Abstract
Precise formation of neuronal circuits requires the coordinated development of the different components of the circuit. Here, we review examples of coordination at multiples scales of development in one of the best-studied systems for neural patterning and circuit assembly, the Drosophila visual system, from coordination of gene expression in photoreceptors to the coordinated patterning of the different neuropiles of the optic lobe.
Introduction:
It is commonplace to say that neurons are the most diverse cell types in the organism and that understanding the mechanisms that permit their integration into circuits is one of the great challenges of modern neuroscience. Robustness of circuit formation requires the specification of the right neuronal types in the right number, which then have to make connections with other neurons sometimes localized at great distances. Thus, coordinated behaviors must exist to allow the matching of neuronal types and number. The Drosophila visual system is a prime model to study morphogenetic events during neuronal development. The retina has been widely studied to uncover the role of signaling pathways in patterning photoreceptors and for neuronal specification. More recently, the optic lobe has been used to uncover the developmental logic of circuit formation by linking the specification of neuronal cell identities to their integration into functional circuits. We review examples of coordinated patterning at multiple levels, from cell intrinsic fate decisions to extrinsic interactions that allow the formation of the retinotopic organization of the visual system, and highlight shared principles in neuronal circuit formation.
Retina organization:
The Drosophila compound eye is composed of ~750 unit eyes called ommatidia (Figure 1A). Each ommatidium is composed of 8 photoreceptors that express different light-sensing Rhodopsin proteins. The six outer photoreceptors (R1-6) express Rh1 and mediate motion detection, similarly to vertebrate rods. The two inner photoreceptors (R7 and R8) are responsible for color vision and express Rhodopsins with different spectral sensitivity that define the subtypes of ommatidia. The two main types of ommatidia are randomly distributed within the retina with a ratio of 65% of the yellow (y) type and 35% of the pale (p) type (Figure 1A and 1B). In the yellow subtype, yR7 expresses UV-sensitive Rh4, whereas yR8, located below R7 within the same ommatidium and thus sees the same point in space, expresses green-sensitive Rh6. In the pale subtype, pR7 expresses a shorter UV-sensitive Rh3 while pR8 expresses blue-sensitive Rh5 (Figure 1B). In addition to pale and yellow ommatidia, a third type is localized in the last row of ommatidia at the dorsal margin of the retina (Figure 1A and 1B). These so-called Dorsal Rim Area (DRA) R7 and R8 both express Rh3 and are important for the detection of the vector of polarized light [1,2].
Figure 1:
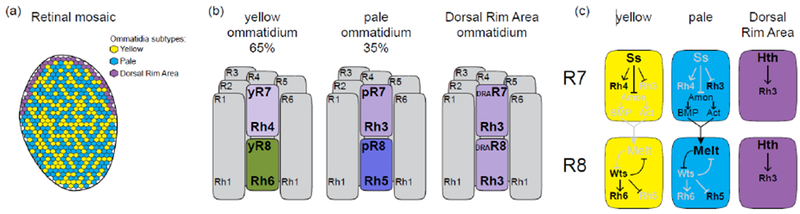
Fate specification in the retina:
(a) Organization of the retinal mosaic. Yellow (y) and pale (p) ommatidia are randomly distributed in the retina, whereas DRA photoreceptors are localized in the last row of ommatidia at the dorsal margin of the retina (b) The three ommatidium subtypes and their Rhodopsin (Rh) expression. (c) Gene regulatory network controlling ommatidia subtype specification.
Establishment and propagation of the retinal mosaic:
The mosaic of photoreceptor subtypes results from the stochastic fate specification of the inner photoreceptors. The initial fate decision is made cell-autonomously in R7: 65% of R7s stochastically turn on the expression of the transcription factor Spineless (Ss) that directs them to adopt the yR7 fate. In the absence of Ss, R7 adopt the default pR7 fate [3,4] (Figure 1C). Within the same ommatidium, R7 and R8 share the same light path and thus see the same point in space. The coupling of Rhodopsin expression between R7 and R8 photoreceptors is essential for color comparison as color opponent processing happens between R7 and R8 of the same ommatidium [5]. Since the initial stochastic fate choice is made by R7, this decision has to be communicated to R8 within the same ommatidium so that inner photoreceptors coordinate their Rhodopsin expression.
