Summary
Objective:
Laser interstitial thermal therapy (LITT) for mesial temporal lobe epilepsy (mTLE) has reported seizure freedom rates between 36% and 78% with at least 1-year of follow-up. Unfortunately, the lack of robust methods capable of incorporating the inherent variability of patient anatomy, the variability of the ablated volumes, and clinical outcomes have limited three-dimensional quantitative analysis of surgical targeting and its impact on seizure outcomes. We therefore aimed to leverage a novel image-based methodology for normalizing surgical therapies across a large multicenter cohort to quantify the effects of surgical targeting on seizure outcomes in LITT for mTLE.
Methods:
This multicenter, retrospective cohort study included 234 patients from 11 centers who underwent LITT for mTLE. To investigate therapy location, all ablation cavities were manually traced on postoperative MRI, which were subsequently non-linearly normalized to a common atlas space. The association of clinical variables and ablation location to seizure outcome were calculated using multivariate regression and Bayesian models, respectively.
Results:
Ablations including more anterior, medial, and inferior temporal lobe structures, which involved greater amygdalar volume, were more likely to be associated with Engel I outcomes. At both 1- and 2-years after LITT, 58.0% achieved Engel I outcomes. A history of bilateral tonic-clonic seizures decreased chances of Engel I outcome. Radiographic hippocampal sclerosis was not associated with seizure outcome.
Significance:
LITT is a viable treatment for mTLE in patients who have been properly evaluated at a comprehensive epilepsy center. Consideration of surgical factors is imperative to the complete assessment of LITT. Based on our model, ablations must prioritize the amygdala and also include hippocampal head, parahippocampal gyrus, and rhinal cortices to maximize chances of seizure freedom. Extending the ablation posteriorly has diminishing returns. Further work is necessary to refine this analysis and define the minimal zone of ablation necessary for seizure control.
Keywords: surgery, stereotactic, amygdalohippocampectomy, ablation, MRI
Introduction
Mesial temporal epilepsy (mTLE) affects the majority of surgical drug-resistant epilepsy (DRE) candidates.1 Although more efficacious than medical therapy alone for DRE, epilepsy surgery remains highly underutilized, in part due to concerns of the morbidity associated with traditional craniotomies.2 While anterior temporal lobectomy (ATL) remains the “gold standard” for the treatment of drug-resistant mTLE, newer therapies such as laser interstitial thermal therapy (LITT) have emerged. As a minimally-invasive procedure with shorter hospitalizations and lower morbidity, including less impairment to cognitive function, LITT has the potential to gain wider acceptance.3–7 To date, single-institution series of LITT have demonstrated 38–78% seizure freedom with at least 1-year follow-up, with upwards of 60–89% seizure freedom in those with radiographic evidence of hippocampal sclerosis (rHS).3–6,8–12
While no studies have identified any significant correlation between ablation volumes and seizure outcomes,3,4,8 laser catheter placement is thought to play a role.4,8,13 As such, we must consider potential differences in surgical technique. Unfortunately, no study has specifically studied the optimal region of ablation for LITT in mTLE. Given the novelty of LITT, no protocol for targeting exists and no single center has the experience to define optimal laser catheter placement. The only study to specifically address this issue revealed that greater mesial hippocampal head ablation correlates with improved seizure outcome.4 Ultimately, the lack of robust methods capable of incorporating the inherent variability of patient anatomy, the variability of the ablated volumes, and clinical outcomes have limited three-dimensional quantitative analysis of surgical targeting and its impact on the likelihood of seizure freedom. As such, specific characteristics of an optimal ablation in three-dimensional space remain unknown.
We therefore aimed to investigate both patient and surgical factors associated with seizure outcome from LITT for mTLE in a large multicenter cohort in order to better understand patient selection, surgical technique, and overall outcome for this procedure.
Methods
Study Design
This multicenter, retrospective cohort study included 234 patients who underwent amygdalohippocampal complex (AHC) LITT for treatment of mTLE across 11 centers in the United States between December 2011 and August 2017. Each participating institution obtained their own Institutional Review Board (IRB) approval for collecting the data and sharing deidentified versions of this data. Given the retrospective nature and the use of only deidentified data for this study, no patient consents were required.
Aggregation, deidentification, normalization, and comparison of data was accomplished with the CranialCloud platform (Neurotargeting LLC; Nashville, TN) and algorithms developed at Vanderbilt University.14,15 The CranialCloud architecture addresses issues of patient privacy by allowing sharing of encrypted and deidentified data between institutions, while retaining potential patient identifiers within each institution, according to respective IRB policies.
Participants
Patients who underwent LITT for mTLE with at least one-year follow-up were included in this study. A total of 274 patients had undergone local multidisciplinary evaluation for DRE, at which time each institution’s epilepsy program had agreed upon the diagnosis of mTLE and determined the appropriateness of AHC LITT. The operative procedure has been previously described and involves stereotactic placement of a laser fiber and MRI thermometry for real-time feedback of the ablation.4,5,13 Given the retrospective nature of this study, each institution performed the procedure according to its own practices, with no effort to standardize across institutions. At least one year of clinical follow-up was available for 234 patients. To critically assess ablation location, patients were required to have a preoperative volumetric 1-mm3 voxel-size T1-weighted MRI of the brain; as well as postoperative volumetric 1-mm3 voxel-size T1-weighted gadolinium-enhanced MRI of the brain, in which the ablation cavity could be clearly identified. A total of 175 MRI pairs were analyzed after excluding patients with insufficient imaging [Figure 1].
