ABSTRACT
Protein prenylation is a crucial post-translational modification largely mediated by two heterodimeric enzyme complexes, farnesyltransferase and geranylgeranyltransferase type-I (GGTase-I), each composed of a shared α-subunit and a unique β-subunit. GGTase-I enzymes are validated drug targets that contribute to virulence in Cryptococcus neoformans and to the yeast-to-hyphal transition in Candida albicans. Therefore, we sought to investigate the importance of the α-subunit, RamB, and the β-subunit, Cdc43, of the A. fumigatus GGTase-I complex to hyphal growth and virulence. Deletion of cdc43 resulted in impaired hyphal morphogenesis and thermo-sensitivity, which was exacerbated during growth in rich media. The Δcdc43 mutant also displayed hypersensitivity to cell wall stress agents and to cell wall synthesis inhibitors, suggesting alterations of cell wall biosynthesis or stress signaling. In support of this, analyses of cell wall content revealed decreased amounts of β-glucan in the Δcdc43 strain. Despite strong in vitro phenotypes, the Δcdc43 mutant was fully virulent in two models of murine invasive aspergillosis, similar to the control strain. We further found that a strain expressing the α-subunit gene, ramB, from a tetracycline-inducible promoter was inviable under non-inducing in vitro growth conditions and was virtually avirulent in both mouse models. Lastly, virulence studies using C. albicans strains with tetracycline-repressible RAM2 or CDC43 expression revealed reduced pathogenicity associated with downregulation of either gene in a murine model of disseminated infection. Together, these findings indicate a differential requirement for protein geranylgeranylation for fungal virulence, and further inform the selection of specific prenyltransferases as promising antifungal drug targets for each pathogen.
KEYWORDS: Aspergillus fumigatus, Candida albicans, prenylation, prenyltransferases, geranylgeranylation, geranylgeranyltransferase-I, protein localization, fungal virulence
Introduction
Protein prenylation is a post-translational modification that increases protein hydrophobicity, promoting protein–protein interactions and protein affinity for cell membranes [1]. This modification is critical for the subcellular localization and function of important proteins, being crucial in various cellular physiological processes. The reaction is mediated by prenyltransferases (PTases) and consists of the transfer of an isoprenoid lipid, derived either from a 15-carbon farnesyl pyrophosphate (FPP) or a 20-carbon geranylgeranyl pyrophosphate (GGPP), to proteins that bear conserved cysteine residues. Three types of prenyltransferases have been identified in eukaryotes: Farnesyltransferase (FTase), Geranylgeranyltransferase type-I (GGTase-I) and type-II (GGTase-II). FTase and GGTase-I similarly recognize a consensus sequence of four amino acids that is usually referred to as the CaaX motif (C = cysteine; aa = two aliphatic amino acids, usually; X = any amino acid) [1–3]. In contrast, protein substrates that are prenylated by GGTase-II harbor CXC or CC recognition motifs [1–3]. Recently, it has been demonstrated that proteins bearing a C-terminus CaaaX sequence may also be subject to prenylation by Ftase enzymes [4].
The CaaX prenyltransferases, GGtase-I and FTase, are conserved heterodimeric zinc metalloenzymes with a common α- subunit and unique β-subunits [5]. The active site is located at the interface of the two domains, and the catalytic reaction consists of the covalent attachment of the isoprenyl group to the sulfur atom of the cysteine residue in the CaaX motif [2,6]. The last amino acid in the CaaX motif partially dictates substrate specificity of the PTase reaction. Classically, GGTases prenylate CaaX motifs in which X is a leucine or isoleucine, whereas substrate proteins ending in a glutamine, methionine, serine, cysteine or alanine are farnesylated [1,7,8]. However, cross-specificity has been reported [9]. After prenylation, the substrate protein can be further processed by proteolysis of the aaX residues and carboxy-methylation [1,10].
Among the important protein substrates that bear a CaaX motif are small GTPases, such as the Ras- and Rho-like proteins, pheromones, lamins and trimeric G-proteins [1,11]. In mammalian cells, these proteins play crucial roles in cell-signaling pathways and are involved in human conditions such as cancer, inflammatory and aging disorders and neurodegenerative diseases [3]. In fungal organisms, knowledge of the protein prenylome is still relatively limited and concentrated on the Ras- and Rho-family proteins. These proteins are involved in fungal growth, morphogenesis, and pathogenesis [12,13]. The absence of prenylation potentially culminates in protein mislocalization, which in turn can drastically impair protein function and have pleiotropic consequences on cellular homeostasis. For this reason, prenylation pathways have been explored as drug targets to limit tumor growth, via inhibition of oncogenic Ras signaling in humans, and to battle infectious diseases, in particular, fungal and parasite infections [6,14–18]. Prenyltransferases are validated targets in mammals and fungi, and several inhibitors were identified and are currently under investigation [3,16,19,20]. Some of these inhibitors were found to be active against fungal enzymes or cells [18,21–24]. Importantly, it has been reported that differences in the tertiary structure of the substrate binding pocket of mammalian versus fungal prenyltransferases may allow the design of inhibitors that could specifically target fungal enzymes, avoiding potential cytotoxicity in host cells [6,24,25].
Protein prenyltransferases have been extensively characterized in fungi, especially yeast organisms. The shared α-subunit of fungal PTases, encoded by RAM2, is essential for the viability of the yeasts Saccharomyces cerevisiae [26], Candida albicans [21] and C. glabrata [27]. The β-subunit of FTase (in yeasts encoded by RAM1 or cpp1+) was found to mediate morphogenesis in Cryptococcus neoformans [28] and Schizosaccharomyces pombe [29]. S. cerevisiae [26,30] and C. neoformans [28] RAM1 mutants also display thermosensitive growth at 37°C. Accordingly, deletion of RAM1 attenuates C. neoformans pathogenicity in a murine model of cryptococcosis [28]. CDC43, which is the β-subunit of GGTase-I, is essential in S. cerevisiae [26], and mediates morphogenesis in C. albicans [31] and thermotolerance, morphology, and virulence in C. neoformans [32]. Recently, we characterized the β-subunit of the farnesyltransferase complex (RamA) in Aspergillus fumigatus and demonstrated that ramA null mutants display attenuated virulence, accompanied by growth defects and decreased conidial viability [33]. In this mutant, localization of RasA was only partially impaired, suggesting that lack of Ras farnesylation could be compensated by GGTase-I activity. However, the role of protein geranylgeranylation has not yet been accessed in filamentous fungi.
Therefore, in this work, we sought to identify and study the β-subunit of GGTase-I (Cdc43) and the shared α-subunit (RamB) in A. fumigatus. We observed that A. fumigatus cdc43 mutants displayed a thermosensitivity phenotype, slow growth, abnormal hyphal morphogenesis, and a defective cell wall. Despite in vitro phenotypes, the Δcdc43 mutant was fully virulent in two models of murine invasive aspergillosis. We also demonstrate that the expression of the α-subunit, RamB, is essential for A. fumigatus viability in vitro and for invasive growth in mouse models of invasive aspergillosis. Lastly, we found that both CDC43 and RAM2 expression are essential for C. albicans pathogenicity in a murine model of invasive candidiasis. Taken into context with our previous reports examining the importance of farnesyltransferase activity in A. fumigatus, our findings here reveal differential requirements for specific prenyltransferases during growth, morphogenesis, and virulence. Further exploration of the roles of the virulence-associated prenylome will identify individual substrate proteins underlying phenotypes associated with farnesyltransferase and geranylgeranyltransferase inhibition, identifying novel factors driving the pathogenesis of invasive aspergillosis.
Materials and methods
Genetic manipulation of A. fumigatus
All strains utilized in this study are listed in Table 1, and oligonucleotides designed for strain construction are listed in Table 2. The generation and transformation of A. fumigatus protoplasts were performed using a previously described protocol [34]. The A. fumigatus genetically engineered KU80∆pyrG strain (ΔakuB) [35] was used as a background for all strains developed in this study. This strain exhibits high rates of homologous recombination due to its deficiency in the non-homologous end joining (NHEJ) DNA repair pathway and is also uracil auxotrophic (pyrG-). The control strain ∆akuB-pyrG+ (Ctrl) was obtained previously [36] by replacing the non-functional pyrG locus of the A. fumigatus KU80∆pyrG strain [35] with the functional homolog from A. parasiticus obtained from plasmid pJW24 [37]. The cdc43 deletion mutant (Δcdc43) was obtained by replacement of the entire ∆akuB cdc43 coding sequence using the A. parasiticus pyrG gene, as previously described [36]. Briefly, 1.5 kb fragments of genomic sequence located immediately upstream and downstream of the putative cdc43 coding locus were first PCR amplified using primer sets P1/P2 and P3/P4. These primers incorporated restriction enzyme sites utilized for sub-cloning of each fragment as flanking arms of the pyrG cassette in vector pJW24. The final deletion cassette was PCR amplified from the resulting vector using the primer set P1/P4 and utilized for transformation (Supp Figure 1). Complementation of Δcdc43 (Δcdc43::cdc43) was achieved by the ectopic integration of the entire cdc43 coding sequence, including 1 kb of upstream and 300 bp of downstream genomic sequence. This fragment was PCR amplified from genomic DNA using primer set P5/P6 and was cloned into a NotI restriction site immediately upstream of a hygromycin resistance cassette in vector pHygR [38]. Primers P5/P7 were then used to amplify the full cdc43 coding locus and downstream hygR cassette for ectopic integration into the Δcdc43 mutant (Supp Figure 1). The ramB (pTetOn-ramB) tetracycline-inducible strain was developed by replacement of the native ramB promoter with the pyrithiamine-based Tet-On cassette from plasmid pCH008 [39] using an overlap extension PCR method. In short, a 1.5 kb fragment of the upstream genomic sequence was first amplified from genomic DNA using primer set P8/P9. In a second reaction, 1.5 kb of the sequence starting at the putative ramB start codon and stretching downstream was PCR amplified from genomic DNA with primer set P10/P11. In a third reaction, the entire pyrithiamine-Tet-On construct was PCR amplified from vector pCH008 using primers P12/P13. Finally, the three resulting fragments from the above reactions were utilized in a single overlap extension PCR reaction using nested primers P14/P15 and the full construct was utilized for transformation (Supp Figure 1). To ensure the expression of the ramB gene, the mutant was maintained on doxycycline (30 µg/ml)-impregnated growth media. All the strains were confirmed by PCR and Southern blot analyses.
