Abstract
To initiate and support locomotion, rhythm generating neurons in the spinal central pattern generator convert descending input into a rhythmic signal which is conveyed to downstream neurons, leading to the recruitment of motor neurons and activation of muscles. Although two genetically-defined neuronal populations have been linked to rhythm generation, a single all-inclusive rhythm generating population has yet to be identified. Here, we consolidate recent work aimed at identifying rhythm generating neurons, summarize the evidence for the involvement of two neuronal populations in rhythm generation, describe the challenges in identifying a marker for rhythm generating neurons, and discuss potential directions to take in integrating spinal rhythm generating neurons into recently identified speed-dependent locomotor circuits.
Keywords: spinal cord, rhythm generation, locomotion, interneurons
Introduction
Although activated and modulated by descending controls and afferent inputs, hindlimb locomotion is generated by central pattern generators (CPGs) in the spinal cord. The way by which the spinal network is organized to convert an activation signal into a rhythmic and coordinated activation of motor neurons and muscles has been hypothesized since the early 1900s. Most of the current conceptual frameworks include rhythm generating neurons, the focus of this review. The number of rhythm generating populations, their arrangement in the network, the mechanisms by which they generate the rhythm, and their dedication to rhythm generation or their diversity in function vary.
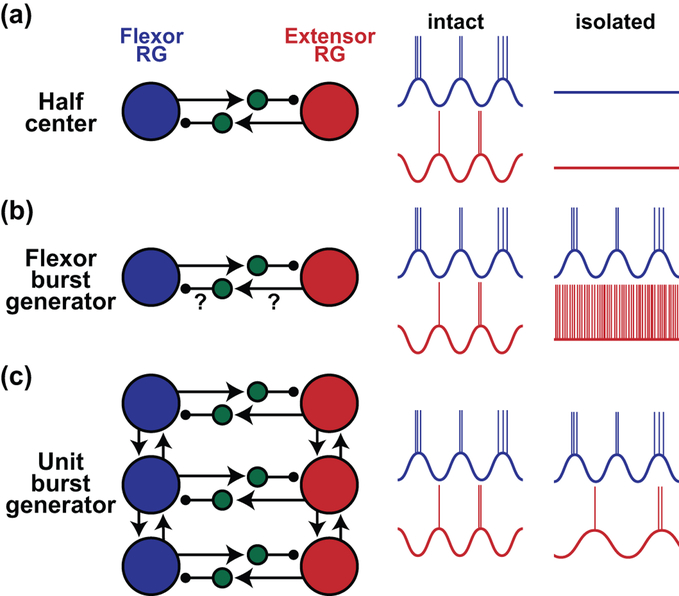
In the classic half-center model [1,2], each side of the cord contains both a flexor and an extensor half-center, which are symmetric and mutually exclusive in their excitation. Mutual inhibition between the two populations of rhythm generating half-centers, together with adaptation in firing, sets the rhythm (Figure 1). Two more recent variations of the half-center model include the flexor burst model and the unit burst model. In the flexor burst model, the flexor and extensor centers are asymmetric [3–8]. The extensor rhythm generating neurons are at a higher activity level than flexor rhythm generating neurons and are tonically active. The intrinsic rhythmicity of the flexor rhythm generating neurons brings the extensor rhythm generating neurons into a rhythmic alternation based on inhibitory connections from flexor to extensor rhythm generating neurons [5–9]. The unit burst model is a more flexible arrangement with half-centers repeated for each joint and capable of operating independently, with different populations of rhythm generating neurons active based on locomotor task [10,11]. Recent work testing these frameworks computationally showed that having either one, both, or neither of the rhythm generating centers intrinsically rhythmic could support locomotion with feasibility dependent on whether the drive to rhythm generating centers is equal or unequal in distribution [12]. The suggestion that the organizational models are not necessarily mutually exclusive and may operate during different states or locomotor behaviors [12] makes it more challenging to determine specific neurons and mechanisms involved.
Figure 1: Conceptual frameworks for rhythm generation and hypothesized operation.
A. Half-center [1,2]. Flexor and extensor rhythm generating neurons are mutually inhibited by interposed inhibitory interneurons. Excitatory connections are shown with arrows and inhibitory with circles. In normal operation, flexor and extensor rhythm generating neurons alternate. In this model, rhythm generating neurons are not intrinsically rhythmic and do not oscillate when synaptically isolated. B. Flexor burst generator [3–8]. Flexor rhythm generating neurons are inherently rhythmic but extensor rhythm generating neurons are not. Activation of flexor rhythm generating neurons leads to the rhythmic inhibition of extensor rhythm generating neurons via interposed inhibitory interneurons. Coordinating connections from extensor rhythm generating neurons to flexor rhythm generating neurons are either less strong or non-existent. If rhythm generating populations were to be isolated, flexor rhythm generating neurons would be rhythmic and extensor rhythm generating neurons would be tonic or silent. C. Unit burst generator [10,11]. There are repeating modules of flexor and extensor rhythm generating populations for each joint. Coordinating connections in between flexor and extensor modules and populations at different joints are both excitatory and inhibitory to allow for flexibility in motor pattern. Note that only excitatory connections between modules of different joints are shown. When isolated, both populations oscillate but flexor populations may oscillate at higher frequency than extensor populations.
