Abstract
Background
This is the second substantive update of this review. It was originally published in 1998 and was previously updated in 2009. Elevated blood pressure (known as 'hypertension') increases with age ‐ most rapidly over age 60. Systolic hypertension is more strongly associated with cardiovascular disease than is diastolic hypertension, and it occurs more commonly in older people. It is important to know the benefits and harms of antihypertensive treatment for hypertension in this age group, as well as separately for people 60 to 79 years old and people 80 years or older.
Objectives
Primary objective
• To quantify the effects of antihypertensive drug treatment as compared with placebo or no treatment on all‐cause mortality in people 60 years and older with mild to moderate systolic or diastolic hypertension
Secondary objectives
• To quantify the effects of antihypertensive drug treatment as compared with placebo or no treatment on cardiovascular‐specific morbidity and mortality in people 60 years and older with mild to moderate systolic or diastolic hypertension
• To quantify the rate of withdrawal due to adverse effects of antihypertensive drug treatment as compared with placebo or no treatment in people 60 years and older with mild to moderate systolic or diastolic hypertension
Search methods
The Cochrane Hypertension Information Specialist searched the following databases for randomised controlled trials up to 24 November 2017: the Cochrane Hypertension Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE Ovid (from 1946), Embase (from 1974), the World Health Organization International Clinical Trials Registry Platform, and ClinicalTrials.gov. We contacted authors of relevant papers regarding further published and unpublished work.
Selection criteria
Randomised controlled trials of at least one year's duration comparing antihypertensive drug therapy versus placebo or no treatment and providing morbidity and mortality data for adult patients (≥ 60 years old) with hypertension defined as blood pressure greater than 140/90 mmHg.
Data collection and analysis
Outcomes assessed were all‐cause mortality; cardiovascular morbidity and mortality; cerebrovascular morbidity and mortality; coronary heart disease morbidity and mortality; and withdrawal due to adverse effects. We modified the definition of cardiovascular mortality and morbidity to exclude transient ischaemic attacks when possible.
Main results
This update includes one additional trial (MRC‐TMH 1985). Sixteen trials (N = 26,795) in healthy ambulatory adults 60 years or older (mean age 73.4 years) from western industrialised countries with moderate to severe systolic and/or diastolic hypertension (average 182/95 mmHg) met the inclusion criteria. Most of these trials evaluated first‐line thiazide diuretic therapy for a mean treatment duration of 3.8 years.
Antihypertensive drug treatment reduced all‐cause mortality (high‐certainty evidence; 11% with control vs 10.0% with treatment; risk ratio (RR) 0.91, 95% confidence interval (CI) 0.85 to 0.97; cardiovascular morbidity and mortality (moderate‐certainty evidence; 13.6% with control vs 9.8% with treatment; RR 0.72, 95% CI 0.68 to 0.77; cerebrovascular mortality and morbidity (moderate‐certainty evidence; 5.2% with control vs 3.4% with treatment; RR 0.66, 95% CI 0.59 to 0.74; and coronary heart disease mortality and morbidity (moderate‐certainty evidence; 4.8% with control vs 3.7% with treatment; RR 0.78, 95% CI 0.69 to 0.88. Withdrawals due to adverse effects were increased with treatment (low‐certainty evidence; 5.4% with control vs 15.7% with treatment; RR 2.91, 95% CI 2.56 to 3.30. In the three trials restricted to persons with isolated systolic hypertension, reported benefits were similar.
This comprehensive systematic review provides additional evidence that the reduction in mortality observed was due mostly to reduction in the 60‐ to 79‐year‐old patient subgroup (high‐certainty evidence; RR 0.86, 95% CI 0.79 to 0.95). Although cardiovascular mortality and morbidity was significantly reduced in both subgroups 60 to 79 years old (moderate‐certainty evidence; RR 0.71, 95% CI 0.65 to 0.77) and 80 years or older (moderate‐certainty evidence; RR 0.75, 95% CI 0.65 to 0.87), the magnitude of absolute risk reduction was probably higher among 60‐ to 79‐year‐old patients (3.8% vs 2.9%). The reduction in cardiovascular mortality and morbidity was primarily due to a reduction in cerebrovascular mortality and morbidity.
Authors' conclusions
Treating healthy adults 60 years or older with moderate to severe systolic and/or diastolic hypertension with antihypertensive drug therapy reduced all‐cause mortality, cardiovascular mortality and morbidity, cerebrovascular mortality and morbidity, and coronary heart disease mortality and morbidity. Most evidence of benefit pertains to a primary prevention population using a thiazide as first‐line treatment.
Keywords: Aged; Aged, 80 and over; Humans; Middle Aged; Antihypertensive Agents; Antihypertensive Agents/therapeutic use; Hypertension; Hypertension/drug therapy; Coronary Disease; Coronary Disease/prevention & control; Randomized Controlled Trials as Topic; Stroke; Stroke/prevention & control
Plain language summary
Pharmacotherapy for hypertension in adults 60 years or older
Review question
This is the second update of this review, first published in 1998 and first updated in 2009. We wanted to study the benefits and harms of using blood pressure‐lowering drugs in adults 60 years or older with raised blood pressure.
Search date
We searched the available medical literature to find all trials that compared drug treatment versus placebo or no treatment to examine this question. Data included in this review are up‐to‐date as of November 2017.
Background
High blood pressure, which is common among elderly people 60 years or older, increases the risk of heart attack and stroke.
Study characteristics
We found 16 studies that randomly assigned 26,795 patients 60 years or older with high blood pressure to antihypertensive drug therapy or to placebo or untreated control for a mean duration of 4.5 years.
Key results
Blood pressure‐lowering drug therapy in people with hypertension 60 years and older reduced death, strokes, and heart attacks. Benefit was similar if both upper and lower blood pressure numbers were elevated and if only the upper number was elevated. First‐line treatment used in most studies was a thiazide. More patients withdrew from the studies owing to side effects of these drugs. The magnitude of benefit in cardiovascular mortality and morbidity observed was probably greater among 60‐ to 79‐year‐old patients than in very elderly patients 80 years or older.
Conclusions
Blood pressure‐lowering drug treatment for healthy persons (60 years or older) with raised blood pressure reduces death, heart attacks, and strokes.
Quality of evidence
Review authors graded the quality of evidence as high for reduction in death and as moderate for reduction in stroke and heart attacks.
Summary of findings
Summary of findings for the main comparison. Antihypertensive drug compared to placebo or no treatment in adults 60 years or older.
| Antihypertensive drug therapy compared to placebo or no treatment in adults 60 years or older | ||||||
| Patient or population: adults 60 years or older with primary hypertension Setting: outpatient Intervention: antihypertensive drug therapy Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) |
Relative effect
(95% CI) Fixed‐effect model |
No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control |
Risk with antihypertensive drug therapy |
|||||
| Total mortality Mean duration of 3.8 years |
110 per 1000 | 100 per 1000 (93 to 106) |
RR 0.91 (0.85 to 0.97) | 25,932 (13 studies) | ⊕⊕⊕⊕ HIGH | ARR = 1% NNTB = 100 |
| Cardiovascular mortality and morbidity Mean duration of 3.7 years |
136 per 1000 | 98 per 1000 (92 to 104) |
RR 0.72 (0.68 to 0.77) | 26,747 (15 studies) | ⊕⊕⊕⊝ MODERATEa | ARR = 3.8% NNTB = 27 |
| Cerebrovascular mortality and morbidity Mean duration of 3.7 years |
52 per 1000 | 34 per 1000 (31 to 39) |
RR 0.66 (0.59 to 0.74) | 26,042 (13 studies) | ⊕⊕⊕⊝ MODERATEa | ARR = 1.8% NNTB = 56 |
| Coronary heart disease mortality and morbidity Mean duration of 2.9 years |
48 per 1000 | 37 per 1000 (33 to 42) |
RR 0.78 (0.69 to 0.88) | 24,559 (11 studies) | ⊕⊕⊕⊝ MODERATEa | ARR = 1.1% NNTB = 91 |
| Withdrawals due to adverse effects Mean duration of 4.6 years |
54 per 1000 | 157 per 1000 (138 to 178) |
RR 2.91 (2.56 to 3.30) | 11,310 (4 studies) | ⊕⊕⊝⊝ LOWb,c | ARI = 10.3% NNTH = 10 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ARI: absolute risk increase; ARR: absolute risk reduction; CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat for an additional harmful outcome; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded due to study limitations (incomplete outcome reporting and selective outcome reporting).
bDowngraded due to high risk of selective reporting bias, as only 4 out of 16 included RCTs reported this outcome.
cDowngraded due to inconsistency (I² > 50%).
Background
Description of the condition
Blood pressure increases with age, and the rate of rise is greater over the age of 60. As a result, the number of people with elevated blood pressure (known as 'hypertension') increases with age. Systolic blood pressure is more strongly associated with cardiovascular disease than is diastolic blood pressure, particularly in older people. Isolated systolic hypertension occurs more commonly in older people. Older people also accumulate higher rates of other risk factors for cardiovascular disease such as obesity, left ventricular hypertrophy, sedentary lifestyle, hyperlipidaemia, and diabetes.
Hypertension is a major risk factor for cardiovascular disease in older adults. Hypertension is present in 69% of patients with a first myocardial infarction; in 77% of those with a first stroke; in 74% of those with congestive heart failure; and in 60% of those with peripheral arterial disease (Aronow 2015).
Uncontrolled high blood pressure can lead to heart attack, stroke, aneurysm (life‐threatening if ruptured), heart failure, kidney damage, and vision loss (due to thickened, damaged, or torn blood vessels in the eye).
Description of the intervention
Changing lifestyle ‐ eating a healthy diet with less salt, exercising regularly, quitting smoking, limiting alcohol intake, and maintaining a healthy weight ‐ can help to control high blood pressure. When these lifestyle changes are not enough, treatment with antihypertensive drugs is recommended. Practitioners use several classes of antihypertensive drugs such as diuretics, angiotensin‐converting enzyme (ACE) inhibitors, angiotensin‐receptor blockers (ARBs), beta blockers, and calcium channel blockers to lower blood pressure. They also use other medications to treat high blood pressure, including alpha blockers, alpha‐beta blockers, centrally acting drugs, vasodilators, and aldosterone antagonists.
How the intervention might work
Following are the mechanisms of action of the most commonly used antihypertensive drug classes.
Thiazide and thiazide‐like diuretics lower blood pressure over the long term through a mechanism of action that is not fully understood (Zhu 2005). After long‐term use, thiazides lower peripheral resistance. The mechanism of these effects is uncertain, as it may involve effects on 'whole body', renal autoregulation, or direct vasodilator actions (Hughes 2004). Thiazides act on the kidney to inhibit reabsorption of sodium (Na+) and chloride (Cl‐) ions from the distal convoluted tubules in the kidneys by blocking the thiazide‐sensitive Na+‐Cl‐ symporter (Duarte 2010).
Beta blockers are competitive antagonists that block the receptor sites for epinephrine (adrenaline) and norepinephrine on adrenergic beta receptors. Some block activation of all types of beta‐adrenergic receptors (β1, β2, and β3), and others are selective for one of the three types of beta receptors (Frishman 2005).
ACE inhibitors block the conversion of angiotensin I (AI) to angiotensin II (AII) and thus decrease the actions of angiotensin II. The end result consists of lowered arteriolar resistance and increased venous capacity; decreased cardiac output, cardiac index, stroke work, and volume; lowered resistance in blood vessels of the kidneys; and increased excretion of sodium in the urine. Renin and AI are increased in concentration in the blood as a result of negative feedback on conversion of AI to AII. Levels of AII and aldosterone are decreased. Bradykinin is increased because ACE is responsible for inactivation of bradykinin.
Angiotensin‐receptor blockers (ARBs) block the activation of angiotensin II AT1 receptors. Blockage of AT1 receptors directly causes vasodilation, reduces secretion of vasopressin, and reduces production and secretion of aldosterone.
Calcium channel blockers block the calcium channel and inhibit calcium ion influx into vascular smooth muscle and myocardial cells. They reduce blood pressure through various mechanisms including vasodilation, reduction in the force of contraction of the heart, slowing of the heartbeat, and direct reduction of aldosterone production.
Alpha1‐adrenergic receptor blockers inhibit the binding of norepinephrine (noradrenaline) to α1 receptors on vascular smooth muscle cells. The primary effect of this inhibition is vasodilation, which decreases peripheral vascular resistance, leading to decreased blood pressure.
Central sympatholytic drugs reduce blood pressure mainly by stimulating central α2‐adrenergic receptors in the brainstem centres, thereby reducing sympathetic nerve activity and neuronal release of norepinephrine to the heart and the peripheral circulation.
Vasodilators act directly on the smooth muscle of arteries to relax their walls so blood can move more easily through them.
Why it is important to do this review
Most of the early trials evaluating antihypertensive drug therapy were conducted in lower‐risk people younger than 60 years. The first definitive clinical trial evidence supporting blood pressure‐lowering treatment was produced in the mid‐1980s. Before that time, policy makers and clinicians were reluctant to recommend treatment, particularly for the elderly; some regarded systolic hypertension as a natural feature of aging, and others feared excessive harm from blood pressure lowering in this age group.
When all drug therapies are included in one review, the underlying assumption is that the benefits of lowering blood pressure are independent of the mechanism by which this is achieved. This assumption has not been proven, and it is likely that different drugs lowering blood pressure by different mechanisms will have effects that are independent of the blood pressure‐lowering effect. A drug that lowers blood pressure could have pharmacological and physiological actions independent of blood pressure lowering, and these other actions (both known and unknown) could enhance or negate effects on health outcomes associated with the decrease in blood pressure. This possibility is supported by an analysis suggesting that blood pressure lowering explains only about 50% of the treatment effect in antihypertensive trials (Boissel 2005).
It is important to know and compare the benefits and harms of antihypertensive drug therapy in different age groups of patients with hypertension ‐ 18 to 59 years old; and 60 years or older. Our aim is to document the best available evidence for adult patients 60 years or older. A meta‐analysis in patients 80 years or older from earlier trials by Gueyffier 1999 showed a trend towards increased mortality. Therefore we planned subgroup analyses of patients 60 to 79 years old, and 80 year or older. This is the second substantive update of this review. It was originally published as Mulrow 1998, and the first update was published as Musini 2009.
A Cochrane Review titled "Pharmacotherapy for hypertension in adults age 18 to 59 years old" was published recently (Musini 2017). A Cochrane Review titled "First line drugs for hypertension" has recently been updated (Wright 2018).
Objectives
Primary objective
To quantify the effects of antihypertensive drug treatment as compared with placebo or no treatment on all‐cause mortality in people 60 years and older with hypertension defined as blood pressure greater than 140/90 mmHg.
Secondary objectives
To quantify the effects of antihypertensive drug treatment as compared with placebo or no treatment on cardiovascular‐specific morbidity and mortality in people 60 years and older with hypertension defined as blood pressure greater than 140/90 mmHg.
To quantify the rate of withdrawal due to adverse effects of antihypertensive drug treatment as compared with placebo or no treatment in people 60 years and older with hypertension defined as blood pressure greater than 140/90 mmHg.
Methods
Criteria for considering studies for this review
Types of studies
We included only parallel‐group randomised controlled trials (RCTs) of at least one year's duration. Trials must have included a control group that received a placebo or received no antihypertensive treatment. We excluded trials that compared two specific antihypertensive treatments without a placebo or an untreated control.
We excluded trials using other than randomised allocation methods such as alternate allocation, week of presentation, or retrospective controls.
Types of participants
Trials must include only people 60 years of age or older or must separately report outcomes for people 60 or older. Researchers must measure blood pressure using the proper technique at least two times with the participant resting for at least five minutes. Participants must have a systolic blood pressure of at least 140 mmHg and/or a diastolic blood pressure of at least 90 mmHg at baseline.
Types of interventions
Acceptable antihypertensive drug treatments include angiotensin‐converting enzyme inhibitors, angiotensin‐receptor antagonists, beta‐adrenergic blockers, combined alpha and beta blockers, calcium channel blockers, diuretics, alpha‐adrenergic blockers, central sympatholytics, direct vasodilators, and peripheral adrenergic antagonists. Investigators could have administered drugs alone or in combination or in fixed or stepped up regimens.
Types of outcome measures
Primary outcomes
All‐cause mortality
Secondary outcomes
-
Cardiovascular morbidity and mortality* including total stroke, total coronary heart disease, hospitalisation or death from congestive heart failure, and other significant vascular deaths such as ruptured aneurysm
This does not include angina, transient ischaemic attacks, surgical or other procedures, or accelerated hypertension
Cerebrovascular morbidity and mortality including fatal and non‐fatal stroke
Coronary heart disease (CHD) morbidity and mortality including fatal and non‐fatal myocardial infarctions and sudden or rapid cardiac death
Withdrawal due to adverse effects
The original review reported data using different definitions of cardiovascular mortality and morbidity as defined in each individual included study (Mulrow 1994). Refer to Characteristics of included studies.
Please note that for this second substantive update, we have modified the definition of cardiovascular mortality and morbidity to exclude transient ischaemic attacks (TIAs) as much as possible (because we judge TIA to be a subjective and less serious outcome). However, when it was not possible to exclude TIA from the total cardiovascular outcome as reported in Mulrow 1998, we report overall effect size in two ways ‐ by including these studies and by deselecting them. We have standardised this update in terms of outcomes and trial identification for consistency with the two complementary reviews (Musini 2017; Wright 2018).
Search methods for identification of studies
Electronic searches
The Cochrane Hypertension Information Specialist conducted systematic searches of the following databases for RCTs without language, publication year, or publication status restrictions.
Cochrane Hypertension Specialised Register via the Cochrane Register of Studies (CRS Web; searched 24 November 2017).
Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies (CRS Web; searched 24 November 2017).
MEDLINE Ovid (from 1946 onwards), MEDLINE Ovid Epub Ahead of Print, and MEDLINE Ovid In‐Process & Other Non‐Indexed Citations (searched 24 November 2017).
Embase Ovid (searched 24 November 2017).
ClinicalTrials.gov (www.clinicaltrials.gov) (searched 24 November 2017).
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch) (searched 24 November 2017).
The Cochrane Hypertension Information Specialist modelled subject strategies for databases using the search strategy designed for MEDLINE. When appropriate, we combined these strategies with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs (as described in the Cochrane Handbook for Systematic Reviews of Interventions, Box 6.4.b) (Higgins 2011a). We translated the MEDLINE search strategy for use with other databases using the appropriate controlled vocabulary, as applicable (Appendix 1). We applied no language restrictions.
The databases searched in the original review and in the first update are presented in Appendix 2.
Searching other resources
We used previously published meta‐analyses on treatment of hypertension to identify references to trials (Davidson 1987; Staessen 1988; Collins 1990; Staessen 1990a; Staessen 1990b; Leonetti 1992; Thijs 1992; Celis 1993; MacMahon 1993; Insua 1994; Thijs 1994; Pearce 1995; Gueyffier 1996; Psaty 1997; Gueyffier 1999; Quan 1999; Wright JM 1999; BPLTTC 2000; Nikolaus 2000; Psaty 2003; Turnbull 2003; Kang 2004; BBLTTC 2005; Musini 2008; Law 2009; Goeres 2014; Thomopoulos 2014; Sundstrom 2015; Zanchetti 2015; Parsons 2016; Tan 2016; Thomopoulos 2016; Kızılırmak 2017; Wiysonge 2017).
We contacted experts in the field to identify any other trials that we may have missed in our search. We checked the reference lists of included studies and contacted relevant individuals for information about unpublished or ongoing studies. The first version of this review did not provide a study flow diagram. However, the review authors listed 25 studies as excluded with reasons.
Data collection and analysis
Selection of studies
We rejected articles on the initial screening if we could determine from the title or the abstract that the article was not a report of a randomised controlled trial, or that there was no possibility that the trial would fit the requirements of this review. Of the articles selected for further review, two review authors (VM and AT) independently assessed whether they would be included or excluded.
Data extraction and management
We abstracted data using a standard data abstraction form; dual abstraction of data from the original reports of trial results by two independent reviewers (VM and AT); and disagreements resolved by discussion. Published results of these meta‐analyses as well as data from additional trials included in the updated review were compared by two review authors (VM and AT). Any disagreements were resolved by consensus (JMW and KB).
The actual endpoints represented by each outcome measure for each study are listed under the "Outcomes" heading of the Characteristics of included studies table. Within each study, the definition of endpoints for each outcome measure is identical between treatment and control groups. The individual non‐fatal outcomes included in the composite endpoint were included as counted by the trialists of each study. Many trials did not report on how events were counted after patients were censored. Refer to personal communication with the author of HYVET 2008 in the risk of bias table to find out how events were counted in that trial.
In this update, we obtained data for Kuramoto 1981,Sprackling 1981,STOP 1991, and VA‐II 1970 from the original Mulrow 1998 and Mulrow 2000 reviews, and the cardiovascular mortality and morbidity outcome definition in these studies did not include transient ischaemic attack. However, data for ATTMH 1981 and Coope 1986 studies included TIA in total cardiovascular outcomes in the original Mulrow 1998 and Mulrow 2000. Therefore in this review, we report overall results for total cardiovascular outcome including these two studies, as well as excluding them from the overall analysis. We excluded TIA data from total cardiovascular mortality and morbidity for several additional studies (SHEP‐P 1989; SHEP 1991; Syst‐Eur 1991 ).
Data for the 60‐ to 64‐year‐old patient subgroup from MRC‐TMH 1985 were obtained by personal communication with Francois Gueyffier from the INDANA Group (Gueyffier 1999). The cardiovascular mortality and morbidity outcome in this study does not include heart failure.
Trial characteristics are detailed in the table Characteristics of included studies. Trials that were excluded are listed in the Characteristics of excluded studies table, and the reasons for exclusion are provided.
Assessment of risk of bias in included studies
Two review authors (VM and AT) independently assessed risk of bias of each included trial; a third review author (JMW) adjudicated any disagreements. We assessed risk of bias according to Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We assessed seven domains: randomisation and allocation concealment to assess selection bias; blinding of participants and physician to assess performance bias; blinding of the outcome assessor to assess detection bias; incomplete outcome reporting to assess attrition bias; and selective reporting of outcomes to assess selective reporting bias. We added a category ‐ industry‐sponsored bias ‐ to assess whether the study was funded by the manufacturer and conflict of interest was present, which we assessed as high risk of bias, since researchers may overestimate treatment effect (Lundh 2017).
'Summary of findings' table
We used GRADEpro GDT software to prepare the 'Summary of findings' table (GRADEpro GDT). We decided to include all clinically relevant primary and secondary outcomes such as total mortality, total cardiovascular events, total stroke, total coronary heart disease, and withdrawal due to adverse events.
We considered five factors in grading the overall quality of evidence: limitations in study design and implementation, indirectness of evidence, unexplained heterogeneity or inconsistency of results, imprecision in results, and high probability of publication bias. This approach specifies four levels of quality: high‐, moderate‐, low‐, and very low‐quality evidence. The highest quality rating applies to randomised trial evidence. We downgraded the quality rating by one level for each factor, up to a maximum of three levels for all factors. If we noted severe problems for any one factor (when assessing limitations in study design and implementation, in concealment of allocation, loss of blinding, or attrition over 50% of participants during follow‐up), randomised trial evidence may fall by two levels due to that factor alone.
Measures of treatment effect
We used Review Manager 5.3 for data synthesis and analyses (RevMan 2014). We based quantitative analyses of outcomes on intention‐to‐treat results. We used risk ratios (RRs) with 95% confidence intervals (CIs) to combine outcomes across trials using the fixed‐effect model. If there was a statistically significant difference in any outcome measure, we presented an absolute risk reduction (ARR), along with the number needed to treat for an additional beneficial (NNTB) or harmful (NNTH) outcome, in the 'Summary of findings' table. This estimate, with 95% confidence intervals (CI), is considered the best point estimate of the average benefit.
Unit of analysis issues
We included only randomised parallel‐group studies in the review. Randomised patients who started treatment in the control group or stopped treatment in the treatment group were still analysed in the treatment group to which they were originally randomised. For all outcome measures reported, we used data from each trial at the end of the follow‐up period mentioned in each trial, which varied from one to six years. Both pilot studies ‐ HYVET P 2003 and SHEP‐P 1989 ‐ had no overlap of participants with the main studies ‐ HYVET 2008 and SHEP 1991, respectively.
Dealing with missing data
When participants were lost to follow‐up, we used data as reported for participants who were followed until end of study in the analyses. Refer to how data were accounted for and included in each study under assessment of attrition bias in the Risk of bias in included studies.
When the primary trials did not report outcomes with exact definitions as listed above, we categorised data to minimise missing data while maintaining the intended study measures. For example, the Medical Research Council Trial of Treatment of Hypertension in Older Adults ‐ MRC‐O 1992 ‐ includes "deaths due to hypertension" in its definition of "cardiovascular events". The broad label "deaths due to hypertension" is not included in the standard definition for "cardiovascular morbidity and mortality" listed above. We included MRCOA's results in the cardiovascular morbidity and mortality outcome measure because "deaths due to hypertension" was congruous with the concept of cardiovascular morbidity and mortality. The alternative ‐ omitting MRCOA's data ‐ would result in a more reliable measure but at the expense of accuracy of the effect estimate. The number of differences in definitions was small and is unlikely to affect results. Supporting this assumption, previous meta‐analyses found homogeneity of risk reduction among outcome measures suggesting differences in outcome definition were unlikely causes of bias.
Similarly, despite the statement in EWPHBPE 1989 that "The intention‐to‐treat analysis was restricted to the cause and date of death because data on non‐fatal events in patients who dropped out from randomised treatment were not available", we still included data on cardiovascular and cerebrovascular and coronary heart disease mortality and morbidity as was previously done in the "First‐line drugs for hypertension" review (Wright 2018).
One of the trials first included in the 2009 update ‐ HYVET P 2003 ‐ was not conducted according to the standards of Good Clinical Practice Guidelines and did not collect data on serious adverse events, non‐fatal myocardial infarction (MI) or heart failure (personal communication with the author). However, data on cardiovascular mortality and morbidity were reported in the trial and are included in the meta‐analysis. The cardiovascular mortality and morbidity outcome in HYVET P 2003 includes fatal and non‐fatal stroke, fatal MI, other fatal ischaemic heart disease, sudden death, fatal congestive heart failure, fatal atherosclerosis, fatal pulmonary embolism, fatal hypertension, and fatal aortic aneurysm but does not include TIA.
Two trials ‐ ATTMH 1981 and Coope 1986 ‐ included TIA in total cardiovascular outcome, and we have reported overall effect size by including these two studies as well as by excluding them from the analysis.
Assessment of heterogeneity
We tested heterogeneity of treatment effect between trials using a standard Chi² statistic, and we used the I² statistic to estimate the amount of heterogeneity. We used the fixed‐effect model to obtain summary statistics of pooled trials in patients 60 years or older. In case heterogeneity was found to be significant, we planned to perform sensitivity analyses using the random‐effects model. Subgroup analyses were compared using the fixed‐effect model.
Assessment of reporting biases
Several RCTs in adults 18 years or older with hypertension met the minimum inclusion criteria. However they did not report data separately in patients 60 years or older. Table 2 lists these 15 studies.
1. RCTs meeting the minimum inclusion criteria but not providing data in patients ≥ 60 with hypertension.
| ACTIVE I 2011 | Randomised trial comparing irbesartan 300 mg/d or double‐blind placebo in patients 55 years or older for a mean follow‐up of 4.1 years. 52% of patients had hypertension at baseline. Data are not available for hypertensive patients 60 years or older |
| Barraclough 1973 | Single‐blind randomised placebo‐controlled trial in patients 45 to 69 years old for a mean follow‐up of 1.5 years. Data for 60‐ to 69‐year‐old patients are not available |
| Bull 2015 | Randomised double‐blind placebo‐controlled trial in adult patients 18 years or older with moderate or severe asymptomatic aortic stenosis. 32% of patients had hypertension at baseline. Participants were randomised to ramipril 10 mg daily or placebo for 1 year. Data are not reported separately in hypertensive subgroup of patients 60 years or older |
| DREAM 2006 | Double‐blind RCT in participants 30 years or older without cardiovascular disease but with impaired fasting glucose levels (after an 8‐hour fast) or impaired glucose tolerance. Participants were randomised to ramipril (up to 15 mg per day) or placebo (and rosiglitazone or placebo) and were followed for a median of 3 years. 43.7% of patients at baseline had a history of hypertension. Data are not reported for patients with hypertension who were 60 years or older |
| DUTCH ‐TIA 1993 | Randomised double‐blind trial comparing atenolol 50 mg daily to placebo in patients 65 years or older who had a TIA for a mean follow‐up period of 2.6 years. Data for those 60 and over with hypertension at baseline were not available |
| HOPE‐HYP 2000 | Double‐blind RCT in patients 55 years or older with previous coronary artery disease, cerebrovascular disease, or peripheral vascular disease or diabetes plus one additional risk factor. Participants randomised to ramipril 2.5 mg titrated up to 0 mg/d or placebo. Average follow‐up was 4.5 years. Data are not reported separately for people 60 years or older with hypertension |
| IPPPSH 1985 | Randomised trial in 40‐ to 64‐year‐old patients with hypertension randomised to oxprenolol or placebo for a mean follow‐up of 3.5 years. Data are not reported separately for participants 60 to 64 years of age |
| Materson 1993 | Randomised double‐blind placebo‐controlled study of male veterans 21 years or older with DBP of 95 to 109 mmHg. Participants randomised to placebo or to 1 of the 6 drugs ‐ HCTZ 12.5 to 50 mg/d; atenolol 25 to 100 mg/d; captopril 25 to 100 mg/d; clonidine 0.2 to 0.6 mg/d; sustained preparation of diltiazem 120 to 360 mg/d; or prazosin 4 to 20 mg/d ‐ for a period of 1 year. Morbidity and mortality outcomes not reported for different drug classes nor for patients 60 years or older |
| PATS 1995 | Randomised double‐blind placebo‐controlled trial conducted in Chinese patients with mean age 60 ± 8 years. Participants were randomised to indapamide 2.5 mg/d or placebo. Data are not reported separately for patients 60 years or older |
| TEST 1995 | Randomised double‐blind placebo‐controlled trial conducted in 720 Swedish patients > 40 years old, within 3 weeks of a stroke or transient ischaemic attack with a mean follow‐up period of 30 months. Data are not reported separately for patients 60 years or older |
| TOMHS 1995 | Four‐year double‐blind placebo‐controlled randomised trial in patients with mild hypertension (average blood pressure, 140/91 mmHg) aged 45 to 69 years. Participants randomised to receive nutritional‐hygienic intervention plus 1 of 6 treatments: (1) placebo; (2) diuretic (chlorthalidone); (3) beta blocker (acebutolol); (4) alpha 1 antagonist (doxazosin mesylate); (5) calcium antagonist (amlodipine maleate); or (6) angiotensin‐converting enzyme inhibitor (enalapril maleate). Morbidity and mortality events were not reported separately for the different drug treatments. Corresponding author was contacted, but data for 60‐ to 69‐year‐old patients were not provided |
| UKPDS 39 1998 | Randomised controlled open‐label trial conducted in newly diagnosed patients 25 to 65 years old with type 2 diabetes mellitus and hypertension. Participants were randomised to captopril or atenolol or placebo and were followed for 8.4 years. Data for patients 60 to 65 years old are not reported separately |
| VA Coop 1962 | Randomised double‐blind placebo‐controlled study of 1 year's duration in 759 hypertensive patients. The study recruited men less than 70 years old. This study did not report results separately for those 60 to 69 years old |
| VA‐I 1967 | Randomised double‐blind placebo‐controlled trial conducted in ambulatory patients in the USA with mean age 51 years. Age range not reported. Participants were randomised to hydrochlorothiazide 100 mg plus reserpine 0.2 mg plus hydralazine 75 mg or 150 mg or placebo. Mean follow‐up was 1.5 years. Data for patients 60 years or older are not reported separately |
| Wolf 1966 | Double‐blind placebo‐controlled trial conducted in ambulatory patients in the USA with mean age 50 years. Participants were randomised to reserpine 0.25 mg t.i.d., chlorothiazide 0.5 g b.i.d., or hydrochlorothiazide 25 mg q.i.d. plus guanethidine if needed or placebo. Mean follow‐up was 2 years. Data for patients 60 years or older are not reported separately |
DBP: diastolic blood pressure.
HCTZ: hydrochlorothiazide.
RCT: randomised controlled trial.
TIA: transient ischaemic attack.
We had planned to use a funnel plot to assess the possibility of publication bias for outcomes that were reported in 10 or more studies. A test for funnel plot asymmetry (small‐study effects) formally examines whether the association between estimated intervention effects and a measure of study size is greater than might be expected to occur by chance, one cause of which is publication bias.
Data synthesis
We used Review Manager 5.3 to perform data synthesis and analyses (RevMan 2014). We presented dichotomous outcomes as RRs with 95% CIs using a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
A meta‐analysis in patients 80 years or older from earlier trials ‐ Gueyffier 1999 and Bejan‐Angoulvant 2010 ‐ showed a trend towards increased mortality. Therefore, we planned analyses in subgroups of patients 60 to 79 years old and 80 years or older and assessed differences among subgroups using an interaction test. Furthermore, two randomised trials ‐ HYVET P 2003 and HYVET 2008 ‐ were specifically done in the 80 years or older group of patients and were included in the first update. A Cochrane Review on the specific age group of young adults (18 to 59 years) has been recently published (Musini 2017), and the Cochrane Review on "First line drugs for hypertension" in adult patients 18 years or over has been recently updated (Wright 2018).
When heterogeneity was estimated to be significant (I² > 50%), we attempted to identify trials that would contribute to heterogeneity and to explore their population characteristics, baseline blood pressure (BP), blinded or open‐label study design, use of antihypertensive drugs as fixed dose or stepped up therapy, or response to placebo that would possibly explain the reason for heterogeneity.
Sensitivity analysis
To test for robustness of results, we conducted several sensitivity analyses. We analysed data using random‐effects models. Other sensitivity analyses included restricting meta‐analysis to trials that were blinded (participant and/or provider) and to trials that contained a placebo control only. We also analysed results when removing trials that had enrolled populations restricted to persons who had previously suffered a stroke. We analysed results of trials restricted to persons with isolated systolic hypertension both as a separate group and combined with trials also assessing persons with both systolic and diastolic hypertension.
Results
Description of studies
See Figure 1 for the PRISMA flow diagram.
1.
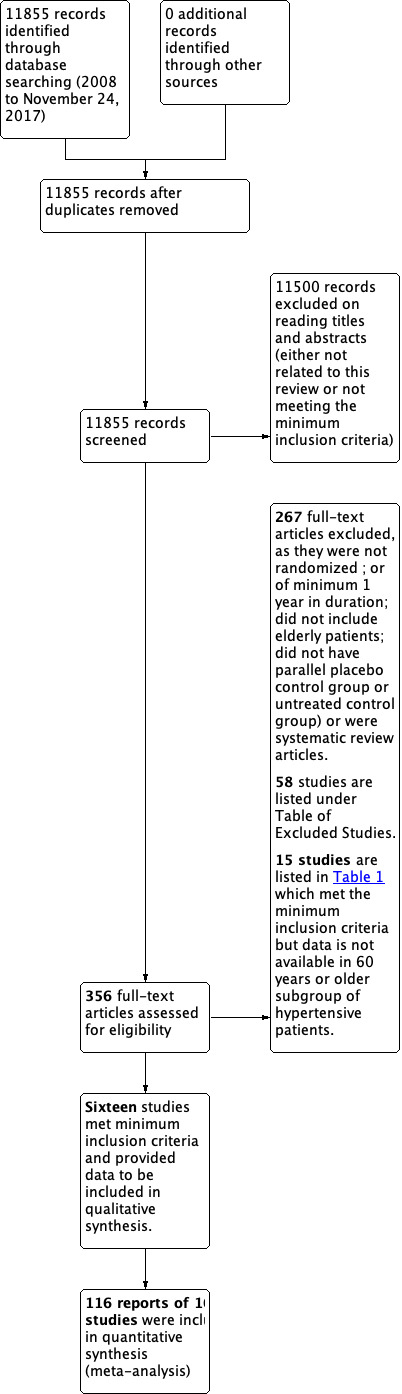
Study flow diagram.
Results of the search
The updated search strategy until November 2017 resulted in 11,855 new citations. Titles and abstracts were screened, and 11,500 were excluded. The remaining 356 full‐text articles were retrieved. None of them met the minimum inclusion criteria mostly because they did not have a placebo or no treatment comparison group; were not one year in duration; or did not have a true no treatment control group.
The Mulrow 1998 original review included 15 studies. In the first update, 13 of the original studies were included and 2 studies ‐ CASTEL 1994 and HDFP 1984 ‐ were excluded, as explained in Characteristics of excluded studies. In addition to the 13 studies in the original review, the first update included 2 new studies ‐ HYVET 2008 and HYVET P 2003 ‐ which exclusively studied patients 80 years or older with hypertension.
For this update, we were able to add the MRC‐TMH 1985 study because data on clinical outcomes for participants 60 to 64 years old were kindly provided by Francois Gueyffier from the INDANA Group (Gueyffier 1999).
A total of 116 reports of 16 studies met the inclusion criteria and are included in this second update. Table 2 lists 15 additional studies that meet the minimum inclusion criteria but do not provide aggregate data for the 60 years or older subgroup of participants. A future update may consider adapting the protocol and requesting this data for individual patient data (IPD) analysis.
Included studies
Sixteen trials (N = 26,795) in healthy ambulatory adults 60 years or older with moderate to severe systolic and/or diastolic hypertension (average 182/95 mmHg) met the inclusion criteria. Most of these trials evaluated first‐line thiazide diuretic therapy for a mean treatment duration of 3.8 years (Table 3).
