Abstract
Contextual fear conditioning relies upon a network of cortical and subcortical structures, including the hippocampus and the retrosplenial cortex (RSC). However, the contribution of the hippocampus is parameter-dependent. For example, with “weak” training procedures, lesions of the hippocampus produce both retrograde and anterograde context amnesia. However, with “strong” training procedures (e.g., more trials and/or higher levels of footshock), lesions of the hippocampus produce retrograde context amnesia but not anterograde amnesia [1]. Likewise, prior studies have shown that with weak training, RSC lesions produce both retrograde and anterograde context amnesia [2]. The purpose of the current study was to examine the effects of RSC damage on contextual fear conditioning following strong training. In Experiment 1, lesions of the RSC resulted in both retrograde and anterograde context amnesia following strong training using the same unsignaled fear conditioning procedures described by Wiltgen et al. [1]. In Experiment 2, using a signaled fear conditioning procedure, we replicated these effects on context memory observing both retrograde and anterograde context amnesia. In contrast, there were no lesion effects on tone-fear memory. Thus, unlike lesions of the hippocampus, lesions of RSC produce both retrograde and anterograde context amnesia even when rats undergo strong fear conditioning. These findings suggest that the RSC has an essential role in contextual fear conditioning and that other systems or pathways are unable to compensate for the loss of RSC function.
Keywords: over-training, memory, parahippocampal, associative learning
1. Introduction
Contexts are generally defined as the physical and temporal environments in which an event occurs, and are composed of a variety of visual, auditory, and tactile cues [3]. It is generally assumed that during contextual fear conditioning, individual features in the environment are linked together to form a unitary representation of the context that can be associated with an outcome or event, such as a footshock [4–6]. A wealth of research has demonstrated that this so-called “configural” fear learning process is dependent on the hippocampus [7–10]. Interestingly, however, lesions of the hippocampus do not always result in impairments in contextual fear conditioning [1,7,11,12]. For example, although hippocampal lesions carried out after training result in retrograde amnesia over a wide-range of parameters, pre-training lesions only produce an effect under certain conditions [1, 12, 13]. Specifically, as systematically demonstrated by Wiltgen et al. [1], pre-training neurotoxic lesions of hippocampus impair context fear memory when only a single shock is delivered during conditioning (“weak training”), but context memory remains intact when the shock is presented several times (“strong training”).
These findings support the notion that under some conditions, an alternative region or pathway is able to fully compensate for hippocampal damage [1, 14]. An interesting, but as yet untested corollary of this hypothesis is that unlike hippocampus, this other brain structure(s) may be essential for context fear memory regardless of the training parameters or timing of the damage. Based on its connectivity, the retrosplenial cortex (RSC) may be particularly well positioned as a structure that is critical for context fear memory. The RSC is situated at the interface between sensory cortical areas (e.g., visuo-spatial regions) and multiple components of the hippocampal/parahippocampal memory system [15–17]. Further, a host of recent behavioral findings indicate that the RSC is important for contextual fear memory. For example, permanent lesions of the RSC carried out either before or after training consistently impair the subsequent expression of contextual fear memory, as evidenced by a significant reduction in fear-related freezing behavior when a lesioned rat is returned to the place that it previously experienced a footshock [2,17–19]. Post-training lesions of the RSC also impairs the context pre-exposure facilitation effect [20], a phenomenon that is considered to be a strong test of configural context learning [5,14]. In addition, RSC neurons are known to be active during contextual fear conditioning [19]. Furthermore, transient pharmacological manipulations that decrease neural activity in RSC impair acquisition [21] and retrieval [22] of contextual fear memories.
If the RSC has an essential role in contextual learning and memory, we predict the effects of RSC lesions would be invariable over a range of training parameters. Specifically, damaging RSC prior to training would be expected to impair context fear conditioning under weak training conditions (like hippocampal lesions), as well as under strong training conditions (unlike hippocampal lesions). Although it has been previously demonstrated that pre-training RSC manipulations do indeed impair context fear conditioning using relatively weak training procedures [2,17–20,22], there has been little research on the effects of RSC lesions using stronger training procedures. To address this, Experiment 1 tested the effects of neurotoxic RSC lesions using the same unsignaled contextual fear conditioning procedures that Wiltgen et al. [1] used to demonstrate that post-training, but not pre-training, lesions of hippocampus impair context fear memory when rats are conditioned using several shock presentations. Experiment 2 expanded on the results of Experiment 1 by testing the effects of RSC damage on both context and cue-specific fear memory using a different strong training procedure in which a tone was paired with footshock.
2. EXPERIMENT 1
Experiments 1A and 1B were purposefully designed to be conceptually and procedurally identical to Wiltgen et al. [1], as illustrated in Figure 1. Accordingly, in Experiment 1A, rats were fear conditioned in Context A using a 10-shock procedure. The next day, rats received either sham lesions or neurotoxic RSC lesions. Following recovery from surgery, context fear memory was assessed by re-exposing rats to Context A in the absence of shock. In Experiment 1B, the same rats were then re-trained in the same context (i.e., presentations of shock in Context A), as in Wiltgen et al. [1]. Contextual fear memory was assessed two weeks later (equal to the recovery time in Experiment 1A) by returning the rats to Context A. Thus, Experiment 1A tested the effects of post-training lesions of RSC on contextual fear memory (i.e., retrograde memory) while Experiment 1B examined the effect of pre-training lesions on contextual fear memory (i.e., anterograde memory).
Figure 1.
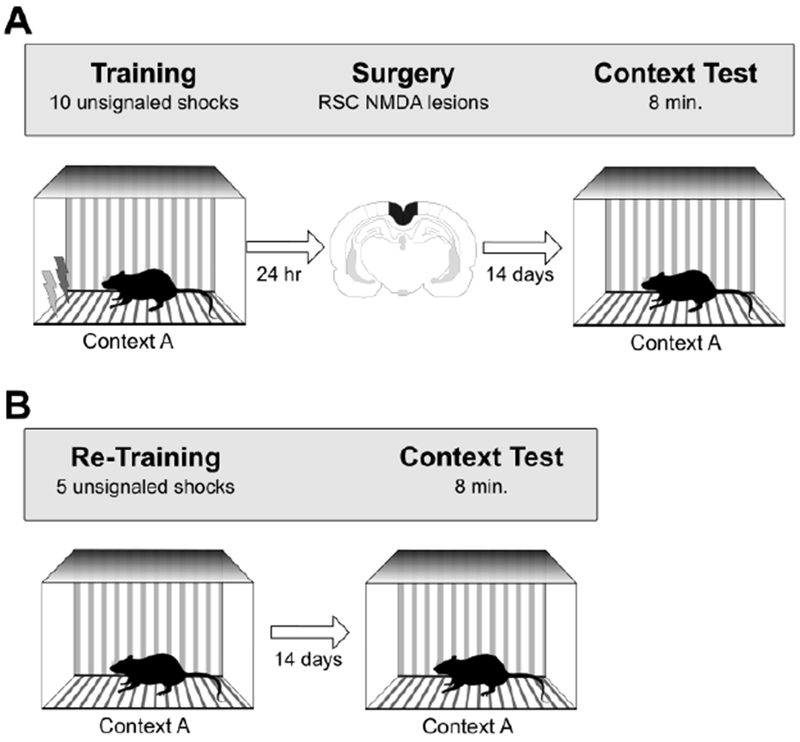
Behavioral procedures for Experiment 1A (panel A) and Experiment 1B (panel B). See text for additional details.