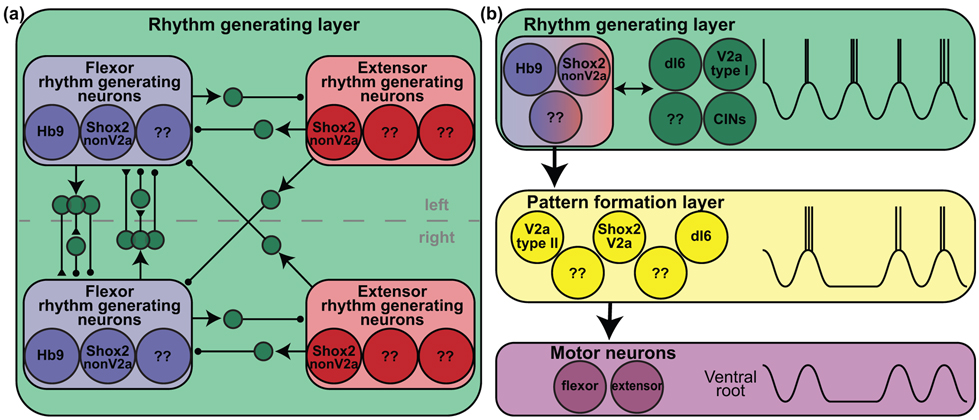
Regardless of framework, the spinal components of locomotion orchestrated by CPGs are often separated into rhythm and pattern. Rhythm is the timing or regularity of the neural oscillations or motor output. Pattern is the coordinated recruitment of motor neurons in sequence, often characterized as the coordination between left and right sides of the body and between flexor and extensor musculature within a limb. In two-layer models of mammalian locomotion, there is a rhythm generating layer and a pattern formation layer [13]. However, even in the two-layer model, rhythm and pattern are rather intertwined since left-right coordination and flexor-extensor alternation are determined by the rhythm generating layer [13,14]. Within the rhythm generating layer, there are both rhythm generating neurons and other neurons involved in coordinating the rhythm generating populations. These include both flexor rhythm generating neurons and extensor rhythm generating neurons minimally on each side of the cord, and possibly repeated for each joint [6,10,11,14]. These rhythm generating neurons are local, ipsilaterally-projecting, excitatory interneurons that can convert tonic input into rhythmic output and provide the necessary rhythmic drive to other downstream neurons, in both the rhythm generating and pattern forming layers. Additionally in the rhythm generating layer, there are other neurons which do not contribute to the rhythm but project ipsilaterally to maintain coordination of the flexor and extensor rhythm generating kernels and commissurally to coordinate kernels on opposite sides of the cord (Figure 2A). Left-right and flexor-extensor coordination at the top level, or rhythm generating level, allows for stable coordination. If this coordination was executed exclusively at the last order level or any level downstream from the rhythm generating layer, it would be possible for competing rhythms to exist in the circuit and the activity of certain rhythm generating populations would need to be entirely overridden. Well-coordinated rhythm generating centers allow for the recruitment of downstream patterning neurons under the rhythm set by the rhythm generating layer. Motor neurons are then recruited by the neurons in the pattern forming layer organized in modules or synergies. This review will focus on rhythm generating neurons so we will first go through how they are separated from pattern formation layer neurons and then from other neurons in the rhythm generating layer.
Figure 2: Components of the rhythm generating layer and use of deletions to separate rhythm and pattern.
A. The rhythm generating layer (green box) contains flexor rhythm generating neurons (blue box) and extensor rhythm generating neurons (red box) on both sides of the spinal cord (midline denoted by dashed line). Flexor rhythm generating neurons (blue) likely include Hb9, Shox2 non-V2a, and other unidentified populations. Extensor rhythm generating neurons (red) include other Shox2 non-V2a and unidentified neurons. The rhythm generating populations are coordinated by populations of excitatory and inhibitory neurons in between them. Several of the coordinating populations have been identified and hypothesized [6,9,22,67]. Excitatory connections from heterogeneous populations are shown with arrows. Other excitatory connections are denoted with triangles and inhibitory connections with circles. Connectivity structure is based on [8]. B. Neuronal activity during spontaneous deletions has been used to categorize neurons as belonging to the rhythm generating layer or patterning formation layer. All neurons in the rhythm generating layer (green box), including rhythm generating neurons (blue and red) and neurons coordinating left-right and flexor-extensor rhythm generating cores, should maintain activity during deletions. Coordinating neurons (green) categorized in this way include dI6, V2a type I, and unspecified commissural interneurons, CINs [5,17]. There are interconnections between core rhythm generating neurons and other neurons in this layer (bidirectional arrow). The core rhythm generating neurons drive the neurons in the pattern formation layer (yellow box). Neurons in the pattern formation layer (yellow) do not maintain activity during deletions. Patterning neurons categorized by lack of activity include V2a type II (which may be Shox2 V2a) and dI6 neurons [5,9,17]. Both rhythm and pattern layers likely contain several other populations. Motor neurons (purple) are not active during deletions [5,15].
How are neurons involved in rhythm and pattern distinguished?
Activity of individual neurons during non-resetting deletions has been a way in which neurons can be categorized as part of the rhythm generating layer or patterning layer [5,13–18]. However, this method does not differentiate between rhythm generating neurons and other neurons in the rhythm generating layer involved in coordinating flexor-extensor and left-right alternation (Figure 2B). Non-resetting deletions occur spontaneously during both locomotion in decerebrate cat and fictive locomotion in isolated rodent spinal cords [5,13–15]. These are characterized by an absence or failure of motor activity (action potentials or ENG/EMG burst) in a motor neuron pool or muscle (and synergist pools/muscles) that occurs within the timing expected based on the ongoing locomotion [5,15]. The rhythm or timing of the motor bursts around the deletion is consistent except for the failed burst(s) and flexor-extensor and left-right alternation are maintained aside from the synergist motor pools where the activity is absent [5,15]. This suggests that there are neurons that are keeping the timing or the rhythm through the deletion, the rhythm generating neurons. Additionally, all neurons within the rhythm generating layer would be expected to maintain rhythmicity during deletions in order to keep both the timing and overall pattern. During deletions, motor neurons do not fire action potentials and receive little or no rhythmic drive during the deleted burst [5], therefore neurons in the patterning layer which recruit them are expected to be silent (no action potentials) during the deletion. Thus, interneurons which fall silent during deletions can be inferred to be in the patterning layer but neurons belonging to the rhythm generating layer maintain activity.
Recordings from unidentified interneurons show an approximate 50/50 split in rhythm and pattern layer neurons classified by activity during deletions, with rhythm generating layer neurons occupying more medial locations and patterning layer neurons tending to be more lateral [5,17]. Commissural interneurons, mainly located ventromedially in the ventral horn, have previously been shown to maintain activity during deletions which fits well with data demonstrating that spontaneous deletions mainly affect only one side of the spinal cord [5]. Many commissural interneurons are thought to be in the rhythm generating layer, downstream from either the left or right rhythm generating kernel, exciting or inhibiting the opposite rhythm generating kernel, and required for appropriate coordination between left and right sides.
Some of the transcription factor defined ventral horn populations have also been classified in this manner. The excitatory, ipsilaterally-projecting V2a neurons, for example, have been separated into two groups based on activity during deletions and these groups fit with anatomical and computational data describing at least two populations based on connectivity and functional ablation studies. V2a type I neurons continue to oscillate during deletions so are classified as part of the rhythm generating layer [5]. These neurons presumably connect to V0V commissural interneurons [19,20], where they act to secure left and right alternation, particularly at higher locomotor speeds [6,21–23]. V2a type II interneurons fall silent during deletions, and therefore are not in the rhythm generating layer [5]. This neuronal population likely includes those co-expressing the transcription factor Shox2 which are thought to be last order interneurons [23,24]. Similarly, dI6 interneurons are in both rhythm and patterning layers based on activity during deletions [16,18]. The dI6 interneurons are also heterogeneous in terms of their projections including subsets of ipsilaterally projecting last-order neurons and commissural interneurons, a portion of which expresses WT1 and projects to other contralaterally projecting interneurons [18,25]. Lastly, although the activity of excitatory V3 neurons during deletions has not yet been reported, they have recently been shown to have discrete connections providing information flow unidirectionally from ventromedial to ventrolateral V3 neurons and bidirectionally from ventrolateral V3 neurons to motor neurons [26]. Based on connectivity and location, the medial and lateral V3 populations are likely to be divisible by rhythm and pattern as well but this is yet to be tested. Thus, most, if not all, of the transcription factor defined populations likely have discrete subsets of neurons involved in the rhythm and the patterning layers.