Figure 1 –
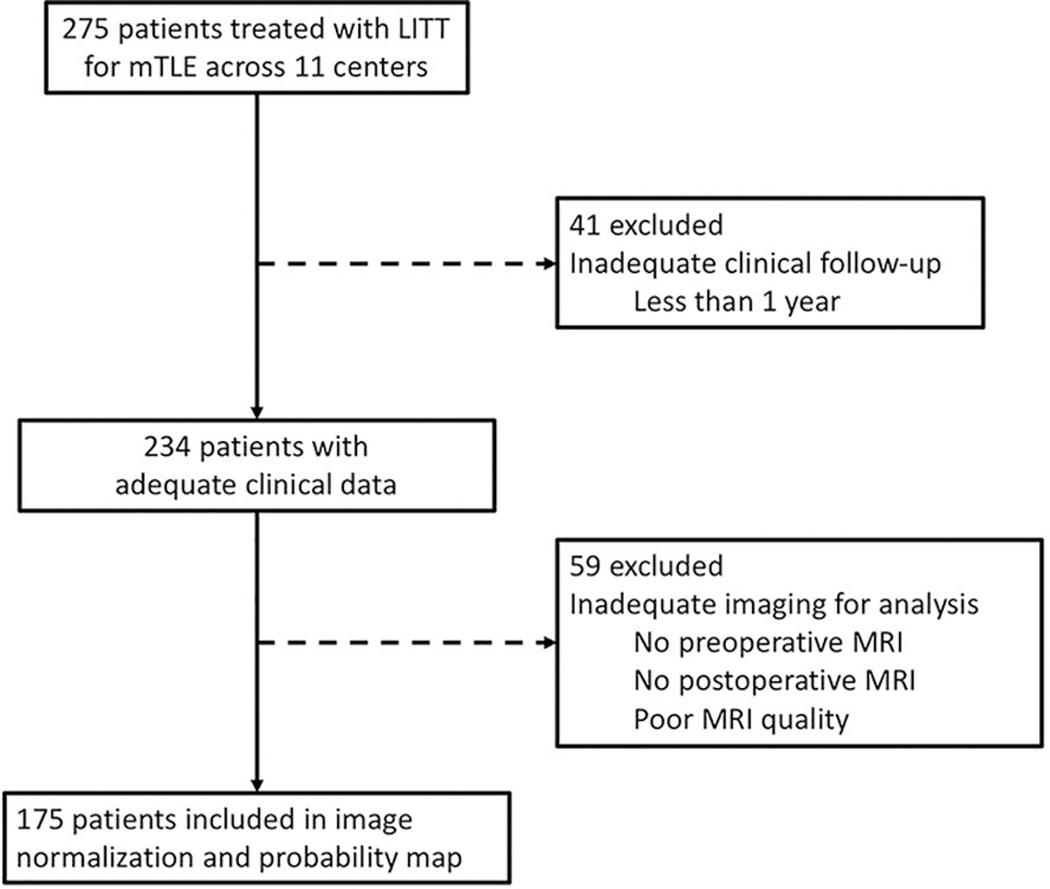
Flowchart of subject selection for analysis of clinical outcomes and calculation of the Engel I outcomes probability map in normalized atlas space.
Data Collection
For 234 subjects, clinical data were obtained retrospectively from each participating institution. Demographic data consisted of sex and handedness. Clinical variables consisted of laterality of mTLE, presence of rHS on MRI, presence of a lesion aside from rHS on MRI (dual pathology), concordant positron emission tomography (PET) temporal hypometabolism, seizure type (focal aware seizures (FAS), focal impaired awareness seizures (FIAS), focal to bilateral tonic-clonic (FTC) seizures), concordance of video-EEG, and use of intracranial EEG (iEEG) monitoring prior to LITT. Radiographic interpretations regarding the presence of rHS or dual pathology were initially performed by a neuroradiologist and confirmed by a consensus decision at each institution’s multidisciplinary surgical epilepsy conference. Similarly, to define the region of seizure onset, the interpretation of long-term video-EEG was agreed upon during this same conference. Clinical outcomes consisted of seizure outcomes (Engel score) at 6-month intervals until their most recent follow-up, the presence of intracranial hemorrhage on postoperative imaging, and reported postoperative complications. The retrospective nature of this study and the variability in neuropsychological panels performed at each institution prevented collection of standardized quantitative cognitive and mood assessments across the cohort.
Ablation Cavity Quantification
All ablation cavities were manually traced by an investigator blinded to seizure outcomes. By convention, the ring of gadolinium enhancement was included within the segmented ablation volume, as this enhancement represents blood-brain barrier breakdown. Since this postoperative sequence is acquired immediately after completion of ablation, there is no time-dependent variability in the radiographic appearance of the ablation between patients.13
Image Normalization
In order to compare the specific location of the ablation across patients, pre- and post-operative scans for each subject underwent non-linear registration to a common reference space derived from 7-Tesla MRI. We have previously described and validated this method to have a mean error of 1.34-mm (~1 voxel).15
Volume of Ablation
The extent to which the amygdala and hippocampus were ablated was calculated for each subject in common atlas space. Once normalized, the manually segmented ablation cavity was superimposed over anatomical structures, which allowed for the calculation of the percentage of amygdala and hippocampus ablated. [Figure 2]
Figure 2 –
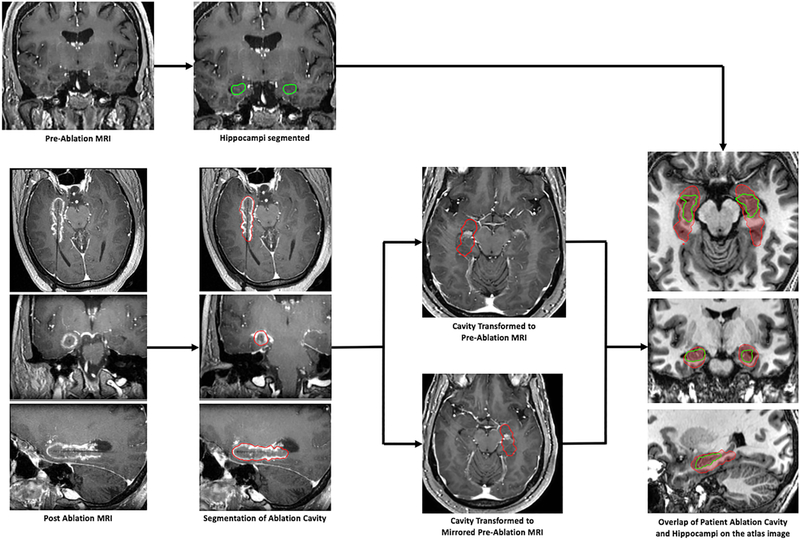
Workflow for image-processing. For each patient, the ablation cavity was manually segmented according to previously-described methods.13 Preoperative and postoperative images along with the manually-segmented ablation cavity were normalized to a common reference space.15 Once completed for the entire cohort, a critical population-based analysis of ablation volumes and locations could be performed.
