ABSTRACT
Tuberculosis (TB), which is primarily caused by the major etiologic agent Mycobacterium tuberculosis (Mtb), remains a serious infectious disease worldwide. Recently, much effort has been made to develop novel/improved therapies by modulating host responses to TB (i.e., host-directed therapy). Autophagy is an intracellular catabolic process that helps maintain homeostasis or the removal of invading pathogens via a lysosomal degradation process. The activation of autophagy by diverse drugs or agents may represent a promising treatment strategy against Mtb infection, even to drug-resistant strains. Important mediators of autophagy activation include vitamin D receptor signaling, the AMP-activated protein kinase pathway, sirtuin 1 activation, and nuclear receptors. High-throughput approaches have identified numerous natural and synthetic compounds that enhance antimicrobial defense against Mtb infection through autophagy. In this review, we discuss the current knowledge of, advancements in, and perspectives on new therapeutic strategies targeting autophagy against TB. Understanding the mechanisms and key players involved in modulating antibacterial autophagy will provide innovative improvements in anti-TB therapy via an autophagy-targeting approach.
Abbreviations: TB: Tuberculosis; Mtb: Mycobacterium tuberculosis; HDT: host-directed therapy; MDR: multidrug resistant; XDR: extensively drug resistant; LAP: LC3-associated phagocytosis; ROS: reactive oxygen species; VDR: vitamin D receptor; TFEB: transcription factor EB; ERRα: estrogen-related receptor α; PGC1α: PPARγ coactivator-1 α
KEYWORDS: Mycobacterium tuberculosis, xenophagy, LC3-associated phagocytosis, host-directed therapy, innate immunity, tuberculosis
Introduction
The autophagy process plays a fundamentally important basal housekeeping function in diverse physiological conditions. Autophagy activation is required for the maintenance of cellular homeostasis and survival by providing energy building blocks during a variety of stresses via the cell autonomous digestion of intracytoplasmic cargo (i.e., large macromolecular aggregates and damaged organelles). Autophagy is also critical for the regulation of a wide range of immune responses including innate immunity, inflammation, and macrophage antibacterial defenses [1,2].
Human TB is an infectious disease with high morbidity and mortality and remains a global threat. The standard treatment for TB is a regimen of frontline combination chemotherapy with multiple antibiotics for at least 6 months. Current anti-TB therapy has many limitations such as prolonged treatment duration, drug toxicity, and potential risk for the development of drug-resistant strains if patients are noncompliant. Therefore, there is an urgent need to develop new therapeutic drugs to control infection more effectively [3]. Host-directed therapy (HDT) may be useful for fighting bacterial infections to improve the treatment efficiency for TB [4,5]. In addition, HDT-TB may be applicable to the treatment of multidrug (MDR)- or extensively drug (XDR)-resistant TB via therapeutic targeting of numerous clinically relevant biological pathways in hosts [6,7]. Based on the modulation of pathological or protective responses in hosts, a variety of components/pathways of the immune system are central players that contribute to therapeutic targets for HDT against TB. Candidate targets include modulators of pathologic inflammation, antimicrobial effectors, and drugs/reagents for the maintenance of homeostasis [3,7]. Because autophagy is critical for maintaining intracellular homeostasis and acts as a crucial immune arm, autophagy modulators/molecules could represent promising candidates in the context of HDT against TB with or without adjunctive agents for standard therapeutics [4,8].
In this review, we focus on current advances in the identification of autophagy-activating agents exhibiting antibacterial activity as potential therapeutics to eradicate Mtb infection. More attention should be paid to the identification of key players and mechanisms by which autophagy-activating agents target bacteria to enhance antimicrobial responses. Finally, we discuss the challenges and perspectives of autophagy-adjunctive therapeutics for their clinical use.
Overview of autophagy
Autophagy (herein, macroautophagy) is an intracellular homeostatic process through which damaged cellular components and organelles are recycled and degraded during conditions of cellular stress [9]. The detailed mechanisms of autophagy have been described in numerous reports and review articles [10–13] and in this review, we briefly mention the general features of macroautophagy (Figure 1). The canonical autophagy pathway includes three different types of autophagy processes based on how the cargo is targeted and delivered to the lysosomes: macroautophagy, microautophagy, and chaperone-mediated autophagy. Among them, macroautophagy (usually referred to as autophagy) is triggered by numerous stress signals including starvation, hypoxia, damage to intracellular organelles, and microbial infection. In the initiation stage, the early phagophores (isolation membrane) are formed from the endoplasmic reticulum–mitochondria contact sites [14,15]. Numerous autophagy -related genes (ATGs) are involved in each step of the autophagy pathway. The ATG1/ULK complex and class III PI3K complex play essential roles in the formation of autophagosomes during the initiation stage [16,17]. The phagophores expand around the intracytoplasmic cargo and eventually grow into double membrane autophagosomes. The elongation and ultimate closure of autophagosomes are mediated through core machinery (i.e., a group of ubiquitin-like protein conjugation systems) such as the ATG5-ATG12-ATG16L1 complex and the ATG8 family, which is composed of microtubule-associated light chain 3 (LC3) [16,17]. Next, autophagosomes mature into autolysosomes by fusing with endosomes/lysosomes for the degradation of cytoplasmic cargo. This step depends on several non-ATG components including the endosomal sorting complex required for transport, soluble N-ethylmaleimide-sensitive factor attachment protein receptors, and RAB proteins [18,19].
Figure 1.
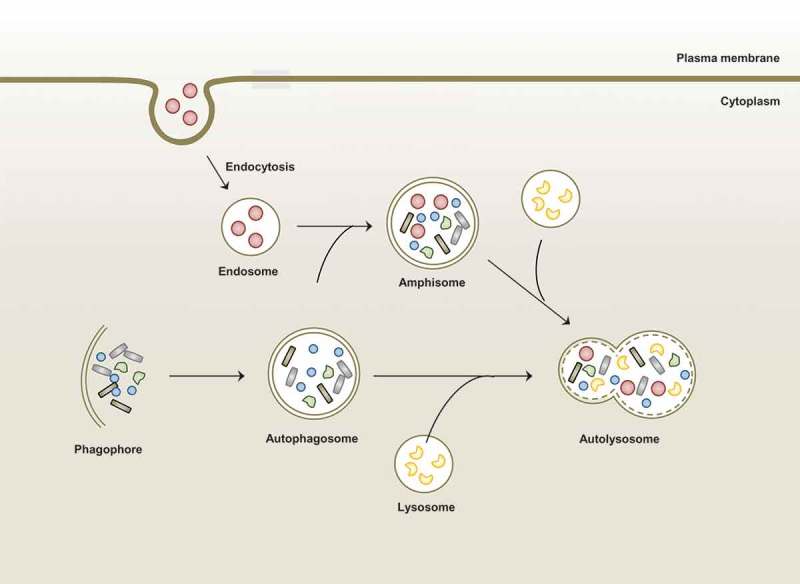
Overview of macroautophagy.
