Abstract
Background
Arteriovenous fistulae (AVFs) are the preferred form of hemodialysis vascular access, but maturation failures occur frequently, often resulting in prolonged catheter use. We sought to characterize AVF maturation among a national sample of prevalent hemodialysis patients in the United States.
Study Design
Nonconcurrent observational cohort study.
Setting & Participants
Prevalent hemodialysis patients having had at least one new AVF placed during 2013, as identified via Medicare claims data in the United States Renal Data System (USRDS).
Predictors
Demographics, geographic location, dialysis vintage, comorbidities.
Outcomes
Successful maturation following placement defined by subsequent use identified using monthly CROWNWeb data.
Measurements
Rates of AVF maturation were compared across strata of predictors. Patients were followed until the earliest evidence of death, AVF maturation, or the end of 2014.
Results
In the study period, 45,087 new AVFs were placed in 39,820 prevalent hemodialysis patients. No evidence of use was identified for 36.2% of AVFs. Only 54.7% of AVFs were used within four months of placement, with maturation rates varying considerably across end-stage renal disease (ESRD) networks. Older age was associated with lower AVF maturation rates. Female sex, black race, some comorbidities (cardiovascular disease, peripheral artery disease, diabetes, needing assistance, or institutionalized status), dialysis vintage >1 year, and catheter or arteriovenous graft use at ESRD incidence were also associated with lower rates of successful AVF maturation. In contrast, hypertension and prior AVF placement at ESRD incidence were associated with higher rates of successful AVF maturation.
Limitations
This study relies on administrative data, with monthly recording of access use.
Conclusions
We identified numerous associations between AVF maturation and patient-level factors in a recent national sample of United States hemodialysis patients. After accounting for these patient factors, we observed substantial differences in AVF maturation across some ESRD Networks—indicating a need for additional study of the provider, practice, and regional factors that explain AVF maturation.
Keywords: Arteriovenous anastomosis, Arteriovenous fistula (AVF), AVF maturation, Arteriovenous shunt, Cannulation, Registry, Renal dialysis, Risk factors, US Renal Data System (USRDS), Vascular access, fistula first, end-stage renal disease (ESRD), hemodialysis (HD)
Introduction
Compared to other forms of vascular access, arteriovenous fistulas (AVFs) are viewed as the best vascular access for most long-term hemodialysis patients, displaying better long-term outcomes and lower rates of thrombosis, infection, hospitalization, and mortality.1–5 Despite many potential advantages of the AVF, the United States (US) has historically relied heavily on arteriovenous grafts (AVGs) and central venous catheters. More recently, US clinical guidelines, such as the National Kidney Foundation - Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) and the Fistula First Breakthrough Initiative (FFBI) have prioritized AVF over AVG, attempting to minimize central venous catheters.6, 7 In the United States, AVF use among prevalent long-term dialysis patients increased from 32% in 2003 to 65% in 2014.8 Despite these efforts, 80% of incident dialysis patients initiate with a catheter, with only a quarter of those patients having maturing AVF or AVG in place.
Successful establishment of an optimally functioning AVF is a highly desirable outcome that can directly improve patient outcomes and lower the cost of care. However, despite gradual improvement in historically low rates of AVF placement in the US, suboptimal AVF maturation rates are increasingly problematic.8,9 Prior work has credited the improvement to the key roles of preoperative planning and surgical techniques,10–12 as well as the dedication and training of those responsible for both vascular access monitoring13 and placement.14 Motivated by this topic’s critical importance and paucity of relevant national data, we sought to characterize time-to-first use of AVF after surgical placement as a surrogate of successful ‘maturation.’ In particular, we explored factors associated with time-to-first successful AVF using newer data from CROWNWeb available from the United States Renal Data System (USRDS). CROWNWeb is a web-based data collection system that was implemented across all Medicare-certified dialysis facilities throughout the US in June 2012, and has replaced the Standard Information Management System (SIMS). CROWNWeb incorporates a number of clinical data elements, including monthly information on dialysis vascular access usage. We previously brought attention to the much longer times to first cannulation of AVF in the US compared to other countries.15 Now, we hypothesize that, in addition to patient-level factors, regional differences may exist with respect to time to AVF maturation, and that rates of successful AVF maturation might be reflective of national practice variations.
Methods
Study Population and Data Sources
The study population included 39,820 hemodialysis patients with AVF placement in 2013, as identified in Medicare claims. The Centers for Medicare & Medicaid Services (CMS) Medical Evidence Form 2728 was used to ascertain dialysis initiation date and comorbidities at dialysis incidence. All Medicare claims among prevalent hemodialysis patients in 2013 were explored to identify procedure codes for AVF placements. Monthly CROWNWeb data for the study period of January 1, 2013, to December 31, 2014, were analyzed to determine vascular access ‘in use.’ In order to be included in the analyses, patients were required to have vascular access use data in CROWNWeb following the fistula placement procedure. Patients were excluded if such vascular access use follow-up data were not available at any point during the study period. We did not formally censor the patients at modality switch. However, as the patient is not in CROWNWeb after a modality switch, they would be treated as “lost to follow-up,” so should not overly influence the outcome. In the merged CROWNWeb-Medicare dataset, 1.12% of records were missing data on access type. The analyses were limited to vascular accesses placed among prevalent hemodialysis patients, as non-dialysis-dependent patients would not need to have their AVF cannulated, and likely would not have the same clinical urgency for timely AVF use. Medicare claims and CROWNWeb data were linked via a patient identifier, allowing us to determine the first month in which the AVF was being used for HD (defined by successful 2-needle cannulation) subsequent to the AVF placement date, which reflects clinical AVF maturation.
