Abstract
Background:
Magnetic resonance imaging (MRI) studies have shown differences in volume and structure in the brains of individuals with alcohol use disorder (AUD). Most research has focused on neuropathological effects of alcohol that appear after years of chronic alcohol misuse. However, few studies have investigated white matter (WM) microstructure and diffusion MRI-based (DWI) connectivity during early stages of AUD. Therefore, the goal of this work was to investigate WM integrity and structural connectivity in emerging adulthood AUD subjects using both conventional DWI metrics and a novel connectomics approach.
Methods:
Twenty-two AUD and eighteen controls (CON) underwent anatomical and diffusion MRI. Outcome measures were scalar diffusion metrics and structural network connectomes. Tract Based Spatial Statistics was used to investigate group differences in diffusion measures. Structural connectomes were used as input into a community structure procedure to obtain a co-classification index matrix (an indicator of community association strength) for each subject. Differences in co-classification and structural connectivity (indexed by streamline density) were assessed via the Network Based Statistics Toolbox.
Results:
AUD had higher FA values throughout the major WM tracts, but also had lower FA values in WM tracts in the cerebellum and right insula (pTFCE < 0.05). Mean diffusivity was generally lower in the AUD group (pTFCE < 0.05). AUD had lower co-classification of nodes between ventral attention and default mode networks, and higher co-classification between nodes of visual, default mode, and somatomotor networks. Additionally, AUD had higher fiber density between an adjacent pair of nodes within the default mode network.
Conclusion:
Our results indicate that emerging adulthood AUD subjects may have differential patterns of FA and distinct differences in structural connectomes compared to CON. These data suggest that such alterations in microstructure and structural connectivity may uniquely characterize early stages of AUD and/or a predisposition for development of AUD.
Keywords: Alcohol Use Disorder, connectivity, neuroimaging, DTI, white matter
Introduction
Alcohol use disorder (AUD) is the most common addictive disorder and is associated with significant morbidity, mortality, social, and economic burden (WHO, 2014). Despite decades of research, a complete understanding of the disease processes remains incomplete, which hinders the development of successful treatments for AUD. One strategy to aid in our knowledge is to study younger individuals with AUD in order to better characterize the brain during the earliest stages of the disorder. Additionally, AUD is a significant health problem in young adults. According to the 2016 National Survey on Drug Use and Health (NSDUH), 12% of individuals aged 21– 25 years old report heavy alcohol use (binge drinking on five or more days in the past month), yet only 5.6% of those 35 and older report heavy alcohol use (NSDUH, 2016). Furthermore, among college aged students (age 18–25), studies show that between 10 – 20% meet the criteria for an alcohol use disorder; however, in the general population, only around 5% meet the criteria for AUD (Blanco et al., 2008, NSDUH, 2016). These epidemiological reports demonstrate that AUD in young adults is already a significant problem. Although a growing body of literature exists on the effects of drinking (and risk for AUD) on adolescent (age 13–17) brains (Nguyen-Louie et al., 2018, Tapert et al., 2003) (also, see below), little is known about brain structure in young adults (~18–25 years old) with AUD. Late adolescence/emerging adulthood is a critical neurodevelopmental window that involves maturation of reward-related regions and cortical areas involved with executive function (for review see Bava & Tapert 2010). As this age group enters a collegiate environment, the potential for greater exposure to alcohol increases. In turn, this increases the vulnerability for development of AUD and potential concomitant adverse consequences on brain development during this time period. Thus, there is a critical need to better understand the effects of AUD on the brain in younger individuals, as this may ultimately facilitate the ability to identify those at risk for sustained AUD. In turn, such knowledge could permit development of interventions to prevent extensive damage and/or persistence of AUD throughout adulthood.
Diffusion weighted imaging (DWI) is a neuroimaging technique that allows a non-invasive investigation of white matter (WM) microstructure. In preclinical studies, it is well known that alcohol leads to myelination injury (De Bellis et al., 2008). Similarly, DWI studies have demonstrated that AUD is associated with deleterious effects on WM microstructure in recently detoxified individuals who reported, on average, two decades of alcohol use (Alhassoon et al., 2012, Konrad et al., 2012). While widespread deficits in WM tracts have been observed, damage has most consistently been demonstrated in the corpus callosum, frontal forceps, internal and external capsules, fornix, superior cingulate and longitudinal fasciculi (Pfefferbaum et al., 2009, Pfefferbaum et al., 2014, Buhler and Mann, 2011, Yeh et al., 2009). However, data on the consequences of alcohol misuse on WM integrity in college-age individuals (18–24 years of age) are extremely limited and equivocal. At a 2-year follow-up, 20–21 year-old binge drinkers had no changes in DTI-based metrics over time, and were not different from controls (Correas et al., 2016). In addition, while lower FA has been reported in binge drinkers ~17 years of age (Jacobus et al., 2009), higher FA has been observed in individuals with AUD of similar age (De Bellis et al., 2008). Thus, additional work is needed to better understand the ramifications of hazardous drinking in college-age young adults.
Studies that employ DWI typically adopt a diffusion tensor model and quantify WM integrity utilizing fractional anisotropy (FA), which is a metric that represents the degree anisotropy of water diffusion. In the WM, lower FA values are indicative of less restriction in water movement, which suggests a disruption in the microstructural environment (Soares et al., 2013). In addition to FA, the full diffusion tensor shape can also be described with other scalar metrics such as mean, axial, and radial diffusivity (MD, AD, and RD, respectively; (Alexander et al., 2007)).
Chronic alcohol misuse has been associated with a reduction in DWI-based measures of WM integrity (Pfefferbaum et al., 2009, Zahr and Pfefferbaum, 2017), whereas, in adolescent populations, higher measurements of WM integrity are typically reported, which are thought to reflect a predisposition to development of alcohol use disorder (Silveri et al., 2016). Few studies have sought to investigate the effects of alcohol on WM microstructure in emerging adulthood (ages 18–24), when persistent hazardous drinking behaviors are typically established. Increased understanding of the neurobiological consequences of AUD in this critical development period could bridge the gap between findings from adolescent and adult AUD research. Additionally, most DWI studies have been conducted in detoxified/abstinent AUD subjects; therefore, the present study makes a further contribution to the limited body of knowledge on WM microstructural differences in currently drinking, non-treatment seeking AUD subjects.