Although the activity of commissural interneurons and subsets of V2a and dI6 interneurons persists during deletions, genetic ablation/silencing experiments of specific commissural populations or the V2a interneurons result in prominent left-right coordination deficits with little to no effect on rhythm [19,22,25,27–29]. Therefore, these neurons are thought to be in the rhythm generating layer, coordinating core rhythm generating neurons (i.e. left and right), but are not likely to be rhythm generating. This leaves the question of which neurons comprise the rhythm generating core.
Which neurons are rhythm generating?
In the search for rhythm generating neurons, there are several attributes that one would expect of the neurons involved. Pharmacology, ablation, and optogenetics experiments have demonstrated that locomotor rhythm generating neurons are excitatory, ipsilaterally projecting interneurons in the intermediate and/or ventral horn (reviewed in [23,30]). The only populations of neurons defined as belonging to the cardinal classes of spinal neurons that meet these criteria are the V2a and subsets of the V3 population. It is expected that rhythm generating neurons are rhythmically active during locomotion and the activity of at least a subset of these neurons should precede the motor burst [31]; however, this is not necessarily the case for the entire population since motor neuron activity, and therefore drive to motor neurons, is not precisely synchronized. Further, their activation should be sufficient to generate locomotor-like activity and inactivation of these neurons should prevent locomotion or, if part of the rhythm generating population remains, the frequency of the rhythm will be substantially reduced [9,23].
Genetic silencing or ablating the cardinal classes containing at least a subset of ventral, excitatory, ipsilaterally projecting neurons has not resulted in disruption of the rhythm [19,22,23,29]. However, two other identifiable populations, Hb9 and Shox2, have been implicated by experiments in which the vesicular glutamate transporter 2 (vGluT2) was removed from these populations, thereby synaptically silencing them. In both cases, the result was a reduction in locomotor frequency with no effect on patterning [24,32]. Therefore, both populations may contribute to rhythm generation but neither is solely responsible. Other evidence is summarized for each of these populations below.
Hb9 interneurons:
Hb9 is expressed transiently during development in many ventral horn neurons and potentially dorsal horn neurons but expression persists only in motor neurons and a small population of ventral medial neurons found in lower thoracic and upper lumbar segments [33–35]. Spinal interneurons expressing the transcription factor Hb9 were first proposed to be rhythm generating neurons as they met many of the criteria expected for a rhythm generating population [30]. In addition to being excitatory and ipsilaterally-projecting, these interneurons are interconnected (by electrical synapses), fire rhythmically during drug-evoked locomotion, and remain rhythmically active when synaptically isolated [34–38]. They receive direct input from low-threshold afferents which when activated during the flexor phase of fictive locomotion results in resetting of the rhythm and the activity of the Hb9 neurons remains phase-locked to motor bursts [39], suggesting a role for Hb9 neurons in controlling the timing of locomotor rhythm. Acute activation and inactivation experiments would be impossible to interpret as the transcription factor Hb9 is also expressed in motor neurons [40]. Selective vGluT2 deletion experiments partly circumvented this problem; however, vGluT2 was also removed from dorsal horn and other ventral horn populations, in addition to the ventromedial population. Additionally, vGluT2 may be expressed in motor neurons [41,42], which provide feedback to the rhythm generator [43]. To rule out the possibility that motor neurons or dorsal neurons labeled by the Hb9Cre line were responsible for the frequency effects seen, similar experiments were performed in ChATcre and Lbx1cre mice. In both cases, there was no effect on the rhythm [32]. This suggests that ventral excitatory Hb9 neurons, which include the canonical ventromedial Hb9 neurons, were responsible for the frequency effects and may participate in rhythm generation.
Shox2 nonV2a interneurons:
Shox2 is transiently expressed by neurons that predominantly settle in lamina VII and partly overlap with the V2a population. Shox2 expression is only maintained in a small percentage of these neurons at P0 [24] and persists in some V2a neurons to adulthood [44]. Many Shox2 neurons display rhythmic activity during locomotion [24] and are interconnected by electrical synapses [45]. The reduction in frequency seen in vGluT2 deletion experiments was attributed to the Shox2 nonV2a neurons, defined as those that developmentally express Shox2 but do not express Chx10, as ablation of Shox2 V2a neurons did not affect the rhythm [24]. Acute disruption of Shox2 interneuron activity, similar to the chronic vGluT2 effect, resulted in a reduction in locomotor frequency, with no change in the locomotor pattern [24]. However, as the rhythm remains after the disturbance, Shox2 neurons are not the sole rhythm generating neurons. Like the Hb9 neurons, acute activation experiments for Shox2 neurons would be difficult to interpret as Shox2 is expressed in populations of primary sensory neurons and stimulation of dorsal roots can initiate locomotion.
The overlap between Hb9 and Shox2 nonV2a neurons is minimal [32]; therefore, it is unlikely to be the same neurons mediating the observed effects. More direct genetic manipulation experiments targeting exclusively the ventral excitatory Hb9 interneuron population and the Shox2 nonV2a interneuron population will be necessary to say definitively but available data suggests that both populations are involved in rhythm generation.
Why has a single marker for all rhythm generating neurons remained elusive?
Although the possibility of a single marker that includes all and is exclusive to rhythm generating neurons is an attractive idea, there are several reasons why the identification of such marker has remained a challenge. First, there would have to be a single rhythm generating population. Although there are likely to be dedicated rhythm generating neurons, they are unlikely to be a homogenous population. Distinct rhythm generating populations may be active at different speeds [46] or in different contexts and these may or may not have a common marker. Those associated with the rhythmic control of different muscle groups or movement around different joints may be unique populations. Further, compensatory mechanisms for the loss of a rhythmogenic population involving distinct neurons and pathways are likely as there are several ways by which it is possible to generate rhythm [47]. vGluT2 knockout mice, for example, locomote without excitatory transmission from spinal neurons [48], although this is not likely to occur in normal circumstances [49]. Acute disruptions make compensation less likely; however, it is unclear how quickly reorganization can occur. Finally, recent work distinguishing subpopulations within the cardinal classes highlights the complexity of the transcription factor code. For example, the V1 class is arguably the most straightforward to separate based on function. Renshaw cells and Ia interneurons are clearly defined in terms of circuit connectivity and function. However, there is still no single transcription factor which can be used to identify either of these two groups [50]. On the other hand, the V1 population may be made up of more than 50 molecularly-defined subpopulations [50]. The molecular markers distinguishing the V1 subtypes are not particularly specific to this cardinal class; therefore, combinations of transcription factors are necessary to identify unique neuronal populations [44,50,51]. Altogether, it is possible that there is no single, unique transcription factor that specifies rhythm generating fate in the spinal cord. However, with two candidate populations, it is conceivable that a functionally guided search for new markers may yield a transcription factor or other molecular marker that is common to all rhythm generating neurons.