Statistical Analysis
AVF placement was identified through inpatient, outpatient, and physician and supplier Medicare claims using the following International Classification of Diseases 9th Revision (ICD-9) procedure codes: 36818, 36819, 36820, 36821, and 36825. Subsequent first use of the placed fistula, defined in CROWNWeb as successful access use with both input and output needles, was obtained from CROWNWeb through the end of 2014. As data on direct clinical assessment is not available in the databases, whether, and when, maturation occurred was determined using the date of AVF placement in Medicare claims and the date of first use of the AVF in CROWNWeb. If CROWNWeb data indicated the AVF was used following the placement, without evidence of any intervening new AVF or AVG placements, the fistula was considered to have successfully matured for use.
A patient could contribute more than one record to the analysis if he or she had more than one AVF placed. For each patient-AVF record, follow-up started at placement and finished at the earliest of maturation, death, subsequent AVF placement, or end of the study period (December 31, 2014). The set-up is consistent with the classical competing risks structure;16 in our case, the competing risks are maturation, death, or subsequent AVF or AVG placements. We analyzed the cause-specific hazard of maturation16, 17, which amounts to the AVF maturation rate among patients who are alive and have not had a subsequent AVF or AVG placement. Naturally, for a given patient-AVF-placement combination (i.e., for a given record used in our analysis), successful AVF maturation (per our above described definition) can only occur prior to death and prior to subsequent AVF or AVG placement. From this perspective, the cause-specific hazard of maturation, which we refer to as the “maturation rate,” estimates the rate of AVF maturation counting only the follow-up time when maturation could actually occur.
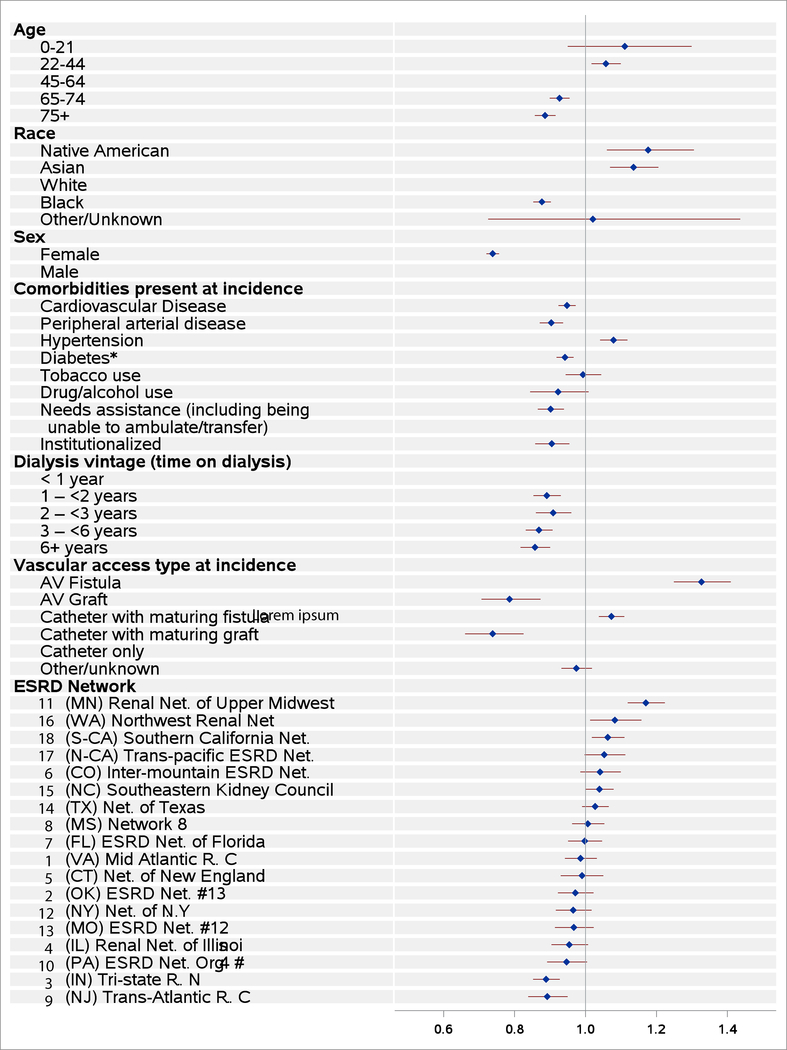
The maturation rate (cause-specific hazard of maturation) was modeled using cause-specific hazards models. The model included the following covariates: age, race, sex, comorbidities at incidence (for results shown in Figure 2 and Table S1), dialysis vintage (time since declaration of ESRD, or time on dialysis) at AVF creation, vascular access type in use at incidence, and ESRD Network region. We used a robust (sandwich estimator) for the variance to take care of the correlation across records with patient. The relationship of each covariate on the outcome was estimated by its cause-specific hazard ratio (HR). For example, the HR for females would represent the maturation rate for females, divided by the maturation rate for males, with the comparison being between a hypothetical female and male, with all other covariates being equal. A HR >1 reflects faster maturation, while HR <1 corresponds to slower maturation rates. Statistical significance was defined as p <0.05.
Figure 2.
Forest plot of cause-specific hazard ratios of time to first AVF use.
As a supplementary descriptive analysis, we present cumulative incidence curves for AVF maturation. Within each panel of the supplementary figures, the curves were generated by running separate Fine and Gray models18, stratified by the panel-specific patient characteristic of interest. Note that this model is the Cox regression analog applicable to the hazard function corresponding to cumulative incidence. In contrast to the afore-described models for the cause-specific maturation hazard, subjects essentially continued follow-up after death or graft placement, to get the risk sets to align with cumulative incidence computation. For the purposes of creating the US map, cause-specific hazard ratios by ESRD Network were calculated using separate Cox regression models with the geographic indicator as the only adjustor.
Statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). This study was performed under the USRDS Coordinating Center contract with the NIH-NIDDK; research as part of the contract has been approved by the University of Michigan Institutional Review Board (HUM0086162). As data for the USRDS components are collected by federal mandate, there are no individual patient consent requirements.