Statistical Analysis of Clinical Variables
Two binary indicator variables were derived to identify whether a patient had (1) an Engel I outcome or (2) either Engel I or II outcomes at 6-, 12-, 18-, 24-months, and at last follow-up. Univariate logistic regression was performed using the aforementioned demographic variables, clinical variables, and complications as independent variables. Missing data was removed prior to performing the regressions. Odds ratios (OR) were used to estimate the effect of candidate independent variables on either freedom from disabling seizures or being almost seizure free (p<0.05). Multivariate logistic regression was subsequently performed.
Statistical Analysis of Ablation Location
Each ablation cavity was duplicated and mirrored to the contralateral side such that regardless of the side of ablation, each patient would contribute to ablation locations over an AHC in atlas space. [Figure 2] The reliability of the mirrored image is only possible through the utilization of our validated non-linear image normalization algorithm. An aggregate ablation map was first generated corresponding to the frequency each voxel was ablated across the entire cohort.
To analyze the role of the ablation location in seizure outcome, a positive predictive value (PPV) map (association between Engel I outcomes and the ablation of a voxel) and a negative predictive value (NPV) map (association between Engel II-IV outcomes and not ablating a voxel) were calculated. The details of this statistical analysis are delineated in the Supporting Information; but in short, Bayesian models were generated for each ablated voxel to quantify the probability of Engel I outcome at last follow-up. Each voxel was treated independently because generation of models with dependencies on either patient or outcome groups would not only be exceedingly complex to generate, but also difficult to clearly interpret.
Finally, in order to translate these findings, a theoretical favorable ablation zone with the dimensions of a typical ablation cavity was generated. Since the highest NPVs represent voxels associated with the most significant chance of persistent seizures if not ablated, all values greater than 50% were included in this theoretical zone. This zone was then translated anteriorly and medially as to maximize inclusion of the highest PPVs. Although multiple theoretical unfavorable ablation zones may exist, such a zone was generated by excluding all NPVs less than 80% and minimizing inclusion of the highest PPVs.
Results
Seizure Outcomes
At 1-year postoperatively, 134 of 231 patients (58.0%) achieved Engel I outcomes; and 178 patients (77.1%) achieved either Engel I or II outcomes. At 18-months, 82 of 161 patients (50.9%) achieved Engel I outcomes; and 118 patients (73.3%) achieved either Engel I or II outcomes. At 2-years, 96 of 167 patients (57.5%) achieved Engel I outcomes; and 134 patients (80.2%) achieved either Engel I or II outcomes. At last follow-up of at least 1-year (30±14 months, 12–75 months), 134 of 234 patients (58.0%) achieved Engel I outcomes; and 180 patients (76.9%) achieved either Engel I or II outcomes.
Given the retrospective nature of this study, seizure outcomes were not available at all time intervals for all patients. In combination with limited follow-up in some patients, this limitation resulted in different cohort sizes at each time point. Statistical analyses of demographic information and clinical details relative to seizure outcomes is summarized in Table 1.
Table 1 –
Patient demographics and characteristics relative to seizure outcomes. Reported are p-values for the univariate analysis between each variable and seizure outcome. Significant values (p<0.05) are denoted by bold font.