While DWI is in widespread use in research and clinical examinations of WM microstructural defects, traditional metrics (e.g., FA) may not be sensitive enough to detect structural differences in early AUD. However, network science-based connectomics methodology may offer a novel insight into microstructural deficits in early AUD. These methods are applied to data obtained from DWI tractography-modeled brain networks of nodes (regions) and edges (connections). One such network method is modularity, which represents the degree of segregation of a network into groups of interconnected nodes called communities (Newman, 2006; Sporns and Betzel, 2016). Putative differences in community structure between emerging adult AUD subjects and healthy individuals may represent a predisposition or an early consequence of AUD that has yet to be investigated.
The purpose of the present study was to investigate the WM integrity in young adults with or without AUD using both traditional metrics and a novel community detection approach. We hypothesized that, similar to older cohorts, younger AUD subjects would display significant differences in WM microstructure and altered connectivity due to the development of and/or consequences of AUD.
Materials and Methods
Subjects
All study procedures were approved by the Institutional Review Board at Indiana University. Informed consent was obtained from all subjects prior to study. Twenty-two subjects with alcohol use disorder (AUD) and eighteen healthy controls (CON) were recruited from the community as part of a larger study as described in (Finn et al., 2015). To qualify, participants had to be 18 – 30 years old, have at least 6th grade level of English comprehension, had consumed alcohol previously, and had no history of psychiatric illness or head trauma. AUD subjects had a current lifetime diagnosis of AUD. Four AUD participants also had a past lifetime diagnosis of a Substance Use Disorder other than alcohol; one AUD participant met criteria for marijuana use disorder. Severity of lifetime alcohol problems was measured using the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) (Bucholz et al., 1994), as the total count of all positive responses to all SSAGA questions in the Alcohol Diagnosis section (Cheng et al., 2018). The score range across all subjects was 0–99. Recent alcohol consumption was quantified as the total number of self-reported drinks in the last 2 weeks. Prior to study day, subjects were asked to abstain from alcohol for 12 hours. A breath alcohol test confirmed sobriety on scan day (Alco-Sensor IV, Intoximeters, St. Louis, MO).
Imaging
Data were acquired on a Siemens 3T Trio-Tim (Siemens, Erlangen, Germany). Diffusion weighted data were collected with a single-shell (b=1000 s/mm2) 2D acquisition; 64 diffusion directions and 8 b = 0 volumes; A-P phase encoding; 128×128 matrix; 72 slices; 2 × 2 × 2 mm3 voxels, iPAT factor = 2. A T1-weighted 3D anatomic sequence was also acquired (Field of view = 192×168 matrix, 160 sagittal slices, and 1.3 × 1.3 × 1.3 mm3 voxels).
Image processing
An in-house Matlab-based pipeline that utilizes FSL (Version 5.0.9) (Jenkinson et al., 2012) and Camino (Cook et al., 2006) was applied for image processing. Each subjects’ anatomic T1-weighted image was denoised (Coupé et al., 2008), skull stripped (FSL bet), and segmented according to tissue-type (FSL FAST). Diffusion weighted data were first denoised with local principal component analysis filter (Manjón et al., 2013), then the eight b0 volumes were registered to the first volume and averaged. The data were then corrected for motion, eddy currents, and registered to each subject’s anatomic space. This image processing procedure has been previously published in detail elsewhere (Chumin et al., 2018). At each voxel, tensor estimation was done with multi-tensor fitting in Camino in which voxels were classified as isotropic, anisotropic Gaussian (single tensor), or nonGaussian (multi-tensor). Scalar metrics of diffusion including FA, MD, AD, and RD were derived from tensor data. Streamline tractography was carried out in Camino with Fiber Assignment by Continuous Tracking algorithm in each subject’s anatomic space. Relevant tractography parameters were: 1 seed per voxel at the interface of gray matter (GM) and WM (obtained from the overlap of dilated GM and WM tissue masks), with an additional seed placed if a streamline encountered a multi-tensor voxel, whereupon each streamline followed one of the tensor directions; step size 1 voxel; maximum turning angle of 45 degrees over 5 steps. Streamlines terminated upon reaching another seed voxel at the GM/WM interface. After tracking, a length filter was applied to discard very short (<8mm) or extremely long (>180mm) streamlines. Throughout the processing, data were visually inspected for proper alignment and quality.
Tract-Based Spatial Statistics (TBSS)
FA maps for each subject were generated in subjects’ anatomical space and analyzed via Tract-Based Spatial Statistics (TBSS) (Smith et al., 2006). These data were nonlinearly registered onto the FMRIB58 template FA image (FSL’s FNIRT). The mean FA volume was created and thinned to generate a skeleton representation of core WM tracts. Each subject’s FA data were then projected onto the skeleton and used for subsequent analysis.
Structural Network Assembly
For each subject, we generated a structural connectivity network matrix, where the cortical nodes were based on a previously published brain parcellation (Shen et al., 2013). For the subcortical nodes, we implemented regions defined by Mawlawi et al. (2001) for the striatum and by Behrens et al. (2003) for the thalamus. The thalamic regions were further consolidated from 7 to 4 regions (pre-motor, primary motor, and sensory input regions were combined, and occipital and temporal regions were combined) per hemisphere to ensure sufficiently sized regions for tractography analysis. For all region pairs, number of streamlines and average seed surface area were extracted from the whole-brain tractogram with the Camino conmat function. The number of streamlines matrix was then thresholded to zero any values ≤ 2 to minimize the influence of false positive connections (Maier-Hein et al., 2017). Finally, network edges were quantified as fiber density (number of streamlines / average seed surface of connected regions; Hagmann et al. (2008)) between any pair of connected regions. Global metrics of network connectivity were calculated from the fiber density matrices with the Brain Connectivity Toolbox (Rubinov and Sporns, 2010).
Community Structure (From Hierarchical Consensus Clustering)
For each subject’s structural connectivity network matrix (see above), a set of 1000 modularity partitions was generated with methodology that is part of a novel Multiresolution Consensus Clustering procedure developed by Jeub et al. (2018). This method samples the full resolution range of resolution of modularity across a given number of partitions. A co-classification (CA) matrix was then generated, where edge weights are the frequency with which two nodes belong to the same module across the identified ensemble of partitions.
