Abstract
HIV pre-exposure prophylaxis (PrEP) is an increasingly important part of the HIV prevention armamentarium. Issues with PrEP, however, include access. We propose that one way to surmount this issue would be to have nurses provide PrEP. Although clinical guidelines exist for PrEP, they are overwhelmingly not targeted to nonprescriber clinicians. In this article, we overview current U.S. and Canadian PrEP guidelines and provide explicit guidance about how nurses can provide PrEP, including the clinical pathways and medical directives we use in our clinic. We call nurse-led provision of PrEP, PrEP-RN (Pre-Exposure Prophylaxis–Registered Nurse) and feel it may be an important step forward in HIV prevention.
Key words: community, HIV, nursing, nurse-led, PrEP
The daily use of antiretroviral medications by HIV-uninfected persons to reduce the risk of HIV acquisition is a prevention strategy known as pre-exposure prophylaxis (PrEP; Centers for Disease Control and Prevention, 2018a). Currently, the only medication approved for PrEP in the United States and Canada is the fixed combination of 300 mg emtricitabine (FTC) plus 200 mg tenofovir disoproxil fumarate (TDF; Centers for Disease Control and Prevention, 2018a; Tan et al., 2017). Clinical trials conducted among men who have sex with men (MSM) found up to 96% reductions in HIV seroconversion among participants who used at least 4 doses of FTC/TDF per week, compared with controls (Centers for Disease Control and Prevention, 2018a; Grant et al., 2010; McCormack et al., 2015). Population data from London, San Francisco, and New York City, meanwhile, have shown significant decreases in HIV incidence correlating with intentional PrEP rollout in these cities (Brown et al., 2017; New York City Department of Health, 2018; San Francisco Department of Health, 2016). These findings are noteworthy given ongoing HIV diagnoses in Canada and areas of the United States during the same periods among MSM (Centers for Disease Control and Prevention, 2016; Haddad, Li, Totten, & McGuire, 2018). Considering the contrast between decreased HIV diagnoses in cities with targeted PrEP programs and increases in cities without such rollout, PrEP may have individual and population HIV prevention benefits.
Despite its efficacy, PrEP delivery faces challenges. In addition to cost, access to providers who prescribe PrEP is limited (Hannaford et al., 2018; Silapaswan, Krakower, & Mayer, 2017; Turner, Roepke, Wardell, & Teitleman, 2018). For example, a 2016 study in Washington State found that, of the 735 state medical providers, one in three providers were unaware of PrEP (Wood et al., 2018). For patients with providers, however, barriers still exist. First, sex and sexual orientation are topics that patients and providers frequently avoid. In a recent survey of MSM in London-Middlesex Ontario, 28.9% had not disclosed their sexual orientation to their primary care providers, and 26.6% indicated that these providers had assumed they were heterosexual (Coleman et al., 2017). Similarly, in a study of family physicians in the United States, Ojile, Sweet, and Kallail (2017) found only 48% “always or frequently” took a sexual history on new patients, only 34% asked about a patient's safer sex practices, and only 28% asked whether a patient had sex with men, women, or both. In a commentary on the Canadian guidelines, Nelson et al. (2019) commented that “Black MSM are much less likely than White MSM to disclose their same-gender sexual practices to healthcare providers” (p.1). An unwillingness to talk about sexual practices precludes discussions of HIV prevention.
Another barrier to prescribing FTC/TDF is a lack of provider knowledge about PrEP, including the medication and required follow-up (Petroll et al., 2017; Scholl, 2016), despite the availability of Centers for Disease Control and Prevention, Canadian, European, and World Health Organization guidelines (Centers for Disease Control and Prevention, 2018a; European AIDS Clinical Society, 2017; Tan et al., 2017; World Health Organization, 2015). Indeed, a survey of 525 primary care and specialist physicians in 10 U.S. cities similarly found that only 28% of primary care providers felt comfortable prescribing PrEP (Petroll et al., 2017). Additional barriers included concerns about medication side effects, adherence, drug resistance, resources required for follow-up, and potential risk compensation for those using PrEP (Ojile et al., 2017; Scholl, 2016; Zablotska et al., 2018).
Proposal
To address the lack of access to PrEP, we propose that registered nurses (RNs) provide PrEP in sexually transmitted infection (STI) and HIV testing clinics, an approach we call Pre-Exposure Prophylaxis–Registered Nurse (PrEP-RN). To accomplish this, we suggest that medical directives and established pathways to interpret laboratory findings be created to allow RNs to provide PrEP, thereby increasing the number of health care professionals who provide this intervention. We detail one example of such pathways for serum creatinine (SeCr) and estimated glomerular filtration rate (eGFR) testing, which could help others incorporate PrEP into STI clinics, settings where staff may be less familiar with the results, parameters, and monitoring for creatinine and eGFR.
Our rationale for PrEP-RN is that (a) PrEP is highly efficacious; (b) PrEP access is currently limited; (c) PrEP delivery by nurses could produce cost-savings compared with delivery by generalist and specialist physicians; (d) PrEP monitoring should include regular STI testing, which is already provided by RNs in STI clinics; and (e) many patients who access STI clinics are prime candidates for PrEP. Regarding the last point, in some studies, more than 1 in 20 men were diagnosed with HIV within 1 year of a syphilis diagnosis (Pathela, Braunstein, Blank, Shepard, & Schillinger, 2015). In other studies, rectal gonorrhea and Chlamydia infection corresponded to increased risk of HIV acquisition (Hull, Edward, & Kelley, 2017). A study in Vancouver, specifically, identified an HIV incidence rate in MSM diagnosed with syphilis and rectal gonorrhea of 17.3/100 persons (Hull et al., 2017). Thus, PrEP-RN links the right patients to the right providers for provision of PrEP at what should be lower costs. Finally, RNs are more numerous compared with physicians and nurse practitioners (Canadian Institutes of Health Information, 2017), and already work in or operate many STI clinics in Canada and the United States. PrEP-RN thus uses an existing workforce experienced in sexual health to deliver PrEP in STI clinics, which are settings already visited by persons at high risk for HIV (Aghaizu et al., 2018; Johnsen, Thimm, Singer, & Page, 2017).
