therapeutic efficacy of doxorubicin-transferrin conjugate and expression of P-glycoprotein in chronic erythromyeloblastoid leukemia cells sensitive and resistant to doxorubicin. Cellular Oncology (Dordrecht), 37(6), 421–428. 10.1007/s13402-014-0205-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagami T, Foltz WD, Ernsting MJ, Lee CM, Tannock IF, May JP, et al. (2011). MRI monitoring of intratumoral drug delivery and prediction of the therapeutic effect with a multifunctional thermosensitive liposome. Biomaterials, 32(27), 6570–6578. 10.1016/j.biomaterials.2011.05.029. [DOI] [PubMed] [Google Scholar]
- Taratula O, Kuzmov A, Shah M, Garbuzenko OB, &Minko T (2013). Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA. Journal of Controlled Release, 171(3), 349–357. 10.1016/j.jconrel.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatiparti K, Sau S, Kashaw SK, & Iyer AK (2017). siRNA delivery strategies: A comprehensive review of recent developments. Nanomaterials (Basel), 7(4), pii: E77. 10.3390/nano7040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telli ML, & Carlson RW (2009). First-line chemotherapy for metastatic breast cancer. Clinical Breast Cancer, 2, S66–72. [DOI] [PubMed] [Google Scholar]
- Usuda J, Tsutsui H, Honda H, Ichinose S, Ishizumi T, Hirata T, et al. (2007). Photodynamic therapy for lung cancers based on novel photodynamic diagnosis using talaporfin sodium (NPe6) and autofluorescence bronchoscopy. Lung Cancer, 58(3), 317–323. 10.1016/j.lungcan.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Vankayala R, Huang YK, Kalluru P, Chiang CS, &Hwang KC (2014). First demonstration of gold nanorods-mediated photodynamic therapeutic destruction of tumors via near infra-red light activation. Small, 10(8), 1612–1622. 10.1002/smll.201302719. [DOI] [PubMed] [Google Scholar]
- Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, & Kopple KD (2002). Molecular properties that influence the oral bioavailability of drug candidates. Journal of Medicinal Chemistry, 45(12), 2615–2623. [DOI] [PubMed] [Google Scholar]
- Wang TY, Choe JW, Pu K, Devulapally R, Bachawal S, Machtaler S,et al. (2015). Ultrasound-guided delivery of microRNA loaded nanoparticles into cancer. Journal of Controlled Release, 203, 99–108. 10.1016/j.jconrel.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Chui WK, & Ho PC (2010). Integrin targeted drug and gene delivery. Expert Opinion on Drug Delivery, 7(2), 159–171. 10.1517/17425240903468696. [DOI] [PubMed] [Google Scholar]
- Wang M, Geilich BM, Keidar M, &Webster TJ (2017). Killing malignant melanoma cells with protoporphyrin IX-loaded polymersome-mediated photodynamic therapy and cold atmospheric plasma. International Journal of Nanomedicine, 12, 4117–4127. 10.2147/IJN.S129266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Kievit FM, Sham JG, Jeon M, Stephen ZR, Bakthavatsalam A, et al. (2016). Iron-oxide-based nanovector for tumor targeted siRNA delivery in an orthotopic hepatocellular carcinoma xenograft mouse model. Small, 12(4), 477–487. 10.1002/smll.201501985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Li C, Cheng J, & Yuan Z (2016). Recent advances on inorganic nanoparticle- based cancer therapeutic agents. International Journal of Environmental Research and Public Health, 13(12), 1182 10.3390/ijerph13121182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lu Z, Wientjes MG, & Au JL (2010). Delivery of siRNA therapeutics: Barriers and carriers. The AAPS Journal, 12(4), 492–503. 10.1208/s12248-010-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Qiao R, Tang N, Lu Z, Wang H, Zhang Z, et al. (2017). Active targeting theranostic iron oxide nanoparticles for MRI and magnetic resonance-guided focused ultrasound ablation of lung cancer. Biomaterials, 127, 25–35. 10.1016/j.biomaterials.2017.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tam YY, Chen S, Zaifman J, van der Meel R, Ciufolini MA, et al. (2016). The Niemann–Pick C1 inhibitor NP3.47 enhances gene silencing potency of lipid nanoparticles containing siRNA. Molecular Therapy, 24(12), 2100–2108. 10.1038/mt.2016.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Tao H, Cheng L, & Liu Z (2011). Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials, 32(26), 6145–6154. 10.1016/j.biomaterials.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yu L, Han L, Sha X, & Fang X (2007). Difunctional pluronic copolymer micelles for paclitaxel delivery: Synergistic effect of folate-mediated targeting and pluronic-mediated overcoming multidrug resistance in tumor cell lines. International Journal of Pharmaceutics, 337(1–2), 63–73. 10.1016/j.ijpharm.2006.12.033. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Zhang H, Yang Y, Xie XY, Yang YF, Li Z,et al. (2016). Preparation, characterization, and efficacy of thermosensitive liposomes containing paclitaxel. Drug Delivery, 23(4), 1222–1231. 10.3109/10717544.2015.1122674. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhao P, Su W, Wang S, Liao Z, Niu R, et al. (2010). PLGA/polymeric liposome for targeted drug and gene co-delivery. Biomaterials, 31(33), 8741–8748. 10.1016/j.biomaterials.2010.07.082. [DOI] [PubMed] [Google Scholar]