Table 1.
A. fumigatus strains utilized in this study.
Table 2.
Oligonucleotides used for genetic manipulation of A.fumigatus.
| Primer | Sequence | Purpose |
|---|---|---|
| P1 | TTTTGCGGCCGCCAGGCTGAACGGTTTATGGG | Subcloning of 1.5kb upstream flanking region of cdc43 in pJW24; amplification of cdc43 deletion cassette |
| P2 | TTTTGCGGCCGCTGACAATGTTGAATCTGCTCG | Subcloning of 1.5kb upstream flanking region of cdc43 in pJW24 |
| P3 | TTTTCTCGAGTGCTCTAAATTATAGTGGACGGG | Subcloning of 1.5kb downstream flanking region of cdc43 in pJW24 |
| P4 | TTTTGGTACCTGGTCAAGAAAG GAGAAGAAGC | Subcloning of 1.5kb downstream flanking region of cdc43 in pJW24; amplification of cdc43 deletion cassette |
| P5 | TTTTGCGGCCGCGCACAACTCAATAAAGGCTTCCAC | Subcloning of cdc43 coding sequence in pHygR; amplification of cdc43 complementation cassette |
| P6 | TTTTGCGGCCGCTAATAGTGGTCTGGTGGATGGCTTG | Subcloning of cdc43 coding sequence in pHygR |
| P7 | CGAGCTCCCAAATCTGTCCAG | Amplification of cdc43 complementation cassette |
| P8 | CACACAAGACACCTCCGCC | Amplification of the 1.5 kb upstream flanking region of ramB |
| P9 | CAGCGCGAGTGTGCTGAGTAAGCTGGGACGGAATAATGATTGGTG | Amplification of the 1.5 kb upstream flanking region of ramB |
| P10 | CCCGCTTGAGCAGACATCACATGGAAGGGAAATACTCGTCCGATC | Amplification of the 1.5 kb region of ramB from the start codon |
| P11 | ATCCAAATCCGTACCGTTTGA ATATCC | Amplification of the 1.5 kb region of ramB from the start codon |
| P12 | TTACTCAGCACACTCGCGCTG | Amplification of the Pyrithiamine-Tet-On cassette from pCH008 |
| P13 | GTGATGTCTGCTCAAGCGGG | Amplification of the Pyrithiamine-Tet-On cassette from pCH008 |
| P14 | CCAACGGGACCTGTGGC | Overlap extension PCR for ramB promoter replacement cassette |
| P15 | GCATAAATGACCTCGCTCCAGC | Overlap extension PCR for ramB promoter replacement cassette |
C. albicans strains
The C. albicans strains with tetracycline repressible expression of either CDC43 or RAM2, were kindly provided by Merck Sharp & Dohme Corp. Their construction has been previously described [40].
Growth conditions and analyses
Strains were cultured in agar Glucose Minimum Media (GMM) [41] for a minimum of 3 days at 37°C and conidia were harvested in sterile distilled water, filtered in miracloth, washed at least twice, and concentration was determined using a hemocytometer. Where indicated, Yeast Peptone Dextrose (YPD, with 1% yeast extract, 2% peptone, 2% dextrose) and Potato Dextrose Agar (PDA) were utilized. Because the Δcdc43 mutants displayed decreased ability to produce conidia, except for the radial growth and cell wall stress analysis with CFW and CR, this strain was inoculated onto GMM with ammonium tartrate as the nitrogen source, instead of Na2NO3, and incubated for at least 5 days at 30°C.
Conidia (5x103) from each strain were point inoculated onto 60 mm diameter agar plates containing the indicated media and incubated at the designated temperatures (27°C to 45°C). Colony diameter was measured every 24 h over 96 total hours of incubation at 37°C. Experiments were performed three times, using conidia derived from independent cultures, and statistical analyses were done using two-way ANOVA with Tukey’s test for multiple comparison (GraphPad Prism 7.00). For growth analysis of the pTetOn-ramB strain, 6-well plates containing GMM agar supplemented with a range of doxycycline concentrations (1, 5, 10, 20 and 30 µg/ml) were point inoculated with conidia (5x103) and incubated for 3 days at 37°C. For analysis of micromorphology at 30°C and 37°C, conidia (105) were inoculated on sterile coverslips submerged in GMM broth (3 ml) in six-well plates. After the designated time points, coverslips were mounted onto slides and visualized by bright field microscopy. Germination rates were determined after 5, 7, 10, 12, 15, and 24 h of incubation. At least 100 conidia were examined, and the percentage of conidia that produced a visible germ tube was determined at each time point. Analyses were performed six times using conidia derived from independent cultures. Statistical differences were analyzed using two-way ANOVA with Tukey’s test for multiple comparison (GraphPad Prism 7.00).
All C. albicans strains were routinely grown in YPD broth or on YPD agar plates at 30°C, unless otherwise stated.
Cell wall stress analysis
Growth in the presence of cell wall stressing agents was evaluated by inoculating 10-fold serial dilutions ranging from 102 to 105 conidia (5 µl) onto GMM agar containing Congo Red (CR) at 20 µg/ml or Calcofluor White (CFW) at 150 µg/ml. Colonies were examined after 48-h incubation at 37°C. Susceptibility to chitin and β-glucan biosynthesis inhibition was assessed through a broth microdilution assay with Nikkomycin Z and Caspofungin, respectively, following the guidelines of the document M38-A2 of CLSI [42] with adaptations. For chitin biosynthesis inhibition, conidia (2x104) were inoculated into GMM broth with ascending doses of the Nikkomyzin Z (ranging from 0.1 to 100 µg/ml) and incubated for 24 h at 37°C. For β-glucan biosynthesis inhibition, conidia (4x104) were inoculated into RPMI with ascending doses of Caspofungin (ranging from 8 to 0.01 µg/ml) and incubated for 24 h at 35°C. Minimum inhibitory concentration (MIC) of Nikkomycin Z, with complete inhibition of growth, and Minimum effective concentration (MEC) of Caspofungin, corresponding to the lowest concentration leading to abnormal hyphal growth, were determined after microscopic visualization.
Analysis of cell wall β-glucan content
For visualization of β-glucan in the cell wall by fluorescence microscopy, conidia (2x107) were grown in filtered GMM broth (10 ml) and incubated at 30°C or 37°C in a rotatory shaker (250rpm). When germ tubes were visible, germlings were harvested by centrifugation, washed in sterile PBS twice, and incubated in a PBS solution with 10 µg/ml of Fc-hDectin-1a (Invivogen), which is an engineered soluble human Dectin-1 receptor fused with a human IgG1 Fc domain. Fc-hDectin-1a binds to β-glucan residues through the Dectin-1 domain, and also allows binding of a tagged secondary antibody to the IgG1 Fc-domain. After 1 h of incubation with Fc-hDectin-1a at 4°C, samples were washed twice with PBS and incubated with a 1:200 dilution of a FITC-conjugated anti-human-IgGFc (Thermofisher). Samples were once again incubated for 1 h at 4°C, washed twice with PBS and mounted for visualization of β-glucan using the GFP filter in a Nikon NiU microscope equipped with a Nikon DS-Qi1Mc camera. Images were captured using Nikon Elements software (v4.0).
Quantification of total β-glucan was performed as previously described [41,43]. Briefly, conidia (2x108) were inoculated into 100 ml of GMM broth and allowed to grow overnight at 27°C, 30°C, or 37°C. Hyphae were harvested by filtration with miracloth, washed in 0.1 M NaOH, and lyophilized for 16 h. Dry weight was recorded and samples were disrupted in a bead-beater for 3x 1-min cycles each followed by 1 min on ice. The resulting powder was resuspended in 1 M NaOH for a final concentration of 20 mg/ml and incubated at 52°C for 30 min. Fifty-microliter triplicates from each sample were aliquoted into a masked, 96-well fluorescence plate and mixed with 185 µl of aniline blue staining solution (183 mM glycine, 229 mM NaOH, 130 mM HCl, and 618 mg/l aniline blue, pH 9.9, incubated overnight in the dark to decolorization). The samples were then incubated for an additional 30 min at 52°C, and allowed to cool down at room temperature for 30 min. Fluorescence readings were performed at 405 nm excitation and 460 nm emission, and a standard curve (twofold dilutions ranging from 160 µg/ml to 2.5 µg/ml) with Curdlan, a β-1,3-glucan analog, was used in order to normalize values. Experiments were performed using samples derived from three independent mycelia cultures, and statistical differences were analyzed using two-way ANOVA with Tukey’s test for multiple comparison (GraphPad Prism 7.00).