Statistical Analyses
Group subject demographics were assessed with independent-samples t-statistic or chi-squared tests, and results at p < 0.05 were considered statistically significant. Group differences in FA images from TBSS were interrogated with permutation testing in FSL’s randomise. Contrasts were generated after 10,000 permutations with Threshold-Free Cluster Enhancement (TFCE) for multiple comparisons correction across all skeleton voxels (Smith and Nichols, 2009). Significant clusters from all contrasts were ordered by size and thresholded at the largest 1% (corresponding to > 500 voxels in size) of clusters across all comparisons (8 contrasts with a total of 1469 clusters that range 1 to 12892 voxels in size). FSL’s cluster tool was used to extract mean cluster FA. Comparisons of continuous measures of subject characteristics and global metrics of network connectivity were conducted with independent samples t-tests. Chi-squared tests were used to test for differences in categorical variables. The Network Based Statistics (NBS; Zalesky et al. (2010)) Toolbox was used to compare the fiber density and CA network matrices between groups. False Discovery Rate (FDR) after 5,000 permutations was used to control for multiple comparisons.
Results
Subjects Characteristics
Subject characteristics are presented in Table 1. AUD and CON were well matched, with no significant differences in age, gender, education, or smoking status. In the AUD group, the mean age of AUD onset was 17.23 ± 1.4, and mean age at first drink was 14.82 ± 1.47, compared to 18 ± 1.91 in CON. Additionally, AUD reported significantly higher lifetime alcohol problem counts (p < 1 × 10−5) and total number of drinks in the two weeks prior to interview (p < 1 × 10−10) compared to CON.
Table 1.
Subject Characteristics.
| CON (n = 18) |
AUD (n = 22) |
p-value | |
|---|---|---|---|
| Age | 22.39 ± 3.35 | 22.73 ± 2.73 | 0.73 |
| Gender | 9 F | 8 F | 0.36 |
| Cigarette use (n) | 4 | 8 | 0.33 |
| Education (years) | 14.17 ± 1.65 | 14.18 ± 1.22 | 0.97 |
| Total Drinks (Last 2 Weeks) | 6.22 ± 6.75 | 66.32 ± 49.49 | 9.68 × 10−6 |
| Lifetime Alcohol Problems | 2.50 ± 3.19 | 48.10 ± 20.93 | 3.99 × 10−11 |
| Age at first drink | 18 ± 1.91 | 14.82 ± 1.47 | 2.15 × 10−6 |
| Age of AUD onset | n/a | 17.23 ± 1.41 |
Data are mean ± standard deviation. M: Male; F: Female. n/a: not applicable.
TBSS: AUD vs. CON
Young individuals with AUD showed higher FA values compared to CON throughout the WM skeleton (pTFCE < 0.05; Figure 1). This apparent difference in FA is likely a function of lower RD (Figure 2C; Figure S1A) in the AUD group. MD was also reduced in AUD (pTFCE < 0.05; Figure 2A), predominantly in areas where FA differences are absent; these differences in MD were likely due to significantly reduced AD (pTFCE < 0.05; Figure 2B). Concurrently, AUD subjects exhibited lower FA values in WM tracts associated with the right insular cortex and in cerebellar WM (pTFCE < 0.05; Figure 1). Mean FA and MD values and anatomical position for clusters > 500 voxels are shown in Figures 3 and 4, respectively. Mean AD and RD values from clusters with significant differences in FA or MD are shown in Figures S1 and S2, respectively.
Figure 1.
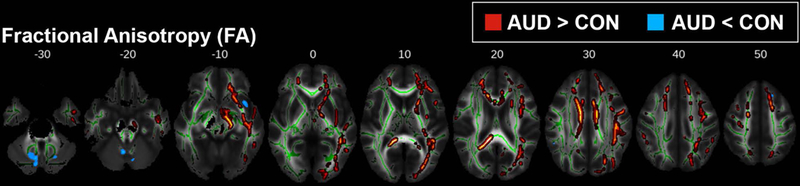
TBSS results for fractional anisotropy (FA). Areas where young adult alcohol use disorder (AUD) subjects had either higher (red-yellow) or lower (blue) FA compared to controls (CON) (pTFCE < 0.05). Group differences are superimposed on the mean FA skeleton (green); the mean FA image of the sample is the underlay. For visualization purposes, significant voxels within the WM skeleton were thickened with tbss_fill.
Figure 2.
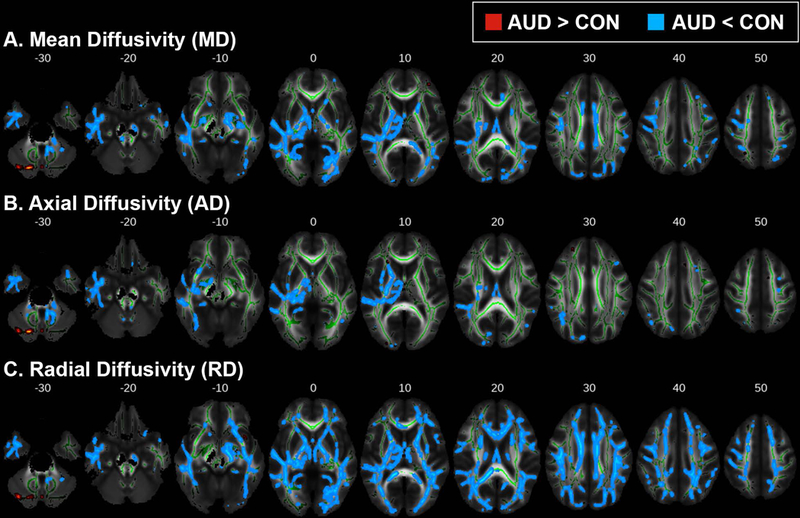
TBSS results for (A) mean diffusivity (MD), (B) axial diffusivity (AD), and (C) radial diffusivity (RD). Areas where alcohol use disorder (AUD) subjects had either higher (red-yellow) or lower (blue) scalar diffusion measures compared to controls (CON) (pTFCE < 0.05). Group differences are superimposed on the mean FA skeleton (green); the mean FA image of the sample is the underlay. For visualization purposes, significant voxels within the WM skeleton were thickened with tbss_fill.
Figure 3.
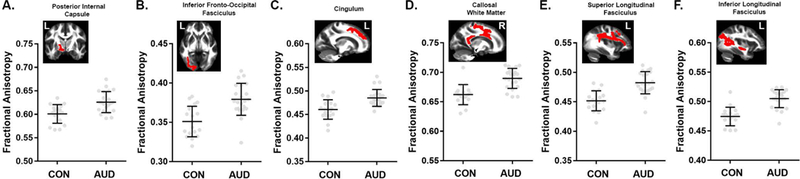
Comparisons of mean fractional anisotropy (FA) for largest 1% of clusters (> 500 voxels). TBSS results (pTFCE < 0.05) showed higher FA in alcohol use disorder (AUD) subjects compared to controls (CON) in clusters encompassing the (A) posterior internal capsule, (B) inferior fronto-occipital, (C) cingulum, (D) callosal, (E) superior longitudinal, and (F) inferior longitudinal tracts. Mean FA images show the anatomic position of each cluster. Data are mean ± standard deviation (black), with individual data points in gray filled circles. Tract labels were obtained from the Johns Hopkins University white-matter tractography atlas available in FSL.