2.1. Materials and Methods
2.1.1. Subjects
The subjects were 16 naïve adult male Long Evans rats, obtained from Harlan/Envigo Laboratories (Indianapolis, IN), and were ~60 days old upon arrival. Rats were housed individually and allowed at least 6 days to acclimate to the vivarium prior to surgery. Food was available ad libitum (Purina standard rat chow; Nestle Purina, St. Louis, MO). Throughout the study, rats were maintained on a 14:10 light-dark cycle and monitored and cared for in compliance with association for Assessment and Accreditation of laboratory Animal Care guidelines and the Dartmouth College Institutional Animal Care and Use Committee.
2.1.2. Surgery
Subjects were anesthetized with isoflurane gas (1.5 - 3% in oxygen) and placed in a Kopf stereotaxic apparatus. The skin was retracted, and holes were drilled through the skull above each of the intended lesion sites based on the rat brain atlas of Paxinos and Watson [23]. Eight rats received bilateral infusions of 0.09M NMDA at the stereotaxic coordinates indicated in Table 1. Eight control rats received sham lesions consisting of a craniotomy and shallow, non-puncturing burr holes to minimize damage to the underlying cortex.
Table 1:
Coordinates for RSC lesions in Experiment 1
| A/P | M/L | D/V | Volume (μL) |
|---|---|---|---|
| −2.2 | −0.5 | −2.0 | 0.28 |
| −3.9 | −0.5 | −2.0 | 0.28 |
| −5.5 | −0.5 | −2.6 | 0.20 |
| −5.5 | −1.0 | −1.8 | 0.28 |
| −6.7 | −1.1 | −2.2 | 0.28 |
| −8.0 | −1.3 | −1.8 | 0.20 |
(Measurements in mm with respect to bregma (A/P, ML) and brain surface (D/V).
2.1.3. Behavioral apparatus
One set of four conditioning chambers served as Contexts A. All boxes were of the same standard design (Med Associates, Inc., St. Albans, VT, ENV-007; 24 cm W × 30.5 cm L × 29 cm H) and each was housed in its own sound attenuation chamber (Med Associates, ENV-017M; 66 cm W × 56 cm H × 56 cm D) and outfitted with an exhaust fan to provide airflow and background noise (~68 dB). Each chamber was outfitted with a food cup, recessed in the center of the front wall, and a retractable lever (Med Associates, ENV-112CM) positioned to the right of the food cup (not used in the experiment). Each chamber had a panel light (Med Associates, ENV-221M) mounted approximately 16 cm above the grid floor centered over the food cup, and a house light (Med Associates, ENV-215M) mounted approximately 24 cm above the grid floor on the back wall of the chamber. A speaker (Med Associates, ENV-224AM) was located 20 cm above and to the right of the food cup (not used in this experiment). The unconditioned stimulus was a 1-mA, 2-sec constant-current shock delivered through the grid floor.
The sidewalls and ceiling were made of clear acrylic plastic and the front and rear walls were made of brushed aluminum. The grid floor was stainless steel rods (5-mm diameter) spaced 1.5 cm apart (center-to-center). The chambers were illuminated with a 2.8-W bulb (with a red cover), mounted to the ceiling of the sound attenuating chamber. To provide a distinct olfactory cue in Context A, 3 ml of Pine-Sol (Clorox, Co., Oakland, CA) was placed in the underlying metal tray floor within each chamber before each session.
2.1.4. Behavioral procedures
Experiment 1A
Rats were fear conditioned using the procedures described by Wiltgen et al. [1], and thus received 10 presentations of the 1-mA, 2-sec foot shock. The first trial began 2 min after rats were placed in the chamber and the interval between shocks was 64 sec. The following day subjects received either RSC or sham surgeries. Two weeks later, as in the Wiltgen et al. study [1], all subjects were returned to Context A for an 8-min context test session during which no shocks were delivered.
Experiment 1B
One day after the context test session in Experiment 1A, the same rats were re-trained in Context A using 5 presentations of the same shock duration and amplitude. Two weeks later, all rats were re-exposed to Context A for an 8-min test session.
2.1.5. Behavioral observations and data analysis
Freezing served as the index of conditioned fear and was operationally defined as total motor immobility except for breathing [24,25]. On the training day, the incidence of freezing behavior was recorded during the 64-sec period prior to the first trial (baseline freezing) and during the 64-sec period following the final trial of conditioning (post-shock freezing). The rats’ behavior was scored every 8 sec during the 64-sec baseline period and post-shock epochs, and during 64-sec blocks of the context test sessions. For each rat, the frequency of freezing behavior was converted to a percentage of total observations for baseline, post-shock, and context test sessions. The data were then used to calculate the mean percentage freezing during these time periods and independent measures t-test were used to test for group differences in freezing behavior (alpha = 0.05). A single primary observer, blind to lesion condition, scored all of the behavioral data and a second observer scored the data to assess objectivity. Observations across observers were highly correlated (r = 0.9).
2.1.6. Lesion verification and analysis
After the behavioral procedures were completed, rats were deeply anesthetized with an overdose of sodium pentobarbital and transcardially perfused with 0.9% saline for 2 min, followed by 10% buffered formalin for 6 min. Coronal brain sections (60 μm) were collected using a freezing microtome and were Nissl-stained using thionin. Using a compound microscope (Axioskop I, Zeiss, Inc.), we identified gross tissue damage as necrosis, missing tissue, or marked thinning of the cortex. Outlines of the lesions were drawn onto digital images adapted from Paxinos and Watson [23] at six levels along the rostro-caudal extent of the RSC (−1.8, −3.0, −4.2, −5.4, −6.6, and −7.8 mm from bregma) and overlaid using ImageJ. At each level, area measurements were made with Stereoinvestigator software (Microbrightfield, Inc. Williston, VT) and an Axiokop I microscope (Zeiss, Inc., Elmsford, NY) using the Cavalieri estimator probe with 100μm grid spacing. Measurements included the total area of the target region and the area of the target region that exhibited gross tissue damage. From these measurements, we report the average percentage of RSC that was damaged. In addition, we report the average percentage of sections across the rostro-caudal plane that exhibited RSC damage (~25 sections collected for each rat), the average percentage of sections with damage outside the RSC, and the number of rats with damage to regions outside the RSC.