Quantification of cell wall total chitin
Total chitin quantification was also performed as described by Fortwendel et. al. (2009) with adaptations [41]. Lyophilized hyphae were weighed, resuspended in 3 ml of 1 M KOH, and autoclaved for 60 min at 121°C. Samples were cooled to room temperature, 8 ml of ice-cold 75% ethanol were added and the resulting suspension was vortexed until a single phase was obtained. After incubation on ice for 15 min, 300 µl of a 13.3% (wt/vol) Celite 545 (C212; Fisher Chemicals) suspension was added and the tubes were centrifuged at 1,500 x g for 5 min at 2°C. The precipitates were washed with 10 ml of ice-cold 40% ethanol, followed by two washes in 10 ml ice-cold water. The final pellet from each sample was resuspended in equal volumes (500 µl) of water, 5% (wt/vol) NaNO2, and of 5% (wt/vol) KHSO4. A standard curve with glucosamine (twofold serial dilutions ranging from 100 µg/ml to 6.25 µg/ml) in water was also treated with equal volumes of 5% NaNO2 and 5% KHSO4. Tubes were mixed three times for a 15-min period, followed by centrifugation at 1,500 x g for 2 min at 2°C. Aliquots (600 µl) were mixed with 0.2 ml of 12.5% (wt/vol) NH4 sulfamate and mixed vigorously each minute for 5 min, followed by the addition of 0.2 ml of 3-methylbenzthiazolin-one-2-hydrazone (5 mg/ml) and incubation at 130°C for 3 min. Tubes were allowed to cool, and 0.2 ml of 0.83% (wt/vol) ferric chloride was added, followed by incubation at room temperature for 25 min. Absorbance readings were performed at 650 nm and glucosamine standard curve values were used in order to normalize values. Chitin levels were reported as glucosamine equivalents per mg of hyphae. Experiments were performed using samples derived from three independent mycelia cultures, and statistical differences were analyzed using unpaired t-test (GraphPad Prism 7.00).
Virulence studies
All animal experiments were done according to protocols approved by the University of Tennessee Institutional Animal Care and Use Committee. For the virulence studies using A. fumigatus strains, six-week-old female CF-1 mice (Harlan Laboratories) were immunosuppressed using two different protocols previously described [33]. For both the non-neutropenic and neutropenic models of invasive aspergillosis, mice were subcutaneously injected with 40 mg/kg of triamcinolone acetonide one day prior to infection. For the neutropenic model, mice were additionally administered intraperitoneal injections with 150 mg/kg of cyclophosphamide 3 days prior infection and again on every third day thereafter for a total of 3 to 5 injections, depending on the health of the animal. On Day 0, mice were transiently anesthetized with 3.5% isoflurane and intranasal inoculation was performed with 105 freshly harvested conidia in 20 µl of pyrogen-free saline solution. Mortality was monitored for 13–15 days, depending on the health of the animals. Survival data were plotted on a Kaplan–Meier curve and analyzed by Log-rank (Mantel-Cox) test using GraphPad Prism 7.00. For histology analysis, mice were immunosuppressed as above, inoculated with conidia and euthanized 3 days after infection. Lungs were inflated with 10% buffered formalin solution and fragments of superior, middle and inferior right lobes were further processed for paraffin-embedding. Slides containing 5 μm sections of lung tissue were stained with hematoxylin and eosin and Grocott’s methenamine silver (GMS). The presence of fungal hyphae was assessed by light microscopy.
The mouse model of hematogenously disseminated candidiasis was performed as follows. Groups of 6–8-week-old female BALB/c mice (Charles River Laboratories) were randomly assigned to one of two treatment groups three days before infection. Group 1 (-Dox) was provided 20 g of DietGel food per day, per cage of 4 mice, while group 2 (+Dox) was provided the same diet supplemented with 2 mg/ml of doxycycline hyclate. Treatment was maintained daily for the duration of the experiment (up to 16 days post-infection). Each C. albicans strain was grown overnight in YPD cultures at 30°C (200 rpm). Cells were washed twice in sterile endotoxin-free phosphate-buffered saline (PBS) and cell density determined using a hemocytometer. Each strain was then diluted to 5 × 106 cells/ml in sterile PBS, and 0.1 ml of each cell suspension inoculated into the lateral tail vein of mice from each treatment group. Viable cell counts of each inoculum were confirmed by plating appropriate dilutions onto YPD agar plates and counting the number of colonies formed after 48 h. Mice were monitored for 16 days and those showing distress euthanized. Survival data were plotted on a Kaplan–Meier curve and analyzed by Log-rank (Mantel-Cox) test using GraphPad Prism 7.00.
Results
A. fumigatus cdc43 mediates thermotolerance and polarized growth
To first examine the role of protein geranylgeranylation in A. fumigatus growth and virulence, we initiated this study by identifying the GGTase-I β-subunit. Mutation of this subunit is expected to cripple protein geranylgeranylation whereas farnesyltransferase activity remains intact. A BLAST search analysis utilizing the S. cerevisiae Cdc43p protein sequence performed through the Aspergillus genome database (aspergillusgenome.org) revealed a single homologous gene for the GGTase-I β-subunit, Afu6g06710 (29.9% amino acid identity), hereafter called cdc43. A cdc43 deletion strain (Δcdc43) was generated by replacement of the entire coding region in the uracil-auxotrophic ΔakuB strain with a selection cassette containing A. parasiticus pyrG. In order to confirm that phenotypic observations in the cdc43 null mutants were due to gene loss, a complemented strain (Δcdc43:cdc43) was achieved by ectopic integration of the cdc43 coding locus, including 1 kb of upstream and 300 bp of downstream genomic sequence to function as native promoter and terminator. All manipulations were confirmed by PCR and Southern Blot analyses.
The phenotypic characterization of the A. fumigatus cdc43 null mutant was initiated by evaluating its ability to grow in defined minimal media at 27°C, 37°C and 45°C. Compared to the wild type and complemented strains, the Δcdc43 mutant displayed defective growth at all temperatures (Figure 1(a)). However, at 37°C and 45°C, the Δcdc43 mutant displayed increasingly reduced growth and loss of conidiation (Figure 1(a,b)). Culture of the mutant on rich media did not rescue growth rates at 27°C, suggesting nutritional deficiency was not underlying reduced growth of Δcdc43 (Figure 1(c,d)). In contrast, culture on rich media was found to exacerbate growth defects during culture at both 37°C and 45°C (Figure 1(c,d)).
Figure 1.
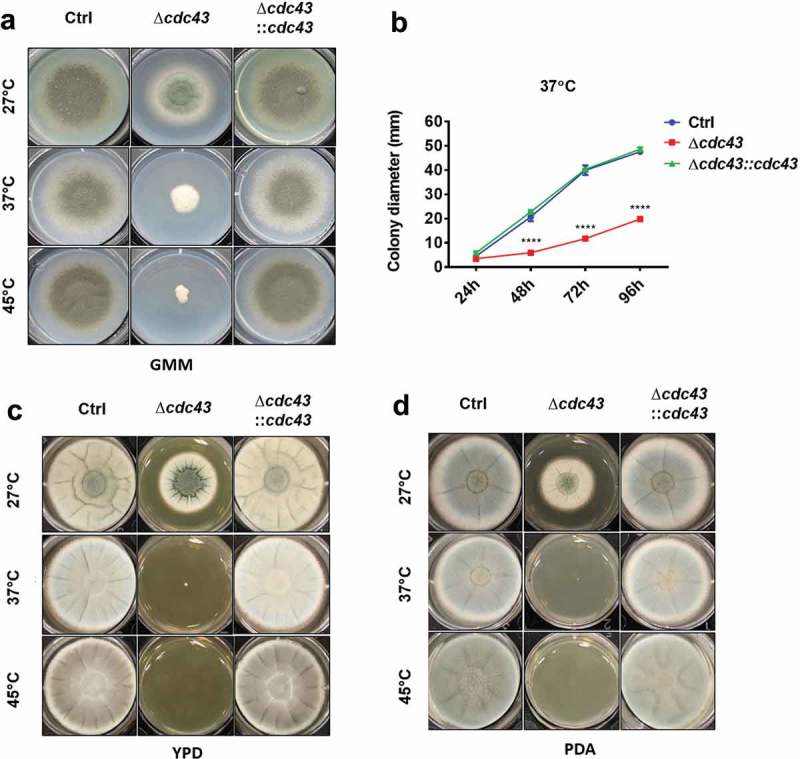
Protein geranylgeranylation promotes A. fumigatus thermotolerant growth.
Conidia (5x103) obtained from control (Ctrl), Δcdc43 or complemented (Δcdc43:cdc43) strains were spot inoculated (5 µl) onto GMM (A), YPD (C), or PDA (D) agar. Growth and development of colony morphology at 27ºC, 37ºC, and 45ºC were monitored and images represent growth and colony development at the time that the control strain reached the plate boundary under each condition. Colony diameters (B) in GMM agar plates were measured every 24 h over 96 h of incubation at 37ºC. Mean and standard deviation (SD) of colonies derived from three independent mycelia cultures are represented. Asterisks indicate statistical differences in comparison with control strain (Ctrl) using two-way ANOVA with Tukey’s test for multiple comparison; ****p < 0.001.