2. Details of studies meeting the inclusion criteria.
| Number |
Study (N = randomised 60 years or older) Blinding |
Baseline SBP/DBP |
Mean age (range), years |
Control group | Antihypertensive drug treatment used | Outcomes reported |
| 1 |
ATTMH 1981 (N = 582) Double‐blind (identified as ANBP 1981 in original Mulrow review) |
165/101 | 64 (60 to 69) |
Placebo | First‐line ‐ chlorothiazide 500 mg, second‐line ‐ dose increased to 1000 mg, or addition of methyldopa, propranolol, or pindolol. Third‐line drugs added were hydralazine or clonidine | Mortality Cardiovascular mortality and morbidity |
| 2 |
Carter 1970 (N = 48) Open‐label |
Not reported | 69 (60 to 79) |
Observation (untreated control group) |
Bendrofluazide (93%), methyldopa, and debrisoquine | Mortality |
| 3 |
Coope 1986a (N = 884) Open‐label (identified as HEP 1986 in original Mulrow review) |
196/99 | 69 (60 to 79) |
Observation | First‐line ‐ atenolol 100 mg daily; second‐line ‐ bendrofluazide 5 mg daily; third‐line ‐ methyldopa 500 mg daily; fourth‐line ‐ any recognised therapy In the last 2 years of the trial, several participants were treated with nifedipine retard 20 mg morning and night |
Mortality Cardiovascular mortality and morbidity Cerebrovascular mortality and morbidity CHD mortality and morbidity |
| 4 |
EWPHBPE 1989 (N = 840) Double‐blind |
183/101 | 72 (60 to 97) |
Placebo | First‐line ‐ hydrochlorothiazide 25 to 50 mg + triamterene 50 to 100 mg daily; second‐line ‐ methyldopa 250 to 2000 mg daily | Mortality Cardiovascular mortality and morbidity Cerebrovascular mortality and morbidity CHD mortality and morbidity |
| 5 |
HSCSG 1974 (N = 200) Double‐blind (identified as HTN‐COOP 1976 in original Mulrow review) |
167/100 | Not reported (60 to 75) |
Placebo | Deserpidine 1 mg plus methyclothiazide 10 mg | Cardiovascular mortality and morbidity Cerebrovascular mortality and morbidity CHD mortality and morbidity |
| 6 |
HYVET 2008 (N = 3845) Double‐blind 80 years or older |
173/91 | 84 (80 to 105) |
Placebo | First‐line‐ indapamide 1.5 mg daily; second‐line ‐ perindopril 2 mg daily; third‐line ‐ perindopril 4 mg daily | Mortality Cardiovascular mortality and morbidity Cerebrovascular mortality and morbidity CHD mortality and morbidity |
| 7 |
HYVET P 2003 (N = 1283) Open‐label 80 years or older |
182/100 | 84 (80 to 96) |
Observation | First‐line ‐ diuretic (usually bendrofluazide 2.5 mg), an ACE inhibitor (usually lisinopril 2.5 mg), or no treatment; second‐line ‐ involved doubling the dose of the first drug; third‐line ‐ involved adding diltiazem slow‐release 120 mg daily; fourth‐line ‐ involved adding diltiazem slow‐release 240 mg daily | Mortality Cardiovascular mortality and morbidity Cerebrovascular mortality and morbidity CHD mortality and morbidity |
| 8 |
Kuramoto 1981 (N = 91) Double‐blind |
169/86 | 76 (> 60) |
Placebo | First‐line ‐ trichlormethiazide 1 to 4 mg; 80% monotherapy; second‐line ‐ reserpine (0.3 mg), methyldopa (125 to 500 mg), and hydralazine (50 to 100 mg) added |
Mortality Cardiovascular mortality and morbidity Cerebrovascular mortality and morbidity CHD mortality and morbidity |
| 9 |
MRC‐O 1992 (N = 4396) Single‐blind |
184/91 | 70 (60 to 74) |
Placebo | Diuretic arm: First‐line ‐ hydrochlorothiazide 25 mg or 50 mg + amiloride 2.5 mg or 5 mg daily; second‐line ‐ atenolol 50 mg daily; third‐line ‐ nifedipine up to 20 mg daily; fourth‐line ‐ other drugs Beta blocker arm: First‐line ‐ atenolol 50 mg daily; second‐line ‐ hydrochlorothiazide 25 mg or 50 mg + amiloride 2.5 mg or 5 mg daily; third‐line ‐ nifedipine up to 20 mg daily; fourth‐line ‐ other drugs |
Mortality Cardiovascular mortality and morbidity Cerebrovascular mortality and morbidity CHD mortality and morbidity |
| 10 |
MRC‐TMH 1985 60‐ to 64‐year‐old subgroup (N = 2813) Single‐blind |
Not reported in this subgroup | Not reported (60 to 74) |
Placebo | Bendrofluazide 10 mg daily, propranolol 80 to 240 mg daily; methyldopa added if required | Mortality Cardiovascular mortality and morbidity Cerebrovascular mortality and morbidity CHD mortality and morbidity |
| 11 |
SHEP 1991 (N = 4736) Double‐blind |
170/77 | 72 (60 or older) |
Placebo | First‐line ‐ chlorthalidone 12.5 or 25 mg daily; second‐line ‐ atenolol 25 or 50 mg or reserpine 0.05 or 0.10 mg daily | Mortality Cardiovascular mortality and morbidity Cerebrovascular mortality and morbidity CHD mortality and morbidity |
| 12 |
SHEP‐P 1989 (N = 551) Double‐blind |
172/75 | 72 (60 or older) |
Placebo | Fisrt‐line ‐ chlorthalidone 25 to 50 mg daily (87%); second‐line ‐ hydralazine 25 mg twice daily, reserpine 0.05 mg twice daily, or metoprolol 50 mg twice daily (13%) | Mortality Cardiovascular mortality and morbidity Cerebrovascular mortality and morbidity CHD mortality and morbidity |
| 13 |
Sprackling 1981a (N = 123) Open‐label |
199/106 | 81 (60 or older) |
Observation | Methyldopa 250 mg twice daily | Mortality Cardiovascular mortality and morbidity |
| 14 |
STOP 1991a (N = 1627) Double‐blind |
195/102 | 76 (70 to 84) |
Placebo | First‐line ‐ atenolol 50 mg daily or hydrochlorothiazide 25 mg + amiloride 2.5 mg daily, or metoprolol 100 mg daily, or pindolol 5 mg daily; second‐line ‐ patients on a beta blocker received diuretics, and those on diuretics received a beta blocker | Mortality Cardiovascular mortality and morbidity Cerebrovascular mortality and morbidity CHD mortality and morbidity |
| 15 |
Syst‐Eur 1991 (N = 4695) Double‐blind |
174/86 | 70 (60 or older) |
Placebo | First‐line ‐ nitrendipine 10 mg daily, 10 mg BID, 20 mg BID; second‐line ‐ enalapril 5 mg, 10 mg, 20 mg daily in evening and/or hydrochlorothiazide 12.5 to 25 mg/d in morning |
Mortality Cardiovascular mortality and morbidity Cerebrovascular mortality and morbidity CHD mortality and morbidity |
| 16 |
VA‐II 1970 (N = 81) Double‐blind |
176/103 | Not reported (60 to 75) |
Placebo | First‐line ‐ HCTZ 100 mg plus reserpine 0.2 mg; second‐line ‐ hydralazine 75 to 150 mg | Cardiovascular mortality and morbidity Cerebrovascular mortality and morbidity CHD mortality and morbidity |
| A total of 16 trials | N = 26,795 | SBP ranged from 165 to199 mmHg and DBP from 75 to 106 mmHg |
Mean age 64 to 84 years; age ranged from 60 to 105 years |
12 placebo‐controlled studies; 4 studies with observation as control group |
Drugs used included thiazides, beta blockers, calcium channel blockers, ACE inhibitors, methyldopa, reserpine, hydralazine, and clonidine |
aStudies with baseline SBP > 190 mmHg.
ACE: angiotensin‐converting enzyme.
CHD: coronary heart disease.
DBP: diastolic blood pressure.
SBP: systolic blood pressure.
For most participants included in this review, mean age ranged from 64 to 84 years. Two trials did not report mean age (MRC‐TMH 1985; VA‐II 1970). Four trials originally included both younger and older persons (ATTMH 1981; Carter 1970; HSCSG 1974; MRC‐TMH 1985). Only data on those older than 60 are reported from these trials. The average age across trials was 73.8 years. Seven trials evaluated participants over 60 years of age (EWPHBPE 1989; Kuramoto 1981; MRC‐O 1992; SHEP 1991; SHEP‐P 1989; Sprackling 1981; Syst‐Eur 1991). The Swedish Trial in Old Patients with Hypertension specifically evaluated people over age 70 (STOP 1991). The HYVET P 2003 and HYVET 2008 trials studied patients 80 years or older. In all, 14,663 participants (54.7%) were female.
Most trials were conducted in Western industrialised countries ‐ USA (20%), UK (32%), European multi‐site trials (40%), Sweden (5%), Australia (2%), and Japan (< 1%) ‐ and evaluated first‐line diuretics (ATTMH 1981;Carter 1970; EWPHBPE 1989; HYVET 2008; HYVET P 2003; Kuramoto 1981;MRC‐O 1992;MRC‐TMH 1985; SHEP 1991; SHEP‐P 1989; VA‐II 1970). HYVET P 2003 recruited patients from Bulgaria (88%), Spain (3%), Romania (3%), UK (2.5%), and Poland (1.5%), and from other countries in smaller numbers (Finland, Lithuania, Ireland, Greece, and Serbia). HYVET 2008 recruited patients from Western Europe (2.2%), Eastern Europe (55.8%), China (39.6%), Australasia (0.5%), and Tunisia (1.9%).
Five trials evaluated beta blocker therapies (Coope 1986; MRC‐O 1992; MRC‐TMH 1985; STOP 1991). HYVET P 2003 evaluated thiazides as well as ACE inhibitors versus placebo. No randomised controlled trial comparing alpha‐adrenergic blockers or angiotensin‐receptor blockers to placebo or untreated controls was identified.
The four trials based in the USA reported ethnicity as African American: SHEP 1991 (14%); SHEP‐P 1989 (18% non‐white); VA‐II 1970 (41%); and HSCSG 1974 (78%). All participants in ATTMH 1981 and STOP 1991 were white. Ten trials did not report ethnicity (Carter 1970; Coope 1986; EWPHBPE 1989; HYVET P 2003; HYVET 2008; Kuramoto 1981; MRC‐O 1992; MRC‐TMH 1985; Sprackling 1981Syst‐Eur 1991).
Study populations predominantly consisted of ambulatory patients recruited from the community or from primary care facilities. A small proportion (6%) of patients were recruited from hospitals or homes for the aged. Studies did not consistently report data on pre‐existing conditions among participants; available data follow. Two studies were limited to stroke survivors (Carter 1970; HSCSG 1974). Six other trials reported the baseline prevalence of stroke. The sample size‐based weighted average prevalence across these six trials was 3.6%:SHEP‐P 1989 (1%), SHEP 1991 (1.4%), Syst‐Eur 1991 (3.5%), Sprackling 1981 (11.3%), HYVET P 2003 (4.5%), and HYVET 2008 (6.8%). Six trials reported the baseline prevalence of myocardial infarction. Average prevalence across trials was 2.3%: ATTMH 1981 (0.5%), Syst‐Eur 1991 (1.2%), SHEP‐P 1989 (4%), SHEP 1991 (4.9%), HYVET P 2003 (3.0%), and HYVET 2008 (3.1%). Two studies excluded patients with diabetes (ATTMH 1981;MRC‐O 1992), while three other trials reported the baseline prevalence. Average prevalence across trials was 9.2%: HYVET 2008 (6.8%), SHEP 1991 (10.1%), and HSCSG 1974 (36%). Two trials reported the baseline prevalence of hyperlipidaemia: HSCSG 1974 (22%) and ATTMH 1981 (62.2%). Ten trials reported the baseline prevalence of smoking. Average prevalence across trials was 12.1%: HYVET P 2003 (4.2%), HYVET 2008 (6.6%), Syst‐Eur 1991 (7.3%), SHEP‐P 1989 (11%), SHEP 1991 (12.7%), EWPHBPE 1989 (16.4%), ATTMH 1981 (17.5%), MRC‐O 1992 (17.5%), Coope 1986 (24%), and HSCSG 1974 (60%). Only HSCSG 1974 reported data on prevalence of obesity (29%).
Entry diastolic blood pressure criteria also have varied somewhat from trial to trial. However, trials in older persons have not routinely included patients with higher diastolic blood pressure than trials in younger persons. All trials except Carter 1970 and a subgroup in MRC‐TMH 1985 reported mean systolic blood pressure (SBP) and diastolic blood pressure (DBP) at baseline. SHEP‐P 1989, SHEP 1991, and Syst‐Eur 1991 restricted recruitment to persons with isolated systolic hypertension, defined as SBP 160 to 219 mmHg and DBP < 90 mmHg (SHEP 1991; SHEP‐P 1989), or as DBP < 95 mmHg (Syst‐Eur 1991).
Mean blood pressure at entry in the three isolated systolic hypertension trials was 172/81 mmHg. Two studies recruited persons with isolated systolic hypertension, diastolic hypertension, or systo‐diastolic hypertension (Carter 1970; Coope 1986). Kuramoto 1981 and MRC‐O 1992 recruited patients with isolated systolic hypertension or systo‐diastolic hypertension. HYVET P 2003 recruited patients with systolic and/or diastolic hypertension (SBP > 140 mmHg and DBP 90 to 109 mmHg). HYVET 2008 recruited patients with persistent hypertension defined as SBP of 160 to 199 mmHg and DBP < 110 mmHg. In all, 32.5% of patients in HYVET 2008 had isolated systolic hypertension. The remainder of the studies required that patients' DBP be at least 90 mmHg. Mean BP at entry was 182/95 mmHg.
See the "Participants" heading in the Characteristics of included studies table for a complete description of each study's blood pressure inclusion criteria. The mean sitting SBP/DBP in HYVET P 2003 was 182/99.6 mmHg, and in HYVET 2008 173/90.8 mmHg.
Thirteen of the 16 trials instituted a stepped care approach to hypertension treatment. In more than 70% of trials, a thiazide diuretic was the first‐line drug used for the treatment group. Seven trials started the treatment group exclusively on a thiazide diuretic (ATTMH 1981;Carter 1970; EWPHBPE 1989; HYVET 2008; Kuramoto 1981;SHEP‐P 1989; SHEP 1991). Coope 1986,MRC‐TMH 1985, and STOP 1991 started the treatment group on a diuretic or a beta blocker. MRC‐O 1992 randomised the treatment group to two arms ‐ one initially receiving diuretics, and the other initially receiving a beta blocker. Syst‐Eur 1991 started the treatment group on a calcium channel blocker. HYVET P 2003 started one treatment arm on a diuretic, and the other treatment arm on an ACE inhibitor. Second‐ and third‐line drugs included diuretics, beta blockers, centrally acting antiadrenergic agents, peripherally acting antiadrenergic agents, vasodilators, converting‐enzyme inhibitors, and calcium channel blockers. See the "Interventions" heading in the Characteristics of included studies table for a complete description of each study's drug treatment protocol.
Four trials maintained participants on a particular therapeutic regimen (i.e. not stepped care) throughout the study. VA‐II 1970 treated participants with a combination diuretic ‐ centrally acting antiadrenergic agent (hydrochlorothiazide/reserpine) ‐ plus a vasodilator (hydralazine). HSCSG 1974 treated participants with a diuretic (methyclothiazide) and a peripherally acting antiadrenergic agent (deserpidine). Sprackling 1981 treated participants with a centrally acting antiadrenergic agent (methyldopa). MRC‐TMH 1985 treated participants with a fixed dose of bendrofluazide10 mg or propranolol 80 to 240 mg and added methyldopa if required.
HYVET P 2003 randomised participants to three groups: no treatment, diuretic‐based treatment (usually bendroflumethiazide 2.5 mg), and an ACE inhibitor (ACEI)‐based regimen (usually lisinopril 2.5 mg). To attain target blood pressure (sitting SBP < 150 mmHg and sitting DBP < 80 mmHg) in the actively treated groups, the dose of diuretic or ACEI could be doubled (step 2); diltiazem slow release 120 mg could be added (step 3); or diltiazem slow release 240 mg could be added (step 4).
HYVET 2008 randomised participants to either indapamide sustained release 1.5 mg or matching placebo. To reach target blood pressure (SBP < 150 mmHg and DBP < 80 mmHg), perindopril 2 mg or 4 mg or matching placebo could be added.
Length of study follow‐up ranged from relatively short ‐ 1 year in HYVET P 2003 or 2 years in STOP 1991,Syst‐Eur 1991, and HYVET 2008 ‐ to relatively long ‐ the rest of the trials lasted three to six years. All trials were multi‐site studies except for Carter 1970 and Kuramoto 1981. The mean duration of treatment was 4.5 years in adults 60 years or older; 4 years among 60‐ to 79‐year‐olds; and 2.8 years in patients 80 years of age or older. The mean duration of treatment was 3.2 years in trials with isolated systolic hypertension in patients 60 years or older.
Twelve trials were placebo controlled (ATTMH 1981; EWPHBPE 1989; HSCSG 1974; HYVET 2008; Kuramoto 1981;MRC‐O 1992; MRC‐TMH 1985; SHEP‐P 1989; SHEP 1991; STOP 1991; Syst‐Eur 1991; VA‐II 1970). In four trials, the control group received no treatment (Carter 1970; Coope 1986; HYVET P 2003; Sprackling 1981).
Studies included in this review allowed participants in the control group to receive antihypertensive therapy because their blood pressure exceeded pre‐set "escape" criteria. Also, a portion of participants assigned to the treatment group stopped taking their assigned medication because they had adverse drug effects, or because they achieved normal blood pressure. Percentages of participants assigned to the control group who were receiving antihypertensive medication by the end of the trial were as follows: Coope 1986 9%; Kuramoto 1981 17%; STOP 1991 23%; Syst‐Eur 1991 27%; ATTMH 1981 35%; SHEP‐P 1989 40%; SHEP 1991 44%; MRC‐O 1992 53%; EWPHBPE 1989 > 35%; HYVET P 2003 0.8%; and HYVET 2008 0.6%. The remaining five trials did not report such data. Percentages of participants assigned to the treatment group who had ceased taking antihypertensive medication by the end of the trial were as follows: ATTMH 1981 33%; Coope 1986 5%; EWPHBPE 1989 > 35%; HYVET P 2003 4%; HYVET 2008 0.5%; MRC‐O 1992 ‐ diuretic arm 48%; MRC‐O 1992 beta blocker arm 63%; SHEP 1991 10%; SHEP‐P 1989 30%; STOP 1991 16%; and Syst‐Eur 1991 18%. The remaining five trials did not report such data. However, those in the control group who started treatment and those in the treatment group who stopped treatment were still analysed in the treatment group to which they were randomised (intention‐to‐treat analyses).
Excluded studies
Two RCTs ‐ HDFP 1984 and CASTEL 1994 ‐ were included in the original 1998 version of this review but were excluded from the first update, as the control group in these studies was not an untreated or placebo control group. HDFP 1984 was excluded because it provided a multi‐factorial intervention, and CASTEL 1994 was excluded because the control group was receiving non‐specific antihypertensive therapy from their personal physician.
A total of 58 studies were excluded from this update and are listed with reasons for exclusion under Characteristics of excluded studies.
Risk of bias in included studies
Refer to Figure 2 and Figure 3 for visual summaries of the risk of bias assessment.
2.
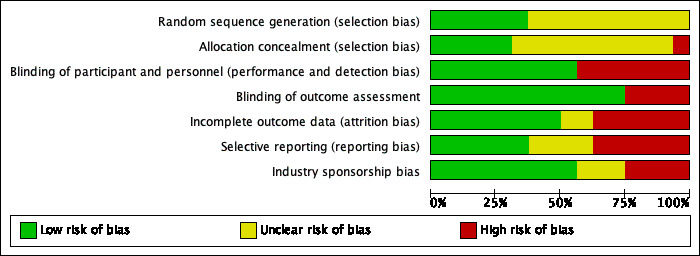
Methodological quality graph: Each methodological quality item presented as percentages across all included studies.
3.
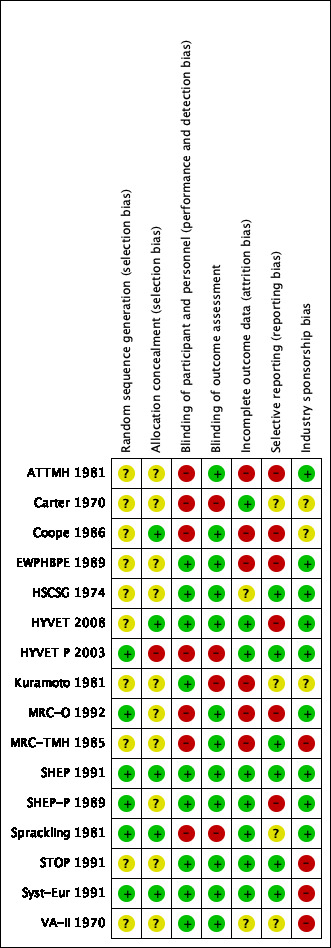
Methodological quality summary of each included trial.
Allocation
In older trials, lack of reporting of the method used for randomisation or allocation concealment is common, and we assessed risk of bias in these trials as unclear.
Randomisation was assessed as having low risk of bias in six trials (HYVET P 2003; MRC‐O 1992; SHEP 1991; SHEP‐P 1989; Sprackling 1981; Syst‐Eur 1991), and as having unclear risk of bias in 10 trials (ATTMH 1981; Carter 1970; Coope 1986; EWPHBPE 1989; HSCSG 1974; HYVET 2008; Kuramoto 1981; MRC‐TMH 1985; STOP 1991; VA‐II 1970).
Allocation concealment was assessed as having low risk of bias in five trials (Coope 1986; HYVET 2008; SHEP 1991; Sprackling 1981; Syst‐Eur 1991), as having unclear risk of bias in 10 trials (ATTMH 1981; Carter 1970; EWPHBPE 1989; HSCSG 1974; Kuramoto 1981; MRC‐O 1992; MRC‐TMH 1985; SHEP‐P 1989; STOP 1991; VA‐II 1970), and as having high risk of bias in one trial (HYVET P 2003).
Blinding
Blinding of participants and personnel was assessed as having low risk of bias in nine trials (EWPHBPE 1989; HSCSG 1974; HYVET 2008; Kuramoto 1981; SHEP 1991; SHEP‐P 1989; STOP 1991; Syst‐Eur 1991; VA‐II 1970), and as having high risk of bias in seven trials (ATTMH 1981; Carter 1970; Coope 1986; HYVET P 2003; MRC‐O 1992; MRC‐TMH 1985; Sprackling 1981).
Blinding of outcome assessors was assessed as having low risk of bias in twelve trials (ATTMH 1981; Coope 1986; EWPHBPE 1989; HSCSG 1974; HYVET 2008; MRC‐O 1992; MRC‐TMH 1985; SHEP 1991; SHEP‐P 1989; STOP 1991; Syst‐Eur 1991), and as having high risk of bias in four trials (Carter 1970; HYVET P 2003; Kuramoto 1981; Sprackling 1981).
Incomplete outcome data
Providing incomplete outcome data was assessed as having low risk of bias in eight trials (Carter 1970; HYVET 2008; HYVET P 2003; SHEP 1991; SHEP‐P 1989; Sprackling 1981; STOP 1991; Syst‐Eur 1991), as having unclear risk of bias in two trials (HSCSG 1974; VA‐II 1970), and as having high risk of bias in six trials (ATTMH 1981; Coope 1986; EWPHBPE 1989; Kuramoto 1981; MRC‐O 1992; MRC‐TMH 1985).
Selective reporting
Selective reporting was assessed as having low risk of bias in seven trials (Carter 1970; HSCSG 1974; HYVET P 2003; MRC‐TMH 1985; SHEP 1991; STOP 1991; Syst‐Eur 1991), unclear risk in three trials (Kuramoto 1981; Sprackling 1981; VA‐II 1970), and high risk in six trials (ATTMH 1981; Coope 1986; EWPHBPE 1989; HYVET 2008; MRC‐O 1992; SHEP‐P 1989).
Other potential sources of bias
Industry sponsorship was assessed as having low risk of bias in nine trials (ATTMH 1981; EWPHBPE 1989; HSCSG 1974; HYVET 2008; HYVET P 2003; MRC‐O 1992; SHEP 1991; SHEP‐P 1989; Sprackling 1981), unclear risk of bias in three trials (Carter 1970; Coope 1986; Kuramoto 1981), and high risk of bias in four trials (MRC‐TMH 1985; STOP 1991; Syst‐Eur 1991; VA‐II 1970).
Effects of interventions
See: Table 1
Analyses were performed on the combined results of all 16 studies. The three trials that included only people with isolated systolic hypertension were included in the overall analyses and were also analysed separately (SHEP‐P 1989; SHEP 1991; Syst‐Eur 1991).
EWPHBPE 1989 reported intention‐to‐treat data for mortality only; the morbidity data reported from EWPHBPE 1989 did not undergo intention‐to‐treat analysis. The occurrence of any trial endpoint in ATTMH 1981 terminated participation in the study. Thus, true intention‐to‐treat data for ATTMH 1981 are available only for combined cardiovascular morbidity and mortality. We decided to include data for all outcomes from both EWPHBPE 1989 and ATTMH 1981, similar to what was done in the Cochrane Review titled "First line drugs for hypertension" (Wright 2018).
Individual differences in patient characteristics or disease severity are associated with different levels of risk to experience an adverse event. In the aggregate, these individual differences contribute to the proportion of patients we expect to experience an event within a population. Variation in level of risk in different patient populations, both within and between clinical trials, is often associated with variability in treatment outcomes (Ioannidis 1997; Schmid 1998). This average population risk is unknown but contributes to the proportion of events experienced by a placebo control group in a randomised trial. We use the term 'control rate' to describe the probability that a member of the control group experiences the adverse event, and we use this sample value to estimate the aggregate population risk for patients enrolled in a clinical trial.
In adults 60 years or older
All‐cause mortality
In the 13 trials reporting mortality data for people 60 years or older, treatment caused a significant reduction in all‐cause mortality (risk ratio (RR) 0.91, 95% confidence interval (CI) 0.85 to 0.97; participants = 25,932; studies = 13; I² = 8%). See Analysis 1.1.
1.1. Analysis.
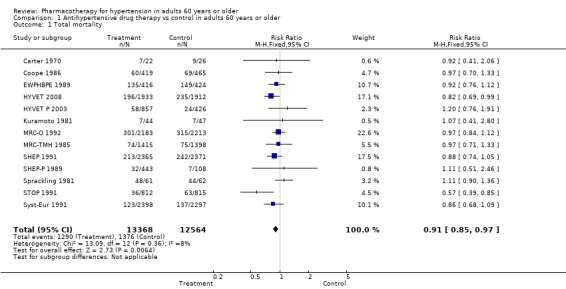
Comparison 1 Antihypertensive drug therapy vs control in adults 60 years or older, Outcome 1 Total mortality.
Mortality was significantly reduced due to reductions in fatal stroke and fatal CHD. See Analysis 1.2 and Figure 4.
1.2. Analysis.
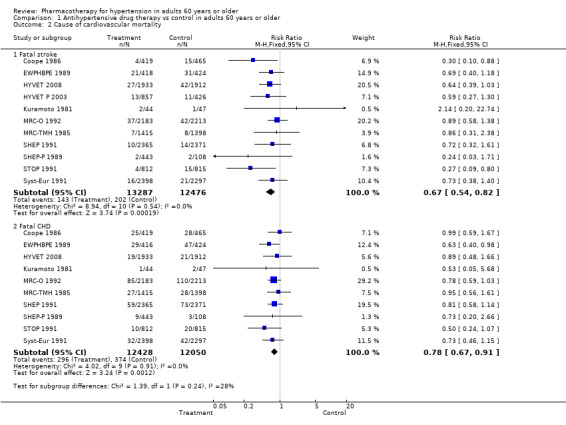
Comparison 1 Antihypertensive drug therapy vs control in adults 60 years or older, Outcome 2 Cause of cardiovascular mortality.
4.
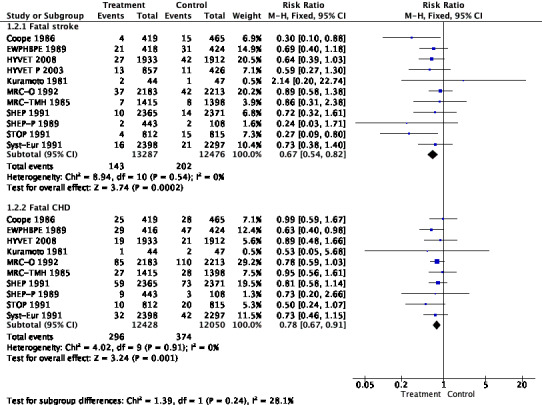
Forest plot of comparison: 1 Antihypertensive drug therapy vs control in adults 60 years or older, outcome: 1.2 Cause of cardiovascular mortality.
For subgroups, refer to Analysis 2.1. Investigating treatment effects between the two subgroups showed no significant differences. Tests for subgroup differences showed the following: Chi² = 2.61, df = 1 (P = 0.11), I² = 61.7%.
2.1. Analysis.
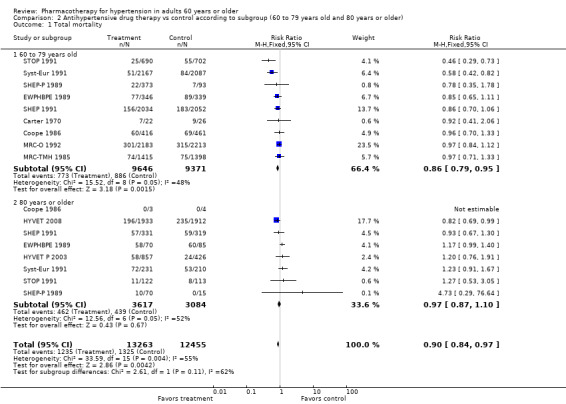
Comparison 2 Antihypertensive drug therapy vs control according to subgroup (60 to 79 years old and 80 years or older), Outcome 1 Total mortality.
People 60 to 79 years old (RR 0.86, 95% CI 0.79 to 0.95; participants = 19,017; studies = 9; I² = 48%); all‐cause mortality was significantly reduced in the 60‐ to 79‐year‐old subgroup.
People 80 years or older (RR 0.97, 95% CI 0.87 to 1.10; participants = 6701; studies = 8; I² = 52%).
Baseline risk of mortality in the control group among patients 60 to 79 years old ranged from 4% in Syst‐Eur 1991 to 34.6% in Carter 1970, and in the 80 years or older group from 0% in SHEP‐P 1989 to 71% in SHEP 1991.
Cardiovascular mortality and morbidity (M&M)
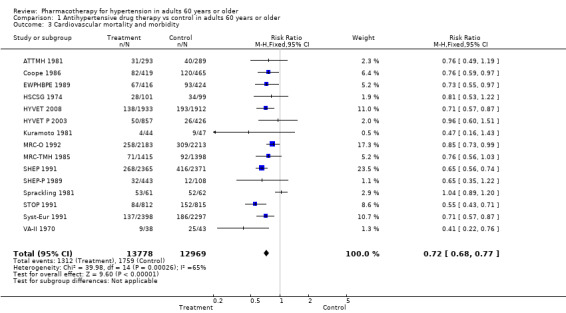
Five studies independently reached statistical significance (HYVET 2008; MRC‐O 1992; SHEP 1991; STOP 1991; Syst‐Eur 1991).
For the 15 trials reporting cardiovascular mortality and morbidity data in people 60 years of age or older, treatment caused a significant reduction with the fixed‐effect model (RR 0.72, 95% CI 0.68 to 0.77; participants = 26,747; studies = 15; I² = 65%) and with the random‐effects model (RR 0.74, 95% CI 0.66 to 0.83; participants = 26,747; studies = 15; I² = 65%). See Analysis 1.3.
1.3. Analysis.
Comparison 1 Antihypertensive drug therapy vs control in adults 60 years or older, Outcome 3 Cardiovascular mortality and morbidity.
Excluding the two studies that included TIA in the definition of the outcome measure revealed a similar reduction in overall cardiovascular mortality and morbidity (RR 0.72, 95% CI 0.67 to 0.77) (ATTMH 1981; Coope 1986).
For subgroups, see Analysis 2.2. Investigating treatment effects between the two subgroups showed no significant difference. Tests for subgroup differences showed the following: Chi² = 0.49, df = 1 (P = 0.49), I² = 0%.
2.2. Analysis.
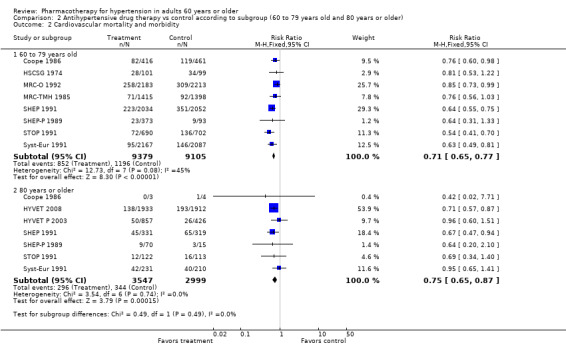
Comparison 2 Antihypertensive drug therapy vs control according to subgroup (60 to 79 years old and 80 years or older), Outcome 2 Cardiovascular mortality and morbidity.
Patients 60 to 79 years old (RR 0.71, 95% CI 0.65 to 0.77; participants = 18,484; studies = 8; I² = 45%).
Patients 80 years or older (RR 0.75, 95% CI 0.65 to 0.87; participants = 6546; studies = 7; I² = 0%).
Cardiovascular mortality and morbidity were significantly reduced in both subgroups.
The test for subgroup differences indicates that there was no statistically significant subgroup effect (P = 0.49), suggesting that age does not modify the effect of antihypertensive treatment in comparison to placebo or no treatment. However, smaller numbers of trials and participants (seven trials in 6546 participants) contributed data to the 80 years or older subgroup than to the 60‐ to 79‐year‐old subgroup (eight trials in 18,484 participants), showing no heterogeneity between the two subgroups and 95% CI overlap.
Cerebrovascular mortality and morbidity
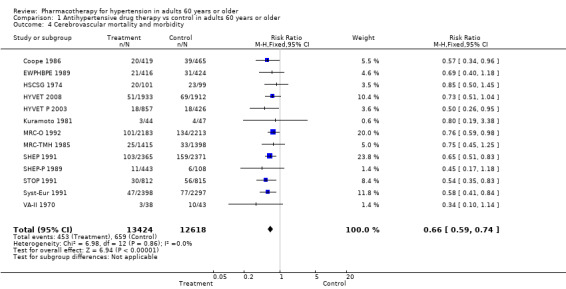
Cerebrovascular mortality and morbidity were significantly reduced among patients 60 years of age or older (RR 0.66, 95% CI 0.59 to 0.74; participants = 26,042; studies = 13; I² = 0%). See Analysis 1.4.
1.4. Analysis.
Comparison 1 Antihypertensive drug therapy vs control in adults 60 years or older, Outcome 4 Cerebrovascular mortality and morbidity.
For subgroups, see Analysis 2.3. Investigating treatment effects between the two subgroups showed no significant difference. Tests for subgroup differences showed the following: Chi² = 0.00, df = 1 (P = 0.98), I² = 0%.
2.3. Analysis.
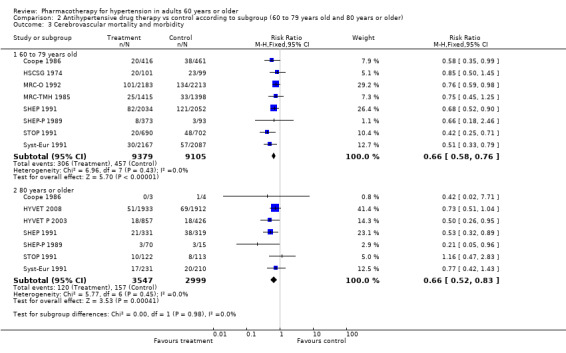
Comparison 2 Antihypertensive drug therapy vs control according to subgroup (60 to 79 years old and 80 years or older), Outcome 3 Cerebrovascular mortality and morbidity.
Patients 60 to 79 years old (RR 0.66, 95% CI 0.58 to 0.76; participants = 18,484; studies = 8; I² = 0%).
Patients 80 years of age or older (RR 0.66, 95% CI 0.52 to 0.83; participants = 6546; studies = 7; I² = 0%).
Cerebrovascular mortality and morbidity were significantly reduced in both subgroups.
The test for subgroup differences indicated that there was no statistically significant subgroup effect (P = 0.98), suggesting that age does not modify the effect of antihypertensive treatment in comparison to placebo or no treatment. However, smaller numbers of trials and participants (seven trials in 6546 participants) contributed data to the 80 years or older subgroup than to the 60‐ to 79‐year‐old subgroup (eight trials in 18,502 participants), showing no heterogeneity between the two subgroups and similar 95% CIs.
Coronary heart disease mortality and morbidity
Coronary heart disease mortality and morbidity were significantly reduced in patients 60 years of age or older (RR 0.78, 95% CI 0.69 to 0.88; participants = 24,559; studies = 11; I² = 0%). See Analysis 1.5.