In this article, we review the literature about PrEP, and, building on U.S. and Canadian guidelines, as well as our own research, put forward a model for how nurses can provide PrEP in STI clinics. In addition to increasing access to PrEP, we posit that PrEP-RN, by including regular STI screenings, could enhance STI prevention and reduce onward transmission. Although other models of RN-led PrEP exist, none have explicitly described how these clinics operate (e.g., Sharma et al., 2018). We provide a detailed account of our PrEP-RN clinic to allow others to build on our work and implement it elsewhere. Although PrEP-RN is exclusively based on the Canadian and U.S. PrEP guidelines, our goal is to encourage other countries to implement the protocols we developed for a community-based STI clinic in Ottawa, Canada.
The Intervention: Pre-exposure Prophylaxis–Registered Nurse Clinical Protocol
Target Population for Pre-exposure Prophylaxis–Registered Nurse
In agreement with U.S. and Canadian guidelines (Centers for Disease Control and Prevention, 2018a; Tan et al., 2017), we feel PrEP should be readily available, and actively targeted, to individuals at highest risk of HIV infection, which should be determined by and tailored to local HIV epidemiology. Based on such data at the national levels in the United States and Canada, this would include the following: MSM and transgender persons who engage in condomless anal sex and were diagnosed with infectious syphilis or a rectal STI in the past 12 months, HIV uninfected individuals (male, female, or transgender) with a sexual partner living with a detectable HIV viral load, and individuals who share injection drug use paraphernalia with persons at risk of HIV infection; persons who use postexposure prophylaxis (PEP) for sexual exposures should also be offered PrEP (Centers for Disease Control and Prevention, 2018a; Tan et al., 2017). Although the U.S. and Canadian guidelines recommend PrEP for persons who use PEP more than once, we feel a single use warrants an offer of PrEP (Centers for Disease Control and Prevention, 2018a; Tan et al., 2017). Of 84 MSM who sought and completed a single course of PEP in our nurse-led PEP program, 9% went on to acquire HIV within 12 months due to ongoing risks and exposures (O'Byrne, MacPherson, & Orser, 2018). Notably, these diagnoses were not PEP failures but the outcome of subsequent HIV exposures (O'Byrne et al., 2018). Transgender persons, irrespective of secondary risk factors for HIV acquisition, should be considered a priority group for PrEP because, in the United States and Canada, the rates of HIV acquisition are higher in this group compared with other populations that are affected by HIV (Centers for Disease Control and Prevention, 2018b; 2018c).
In addition, we believe that all individuals should maintain ownership of their sexual health and have the right to obtain prevention strategies that meet their needs. In view of this, we believe PrEP should be available to all individuals who feel they are at reasonable risk of HIV acquisition, irrespective of risk factors. This may include persons with serodiscordant sex partners, anyone who is sexually active within a group affected by HIV (e.g., MSM; individuals in African, Caribbean, Black [ACB] communities), persons from HIV endemic regions, persons who share injection drug equipment, and others based on local HIV epidemiologic data. As part of this, despite lack of acknowledgement in current guidelines, we feel consideration should go toward offering PrEP to ACB communities. In the United States, in 2016, ACB persons accounted for 8% of all HIV diagnoses, and the rate of HIV infection among ACB women in the United States was disproportionately higher than female population of other ethnicities (Centers for Disease Control and Prevention, 2018c). In these cases, providers should consider offering PrEP, without needing to identify additional risk criteria. Knowing that many people feel uncomfortable discussing sexual practices with health care providers (and that this may be exacerbated in ACB groups; Nelson et al., 2019), allowing broader access to PrEP removes possible barriers that could arise by requiring that patients disclose such activities. We suggest, as well, that persons should be eligible for PrEP before they acquire an STI rather than waiting until after, as suggested by current guidelines. Conversely, we feel that members of the groups most affected by HIV should be offered PrEP after STI diagnoses, even if they do not disclose being MSM or report having a partner who is living with HIV. Table 1 and Figure 1 lists our proposed inclusion criteria for PrEP.
Table 1.
Pre-exposure Prophylaxis Inclusion Criteria

Figure 1.
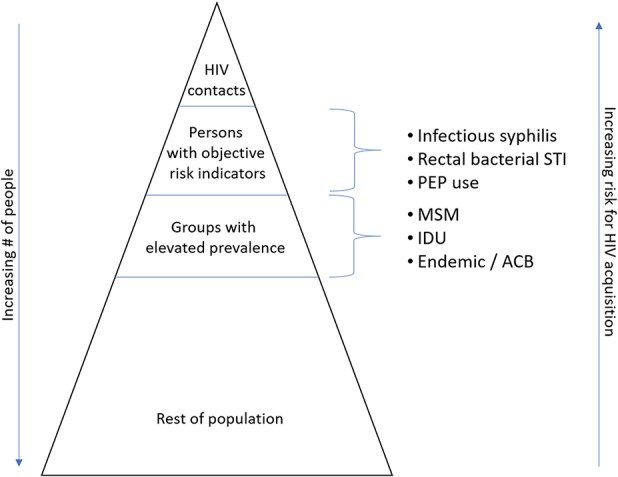
HIV risk stratification. Note. ACB = African, Caribbean, Black communities; IDU = injection drug use; MSM = men who have sex with men; PEP = postexposure prophylaxis; STI = sexually transmitted infection.
Patient Recruitment
For a PrEP program to be effective, those who would benefit from it must be aware it exists and must know how and where to access it (Mosely et al., 2018). There are several avenues to increase PrEP awareness, with mass advertising being one, albeit limited, approach (Liu et al., 2014). As a first limitation, to date, mass advertising has primarily targeted risk groups. Although this strategy allows culturally appropriate messaging, it may be undermined by reach. For example, not all MSM identify as gay or access gay media; this limitation could be compounded for transgender and ACB persons who may not access these media, in part, due to the possible lack of attachment to mainstream gay culture. Second, although advertising may increase awareness of PrEP, it does not address individual need. One study found that MSM, for example, often underestimated their risk of HIV Infection, which was not something advertising necessarily would affect (Sullivan & Stephenson, 2018). Research involving ACB, meanwhile, has identified that many Black women who were diagnosed with HIV were unaware their sexual partners were living with HIV, meaning that, in high HIV prevalence groups, PrEP should be considered even without knowledge that a sexual partner has HIV (Nelson et al., 2019).