| Entire Cohort | Association with Engel I outcome | Association with Engel I or II outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 234 | p-value | p-value | ||||||||
| μ ± σ | range | 6 months |
12 months |
18 months |
24 months |
6 months |
12 months |
18 months |
24 months |
|
| Age [years] | 42 ± 15 | (7 – 82) | 0.005 | 0.213 | 0.477 | 0.652 | 0.020 | 0.001 | 0.053 | 0.093 |
| Follow-up[months] | 30 ± 14 | (12 – 75) | ||||||||
| n | % | |||||||||
| Gender | ||||||||||
| Female | 124 | 53.0% | 0.984 | 0.636 | 0.185 | 0.534 | 0.340 | 0.210 | 0.729 | 0.643 |
| Handedness† | ||||||||||
| Right | 161 | 83.4% | ||||||||
| Left | 29 | 15.0% | 0.533 | 0.218 | 0.324 | 0.438 | 0.611 | 0.656 | 0.929 | 0.679 |
| Ambidextrous | 3 | 1.6% | ||||||||
| Side of LITT | ||||||||||
| Left | 136 | 58.1% | 0.604 | 0.732 | 0.719 | 0.953 | 0.804 | 0.691 | 0.690 | 0.440 |
| rHS on MRI | ||||||||||
| Yes | 172 | 73.5% | 0.048 | 0.289 | 0.225 | 0.613 | 0.232 | 0.587 | 0.751 | 0.969 |
| Dual Pathology on MRI† | ||||||||||
| Yes | 49 | 21.1% | 0.504 | 0.454 | 0.043 | 0.171 | 0.322 | 0.933 | 0.206 | 0.537 |
| PET† | ||||||||||
| Ipsilateral | 139 | 73.5% | 0.749 | 0.423 | 0.140 | 0.262 | 0.879 | 0.670 | 0.660 | 0.924 |
| Negative | 29 | 15.3% | ||||||||
| Bilateral | 16 | 8.5% | ||||||||
| Contralateral | 3 | 1.6% | ||||||||
| Seizure Type* | ||||||||||
| FIAS | 223 | 95.3% | 0.311 | 0.392 | 0.506 | 0.158 | 0.800 | 0.701 | 0.369 | 1.000 |
| FTC | 107 | 45.7% | 0.841 | 0.506 | 0.490 | 0.106 | 0.016 | 0.007 | 0.109 | 0.030 |
| FAS | 38 | 16.2% | 0.684 | 0.708 | 0.327 | 0.870 | 0.206 | 0.074 | 0.078 | 0.346 |
| EEG Localization† | ||||||||||
| Ipsilateral Temporal | 180 | 83.7% | 0.427 | 0.175 | 0.302 | 0.069 | 0.630 | 0.379 | 0.551 | 0.618 |
| Bitemporal | 20 | 9.3% | ||||||||
| Multifocal | 5 | 2.3% | ||||||||
| Non-localized | 6 | 2.8% | ||||||||
| Ipsilateral Extratemporal | 3 | 1.4% | ||||||||
| Contralateral Temporal | 1 | 0.5% | ||||||||
| Invasive Monitoring | ||||||||||
| Yes | 48 | 20.5% | 0.978 | 0.959 | 0.475 | 0.942 | 0.749 | 0.696 | 0.564 | 0.950 |
Since patients may have more than one seizure types, each patient could belong to multiple seizure type categories. For this reason, the total percentage of patients for this variable is greater than 100%.
Variables with missing data. The numbers and percentages reported reflect the number of patients missing data.
There were no significant differences in seizure outcomes between the rHS and non-rHS subgroups after 6-months follow-up [Figure 3]. Even after excluding patients with dual pathology from this rHS subgroup, no significant difference was seen at any time point (p≥0.078). Implementation of iEEG was not associated with seizure outcome (p≥0.475); but since iEEG monitoring in patients without rHS may confirm isolated mesial onset and the use of iEEG was disproportionately high in the non-rHS group (n = 33, p<0.001), further analysis was performed by combining non-rHS patients who underwent iEEG with the rHS subgroup. This cohort of rHS patients plus non-rHS patients with iEEG confirmation of mesial onset did not experience greater rates of Engel I outcomes either (p≥0.239).
Figure 3 –
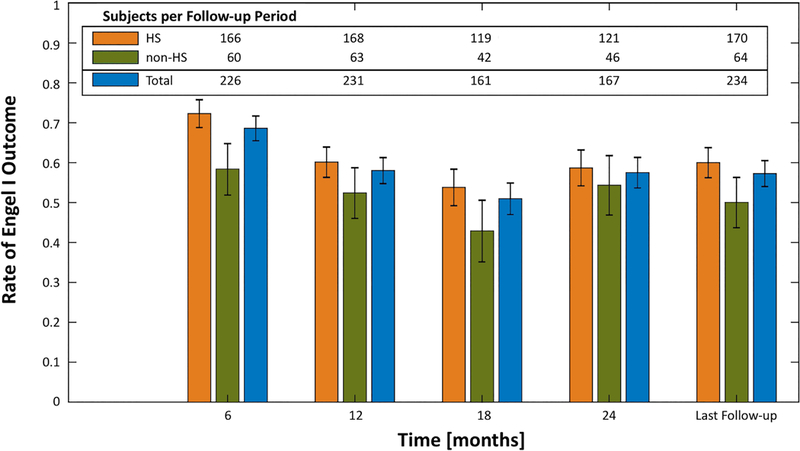
Rates of Engel I outcome at 6-month epochs for the entire cohort, the rHS subgroup, and the non-rHS subgroup. Error bars represent the standard error. Univariate regression demonstrated no significant difference in the 95% confidence intervals between the rHS and non-rHS subgroups. Seizure outcomes were durable between 12-months after LITT and at last follow-up.
Patients with a history of FTC were less likely to demonstrate Engel I outcomes at last follow-up (OR=0.52, 95%CI=0.27–0.98, p≤0.042) and either Engel I or II outcomes at 6-months, 12-months, and last-follow-up (OR=0.31–0.38, 95%CI=0.14–0.83, p≤0.014).
Age was not associated with long-term Engel I or II outcomes. At no time point was sex, handedness, side of ablation, side of MTLE, dual pathology, ablation of the dominant hemisphere, concordant PET hypometabolism, or concordance of video-EEG significantly associated with seizure outcomes.
Complications
Postoperative hemorrhage was identified in three patients (1.3%), of which one was associated with clinical symptoms (transient double vision). A total of 42 complications were recorded for 35 patients (15.0%); of which 8 were transient neurologic deficits and 34 were persistent at last follow-up. Visual disturbances were most common (5.1%), followed by worsening of a preexisting affective disorder (4.3%). Given the retrospective nature of the study, details regarding the severity of symptoms were not consistently available. Similarly, language and memory deficits could not quantified and are likely underestimated. The one death in this cohort was attributed to sudden unexplained death in epilepsy (SUDEP) occurring 12 months postoperatively. Recorded complications are detailed in the Supporting Information.
The presence of a postoperative complication was associated with a lower chance of Engel I outcomes at 24-months and at last follow-up (OR=0.18–0.26, 95%CI=0.04–0.86, p≤0.026).
Volume of Ablation for Mesial Structures
Multivariate regression analysis demonstrated that more extensive amygdalar ablation was associated with Engel I outcomes at 6-, 12-, 18-months, and at last follow-up (OR=1.60–1.77 per additional percent ablated, p≤0.040); and increasing hippocampal ablation was associated with a decreased chance of Engel I outcomes at 6-, 18-, and 24-months (OR=0.04 per additional percent ablated, p≤0.040).
