Abstract
Objectives:
Sleep disturbance and chronic pain are related. The present study evaluated both direct and indirect (mediated) pathways through which sleep disturbance might be related to chronic pain intensity and function.
Methods:
Eighty-seven individuals (64% female) with chronic low back pain but not using opioids daily completed questionnaires assessing their sleep disturbance, chronic pain intensity, function, depression, anxiety, positive affect, and catastrophizing.
Results:
Greater sleep disturbance was associated with greater pain intensity, worse function, greater emotional distress, lower positive affect and higher levels of catastrophizing. Cross-sectional mediation analyses revealed that the positive associations between sleep disturbance and chronic pain intensity were conveyed statistically not only via significant indirect effects of elevated emotional distress, lower positive affect, and greater catastrophizing associated with sleep disturbance, but also by significant direct effects of sleep disturbance on chronic pain intensity. Similarly, we found that the associations between sleep disturbance and impaired function were conveyed statistically not only via significant indirect effects of elevated chronic pain intensity associated with sleep disturbance, but also via significant direct effects of sleep disturbance on function.
Discussion:
Sleep disturbance was related significantly with chronic pain intensity and function via both direct and indirect pathways. These results are consistent with an emerging literature highlighting the potential significance of sleep disturbance in chronic pain patients, and provide further support for addressing sleep disturbance in the assessment and management of chronic pain.
Keywords: chronic pain, function, pain, sleep, mediation
Introduction
Chronic pain is often associated with poor quality of life, negative affect, and functional impairment. For example, in a recent sample of chronic low back pain patients recruited from primary care clinics (n=423), 22% reported clinically relevant depressive symptoms and 54% reported significant functional impairment [1]. Many chronic pain patients also report sleep disturbance. In samples of chronic low back pain patients recruited from outpatient clinics, 44–53% reported clinically significant insomnia symptoms (Insomnia Severity Index score ≥ 15) [2, 3].
It has been proposed that sleep disturbance and pain may have a reciprocal relationship [4], such that poor sleep during the night may worsen pain later in the day and vice versa. A comprehensive review on the association between sleep and pain, however, suggests that sleep disturbance at Time 1 is a stronger and more reliable predictor of pain at Time 2 than the reverse [4]. The former direction of causality raises question about whether sleep disturbance exerts mostly direct effects on chronic pain intensity or whether sleep disturbance acts on pain indirectly through its influence on pain-related psychosocial factors (e.g., depression).
On one level there are results emphasizing direct effects. Findings showed in a heterogeneous sample of chronic pain patients (~50% with chronic low back pain) that sleep problems were independently associated with chronic pain intensity, even after accounting for levels of depression and catastrophizing [5]. Similarly, an earlier study also in a heterogeneous sample of chronic pain patients (~50% with chronic low back pain) reported that sleep disturbance predicted disability independent of pain and depression [6]. These findings suggest that sleep disturbance may indeed exert direct effects on chronic pain intensity and functional impairment independent of sleep-related influences on psychosocial status.
Other studies are consistent with the possibility that various aspects of psychosocial functioning might act as mediators through which sleep disturbance is linked indirectly to chronic pain intensity. That is, sleep disturbance may worsen pain by increasing negative or reducing positive emotions, which then in turn impacts pain intensity. For example, results show that depressive and anxious symptoms are positively associated with both chronic pain intensity and sleep disturbance in chronic low back pain patients [3]. Other findings reveal that sleep disruption in healthy subjects (forced awakenings or partial sleep restriction) reduces positive affect and, in parallel, increases generalized body pain and back pain reports [7, 8]. We have also previously reported that pain catastrophizing in chronic low back pain patients was positively associated not only with chronic pain intensity, but also with poorer sleep quality [9]. Patterns of intercorrelations like these support psychosocial factors as potential mediators of links between sleep and chronic pain intensity. Findings from previous studies employing mediation analyses also suggest indirect effects. One study of a heterogeneous sample of chronic pain patients (~19% chronic low back pain) found that depression and anxiety mediated the relationship between sleep quality and pain intensity, with minimal direct effects of sleep quality on pain intensity [10]. Further, two prior studies, again in heterogeneous samples of chronic pain patients, found, using a hierarchical multiple regression approach, that sleep duration and/or sleep quality no longer significantly predicted function or disability once the effects of chronic pain intensity and depression/catastrophizing were statistically controlled; findings consistent with the presence of indirect (mediated) effects [5][11]. To our knowledge, previous studies have not used state-of-the-art bootstrapped mediation analysis to simultaneously parse out the influence of direct versus indirect effects of sleep disturbance on function in chronic pain patients. In the current work, we hypothesized that sleep disturbance may act in part directly on chronic pain and functional outcomes, but that sleep disturbance may also do so indirectly by elevating negative affect and pain catastrophizing, and reducing positive affect (for pain intensity) and by increasing pain intensity (for function).