How do rhythm generating neurons generate rhythm?
The hallmark of a rhythm generating population is that it is capable of converting a tonic input into a rhythmic signal. Precisely how this occurs in locomotor rhythm generating neurons is unknown but with two identified rhythm generating populations, it is possible to explore. Rhythm generation likely results from a combination of conditionally-active pacemaker properties intrinsic to individual rhythm generating neurons and emergent network properties defined by neuronal circuit connectivity dynamics. Experimentally distinguishing between these mechanisms is not trivial, even with a population to target. One can isolate a neuron to determine if it is capable of oscillating as a pacemaker; however, just because a neuron can act like a pacemaker, does not necessarily mean this is how it typically functions. Contributions of circuit connectivity are difficult to demonstrate but essential elements may be possible to determine experimentally.
NMDA, persistent sodium (INaP), and T-type calcium (IT) currents could support pacemaker properties and have been implicated in locomotor-like activity [35,38,52–56]. The ventromedial Hb9 neurons, a subset of the neurons silenced in the Hb9-vGlut2 deletion experiments, have been shown to display large inward currents in response to ramps [57] and post-inhibitory rebound dependent on hyperpolarization voltage and time [35], which are hallmarks of persistent inward and T-type Ca2+ currents, respectively. Blockade of either current results in a reduction in the amplitude and frequency of Hb9 membrane oscillations [38,56]. Given that upregulation of INaP decreases locomotor frequency [53] and T-type Ca2+ oscillations emerge in high concentrations of NMDA [38,55,56], it does not seem that either of these currents are solely responsible for promoting rhythmic activity in the locomotor CPG.
The potential importance of conditional pacemaker properties has been supported by the observation that changes in Ca2+ and K+ concentrations proceed the development of drug-evoked locomotion and mimicking these concentration changes alone, in the absence of drugs, is sufficient to produce locomotor-like bursting [57]. It is possible that these ionic changes amplify certain currents (i.e. INaP) and activate pacemaker properties in rhythm generating neurons [57]. Whether or not the presence of intrinsic properties which are capable of supporting cellular oscillations are necessarily suggestive of rhythm generating status is another question. Several of these properties, including INaP, IT, and Ih, are present in large subsets of interneurons, even in those that are not considered to be rhythm generating [18,53,58,59]. Both the flexor generator model and the unit burst generator depend on at least one population capable of generating the rhythm autonomously without the requirement of inhibition, which would suggest at least a subset of neurons would have pacemaker or conditional pacemaker properties [11,12].
It is also possible that intrinsic currents in a single neuron are not strong enough to initiate oscillations; however, the same currents may synchronize a larger interconnected population and lead to oscillations [60,61]. A high degree of electrical coupling has been found between GFP+ neurons in Hb9-GFP mice [36,37] and preferentially between subsets of Shox2 neurons defined by presence/absence of Chx10 [45]. Gap junctional blockers not only decrease the interconnectivity of rhythm generating neurons but they also decrease the frequency of drug-evoked locomotion [45], suggesting a possible contribution of interneuronal connectivity to rhythm generation. A similar hypothesis is the group pacemaker hypothesis where recurrent excitatory interactions between rhythm generating neurons allow for the activation of currents and generation of a burst, once activity levels reach a threshold [62,63]; however recent evidence in the respiratory system suggests that bursts are not required for rhythm but synchronizations of activity among smaller subsets of neurons are sufficient [64,65]. Whether a similar mechanism is involved in locomotion remains to be determined.
How are rhythm generating neurons organized and how are these circuits selected by descending control systems?
The organization of rhythm generating neurons has yet to be determined. Rhythm generating neurons appear to be a heterogeneous population which may suggest that subsets of neurons are responsible for different locomotor speeds or contexts. In zebrafish, speed-dependent modules of rhythm generating neurons are activated at different locomotor frequencies [46,66]; however, without limbs, there may not be separate rhythm and patterning layers and the levels of control are likely fewer. In rodents, modular organization is apparent at the level of the commissural interneurons, proposed to be in the rhythm generating layer [6,22,23,67]. Distinct commissural interneuron populations are activated at increasing locomotor speeds and are implicated in different gaits [6,22,23,67]. Specifically, the V0D population of inhibitory commissural interneurons is active at low speeds of locomotion and the V0v population of excitatory commissural interneurons takes over at medium speeds corresponding to trot [6,22,67]. It is possible that these commissural populations are recruited by distinct rhythm generating neurons arranged in modules.
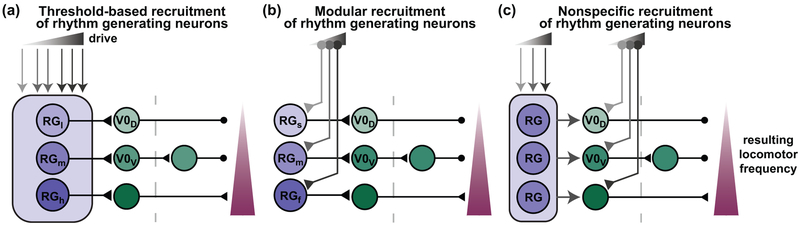
Relatedly, the recruitment of rhythm generating neurons by descending systems is currently unknown but is essential to a complete understanding of locomotor function. Several recent papers have demonstrated the participation of supraspinal structures in the control of locomotor speed [68–70]. The cuneiform nuclei (CnF) and pedunculopontine nuclei (PPN) which control high and low speeds, related to escape and exploration, respectively [69,70], do not project directly to the spinal cord but through the reticular nuclei, specifically the glutamatergic neurons of the lateral paragigantocellular nucleus (LPGi) [68]. Further, unilateral stimulation of the LPGi is sufficient to generate bilateral locomotion and increase the speed of ongoing activity [68] but different stimulus durations delivered to opposite sides of the ventral caudal medulla modulate the frequency of the rhythm and lead to motor outputs in vitro consistent with those expected during turning [71]. The level(s) of the CPG that is (are) controlled by reticulospinal inputs is currently unknown but there are several possibilities. First, common reticulospinal inputs may select rhythm generating neurons based on differences in neuronal activation threshold (Figure 3A), with lower threshold rhythm generating neurons being recruited by weak reticulospinal input and those with higher thresholds recruited by stronger reticulospinal input. Another possibility is that the system is fully modular, with specific reticulospinal populations activated by different regions of the mesencephalic locomotor region based on functional context and these specific reticulospinal populations select speed-dependent rhythm generating modules (Figure 3B). Lastly, specific reticulospinal populations may select gait at the level of the neurons coordinating the left and right sides of the cord at different speeds (Figure 3C, [7,8,22,67]). In this case, a common drive may activate rhythm generating neurons at the appropriate frequency.