The mechanism that allows this coordination was first identified in the sevenless mutant that lacks all R7s, in which most of the R8s acquire the default yellow R8 fate suggesting that coordinated Rhodopsin expression is achieved through a signal sent by pR7 to R8 [6,7]. The nature of this signal remained elusive for a long time, but it was recently shown that both the BMP and Activin pathways act non-redundantly in parallel to promote the pR8 fate [8]. Interestingly, the expression of the different BMP and activin ligands involved in the process is not restricted to pR7s, instead the specificity seems to come, at least in part, from the expression of the TGFyβ processing factor Amon that is only present in pR7s [8]. Once instructed by R7, the pale versus yellow fate in R8 is controlled by two cross repressing factors: Warts a kinase member of the Hippo pathway is exclusively expressed in yR8 and promotes the yR8 fate through the co-activator Yorkie [9,10]. In pR8, Melted represses Warts and specifies the pale R8 fate [9]. This bistable transcriptional feedback loop between Warts and Melted ensures that only one of the two factors is expressed in a given R8 and thus creates two mutually exclusive cell fates [9,11] (Figure 1C).
Thus, photoreceptor fate decision is the result of sequential steps that each requires coordinated patterning. The initial fate decision is made at the level of a single transcription factor. Then, this decision is transmitted by signaling pathways to R8 where a complex gene regulatory network ensures that a single Rhodopsin is expressed whereas the alternative fate is repressed.
Matching neuronal types and number across neuropiles:
Information from the retina is sent to the optic lobes, which are composed of 4 distinct neuropiles that either receive direct inputs from photoreceptors or from second order neurons. These neuropiles process distinct visual features before sending information to the central brain to produce appropriate behaviors. The topographic organization of neurons is preserved along the different neuropiles of the visual system (Figure 2A), a process called retinotopy that is required to maintain the spatial information of the perceived stimuli in the downstream processing centers. Distinct developmental strategies are employed in the different neuropiles to coordinate the assembly of the successive topographic maps.
Figure 2:
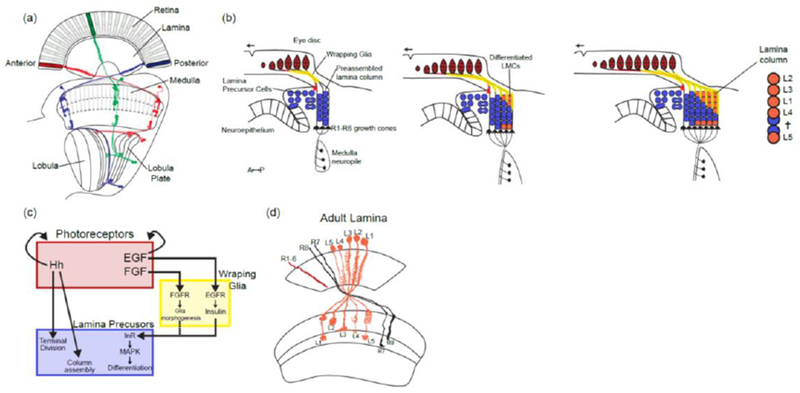
Building the retinotopic map in the lamina:
(a) Retinotopic organization of the visual system. Colors represent three different retinotopic points along the A-P axis. Examples of neurons from the different neuropiles that are retinotopically organized (L2 in the lamina, Mi1 in the medulla and T4 and T5 in the lobula complex).
(b) Sequence of lamina differentiation during larval development: the entry of photoreceptor axons into the lamina lead to the division of Lamina Precursors Cells (LPCs) and their assembly into lamina columns. This is followed by the entry of wrapping glia cell processes that will direct the differentiation of LPCs into differentiated lamina monopolar cells. As the eye disc grows more photoreceptor axons (in red) and glial cells enter the lamina leading to more columns being assembled posteriorly following a front of differentiation. Note that L5 neurons at the bottom of the lamina follow a different sequence than the other lamina neurons and do not depend on signaling from wrapping glia.
(c) Schematic of the signaling activities required for the formation and differentiation of lamina neurons. (d) Schematic of the adult lamina. Note that each lamina cartridge is composed of one copy of each of the 5 lamina monopolar cells and 6 outer photoreceptors (R1-6).