Results
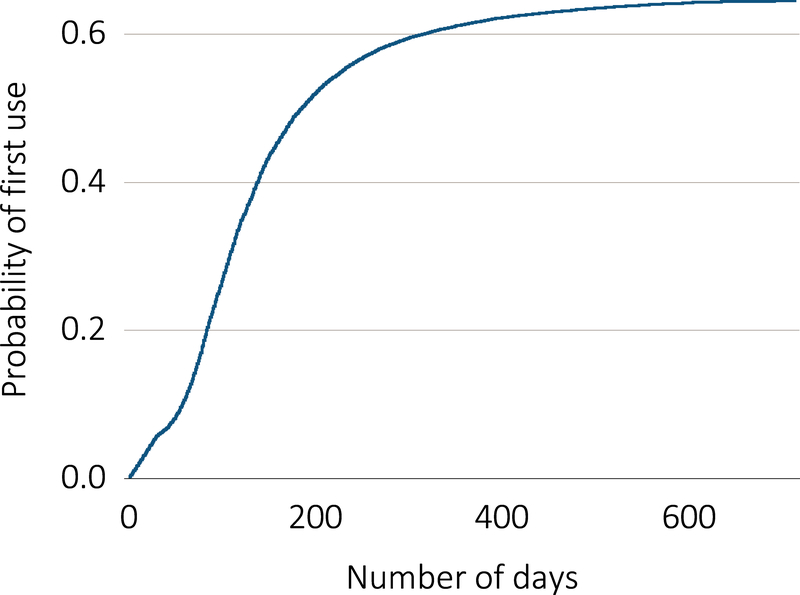
Among 39,820 prevalent hemodialysis patients, 45,087 AVF placements were identified in Medicare claims and had follow-up data in CROWNWeb (Table 1). A mean of 1.13 (95% CI, 1.128–1.13) AVFs were placed per patient, with 79.5% of patients contributing one AVF, 17.8% contributing two AVFs, and 2.7% contributing three or more AVFs. Diabetic and hypertensive kidney disease were the most commonly assigned primary causes of ESRD in the study population. Cumulative probability of successful AVF first use is shown in Figure 1. No evidence of use during the study period (ending December 31, 2014) was found for 36.2% of the AVF placements, with 12.5% and 4.7% of patients subsequently undergoing another AVF or an AVG placement, respectively, and 16.2% of patients were no longer followed in CROWNWeb due to death prior to access use. Among successfully used AVF, the median time to first reported use in CROWNWeb was 111 days. Of those successfully utilized, 54.7% were accessed within four months, with another 23.1% accessed by six months (Table 2). Of these successfully used AVFs, 83.7% were recorded as in use the month following initial use, with 95.4% having at least two months of consecutive use during the study period.
Table 1.
Patient characteristics
| Value | ||
|---|---|---|
| No. of AVF placements per patient | 1.13** | |
| Age category* | ||
| 0–21 y | 210 (0.5%) | |
| 22–44 | 4,832 | 12.1% |
| 45–64 | 15,325 | 38.5% |
| 65–74 | 10,596 | 26.6% |
| 75+ | 8,857 | 22.2% |
| Race | ||
| Native American | 421 | 1.1% |
| Asian | 1,637 | 4.1% |
| Black | 12,546 | 31.5% |
| White | 25,172 | 63.2% |
| Other/Unknown | 44 | 0.1% |
| Sex | ||
| Male | 22,703 | 57.0% |
| Female | 17,117 | 43.0% |
| Primary Cause of ESRD | ||
| Diabetes | 18,617 | 46.8% |
| Hypertension | 11,999 | 30.1% |
| Glomerulonephritis | 3,552 | 8.9% |
| Cystic kidney | 645 | 1.6% |
| Other urologic | 584 | 1.5% |
| Other cause | 3,256 | 8.2% |
| Unknown cause | 1,167 | 2.9% |
| ESRD Network | ||
| 1 | 1,238 | 3.1% |
| 2 | 1,838 | 4.6% |
| 3 | 1,460 | 3.7% |
| 4 | 1,436 | 3.6% |
| 5 | 2,468 | 6.2% |
| 6 | 4,105 | 10.3% |
| 7 | 2,352 | 5.9% |
| 8 | 2,557 | 6.4% |
| 9 | 3,052 | 7.7% |
| 10 | 1,650 | 4.1% |
| 11 | 2,653 | 6.7% |
| 12 | 1,614 | 4.1% |
| 13 | 1,971 | 4.9% |
| 14 | 4,015 | 10.1% |
| 15 | 1,616 | 4.1% |
| 16 | 1,150 | 2.9% |
| 17 | 1,824 | 4.6% |
| 18 | 2,821 | 7.1% |
N = 39,820. Except as indicated, values given as count (percentage).
Age as of January 1, 2013
mean
AVF, arteriovenous fistula; ESRD, end-stage renal disease
Figure 1.
Cumulative probability of first-use of AVFs placed among prevalent hemodialysis patients in the United States in 2013.
Table 2.
Time from AVF placement by Medicare claims, to first reported use of AVF in CROWNWeb data
| Time Between AVF Placement and First Reported Use | Count (Column Percentage) | |
|---|---|---|
| 0 - <2 months | 4,903 (17.1%) | |
| 2 - <4 months | 10,799 | 37.6 |
| 4 - <6 months | 6,630 | 23.1 |
| 6 - <8 months | 2,984 | 10.4 |
| 8 - <10 months | 1,524 | 5.3 |
| 10+ months | 1,901 | 6.6 |
AVF, arteriovenous fisula
Older age groups were incrementally associated with longer time to maturation and lower rates of successful AVF use, while patients who were ≤44 years of age had the shortest maturation times and the highest rate of successful AVF use (Figure 2, Figure S1, Table S1). Similarly, males were observed to have earlier and more successful AVF maturation than females. Asians and Native Americans were more likely to experience successful AVF maturation than whites, while blacks had the lowest maturation rates. Dialysis vintage ≥1 year was associated with greater difficulty in successfully establishing an AVF. Greater successful maturation was seen for AVF placements in prevalent dialysis patients who at HD initiation had used an AVF or a catheter but had a maturing AVF in place, compared to AVF placements in prevalent HD patients who initiated HD with a catheter only. AVF maturation was poorest for AVF placements in prevalent patients who had an AVG, either in use or maturing with a catheter in place, at HD initiation. Cardiovascular disease, peripheral arterial disease, diabetes, institutionalization, and poor functional status were associated with a slower rate of AVF maturation, while a diagnosis of hypertension was associated with a somewhat greater maturation rate (Figure 2, Figure S2, Table S1).