Figure 3: Potential points of descending control of coordinated locomotion at different speeds.
A. Threshold-based recruitment of rhythm generating neurons. Reticulospinal drive is distributed to all rhythm generating neurons. The low threshold rhythm generating neurons (RGl) are activated by weak inputs (light gray) and activate inhibitory (V0D) commissural neurons which function at low speeds for walking. Increased descending drive would recruit medium threshold rhythm generating neurons (RGm) which may activate a distinct set of commissural interneurons, such as the disynaptic inhibitory pathway (via V0v neurons) that is the main pathway for trot, an intermediate speed gait. High threshold rhythm generating (RGh) neurons may be activated only when descending drive is strongest and control excitatory commissural neurons which synchronize the left and right rhythm generating centers in the fast bounding gait. Commissural pathways activated at distinct gaits have been previously described [6–8,22,67]. Arrows denote drive to or from a population, triangles are excitatory synapses, and circles are inhibitory synapses. B. Modular recruitment of rhythm generating neurons. Instead of being recruited based on threshold, separate modules of slow (RGs), medium (RGm), and fast (RGf) rhythm generating neurons are activated by distinct reticulospinal populations. Each rhythm generating module would be related to a speed-dependent commissural pathway. Therefore, selective activation of particular modules would determine speed and locomotor gait. C. Nonspecific recruitment of rhythm generating neurons, selection of commissural coordinating neurons. Increasing reticulospinal drive increases the oscillatory frequency of the rhythm generating neurons but rhythmic drive to commissural populations is subthreshold. The speed-dependent commissural populations are selectively recruited by specific reticulospinal inputs, which allow oscillatory inputs from the rhythm generating neurons to bring them to threshold. In this case, lower speed circuits may also be actively inhibited at higher speeds (not shown).
In addition to activation, the brainstem exerts tonic inhibitory controls as removal of the brainstem in isolated neonatal preparations results in increased locomotor frequency [72]. Both descending reticulospinal inhibition [68] and descending excitation of local circuit inhibitory neurons [73] have been demonstrated. The latter pathway acts on rhythm generating neurons [73] and may provide an additional criterion by which to identify rhythm generating neurons. It is entirely possible that in addition to or instead of activation patterns shown in Figure 3, there are opposite descending inhibitory controls. The precise ways by which these inhibitory pathways integrate with or run parallel to the activation pathways in order to control the spinal locomotor circuits remains to be directly tested.
Conclusions and Future Directions
Many open questions remain regarding locomotor rhythm generation, including the selection or recruitment of rhythm generating neurons by descending pathways, the mechanisms underlying rhythm generation, and the ways by which the rhythm generating neurons are precisely organized within the network architecture of the CPG. Although there is no single, all-inclusive marker for rhythm generating neurons, two defined groups of interneurons linked to rhythm generation, Hb9 and Shox2 nonV2a interneurons, may provide an entry point by which to answer these questions. The mechanisms by which locomotor rhythm is generated remain to be identified and may depend on condition or state. Therefore, recording a combination of single cell (i.e. whole cell patch clamp), population activity (i.e. Ca2+ imaging), and motor output to assess cellular currents/oscillations, network activation patterns, and locomotor activity simultaneously will likely be necessary. Circuit connectivity experiments using dual recordings [36,37,45] or alternative approaches such as channelrhodopsin-assisted circuit mapping or glutamate uncaging [26] will allow for the testing of precise connectivity proposed based on computational modeling and population silencing experiments. These strategies, together with chemo- and optogenetic activation/silencing of descending inputs, may reveal the recruitment, control, and maintenance of the rhythm. In addition to descending inputs to and downstream connections from rhythm generating neurons, determining the afferent input to these neurons and the involvement of rhythm generating neurons in the rhythmic gating of sensory pathways [74,75] will be essential to complete the circuit. As new ways to reliably identify and label more restricted populations of neurons become available, such combinatorial approaches will make it possible to directly test both new and longstanding hypotheses, bringing us closer to revealing the mechanisms and connectivity structures underlying rhythm generation and the functional connectivity of the CPG.
Highlights.
Spinal rhythm generating neurons initiate and support locomotion by converting descending drive into a rhythmic signal.
There is no single all-inclusive marker for rhythm generating neurons.
Hb9 and Shox2 nonV2a neurons are proposed to participate in rhythm generation.
Rhythm generating mechanism and precise connectivity structure of the spinal locomotor circuitry are outstanding questions.
Acknowledgements
We are grateful to Ole Kiehn, Ilya Rybak, D. Leonardo Garcia Ramirez, Erik Li, and Nick Stachowski for discussions and comments on the manuscript. Our work is supported by the National Institutes of Health (R01 NS095366 and R01 NS104194).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown TG: On the nature of the fundamental activity of the nervous centres; together with an analysis of the conditioning of rhythmic activity in progression and a theory of the evolution of function in the nervous system. J Physiol 1914, 48:18–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jankowska E, Jukes MG, Lund S, Lundberg A: The effect of DOPA on the spinal cord. 6. Half-centre organization of interneurones transmitting effects from the flexor reflex afferents. Acta Physiol Scand 1967, 70:389–402. [DOI] [PubMed] [Google Scholar]
- 3.Pearson KG, Duysens J: Function of segmental reflexes in the control of stepping in cockroaches and cats In Neural Control of Locomotion. Advances in Behavioral Biology. Edited by H RM, G S, S PSG, S DG: Springer; 1976:519–537. vol 18.] [Google Scholar]
- 4.Duysens J, De Groote F, Jonkers I: The flexion synergy, mother of all synergies and father of new models of gait. Front Comput Neurosci 2013, 7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong G, Shevtsova NA, Rybak IA, Harris-Warrick RM: Neuronal activity in the isolated mouse spinal cord during spontaneous deletions in fictive locomotion: insights into locomotor central pattern generator organization. J Physiol 2012, 590:4735–4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shevtsova NA, Talpalar AE, Markin SN, Harris-Warrick RM, Kiehn O, Rybak IA: Organization of left-right coordination of neuronal activity in the mammalian spinal cord: Insights from computational modelling. J Physiol 2015, 593:2403–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danner SM, Wilshin SD, Shevtsova NA, Rybak IA: Central control of interlimb coordination and speed-dependent gait expression in quadrupeds. J Physiol 2016, 594:6947–6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danner SM, Shevtsova NA, Frigon A, Rybak IA: Computational modeling of spinal circuits controlling limb coordination and gaits in quadrupeds. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rybak IA, Dougherty KJ, Shevtsova NA: Organization of the Mammalian Locomotor CPG: Review of Computational Model and Circuit Architectures Based on Genetically Identified Spinal Interneurons(1,2,3). eNeuro 2015, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grillner S: Control of locomotion in bipeds, tetrapods, and fish In Handbook of Physiology, The Nervous System, Motor Control. Edited by; 1981. [Google Scholar]
- 11.Hagglund M, Dougherty KJ, Borgius L, Itohara S, Iwasato T, Kiehn O: Optogenetic dissection reveals multiple rhythmogenic modules underlying locomotion. Proc Natl Acad Sci U S A 2013, 110:11589–11594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ausborn J, Snyder AC, Shevtsova NA, Rybak IA, Rubin JE: State-dependent rhythmogenesis and frequency control in a half-center locomotor CPG. J Neurophysiol 2018, 119:96–117.**This computational modeling study examines the feasibility of three different operating regimes hypothesized for the spinal central pattern generator (CPG): 1) neither the flexor nor extensor centers have intrinsic bursting capacities, 2) rhythmogenesis is flexor-driven, or 3) both centers have intrinsic rhythmicity. All three regimes were found to be plausible within the same network with differing levels of external drive received by flexor and extensor populations, demonstrating that they are not mutually exclusive but dependent on condition or context.
