Structured Abstract
Importance
Mitochondrial DNA (mtDNA) disorders have emerged as major cause of inherited neurological disease. Despite being well recognized for over two decades, the clinical presentation continues to broaden. The phenotypic heterogeneity is partly due to different percentage levels of mutant mtDNA heteroplasmy in different tissues, but the factors influencing this are poorly understood.
Observations
A case report describing monozygotic male twins with ptosis, optic atrophy, and recent onset intractable myoclonic epilepsy. The assessment of respiratory chain enzyme activities in muscle from one twin revealed a severe and isolated defect involving mitochondrial complex I. MtDNA sequencing revealed a pathogenic m.14487T>C MTND6 mutation, which was present at very high levels of heteroplasmy (84%) in muscle, and lower levels in blood (15%), urinary epithelium (75%), and buccal mucosa (58%). Of particular interest, his identical twin was found to harbor very similar levels of the m.14487T>C mutation in his blood, urine, buccal mucosa and hair follicle DNA samples, whilst the presence of low levels in the mother’s tissues confirmed maternal transmission.
Conclusions and relevance
m14487T>C can also cause the unusual combination of optic atrophy, ptosis, and encephalomyopathy leading to intractable seizures. Near-identical heteroplasmy levels in different tissues in both siblings supports a nuclear genetic mechanism controlling the tissue segregation of mtDNA mutations.
Introduction
Pathogenic mitochondrial DNA (mtDNA) mutations were first described over 25 years ago, and mtDNA sequencing has been a mainstream diagnostic test for over a decade. It is therefore remarkable that new phenotypes continue to be identified that defy clinical paradigms. Here we describe two monozygotic twins with bilateral optic neuropathy and ptosis, preceding a fluctuating leukoencepalopathy with myoclonus and intractable seizures. Despite strong clinical clues, the underlying gene defect was not in the expected place, and was only identified through a systematic clinical approach and respiratory chain biochemistry.
Report of a case
Monozygotic male twins were born at 38.5 weeks through spontaneous vaginal delivery, with no perinatal problems. Mild bilateral ptosis was noticed at birth, but they were otherwise well and had normal development. There was no relevant family history.