To examine the effects of temperature on hyphal morphogenesis, micromorphology was analyzed in GMM broth at 30°C and 37°C. Conidia of the cdc43 null mutant displayed delayed development of germ tubes accompanied by continuous isotropic swelling, resulting in unpolarized conidia with diameters measuring 2 times, at 30°C (Figure 2(a), 12 h), and 3 times, at 37°C (Figure 2(b), 12 h), the diameter of the control strain (Ctrl) swollen conidia. At 30°C, the Δcdc43 mutant formed wide, slow-growing germlings within 15 h of culture and eventually generated hyper-branched hyphae by 24 h (Figures 2(a), 15 h and 24 h). These phenotypes were further exacerbated at 37°C (Figures 2(b), 15 h and 24 h). Quantification of germ tube formation revealed an approximately 5-h delay in the Δcdc43 mutant at either temperature (Figure 2(c,d)). Moreover, by the end of 24 h of growth at 37°C, only 66.96% (±13.14%) of cdc43 null mutant conidia were able to develop germ tubes (Figure 2(d)). The cdc43 complemented strain (Δcdc43:cdc43) was indistinguishable from the control strain in terms of radial growth and germination rates. These data suggest that protein geranylgeranylation mediates A. fumigatus thermotolerance and establishment of polarized growth.
Figure 2.

Lack of GGTase-I impairs A. fumigatus establishment of polarized growth.
Conidia (105) obtained from control (Ctrl), Δcdc43 or complemented (Δcdc43:cdc43) strains were inoculated on sterile coverslips submerged in GMM broth (3 ml) in six-well plates. At the designated time points, coverslips were mounted onto slides and visualized by bright field microscopy. Micromorphology of germlings at 30ºC (A) and 37ºC (B) were evaluated after 12 h, 15 h, and 24 h (scale bar = 50 µm). Germ tube formation rates at 30ºC (C) and 37ºC (D) were determined by examining at least 100 conidia. Mean and standard deviation (SD) of five independent experiments are represented, and asterisks indicate statistical differences in comparison with control strain (Ctrl) using two-way ANOVA with Tukey’s test for multiple comparison; **p < 0.01, ***p < 0.001 and ****p < 0.0001.
GGtase-I activity is important for A. fumigatus cell wall stress response and composition
The ability of the cdc43 isogenic set to respond to cell wall stress was evaluated by spot assay using GMM agar impregnated with the cell wall stress agents, Congo Red (CR) or Calcofluor White (CFW). We observed that Δcdc43 displayed increased sensitivity to both agents, indicated by loss of colony growth at lower inoculums (Figure 3(a)). Additionally, submerged culture in the presence of the chitin synthesis inhibitor Nikkomycin Z revealed dose-dependent inhibition of Δcdc43 growth at concentrations producing little-to-no effect in the control strain (Figure 3(b)). At the highest concentration of Nikkomycin Z, conidia of the Δcdc43 mutant only grew isotropically as hyphal production was completely abolished (Figure 3(b)). Susceptibility to β-glucan synthesis inhibition by Caspofungin was also evaluated through a broth microdilution assay and revealed an increase in susceptibility by twofold dilutions (0.06 µg/ml) for Δcdc43 mutants in comparison to the control strain (0.25 µg/ml). Complementation of cdc43 deletion recovered the ability to grow in the presence of CR and CFW (Figure 3(a)), and also recapitulated control strain growth in the presence of Nikkomycin Z and Caspofungin (data not shown).
Figure 3.
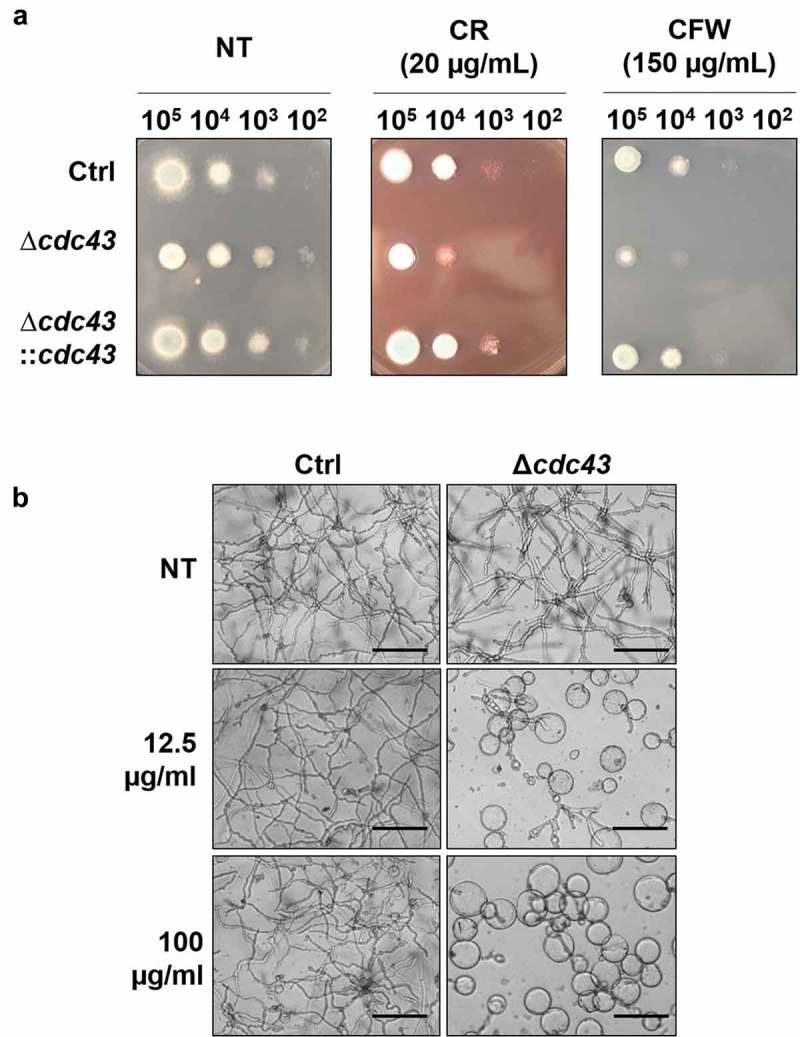
Loss of cdc43 alters response to cell wall disrupting compounds.
(A) Conidia (105 to 102) obtained from control (Ctrl), Δcdc43 or complemented (Δcdc43:cdc43) strains (5 µl) were spot inoculated onto GMM agar plates impregnated with Congo Red (CR) or Calcofluor White (CFW). Colony size was examined after 48 h at 37ºC. (B) Micrographs of hyphae grown for 48 h at 37ºC in GMM broth with Nikkomycin Z (scale bar = 50 µm). Samples incubated in media with no compound (NT) were used as a control.
A putative protein substrate of GGTase-I enzyme activity is RhoA, a small GTPase that plays a dual role in the activation of cell wall stress responses and in β-glucan biosynthesis (Figure 4(a)). Mislocalization of RhoA through the loss of GGTase-I activity would be predicted to result in reduced hyphal β-glucan content. Therefore, we evaluated cell wall distribution and composition by analyzing the localization and content of β-glucan and chitin in both the control and the Δcdc43 strains under non-stress culture conditions. Fluorescence staining of germlings for β-glucan visualization using a recombinant, soluble Dectin-1 probe revealed an irregular staining pattern of nascent germlings in the Δcdc43 strain (Figure 4(b)). Positive staining for β-glucan was also noted in the germinating conidium on the Δcdc43 mutant when grown at 37°C, whereas the original conidium was nearly devoid of β-glucan signal in the control strain at 37°C and in either strain at 30°C (Figure 4(a)). These findings suggest that deposition of β-glucan in the cell wall is altered in the Δcdc43 mutant, especially during growth at higher temperatures. In contrast, fluorescence staining for chitin distribution using calcofluor white revealed no alteration of localization of this polysaccharide (data not shown). Total β-glucan quantification in hyphae grown at 37°C, 30°C and 27°C revealed significantly lower amounts of this polysaccharide in the cdc43 null mutant at 30°C (Figure 4(c)). Although β-glucan content trended lower at 27°C and 37°C the differences were not statistically significant due to assay variability (Figure 4(c)). In contrast, total chitin quantification did not show significant differences among the strains at lower temperatures and only produced significantly different results at 37°C (Figure 4(d)). The impaired β-glucan composition of the cell wall and the increased susceptibility to cell wall perturbing agents suggest that protein geranylgeranylation is important for substrate proteins to function in A. fumigatus cell wall biosynthesis, assembly and/or stress response.
Figure 4.
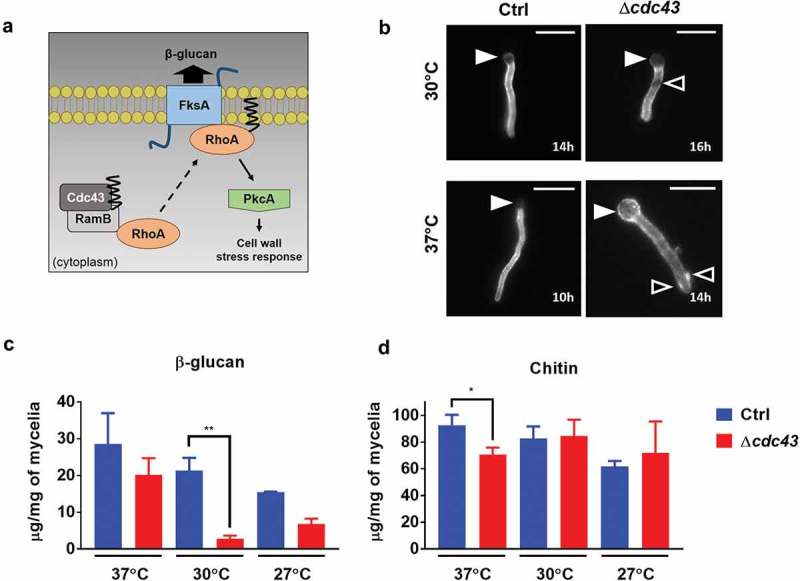
Protein geranylgeranylation is required for cell wall β-glucan composition.