- 13.McCrea DA, Rybak IA: Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev 2008, 57:134–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rybak IA, Shevtsova NA, Lafreniere-Roula M, McCrea DA: Modelling spinal circuitry involved in locomotor pattern generation: insights from deletions during fictive locomotion. J Physiol 2006, 577:617–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lafreniere-Roula M, McCrea DA: Deletions of rhythmic motoneuron activity during fictive locomotion and scratch provide clues to the organization of the mammalian central pattern generator. J Neurophysiol 2005, 94:1120–1132. [DOI] [PubMed] [Google Scholar]
- 16.Dyck J, Lanuza GM, Gosgnach S: Functional characterization of dI6 interneurons in the neonatal mouse spinal cord. J Neurophysiol 2012, 107:3256–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griener A, Dyck J, Gosgnach S: Regional distribution of putative rhythm-generating and pattern-forming components of the mammalian locomotor CPG. Neuroscience 2013, 250:644–650. [DOI] [PubMed] [Google Scholar]
- 18.Griener A, Zhang W, Kao H, Haque F, Gosgnach S: Anatomical and electrophysiological characterization of a population of dI6 interneurons in the neonatal mouse spinal cord. Neuroscience 2017, 362:47–59. [DOI] [PubMed] [Google Scholar]
- 19.Crone SA, Quinlan KA, Zagoraiou L, Droho S, Restrepo CE, Lundfald L, Endo T, Setlak J, Jessell TM, Kiehn O, et al. : Genetic ablation of V2a ipsilateral interneurons disrupts left-right locomotor coordination in mammalian spinal cord. Neuron 2008, 60:70–83. [DOI] [PubMed] [Google Scholar]
- 20.Dougherty KJ, Kiehn O: Functional organization of V2a-related locomotor circuits in the rodent spinal cord. Ann N Y Acad Sci 2010, 1198:85–93. [DOI] [PubMed] [Google Scholar]
- 21.Crone SA, Zhong G, Harris-Warrick R, Sharma K: In mice lacking V2a interneurons, gait depends on speed of locomotion. J Neurosci 2009, 29:7098–7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talpalar AE, Bouvier J, Borgius L, Fortin G, Pierani A, Kiehn O: Dual-mode operation of neuronal networks involved in left-right alternation. Nature 2013, 500:85–88. [DOI] [PubMed] [Google Scholar]
- 23.Kiehn O: Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci 2016, 17:224–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dougherty KJ, Zagoraiou L, Satoh D, Rozani I, Doobar S, Arber S, Jessell TM, Kiehn O: Locomotor rhythm generation linked to the output of spinal shox2 excitatory interneurons. Neuron 2013, 80:920–933.** The authors identified a population of interneurons which expresses the transcription factor Shox2. Shox2 neurons are excitatory, ipsilaterally projecting, partly overlap with the V2a population, and form connections with each other, as well as with motor neurons and commissural INs. Additionally, the majority of Shox2 INs are rhythmically active during fictive locomotion. Chronic synaptic silencing or acute inactivation of Shox2 neurons resulted in a reduction in locomotor frequency. Ablating the Shox2 INs that co-express Chx10 did not have a significant effect on locomotor-like activity. Together, this suggests that spinal neurons exclusively expressing Shox2 (Shox2 non-V2a) are involved in rhythm generation.
- 25.Haque F, Rancic V, Zhang W, Clugston R, Ballanyi K, Gosgnach S: WT1-Expressing Interneurons Regulate Left-Right Alternation during Mammalian Locomotor Activity. J Neurosci 2018, 38:5666–5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chopek JW, Nascimento F, Beato M, Brownstone RM, Zhang Y: Sub-populations of Spinal V3 Interneurons Form Focal Modules of Layered Pre-motor Microcircuits. Cell Rep 2018, 25:146–156 e143.**This study uses glutamate uncaging to probe circuit connectivity of the V3 interneuron population. Ventromedial V3 interneurons are interconnected and have inputs to other V3 interneurons that reside laterally. Ventrolateral V3 interneurons and motor neurons are bidirectional connected. The authors suggest that the ventral V3 interneurons form layered microcircuits in the thoracolumbar spinal cord.
- 27.Lanuza GM, Gosgnach S, Pierani A, Jessell TM, Goulding M: Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron 2004, 42:375–386. [DOI] [PubMed] [Google Scholar]
- 28.Andersson LS, Larhammar M, Memic F, Wootz H, Schwochow D, Rubin CJ, Patra K, Arnason T, Wellbring L, Hjalm G, et al. : Mutations in DMRT3 affect locomotion in horses and spinal circuit function in mice. Nature 2012, 488:642–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Narayan S, Geiman E, Lanuza GM, Velasquez T, Shanks B, Akay T, Dyck J, Pearson K, Gosgnach S, et al. : V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron 2008, 60:84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brownstone RM, Wilson JM: Strategies for delineating spinal locomotor rhythm-generating networks and the possible role of Hb9 interneurones in rhythmogenesis. Brain Res Rev 2008, 57:64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwan AC, Dietz SB, Webb WW, Harris-Warrick RM: Activity of Hb9 interneurons during fictive locomotion in mouse spinal cord. J Neurosci 2009, 29:11601–11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caldeira V, Dougherty KJ, Borgius L, Kiehn O: Spinal Hb9::Cre-derived excitatory interneurons contribute to rhythm generation in the mouse. Sci Rep 2017, 7:41369.*Hb9 neurons had been hypothesized to take part in locomotor rhythm generation but expression of Hb9 in motor neurons precluded population ablation/silencing experiments. Here, the authors synaptically silence glutamatergic Hb9 neurons by deleting vGluT2 from them. This resulted in a reduction in locomotor frequency with no change in flexor-extensor and left-right alternation. When vGluT2 was removed from motor neurons selectively, however, there were no changes in locomotor frequency. This suggests that Hb9 neurons are involved in rhythm generation.