Ablation Location
The aggregate ablation map for 175 patients illustrates that all ablations were centered around the long-axis of the AHC [Figure 4]. The overall diameter of the ellipsoid representing all possible ablated voxels measures approximately 30-mm in the coronal plane. Since the ablation is roughly centered around the laser catheter and has an average diameter of 15-mm, we can estimate a maximal difference in implanted probe position of approximately 15-mm. This finding highlights the degree of variability that currently exists in AHC targeting for LITT.
Figure 4 –
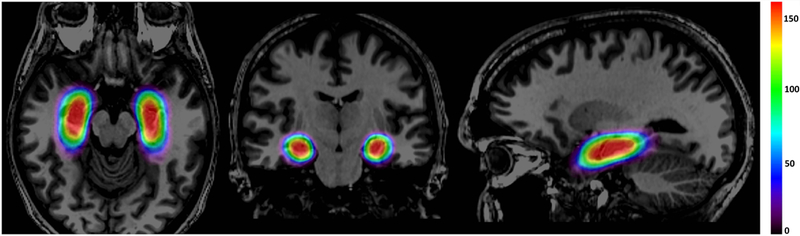
Heat map of the distribution of ablations in 175 patients treated across 11 comprehensive epilepsy centers. Effectively all ablations (red) were centered around the long-axis of the AHC and extended posteriorly to the level of the lateral mesencephalic sulcus. Variation in ablation location is represented by the less frequently ablated regions (green and blue) extending from this central core.
The PPV and NPV maps for Engel I outcomes associated with each voxel are shown in Figure 5. The theoretical favorable and unfavorable ablations are delineated in Figure 6. Ablation of more mesial, anterior, and inferior structures of the temporal lobe – including the amygdala, hippocampal head, parahippocampal gyrus, entorhinal cortex, and perirhinal cortex – were associated with higher rates of Engel I outcomes. Excluding these structures from the ablation and focusing only around the hippocampal body and tail was associated with persistent seizures. Ablations extending posteriorly beyond the coronal plane in line with the lateral mesencephalic sulcus were less likely to be associated with Engel I outcomes and avoiding these posterior voxels was not associated with high rates of persistent seizures.
Figure 5 –
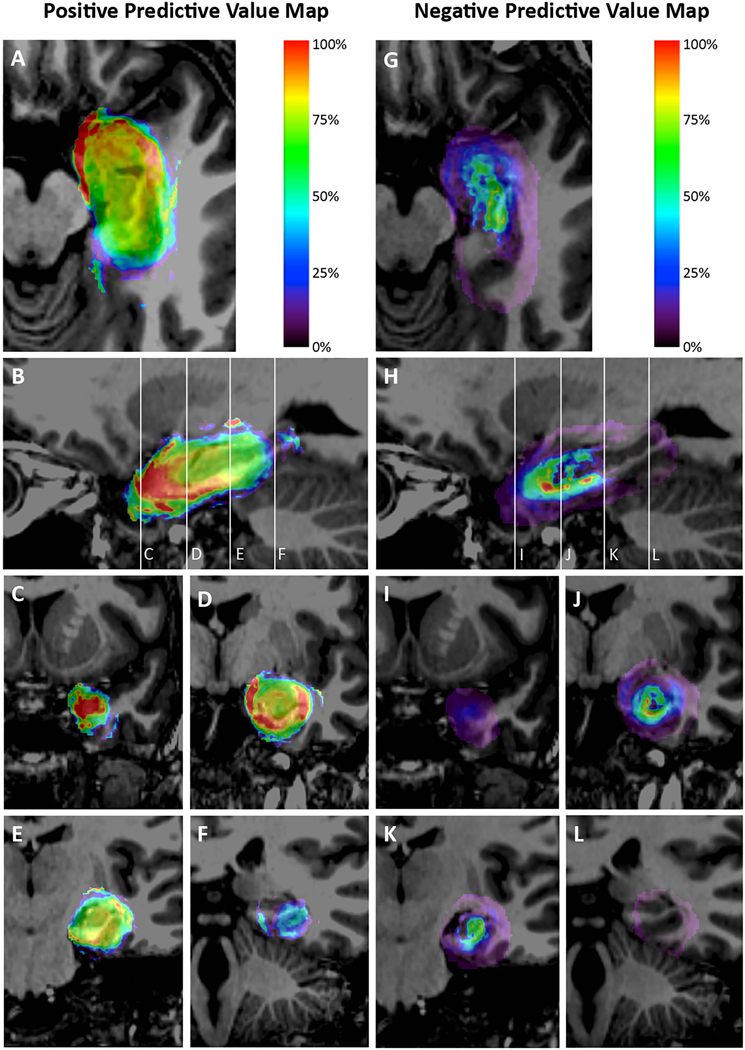
Maps representing the positive predictive value (PPV) [A-F] and negative predictive value (NPV) [G-L] of Engel I outcome associated with at least 1-year follow-up for each voxel ablated in normalized atlas space for 175 patients. Panels A and G represent axial views; Panels B and H represent sagittal views; and Panels C-F and I-L represent coronal views through the temporal pole, hippocampal head and posterior amygdala, hippocampal body, and hippocampal tail as represented by the reference lines on the sagittal image. Voxels were assigned a color if it was involved in the ablation of at least 4 patients. Each voxel was analyzed independently. Both maps demonstrate the importance of targeting the anterior, medial, and inferior structures in the mesial temporal lobe. Ablations extending posteriorly beyond the coronal plane in line with the lateral mesencephalic sulcus were less likely to be associated with Engel I outcomes.