The aim of the current study was to use a bootstrapped mediation analysis approach to systematically examine both direct and indirect pathways by which sleep disturbance may affect chronic pain intensity and functional status in a more homogenous sample than in past work (people with chronic low back pain) and in individuals not using daily opioids (a factor that might confound analyses). The goals were to (1) replicate previous findings where psychosocial distress mediates the effect of sleep disturbance on pain intensity, and (2) examine the less studied question of whether chronic pain intensity mediates the effect of sleep disturbance on functional impairment. We first examined zero-order correlations among sleep disturbance, chronic pain intensity, psychosocial factors (depression, anxiety, positive affect, pain catastrophizing), and functional status. Then, we evaluated the degree to which the total effects of sleep disturbance on chronic pain intensity were comprised of direct effects and/or indirect effects of sleep disturbance via its association with psychosocial factors (see Figure 1). Finally, we evaluated the degree to which the total effects of sleep disturbance on functional impairment reflected direct effects versus indirect effects of sleep disturbance via its associations with chronic pain intensity (see Figure 2).
Figure 1.
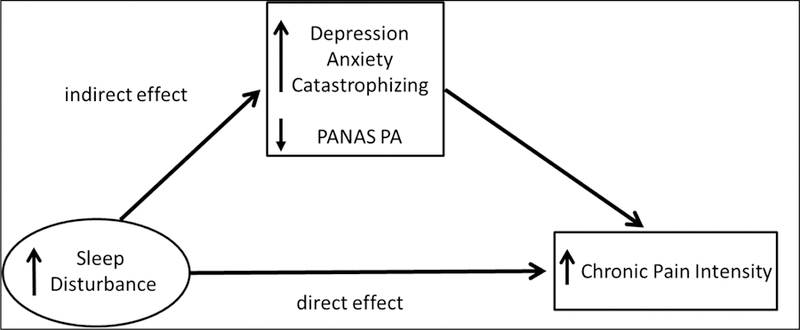
Conceptual mediation model in which the effects of sleep disturbance on chronic pain intensity are mediated by psychosocial factors.
Figure 2.
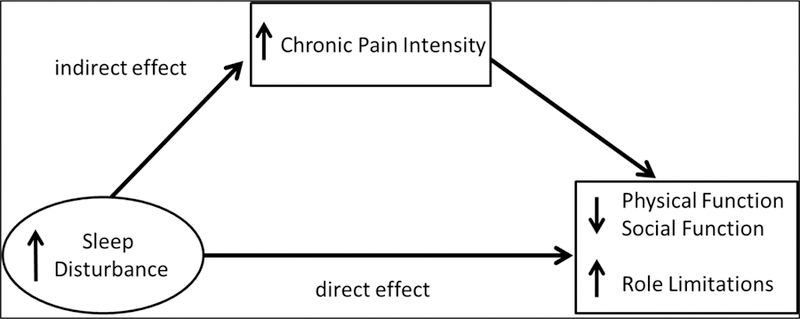
Conceptual mediation model in which the effects of sleep disturbance on functional outcomes are mediated by chronic pain intensity.
Materials and Methods
Design
The primary study used a three session, double-blind, crossover design with administration of an opioid antagonist (naloxone), an opioid analgesic (morphine), or saline placebo in each session. Results reported below were cross-sectional, reflecting measures obtained at the initial baseline screening visit (prior to any drug administration or laboratory procedures). Identical data collection procedures were employed in a closely coordinated fashion at two sites (Vanderbilt University Medical Center and Rush University Medical Center).
Participants
Participants were 87 individuals with chronic low back pain who were recruited through on-line advertisements on the Vanderbilt University e-mail recruitment system, the Rush University Pain Clinic, advertisements in local print media, or posted flyers. Inclusion criteria were: 1) age between 18–55 years; 2) no self-reported history of cardiovascular disease, hypertension, liver, kidney disorders; diabetes, or seizure disorder; 3) no self-reported history of posttraumatic stress disorder, bipolar disorder, psychotic disorder, alcohol or drug dependence; 4) no use of anti-hypertensive medications; 5) no daily use of opioid analgesics; 6) chronic daily low back pain of at least 3 months duration, and 7) an average past month severity of at least 3 on a 0–10 verbal numeric pain intensity scale. Individuals with chronic pain related to malignancy, autoimmune disorders, or fibromyalgia were excluded. Potential subjects who were pregnant (determined by urine pregnancy screens) were excluded to avoid fetal exposure to naloxone and morphine administered as part of the primary study. Thirteen subjects reported occasional use of as-needed opioid analgesics, but none used any opioids in the 3 days preceding the study procedures described below (confirmed via urine opiate screen). Three subjects reported use of antidepressant medications. Subjects were compensated $375 for their time upon completion of all study procedures. See Table 1 for sample characteristics.
Table 1.