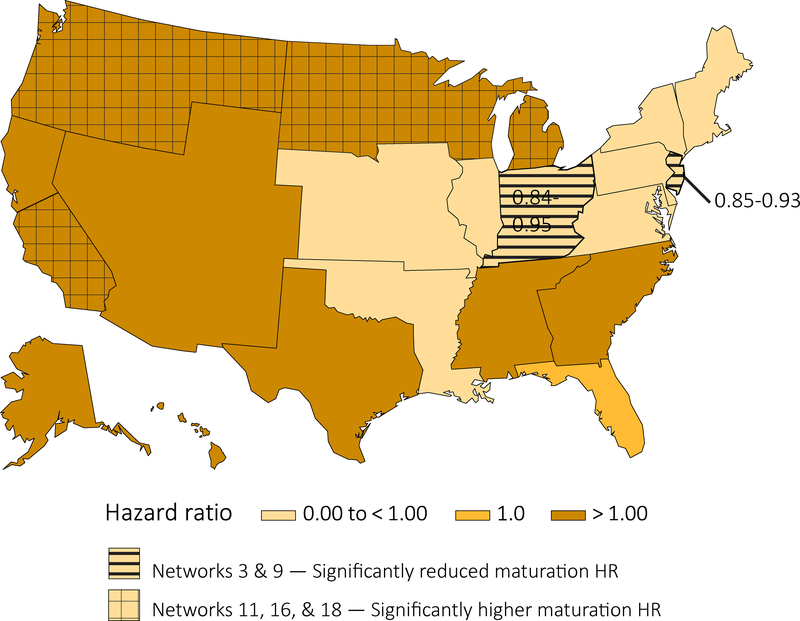
AVF maturation rates differed considerably across ESRD Networks in analyses adjusted for patient case-mix differences (Figures 2–3, Table S1). Network 11 (Minnesota, Michigan, North Dakota, South Dakota, and Wisconsin) displayed the highest adjusted AVF maturation rate, followed by Networks 16 (Alaska, Idaho, Montana, Oregon, and Washington) and 18 (Southern California). In contrast, Networks 3 (New Jersey, Puerto Rico, and the Virgin Islands) and 9 (Indiana, Kentucky, and Ohio) displayed AVF maturation rates that were significantly lower (p<0.05) than the national average.
Figure 3.
Cause-specific hazard ratios for AVF maturation from univariate Cox regression model with ESRD Networks.
Discussion
AVF use rates among prevalent dialysis patients in the US rose from 32% in 2003 to 65% in 2014.8 However, these improvements did not occur without substantial effort, as there are a number of barriers to successfully establishing AVF. AVF often require several weeks to mature, and even after that time, can subsequently fail. NKF-KDOQI recommends timely referral to allow time to complete preoperative vein mapping, placement, and maturation.6 However, patients with shorter duration of nephrology care prior to initiation of dialysis are unlikely to have a usable AVF at initiation for logistic reasons. Hence, 60% of US incident dialysis patients have only a catheter at initiation, while another 20% have a maturing AVF or AVG requiring a catheter at initiation. Furthermore, progression of chronic kidney disease (CKD) to ESRD requiring dialysis can be difficult to predict,20, 21 thresholds for dialysis initiation vary,22, 23 and patients may express reluctance toward access placement consistent with clinical expediency.24 Timely vascular access planning and placement is thus fraught with multiple barriers. Similar issues occur for kidney transplant patients with failing allografts25, 26, which suggests that many of these problems may stem from more than simply a lack of early nephrology or surgical care. For example, despite high rates of hospitalized central venous catheter-related infections27, 28 and pre-existing nephrology care for 93.5% of the patients with failing kidney transplants, 65% of such patients returned to dialysis utilizing a catheter. In the case of patients with cystic kidney disease, who often experience earlier referral for nephrology care and may be less likely to have utilized arm veins for other medical care, a greater proportion—43.6%—were observed to have started hemodialysis with an AVF, with 52.5% utilizing a catheter at initiation.8
In our study, a number of comorbidities were associated with prolonged time to, or failure of, AVF usage and maturation. We found that older age, need for assistance, and institutionalization were associated with prolonged AVF maturation and higher failure. Interestingly, using Medicare billing data from patients ≥66 years of age, Woo et al. found that, even though mortality was somewhat lower in patients using an AVF compared to AVG (28.2% versus 29.9%, p = 0.03), the one-year repeat fistula, graft, or catheter-free survival was lower after AVF creation than AVG creation; 44.4% of AVF patients required a subsequent access, while only 33.7% of AVG patients did (p < 0.001).29
The Dialysis Outcomes and Practice Patterns Study (DOPPS) has demonstrated that both older age4 and lower functional status30 are also associated with early mortality following hemodialysis initiation. Furthermore, elderly nursing home residents are observed to experience a sustained decline in functional status after initiation of dialysis, 31 further compounding maturation difficulty. As there is high mortality during the first few months of hemodialysis,8 and older patients on hemodialysis tend to be more frail,30 a focus on this group in future investigations is warranted. It has been proposed that elderly patients may benefit from more liberal use of AVG placement, as AVGs are associated with earlier catheter removal and fewer catheter days in the first year following dialysis initiation.33 However, it is possible that there is additional unaccounted for time following AVG failure, as AVGs have historically had shorter functional life expectancies than AVFs.34–37 However, there have been recent improvements in secondary patency for AVGs, which suggests that interventional techniques may improve their viability as an access option38. However, given that “steal syndrome”---more common with AVGs39, 40---can result in significant morbidity and loss of quality of life, it remains unclear whether such a policy approach is reasonable. A patient-centered approach to preoperative planning and intraoperative plan modifications that take into account the likelihood of AVF success may have improved utility and outcomes.41–43
Consistent with previous studies,8, 25, 34, 42, 44, 45 we found that for females, blacks, and those with peripheral arterial disease, there was more difficulty successfully establishing AVF. Interestingly, hypertension was associated with higher rates of successful AVF maturation. While our study focused on prevalent patients, Zarkowsky et al., similarly found that female gender, black race, or presence of peripheral arterial disease decreased the likelihood of initiating hemodialysis with an AVF. In their study, they also observed that hypertension is associated with greater likelihood of achieving a successful AVF at dialysis start.45 In that study, as well as in ours, diabetes was associated with lower AVF maturation. However, the prospective Hemodialysis Fistula Maturation Study recently reported lower frequency of early thrombosis among diabetic patients, suggesting that screening and preoperative planning may allow for improved outcomes among diabetics. The regional variation with respect to time to AVF maturation may reflect known geographic variation in timing of provider visits and first cannulation attempts.46, 47 However, the geographic heterogeneity with respect to AVF maturation requires further investigation, and may reveal unrecognized practice variations.