Figure 4.
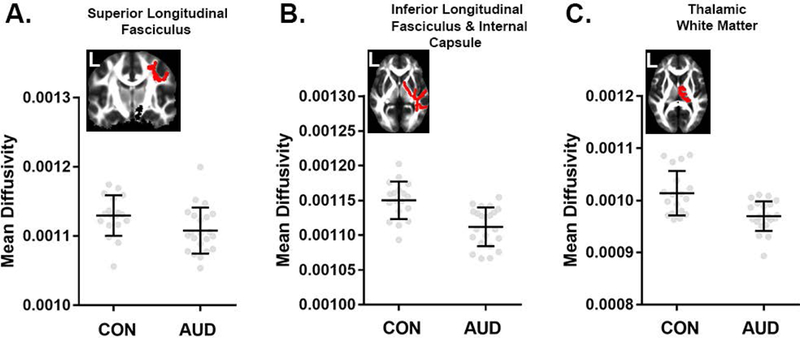
Comparisons of mean diffusivity (MD) for largest 1% of clusters (> 500 voxels). TBSS results (pTFCE < 0.05) showed reduced MD in portions of the (A) superior longitudinal, (B) inferior longitudinal and internal capsule, as well as (C) thalamic white matter. Mean FA images from the sample show the anatomic position of each cluster in red. Data are mean ± standard deviation (black), with individual data points in gray filled circles. Tract labels were obtained from the Johns Hopkins University white-matter tractography atlas in FSL.
Network Metrics of AUD vs. CON Structural Connectivity
Comparisons of global measures of structural connectivity showed increased network strength (pFDR < 0.02) and global efficiency (pFDR < 0.04) in AUD, while network density, degree, and clustering coefficient did not differ (Figure 5). AUD had higher fiber density in a single connection between a node in the middle frontal gyrus and a node in the superior frontal gyrus (Figure 6A). In AUD, co-classification (CA) was greater between nodes in middle frontal and pre/postcentral gyri (Figure 6B, left), between nodes in the cuneus and the middle temporal gyrus, and between supplementary motor and middle occipital areas (Figure 6B, right). AUD also had lower CA between nodes in the middle temporal gyrus and inferior insula (Figure 6C).
Figure 5.
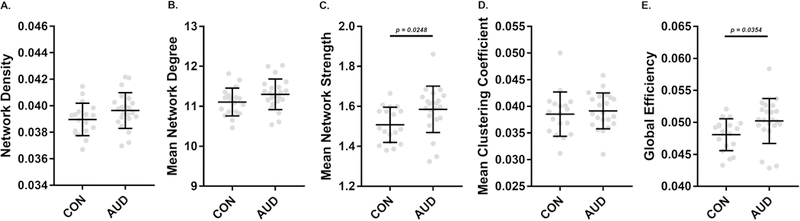
Characterization of networks from tractography-based structural connectomes (A,B,C) and comparisons of measures of network segregation and integration (D,E). Structural networks of CON and AUD groups were similar in density (A) and mean degree (B), while mean strength of edges was significantly (p = 0.0248) higher in the AUD group. Clustering coefficient, a measure of network segregation did not differ between groups, while global efficiency, a network integration metric, was significantly (p = 0.0354) higher in the AUD group. P-values for independent-samples t-test are indicated when significant.
Figure 6.
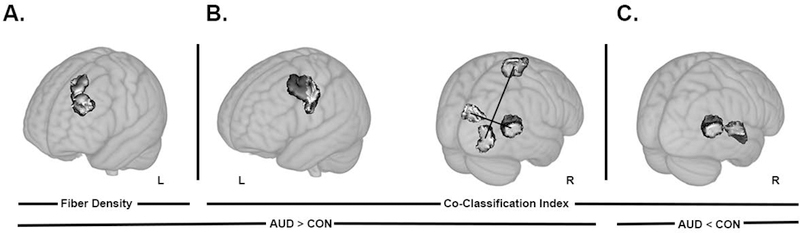
Results from the network based statistics comparison of fiber density and co-classification (pFDR < 0.05). (A) Higher fiber density in alcohol use disorder (AUD) subjects compared to controls (CON) was found between nodes located in the middle frontal and superior frontal gyri. (B) Co-classification index (measure of strength of association of a pair of nodes in an ensemble of community structure partitions obtained from modularity), was higher in AUD compared to CON in edges that connect middle frontal gyrus and pre/postcentral gyrus (left) node pairs, cuneus and middle temporal gyrus (right), and supplementary motor area and middle occipital gyrus (right). (C) One edge that connected nodes in the cuneus and inferior insula showed lower co-classification in AUD compared to CON. Renders are on a Montreal Neurological Institute 1mm template included in FSL, visualized with MRIcroGL software. Node pairs with edges that differed between groups are overlaid in shaded gray. A black line was used to specify the connected pair, when multiple pairs are presented on a single underlay. R and L represent right and left hemisphere, respectively.
Discussion
Studies employing DWI frequently focus on alcoholism in detoxified/abstinent older adults, but few DWI studies have investigated the WM differences in currently-drinking young adults with AUD. Emerging adulthood is a critical period for alcohol use as it represents the time period when one reaches the legal drinking age with greater access to alcohol use. This, coupled with increasing social acceptance of heavy drinking at this age, means that emerging adulthood is likely an important stage for the development and/or maintenance of AUD (Jennison, 2004). The present study demonstrates that there are alterations in WM microstructure in young adults with AUD. Additionally, our investigation of structural network modularity utilizing a novel connectomics based approach yielded differences in community structure in the brains of young AUD subjects. This study represents one of the first investigations of WM differences in an emerging adulthood AUD sample using a network science based analysis in addition to traditional DWI metrics.