Sample Characteristics (N = 87)
| Characteristic | Mean ± SD or % |
|---|---|
| Gender (% female) | 64.4 |
| Race: | |
| Caucasian (%) | 60.9 |
| African-American (%) | 32.2 |
| Ethnicity: | |
| Non-Hispanic (%) | 96.5 |
| Age (years) | 40.0 ± 11.49 |
| Neuropathic Signs Present (%) | 54.0 |
| Pain Duration (months) | 116.5 ± 104.33 |
| PROMIS Sleep Disturbance (T score) | 54.0 ± 9.14 (38–77) |
| Chronic Pain Intensity Measures: | |
| MPQ-Sensory | 12.9 ± 6.81 (3–32) |
| MPQ-Affective | 2.9 ± 3.22 (0–12) |
| VAS Pain Intensity | 55.1 ± 22.8 (12–100) |
| Psychosocial Measures: | |
| BDI | 7.0 ± 8.69 (0–37) |
| STAI | 37.1 ± 12.16 (20–70) |
| PANAS-PA | 33.0 ± 8.39 (10–48) |
| CATS | 15.8 ± 12.74 (0–48) |
| Functional Measures: | |
| Rand 36 – Physical Functioning | 68.7 ± 26.80 (11–100) |
| Rand 36 – Social Functioning | 75.6 ± 26.20 (0–100) |
| Rand 36 – Physical Role Limitations | 54.9 ± 40.32 (0–100) |
| Rand 36 – Emotional Role Limitations | 74.3 ± 39.93 (0–100) |
Note: For sleep, pain, psychosocial, and functional measures, observed ranges are provided parenthetically after the Mean ± SD values. MPQ = McGill Pain Questionnaire–Short Form (Past Month), VAS = Visual Analog Scale, BDI = Beck Depression Inventory, STAI = State Trait Anxiety Inventory, PANAS-PA = Positive Affect Subscale of the Positive and Negative Affect Schedule, CATS = Pain Catastrophizing Scale
Measures
Sleep Disturbance
Sleep disturbance was assessed using the PROMIS Sleep Disturbance – Short Form 8a [12], an 8 item instrument assessing sleep quality, sleep depth and restoration associated with sleep. A score of 50 represents the average score in the normative sample, with higher scores indicating greater sleep disturbance. The PROMIS Sleep Disturbance measure has been shown to have strong psychometric properties, and demonstrates better measurement precision than other commonly used self-report sleep measures [12].
Chronic Pain Intensity Measures
Chronic pain intensity was assessed using the McGill Pain Questionnaire-Short Form (MPQ) [13]. Instructions were modified to refer specifically to the low back pain experienced “over the past month.” The MPQ is a well-validated measure that allows separate assessment of the sensory (MPQ-Sensory) and affective (MPQ-Affective) qualities of pain [13]. The MPQ also includes a visual analog pain intensity scale (VAS), anchored with “no pain” and “worst possible pain”, which was used to assess overall chronic pain intensity in the past month.
Psychosocial Measures
The Beck Depression Inventory (BDI) [14] was used to assess recent depressive symptoms. Higher scores on the BDI indicate greater depressive symptoms, with scores of 10 or more indicating at least mild depressive symptoms. The trait form of the State-Trait Anxiety Inventory (STAI) [15] was used to measure the tendency to experience anxiety symptoms. Higher scores on the STAI indicate greater tendency to experience anxiety symptoms. Both the BDI and STAI are commonly used self-report measures which have well-established psychometric properties.
To provide a complementary measure of affect, the Positive and Negative Affect Schedule (PANAS) [16] was used. The PANAS contains brief negative affect (NA) and positive affect (PA) subscales, each consisting of 10 items. Higher scores indicate greater global negative and positive affect, respectively. Both PANAS subscales have shown good psychometric characteristics [16]. For the current study, only the PANAS-PA scale was used, given the high degree of overlap between the PANAS-NA subscale and the BDI and STAI.
Pain catastrophizing was assessed using the Pain Catastrophizing Scale (PCS) [17]. The PCS is a 13-item measure which assesses the degree to which individuals have negative thoughts and feelings when experiencing pain. The PCS has been shown to have strong psychometric characteristics [17]. Higher scores indicate greater pain catastrophizing.
Functional Measures
To assess functional status, we administered four functional subscales of the Rand-36 Health Survey [18]. The Rand-36 consists of the same 36 items included in the SF-36 questionnaire frequently employed in medical outcomes research [19]. The Rand-36, however, employs a simpler unweighted scoring system [18]. Subscales administered were: Physical Functioning, Social Functioning, Role Limitations due to Physical Health, and Role Limitations due to Emotional Problems. Subscale scores range from 0–100, with lower scores on each of these subscales indicating greater functional impairment.
Procedure
All procedures were conducted at the Vanderbilt General Clinical Research Center or a dedicated research room at the Rush University Pain Center. All procedures were approved by the Institutional Review Boards at the respective institutions. After providing informed consent, subjects completed a packet of questionnaires, including information regarding demographics, as well as the chronic pain, psychosocial status, and functional measures described above.