Macroautophagy is one of autophagic process in which is induced by stimuli or stress such as starvation and infection. Autophagy sequesters aggregated proteins, damaged organelles, and microbes from cytoplasm through formation of double-membraned structure (autophagosome). These autophagosomes fuse with lysosome or endosome that contains endocytic compartments to form amphisome or autolysosome. Finally, cargos are degraded in autolysosome for the maintenance of cellular homeostasis.
Overview of autophagy during mycobacterial infection
Mtb has developed several strategies to counteract autophagy, a well-known cellular response to various stresses including infections [20]. For example, Mtb Eis inhibits macrophage autophagy and cell death via a reactive oxygen species (ROS)-dependent pathway [21]. In addition, 6-kDa early secretory antigen target (ESAT6), a major ESX-1-mediated secretory protein, plays a role in the suppression of late-stage autophagy in human dendritic cells [22]. Since autophagy has emerged as a crucial protective process to restrict Mtb growth in host cells [23–25], it is worthwhile to focus on the function of autophagy against mycobacterial infection.
Traditionally, canonical autophagy activation is considered nonspecific, and is involved in cell survival and homeostasis. Noncanonical autophagy includes selective autophagy, which targets a variety of components, macromolecules, and intracellular microbes, as well as LC3-associated phagocytosis (LAP) [26]. When the autophagic machinery targets intracellular pathogens, it is called “xenophagy,” a form of selective autophagy. Both the canonical and noncanonical autophagy pathways play pivotal roles in host defense and innate immune responses against intracellular pathogens [2,9]. There are numerous elegant reviews for the mechanism, function, and regulation of canonical autophagy [12,27,28]. Thus we here discuss a brief summary of xenophagy and LAP in terms of mycobacterial infection (Figure 2).
Figure 2.
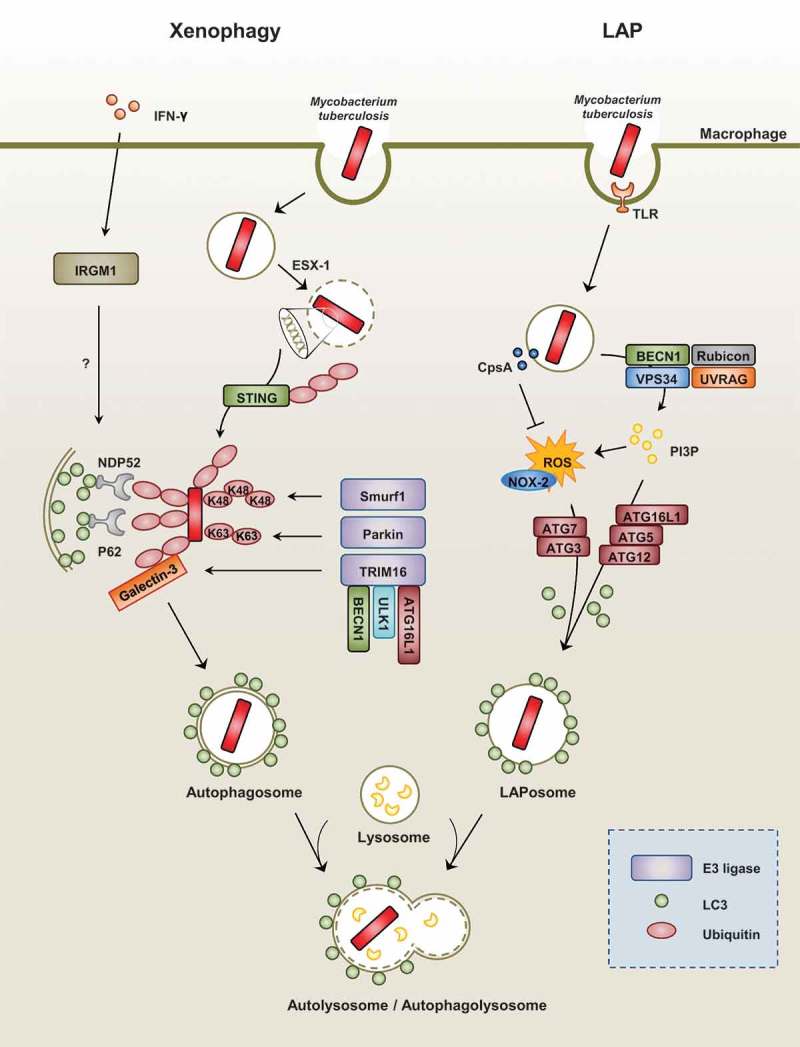
Xenophagy versus LAP during Mtb infection.
(Left) During mycobacterial infection with Mtb, xenophagy is triggered by the cytoplasmic release of bacteria via its ESX-1 system. Next, the recognition of extracellular bacterial DNA by the STING-dependent pathway allows for the ubiquitination of bacteria. In addition, ubiquitin ligases such as Parkin and Smurf1 allow for the ubiquitination of different chain linkages (K63 and K48, respectively). TRIM16 cooperates with Galectin-3, ATG16L1, ULK1, and BECN1 for the subsequent ubiquitination of bacteria and autophagy activation. These ubiquitin chains recruit autophagy adaptors such as NDP52 and p62, which link to LC3 of the autophagosomal membranes. The exact function of IFN-γ-dependent IRGM1 (LRG47) in the regulation of selective autophagy requires further clarification.
(Right) LAP is an LC3-conjugation process onto the single-membrane phagosome (LAPosome). It is triggered by pathogenic microbes, such as Mtb, through numerous receptors, including toll-like receptor signals. During the LAP process, recruitment of the class III PI3-kinase complex (composed of VPS34, Beclin-1, UVRAG, and Rubicon) increases the production of phosphatidylinositol-3-phosphate (PI3P), which is needed to stabilize the NOX2 complex for the production of reactive oxygen species and the recruitment of autophagic proteins (ATG5, ATG12, ATG16L, ATG7, and ATG3) for the conjugation of lipidated LC3-II to the LAPosomal membrane. Mtb protein CpsA is required for bacteria to block NADPH oxidase activity in order to evade killing by LAP.
Selective autophagy (xenophagy) targeting mycobacteria
During mycobacterial infection, xenophagy against Mtb infection is triggered by the cytoplasmic release of bacteria through its ESX-1 system. The STING-dependent cytosolic pathway and autophagic receptors p62 and NDP52 play a critical role in the xenophagic elimination of Mtb [29]. The subsequent ubiquitination and host defense against Mtb are dependent on the ubiquitin ligase Parkin [30]. The ubiquitin ligase Smurf1, which functions primarily in K48-linked ubiquitination, was recently found to play an essential role in the activation of selective autophagy, host defense against Mtb, and lung inflammation [31]. In addition, TRIM16, in cooperation with Galectin-3 and ATG16L1, is required for ubiquitination and autophagy in response to lysosomal damage, and contributes to autophagic protection against Mtb infection [32].