Alterations in FA, MD, AD, and RD values reflect differences in axonal diameters, density, and myelination (Alexander et al., 2011, Alexander et al., 2007, Feldman et al., 2010). Consistently, the literature has reported that decrements in WM integrity are associated with alcohol use (for review see Zahr and Pfefferbaum (2017)) and higher WM integrity is associated with better cognitive functioning (Elofson et al., 2013). However, our results show that young AUD subjects have elevated FA, with lower MD, RD, and AD throughout major WM tracts of the TBSS FA-skeleton. This suggests that young AUD may have differential axonal diameters, density, and/or myelination compared to CON subjects. The present findings are consistent with several reports that observed higher FA in at-risk adolescent populations and adolescent-onset AUD (De Bellis et al., 2008, Tapert and Schweinsburg, 2005, Bava et al., 2009). There are several potential explanations for these unexpected results. It is possible that accelerated myelination of callosal tracts may present a vulnerability for adolescent AUD (De Bellis et al., 2008). Alternatively, such WM changes might reflect compensatory responses that occur during the early progression of AUD (Bava et al., 2009). Indeed, fMRI studies have shown that subtle reorganization of functional activation may occur during the progression of AUD with increasing use (Tapert et al., 2004). Taken together, these findings could suggest that the early stages of AUD may be associated with higher FA in many regions, either as a function of predisposition to AUD (Cardenas et. al., 2013) or as a result of the effects of extensive alcohol exposure during the early stages of AUD. Further work is needed to understand whether or not these neurophysiological signatures are related to behavioral and cognitive endophenotypes in early-stage AUD, and whether they are predictive of sustained alcohol use disorders throughout adulthood.
The lower FA values in WM tracts near the insula indicate potential demyelination or damage in tracts that connect with the insula. The insula is an important neural substrate for reward and addiction (Naqvi et al., 2014, Droutman et al., 2015), and is critical for mediating cue-induced craving that can drive drug use (Naqvi et al., 2014, Droutman et al., 2015). This is supported by rodent models of alcohol addiction, which have shown that disrupting excitatory insular-striatal circuitry decreases alcohol self-administration and increases sensitivity to alcohol (Jaramillo et al., 2018a, Jaramillo et al., 2018b). Decrements in FA in the vicinity of the insula in our young adult AUD sample may lead to impaired ability to detect changes in internal states such as alcohol intoxication (Berk et al., 2015, Migliorini et al., 2013). Additionally, the key role of the insula in salience attribution (Seeley et al., 2007) to the effects of alcohol intoxication could also be altered with deficits of WM adjacent to the insula. In support of this, a recent resting state fMRI study demonstrated that alcohol disrupted the connectivity between the anterior insular cortex and dorsal cingulate cortex (key nodes in the salience network), and that this disrupted connectivity was associated with greater “relaxing” effects of alcohol (Gorka et al., 2018).
To our knowledge, only one other structural connectivity study used graph theory analyses in AUD and demonstrated that the AUD group had significantly weaker connectivity, primarily in the right hemisphere (Zorlu et al., 2017). The edges or tracts of significance included connections of the putamen and hippocampus with other brain regions (Zorlu et al., 2017). We add to these findings by reporting AUD had lower co-classification of nodes between ventral attention (supplementary motor and inferior insula/superior temporal areas) and default mode (superior middle temporal gyrus) networks and higher co-classification between nodes of visual (middle occipital/temporal areas) and somatomotor (supplementary motor area) networks. Additionally, AUD had higher fiber density between a pair of nodes within the default mode (middle frontal and superior frontal areas) network and a pair of nodes between the dorsal attention (precentral/middle frontal area) and somatomotor (precentral/postcentral area) networks.
We recently reported structural connectivity differences in the ventral and dorsal attention networks, frontoparietal network, and default mode network in adult nontreatment-seeking AUD subjects (Chumin et al., 2018). In agreement with our previous findings, here we demonstrate that younger AUD subjects have altered connectivity in the ventral and dorsal attention networks and default mode network in addition to differences in the visual and somatomotor networks. Structural networks generally correspond to functional networks (Bullmore and Sporns, 2009); therefore, alterations to structural networks likely result in disrupted function. Respectively, the dorsal and ventral attention networks mediate voluntary shifts in attention, and detect unexpected, behaviorally-relevant stimuli (Vossel et al., 2014). The default mode network is a set of nodes broadly thought to be active during rest and silent during cognitive activities (Greicius et al., 2003). Importantly, the functions of the dorsal and ventral attention network and default mode network are relevant to alcohol abuse and dependence. Disrupted functional connectivity in the default mode has been reported in heavy drinking populations (Chanraud et al., 2011, Shokri-Kojori et al., 2016). Attentional bias and deficits are also frequently observed in AUD patients (Field and Cox, 2008, Fryer et al., 2013). Disruptions in the somatomotor and visual networks are less frequently associated with AUD; however, altered functional connectivity has been observed in the somatomotor and visual networks during alcohol intoxication (Esposito et al., 2010, Khalili-Mahani et al., 2012). Thus, the functional findings in the literature are consistent with our present structural connectivity data in young adult AUD.
Nodes within the networks referred to above, including the superior frontal cortex, insular cortex, temporal cortex and lateral parietal cortex, have been shown to be highly connected regions with a central position in the overall network (van den Heuvel and Sporns, 2013). These densely connected “hubs” or “rich clubs” play key roles in integrating information between segregated parts of the brain (van den Heuvel and Sporns, 2013). It has been suggested that alterations in these hubs or interconnections could likely lead to severe impairments because of their roles in whole-brain integrative processes (van den Heuvel and Sporns, 2013). For instance, abnormal rich club organization has been reported in structural connectivity analysis of patients with AUD (Zorlu et. al., 2017). The differences seen here may represent a network phenotype associated with a predisposition to develop AUD. However, it should be noted that the neurophysiological and cognitive/behavioral relevance of alterations in hubs and their interconnectedness are not fully understood.
The present study contains some limitations that should be considered. Not all individuals with hazardous drinking behavior in emerging adulthood will continue on to AUD as they age (for review see (O’Malley, 2004)). It is possible that subjects meeting the criteria for AUD in our study may “grow out” or “age out” of the AUD status as they progress through adulthood. Nonetheless, identifying differences in WM integrity and structural connectivity in young adult subjects currently meeting AUD criteria and experiencing the burden of heavy alcohol use is necessary, as these differences could represent areas of lasting neuroanatomical changes that continue regardless of the retention of AUD status. In addition, information on family history of AUD, which is a strong predictor for eventual development of AUD, was not available for this sample, and may be a contributing predisposing factor toward alterations of DWI scalar metrics (for review see (Cservenka, 2016)). Finally, this study utilized a relatively small, retrospective sample; longitudinal research with larger sample sizes is necessary to determine whether differences in scalar DWI metrics, fiber densities, and node associations contribute to the development of AUD, or are a consequence of the early stages of AUD.