(A) The small GTPase, RhoA, is predicted to be prenylated by the GGTase-I complex (composed of the Cdc43-RamB heterodimer) based on analysis of CAAX motif composition [33]. Geranylgeranylation drives RhoA to cellular membranes where it functions as a regulatory subunit of the β-glucan synthase, FksA, complex [59] and activates downstream signaling cascades (via modulation of PkcA activity) to orchestrate cell wall stress responses [63]. (B) Conidia obtained from control (Ctrl) and Δcdc43 strains were inoculated in GMM broth and incubated at 30ºC or 37ºC at 250rpm. At designated time points, germlings were harvested and β-glucan staining was performed using a recombinant Fc-hDectin (10µg/ml) and anti-IgG-FITC (1:200). β-glucan staining of germlings was visualized by fluorescence microscopy (scale bar = 10 µm). Closed arrowheads denote the original germinating conidium and open arrowheads denote areas of patchy staining in hyphae of the Δcdc43 strain. Quantification of total β-glucan (C) and chitin (D) was performed after 18 h of incubation in GMM broth at 27°C, 30ºC or 37ºC with shaking at 250 rpm. Mean and standard deviation (SD) of samples derived from three independent mycelial cultures are represented, and asterisks indicate statistical differences in comparison with control strain (Ctrl) at the same temperature using unpaired t-test; *p < 0.05 and ****p < 0.0001.
GGtase-I activity is not required for A. fumigatus virulence in experimental invasive aspergillosis
Since Cdc43 plays a crucial role in A. fumigatus growth at physiologically relevant temperatures in vitro, and in cell wall composition, we hypothesized that deletion of cdc43 would have a negative impact on pathogenesis during a murine model of invasive aspergillosis. Surprisingly, virulence was not attenuated during infection with the Δcdc43 mutants in both neutropenic (Figure 5(a,b)) and non-neutropenic mice (Figure 5(c,d)). Infected mice displayed similar survival rates in the neutropenic model, reaching 50% of mortality around 5 (Ctrl and Δcdc43::cdc43) and 6 days (Δcdc43) post infection (Figure 5(a)). In the non-neutropenic model, 60% of the mice did not succumb to Δcdc43 infection in comparison to 20% of survival in the control strain, but these rates were not sufficient to establish a statistical difference (Figure 5(c)). Histological analysis of infected lungs in both immunosuppression models demonstrated hyphal growth around bronchioles and tissue invasion within 3 days of infection with all strains (Figure 5(b,d)). Importantly, although hyphae appeared to be stunted, the hyper-branching phenotype previously observed in vitro was not detected in the lung tissue of mice infected with Δcdc43 (Figure 5(b,d) and data not shown).
Figure 5.
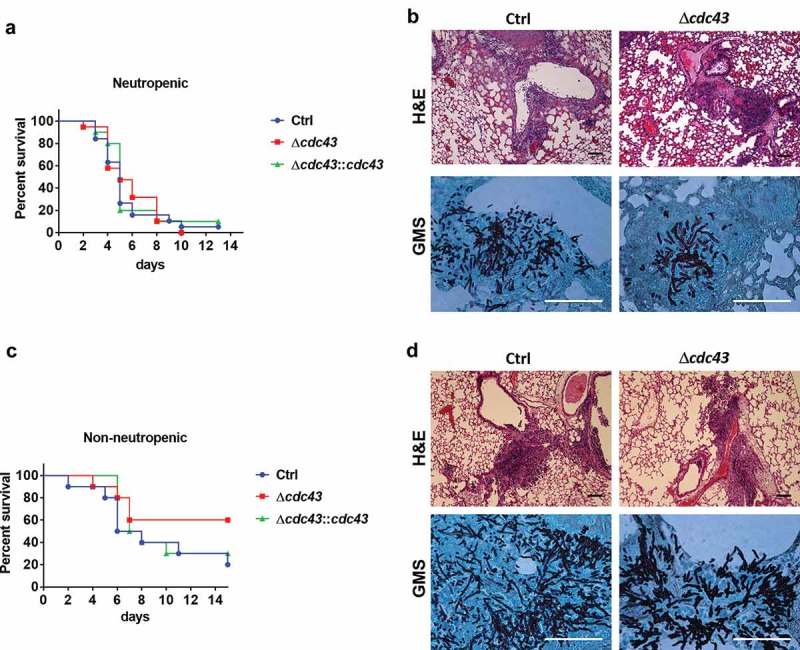
The Δcdc43 mutant retains virulence during experimental invasive aspergillosis.
Neutropenic (A, B) and non-neutropenic (C, D) CF-1 female mice were intranasally inoculated with 105 conidia obtained from control (Ctrl), Δcdc43 or complemented (Δcdc43:cdc43) strains. Survival was monitored for two weeks (A, C) and analyzed using a Log-rank (Mantel-Cox) test. Histology sections of lung tissue stained with hematoxylin and eosin (H&E) and Gomori methenamine silver (GMS) collected 3 days after infection (scale bars = 100 µm) (B, D).
The shared prenyltransferase α-subunit, RamB, is essential for A. fumigatus growth and virulence
Our results showed that lack of GGTase-I activity impacts A. fumigatus growth, morphology and cell wall composition, but not virulence. In contrast, we previously reported that mutants lacking the β-subunit of FTase presented attenuated virulence, in addition to impaired growth rates, decreased conidial viability and abnormal distribution of nuclei during polarized growth [33]. Both prenyltransferases share a common α-subunit, RamB, that is predicted to stabilize the dimeric complex and participate in the catalytic reaction transferring prenyl moieties to substrate proteins [3]. We hypothesized that complete lack of cellular prenylation, imparted by deletion of the α-subunit, would be deleterious in this fungal pathogen. Attempts to create a ramB null mutant were unsuccessful. Therefore, we generated a strain with inducible expression of ramB (pTetOn-ramB) by replacing the native promoter with a tetracycline-inducible promoter (Figure 6(a,b)). Expression of ramB in this strain can be titrated by the addition of increasing amounts of doxycycline to the culture media. After culturing the pTetOn-ramB mutant in GMM media with increasing concentrations of doxycycline (0 to 30 µg/ml), we observed that this strain was completely unable to grow in concentrations lower than 5 µg/ml whereas the control strain was unaffected at all concentrations tested (Figure 6(c)). These data support that ramB is an essential gene during A. fumigatus in vitro growth.
Figure 6.
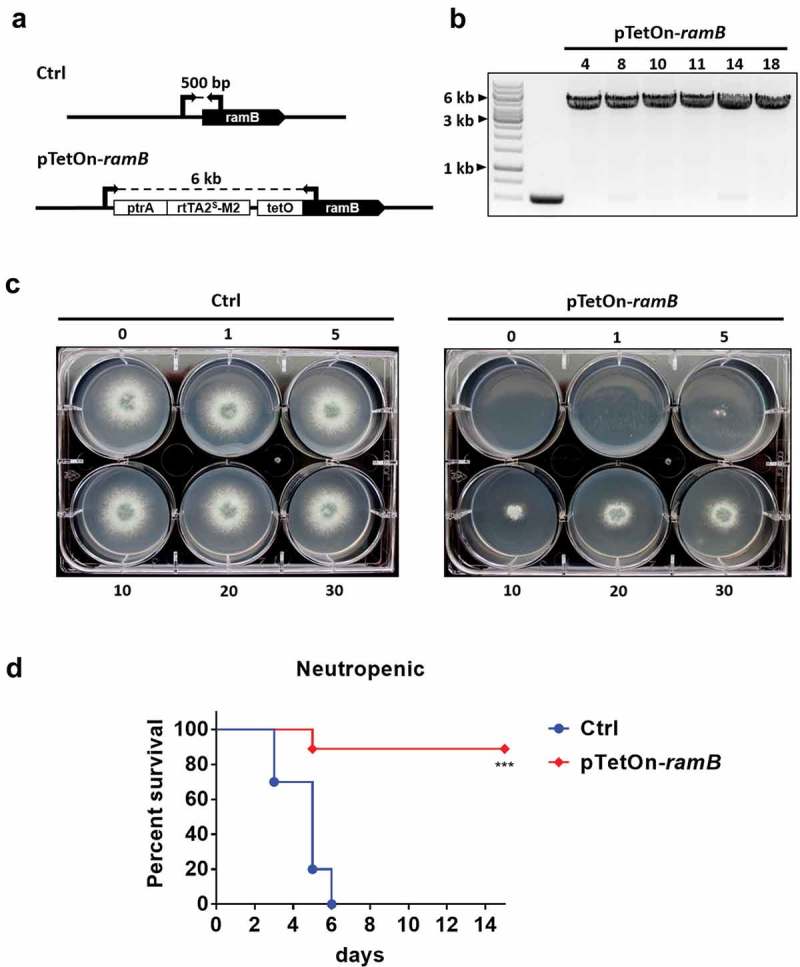
Protein prenylation is essential for A. fumigatus viability and virulence.