Earlier studies have demonstrated the importance of murine immunity-related p47 guanosine triphosphatase family M protein 1 (IRGM1) (also known as LRG47) in IFN-γ-dependent host defense against several intracellular infections, including mycobacterial infection [33]. In addition, IRGM1 and human IRGM (the human homolog of mouse Irgm/Lrg47) have roles in the autophagic elimination of mycobacteria [34,35] and contribute genetically to Crohn’s disease and TB [25,36,37]. Recent studies have revealed the molecular mechanisms by which IRGM functions in the regulation of autophagy and the promotion of antimicrobial effects. IRGM works by stabilizing AMP-activated protein kinase (AMPK) and maintaining and associating with autophagy factors ULK1, ATG14, and ATG16L1 [38]. However, the exact contribution of IRGM to the regulation of selective autophagy during mycobacterial infection requires further clarification. In addition, the IRGM/murine ortholog IRGM1 may play multiple regulatory functions in addition to autophagy in terms of infection and inflammation [39].
Furthermore, a recent study by Kimmey et al. showed that multiple autophagy-related genes did not contribute to host resistance to Mtb infection in mouse models in vivo [40]. Importantly, the authors could not rule out that autophagy restricts the mycobacterial growth [40]. Future studies are warranted which pathways/factors are critically required for antimicrobial responses by autophagy activation in human cells as well as in mouse models.
LAP and mycobacterial infection
LAP is an LC3 conjugation process onto the phagosomal membrane during the phagocytosis of pathogenic microbes or apoptotic cells [41–44]. As a noncanonical autophagy pathway, LAP does not lead to the formation of double membrane structures [45,46]. Upon Toll-like receptor (TLR) signaling, the autophagic proteins LC3 and Beclin-1 are directly recruited to the single-membrane phagosome and promote phagosomal maturation and intracellular bacterial killing [45]. Indeed, LAP is triggered by the recognition of pathogenic microbes or dead cells through numerous receptors including Fc-receptors, TLRs, the C-type lectin receptor Dectin-1, or the phosphatidylserine receptor T cell immunoglobulin mucin-4 [41,47,48]. LAP does not depend on the activity of the core pre-initiation complex of conventional autophagy machinery but rather involves the function of certain autophagic proteins including BECN1, ATG5, ATG7, and ATG3 [49,50]. Importantly, RUBCN/Rubicon is a critical regulator of LAP and a distinguished molecule in the canonical autophagy pathway [51]. During the LAP process, the phagocytosed cargo within a single-membrane phagosome, called the LAPosome, is decorated with PI3P produced by the class III PI3-kinase complex (VPS15, VPS34, Beclin-1, UVRAG, and Rubicon). RUBCN and PI3P are required for the stabilization of CYBB/NOX-2 to generate ROS and the conjugation of lipidated LC3-II to the LAPosomal membrane to enhance phagosomal maturation [50,51]. Since Mtb activates numerous PRRs [52], LAP is being thought to be essential for the regulation of antimicrobial an inflammatory responses during mycobacterial infection. However, recent study showed that Mtb protein CpsA enabled bacteria to evade NADPH oxidase-dependent ROS signaling and LAP [53]. Still, it remains unclear what pathway is mainly involved (i.e., either LAP or xenophagy or both) upon Mtb infection from the perspective of host defense.
Potential anti-TB reagents that target autophagy
As mentioned above, HDT is an emerging concept for emerging and re-emerging infectious diseases including TB via the enhancement of protective immunity [5]. Here we discuss the potential drug candidates that target autophagy for the development of HDT-TB. For clear understanding, we also summarized drug types and their mechanisms in Table 1 [54–76].
Table 1.
Potential adjunctive anti-TB therapeutics targeting autophagy.
| Candidates | Mechanisms | References |
|---|---|---|
| Vitamin D receptor signaling | ||
| 1,25-dihydroxyvitamin D3 | Induction of cathelicidin, autophagy gene transcription IL1R signaling and DEFB4/HBD2 induction |
[54, 57] |
| Mycobacterial LpqH antigen | Functional VDR activation through TLR2/1/CD14-Ca2+-AMPK-p38 MAPK signaling | [55] |
| 4-phenylbutyrate | LL-37 expression, P2RX7 receptor signaling, intracellular calcium influx and AMPK pathways | [56] |
| IFN-γ | Crosstalk with vitamin D-dependent pathway | [58,59] |
| Nuclear receptors and agonists | ||
| NR1D1 | Increase of MAPL1LC3-II and LAMP1 | [60] |
| PPAR-α | Upregulation of autophagy and lysosomal genes | [61] |
| ESRRA | Transcriptional and post-tranaslational regulation of autophagy genes/protein | [62] |
| AMPK pathway | ||
| Calcium-mobilizing agents | Ca(2+)/calmodulin-dependent kinase kinase-β (CaMKKβ)-mediated AMPK activation | [63] |
| AICAR | AMPK-PGC1α-mediated autophagy-related gene transcription | [64] |
| Cyclic peptides Ohmyungsamycins A/B |
AMPK-mediated autophagy activation and regulation of inflammation | [65] |
| Phytochemicals; steroid glycoside chemicals | AMPK-ULK1-dependent pathway | [66] |
| Resveratrol | Activation of AMPK and sirtuin 1 | [67,68] |
| Small molecules/Chemicals | ||
| Gefitinib | Inhibition of p38 MAPK pathway; activation of autophagy | [69] |
| Fluoxetine | Increased secretion of TNF-α; induction of autophagy | [69] |
| Baicalin | Modulation of PI3K/Akt/mTOR pathway | [70] |
| Loperamide, Verapamil | Induction of autophagy; mechanisms are unknown | [71] |
| Src kinase inhibitors | Activation of autophagy; mechanisms are unknown | [72] |
| Carbamazepine | Inositol triphosphate (IP3) depletion; mTOR-independent mechanisms | [73] |
| Antibiotics | ||
| Isoniazid, Pyrazinamide | Bacterial hydroxyl radicals and cellular ROS-dependent mechanisms | [74] |
| Thiostrepton | ER stress-mediated autophagy activation | [75] |
| Ambroxol | Induction of autophagy; mechanisms are unknown | [76] |
Vitamin D3 and VDR signaling
The impact of vitamin D and vitamin D receptor (VDR) signaling has been most extensively studied in the context of autophagy and host defense. Innate immune activation and VDR signaling are tightly linked in innate immune cells to converge into antimicrobial host defenses against Mtb infection [77]. Liu et al. [78] demonstrated that, in human monocytes/macrophages, activation of VDR signaling leads to the induction of cathelicidin, a cationic antimicrobial protein with killing effects against Mtb. Further work by Yuk et al. [54] showed that 1,25-D3 enhances the activation of antibacterial autophagy and the elimination of intracellular Mtb in human monocytes/macrophages via cathelicidin induction and autophagy gene activation. Interestingly, TLR signaling activation during Mtb infection triggers a complicated intracellular signaling pathway that induces the expression of the Cyp27b1 gene (1α-hydroxylase) in human monocytes/macrophages, thereby activating functional VDR signaling to amplify cathelicidin induction and antimicrobial responses [55,79]. Furthermore, 4-phenylbutyrate (PBA), given alone or in combination with vitamin D3, overcomes the Mtb-induced inhibition of cathelicidin LL-37 expression and enhances anti-mycobacterial effects [56,80]. Rekha [56] found that PBA-induced autophagy activation is mediated through numerous intracellular signaling pathways including LL-37 expression, P2RX7 receptor signaling, and the intracellular calcium and AMPK pathways. Importantly, Fabri et al. [59] showed that supplementation of vitamin D-deficient serum with 25-hydroxyvitamin D3 recovered IFN-γ-mediated autophagy and phagosome-lysosome fusion in human macrophages, which suggests the importance of sufficient levels of vitamin D for the maintenance of protective immunity against Mtb infection. However, there are conflicting results regarding the therapeutic effects of vitamin D3, presumably due to the variation in individual levels of basal vitamin D, treatment dose and duration, and the clinical stages of disease [3]. Further studies with proper clinical settings will clarify our understanding of the beneficial effects of vitamin D in terms of protection and therapeutics against TB.