- Watts JK, & Corey DR (2012). Silencing disease genes in the laboratory and the clinic. The Journal of Pathology, 226(2), 365–379. 10.1002/path.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss WA, Taylor SS, & Shokat KM (2007). Recognizing and exploiting differences between RNAi and small-molecule inhibitors. Nature Chemical Biology, 3(12), 739–744. 10.1038/nchembio1207-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen HY, Dong HQ, Xie WJ, Li YY, Wang K, Pauletti GM, et al. (2011). Rapidly disassembling nanomicelles with disulfide-linked PEG shells for glutathione- mediated intracellular drug delivery. Chemical Communications, 47(12), 3550–3552. 10.1039/c0cc04983b. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang W, Chen Y, Huang K, Shuai X, Chen Q, et al. (2010). The investigation of polymer-siRNA nanoparticle for gene therapy of gastric cancer in vitro. International Journal of Nanomedicine, 5, 129–136.www.clinicaltrial.gov website accessed on June 28, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Killinger B, Moszczynska A, & Merkel OM (2016). Targeted delivery of siRNA to transferrin receptor overexpressing tumor cells via peptide modified polyethylenimine. Molecules, 21(10), pii: E1334. 10.3390/molecules21101334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Kim NH, Nadithe V, Schalk D, Thakur A, Kilic A, et al. (2016). Targeted delivery of siRNA to activated T cells via transferrin-polyethylenimine (Tf-PEI) as a potential therapy of asthma. Journal of Controlled Release, 229, 120–129. 10.1016/j.jconrel.2016.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Singh A, & Amiji MM (2014). Redox-responsive targeted gelatin nanoparticles for delivery of combination wt-p53 expressing plasmid DNA and gemcitabine in the treatment of pancreatic cancer. BMC Cancer, 14, 75 10.1186/1471-2407-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Xia Y, Wang CH, & Pack DW (2012). Monodisperse double-walled microspheres loaded with chitosan-p53 nanoparticles and doxorubicin for combined gene therapy and chemotherapy. Journal of Controlled Release, 163(2), 130–135. 10.1016/j.jconrel.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T, Wang L, Yin L, Zhou J, & Huo M (2015). Co-delivery of hydrophobic paclitaxel and hydrophilic AURKA specific siRNA by redox-sensitive micelles for effective treatment of breast cancer. Biomaterials, 61, 10–25. 10.1016/j.biomaterials.2015.05.022. [DOI] [PubMed] [Google Scholar]
- Yin H, Yang J, Zhang Q, Yang J, Wang H, Xu J, et al. (2017). iRGD as a tumorpenetrating peptide for cancer therapy (Review). Molecular Medicine Reports, 15(5), 2925–2930. 10.3892/mmr.2017.6419. [DOI] [PubMed] [Google Scholar]
- Yokoyama T, Tam J, Kuroda S, Scott AW, Aaron J, Larson T, et al. (2011). EGFR- targeted hybrid plasmonic magnetic nanoparticles synergistically induce autophagy and apoptosis in non-small cell lung cancer cells. PLoS One, 6(11), e25507. 10.1371/journal.pone.0025507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SS, Razzak R, Bedard E, Guo L, Shaw AR, Moore RB, et al. (2014). Layered gadolinium-based nanoparticle as a novel delivery platform for microRNA therapeutics. Nanotechnology, 25(42), 425102. 10.1088/0957-4484/25/42/425102. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Tokashiki R, Ito H, Shimizu A, Nakamura K, Hiramatsu H, et al. (2008). Therapeutic effects of a new photosensitizer for photodynamic therapy of early head and neck cancer in relation to tissue concentration. Auris Nasus Larynx, 35(4), 545–551. 10.1016/j.anl.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Younes RN, Pereira JR, Fares AL, & Gross JL (2011). Chemotherapy beyond first- line in stage IV metastatic non-small cell lung cancer. Revista Da Associacao Medica Brasileira, 57(6), 686–691. [DOI] [PubMed] [Google Scholar]
- Yu X, Trase I, Ren M, Duval K, Guo X, & Chen Z (2016). Design of nanoparticle- based carriers for targeted drug delivery. Journal of Nanomaterials, 2016, pii: 1087250. 10.1155/2016/1087250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamore PD, Tuschl T, Sharp PA, & Bartel DP (2000). RNAi: Double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell, 101(1), 25–33. 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- Zhang JL, Gong JH, Xing L, Cui PF, Qiao JB, He YJ, et al. (2016). One-step assembly of polymeric demethylcantharate prodrug/Akt1 shRNA complexes for enhanced cancer therapy. International Journal of Pharmaceutics, 513(1–2), 612–627. 10.1016/j.ijpharm.2016.09.070. [DOI] [PubMed] [Google Scholar]