Dialysis vintage >1 year was associated with a lower rate of AVF maturation, as was dialysis access initiation with an AVG. Prevalent HD patients with an AVF at initiation were more likely to develop a future AVF, compared to those with a catheter at initiation, consistent with prior observations.19 As the percentage of prevalent patients with AVFs continues to rise, coupled with a fall in AVG use,8 it is likely that improvements in maintaining the AVFs have been successfully developed. However, given the more recent guidelines for universal preoperative vein mapping for operative planning,6 it is not surprising that those with previous AVFs may have usable veins, while those who previously required an AVG may have had poorer vasculature near the time of HD initiation, with a lower likelihood of later AVF development if subsequently attempted.
We found that only 17.1% of patients with successful AVFs were cannulated by two months, with 54.7% by four months (Table 2). The 2015 USRDS Annual Data Report (ADR) reported a median of 112 days (IQR 74 to 171 days) between AVF placement and first use.8 In contrast, in a 2004 DOPPS report, Saran et al. found that 36% of AVFs in the US were cannulated by two months, which contrasted with 79% in Europe, and 98% in Japan.15 Since that time, there has been increasing AVF in the US.8 It is possible that more tenuous fistulae require even more time to mature, but these differences may also reflect provider practice differences with respect to first cannulation. There are also significant differences between blood flow rates in the US and Japan (400 versus 200 mL/min), which might explain the lower threshold for AVF failure in the US. Furthermore, there are meaningful differences in the comorbidities of the dialysis population, access to predialysis CKD care, and in the organization of ESRD care among these countries.2, 15, 46, 48, 49 These data suggest that there is more to early cannulation than merely early needle placement. Additional attention to training for both the nephrologist and surgeon are likely beneficial.13, 14, 50 Dialysis access coordinators can improve access to care.7, 34, 51 Vascular access placement reimbursement is significantly lower than other vascular operations,52 which likely result in lower priority for scarce operating time. Expanded pre-dialysis CKD and early ESRD care, with expanded patient educational efforts addressing vascular access prior to the surgical evaluation, may also be of benefit7, 47, 53, 54, particularly as patients may be resistant to access placement,24, 55 resulting in patient-related factors delaying work-up, surgical evaluation, and scheduling.
This study has limitations. The USRDS ESRD database relies on administrative data submitted by dialysis providers. As such, it is dependent upon the accuracy and completeness of data submitted by the dialysis community. Furthermore, CROWNWeb accuracy for specific months post-initiation has not been determined. However, at least for incident patients, vascular access data from the CMS Medical Evidence Form 2728 and CROWNWeb frequently agree, with a kappa statistic of 0.88 for the first month of dialysis,56 suggesting that vascular access data reporting is reasonably accurate. With regard to Medicare billing data, CPT coding errors are not uncommon in other procedural specialties.57 As there are a number of CPT codes for dialysis access, there is certainly the possibility of systemic miscoding. Furthermore, CPT codes for AVF placement are not very specific, particularly in regards to anatomic location. For example, for two stage basilic vein transpositions or cephalic vein AVFs that are deep enough to require superficialization or transposition, the CPT code for the second stage falls under the general AVF revision code. In those particular instances they should not have been coded as a new AVF, although such an undetected errors may have been made in some instances. CROWNWeb is limited to ESRD patients on dialysis, with mandatory participation by Medicare-certified dialysis centers but with select participation by other ESRD-care providers.54 The ideal AVF placement should occur prior to dialysis initiation;6, 7 AVFs placed prior to initiation of dialysis are not directly addressed by this study and merit further investigation. In addition, CROWNWeb records access use at monthly intervals, introducing potential bias towards a median of ~15 days longer for maturation by this approach or for inaccurate reporting for those with partial month usage before failure. While it is unlikely that the distribution of monthly temporal differences among patients would have a significant bias, it is not inconceivable. We utilized a modified, ‘functional’ definition of AVF maturation (i.e., time to first use of AVF, as indicated in CROWNWeb), rather than a clinical or radiological determination of true AVF maturity, as the USRDS lacks this type of data pertaining to AVFs. This can be obtained in the setting of a prospective observational study such as the Hemodialysis Fistula Maturation Study. This surrogate definition for AVF maturation necessarily biases the study toward longer time to maturation. Finally, the reason for delay of cannulation cannot be ascertained from the database. For example, if a patient refused cannulation, if the AVF was deep due to high BMI, or the dialysis unit technician was not experienced and had difficulty with cannulation, these would appear as any noncannulated AVF.