2.2. Results and Discussion
2.2.1. Histology
A photomicrograph of a representative RSC lesion is shown in Figure 2A. In Figure 2B, lesion drawings are stacked onto a single atlas image for each of the six coronal sections that were analyzed. Bilateral RSC damage was observed in all of the lesion rats, and the average area of RSC damaged on each section analyzed was 22.3% (SEM = 3.9). Damage to the RSC was present on 95% of the sections collected for each subject, indicating that damage extended throughout the rostro-caudal extent of the RSC. There was minor damage outside the RSC in six of the eight rats (e.g., anterior cingulate cortex, visual cortex, and motor cortex). In contrast to RSC damage, the minor damage outside RSC was present on only 27% (SEM = 7.0) of sections collected. Furthermore, the majority of extra-RSC damage was to visual cortex or motor cortex. Damage to these areas does not impair contextual fear conditioning [13].
Figure 2.
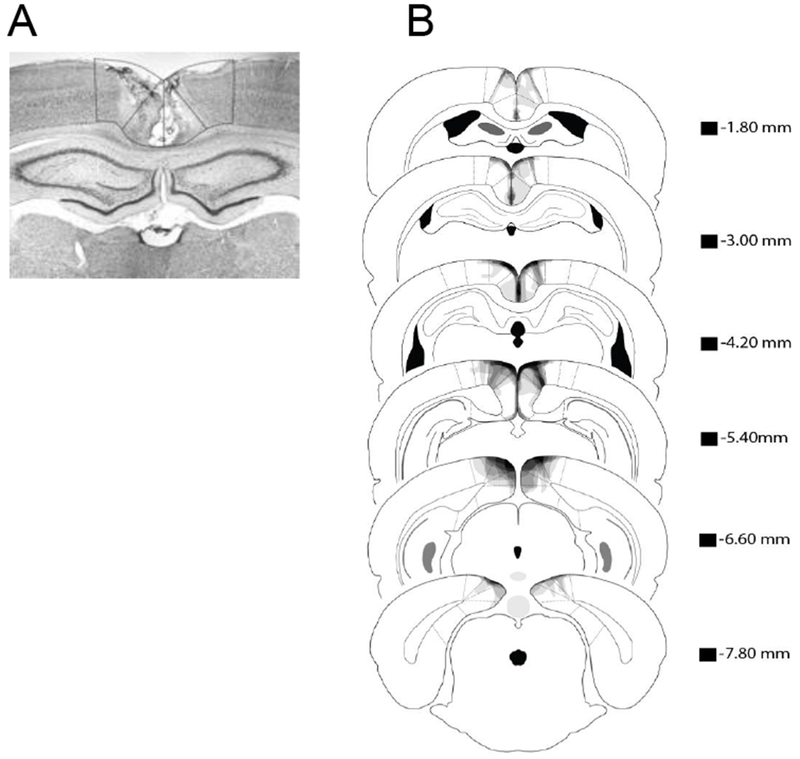
(A) Photomicrograph of a representative RSC lesion. (B) Drawings of lesions at six levels along the rostro-caudal extent of the RSC (−1.8, −3.0, −4.2, −5.4, −6.6, and −7.8 mm posterior to bregma). At each level, lesion drawings were stacked onto a single image. The darkness of an area indicates the number of lesions cases that include that area. Grey boxes (next to the bregma values) represents the expected darkness for overlap from all subjects. M2 = secondary motor cortex, RSCd = retrosplenial dysgranular, RSCg = retrosplenial granula, V2 = secondary visual cortex.
2.2.2. Behavior
Experiment 1A
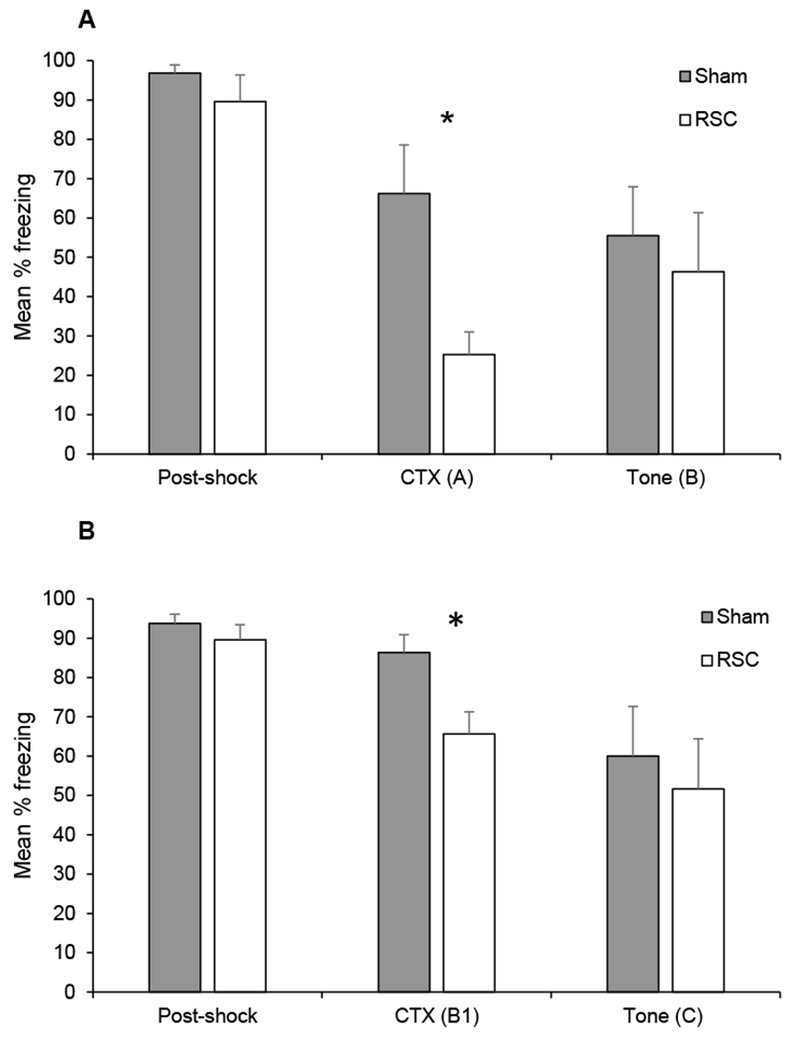
None of the rats in either group exhibited freezing prior to the first shock (i.e., during the baseline period). As shown in Figure 3A, post-shock freezing behavior was comparable between the two sets of rats on the training day (p > 0.1), as expected since rats had not yet undergone any surgery. However, rats that received lesions of the RSC after training froze less during the subsequent context test (CTX A) compared to sham-lesioned animals [t (14) = 3.8, p < 0.002]. These data indicate that damage to RSC, like damage to hippocampus [1], produces retrograde amnesia for context fear that was conditioned using strong training procedures. When considered with the results of a multitude of other post-training lesion and inactivation studies [2,12,13,17,22], this indicates that both RSC and hippocampus are normally involved during contextual fear conditioning.
Figure 3.