In contrast to mass media strategies, a targeted approach would involve ensuring that staff in STI clinics are educated about PrEP and can identify patients who would benefit from it. The goal in doing this would be to ensure that clinicians not only have knowledge in case patients inquire about PrEP but also initiate conversations about PrEP with patients who could benefit from it. To accomplish this provider-driven strategy, we recommend including prompts in clinical record systems (such as on patient visit forms) and visual cues (such as posters) in clinic waiting areas or examination rooms to trigger patients and providers to discuss PrEP. For example, we have included PrEP as a service patients can request on our clinic intake forms and have included a prompt for nurses to discuss PrEP with patients diagnosed with infectious syphilis, a rectal STI, or recent use of PEP (Table 1). We also recommend collaborating with local public health units to ensure a referral process for those who fulfill these criteria. Locally, our public health unit has its nurses offer PrEP referrals to patients diagnosed with syphilis, rectal gonorrhea, or chlamydia, and anyone named as an HIV contact. We suspect that provider-initiated referrals may increase access for those at highest risk compared with current models of patient self-selection for PrEP (Orser & O'Byrne, 2019).
Pre-exposure Prophylaxis–Registered Nurse Delivery and Clinical Visits
As per current guidelines (Centers for Disease Control and Prevention, 2018a; Tan et al., 2017), patients should be seen for an initial visit, with a follow-up after 1 month of PrEP use and every 3 months thereafter (Table 2). Although current guidelines list the indications, assessments, prescriptions, and monitoring required for PrEP, they do not provide step-by-step details about how to execute the guidelines. We provide this information to help clinicians who may be less familiar with PrEP to incorporate this intervention into their care.
Table 2.
Pre-Exposure Prophylaxis–Registered Nurse Visit Schedule
Initial (baseline) visit
In our clinic, the initial visit addresses the patient's interest in PrEP, and her/his risk of HIV infection, level of anxiety about HIV, and whether PrEP is an appropriate intervention. We complete a detailed history to determine the patient's indication for PrEP (Table 1), to identify symptoms of acute HIV infection at the time of the visit and in the preceding 4 weeks (such as fever, chills, sweats, myalgias, rash, lymphadenopathy, pharyngitis, and diarrhea), and to discern the presence of known contraindications to FTC/TDF (HIV-positive, allergy to FTC or TDF, or an eGFR less than 60 mL/min; Tan et al., 2017). Due to high rates of syndemic factors identified in previous PrEP cohorts (Wilton et al., 2018), at this first visit, we also administer screening tools for anxiety, depression, and alcohol and substance use. Because PrEP-RN operates within an STI clinic, we do not provide mental health services but connect patients with referrals and emergency services, as indicated. We also assess if patients had recent potential HIV exposures that warrant PEP, and, if so (and the patient is amenable and has no contraindications), we initiate PEP on site. Prior to patients completing PEP, they complete PrEP assessments, including laboratory work, with the goal of transitioning directly to PrEP after completion of PEP. Finally, we assess how patients will purchase PrEP, including insurance status (private or public), and we work with uninsured patients to qualify for public drug plans. As part of this, we provide the first month and subsequent 3 months of PrEP using stock we purchase, thus allowing immediate initiation and referral for PrEP. Various payment programs are available in different regions, a review of which is beyond the scope of this article.
Our physical examination at the initial visit focuses on identifying signs of genitourinary infection (e.g., discharge, lesions, or pain), rectal infection (anal pain, tenesums, or discharge), or seroconversion (local or generalized rashes, and cervical, axillary, epitrochlear, or inguinal lymphadenopathy). The examination also includes an inspection of the oral mucosa for signs of primary or secondary syphilis.
During the examination, we test for gonorrhea and chlamydia (oral and rectal swabs and urine, all as nucleic acid amplification tests). We also request serology for syphilis, hepatitis A and C antibodies, and hepatitis B surface antigen and surface and core antibodies. We perform HIV testing using fourth-generation serology, and a third-generation point-of-care test. We perform both tests because, in our previous nurse-led PEP program, we identified a small number of persons who were unaware they were already living with HIV at initial assessment (O'Byrne, MacPherson, Roy, & Orser, 2017), which allowed immediate referral to care. Finally, we perform SeCr testing to calculate an eGFR using the Chronic Kidney Disease Epidemiology Collaboration formula (Zhu et al., 2014). We use this equation because it is more accurate than other equations (Gamma-Dynacare Medical Laboratories, 2015; Palacio-Lacambra et al., 2018). Finally, to ensure testing, our PrEP-RN nurses collect specimens on-site, rather than sending patients to laboratories.
At the first visit, we also provide risk reduction counseling tailored to individual need and risk (e.g., condoms, safer drug use equipment, overdose prevention training, and Naloxone kits). Regarding condom use, we emphasize the paucity of evidence for exclusive PrEP use without condoms, and that, in the extant trials, participants reported condom use approximately 75% of the time while on PrEP. We also counsel about PEP for missed FTC/TDF doses. Based on pharmacokinetic data, which suggest that four tablets of FTC/TDF per week corresponds with equal prevention outcomes to consistent daily use (Grant et al., 2014), plus other studies showing TDF's intracellular half-life of 72 hours (Louissant et al., 2013), we recommend that patients consider PEP if they have both of the following: a sexual contact that could warrant PEP according to guidelines (Centers for Disease Control and Prevention, 2018a; Tan et al., 2017) and missing at least two consecutive daily doses of FTC/TDF. At this visit, we also explore if patients have questions or concerns, and we address these points. Counseling is guided by patient need.