We have characterized AVF maturation in a recent national US sample and identified important associations with multiple patient-level factors utilizing the newly added CROWNWeb component of the USRDS database. Longer AVF maturation times are associated with a number of risk factors such as age, race, comorbidities including cardiovascular or peripheral arterial disease, need for assistance, dialysis vintage, and previous access history. After accounting for these patient factors, substantial differences in AVF maturation were seen across some ESRD Networks. Research is urgently required into the importance of patient, provider, region, and practice factors that could improve AVF placement rates and successful maturation, decrease central venous catheter use, and improve patient outcomes.
Supplementary Material
Figure S1. Adjusted probability of first use of AVF placed in 2013 by age, race, sex, dialysis vintage, and vascular access type at incidence.
Figure S2. Adjusted probability of first use of AVF placed in 2013 by comorbidity at incidence.
Table S1. Cause-specific HRs of time to first AVF use (with follow-up through the end of 2014).
Acknowledgements
The authors would like to thank Ruth Shamraj and Janet Leslie for editing the manuscript.
Support: The data reported here have been supplied by the United States Renal Data System (USRDS), which is funded by the National Institute of Digestive and Diabetes and Kidney Diseases (NIDDK), through National Institutes of Health (NIH) contract HHSN276201400001C. This study was performed under the USRDS Coordinating Center contract with the NIH-NIDDK; the work was conducted by the Coordinating Center, with the manuscript as originally submitted approved by NIDDK (NIDDK project officers are Kevin C. Abbott, MD, MPH and Lawrence Y.C. Agodoa, MD). Except as indicated, the funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The USRDS Coordinating Center is located at the University of Michigan Kidney Epidemiology and Cost Center, in partnership with Arbor Research Collaborative for Health, Ann Arbor, Michigan. The USRDS director is Rajiv Saran, MD, MRCP, MS, Professor of Medicine and Epidemiology at the University of Michigan, and the co-deputy directors are Vahakn Shahinian, MD, MS Associate Professor of Medicine at the University of Michigan, and Bruce Robinson, MD, Vice President, Arbor Research Collaborative for Health.
Financial Disclosure: Dr. Pisoni is a senior research scientist for the non-profit research organization Arbor Research Collaborative for Health, which has designed and carries out the Dialysis Outcomes and Practice Patterns Study (DOPPS) Program. The DOPPS Program is supported by Amgen, Kyowa Hakko Kirin, AbbVie, Sanofi Renal, Baxter Healthcare, and Vifor Fresenius Medical Care Renal Pharma. Additional support for specific projects and countries is provided by Keryx Biopharmaceuticals, Merck Sharp & Dohme, Proteon Therapeutics, Relypsa, and F. Hoffmann-LaRoche; in Canada by Amgen, BHC Medical, Janssen, Takeda, and the Kidney Foundation of Canada (for logistics support); in Germany by Hexal, DGfN, Shire, and the WiNe Institute; and for PDOPPS in Japan by the Japanese Society for Peritoneal Dialysis (JSPD). Proteon Therapeutics specifically has provided funding support for vascular access-related research in the DOPPS. All support is provided without restrictions on publications. Grants are made to Arbor Research Collaborative for Health, and not to individual investigators.
Footnotes
Disclaimer: The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the United States government.
Supplementary Material
Supplementary Material Descriptive Text for Online Delivery
Supplementary Figure S1 (PDF). Adjusted probability of first use of AVF placed in 2013 by age, race, sex, dialysis vintage, and vascular access type at incidence.
Supplementary Figure S1 (PDF). Adjusted probability of first use of AVF placed in 2013 by comorbidity at incidence.
Supplementary Item S1 (PDF). Cause-specific HRs of time to first AVF use (with follow-up through the end of 2014).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ravani P, Palmer SC, Oliver MJ, et al. Associations between Hemodialysis Access Type and Clinical Outcomes: A Systematic Review. Journal of the American Society of Nephrology. 2013;24(3):465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pisoni RL, Arrington CJ, Albert JM, et al. Facility Hemodialysis Vascular Access Use and Mortality in Countries Participating in DOPPS: An Instrumental Variable Analysis. American Journal of Kidney Diseases. 2009;53(3):475–491. [DOI] [PubMed] [Google Scholar]
- 3.Dalrymple LS, Mu Y, Nguyen DV, et al. Risk Factors for Infection-Related Hospitalization in In-Center Hemodialysis. Clinical journal of the American Society of Nephrology: CJASN. 2015;10(12):2170–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradbury BD, Fissell RB, Albert JM, et al. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clinical journal of the American Society of Nephrology: CJASN. 2007;2(1):89–99. [DOI] [PubMed] [Google Scholar]
- 5.Malas MB, Canner JK, Hicks CW, et al. Trends in incident hemodialysis access and mortality. JAMA Surg. 2015;150(5):441–448. [DOI] [PubMed] [Google Scholar]
- 6.Vascular Access Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48 Suppl 1(S176–S247. [DOI] [PubMed] [Google Scholar]
- 7.Vassalotti JA, Jennings WC, Beathard GA, et al. Fistula first breakthrough initiative: targeting catheter last in fistula first. Semin Dial. 2012;25(3):303–310. [DOI] [PubMed] [Google Scholar]