1.5. Analysis.
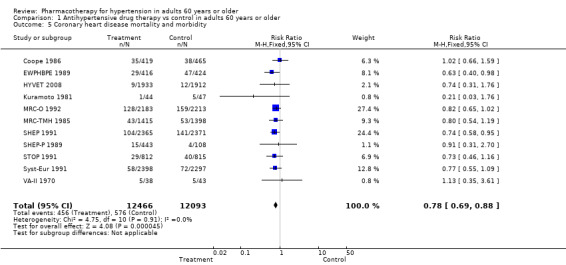
Comparison 1 Antihypertensive drug therapy vs control in adults 60 years or older, Outcome 5 Coronary heart disease mortality and morbidity.
For subgroups, see Analysis 2.4. Investigating treatment effects between the 2 subgroups showed no significant difference. Tests for subgroup differences showed the following: Chi² = 0.03, df = 1 (P = 0.86), I² = 0%.
2.4. Analysis.
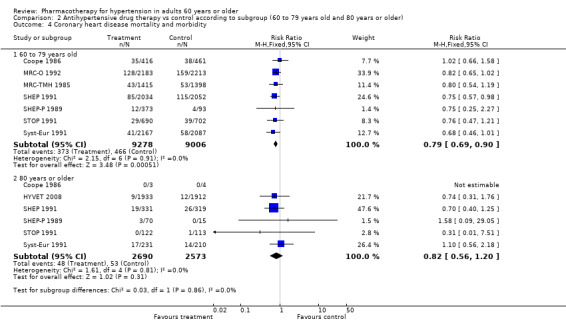
Comparison 2 Antihypertensive drug therapy vs control according to subgroup (60 to 79 years old and 80 years or older), Outcome 4 Coronary heart disease mortality and morbidity.
Patients 60 to 79 years old (RR 0.79, 95% CI 0.69 to 0.90; participants = 18,284; studies = 7; I² = 0%): coronary heart disease mortality and morbidity were significantly reduced in the 60‐ to 79‐year‐old subgroup.
Patients 80 years or older (RR 0.82, 95% CI 0.56 to 1.20; participants = 5263; studies = 6; I² = 0%).
The test for subgroup differences indicated that there was no statistically significant subgroup effect (P = 0.86), suggesting that age does not modify the effect of antihypertensive treatment in comparison to placebo or no treatment. However, smaller numbers of trials and participants (six trials in 5263 participants) contributed data to the 80 years or older subgroup than to the 60‐ to 79‐year‐old subgroup (seven trials in 18284 participants), showing no heterogeneity between the two subgroups and 95% CI overlap.
Withdrawals due to adverse effects
The numbers of participants who dropped out of trials due to adverse drug effects often were not reported. The five trials that did report these data showed a significant increase in withdrawals due to adverse effects (RR 2.91, 95% CI 2.56 to 3.30; participants = 11,310; studies = 4; I² = 97%). The effect size was significant even when MRC‐O 1992 with high risk of performance and detection bias as well as selective reporting bias was excluded from analyses (RR 1.71, 95% CI 1.45 to 2.00; participants = 6914; studies = 4; I² = 55%).
The number of people withdrawing from therapy due to adverse effects varied from study to study. On average, treating 17 participants in SHEP 1991 resulted in one withdrawal, whereas in MRC‐O 1992, treating nine participants with a diuretic and four with a beta blocker resulted in one withdrawal. In MRC‐O 1992, unblinded physicians made decisions regarding severity of side effects and continuation of therapy; 176 of those in the beta blocker group were withdrawn because of bradycardia. Withdrawal data for participant subgroups 60 to 79 years old and 80 years and older were not reported separately.
Sensitivity analyses
When we excluded studies with SBP ≥ 190 mmHg (Coope 1986; Sprackling 1981; STOP 1991), studies with observation as a control (Carter 1970; Coope 1986; HYVET P 2003), or studies with high risk of performance and detection bias (ATTMH 1981; Carter 1970; Coope 1986; HYVET P 2003; MRC‐O 1992; MRC‐TMH 1985; Sprackling 1981), the overall risk ratio for both mortality and cardiovascular mortality and morbidity was similar. Results were similar when the fixed‐effect or the random‐effects model was used. In the three trials restricted to persons with isolated systolic hypertension, reported benefits were similar.
Discussion
Summary of main results
This systematic review provides the best available evidence for antihypertensive treatment for people with elevated blood pressure who are at least 60 years of age. It is important to appreciate that the populations studied had relatively high systolic blood pressure: an average of 172/81 mmHg in the isolated systolic hypertension trials and an average of 182/95 mmHg in the other trials. The reason that diastolic blood pressure (DBP) is lower than expected is that for two trials, mean baseline DBP was 91 mmHg (HYVET 2008; MRC‐O 1992), for two trials 86 mmHg (Kuramoto 1981; Syst‐Eur 1991), and for one study 77 mmHg (SHEP 1991). DBP at baseline was not reported in two studies (Carter 1970; MRC‐TMH 1985).
In this population, antihypertensive drug treatment was associated with a modest reduction in all‐cause mortality (high‐quality evidence; risk ratio (RR) 0.91, 95% confidence interval (CI) 0.85 to 0.97). This represents an absolute risk reduction (ARR) in deaths from 110 to 100 events per 1000 participants over an average duration of 3.8 years (ARR = 1%; number needed to treat for an additional beneficial outcome (NNTB) = 100) (Table 1). The reduction in mortality among adults 60 years or older was due to a significant reduction in fatal stroke and in fatal myocardial infarction (MI) (Figure 4).
Cardiovascular mortality and morbidity were significantly reduced (RR 0.72, 95% CI 0.68 to 0.77). This represents an absolute reduction from 136 to 98 events per 1000 participants for a mean duration of treatment of 3.7 years (ARR = 3.8%; number needed to treat for an additional beneficial outcome = 27) (Table 1). This is a smaller ARR than the 4.3% reported in the first update of this review. This smaller ARR is due to the fact that we have added data from the MRC‐TMH 1985 trial, which studied patients with mild to moderate elevations in blood pressure (BP), and to the fact that we excluded transient ischaemic attack (TIA) from this outcome. The overall ARR of 3.8% as seen here is less than that found for first‐line low‐dose thiazides of 3.9%, which includes adults of all ages. To assess benefit from first‐line thiazides in adults 60 and over, we deselected all trials that did not provide first‐line thiazides. In that analysis, the effect on total cardiovascular events with first‐line thiazides in adults 60 and over from eight trials in 10,926 people was RR 0.67 (95% CI 0.61 to 0.74) with ARR 5.1% and number needed to treat for an additional beneficial outcome = 20 over 3.7 years.
The test of interaction between the two subgroups showed no significant differences for any outcome measure. The subgroup analysis of treatment among patients 60 to 79 years or older showed significant benefit in terms of all outcomes, that is, all‐cause mortality (ARR = 1.4%; number needed to treat for an additional beneficial outcome = 72); cardiovascular mortality and morbidity (ARR = 3.8%; number needed to treat for an additional beneficial outcome = 27); cerebrovascular mortality and morbidity (ARR = 1.7%; number needed to treat for an additional beneficial outcome = 59); and coronary heart disease mortality and morbidity (ARR = 1.1%; number needed to treat for an additional beneficial outcome = 91). See Table 4. However, in the 80 years or older subgroup, a significant risk reduction was observed in cardiovascular mortality and morbidity from 115 to 86 (75 to 100) events per 1000 participants (ARR= 2.9%; number needed to treat for an additional beneficial outcome = 35) with a mean duration of 2.2 years. This was mostly due to a decrease in cerebrovascular mortality and morbidity from 52 to 35 (27 to 43) per 1000 participants (ARR = 1.7%; number needed to treat for an additional beneficial outcome = 59). See Table 5.
3. Antihypertensive drug therapy compared to control in adults 60 to 79 years old with hypertension.
| Antihypertensive drug therapy compared to control in adults 60 to 79 years old with hypertension | ||||||
| Patient or population: healthy ambulatory adults 60 to 79 years old with hypertension Setting: outpatient Intervention: antihypertensive drug therapy Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) |
Relative effect
(95% CI) Fixed‐effect model |
No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control |
Risk with antihypertensive drug therapy |
|||||
| Total mortality Mean duration of 4.4 years |
95 per 1000 | 81 per 1000 (75 to 90) | RR 0.86 (0.79 to 0.95) | 19,017 (9 studies) | ⊕⊕⊕⊕ HIGH | ARR = 1.4% NNTB = 72 |
| Cardiovascular mortality and morbidity Mean duration of 4.2 years |
131 per 1000 | 93 per 1000 (85 to 101) | RR 0.71 (0.65 to 0.77) | 18,484 (8 studies) | ⊕⊕⊕⊝ MODERATEa | ARR = 3.8% NNTB = 27 |
| Cerebrovascular mortality and morbidity Mean duration of 4.2 years |
50 per 1000 | 33 per 1000 (29 to 38) | RR 0.66 (0.58 to 0.76) | 18,484 (8 studies) | ⊕⊕⊕⊝ MODERATEa | ARR = 1.7% NNTB = 59 |
| Coronary heart disease mortality and morbidity Mean duration of 4.2 years |
52 per 1000 | 41 per 1000 (36 to 47) | RR 0.79 (0.69 to 0.90) | 18,284 (7 studies) | ⊕⊕⊕⊝ MODERATEa | ARR = 1.1% NNTB = 91 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ARR: absolute risk reduction; CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded due to study limitations (incomplete outcome reporting and selective outcome reporting).
4. Antihypertensive drug therapy compared to control in adults 80 years or older with hypertension.
| Antihypertensive drug therapy compared to control in adults 80 years or older with hypertension | ||||||
| Patient or population: healthy ambulatory adults 80 years or older with hypertension Setting: outpatient Intervention: antihypertensive drug therapy Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) |
Relative effect
(95% CI) Random‐effects model |
No. of participants (studies) |
Certainty of the evidence (GRADE) |
Comments | |
| Risk with control |
Risk with antihypertensive drug therapy |
|||||
| Total mortality Mean duration of 2.3 years |
142 per 1000 | 138 per 1000 (124 to 157) |
RR 0.97 (0.87 to 1.10) | 6701 (8 studies) | ⊕⊕⊝⊝ LOWa,b | Not significant |
| Cardiovascular mortality and morbidity Mean duration of 2.2 years |
115 per 1000 | 86 per 1000 (75 to 100) | RR 0.75 (0.65 to 0.87) | 6546 (7 studies) | ⊕⊕⊕⊝ MODERATEb | ARR = 2.9% NNTB = 35 |
| Cerebrovascular mortality and morbidity Mean duration of 2.2 years |
52 per 1000 | 35 per 1000 (27 to 43) | RR 0.66 (0.52 to 0.83) | 6546 (7 studies) | ⊕⊕⊕⊝ MODERATEb | ARR = 1.7% NNTB = 59 |
| Coronary heart disease mortality and morbidity Mean duration of 2.5 years |
21 per 1000 | 17 per 1000 (12 to 25) | RR 0.82 (0.56 to 1.20) | 5263 (6 studies) | ⊕⊕⊕⊝ MODERATEb | Not significant |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ARR: absolute risk reduction; CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded due to inconsistency and wide confidence interval.
bDowngraded due to study limitations ‐ high risk of selective reporting bias in HYVET 2008 study.
Overall completeness and applicability of evidence
The evidence is applicable to healthy ambulatory adults 60 years of age or older (mean age 73.4 years) from Western industrialised countries with moderate to severe systolic and/or diastolic hypertension (average 182/95 mmHg). Most of these trials evaluated first‐line thiazide diuretic therapy for a mean treatment duration of 3.8 years.
Trials involving older people could have varied systematically from those including younger people. Trials that included younger people were published before 1987 (ATTMH 1981; HSCSG 1974; Oslo 1986; USPHSHCSG 1977; VA‐II 1970; VANHLBI 1978). Six large trials involving older people were published after 1990 (HYVET 2008; HYVET P 2003; MRC‐O 1992; SHEP 1991; STOP 1991; Syst‐Eur 1991). Although first‐line beta blockers and thiazide diuretics were used in most trials, recent large trials in older people have usually used either lower doses of thiazides or combinations with potassium‐sparing agents. As a result, they may be associated with less toxic adverse effects. The most recent trial in the very elderly studied first‐line indapamide sustained release 1.5 mg (HYVET 2008). If blood pressure remained at systolic blood pressure (SBP) > 150 mmHg and diastolic blood pressure (DBP) > 80 mmHg, perindopril 2 mg or 4 mg could be added to the active treatment arm. This trial showed a significant reduction in mortality (RR 0.82, 95% CI 0.69 to 0.99; ARR = 2.2%; NNTB = 48 for 2 years) and in total cardiovascular events (RR 0.71, 95% CI 0.57 to 0.87) with low doses of two antihypertensive drugs. The other trials in the very elderly used higher doses of more antihypertensive drugs and showed a trend towards increased total mortality. Although it was not possible for us to conduct a meta‐regression analysis, when heterogeneity was explored by Bejan‐Angoulvant using meta‐regression in patients 80 years of age or older based on the same eight randomised controlled trials (RCTs) in 6701 participants included in our review (Coope 1986; EWPHBPE 1989; HYVET 2008; HYVET P 2003; SHEP 1991; SHEP‐P 1989; Syst‐Eur 1991; STOP 1991), results suggested that reduction in mortality was achieved in trials with the least reductions in BP and with the lowest intensity of therapy (Bejan‐Angoulvant 2010). This has led to the recommendation that people over age 80 should be treated with low doses of a thiazide and angiotensin‐converting enzyme (ACE) inhibitor, as were used in the only trial associated with a reduction in mortality (Bejan‐Angoulvant 2010; HYVET 2008). These observations suggest that less aggressive treatment is probably a good approach in the very elderly. This approach needs to be tested in younger populations as well.
A Cochrane Review in adults 18 to 59 years old is based on seven studies in 17,327 patients with primary hypertension (Musini 2017). Mean blood pressure at baseline was 160/98 mmHg, and mean duration of treatment was five years. There was no significant reduction in mortality (RR 0.94, 95% CI 0.77 to 1.13) nor in coronary heart disease (RR 0.99, 95% CI 0.82 to 1.19). However, a significant decrease was seen in cardiovascular mortality and morbidity (RR 0.78, 95% CI 0.67 to 0.91), with an ARR of 0.9% (NNTB = 112), which was mainly due to a significant decrease in cerebrovascular mortality and morbidity (RR 0.46, 95% CI 0.34 to 0.64), with an ARR of 0.7% (NNTB = 143). The magnitude of ARR in younger adults 18 to 59 years old is much lower (ARR = 0.9%) than in adults 60 years or older (ARR = 3.8%) and in adults 60 years or older treated with first‐line thiazides (ARR = 5.1%).
The magnitude of benefit depends on multiple factors including the baseline risk of cardiovascular complications of hypertension (Gueyffier 1997). People with more cardiovascular risk factors (e.g. diabetes, family history of heart disease, left ventricular hypertrophy) have a greater likelihood of a reduction in cardiovascular events by antihypertensive therapy. The five‐year absolute morbidity and mortality benefit of antihypertensive therapy is greater for older than for younger adults (Collins 1990; Mulrow 1994; Musini 2017). The main reason for this greater absolute benefit is that older people are at higher absolute risk of a cardiovascular event when compared with younger people (Alderman 1981; Alderman 1993; Browner 1989). Risk factors include pre‐existing cardiovascular disease and systolic hypertension (Applegate 1992; Mann 1992).
The numbers of participants who dropped out of trials due to adverse drug effects often were not reported. The four trials that did report these data showed a significant increase in withdrawals due to adverse effects in 1000 participants. The absolute risk increase (low‐quality evidence due to substantial heterogeneity) was 10.3%, and the number needed to treat to cause one event was 10. Separate data for withdrawals due to adverse effects were not available for very elderly patients. The Musini 2017 review in younger adults aged 18 to 59 years also showed a significant increase in withdrawals due to adverse effects (RR 4.82, 95% CI 1.67 to 13.92), but the absolute increase was of a lower magnitude (3.8%) and the number needed to treat to cause one event was 27.
Studies included in this review allowed participants in the control group to receive antihypertensive therapy because their blood pressure exceeded pre‐set "escape" criteria. Also, a portion of participants assigned to the treatment group stopped taking their assigned medication because they showed adverse drug effects, or because they achieved normal blood pressure. The degree to which participants cross over from one group to the other dilutes the results of the study. The percentage of participants assigned to the control group who were receiving antihypertensive medication by the end of the trial ranged from 9% to 53%. The percentage of participants assigned to the treatment group that had ceased taking antihypertensive medication by the end of the trial ranged from 0.5% to 63%. The impact of this cross‐over from one group to the other on the magnitude of overall effect size for all outcomes is not known.
Control rates
Control rates provide insight regarding baseline risk of study populations and can explain the differences in outcomes between individual trials. Total mortality rates in the control groups ranged from 3 to 71%. Trials with relatively low rates included ATTMH 1981 (3%), HYVET P 2003 (5.2%), MRC‐TMH 1985 (5.4%), Syst‐Eur 1991 (6%), SHEP‐P 1989 (6.5%), STOP 1991 (7.7%), and SHEP 1991 (10.2%). Trials with moderate rates included HYVET 2008 (12.3%), MRC‐O 1992 (14.2%), Coope 1986 (14.8%), and Kuramoto 1981 (14.9%). Trials with relatively high rates included Carter 1970 (34.6%), EWPHBPE 1989 (35.1%), and Sprackling 1981 (71%). VA‐II 1970 and HSCSG 1974 did not report total mortality, but reported the second and third highest event rates (behind Sprackling 1981) in cardiovascular morbidity and mortality (VA‐II 1970 58.1%, HSCSG 1974 34.3%, Sprackling 1981 83.9%).
The 95% CI for the RR of total mortality for Sprackling 1981 ‐ 1.11 (0.90 to 1.36) ‐ did not overlap with the 95% CI of the STOP 1991 trial ‐ 0.46 (0.29 to 0.73) ‐ in the 60‐ to 79‐year‐old subgroup. Differences in control rates are likely due to differing baseline characteristics in recruited patients. For example, all participants in Carter 1970 and in HSCSG 1974 were stroke survivors. Those in Sprackling 1981 and Kuramoto 1981 resided in a home for the aged; patients in Carter 1970 and VA‐II 1970 were recruited from hospitals (but followed up in clinics); and participants in EWPHBPE 1989 were recruited from geriatric hospitals, physicians' offices, and homes for the aged.
Additional explanations for differing control rates include variation in definitions of trial endpoints, cross‐over rates, and follow‐up durations. Although we attempted to standardise outcome definitions as much as possible (see Methods section), truly uniform definitions between trials were not possible. Trials had cross‐over rates ranging from 9% to 62% (see Characteristics of included studies) and follow‐up durations ranging from one to six years.
Because most data were based on healthy ambulatory patients 60 years or older and included only a small percentage of randomised participants with stroke or MI at baseline, participants with significant competing comorbidity and complicated medical regimens may show poorer compliance, less benefit, and more adverse effects compared to trial participants.
Limitations and generalisations
The most appropriate way to match expected magnitude of benefit to patients with particular constellations of risk factors is to perform individual patient‐based meta‐analyses (Gueyffier 1997). This was not possible in this review. Moreover, our aggregate results refer to generally expected benefit for hypertensive patients 60 years or older and are not tailored specifically to patients with particular risk factors. Our average results refer primarily to a primary prevention population with moderate to severe systolic or systo‐diastolic hypertension treated with a first‐line thiazide. Data for other first‐line drugs were insufficient, and the objective of this review was not to compare different first‐line drugs, which has been done by other systematic reviews (Psaty 1997; Psaty 2003; Wright JM 1999; Wright 2018).
Actual estimates of benefits and harms of treating adults 60 years or older with hypertension, derived from trials with highly selected participants, are not readily generalisable to clinical practice and, strictly speaking, trial results cannot be generalised to such patients. Many patients would not meet eligibility criteria or, if offered the chance, would not have enrolled in a clinical trial. In practice, clinicians are of course willing to offer treatment to patients who may not have been eligible for a trial, or who, if eligible, would have refused participation, but we should approach these generalisations with forethought. Without extra care and visits provided in many trials, even our "eligible" patients may be less compliant than trial participants. Patients with significant competing comorbidities and complicated medical regimens may also have poorer compliance, less benefit, and more adverse effects compared to participants in trials. For example, in an octogenarian with orthostasis and recurring falls related to antihypertensive therapy, harms likely exceed benefits. On the other hand, clinicians should not always assume that less benefit would be seen in "real‐life" clinical settings. A person who is at high immediate risk of suffering a cardiovascular event and who does not have other competing illnesses may have a higher benefit‐to‐harm ratio than the average trial participant.
Quality of the evidence
Risk of bias was assessed using the Cochrane risk of bias tool, and findings demonstrated that approximately 60% of trials had evidence of unclear risk of selection bias and 30% had high risk of selective reporting bias; also, approximately 50% of trials did not deal with missing or incomplete outcome data appropriately. In other words, 50% of trials could have censored outcome data for patients after they had their first event. In addition, in 30% of trials, when outcome data were not available, it appeared the assumption was that an event did not occur in that patient. See Figure 2 and Figure 3. The implications are that available outcome data used in the meta‐analyses may be incomplete. It is difficult to determine whether this bias would favour treatment or control. What can be said is that reported event rates are underestimates and calculated effect sizes for outcomes (other than death as the first event) may be inaccurate. However, deselecting trials with high risk of performance and detection bias for the cardiovascular mortality and morbidity outcome increased the effect size from RR 0.72 (95% CI 0.68 to 0.77) to RR 0.66 (95% CI 0.61 to 0.72). Deselecting trials with high risk of attrition bias increased the effect size to RR 0.68 (95% CI 0.63 to 0.74). Deselecting trials with high risk of selective reporting bias also increased the effect size (RR 0.68, 95% CI 0.63 to 0.75). However, we have graded certainty for the body of evidence for cardiovascular mortality and morbidity in patients 60 years or older as moderate due to study limitations.
Overall certainty for grading of evidence was high for all‐cause mortality and moderate for cardiovascular mortality and morbidity outcomes in patients 60 years or older. The interpretation of high certainty is that we are very confident that the true effect lies close to that of the estimate of the effect. The interpretation of moderate certainty is that we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty evidence was noted for withdrawal due to adverse effects. The interpretation of low‐certainty evidence is that we have very little confidence in the effect estimate and the true effect is likely to be substantially different from the estimate of the effect.
Potential biases in the review process
Obtaining data from all available trials in adults with hypertension may improve the effect estimate and may yield evidence of high certainty. Most trials in patients with hypertension include adults 18 years or older but do not report data in subgroups based on age (18 to 59 years old; 60 to 79 years old; and the very elderly 80 years or older). Although 15 additional trials met the minimum inclusion criteria of this review, data from these studies for patients 60 years or older could not be included (see Table 2). We would like to encourage researchers to provide access to data on subgroups of older patients in trial populations, either by providing aggregate data for participants age 60 years or older, or by providing individual patient data, so we can include these trials in a future update.
The main limitation of this meta‐analysis is the heterogeneity of the studies included. Other limitations common to all meta‐analyses include publication bias and lack of individual patient data.
Agreements and disagreements with other studies or reviews
The Pearce 1995 systematic review evaluated short‐term benefit of drug treatment for hypertension or placebo in the elderly 60 years or older for stroke, major coronary events, and mortality rates. These review authors included eight RCTs in 15,990 patients treated on average for 4.9 years (ATTMH 1981;Coope 1986;EWPHBPE 1989;HDFP 1984;MRC‐O 1992; SHEP 1991; SHEP‐P 1989;STOP 1991). Mean baseline blood pressure was 179/90 mmHg, with a mean treatment effect of 15/6 mmHg. Reduction was observed in fatal or non‐fatal major coronary events of 0.82 (0.73 to 0.92); fatal or non‐fatal stroke 0.65 (0.57 to 0.75); and death from any cause 0.85 (0.78 to 0.92) (P < 0.005 for each). These review authors concluded, similarly to us, that antihypertensive treatment in the elderly prevents major coronary events and stroke and prolongs life, with significant treatment effects observed within five years.
Quan 1999 systematically reviewed 11 RCTs from 1966 to 1998 in > 100 women with hypertension that compared single or multiple antihypertensive treatment versus placebo or standard care to evaluate cardiovascular mortality and morbidity outcomes according to gender, race, or both (ATTMH 1981; CASTEL 1994; Coope 1986; EWPHBPE 1989; HDFP 1984; MRC‐O 1992; MRC‐TMH 1985; SHEP 1991; SHEP‐P 1989; STOP 1991; Syst‐Eur 1991). In women aged 55 years or older (90% white), hypertension treatment resulted in a 38% risk reduction in fatal and non‐fatal cerebrovascular events (95% CI 27% to 47%; 5‐year NNTB 78), a 25% reduction in fatal and non‐fatal cardiovascular events (95% CI 17% to 33%; 5‐year NNTB 58), and a 17% reduction in cardiovascular mortality (95% CI 3% to 29%; 5‐year NNTB 282). Review authors concluded that hypertension treatment lowers the relative and absolute risk of cardiovascular morbidity and mortality in women aged 55 years and older and in African American women of all ages.
The Goeres 2014 systematic review included 19 studies (N = 55,489) from 1996 to 2014, consisting of seven studies comparing antihypertensive treatment to placebo or no treatment (N = 17,206) (HYVET P 2003; HYVET 2008; MRC‐O 1992; SHEP 1991; SHEP‐P 1989; STOP 1991; Syst‐Eur 1991), along with 12 head‐to‐head comparator trials (N = 38,283). Review authors concluded that older adults ≥ 65 years had decreased cardiovascular mortality and morbidity with antihypertensive treatment compared with no treatment. There was enormous heterogeneity in these studies, and reporting of harms stratified by age was lacking. They also concluded that current evidence was insufficient to determine the safest, most beneficial hypertension regimen for older adults.
Parsons 2016 conducted a systematic review of nine randomised placebo‐controlled trials in which hypertensive patients with mean age ≥ 65 years received antihypertensive or control treatment for a minimum duration of 2 years (FEVER 2011; HYVET 2008; MRC‐O 1992; SCOPE 2003; SHEP 1991; STONE 1996; STOP 1991; Syst‐China 1993; Syst‐Eur 1991). They concluded that antihypertensive treatment reduced the risk of stroke (RR 0.67, 95% CI 0.57 to 0.79) including fatal and non‐fatal stroke and transient ischaemic attacks. Reduction in dementia and cognitive decline was not significant.
Bejan‐Angoulvant 2010 explored heterogeneity using meta‐regression in patients 80 years or older based on the same eight RCTs in 6701 patients included in our review (Coope 1986; EWPHBPE 1989; HYVET 2008; HYVET P 2003; SHEP 1991; SHEP‐P 1989; STOP 1991; Syst‐Eur 1991). There was significant heterogeneity between HYVET 2008 and other trials. Mean SBP at entry was 173 mmHg in the HYVET 2008 population and about 180 mmHg in the remaining trials. In the HYVET 2008 study, the percentage of patients with history of diabetes was lower (6.9% vs 14%), history of previous stroke was higher (6.8% vs 4%), and previous hypertension treatment was more frequent (65% vs 34%) in comparison with the other included trials. Other trials recruited most patients from Europe and the USA. However, more than one‐third of the HYVET 2008 population was recruited in China. Chinese patients had significantly lower body mass index (BMI), lower sitting DBP, lower total cholesterol, higher high‐density lipoprotein (HDL) cholesterol, and better renal function. Previous episodes of myocardial infarction and congestive heart failure were significantly fewer among Chinese. The estimated annual mortality rate in control groups varied from 3.4% in STOP 1991 to 15.4% in EWPHBPE 1989 and to 6% in HYVET 2008. Despite meta‐analysis of the best evidence for patients 80 years or older showing reduction in stroke and heart failure, a reduction in mortality was not observed. This heterogeneity was not explained by differences in follow‐up duration between trials. The meta‐regression suggested that reduction in mortality was achieved in trials with the least blood pressure (BP) reduction and the lowest intensity of therapy. This has led to the recommendation that people over 80 should be treated with low doses of a thiazide and angiotensin‐converting enzyme (ACE) inhibitor, as was used in the only trial associated with a reduction in mortality (HYVET 2008). These observations suggest that less aggressive treatment is probably a good approach in the very elderly.
Authors' conclusions
Implications for practice.
Antihypertensive treatment in people aged 60 and older with moderate to severe systolic and/or diastolic hypertension reduces total mortality and total cardiovascular morbidity and mortality. The absolute risk reduction in cerebrovascular mortality and morbidity over 3.7 years was greater (1.8%; number needed to treat for an additional beneficial outcome (NNTB) = 56) than for coronary heart disease mortality and morbidity (1% with NNTB = 100). Evidence of benefit pertains mostly to a primary prevention population and to first‐line treatment with a thiazide.
This comprehensive systematic review provides additional evidence that the reduction in mortality observed was mostly due to reduction in the 60‐ to 79‐year‐old patient subgroup (high‐quality evidence; risk ratio (RR) 0.86, 95% confidence interval (CI) 0.79 to 0.95). Although cardiovascular mortality and morbidity were significantly reduced in both subgroups 60 to 79 years old (moderate‐quality evidence; RR 0.71, 95% CI 0.65 to 0.77) and 80 years or older (moderate‐quality evidence; RR 0.75, 95% CI 0.65 to 0.87), the magnitude of absolute risk reduction was probably greater in 60‐ to 79‐year‐old patients (3.8% vs 2.9%). The reduction in total cardiovascular mortality was primarily due to a reduction in cerebrovascular mortality and morbidity.
Meta‐regression by Bejan‐Angoulvant 2010 based on the same eight randomised studies included in this review suggested that reduction in mortality was achieved in trials with the least BP reductions and the lowest intensity of therapy. This has led to the recommendation that people over 80 should be treated with low doses of a thiazide and ACE inhibitor, as was used in the only trial associated with a reduction in mortality (HYVET 2008). These observations suggest that less aggressive treatment is probably a good approach in the very elderly.
Implications for research.
Individual patient‐based meta‐analyses of data from existing trials should be used to derive evidence for the treatment of specific subgroups of hypertensive patients such as persons with diabetes, functional impairment, or recent stroke, or persons of African descent. Trials are needed in people with mild hypertension, resting BP 140‐159/90‐99. Further long‐term RCTs are needed to investigate which first‐line drug is best for patients 60 years or older, and to study different approaches to treatment (e.g. an RCT comparing the use of two drugs at low dose (as in the HYVET 2008 trial) vs traditional antihypertensive therapy using three to four drugs in maximal doses).
We would like to encourage researchers to provide access to data on subgroups of older patients in trial populations, either by providing aggregate data for participants age 60 years or older, or by providing individual patient data to enable such analyses by review authors in the future. Without access to data on the age group of interest in all trials, we are limited to analysing a subset of trials that do provide these data.
More randomised controlled trials need to be done in the specific age group of hypertensive patients 60 to 79 years old, and particularly in the age group 80 years or older.
Feedback
Comment on the conclusion
Summary
While reading your interesting review in the Cochrane Library: "Pharmacotherapy for hypertension in the elderly", we were particularly interested in a statement made in the Main results of the abstract:" The average prevalence of cardiovascular risk factors, cardiovascular disease, and competing co‐morbid diseases was lower among trial participants than the general population of hypertensive elderly persons." We would very much like to know how you came to that conclusion. After carefully reading the full review, we were not able to find this statement mentioned in any other part of the review. Could you please provide how you validated this statement and what references were used to validate this statement?
Reply
We have deleted that statement in the current/updated version of this review.
Contributors
Saba T.A. and Berger Ch. Fifth year Pharmacy Students Department of pharmacology University of Lausanne Switzerland We certify that we have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of our criticisms.
Conclusions are flawed, 28 October 2008
Summary
As stated in the title and objectives: The purpose of this SR was to provide a comprehensive overview of trial evidence regarding benefits of "anti‐hypertensive drug" therapy in elders. This systematic review can be criticized mainly because it includes the HDFP trial (in which patients were randomized to two different treatment strategies, i.e. stepped care vs. referred care. In other words, in this trial not only the type of pharmacological agents were different in both groups, but also non‐pharmacological interventions. Thus, it is not possible to be certain if the difference in outcomes was due to pharmacological or to non‐pharmacological interventions) and CASTEL trial (similar design as that of HDFP) and pooled these trials along with true placebo control trials. Thus, when calculating total mortality, the weight given to those two trials in combination is even greater than that given to the biggest placebo‐control trial, SHEP trial. If those two trials were removed the benefit disappears. Therefore, the conclusions of this systematic review are flawed.
Reply
We have excluded HDFP 1982 trial in the current/updated version of this review.
Contributors
Marco Perez Occupation MD/research Department of Anesthesiology, Pharmacology & Therapeutics University of British Columbia Vancouver, BC Canada I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
What's new
| Date | Event | Description |
|---|---|---|
| 22 January 2020 | Amended | corrected minor error in Methods section |
History
Protocol first published: Issue 3, 1998 Review first published: Issue 3, 1998
| Date | Event | Description |
|---|---|---|
| 6 June 2018 | New search has been performed | Search has been updated until 24 November 2017 One new study has been added to this update: MRC‐TMH 1985 Included studies have been identified using the same names as used in the "First line drugs for hypertension" (Wright 2018) and "Pharmacotherapy for hypertension in adults 18 to 59 years" (Musini 2017) Cochrane Reviews The definition of cardiovascular mortality and morbidity outcome was modified in this update in keeping with the definition used in the 2 similar reviews mentioned above; the revised outcome does not include transient ischaemic attacks. However, when it was not possible to exclude transient ischaemic attacks from the outcome in some studies, we reported the overall effect size while including these studies and while deselecting them Risk of bias of all studies has been assessed. Overall grading of evidence for adults 60 years or older is documented in a "Summary of findings" table In the "Additional tables" section, overall grading of evidence is provided separately for the age groups 60 to 79 years and 80 years or older |
| 6 June 2018 | New citation required but conclusions have not changed | The title of the review has been changed from "Pharmacotherapy for hypertension in elderly" to "Pharmacotherapy for hypertension in adults 60 years or older". Numerous changes were made to methods and reporting |
| 27 October 2009 | Amended | Corrected denominator of the STOP trial for total mortality from 22 to 122 in the hypertension in the very elderly subgroup |
| 11 August 2009 | New citation required and conclusions have changed | Prepared substantive update; review authors and conclusions have changed |
| 11 August 2009 | Feedback has been incorporated | Excluded HDFP trial because it is a multi‐interventional study |
| 28 October 2008 | Feedback has been incorporated | New feedback received 28 October 2008 |
| 13 August 2008 | Amended | Converted to new review format |
| 5 June 2006 | Amended | Minor update prepared |
| 17 November 2004 | Feedback has been incorporated | Feedback added |
Notes
This systematic review was substantially updated for the first time in 2009 by a new team of authors. The 2009 update included two additional trials ‐ HYVET P 2003 and HYVET 2008 ‐ and excluded two previously included trials ‐ HDFP 1984 and CASTEL 1994. A meta‐analysis of data for the very elderly (80 years or older) was added.
The updated search from 2009 until November 2017 did not result in any new randomised trial meeting the minimum inclusion criteria. However, this update includes data from one additional study because we were able to get data for the 60 and over age group. We were able to obtain data on clinical outcomes for 60‐ to 64‐year‐old patients from MRC‐TMH 1985, Francois Gueyffier and the INDANA database.
For ATTMH 1981 and Coope 1986, cardiovascular mortality and morbidity data were available in the original Mulrow 1998, but the reported outcome included transient ischaemic attacks. Despite this, we decided to report these trials and have done a sensitivity analysis excluding them.
An error in mortality data entry for HYVET P 2003 has been corrected in the second update. It is changed for the treatment group from 57/857 to 58/857, and for the control group from 22/426 to 24/426. An additional three patients died after randomisation.
Acknowledgements
We would like to acknowledge the authors of the previous (1998) version of this systematic review: Cynthia Mulrow, Joseph Lau, John Cornell, and M Brand.
We would like to acknowledge Stephen Adams for retrieving innumerable articles needed to complete this review. We would also like to acknowledge Marcia Reinhart for partial screening of titles and abstracts to identify trials meeting inclusion/exclusion criteria. Finally, we acknowledge the assistance of the Cochrane Hypertension Group.