Induction of lamina neurons by photoreceptors to build retinotopy in the lamina:
The lamina is the first neuropile of the optic lobe: it is organized in ~750 cartridges that correspond to the ~750 ommatidia of the retina. Each cartridge receives inputs from R1-R6 that see the same point of the visual field. This information is received directly or indirectly by the five Lamina Monopolar Cells (LMCs) that project retinotopically to the medulla for further processing (Figure 2D).
The development of photoreceptors occurs in a sequential manner, where single rows of ommatidia are added anteriorly to the previous ones [12] (Figure 2A). The development of the lamina happens synchronously with the development of the retina, as lamina columns are added similarly anteriorly from a pool of Lamina Precursor Cells. The incoming photoreceptors axons tightly coordinate the development of the lamina and are absolutely necessary for its formation [13,14]: Hedgehog secretion from photoreceptor axons induces lamina precursor cells to enter S phase, undergo final division and terminally differentiate into post mitotic neuronal precursors that will preassemble into lamina columns [15–17] (Figure 2B and 2C). Because photoreceptor axons enter sequentially in the developing lamina as they are produced, lamina columns are assembled concomitantly to photoreceptor production.
As columns assemble with the proper number of lamina precursor cells, a second signal allows their differentiation into the five types of LMCs: Photoreceptor axons secrete the EGF ligand Spitz that is absolutely required for the induction of LMCs [18]. However, rather than directly inducing LMC fate, EGF instead activates a second signal in wrapping glia that themselves migrate from the optic stalk along photoreceptor axons: These wrapping glia secrete Insulin-like peptides (Ilps) that trigger lamina precursor cells to differentiate into LMCs through Insulin Receptor (InR) signaling via MAPK [19] (Figure 2B and 2C). Interestingly, wrapping glia morphogenesis and migration requires photoreceptors in two ways: they migrate from the optic stalk to the lamina along the photoreceptor axons in response to FGF signaling from photoreceptors [20], and they respond to the EGF signal that is also secreted from photoreceptors by secreting lips [18,19]. One can only speculate about the sequential activity of these signaling molecules and the requirement of wrapping glia in this process. One tempting hypothesis is that the involvement of wrapping glia creates a delay between the role of Hh in column formation and the specification of LMCs by InR signaling. Indeed there is a lag between photoreceptors innervation of the lamina and the progressive penetration of wrapping glia ensheathing photoreceptors axons into the developing lamina [19,21].
Thus, these mechanisms permit the production of the right number of lamina cartridges at a 1:1 ratio with ommatidia, and the correct number of each of the five LMCs in the right spatial position.
Coordination between photoreceptor and medulla development:
The second neuropile of the visual system, the medulla, receives inputs from the inner photoreceptors R7 and R8 and from the five LMCs. Similarly to the lamina that is organized in cartridges, the medulla is organized in columns corresponding to the ~750 ommatidia of the retina. However, its organization is more complex: it contains ~ 40,000 neurons of more that 80 types that project either locally within the medulla, or to other neuropiles [22]. These medulla neurons can be broadly classified into 2 categories: uni-columnar neurons have processes restricted to a single column and are present in every column (thus at a 1:1 stoichiometry with ommatidia) (Figure 3F). Multi-columnar neurons arborize in multiple columns and have a lower stoichiometry to photoreceptors that is variable depending on the neuronal type (from 5 to 300 neurons per optic lobe, [22,23]). Thus, columns and cartridges can be seen as parallel repetitive local microcircuits composed of neurons that compute the information from a single pixel of the visual field (1 ommatidium) whereas multi-columnar neurons integrate information from multiple columns (Figure 3F).