In summary, we utilized traditional DWI metrics and a novel connectomics-based approach to examine WM differences in young adult AUD subjects. We demonstrated that young AUD subjects had distinct differences in microstructure with both higher WM integrity in many tracts and lower WM integrity in others. The connectomics analysis also revealed altered structural connectivity in this young adult AUD sample. The presence of these differences indicates that alterations in WM may not only appear after years of chronic alcohol abuse, but may in fact emerge during the early stages of AUD. The connectomics approach also detected structural connectivity differences in regional and global network connectivity measures in these emerging adulthood AUD subjects, and suggests that DWI studies investigating WM microstructure in AUD populations may benefit from utilizing this complementary, network science-based approaches. The present findings demonstrate WM microstructural structural connectome patterns that may be important for further understanding the early stages of AUD. This is clinically relevant, as young adult AUD represents a crucial time period for intervention to prevent maintenance of AUD throughout adulthood and subsequent potentially damaging changes to brain tissue.
Supplementary Material
Acknowledgments
Supported by NIAAA R01 AA13650 (PF), the Indiana Clinical and Translational Sciences Institute (HC), and NIAAA F31 AA025518 (EJC).
Footnotes
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- ALEXANDER AL, HURLEY SA, SAMSONOV AA, ADLURU N, HOSSEINBOR AP, MOSSAHEBI P, TROMP DPM, ZAKSZEWSKI E & FIELD AS 2011. Characterization of Cerebral White Matter Properties Using Quantitative Magnetic Resonance Imaging Stains. Brain Connect, 1, 423–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALEXANDER AL, LEE JE, LAZAR M & FIELD AS 2007. Diffusion Tensor Imaging of the Brain. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics, 4, 316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALHASSOON OM, SORG SF, TAYLOR MJ, STEPHAN RA, SCHWEINSBURG BC, STRICKER NH, GONGVATANA A & GRANT I 2012. Callosal white matter microstructural recovery in abstinent alcoholics: a longitudinal diffusion tensor imaging study. Alcohol Clin Exp Res, 36, 1922–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAVA S, FRANK LR, MCQUEENY T, SCHWEINSBURG BC, SCHWEINSBURG AD & TAPERT SF 2009. Altered white matter microstructure in adolescent substance users. Psychiatry Research: Neuroimaging, 173, 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAVA S & TAPERT SF 2010. Adolescent Brain Development and the Risk for Alcohol and Other Drug Problems. Neuropsychology Review, 20, 398–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEHRENS TEJ, JOHANSEN-BERG H, WOOLRICH MW, SMITH SM, WHEELER-KINGSHOTT CAM, BULBY PA, BARKER GJ, SILLERY EL, SHEEHAN K, CICCARELLI O, THOMPSON AJ, BRADY JM & MATTHEWS PM 2003. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience, 6, 750–757. [DOI] [PubMed] [Google Scholar]
- BERK L, STEWART JL, MAY AC, WIERS RW, DAVENPORT PW, PAULUS MP & TAPERT SF 2015. Under pressure: adolescent substance users show exaggerated neural processing of aversive interoceptive stimuli. Addiction, 110, 2025–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLANCO C, OKUDA M, WRIGHT C, HASIN DS, GRANT BF, LIU SM & OLFSON M 2008. Mental Health of College Students and Their Non-college-attending Peers: Results from the National Epidemiologic Study on Alcohol and Related Conditions. Arch Gen Psychiatry, 65, 1429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCHOLZ KK, CADORET R, CLONINGER CR, DINWIDDIE SH, HESSELBROCK VM, NURNBERGER JI, REICH T, SCHMIDT I & SCHUCKIT MA 1994. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. Journal of Studies on Alcohol, 55, 149–158. [DOI] [PubMed] [Google Scholar]
- BUHLER M & MANN K 2011. Alcohol and the human brain: a systematic review of different neuroimaging methods. Alcohol Clin Exp Res, 35, 1771–93. [DOI] [PubMed] [Google Scholar]
- BULLMORE E & SPORNS O 2009. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci, 10, 186–98. [DOI] [PubMed] [Google Scholar]
- CARDENAS VA, GREENSTEIN D, FOUCHE J-P, FERRETT H, CUZEN N, STEIN DJ & FEIN G 2013. Not lesser but Greater fractional anisotropy in adolescents with alcohol use disorders. NeuroImage: Clinical, 2, 804–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. (2017). 2016 National Survey on Drug Use and Health: Detailed Tables. Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- CHANRAUD S, PITEL AL, PFEFFERBAUM A & SULLIVAN EV 2011. Disruption of Functional Connectivity of the Default-Mode Network in Alcoholism. Cereb Cortex, 21, 2272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHENG H, KELLAR D, LAKE A, FINN P, REBEC GV, DHARMADHIKARI S, DYDAK U, & NEWMAN S 2018. Effects of Alcohol Cues on MRS Glutamate Levels in the Anterior Cingulate. Alcohol and Alcoholism, 53, 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUMIN EJ, GONI J, HALCOMB ME, DURAZZO TC, DZEMIDZIC M & YODER KK 2018. Differences in White Matter Microstructure and Connectivity in Nontreatment-Seeking Individuals with Alcohol Use Disorder. Alcohol Clin Exp Res, 42, 889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOK PA, BAI Y, NEDJATI-GILANI S, SEUNARINE KK, HALL MG, PARKER GJ & ALEXANDER DC 2006. Camino: Open-Source Diffusion-MRI Reconstruction and Processing. 14th Scientific Meeting of the International Society for Magnetic Resonance in Medicine, p. 2759. [Google Scholar]