Appendices
Appendix 1. Search strategies
Database: Cochrane Hypertension Specialised Register via Cochrane Register of Studies (CRS‐Web) Search date: 24 November 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 (loop OR ceiling) NEXT (diuretic OR diuretics) AND INSEGMENT #2 (amiloride or benzothiadiazine or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide or thiazides) AND INSEGMENT #3 (chlorthalidone or chlortalidone or phthalamudine or chlorphthalidolone or oxodoline or thalitone or hygroton or indapamide or metindamide) AND INSEGMENT #4 #1 OR #2 OR #3 AND INSEGMENT #5 "angiotensin converting enzyme" NEXT inhibit* AND INSEGMENT #6 ace NEAR3 inhibit* AND INSEGMENT #7 acei AND INSEGMENT #8 (alacepril or altiopril or ancovenin or benazepril or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril or epicaptopril or fasidotril or fosinopril or foroxymithine or gemopatrilat or idapril or imidapril or indolapril or libenzapril or lisinopril or moexipril or moveltipril or omapatrilat or pentopril* or perindopril or pivopril or quinapril or ramipril or ramiprilat or rentiapril or saralasin or s nitrosocaptopril or spirapril or temocapril or teprotide or trandolapril or utibapril or zabicipril or zofenopril) AND INSEGMENT #9 #5 OR #6 OR #7 OR #8 AND INSEGMENT #10 angiotensin NEAR3 (receptor antagonist* OR receptor block*) AND INSEGMENT #11 (arb OR arbs) AND INSEGMENT #12 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan) AND INSEGMENT #13 #10 OR #11 OR #12 AND INSEGMENT #14 (amlodipine or amrinone or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil) AND INSEGMENT #15 calcium NEAR2 (antagonist* OR block* OR inhibit*) AND INSEGMENT #16 #14 OR #15 AND CENTRAL:TARGET #17 (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa):ti,ab,kw AND CENTRAL:TARGET #18 (reserpine or serpentina or rauwolfia or serpasil):ti,ab,kw AND CENTRAL:TARGET #19 (clonidine or adesipress or arkamin or caprysin or catapres$ or catasan or chlofazolin or chlophazolin or clinidine or clofelin$ or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin$ or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or m‐5041t or normopresan or paracefan or st‐155 or st 155 or tesno timelets) AND INSEGMENT #20 (hydralazin* or hydrallazin* or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or 1‐hydrazinophthalazine or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat) AND INSEGMENT #21 #17 OR #18 OR #19 OR #20 AND INSEGMENT #22 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol) AND INSEGMENT #23 beta NEAR2 (adrenergic or antagonist or antagonists or blocker or blockers or blocking or receptor or receptors) AND INSEGMENT #24 #22 OR #23 AND INSEGMENT #25 (alfuzosin or bunazosin or doxazosin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin) AND INSEGMENT #26 adrenergic NEAR2 (alpha OR antagonist OR antagonists) AND INSEGMENT #27 (adrenergic or alpha or receptor or receptors) NEAR2 (blocker or blockers or blocking) AND INSEGMENT #28 #25 OR #26 OR #27 AND INSEGMENT #29 #4 OR #9 OR #13 OR #16 OR #21 OR #24 OR #28 AND INSEGMENT #30 hypertens* AND INSEGMENT #31 (elevate* OR high* OR rais*) NEAR2 blood pressure AND INSEGMENT #32 #30 OR #31 AND INSEGMENT #33 RCT:DE AND INSEGMENT #34 Review:MISC2 AND INSEGMENT #35 #33 OR #34 AND INSEGMENT #36 #29 AND #32 AND #35 AND INSEGMENT
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: Cochrane Central Register of Controlled Trials via Cochrane Register of Studies (CRS‐Web) Search date: 24 November 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 MESH DESCRIPTOR Thiazides EXPLODE ALL AND CENTRAL:TARGET #2 MeSH DESCRIPTOR Sodium Chloride Symporter Inhibitors EXPLODE ALL AND CENTRAL:TARGET #3 MeSH DESCRIPTOR Sodium Potassium Chloride Symporter Inhibitors EXPLODE ALL AND CENTRAL:TARGET #4 ((loop or ceiling) next (diuretic or diuretics)):ti,ab AND CENTRAL:TARGET #5 (amiloride or benzothiadiazine or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide or thiazides):ti,ab AND CENTRAL:TARGET #6 (chlorthalidone or chlortalidone or phthalamudine or chlorphthalidolone or oxodoline or thalitone or hygroton or indapamide or metindamide):ti,ab AND CENTRAL:TARGET #7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 AND CENTRAL:TARGET #8 MeSH DESCRIPTOR Angiotensin‐Converting Enzyme Inhibitors EXPLODE ALL AND CENTRAL:TARGET #9 "angiotensin converting enzyme" next inhibit*:ti,ab AND CENTRAL:TARGET #10 ace near3 inhibit*:ti,ab AND CENTRAL:TARGET #11 acei:ti,ab AND CENTRAL:TARGET #12 (alacepril or altiopril or ancovenin or benazepril or captopril or ceranapril or ceronapril or cilazapril or deacetylalacepril or delapril or derapril or enalapril or epicaptopril or fasidotril or fosinopril or foroxymithine or gemopatrilat or idapril or imidapril or indolapril or libenzapril or lisinopril or moexipril or moveltipril or omapatrilat or pentopril* or perindopril or pivopril or quinapril or ramipril or ramiprilat or rentiapril or saralasin or s nitrosocaptopril or spirapril or temocapril or teprotide or trandolapril or utibapril or zabicipril or zofenopril):ti,ab AND CENTRAL:TARGET #13 #8 OR #9 OR #10 OR #11 OR #12 AND CENTRAL:TARGET #14 MeSH DESCRIPTOR Angiotensin Receptor Antagonists EXPLODE ALL AND CENTRAL:TARGET #15 angiotensin near3 (receptor antagonist* or receptor block*):ti,ab AND CENTRAL:TARGET #16 (arb OR arbs):ti,ab AND CENTRAL:TARGET #17 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan):ti,ab AND CENTRAL:TARGET #18 #14 OR #15 OR #16 OR #17 AND CENTRAL:TARGET #19 MeSH DESCRIPTOR Calcium Channel Blockers EXPLODE ALL AND CENTRAL:TARGET #20 (amlodipine or amrinone or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil):ti,ab AND CENTRAL:TARGET #21 calcium near2 (antagonist* or block* or inhibit*):ti,ab AND CENTRAL:TARGET #22 #19 OR #20 OR #21 AND CENTRAL:TARGET #23 (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa):ti,ab,kw AND CENTRAL:TARGET #24 (reserpine or serpentina or rauwolfia or serpasil):ti,ab,kw AND CENTRAL:TARGET #25 (clonidine or adesipress or arkamin or caprysin or catapres$ or catasan or chlofazolin or chlophazolin or clinidine or clofelin$ or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin$ or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or m‐5041t or normopresan or paracefan or st‐155 or st 155 or tesno timelets):ti,ab,kw AND CENTRAL:TARGET #26 MeSH DESCRIPTOR Hydralazine EXPLODE ALL AND CENTRAL:TARGET #27 (hydralazin* or hydrallazin* or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or 1‐hydrazinophthalazine or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat):ti,ab,kw AND CENTRAL:TARGET #28 #23 OR #24 OR #25 OR #26 OR #27 AND CENTRAL:TARGET #29 MeSH DESCRIPTOR Adrenergic beta‐Antagonists EXPLODE ALL AND CENTRAL:TARGET #30 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol):ti,ab AND CENTRAL:TARGET #31 beta near2 (adrenergic or antagonist or antagonists or blocker or blockers or blocking or receptor or receptors):ti,ab AND CENTRAL:TARGET #32 #29 OR #30 OR #31 AND CENTRAL:TARGET #33 MeSH DESCRIPTOR Adrenergic alpha‐Antagonists EXPLODE ALL AND CENTRAL:TARGET #34 (alfuzosin or bunazosin or doxazosin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin):ti,ab AND CENTRAL:TARGET #35 adrenergic near2 (alpha or antagonist or antagonists):ti,ab AND CENTRAL:TARGET #36 (adrenergic or alpha or receptor or receptors) near2 (blocker or blockers or blocking):ti,ab AND CENTRAL:TARGET #37 #33 OR #34 OR #35 OR #36 AND CENTRAL:TARGET #38 MeSH DESCRIPTOR Hypertension AND CENTRAL:TARGET #39 hypertens*:ti,ab AND CENTRAL:TARGET #40 (elevate* OR high* OR raise*) NEAR2 blood pressure:ti,ab AND CENTRAL:TARGET #41 #38 OR #39 OR #40 AND CENTRAL:TARGET #42 #7 OR #13 OR #18 OR #22 OR #28 OR #32 OR #37 AND CENTRAL:TARGET #43 #41 AND #42 AND CENTRAL:TARGET
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: Ovid MEDLINE(R) 1946 to Present With Daily Update Search date: 24 November 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp thiazides/ 2 exp sodium chloride symporter inhibitors/ 3 exp sodium potassium chloride symporter inhibitors/ 4 ((ceiling or loop) adj diuretic?).tw. 5 (amiloride or benzothiadiazine or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide?).tw. 6 (chlorthalidone or chlortalidone or phthalamudine or chlorphthalidolone or oxodoline or thalitone or hygroton or indapamide or metindamide).tw. 7 or/1‐6 8 exp angiotensin‐converting enzyme inhibitors/ 9 angiotensin converting enzyme inhibit$.tw. 10 (ace adj2 inhibit$).tw. 11 acei.tw. 12 (alacepril or altiopril or ancovenin or benazepril$ or captopril or ceranapril or ceronapril or cilazapril$ or deacetylalacepril or delapril or derapril or enalapril$ or epicaptopril or fasidotril$ or foroxymithine or fosinopril$ or gemopatrilat or idapril or imidapril$ or indolapril or libenzapril or lisinopril or moexipril$ or moveltipril or omapatrilat or pentopril$ or perindopril$ or pivopril or quinapril$ or ramipril$ or rentiapril or saralasin or s nitrosocaptopril or spirapril$ or temocapril$ or teprotide or trandolapril$ or utibapril$ or zabicipril$ or zofenopril$ or Aceon or Accupril or Altace or Capoten or Lotensin or Mavik or Monopril or Prinivil or Univas or Vasotec or Zestril).tw. 13 or/8‐12 14 exp Angiotensin Receptor Antagonists/ 15 (angiotensin adj3 (receptor antagon$ or receptor block$)).tw. 16 arb?.tw. 17 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan or Atacand or Avapro or Benicar or Cozaar or Diovan or Micardis or Teveten).tw. 18 or/14‐17 19 exp calcium channel blockers/ 20 (amlodipine or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil or Cardizem CD or Dilacor XR or Tiazac or Cardizem Calan or Isoptin or Calan SR or Isoptin SR Coer or Covera HS or Verelan PM).tw. 21 (calcium adj2 (antagonist? or block$ or inhibit$)).tw. 22 or/19‐21 23 (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa).mp. 24 (reserpine or serpentina or rauwolfia or serpasil).mp. 25 (clonidine or adesipress or arkamin or caprysin or catapres$ or catasan or chlofazolin or chlophazolin or clinidine or clofelin$ or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin$ or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or m‐5041t or normopresan or paracefan or st‐155 or st 155 or tesno timelets).mp. 26 exp hydralazine/ 27 (hydralazin$ or hydrallazin$ or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or 1‐hydrazinophthalazine or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat).tw. 28 or/23‐27 29 exp adrenergic beta‐antagonists/ 30 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol).tw. 31 (beta adj2 (adrenergic? or antagonist? or block$ or receptor?)).tw. 32 or/29‐31 33 exp adrenergic alpha antagonists/ 34 (alfuzosin or bunazosin or doxazosin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin).tw. 35 (adrenergic adj2 (alpha or antagonist?)).tw. 36 ((adrenergic or alpha or receptor?) adj2 block$).tw. 37 or/33‐36 38 hypertension/ 39 hypertens$.tw. 40 ((high or elevat$ or rais$) adj2 blood pressure).tw. 41 or/38‐40 42 randomized controlled trial.pt. 43 controlled clinical trial.pt. 44 randomized.ab. 45 placebo.ab. 46 clinical trials as topic/ 47 randomly.ab. 48 trial.ti. 49 or/42‐48 50 animals/ not (humans/ and animals/) 51 Pregnancy/ or Hypertension, Pregnancy‐Induced/ or Pregnancy Complications, Cardiovascular/ or exp Ocular Hypertension/ 52 (pregnancy‐induced or ocular hypertens$ or preeclampsia or pre‐eclampsia).ti. 53 49 not (50 or 51 or 52) 54 (7 or 13 or 18 or 22 or 28 or 32 or 37) and 41 and 53 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: Embase <1974 to 2017 November 22> Search date: 24 November 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp thiazide diuretic agent/ 2 exp loop diuretic agent/ ) 3 ((loop or ceiling) adj diuretic?).tw. 4 (amiloride or benzothiadiazine or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide?).tw. 5 (chlorthalidone or chlortalidone or phthalamudine or chlorphthalidolone or oxodoline or thalitone or hygroton or indapamide or metindamide).tw. 6 or/1‐5 7 exp dipeptidyl carboxypeptidase inhibitor/ 8 angiotensin converting enzyme inhibit$.tw. 9 (ace adj2 inhibit$).tw. 10 acei.tw. 11 (alacepril or altiopril or ancovenin or benazepril$ or captopril or ceranapril or ceronapril or cilazapril$ or deacetylalacepril or delapril or derapril or enalapril$ or epicaptopril or fasidotril$ or foroxymithine or fosinopril$ or gemopatrilat or idapril or imidapril$ or indolapril or libenzapril or lisinopril or moexipril$ or moveltipril or omapatrilat or pentopril$ or perindopril$ or pivopril or quinapril$ or ramipril$ or rentiapril or saralasin or s nitrosocaptopril or spirapril$ or temocapril$ or teprotide or trandolapril$ or utibapril$ or zabicipril$ or zofenopril$ or Aceon or Accupril or Altace or Capoten or Lotensin or Mavik or Monopril or Prinivil or Univas or Vasotec or Zestril).tw. 12 or/7‐11 13 exp angiotensin receptor antagonist/ 14 (angiotensin adj3 (receptor antagon$ or receptor block$)).tw. 15 arb?.tw. 16 (abitesartan or azilsartan or candesartan or elisartan or embusartan or eprosartan or forasartan or irbesartan or losartan or milfasartan or olmesartan or saprisartan or tasosartan or telmisartan or valsartan or zolasartan or Atacand or Avapro or Benicar or Cozaar or Diovan or Micardis or Teveten).tw. 17 or/13‐16 18 calcium channel blocking agent/ 19 (amlodipine or aranidipine or barnidipine or bencyclane or benidipine or bepridil or cilnidipine or cinnarizine or clentiazem or darodipine or diltiazem or efonidipine or elgodipine or etafenone or fantofarone or felodipine or fendiline or flunarizine or gallopamil or isradipine or lacidipine or lercanidipine or lidoflazine or lomerizine or manidipine or mibefradil or nicardipine or nifedipine or niguldipine or nilvadipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or semotiadil or terodiline or tiapamil or verapamil or Cardizem CD or Dilacor XR or Tiazac or Cardizem Calan or Isoptin or Calan SR or Isoptin SR Coer or Covera HS or Verelan PM).tw. 20 (calcium adj2 (antagonist? or block$ or inhibit$)).tw. 21 or/18‐20 22 (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa).mp. 23 (reserpine or serpentina or rauwolfia or serpasil).mp. 24 (clonidine or adesipress or arkamin or caprysin or catapres$ or catasan or chlofazolin or chlophazolin or clinidine or clofelin$ or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin$ or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or m‐5041t or normopresan or paracefan or st‐155 or st 155 or tesno timelets).mp. 25 hydralazine/ 26 (hydralazin$ or hydrallazin$ or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or 1‐hydrazinophthalazine or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat).tw. 27 or/22‐26 28 exp beta adrenergic receptor blocking agent/ 29 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol).tw. 30 (beta adj2 (adrenergic? or antagonist? or block$ or receptor?)).tw. 31 or/28‐30 32 exp alpha adrenergic receptor blocking agent/ 33 (alfuzosin or bunazosin or doxazosin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin).tw. 34 (adrenergic adj2 (alpha or antagonist?)).tw. 35 ((adrenergic or alpha or receptor?) adj2 block$).tw. 36 or/32‐35 37 exp hypertension/ 38 (hypertens$ or antihypertens$).tw. 39 ((high or elevat$ or rais$) adj2 blood pressure).tw. 40 or/37‐39 41 double blind$.mp. 42 placebo$.tw. 43 blind$.tw. 44 or/41‐43 45 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 46 Pregnancy/ or Hypertension, Pregnancy‐Induced/ or Pregnancy Complications, Cardiovascular/ or exp Ocular Hypertension/ 47 (pregnancy‐induced or ocular hypertens$ or preeclampsia or pre‐eclampsia).ti. 48 44 not (45 or 46 or 47) 49 (6 or 12 or 17 or 21 or 27 or 31 or 36) and 40 and 48 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: ClinicalTrials.gov Search date: 24 November 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Other Terms: randomized Study Type: Interventional Studies Condition / Disease: hypertension Intervention / Treatment: Antihypertensive Agents ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Database: WHO International Clinical Trials Registry Platform (ICTRP) Search date: 24 November 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ antihypertens* AND hypertens* AND randomized antihypertens* AND high blood pressure AND randomized
Appendix 2. Databases searched and search findings in original review and the first update
For the first update of this review in 2009, we searched the following sources: Ovid MEDLINE (to December 2008), Ovid Embase (to December 2008), and CENTRAL (2008 Issue 4). In 2008, the updated search of MEDLINE up to December 2008 identified 162 citations. Two review authors (VM and AT) screened the titles and abstracts on this list independently for inclusion, which resulted in retrieval of 31 full papers. One review author then screened the 31 full papers and considered a further three RCTs for potential inclusion in the review (ADVANCE 2007;Jikei 2007;SCOPE 2003). Three review authors discussed and reached consensus that the three RCTs did not meet the inclusion criteria and excluded them from the review. A search of CENTRAL up to June 2009 revealed only one additional citation (HYVET‐Cog 2008) that was not identified in the MEDLINE search. We retrieved the HYVET‐Cog 2008 study but concluded that this substudy of the HYVET 2008 trial did not provide any additional data for analysis. We searched Embase up to December 2008 and identified six new citations; however a review of titles and abstracts revealed that none of these met the inclusion criteria for this review.
The second updated search in November 2017 yielded 11,855 citations. We screened the titles and abstracts of these citations and found 11,500 to be irrelevant. We requested the full text of 355 citations, but none of these studies met the minimum inclusion criteria.
The previous version of this review included a search of two Japanese databases: JMEDICINE in the previous review from 1981 to 1995; and JAPIC‐DOC from 1973 to 1995 with the keywords Hikaku‐Shiken (comparative studies), Nijuu‐Mouken‐Ho (double‐blind method), and Hontaisei‐Koketsuatsu (hypertension). This search produced 46 articles, of which 34 were reports of RCTs. S Lee‐Borges translated the titles of the 34 RCTs into English, along with the abstracts of three possibly relevant trials. None of these studies met the inclusion criteria of this review.
Data and analyses
Comparison 1. Antihypertensive drug therapy vs control in adults 60 years or older.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 13 | 25932 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.85, 0.97] |
| 2 Cause of cardiovascular mortality | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Fatal stroke | 11 | 25763 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.54, 0.82] |
| 2.2 Fatal CHD | 10 | 24478 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.67, 0.91] |
| 3 Cardiovascular mortality and morbidity | 15 | 26747 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.68, 0.77] |
| 4 Cerebrovascular mortality and morbidity | 13 | 26042 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.59, 0.74] |
| 5 Coronary heart disease mortality and morbidity | 11 | 24559 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.69, 0.88] |
| 6 Withdrawal due to adverse effects | 4 | 11310 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.91 [2.56, 3.30] |
1.6. Analysis.
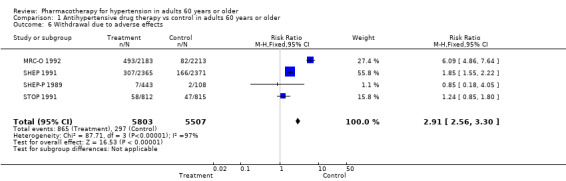
Comparison 1 Antihypertensive drug therapy vs control in adults 60 years or older, Outcome 6 Withdrawal due to adverse effects.
Comparison 2. Antihypertensive drug therapy vs control according to subgroup (60 to 79 years old and 80 years or older).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 11 | 25718 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.84, 0.97] |
| 1.1 60 to 79 years old | 9 | 19017 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.79, 0.95] |
| 1.2 80 years or older | 8 | 6701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.87, 1.10] |
| 2 Cardiovascular mortality and morbidity | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 60 to 79 years old | 8 | 18484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.65, 0.77] |
| 2.2 80 years or older | 7 | 6546 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.65, 0.87] |
| 3 Cerebrovascular mortality and morbidity | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 60 to 79 years old | 8 | 18484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.58, 0.76] |
| 3.2 80 years or older | 7 | 6546 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.52, 0.83] |
| 4 Coronary heart disease mortality and morbidity | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 60 to 79 years old | 7 | 18284 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.69, 0.90] |
| 4.2 80 years or older | 6 | 5263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.56, 1.20] |
Comparison 3. Antihypertensive drug therapy vs control in adults 60 years or older with isolated systolic hypertension.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 60 to 79 years old | 3 | 8806 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.65, 0.92] |
| 1.2 80 years or older | 3 | 1176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.88, 1.38] |
| 2 Cardiovascular morbidity and mortality | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 60 to 79 years old | 3 | 8806 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.56, 0.73] |
| 2.2 80 years or older | 3 | 1176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.60, 0.99] |
| 3 Cerebrovascular morbidity and mortality | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 60 to 79 years old | 3 | 8806 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.50, 0.79] |
| 3.2 80 years or older | 3 | 1176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.40, 0.86] |
| 4 Coronary heart disease morbidity and mortality | 3 | 9982 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.62, 0.91] |
| 4.1 60 to 79 years old | 3 | 8806 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.58, 0.90] |
| 4.2 80 years or older | 3 | 1176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.56, 1.32] |
| 5 Withdrawal due to adverse effects 60 years or older | 2 | 5287 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.53, 2.19] |
3.1. Analysis.
Comparison 3 Antihypertensive drug therapy vs control in adults 60 years or older with isolated systolic hypertension, Outcome 1 Total mortality.
3.2. Analysis.
Comparison 3 Antihypertensive drug therapy vs control in adults 60 years or older with isolated systolic hypertension, Outcome 2 Cardiovascular morbidity and mortality.
3.3. Analysis.
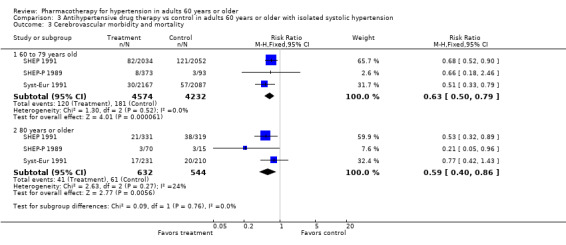
Comparison 3 Antihypertensive drug therapy vs control in adults 60 years or older with isolated systolic hypertension, Outcome 3 Cerebrovascular morbidity and mortality.
3.4. Analysis.
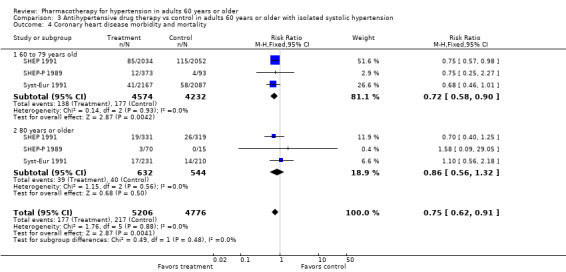
Comparison 3 Antihypertensive drug therapy vs control in adults 60 years or older with isolated systolic hypertension, Outcome 4 Coronary heart disease morbidity and mortality.
3.5. Analysis.
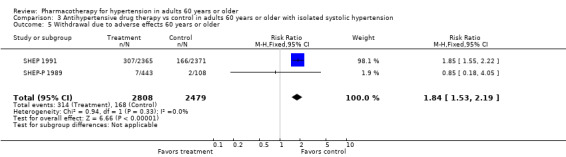
Comparison 3 Antihypertensive drug therapy vs control in adults 60 years or older with isolated systolic hypertension, Outcome 5 Withdrawal due to adverse effects 60 years or older.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ATTMH 1981.
| Methods | Randomised placebo‐controlled single‐blind trial conducted at 4 centres in Australia | |
| Participants | 582 ambulatory Caucasian patients, mean age 50.5 years, range 30 to 69 years. Male 37%. Baseline SBP/DBP was 157/100.5 mmHg and pulse pressure was 57 mmHg Inclusion criteria: SBP < 200 mmHg and DBP 95 to 110 mmHg Follow‐up: 4 years Target BP: < 90 mmHg, which was lowered to < 80 mmHg after 2 years |
|
| Interventions |
Treatment Step 1 ‐ chlorothiazide 500 mg daily Step 2 ‐ chlorothiazide 500 mg twice daily or methyldopa or propranolol or pindolol Step 3 ‐ hydralazine or clonidine added Control: placebo |
|
| Outcomes |
Mortality Cardiovascular mortality and morbidity (M&M) ‐ includes CHD M&M and cerebrovascular M&M Cerebrovascular M&M ‐ fatal stroke, non‐fatal cerebrovascular haemorrhage, or thrombosis CHD M&M ‐ CHD mortality; non‐fatal myocardial infarction CHF (patients were censored after the first outcome, so data are limited to first outcome in each category) Dropouts due to side effects: not stated Quality of life or functional status outcomes: not reported |
|
| Notes | "Of the 104,171 subjects screened, 3931 were randomised. The number eligible to start tablets previously defined as trial population was 3427 (3.3%) of originally screened population" "Thus, 504 subjects originally randomised, who at no time throughout the trial became eligible for tablets, were eliminated" "There were 62 subjects in active group and 46 in the placebo group who by mistake did not start tablets within 4 months of becoming eligible. As required by the study design, they were included in the trial population but withdrawn from the regimen after the 4‐month period of grace" "About one third of the trial population prematurely stopped the regimen they had been randomised. Those who stopped had a higher proportion of smokers (29% vs 23%) and a higher proportion of women (42% vs 34%)" Lost to follow‐up: 2.1% Percentage not on assigned therapy at study end: placebo group 35%; treatment group 33% Difference in blood pressure at end of study (Treatment ‐ Control) diastolic: ‐6.7 (systolic not stated) Cardiovascular mortality and morbidity data are available in the original Mulrow review but include all strokes, MI, sudden death, heart failure, and TIA Data on mortality, cardiovascular mortality and morbidity without TIA, cerebrovascular mortality and morbidity, coronary heart disease mortality and morbidity, and withdrawal due to adverse effects are not available for the 60‐ to 69‐year age group ATTMH 1981 is identified as ANBP 1981 in Mulrow 1998, and as Australia in Mulrow 1994 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "eligible subjects who agreed to enter the study were randomly allocated, with stratification by age and sex, to one of the two trial regimens, to take either pharmacologically active tablets, the "active group", or placebo tablets, the "placebo group"" Comment: method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation was not reported |
| Blinding of participant and personnel (performance and detection bias) | High risk | Single‐blind study in which participants were not aware of which treatment they received "Placebo tablets were identical in appearance to the active tablets" "The study centre staff knew the trial regimen of each subject, and this information was available, on request, to a subject’s local doctor. An ethics committee was kept aware of all aspects of the trial including the progressive distribution of trial endpoints between the groups" Comment: single‐blind study in which treating physicians were not blinded |
| Blinding of outcome assessment | Low risk | Quote: "during the trial the members were not aware of the distribution of trial end‐points between active and placebo groups until the day the decision was taken to stop, except that one member was on the ethics committee and three members prepared the data on which the decision to stop was based. A trial end‐point committee, unaware of the subject’s treatment group and blood‐pressure, made the final decision on acceptance of a trial end‐point. An ECG committee, similarly "blind", reported on all electrocardiographic tracings" Comment: outcome assessors were blinded to treatment groups |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "The occurrence of any trial end‐point (table 11) terminated the subject’s participation in the study" "There were more withdrawals initiated by subjects’ doctors in the placebo than in the active group. Of the 88 subjects lost to follow‐up, 42 were in the active and 46 in the placebo group" Comment: outcome data after termination of patient's participation due to occurrence of trial endpoint were not reported |
| Selective reporting (reporting bias) | High risk | All stated outcomes were reported. However, occurrence of any trial endpoint terminated the individual's participation in the study, so follow‐up of these patients was not done, and outcome data were missing for the entire duration of the trial |
| Industry sponsorship bias | Low risk | Study was initiated and administered by the National Health Foundation of Australia. It was jointly sponsored by the National Health and Medical Research Council of Australia, the Life Insurance Medical Research Fund of Australia and New Zealand, the Raine Medical Research Foundation of Western Australia, the Ramaciotti Foundation, and the Victorian government |
Carter 1970.
| Methods | Randomised single‐site open‐label study conducted in UK Patients were stroke survivors admitted to the hospital and followed in clinics |
|
| Participants | 99 participants, of which 71 were aged 60 to 79 years; 54% men; age range 40 to 79; mean 69 years; race/ethnicity not reported; mean BP at entry not reported N = 71; age 60 to 79 Pre‐existing factors: stroke 100%; BP entry criteria: SBP > 160 mmHg and DBP < 110 mmHg, or DBP ≥ 110 mmHg irrespective of SBP Exclusion criteria: cerebral haemorrhage; embolism; tumour; accelerated hypertension; "those with an obvious need for hypotensive therapy"; left ventricular failure; congestive cardiac failure; gross radiological cardiac enlargement; various cardiac arrhythmias or evidence of renal failure Mean follow‐up: 4.0 years |
|
| Interventions | Treatment: first choice ‐ thiazide diuretic (dose or type of thiazide was not specified; assumed to be high‐dose thiazide); second choice ‐ methyldopa; third choice ‐ bethanidine, debrisoquine, or guanethidine Control: observation without placebo | |
| Outcomes | Total mortality: death from all causes Stroke; coronary heart disease; congestive heart failure Dropouts due to side effects: not reported Quality of life or functional status outcomes: not reported |
|
| Notes | Percentage of patients not on assigned therapy at study end: not reported Difference in blood pressure at study end: not reported Data on mortality were available in 60‐ to 79‐year age group from the Mulrow 1998 review. Data on cardiovascular mortality and morbidity; cerebrovascular mortality and morbidity; and coronary heart disease mortality and morbidity, and withdrawals due to adverse effects were not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "placed at random into treated (50) or control (49) groups. The two groups matched reasonably closely with regard to numbers, age, sex, and severity of hypertension" Comment: method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Method for allocation concealment was not mentioned |
| Blinding of participant and personnel (performance and detection bias) | High risk | Study does not state blinding of participants or personnel. Treating physicians were aware of the treatment prescribed |
| Blinding of outcome assessment | High risk | Study does not mention blinding of the outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "2 out of 99 patients (2%) have been lost to follow‐up, a treated man aged 65 and untreated women of 70 ‐ so results are available for 49 treated and 48 untreated patients" Comment: the attrition rate is extremely low, and although reason for loss to follow‐up was not mentioned, it could not have affected the outcome analysis |
| Selective reporting (reporting bias) | Unclear risk | Protocol is not available to confirm reporting bias. Mortality rate and recurrence rate of strokes mentioned, as study objectives were reported in the results section "Figures for minor strokes or transient cerebral ischaemic attacks are not available" |
| Industry sponsorship bias | Unclear risk | "Part of the expenses of this research project was covered by a grant from the clinical research subcommittee of the North West Metropolitan Regional Hospital Board" |
Coope 1986.
| Methods | Randomised multi‐site study in England and Wales | |
| Participants | Primary care setting (physicians' offices)
N = 884 patients 60 years or older: 419 in treatment group and 465 in control group Female = 69.5%; age range 60 to 79; mean 68.8 years; race not stated; mean blood pressure at entry 196/99 mmHg Pre‐existing factors: smoking 24% Inclusion criteria: BP entry criteria: systolic BP 170 to 280 mmHg and/or diastolic BP 105 to 120 mmHg Exclusions: atrial fibrillation, A‐V heart block, ventricular failure, bronchial asthma, diabetes mellitus (needing pharmacological treatment), any serious concomitant disease limiting the prospect of fruitful living, untreated hypertension with levels persistently above 280 mmHg systolic or 120 mmHg diastolic, patients already being treated for hypertension (within 3 months), dementia Follow‐up: 4.4 years (range 1 to 10 years) |
|
| Interventions |
Treatment Step 1 ‐ atenolol 100 mg daily Step 2 ‐ bendrofluazide 5 mg daily Step 3 ‐ methyldopa 500 mg daily Step 4 ‐ any recognised therapy In last 2 years of the trial, several participants were treated with nifedipine retard 20 mg morning and night Control: observation without placebo |
|
| Outcomes | Total mortality ‐ death from all causes Cardiovascular mortality ‐ fatal coronary artery attacks; fatal strokes and fatal ruptured aneurysms CHD mortality ‐ fatal myocardial infarctions; sudden death Cerebrovascular mortality ‐ fatal strokes Cardiovascular M&M ‐ CHD M&M; cerebrovascular M&M; ventricular failure CHD M&M ‐ fatal and non‐fatal myocardial infarctions Cerebrovascular M&M ‐ fatal strokes; major strokes and minor strokes Dropouts due to side effects: not stated Quality of life or functional status outcomes: symptom questionnaires |
|
| Notes | 70% of participants in treatment group were on atenolol, 60% on bendrofluazide, 7% on bendrofluazide only, and 5% on no treatment throughout most of the study. In the control group, 2% of participants were on antihypertensive treatment as BP above 280/120. 7% put on diuretics because of ventricular failure Percentage of participants not on assigned therapy at study end: control group 9%; treatment group 5% Difference in SBP/DBP at end of study in (treatment – control group): ‐18/‐11 mmHg Information was obtained for subgroups from publications using individual patient data from the INDANA database (Gueyffier 1999). Data on mortality were available for the 60‐ to 79‐year age group. Cardiovascular mortality and morbidity in the original Mulrow review included all strokes, MI, sudden death, heart failure, and TIA. However, data on cardiovascular mortality and morbidity without TIA, cerebrovascular mortality and morbidity, and CHD mortality and morbidity were not available for 60‐ to 79‐year‐old participants Coop 1986 is identified as HEP 1986 in original Mulrow 1998, and as Coope and Warrender in Mulrow 1994 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were randomised to treatment or control group on a 50:50 basis without stratification” Comment: method of randomisation was not described |
| Allocation concealment (selection bias) | Low risk | "Randomisation was achieved by opening an opaque envelope supplied in sequence from the trial administrative centre that gave instructions for allocation to the treatment or control group” |
| Blinding of participant and personnel (performance and detection bias) | High risk | Patients and providers were not blinded |
| Blinding of outcome assessment | Low risk | “Medical records of all patients in the treatment and control groups were reviewed by the trial nurses continuously throughout the trial, and every six months they were examined by 2 investigators from the trial administrative centre” “All deaths and major recordable events were reviewed by pilot committee who adjudicated on the status of these events while being unaware of the treatment the patient was receiving” “All ECGs were classified by an experienced coder also blinded to treatment patient was receiving” Assessors of morbidity and mortality outcomes were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Analysis was “intention‐to‐treat” basis. Incomplete outcome data for participants who reached age of 80 years and were in the trial for 5 years “No events that occurred after patients left the practice have been reported in this paper” Percentage of participants lost to follow‐up was not stated |
| Selective reporting (reporting bias) | High risk | "Once the patients reached the age of 80 and had been in the study for 5 years they were excluded from further analyses" "Patients who left the practices were excluded at that time" "For this paper, however, no events that occurred to the patient after leaving the practices were included in the analysis" "A fatal event cancelled out non‐fatal events of the same kind" "In the case of stroke, the most serious, major, minor, or transient ischemic attack was counted" |
| Industry sponsorship bias | Unclear risk | No information provided; therefore unable to judge |
EWPHBPE 1989.