Nuclear receptors and agonists
Several nuclear receptor agonists have promising effects on host autophagy and antimicrobial defense in mycobacterial infection. Chandra et al. [60] showed that nuclear receptor subfamily 1, group D, member 1 (NRD1), an adopted orphan nuclear receptor, is involved in the enhancement of antimycobacterial effects and autophagy in human macrophages. NR1D1 contributes to the activation of autophagy and lysosomal biogenesis via an increase in MAP1LC3-II and the lysosomal protein LAMP1.
In two recent studies, our group also examined autophagy and the antimycobacterial effects of orphan nuclear receptors. First, activation of the adopted orphan nuclear receptor peroxisome proliferator-activated receptor α (PPARα) led to the upregulated colocalization of Mtb phagosomes and autophagosomes, as well as lysosomal biogenesis and lipid catabolism, thereby enhancing antimycobacterial effects in macrophages. PPARα agonists have positive transcriptional regulatory effects on numerous autophagy and lysosomal genes, particularly transcription factor EB (TFEB), which is critically involved in antimicrobial effects and controlling macrophage inflammatory responses during mycobacterial infection [61]. In addition, the orphan nuclear receptor estrogen-related receptor α (ERRα), a critical regulator of metabolic homeostasis, plays an essential role in the activation of autophagy and increased anti-mycobacterial responses via transcriptional and post-translational modifications of essential autophagy genes/proteins. Through a positive feed-forward loop between ERRα and SIRT1, ERRα overexpression promotes the deacetylation of several autophagy-related proteins including ATG5, BECN1, and ATG7. Either ERRα or SIRT1 activation contributes to the enhancement of phagosomal acidification and antimicrobial responses against Mtb infection, thus demonstrating the critical role of ERRα in the innate host defense during mycobacterial infection [62]. It is interesting to note that all of the nuclear receptors described above (NR1D1, PPARα, and ERRα) have crucial functions in various biological processes, particularly energy metabolism. Thus, future studies are required to understand the ultimate roles of various nuclear receptors in terms of host susceptibilities or defense during infection in the context of metabolic diseases.
Targeting the AMPK and SIRT1 pathways
AMPK, a key metabolic and energy regulator that functions by sensing the AMP/ATP ratio, plays essential roles in the coordination of anabolic and catabolic activity via the phosphorylation of multiple proteins [81]. Numerous studies have shown the positive action of AMPK signaling in autophagy activation. Activated AMPK promotes autophagy by inhibiting mTOR, a suppressor of autophagy induction, or by activating ULK1/2, autophagy initiators [82,83]. Mtb infection of macrophages leads to robust activation of mTOR signaling but only a slight increase in AMPKα phosphorylation [64]. Indeed, the AMPK pathway enhances antibacterial autophagy induced by numerous reagents or drugs [24,84]. A variety of calcium-mobilizing agents activate autophagy through the AMPK pathway via Ca (2+)/calmodulin-dependent kinase kinase-β signaling [63]. In human monocytes/macrophages, vitamin D signaling activates autophagy and antimicrobial defense against Mtb infection via AMPK activation [55,56]. In addition, the AMPK-PPARγ coactivator-1 α (PGC1α) pathway plays a critical role in the promotion of autophagy-related gene transcription to enhance antibacterial autophagy and phagosomal acidification in macrophages during mycobacterial infection. Importantly, AMPK-PGC1α signaling contributes to increased mitochondrial biogenesis and respiratory function in Mtb-infected macrophages, suggestive of mitochondrial support by the same pathway that triggers autophagy during infection [64]. In addition, newly identified cyclic peptides ohmyungsamycins A and B promote phagosomal maturation and antimicrobial responses against Mtb infections by activating the AMPK pathway [65]. Recent studies that have used analytical chemistry approaches have identified several natural chemicals that enhance autophagy via an AMPK-ULK1-dependent pathway and that play roles in cognitive behaviors in mouse models of Alzheimer’s disease [66]. The SIRT1 activator resveratrol (RSV) triggers the production of intracytoplasmic calcium and activates AMPK to promote the autophagy-dependent lysosomal degradation of amyloid-β [67]. We recently showed that RSV facilitates the colocalization of mycobacterial phagosomes with autophagosomes, as well as the antimicrobial effects against Mtb in macrophages [65]. Vingtdeux [68] screened a chemical library with structural similarities to RSV and identified potent autophagy-activating agents that facilitate the activation of AMPK but inhibit the mTOR pathway. Although the drugs mentioned above showed promising activity in amyloid-β clearance, whether these drugs enhance the autophagic clearance of intracellular Mtb remains unknown.
Autophagy-targeting small molecules
A variety of small molecules of natural and synthetic origin may be promising targets for HDT-TB by targeting autophagy. Stanley [69], using a small molecule-based chemical biological approach, showed that gefitinib (Iressa, ZD-1839), which targets epidermal growth factor receptor, effectively inhibits the replication of Mtb in macrophages and in vivo, inhibits phosphorylation of the p38 MAPK pathway, and induces autophagy to enhance intracellular Mtb clearance. In that study, fluoxetine, a selective serotonin reuptake inhibitor, also exhibited restrictive activity against intracellular mycobacteria and induced autophagy, presumably via the increased secretion of the pro-inflammatory cytokine tumor necrosis factor alpha in Mtb-infected macrophages. Baicalin, an herbal medicine, induces autophagy via the PI3K/Akt/mTOR pathway, and exhibits antimicrobial and anti-inflammatory effects against Mtb infection, which suggests that it may be a new candidate for improved TB treatment [70]. Furthermore, a recent study that evaluated potential anti-mycobacterial agents, including loperamide and verapamil, showed that these drugs induce autophagy, which is accompanied by significant inhibition of intracellular Mtb survival in murine alveolar macrophages [71]. In our earlier study [74], we found that the standard anti-TB drugs isoniazid and pyrazinamide promoted autophagy activation, which was required for effective therapeutic responses in macrophages and fly models during mycobacterial infection. A very recent paper from Choi et al. [76] also demonstrated that the lead compound ambroxol induced autophagy in vitro and in vivo, promoting mycobacterial killing, in murine macrophages. Interestingly, ambroxol showed potentiating rifampin activity in a murine model of Mtb infection.