Statistical Analysis
All analyses were conducted using IBM SPSS for Windows Version 24 (SPSS Inc., Chicago, IL). First, Pearson zero-order correlations were generated to examine relationships among the targeted variables. Next, evaluation of hypothesized statistical mediation models was carried out. Primary analyses were conducted in two sets, reflecting different hypothetical causal paths. The first (see conceptual model in Figure 1) tested models in which the influence of sleep disturbance on chronic pain intensity was due to indirect effects of each of the psychosocial variables. The second (see conceptual model in Figure 2) tested models in which the influence of sleep disturbance on functional measures was due to indirect effects of each of the chronic pain intensity measures. As described by Preacher and Hayes [20], two criteria were necessary to demonstrate mediation. Using the sleep disturbance and chronic pain intensity association as an example, to support mediation there needed to be: 1) a significant association between PROMIS Sleep Disturbance scores and chronic pain intensity measures (i.e., there was an effect to be mediated), and 2) a significant indirect effect of PROMIS Sleep Disturbance scores on chronic pain intensity via the psychosocial measure tested. Where criterion 1 was met, the approach of Preacher and Hayes [20] was used to test the significance of the indirect effect. To provide a conservative test for significance of direct effects of sleep disturbance on pain intensity and functional outcomes independent of all mediators examined, secondary analyses tested two multiple mediation models. The first was a model in which the effects of sleep disturbance on chronic pain intensity were mediated simultaneously by BDI, STAI, PANAS-PA, and CATS. The second was a model in which the effects of sleep disturbance on functional measures were mediated simultaneously by MPQ-Sensory, MPQ-Affective, and VAS intensity measures. Given the cross-sectional nature of the data, results of mediation analyses reflected statistical mediation only, and were unable to evaluate temporal precedence. Phrases used below describing effects “conveyed via” or “through” mediators are used to maximize readability, but should not be interpreted as indicating causality.
Direct and indirect effects were examined for all models above using bootstrapped mediation analysis [20] via custom SPSS dialogue (the PROCESS Procedure; http://www.processmacro.org/index.html). Bootstrapping is a nonparametric re-sampling procedure that does not assume normality for the indirect effect, or a*b path coefficient. This nonparametric feature differs from the ‘causal steps’ approach to indirect effects (mediation) testing [21]. Bootstrapping involves random and repeated sub-sampling of the data to derive an estimate of the a*b (indirect effect) path coefficient and a 95% confidence interval around the bootstrapped indirect effect parameter. If the 95% confidence interval for the indirect effect generated by the model does not include zero, then the hypothesized indirect (mediation) effect is significant at the p<0.05 level. Mediation analyses specified Model 4 and k = 10,000 bootstraps. Percentile bootstrapping was used to derive path coefficients and corresponding 95% confidence intervals for indirect effects. Based on empirically-derived guidelines provided by Fritz and MacKinnon [22], the study sample size (n=87) was sufficient to detect indirect (mediated) effects with greater than 0.80 power, assuming at least a moderate effects size for the association between sleep and the targeted mediator and the association between the mediator and the pain or functional outcome. Such effect sizes would likely be required for these mediated effects to be clinically meaningful.
Results
Preliminary Analyses
Table 1 indicates that the sample was comprised of individuals who had experienced chronic low back pain for an average of almost 10 years, and who on average reported moderate pain intensity on the VAS measure. The mean level of sleep disturbance in the sample was comparable to the mean observed in the PROMIS normative sample (i.e., T=50), although sleep disturbance levels were elevated at least one standard deviation above this mean in 30.3% of study participants (i.e., T score ≥ 60). Participants did not, on average, report a high level of functional impairment (Rand-36) or psychosocial distress (BDI, STAI), consistent with the nature of the sample (i.e., not using opioid analgesics regularly, the majority not being treated at a tertiary care chronic pain clinic).
Zero-Order Correlations
Zero-order correlations between sleep disturbance and chronic pain intensity measures, and measures of psychosocial status and function, are summarized in Table 2. Higher levels of sleep disturbance on the PROMIS measure were correlated positively with all three measures of chronic back pain intensity (medium to large effect sizes). Higher sleep disturbance scores were also associated with greater depression (BDI), anxiety (STAI), and catastrophizing (CATS), lower global positive affect (PANAS-PA), and worse functioning on all four Rand-36 subscales examined. Hypothesized mediators in both proposed models were associated as expected with targeted outcomes. Elevated depression (BDI), anxiety (STAI), and catastrophizing (CATS), and lower positive affect (PANAS-PA), were all significantly correlated in the expected direction with chronic pain intensity outcomes (MPQ-Sensory, MPQ-Affective, VAS intensity). Greater chronic pain intensity on all three of these measures was also significantly associated with worse function on all four Rand-36 subscales.
Table 2.