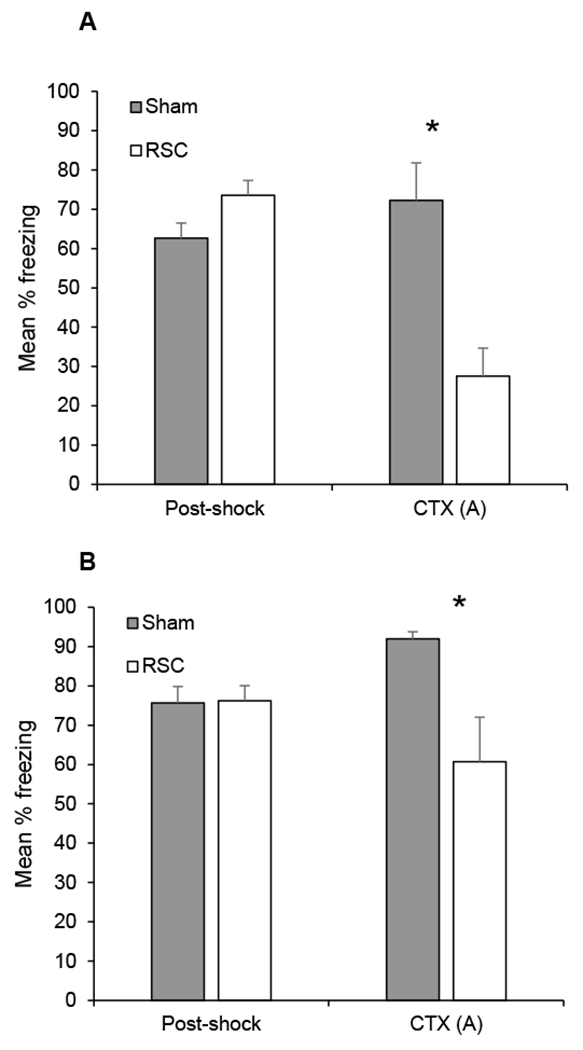
Mean percentage freezing (± SEM) during the training session in Context A (post-shock) and the context test (CTX) in Experiment 1A (panel A) and Experiment 1B (panel B). In Experiment 1A, training took place before surgery. After recovery from surgery, context fear was assessed in Context A (CTX A). In Experiment 1B, re-training occurred in Context A and context fear was assessed in Context A. *p < .05.
Experiment 1B
The primary results from the re-training session in Context A and the subsequent context test session are presented in Figure 3B. During the re-training session in Context A, sham-lesioned groups exhibited more freezing during the baseline period than RSC-lesioned rats [sham-lesion, mean = 46 ± 12%; RSC-lesion, mean = 13 ± 7%, t (14) = 2.4, p < 0.03], perhaps reflecting more context generalization in sham vs. RSC-lesioned rats. Importantly, however, post-shock freezing did not differ between RSC and sham-lesioned rats (p > 0.9), indicating that RSC-lesioned rats could acquire the conditioned response, consistent with prior studies [2,18]. However, rats with RSC lesions froze less during the Context A test session than sham-lesioned rats [t (14) = 2.7, p < 0.02]. Thus, unlike hippocampal lesions [1], the effects of pre-training RSC damage were not overcome by strong training. These findings support the notion that the RSC, unlike hippocampus, is essential for contextual fear memory even when strong conditioning procedures used. That is, while other brain structures or systems can compensate for hippocampal damage, this does not appear to be true in the case of RSC damage.
3. EXPERIMENT 2
If the RSC has an indispensable role in contextual fear learning and memory, we would expect RSC lesions to affect context memory regardless of whether context conditioning occurs in the ‘foreground,’ as in Experiment 1 (i.e., shock is not signaled by any discrete cue), or in the ‘background’ (e.g., shock is signaled by a tone [26]). Moreover, RSC lesions would be expected to impact context memory regardless of the specific parameters used to produce strong conditioning. To test this, Experiment 2 used a different training regimen and examined the effects of post-training and pre-training RSC lesions on context fear memory when shock was signaled by a tone. The inclusion of a tone during conditioning also allowed us to determine if the effect of RSC damage on fear memory resulting from strong training procedures is specific to context memory or also extends to cue-specific memory.
Experiments 2A and 2B were thus designed to be conceptually similar to Experiments 1A and 1B and Wiltgen et al. [1] except that shock was signaled by a tone (Figure 4). In Experiment 2A, rats received sham or RSC lesions one day after conditioning in Context A. Two weeks later, context fear memory was assessed by re-exposing rats to Context A in the absence of shock. Four weeks after the context test, fear to the tone was assessed by presenting the tone in a new context (Context B; no shock). The rationale for waiting four weeks between the context and tone test was to provide a rigorous test for determining if RSC damage affected tone-fear memory. This is because prior studies have shown that under certain circumstances, tone fear-memory is sensitive to post-training RSC damage at remote time points [17].
Figure 4.
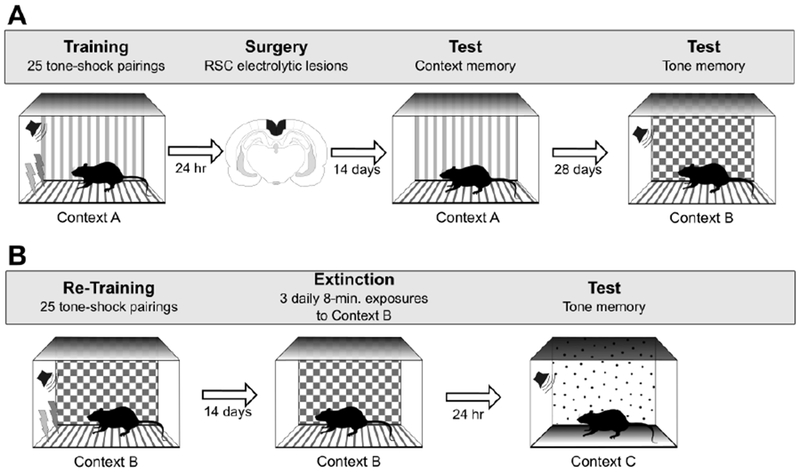
Behavioral procedures for Experiment 2A (panel A) and Experiment 2B (panel B). See text for additional details.
In Experiment 2B, the same rats were re-trained using the same tone-shock pairings and shock parameters, but in Context B. Contextual fear memory was assessed in Context B two weeks later and cue-specific fear memory was tested the next day in a new context (Context C) to isolate tone-specific freezing. Thus, Experiment 2B examined the effect of pre-training RSC lesions on context and cue-specific fear memory.
3.1. Materials and Methods
3.1.1. Subjects
The subjects were 16 naive adult male Long Evans rats, obtained from Harlan/Envigo Laboratories (Indianapolis, IN), and were about ~60 days old upon arrival. Rats were housed and maintained as described in Experiment 1.