(A) Schematic representation of pTetOn promoter replacement for ramB. The pTetOn cassette was previously described and contains the pyrithiamine (ptrA) resistance marker [39]. Arrows indicate areas where screening primers anneal to the genomic template. (B) Homologous integration of the pTetOn cassette was confirmed by PCR. (C) pTetOn-ramB growth in GMM media at 37°C was dependent on the presence of doxycycline in the growth media. 104 conidia from each strain were spot inoculated onto GMM with the indicated concentrations of doxycycline and incubated at 37°C for 48 h. Numbers on each well indicate concentration (µg/ml) of doxycycline. (D) Neutropenic CF-1 female mice were intranasally inoculated with 105 conidia obtained from control (Ctrl) or the tetracycline-inducible ramB (pTetOn-ramB) strains. Survival was monitored along 14 days and analyzed using Log-rank (Mantel-Cox) test. Asterisks indicate a statistically significant difference in comparison with the control group (Ctrl); ***p < 0.001.
In order to evaluate the importance of ramB expression during invasive growth in vivo, we infected mice with the control and pTetOn-ramB strains in both neutropenic and non-neutropenic mouse models. The pTetOn-ramB mutant displayed markedly attenuated virulence in the neutropenic (Figure 6(d)) model and was completely avirulent in the non-neutropenic model of invasive aspergillosis (Supplemental Figure 2A). Histological analyses did not reveal the presence of hyphae or tissue damage in the lungs within 3 days of infection with the pTetOn-ramB mutant in both immunosuppression models (Supplemental Figure 2B). Therefore, we conclude that ramB is essential for growth in vitro and to cause invasive aspergillosis in mice.
Both α- and β-subunits of GGTase-I are required for C. albicans virulence in murine model of disseminated infection
Our studies with A. fumigatus established that ramB, but not cdc43, was essential for virulence. Similarly, the shared prenyltransferase α-subunit homolog, RAM2, was also found to be required for C. glabrata to infect mice [27]. In C. albicans, the α-subunit of prenyltransferase is essential for viability [21], whereas lack of CDC43 has been shown to affect cell morphology and filamentous growth in vitro [31]. However, the importance of prenyltransferase activity for C. albicans to cause disease within its mammalian host has not yet been determined. We therefore evaluated the virulence of two C. albicans strains, one with tetracycline-repressible expression of CDC43 (PTETO-CDC43), and the other with tetracycline-repressible expression of RAM2 (PTETO-RAM2) [40], in a murine model of hematogenously disseminated candidiasis. The essentiality of RAM2 (but not CDC43), was supported by the fact that the PTETO-RAM2 strain was unable to grow on YPD + 5 µg/ml doxycycline agar (data not shown). BALB/c mice were next assigned to one of two treatment groups, one provided a gel food supplemented with doxycycline, the other provided gel food alone. After 3 days, half the mice in each treatment group were infected with PTETO-CDC43, and the other half with PTETO-RAM2, via the lateral tail vein (n = 7 per experimental group). In the absence of doxycycline, all 7 mice infected with PTETO-CDC43, and 5 of 7 infected with the PTETO-RAM2 strain succumbed to infection (Figure 7(a,b)). However, only 1 of 7 PTETO-CDC43 infected mice, and none of the PTETO-RAM2 infected mice succumbed to infection in the doxycycline treatment group. These findings indicate that the expression of both CDC43 and RAM2 is required for C. albicans pathogenesis.
Figure 7.
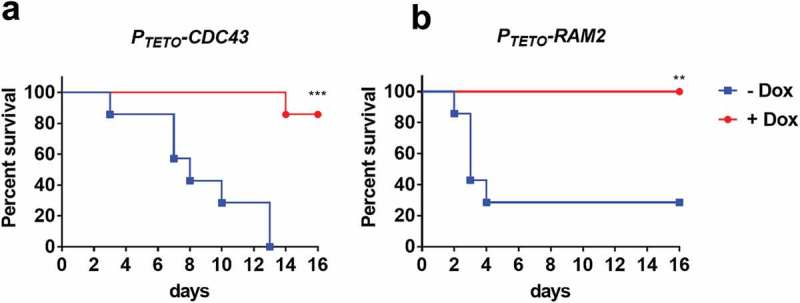
Both CDC43 and RAM2 are essential for Candida albicans virulence in a mouse model of disseminated infection.
Female BALB/c mice (6–8 week old) were randomly assigned into two treatment groups, one provided a gel food formulation supplemented with doxycycline (2 mg/ml) (+ Dox), the second gel food alone (- Dox). Half the mice in either group were then inoculated with 5 × 105 yeast cells of C. albicans strain PTETO-CDC43 (A), and the other half with strain PTETO-RAM2 (B) via tail vein injection (n = 7 per experimental group). Mice were monitored for 16 days, and those showing signs of distress humanely euthanized. Survival of mice in either treatment group was then compared using the Log-rank (Mantel-Cox) test, and asterisks indicate a statistically significant difference among the groups; **p < 0.01 and ***p < 0.001.
Discussion
The contributions of protein prenylation in eukaryotic cell biology are continuously under investigation, and the demonstration that CaaaX-residues can also be prenylated [4] expanded even more the repertoire of possible prenylated proteins. Prenylation controls the localization of several proteins that are crucial for the viability of fungal pathogens. In silico analyses revealed that A. fumigatus encodes 28 putative prenylated CaaX proteins [33]. Among them are important regulators of cell growth and development, including small GTPases such as Ras- and Rho- family proteins. We had previously shown that protein farnesylation promotes A. fumigatus growth, nuclear distribution, conidial viability and virulence [33]. In this study, we analyzed the contribution of protein prenylation in general, and specifically protein geranylgeranylation, to the growth and virulence of A. fumigatus.
We started by creating and characterizing a strain that lacked the cdc43 gene, that encodes the β-subunit of geranylgeranyltransferase type-I. Since A. fumigatus is a thermotolerant organism [44,45], we analyzed if the absence of GGTase-I would have an impact on the ability of this pathogen to grow at host physiological, or greater, temperatures. The Δcdc43 mutant presented reduced hyphal growth and thermo-sensitivity, especially while growing in rich media at 45°C. Impaired ability to grow at 37°C was also observed after disruption of RAM1 in S. cerevisiae [26] and RAM1 [28] and CDC43 [32] in C. neoformans. In fact, RAS1, a farnesylated protein, has been shown to directly control C. neoformans thermotolerant growth [46]. We speculate that protein geranylgeranylation is crucial for A. fumigatus heat shock response, especially under conditions where metabolic activity is intensified, such as when resources are abundant. We compared the list of A. fumigatus putative prenylated proteins [33] with a list of proteins that were found regulated by Albrecht et. al. (2010) during a temperature shift from 30°C to 48°C [45], however, we found no matches. A. fumigatus null mutants for rho2 and rho4 were found sensitive to growth at 48°C [47], and these proteins are potentially prenylated. It is likely, therefore, that the cell wall and temperature-sensitive phenotypes are directly linked. Two other genes identified as mediators of thermotolerance in A. fumigatus, cgrA [48] and thtA [49] do not have a predicted CaaX motif. Although this suggests that these proteins are not likely to be directly impacted by GGTase-I activity, we cannot rule out that they may be indirectly associated with the thermosensitive phenotype observed here.
We observed that lack of GGTase-I had an impact on fungal cell morphology. The Δcdc43 mutant presented larger swollen conidia as well as wide, hyper-branched hyphae. These phenotypes were exacerbated during growth at 37°C. In a similar way, C. neoformans [32] and S. cerevisiae CDC43 mutants presented altered morphology, such as failure to bud and continuous cell growth at 37°C, what suggests abnormal control of cell cytokinesis. Accordingly, S. cerevisiae mutants for CDC42 [50], which is a substrate for GGTase-I, present similar altered morphology. A. fumigatus has a CDC42 homolog that is a putative substrate of GGTase-I [33], but this gene has not yet been studied. CDC42 is involved in the morphogenesis of S. cerevisiae [51], C. albicans [52], C. neoformans [53], A. niger [54] and A. nidulans [55,56]. In addition, RacA is also a potentially prenylated protein, and mediates polarized growth in A. fumigatus [57]. As with the Δcdc43 strain, racA null mutants present impaired ability to conidiate in GMM [57,58]. The lack of this protein in A. niger produces hyper-branching hyphae, and double deletion of racA and cdc42 leads to malformed germlings during growth in GMM [54]. Taking this into consideration, the A. fumigatus homolog for CDC42 and RacA are likely substrate proteins that have altered function by the absence of GGTase-I activity in the Δcdc43 mutant.
The disruption of cdc43 created mutants hypersensitive to cell wall disrupting agents, namely Congo Red, Calcofluor White, Nikkomycin Z, and Caspofungin, indicating defects in cell wall biosynthesis, assembly and/or integrity. In fact, analysis of cell wall β-glucan by both fluorescence microscopy and quantification using the aniline blue assay revealed the altered distribution and reduced amount of this polysaccharide in the Δcdc43 strain. β-glucan is synthesized from glucose by the β-1,3-glucan synthase complex, constituted by the catalytic subunit FksA, and the regulatory subunit RhoA [59–61]. In S. cerevisiae, prenylation of Rho1p is required for the activation of β-1,3-glucan synthase [61]. We predict that RhoA is at least partially mislocalized in the Δcdc43 mutant which, in turn, impairs cell wall β-glucan production by inhibiting activation of the β-1,3-glucan synthase complex. A. fumigatus rho2 and rho4 null mutants also display sensitivity to CR and CFW [47]. The hypersensitivity of the Δcdc43 mutant to Caspofungin may further suggest that FksA activity is reduced potentially as a result of RhoA mislocalization. Also, among the possible prenylated proteins in A. fumigatus are two uncharacterized genes [33] that have homology to a S. cerevisiae and C. albicans chitin-synthase regulator (CHR4). In these fungal species, lack of CHS4 reduces cell wall chitin content. However, we did not identify changes in chitin levels after visualization of hyphae stained with CFW by fluoresce microscopy, nor by quantification of glucosamine-equivalents in hyphal mats. Even though chitin content is normal, we did note hypersensitivity of Δcdc43 to chitin synthesis inhibition by Nikkomycin Z. A possible explanation is that, since β-glucan content is impaired in this mutant, chitin is the major component stabilizing the cell wall such that inhibition of chitin synthesis by Nikkomycin Z became completely deleterious to polarized morphogenesis. In addition, the predicted GGTase-I substrates RhoA, Rho2, and Rho4 are known to participate in the cell wall integrity signaling, which is crucial to counter stress agents such as CFW, CR and Caspofungin [47]. Further studies defining prenyltransferase substrate specificity and Rho protein localization in the Δcdc43 background will be required to pursue this possibility.