Several studies have proven the effectiveness of HDT-TB against drug-resistant strains. A recent study showed that inhibiting Src kinase activity significantly suppresses the intracellular survival of Mtb H37Rv, particularly the MDR and XDR strains of Mtb [72]. Importantly, in the same study, Src inhibition was effective for infection of the XDR-Mtb strain in a guinea pig models, which suggests that it may also be a promising agent for HDT-TB therapeutics. Importantly, persistent activation of Src kinase results in the activation of mTOR, a well-known autophagy repressor, whereas the inhibition of Src kinase leads to a partial rescue of defective autophagy [85]. A study that screened U.S. Food and Drug Administration-approved drugs showed that the anti-convulsant drug carbamazepine leads to the induction of autophagy via inositol triphosphate depletion and AMPK activation [73]. In that same study, the drug also significantly inhibited highly virulent MDR-TB in the lungs of infected mice. These findings shed light on potential therapeutic strategies using drug repositioning in the field of HDT-TB.
Challenges and perspectives of autophagy-adjunctive therapeutics
Autophagy-adjunctive therapeutics is an emerging concept for the improved treatment of TB. Although it is a promising strategy, even for controlling drug-resistant strains, according to in vitro and preclinical studies [72,73,86], current data on its clinical use are insufficient, and more evidence is need in future studies.
Challenges of autophagy-adjunctive therapeutics for clinical use
In Table 2 [87–96], we summarized recent clinical studies on vitamin D and autophagy-adjunctive therapy for patients with pulmonary TB. As previously discussed, vitamin D treatment has diverse biological effects on host defense in TB including autophagy activation. Mily et al. [95] demonstrated that adjunctive therapy with vitamin D alone or in combination with PBA to standard short-course therapy enhanced clinical recovery, thus representing a possibility for HDT-TB. Salahuddin et al. [91] revealed that supplementation with high doses of vitamin D3 accelerated clinical and radiological improvements in all TB patients and increased host immune activation in patients with deficient baseline serum levels of vitamin D. Despite these promising results, vitamin D-based therapy remains controversial. Other studies [87,88] have shown that vitamin D3 supplementation does not reduce the time period of sputum culture conversion. In addition, high-dose vitamin D3 regimens safely correct vitamin D3 deficiency without improving the rate of sputum Mtb clearance [89]. Inconsistent results regarding vitamin D-based HDT-TB may be due to the basal levels of vitamin D in the patient population, VDR polymorphisms, and geographic and/or ethnic variation associated with TB susceptibility [78,97,98]. In addition, it is difficult to show the comparative effects of randomized clinical trials in which standard TB drugs are given together with the adjuvant drug (e.g., vitamin D) or placebo. In fact, this issue may explain the null effect on the primary endpoint (sputum-conversion) found in many studies. Future studies on personalized medicine should be conducted to clarify the effective clinical use of vitamin D as a supplement for TB, MDR-TB, and latent TB infection. At present, few clinical trials have examined potential treatments for TB other than vitamin D. Trials of repositioning drugs for combating TB are ongoing. Repositioned drugs such as gefitinib, fluoxetine, loperamide, and verapamil could represent promising candidates and should be validated in well-designed clinical studies as adjuvant therapeutics for TB.
Table 2.
Clinical studies of autophagy-adjunctive therapeutics for tuberculosis.
| Study name | Phase | Results | Clinical trials. gov Identifier* | References | |
|---|---|---|---|---|---|
| Vitamin D replacement | |||||
| Trial of Adjunctive Vitamin D in TB Treatment | III | Vitamin D did not significantly affect time to sputum culture conversion | NCT 419068 | [87] | |
| A Clinical Trial to Study the Effect of Vitamin D to Treatment in New Pulmonary TB Patients | II | Vitamin D supplementation did not reduce time to sputum culture conversion | NCT 366470 | [88] | |
| Impact of Vitamin D supplementation on Host Immunity to Mtb and Response to Treatment | II | A high-dose vitamin D3 regimen safely corrected vitamin D deficiency but did not improve the rate of sputum Mtb clearance over 16 weeks in this pulmonary TB cohort | NCT 918086 | [89] | |
| Replacement of Vitamin D in Patients With Active TB | II | High doses of vitamin D accelerated clinical, radiographic improvement in all TB patients and increased immune activation in patients with deficient serum vitamin D levels | NCT 1130311 | [90,91] | |
| Role of Vitamin D in Innate Immunity to TB | II | Vitamin D supplementation for 6 months had significant favorable effects on serum 25(OH) D concentrations and on growth in stature | NCT 1244204 | [92] | |
| Effects of Vitamin D Supplementation on Antimycobacterial Immunity | II | A single oral dose of 2.5 mg vitamin D significantly enhanced the ability of participants' whole blood to restrict BCG-lux luminescence in vitro | NCT 00157066 | [93] | |
| Vitamin D + other replacement | |||||
| L-arginine and Vitamin D Adjunctive Therapy in Pulmonary TB | III | Neither vitamin D nor L-arginine supplementation affected TB outcomes | NCT 677339 | [94] | |
| Clinical Trial of PBA and Vitamin D in TB | II | Adjunct therapy with PBA+vitamin D3 or vitamin D3 or PBA to standard short-course therapy demonstrated beneficial effects towards clinical recovery | NCT 01580007 | [95] | |
| Drug repositioning | |||||
| HDT-TB (Everolimus, Auranofin) | II | Enrolling | NCT 2968927 | ||
| Doxycycline in human pulmonary TB | II | Enroll: completed | NCT 2774993 | [96] | |
BCG, Bacille Calmette-Guérin * Further details for trial with NCT numbers can be accessed at http://clinicaltrials.gov.
In addition, there are several issues associated with the failure of TB treatment due to drug resistance, poor compliance, and severe side effects. Therefore, the enrollment of carefully selected and appropriate patients for specific drug regimens may be one of the most important factors when evaluating autophagy-adjunctive therapeutics as new and improved therapeutic options for refractory TB. In addition, there is a lack of studies on drug delivery and treatment duration in autophagy-adjunctive therapy combined with conventional antibiotics treatment. It is necessary to deliver the drug to the affected tissues/organs to avoid nontarget cell exposure and unwanted side effects [8].
Limitation and perspectives of autophagy-adjunctive therapeutics
A major limitation of this review is the limited information obtained from the in vitro studies demonstrating that autophagy activation is beneficial for inhibiting Mtb survival in host cells. In addition, there might be off-target effects by autophagy-modulating small molecules or agents. Studies using gene-manipulated mouse models of autophagy have provided essential knowledge for understanding how autophagy functions against mycobacterial infection. There is evidence showing the beneficial effects of GTPase IRGM on cell-autonomous protective immunity against mycobacterial infection in murine and human cells [34,35]. However, there is clear evidence of species-specific differences. For example, the major axis of autophagy and host defense involving vitamin D and cathelicidin is not functional in mouse models [99]. Future studies of autophagy-targeted anti-mycobacterial responses in vivo are warranted for the development of new treatments against TB and the assessment of off-target effects as well as species-specific similarities and differences.