- 8.Saran R, Li Y, Robinson B, et al. US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. American Journal of Kidney Diseases. 2016;67(3, Supplement 1):A7–A8, S1-S434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dember LM, Beck GJ, Allon M, et al. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: a randomized controlled trial. Jama. 2008;299(18):2164–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong CS, McNicholas N, Healy D, et al. A systematic review of preoperative duplex ultrasonography and arteriovenous fistula formation. J Vasc Surg. 2013;57(4):1129–1133. [DOI] [PubMed] [Google Scholar]
- 11.Ene-Iordache B, Cattaneo L, Dubini G, Remuzzi A. Effect of anastomosis angle on the localization of disturbed flow in ‘side-to-end’ fistulae for haemodialysis access. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2013;28(4):997–1005. [DOI] [PubMed] [Google Scholar]
- 12.Konner K, Lomonte C, Basile C. Placing a primary arteriovenous fistula that works--more or less known aspects, new ideas. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2013;28(4):781–784. [DOI] [PubMed] [Google Scholar]
- 13.Asif A, Beathard GA. We Need to Train the Trainers. Clinical journal of the American Society of Nephrology: CJASN. 2015;10(10):1711–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saran R, Elder SJ, Goodkin DA, et al. Enhanced training in vascular access creation predicts arteriovenous fistula placement and patency in hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study. Annals of surgery. 2008;247(5):885–891. [DOI] [PubMed] [Google Scholar]
- 15.Saran R, Dykstra DM, Pisoni RL, et al. Timing of first cannulation and vascular access failure in haemodialysis: an analysis of practice patterns at dialysis facilities in the DOPPS. Nephrology Dialysis Transplantation. 2004;19(9):2334–2340. [DOI] [PubMed] [Google Scholar]
- 16.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. 2nd ed. Hoboken, New Jersey: Wiley-Interscience; 2002. [Google Scholar]
- 17.Prentice RL, Kalbfleisch JD, Peterson AV Jr., Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34(4):541–554. [PubMed] [Google Scholar]
- 18.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American statistical association. 1999;94(446):496–509. [Google Scholar]
- 19.Pisoni RL, Young EW, Dykstra DM, et al. Vascular access use in Europe and the United States: results from the DOPPS. Kidney Int. 2002;61(1):305–316. [DOI] [PubMed] [Google Scholar]
- 20.Marks A, Fluck N, Prescott GJ, et al. Looking to the future: predicting renal replacement outcomes in a large community cohort with chronic kidney disease. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2015;30(9):1507–1517. [DOI] [PubMed] [Google Scholar]
- 21.Lennartz CS, Pickering JW, Seiler-Mussler S, et al. External Validation of the Kidney Failure Risk Equation and Re-Calibration with Addition of Ultrasound Parameters. Clinical journal of the American Society of Nephrology: CJASN. 2016;11(4):609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slinin Y, Guo H, Li S, et al. Provider and care characteristics associated with timing of dialysis initiation. Clinical journal of the American Society of Nephrology: CJASN. 2014;9(2):310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Hare AM, Wong SP, Yu MK, et al. Trends in the Timing and Clinical Context of Maintenance Dialysis Initiation. Journal of the American Society of Nephrology: JASN. 2015;26(8):1975–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casey JR, Hanson CS, Winkelmayer WC, et al. Patients’ perspectives on hemodialysis vascular access: a systematic review of qualitative studies. Am J Kidney Dis. 2014;64(6):937–953. [DOI] [PubMed] [Google Scholar]
- 25.Chan MR, Oza-Gajera B, Chapla K, et al. Initial vascular access type in patients with a failed renal transplant. Clinical journal of the American Society of Nephrology: CJASN. 2014;9(7): 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang JC, Al-Jaishi A, Perl J, Garg AX, Moist LM. Hemodialysis Arteriovenous Vascular Access Creation After Kidney Transplant Failure. Am J Kidney Dis. 2015;66(4):646–654. [DOI] [PubMed] [Google Scholar]
- 27.Woodside KJ, Schirm ZW, Noon KA, et al. Fever, infection, and rejection after kidney transplant failure. Transplantation. 2014;97(6):648–653. [DOI] [PubMed] [Google Scholar]
- 28.Perl J, Zhang J, Gillespie B, et al. Reduced survival and quality of life following return to dialysis after transplant failure: the Dialysis Outcomes and Practice Patterns Study. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2012;27(12):4464–4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woo K, Goldman DP, Romley JA. Early Failure of Dialysis Access among the Elderly in the Era of Fistula First. Clinical journal of the American Society of Nephrology: CJASN. 2015;10(10):1791–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jassal SV, Karaboyas A, Comment LA, et al. Functional Dependence and Mortality in the International Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2016;67(2):283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. The New England journal of medicine. 2009;361(16):1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiremath S, Knoll G, Weinstein MC. Should the arteriovenous fistula be created before starting dialysis?: A decision analytic approach. PLoS One. 2011;6(12):e28453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leake AE, Yuo TH, Wu T, et al. Arteriovenous grafts are associated with earlier catheter removal and fewer catheter days in the United States Renal Data System population. J Vasc Surg. 2015;62(1):123–127. [DOI] [PubMed] [Google Scholar]
- 34.Kalman PG, Pope M, Bhola C, Richardson R, Sniderman KW. A practical approach to vascular access for hemodialysis and predictors of success. J Vasc Surg. 1999;30(4):727–733. [DOI] [PubMed] [Google Scholar]
- 35.Leermakers JJ, Bode AS, Vaidya A, van der Sande FM, Evers SM, Tordoir JH. Cost-effectiveness of vascular access for haemodialysis: arteriovenous fistulas versus arteriovenous grafts. European journal of vascular and endovascular surgery: the official journal of the European Society for Vascular Surgery. 2013;45(1):84–92. [DOI] [PubMed] [Google Scholar]