After characterizing the importance of protein geranylgeranylation in A. fumigatus, we sought to evaluate the impact of protein prenylation in general. The ramB gene encodes the α-subunit of both FTase and GGTase-I. Lack of RamB is expected to completely abolish CaaX protein prenylation and cause the concurrent mislocalization of all substrate proteins. Accordingly, we were unable to delete ramB. Replacement of the native promoter with a tetracycline inducible promoter (TetOn) generated a strain completely dependent on the presence of doxycycline to survive. These findings are in accordance with other studies showing that ramB homologs are essential for S. cerevisiae [26], C. albicans [21] and C. glabrata [27] viability. We assume that inhibition of ramB expression severely impacts pathways that depend on protein prenylation, such as signal transduction mediated by Ras and Rho-proteins. Lack of prenylation likely affects a range of essential cellular processes, such as growth, differentiation and establishment of polarity.
The temperature sensitive phenotype and the inability to maintain proper polarity indicated that the A. fumigatus Δcdc43 mutant would likely present attenuated virulence. Surprisingly, our virulence studies suggested that complete lack of GGTase-I activity did not affect A. fumigatus ability to grow in the host environment. The same was observed in thtA mutants and six other mutants that presented normal growth at 25°C, but failed to grow at 48°C, and presented no reduction in virulence in the murine model of invasive aspergillosis [49]. In addition, an A. fumigatus racA deletion strain was as virulent as Af293 in both neutropenic and non-neutropenic models of invasive aspergillosis, despite loss of polarity and impaired growth phenotypes in vitro [57]. In fact, little-to-no morphological changes were identified in the Δcdc43 hyphae growing in the lung tissue compared to the control strain. These data could indicate that, once inside the mammalian host, A. fumigatus has a reduced requirement for protein geranylgeranylation. However, we cannot currently rule out the possibility that the altered cell wall of the Δcdc43 mutant leads to differences in the immune response that impact this outcome.
CDC43 was previously described as a non-essential gene in C. albicans, although null mutants displayed abnormal morphology and decreased the ability to form hyphae [31]. In this study, using a tetracycline-repressible strain, we demonstrated that suppression of C. albicans CDC43 expression resulted in increased survival of mice in a murine model of invasive candidiasis. These data establish that whereas geranylgeranylation is not required for A. fumigatus virulence, it is crucial for C. albicans virulence. We also demonstrated that the α-subunit of both FTase and GGTase-I is crucial for fungal growth and ability to infect the host. The A. fumigatus pTetOn-ramB strain presented no virulence in both neutropenic and non-neutropenic models of invasive aspergillosis. Similarly, suppression of RAM2 expression is sufficient to render C. albicans avirulent, establishing that prenylation is essential for pathogenicity.
The data reported here supports the exploration of fungal protein prenylation as a target for antifungal drug development. In vitro effectiveness of some prenyltransferase inhibitors have been investigated, and revealed antifungal activity against C. neoformans [23,24], Aspergillus spp. and Candida spp. [18]. Additional reports indicate that these inhibitors are not active against the whole fungal cell, although they are able to inhibit purified prenyltransferases. These discrepant results are possibly due to the inability of the tested compounds to enter the fungal cell or may result from potential cross-prenylation of substrate proteins due to promiscuous substrate specificity [9,21,31,62]. To the best of our knowledge, no in vivo studies analyzing antifungal efficacy of protein prenyltransferase inhibitors have been conducted. Virulence studies performed with prenyltransferase mutants demonstrate their importance for in vivo growth of fungal pathogens. In C. neoformans [28] and A. fumigatus [33], lack of farnesyltransferase activity diminishes virulence. In addition, mice infected with C. glabrata mutants that harbor a tetracycline-repressible RAM2 display lower numbers of viable fungal cells in the kidneys when treated with doxycycline [27]. The fact that the α-subunit of the CaaX prenyltransferases is crucial for pathogenicity of C. albicans, C. glabrata, and A. fumigatus is encouraging. Although a well-conserved pathway in both mammalian and fungal cells, important structural differences [6,17,24] raise the possibility of specifically targeting the fungal enzymes, avoiding undesired side effects during the antifungal therapy. Simultaneous inhibition of FTase and GGTase-I activity or the impairment of the dimerization of the α-subunit with the β-subunits of prenyltransferases seem to be a promising strategy to combat fungal infections in a broader spectrum. Therefore, further investigations in the development or repurposing of inhibitors of prenylation will be valuable in the fight against invasive fungal diseases.
Funding Statement
This work was supported by the National Institutes of Health (NIH) grants [R01 AI106925 and R21 AI139388] awarded to JRF, and [R01AI099080] awarded to GEP. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments
The authors would like to thank Merck Sharp & Dohme Corp, who kindly provided the tetracycline-repressible strains of C. albicans used in this study to GEP.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental material
Supplemental data for this article can be accessed here.
References
- [1].Zhang FL, Casey PJ.. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269. [DOI] [PubMed] [Google Scholar]
- [2].Omer CA, Gibbs JB. Protein prenylation in eukaryotic microorganisms: genetics, biology and biochemistry. Mol Microbiol. 1994;11:219–225. [DOI] [PubMed] [Google Scholar]
- [3].Wang M, Casey PJ. Protein prenylation: unique fats make their mark on biology. Nat Rev Mol Cell Biol. 2016;17:110–122. [DOI] [PubMed] [Google Scholar]
- [4].Blanden MJ, Suazo KF, Hildebrandt ER, et al. Efficient farnesylation of an extended C-terminal C(x)3X sequence motif expands the scope of the prenylated proteome. J Biol Chem. 2018;293:2770–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Seabra MC, Reiss Y, Casey PJ, et al. Protein farnesyltransferase and geranylgeranyltransferase share a common α subunit. Cell. 1991;65:429–434. [DOI] [PubMed] [Google Scholar]
- [6].Mabanglo MF, Hast MA, Lubock NB, et al. Crystal structures of the fungal pathogen Aspergillus fumigatus protein farnesyltransferase complexed with substrates and inhibitors reveal features for antifungal drug design. Protein Sci. 2014;23:289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Scott Reid T, Terry KL, Casey PJ, et al. Crystallographic analysis of CaaX prenyltransferases complexed with substrates defines rules of protein substrate selectivity. J Mol Biol. 2004;343:417–433. [DOI] [PubMed] [Google Scholar]
- [8].Moores SL, Schaber MD, Mosser SD, et al. Sequence dependence of protein isoprenylation. J Biol Chem. 1991;266:14603–14610. [PubMed] [Google Scholar]
- [9].Trueblood CE, Ohya Y, Rine J. Genetic evidence for in vivo cross-specificity of the CaaX-box protein prenyltransferases farnesyltransferase and geranylgeranyltransferase-I in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:4260–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Casey PJ, Seabra MC. Protein Prenyltransferases. J Biol Chem. 1996;271:5289–5292. [DOI] [PubMed] [Google Scholar]
- [11].Schafer WR, Rine J. Protein prenylation: genes, enzymes, targets, and functions. Annu Rev Genet. 1992;26:209–237. [DOI] [PubMed] [Google Scholar]
- [12].Fortwendel JR. Ras-mediated signal transduction and virulence in human pathogenic fungi. Fungal genomics Biol. 2012;2:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Harris SD. Cdc42/Rho GTPases in fungi: variations on a common theme. Mol Microbiol. 2011;79:1123–1127. [DOI] [PubMed] [Google Scholar]
- [14].Philips M, Cox A. Geranylgeranyltransferase I as a target for anti-cancer drugs. J Clin Invest. 2007;117:1223–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xu N, Shen N, Wang XX, et al. Protein prenylation and human diseases: a balance of protein farnesylation and geranylgeranylation. Sci China Life Sci. 2015;58:328–335. [DOI] [PubMed] [Google Scholar]
- [16].Shen M, Pan P, Li Y, et al. Farnesyltransferase and geranylgeranyltransferase I: structures, mechanism, inhibitors and molecular modeling. Drug Discov Today. 2015;20:267–276. [DOI] [PubMed] [Google Scholar]
- [17].Alspaugh JA. Targeting protein localization for anti-infective therapy. Virulence. 2017;8:1105–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Qiao J, Gao P, Jiang X, et al. In vitro antifungal activity of farnesyltransferase inhibitors against clinical isolates of Aspergillus and Candida. Ann Clin Microbiol Antimicrob. 2013;12:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Palsuledesai CC, Distefano MD. Protein prenylation: enzymes, therapeutics, and biotechnology applications. ACS Chem Biol. 2015;10:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ochocki JD, Distefano MD. Prenyltransferase Inhibitors: treating human ailments from cancer to parasitic infections. Medchemcomm. 2013;4:476–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Song JL, White TC. RAM2: an essential gene in the prenylation pathway of Candida albicans. Microbiology. 2003;149:249–259. [DOI] [PubMed] [Google Scholar]