Zero-order correlations
| Measure | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. PROMIS Sleep | 0.44 | 0.39 | 0.51 | 0.44 | 0.44 | −0.33 | 0.52 | −0.67 | −0.46 | −0.44 | −0.44 |
| 2. MPQ-Sensory | -- | 0.69 | 0.77 | 0.48 | 0.37 | −0.27 | 0.41 | −0.46 | −0.46 | −0.38 | −0.31 |
| 3. MPQ-Affective | -- | -- | 0.58 | 0.52 | 0.49 | −0.43 | 0.50 | −0.47 | −0.60 | −0.47 | −0.43 |
| 4. VAS Pain Intensity | -- | -- | -- | 0.43 | 0.28 | −0.31 | 0.48 | −0.58 | −0.45 | −0.43 | −0.36 |
| 5. BDI | -- | -- | -- | -- | 0.75 | −0.71 | 0.67 | −0.43 | −0.63 | −0.33 | −0.52 |
| 6. STAI | -- | -- | -- | -- | -- | −0.76 | 0.54 | −0.34 | −0.61 | −0.36 | −0.52 |
| 7. PANAS-PA | -- | -- | -- | -- | -- | -- | −0.53 | 0.36 | 0.57 | 0.36 | 0.33 |
| 8. CATS | -- | -- | -- | -- | -- | -- | -- | −0.62 | −0.69 | −0.52 | −0.63 |
| 9. Rand 36 – Physical Functioning | -- | -- | -- | -- | -- | -- | -- | -- | 0.71 | 0.67 | 0.51 |
| 10. Rand 36 – Social Functioning | -- | -- | -- | -- | -- | -- | -- | -- | -- | 0.66 | 0.66 |
| 11. Rand 36 – Physical Role Limitations | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 0.53 |
| 12. Rand 36 – Emotional Role Limitations | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
Note: All correlations are significant at p <0.05. MPQ = McGill Pain Questionnaire–Short Form (Past Month), VAS = Visual Analog Scale, BDI = Beck Depression Inventory, STAI = State Trait Anxiety Inventory, PANAS-PA = Positive Affect Subscale of the Positive and Negative Affect Schedule, CATS = Pain Catastrophizing Scale
Tests of Mediation
The results of zero order correlation analyses indicated that preconditions for testing mediation were met for all associations between sleep disturbance and the chronic pain and functional outcomes. Results of the first series of mediation analyses testing whether the association between sleep disturbance and chronic pain intensity was mediated by psychosocial status (with each potential mediator tested in a separate model) are summarized in Table 3. Significant indirect effects of sleep disturbance on all three chronic pain intensity outcomes via depression levels (BDI) were observed (i.e., the bootstrap confidence intervals did not include zero). Similarly, significant indirect (mediated) effects of sleep disturbance on both MPQ-Affective and VAS pain intensity ratings via catastrophizing (CATS) were also noted. The mediation model focused on the MPQ-Affective pain rating outcome (i.e., pain-related suffering) was the only one to reveal significant indirect effects of sleep disturbance conveyed statistically through elevated anxiety (STAI) and reduced positive affect levels (PANAS-PA). Direct effects of sleep disturbance on chronic pain intensity outcomes were significant for 10 of the 12 models tested, indicating partial rather than full mediation by the psychosocial variables evaluated. That is, greater sleep disturbance is associated with greater chronic pain intensity both through the mediating effects of psychosocial distress and catastrophizing, as well as through associations with pain intensity that are independent of psychosocial factors.
Table 3.
Summary of Direct and Indirect Effects for Models in which Associations Between Sleep Disturbance and Chronic Pain Intensity Are Mediated by Psychosocial Factors.
| Bootstrap 95% Confidence Intervals forIndirect Effects |
||||
|---|---|---|---|---|
| Outcome | Mediator | Direct Effect of Sleep Disturbance | Lower | Upper |
| MPQ-Sensory | BDI | p = .006 | 0.023 | 0.227 |
| STAI | p = .002 | −0.016 | 0.174 | |
| PANAS-PA | p < .001 | −0.044 | 0.109 | |
| CATS | p = .005 | −0.017 | 0.220 | |
| MPQ-Affective | BDI | p = .059 | 0.017 | 0.133 |
| STAI | p = .037 | 0.014 | 0.118 | |
| PANAS-PA | p = .009 | 0.006 | 0.083 | |
| CATS | p = .102 | 0.019 | 0.138 | |
| VAS Intensity | BDI | p < .001 | 0.067 | 0.543 |
| STAI | p < .001 | −0.151 | 0.320 | |
| PANAS-PA | p < .001 | −0.055 | 0.322 | |
| CATS | p < .001 | 0.108 | 0.693 | |
Note: Each row represents a unique analysis testing a single mediator. Bootstrap 95% confidence intervals that do not contain zero are significant at p<0.05 (in bold). MPQ = McGill Pain Questionnaire–Short Form (Past Month), BDI = Beck Depression Inventory, STAI = State Trait Anxiety Inventory, PANAS-PA = Positive Affect subscale of the Positive and Negative Affect Schedule, CATS = Pain Catastrophizing Scale
The second series of mediation analyses (Table 4) evaluated the hypothesis that the effects of sleep disturbance on functional outcomes were mediated by chronic pain intensity (with each potential mediator tested in a separate model). Significant indirect effects of sleep disturbance on the four functional (Rand-36) outcomes were observed for all three hypothesized chronic pain intensity mediators. Indirect (mediated) effects were most consistently observed for the MPQ-Affective measure. These findings support a model in which the influence of greater sleep disturbance on higher functional impairment is conveyed in part via the indirect effects of sleep disturbance on higher chronic pain intensity. Nonetheless, significant direct effects for all 12 models tested indicate partial rather than full mediation. In other words, greater sleep disturbance is associated with elevated functional impairment in part due to the association between sleep disturbance and increased chronic pain intensity, but also through direct influences of sleep disturbance independent of pain intensity.