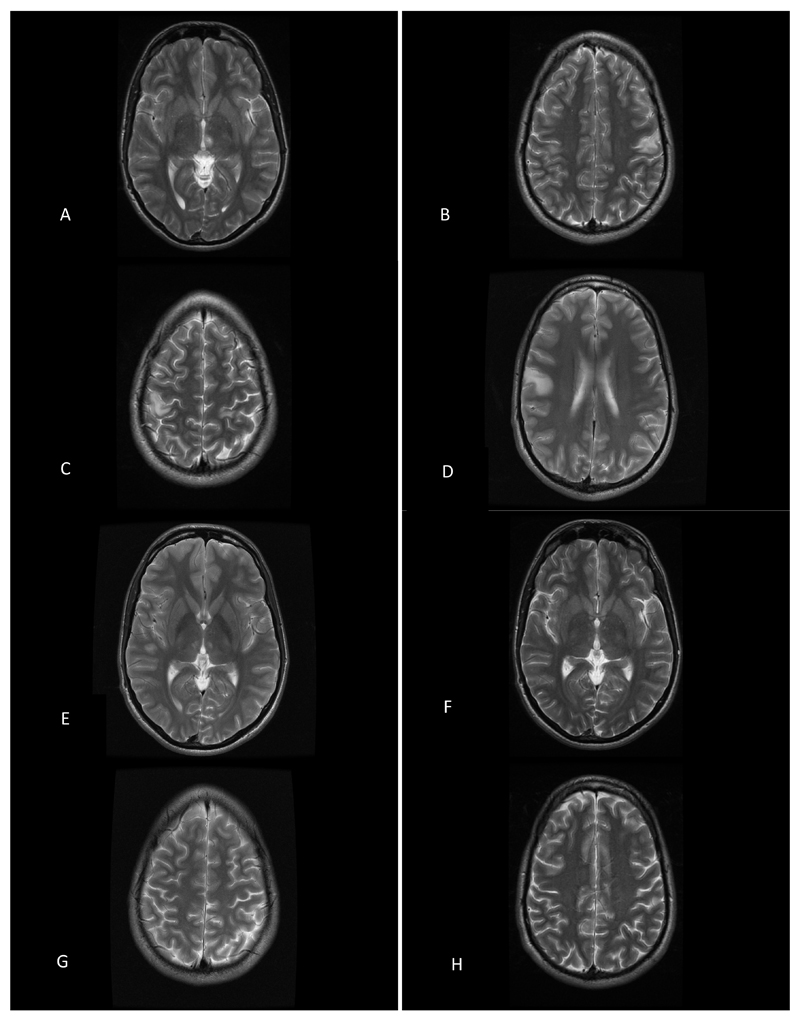
Twin 1 presented aged 14-years with visual acuity (VA) of 6/9 on the right and 6/24 on the left. His left visual field was restricted peripherally secondary to a previous retinal detachment that was surgically treated successfully and restricted visual fields. An MRI brain scan was normal. At the age of 18 he experienced intermittent tingling in his right hand and twitching movements of his fingers and arm. At the age 20 he had two generalized tonic clonic seizures, followed by persistent jerking of his right upper limb. Brain MRI showed lesions in the postcentral gyrus bilaterally and in the left posteriomedial thalamus (Fig.1). An EEG showed an alpha rhythm of 9-11 Hz, with some theta waves posteriorly and multifocal spike-wave discharges predominantly over the left centroparietal region. Repeat imaging, showed regression of the lesion in the left thalamus but a new mirror lesion in the right thalamus. Two and a half years after his first scan there was a new left superior temporal lesion, and a new cingulate lesion. Nearly two years later, these lesions had resolved but there was a new small (7mm) lesion in the right posterior frontal region.
Figure 1. Serial brain MR imaging from Twin 1.
MR findings from Twin 1 demonstrating a fluctuating leucoencephalopathy on serial imaging. Initial brain imaging demonstrated a left posteromedial thalamic lesion and bilateral cortical lesions (A-D, arrows). Repeat imaging 8 months later, revealed a right posteromedial thalamic lesion and a new right superior temporal lesion (E, arrow). Repeat imaging, nearly 2 years later, revealed resolution of the cortical and thalamic lesions (F-H).
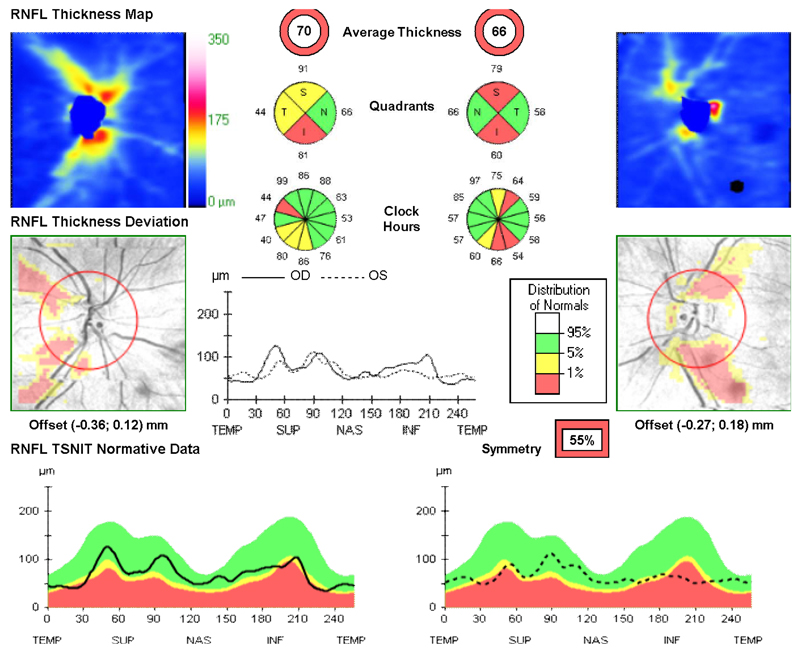
On examination aged 23-years, he had poor color vision, and VA had deteriorated further to 6/36 (left) and 6/60 (right). He had bilateral optic atrophy with thinning of the retinal nerve fibre layer on optical coherence tomography (OCT, Fig.2a). Bilateral ptosis was evident with no ophthalmoparesis. The rest of the cranial nerve examination was normal. He had brisk symmetrical reflexes, and downgoing plantars. There was continuous myoclonic jerking of his right upper limb.