3.1.2. Surgery
Rats were prepared for surgery as described in Experiment 1. Eight rats received bilateral electrolytic lesions (2 mA, 15 sec at each site) of the RSC using the coordinates shown in Table 2. Eight control rats received sham lesions as in Experiment 1. Electrolytic lesions were used in this experiment because this approach typically results in more complete damage compared to neurotoxic lesions [2,17]. In addition, electrolytic lesions may damage fibers of passage as well as neurons, and impair communication between regions other than RSC. Thus, we reasoned that using this more aggressive lesion approach would maximize the chances of observing any effects on tone-fear memory. That is, if the electrolytic lesions were without effect, we could be confident that RSC damage does not impact tone-fear memory when strong training procedures are used.
Table 2:
Coordinates for RSC lesions in Experiment 2
| A/P | M/L | D/V |
|---|---|---|
| −2.0 | ± 0.5 | − 2.0 − 2.7 |
| − 3.5 | ± 0.4 | − 2.0 − 2.7 |
| − 5.0 | ± 0.4 | − 2.0 − 2.7 |
| ± 1.0 | − 2.0 | |
| − 6.5 | ± 0.8 | − 2.0 − 2.8 |
| − 6.5 | ± 1.5 | − 3.4 |
| − 8.0 | ± 1.6 | − 2.5 |
| ± 2.4 | − 3.1 | |
| −9.0 | ± 3.4 | − 4.0 |
Measurements in mm with respect to bregma (A/P, ML) and brain surface (D/V).
3.1.3. Behavioral apparatus
The first set of chambers were identical to those described in Experiment 1. A second set of chambers were used where the grids of the floor were staggered such that odd- and even-numbered grids were mounted in two separate planes, one 0.5 cm above the other. The staggered grid floor provided a distinct tactile feature. These chambers were illuminated by the house light, as well as the panel light. The ceiling, back wall, and door were covered with laminated black and white checkerboard paper (1-cm squares) to provide distinct visual cues. All other details were identical to the first set of chambers.
Because these two sets of chambers were located within the same room of the laboratory, in order to prevent diffusion of the olfactory cues we used one olfactory cue for Context A sessions, and a second olfactory cue for Context B sessions. During Context A sessions, 3 ml of 8% coconut solution was placed in the chamber tray below the grid floor (McCormick & Co Inc., Hunt Valley, MD), and for Context B sessions, 3 ml of Pine-Sol (Clorox, Co., Oakland, CA).
The third context (Context C) was created by modifying all 8 chambers. For Context C, manila file folders were used to cover the grid floor, ceiling, front and back walls of the chamber (replaced between rats). Approximately 0.5 grams of Vicks Vaporub (Proctor & Gamble, Cincinnati, Ohio) was smeared along the chamber tray below the grid floor to provide a distinct olfactory cue. The unconditioned stimulus was a 1.0-mA, 1-sec constant-current shock delivered through the grid floor. A speaker was located 15 cm above and to the right of the food cup and used to deliver a 1500 Hz tone (78 dB) for 10 sec (the conditioned stimulus, CS).
3.1.4. Behavioral procedures
Experiment 2A
Rats were fear conditioned with the strong training procedures used by Maren [27]. The training session consisted of 25 presentations of the tone (10 sec) co-terminating with 1 mA, 1-sec foot shock. The first trial began 3 minutes after rats were placed in the chamber, and the interval from shock to the next tone (inter-trial interval, ITI) was 64 sec. The following day subjects received either RSC or sham surgeries. Two weeks later, as in the Wiltgen et al. study [1], all subjects received an 8-min context test, and no shocks were delivered. Finally, memory for the tone was tested 4 weeks later in Context B. During the tone test, the 10-sec tone was presented 20 times with an ITI of 30 sec. No shocks were delivered during the final test session. The first trial began 30 sec after placement into the chamber.
Experiment 2B
The rats from Experiment 2A were then re-trained in Context B using the identical procedures as Experiment 2A. Two weeks later, all rats were exposed to Context B for 8 min. At this point, since all rats were exposed to a total of 50 shocks (albeit 25 in Context A, and 25 in Context B), we elected to expose rats to Context B for an additional two subsequent days in an effort to fully extinguish any generalized or conditioned fear to Context B. The next day, tone retrieval was tested in Context C, using the same procedure as Experiment 2A.
3.1.5. Behavioral observations and data analysis
As in Experiment 1, freezing served as the index of conditioned fear. On the training day, the incidence of freezing behavior was recorded during the 64-sec period prior to the first trial (baseline freezing) and during the 64-sec period following the final trial of conditioning (post-shock freezing). The rats’ behavior was scored every 8 sec during the 64-sec baseline and post-shock epochs, as well as the context test sessions. For the tone test sessions, freezing was scored every 2 sec during each 10-sec presentation of the tone. We also scored freezing during the 64 sec period prior to the onset of the first tone presentation to assess baseline freezing in the context in which the tone was tested. As in Experiment 1, the frequency of freezing behavior was converted to a percentage of total observations and the data were used to calculate the mean percentage freezing during baseline, post-shock, context test, and tone test sessions. Behavioral observations were made, and data were analyzed, as described in Experiment 1.
3.1.6. Lesion verification and analysis
After the behavioral procedures were completed, rats were deeply anesthetized, and tissues sections were processed as described in Experiment 1. Area measurements to determine extent of damage was analyzed using the ImageJ overlaid lesion tracings.
3.2. Results
3.2.1. Histology
Figure 5A shows a photomicrograph of a representative RSC lesion. In Figure 5B, lesion drawings are stacked onto a single atlas image for each of the six coronal sections that were analyzed. Two RSC lesioned rats were excluded due to significant extra-RSC damage. Bilateral RSC damage was observed in all remaining rats, and the average area of RSC damaged on each section analyzed was 46.5% (SEM = 7.3). Damage to the RSC was present on 100% of the sections collected for each subject, indicating that damage extended throughout the rostro-caudal extent of the RSC. There was minor damage outside the RSC in all rats (e.g., anterior cingulate, visual cortex, motor cortex, cingulum bundle, forceps major corpus callosum). In contrast to RSC damage, which was present on all sections, the minor damage outside RSC was present on only 39.4% (SEM = 8.8) of sections collected. Furthermore, the majority of extra-RSC damage was to neocortex (visual cortex, motor cortex). Damage to these areas does not impair contextual fear conditioning [13].
Figure 5.