| Methods | Multi‐site randomised placebo‐controlled double‐blind trial conducted in Europe stratified by age, sex, presence or absence of cardiovascular complications, and site Study setting: hospitals (geriatric); physician offices; nursing homes |
|
| Participants | 840 ambulatory patients 60 years or older Geographic region: Europe (Belgium 25%, United Kingdom 19%, Finland 17%, France 14%, Italy 7%, The Netherlands 7%, Ireland 6%, Portugal 3%, Norway 2%, West Germany 1% Ambulatory elderly patients: N = 840 (69.8% female); age range 60 to 97; mean 72.0 years; ethnicity not reported Baseline SBP/DBP was 183/101 mmHg; pulse pressure was 82 mmHg Inclusion criteria: SBP 160 to 239 mmHg and DBP 90 to 119 mmHg; mean blood pressure at entry 182/101 mmHg; pre‐existing factors: smoking 16.4%. Blood pressure (BP) entry criteria: systolic BP 160 to 239 mmHg and diastolic BP 90 to 119 mmHg Exclusion criteria: curable causes of high blood pressure; certain complications of hypertension (i.e. retinopathy grade III or IV, congestive heart failure, history of cerebral or subarachnoid haemorrhage); concurrent disease such as hepatitis or cirrhosis, gout, malignancy, and diabetes mellitus requiring insulin treatment Follow‐up: 7 years. Average follow‐up: placebo 4.63 years; treatment 4.69 years |
|
| Interventions |
Treatment Step 1 ‐ hydrochlorothiazide 25 to 50 mg + triamterene 50 to 100 mg daily Step 2 ‐ methyldopa 250 to 2000 mg daily Control: placebo |
|
| Outcomes | Total mortality ‐ death from any cause
Cardiovascular mortality ‐ CHD mortality plus cerebrovascular mortality CHD (coronary heart disease) mortality ‐ fatal myocardial infarction and ischaemic heart disease, sudden death and fatal arrhythmia, fatal heart failure Cerebrovascular mortality ‐ fatal stroke Dropouts due to side effects: not stated Quality of life or functional outcomes: not stated |
|
| Notes | Percentage not on assigned therapy at study end: placebo group > 35%; treatment group > 35% Difference in blood pressure at study end (Treatment ‐ Control) systolic/diastolic: ‐22/‐10 mmHg Information was obtained for subgroups from publications using individual patient data from the INDANA database (Gueyffier 1999) Data were available for mortality outcomes only but not for cardiovascular events, cerebrovascular events, or CHD events in 60 to 79 or 80 years or older subgroups; therefore overall data for 60 years or older available for the first‐line drugs for hypertension review have been used. Cardiovascular mortality and morbidity outcome does not include TIA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the 840 patients were randomised to placebo (n = 424) or active treatment (n = 416). The placebo and active treatment groups were similar in sex ratio, age, sitting blood pressure at randomisation, weight, and percentage with cardiovascular complications on admission to the trial" Comment: stratified randomisation was utilised but method of random allocation was not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported |
| Blinding of participant and personnel (performance and detection bias) | Low risk | Quote: "a double‐blind randomised placebo controlled trial of antihypertensive treatment was conducted in patients over the age of 60" "Tablets and matching placebos are identical in shape, taste and colour" Comment: both patients and physicians were blinded |
| Blinding of outcome assessment | Low risk | Quote: "data were sent to the coordinating office every three months on specially designed forms, and deaths and other terminating events were classified independently by two investigators into previously agreed categories. These investigators were not aware of the treatment group to which the patients had been assigned. After leaving the double‐blind part of the trial, the surviving patients were followed up until July 1984, but only date and cause of death were recorded" Comment: outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "the intention‐to‐treat analysis was restricted to the cause and date of death because data on non‐fatal events in patients who dropped out from randomised treatment were not available" "During randomised treatment 128 patients defaulted from follow‐up and 52 refused to continue their randomised treatment for various reasons but continued to attend. 38 patients were withdrawn from randomised treatment because of serious intercurrent illnesses (mainly neoplasms). Withdrawal was less frequent in the actively treated group" "One centre with 21 patients withdrew from the trial before its end. In another centre the double‐blind phase was terminated in 29 patients, each followed for 5 yr, because this was the duration to which the patients had agreed. 11 patients were withdrawn from randomised treatment by the local investigators owing to a moderate increase in blood pressure that did not, however, reach the previously established study‐terminating criteria. Similarly, 17 patients were withdrawn by the local investigators on discovery that the patients were no longer hypertensive during a brief period without treatment. In 6 patients the treatment code was broken‐eg, at the request of an anaesthetist. 2 patients had treatment stopped in error and 2 others were withdrawn because the double‐blind drug supply ‐was not available. There were 291 patients still in the double‐blind part of the trial when it was stopped in the summer of 1984" "Both analyses on randomised treatment in the double‐blind part of the trial (on‐randomised‐treatment or per‐protocol analysis) and an overall intention‐to‐treat analysis were performed. The latter was confined to mortality owing to the difficulty in determining morbidity outside the period of double‐blind follow‐up" Comment: 16.3% of patients in the placebo group and 14.2% of those in the treatment group were lost to follow‐up. Data on non‐fatal events in patients who dropped out of the trial were not available |
| Selective reporting (reporting bias) | High risk | Participants were censored if they had "one of the specific study terminating events, including death, non‐fatal cerebral or subarachnoid haemorrhage, development of hypertensive retinopathy grade III or IV, dissecting aneurysm, congestive heart failure not controllable without diuretics or antihypertensive drugs, hypertensive encephalopathy, severe increase in left ventricular hypertrophy, and a rise in blood pressure exceeding the defined limits" Comment: although all terminating fatal events (cardiovascular, non‐cardiovascular, non‐renal, renal, and other causes) as well as non‐fatal, morbid cardiovascular terminating events and non‐fatal, non‐morbid cardiovascular terminating events were reported in the results section, censoring of participants led to high risk of bias |
| Industry sponsorship bias | Low risk | Quote: "this study is supported by the Belgian Hypertension Committee and the World Health Organization. Tablets of alpha methyldopa and placebo were supplied by Merck, Sharp and Dohme; capsules of hydrochlorothiazide and triamterene by Smith, Kline and French" Comment: conflict of interest was not reported; however, this study was not funded by the manufacturer |
HSCSG 1974.
| Methods | Randomised double‐blind placebo controlled trial conducted in USA with a 6‐week drug run‐in phase | |
| Participants | 452 ambulatory stroke survivors with mild to moderate hypertension, 80% African American, mean age 59 years, range < 75 years, 60% men. Baseline SBP/DBP 167/100 mmHg, pulse pressure 67 mmHg. 80% of participants had completed stroke in the year before randomisation. 16% had mixtures of completed stroke and TIA, and 4% had only TIAs Inclusion criteria: SBP ≥ 140 to 220 mmHg and DBP 90 to 115 mmHg and stroke or TIA or both in previous year. Ambulatory, capable of long‐term attendance at treatment clinic, < 75 years of age, and no concomitant disease that might be influenced adversely by prolonged treatment with drug or placebo Follow‐up: 3 years |
|
| Interventions | Treatment: deserpidine 0.5 mg and methyclothiazide 5 mg in a single tablet twice daily Control: no treatment | |
| Outcomes | Cerebrovascular morbidity and mortality (M&M) ‐ fatal and non‐fatal stroke Dropouts due to side effects: for entire study group (i.e. these data were not reported for the > 60 age subgroup) Quality of life or functional status outcomes: not reported | |
| Notes | Definition of stroke used in the trial – “A marked increase in frequency of TIAs (twice the weekly pre‐randomization level of occurrence and more than four per week), or a deterioration of more than eight points in the neurological score, also qualified as a stroke endpoint” A stroke endpoint was defined by the same criteria for entry into the study. It was confirmed by the majority of a committee consisting of 2 members outside of the study and the Central Registry neurologist. A marked increase in frequency of TIAs (twice the weekly prerandomisation level of occurrence and more than 4 per week) or a deterioration of more than 8 points in the neurological score also qualified as a stroke endpoint The scoring system of residual deficits by the neurologist was based on a total of 100 points and allowed a maximum of 35 points for level of consciousness and mentation, 9 points for cranial nerve function, 30 points for motor system, 3 points for reflexes, 3 points for sensory function, and 20 points for "health and performance" function "The study was terminated earlier than planned when it became evident that further follow‐up would not significantly affect the results. All patients without endpoints were under observation for at least one year; the mean follow‐up period for all individuals including those with end points was 27.4 months, and for those not having endpoints, it was 38.6 months" "Forty‐nine who entered the drug trial were not subsequently randomized" Difference in blood pressure at study end (Treatment ‐ Control) systolic/diastolic: ‐27/‐12 mmHg; estimated from graphical presentation of data and for entire study group (i.e. these data were not reported for the > 60 age subgroup) Data on cardiovascular mortality and morbidity (definition not provided), cerebrovascular events, and CHD events for the 60‐ to 75‐year subgroup were available in the original Mulrow 1998 review. Data on mortality were not available Dropouts due to side effects: for entire study group (i.e. these data were not reported for the > 60 age subgroup) during 6‐week pre‐trial run‐in phase with treatment drugs 1.4%; during post‐randomisation period on treatment drugs 3% HSCSG 1974 is identified as HTN COOP 1974 in original Mulrow 1998 review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "a prospective double blind cooperative study was undertaken to determine the influence of treatment on the prognosis in stroke survivors with mild to moderate hypertension. If no intolerable side effects occurred, the patient was placed on a regimen of two tablets daily of randomized medication” "To ensure that drug and placebo were balanced among groups with characteristics of possible prognostic importance, patients were divided into cells based on these characteristics, and drug or placebo was prescribed to maintain a balance within these cells. The characteristics for which this randomization was conducted were sex, race, diastolic blood pressure above or below 100 mm Hg, and the four stroke categories" "Although no effort was made to assure an equal distribution of drug‐treated and placebo‐treated patients within each clinic, the drug‐placebo ratio differed appreciably in only two of the ten clinics" "No statistically significant differences were noted in the frequency of abnormalities in the laboratory findings, ECGs, and chest X ray films between the drug and placebo groups" Comment: the method for random sequence generation was not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Quote: "the biostatistical section was responsible for assignment of patients to drug or placebo regimens, distribution of medication by mail to the individual clinics, data preparation, coding, and analysis" “For use in an emergency, a sealed envelope held by a disinterested person at the local clinic identified the type of medication the patient was receiving" Comment: the method for allocation concealment was not reported |
| Blinding of participant and personnel (performance and detection bias) | Low risk | Quote: "a prospective double blind cooperative study" "Neither the doctor nor the patient was aware of whether placebo or drug had been supplied. For use in an emergency, a sealed envelope held by a disinterested person at the local clinic identified the type of medication the patient was receiving" Comment: participants and treating physicians were blinded |
| Blinding of outcome assessment | Low risk | Quote: "the report of the stroke event and the neurological findings were submitted to Central Registry for confirmation. A stroke endpoint was defined by the same criteria for entry into the study. It also was confirmed by a majority of a committee consisting of two members outside of the study and the Central Registry neurologist" "Similarly, any medical event justifying removal of the patient from the study was carefully reviewed and classified into cardiovascular and non‐cardiovascular categories. The events of a cardiovascular nature were confirmed by an outside cardiologist" Comment: outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "five‐hundred one patients were exposed to pre randomization drug trial. Forty‐nine who entered the drug trial were not subsequently randomized" Of the 452 participants randomised, total withdrawals are not reported "The study was terminated earlier than planned (3 years follow up) when it became evident that further follow‐up would not significantly affect the results. All patients without endpoints were under observation for at least one year; the mean follow‐up period for all individuals including those with endpoints was 27.4 months, and for those not having endpoints, it was 38.6 months" "The high degree of cooperation over the long period of the observation is worthy of comment. Only 30 patients (7%) of those randomised were unreliable" Comment: attrition rate was not mentioned, and how data for these patients were analysed was not reported |
| Selective reporting (reporting bias) | Low risk | Comment: protocol is not available. Cerebrovascular and cardiovascular outcomes, blood pressure measurements, drug intolerance, and laboratory measurements were reported |
| Industry sponsorship bias | Low risk | This investigation was supported by grants from the National Institute of Neurological Diseases and Stroke |
HYVET 2008.
| Methods | Randomised double‐blind placebo‐controlled multi‐site outpatient study conducted in Western Europe (86 patients), Eastern Europe (2144), China (1526), Australasia (19), and Tunisia (70) | |
| Participants | 3845 participants (61% women); age range 80 to 105; mean age = 84 years Pre‐existing factors: cardiovascular disease = 12.0%; hypertension = 89.9%; antihypertensive treatment = 64%; stroke = 6.8%; myocardial infarction = 3.1%; diabetes = 6.8%; heart failure = 2.9%; smoking = 6.5% Blood pressure (BP) entry criteria: mean of the 4 systolic blood pressure measurements taken at the second and third visits (2 at each visit) was between 160 and 199 mmHg. Baseline BP 173.0/90.8 mmHg. Pulse pressure 82.2 mmHg. Target BP was < 150/80 mmHg Exclusion criteria: accelerated hypertension (retinal haemorrhage, exudates, or papilledema); overt clinical congestive heart failure requiring treatment with diuretic, vasodilator, or ACE inhibitor; renal failure; documented cerebral or subarachnoid haemorrhage; condition expected to severely limit survival (e.g. terminal illness), inability to stand up, requiring BP‐lowering treatment for reasons other than hypertension (e.g. angina, peripheral); ischaemia, gout; renal artery stenosis; dementia (Mental Test score < 7/10) Follow‐up: 2.1 years (median 1.8 years) |
|
| Interventions |
Treatment Step 1 ‐ indapamide 1.5 mg daily Step 2 ‐ perindopril 2 mg daily Step 3 ‐ perindopril 4 mg daily Control ‐ identical appearing placebos for each step |
|
| Outcomes | Total stroke, total coronary artery disease, total mortality, total cardiovascular events (including CHF) Dropouts due to side effects: not reported Quality of life or functional status outcomes: not reported |
|
| Notes | Difference in blood pressure at study end (Treatment ‐ Control) systolic/diastolic: sitting ‐15.0/‐6.1 mmHg, standing ‐14.7/‐5.4 mmHg. Percentage of participants not on assigned therapy at study end: active treatment 0.8%, placebo 0.6%. Corresponded with the study author to request missing information Data on mortality, cardiovascular mortality and morbidity (includes CHF but excludes TIA), cerebrovascular mortality and morbidity, and CHD mortality and morbidity are available for the 80 years or older subgroup |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation: sequence generation was not reported. Randomisation was stratified according to age (80 to 89 years and 90 years or older) and sex; permuted blocks of 4 and 6 of any 10 participants were used to ensure roughly equal assignment to each of the 2 groups within large centres Comment: method used for randomisation was not mentioned |
| Allocation concealment (selection bias) | Low risk | An interactive voice response system (IVRS) was employed to tell the investigator which 6‐month drug pack to prescribe |
| Blinding of participant and personnel (performance and detection bias) | Low risk | The main trial was a randomised double‐blind placebo‐controlled trial Comment: patients and providers were blinded |
| Blinding of outcome assessment | Low risk | Quote: "the Endpoint Committee will provide an objective blinded evaluation of previously defined end‐points" "All events that were possible end points were reviewed by an independent committee, unaware of the group assignment, using predefined definitions from the protocol" Comment: outcome assessment was done in an independent manner and outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Percentage lost to follow‐up: active treatment 0.3%, placebo 0.6%. Reported on the number of participants lost to follow‐up (16 patients) "...vital status was unknown in 17 patients..." "The primary analysis was performed according to the intention‐to‐treat principle" Comment: small losses to follow‐up; ITT analysis was used |
| Selective reporting (reporting bias) | High risk | All primary and secondary outcomes mentioned in the objectives were reported in the results. Cannot extract the number of participants in each group who had non‐fatal myocardial infarction Correspondence with the study author Question: "the serious adverse events noted in the publication...are the numbers the total serious adverse events OR was the first event counted and analysed? Answer: it is the total number of SAEs. Patients could contribute more than one SAE" Question: "if a patient had an event after being censored, were those events counted? If not, is it possible to see that data? Answer:it would depend on the event. If it was a recurrent endpoint, then it was not counted (e.g. a further non‐fatal stoke). If the event was a new endpoint (e.g. a fatal MI in someone who had previously had a non‐fatal stroke), then it was counted" |
| Industry sponsorship bias | Low risk | Quote: "supported by grants from the British Heart Foundation and the Institut de Recherches Internationales Servier. Drs Beckett and Peters and Mr Banya report receiving grant support from the Institut de Recherches Internationales Servier; Dr Staessen, consulting fees from Pfizer, Tanabe, Daiichi‐Sankyo, and Sigma‐Tau and speakers’ fees from Pfizer, Tanabe, and Bayer; Dr Anderson, consulting fees from Boehringer Ingelheim and Servier and speakers’ fees from Boehringer Ingelheim, Servier, AstraZeneca, and Sanofi‐Aventis; Dr Forette, consulting fees from Wyeth Elan, Sanofi‐Aventis, and Bristol‐Myers Squibb and speakers’ fees from Servier, AstraZeneca, and Sanofi‐Aventis; Dr Rajkumar, speakers’ fees from Schering‐Plough, Merck Sharp & Dohme, and Menarini; and Dr Bulpitt, consulting fees from Imperial College Consulting, a consultancy funded by a grant from the Institut de Recherches Internationales Servier" "No other potential conflict of interest relevant to this article was reported" Comment: some of the doctors received consulting fees and speakers' fees from the pharmaceutical companies, though researchers received grants from the British Heart Foundation and the Institut de Recherches Internationales Servier |
HYVET P 2003.
| Methods | Randomised open multi‐site trial conducted in Europe. Most patients enrolled were from Bulgaria 1130 (88%), with 39 (3%) from Spain, 39 (3%) from Romania, 32 (2.5%) from the UK, 20 (1.5%) from Poland, and smaller numbers from Finland, Lithuania, Ireland, Greece, and Serbia | |
| Participants | Study setting: both primary and secondary care
1283 participants (63% women); age range 79.5 to 96.1; mean age = 84 years; race: not stated Blood pressure (BP) entry criteria: systolic blood pressure (average of 4 readings) 160 to 219 mmHg, diastolic blood pressure 95 to 109 mmHg (later changed to 90 to 109 mmHg), and standing systolic blood pressure > 140 mmHg (average of 2 readings). Mean blood pressure at entry: systolic blood pressure averaged 181.5 ± 11.3 mmHg (range 160 to 217 mmHg) and entry diastolic pressure averaged 99.6 ± 3.4 mmHg (range 90 to 114 mmHg). Pulse pressure was 82 mmHg. Target blood pressure was < 150/80 mmHg Pre‐existing factors: patients were not obese, with an average body mass index of 25 kg/m²; 48% had been previously treated, 3.0% had a previous myocardial infarction, 4.5% had a previous stroke, and 20.7% drank more than 1 unit of alcohol per day Smoking: 4.2% Target blood pressures were sitting systolic pressure < 150 mmHg plus sitting diastolic pressure < 80 mmHg Exclusion criteria: serum creatinine > 150 mol/L, accelerated hypertension, congestive heart failure requiring treatment, inability to stand, cerebral or subarachnoid haemorrhage in past 6 months, need for blood pressure‐decreasing treatment because of angina etc., presence of gout, renal artery stenosis, dementia (abbreviated mental test score 7/10 (4)), and a condition expected to limit survival severely Follow‐up: 13 months |
|
| Interventions |
Treatment Step 1 ‐ diuretic (usually bendrofluazide 2.5 mg), an ACE inhibitor (usually lisinopril 2.5 mg), or no treatment Step 2 ‐ involved doubling the dose of the first drug Step 3 ‐ involved adding diltiazem slow‐release 120 mg daily Step 4 ‐ involved adding diltiazem slow‐release 240 mg daily Control ‐ no treatment |
|
| Outcomes | Total stroke, total mortality, cardiovascular mortality, cardiac mortality, sitting systolic BP and diastolic BP Dropouts due to side effects: not reported Quality of life or functional status outcomes: not reported |
|
| Notes | "As the trial was a pilot trial with limited numbers and a short period of follow‐up, interim analyses were not performed. Similarly, although power calculations are published, they are not relevant to the pilot trial. All analyses are presented on an intention‐to‐treat basis" "The main weaknesses of the pilot trial were that it was an open study and also was not conducted to the standards of Good Clinical Practice. The problem with the use of an open design is that both patient and investigator know the treatment given. This can lead to bias in several different ways. Investigator bias may affect what is written on a death certificate: for example, if the patient has both a myocardial infarction and a stroke before death, the investigator may tend to record a stroke as the underlying cause of death if the patient is receiving no treatment and blood pressure is high" Percentage of patients not on assigned therapy at study end: diuretic 97%, ACEI 96%, no treatment 99.2% Difference in blood pressure at study end (Treatment ‐ Control): sitting BP difference between diuretic/ACEI and no treatment ‐23/‐11 mmHg; standing BP difference between diuretic and no treatment ‐23/‐11 mmHg; and difference between ACEI and no treatment ‐24/‐12 mmHg Data on mortality, cardiovascular mortality and morbidity (includes fatal and non‐fatal stroke, fatal MI, other fatal ischaemic heart disease, sudden death, fatal congestive heart failure, fatal atherosclerosis, fatal pulmonary embolism, fatal hypertension, fatal aortic aneurysm but does not include TIA), cerebrovascular mortality and morbidity (includes fatal and non‐fatal stroke), and CHD mortality and morbidity (includes fatal MI, sudden death, and death due to other ischaemic heart disease and congestive heart failure) are available for the 80 years or older subgroup |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "in the pilot trial, patients older than 80 years and with hypertension were allocated randomly but equally to groups to receive a diuretic‐based regimen, an angiotensin‐converting enzyme (ACE)‐based regimen or to no treatment" "The unit of randomisation was the individual and the SAS Random Allocation of Treatments Balanced in Blocks Program was used to generate the schedule." Restricted random allocation to groups was used to ensure equal allocation per group within each centre and allocation to groups was performed centrally. Stratified into four groups on the basis of sex and age (80–89 years and ≥ 90 years)" Comment: randomisation done; baseline characteristics similar in all treatment groups |
| Allocation concealment (selection bias) | High risk | Quote: "restricted random allocation to groups was used to ensure equal allocation per group within each centre and allocation to groups was performed centrally" "The pilot HYVET trial was an open design that worked well, but concerns were expressed that only the results of a double‐blind trial conducted to Good Clinical Practice guidelines would be acceptable in the 21st century" (page 2409) Comment: method used for allocation concealment was not specified; this probably was not done, as it was an open‐label pilot study |
| Blinding of participant and personnel (performance and detection bias) | High risk | "The trial recruited individuals from both primary and secondary care and was of an open design" Comment: patients and providers were not blinded |
| Blinding of outcome assessment | High risk | Outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Of the 1283 patients who were assigned to groups, only 27 (2.1%) were lost to follow‐up (had no end‐of‐trial information)" (diuretic 2%, ACEI 2%, no treatment 2%) "Of the 426 patients allocated randomly to a diuretic‐based treatment, 385 (88.5%) were alive and provided information at the end of the trial. The corresponding numbers were 397 (89.8%) for ACE based treatment and 394 (90.1%) for no treatment" "Both the investigators’ and the patients’ knowledge of treatment may affect the withdrawal rates, for example favouring the removal from the trial of a patient who is receiving no treatment but has high blood pressure that approaches but does not exceed a terminating outcome" Comment: number of participants lost to follow‐up low and reasons for attrition not mentioned, although small attrition could not have affected the outcome |
| Selective reporting (reporting bias) | Low risk | Quote: "the main endpoints of the trial were stroke events, total mortality and cardiovascular, cardiac and stroke mortality" "As this was an open study, the randomised treatment could be continued after a non‐fatal event" Comment: all endpoints were reported in the results section |
| Industry sponsorship bias | Low risk | Quote: "the pilot trial was supported by the British Heart Foundation" |
Kuramoto 1981.
| Methods | Randomised double‐blind placebo‐controlled single‐site study conducted in ambulatory patients in a home for the aged in Tokyo, Japan | |
| Participants | 91 patients 60 years or older; 45% female; mean age 76.1 years; race not stated. Pre‐existing factors not reported. Blood pressure (BP) entry criteria not clearly stated. Mean blood pressure at entry: 169/86 mmHg (isolated systolic hypertension in 44% of participants). Pulse pressure 83 mmHg Inclusion criteria were SBP/DBP 160/90 mmHg to < 200/110 mmHg Exclusion criteria were not mentioned Follow up: 2.7 years |
|
| Interventions | Treatment: trichlormethiazide 1 to 4 mg (80% monotherapy) Reserpine (0.3 mg), methyldopa (125 to 500 mg), and hydralazine (50 to 100 mg) added Control: placebo |
|
| Outcomes | Mortality, stroke, CHD, CHF Cardiovascular mortality and morbidity includes fatal and non‐fatal stroke, and cerebral haemorrhage fatal and non‐fatal MI, plus CHF with arrhythmia Systolic BP and diastolic BP Dropout due to side effects: not reported Quality of life or functional status outcomes: not reported |
|
| Notes | Difference in blood pressure at study end (Treatment ‐ Control) systolic/diastolic: 20/7 mmHg (Mulrow 1994) Data on mortality, cardiovascular mortality and morbidity (does not include TIA), cerebrovascular mortality and morbidity, and CHD mortality and morbidity are available for 60 or older patients and not for 60 to 79 years and 80 years or older subgroups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "described as randomised double blind placebo controlled study" "The matched pair group was selected by the age, sex, and blood pressure levels during the drug‐off control period of about 1 year" Comment: method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation of individuals within matched pairs to treatment and control groups made by a blinded statistical co‐ordinator; thought to be randomised but not entirely clear (unpublished information as per personal conversation with author from Mulrow 1998) |
| Blinding of participant and personnel (performance and detection bias) | Low risk | Quote: "a double‐blind study utilizing 87 out‐patients has been conducted in the Baltimore City Hospitals hypertension clinic to examine the feasibility and value of maintaining patients with essential hypertension on effective long‐term hypotensive therapy" Comment: although study does not state whether patients and physicians were blinded, patients and providers were blinded (unpublished information as per personal conversation with author from Mulrow 1998) |
| Blinding of outcome assessment | High risk | Outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "patients were excluded from the trial when the blood pressure exceeded 200/110, and appearance of cerebrovascular or cardiac complications, other diseases which needed hospital admission, death, or moving out from home were considered to be drop out" "As a whole 9 out of 41 cases or 22.0% in the placebo group, and 4 out of 38 cases or 10.5% in the drug group, dropped out by cerebrovascular or cardiac complications. In addition to the cerebrovascular and cardiac complications, dropouts due to blood pressure elevation were observed in 8 cases in the placebo group, and total dropouts in the placebo group were 17 cases or 41.5%. This incidence was significantly higher than that in the drug treated group (Table IV)" "Six cases of dropout due to moving out from the home were observed in both groups, and follow‐up cases were 38 in the drug group and 41 in the placebo group" For blood pressure measurements, the number of follow‐up cases in the placebo group decreased markedly from 47 to 32, 24, 13, and 7 at the end of each year. The number of follow‐up cases at the end of each year in the drug group declined from 44 to 32, 26, 25, and 22 due to dropouts Comment: follow‐up of participants was incomplete |
| Selective reporting (reporting bias) | Unclear risk | Protocol is not available Comment: insufficient information to judge selective reporting bias |
| Industry sponsorship bias | Unclear risk | Comment: no mention about source of funding and conflict of interest |
MRC‐O 1992.
| Methods | Randomised single‐blind placebo‐controlled multi‐site study conducted in general practice setting in England, Scotland, and Wales | |
| Participants | 4396 ambulatory patients 60 years or older; age range 60 to 74; mean 70.3 years; male 42%; 58% female; race not reported. Mean blood pressure at entry: 184/91 mmHg; pulse pressure 94 mmHg
Inclusion criteria: BP entry criteria: systolic BP 160 to 209 mmHg and diastolic BP < 115 mmHg Exclusion criteria: known or suspected secondary hypertension; taking antihypertensive drugs; cardiac failure or any other accepted indication for antihypertensive treatment; receiving treatment for angina pectoris; history of myocardial infarction or stroke within preceding 3 months; impaired renal function; diabetes; asthma; serious intercurrent disease, including malignancy, known to be present at time of examination; serum potassium concentration ≤ 3.4 mmol/L or > 5.0 mmol/L Pre‐existing risk factors: myocardial infarction: excluded if within last 3 months; stroke: excluded if within last 3 months; diabetes: excluded; smoking: 17.5% Follow‐up: 5.8 years |
|
| Interventions |
Diuretic arm Step 1 ‐ hydrochlorothiazide 25 mg or 50 mg + amiloride 2.5 mg or 5 mg daily Step 2 ‐ atenolol 50 mg daily Step 3 ‐ nifedipine up to 20 mg daily Step 4 ‐ other drugs Beta blocker arm Step 1 ‐ atenolol 50 mg daily Step 2 ‐ hydrochlorothiazide 25 mg or 50 mg + amiloride 2.5 mg or 5 mg daily Step 3 ‐ nifedipine up to 20 mg daily Step 4 ‐ other drugs Control ‐ matching placebo |
|
| Outcomes | Mortality, stroke, CHD, systolic BP, diastolic BP Dropouts due to side effects Quality of life or functional outcomes |
|
| Notes | Percentage not on assigned therapy at study end (including withdrawals and losses to follow‐up): placebo group 53%; diuretic arm 48%; beta blocker arm 63% Difference in blood pressure at study end (Treatment ‐ Control) ‐ systolic/diastolic: ‐6.3/‐5.9 mmHg Dropouts due to side effects: control group 82 (3.7%); diuretic arm 160 (14.8%); beta blocker arm 333 (30.2%) Quality of life or functional outcomes: no perceptible negative effect of treatment compared to control on measures of cognitive function Data on mortality, cardiovascular mortality and morbidity (does not include TIA), cerebrovascular mortality and morbidity, and CHD mortality and morbidity are available for 60‐ to 74‐year‐old patients |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "all trial entrants were randomly allocated in equal proportions to one of the four treatment categories...." Randomisation was stratified by gender and site; at each site, participants were assigned to therapy based on computer‐generated lists Comment: baseline characteristics were similar |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participant and personnel (performance and detection bias) | High risk | Quote: "this trial was single‐blind; patients did not know in which treatment group they were in; but the doctors and nurses" (page 406) Comment: patients were blinded; providers were not blinded |
| Blinding of outcome assessment | Low risk | Quote: "the records of all patients were "flagged" at Southport NHS center register to ensure notification of death. The diagnostic evidence for each terminating event was assessed by the arbitrator, blind to the treatment regimen. World Health Organization criteria for classification of strokes and coronary events were used. All available documentation was reviewed, including copies of general practitioners' notes, hospital inpatient and outpatient notes, electrocardiographic recordings, necropsy findings, and death certificates" "Data on terminating events were analysed after every 5000 patient years and were reviewed by an independent monitoring and ethics committee" Comment: outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "over five and a half years about 25% of people were lost to follow up. The cumulative percentage of people who stopped taking their randomised treatment, including both those withdrawn but continuing on follow up and those lost to follow up, 48% of the diuretic group, 63% of the beta‐blocker group, and 53% of the placebo group" "Overall, the beta‐blocker group had significantly more withdrawals than diuretic groups" Comment: loss to follow‐up was high; only selected reasons for the beta blocker group were provided. Reasons pertinent to respective groups were not mentioned. Insufficient detail was provided to determine if intention‐to‐treat analysis was carried out correctly |
| Selective reporting (reporting bias) | High risk | "A patient's participation in a trial ended with a stroke, whether non‐fatal or fatal; coronary events; other cardiovascular events, and death from any cause" "If a patient had a non‐fatal event followed by a fatal event in the same category, only the fatal event was included in the analyses. If a patient had two events in different categories, for example, a non‐fatal stroke then a coronary event (fatal or non‐fatal), then both were included" Morbidity and mortality data were reported as stated in the objectives |
| Industry sponsorship bias | Low risk | Quote: "the trial was supervised by an MRC working party and coordinated by the MRC Epidemiology and Medical Care Unit at Northwick Park Hospital, Harrow" The source of funding for carrying out the trial was not mentioned, nor was the relation of investigators or any member of the MRC working party to the manufacturers/suppliers of medications for the trial |
MRC‐TMH 1985.
| Methods | Randomised single‐blind trial comparing 2 treatments and placebo in ambulatory young patients in England, Scotland, and Wales | |
| Participants | 17,354 participants (8306 male and 9048 female) with mean age 52 years; range 35 to 64 years Ethnicity not reported. Male 52%. Baseline mean SBP/DBP 161.4/98.2 mmHg; pulse pressure 63 mmHg Inclusion criteria: SBP < 200 mmHg and DBP 90 to 109 mmHg Patients in 60‐ to 64‐year‐old age group ‐ thaizide = 686; beta blocker = 729; placebo = 1398 Exclusion criteria: secondary hypertension; taking antihypertensive treatment; normally accepted indications for antihypertensive treatment (such as congestive cardiac failure) present; myocardial infarction or stroke within the previous 3 months; presence of angina, intermittent claudication, diabetes, gout, bronchial asthma, serious intercurrent disease, or pregnancy Follow‐up: 5 years |
|
| Interventions | Treatment arms: bendrofluazide 10 mg daily or propranolol 80 to 240 mg daily. Methyldopa could be added if required Control: placebo Note: 288 participants were randomly assigned to observation only, taking no tablets, and were merged with placebo |
|
| Outcomes | Mortality, stroke, CHD, systolic BP, diastolic BP No congestive heart failure data |
|
| Notes | Data for the 60‐ to 64‐year‐old subgroup were obtained from the INDANA database through personal communication with Francois Gueyffier The definition of total cardiovascular events did not include heart failure |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly allocated at entry... Randomisation was in stratified blocks of eight within each sex, 10 year age group, and clinic" Comment: no information was provided for sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No description of method for allocation concealment was provided |
| Blinding of participant and personnel (performance and detection bias) | High risk | Quote: "four treatments: the thiazide diuretic bendrofluazide; placebo tablets that looked like bendrofluazide; the beta blocker propranolol; and placebo tablets that looked like propranolol. The two placebo groups were treated as one in all analyses" Quote: "when the protocol was written, it was judged unreasonable to ask general practitioners to undertake such adjustments in a double blind study, and the trial was therefore single blind only" Comment: participant was blinded but not the physician |
| Blinding of outcome assessment | Low risk | Quote: "the evidence on which the diagnosis of each terminating event was based was assessed by an arbitrator ignorant of the treatment regimen... The arbitrator used WHO criteria for classification" "All events were assessed by an independent arbiter who was blind to the treatment regimen" "Each electrocardiogram tracing was read by two observers who were blind to the treatment regimen; the second reader was also blind to the first reader's coding. If these two readers disagreed, a third reader was used" Comment: adjudication was independent and blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "all analyses presented here are based on randomised treatment ("intention to treat") categories. Thus data for all participants are presented as if the individual was still in the treatment group to which he was originally randomised, although substantial percentages of patients (see below) were in fact withdrawn from their randomly allocated regimen during follow up" Quote: "the total five and a half year cumulative percentages of men who stopped taking their randomised treatment, including both those withdrawn from their randomly allocated regimen but continuing on follow up and those lapsing from the trial, were 43% of the bendrofluazide group, 42% of the propranolol group, and 47% of the placebo group. For women the figures were 33%, 40%, and 40% respectively. The cumulative percentages of people not taking either primary active drug by five and a half years were smaller: 33% of men originally randomised to bendrofluazide and 34% of men randomised to propranolol and 28% and 31% respectively of women" Quote: "events terminating a patient’s participation were: stroke, whether fatal or non‐fatal; coronary events, including sudden death thought to be due to a coronary cause, death known to be due to myocardial infarction, and non‐fatal myocardial infarction; other cardiovascular events, including deaths due to hypertension (ICD 400‐404) and to rupture or dissection of an aortic aneurysm; and death from any other cause. Clinic staff reported these events to the coordinating centre. The records of all patients who suffered non‐fatal terminating events and of any others who lapsed from the trial, whatever the reason, were “flagged” at the Southport NHS central register to ensure notification of death)" Comment: myocardial infarction and stroke were reasons for terminating study follow‐up, except for death flagging. This induces a censoring attrition bias, limited to the occurrence of non‐fatal events, myocardial infarction, or stroke |
| Selective reporting (reporting bias) | Low risk | No information about prespecified outcomes is available on which to make this assessment. However the aim of the study was to study mortality and morbidity, which have been reported |
| Industry sponsorship bias | High risk | Conflict of interest was not reported "The working party thanks the general practitioners and nurses collaborating in the trial; the staff at the coordinating centre; the staff of the Wolfson Research Laboratories, Queen Elizabeth Medical Centre, Birmingham, for carrying out the biochemical analyses; Duncan, Flockhart and Co Ltd for tablets of bendrofluazide and placebo; Imperial Chemical Industries Ltd for financial support and for tablets of propranolol and placebo; Ciba Laboratories for supplies of guanethidine; and Merck Sharp and Dohme Ltd for a mobile screening unit, funds for its staffing, and supplies of methyldopa" |
SHEP 1991.
| Methods | Randomised double‐blind placebo‐controlled multi‐site study in community ambulatory patients conducted in USA | |
| Participants | 4736 participants; 55.8% female; age range 60 to > 80; mean 72 years; male 43%; race: white non‐Hispanic (79.2%), black (13.8%), Hispanic (1.8%), Asian (4.3%), other (0.9%); mean blood pressure at entry 170/77 mmHg
Pre‐existing risk factors: myocardial infarction 4.9%; stroke 1.4%; diabetes 10.1%; smoking 12.7% Blood pressure (BP) entry criteria: systolic BP 160 to 219 mmHg and diastolic BP < 90 mmHg. Baseline mean SBP/DBP was 170/77 mmHg and pulse pressure was 93 mmHg Exclusion criteria: history and/or signs of major cardiovascular diseases likely to require pharmacologic and other treatment (e.g. previous myocardial infarction, coronary artery surgery, major arrhythmias, conduction defect, recent stroke, carotid artery disease, history of transient ischaemic attack (TIA) with bruit matched with TIA localisation, 2 or more TIAs and signs or symptoms in a single neurological distribution); other major diseases (e.g. cancer, alcoholic liver disease, established renal dysfunction) with competing risk factors for the primary endpoint ‐ stroke; presence of medical management problems (e.g. insulin‐dependent diabetes, history of dementia, evidence of alcohol abuse); bradycardia; people maintained on beta blockers, diuretics, other antihypertensive drugs, anticoagulants, or experimental drugs on recommendation of their physicians Follow‐up: 4.5 years |
|
| Interventions |
Treatment Step 1 ‐ chlorthalidone 12.5 or 25 mg daily Step 2 ‐ atenolol 25 or 50 mg or reserpine 0.05 or 0.10 mg daily Control ‐ placebo |
|
| Outcomes | Mortality, stroke, CHD, CHF, systolic BP, diastolic BP Dropouts due to side effects Quality of life or functional outcomes |
|
| Notes | Percentage not on assigned therapy at study end: placebo group 44% and treatment group 10% Difference in blood pressure at study end (Treatment ‐ Control) systolic/diastolic: ‐11.1/‐3.4 mmHg Dropouts due to side effects: control group 7%; treatment group 13% Quality of life or functional outcomes: no perceptible negative effect of treatment compared to control on measures of cognitive, physical, and emotional function Information was obtained for subgroups from publications using individual patient data from the INDANA database (Gueyffier 1999) Data on mortality, cardiovascular mortality and morbidity (does not include TIA), cerebrovascular mortality and morbidity, and CHD mortality and morbidity are available for 60 to 79 years and 80 years or older subgroups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “stratified randomization by antihypertensive drug treatment status at initial contact and by center produced two SHEP groups—assigned to active treatment and placebo—comparable at baseline” "Each randomisation was carried out by telephone" “Both treatment groups were generally comparable to the several traits assessed” Comment: randomisation was adequately done and baseline characteristics of 2 groups were well matched |
| Allocation concealment (selection bias) | Low risk | Quote: "the random assignment to one of the two study groups was to be made by the Coordinating Center and transmitted to the clinical center by telephone after verification of eligibility (inclusion and exclusion criteria). Each participant was to be assigned a drug bottle number for the first step and dosage of the treatment program. A randomization report was then to be mailed to each clinical center" Comment: participants were randomly allocated by co‐ordinating centre, and allocation concealment seems to have been performed adequately |
| Blinding of participant and personnel (performance and detection bias) | Low risk | Quote: "SHEP was a long term, multicenter, randomized, double‐blind, placebo controlled trial sponsored by the National Heart, Lung and Blood Institute and National Institute of Ageing" "Participants were to be randomized at each center to either chlorthalidone or matching placebo in a double‐blind manner" “Drug dosage was doubled (including matching placebo) for participants failing to achieve the SBP goal at follow‐up visits” Comment: both participants and treating physicians were not aware of the treatment given |
| Blinding of outcome assessment | Low risk | Quote: “occurrence of study events listed above was confirmed by a coding panel of three physicians blind to randomization allocation” "The SHEP endpoint committee, which was masked to results by treatment group and individual participant treatment assignment, coded strokes, causes of death, and selected nonfatal outcomes. Documented criteria [1, 2a, 2b] were used in assessing outcomes. At each of its meetings, the DSMB was satisfied that the ascertainment of outcomes was not biased" "The progress of the study and the safety of the participants were reviewed on a regular basis by an independent data and safety monitoring board" (page 982; Probstfield et al 1989) Comment: morbidity and mortality outcome assessment was carried out independently |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "by July 1990, there were 661 initial reports of strokes and deaths. Of these, 90.3%, or 587, had complete information of which 579 had been coded by the endpoint committee. By December 1990, there were 721 reports of strokes and deaths, and 94.9%, or 684, had complete information and 666 had been coded. Primary outcome determination was complete for 99.8% of the participants" “All analyses are to be based on participants’ original treatment group assignment (i.e. the “intention to treat” principle)" Comment: there was complete follow‐up of 99.8% of patients; therefore assessed as low risk of bias |
| Selective reporting (reporting bias) | Low risk | Comment: primary endpoints such as non‐fatal and fatal stroke over a 5‐year period; secondary endpoints such as non‐fatal myocardial infarction and fatal coronary heart disease and major CVD morbidity and mortality were reported |
| Industry sponsorship bias | Low risk | Quote: "the SHEP trial was supported by contracts with the National Heart, Lung and Blood Institute and the National Institute on Aging. Drugs were supplied by the Lemmon Co., Sellersville, Pa; Wyeth laboratories/Ayerst laboratories and AH Robims Co.; Richmond Va; Stuart Pharmaceuticals, Welmington, Del. It is pleasure to acknowledge the contribution of the investigators and the staff at the 16 clinical centers and coordination and service centers of the SHEP Cooperative Research Group" Comment: study was sponsored by the NHLBI; no conflict of interest was declared |
SHEP‐P 1989.