Figure 3:
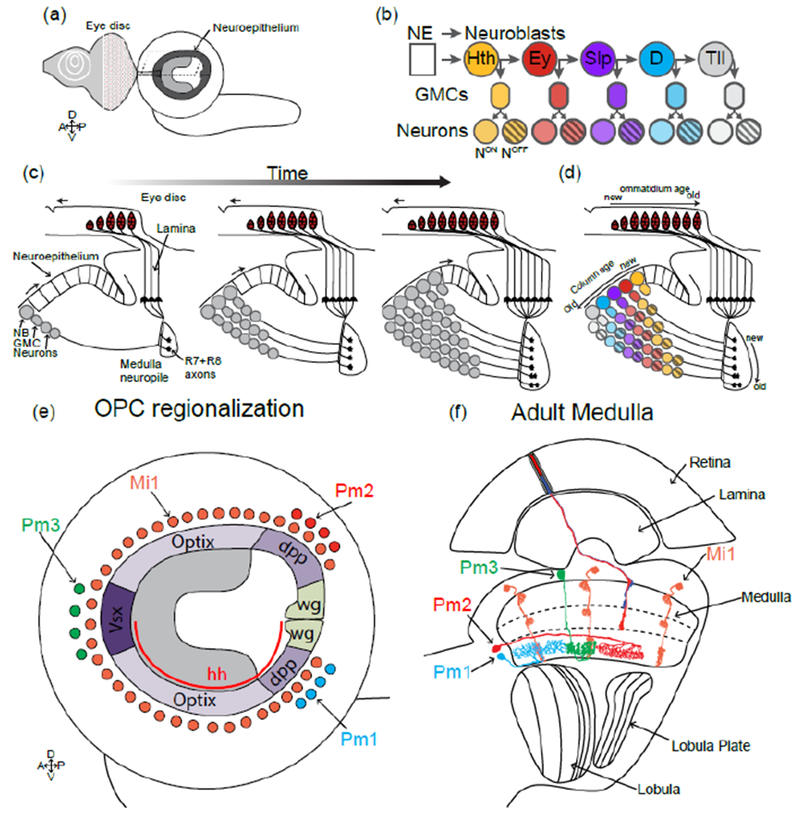
Medulla development:
(a) Representation of the larval brain showing the OPC lying on the lateral surface of the brain, below the eye disc.
(b) In the OPC neuroepithelium cells are converted into neuroblasts that express a temporal sequence of transcription factors that generates neuronal diversity. During each window, different subtypes of neurons are produced (represented in colors). A Notch binary fate decision further increases diversity of each progeny by producing two distinct fates (striped vs non-striped neurons).
(c) Cross section of the developing larval optic lobe showing the sequential formation of the medulla. The first neuroblast produces the most posterior medulla column. Over time more neuroepithelial cells are converted into neuroblasts that will add columns to the medulla anteriorly. (d) Overlay of the temporal patterning of neuroblasts and the sequential production of medulla columns.
(e and f) Larval (e) and adult (f) schematics of the medulla representing how the regionalization of the OPC increases neuronal diversity. The uni-columnar neuron Mi1 is produced all along the OPC independently of the spatial compartments, whereas the three multi-columnar neurons Pm1,2 and 3 are only produced by progenitors from specific domains.
Contrary to the lamina, the specification of medulla neurons does not require photoreceptor innervation [17], although photoreceptors are important for the maintenance of medulla neurons, as in the absence of the eye most medulla neurons do form but the medulla degenerates later during pupal development [24,25]. The medulla develops synchronously with photoreceptors and with the lamina from a single neuroepithelium, the Outer Proliferation Center (OPC), during late larval and early pupal stages (Figure 3A). A proneuronal wave that is concomitant to (but independent from) the entry of photoreceptor axons in the medulla leads to the sequential conversion of single rows of neuroepithelial cells into neuroblasts (Figure 3C), the Drosophila neural stem cells. These neuroblasts then divide multiple times asymmetrically to renew themselves and produce Ganglion Mother Cells that divide once more to produce two neurons (Figure 3B and C). Over these multiple cell divisions, neuroblasts sequentially express different transcription factors that govern the type of neurons produced by the neuroblast during each temporal window [26,27] (Figure 3B). Thus, overtime, a single neuroblast produces a variety of distinct neuronal types (Figure 3D). The OPC neuroepithelium is progressively transformed into neuroblasts that undergo the same temporal series until the entire neuroepithelium is consumed. Thus, similarly to the lamina, the medulla is built sequentially and concomitantly to the photoreceptors, with early born medulla neurons connecting to photoreceptors from the posterior part of the eye and late born to photoreceptors from the anterior part of the eye (Figure 3B-D).
The OPC is also divided in different compartments defined by the expression of spatially restricted transcription factors along the dorso-ventral axis, thus producing neuroblasts with different spatial identity [28] (Figure 3E). This division increases the diversity of neuronal types produced: Although uni-columnar neurons are produced by every neuroblast independently of its spatial identity, multi-columnar neurons are only produced (in smaller numbers) from spatially restricted progenitors [28] (Figure 3E).