- 33.Lee SK, Jurata LW, Funahashi J, Ruiz EC, Pfaff SL: Analysis of embryonic motoneuron gene regulation: derepression of general activators function in concert with enhancer factors. Development 2004, 131:3295–3306. [DOI] [PubMed] [Google Scholar]
- 34.Hinckley CA, Hartley R, Wu L, Todd A, Ziskind-Conhaim L: Locomotor-like rhythms in a genetically distinct cluster of interneurons in the mammalian spinal cord. J Neurophysiol 2005, 93:1439–1449. [DOI] [PubMed] [Google Scholar]
- 35.Wilson JM, Hartley R, Maxwell DJ, Todd AJ, Lieberam I, Kaltschmidt JA, Yoshida Y, Jessell TM, Brownstone RM: Conditional rhythmicity of ventral spinal interneurons defined by expression of the Hb9 homeodomain protein. J Neurosci 2005, 25:5710–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hinckley CA, Ziskind-Conhaim L: Electrical coupling between locomotor-related excitatory interneurons in the mammalian spinal cord. J Neurosci 2006, 26:8477–8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson JM, Cowan AI, Brownstone RM: Heterogeneous electrotonic coupling and synchronization of rhythmic bursting activity in mouse Hb9 interneurons. J Neurophysiol 2007, 98:2370–2381. [DOI] [PubMed] [Google Scholar]
- 38.Ziskind-Conhaim L, Wu L, Wiesner EP: Persistent sodium current contributes to induced voltage oscillations in locomotor-related hb9 interneurons in the mouse spinal cord. J Neurophysiol 2008, 100:2254–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hinckley CA, Wiesner EP, Mentis GZ, Titus DJ, Ziskind-Conhaim L: Sensory modulation of locomotor-like membrane oscillations in Hb9-expressing interneurons. J Neurophysiol 2010, 103:3407–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM: Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell 1996, 84:309–320. [DOI] [PubMed] [Google Scholar]
- 41.Mentis GZ, Alvarez FJ, Bonnot A, Richards DS, Gonzalez-Forero D, Zerda R, O’Donovan MJ: Noncholinergic excitatory actions of motoneurons in the neonatal mammalian spinal cord. Proc Natl Acad Sci U S A 2005, 102:7344–7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishimaru H, Restrepo CE, Ryge J, Yanagawa Y, Kiehn O: Mammalian motor neurons corelease glutamate and acetylcholine at central synapses. Proc Natl Acad Sci U S A 2005, 102:5245–5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falgairolle M, Puhl JG, Pujala A, Liu W, O’Donovan MJ: Motoneurons regulate the central pattern generator during drug-induced locomotor-like activity in the neonatal mouse. Elife 2017, 6.*This study utilized optogenetics in isolated neonatal mouse spinal cords to manipulate the activity of motor neurons during ongoing fictive locomotion. Dampening motor neuron activity resulted in a reduction in the locomotor frequency and a disruption in pattern. Enhancement of motor neuron activation increased the locomotor frequency, independent of cholinergic transmission but mediated, at least partly, by glutamatergic transmission. These experiments demonstrate that motor neurons provide feedback to the spinal central pattern generator.
- 44.Hayashi M, Hinckley CA, Driscoll SP, Moore NJ, Levine AJ, Hilde KL, Sharma K, Pfaff SL: Graded Arrays of Spinal and Supraspinal V2a Interneuron Subtypes Underlie Forelimb and Hindlimb Motor Control. Neuron 2018, 97:869–884 e865.*Using population and single cell RNAseq comparisons, this study explores the V2a neuronal diversity which may support functional diversity at lumbar and cervical spinal segments. The authors found two types of V2a interneurons in opposite gradients from cervical to lumbar segments. Type I neurons, more prevalent in the lumbar cord, maintain Chx10 expression and form dense connections with local motor neurons. Type II neurons are more prominent in cervical cord, downregulate Chx10 expression, and project to the rostral brainstem. Each type is made up of several neuronal subtypes with transcription factors common to other ventral classes.
- 45.Ha NT, Dougherty KJ: Spinal Shox2 interneuron connectivity related to function and development. Elife 2018, 7:e42519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McLean DL, Dougherty KJ: Peeling back the layers of locomotor control in the spinal cord. Curr Opin Neurobiol 2015, 33:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris-Warrick RM: General principles of rhythmogenesis in central pattern generator networks. Prog Brain Res 2010, 187:213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talpalar AE, Endo T, Low P, Borgius L, Hagglund M, Dougherty KJ, Ryge J, Hnasko TS, Kiehn O: Identification of minimal neuronal networks involved in flexor-extensor alternation in the mammalian spinal cord. Neuron 2011, 71:1071–1084. [DOI] [PubMed] [Google Scholar]
- 49.Hagglund M, Borgius L, Dougherty KJ, Kiehn O: Activation of groups of excitatory neurons in the mammalian spinal cord or hindbrain evokes locomotion. Nat Neurosci 2010, 13:246–252. [DOI] [PubMed] [Google Scholar]
- 50.Bikoff JB, Gabitto MI, Rivard AF, Drobac E, Machado TA, Miri A, Brenner-Morton S, Famojure E, Diaz C, Alvarez FJ, et al. : Spinal Inhibitory Interneuron Diversity Delineates Variant Motor Microcircuits. Cell 2016, 165:207–219.**The authors demonstrated that the V1 class of spinal inhibitory interneurons can be divided into 50 subtypes based on transcription factor expression. Four transcription factors (FoxP2, MafA, Pou6f2, and Sp8) labeled non-overlapping groups or clades of V1 interneurons. Each clade settles at a distinct position in the spinal cord, related to the type of sensory input the group receives. Factors identifying clades are also present in other ventral interneuron classes, highlighting the need for combinatorial and intersectional strategies to specify more discrete functional populations.