Figure 6 –
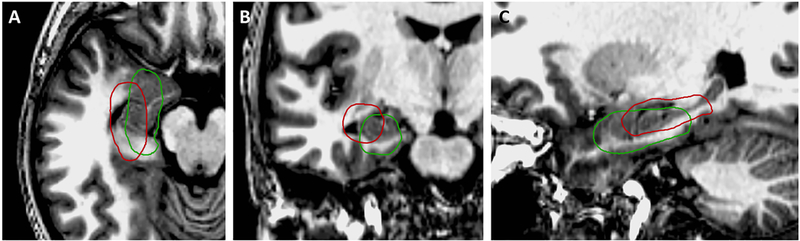
Theoretical favorable (green) and unfavorable (red) ablation locations based on the PPV and NPV maps. Both ablations are of roughly the same volume, but are located and oriented differently within the mesial temporal structures. The theoretical favorable ablation is located more anteriorly, medially, and inferiorly to cover the high probability voxels for both the PPV and NPV maps. This ablation covers the amygdala, hippocampus, parahippocampal gyrus, and rhinal cortices. The theoretical suboptimal ablation is located more posteriorly, laterally, and superiorly to exclude the high probability voxels for both the PPV and NPV maps. This ablation covers the posterolateral amygdala and hippocampus, but misses a large part of the amygdala, the mesial hippocampal head, parahippocampal gyrus, and rhinal cortices.
Discussion
To date, small single-institution series have suggested that AHC LITT is a viable treatment for drug-resistant mTLE.3–6,8–12 Unfortunately, without consideration of specific therapy location, which is subject to variability in patient anatomy and the surgery itself, these studies are limited in their ability to reliably report clinical outcomes. In this study we have: (1) proposed a novel robust methodology using non-linear normalization and statistical models to study complex brain therapies such as LITT for mTLE; (2) applied it to previously existing data in the largest and most comprehensive multicenter study to date; (3) demonstrated in 234 patients with at least 1-year follow-up that 58.0% achieved Engel I outcomes and 76.9% achieved either Engel I or II outcomes at the time of last follow-up; and (4) present the first three-dimensional model of a favorable laser ablation zone.
Patient Factors
The presence of rHS on MRI has been associated with favorable surgical outcomes.6,9,12,16 Our findings, however, agree with recent studies, which have suggested that LITT is effective in properly selected patients with mTLE, regardless of isolated rHS.11,17 In truth, the presence of rHS should not be viewed as a singular epilepsy phenotype, but rather an attribute of distinct MTLE subtypes.18 As such, the importance of a thorough preoperative assessment leveraging multiple modalities used to localize seizure onset emphasizes the need for LITT candidates to be evaluated at an experienced comprehensive epilepsy center.
In line with prior analyses of epilepsy surgery,19 FTC seizures were negatively associated with seizure outcome. Particular caution should therefore be exercised when performing AHC LITT in patients with a history of FTC seizures, as they were 62% less likely to become almost seizure free. This finding can be explained by the fact that patients with FTC are more likely to have lateral neocortical rather than mesial onset.20
A shorter duration of epilepsy at the time of surgical intervention has also been described as a positive prognostic indicator.21,22 Unfortunately, given the retrospective nature of this study, such data was not reliably available from all participating centers and was therefore not included in our analysis.
Surgical Factors
An appropriate surgical approach is critical to optimizing clinical outcomes in properly selected patients. The extent of surgical resection has remained controversial. Studies have emphasized the need for sufficient hippocampal resection to maximize chances of seizure freedom.23,24 At the same time, selective amygdalohippocampectomy has been shown to provide outcomes comparable to standard anterior temporal lobectomy;25,26 and the extent of hippocampal resection has been found to have no bearing on seizure freedom.26–28 Prior studies of LITT for mTLE have demonstrated no relationship between the ablation volumes and seizure outcomes.3,4,8
When considering these recommendations, one must acknowledge the considerable complexity of accurately measuring of surgical resections. After a craniotomy, tissue manipulation, removal of brain tissue, loss of cerebrospinal fluid, gravity, and use of osmotic therapy distort patient anatomy on postoperative imaging.29 As such, measurements on postoperative images remain challenging for procedures like a temporal lobectomy. The minimally-invasive nature LITT, however, results in minimal anatomical distortion and affords more accurate quantification of the intervention.
Multivariate regression analysis revealed that more extensive amygdalar ablation was associated with greater chances of Engel I outcomes at almost every time point. While more extensive hippocampal ablation was negatively associated with Engel I outcomes at almost every time point, this association was modest (OR=0.04 per additional percent ablation) relative to those seen with volumes of amygdala ablated (OR=1.60–1.77 per additional percent ablated). This finding may suggest that beyond some threshold volume of hippocampal ablation, further ablation may be counterproductive. In order to ablate greater volumes of the hippocampus, the laser trajectory would also have to involve the hippocampal body and tail posteriorly – consequently compromising ablation of the hippocampal head and amygdala. Further analysis is required to fully investigate this possibility.
Our investigation was therefore extended beyond ablation volumes with the goal of better understanding optimal targeting for LITT in mTLE. The requirement of cannulating the curved AHC with a straight laser catheter forces a compromise on ablation coverage. With a single laser trajectory, one must decide whether the amygdala and hippocampal head, entorhinal cortex and parahippocampal gyrus, versus hippocampal tail should be prioritized. Little data currently exists to guide surgeons in their decision making, which is the likely reason significantly different therapy locations exist between patients – we estimated a maximal targeting difference of approximately 15-mm [Figure 4].