It’s not entirely clear how the 1:1 matching between photoreceptors and uni-columnar neurons is established. The preferred hypothesis is that a single neuroblast forms a single column by producing the entire repertoire of uni-columnar neurons of a given column [28] (Figure 3D). Thus, the number of neuroblasts should perfectly match the number of columns (and thus photoreceptors). For multi-columnar neurons, it is more difficult to conceive that a perfect matching would be possible without direct interaction with photoreceptors, as for lamina neurons. One alternative hypothesis is that this matching is obtained by producing more neurons than columns during development and that supernumerary neurons are culled by apoptosis during later stages. Such direct coordination has not yet been uncovered but at least some aspects of medulla neurons development is coordinated with photoreceptors. For instance, photoreceptors directly regulate the size of the receptive field of their postsynaptic targets through Activin signaling [29].
Retinotopy in the lobula complex:
The final two neuropiles of the visual system, lobula and lobula plate, both receive inputs from the medulla and process information before it is sent to the central brain. Both structures are retinotopically built, but only the lobula plate is thought to have intrinsically retinotopic neurons (equivalent to the uni-columnar neurons of the medulla that are present as one per ommatidium). Very little is known about the development of the lobula but recent work has highlighted how the retinotopic map is built in the lobula plate [30–32]. The lobula plate is composed of two main classes of retinotopic neurons: T4 neurons that receive inputs from the ON motion pathway (that detect bright moving edges in the visual field), and T5 neurons that correspondingly receive inputs from the OFF motion pathway (dark moving edges) [33] (Figure 4A). T4 and T5 can each be further subdivided into four subclasses that each projects into one of the four distinct layers of the lobula plate that each responds to motion in one of the 4 cardinal directions (front-to-back, back-to-front, upward or downward motion) [33] (Figure 4A). Thus, the four T4 and four T5 cell types each have to build their own parallel retinotopic maps in the lobula plate such that each of the ~750 lobula plate columns contains one copy of each neuronal subtype.
Figure 4:
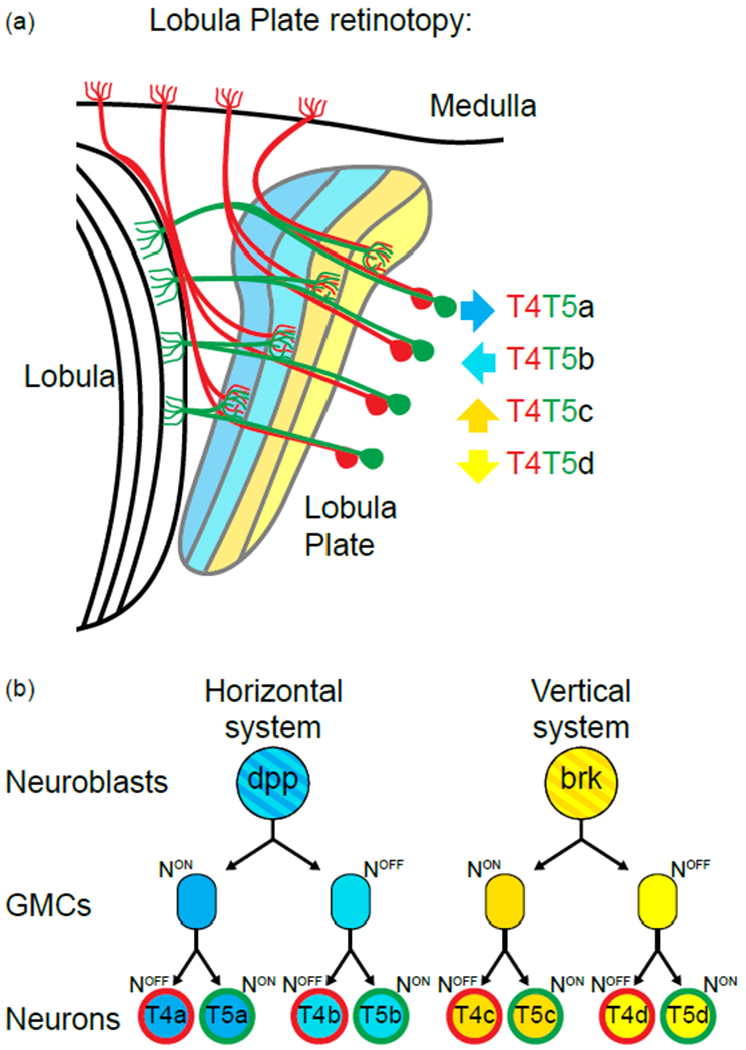
Retinotopic formation in the lobula plate:
(a) Schematic of the lobula plate and the 4 subtypes of T4 (in red) and T5 neurons (in green). Each subtype targets to a different layer of the lobula plate neuropile (color coded for their response to motion direction, arrow on the side
(b) Two neuroblasts give rise to the 4 T4s and T5s of a single column. Both go through two rounds of Notch mediated asymmetric decision and give rise to the 2 T4s and T5s of either the horizontal system for the Dpp+ neuroblast or of the vertical system for the Brk+ neuroblast.