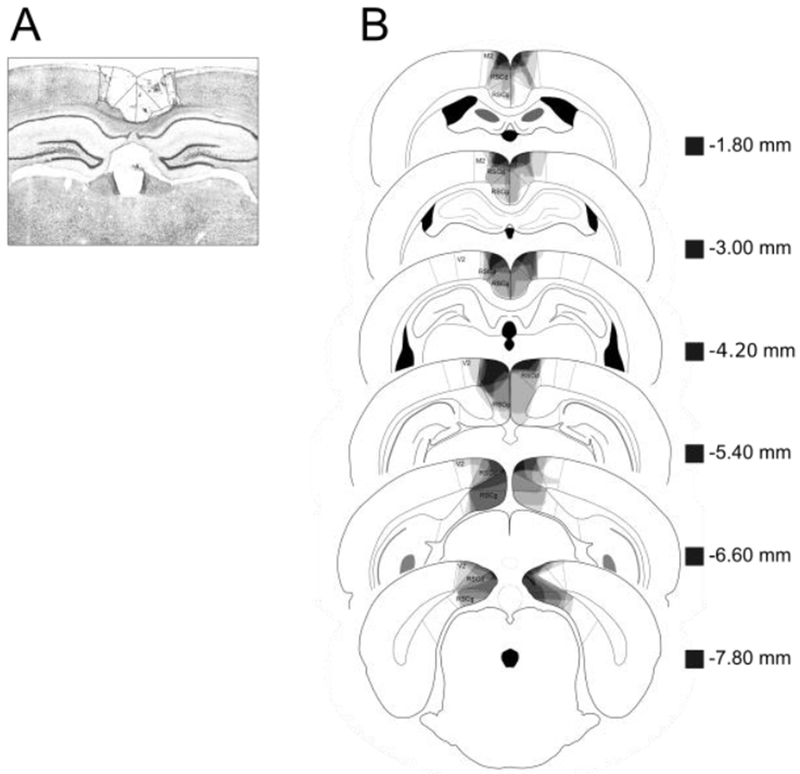
(A) Photomicrograph of a representative RSC lesion. (B) Drawings of lesions at six levels along the rostro-caudal extent of the RSC (−1.8, −3.0, −4.2, −5.4, −6.6, and −7.8 mm posterior to bregma). At each level, lesion drawings were stacked onto a single image. The darkness of an area indicates the number of lesions cases that include that area. Grey boxes (next to the bregma values) represents the expected darkness for overlap from all subjects. M2 = secondary motor cortex, RSCd = retrosplenial dysgranular, RSCg = retrosplenial granula, V2 = secondary visual cortex.
3.2.2. Behavior
Experiment 2A
The results of Experiment 2A are presented in Figure 6A. Baseline freezing was 0% in both groups, and freezing did not differ between groups during the post-shock period (p > 0.3), as expected since rats had not yet undergone surgery. However, rats with post-training lesions of the RSC froze less during the context test (CTX A) than sham-lesioned rats [t (12) = 2.7, p < 0.02], replicating the findings in Experiment 1A. During the tone test session in Context B, there were low levels of freezing prior to the first tone presentation [sham-lesion, mean = 17 ± 13%; RSC-lesion, mean = 0 ± 0%; p > 0.2]. The groups did not differ in their levels of freezing during the tone test in Context B (p > 0.5) as shown in Figure 6A.
Figure 6.
Mean percentage freezing (± SEM) during the training session (post-shock) and the context (CTX) and tone tests in Experiment 2A (panel A) and Experiment 2B (panel B). In Experiment 2A, training took place before surgery. After recovery from surgery, context fear was assessed in Context A (CTX A) and tone-specific fear was assessed in Context B. In Experiment 2B, re-training occurred in Context B, context fear was assessed in Context B (CTX B1) and tone-specific fear was assessed in Context C. *p < .05
Experiment 2B
The results from the re-training session, first context test session and tone test session are presented in Figure 6B. During the re-training session, both groups of rats exhibited very little freezing prior to the first shock (i.e. baseline period; sham-lesion, mean = 3.1%; RSC-lesion, 0%, p > 0.2), and post-shock freezing did not differ between the groups (p > 0.2). During the first test session in Context B, rats with RSC-lesions froze less than sham-lesioned rats [t (12) = 2.9, p < 0.02] as shown in Figure 6B, replicating the results of Experiment 1B. During the second and third context exposures to Context B (not shown), the groups did not differ [lowest p > 0.2]. During the second test in Context B, the mean percentage freezing was 68.2 ± 9.5% and 57.8 ± 13.0% for sham- and RSC-lesioned rats, respectively. During the third test in Context B, the mean percentage freezing was 42.4 ± 10.6% and 25.8 ± 5.5% for sham- and RSC-lesioned rats, respectively. During the tone test session in Context C, there was very little freezing in either group prior to the first tone presentation [sham-lesion, mean = 5 ± 5%; RSC-lesion, mean = 0 ± 0 %; p > 0.3]. The groups did not differ during the tone test in Context C [p > 0.5), as shown in Figure 6B.
4. General Discussion
Previous experiments have demonstrated that the RSC contributes to contextual fear conditioning, and it has been suggested that the RSC might work in concert with the hippocampus to produce and represent a configural representation of the context [20,28]. For example, post-training damage to the RSC [20] attenuates the context pre-exposure facilitation effect, a strong test of configural context learning that requires the hippocampus [5,9]. Further, when hippocampal CA1 cells are inactivated, context memory retrieval is impaired and activity in the RSC is reduced [28]. This suggests a functional connection between the hippocampus and the RSC during retrieval of contextual fear conditioning.
Although the hippocampus has a central role in contextual fear conditioning [4,5,14,29] there is evidence that its role is parameter dependent [1, 11]. For example, when only 1 shock is delivered, lesions of the hippocampus produce both retrograde and anterograde context amnesia (Wiltgen et al. [1]; Experiment 1). However, when more shocks are delivered, only retrograde amnesia is observed (Wiltgen et al. [1]; Experiment 3). If the contributions of RSC and hippocampus to context memory are functionally inseparable, then it might be expected that a similar pattern of results would emerge following RSC lesions. Indeed, with limited training, lesions of the RSC also produce retrograde and anterograde context amnesia [2,22]. With respect to strong conditioning, we found in the present study that, like lesions of the hippocampus, lesions of the RSC produced retrograde context amnesia (Experiments 1A and 2A). However, RSC lesions also produced anterograde context amnesia (Experiments 1B and 2B). This latter result suggests a functional dissociation from the hippocampus, since hippocampal lesions do not produce anterograde amnesia with strong training (Wiltgen et al. [1]; Experiment 3).
To explain the parametric dependence of hippocampal involvement in contextual fear conditioning, Fanselow [14], suggested that there are primary and alternative pathways capable of mediating fear behavior. The hippocampus is part of the primary system and it typically used by animals to form context memories. When the hippocampus is damaged prior to training, the alternative pathway is able to compensate. However, the alternative pathway is not as efficient as the primary pathway, which explains the parametric dependence of anterograde amnesia; pre-training hippocampal lesions produce anterograde amnesia with weak or limited training, but not with strong training [1]. Strong training procedures allow the less efficient alternative pathway to fully compensate for damage to the primary pathway. Fanselow [14] further suggested that the primary system dominates learning when the hippocampus is intact, which explains why hippocampal lesions produce retrograde context amnesia with both weak and strong training procedures.