Given their complementary nature, PPV and NPV maps must be considered along with the map of ablation distributions. Of note, caution must be exercised at the periphery of these maps, which may be comprised of no more than half the cohort and is susceptible to extreme values. It is also important to remember that statistics were calculated independently for each voxel – resulting in artificial regional clusters with similar probability values. Given the capabilities of LITT and the consequent range of ablation volumes included in this analysis, we can only generalize our findings to ablations of comparable shapes and sizes. One cannot conclude that ablation of only high PPV or NPV regions in isolation will maximize chances of Engel I outcomes. Instead, as we have demonstrated in Figure 6, we can infer that inclusion of these regions within a typical ablation cavity will maximize chances of Engel I outcomes; while exclusion of these regions may be detrimental to chances of Engel I outcomes.
We have demonstrated that patients have the greatest opportunity for Engel I outcomes when the ablation includes anteromesial temporal lobe structures including the amygdala, which coincides with our findings on amygdalar ablation volumes. Further examination of the maps highlights the importance of targeting the mesial hippocampus, parahippocampal gyrus, entorhinal cortex, and perirhinal cortex – as missing these structures frequently resulted in persistent seizures. The rhinal cortices are known to be highly epileptogenic and intensely interconnected within the limbic network;30 and Jermakowicz et al has previously described the importance of ablating the mesial hippocampal head.4
Extending the ablation beyond the coronal plane in line with the lateral mesencephalic sulcus yields diminishing returns. More importantly, ablations that extend further posteriorly have been associated with damage to the optic radiations with resultant visual field deficits.31 The lack of significant benefit with ablation of the hippocampal tail posteriorly may be related to the negative relationship between hippocampal ablation volumes and seizure outcome – as ablation of the hippocampal tail with a straight laser catheter necessitates a compromise of hippocampal head coverage.
Overall, this study sheds new light on previously published results by demonstrating the importance of therapy location in LITT. Specifically, the wide range of seizure freedom rates published to date may stem not only from the fact that these studies may have been underpowered, but also from differences in ablation locations. In order to maximize the opportunity for seizure freedom, properly selected patients must undergo LITT of the appropriate mesial structures. Previously, variability in surgical targeting has not been consistently taken into consideration – an approach analogous to analyzing the efficacy of a new drug without factoring in drug serum levels or even the administered dosages. Ultimately, ablating the necessary anatomical structures is more important than the total amount of tissue ablated with LITT. That being said, a significant amount of work is still needed to further elucidate the nuances of ablation location and its impact on clinical outcomes. With strict criteria of therapy delivery, LITT may be associated with higher rates of seizure control for appropriately selected patients with mTLE.
Complications
The observed overall complication rate of 15.0% is comparable to existing has received on surgery for mTLE.32,33 The 1.3% rate of radiographic hemorrhage, 0.4% rate of symptomatic hemorrhage, and 0.4% rate of permanent deficit from hemorrhage is comparable to reported rates in stereotactic neurosurgical procedures.34 The most common complications observed were affective disorders and visual disturbances. Comorbid mood disturbances in epilepsy patients with worsening of symptoms after temporal lobe surgery have been described.35 Unfortunately, the retrospective nature of this study limited our ability to quantify and critically evaluate neuropsychological changes in a large multicenter cohort. It is also unknown if these complications would resolve as patients are followed beyond the time constraints of this study. Visual disturbances such as diplopia and visual field loss have also been commonly described complications of temporal lobe surgery.10,29,36,37 Visual complications in 5.1% of this cohort is lower than what has been reported for both LITT and temporal lobe resections. Interestingly, the presence of a postoperative complication was associated with poorer seizure outcome. Given the importance of ablation location on seizure outcomes, suboptimal ablation may result in both a decreased opportunity for seizure freedom and increased complications.
Limitations
The retrospective nature of this study is its major limitation. Specific data points were obtained from each center through a chart review to develop a uniform data set for statistical analysis. As such, the analysis is subject to both recall and misclassification bias. Complications, in particular, may have been underreported as minor complications not documented in the medical record would have been missed. Similarly, the details of each complication were not consistently available, which limits a clear interpretation of its impact.
As previously noted, seizure outcome data was only recorded in 6-month epochs, rather than at every available time point. Furthermore, outcome data was not consistently available for all epochs, which explains the presence of larger numbers of subjects at later time points. As a result of such missing data, a formal Kaplan-Meier analysis could not be properly performed – as it would serve as a skewed representation of a small subgroup of the entire cohort. Instead, we have reported the proportion of patients with Engel I outcomes at each 6-month time point after LITT.
This study design also precluded critical assessment the effect of LITT on neuropsychological outcomes. Both the temporal pole and basal temporal regions have been associated with category-specific naming deficits and a more inferior ablation could lessen the described cognitive benefits of LITT.7,38 Further analysis of the specific effects of LITT location on cognitive and mood outcomes are necessary.
The image-based analysis depends entirely on the accuracy of the non-linear registration algorithm. Although we chose to accept the mean error of 1.34-mm (~1 voxel), this certainly serves as a potential source of error. Ultimately, we believe that this compromise does not significantly affect the overall results of the image-based analysis presented here.
Finally, it is again important to note that the calculation of PPV and NPV maps treated each voxel independently and did not group voxels by patients or outcomes. As delineated above, this limits the generalizability of our findings and mandates a degree of caution in the interpretation of the resulting probability maps.
Conclusion
This work represents the first multicenter study of LITT for mTLE; and the largest LITT series to date with long-term follow-up of seizure outcome. The Engel I outcome seen in 58% of the cohort is comparable to what has been published to date; and the persistence of this seizure outcome at 2-years demonstrates the durability of this therapy. Radiographic evidence of hippocampal sclerosis was not associated with seizure outcome. Patients presenting with a history of FTC are less likely to experience either Engel I or II outcomes. Consideration of surgical factors – with a focus on ablation location more so than ablation volumes alone – is imperative to the complete assessment of LITT. Our novel method of image analysis in this large multicenter cohort has revealed that ablations prioritizing the amygdala, but also including the hippocampal head, parahippocampal gyrus, and rhinal cortices are associated with a greater chance of seizure freedom; while extending the ablation to the hippocampal tail yields diminishing returns. While this serves as the first step towards standardizing targeting for LITT in mTLE, further work with the incorporation of more complete complications data is necessary to refine this analysis and define the minimal zone of ablation necessary to maximize seizure control and minimize associated morbidity.