Pre-exposure Prophylaxis–Registered Nurse initiation visit
Details about when a patient should start PrEP are missing from extant guidelines. We recommend a visit shortly after the initial assessment to review results and provide PrEP. We complete this visit either in person or by phone after obtaining HIV, hepatitis B, and creatinine test results, which occurs 1–2 weeks after the first visit. At this visit, we review baseline test results with the patient and address any questions or concerns regarding PrEP and sexual risk reduction. Provided the patient is HIV-negative by laboratory testing, has no serologic evidence of acute or chronic hepatitis B infection, and has an eGFR greater than 60 mL/min, we provide a prescription for 1 month of FTC/TDF. See Figure 2 for a pathway nurses can use to review baseline eGFR and creatinine results. If the patient has a negative HIV test result, but symptoms suggesting acute HIV infection, we defer PrEP initiation and repeat HIV testing, scheduling another follow-up to review these results. At this point, we also address vaccination status. If serology reveals the patient is not immune to hepatitis A or B, vaccination is offered. Following the Canadian National Advisory Committee on Immunizations (2018), we also offer vaccination against human papillomavirus to all MSM regardless of age.
Figure 2.
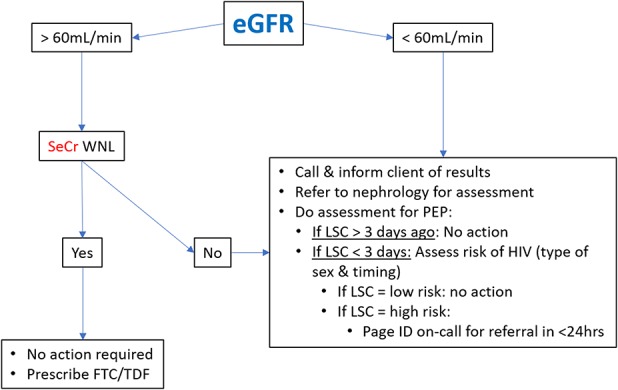
Estimated glomerular filtration rate management pathway for first visit. Note. eGFR = estimated glomerular filtration rate; FTC/TDF = 300 mg emtricitabine (FTC) plus 200 mg tenofovir disoproxil fumarate; ID = infectious diseases; LSC = last sexual contact; PEP = postexposure prophylaxis; SeCr = serum creatinine; WNL = within normal limits.
An alternative to the two-visit PrEP initiation approach is to start PrEP at the first visit and to discontinue PrEP if, based on laboratory results from the first visit, a patient has an eGFR lower than 60 mL/min, a reactive HIV test, or confirmed or possible hepatitis B infection. The benefits to this approach are that it eliminates a second visit and the patient initiates PrEP without delays, when additional HIV exposures and seroconversion could occur. The downsides to immediate initiation are that, if the patient were living with HIV, PrEP is inadequate as therapy and could facilitate the development of antiretroviral drug resistance, although this concern might be limited based on what is often a short turnaround time for HIV results. Performing a point-of-care HIV test could further minimize this risk. Another risk is the possibility of an acute hepatitis flare in persons with hepatitis B infection who use FTC/TDF as PrEP. This strategy is, nevertheless, an option and should be considered for PrEP-RN. The challenge for PrEP-RN would be that nonprescriber nurses, who work under medical directives, would be executing these initiations and discontinuations of PrEP. We, accordingly, opt for the two-visit approach.
1-Month visit
Patients return to clinic for their first follow-up visit after being on PrEP for 1 month. At that time, we answer questions and assess for medication side effects. Although side effects from FTC/TDF are usually relatively minor and often transient when they occur, they can include nausea, bloating, and diarrhea (Gilead Sciences, 2017; Merck Canada, Inc., 2017). Due to the potential for renal proximal tubular toxicity from TDF (Gilead Sciences, 2017), SeCr is repeated to monitor kidney function and is compared with baseline. HIV serology is also repeated. Finally, we review medication adherence, which we assess via self-report. Based on the iPrEx study findings that four FTC/TDF tablets per week corresponded with optimal HIV prevention outcomes (Anderson et al., 2012), we defined “good adherence” as a self-report of at least four tablets of FTC/TDF per week, with no more than 1 consecutive day of missed medication. All PrEP studies have underscored the importance of adherence with sharp reductions in efficacy when individuals do not take FTC/TDF as prescribed (Grant et al., 2014). If the patient wants to continue PrEP, has no or only minor side effects, good medication adherence, and there is no evidence of renal toxicity, we provide a 3-month prescription for FTC/TDF.
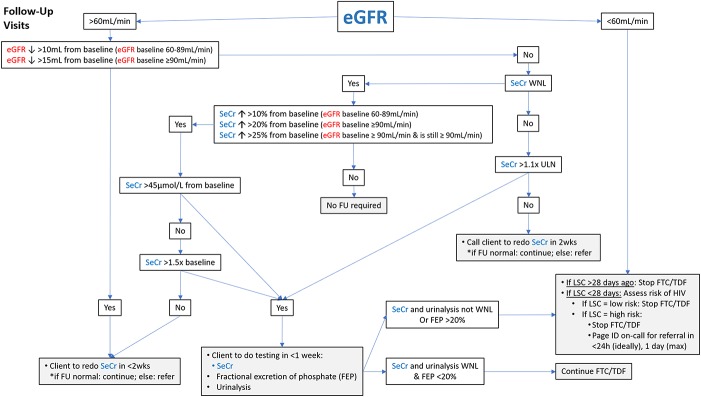
Figure 3 details a pathway nurses can use to interpret follow-up eGFR and SeCr results for patients taking FTC/TDF as PrEP. Appropriate follow-up and referrals are also detailed in this pathway. It is important to note that this pathway uses the Chronic Kidney Disease Epidemiology equation, and the values should not be interpreted using Cockcroft-Gault or Modification of Diet in Renal Disease equations. Doing so could result in needless PrEP discontinuation.
Figure 3.
Estimated glomerular filtration rate management pathway for follow-up visits. Note. eGFR = estimated glomerular filtration rate; FEP = fractional excretion of phosphate; FTC/TDF = 300 mg emtricitabine (FTC) plus 200 mg tenofovir disoproxil fumarate; FU = follow-up; ID = infectious diseases; LSC = last sexual contact; PEP = pre-exposure prophylaxis; SeCr = serum creatinine; ULN = upper limit of normal; WNL = within normal limits.
Subsequent visits every 3 months
The next visit is scheduled 3 months later and every 3 months thereafter. At each of these visits, we review ongoing need for PrEP, medication adherence, and symptoms, and we perform relevant physical examinations and STI testing (for gonorrhea, chlamydia, and syphilis); we also collect blood for HIV antibody and creatinine testing. We inquire if patients have questions or concerns, and address these if present. We provide prescriptions for 3 months of FTC/TDF per visit, without extensions or renewals. We use the same eGFR and SeCr pathway and follow up at these visits. Identified STIs are treated per guidelines (Centers for Disease Control and Prevention, 2015a; Public Health Agency of Canada, 2018), without PrEP interruption.