- CORREAS A, CUESTA P, LÓPEZ-CANEDA E, RODRÍGUEZ HOLGUÍNS, GARCÍA-MORENO LM, PINEDA-PARDO JA, CADAVEIRA F & MAESTÚ F 2016. Functional and structural brain connectivity of young binge drinkers: a follow-up study. Scientific Reports, 6, 31293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COUPÉ P, YGER P, PRIMA S, HELLIER P, KERVRANN C & BARILLOT C 2008. An Optimized Blockwise Nonlocal Means Denoising Filter for 3-D Magnetic Resonance Images. Medical Imaging, IEEE Transactions on, 27, 425–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSERVENKA A 2016. Neurobiological phenotypes associated with a family history of alcoholism. Drug and Alcohol Dependence, 158, 8–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE BELLIS MD, VAN VOORHEES E, HOOPER SR, GIBLER N, NELSON L, HEGE SG, PAYNE ME & MACFALL J 2008. Diffusion tensor measures of the corpus callosum in adolescents with adolescent onset alcohol use disorders. Alcohol Clin Exp Res, 32, 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE HAAN W, MOTT K, VAN STRAATEN ECW, SCHELTENS P & STAM CJ 2012. Activity Dependent Degeneration Explains Hub Vulnerability in Alzheimer’s Disease. PLOS Computational Biology, 8, e1002582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DROUTMAN V, READ SJ & BECHARA A 2015. Revisiting the role of the insula in addiction. Trends Cogn Sci, 19, 414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELOFSON J, GONGVATANA W & CAREY KB 2013. Alcohol Use and Cerebral White Matter Compromise in Adolescence. Addict Behav, 38, 2295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESPOSITO F, PIGNATARO G, DI RENZO G, SPINALI A, PACCONE A, TEDESCHI G & ANNUNZIATO L 2010. Alcohol increases spontaneous BOLD signal fluctuations in the visual network. Neuroimage, 53, 534–43. [DOI] [PubMed] [Google Scholar]
- FELDMAN HM, YEATMAN JD, LEE ES, BARDE LHF & GAMAN-BEAN S 2010. Diffusion Tensor Imaging: A Review for Pediatric Researchers and Clinicians. J Dev Behav Pediatr, 31, 346–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIELD M & COX WM 2008. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend, 97, 1–20. [DOI] [PubMed] [Google Scholar]
- FINN PR, GUNN RL & GERST KR 2015. The Effects of a Working Memory Load on Delay Discounting in Those with Externalizing Psychopathology. Clinical psychological science : a journal of the Association for Psychological Science, 3, 202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRYER SL, JORGENSEN KW, YETTER EJ, DAURIGNAC EC, WATSON TD, SHANBHAG H, KRYSTAL JH & MATHALON DH 2013. Differential brain response to alcohol cue distractors across stages of alcohol dependence. Biological Psychology, 92, 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORKA SM, PHAN KL & CHILDS E 2018. Acute calming effects of alcohol are associated with disruption of the salience network. Addict Biol, 23, 921–930. [DOI] [PubMed] [Google Scholar]
- GREICIUS MD, KRASNOW B, REISS AL & MENON V 2003. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences, 100, 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGMANN P, CAMMOUN L, GIGANDET X, MEULI R, HONEY CJ, WEDEEN VJ & SPORNS O 2008. Mapping the Structural Core of Human Cerebral Cortex. PLoS Biol, 6, e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBUS J, MCQUEENY T, BAVA S, SCHWEINSBURG BC, FRANK LR, YANG TT & TAPERT SF 2009. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicology and Teratology, 31, 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARAMILLO AA, RANDALL PA, STEWART S, FORTINO B, VAN VOORHIES K & BESHEER J 2018a. Functional role for cortical-striatal circuitry in modulating alcohol self-administration. Neuropharmacology, 130, 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARAMILLO AA, VAN VOORHIES K, RANDALL PA & BESHEER J 2018b. Silencing the insular-striatal circuit decreases alcohol self-administration and increases sensitivity to alcohol. Behav Brain Res, 348, 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON M, BECKMANN CF, BEHRENS TEJ, WOOLRICH MW & SMITH SM 2012. FSL. NeuroImage, 62, 782–790. [DOI] [PubMed] [Google Scholar]
- JENNISON KM 2004. The Short‐Term Effects and Unintended Long‐Term Consequences of Binge Drinking in College: A 10‐Year Follow‐Up Study. The American Journal of Drug and Alcohol Abuse, 30, 659–684. [DOI] [PubMed] [Google Scholar]
- JEUB LGS, SPORNS O & FORTUNATO S 2018. Multiresolution Consensus Clustering in Networks. Scientific Reports, 8, 3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHALILI-MAHANI N, ZOETHOUT RM, BECKMANN CF, BAERENDS E, DE KAM ML, SOETER RP, DAHAN A, VAN BUCHEM MA, VAN GERVEN JM & ROMBOUTS SA 2012. Effects of morphine and alcohol on functional brain connectivity during “resting state”: a placebo-controlled crossover study in healthy young men. Hum Brain Mapp, 33, 1003–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONRAD A, VUCUREVIC G, LORSCHEIDER M, BERNOW N, THUMMEL M, CHAI C, PFEIFER P, STOETER P, SCHEURICH A & FEHR C 2012. Broad disruption of brain white matter microstructure and relationship with neuropsychological performance in male patients with severe alcohol dependence. Alcohol Alcohol, 47, 118–26. [DOI] [PubMed] [Google Scholar]
- LE BERRE AP, FAMA R & SULLIVAN EV 2017. Executive Functions, Memory, and Social Cognitive Deficits and Recovery in Chronic Alcoholism: A Critical Review to Inform Future Research. Alcohol Clin Exp Res, 41, 1432–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAIER-HEIN KH, NEHER PF, HOUDE J-C, CÔTÉ M-A, GARYFALLIDIS E, ZHONG J, CHAMBERLAND M, YEH F-C, LIN Y-C, JI Q, REDDICK WE, GLASS JO, CHEN DQ, FENG Y, GAO C, WU Y, MA J, RENJIE H, LI Q, WESTIN C-F, DESLAURIERS-GAUTHIER S, GONZÁLEZ JOO, PAQUETTE M, ST-JEAN S, GIRARD G, RHEAULT F, SIDHU J, TAX CMW, GUO F, MESRI HY, DÁVID S, FROELING M, HEEMSKERK AM, LEEMANS A, BORÉ A, PINSARD B, BEDETTI C, DESROSIERS M, BRAMBATI S, DOYON J, SARICA A, VASTA R, CERASA A, QUATTRONE A, YEATMAN J, KHAN AR, HODGES W, ALEXANDER S, ROMASCANO D, BARAKOVIC M, AURÍA A, ESTEBAN O, LEMKADDEM A, THIRAN J-P, CETINGUL HE, ODRY BL, MAILHE B, NADAR MS, PIZZAGALLI F, PRASAD G, VILLALON-REINA JE, GALVIS J, THOMPSON PM, REQUEJO FDS, LAGUNA PL, LACERDA LM, BARRETT R, DELL’ACQUA F, CATANI M, PETIT L, CARUYER E, DADUCCI A, DYRBY TB, HOLLAND-LETZ T, HILGETAG CC, STIELTJES B & DESCOTEAUX M 2017. The challenge of mapping the human connectome based on diffusion tractography. Nature Communications, 8, 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANJÓN JV, COUPÉ P, CONCHA L, BUADES A, COLLINS DL & ROBLES M 2013. Diffusion Weighted Image Denoising Using Overcomplete Local PCA. PLoS ONE, 8, e73021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAWLAWI O, MARTINEZ D, SLIFSTEIN M, BROFT A, CHATTERJEE R, HWANG D, HUANG Y, SIMPSON N, NGO K, HEERTUM R & LARUELLE M 2001. Imaging Human Mesolimbic Dopamine Transmission With Positron Emission Tomography: I. Accuracy and Precision of D2 Receptor Parameter Measurements in Ventral Striatum. Journal of Cerebral Blood Flow & Metabolism, 21, 1034–1057. [DOI] [PubMed] [Google Scholar]