Table 4.
Summary of Direct and Indirect Effects for Models in which Associations Between Sleep Disturbance and Functional Outcomes Are Mediated by Chronic Pain Intensity.
| Bootstrap 95% Confidence Intervals forIndirect Effects |
||||
|---|---|---|---|---|
| Outcome | Mediator | Direct Effect of Sleep Disturbance | Lower | Upper |
| Rand 36-Physical Function | MPQ-Sensory | p < .001 | −0.587 | 0.002 |
| MPQ-Affective | p < .001 | −0.626 | −0.029 | |
| VAS Intensity | p < .001 | −0.866 | −0.203 | |
| Rand 36-Social Function | MPQ-Sensory | p = .004 | −0.827 | −0.062 |
| MPQ-Affective | p = .006 | −0.960 | −0.220 | |
| VAS Intensity | p = .008 | −0.861 | −0.130 | |
| Rand 36-Physical Role Limitations | MPQ-Sensory | p = .004 | −0.929 | −0.005 |
| MPQ-Affective | p = .005 | −1.081 | −0.208 | |
| VAS Intensity | p = .013 | −1.239 | −0.107 | |
| Rand 36-Emotional Role Limitations | MPQ-Sensory | p = .001 | −0.819 | 0.215 |
| MPQ-Affective | p = .004 | −1.038 | −0.087 | |
| VAS Intensity | p = .005 | −0.968 | 0.066 | |
Note: Each row represents a unique analysis testing a single mediator. Bootstrap 95% confidence intervals that do not contain zero are significant at p<.05 (in bold). MPQ = McGill Pain Questionnaire–Short Form (Past Month)
In secondary analyses, we conducted multiple mediation tests to provide a conservative test of the direct effects of sleep disturbance on both chronic pain intensity and functional outcomes independent of the multiple mediators (entered jointly) identified in primary analyses. Results indicated significant direct effects of sleep disturbance on MPQ-Sensory ratings (t = 2.41, p = 0.0183) and VAS intensity ratings (t = 3.46, p = 0.009) independent of the multiple mediators (BDI, STAI, PANAS-PA, and CATS). In addition, the total indirect (mediated) effect via the multiple mediators was significant for VAS intensity (95% CI for Total Indirect Effect: 0.030, 0.6797), but not for MPQ-Sensory ratings (95% CI for Total Indirect Effect: −0.003, 0.2667). For the MPQ-Affective outcome, the direct effect independent of the multiple mediators was not significant (t = 1.066, p = 0.29), but there was a significant indirect (mediated) effect via the multiple mediators (95% CI for Total Indirect Effect: 0.0287, 0.1662).
For the functional outcomes, results of these secondary analyses indicated significant direct effects of sleep disturbance on Rand-36 Functional Limitations (t = −5.374, p <.001), Rand-36 Social Functioning (t = −2.401, p = 0.0186), Rand-36 Physical Role Limitations (t=−2.271, p = 0.026), and Rand-36 Emotional Role Limitations (t= −2.69, p= 0.0086) independent of the multiple mediators (MPQ-Sensory, MPQ-Affective, VAS Intensity). In addition, the total indirect (mediated) effects via the multiple mediators were significant for Rand-36 Physical Functioning (95% CI for Total Indirect Effect: −0.9722, −0.168), Rand-36 Social Functioning (95% CI for Total Indirect Effect: −1.0744, −0.1995), and Rand-36 Physical Role Limitations (95% CI for Total Indirect Effect: −1.456, −0.1738), but not for Rand-36 Emotional Role Limitations (95% CI for Total Indirect Effect: −1.1668, 0.0126).
Discussion
Evidence strongly suggests that sleep disturbance, chronic pain intensity, and functional impairment are related, either directly and/or via intermediate factors. The present study examined these direct and indirect (mediated) pathways through which sleep disturbance might be related to chronic pain intensity and functional impairment. Findings support both direct and indirect associations. Greater sleep disturbance was associated with greater pain intensity, greater pain-related dysfunction and greater psychosocial distress and catastrophizing [3, 9]. Given the pattern of observed associations, we tested two series of mediation models. The first model tested whether previously reported links between sleep disturbance and greater pain intensity were mediated by negative psychosocial status associated with sleep disturbance. Results as a whole suggested that positive associations between sleep disturbance and chronic pain intensity were all conveyed statistically in part via the indirect effects of depressive symptoms, anxiety, catastrophizing, and positive affect (inverse) associated with sleep disturbance. This pattern showed consistency particularly for depression, and to a lesser degree catastrophizing, across multiple indices of chronic pain intensity. The most consistent mediation effects for psychosocial status were noted for affective chronic pain ratings (MPQ-Affective). Overall, 7 of 12 mediation models that were evaluated indicated significant indirect (mediated) effects of sleep disturbance via psychosocial status. That is, sleep disturbance may exert deleterious effects on pain by increasing negative affect and catastrophizing and by reducing positive affect, which may in turn lead to increased pain intensity. This result is consistent with previous reports of psychosocial distress mediating the relationship between sleep and pain [10, 23]. This result also adds to recent literature suggesting that psychological therapies, such as cognitive behavioral therapy, may improve chronic pain intensity in part by reducing psychosocial distress [24]. However, the fact that 10 of the 12 mediation models tested also revealed significant direct effects indicated that psychosocial status partially rather than fully mediated most of the observed associations between sleep disturbance and chronic pain intensity. Thus, sleep disturbance may worsen pain intensity via other mechanisms not tapped by psychosocial factors, such as increased inflammation [25].