- 36.Lok CE, Sontrop JM, Tomlinson G, et al. Cumulative patency of contemporary fistulas versus grafts (2000–2010). Clinical journal of the American Society of Nephrology: CJASN. 2013;8(5):810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schild AF, Perez E, Gillaspie E, Seaver C, Livingstone J, Thibonnier A. Arteriovenous fistulae vs. arteriovenous grafts: a retrospective review of 1,700 consecutive vascular access cases. The journal of vascular access. 2008;9(4):231–235. [PubMed] [Google Scholar]
- 38.Allemang MT, Schmotzer B, Wong VL, et al. Arteriovenous grafts have higher secondary patency in the short term compared with autologous fistulae. American journal of surgery. 2014;208(5):800–805. [DOI] [PubMed] [Google Scholar]
- 39.Morsy AH, Kulbaski M, Chen C, Isiklar H, Lumsden AB. Incidence and characteristics of patients with hand ischemia after a hemodialysis access procedure. The Journal of surgical research. 1998;74(1):8–10. [DOI] [PubMed] [Google Scholar]
- 40.Leon C, Asif A. Arteriovenous access and hand pain: the distal hypoperfusion ischemic syndrome. Clinical journal of the American Society of Nephrology: CJASN. 2007;2(1):175–183. [DOI] [PubMed] [Google Scholar]
- 41.Rosas SE, Feldman HI. Synthetic vascular hemodialysis access versus native arteriovenous fistula: a cost-utility analysis. Annals of surgery. 2012;255(1):181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farber A, Imrey PB, Huber TS, et al. Multiple preoperative and intraoperative factors predict early fistula thrombosis in the Hemodialysis Fistula Maturation Study. J Vasc Surg. 2016;63(1):163–170 e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jennings WC, Landis L, Taubman KE, Parker DE. Creating functional autogenous vascular access in older patients. J Vasc Surg. 2011;53(3):713–719; discussion 719. [DOI] [PubMed] [Google Scholar]
- 44.Pisoni RL, Zepel L, Port FK, Robinson BM. Trends in US Vascular Access Use, Patient Preferences, and Related Practices: An Update From the US DOPPS Practice Monitor With International Comparisons. Am J Kidney Dis. 2015;65(6):905–915. [DOI] [PubMed] [Google Scholar]
- 45.Zarkowsky DS, Arhuidese IJ, Hicks CW, et al. Racial/Ethnic Disparities Associated With Initial Hemodialysis Access. JAMA Surg. 2015;150(6):529–536. [DOI] [PubMed] [Google Scholar]
- 46.Moist LM, Bragg-Gresham JL, Pisoni RL, et al. Travel time to dialysis as a predictor of health-related quality of life, adherence, and mortality: The dialysis outcomes and practice patterns study (DOPPS). American Journal of Kidney Diseases. 2008;51(4):641–650. [DOI] [PubMed] [Google Scholar]
- 47.Erickson KF, Mell M, Winkelmayer WC, Chertow GM, Bhattacharya J. Provider Visits and Early Vascular Access Placement in Maintenance Hemodialysis. Journal of the American Society of Nephrology: JASN. 2015;26(8):1990–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asano M, Thumma J, Oguchi K, et al. Vascular access care and treatment practices associated with outcomes of arteriovenous fistula: international comparisons from the Dialysis Outcomes and Practice Patterns Study. Nephron. Clinical practice. 2013;124(1–2):23–30. [DOI] [PubMed] [Google Scholar]
- 49.Ethier J, Mendelssohn DC, Elder SJ, et al. Vascular access use and outcomes: an international perspective from the Dialysis Outcomes and Practice Patterns Study. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2008;23(10):3219–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huber TS. A call to action for pre-end-stage renal disease care. JAMA Surg. 2015;150(5):449. [DOI] [PubMed] [Google Scholar]
- 51.Polkinghorne KR, Seneviratne M, Kerr PG. Effect of a vascular access nurse coordinator to reduce central venous catheter use in incident hemodialysis patients: a quality improvement report. Am J Kidney Dis. 2009;53(1):99–106. [DOI] [PubMed] [Google Scholar]
- 52.Martin JD, Warble PB, Hupp JA, et al. A real world analysis of payment per unit time in a Maryland Vascular Practice. J Vasc Surg. 2010;52(4):1094–1098; discussion 1098–1099. [DOI] [PubMed] [Google Scholar]
- 53.Allon M, Dinwiddie L, Lacson E Jr., et al. Medicare reimbursement policies and hemodialysis vascular access outcomes: a need for change. Journal of the American Society of Nephrology: JASN. 2011;22(3):426–430. [DOI] [PubMed] [Google Scholar]
- 54.Goodkin DA, Pisoni RL, Locatelli F, Port FK, Saran R. Hemodialysis Vascular Access Training and Practices Are Key to Improved Access Outcomes. American Journal of Kidney Diseases. 2010;56(6):1032–1042. [DOI] [PubMed] [Google Scholar]
- 55.Fissell RB, Fuller DS, Morgenstern H, et al. Hemodialysis patient preference for type of vascular access: variation and predictors across countries in the DOPPS. The journal of vascular access. 2013;14(3):264–272. [DOI] [PubMed] [Google Scholar]
- 56.Mukhopadhyay P, Pearson J, Woodside KJ, et al. Vascular access at dialysis initiation in the United States Renal Data System (USRDS): strong agreement between CMS 2728 medical evidence form and CROWNWeb. Journal of the American Society of Nephrology: JASN. 2015;26(Abstract Edition). [Google Scholar]
- 57.Duszak R, Blackham WC, Kusiak GM, Majchrzak J. CPT coding by interventional radiologists: a multi-institutional evaluation of accuracy and its economic implications. J Am Coll Radiol. 2004;1(10):734–740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Adjusted probability of first use of AVF placed in 2013 by age, race, sex, dialysis vintage, and vascular access type at incidence.
Figure S2. Adjusted probability of first use of AVF placed in 2013 by comorbidity at incidence.
Table S1. Cause-specific HRs of time to first AVF use (with follow-up through the end of 2014).