Since lesions of the RSC produce retrograde amnesia with both weak training and strong training procedures, this suggests that the RSC is part of the primary pathway that dominates learning in the intact brain, consistent with other data suggesting that the hippocampus and RSC function together to produce and maintain context representations [20,28]. However, the finding that RSC lesions produce anterograde damage with strong training while hippocampal lesions do not, suggests an especially fundamental role for the RSC in contextual fear conditioning. One possibility is that the RSC provides critical information to both the primary and alternative pathways capable of mediating context fear. Damage to the RSC would impact both systems, explaining why both retrograde and anterograde amnesia are observed.
The perspective that the RSC provides critical information to both primary and alternative pathways capable of fear learning suggests that RSC damage should consistently disrupt context fear memory. In line with this, the present study showed that RSC damage produced both anterograde and retrograde context amnesia regardless of whether context conditioning occurred in the presence or absence of a tone paired with shock. In addition, the same pattern of effects on context memory was observed when different shock values and training trials were used. Based on this perspective, we might also expect to find RSC lesion induced behavioral deficits in other cases where lesions of the hippocampus do not produce behavioral impairments. For example, Lehman et al. [11] have demonstrated that when shocks are distributed across multiple sessions, lesions of the hippocampus do not produce retrograde amnesia (for a discussion, see [5,14]). With these parameters, the role of the RSC is currently unknown. In a similar fashion, it remains to be fully determined the extent to which RSC damage can be overcome.
In Experiment 2, neither anterograde nor retrograde impairments were observed for cue-specific fear, despite using more aggressive lesion methods. This indicates that the effect of RSC damage was specific to contextual memory and did not produce a general impairment in learning and memory. In addition, the findings from Experiment 2A provide new insight into the role of the RSC in the storage/retrieval of auditory fear memories. Although the RSC has often been found to be unnecessary for the retrieval of auditory fear memories [2,18,21,22,30] more recent evidence suggests that the RSC is necessary for trace fear conditioning and extinction [21,30] and the retrieval of remotely acquired auditory fear memories [17]. For example, Todd et al. [17, Experiment 1] found that RSC lesions impaired retrieval of an auditory fear memory when lesions were made remotely (28 days after training), suggesting that the RSC has a role in the long-term storage of auditory fear memories. In contrast, when lesions were made 1 day after training, and testing occurred 28 days later (Experiment 2A), RSC lesions had no impact on auditory fear. Taken together with the results of Todd et al. [17], this suggests that the RSC must be intact during the consolidation interval in order for storage/retrieval of the remotely acquired auditory fear memory to become RSC dependent. One caveat is that the present Experiment 2A utilized a strong training procedure, whereas prior experiments included only 3 tone-shock pairings [17].
The experimental design used in Experiments 1 and 2 was purposefully chosen to replicate the one used by Wiltgen et al. [1] so that we could compare the pattern of effects following RSC damage to those hippocampal lesions. Thus, anterograde context amnesia (Experiments 1B and 2B) was assessed using the same rats that had previously been used to assess retrograde amnesia (Experiments 1A and 2A). One downside of this approach is that interpreting the anterograde effects could be complicated by the possibility that different mechanisms may sometimes underlie re-acquisition and initial acquisition [31]. Although this is an important consideration in the present study and in the one by Wiltgen et al. [1], it is important to note that RSC lesions produced anterograde amnesia, while hippocampal lesions did not. That is, the differential pattern of results, regardless of whether they reflect the involvement of alternative (re)acquisition mechanisms, reveals a fundamental difference between the contributions of hippocampus and RSC to contextual fear memory when strong training procedures are used. Moreover, Kwapis and colleagues [21] have previously showed in behaviorally-naïve rats that perturbations of RSC disrupt contextual fear memory using strong training procedures, consistent with the present results. Thus, regardless of whether rats are behaviorally-naïve or not, the anterograde amnesia resulting from manipulations of RSC cannot be overcoming by strong training. Nevertheless, future studies might employ strong training procedures with behaviorally-naïve animals to eliminate the potential for reconsolidation effects after retraining.
Another potential issue with the use of the Wiltgen et al. [1] design is that prior experience could have led to a reduction in freezing behavior observed in RSC-lesioned rats in Experiments 1B and 2B. This seems unlikely for several reasons. First, RSC-lesioned rats reacquired the conditioned freezing similarly to control rats in both experiments. Second, the re-acquisition context used in Experiment 2B was different from the original training context used in Experiment 2A. Third, as mentioned above, a prior study using behaviorally-naïve rats showed that blocking protein synthesis in RSC prior to fear conditioning with strong conditioning procedures impaired context memory after [21], consistent with the results of Experiments 1B and 2B. In contrast, hippocampal manipulations carried out prior to strong training did not impact context memory regardless of whether the rats were behaviorally naive (Wiltgen et al. [1]; Experiments 1 and 2) or had prior training (Wiltgen et al. [1]; Experiment 3). Finally, it should be noted that the design used here and by Wiltgen et al. [1] tested rats two weeks after training. This is important to consider since RSC has been shown to be involved in remote (i.e., 28 day old) contextual fear memory [17] and so the behavioral procedures may be particularly sensitive to RSC damage.
The RSC contributes to contextual information processing, and recent work from our laboratory suggests that it is critical for configural processing of the context, a process that also requires the hippocampus [20]. Here, we extended this work by testing the importance of the RSC for context memory under additional parameters to determine how extensive the role of the RSC is in processing contextual information. In the current experiments, damage to the RSC produced both retrograde and anterograde context amnesia with two different strong training procedures. In contrast, prior studies have found that lesions of the hippocampus produce only retrograde amnesia when a strong training procedure is used [1]. We suggest that the RSC is fundamental to contextual fear learning and provides information to both primary and alternative fear pathways [14].
Highlights.