Figure 2. Bilateral optic atrophy in Twin 1.
Marked global thinning of the peripapillary retinal nerve fibre layer in Twin 1. OCT measurements were obtained with the Topcon 3D OCT-2000™ platform (Topcon Medical Systems, Oakland, NJ). The analysis software automatically selects the appropriate normative range for the patient and the peripapillary retinal nerve fiber layer measurements (dark traces) are represented within color-coded distribution centiles (bottom panel): (i) red < 1%, (ii) yellow 1-5%, and (iii) green 5-95%.
Twin 2 presented aged 21-years, with intermittent numbness and tingling affecting his right hand. At the time, brain MRI, EEG and nerve conduction studies were normal. He had his first generalized tonic clonic seizure at the age of 23, then experienced episodes of right hemiparasthesiae as he was going into sleep or as he was waking. He subsequently developed visual symptoms, which consisted of seeing red dots; flashing blotches, and eventually obscured central vision. He had a reduced VA (6/9 bilaterally). Visual evoked potentials and electroretinography were consistent with a bilateral optic neuropathy secondary to retinal ganglion cell dysfunction. At the age of 24, he suffered clusters of partial seizures affecting his right hand about once per month. Brain MRI showed two small, predominantly cortical lesions on FLAIR in the right superior temporal gyrus and posterior frontal region. Six months later, two new lesions had appeared in the post-central gyrus on either side. On examination aged 26-years, his VA was 6/9 (left) and 6/18 (right). He had markedly reduced color vision in both eyes and bilateral dense central scotomas. Fundal examination revealed bilateral optic atrophy. OCT imaging confirmed significant thinning of the peripapillary retinal nerve fiber layer bilaterally (eFigure.1). He had bilateral ptosis with symmetrical bilateral restriction of eye movements, intermittent jerky movements of his right arm, but neurological examination of the limbs was otherwise normal.
Clinical investigations and molecular genetics
Given the familial optic atrophy, twin 1 was initially tested for common mtDNA mutations. Selective sequencing of blood mtDNA was negative for the m.11778A>G, m.3460A>G, and m.14484T>C Leber Hereditary Optic Neuropathy (LHON) mutations. Given the fluctuating leukoencephalopathy, m.3243A>G and POLG1 were also sequenced, and the familial ptosis prompted m.8344A>G, PEO1, ANT1, RRM2B and POLG2 sequencing, all of which were normal. He subsequently underwent a muscle biopsy which showed no histochemical evidence of mitochondrial dysfunction. However, measurement of the individual respiratory chain enzyme activities revealed an isolated deficiency in mitochondrial complex I (21% of controls), with normal activities of complexes II, III and IV. Sequencing the entire mitochondrial genome in muscle DNA revealed the m.14487T>C (p.Met63Val) MTND6 gene mutation, a recognized cause of isolated complex I deficiency1–9. Quantitative pyrosequencing showed the mutation to be present at levels of 84% in skeletal muscle, 15% in blood, 75% in urinary epithelium, 58% in buccal mucosa, and 86% in hair follicles. His identical twin harbored the similar mutation at levels of 17% in blood, 71% in urine and, 57% in buccal mucosa, and 74% in hair follicles while their unaffected mother had significantly lower mutation levels (5% in blood, 25% in urine, 30% in buccal mucosa, and 34% in hair follicles).