The lobula plate develops from a neuroepithelium, the Inner Proliferation Center (IPC) that lies below the OPC in the larval brain. The IPC produces migrating progenitors that become neuroblasts that subsequently produce all T4 and T5 [34]. Similarly to the OPC, the IPC can be divided in two compartments that each produces half of the T4-T5 subtypes: one producing the cells of the horizontal system (front-to-back and back-to-front) and the other cells of the vertical system (up and down) [30,31].
Neuroblasts originating from either compartment undergo two rounds of asymmetric cell divisions that lead to the production of the four neurons of the horizontal system, or the four neurons of the vertical system (Figure 4B). Interestingly the four neurons originating from a single NB innervate the same lobula plate column [31].
Thus, a neuroblast, or more precisely two neuroblasts, form the neurons that innervate a single column of the lobula plate, as was proposed for the medulla [28]. As for other parts of the visual system, the lobula plate is built sequentially where first born neurons innervate part of the retinotopic map corresponding to the posterior part of the eye while later born neurons innervate regions that correspond to the anterior part of the eye [31].
Thus, the intrinsic properties of the developmental program allow for the numerical and spatial matching of the different neurons of the same microcircuit: There is no absolute need for direct coordination to match the number and the identity of the different components of the circuit.
Conclusion:
Along the different regions of the visual system, different mechanisms allow the numerical and retinotopic matching of neuronal subtypes so that a 1:1 stoichiometry is established within and across neuropiles. Coordination of R7 and R8 fates, and the formation of the lamina are due to the direct instruction from photoreceptors, or via glial cells, through signaling activity. In the medulla and lobula plate it is at least partly due to the intrinsic properties of neuronal progenitors and their temporal production that generates the right number of each cell type for each column. Finally, within each structure, neurons are born sequentially along one axis (Posterior-> Anterior in reference to the retina). The different neuropiles are born concomitantly and neurons born synchronically innervate the same column. It is not clear whether the concomitant formation of the different neuropiles is instructed by signals from the other structures because perturbing the temporal formation of parts of the circuit has been technically challenging. However, one tempting hypothesis is that temporal mechanisms might directly instruct the formation of retinotopic maps and might not use topographic gradients of growth factors like Ephrins [35] that provide positional retinotopic information in the vertebrate visual system.
Highlights.
The Drosophila visual system is an emerging model for neural circuit formation
The visual system is composed of repetitive units that are repeted across neuropiles
Coordination of patterning is required for the precise formation of circuits
Different strategies are used for the formation of retinotopic maps across neuropiles
Acknowledgments
Funding
This work was supported by NIH grant R01 EY13010 to C.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Wernet MF, Labhart T, Baumann F, Mazzoni EO, Pichaud F, Desplan C: Homothorax switches function of Drosophila photoreceptors from color to polarized light sensors. Cell 2003, 115:267–279. [DOI] [PubMed] [Google Scholar]
- 2.Wernet MF, Velez MM, Clark DA, Baumann-Klausener F, Brown JR, Klovstad M, Labhart T, Clandinin TR: Genetic dissection reveals two separate retinal substrates for polarization vision in drosophila. CurrBiol 2012, 22:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wernet MF, Mazzoni EO, Çelik A, Duncan DM, Duncan I, Desplan C: Stochastic spineless expression creates the retinal mosaic for colour vision. Nature 2006, 440:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston RJ, Desplan C: Interchromosomal communication coordinates intrinsically stochastic expression between alleles. Science (80-) 2014, 343:661–665. [DOI] [PMC free article] [PubMed] [Google Scholar]; ••The establishment of the retinal mosaic is controled by the stochastic expression of the transcription factor Spineless (Ss). The authors show how the stochastic expression of ss is established at the level of individual ss alleles and how interchromosomal communication is required for coordinating the expression of both ss alleles.