- Zhang X, Liu N, Shao Z, Qiu H, Yao H, Ji J, et al. (2017). Folate-targeted nanoparticle delivery of androgen receptor shRNA enhances the sensitivity of hormone-independent prostate cancer to radiotherapy. Nanomedicine, 13(4), 1309–1321. 10.1016/j.nano.2017.01.015. [DOI] [PubMed] [Google Scholar]
- Zhang C, Pan D, Li J, Hu J, Bains A, Guys N, et al. (2017). Enzyme-responsive peptide dendrimer-gemcitabine conjugate as a controlled-release drug delivery vehicle with enhanced antitumor efficacy. Acta Biomaterialia, 55, 153–162. 10.1016/j.actbio.2017.02.047. [DOI] [PubMed] [Google Scholar]
- Zhang J, Qian Z, & Gu Y (2009). In vivo anti-tumor efficacy of docetaxel-loaded thermally responsive nanohydrogel. Nanotechnology, 20(32). 325102. 10.1088/0957-4484/20/32/325102. [DOI] [PubMed] [Google Scholar]
- Zhang J, & Saltzman M (2013). Engineering biodegradable nanoparticles for drug and gene delivery. Chemical Engineering Progress, 109(3), 25–30. [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang T, Yang L, Liu C, Wang C, Liu H, et al. (2012). General route to multifunctional uniform yolk/mesoporous silica shell nanocapsules: A platform for simultaneous cancer-targeted imaging and magnetically guided drug delivery. Chemistry, 18(39), 12512–12521. 10.1002/chem.201200030. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang H, Zhu X, Zhang X, Chen Q, Chen J, et al. (2017). Visible-light-sensitive titanium dioxide nanoplatform for tumor-responsive Fe2 + liberating and artemisinin delivery. Onco Target, 8(35), 58738–58753. 10.18632/oncotarget.17639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, & Huang L (2014). Lipid nanoparticles for gene delivery. Advances in Genetics, 88, 13–36. 10.1016/B978-0-12-800148-6.00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Li F, Li Y, Wang H, Ren H, Chen J, et al. (2015). Co-delivery ofHIF1alpha siRNA and gemcitabine via biocompatible lipid-polymer hybrid nanoparticles for effective treatment of pancreatic cancer. Biomaterials, 46, 13–25. 10.1016/j.biomaterials.2014.12.028. [DOI] [PubMed] [Google Scholar]
- Zhao D, Wu J, Li C, Zhang H, Li Z, & Luan Y (2017). Precise ratiometric loading of PTX and DOX based on redox-sensitive mixed micelles for cancer therapy. Colloids and Surfaces B: Biointerfaces, 155, 51–60. 10.1016/jxolsurfb.2017.03.056. [DOI] [PubMed] [Google Scholar]
- Zheng M, Zhong Z, Zhou L, Meng F, Peng R, & Zhong Z (2012). Poly(ethylene oxide) grafted with short polyethylenimine gives DNA polyplexes with superior colloidal stability, low cytotoxicity, and potent in vitro gene transfection under serum conditions. Biomacromolecules, 13(3), 881–888. 10.1021/bm2017965. [DOI] [PubMed] [Google Scholar]
- Zhou H, Hou X, Liu Y, Zhao T, Shang Q, Tang J, et al. (2016). Superstable magnetic nanoparticles in conjugation with near-infrared dye as a multimodal theranostic platform. ACS Applied Materials & Interfaces, 8(7), 4424–4433. 10.1021/acsami.5b11308. [DOI] [PubMed] [Google Scholar]
- Zhu WJ, Yang SD, Qu CX, Zhu QL, Chen WL, Li F, et al. (2017). Low-density lipoprotein-coupled micelles with reduction and pH dual sensitivity for intelligent co-delivery of paclitaxel and siRNA to breast tumor. International Journal of Nanomedicine, 12, 3375–3393. 10.2147/IJN.S126310. [DOI] [PMC free article] [PubMed] [Google Scholar]
FURTHER READING
- Bhattacharjee H, Balabathula P, & Wood GC (2010). Targeted nanoparticulate drug-delivery systems for treatment of solid tumors: A review. Therapeutic Delivery, 1(5), 713–734. [DOI] [PubMed] [Google Scholar]
- Greish K (2007). Enhanced permeability and retention of macromolecular drugs in solid tumors: A royal gate for targeted anticancer nanomedicines. Journal of Drug Targeting, 15(7–8), 457–464. 10.1080/10611860701539584. [DOI] [PubMed] [Google Scholar]
- Jiang XC, & Gao JQ (2017). Exosomes as novel bio-carriers for gene and drug delivery. International Journal of Pharmaceutics, 521(1–2), 167–175. [DOI] [PubMed] [Google Scholar]
- Maeda H, Bharate GY, & Daruwalla J (2009). Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. European Journal of Pharmaceutics and Biopharmaceutics, 71(3), 409–419. https://doi.org/10.1016Zj.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Singh R, & Lillard JW Jr. (2009). Nanoparticle-based targeted drug delivery. Experimental and Molecular Pathology, 86(3), 215–223. 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Feng J, & Chen J (2016). External-stimuli responsive systems for cancer theranostic. Asian Journal of Pharmaceutical Sciences, 11, 585–595. [Google Scholar]