It would also be interesting to identify the actual effector arms in autophagy-mediated killing of bacteria. Ponpuak et al. [100] described the existence of a novel antimicrobial peptide that can directly kill Mtb. They successfully demonstrated that the autophagic adaptor protein p62 is involved in the transport of rpS30 peptide to the mycobacterial phagosome and in the autophagy prevention of tubercle bacilli. However, the degree to which rpS30 or cathelicidin is actually involved in bacterial killing in autophagy-mediated HDTs is still unknown. We also believe that an acidic environment in phagolysosomal acidification can directly damage live Mtb [101] and that numerous lysosomal enzymes are involved in such circumstances. Thus, future studies are needed to investigate this aspect further, and it would be promising to identify new antimicrobial peptides that directly target Mtb to advance autophagy-based HDT studies.
Concluding remarks
During recent years, numerous advances have been made in our understanding of the discovery and functional characterization of autophagy-based antimicrobial agents and pathways. Despite the potential advantages of autophagy-activating agents for HDT against TB, many challenges remain with regard to the detailed molecular mechanisms by which the activation of autophagy enhances the limitation of Mtb replication in host cells. There is a possibility that the different pathways/mechanisms involved may participate in the enhancement of autophagic clearance of intracellular Mtb depending on diverse autophagy-activating agents. The known HDT drugs play important roles in pathologic inflammation, phagolysosomal fusion, lysosomal functions, and the antimicrobial activity of host cells infected with Mtb infection. Although autophagy-activating agents may act as therapeutic candidates for HDT-TB, it is possible that many other biological pathways, in addition to autophagy, contribute to the host defense against TB infection. For example, the most widely used reagent for HDT-TB is vitamin D, a therapeutic reagent for TB used before the antibiotic era [102]. In addition to its autophagy-activating ability, vitamin D has pleiotropic effects on infected cells and/or tissues to promote direct antimicrobial defense via cathelicidin and by regulating inflammation. Another challenge in the field relates to clinical applications, namely, whether drug administration could be targeted to disease sites or whether autophagy-adjunctive therapies could reduce the duration of antibiotic treatment. Continuous and future studies on autophagy-based HDT therapeutic candidates will not only provide insight into the antibacterial function of autophagy, but may also contribute to potential therapeutic improvements against TB.
Funding Statement
This work was supported by the National Research Foundation of Korea [2015K2A2A6002008];National Research Foundation of Korea [2017R1A5A2015385].
Acknowledgments
We are indebted to current and past members of our laboratory for discussions and investigations that contributed to this article. We apologize to colleagues whose work and publications could not be referenced owing to space constraints.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Pareja ME, Colombo MI.. Autophagic clearance of bacterial pathogens: molecular recognition of intracellular microorganisms. Front Cell Infect Microbiol. 2013;3:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bah A, Vergne I. Macrophage autophagy and bacterial infections. Front Immunol. 2017;8:1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kolloli A, Subbian S. Host-directed therapeutic strategies for tuberculosis. Front Med (Lausanne). 2017;4:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kimmey JM, Stallings CL. Bacterial pathogens versus autophagy: implications for therapeutic interventions. Trends Mol Med. 2016. December;22(12):1060–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kaufmann SHE, Dorhoi A, Hotchkiss RS, et al. Host-directed therapies for bacterial and viral infections. Nat Rev Drug Discov. 2018. January;17(1):35–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zumla A, Maeurer M. Host-Directed therapies for tackling multi-drug resistant tuberculosis: learning from the Pasteur-Bechamp debates. Clin Infect Dis. 2015. November 1;61(9):1432–1438. [DOI] [PubMed] [Google Scholar]
- [7].Zumla A, Rao M, Dodoo E, et al. Potential of immunomodulatory agents as adjunct host-directed therapies for multidrug-resistant tuberculosis. BMC Med. 2016. June;15(14):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gupta A, Misra A, Deretic V. Targeted pulmonary delivery of inducers of host macrophage autophagy as a potential host-directed chemotherapy of tuberculosis. Adv Drug Deliv Rev. 2016. July;1(102):10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Y GL M, Zitvogel L, Kroemer G. Autophagy and cellular immune responses. Immunity. 2013;39:211–227. [DOI] [PubMed] [Google Scholar]
- [10].Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000. December 1;290(5497):1717–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mizushima N, Levine B, Cuervo AM, et al. Autophagy fights disease through cellular self-digestion. Nature. 2008. February 28;451(7182):1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008. January 11;132(1):27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ. 2005. November;12(Suppl 2):1509–1518. [DOI] [PubMed] [Google Scholar]
- [14].Axe EL, Walker SA, Manifava M, et al. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol. 2008. August 25;182(4):685–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hamasaki M, Furuta N, Matsuda A, et al. Autophagosomes form at ER-mitochondria contact sites. Nature. 2013. March 21;495(7441):389–393. [DOI] [PubMed] [Google Scholar]
- [16].Feng Y, He D, Yao Z, et al. The machinery of macroautophagy. Cell Res. 2014. January;24(1):24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shibutani ST, Yoshimori T. A current perspective of autophagosome biogenesis. Cell Res. 2014. January;24(1):58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lata S, Schoehn G, Solomons J, et al. Structure and function of ESCRT-III. Biochem Soc Trans. 2009. February;37(Pt(1)):156–160. [DOI] [PubMed] [Google Scholar]
- [19].Furuta N, Yoshimori T, Amano A. Mediatory molecules that fuse autophagosomes and lysosomes. Autophagy. 2010. April;6(3):417–418. [DOI] [PubMed] [Google Scholar]
- [20].de Chastellier C. The many niches and strategies used by pathogenic mycobacteria for survival within host macrophages. Immunobiology. 2009;214(7):526–542. [DOI] [PubMed] [Google Scholar]
- [21].Shin DM, Jeon BY, Lee HM, et al. Mycobacterium tuberculosis eis regulates autophagy, inflammation, and cell death through redox-dependent signaling. PLoS Pathog. 2010. December 16;6(12):e1001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Romagnoli A, Etna MP, Giacomini E, et al. ESX-1 dependent impairment of autophagic flux by Mycobacterium tuberculosis in human dendritic cells. Autophagy. 2012. September;8(9):1357–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007. October;7(10):767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fabri M, Realegeno SE, Jo EK, et al. Role of autophagy in the host response to microbial infection and potential for therapy. Curr Opin Immunol. 2011. February;23(1):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Songane M, Kleinnijenhuis J, Netea MG, et al. The role of autophagy in host defence against Mycobacterium tuberculosis infection. Tuberculosis (Edinb). 2012. September;92(5):388–396. [DOI] [PubMed] [Google Scholar]
- [26].Sil P, Muse G, Martinez J. A ravenous defense: canonical and non-canonical autophagy in immunity. Curr Opin Immunol. 2017. November;7(50):21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mizushima N. Autophagy: process and function. Genes Dev. 2007. November 15;21(22):2861–2873. [DOI] [PubMed] [Google Scholar]