Comment
The pathogenicity of the m.14487T>C mutation has been previously confirmed because: 1) it has never been reported as a polymorphism in >10,000 control subjects.10 2) It is predicted to cause an amino acid substitution of a highly conserved methionine to valine (p.Met63Val) in the ND6 protein associated with an isolated complex I defect. 3) Transmitochondrial cybrid studies have demonstrated that the complex I defect correlates with the mutant heteroplasmy levels7, 9 4) The mutation is heteroplasmic, and segregates with a clinical phenotype. 5) It is a recurrent cause of mitochondrial disease, associated with several clinical presentations including severe infantile and childhood-onset Leigh’s syndrome and Leigh’s-like encephalopathy1–8 and progressive dystonia9, juvenile-onset Leigh’s syndrome with optic atrophy, ataxia and epilepsy4. Low levels of m.14487T>C mutation heteroplasmy were associated with a variety of phenotypes in a Belgian family that included subclinical LHON, migraine with aura, bilateral sensorineural hearing loss or type II diabetes mellitus1.
The twins illustrate several important clinical points. First, the combination of optic atrophy and ptosis/PEO is not typical for a mtDNA disorder. It is usually seen in patients with a Mendelian disorder due to mutations in OPA1. Second, a fluctuating encephalopathy with intractable epilepsy is usually seen in patients with an intra-mitochondrial protein translation, commonly due to either a mtDNA mutation affecting a tRNA gene, or to mutations in POLG where secondary mtDNA deletions contribute to the phenotype. A mtDNA protein-coding mutation, such as the one found in the twins we describe here, would be most unusual in both clinical contexts. Although additional clinical features have been described in patients with optic atrophy due to the three common LHON mutations in MTND1, MTND4 and MTND6, including psychiatric disturbances, spastic dystonia, ataxia, and juvenile onset encephalopathy, these extra-ocular manifestations are individually rare. The complex phenotype displayed by the two monozygotic twins in this report has not been described before, adding to the spectrum of “LHON plus” syndromes (reviewed in 11). Likewise, mutations in MTND5 have been associated with MELAS or with overlap syndromes of MELAS and Leigh syndrome, MELAS and LHON, and MELAS and MERRF.12 Finally, the imaging findings were unusual for a complex I defect, particularly when due to a mtDNA mutation.13,14
Finally, it is striking that both twins had almost identical heteroplasmy levels in four tissues, which were different to their mother. Genetic analysis with 16 highly informative microsatellite markers confirmed that the twins were identical, and thus arose from the same oocyte. It is therefore highly likely that they both inherited the same mutation load from their mother. The near identical levels in other tissues strongly suggests that tissue-specific segregation of mtDNA levels is tightly regulated, and identical levels in identical twins is in keeping with recent animal studies implicating a nuclear-genetic control mechanism.15 These findings contrast with identical twins harboring a mtDNA deletion,16 where strikingly different levels of mtDNA heteroplasmy could have arisen through unequal partitioning of genomes during development. This does not appear to be the case for single nucleotide variants, based on a recent study in twins.17
These findings stress the importance of taking a systematic, algorithmic-based approach when investigating suspected mitochondrial disease. Even in this era of high-throughput genomics, a muscle biopsy and subsequent biochemical analysis should be considered in patients who do not harbor a common mutation in mtDNA or a relevant nuclear gene, because these well-established clinical investigations may lead directly to the causal gene.
Acknowledgments
Study sponsorship: The Wellcome Trust
Footnotes
All of the authors report no financial disclosures.