- MIGLIORINI R, STEWART JL, MAY AC, TAPERT SF & PAULUS MP 2013. What do you feel? Adolescent drug and alcohol users show altered brain response to pleasant interoceptive stimuli. Drug and Alcohol Dependence, 133, 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAQVI NH, GAZNICK N, TRANEL D & BECHARA A 2014. The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann N Y Acad Sci, 1316, 53–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWMAN MEJ 2006. Modularity and community structure in networks. Proceedings of the National Academy of Sciences, 103, 8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NGUYEN-LOUIE TT, SIMMONS AN, SQUEGLIA LM, ALEJANDRA INFANTE M, SCHACHT JP & TAPERT SF 2018. Earlier alcohol use onset prospectively predicts changes in functional connectivity. Psychopharmacology, 235, 1041–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’MALLEY PM 2004. Maturing out of problematic alcohol use. Alcohol Research & Health, 28, 202–204. [Google Scholar]
- PFEFFERBAUM A, ROSENBLOOM M, ROHLFING T & SULLIVAN EV 2009. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol Psychiatry, 65, 680–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFEFFERBAUM A, ROSENBLOOM MJ, CHU W, SASSOON SA, ROHLFING T, POHL KM, ZAHR NM & SULLIVAN EV 2014. White Matter Microstructural Recovery with Abstinence and Decline with Relapse in Alcoholism: Interaction with Normal Aging Revealed with Longitudinal DTI. Lancet Psychiatry, 1, 202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBINOV M, SPORNS O 2010. Complex network measures of brain connectivity: Uses and interpretations. NeuroImage, 52, 1059–1069. [DOI] [PubMed] [Google Scholar]
- SEELEY WW, MENON V, SCHATZBERG AF, KELLER J, GLOVER GH, KENNA H, REISS AL & GREICIUS MD 2007. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. The Journal of neuroscience : the official journal of the Society for Neuroscience, 27, 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEN X, TOKOGLU F, PAPADEMETRIS X & CONSTABLE RT 2013. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. NeuroImage, 82, 403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHI F, WANG L, PENG Z, WEE C-Y & SHEN D 2013. Altered Modular Organization of Structural Cortical Networks in Children with Autism. PLOS ONE, 8, e63131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOKRI-KOJORI E, TOMASI D, WIERS CE, WANG GJ & VOLKOW ND 2016. Alcohol affects brain functional connectivity and its coupling with behavior: greater effects in male heavy drinkers. Molecular Psychiatry, 22, 1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVERI MM, DAGER AD, COHEN-GILBERT JE & SNEIDER JT 2016. Neurobiological signatures associated with alcohol and drug use in the human adolescent brain. Neuroscience & Biobehavioral Reviews, 70, 244–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH SM, JENKINSON M, JOHANSEN-BERG H, RUECKERT D, NICHOLS TE, MACKAY CE, WATKINS KE, CICCARELLI O, CADER MZ, MATTHEWS PM & BEHRENS TEJ 2006. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage, 31, 1487–1505. [DOI] [PubMed] [Google Scholar]
- SMITH SM & NICHOLS TE 2009. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44, 83–98. [DOI] [PubMed] [Google Scholar]
- SOARES JM, MARQUES P, ALVES V & SOUSA N 2013. A hitchhiker’s guide to diffusion tensor imaging. Front Neurosci, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPORNS O & BETZEL RF 2016. Modular Brain Networks. Annu Rev Psychol, 67, 613–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAPERT SF, CHEUNG EH, BROWN GG & et al. 2003. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Archives of General Psychiatry, 60, 727–735. [DOI] [PubMed] [Google Scholar]
- TAPERT SF & SCHWEINSBURG AD 2005. The human adolescent brain and alcohol use disorders. Recent Dev Alcohol, 17, 177–97. [DOI] [PubMed] [Google Scholar]
- TAPERT SF, SCHWEINSBURG AD, BARLETT VC, BROWN SA, FRANK LR, BROWN GG & MELOY MJ 2004. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol Clin Exp Res, 28, 1577–86. [DOI] [PubMed] [Google Scholar]
- VAN DEN HEUVEL MP & SPORNS O 2013. Network hubs in the human brain. Trends in Cognitive Sciences, 17, 683–696. [DOI] [PubMed] [Google Scholar]
- VOSSEL S, GENG JJ & FINK GR 2014. Dorsal and Ventral Attention Systems: Distinct Neural Circuits but Collaborative Roles. The Neuroscientist, 20, 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO 2014. Global status report on alcohol and health, World Health Organization. [Google Scholar]
- YEH PH, SIMPSON K, DURAZZO TC, GAZDZINSKI S & MEYERHOFF DJ 2009. Tract-Based Spatial Statistics (TBSS) of diffusion tensor imaging data in alcohol dependence: abnormalities of the motivational neurocircuitry. Psychiatry Res, 173, 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAHR NM & PFEFFERBAUM A 2017. Alcohol’s Effects on the Brain: Neuroimaging Results in Humans and Animal Models. Alcohol Research : Current Reviews, 38, 183–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZALESKY A, FORNITO A & BULLMORE ET 2010. Network-based statistic: Identifying differences in brain networks. NeuroImage, 53, 1197–1207. [DOI] [PubMed] [Google Scholar]
- ZORLU N, CAPRAZ N, OZTEKIN E, BAGCI B, DI BIASE MA, ZALESKY A, GELAL F, BORA E, DURMAZ E, BESIROGLU L & SARICICEK A 2017. Rich club and reward network connectivity as endophenotypes for alcohol dependence: a diffusion tensor imaging study. Addict Biol. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