| Methods | Randomised double‐blind placebo‐controlled multi‐site study in community ambulatory patients in USA | |
| Participants | 551 participants; 63% female; age range: > 60 (15% > 80); mean 72 years; race: white (82%); non‐white (18%); male (37%); mean blood pressure at entry 172/75 mmHg; pulse pressure 93 mmHg. Pre‐existing risk factors: myocardial infarction 4%; stroke 1%; smoking 11%
Inclusion criteria: SBP 160 to 219 mmHg and DBP < 90 mmHg Exclusion criteria: coronary bypass surgery within 2 years; heart attack within 6 months; stroke with residua; current treatment with antihypertensive drugs, insulin, or anticoagulants; allergy to study medications; specified arrhythmias or a pacemaker; uncontrolled congestive heart failure; serum creatinine level 2.0 mg/dL or more; alcohol abuse; cancer or other life‐threatening disease; chronic obstructive pulmonary disease; peripheral vascular disease with tissue injury; senile dementia; residence in a nursing home; carotid bruit with history of transient ischaemic attacks; history of malignant hypertension Follow‐up: 3 years |
|
| Interventions |
Treatment Step 1 ‐ chlorthalidone 25 to 50 mg daily (87%) Step 2 ‐ randomised to hydralazine 25 mg twice daily, reserpine 0.05 mg twice daily, or metoprolol 50 mg twice daily (13%) Control ‐ placebo |
|
| Outcomes | Mortality, CHD, stroke, CHF, systolic BP, diastolic BP Dropouts due to side effects reported at 12 months Quality of life or functional outcomes not reported |
|
| Notes | Percentage not on assigned therapy at study end: placebo group 40% and treatment group 30%. Difference in blood pressure at study end (Treatment ‐ Control) systolic/diastolic: ‐17/‐5 mmHg. Dropouts due to side effects (at 12 months; data not reported for end of study): control group 2 (1.8%); treatment group 7 (1.6%) Information was obtained for subgroups from publications using individual patient data from the INDANA database (Gueyffier 1999) Data on mortality, cardiovascular mortality and morbidity (does not include TIA), cerebrovascular mortality and morbidity, and CHD mortality and morbidity are available for 60 to 79 years and 80 years or older subgroups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “the pilot study of the Systolic Hypertension in the Elderly Program was a randomized, double‐blind, placebo‐controlled trial of drug therapy for isolated systolic hypertension” (Perry et al Stroke 1989; 20: page 4) “Each randomization was carried out by telephone between the clinic staff and the coordinating center data manager, who checked that eligibility criteria were met before assigning the participant to chlorthalidone or placebo. We used an adaptive randomization procedure that varied treatment assignment probabilities by 10% in one or the other direction in order to balance the step I study groups within race, sex, age and baseline systolic BP strata” Comment: randomisation was carried out in a proper manner, and baseline characteristics were matching, although minor differences were seen in the medical history and physical examination, which were relatively small and could not affect the outcome |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participant and personnel (performance and detection bias) | Low risk | Quote: "the pilot study of systolic hypertension in the Elderly Program (SHEP‐PS) was a randomized, double‐blind, placebo‐controlled trial, following participants for an average of 34 months" "Upon randomization into the study, participants entered the step‐up protocol and received 25 mg/day of chlorthalidone or placebo (supplied as identical capsules by USV Pharmaceutical Corp)" “Participants receiving step I placebo who had not reached goal underwent a dummy randomization, and all received step II placebo twice daily. Twelve weeks later, the dosage for participants who still had not reached goal was doubled” Comment: participants and treating physicians were blinded |
| Blinding of outcome assessment | Low risk | Quote: "when the necessary documentation for a morbid event was assembled at the Coordinating Center, it was copied and mailed to the three members of the Morbidity and Mortality Committee (a neurologist and two internists). Working independently and without knowledge of the participant's treatment group assignment, each member made a diagnosis based on the criteria of Table 1. The diagnosis of "no event" was also acceptable and was the final diagnosis for five suspected morbid events. A diagnosis was accepted when the three members agreed unanimously" Comment: outcome assessment was done in an independent manner; outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “at the end of SHEP‐PS, the vital status of all participants was known; 512 were alive” “Analysis was by intention to treat according to randomization to Step I medication (chlorthalidone or placebo), regardless of whether a Step II medication was added subsequently” "We specified an “intention to treat” rule (with study groups divided by the randomized assignment regardless of subsequent crossovers) and a plan for replacing any missing annual visit BP with the last available value" Comment: there was no loss to follow‐up |
| Selective reporting (reporting bias) | High risk | All cardiovascular events such as stroke, left ventricular failure, transient ischaemic attack, myocardial infarction, sudden death, angina pectoris, coronary artery surgery, and peripheral vascular disease were reported in the results section "For any participant who had two or more events, one was designated the study event based on a hierarchal classification headed by death followed by four categories of nonfatal events in rank order of stroke, other hypertensive events, atherosclerotic events, and non‐cardiovascular events. When there were two events in one category, the event that occurred first was used" Comment: not all events were reported if they occurred in the same category |
| Industry sponsorship bias | Low risk | Quote: "sponsorship: this study was supported by the National Heart, Lung and Blood Institute: The National Institute of Ageing; in part by the National Institute of Mental Health" Comment: conflict of interest was declared and source of funding was not provided; because the study is not industry sponsored, we assessed it as having low risk of bias |
Sprackling 1981.
| Methods | Randomised open‐label multi‐site study in Nottinghamshire, England | |
| Participants | Study setting: welfare homes for the elderly
123 participants; 74% female; age > 60 years (range not reported); mean age 80.7 years; 83% of patients were over 74 years; race: not stated Mean blood pressure at entry 199/106 mmHg; pre‐existing factors: stroke 11.3% Inclusion criteria: elderly patients with BP entry criteria: diastolic BP ≥ 100 mmHg Exclusion criteria: not reported Follow‐up: not clearly stated; approximately 4 to 5 years |
|
| Interventions | Treatment: methyldopa 250 mg twice daily Control: observation without placebo | |
| Outcomes | Total mortality: death from all causes Cardiovascular morbidity and mortality: myocardial infarction, stroke, heart failure, or deterioration of pre‐existing heart failure (does not include TIA) Dropouts due to side effects | |
| Notes | Dropouts due to side effects: control group not stated (implied 0%); treatment group 9 (15%) Difference in blood pressure at study end (Treatment ‐ Control) systolic/diastolic: ‐18.4/‐7.8 mmHg Quality of life or functional status outcomes: not reported Data on mortality and cardiovascular mortality and morbidity (did not include TIA) were available for 60 years or older patients in 2009 update and were used in this update as well Data on cerebrovascular mortality and morbidity and coronary heart disease mortality and morbidity are not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “123 subjects were randomly allocated to simple observation or to treatment with methyldopa” “To avoid seasonal and interhome bias, because entry to the trial occurred over a two‐year period, a block of 24 sealed envelopes was prepared for each of the seven homes. Each envelope contained a randomly generated instruction to "observe" or to "treat." The computer program used had instructions to truncate runs of consecutive assignments longer than four. After a resident had been found eligible for the study and a case record completed the next envelope in the sequence for that home was opened and the regimen therein followed" Comment: randomisation was carried out in a proper manner |
| Allocation concealment (selection bias) | Low risk | "A block of 24 sealed envelopes was prepared for each of the seven homes" Allocation assignment distributed in sealed envelopes; stratified by site |
| Blinding of participant and personnel (performance and detection bias) | High risk | Open‐label study; patients and providers were not blinded |
| Blinding of outcome assessment | High risk | Outcome assessor was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up: 2% 60 participants were randomised to each group but "the blood pressures at the first routine visit after 6 months from the entry to the trial were available in 36 surviving treated patients and 39 surviving observed patients" |
| Selective reporting (reporting bias) | Unclear risk | Unable to judge |
| Industry sponsorship bias | Low risk | Work was initiated under the auspices of the Nottingham Old Age Project, which was funded by the Nuffield Foundation, to which we are grateful for support |
STOP 1991.
| Methods | Prospective randomised double‐blind placebo‐controlled multi‐site study in Sweden | |
| Participants | Study setting: primary care
1627 participants 70 to 84 years old: 812 to treatment and 815 to placebo Mean 75.6 years; female = 63%; race white; mean blood pressure at entry 195/102 mmHg Pre‐existing risk factors: not reported Inclusion criteria: BP entry criteria: systolic BP 180 to 230 mmHg and diastolic BP ≥ 90 mmHg or diastolic BP 105 to 120 mmHg, irrespective of systolic BP Exclusion criteria: isolated systolic hypertension (180 mmHg or higher with diastolic below 90 mmHg); orthostatic hypotension (more than 30 mmHg fall in systolic blood pressure on standing); contraindications to any of the drugs; myocardial infarction or stroke in previous 12 months; angina pectoris requiring treatment with drugs other than glyceryl trinitrate; other severe or incapacitating illnesses; unwillingness to take part Follow‐up: 2.1 years |
|
| Interventions |
Treatment Step 1 ‐ atenolol 50 mg daily, or hydrochlorothiazide 25 mg + amiloride 2.5 mg daily, or metoprolol 100 mg daily, or pindolol 5 mg daily Step 2 ‐ patients on a beta blocker received diuretics and those on diuretics received a beta blocker Control ‐ placebo |
|
| Outcomes | Total mortality: death from all causes Cardiovascular mortality ‐ fatal myocardial infarction; fatal stroke; sudden death; fatal congestive heart failure and fatal cardiovascular events not covered by above definitions (example, ruptured aortic aneurysm) CHD M&M ‐ fatal or non‐fatal myocardial infarction Cerebrovascular M&M ‐ fatal or non‐fatal stroke Dropouts due to side effects | |
| Notes | Withdrawal due to adverse events: control group 47 (5.7%); treatment group 58 (7.1%) Percentage of patients not on assigned therapy at study end: placebo group 23%; treatment group 16% Quality of life or functional status outcomes: not reported Difference in SBP/DBP at study end (Treatment ‐ Control) systolic/diastolic: ‐27.0/‐10.0 mmHg Information was obtained for subgroups from publications using individual patient data from the INDANA database (Gueyffier 1999) Data on mortality, cardiovascular mortality and morbidity (does not include TIA), cerebrovascular mortality and morbidity, and CHD mortality and morbidity are available for 60 to 79 years and 80 years or older subgroups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation and allocation was not described; no other information is provided |
| Allocation concealment (selection bias) | Unclear risk | Method of randomisation and allocation was not described; no other information is provided |
| Blinding of participant and personnel (performance and detection bias) | Low risk | Described as double‐blind “Placebo tablets were identical in shape, taste and colour to the active medication” Participants and providers were blinded |
| Blinding of outcome assessment | Low risk | “Endpoints were evaluated by an independent endpoint committee, unaware of the treatment given or blood pressure” Outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention to treat analysis used “No patient was lost to follow‐up” |
| Selective reporting (reporting bias) | Low risk | All endpoints stated in the study were reported |
| Industry sponsorship bias | High risk | Study was supported by Astra/Hassle, ICI Pharma, Merck Sharpe & Dohme (Sweden), Sandoz, and the Swedish County Councils “Data auditing in accordance with the recommendations of the US Food and Drug Administration was carried out at randomly selected health centres by an independent reviewer. The survey found no deviations from the protocol of a kind that could affect the main purpose of the trial and that the study had been carried out in a scientifically correct manner, so its final result should be reliable" |
Syst‐Eur 1991.
| Methods | Randomised double‐blind placebo‐controlled multi‐site study conducted in ambulatory community‐based patients from referral clinic in Europe (23 countries across Western and Eastern Europe, mainly from Finland, Bulgaria, the Russian Federation, Belgium, Italy, Israel, UK, France, Estonia, Lithuania, Spain, Poland, and Romania) | |
| Participants | 4695 participants; 66.8% female; age range ≥ 60; mean 70.3 years; race: not reported; male 31% Mean blood pressure at entry: 174/86 mmHg Pre‐existing risk factors: myocardial infarction 1.2%; stroke 3.5%; smoking 7.3% BP target: reduce systolic by > 20 mmHg or < 150 mmHg Inclusion criteria: SBP 160 to 219 mmHg and DBP < 95 mmHg Exclusion criteria: hypertension secondary to a disorder that needed specific medical or surgical treatment; retinal haemorrhage or papilledema; congestive heart failure; dissecting aortic aneurysm; serum creatinine concentration at presentation of 180 micromols/L or more; history of severe nosebleeds, stroke, or myocardial infarction in the year before the study; dementia; substance abuse; any disorder prohibiting a sitting or standing position; any severe concomitant cardiovascular or non‐cardiovascular disease Follow‐up: 2.5 years; average follow‐up: 2 years (median) |
|
| Interventions |
Treatment Step 1 ‐ nitrendipine 10 mg daily, 10 mg BID, 20 mg BID Step 2 ‐ enalapril 5 mg, 10 mg, 20 mg daily in evening and/or hydrochlorothiazide 12.5 to 25 mg/d in morning Control ‐ placebo |
|
| Outcomes | Mortality, stroke, CHD, CHF, systolic BP, diastolic BP | |
| Notes | Percentage not on assigned therapy at study end (2 years) including open follow‐up and losses to follow‐up: placebo group 27%, treatment group 18% Percentage receiving nitrendipine fell from 80% in year 1 to 50% in year 4 Difference in blood pressure at end of study (Treatment ‐ Control) systolic/diastolic: ‐10.1/‐4.5 mmHg at 2 years Information was obtained for subgroups from publications using individual patient data from the INDANA database Data on mortality, cardiovascular mortality and morbidity (does not include TIA), cerebrovascular mortality and morbidity, and CHD mortality and morbidity are available for 60 to 79 years and 80 years or older subgroups from the INDANA database (Gueyffier 1999) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomized to double‐blind treatment with active medication or placebo by means of a computerized random function” Randomisation was stratified by centre, sex, and previous cardiovascular complications. Group allocation determined by computerised random function Comment: randomisation was properly done |
| Allocation concealment (selection bias) | Low risk | Quote: "all bottles with study medication are identified by a unique number, allowing persons with access to the code to distinguish between placebo and active medication.....The responsible officer at the RDDC is instructed by the Coordinating Office whether the patient should receive placebo or active medication. The officer then writes the patient identification number on the labels of the bottles with the study medications and ships a one‐year supply to the local investigator. Under no circumstances is the officer at the RDDC allowed to disclose a patient's code. The physician, who proposed the patient for entry into the trial, receives the patient's identification number and a sealed envelope with patient's code from the Coordinating Office. This envelop will be collected at the end of study, and can only be opened in a medical emergency that cannot be dealt otherwise. The investigator verifies whether the patient identification number on the label of each medicine bottle corresponds with the number given by the Coordinating Office" Comment: allocation of treatment was concealed via proper methods |
| Blinding of participant and personnel (performance and detection bias) | Low risk | Quote: "Sys‐Eur is conducted as a double‐blind placebo controlled multicentre trial" "In the active treatment, tablets with 20 mg nitrendipine, 10 mg enalapril, and 25 mg hydrochlorothiazide were used. The matching placebos in the control patients do not contain any active substance" "Placebo tablets were identical to the study drugs, with a similar schedule" Comment: both patients and physicians were unaware of treatment provided |
| Blinding of outcome assessment | Low risk | Quote: "the endpoint committee, which was unaware of the patients’ treatment status, identified all major endpoints by reviewing the patients’ files and other source documents, or by requesting detailed written information from the investigators, or by both approaches" "All other events were checked at the coordinating office by doctors who were unaware of the treatment‐group status" Comment: outcome assessment was carried out in an independent manner, and outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "in patients who do not continue to attend clinics (non‐supervised open follow‐up), the following information is obtained by writing, telephone or personal contact either from the patients themselves or where appropriate, their General Practitioner, family members, or via office of vital statistics: vital status, if deceased cause of death, information on current medical treatment; and the incidence of non‐morbid fatal events" "Patients without any report within the year before the trial stopped were counted as lost to follow‐up" Comment: follow‐up as complete as possible; losses to follow‐up: 2% at 2 years |
| Selective reporting (reporting bias) | Low risk | Comment: all fatal and non‐fatal cerebrovascular and cardiovascular outcomes were reported |
| Industry sponsorship bias | High risk | Quote: "the trial was sponsored by Bayer AG, Wuppertal, Germany. The National Fund for Scientific Research, Brussels, Belgium, provided additional support. The study medication was donated by Bayer AG and Merck Sharpe & Dohme Inc, West Point, Pa. The Syst‐Eur trial, initiated by Antoon Amery, MD, who died on November 2, 1994, was a concerted action of the BIOMED Research Program sponsored by the European Union. The trial was carried out in consultation with the World Health Organization, International Society of Hypertension, European Society of Hypertension, and World Hypertension League" Comment: conflict of interest was not declared |
VA‐II 1970.
| Methods | Multi‐site study Randomisation: stratified by diastolic blood pressure (i.e. 90 to 114 mmHg and 115 to 129 mmHg); group allocation determined by sealed envelope containing randomised assignment. Assignment was determined by a statistician utilising a random number table Patients blinded; providers blinded |
|
| Participants | Geographic region: United States of America
Study setting: recruited from Veterans Affairs hospitals and seen in outpatient clinics
n = 81 (0% female) Age range 60 to 75; mean not reported Race: white (57.6%), black (41.3%), Asian (1.1%); for entire study group (i.e. these data not reported for > 60 years subgroup) Mean blood pressure at entry: 176/103 mmHg; pre‐existing factors: not reported Blood pressure (BP) entry criteria: diastolic BP 90 to 114 mmHg Exclusions: severe hypertension; surgically curable hypertension; uremia; concomitant fatal diseases such as carcinoma; haemorrhages, exudates, or papilledema in the optic fundi; history of cerebral or subarachnoid haemorrhage; dissecting aneurysm; congestive heart failure resistant to digitalis and mercurial diuretics; patients who wished to return to the care of their private physicians; patients unable to attend clinic regularly; patients of dubious reliability such as alcoholics, vagrants, and poorly motivated patients |
|
| Interventions |
Treatment Step 1 ‐ hydrochlorothiazide 50 mg and reserpine 0.1 mg twice daily Step 2 ‐ hydralazine 25 mg 3 times daily up to 150 mg/d Control ‐ placebo Average follow‐up: 3.3 years |
|
| Outcomes | Coronary heart disease (CHD) morbidity and mortality (M&M) ‐ myocardial infarction or sudden death Cerebrovascular M&M ‐ cerebrovascular accidents Cardiovascular M&M ‐ CHD M&M plus cerebrovascular M&M plus congestive heart failure and aneurysms Dropouts due to side effects: for entire study group (i.e. these data not reported for > 60 years of age subgroup) Control group: 3.1%; treatment group: 5.9% Quality of life or functional status outcomes: not reported | |
| Notes | Difference in blood pressure at study end (Treatment ‐ Control) diastolic: ‐17 mmHg; systolic: ‐27 mmHg % lost to follow‐up: 14.7% for entire study group (i.e. these data not reported for > 60 years of age subgroup) % not on assigned therapy at study end: not reported "The study was terminated in the subgroup of 143 patients whose diastolic blood pressures averaged 115 through 129 mm Hg prior to randomization. Termination of the study of this group as previously reported was necessitated by the high incidence of morbid events in the control as compared to the treated patients, demonstrating at a relatively early date a highly significant (P < 0.001) effect of treatment" "Many uncooperative and unreliable patients were identified and eliminated from the trial on the basis of pill counts, urine fluorescence test results, and irregularity of clinic attendance during a pre randomization observation period. Treatment obviously would not have been as effective in a group of patients less carefully selected with regard to their desire to cooperate. The population was further limited in that it excluded female patients and patients with labile hypertension whose diastolic blood pressures averaged lower than 90 mm Hg during the fourth through the sixth day of hospitalization" This study is identified as VA COOP 1970 in the Mulrow 1998 review; the Mulrow 1994 publication; and Musini 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "three hundred and eighty male hypertensive patients with diastolic blood pressures averaging 90 to 114 mm Hg were randomly assigned to either active antihypertensive agents or placebos" Comment: method used for random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "accepted patients were then randomly assigned double‐blind to either active drugs or placebos" (page 1144) Comment: method used for allocation concealment not reported |
| Blinding of participant and personnel (performance and detection bias) | Low risk | Quote: "accepted patients were then randomly assigned double‐blind to either active drugs or placebos" "Active drugs consisted of two types of tablets, one being a combination tablet containing 50 mg hydrochlorothiazide and 0.1 mg reserpine which was given twice daily. The other was 25 mg of hydralazine hydrochloride given three times daily. The latter medication was raised to 50 mg three times daily if the diastolic blood pressure remained at 90 mm Hg or higher. Obviously, practically all of the patients in the placebo group had their "doses" raised to this level" "Patients in the control group received placebos identical in taste and appearance to the active drugs" "In order to avoid losses to protocol because of side effects presumably caused by one or the other of the two agents, provision was made to permit substitution of a tablet which contained either reserpine or hydrochlorothiazide alone and omitted the offending medication. These special tablets were made available on request of a participating physician. Similar appearing placebo tablets were made available for the control patients and the physician did not know whether the substitution represented active drugs or placebos" Comment: trial was double‐blinded whereby participants and physicians were not aware of the treatment allocated to either group |
| Blinding of outcome assessment | Low risk | Quote: "the records of the patients reported as having assessable morbid events were reviewed by two consulting physicians who had not participated in the trial" "All available data pertaining to each organic complication, except the type of protocol treatment and the level of blood pressure, were presented to the reviewers and their decisions regarding the occurrence and classification of an event according to the definitions given in the protocol (see list of assessable events at the end of the communication) were accepted as final" Comment: outcome assessors were probably blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "fifty‐six or 15% of the 380 randomized patients were classified as drop‐outs during the course of the trial. Of this number 27 had been randomized to receive placebos and 29 to receive active drugs. The average period of follow up prior to dropping out was 17.6 months with a range from less than 1 month to 49 months" "Thus, the earliest entrants were observed for 5.5 years and the latest entrants for a minimum of 1 year. The average potential duration of observation, disregarding losses and terminations, was 3.9 years for the control group and 3.7 years for the treated patients. However, because of the losses and terminations due to elevated diastolic blood pressure described below, the actual duration of post randomization observation was 3.3 years for the control group and 3.2 years for the treated patients" Comment: reasons for dropouts were mentioned, although the reasons were not given separately for the 2 groups. How data for these patients were analysed is not reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol is not available Mortality (various causes of death) and morbidity (various terminating morbid events other than death) data were reported |
| Industry sponsorship bias | High risk | COI has not been reported "The special medications used in this investigation were prepared by William E. Wagner, MD, of Ciba Pharmaceutical Co., Summit, NJ |
ACE: angiotensin‐converting enzyme.
ACEI: angiotensin‐converting enzyme inhibitor.
BP: blood pressure.
CHD: coronary heart disease.
CHF: congestive heart failure.
CVD: cardiovascular disease.
DBP: diastolic blood pressure.
ECG: electrocardiogram.
ITT: intention‐to‐treat.
IVRS: interactive voice response system.
M&M: morbidity and mortality.
MI: myocardial infarction.
SAE: serious adverse event.
SBP: systolic blood pressure.
TIA: transient ischaemic attack.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ADVANCE 2007 | Randomised controlled trial that includes 11,140 adults with type 2 diabetes at elevated risk of vascular disease. Following 6 weeks on open‐label perindopril‐indapamide combination, eligible individuals were randomised to continued perindopril‐indapamide or matching placebo, and to an intensive gliclazide MR‐based glucose control regimen (aiming for HbA1c of 6.5% or lower) or usual guidelines‐based therapy for a mean of 4·3 years of follow‐up. More than 75% of patients had hypertension at baseline. Excluded because control group included non‐specific antihypertensive therapy |
| ALLHAT 1996 | Head‐to‐head comparison of different drug therapies without a non‐drug control group |
| BENEDICT 2004 | Multi‐centre DBRCT in 1204 patients, 40 years of age or older who had hypertension and a known history of type 2 diabetes mellitus. Eligible patients were randomly assigned to receive one of the study treatments: the non‐dihydropyridine calcium channel blocker verapamil (in a sustained‐release formulation, at a dose of 240 mg per day), the ACE inhibitor trandolapril (2 mg per day), the combination of verapamil (in a sustained‐release formulation, 180 mg per day) plus trandolapril (2 mg per day), or placebo for a median follow‐up of 3.6 years. Additional antihypertensive drugs were allowed, to achieve the target blood pressure of 120/80 mmHg. At baseline, 56% of patients in placebo group received antihypertensive medications; this was increased to 67% of patients at end of follow‐up. Excluded because there is no true placebo group |
| BENEDICT A 2006 | Randomised double‐blind placebo‐controlled study in 590 hypertensive patients (age 30 to 70 years) with type 2 diabetes and microalbuminuria. Patients were randomly assigned to receive irbesartan at a dose of 150 mg once daily, irbesartan at a dose of 300 mg once daily, or matching placebo once daily. There is no true placebo group, as 56% of patients in the placebo group were receiving blood pressure–lowering therapy at the end of 2 years of follow‐up |
| Berglund 1981 | Drug‐drug comparison of bendrofluazide 2.5 mg vs propranolol 160 mg with no placebo or untreated control group |
| CASTEL 1994 | This study was included in the original Mulrow 1998 review. However we excluded it in the first update, as it is a drug‐drug comparison with no placebo or untreated control group. Control group included non‐specific antihypertensive therapy |
| DIABHYCAR 2004 | This is a randomised double‐blind parallel‐group trial comparing ramipril (1.25 mg/d) with placebo (on top of usual treatment) for cardiovascular and renal outcomes for at least 3 years in 4937 patients with type 2 diabetes and high urinary albumin excretion. 56% of patients had hypertension at baseline. There is no true placebo control group |
| EUROPA 2003 | Randomised double‐blind trial conducted in 13,655 patients with previous myocardial infarction (64%), angiographic evidence of coronary artery disease (61%), coronary revascularisation (55%), or a positive stress test only (5%). After a run‐in period of 4 weeks, in which all patients received perindopril, 12,218 patients were randomly assigned perindopril 8 mg once daily (n = 6110) or matching placebo (n = 6108). Mean follow‐up was 4·2 years. There is no true placebo group |
| Fuchs 2011 | Randomised double‐blind clinical trial, controlled by placebo in people 30 to 70 years of age with pre‐hypertension. Excluded as people did not have hypertension |
| Generic 2010 | Single‐centre randomised double‐blind placebo‐controlled cross‐over trial comparing effects of moexipril and placebo on insulin sensitivity and 24‐hour blood pressure control in postmenopausal women with essential hypertension. It is not 1 year in duration |
| GENRES 2007 | Prospective randomised double‐blind placebo‐controlled cross‐over study in 208 moderately hypertensive Finnish men (aged 35 to 60 years) treated with 4 weeks of antihypertensive drugs with 4 weeks placebo in between treatment periods. This study does not meet the minimum duration of 52 weeks criterion |
| GLANT 1995 | This study employed alternate allocation (i.e. not random allocation). Drug‐drug comparison of delapril 30 to 120 mg vs several dihydropyridine CCBs with no placebo or untreated control group |
| HAPPHY 1987 | This study is a drug‐drug comparison of bendrofluazide 5 mg or HCTZ 50 mg vs atenolol 100 mg or metoprolol 200 mg with no placebo or untreated control group |
| HDFP 1984 | Based on comments received regarding improper inclusion of this trial in the previous systematic review, we excluded this study because the intervention was multi‐factorial. Treated group included various lifestyle measures in addition to antihypertensive drug therapy. Control group was given usual care and did not necessarily consist of untreated controls |
| Hood 2007 | Placebo‐controlled double‐blind randomised cross‐over trial. Patients received 10 cycles of double‐blind treatment comprising spironolactone 50 to 100 mg, amiloride 20 to 40 mg, bendroflumethiazide 2.5 to 5 mg at the 2 doses shown, losartan 100 mg, and placebo. Order of drugs and doses were randomised, except that higher doses of diuretic and placebo were administered in alternate cycles, and the 2 doses of each diuretic were separated by at least 3 intervening cycles. Each cycle of treatment lasted 5 weeks. There were no washout periods, and the entire study lasted 44 weeks for each patient. Study treatment was not given for minimum duration of 1 year |
| HOPE 3 2016 | Double‐blind RCT of 12,705 women 65 years or older and men 55 years or older with at least 1 CV risk factor, no known CV disease, and without any clear indication or contraindication to the study drugs. Patients were randomised to rosuvastatin 10 mg/d or placebo and to candesartan/ hydrochlorothiazide 16/12.5 mg/d or placebo (22 factorial design) and were followed for a mean of 5.8 years. Persons with a history of hypertension could be enrolled if blood pressure was adequately controlled (in the assessment of the recruiting physician) with lifestyle or drugs other than an ARB, ACE inhibitor, or thiazides. Only 38% of patients had hypertension at baseline; 29% were taking antihypertensive agents (other than ARBs, ACE inhibitors, or thiazides). Participants were allowed open‐label use of ARBs, ACE inhibitors, thiazides, and other blood pressure‐lowering drugs; therefore it was excluded |
| HOT 1995 | RCT that evaluates the effects of achieving prespecified levels of diastolic blood pressure control with all patients receiving antihypertensive treatment. There is no true placebo control group |
| IDM 2001 | This RCT is excluded, as there is no true placebo control group. Patients in the control group (56%) received other antihypertensive drugs |
| IDNT 2003 | Randomised double‐blind placebo‐controlled trial with median follow‐up of 2.6 years in 1715 adults with type 2 diabetic nephropathy and hypertension treated with irbesartan, amlodipine, or placebo. The placebo group received an average of 3.3 non‐study drugs, and the other 2 groups received an average of 3.0 drugs. There was no true placebo control group |
| IMAGINE 2008 | Double‐blind placebo‐controlled study of 2553 patients after CABG who were randomly assigned to quinapril, target dose 40 mg/d, or placebo, and were followed up to a maximum of 43 months. 47% had hypertension at baseline; baseline SBP/DBP was 122/70 mmHg. There was no true placebo control group |
| Imai 2011 | RCT in 577 patients treated with antihypertensive therapy (73.5% (n = 424) received concomitant ACEI) who were given either once‐daily olmesartan (10 to 40 mg) (n = 288) or placebo (n = 289) over 3.2 ± 0.6 years (mean ± SD). 282 received olmesartan and 284 received placebo in addition to conventional antihypertensive therapy. There was no true placebo control group |
| INSIGHT 1996 | This RCT is excluded as there was no placebo or untreated control group |
| Jikei 2007 | This RCT did not truly randomise patients to treatment arms; control group included non‐specific antihypertensive therapy |
| Kondo 2003 | This RCT included patients with a history of coronary intervention and no significant coronary stenosis on follow‐up angiography 6 months after intervention. Patients were randomly assigned to a candesartan group (n = 203; baseline treatment plus candesartan 4 mg/d) or a control group (n = 203; baseline treatment alone). No placebo tablets were administered in the control group |
| Kuramoto 1994 | RCT with head‐to‐head comparison of different drug therapies (nicardipine vs trichlormethiazide) without a non‐drug control group |
| Lewis 1993 | Randomised controlled trial in 207 comparing captopril with placebo in patients with insulin‐dependent diabetes mellitus. 75.5% of patients were hypertensive at baseline. Median follow‐up was 1.7 years. Patients receiving CCB or ACE inhibitors were eligible provided their blood pressure could be maintained with BP goals required by the trial. There is no true placebo group, and not all patients had hypertension at baseline |
| Lewis 2001 | RCT that was excluded as there was no true placebo control group; average of 3.3 antihypertensive drugs received per patient during the study |
| MacMahon 2000 | DBRCT in patients aged 75 years or younger if they had a hospital diagnosis (within 5 years of enrolment) of any of the following: acute myocardial infarction (MI), angina with coronary disease confirmed by angiography or exercise electrocardiogram, transient ischaemic attack (TIA), or intermittent claudication. Patients (N = 617) were randomised to ramipril 5 mg or 10 mg daily or placebo for a duration of 4 years. At baseline, 42% of patients were on beta blocker and 25% on calcium antagonists. The percentage of patients at baseline with hypertension has not been reported. Average BP at entry was 133/79 mmHg |
| MAPHY 1988 | Represents a subgroup of the patients included in the HAPPHY trial. RCT was excluded as drug‐drug comparison of bendrofluazide 5 mg or HCTZ 50 mg vs atenolol 100 mg or metoprolol 200 mg with no placebo or untreated control group |
| MIDAS 1996 | RCT that was excluded as it is a drug‐drug comparison of HCTZ 25 mg vs isradipine 5 mg with no placebo or untreated control group |
| Morgan 1980 | RCT that was excluded as allocation to the 4 study groups (no treatment, reduced salt intake, thiazide diuretic, beta blocker) was non‐random (i.e. "based on their week of presentation at the clinic") |
| NAVIGATOR 2010 | DBRCT in 9306 patients with impaired glucose tolerance and established cardiovascular disease or cardiovascular risk factors to receive valsartan (up to 160 mg daily) or placebo (and nateglinide or placebo) in addition to lifestyle modification. 77.5% of patients were hypertensive at baseline. Use of diuretics and calcium channel blockers was similar in the 2 groups. At the last study visit, 20.4% of patients in the valsartan group and 24.0% of those in the placebo group were receiving an open‐label renin–angiotensin inhibitor. There was no placebo or untreated control group |
| NICOLE 2003 | DBRCT in 9306 patients with impaired glucose tolerance and established cardiovascular disease or cardiovascular risk factors to receive valsartan (up to 160 mg daily) or placebo (and nateglinide or placebo) in addition to lifestyle modification. 77.5% of patients were hypertensive at baseline. At the last study visit, 20.4% of patients in the valsartan group and 24.0% of those in the placebo group were receiving an open‐label renin–angiotensin inhibitor. There was no placebo or untreated control group |
| NORDIL 2000 | RCT that was excluded as there is no placebo or untreated control group |
| Oslo 1986 | Open randomised trial conducted in ambulatory young male patients 40 to 49 years old randomised to treatment or no treatment in Norway. This trial does not include patients 60 years or older |
| PEACE 2004 | DBPCT in which 8290 patients with stable coronary artery disease and normal or slightly reduced left ventricular function were randomly assigned to receive either trandolapril at a target dose of 4 mg per day (4158 patients) or matching placebo (4132 patients) for a median follow‐up of 4.8 years. 45.5% of patients were hypertensive at baseline. 68.6% of the treated group and 77.7% of the placebo group were taking the target dose of 4 mg of trandolapril or placebo, respectively, per day. There was no placebo or untreated control group |
| Pool 2007 | An 8‐week multi‐centre randomised double‐blind placebo‐controlled parallel‐group trial that compared the efficacy and tolerability of the combination of valsartan/HCTZ at doses up to 320 mg/25 mg with monotherapy of both drugs. Does not meet the minimum inclusion criterion of 52 weeks' duration |
| PRoFESS 2008 | Multi‐centre trial in 20,332 patients who recently had an ischaemic stroke and were randomly assigned to receive telmisartan (80 mg daily) and placebo for a mean follow‐up of 2.5 years. 74% of patients at baseline had a history of hypertension. By the end of the study, the use of diuretics, ACE inhibitors, calcium channel blockers, and beta blockers was more frequent in the placebo group than in the telmisartan group. There was no placebo or untreated control group in this study |
| PROGRESS 2001 | RCT that included less than 50% of patients with elevated blood pressure with about 50% of patients receiving other antihypertensive therapy at baseline and throughout the trial. There was no placebo or untreated control group |
| QUIET 2001 | RCT in which most patients did not have elevated blood pressure. 25% of patients were receiving a beta blocker. There was no placebo or untreated control group |
| REIN 1997 | Prospective double‐blind trial in 352 patients classified according to baseline proteinuria and randomly assigned to ramipril or placebo plus conventional antihypertensive therapy targeted at achieving diastolic blood pressure under 90 mmHg. There was no placebo or untreated control group |
| RENAAL 2001 | Double‐blind randomised placebo‐controlled study designed to evaluate the renoprotective effects of losartan in 1513 patients with type 2 diabetes and nephropathy. Not all included patients had hypertension at baseline. Patients with hypertension in this trial received open‐label diuretics, CCBs, alpha or beta blockers, centrally acting drugs, or a combination in the control group There was no placebo or untreated control group |
| ROAD 2007 | Prospective randomised open blinded endpoint (PROBE) study with median follow‐up of 3.7 years in 360 patients with chronic renal insufficiency. They were randomly assigned to 4 groups. Patients received open‐label treatment with a conventional dosage of benazepril (10 mg/d), individual up‐titration of benazepril (median 20 mg/d; range 10 to 40), a conventional dosage of losartan (50 mg/d), or individual up‐titration of losartan (median 100 mg/d; range 50 to 200). There was no placebo or untreated control group |
| ROADMAP 2011 | DBRCT in 4447 patients with type 2 diabetes comparing olmesartan 40 mg once daily or placebo for a median duration of 3.2 years. 82% of patients had hypertension at baseline. Additional antihypertensive drugs (except angiotensin‐converting enzyme inhibitors or ARBs) were used as needed to lower blood pressure to less than 130/80 mmHg. There was no placebo or untreated control group |
| SCAST 2015 | Randomised placebo‐controlled double‐masked trial in 2029 patients presenting within 30 hours of acute ischaemic or haemorrhagic stroke and with high systolic blood pressure (> 140 mmHg). Patients were treated with candesartan or placebo for 7 days, with doses increasing from 4 to 16 mg once daily during the first 3 days, and were followed for 6 months. Minimum duration of 1 year criterion is not met |
| SCAT 2000 | Multi‐centre randomised double‐blind placebo‐controlled trial in 460 patients: 230 received simvastatin and 230 received a simvastatin placebo; 229 received enalapril and 231 received an enalapril placebo (some patients received both drugs and some received a double placebo). Over 60% did not have hypertension, and about half were taking beta blockers at baseline and throughout. There was no true placebo control group |
| Scheimder 2012 | Randomised double‐blind multi‐centre placebo‐controlled study. 1124 patients were randomised to aliskiren 150 mg, hydrochlorothiazide 12.5 mg, or placebo once daily. Forced titration (to aliskiren 300 mg or hydrochlorothiazide 25 mg) occurred at week 3; at week 6, patients receiving placebo were reassigned (1:1 ratio) to aliskiren 300 mg or hydrochlorothiazide 25 mg. From week 12, amlodipine 5 mg was added, and it was titrated to 10 mg from week 18 for patients whose BP remained uncontrolled. There was no true placebo control group |
| SCOPE 2003 | Study of 4964 patients aged 70 to 89 years, with systolic blood pressure 160 to 179 mmHg and/or diastolic blood pressure 90 to 99 mmHg, and a Mini Mental State Examination (MMSE) test score > 24. Patients were assigned randomly to receive the angiotensin receptor blocker candesartan or placebo, with open‐label active antihypertensive therapy added as needed. As a consequence, active antihypertensive therapy was extensively used in the control group (84% of patients). Mean follow‐up was 3.7 years. There was no placebo or untreated control group |
| SHELL 1994 | Randomised study was excluded as it is a head‐to‐head comparison of different drug therapies without a non‐drug control group |
| STONE 1996 | Single‐blind trial in 1632 patients aged 60 to 79 years, alternatively allocated by entry order numbers to either nifedipine or placebo with a mean follow‐up of 30 months. No randomised allocation |
| STOP‐2 1993 | This study was excluded as it is a head‐to‐head comparison of different drug therapies without a non‐drug control group |
| Strandberg 1991 | This study was excluded as the treatment group had multiple interventions. Control group was given usual treatment and there was no untreated control |
| Syst‐China 1993 | This study was excluded as allocation to treatment and control groups was not random (i.e. alternate allocation was employed) |
| TRANSCEND 2008 | 5926 patients intolerant to ACE inhibitors with cardiovascular disease or diabetes with end‐organ damage were randomised to receive telmisartan 80 mg/d (n = 2954) or placebo (n = 2972). 76.4% of patients had hypertension at baseline. Other non‐study blood pressure‐lowering agents were used more frequently in the placebo group than in the telmisartan group by the end of the study (telmisartan vs placebo—diuretics: 888 (33.7%) vs 1059 (40.0%); P < 0·0001; calcium channel blockers: 1003 (38.0%) vs 1215 (45.9%); P < 0·0001; β blockers: 1492 (56.6%) vs 1561 (59.0%); P = 0.081; α blockers: 140 (5.3%) vs 197 (7.5%); P = 0·002). There was no placebo or untreated control group |
| USPHSHCSG 1977 | Randomised double‐blind placebo‐controlled trial conducted in young ambulatory patients 21 to 55 years old in the USA. This trial did not include patients 60 years of age or older |
| VACS 1982 | This study was excluded as it is a drug‐drug comparison of HCTZ 50 mg vs propranolol 80 mg with no placebo or untreated control group |
| VANHLBI 1978 | Randomised double‐blind placebo‐controlled trial conducted in ambulatory patients 21 to 50 years old in the USA. This trial did not include patients 60 years of age or older |
| VHAS 1997 | This study was excluded as it is a drug‐drug comparison of chlorthalidone 25 mg vs verapamil 240 mg with no placebo or untreated control group |
| White 1995 | This study was excluded as it is a drug‐drug comparison of different drug therapies without a non‐drug control group |
ACE: angiotensin‐converting enzyme.