- [28].Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010. May;221(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Watson RO, Manzanillo PS, Cox JS. Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell. 2012. August 17;150(4):803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Manzanillo PS, Ayres JS, Watson RO, et al. The ubiquitin ligase parkin mediates resistance to intracellular pathogens. Nature. 2013. September 26;501(7468):512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Franco LH, Nair VR, Scharn CR, et al. The ubiquitin ligase Smurf1 functions in selective autophagy of Mycobacterium tuberculosis and anti-tuberculous host defense. Cell Host Microbe. 2017. January 11;21(1):59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chauhan S, Kumar S, Jain A, et al. TRIMs and Galectins globally cooperate and TRIM16 and Galectin-3 co-direct autophagy in endomembrane damage homeostasis. Dev Cell. 2016. October 10;39(1):13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Feng CG, Collazo-Custodio CM, Eckhaus M, et al. Mice deficient in LRG-47 display increased susceptibility to mycobacterial infection associated with the induction of lymphopenia. J Immunol. 2004. January 15;172(2):1163–1168. [DOI] [PubMed] [Google Scholar]
- [34].Gutierrez MG, Master SS, Singh SB, et al. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004. December 17;119(6):753–766. [DOI] [PubMed] [Google Scholar]
- [35].Singh SB, Davis AS, Taylor GA, et al. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science. 2006. September 8;313(5792):1438–1441. [DOI] [PubMed] [Google Scholar]
- [36].The Wellcome Trust Case Control Consortium Genome-wide association study of CNVs in 16,000 cases of eight common diseases and 3,000 shared controls. Nature. 2010. April 1;464(7289):713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].The Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007. June 7;447(7145):661–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chauhan S, Mandell MA, Deretic V. IRGM governs the core autophagy machinery to conduct antimicrobial defense. Mol Cell. 2015. May 7;58(3):507–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Matsuzawa T, Kim BH, Shenoy AR, et al. IFN-gamma elicits macrophage autophagy via the p38 MAPK signaling pathway. J Immunol. 2012. July 15;189(2):813–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kimmey JM, Huynh JP, Weiss LA, et al. Unique role for ATG5 in neutrophil-mediated immunopathology during M. tuberculosis infection. Nature. 2015. December 24;528(7583):565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Martinez J, Almendinger J, Oberst A, et al. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc Natl Acad Sci U S A. 2011. October 18;108(42):17396–17401. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [42].Galluzzi L, Baehrecke EH, Ballabio A, et al. Molecular definitions of autophagy and related processes. Embo J. 2017. July 3;36(13):1811–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mitchell G, Isberg RR. Innate immunity to intracellular pathogens: balancing microbial elimination and inflammation. Cell Host Microbe. 2017. August 9;22(2):166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Panneerdoss S, Viswanadhapalli S, Abdelfattah N, et al. Cross-talk between miR-471-5p and autophagy component proteins regulates LC3-associated phagocytosis (LAP) of apoptotic germ cells. Nat Commun. 2017. September 19;8(1):598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sanjuan MA, Dillon CP, Tait SW, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007. December 20;450(7173):1253–1257. [DOI] [PubMed] [Google Scholar]
- [46].Mehta P, Henault J, Kolbeck R, et al. Noncanonical autophagy: one small step for LC3, one giant leap for immunity. Curr Opin Immunol. 2014;26:69–75. [DOI] [PubMed] [Google Scholar]
- [47].Henault J, Martinez J, Riggs JM, et al. Noncanonical autophagy is required for type I interferon secretion in response to DNA-immune complexes. Immunity. 2012. December 14;37(6):986–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ma J, Becker C, Lowell CA, et al. Dectin-1-triggered recruitment of light chain 3 protein to phagosomes facilitates major histocompatibility complex class II presentation of fungal-derived antigens. J Biol Chem. 2012. October 5;287(41):34149–34156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bandyopadhyay U, Overholtzer M. LAP: the protector against autoimmunity. Cell Res. 2016. August;26(8):865–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sprenkeler EG, Gresnigt MS. van de Veerdonk FL. LC3-associated phagocytosis: a crucial mechanism for antifungal host defence against Aspergillus fumigatus. Cell Microbiol. 2016. September;18(9):1208–1216. [DOI] [PubMed] [Google Scholar]
- [51].Martinez J, Malireddi RK, Lu Q, et al. Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat Cell Biol. 2015. July;17(7):893–906. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [52].Ferwerda G, Girardin SE, Kullberg BJ, et al. NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog. 2005. November;1(3):279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Koster S, Upadhyay S, Chandra P, et al. Mycobacterium tuberculosis is protected from NADPH oxidase and LC3-associated phagocytosis by the LCP protein CpsA. Proc Natl Acad Sci U S A. 2017. October 10;114(41):E8711–E8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yuk JM, Shin DM, Lee HM, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009. September 17;6(3):231–243. [DOI] [PubMed] [Google Scholar]
- [55].Shin DM, Yuk JM, Lee HM, et al. Mycobacterial lipoprotein activates autophagy via TLR2/1/CD14 and a functional vitamin D receptor signalling. Cell Microbiol. 2010. November;12(11):1648–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Rekha RS, Rao Muvva SS, Wan M, et al. Phenylbutyrate induces LL-37-dependent autophagy and intracellular killing of Mycobacterium tuberculosis in human macrophages. Autophagy. 2015;11(9):1688–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Verway M, Bouttier M, Wang TT, et al. Vitamin D induces interleukin-1beta expression: paracrine macrophage epithelial signaling controls M. tuberculosis infection. PLoS Pathog. 2013;9(6):e1003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Edfeldt K, Liu PT, Chun R, et al. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc Natl Acad Sci U S A. 2010. December 28;107(52):22593–22598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fabri M, Stenger S, Shin DM, et al. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci Transl Med. 2011. October 12;3(104):104ra102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chandra V, Bhagyaraj E, Nanduri R, et al. NR1D1 ameliorates Mycobacterium tuberculosis clearance through regulation of autophagy. Autophagy. 2015. November 2;11(11):1987–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kim YS, Lee HM, Kim JK, et al. PPAR-alpha activation mediates innate host defense through induction of TFEB and lipid catabolism. J Immunol. 2017. April 15;198(8):3283–3295. [DOI] [PubMed] [Google Scholar]
- [62].Kim SY, Yang CS, Lee HM, et al. ESRRA (estrogen-related receptor alpha) is a key coordinator of transcriptional and post-translational activation of autophagy to promote innate host defense. Autophagy. 2018;14(1):152–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hoyer-Hansen M, Bastholm L, Szyniarowski P, et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007. January 26;25(2):193–205. [DOI] [PubMed] [Google Scholar]