If, while on PrEP, a patient's HIV test indicates new HIV infection, we recommend urgent consultation with, and transfer of care to, an infectious diseases specialist. Centers for Disease Control and Prevention (2018a) guidelines suggest, in these cases, addition of a third medication, such as dolutegravir 50 mg daily. Other experts suggest FTC/TDF discontinuation and immediate switch to three new antiretroviral agents. In either case, nurses must urgently consult with an infectious diseases physician before proceeding.
Medication Prescription
In alignment with U.S. and Canadian guidelines, we recommend PrEP be taken as one daily tablet of FTC/TDF for continuous use. So-called “on demand” or intermittent FTC/TDF use (taken as two tablets 2–24 hours before sex and one tablet daily for 2 days after) has been studied in one trial, and it was found to be effective (Molina et al., 2015). However, participants in that trial used “on demand” PrEP weekly, taking a median of 15 tablets per month corresponding to the minimum dosing required for efficacy in trials examining continuous daily PrEP (Molina et al., 2015). It is unclear then whether “on demand” PrEP is generalizable to individuals using this approach less than weekly (Centers for Disease Control and Prevention, 2015b; Molina et al., 2015). We, consequently, recommend PrEP-RN provide only daily FTC/TDF without modification pending further data.
To ensure appropriate follow-up, we recommend patients return to clinic every 3 months for assessment (history, examination, investigations) and to receive prescriptions. This aligns with current Canadian and U.S. guidelines (Centers for Disease Control and Prevention, 2018a; Tan et al., 2017). Because, in PrEP-RN, FTC/TDF prescriptions are provided under medical directive, we advise against prescription repeats beyond 3 months and discourage renewals by fax or e-mail for patients who fail to show for appointments.
We provide FTC/TDF prescriptions as follows. After the first visit, a nurse practitioner prepares prescriptions that the nurses give to patients at subsequent visits if specific conditions are met; these are that the patient wishes to continue PrEP, is asymptomatic, has laboratory results from the previous visit that are appropriate for PrEP continuation, and, if already on PrEP, is taking the medication as prescribed without any or only minimal side effects. Practically, we accomplish this by preparing one prescription for 1 month of PrEP and four prescriptions for 3 months each. Our prescribers date and sign these prescriptions at the initiation visit and leave them in the patient's file. The prescriptions are fully completed with patient name, date of birth, and address. A record indicates the number of these prescriptions on file. At follow-up visits, when the nurse assesses and ensures the patient is willing, safe, and able to continue PrEP, she/he gives the patient the prescription, and marks that she/he provided the prescription on a documentation form. For patients who obtain PrEP from our on-site stock, no prescription is required, and the nurse dispenses medication under the initial order.
At subsequent visits, nurses review the foregoing items and, under medical directive, authorize the next cycle of PrEP if their assessments are unremarkable; that is, the nurses give the patient the prepared prescription that is appropriate to the visit. The nurses then document their assessment findings, the testing performed at the follow-up visit, that they authorized further PrEP, that they gave a PrEP prescription, and the counseling provided. The nurses then book the patients' next follow-up visits.
Missed Appointments
Pre-exposure prophylaxis delivery which involves six to seven appointments in the first year and four visits per year in subsequent years (every 3 months), creates a clinical situation with potential for missed appointments. Although prescribers can make extensions and modifications based on clinical judgment, in PrEP-RN, because nurses provide PrEP under medical directive, this decision must be made in advance. One option would be to have nurses consult a prescriber for each case. This would likely be the most tailored and patient-specific choice but would also be most time-consuming and would require a prescriber who is comfortable extending PrEP beyond the follow-up period indicated in current guidelines. This approach may also seem arbitrary, where one patient is extended and another not.
An alternative approach would be to permit an established number of missed visits. In our PrEP-RN clinic, we proceed as such and inform patients at the initial visit that if they miss appointments, we will not renew their PrEP and will refer them to a provider at another clinic for continuation. We also inform patients that this may result in a short discontinuation of PrEP between when our prescription expires and when they reinitiate with another provider. As part of this discussion, at the baseline visit, we have patients initial a clinical consent form listing these points. This form serves as a clinical contract, informing patients of our obligations (to ensure safety, prescribe, remind patients of appointments) and their obligations (to attend appointments). Clinically, we modify such discontinuations based on nurses' clinical judgments and provide more support to patients with greater syndemic factors. As well, to enhance care retention, we contact patients by phone or text message (based on their preference), the work day preceding their appointments, to remind them about the visit. At that point, rescheduling of appointments to a different day is possible and does not constitute a missed visit. After missed visits, we contact the patient up to twice to rebook him/her for a new appointment or to offer a referral to an alternate clinic for PrEP. We consider patients lost to follow-up if they do not respond to our calls after two attempts.
Duration of Clinical Services
Depending on resources and local objectives, PrEP-RN could provide ongoing follow-up and care to patients on PrEP or be used solely to identify and initiate high-risk patients on PrEP. In the latter case, after a short-term follow-up period, patients could be referred to an external provider for continuation of care. We selected the latter option for our PrEP-RN clinic, with services being provided for 6–12 months. This time frame allows for follow-up testing in the event of an STI infection, completion of vaccinations, and engagement in programs to address syndemic conditions. When transferring to primary care, we do so with specific instructions on how to prescribe and monitor PrEP. To increase capacity in primary care providers, we send consult notes to the providers, detailing our initiation, monitoring steps, parameters, and how the clinician can provide this care. We also provide contact details for these providers to speak with our nurse practitioners.