References
- 1).Dermaut B, Seneca S, Dom L, et al. Progressive myoclonic epilepsy as an adult-onset manifestation of Leigh syndrome due to m.14487T>C. J Neurol Neurosurg Psychiatry. 2010;81:90–93. doi: 10.1136/jnnp.2008.157354. [DOI] [PubMed] [Google Scholar]
- 2).Esteitie N, Hinttala R, Wibom R, et al. Secondary metabolic effects in complex I deficiency. Ann Neurol. 2005;58(4):544–552. doi: 10.1002/ana.20570. [DOI] [PubMed] [Google Scholar]
- 3).Lebon S, Chol M, Benit P, et al. Recurrent de novo mitochondrial DNA mutations in respiratory chain deficiency. J Med Genet. 2003;40(12):896–899. doi: 10.1136/jmg.40.12.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Leshinsky-Silver E, Shuvalov R, Inbar S, Cohen S, Lev D, Lerman-Sagie T. Juvenile Leigh syndrome, optic atrophy, ataxia, dystonia, and epilepsy due to T14487C mutation in the mtDNA-ND6 gene: a mitochondrial syndrome presenting from birth to adolescence. J Child Neurol. 2011;26(4):476–481. doi: 10.1177/0883073810384615. [DOI] [PubMed] [Google Scholar]
- 5).Malfatti E, Bugiani M, Invernizzi F, et al. Novel mutations of ND genes in complex I deficiency associated with mitochondrial encephalopathy. Brain. 2007;130:1894–1904. doi: 10.1093/brain/awm114. [DOI] [PubMed] [Google Scholar]
- 6).Naess K, Freyer C, Bruhn H, et al. MtDNA mutations are a common cause of severe disease phenotypes in children with Leigh syndrome. Biochim Biophys Acta. 2009;1787(5):484–490. doi: 10.1016/j.bbabio.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 7).Ugalde C, Triepels RH, Coenen MJ, et al. Impaired complex I assembly in a Leigh syndrome patient with a novel missense mutation in the ND6 gene. Ann Neurol. 2003;54(5):665–669. doi: 10.1002/ana.10734. [DOI] [PubMed] [Google Scholar]
- 8).Wang J, Brautbar A, Chan AK, et al. Two mtDNA mutations 14487T>C (M63V, ND6) and 12297T>C (tRNA Leu) in a Leigh syndrome family. Mol Genet Metab. 2008;96:59–65. doi: 10.1016/j.ymgme.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 9).Solano A, Roig M, Vives-Bauza C, et al. Bilateral striatal necrosis associated with a novel mutation in the mitochondrial ND6 gene. Ann Neurol. 2003;54(4):527–530. doi: 10.1002/ana.10682. [DOI] [PubMed] [Google Scholar]
- 10).National Centre for Biotechnology Information. www.ncbi.nlm.nih.gov.
- 11).Yu-Wai-Man P, Griffiths PG, Chinnery PF. Mitochondrial optic neuropathies-Disease mechanisms and therapeutic strategies. Progress in Retinal and Eye Research. 2011;30:81–114. doi: 10.1016/j.preteyeres.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Niani AB, Jiesheng L, Kaufmann P, et al. Novel Mitochondrial DNA ND5 Mutation in a Patient With Clinical Features of MELAS and MERRF. Arch Neurol. 2005;62:473–476. doi: 10.1001/archneur.62.3.473. [DOI] [PubMed] [Google Scholar]
- 13).Lebre AS, Rio M, Faivre d'Arcier L, et al. A common pattern of brain MRI imaging in mitochondrial diseases with complex I deficiency. J Med Genet. 2011;48(1):16–23. doi: 10.1136/jmg.2010.079624. [DOI] [PubMed] [Google Scholar]
- 14).Koene S, Rodenburg RJ, van der Knaap MS, et al. Natural disease course and genotype-phenotype correlations in Complex I deficiency caused by nuclear gene defects: what we learned from 130 cases. J Inher Metab Dis. 2012;35(5):737–47. doi: 10.1007/s10545-012-9492-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Jokinen R, Marttinen P, Sandell HK, et al. Gimap3 regulates tissue-specific mitochondrial DNA segregation. PLOoS Genet. 2010;6(10):e1001161. doi: 10.1371/journal.pgen.1001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Blakely EL, He L, Taylor RW, et al. Mitochondrial DNA deletion in “identical” twin brothers. J Med Genet. 2004;41:e19. doi: 10.1136/jmg.2003.011296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Andrew T, Calloway CD, Stuart S, et al. A twin study of mitochondrial DNA polymorphisms show that heteroplasmy at multiple sites is associated with mtDNA variant 16093 but not with zygosity. PLOS One. 2011;6(8):e22332. doi: 10.1371/journal.pone.0022332. [DOI] [PMC free article] [PubMed] [Google Scholar]