ACEI: angiotensin‐converting enzyme inhibitor.
ARB: angiotensin‐receptor blocker.
CCB: calcium channel blocker.
CV: cardiovascular.
DBRCT: double‐blind randomised controlled trial.
HbA1c: glycated haemoglobin.
HCTZ: hydrochlorothiazide.
MI: myocardial infarction.
MMSE: Mini Mental State Examination.
MR: modified release.
RCT: randomised controlled trial.
SD: standard deviation.
TIA: transient ischaemic attack.
Differences between protocol and review
Total cardiovascular events (mortality and morbidity) outcome has been re‐defined similar to the "First line drugs for hypertension" Cochrane Review (Wright 2018), and "Pharmocotherapy for hypertension in adults 18 to 59 years" (Musini 2017).
Mulrow et al original review ‐ Mulrow 1998 and Mulrow 2000 and the Musini 2009 update included the definition of cardiovascular mortality and morbidity as "fatal and non‐fatal stroke; fatal and non‐fatal myocardial infarction; sudden or rapid cardiac death; aneurysms, congestive heart failure and transient ischemic attack".
However, for the 2017 update, the definition has been modified to include all components except transient ischaemic attacks. It is defined as "total stroke, total CHD, hospitalisation or death from congestive heart failure and other significant vascular deaths such as ruptured aneurysms". It does not include angina, transient ischaemic attacks, surgical or other procedures, or accelerated hypertension.
This change in definition has led to differences in data for this outcome as compared to the previous update.
SYST‐EUR trial data on cardiovascular mortality and morbidity have been corrected for the treatment group from 160/2398 overall to 137/2398, and for the control group from 216/2297 to 186/2297.
SHEP cardiovascular mortality and morbidity data have been changed for the treatment group from 346/2365 to 268/2365, and for the control group from 519/2371 to 416/2371.
SHEP‐PS cardiovascular mortality and morbidity data have been changed for the treatment group from 33/443 to 32/443, and for the control group from 14/108 to 12/108.
Contributions of authors
Vijaya Musini and Aaron Tejani did an updated search until October 2008 for the second update. They screened titles and abstracts to identify trials meeting inclusion/exclusion criteria.
Vijaya Musini and Lorri Puil did an updated literature search from October 2008 until November 2017. They screened titles and abstracts to identify trials meeting inclusion/exclusion criteria. Lorri Puil contributed to the data analysis and to interpretation and final draft of the review.
Vijaya Musini (VM) confirmed inclusion/exclusion of retrieved articles, extracted data, checked data entry, assessed risk of bias of included studies, and contributed to data analysis and interpretation and to the final draft of the review. VM graded the overall quality of evidence and prepared Summary of findings tables using GradePro software, along with two additional tables.
Aaron Tejani assessed risk of bias assessment of included studies, extracted data, checked data entry, and contributed to data analysis and interpretation and to the final draft of the review.
James Wright and Ken Bassett verified data and resolved differences.
All authors contributed to the writing and interpretation of the review.
Sources of support
Internal sources
-
Department of Anesthesiology, Pharmacology & Therapeutics, University of BC, Canada.
Office space
External sources
-
CIHR grant to the Hypertension Review Group, Canada.
Infrastructure
Declarations of interest
Vijaya Musini: nothing to declare.
Aaron Tejani: nothing to declare.
Ken Bassett: nothing to declare.
Lorri Puil: nothing to declare.
James Wright: nothing to declare.
Edited (no change to conclusions)
References
References to studies included in this review
ATTMH 1981 {published data only}
- A report by the Management Committee. Initial results of the Australian therapeutic trial in mild hypertension. Clinical Science 1979;57(Suppl 5):449s‐52s. [MEDLINE: ] [PubMed] [Google Scholar]
- A report by the Management Committee. The Australian therapeutic trial in mild hypertension. Lancet 1980;1(8181):1261‐7. [PubMed] [Google Scholar]
- A report by the Management Committee. The Australian therapeutic trial in mild hypertension. The Lancet 1980;1:1261‐7. [PubMed] [Google Scholar]
- A report by the Management Committee of the Australian Therapeutic Trial in Mild Hypertension. Untreated mild hypertension. Lancet 1982;1(8265):185‐91. [MEDLINE: ] [PubMed] [Google Scholar]
- A study initiated and administered by the National Heart Foundation of Australia. Treatment of mild hypertension in the elderly. Medical Journal of Australia 1981;2(8):398‐402. [PubMed] [Google Scholar]
- Abernethy JD. The Australian therapeutic trial in mild hypertension. Hypertension 1984;6(5):774‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Abernethy JD. The need to treat mild hypertension. Misinterpretation of results from the Australian trial. JAMA 1986;256(22):3134‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Doyle AE. Cardiovascular morbidity and mortality in mild hypertension: the Australian trial. Journal of Cardiovascular Pharmacology 1985;7(Suppl 2):S10‐3. [85238920] [DOI] [PubMed] [Google Scholar]
- Doyle AE. The Australian therapeutic trial in mild hypertension. Nephron 1987;47(Suppl 1):115‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Parry CA. Australian therapeutic trial in mild hypertension. Lancet 1980;2(8191):425. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Reader R. Australian therapeutic trial in mild hypertension. Medical Journal of Australia 1984;140(13):752‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carter 1970 {published data only}
- Carter AB. Hypotensive therapy in stroke survivors. Lancet 1970;1(7645):485‐9. [DOI] [PubMed] [Google Scholar]
Coope 1986 {published data only}
- Coope J, Warrender TS. Randomized trial of treatment of hypertension in elderly patients in primary care. BMJ 1986;293:1145‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
EWPHBPE 1989 {published data only}
- Amery A, Berthaux P, Birkenhager C, Bulpitt C, Clement D, Shaepdryver A, et al. Antihypertensive therapy in elderly patients. Pilot trial of the European Working Party on High Blood Pressure in the Elderly. Gerontology 1977;23(6):426‐37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Amery A, Berthaux P, Birkenhager W, Boel A, Brixko P, Bulpitt C, et al. Antihypertensive therapy in patients above age 60 years (Fourth interim report of the European Working Party on High Blood Pressure in the Elderly: EWPHE). Clinical Science and Molecular Medicine 1978;55(Suppl 4):263s‐70s. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Amery A, Berthaux W, Birkenhager W, Boel A, Brixko P, Bulpitt C, et al. Antihypertensive therapy in patients above age 60. Third interim report of the European Working Party on High Blood Pressure in the Elderly (EWPHE). Acta Cardiologica 1978;33(2):113‐34. [MEDLINE: ] [PubMed] [Google Scholar]
- Amery A, Birkenhager W, Brixko P, Bulpitt C, Clement D, Deruyttere M, et al. Efficacy of antihypertensive drug treatment according to age, sex, blood pressure, and previous cardiovascular disease in patients over the age of 60. Lancet 1986;2(8507):589‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Amery A, Birkenhager W, Brixko P, Bulpitt C, Clement D, Deruyttere M, et al. Glucose intolerance during diuretic therapy in elderly hypertensive patients. A second report from the European Working Party on High Blood Pressure in the Elderly (EWPHE). Postgraduate Medical Journal 1986;62(732):919‐24. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amery A, Birkenhager W, Brixko P, Bulpitt C, Clement D, Deruyttere M, et al. Mortality and morbidity results from the European Working Party on High Blood Pressure in the Elderly trial. Lancet 1985;1(8442):1349‐54. [DOI] [PubMed] [Google Scholar]
- Amery A, Birkenhager W, Brixko P, Bulpitt C, Clement D, Leeuw P. Influence of antihypertensive drug treatment on morbidity and mortality in patients over the age of 60 years. European Working Party on High Blood Pressure in the Elderly (EWPHE) results: sub‐group analysis on entry stratification. Journal of Hypertension 1986;4(Suppl 6):S642‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Amery A, Birkenhager W, Brixko P, Bulpitt C, Clement D, Leeuw P, et al. Influence of hypotensive drug treatment in elderly hypertensives: study terminating events in the trial of the European Working Party on High Blood Pressure in the Elderly. Journal of Hypertension 1985;3(3):S501‐11. [MEDLINE: ] [PubMed] [Google Scholar]
- Amery A, Schaepdrijver A. European Working Party on High Blood Pressure in the Elderly (EWPHE): organization of a double‐blind multicentre trial on antihypertensive therapy in elderly patients. Clinical Science and Molecular Medicine 1973;45(Suppl 1):71s‐3s. [DOI] [PubMed] [Google Scholar]
- Amery A, Schaepdryver A. The European Working Party on High Blood Pressuire in the Elderly. American Journal of Medicine 1991;90(Suppl 3A):1S‐4S. [MEDLINE: ] [PubMed] [Google Scholar]
- Leeuw PW. Renal function in the elderly: results from the European Working Party on High Blood pressure in the Elderly trial. American Journal of Medicine 1991;90(Suppl 3A):45S‐9S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- European Working Party on High Blood Pressure in the Elderly (EWPHE). An international trial of antihypertensive therapy in elderly patients. Objectives, protocol and organization. Archives Internationales de Pharmacodynamie et de Therapie 1985;275(2):300‐34. [PubMed] [Google Scholar]
- European Working Party on High Blood Pressure in the Elderly (EWPHE). Antihypertensive therapy in the elderly. Ninth interim report. Netherlands Journal of Medicine 1984;27:165‐70. [MEDLINE: ] [PubMed] [Google Scholar]
- European Working Party on High Blood Pressure in the Elderly trial. Discussion after the presentation of the results of the European Working Party on High Blood Pressure in the Elderly trial (EWPHE). Journal of Hypertension 1985;3(Suppl 3):S509‐11. [MEDLINE: ] [Google Scholar]
- Fagard R. Serum cholesterol levels and survival in elderly hypertensive patients: analysis of data from the European Working Party on High Blood Pressure in the Elderly. American Journal of Medicine 1991;90(Suppl 3A):62S‐3S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fagard R, Staessen J, Amery A. Experience with diuretics in the study conducted by the European Working Party on High Blood Pressure in the Elderly (EWPHE). Progress in Pharmacology and Clinical Pharmacology 1992;9:463‐72. [Accession number 1992230803] [Google Scholar]
- Fletcher A, Amery A, Birkenhager W, Bulpitt C, Clement D, Leeuw P, et al. Risks and benefits in the trial of the European Working Party on High Blood Pressure in the Elderly. Journal of Hypertension 1991;9:225‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fletcher AE. Adverse treatment effects in the trial of the European Working Party on High Blood Pressure in the Elderly. American Journal of Medicine 1991;90(Suppl 3A):42S‐4S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- O'Malley K, Cox JP, O'Brien E. Further learnings from the European Working Party on High Blood Pressure in the Elderly (EWPHE) study: focus on systolic hypertension. Cardiovascular Drugs and Therapy 1990;4(Suppl 6):1249‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- O'Malley KO, McCormack P, O'Brien ET. Isolated systolic hypertension: data from the European Working Party on High Blood Pressure in the Elderly. Journal of Hypertension 1988;6(Suppl 1):S105‐8. [PubMed] [Google Scholar]
- Prisant LM, Carr AA. Overview of the findings of the European Working Party on High Blood Pressure in the Elderly. Geriatric Medicine Today 1990;9(5):35‐8. [Accession number 1990224411] [Google Scholar]
- Staeesen J. The determinants and prognostic significance of serum uric acid in elderly patients of the EWPHE trial. American Journal of Medicine 1991;90(Suppl 3A):50S‐4S. [MEDLINE: ] [PubMed] [Google Scholar]
- Staessen J. Mortality and treatment of blood pressure in patients of the EWPHE. American Journal of Medicine 1991;90(Suppl 3A):60S‐1S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Staessen J, Bulpitt C, Clement D, Leeuw P, Fagard R, Fletcher A. Relation between mortality and treated blood pressure in elderly patients with hypertension: report of the European Working Party on High Blood Pressure in the Elderly. BMJ 1989;298:1552‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijs L. Age‐related hypotensive effect of placebo and active treatment in patients older than 60 years. American Journal of Medicine 1991;90(Suppl 3A):24S‐6S. [MEDLINE: ] [PubMed] [Google Scholar]
- Tuomilehto J. Body mass index and prognosis in elderly hypertensive patients: a report from the EWPHE. American Journal of Medicine 1991;90(Suppl 3A):34S‐41S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hoof R. Left ventricular hypertrophy in elderly hypertensive patients: a report from the European Working Party on High Blood Pressure in the Elderly trial. American Journal of Medicine 1991;90(Suppl 3A):55s‐9s. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hoof R, Staessen J, Fagard L, Thijs L, Amery A. The effect of antihypertensive treatment on electrocardiogram voltages in the European Working Party on High Blood Pressure in the Elderly trial. Journal of Cardiovascular Pharmacology 1991;17(Suppl 2):S101‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
HSCSG 1974 {published data only}
- Hypertension‐Stroke Cooperative Study Group. Effect of antihypertensive treatment on stroke recurrence. JAMA 1974;229:409‐18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
HYVET 2008 {published data only}
- Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, et al. Treatment of hypertension in patients 80 years of age or older. New England Journal of Medicine 2008;358(18):1887‐98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bulpitt CJ, Fletcher AE, Amery A, Coope J, Evans JG, Lightowlers S, et al. The Hypertension in the Very Elderly Trial (HYVET). Journal of Human Hypertension 1994;8(8):631‐2. [PubMed] [Google Scholar]
- Bulpitt CJ, Fletcher AE, Amery A, Coope J, Evans JG, Lightowlers S, et al. The Hypertension in the Very Elderly Trial (HYVET): rationale, methodology and comparison with previous trials. Drugs & Aging 1994;5(3):171‐83. [DOI] [PubMed] [Google Scholar]
HYVET P 2003 {published data only}
- Beckett NS, Connor M, Sadler JD, Fletcher AE, Bulpitt CJ, et al. Orthostatic fall in blood pressure in the very elderly: results from the Hypertension in the Very Elderly trial (HYVET) ‐ pilot. Journal of Human Hypertension 1999;13(12):839‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bulpitt CJ, Beckett NS, Cooke J, Dumiktrascu DL, Gil‐Extremera B, Nachev C, et al. Results of the pilot study for the Hypertension in the Very Elderly Trial. Journal of Hypertension 2003;21:2409‐17. [DOI] [PubMed] [Google Scholar]
Kuramoto 1981 {published data only}
- Kuramoto K, Matsushita S, Kuwajima T, Murakami M. Prospective study on the treatment of mild hypertension in the aged. Japanese Heart Journal 1981;22(1):75‐85. [DOI] [PubMed] [Google Scholar]
MRC‐O 1992 {published data only}
- MRC Working Party. Medical Research Council Trial of treatment of hypertension in older adults: principal results. BMJ 1992;304(6824):405‐12. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
MRC‐TMH 1985 {published data only}
- Anonymous. Report of the Medical Research Council Working party on mild to moderate hypertension. Randomised controlled trial of treatment of mild hypertension: design and pilot trial. BMJ 1977;1(6074):1437‐40. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medical Research Council Working Party. MRC trial of treatment of mild hypertension: principal results. British Medical Journal Clinical Research Ed. 1985;291(6488):97‐104. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medical Research Council Working Party. Stroke and coronary heart disease in mild hypertension: risk factors and the value of treatment. BMJ 1988;296(6636):1565‐70. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
- Medical Research Council Working Party on Mild Hypertension. Coronary heart disease in the Medical Research Council trial of treatment of mild hypertension. British Heart Journal 1988;59(3):364‐78. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medical Research Council Working Party on Mild Hypertension. Course of blood pressure in mild hypertension after withdrawal of long term antihypertensive treatment. BMJ 1986;293(6553):988‐92. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peart S. Results of the MRC (UK) trial of drug therapy for mild hypertension. Clinical and Investigative Medicine 1987;10(6):616‐20. [MEDLINE: ] [PubMed] [Google Scholar]
SHEP 1991 {published data only}
- Applegate WB, Davis BR, Black HR, Smith WM, Miller ST, Burlando AJ. Prevalence of postural hypotension at baseline in the Systolic Hypertension in the Elderly Program (SHEP) cohort. Journal of American Geriatric Society 1991;39(11):1057‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Applegate WB, Pressel S, Wittes J, Luhr J, Shekelle RB, Camel GH, et al. Impact of the treatment of isolated systolic hypertension on behavioral variables: results from the systolic hypertension in the elderly program. Archives of Internal Medicine 1994;154(19):2154‐60. [PubMed] [Google Scholar]
- Bearden D, Allman R, McDonald R, Miller S, Pressel S, Petrovich H. Age, race, and gender variation in the utilization of coronary artery bypass surgery and angioplasty in SHEP. SHEP Cooperative Research Group. Systolic Hypertension in the Elderly Program. Journal of the American Geriatric Society 1994;42:1143‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Black HR, Unger D, Burlando A, Wright JC, Pressel SL, Allen R, et al. Systolic Hypertension in the Elderly Program (SHEP). Part 6: Baseline physical examination findings. Hypertension 1991;17(3 Suppl):II‐77‐101. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Borhani NO, Applegate WB, Cutler JA, Davis BR, Furberg CD, Lakatos E, et al. Systolic Hypertension in the Elderly Program (SHEP). Part 1: Rationale and design. Hypertension 1991;17(3 Suppl):II2‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brittain E, Palensky J, Blood J, Wittes J. Blinded subjective rankings as a method of assessing treatment effect: a large sample example from the Systolic Hypertension in the Elderly Program (SHEP). Statistics in Medicine 1997;16(6):681‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Curb JD, Lee M, Jensen J, Applegate W. Systolic Hypertension in the Elderly Program (SHEP). Part 4: Baseline medical history findings. Hypertension 1991;17(3 Suppl):II35‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Curb JD, Pressel SL, Cutler JA, Savage PJ, Applegate WB, Black H, et al. Effect of diuretic based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. JAMA 1996;276(23):1886‐92. [MEDLINE: ] [PubMed] [Google Scholar]
- Davis BR, Wittes J. Pressel S, Berge KG, Hawkins CM, Lakatos E, et al. Statistical considerations in monitoring the Systolic Hypertension in the Elderly Program (SHEP). Controlled Clinical Trials 1993;14(5):350‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Franse LV, Pahor M. Di Bari M, Shorr RI, Wan JY, Somes GW, et al. Serum uric acid, diuretic treatment and risk of cardiovascular events in the Systolic Hypertension in the Elderly Program (SHEP). Journal of Hypertension 2000;18(8):1149‐54. [DOI] [PubMed] [Google Scholar]
- Frost PH, Davis BR, Burlando AJ, Curb JD, Guthrie JP Jr, Isaacson JL, et al. Serum lipids and incidence of coronary heart disease. Findings from the Systolic Hypertension in the Elderly Program (SHEP). Circulation 1996;94(10):2381‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hall WD, Davis BR, Frost P, Hoffmeier M, O'Brien JE, Pace S, et al. Systolic Hypertension in the Elderly Program (SHEP). Part 7: Baseline laboratory characteristics. Hypertension 1991;17(3 Suppl):II102‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hawkins CM. Isolated systolic hypertension, morbidity and mortality: the SHEP experience. American Journal of Geriatric Cardiology 1993;2(5):25‐7. [PubMed] [Google Scholar]
- Hulley SB, Furberg CD, Gurland B, McDonald R, Perry HM, Schnaper HW, et al. Systolic Hypertension in the Elderly Program (SHEP): antihypertensive efficacy of chlorthalidone. American Journal of Cardiology 1985;56(15):913‐20. [DOI] [PubMed] [Google Scholar]
- Kostis JB, Allen R, Berkson DM, Curb JD, Davey J, Grimm RH, et al. Correlates of ventricular ectopic activity in isolated systolic hypertension. American Heart Journal 1994;127(1):112‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kostis JB, Davis BR, Cutler J, Berge KG, Cohen JD, Lacy CR, et al. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. JAMA 1997;278(3):212‐16. [MEDLINE: ] [PubMed] [Google Scholar]
- Kostis JB, Lacy CR, Hall D, Wilson AC, Borhani NO, Krieger SD, et al. The effect of chlorthalidone on ventricular ectopic activity in patients with isolated systolic hypertension. American Journal of Cardiology 1994;74(5):464‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kostis JB, Prineas R, Curb JD, Lee M, Berkson D, Raines J, et al. Systolic Hypertension in the Elderly Program (SHEP). Part 8: Electrocardiographic characteristics. Hypertension 1991;17(3 Suppl):II123‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Menard J, Day M, Chatellier G, Laragh JH. Some lessons from systolic hypertension in the elderly program (SHEP). American Journal of Hypertension 1992;5(5 Pt 1):325‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Newman AB, Tyrrell KS, Kuller LH. Mortality over four years in SHEP participants with a low ankle‐arm index. Journal of the American Geriatrics Society 1997;45(12):1472‐78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Perry HM Jr, Davis BR, Price TR, Applegate WB, Fields WS, Guralnik JM, et al. Effect of treating isolated systolic hypertension on the risk of developing various types and subtypes of stroke. JAMA 2000;284(4):465‐71. [DOI] [PubMed] [Google Scholar]
- Petrovich H, Byington R, Bailey G, Borhani P, Carmody S, Goodwin L, et al. Systolic Hypertension in the Elderly Program (SHEP). Part 2: screening and recruitment. Hypertension 1991;17(3 Suppl):II17‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Probstfield JL. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). Annals of Internal Medicine 1991;115(Suppl 3):65. [PubMed] [Google Scholar]
- Probstfield JL, Applegate WB, Borhani NO, Curb JD, Cutler JA, Davis BR, et al. The Systolic Hypertension in the Elderly Program (SHEP): an intervention trial on isolated systolic hypertension. SHEP Cooperative Research Group. Clinical & Experimental Hypertension ‐ Part A, Theory & Practice 1989;11(5‐6):973‐89. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991;265(24):3255‐64. [PubMed] [Google Scholar]
- Savage PJ, Pressel SL, Curb D, Schron EB, Applegate WB, Black HR, et al. Influence of long‐term, low‐dose, diuretic‐based, antihypertensive therapy on glucose, lipid, uric acid, and potassium levels in older men and women with isolated systolic hypertension: the Systolic Hypertension in the Elderly Program. SHEP Cooperative Research Group. Archives of Internal Medicine 1998;158(7):741‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- The Systolic Hypertension in the Elderly Program (SHEP) Cooperative Research Group. Rationale and design of a randomized clinical trial on prevention of stroke in isolated systolic hypertension. Journal of Clinical Epidemiology 1988;41(12):1197‐208. [DOI] [PubMed] [Google Scholar]
- Vogt TM, Schron E, Pressel S, Wasserthiel‐Smoller S, Eddleman EE, Miller S, et al. Systolic Hypertension in the Elderly Program (SHEP). Part 3: sociodemographic characteristics. Hypertension 1991;17(3 Suppl):II‐24‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wassertheil‐Smoller S, Applegate WB, Berge K, Chang CJ, Davis BR, Grimm R Jr, et al. Change in depression as a precursor of cardiovascular events. SHEP Cooperative Research Group (Systolic Hypertension in the elderly). Archives of Internal Medicine 1996;156(5):533‐61. [MEDLINE: ] [PubMed] [Google Scholar]
- Wasserthell‐Smoller S, Fann C, Allman RM, Black HR, Camel GH, Davis B, et al. Relation of low body mass to death and stroke in the systolic hypertension in the elderly program. The SHEP Cooperative Research Group. Archives of Internal Medicine 2000;160(4):494‐500. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Weiler PG, Camel GH, Chiappini M, Greenlick MR, Hughes GH, Luhr JC, et al. Systolic Hypertension of the Elderly Program (SHEP). Part 9: behavioral characteristics. Hypertension 1991;17(3 Suppl):II152‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wittes J, Davis B, Berge K, Cohen JD, Grimm RH Jr, Hawkins CM, et al. Systolic Hypertension of the Elderly Program (SHEP). Part 10: analysis. Hypertension 1991;17(3 Suppl):II162‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SHEP‐P 1989 {published data only}
- Black DM, Brand RJ, Greenlick M, Hughes G, Smith J. Compliance to treatment for hypertension in elderly patients: the SHEP pilot study. Systolic Hypertension in the Elderly Program. Journal of Gerentology 1987;42(5):552‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Furberg CD, Black DM. The systolic hypertension in the elderly pilot program: methodological issues. European Heart Journal 1988;9(2):223‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hulley SB, Feigal D, Ireland C, Kuller LH, Smith WM. Systolic hypertension in the elderly program (SHEP). The first three months. Journal of the American Geriatrics Society 1986;34(2):101‐105. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hulley SB, Furberg CD, Gurland B, McDonald R, Perry HM, Schnaper HW, et al. Systolic Hypertension in the Elderly Program (SHEP): antihypertensive efficacy of chlorthalidone. American Journal of Cardiology 1985;56(15):913‐20. [DOI] [PubMed] [Google Scholar]
- Perry HM, McDonald RH, Hulley SB, Smith WM, Furberg CD, Greenlick MR. Systolic Hypertension in the Elderly Program, Pilot Study (SHEP‐PS): morbidity and mortality experience. Journal of Hypertension 1986;4(6):S21‐3. [PubMed] [Google Scholar]
- Perry HM, Smith WM, McDonald RH, Black D, Cutler JA, Furberg CD, et al. Morbidity and mortality in the Systolic Hypertension in the Elderly Program (SHEP) Pilot Study. Stroke 1989;20(1):4‐13. [DOI] [PubMed] [Google Scholar]
- Vogt TM, Ireland CC, Black D, Camel G, Hughes G. Recruitment of elderly volunteers for a multicenter clinical trial: the SHEP pilot study. Controlled Clinical Trials 1986;7(2):118‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vogt TM, Ireland CC, Greenlick MR, Hughes GH. Relation of life events to blood pressure control in the SHEP pilot trial. American Journal of Preventive Medicine 1988;4(1):1‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Sprackling 1981 {published data only}
- Sprackling ME, Mitchell JRA, Short AH, Watt G. Blood pressure reduction in the elderly: a randomised controlled trial of methyldopa. BMJ 1981;283:1151‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
STOP 1991 {published data only}
- Dahlof B, Lindholm LH, Hansson L, Schersten B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients With Hypertension (STOP‐Hypertension). Lancet 1991;338(8778):1281‐5. [DOI] [PubMed] [Google Scholar]
Syst‐Eur 1991 {published data only}
- Amery A, Birkenhager W, Bulpitt CJ, Clement D, Leeuw P, Fagard R, et al. Syst‐Eur. A multicentre trial on the treatment of isolated systolic hypertension in the elderly: objectives, protocol and organization. Aging 1991;3(3):287‐302. [DOI] [PubMed] [Google Scholar]
- Birkenhager WH, Staessen J, Gasowski J, Leeuw PW. Effects of antihypertensive treatment on endpoints in the diabetic patients randomized in the Systolic Hypertension in Europe (Syst‐Eur) trial. Journal of Nephrology 2000;13(3):323‐37. [PubMed] [Google Scholar]
- Celis H, Yodfat Y, Thijs L, Clement D, Cozic J, Cort P, et al. Antihypertensive therapy in older patients with isolated systolic hypertension: the Syst‐Eur experience in general practice. The Syst‐Eur Investigators. Family Practice 1996;13(2):138‐43. [PubMed] [Google Scholar]
- Gasowski J, Birkenhager WH, Staessen J, Leeuw PW. Benefit of antihypertensive treatment in diabetic patients enrolled in the systolic hypertension in Europe (Sys‐Eur) trial. Cardiovascular Drugs and Therapy 2000;14:49‐53. [DOI] [PubMed] [Google Scholar]
- Girerd X, Amery A, Birkenhager W, Bulpitt C, Cox J, Leeuw P, et al. SYST‐EUR: a multicenter trial of treatment of systolic hypertension in aged subjects. An initial report [SYST‐EUR un essai multicentrique du traitement de l'hypertension systolique du sujet age. Premier rapport intermediaire]. Archives des Maladies du Coeur et des Vaisseaux 1992;85(8):1243‐7. [PubMed] [Google Scholar]
- Slovick D, Staessen J, Bert P, Bulpitt C, Cort P, Fagard R, et al. Nitrendipine in older patients with isolated systolic hypertension: second progress report on the Syst‐Eur trial. Journal of Human Hypertension 1993;7(4):411‐2. [PubMed] [Google Scholar]
- Slovick DI, Amery A, Birkenhager W, Bulpitt CJ, Cox J, Leeuw P, et al. Syst‐Eur multicenter trial on the treatment of isolated hypertension in the elderly: first interim report. J Hum Hypertens 1993;7(2):201‐3. [PubMed] [Google Scholar]
- Slovick DI, Amery A, Birkenhager W, Bulpitt CJ, Cox J, Leeuw P, et al. Syst‐Eur multicenter trial on the treatment of isolated hypertension in the elderly: first interim report. Journal of Human Hypertension 1993;7(2):201‐3. [PubMed] [Google Scholar]
- Staessen J, Bert P, Bulpitt C. de Cort P, Fagard R, Fletcher A, et al. Nitrendipine in older patients with isolated systolic hypertension: second progress report on the Syst‐Eur trial. Journal of Human Hypertension 1993;7(3):265‐71. [PubMed] [Google Scholar]
- Staessen J, Dekempeneer L, Fagard R, Guo CY, Thijs L, Hoof R, et al. Treatment of isolated systolic hypertension in the elderly. Journal of Cardiovascular Pharmacology 1991;18(Suppl 1):S34‐40. [MEDLINE: ] [PubMed] [Google Scholar]
- Staessen JA, Amery A, Birkenhager W, Bulpitt CJ, Cox J, Leeuw P, et al. Syst‐Eur ‐ a multicenter trial on treatment of isolated systolic hypertension in the elderly: first interim report. Journal of Cardiovascular Pharmacology 1992;19(1):120‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhager WH, et al. Randomised double‐blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst‐Eur) Trial Investigators. Lancet 1997;350(9080):757‐64. [DOI] [PubMed] [Google Scholar]
- Staessen JA, Fagard R, Thijs L. Celis H, Birkenhager WH, Bulpitt CJ, et al. Subgroup and per‐protocol analysis of the randomized European Trial on Isolated Systolic Hypertension in the Elderly. Archives of Internal Medicine 1998;158(15):1681‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Staessen JA, Thijs L, Bieniaszewski L, O'Brien ET, Palatini P, Davidson C, et al. Ambulatory monitoring uncorrected for placebo overestimates long‐term antihypertensive action. Systolic Hypertension in Europe (SYST‐EUR) Trial Investigators. Hypertension 1996;27(3 Pt 1):414‐20. [DOI] [PubMed] [Google Scholar]
- Staessen JA, Thijs L, Clement D, Davidson C, Fagard R, Lehtonen A, et al. Ambulatory pressure decreases on long‐term placebo treatment in older patients with isolated systolic hypertension. Syst‐Eur Investigators. Journal of Hypertension 1994;12(9):1035‐9. [PubMed] [Google Scholar]
- Staessen JA, Thijs L, Mancia G, Parati G, O'Brien ET. Clinical trials with ambulatory blood pressure monitoring: fewer patients needed? Syst‐Eur Investigators. Lancet 1994;344(8936):1552‐6. [DOI] [PubMed] [Google Scholar]
- Thijs L, Dabrowska E, Clement D, Fagard R, Laks T, Mancia G, et al. Diurnal blood pressure profile in older patients with isolated systolic hypertension. The SYST‐EUR Investigators. Journal of Human Hypertension 1995;9(11):917‐24. [PubMed] [Google Scholar]
VA‐II 1970 {published data only}
- Veterans Administration Cooperative Study Group on Antihypertensive Agents. Effects of treatment on morbidity in hypertension. 3. Influence of age, diastolic pressure, and prior cardiovascular disease; further analysis of side effects. Circulation 1972;45(5):991‐1004. [DOI] [PubMed] [Google Scholar]
- Veterans Administration Cooperative Study Group on Antihypertensive Agents. Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA 1970;213(7):1143‐52. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies excluded from this review
ADVANCE 2007 {published data only}
- The ADVANCE Collaborative Group. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007;370:829‐40. [DOI: 10.1016/S0140-6736(07)61303-8] [DOI] [PubMed] [Google Scholar]
ALLHAT 1996 {published data only}
- Davis BR, Cutler JA, Gordon DJ, Furberg CD, Wright JT Jr, Cushman WC, et al. Rationale and design for the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). ALLHAT Research Group. American Journal of Hypertension 1996;9(4 Pt 1):342‐60. [DOI] [PubMed] [Google Scholar]
BENEDICT 2004 {published data only}
- Ruggenenti P, Fassi A, Ilieva AP, Bruno S, Iliev IP, Brusegan V, et al. Preventing microalbuminuria in type 2 diabetes. New England Journal of Medicine 2004;351(19):1941‐51. [DOI] [PubMed] [Google Scholar]