The second series of mediation models evaluated whether associations between sleep disturbance and greater functional impairment were mediated in part through the indirect effects of elevated pain intensity. Nine of the 12 mediation models tested across the four functional measures revealed significant indirect effects. Specifically, the association between sleep disturbance and impaired physical and social functioning, and role limitations due to physical and emotional factors, were all at least partly accounted for by the mediating effects of greater pain intensity. As for the psychosocial mediation models, partial rather than full mediation by pain intensity was suggested.
The overall pattern of study findings suggests that sleep disturbance may negatively impact both chronic pain intensity and functional status independent of a number of plausible theory-driven mediators. These results are consistent with previous reports from more heterogeneous chronic pain samples [5, 6] indicating that sleep disturbance can have direct effects on chronic pain intensity and separate direct effects on function. This interpretation is supported by the significant direct effects observed in multiple mediation models (in secondary analyses). Adding to this prior work, however, these multiple mediation models also suggest that the negative effects of sleep disturbance on chronic pain intensity may derive in part from the adverse effects of sleep disturbance on psychosocial status. Moreover, these models further suggest that sleep disturbance may adversely impact functional status in chronic back pain patients in part because of associations between sleep disturbance and elevated chronic pain intensity. This latter finding is in contrast to previous work suggesting that when sleep duration and sleep quality were considered jointly, neither predicted results of behavioral functional tests once the influence of pain intensity and catastrophizing were controlled [5]. Differing results for functional measures between our work and this prior work may be due to the different sleep measures employed, different functional measures used (broad self-reported function versus specific behavioral function tests), as well as different analytical approaches (mediation analysis versus multiple regression). Moreover, differences could have been due in part to presence of medication confounds in prior work. Due to inclusion criteria of the primary study, no patients in the current study were using daily opioids and none had recently used as-needed opioids (confirmed via urine drug screen).
These results further highlight the potential benefits of screening for and treating sleep disturbance within the context of chronic pain management. Sleep disturbance can result from a variety of sleep disorders such as sleep disordering breathing (sleep apnea) and insomnia, and risk for these can be assessed with various questionnaires [26, 27]. Our results, along with others, raise the possibility that treating chronic pain patients for these causes of sleep disturbance (i.e. continuous positive airway pressure [CPAP], cognitive behavioral therapy for insomnia [CBTI]) could lead to improvements in chronic pain intensity and function, both directly and via improvements in psychosocial status. Indeed, CPAP has been shown to reduce bodily pain, reduce pain sensitivity and improve function in patients with sleep apnea [28, 29], findings consistent with the significant direct effects of sleep disturbance on pain and function reported in the current work. Additionally, CBT-I has been shown to improve sleep and also reduce pain intensity and pain interference in chronic pain patients [30–32]. Only one of these studies examined associations between the degree of improvement in sleep and pain outcomes, and found that the improvement in sleep significantly predicted subsequent decreases in pain [32].
Some limitations of this study should be acknowledged. The first is that the design was cross-sectional, and therefore the results reported describe statistical mediation only. A longitudinal design assessing chronic pain, sleep disturbance, psychosocial status, and function at multiple points over time would be required to demonstrate true causal mediation. This study also conducted a relatively large number of mediation tests, which could potentially have inflated the type I error rate. Risks that the mediation results reported above are due solely to Type I error are mitigated to some extent by the fact that significant results were largely consistent across the three chronic pain outcomes and the four functional measures evaluated (16 of 24 mediation tests were significant). Nonetheless, replication of these findings would be desirable. Finally, sleep disturbance was self-reported and there was no objective assessment of sleep such as can be derived from wrist actigraphy.
In summary, results of this study add to an emerging literature that highlights the potential negative effects of sleep disturbance on chronic pain intensity and function in patients with chronic pain. Here, we contribute to the literature in two ways. First, we showed that the detrimental effects of sleep disturbance on pain and function can be explained partly through the detrimental effects sleep disturbance may have on psychosocial factors, such as depressed mood. Sleep disturbance may worsen mood which in turn may worsen pain. Second, we showed that some of the effects of sleep disturbance on pain and function cannot be explained by ill effects of sleep disturbance on psychosocial factors. Thus, other mechanisms by which poor sleep affects pain and function remain to be determined.
Acknowledgments
Source of Funding
This work was supported by R01-DA031726 and R01-DA037891 from the National Institute on Drug Abuse/NIH.