- 51.Sweeney LB, Bikoff JB, Gabitto MI, Brenner-Morton S, Baek M, Yang JH, Tabak EG, Dasen JS, Kintner CR, Jessell TM: Origin and Segmental Diversity of Spinal Inhibitory Interneurons. Neuron 2018, 97:341–355 e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhong G, Masino MA, Harris-Warrick RM: Persistent sodium currents participate in fictive locomotion generation in neonatal mouse spinal cord. J Neurosci 2007, 27:4507–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tazerart S, Vinay L, Brocard F: The persistent sodium current generates pacemaker activities in the central pattern generator for locomotion and regulates the locomotor rhythm. J Neurosci 2008, 28:8577–8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brocard F, Tazerart S, Vinay L: Do pacemakers drive the central pattern generator for locomotion in mammals? Neuroscientist 2010, 16:139–155. [DOI] [PubMed] [Google Scholar]
- 55.Masino MA, Abbinanti MD, Eian J, Harris-Warrick RM: TTX-resistant NMDA receptor-mediated membrane potential oscillations in neonatal mouse Hb9 interneurons. PLoS One 2012, 7:e47940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson TM, Abbinanti MD, Peck JH, Gilmour M, Brownstone RM, Masino MA: Low-threshold calcium currents contribute to locomotor-like activity in neonatal mice. J Neurophysiol 2012, 107:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brocard F, Shevtsova NA, Bouhadfane M, Tazerart S, Heinemann U, Rybak IA, Vinay L: Activity-dependent changes in extracellular Ca2+ and K+ reveal pacemakers in the spinal locomotor-related network. Neuron 2013, 77:1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dougherty KJ, Kiehn O: Firing and cellular properties of V2a interneurons in the rodent spinal cord. J Neurosci 2010, 30:24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhong G, Droho S, Crone SA, Dietz S, Kwan AC, Webb WW, Sharma K, Harris-Warrick RM: Electrophysiological characterization of V2a interneurons and their locomotor-related activity in the neonatal mouse spinal cord. J Neurosci 2010, 30:170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Butera R, Rubin J, Terman D, Smith J: Oscillatory bursting mechanisms in respiratory pacemaker neurons and networks In Bursting: The Genesis of Rhythm in the Nervous System. Edited by Coombes S, Bressloff PC: World Scientific; 2005:303–346. [Google Scholar]
- 61.Jasinski PE, Molkov YI, Shevtsova NA, Smith JC, Rybak IA: Sodium and calcium mechanisms of rhythmic bursting in excitatory neural networks of the pre-Botzinger complex: a computational modelling study. Eur J Neurosci 2013, 37:212–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rekling JC, Feldman JL: PreBötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol 1998, 60:385–405. [DOI] [PubMed] [Google Scholar]
- 63.Kam K, Worrell JW, Ventalon C, Emiliani V, Feldman JL: Emergence of population bursts from simultaneous activation of small subsets of preBotzinger complex inspiratory neurons. J Neurosci 2013, 33:3332–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kam K, Worrell JW, Janczewski WA, Cui Y, Feldman JL: Distinct inspiratory rhythm and pattern generating mechanisms in the preBotzinger complex. J Neurosci 2013, 33:9235–9245.23719793 [Google Scholar]
- 65.Del Negro CA, Funk GD, Feldman JL: Breathing matters. Nat Rev Neurosci 2018, 19:351–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ampatzis K, Song J, Ausborn J, El Manira A: Separate microcircuit modules of distinct v2a interneurons and motoneurons control the speed of locomotion. Neuron 2014, 83:934–943. [DOI] [PubMed] [Google Scholar]
- 67.Bellardita C, Kiehn O: Phenotypic characterization of speed-associated gait changes in mice reveals modular organization of locomotor networks. Curr Biol 2015, 25:1426–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Capelli P, Pivetta C, Soledad Esposito M, Arber S: Locomotor speed control circuits in the caudal brainstem. Nature 2017, 551:373–377.*The authors demonstrated that glutamatergic neurons in the lateral paragigantocellular nucleus (LPGi) are highly implicated in locomotion, specifically at high locomotor speeds. On the contrary, inhibitory interneurons in multiple regions of the brainstem act to stall locomotion. Anatomically, the LPGi glutamatergic neurons project mainly to the central grey matter of the spinal cord, while the inhibitory neurons project mainly to motor neurons.
- 69.Caggiano V, Leiras R, Goni-Erro H, Masini D, Bellardita C, Bouvier J, Caldeira V, Fisone G, Kiehn O: Midbrain circuits that set locomotor speed and gait selection. Nature 2018, 553:455–460.This study uses optogenetics and chemogenetics to probe neurons of specific transmitter phenotypes in adjacent areas of the mesencephalic locomotor region which cannot be accurately separated with electrical stimulation. The authors showed that activating either the cuneiform nucleus (CnF) or the pedunculoponine nucleus (PPN) was sufficient to drive locomotion. However, only the CnF is able to evoke high-speed synchronous locomotion (i.e gallop and bound). Overall, they suggest that PPN is implicated in explorative behavior and CnF in escape behavior.
- 70.Josset N, Roussel M, Lemieux M, Lafrance-Zoubga D, Rastqar A, Bretzner F: Distinct Contributions of Mesencephalic Locomotor Region Nuclei to Locomotor Control in the Freely Behaving Mouse. Curr Biol 2018, 28:884–901 e883. [DOI] [PubMed] [Google Scholar]
- 71.Oueghlani Z, Simonnet C, Cardoit L, Courtand G, Cazalets JR, Morin D, Juvin L, Barriere G: Brainstem Steering of Locomotor Activity in the Newborn Rat. J Neurosci 2018, 38:7725–7740.*The authors demonstrated that a subset of neurons in the medullary reticulospinal region receive ascending locomotor-related information from the spinal cord in neonatal rats and that these neurons are rhythmically active in-phase with either the ipsilateral flexor or extensor ventral root. Alternating electrical stimulation delivered to the left and right sides of the ventral medulla effectively evoked fictive-locomotion in the lumbar spinal cord and varying the duration of the stimulation on one side, lead to a concomitant change in the duration of ipsilateral flexor-related (and contralateral extensor-related) motor activity. Such left-right asymmetry maybe implicated in turning.
- 72.Jean-Xavier C, Perreault MC: Influence of Brain Stem on Axial and Hindlimb Spinal Locomotor Rhythm Generating Circuits of the Neonatal Mouse. Front Neurosci 2018, 12:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bouvier J, Caggiano V, Leiras R, Caldeira V, Bellardita C, Balueva K, Fuchs A, Kiehn O: Descending Command Neurons in the Brainstem that Halt Locomotion. Cell 2015, 163:1191–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bui TV, Stifani N, Akay T, Brownstone RM: Spinal microcircuits comprising dI3 interneurons are necessary for motor functional recovery following spinal cord transection. Elife 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mayer WP, Akay T: Stumbling corrective reaction elicited by mechanical and electrical stimulation of the saphenous nerve in walking mice. J Exp Biol 2018, 221. [DOI] [PubMed] [Google Scholar]