The Role of the Registered Nurse
In PrEP-RN, nurses counsel patients about PrEP (use, risks, benefits, and side effects), provide risk reduction services and vaccinations, and perform STI/HIV testing and relevant examinations. As is standard practice at our clinics, nurses review laboratory results and contact patients with positive STI/HIV results to ensure treatment and referral to care, as indicated. Following the pathways in Figures 2 and 3, nurses review the eGFR and SeCr results and flag abnormal results or significant changes for review by a nurse practitioner. Additionally, as detailed above, for PrEP-RN, we created a medical directive for FTC/TDF provision. As part of this, nurses in our PrEP-RN clinic execute this order when they encounter patients who fulfill the clinical indications for, and have no contraindications to, FTC/TDF. This increases access, enabling more patients to obtain PrEP and, given the lower per provider cost, does so in a more cost-effective way.
Research in Support of Pre-exposure Prophylaxis–Registered Nurse
Two of our projects support the idea that PrEP-RN is a feasible way to deliver PrEP and concurrently establish STI diagnosis and management.
First, in 2014, we piloted a nurse-led PEP program in two STI/HIV testing clinics in Ottawa, Canada (O'Byrne, MacPherson, Roy, & Kitson, 2015). As part of this, patients who presented to either clinic requesting PEP were immediately assessed by a nurse to determine if they had engaged in practices likely to transmit HIV with partners known to be, or at-risk for being, HIV positive. For patients where PEP was indicated, nurses provided STI/HIV testing, behavioral and medication counseling, and a starter pack of PEP medications consisting of FTC/TDF, one tablet once daily, and Raltegravir 400 mg twice daily. The nurses then referred patients to an HIV specialist for further assessment and continuation of care. Since inception, this program has seen more than 300 patients for PEP. An important finding was that approximately 9% of persons who used the program were subsequently diagnosed with HIV within 12 months of completing PEP (O'Byrne et al., 2017). These diagnoses (all MSM) were not PEP failures, but new exposures for which PEP was not sought (O'Byrne et al., 2018), underscoring the need for linkage to care and transition to PrEP for individuals at risk. The findings also highlighted that, when made readily available, HIV prevention services, such as PEP and PrEP, can link persons at-risk for HIV to appropriate care.
In addition, preliminary data from our first local PrEP clinic (started in 2015) provides empirical evidence for PrEP as a public health STI prevention strategy. Of the 108 patients followed in the clinic, 85% have had routine syphilis screening, and, among those who had this testing, 87% did so every 3 months. By including syphilis testing as part of PrEP care, nine new syphilis cases were identified at an early stage, allowing immediate treatment and prevention of onward transmission. Although PrEP is often positioned exclusively for HIV prevention, we feel it could also contribute to syphilis and other STI management. Routine testing every 3 months ensures a short period between STI acquisition and diagnosis, thus limiting onward transmission. In addition, regular STI screening as part of PrEP care ensures patients complete follow-up for gonorrhea and chlamydia tests of cure and syphilis posttreatment serology to ensure treatment success. Currently, in our city, one in three persons diagnosed with syphilis and not on PrEP do not complete follow-up testing. By linking an individual with a new syphilis diagnosis to enhanced HIV prevention with PrEP, and, in turn, by using PrEP care to ensure syphilis treatment and follow-up, synergistic syphilis and HIV prevention outcomes might be expected.
Conclusion
Pre-exposure prophylaxis is an effective HIV prevention tool with both clinical trial and observational evidence of efficacy. However, cost and access continue to be barriers limiting its use by those at risk. We feel that nurses could enhance access and have detailed such a program called PrEP-RN. Building on our experience with a nurse-led PEP project, we feel this service can be safe, effective, and appropriate and can ideally target individuals most at risk of HIV infection. We also feel that by situating PrEP within STI clinics, patients may access combination prevention strategies, which could yield beneficial HIV prevention outcomes and decrease the likelihood of onward STI transmission. We have established one such clinic, which we hypothesize will match our nurse-led PEP clinic and yield safe, efficient care with correspondingly high levels of patient satisfaction. We have detailed the protocols we developed so that others can modify and transfer these procedures into clinical settings to make PrEP better available and, ultimately, decrease ongoing HIV transmission.
Disclosures
The authors report no real or perceived vested interests related to this article that could be construed as a conflict of interest.
Key Considerations
HIV PrEP is an important HIV prevention strategy.
PrEP can prevent HIV acquisition by more than 96% when taken as prescribed, with condom use and appropriate clinical monitoring.
One barrier to PrEP access is the limited number of health care professionals who provide it.
With appropriate development of directives and clinical pathways, nurses can deliver PrEP, and thereby increase access to this effective intervention.
Acknowledgment
The authors wish to thank the Ontario HIV Treatment Network for funding for this project (Grant # ID 1089, PI: P. O'Byrne).