BENEDICT A 2006 {published data only}
- Parving HH, Lehnert H, Brochner‐Mortensen J, Gomis R, Andersen S, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. New England Journal of Medicine 2001;345(12):870‐8. [DOI] [PubMed] [Google Scholar]
Berglund 1981 {published data only}
- Berglund G, Andersson O. Beta‐blockers of diuretics in hypertension? A six year follow‐up of blood pressure and metabolic side effects. Lancet 1981;1(8223):744‐7. [DOI] [PubMed] [Google Scholar]
CASTEL 1994 {published data only}
- Casiglia E, Spolaore P, Mazza A, Ginocchio G, Colangeli G, Onesto C, et al. Effect of two different therapeutic approaches on total and cardiovascular mortality in a Cardiovascular Study in the Elderly (CASTEL). Japanese Heart Journal 1994;35(5):589‐600. [DOI] [PubMed] [Google Scholar]
DIABHYCAR 2004 {published data only}
- Marre M, Lievre M, Chatellier G, Mann JF, Passa P, Menard J, et al. Effects of low dose ramipril on cardiovascular and renal outcomes in patients with type 2 diabetes and raised excretion of urinary albumin: randomised, double blind, placebo controlled trial (the DIABHYCAR study). BMJ 2004;328(7438):495. [DOI] [PMC free article] [PubMed] [Google Scholar]
EUROPA 2003 {published data only}
- Fox KM, EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double‐blind, placebo‐controlled, multicentre trial (the EUROPA study). Lancet 2003;362(9386):782‐8. [DOI] [PubMed] [Google Scholar]
Fuchs 2011 {published data only}
- Fuchs FD, Fuchs SC, Moreira LB, Gus M, Nobrega AC, Poli‐de‐Figueiredo CE, et al. Prevention of hypertension in patients with pre‐hypertension: protocol for the PREVER‐prevention trial. Trials 2011;12:65. [DOI: 10.1186/1745-6215-12-65] [DOI] [PMC free article] [PubMed] [Google Scholar]
Generic 2010 {published data only}
- Punzi HA, Lewin AJ, Lukic T, Goodin T, Chen W. Efficacy and safety of nebivolol monotherapy in Hispanics with stage I‐II hypertension by obesity status. Journal of Clinical Hypertension 2010;12:536. [Google Scholar]
GENRES 2007 {published data only}
- Hitunen TP, Suonsyrja T, Hannila‐Handelberg T, Paavonen KJ, et al. Predictors of antihypertensive drug responses: initial data from a placebo‐controlled, randomized, cross over study with four antihypertensive drugs. Journal of Hypertension 2009;27:2001‐9. [DOI] [PubMed] [Google Scholar]
GLANT 1995 {published data only}
- Study Group on Long‐term Antihypertensive Therapy. A 12‐month comparison of ACE inhibitor and CA antagonist therapy in mild to moderate essential hypertension ‐ the GLANT study. Study Group on Long‐term Antihypertensive Therapy. Hypertension Research ‐ Clinical & Experimental 1995;18(3):235‐44. [PubMed] [Google Scholar]
HAPPHY 1987 {published data only}
- Wilhelmsen L, Berglund G, Elmfeldt D, Fitzsimons T, Holzgreve H, Hosie J, et al. Beta‐blockers versus diuretics in hypertensive men: main results from the HAPPHY trial. Journal of Hypertension 1987;5(5):561‐72. [DOI] [PubMed] [Google Scholar]
HDFP 1984 {published data only}
- Hypertension Detection and Follow‐up Program Cooperative Group. Effect of stepped care treatment on the incidence of myocardial infarction and angina pectoris. 5‐year findings of the hypertension detection and follow‐up program. Hypertension 1984;6(2 Pt 2):I198‐206. [DOI] [PubMed] [Google Scholar]
Hood 2007 {published data only}
- Hood SJ, Taylor KP, Ashby MJ, Brown MJ. The spironolactone, amiloride, losartan, and thiazide (SALT) double‐blind crossover trial in patients with low‐renin hypertension and elevated aldosterone‐renin ratio. Circulation 2007;116(3):268‐75. [DOI] [PubMed] [Google Scholar]
HOPE 3 2016 {published data only}
- Lonn EM, Bosch J, Lopez‐ Jaramillo P, Zhu J, Liu L, Pais P, et al. Blood‐pressure lowering in intermediate‐risk persons without cardiovascular disease. New England Journal of Medicine 2016;374(21):2009‐20. [DOI] [PubMed] [Google Scholar]
HOT 1995 {published data only}
- Hansson L, Zanchetti A. The Hypertension Optimal Treatment (HOT) Study: 12‐month data on blood pressure and tolerability. With special reference to age and gender. Blood Pressure 1995;4:313‐9. [DOI] [PubMed] [Google Scholar]
IDM 2001 {published data only}
- Parving HH, Lehnert H, Brochner‐Mortensen J, Gomis R, Andersen S, Arner P, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. New England Journal of Medicine 2001;345(12):870‐8. [DOI] [PubMed] [Google Scholar]
IDNT 2003 {published data only}
- Berl T, Hunsicker LG, Lewis JB, Pfeffer MA, Porush JG, Rouleau JL, et al. Cardiovascular outcomes in the Irbesartan diabetic nephropathy trial of patients with type 2 diabetes and overt nephropathy. Annals of Internal Medicine 2003;138(7):542‐9. [DOI] [PubMed] [Google Scholar]
IMAGINE 2008 {published data only}
- Rouleau JL, Warnica WJ, Baillot R, Block PJ, Chocron S, Johnstone D, et al. Effects of angiotensin converting enzyme inhibition in low‐risk patients early after coronary artery bypass surgery. Circulation 2008;117:24‐31. [DOI] [PubMed] [Google Scholar]
Imai 2011 {published data only}
- Imai E, Chan J, Ito S, Yamasaki T, Kobayashi F, Haneda M, et al. Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy: a multicentre, randomised, placebo‐controlled study. Diabetologia 2011;54(12):2978‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
INSIGHT 1996 {published data only}
- Brown MJ, Castaigne A, Ruilope LM, Mancia G, Rosenthal T, Leeuw PW, et al. INSIGHT: International Nifedipine GITS study: intervention as a goal in hypertension treatment. Journal of Human Hypertension 1996;10(Suppl 3):S157‐60. [PubMed] [Google Scholar]
Jikei 2007 {published data only}
- Mochizuki S, Dahlof B, Shimizu M, Ikewaki K, Yoshikawa M, Taniguchi I, et al. Valsartan in a Japanese population with hypertension and other cardiovascular disease (Jikei Heart Study): a randomised, open‐label, blinded endpoint morbidity‐mortality study.[Retraction in Lancet. 2013 Sep 7;382(9895):843; PMID: 24012258]. Lancet 2007;369(9571):1431‐9. [DOI] [PubMed] [Google Scholar]
Kondo 2003 {published data only}
- Kondo J, Sone T, Tsuboi H, Mukawa H, Morishima I, Uesegi M, et al. Effects of low‐dose angiotensin II receptor blocker candesartan on cardiovascular events in patients with coronary artery disease. American Heart Journal 2003;146(6):E20‐25. [DOI] [PubMed] [Google Scholar]
Kuramoto 1994 {published data only}
- Kuramoto K. Treatment of elderly hypertensives in Japan: national intervention cooperative study in elderly hypertensives. The National Intervention Cooperative Study Group. Journal of Hypertension ‐ Supplement 1994;12(6):S35‐40. [PubMed] [Google Scholar]
Lewis 1993 {published data only}
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin‐converting‐enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. New England Journal of Medicine 1993;329(20):1456‐62. [DOI] [PubMed] [Google Scholar]
Lewis 2001 {published data only}
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin‐converting‐enzyme inhibition on diabetic nephropathy. New England Journal of Medicine 2001;345:851‐60. [DOI] [PubMed] [Google Scholar]
MacMahon 2000 {published data only}
- MacMahon S, Sharpe N, Gamble G, Clague A, Mhurchu CN, Clark T, et al. Randomized, placebo‐controlled trial of the angiotensin‐converting enzyme inhibitor, ramipril, in patients with coronary or other occlusive arterial disease. PART 2 Collaborative Research Group. Prevention of Atherosclerosis With Ramipril. Journal of the American College of Cardiology 2000;36(2):438‐43. [DOI] [PubMed] [Google Scholar]
MAPHY 1988 {published data only}
- Wikstrand J, Warnold I, Olsson G, Tuomilehto J, Elmfeldt D, Berglund G. Primary prevention with metoprolol in patients with hypertension. Mortality results from the MAPHY study. JAMA 1988;259(13):1976‐82. [PubMed] [Google Scholar]
MIDAS 1996 {published data only}
- Borhani NO, Mercuri M, Borhani PA, Buckalew VM, Canossa‐Terris M, Carr AA, et al. Final outcome results of the Multicenter Isradipine Diuretic Atherosclerosis Study (MIDAS). JAMA 1996;276(10):785‐91. [PubMed] [Google Scholar]
Morgan 1980 {published data only}
- Morgan TO, Adams WR, Hodgson M, Gibberd RW. Failure of therapy to improve prognosis in elderly males with hypertension. Medical Journal of Australia 1980;2(1):27‐31. [PubMed] [Google Scholar]
NAVIGATOR 2010 {published data only}
- McMurray JJ, Holman RR, Haffner SM, Bethel MA, Holzhauer B, et al. Effect of valsartan on the incidence of diabetes and cardiovascular events. New England Journal of Medicine 2010;362(16):1477‐90. [DOI] [PubMed] [Google Scholar]
NICOLE 2003 {published data only}
- Dens JA, Desmet WJ, Coussement P, Scheerder KD, Kostopoulos K, Kerdsinchal P, et al. Long term effects of nisoldipine on the progression of coronary atherosclerosis and the occurrence of clinical events: the NICOLE study. Heart 2003;89(8):887‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
NORDIL 2000 {published data only}
- Hansson L, Hedner T, Lund‐Johnson P, Kjeldsen SE, Lindholm LH, Syvertsen JO, et al. Randomised trial of effects of calcium antagonists compared with diuretics and beta‐blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Dilitazem (NORDIL) study. Lancet 2000;356(9227):359‐65. [DOI] [PubMed] [Google Scholar]
Oslo 1986 {published data only}
- Leren P, Helgeland A. Oslo Hypertension Study. Drugs 1986;31(1):41‐5. [DOI] [PubMed] [Google Scholar]
PEACE 2004 {published data only}
- Braunwald E, Domanski MJ, Fowler SE, Geller NL, Gersh BJ, Hsia J, et al. Angiotensin converting‐ enzyme inhibition in stable coronary artery disease. New England Journal of Medicine 2004;351:2058‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pool 2007 {published data only}
- Pool JL, Glazer R, Weinberger M, Alvarado R, Huang J, Graff A. Comparison of valsartan/hydrochlorothiazide combination therapy at doses up to 320/25 mg versus monotherapy: a double‐blind, placebo‐controlled study followed by long‐term combination therapy in hypertensive adults. Clinical Therapeutics 2007;29(1):61‐73. [DOI] [PubMed] [Google Scholar]
PRoFESS 2008 {published data only}
- Yusuf S, Diener HC, Sacco RL, Cotton D, Ounpuu S, Lawton WA, et al. Telmisartan to prevent recurrent stroke and cardiovascular events. New England Journal of Medicine 2008;359:1225‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
PROGRESS 2001 {published data only}
- MacMahon S, Neal B, Tzourio C, Rodgers A, Woodward M, Cutler J, et al. Randomised trial of a perindopril‐based blood‐pressure‐lowering regimen among 6105 individuals with previous stroke or transient ischemic attack. Lancet 2001;358(9287):1033‐41. [DOI] [PubMed] [Google Scholar]
QUIET 2001 {published data only}
- Pitt B, O'Neill B, Feldman R, Ferrari R, Schwartz L, Judra H, et al. Evaluation of chronic ACE inhibitor therapy in patients with ischemic heart disease and preserved left ventricular function. American Journal of Cardiology 2001;87:1058‐63. [DOI] [PubMed] [Google Scholar]
REIN 1997 {published data only}
- GISEN Group. Randomised placebo‐controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non‐diabetic nephropathy [Gruppo Italiano di Studi Epidemiologici in Nefrologia]. Lancet 1997;349(9069):1857‐63. [PubMed] [Google Scholar]
RENAAL 2001 {published data only}
- Brenner BM, Cooper ME, Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. New England Journal of Medicine 2001;345(12):861‐9. [DOI] [PubMed] [Google Scholar]
ROAD 2007 {published data only}
- Hou FF, Xie D, Zhang X, Chen PY, Zhang WR, Liang M, et al. Renoprotection of Optimal Antiproteinuric Doses (ROAD) Study: a randomized controlled study of benazepril and losartan in chronic renal insufficiency. Journal of the American Society of Nephrology 2007;18(6):1889‐98. [DOI] [PubMed] [Google Scholar]
ROADMAP 2011 {published data only}
- Haller H, Ito S, Izzo JL Jr, Januszewicz A, Katayama S, Menne J, et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. New England Journal of Medicine 2011;364(10):907‐17. [DOI] [PubMed] [Google Scholar]
SCAST 2015 {published data only}
- Jusufovic M, Sandset EC, Bath PM, Karlson BW, Berge E, Scandinavian Candesartan Acute Stroke Trial Study Group. Effects of blood pressure lowering in patients with acute ischemic stroke and carotid artery stenosis. International Journal of Stroke 2015;10(3):354‐9. [DOI] [PubMed] [Google Scholar]
SCAT 2000 {published data only}
- Teo KK, Burton JR, Buller CE, Plante S, Catellier D, Tymchak W, et al. Long‐term effects of cholesterol lowering and angiotensin converting enzyme inhibition on coronary atherosclerosis. Circulation 2000;102(15):1748‐54. [DOI] [PubMed] [Google Scholar]
Scheimder 2012 {published data only}
- Schmieder RE, Philipp T, Guerediaga J, Gorostidi M, Smith B, et al. Long‐term antihypertensive efficacy and safety of the oral direct renin Inhibitor aliskiren. A 12‐month randomized, double‐blind comparator trial with hydrochlorothiazide. Circulation 2009;119(3):417‐25. [DOI] [PubMed] [Google Scholar]
SCOPE 2003 {published data only}
- Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double‐blind intervention trial. Journal of Hypertension 2003;21(5):875‐86. [DOI: 10.1097/01.hjh.0000059028.82022.89] [DOI] [PubMed] [Google Scholar]
SHELL 1994 {published data only}
- Malacco E, Gnemmi AE, Romagnoli A, Coppini A. Systolic hypertension in the elderly: long‐term lacidipine treatment. Objective, protocol, and organization. SHELL Study Group. Journal of Cardiovascular Pharmacology 1994;23(Suppl 5):S62‐6. [PubMed] [Google Scholar]
STONE 1996 {published data only}
- Gong L, Zhang W, Zhu Y, Zhu J, Kong D, Page V, et al. Shanghai trial of nifedipine in the elderly (STONE). Journal of Hypertension 1996;14(10):1237‐45. [DOI] [PubMed] [Google Scholar]
STOP‐2 1993 {published data only}
- Dahlof B, Hansson L, Lindholm LH, Schersten B, Wester PO, Ekbom T, et al. STOP‐Hypertension 2: a prospective intervention trial of "newer" versus "older" treatment alternatives in old patients with hypertension. Swedish Trial in Old Patients with Hypertension. Blood Pressure 1993;2(2):136‐41. [DOI] [PubMed] [Google Scholar]
Strandberg 1991 {published data only}
- Strandberg TE, Salomaa VV, Naukkarinen VA, Vanhanen HT, Sarna SJ, Miettinen TA. Long‐term mortality after 5‐year multifactorial primary prevention of cardiovascular diseases in middle‐aged men. JAMA 1991;266(9):1255‐9. [PubMed] [Google Scholar]
Syst‐China 1993 {published data only}
- Anonymous. Systolic hypertension in the elderly: Chinese trial (Syst‐China) ‐ second interim report. Chung‐Hua Hsin Hsueh Kuan Ping Tsa Chih [Chinese Journal of Cardiology] 1993;21(3):135‐7,185. [PubMed] [Google Scholar]
- Anonymous. [Systolic hypertension in the elderly: Chinese trial (Syst‐China). Interim report]. [Chinese] Chung‐Hua Hsin Hsueh Kuan Ping Tsa Chih [Chinese Journal of Cardiology] 1992;20(5):270‐5,323. [PubMed] [Google Scholar]
TRANSCEND 2008 {published data only}
- Yusuf S, Teo K, Anderson C, Pogue J, et al. Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators. Effects of the angiotensin‐receptor blocker telmisartan on cardiovascular events in high‐risk patients intolerant to angiotensin converting enzyme inhibitors: a randomised controlled trial. Lancet 2008;372(9644):1174‐83. [DOI] [PubMed] [Google Scholar]
USPHSHCSG 1977 {published data only}
- Smith WM, US Public Health Service Hospitals Cooperative Study Group. Treatment of mild hypertension: results of a ten year intervention trial. Circulation Research 1977;40(5 Suppl 1):98‐105. [PubMed] [Google Scholar]
VACS 1982 {published data only}
- Veterans Administrative Cooperative Study Group on Antihypertensive Agents. Comparison of propranolol and hydrochlorothiazide for the initial treatment of hypertension. II. Results of long‐term therapy. Veterans Administration Cooperative Study Group on Antihypertensive Agents. JAMA 1982;248(16):2004‐11. [PubMed] [Google Scholar]
VANHLBI 1978 {published data only}
- Perry HM Jr, Goldman AI, Lavin MA. Evaluation of drug treatment in mild hypertension: VA‐NHLBI feasibility trial. Annals of the New York Academy of Sciences 1978;304:267‐88. [DOI] [PubMed] [Google Scholar]
VHAS 1997 {published data only}
- Agabiti RE, Dal Palu D, Leonetti G, Magnani G, Pessina A, Zanchetti A. Clinical results of the verapamil in hypertension and atherosclerosis study. Journal of Hypertension 1997;15(11):1337‐44. [DOI] [PubMed] [Google Scholar]
White 1995 {published data only}
- White WB, Stimpel M. Long‐term safety and efficacy of moexipril alone and in combination with hydrochlorothiazide in elderly patients with hypertension. Journal of Human Hypertension 1995;9(11):879‐84. [PubMed] [Google Scholar]
Additional references
ACTIVE I 2011
- The Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE I) Investigators. Irbesartan in patients with atrial fibrillation. New England Journal of Medicine 2011;364:928‐33. [Google Scholar]
Alderman 1981
- Alderman MH, Madhavan S. Management of the hypertensive patient: a continuing dilemma. Hypertension 1981;3:192‐7. [DOI] [PubMed] [Google Scholar]
Alderman 1993
- Alderman MH. Blood pressure management: individualized treatment based on absolute risk and the potential for benefit. Annals of Internal Medicine 1993;119:329‐35. [DOI] [PubMed] [Google Scholar]
Applegate 1992
- Applegate WB, Miller ST, Elam JT, Cushman WC, Derwi D, Brewer A, Graney MJ. Nonpharmacologic intervention to reduce blood pressure in older patients with mild hypertension. Archives of Internal Medicine 1992;152(6):1162‐6. [PubMed] [Google Scholar]
Aronow 2015
- Aronow WS. Treating hypertension and prehypertension in older people: when, whom and how. Maturitas 2015;80:31‐6. [DOI] [PubMed] [Google Scholar]
Barraclough 1973
- Barraclough M, Bainton D, Cochrane AL, et al. Control of moderately raised blood pressure: report of a co‐operative randomized controlled trial. BMJ 1973;3(5877):434‐6. [PMC free article] [PubMed] [Google Scholar]
BBLTTC 2005
- Turnbull F, Neal B, Albert C, Chalmers J, Chapman N, Cutler J, et al. Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of different blood pressure‐lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Archives of Internal Medicine 2005;165(12):1410‐9. [DOI] [PubMed] [Google Scholar]
Bejan‐Angoulvant 2010
- Bejan‐Angoulvant T, Saadatian‐Elahi M, Wright JM, Schron EB, Lindholm LH, Fagard R, et al. Treatment of hypertension in patients 80 years and older: the lower the better? A meta‐analysis of randomized controlled trials. Journal of Hypertension 2010;28(7):1366‐72. [DOI] [PubMed] [Google Scholar]
Boissel 2005
- Boissel JP, Gueyffier F, Boutitie F, Pocock S, Fagard R. Apparent effect on blood pressure is only partly responsible for the risk reduction due to antihypertensive treatments. Fundamentals of Clinical Pharmacology 2005;19:579‐84. [DOI] [PubMed] [Google Scholar]
BPLTTC 2000
- Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood pressure‐lowering drugs: results of prospectively designed overviews of randomised trials. The Lancet 2000;355:1955‐64. [DOI] [PubMed] [Google Scholar]
Browner 1989
- Browner WS, Hulley SB. Implications of hypertension trials: effect of risk status on treatment criteria. Hypertension 1989;13(1):51‐6. [DOI] [PubMed] [Google Scholar]
Bull 2015
- Bull S, Loudon M, Francis JM, Joseph J, Gerry S, Karamitsos TD, et al. A prospective, double‐blind, randomized controlled trial of the angiotensin‐converting enzyme inhibitor Ramipril In Aortic Stenosis (RIAS trial). European Heart Journal Cardiovascular Imaging 2015;16(8):834‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Celis 1993
- Celis H, Fagard R, Staessen J, Thijs L, Amery A. The older hypertensive: assessment and treatment. Netherlands Journal of Medicine 1993;43:S66‐77. [PubMed] [Google Scholar]
Collins 1990
- Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, et al. Blood pressure, stroke, and coronary heart disease. Part 2. Short‐term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 1990;335(8693):827‐38. [DOI] [PubMed] [Google Scholar]
Davidson 1987
- Davidson RA, Caranasos GJ. Should the elderly hypertensive be treated? Evidence from clinical trials. Archives of Internal Medicine 1987;147:1933‐7. [PubMed] [Google Scholar]
DREAM 2006
- DREAM Trial Investigators, Bosch J, Yusuf S, Gerstein HC, Pogue J, Sheridan P, et al. Effect of ramipril on the incidence of diabetes. New England Journal of Medicine 2006;355(15):1551‐62. [DOI] [PubMed] [Google Scholar]
Duarte 2010
- Duarte JD, Cooper‐DeHoff RM. Mechanisms for blood pressure lowering and metabolic effects of thiazide and thiazide‐like diuretics. Expert Reviews on Cardiovascular Therapy 2010;8(6):793‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
DUTCH ‐TIA 1993
- DUTCH ‐TIA Trial Study Group. Trial of secondary prevention with atenolol after transient ischemic attack or non‐disabling ischemic stroke. Stroke 1993;24:543‐8. [DOI] [PubMed] [Google Scholar]
FEVER 2011
- Zhang Y, Zhang X, Liu L, Zanchetti A, Group FS. Is a systolic blood pressure target < 140 mmHg indicated in all hypertensives? Subgroup analyses of findings from the randomised FEVER trial. European Heart Journal 2011;32(12):1500‐8. [DOI] [PubMed] [Google Scholar]
Frishman 2005
- Frishman WH, Cheng‐Lai A, Nawarskas J. Current Cardiovascular Drugs. Fourth. London, UK: Current Science Group, 2005. [Google Scholar]
Goeres 2014
- Goeres LM, Eckstrom E, Lee DS. Pharmacology for hypertension in older adults: a systematic review. Drugs and Aging 2014;31:897‐910. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Gueyffier 1996
- Gueyffier F, Froment A, Gouton M. New meta‐analysis of treatment trials of hypertension: improving the estimate of therapeutic benefit. Journal of Human Hypertension 1996;10:1‐8. [PubMed] [Google Scholar]
Gueyffier 1997
- Gueyffier F, Boutitie F, Boissel JP, Pocock S, Coope J, Cutler J, et al. Effect of antihypertensive drug treatment on cardiovascular outcomes in women and men. A meta‐analysis of individual patient data from randomized, controlled trials. The INDANA Investigators. Annals of Internal Medicine 1997;126(10):761‐7. [DOI] [PubMed] [Google Scholar]
Gueyffier 1999
- Gueyffier F, Bulpitt C, Boissel, et al. for the INDANA Group. Antihypertensive drugs in very old people: a subgroup meta‐analysis of randomized controlled trials. Lancet 1999;353(9155):793‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from handbook.cochrane.org. [Google Scholar]
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8. Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from handbook.cochrane.org. [Google Scholar]
HOPE‐HYP 2000
- Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. The Heart Outcomes Prevention Evaluation Outcomes Study Investigators. New England Journal of Medicine 2000;342(3):145‐53. [DOI] [PubMed] [Google Scholar]
Hughes 2004
- Hughes AD. How do thiazide and thiazide‐like diuretics lower blood pressure?. Journal of the Renin‐Angiotensin‐Aldosterone System 2004;5(4):155‐60. [DOI: 10.3317/jraas.2004.034] [DOI] [PubMed] [Google Scholar]
Insua 1994
- Insua JT, Sacks HS, Lau TS, Lau J, Reitman D, Pagano D, et al. Drug treatment of hypertension in the elderly: a meta‐analysis. Annals of Internal Medicine 1994;121:355‐62. [DOI] [PubMed] [Google Scholar]
Ioannidis 1997
- Ioannidis JP, Lau J. The impact of high‐risk patients on the results of clinical trials. Journal of Clinical Epidemiology 1997;50(10):1089‐98. [DOI] [PubMed] [Google Scholar]
IPPPSH 1985
- The IPPPSH Collaborative Group. Cardiovascular risk and risk factors in a randomized trial of treatment based on the beta‐blocker oxprenolol: the International Prospective Primary Prevention Study in Hypertension (IPPPSH). Journal of Hypertension 1985;3(4):379‐92. [DOI] [PubMed] [Google Scholar]
Kang 2004
- Kang S, Wu FY, An N, Ren M. A systematic review and meta‐analysis of the efficacy and safety of a fixed low‐dose perindopril‐indapamide combination as first‐line treatment of hypertension. Clinical Therapeutics 2004;26(2):257‐70. [DOI] [PubMed] [Google Scholar]
Kızılırmak 2017
- Kızılırmak P, Uresin Y, Ozdemir O, Kilickiran B, et al. Renin‐angiotensin‐aldosterone system blockers and cardiovascular outcomes: a meta‐analysis of randomized clinical trials [Renin‐anjiyotensin‐aldosteron sistemi blokerleri ve kardiyovasküler sonuçları: Randomize klinik çalışmaların meta‐analizi]. Turk Kardiyoloji Dernegi Arsivi 2017;45(1):49‐66. [DOI] [PubMed] [Google Scholar]
Law 2009
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta‐analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leonetti 1992
- Leonetti G, Cuspidi C, Fastidio M, Lonati L, Chianca R. Arterial hypertension as a risk factor in the elderly and its treatment. Journal of Hypertension 1992;10(2):S3‐7. [PubMed] [Google Scholar]
Lundh 2017
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.MR000033.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
MacMahon 1993
- MacMahon S, Rogers A. The effects of blood pressure reduction in older patients: an overview of five randomized controlled trials in elderly hypertensives. Clinical and Experimental Hypertension 1993;15(6):967‐78. [DOI] [PubMed] [Google Scholar]
Mann 1992
- Mann SJ. Systolic hypertension in the elderly. Archives of Internal Medicine 1992;152:1977‐84. [PubMed] [Google Scholar]
Materson 1993
- Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, Kochar MS, et al. Single‐drug therapy for hypertension in men: a comparison of six antihypertensive agents with placebo. New England Journal of Medicine 1993;328(13):914‐21. [DOI] [PubMed] [Google Scholar]
Musini 2008
- Therapeutics Initiative. Treatment of elevated blood pressure in the elderly: less is better. www.ti.ubc.ca/2008/10/31/treatment‐of‐elevated‐blood‐pressure‐in‐the‐very‐elderly‐less‐is‐better (accessed 15 April 2018).
Musini 2017
- Musini VM, Gueyffier F, Puil L, Salzwedel DM, Wright JM. Pharmacotherapy for hypertension in adults aged 18 to 59 years. Cochrane Database of Systematic Reviews 2017, Issue 8. [DOI: 10.1002/14651858.CD008276.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nikolaus 2000
- Nikolaus T, Sommer N, Becker C. Treatment of arterial hypertension with diuretics, beta‐ and calcium channel blockers in old patients. Zeitschrift fur Gerontologie und Geriatrie 2000;33(6):427‐32. [DOI] [PubMed] [Google Scholar]
Parsons 2016
- Parsons C, Murad MH, Andersen S, Mookadam F, Labonte H. The effect of antihypertensive treatment on the incidence of stroke and cognitive decline in the elderly: a meta‐analysis. Future Cardiology 2016;12(2):237‐48. [DOI] [PubMed] [Google Scholar]
PATS 1995
- PATS Collaborating Group. Post‐stroke antihypertensive treatment study. A preliminary result. Chinese Medical Journal 1995;108(9):710‐7. [PubMed] [Google Scholar]
Pearce 1995
- Pearce KA, Furberg CD, Rushing J. Does antihypertensive treatment of the elderly prevent cardiovascular events or prolong life?. Archives of Family Medicine 1995;4(11):943‐50. [DOI] [PubMed] [Google Scholar]
Psaty 1997
- Psaty BM, Smith NL, Siscovick DS, Koepsell TD, et al. Health outcomes associated with antihypertensive therapies used as first‐line agents. A systematic review and meta‐analysis. JAMA 1997;277(9):739‐45. [PubMed] [Google Scholar]
Psaty 2003
- Psaty BM, Lumley T, Furberg CD, Schellenbaum G, Pahor M, Alderman MH, et al. Health outcomes associated with various antihypertensive therapies used as first‐line agents: a network meta‐analysis. JAMA 2003;289(19):2534‐44. [DOI] [PubMed] [Google Scholar]
Quan 1999
- Quan A, Kerlikowske K, Gueyffier F, Boissel JP. Efficacy of treating hypertension in women. Journal of General Internal Medicine 1999;14(12):718‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schmid 1998
- Schmid CH, Lau J, McIntosh MW, Cappelleri JC. An empirical study of the effect of the control rate as a predictor of treatment efficacy in meta‐analysis of clinical trials. Statistics in Medicine 1998;17(17):1923‐42. [DOI] [PubMed] [Google Scholar]
Staessen 1988
- Staessen J, Fagard R, Hoof R, Amery A. Mortality in various intervention trials in elderly hypertensive patients: a review. European Heart Journal 1988;9(2):215‐22. [DOI] [PubMed] [Google Scholar]
Staessen 1990a
- Staessen J, Fagard R, Hoof R, Amery A. Intervention trials in elderly hypertensive patients: a review. Journal of Hypertension 1990;8(4):S63‐7. [PubMed] [Google Scholar]
Staessen 1990b
- Staessen J, Amery A, Fagard R. Isolated systolic hypertension in the elderly. Journal of Hypertension 1990;8(5):393‐405. [DOI] [PubMed] [Google Scholar]
Sundstrom 2015
- Sundstrom J, Arima H, Jackson R, Turnbull F, Rahimi K, Chalmers J, et al. Effects of blood pressure reduction in mild hypertension: a systematic review and meta‐analysis. Annals of Internal Medicine 2015;162(3):184‐91. [DOI] [PubMed] [Google Scholar]
Tan 2016
- Tan XY, Hu JB. ACEIs/ARBs for the prevention of type 2 diabetes in patients with cardiovascular diseases: a systematic review and meta‐analysis. International Journal of Clinical and Experimental Medicine 2016;9(5):7624‐37. [Google Scholar]
TEST 1995
- Eriksson S, Olofsson BO, Wesley PO, et al. Atenolol for secondary prevention after stroke. Cerebrovascular Disease 1995;5:21‐5. [Google Scholar]
Thijs 1992
- Thijs L, Fagard R, Lijnen P, Staessen J, Hoof R, Amery A. A meta‐analysis of outcome trials in elderly hypertensives. Journal of Hypertension 1992;10(10):1103‐9. [DOI] [PubMed] [Google Scholar]
Thijs 1994
- Thijs L, Fagard R, Lijnen P, Staessen J, Hoof R, Amery A. Why is antihypertensive drug therapy needed in elderly patients with systodiastolic hypertension?. Journal of Hypertension 1994;12(6):S25‐34. [PubMed] [Google Scholar]
Thomopoulos 2014
- Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension. 1. Overview, meta‐analyses, and meta‐regression analyses of randomized trials. Journal of Hypertension 2014;32(12):2285‐95. [DOI] [PubMed] [Google Scholar]
Thomopoulos 2016
- Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure‐lowering treatment. 6. Prevention of heart failure and new‐onset heart failure ‐ meta‐analyses of randomized trials. Journal of Hypertension 2016;34(3):373‐84. [DOI] [PubMed] [Google Scholar]
TOMHS 1995
- Elmer PJ, Grimm R, Laing B, Grandits G, Svedsen K, Heel N, et al. Lifestyle intervention: results of the Treatment of Mild Hypertension Study (TOMHS). Preventive Medicine 1995;24(4):378‐88. [DOI] [PubMed] [Google Scholar]
Turnbull 2003
- Turnbull F, Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of different blood‐pressure‐lowering regimens on major cardiovascular events: results of prospectively‐designed overviews of randomised trials. Lancet 2003;362(9395):1527‐35. [DOI] [PubMed] [Google Scholar]
UKPDS 39 1998
- UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998;317(7160):713‐20. [PMC free article] [PubMed] [Google Scholar]
VA Coop 1962
- Veterans Administration Cooperative Study Group on Antihypertensive Agents. A double‐blind control study of antihypertensive agents. II. Further report on the comparative effectiveness of reserpine, reserpine and hydralazine, and three ganglionic blocking agents, clorisondamine, mecamylamine, and pentolinium tartrate. Archives of Internal Medicine 1962;110(2):222‐29. [PubMed] [Google Scholar]
VA‐I 1967
- Veterans Administration Cooperative Study Group on Antihypertensive Agents. Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA 1967;202:1028‐34. [PubMed] [Google Scholar]
Wiysonge 2017
- Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Opie LH. Beta‐blockers for hypertension. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD002003.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolf 1966
- Wolf FW, Lindeman RD. Effects of treatment in hypertension. Results of a controlled study. Journal of Chronic Diseases 1966;19(3):227‐40. [DOI] [PubMed] [Google Scholar]
Wright 2018
- Wright JM, Musini VM, Gill R. First‐line drugs for hypertension. Cochrane Database of Systematic Reviews 2018, Issue 4. [DOI: 10.1002/14651858.CD001841.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright JM 1999
- Wright JM, Lee CH, Chambers KG. Systematic review of antihypertensive therapies: does the evidence assist in choosing a first‐line drug?. Canadian Medical Association Journal 1999;161(1):25‐32. [PMC free article] [PubMed] [Google Scholar]
Zanchetti 2015
- Zanchetti A, Thomopoulos C, Parati G. Randomized controlled trials of blood pressure lowering in hypertension: a critical reappraisal. Circulation Research 2015;116(6):1058‐73. [DOI] [PubMed] [Google Scholar]
Zhu 2005
- Zhu Z, Zhu S, Liu D, Cao T, Wang L, Tepel M. Thiazide‐like diuretics attenuate agonist‐induced vasoconstriction by calcium desensitisation linked to rho kinase. Hypertension 2005;45(2):233‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Mulrow 1994
- Mulrow CD, Cornell JA, Herrera CR, Kadri A, Farnett L, Aguilar C. Hypertension in the elderly: Implications and generalizability of randomized trials. JAMA 1994;272:1932‐8. [PubMed] [Google Scholar]
Mulrow 1998
- Mulrow CD, Lau J, Cornell J, Brand M. Pharmacotherapy for hypertension in the elderly. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD000028] [DOI] [PubMed] [Google Scholar]
Mulrow 2000
- Mulrow CD, Lau J, Cornell J, Brand M. Pharmacotherapy for hypertension in the elderly. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD000028] [DOI] [PubMed] [Google Scholar]
Musini 2009
- Musini VM, Tejani AM, Bassett K, Wright JM. Pharmacotherapy for hypertension in the elderly. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD000028.pub2] [DOI] [PubMed] [Google Scholar]