- 5.Schnaitmann C, Haikala V, Abraham E, Oberhauser V, Thestrup T, Griesbeck O, Reiff DF: Color Processing in the Early Visual System of Drosophila. Cell 2018, 172:318–318.e18. [DOI] [PubMed] [Google Scholar]
- 6.Chou WH, Hall KJ, Wilson DB, Wideman CL, Townson SM, Chadwell LV, Britt SG: Identification of a novel Drosophila opsin reveals specific patterning of the R7 and R8 photoreceptor cells. Neuron 1996, 17:1101–1115. [DOI] [PubMed] [Google Scholar]
- 7.Chou WH, Huber a, Bentrop J, Schulz S, Schwab K, Chadwell L V, Paulsen R, Britt SG: Patterning of the R7 and R8 photoreceptor cells of Drosophila: evidence for induced and default cell-fate specification. Development 1999, 126:607–616. [DOI] [PubMed] [Google Scholar]
- 8.Wells BS, Pistillo D, Barnhart E, Desplan C : Parallel activin and BMP signaling coordinates R7/R8 photoreceptor subtype pairing in the stochastic Drosophila retina. Elite 2017, 6:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikeladze-Dvali T, Wernet MF, Pistillo D, Mazzoni EO, Teleman AA, Chen YW, Cohen S, Desplan C: The growth regulators warts/lats and melted interact in a bistable loop to specify opposite fates in Drosophila R8 photoreceptors. Cell 2005, 122:775–787. [DOI] [PubMed] [Google Scholar]
- 10.Jukam D, Desplan C: Binary regulation of Hippo pathway by Merlin/NF2, Kibra, Lgl, and melted specifies and maintains postmitotic neuronal fate. Dev Cell 2011, 21:874–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jukam D, Xie B, Rister J, Terrell D, Charlton-Perkins M, Pistillo D, Gebelein B, Desplan C, Cook T: Opposite feedbacks in the Hippo pathway for growth control and neural fate. Science (80-) 2013, 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roignant JY, Treisman JE: Pattern formation in the Drosophila eye disc. Int J Dev Biol 2009, 53:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyerowitz M, Kankel DR: Analysis of Visual System Development melanogaster in Drosophilia melanogaster. Dev Biol 1978, 142:112–142. [DOI] [PubMed] [Google Scholar]
- 14.Nässel DR, Geiger G: Neuronal organization in fly optic lobes altered by laser ablations early in development or by mutations of the eye. J Comp Neurol 1983, 217:86–102. [DOI] [PubMed] [Google Scholar]
- 15.Selleck SB, Gonzalez C, Glover DM, White K: Regulation of the G1-S transition in postembryonic neuronal precursors by axon ingrowth. Nature 1992, 355:253–255. [DOI] [PubMed] [Google Scholar]
- 16.Huang Z, Kunes S: Hedgehog, transmitted along retinal axons, triggers neurogenesis in the developing visual centers of the Drosophila brain. Cell 1996, 86:411–422. [DOI] [PubMed] [Google Scholar]
- 17.Selleck SB, Steller H: The influence of retinal innervation on neurogenesis in the first optic ganglion of drosophila. Neuron 1991, 6:83–99. [DOI] [PubMed] [Google Scholar]
- 18.Huang Z, Shilo BZ, Kunes S: A retinal axon fascicle uses Spitz, an EGF receptor ligand, to construct a synaptic cartridge in the brain of Drosophila. Cell 1998, 95:693–703. [DOI] [PubMed] [Google Scholar]
- 19.Fernandes VM, Chen Z, Rossi AM, Zipfel J, Desplan C: Glia relay differentiation cues to coordinate neuronal development in Drosophila. Science (80-) 2017, 357:886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]; ••This study descibes how the retinotopic organisation of the lamina is achieved through the sequential signaling activity of photoreceptors and glial cells.