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- Aghaizu A., Tosswill J., De Angelis D., Ward H., Hughes G., Murphy G., Delpech V. (2018). HIV incidence among sexual health clinic attendees in England: First estimates for black African heterosexuals using a biomarker, 2009–2013. PLoS One, 13(6), 1-14. 10.1371/journal.pone.0197939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P. L., Glidden D. V., Liu A., Buchbinder S., Lama J. R., Guarnira J. V., Grant R. M. (2012). Emtricitabine-tenofovir exposure and pre-exposure prophylaxis efficacy in men who have sex with men. Science Translational Medicine, 4(151),151ra125. 10.1126/scitranslmed.3004006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. E., Mohammed H., Ogaz D., Kirwan P. D., Yung M., Nash S. G., Gill O. N. (2017). Fall in new HIV diagnoses among men who have sex with men (MSM) at selected London sexual health clinics since early 2015: Testing or treatment or pre-exposure prophylaxis (PrEP)? Eye Science, 22(25), 30553. 10.2807/1560-7917.ES.2017.22.25.30553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Institutes of Health Information. (2017). Regulated Nurses, 2017. Retrieved from https://www.cihi.ca/en/regulated-nurses-2017-infographic
- Canadian National Advisory Committee on Immunization. (2018). Canadian immunization guide: Part 4 – active vaccines. Human papillomavirus. Retrieved from https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-9-human-papillomavirus-vaccine.html
- Centers for Disease Control and Prevention. (2015b). CDC statement on IPERGAY trial of pre-exposure prophylaxis (PrEP) for HIV prevention among men who have sex with men. Retrieved from https://www.canada.ca/en/public-health/services/infectious-diseases/sexual-health-sexually-transmitted-infections/canadian-guidelines/sexually-transmitted-infections.html#toc
- Centers for Disease Control and Prevention. (2016). Diagnoses of HIV infection in the United States and dependent areas, 2016. Retrieved from https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2016-vol-28.pdf
- Centers for Disease Control and Prevention. (2018c). HIV among African Americans. Retrieved from https://www.cdc.gov/hiv/group/racialethnic/africanamericans/index.html
- Centers for Disease Control and Prevention. (2018b). HIV and transgender people. Retrieved from https://www.cdc.gov/hiv/pdf/group/gender/transgender/cdc-hiv-transgender-factsheet.pdf
- Centers for Disease Control and Prevention. (2018a). Preexposure Prophylaxis for the prevention of HIV infection in the United States—2017 Updated. Retrieved from https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf
- Centers for Disease Control and Prevention. (2015a). Sexually transmitted diseases treatment guidelines, 2015. Retrieved from https://www.cdc.gov/mmwr/index.html
- Coleman T. A., Bauer G. R., Pugh D., Aykroyd G., Powell L., Newman R. (2017). Sexual orientation disclosure in primary care settings by gay, bisexual, and other men who have sex with men in a Canadian city. Laboratory Hematology, 4(1), 42-54. 10.1089/lgbt.2016.0004 [DOI] [PubMed] [Google Scholar]
- European AIDS Clinical Society. (2017). Guidelines. Retrieved from http://www.eacsociety.org/files/guidelines_8.2-english.pdf
- Gamma-Dynacare Medical Laboratories. (2015). Evaluation of chronic kidney disease: Changes to eGFR and albuminuria reporting. Retrieved from https://www.dynacare.ca/DYN/media/DYN/eng/Notices-Services/2015/CKD_EPI_10-03-2015.pdf
- Gilead Sciences. (2017). Product monograph: Truvada (emtricitabine/tenofovir disoproxil fumarate) tablets (200 mg/300 mg) antiretroviral agent. Retrieved from http://www.gilead.ca/application/files/9814/9797/8029/truvada_pm_english.pdf
- Grant R. M., Anderson P. L., McMahan V., Liu A., Amico K. R., Mehrotra M., Gidden D. V. (2014). Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: A cohort study. The Lancet Infectious Diseases, 14(9), 820-829. 10.1016/S1473-3099(14)70847-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant R. M., Lama J. R., Anderson P. L., McMahan V., Liu A. Y., Vargas L., Glidden D. V. (2010). Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. New England Journal of Medicine, 363(27), 2587-2599. 10.1056/NEJMoa101120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad N., Li J. S., Totten S., McGuire S. (2018). HIV in Canada: Surveillance report, 2017. Retrieved from https://www.canada.ca/content/dam/phac-aspc/documents/services/reports-publications/canada-communicable-disease-report-ccdr/monthly-issue/2018-44/issue-12-december-6-2018/ccdrv44i12a03-eng.pdf [DOI] [PubMed]
- Hannaford A., Lipshie-Williams M., Starrels J. L., Arnsten J. H., Rizzuto J., Cohen P., Patel V. V. (2018). The use of online posts to identify barriers to and facilitators of HIV pre-exposure prophylaxis (PrEP) among men who have sex with men: A comparison to a systematic review of the peer-reviewed literature. AIDS & Behaviour, 22(4), 1080-1095. 10.1007/s10461-017-2011-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull M., Edward J., Kelley L. (2017). PEP/PrEP update—ABC case study. Retrieved from: https://www.catie.ca/sites/default/files/pep-prep-bc-casestudy-03272017.pdf
- Johnsen L. E., Thimm M. A., Singer J. M., Page K. R. (2017). Awareness and interest in pre-exposure prophylaxis (PrEP) among patients receiving services at a public sexually transmitted diseases (STD) clinic in a high prevalence urban setting. Sexually Transmitted Infections, 93(Suppl 2), A79. 10.1136/sextrans-2017-053264.200 [DOI] [Google Scholar]
- Liu A., Cohen S., Follansbee S., Cohan D., Weber S., Sachdev D., Buchbinder S. (2014). Early experiences implementing pre-exposure prophylaxis (PrEP) for HIV prevention in San Francisco. PLoS Medicine, 11(3), e1001613. 10.1371/journal.pmed.1001613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louissant N. A., Cao Y. J., Skipper P. L., Liberman R. G., Tannenbaum S. R., Nimmagadda S., Hendrix C. W. (2013). Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Research and Human Retroviruses, 29(11), 1443-1450. 10.1089/aid.2013.0044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack S., Dunn D., Desai M., Dolling D., Gafos M., Gilson R., Owen N. (2015). Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): Effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet, 387(10013), 53-60. 10.1016/S0140-6736(15)00056-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merck Canada, Inc. (2017). Product monograph: Isentress (Raltegravir tablets 400 mg). Retrieved from https://pdf.hres.ca/dpd_pm/00039853.PDF
- Molina J. M., Capitant C., Spire B., Pialoux G., Cotte L., Charreau I., Delfraissy J. F. (2015). On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. New England Journal of Medicine, 373, 2237-2246. 10.1056/NEJMoa1506273 [DOI] [PubMed] [Google Scholar]