Footnotes
Conflicts of Interest: HJB is a consultant for Natrol, LLC. SB is a consultant for Grunenthal and BioHaven. All other authors report no conflicts of interest.
References
- 1.Jegan NR, Brugger M, Viniol A, et al. Psychological risk and protective factors for disability in chronic low back pain - a longitudinal analysis in primary care. BMC Musculoskelet Disord 2017;18:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bahouq H, Allali F, Rkain H, et al. Prevalence and severity of insomnia in chronic low back pain patients. Rheumatol Int 2013;33:1277–81. [DOI] [PubMed] [Google Scholar]
- 3.Tang NK, Wright KJ, Salkovskis PM. Prevalence and correlates of clinical insomnia co-occurring with chronic back pain. J Sleep Res 2007;16:85–95. [DOI] [PubMed] [Google Scholar]
- 4.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain 2013;14:1539–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts MB, Drummond PD. Sleep problems are associated with chronic pain over and above mutual associations with depression and catastrophizing. Clin J Pain 2016;32:792–9. [DOI] [PubMed] [Google Scholar]
- 6.McCracken LM, Iverson GL. Disrupted sleep patterns and daily functioning in patients with chronic pain. Pain Res Manag 2002;7:75–9. [DOI] [PubMed] [Google Scholar]
- 7.Finan PH, Quartana PJ, Remeniuk B, et al. Partial sleep deprivation attenuates the positive affective system: effects across multiple measurement modalities. Sleep 2017;40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haack M, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain 2005;119:56–64. [DOI] [PubMed] [Google Scholar]
- 9.Gerhart JI, Burns JW, Post KM, et al. Relationships between sleep quality and pain-related factors for people with chronic low back pain: tests of reciprocal and time of day effects. Ann Behav Med 2017;51:365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien EM, Waxenberg LB, Atchison JW, et al. Negative mood mediates the effect of poor sleep on pain among chronic pain patients. Clin J Pain 2010;26:310–9. [DOI] [PubMed] [Google Scholar]
- 11.Naughton F, Ashworth P, Skevington SM. Does sleep quality predict pain-related disability in chronic pain patients? The mediating roles of depression and pain severity. Pain 2007;127:243–52. [DOI] [PubMed] [Google Scholar]
- 12.Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS sleep disturbance and Sleep-Related Impairment item banks. Behav Sleep Med 2011;10:6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melzack R. The short-form McGill Pain Questionnaire. Pain 1987;30:191–7. [DOI] [PubMed] [Google Scholar]
- 14.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:53–63. [DOI] [PubMed] [Google Scholar]
- 15.Spielberger CD, Gorsuch RL, Lushene RE. The State-Trait Anxiety Inventory manual Palo Alto, CA.: Consulting Psychologists Press, 1970. [Google Scholar]
- 16.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 1988;54:1063–70. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess 1995;7:524–532. [Google Scholar]
- 18.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ 1993;2:217–27. [DOI] [PubMed] [Google Scholar]
- 19.Ware JE Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 20.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput 2004;36:717–31. [DOI] [PubMed] [Google Scholar]
- 21.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986;51:1173–82. [DOI] [PubMed] [Google Scholar]
- 22.Fritz MS, Mackinnon DP. Required sample size to detect the mediated effect. Psychol Sci 2007;18:233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koffel E, Krebs EE, Arbisi PA, et al. The unhappy triad: pain, sleep complaints, and internalizing symptoms. Clin Psychol Sci 2016;4:96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dura-Ferrandis E, Ferrando-Garcia M, Galdon-Garrido MJ, et al. Confirming the mechanisms behind cognitive-behavioural therapy effectiveness in chronic pain using structural equation modeling in a sample of patients with temporomandibular disorders. Clin Psychol Psychother 2017;24:1377–1383. [DOI] [PubMed] [Google Scholar]
- 25.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep 2007;30:1145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999;131:485–91. [DOI] [PubMed] [Google Scholar]
- 27.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2001;2:297–307. [DOI] [PubMed] [Google Scholar]
- 28.Zhao YY, Wang R, Gleason KJ, et al. Effect of continuous positive airway pressure treatment on health-related quality of life and sleepiness in high cardiovascular risk individuals with sleep apnea: Best Apnea Interventions for Research (BestAIR) Trial. Sleep 2017;40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khalid I, Roehrs TA, Hudgel DW, et al. Continuous positive airway pressure in severe obstructive sleep apnea reduces pain sensitivity. Sleep 2011;34:1687–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vitiello MV, Rybarczyk B, Von Korff M, et al. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med 2009;5:355–62. [PMC free article] [PubMed] [Google Scholar]
- 31.Jungquist CR, O’Brien C, Matteson-Rusby S, et al. The efficacy of cognitive-behavioral therapy for insomnia in patients with chronic pain. Sleep Med 2010;11:302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith MT, Finan PH, Buenaver LF, et al. Cognitive-behavioral therapy for insomnia in knee osteoarthritis: a randomized, double-blind, active placebo-controlled clinical trial. Arthritis Rheumatol 2015;67:1221–33. [DOI] [PMC free article] [PubMed] [Google Scholar]