- [22].McGeady P, Logan DA, Wansley DL. A protein-farnesyl transferase inhibitor interferes with the serum-induced conversion of Candida albicans from a cellular yeast form to a filamentous form. FEMS Microbiol Lett. 2002;213:41–44. [DOI] [PubMed] [Google Scholar]
- [23].Vallim MA, Fernandes L, Alspaugh JA. The RAM1 gene encoding a protein-farnesyltransferase beta-subunit homologue is essential in Cryptococcus neoformans. Microbiology. 2004;150:1925–1935. [DOI] [PubMed] [Google Scholar]
- [24].Hast MA, Nichols CB, Armstrong SM, et al. Structures of Cryptococcus neoformans protein farnesyltransferase reveal strategies for developing inhibitors that target fungal pathogens. J Biol Chem. 2011;286:35149–35162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hast MA, Beese LS. Structure of protein geranylgeranyltransferase-I from the human pathogen Candida albicans complexed with a lipid substrate. J Biol Chem. 2008;283:31933–31940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].He B, Chen P, Chen SY, et al. RAM2, an essential gene of yeast, and RAM1 encode the two polypeptide components of the farnesyltransferase that prenylates a-factor and Ras proteins. Proc Natl Acad Sci U S A. 1991;88:11373–11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nakayama H, Ueno K, Uno J, et al. Growth defects resulting from inhibiting ERG20 and RAM2 in Candida glabrata. FEMS Microbiol Lett. 2011;317:27–33. [DOI] [PubMed] [Google Scholar]
- [28].Esher SK, Ost KS, Kozubowski L, et al. Relative contributions of prenylation and postprenylation processing in Cryptococcus neoformans pathogenesis. Mol Biol Physiol. 2016;1:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yang W, Urano J, Tamanoi F. Protein farnesylation is critical for maintaining normal cell morphology and canavanine resistance in Schizosaccharomyces pombe. J Biol Chem. 2000;275:429–438. [DOI] [PubMed] [Google Scholar]
- [30].Goodman LE, Judd SR, Farnsworth CC, et al. Mutants of Saccharomyces cerevisiae defective in the farnesylation of Ras proteins. Proc Natl Acad Sci U S A. 1990;87:9665–9669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kelly R, Card D, Register E, et al. Geranylgeranyltransferase I of Candida albicans: null mutants or enzyme inhibitors produce unexpected phenotypes. J Bacteriol. 2000;182:704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Selvig K, Ballou ER, Nichols CB, et al. Restricted substrate specificity for the geranylgeranyltransferase-i enzyme in Cryptococcus neoformans: implications for virulence. Eukaryot Cell. 2013;12:1462–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Norton TS, Al Abdallah Q, Hill AM, et al. The Aspergillus fumigatus farnesyltransferase β-subunit, RamA, mediates growth, virulence, and antifungal susceptibility. Virulence. 2017;8:1401–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yelton MM, Hamer JE, Timberlake WE. Transformation of Aspergillus nidulans by using a trpC plasmid. Genetics. 1984;81:1470–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Da Silva Ferreira ME, Kress MRVZ, Savoldi M, et al. The akuB KU80 mutant deficient for nonhomologous end joining is a powerful tool for analyzing pathogenicity in Aspergillus fumigatus. Eukaryot Cell. 2006;5:207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Al Abdallah Q, Martin-Vicente A, Souza ACO, et al. C-terminus proteolysis and palmitoylation cooperate for optimal plasma membrane localization of RasA in Aspergillus fumigatus. Front Microbiol. 2018;9:562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Calvo AM, Bok J, Brooks W, et al. veA is required for toxin and sclerotial production in Aspergillus parasiticus. Appl Environ Microbiol. 2004;70:4733–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Al Abdallah Q, Ge W, Fortwendel JR. A simple and universal system for gene manipulation in Aspergillus fumigatus: in vitro-assembled Cas9-guide RNA ribonucleoproteins coupled with microhomology repair templates. mSphere. 2017;2:e00446–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Helmschrott C, Sasse A, Samantaray S, et al. Upgrading fungal gene expression on demand: improved systems for doxycycline-dependent silencing in Aspergillus fumigatus. Appl Environ Microbiol. 2013;79:1751–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Roemer T, Jiang B, Davison J, et al. Large-scale essential gene identification in Candida albicans and applications to antifungal drug discovery. Mol Microbiol. 2003;50:167–181. [DOI] [PubMed] [Google Scholar]
- [41].Fortwendel JR, Juvvadi PR, Pinchai N, et al. Differential effects of inhibiting chitin and 1,3-{beta}-D-glucan synthesis in ras and calcineurin mutants of Aspergillus fumigatus. Antimicrob Agents Chemother. 2009;53:476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].CLSI Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard - second edition. CLSI document M38-A2. Wayne (PA): Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- [43].Koenig S, Rühmann B, Sieber V, et al. Quantitative assay of β-(1,3)–β-(1,6)–glucans from fermentation broth using aniline blue. Carbohydr Polym. 2017;174:57–64. [DOI] [PubMed] [Google Scholar]
- [44].Bhabhra R, Askew DS. Thermotolerance and virulence of Aspergillus fumigatus: role of the fungal nucleolus. Med Mycol. 2005;43(Suppl 1):S87–93. [DOI] [PubMed] [Google Scholar]
- [45].Albrecht D, Guthke R, Brakhage AA, et al. Integrative analysis of the heat shock response in Aspergillus fumigatus. BMC Genomics. 2010;11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Alspaugh JA, Cavallo LM, Perfect JR, et al. RAS1 regulates filamentation, mating and growth at high temperature of Cryptococcus neoformans. Mol Microbiol. 2000;36:352–365. [DOI] [PubMed] [Google Scholar]
- [47].Dichtl K, Helmschrott C, Dirr F, et al. Deciphering cell wall integrity signalling in Aspergillus fumigatus: identification and functional characterization of cell wall stress sensors and relevant Rho GTPases. Mol Microbiol. 2012;83:506–519. [DOI] [PubMed] [Google Scholar]
- [48].Bhabhra R, Miley MD, Mylonakis E, et al. Disruption of the Aspergillus fumigatus gene encoding nucleolar protein CgrA impairs thermotolerant growth and reduces virulence. Infect Immun. 2004;72:4731–4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chang YC, Tsai HF, Karos M, et al. THTA, a thermotolerance gene of Aspergillus fumigatus. Fungal Genet Biol. 2004;41:888–896. [DOI] [PubMed] [Google Scholar]
- [50].Adams AEM, Johnson DI, Longnecker RM, et al. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J Cell Biol. 1990;111:131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Johnson DI, Pringle JR. Molecular characterization of CDC42, a Saccharomyces cerevisiae gene involved in the development of cell polarity. J Cell Biol. 1990;111:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ushinsky SC, Harcus D, Ash J, et al. CDC42 is required for polarized growth in human pathogen Candida albicans. Eukaryot Cell. 2002;1:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ballou ER, Nichols CB, Miglia KJ, et al. Two CDC42 paralogues modulate Cryptococcus neoformans thermotolerance and morphogenesis under host physiological conditions. Mol Microbiol. 2010;75:763–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kwon MJ, Arentshorst M, Roos ED, et al. Functional characterization of Rho GTPases in Aspergillus niger uncovers conserved and diverged roles of Rho proteins within filamentous fungi. Mol Microbiol. 2011;79:1151–1167. [DOI] [PubMed] [Google Scholar]
- [55].Virag A, Lee MP, Si H, et al. Regulation of hyphal morphogenesis by cdc42 and rac1 homologues in Aspergillus nidulans. Mol Microbiol. 2007;66:1579–1596. [DOI] [PubMed] [Google Scholar]
- [56].Si H, Rittenour WR, Harris SD. Roles of Aspergillus nidulans Cdc42/Rho GTPase regulators in hyphal morphogenesis and development. Mycologia. 2016;108:543–555. [DOI] [PubMed] [Google Scholar]
- [57].Li H, Barker BM, Grahl N, et al. The small GTPase RacA mediates intracellular reactive oxygen species production, polarized growth, and virulence in the human fungal pathogen Aspergillus fumigatus. Eukaryot Cell. 2011;10:174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Chi MH, Craven KD. RacA-mediated ROS signaling is required for polarized cell differentiation in conidiogenesis of Aspergillus fumigatus. PLoS One. 2016;11:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Beauvais A, Bruneau JM, Mol PC, et al. Glucan synthase complex of Aspergillus fumigatus. J Bacteriol. 2001;183:2273–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Dichtl K, Samantaray S, Aimanianda V, et al. Aspergillus fumigatus devoid of cell wall β-1,3-glucan is viable, massively sheds galactomannan and is killed by septum formation inhibitors. Mol Microbiol. 2015;95:458–471. [DOI] [PubMed] [Google Scholar]
- [61].Inoue SB, Qadota H, Arisawa M, et al. Prenylation of Rho1p is required for activation of yeast 1, 3-beta-glucan synthase. J Biol Chem. 1999;274:38119–38124. [DOI] [PubMed] [Google Scholar]
- [62].Qiao J, Sun Y, Gao L, et al. Lonafarnib synergizes with azoles against Aspergillus spp. and Exophiala spp. Med Mycol. 201. 8;56:452–457. [DOI] [PubMed] [Google Scholar]
- [63].Valiante V, Macheleidt J, Föge M, et al. The Aspergillus fumigatus cell wall integrity signalling pathway: drug target, compensatory pathways and virulence. Front Microbiol. 2015;6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