- 20.Franzdóttir SR, Engelen D, Yuva-Aydemir Y, Schmidt I, Aho A, Klämbt C: Switch in FGF signalling initiates glial differentiation in the Drosophila eye. Nature 2009, 460:758. [DOI] [PubMed] [Google Scholar]
- 21.Rossi AM, Fernandes VM: Wrapping Glial Morphogenesis and Signaling Control the Timing and Pattern of Neuronal Differentiation in the Drosophila Lamina. J Exp Neurosci 2018, 12:117906951875929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischbach KF, Dittrich APM: The optic lobe of Drosophila melanogaster. I. A Golgi analysis of wild-type structure. Cell Tissue Res 1989, 258:441–475. [Google Scholar]
- 23.Nern A, Pfeiffer BD, Rubin GM: Optimized tools for multicolor stochastic labeling reveal diverse stereotyped cell arrangements in the fly visual system. Proc Natl Acad Sci 2015, 112: E2967–E2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischbach KF: Neural cell types surviving congenital sensory deprivation in the optic lobes of Drosophila melanogaster. Dev Biol 1983, 95:1–18. [DOI] [PubMed] [Google Scholar]
- 25.Fischbach KF, Technau GM: Cell Degeneration in the Developing Optic Lobes of the sine oculis and small-optic-lobes Mutants of Drosophila melanogaster. Dev Biol 1984, 104:219–239. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Erclik T, Bertet C, Chen Z, Voutev R, Venkatesh S, Morante J, Celik A, Desplan C: Temporal patterning of Drosophila medulla neuroblasts controls neural fates. Nature 2013, 498:456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki T, Kaido M, Takayama R, Sato M: A temporal mechanism that produces neuronal diversity in the Drosophila visual center. Dev Biol 2013, 380:12–24. [DOI] [PubMed] [Google Scholar]
- 28.Erclik T, Li X, Courgeon M, Bertet C, Chen Z, Baumert R, Ng J, Koo C, Arain U, Behnia R, et al. : Integration of temporal and spatial patterning generates neural diversity. Nature 2017, 541:365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]; ••This paper illustrates how temporal and spatial patterning of neural progenitors increase neural diversity, regulate neuronal stochiometry and play a role in the retinotopic formation of the medulla.
- 29.Ting CY, McQueen PG, Pandya N, Lin TY, Yang M, Venkateswara Reddy O, O’Connor MB, McAuliffe M, Lee CH : Photoreceptor-derived activin promotes dendritic termination and restricts the receptive fields of first-order interneurons in Drosophila. Neuron 2014, 81:830–846. [DOI] [PMC free article] [PubMed] [Google Scholar]; •This paper describes how the photoreceptors R7 and R8 regulate the dendritic field size of their postsynaptic partners through the secretion of Activin.
- 30.Apitz H, Salecker I: Spatio-temporal relays control layer identity of direction-selective neuron subtypes in Drosophila. Nat Commun 2018, 9:2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinto-Teixeira F, Koo C, Rossi AM, Neriec N, Bertet C, Li X, Del-Valle-Rodriguez A, Desplan C: Development of Concurrent Retinotopic Maps in the Fly Motion Detection Circuit. Cell 2018, 173:485–498.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mora N, Oliva C, Fiers M, Ejsmont R, Soldano A, Zhang TT, Yan J, Claeys A, De Geest N, Hassan BA: A Temporal Transcriptional Switch Governs Stem Cell Division, Neuronal Numbers, and Maintenance of Differentiation. Dev Cell 2018, 45:53–66.e5. [DOI] [PubMed] [Google Scholar]
- 33.Maisak MS, Haag J, Ammer G, Serbe E, Meier M, Leonhardt A, Schilling T, Bahl A, Rubin GM, Nern A, et al. : A directional tuning map of Drosophila elementary motion detectors. Nature 2013, 500:212–216. [DOI] [PubMed] [Google Scholar]
- 34.Apitz H, Salecker I: A region-specific neurogenesis mode requires migratory progenitors in the Drosophila visual system. Nat Neurosci 2015, 18:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drescher U, Kremoser C, Handwerker C, Löschinger J, Noda M, Bonhoeffer F: In Vitro Guidance of Retinal Ganglion Cell Axons by RAGS, a 25 kDa Tectal Protein Related to Ligands for Eph Receptor Tyrosine Kinases. Cell 1995, 82:359–370. [DOI] [PubMed] [Google Scholar]