- Mosely T., Khaketla M., Armstrong H. L., Cui Z., Sereda P., Lachowsky N. L., Moore D. A. (2018). Trends in awareness and use of HIV PrEP among gay, bisexual, and other men who have sex with men in Vancouver, Canada, 2012-2016. AIDS and Behavior, 22(11), 3550-3565. 10.1007/s10461-018-2026-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson L. E., James L., Coleman T., Etowa J., Husbands W., Lofters A., Wilson C. L. (2019). A recipe for increasing racial and gender disparities in HIV infection: A critical analysis of the Canadian guideline on pre-exposure prophylaxis and non-occupational post-exposure prophylaxis' responsiveness to the HIV epidemics among women and Black communities. Canadian Journal of Human Sexuality, 27(3), 195-206. 10.3138/cjhs.2018-0043. [DOI] [Google Scholar]
- New York City Department of Health. (2018). Health department launches city's first-ever campaign promoting HIV prevention medication among women. Retrieved from https://www1.nyc.gov/site/doh/about/press/pr2018/pr017-18.page
- Ojile N., Sweet D., Kallail K. J. (2017). A preliminary study of the attitudes of and barriers of family physicians to prescribing HIV preexposure prophylaxis. The Keio Journal of Medicine, 10(2), 40-42. [PMC free article] [PubMed] [Google Scholar]
- Orser L., O'Byrne P. (2019). The role of public health units in the delivery of HIV pre-exposure prophylaxis. Canadian Journal of Public Health, 110(1), 1-4. 10.17269/s41997-018-0141-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Byrne P., MacPherson P. A., Orser L. (2018). Nurse-led PEP program used by men at high risk for HIV seroconversion. The Journal of the Association of Nurses in AIDS Care, 29(4), 550-559. 10.1016/j.jana.2018.02.004 [DOI] [PubMed] [Google Scholar]
- O'Byrne P., MacPherson P. A., Roy M., Kitson C. (2015). Overviewing a nurse-led, community-based HIV PEP program: applying the extant literature in frontline practice. Public Health Nursing, 32(3), 256-265. 10.1111/phn.12123 [DOI] [PubMed] [Google Scholar]
- O'Byrne P., MacPherson P. A., Roy M., Orser L. (2017). Community-based, nurse-led post-exposure prophylaxis: results and implications. International Journal of STD & AIDS, 28(5), 505-511. 10.1177/09564624166584512 [DOI] [PubMed] [Google Scholar]
- Palacio-Lacambra M. E., Comas-Reixach I., Blanco-Grau A., Sune-Negre J. M., Segarra-Medrano A., Montoro-Rosano J. B. (2018). Comparison of Cockgault-Croft, MDRD, and CKD-EPI equations for estimating ganciclovir clearance. British Journal of Clinical Pharmacology, 84(9), 2120-2128. 10.1111/bcp.13647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathela P., Braunstein S. L., Blank S., Shepard C., Schillinger J. A. (2015). The high risk of an HIV diagnosis following a diagnosis of syphilis: A population-level analysis of New York City men. Clinical Infectious Diseases, 61(2), 281-287. 10.1093/cid/civ289 [DOI] [PubMed] [Google Scholar]
- Petroll A. E., Walsh J. L., Owczarzak J. L., McAuliffe T. L., Bogart L. M., Kelly J. A. (2017). PrEP awareness, familiarity, comfort, and prescribing experience among US primary care providers and HIV specialists. AIDS and Behavior, 21(5), 1256-1267. 10.1007/s10461-016-1625-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health Agency of Canada. (2018). Canadian guidelines on sexually transmitted infections. Retrieved from https://www.canada.ca/en/public-health/services/infectious-diseases/sexual-health-sexually-transmitted-infections/canadian-guidelines/sexually-transmitted-infections.html#toc
- San Francisco Department of Health. (2016). HIV Epidemiology annual report—2016. Retrieved from https://www.sfdph.org/dph/files/reports/RptsHIVAIDS/Annual-Report-2016-20170831.pdf
- Scholl E. (2016). Improving outpatient implementation of preexposure prophylaxis in men who have sex with men. Journal of the American Association of Nurse Practitioners, 28(8), 446-452. 10.1002/2327-6924.12344 [DOI] [PubMed] [Google Scholar]
- Sharma M., Chris A., Chan A., Knox D. C., Wilton J., McEwan O., Tan D. H. S. (2018). Decentralizing the delivery of HIV pre-exposure (PrEP) through family physicians and sexual health clinic nurses: A dissemination and implementation study protocol. BMC Health Services, 15, 513. 10.1186/s121913-018-3324-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silapaswan A., Krakower D., Mayer K. H. (2017). Pre-exposure prophylaxis: A narrative review of provider behavior and interventions to increase PrEP Implementation in primary care. Journal of General Internal Medicine, 32(2), 192-198. 10.1007/s11606-016-3899-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S., Stephenson R. (2018). Perceived HIV prevalence accuracy and sexual risk behavior among gay, bisexual, and other men who have sex with men in the United States. AIDS and Behavior, 22(6), 1849-1857. 10.1007/s10461-017-1789-3 [DOI] [PubMed] [Google Scholar]
- Tan D. H. S., Hull M. W., Yoong D., Tremblay C., O'Byrne P., Thomas R., Shafran S. (2017). Canadian guidelines on HIV pre-exposure prophylaxis and non-occupational post-exposure prophylaxis. Canadian Medical Association Journal, 189(47), 1448-1458. 10.1503/cmaj.170494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner L., Roepke A., Wardell E., Teitleman A. M. (2018). Do you PrEP? A review of primary care provider knowledge of PrEP and attitudes on prescribing PrEP. Journal of the American Association of Nurses in AIDS Care, 29(1), 83-92. 10.1016/j.jana.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton J., Noor S. W., Schnubb A., Lawless J., Hart T. A., Grennan T., Tan D. H. S. (2018). High HIV risk and syndemic burden regardless of referral source among MSM screening for a PrEP demonstration project in Toronto, Canada. BMC Public Health, 18, 292. 10.1186/s12889-018-5180-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood B. R., McMahan V. M., Naismith K., Stockton J. B., Delaney L. A., Stekler J. D. (2018). Knowledge, practices, and barriers to HIV preexposure prophylaxis prescribing among Washington State medical providers. Sexually Transmitted Diseases, 45(7), 452-458. 10.1097/QLQ/0000000000000781 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2015). Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Retrieved from http://apps.who.int/iris/bitstream/handle/10665/186275/9789241509565_eng.pdf?sequence=1 [PubMed]
- Zablotska I. B., Selvey C., Guy R., Prince K., Holden J., Schmidt H. M., Grulich A. E. (2018). Expanded HIV pre-exposure prophylaxis (PrEP) implementation in communities in New South Wales, Australia (EPIC-NSW): Design of an open-label, single arm implementation trial. BMC Public Health, 18, 210. 10.1186/s12889-017-5018-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Ye X., Zhu B., Pei X., Wei L., Wu J., Zhao W. (2014). Comparisons between the 2012 new CKD-Epi (chronic kidney disease epidemiology collaboration) equations and other four approved equations. PLOS One, 9(1), e84688. 10.1371/journal.pone.0084688 [DOI] [PMC free article] [PubMed] [Google Scholar]