Damage to retrosplenial cortex produces anterograde and retrograde context amnesia
Strong training procedures cannot overcome anterograde deficits
The results differ from the effects of hippocampal damage using the same procedures
5. Acknowledgements
Research sponsored by NSF Grants IOS-1353137 (D.J.B.) and DGE-1313911 (N.E.D.), and NIH Grants MH112337 (M.C.E.) and DA037202 (D.I.F.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, & Fanselow MS (2006). Context Fear Learning in the Absence of the Hippocampus. Journal of Neuroscience, 26(20), 5484–5491. 10.1523/JNEUROSCI.2685-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Keene CS, & Bucci DJ (2008). Contributions of the retrosplenial and posterior parietal cortices to cue-specific and contextual fear conditioning. Behavioral Neuroscience, 122(1), 89–97. 10.1037/0735-7044.122.1.89 [DOI] [PubMed] [Google Scholar]
- [3].Bouton ME (2010). The multiple forms of “context” in associative learning theory. The mind in context, 233–258. [Google Scholar]
- [4].Fanselow MS (2000). Contextual fear, gestalt memories, and the hippocampus. Behavioural Brain Research, 110(1–2), 73–81. 10.1016/S0166-4328(99)00186-2 [DOI] [PubMed] [Google Scholar]
- [5].Rudy JW (2009). Context representations, context functions, and the parahippocampal-hippocampal system. Learning & Memory, 16(10), 573–585. 10.1101/lm.1494409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rudy JW, Huff NC, & Matus-Amat P (2004). Understanding contextual fear conditioning: Insights from a two-process model. Neuroscience and Biobehavioral Reviews, 28(7), 675–685. 10.1016/j.neubiorev.2004.09.004 [DOI] [PubMed] [Google Scholar]
- [7].Rudy JW, Barrientos RM, & O’Reilly RC (2002). Hippocampal formation supports conditioning to memory of a context. Behavioral Neuroscience, 116(4), 530–538. 10.1037/0735-7044.116.4.530 [DOI] [PubMed] [Google Scholar]
- [8].Rudy JW, & Matus-Amat P (2005). The ventral hippocampus supports a memory representation of context and contextual fear conditioning: implications for a unitary function of the hippocampus. Behavioral Neuroscience, 119(1), 154–163. 10.1037/0735-7044.119.1.154 [DOI] [PubMed] [Google Scholar]
- [9].Matus-Amat P, Higgins EA, Barrientos RM, & Rudy JW (2004). The Role of the Dorsal Hippocampus in the Acquisition and Retrieval of Context Memory Representations, Journal of Neuroscience, 24(10), 2431–2439. 10.1523/JNEUROSCI.1598-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stote DL, & Fanselow MS (2004). NMDA receptor modulation of incidental learning in Pavlovian context conditioning. Behavioral Neuroscience, 118(1), 253–7. 10.1037/0735-7044.118.1.253 [DOI] [PubMed] [Google Scholar]
- [11].Lehmann H, Sparks FT, Spanswick SC, Hadikin C, McDonald RJ, & Sutherland RJ (2009). Making context memories independent of the hippocampus. Learning & Memory, 16(7), 417–20. 10.1101/lm.1385409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Maren S, Aharonov G, & Fanselow MS (1997). Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behavioural Brain Research, 88(2), 261–274. 10.1016/S0166-4328(97)00088-0 [DOI] [PubMed] [Google Scholar]
- [13].Kim JJ, & Fanselow MS (1992). Modality-specific retrograde amnesia of fear. Science, 256(5057), 675–677. 10.1126/science.1585183 [DOI] [PubMed] [Google Scholar]
- [14].Fanselow MS (2010). From contextual fear to a dynamic view of memory systems. Trends in Cognitive Sciences, 14(1), 7–15. 10.1016/j.tics.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].van Strien NM, Cappaert NLM, & Witter MP (2009). The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nature Reviews. Neuroscience, 10(4), 272–82. 10.1038/nrn2614 [DOI] [PubMed] [Google Scholar]
- [16].Sugar J, Witter MP, van Strien NM, & Cappaert NLM (2011). The retrosplenial cortex: intrinsic connectivity and connections with the (para)hippocampal region in the rat. An interactive connectome. Frontiers in Neuroinformatics, 5, 7 10.3389/fninf.2011.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Todd TP, Mehlman ML, Keene CS, Deangeli NE, & Bucci DJ (2016). Retrosplenial cortex is required for the retrieval of remote memory for auditory cues. Learning & Memory, 23, 278–288. 10.1101/lm.041822.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Keene CS, & Bucci DJ (2008b). Neurotoxic lesions of retrosplenial cortex disrupt signaled and unsignaled contextual fear conditioning. Behavioral Neuroscience, 122(5), 1070–1077. 10.1037/a0012895 [DOI] [PubMed] [Google Scholar]
- [19].Robinson S, Poorman CE, Marder TJ, & Bucci DJ (2012). Identification of Functional Circuitry between Retrosplenial and Postrhinal Cortices during Fear Conditioning. Journal of Neuroscience, 32(35), 12076–12086. 10.1523/JNEUROSCI.2814-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Todd TP, DeAngeli NE, Jiang MY, & Bucci DJ (2017). Retrograde amnesia of contextual fear conditioning: Evidence for retrosplenial cortex involvement in configural processing. Behavioral Neuroscience, 131(1), 46 10.1037/bne0000183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kwapis JL, Jarome TJ, Lee JL, & Helmstetter FJ (2015). The retrosplenial cortex is involved in the formation of memory for context and trace fear conditioning. Neurobiology of Learning and Memory, 123(2015), 110–116. http://doi.Org/10.1016/j.nlm.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Corcoran K, Donnan MD, Tronson NC, Guzmán YF, Gao C, Jovasevic V, ... Radulovic J (2011). NMDA Receptors in Retrosplenial Cortex Are Necessary for Retrieval of Recent and Remote Context Fear Memory. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(32), 11655–11659. 10.1523/JNEUROSCI.2107-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Paxinos G, Watson C (2009). Rat rain in stereotaxic corrddinates, 6th ed. Elsevier: New York. [Google Scholar]
- [24].Blanchard RJ, & Blanchard DC (1969). Crouching as an index of fear. Journal of Comparative and Physiological Psychology, 67(3), 370 10.1037/h0026779 [DOI] [PubMed] [Google Scholar]
- [25].Fanselow MS (1980). Conditional and unconditional components of post-shock freezing. The Pavlovian Journal of Biological Science: Official Journal of the Pavlovian, 15(4), 177–182. 10.1007/BF03001163 [DOI] [PubMed] [Google Scholar]
- [26].Phillips RG, & LeDoux JE (1994). Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learning & Memory, 1(1), 34–44. 10.1101/lm.1.1.34 [DOI] [PubMed] [Google Scholar]
- [27].Maren S (1998). Overtraining does not mitigate contextual fear conditioning deficits produced by neurotoxic lesions of the basolateral amygdala. Journal of Neuroscience, 18(8), 3088–3097. 10.1523/JNEUROSCI.18-08-03088.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tanaka KZ, Pevzner A, Hamidi AB, Nakazawa Y, Graham J, & Wiltgen BJ (2014). Cortical Representations Are Reinstated by the Hippocampus during Memory Retrieval.Neuron, 84(2), 347–354. 10.1016/j.neuron.2014.09.037 [DOI] [PubMed] [Google Scholar]
- [29].Maren S (2001). Neurobiology of Pavlovian fear conditioning. Annual review of neuroscience, 24(1), 897–931. 10.1146/annurev.neuro.24.1.897 [DOI] [PubMed] [Google Scholar]
- [30].Kwapis JL, Jarome TJ, Lee JL, Gilmartin MR, & Helmstetter FJ (2014). Extinguishing trace fear engages the retrosplenial cortex rather than the amygdala. Neurobiology of Learning and Memory, 113, 41–54. 10.1016/j.nlm.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Laurent V, Marchand AR, Westbrook RF. (2008) The basolateral amygdala is necessary for learning but not relearning extinction of context conditioned fear. Learn Mem. 15(5):304–14 [DOI] [PMC free article] [PubMed] [Google Scholar]