- [64].Yang CS, Kim JJ, Lee HM, et al. The AMPK-PPARGC1A pathway is required for antimicrobial host defense through activation of autophagy. Autophagy. 2014. May;10(5):785–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kim TS, Shin YH, Lee HM, et al. Ohmyungsamycins promote antimicrobial responses through autophagy activation via AMP-activated protein kinase pathway. Sci Rep. 2017. June 13;7(1):3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Fan Y, Wang N, Rocchi A, et al. Identification of natural products with neuronal and metabolic benefits through autophagy induction. Autophagy. 2017. January 2;13(1):41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Vingtdeux V, Giliberto L, Zhao H, et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem. 2010. March 19;285(12):9100–9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Vingtdeux V, Chandakkar P, Zhao H, et al. Novel synthetic small-molecule activators of AMPK as enhancers of autophagy and amyloid-beta peptide degradation. FASEB J. 2011. January;25(1):219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Stanley SA, Barczak AK, Silvis MR, et al. Identification of host-targeted small molecules that restrict intracellular Mycobacterium tuberculosis growth. PLoS Pathog. 2014. February;10(2):e1003946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhang Q, Sun J, Wang Y, et al. Antimycobacterial and anti-inflammatory mechanisms of Baicalin via induced autophagy in macrophages Infected with Mycobacterium tuberculosis. Front Microbiol. 2017;8:2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Juarez E, Carranza C, Sanchez G, et al. Loperamide restricts intracellular growth of Mycobacterium tuberculosis in lung macrophages. Am J Respir Cell Mol Biol. 2016. December;55(6):837–847. [DOI] [PubMed] [Google Scholar]
- [72].Chandra P, Rajmani RS, Verma G, et al. Targeting drug-sensitive and -resistant strains of Mycobacterium tuberculosis by inhibition of Src family kinases lowers disease burden and pathology. mSphere. 2016. Mar-Apr;1(2):e00043-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Schiebler M, Brown K, Hegyi K, et al. Functional drug screening reveals anticonvulsants as enhancers of mTOR-independent autophagic killing of Mycobacterium tuberculosis through inositol depletion. EMBO Mol Med. 2015. February;7(2):127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kim JJ, Lee HM, Shin DM, et al. Host cell autophagy activated by antibiotics is required for their effective antimycobacterial drug action. Cell Host Microbe. 2012. May 17;11(5):457–468. [DOI] [PubMed] [Google Scholar]
- [75].Zheng Q, Wang Q, Wang S, et al. Thiopeptide antibiotics exhibit a dual mode of action against intracellular pathogens by affecting both host and microbe. Chem Biol. 2015. August 20;22(8):1002–1007. [DOI] [PubMed] [Google Scholar]
- [76].Choi SW, Gu Y, Peters RS, et al. Ambroxol induces autophagy and potentiates rifampin antimycobacterial activity. Antimicrob Agents Chemother. 2018. September;62(9):e01019-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Liu PT, Krutzik SR, Modlin RL. Therapeutic implications of the TLR and VDR partnership. Trends Mol Med. 2007. March;13(3):117–124. [DOI] [PubMed] [Google Scholar]
- [78].Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006. March 24;311(5768):1770–1773. [DOI] [PubMed] [Google Scholar]
- [79].Liu PT, Schenk M, Walker VP, et al. Convergence of IL-1beta and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS One. 2009. June 5;4(6):e5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mily A, Rekha RS, Kamal SM, et al. Oral intake of phenylbutyrate with or without vitamin D3 upregulates the cathelicidin LL-37 in human macrophages: a dose finding study for treatment of tuberculosis. BMC Pulm Med. 2013. April 16;13:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012. March 22;13(4):251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kim J, Kundu M, Viollet B, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011. February;13(2):132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Alers S, Loffler AS, Wesselborg S, et al. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012. January;32(1):2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Hoyer-Hansen M, Jaattela M. AMP-activated protein kinase: a universal regulator of autophagy? Autophagy. 2007. Jul-Aug;3(4):381–383. [DOI] [PubMed] [Google Scholar]
- [85].Pal R, Palmieri M, Loehr JA, et al. Src-dependent impairment of autophagy by oxidative stress in a mouse model of Duchenne muscular dystrophy. Nat Commun. 2014. July;16(5):4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kidwai S, Park CY, Mawatwal S, et al. Dual mechanism of action of 5-nitro-1,10-phenanthroline against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2017. November;61(11):e00969-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Martineau AR, Timms PM, Bothamley GH, et al. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 2011. January 15;377(9761):242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Daley P, Jagannathan V, John KR, et al. Adjunctive vitamin D for treatment of active tuberculosis in India: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2015. May;15(5):528–534. [DOI] [PubMed] [Google Scholar]
- [89].Tukvadze N, Sanikidze E, Kipiani M, et al. High-dose vitamin D3 in adults with pulmonary tuberculosis: a double-blind randomized controlled trial. Am J Clin Nutr. 2015. November;102(5):1059–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Hasan Z, Salahuddin N, Rao N, et al. Change in serum CXCL10 levels during anti-tuberculosis treatment depends on vitamin D status [Short Communication]. Int J Tuberc Lung Dis. 2014. April;18(4):466–469. [DOI] [PubMed] [Google Scholar]
- [91].Salahuddin N, Ali F, Hasan Z, et al. Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis’. BMC Infect Dis. 2013. January 19;13:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Ganmaa D, Giovannucci E, Bloom BR, et al. Vitamin D, tuberculin skin test conversion, and latent tuberculosis in Mongolian school-age children: a randomized, double-blind, placebo-controlled feasibility trial. Am J Clin Nutr. 2012. August;96(2):391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Martineau AR, Wilkinson RJ, Wilkinson KA, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007. July 15;176(2):208–213. [DOI] [PubMed] [Google Scholar]
- [94].Ralph AP, Waramori G, Pontororing GJ, et al. L-arginine and vitamin D adjunctive therapies in pulmonary tuberculosis: a randomised, double-blind, placebo-controlled trial. PLoS One. 2013;8(8):e70032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Mily A, Rekha RS, Kamal SM, et al. Significant effects of oral phenylbutyrate and vitamin D3 adjunctive therapy in pulmonary tuberculosis: A randomized controlled trial. PLoS One. 2015;10(9):e0138340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Xing Y, Liqi Z, Jian L, et al. Doxycycline induces mitophagy and suppresses production of interferon-beta in IPEC-J2 cells. Front Cell Infect Microbiol. 2017;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Gao L, Tao Y, Zhang L, et al. Vitamin D receptor genetic polymorphisms and tuberculosis: updated systematic review and meta-analysis. Int J Tuberc Lung Dis. 2010. January;14(1):15–23. [PubMed] [Google Scholar]
- [98].Wallis RS, Zumla A. Vitamin D as adjunctive host-directed therapy in tuberculosis: A systematic review. Open Forum Infect Dis. 2016. September;3(3):ofw151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Dimitrov V, White JH. Species-specific regulation of innate immunity by vitamin D signaling. J Steroid Biochem Mol Biol. 2016. November;164:246–253. [DOI] [PubMed] [Google Scholar]
- [100].Ponpuak M, Davis AS, Roberts EA, et al. Delivery of cytosolic components by autophagic adaptor protein p62 endows autophagosomes with unique antimicrobial properties. Immunity. 2010. March 26;32(3):329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Gomes MS, Paul S, Moreira AL, et al. Survival of Mycobacterium avium and Mycobacterium tuberculosis in acidified vacuoles of murine macrophages. Infect Immun. 1999. July;67(7):3199–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Luong K, Nguyen LT. Impact of vitamin D in the treatment of tuberculosis. Am J Med Sci. 2011. June;341(6):493–498. [DOI] [PubMed] [Google Scholar]