Supplementary Material
Key Point Box:
Specifics details of surgical targeting must be taken into consideration when assessing the efficacy of a surgical intervention.
We introduce a novel technique to normalize and quantify LITT across subjects from multiple centers in three-dimensional atlas space.
LITT for mTLE should prioritize ablation of the amygdala, hippocampal head, parahippocampal gyrus, and rhinal cortices.
Ablations posterior to the lateral mesencephalic sulcus yields diminishing returns and has been associated with increased complications.
Further work is needed to further elucidate the nuances of ablation location and its impact on seizure and non-seizure outcomes.
Acknowledgements
Study funded by NIH (R01 NS095291)
Disclosure of Conflicts of Interest:
Dr. Wu has received grants and consulting fees from Medtronic, Inc., and consulting fees from Neuropace, Inc. Dr. Sharan has received consulting fees from Cerebral Therapeutics, and is a board member of Neurotargeting, LLC. Dr. Sperling has received grants from Medtronic, Inc., grants from UCB Pharma, grants from Takeda, grants from Eisai, grants from Neurelis, grants from SK Life Science, grants from Pfizer, grants from Accorda, grants from Sunovion, grants from Engage, grants from Cavion, grants from Marinus, grants from Upsher-Smith, and grants from Brain Sentinel. Dr. Ojemann has a patent US9522081B2 issued for “Methods and devices for brain cooling for treatment and/or prevention of epileptic seizures”; and is a member and advisory board member of the Epilepsy Foundation of Washington. Dr. Miller has received grants from Medtronic Inc. Dr. Sheth has received consulting fees from Medtronic, Inc., and consulting fees from Koh Young. Dr. Popli has received grants from Medtronic, Inc. Dr. Mehta has received consulting fees from Medtronic, Inc. Dr. Englot has received consulting fees from Medtronic, Inc. Dr. Neimat has received consulting fees from Monteris Inc. Dr. Konrad has received grants from Medtronic, Inc., grants from Microtransponder, and is a co-founder and board member of Neurotargeting, LLC. Dr. Air has received grants from Medtronic Inc. Dr. Schwalb has received grants from Medtronic, Inc., and consulting fees from Guidant, LLC. Dr. Dawant has received grants from the NIH and is a co-founder and board member of Neurotargeting, LLC. Dr. D’Haese has received grants from the NIH and is a co-founder and CEO of Neurotargeting, LLC.
Dr. Wu, Dr. Sharan, Dr. Sperling, Dr. Jagid, Dr. Ko, Dr. Ojemann, Dr. Miller, Dr. McKhann, Dr. Laxton, Dr. Couture, Dr. Popli, Dr. Mehta, Dr. Halpern, Dr. Air, and Dr. Schwalb, also report serving as an investigator in the current Stereotactic Laser Ablation for Temporal Lobe Epilepsy (SLATE) trial (Medtronic) for laser ablation in temporal lobe epilepsy. No financial compensation received. No patients in this study are part of the SLATE trial (no patient overlap).
Dr. Jermakowicz, Ms. Chakravorti, Dr. Cajigas, Dr. Matias, Dr. Buckley, Dr. Youngerman, Mr. Smith, Dr. Ho, Mr. Neal, Dr. Vale, and Dr. Holloway have nothing to disclose.
Footnotes
Ethical Publication Statement:
We confirm that we have read the Journal’s position on issues involved in ethical publica- tion and affirm that this report is consistent with those guidelines.
References
- 1.Téllez-Zenteno JF, Hernández-Ronquillo L. A review of the epidemiology of temporal lobe epilepsy. Epilepsy Res Treat 2012; 2012: 630853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiebe S, Jetté N. Epilepsy surgery utilization: who, when, where, and why? Curr Opin Neurol 2012; 25: 187–193. [DOI] [PubMed] [Google Scholar]
- 3.Kang JY, Wu C, Tracy J, et al. Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia 2016; 57: 325–334. [DOI] [PubMed] [Google Scholar]
- 4.Jermakowicz WJ, Kanner AM, Sur S, et al. Laser thermal ablation for mesiotemporal epilepsy: Analysis of ablation volumes and trajectories. Epilepsia 2017; 58: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willie JT, Laxpati NG, Drane DL, et al. Real-Time Magnetic Resonance-Guided Stereotactic Laser Amygdalohippocampotomy for Mesial Temporal Lobe Epilepsy. Neurosurgery 2014; 74: 569–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gross RE, Stern MA, Willie JT, et al. Stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Ann Neurol 2018; 83: 575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drane DL, Loring DW, Voets NL, et al. Better object recognition and naming outcome with MRI-guided stereotactic laser amygdalohippocampotomy for temporal lobe epilepsy. Epilepsia 2015; 56: 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vakharia VN, Sparks R, Li K, et al. Automated trajectory planning for laser interstitial thermal therapy in mesial temporal lobe epilepsy. Epilepsia 2018; 59: 814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao JX, Wu S, Lacy M, et al. Stereotactic EEG-guided laser interstitial thermal therapy for mesial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 2018; 89: 542–548. [DOI] [PubMed] [Google Scholar]
- 10.Grewal SS, Zimmerman RS, Worrell G, et al. Laser ablation for mesial temporal epilepsy: a multi-site, single institutional series. J Neurosurg Epub 2018. July 1. [DOI] [PubMed] [Google Scholar]
- 11.Donos C, Breier J, Friedman E, et al. Laser ablation for mesial temporal lobe epilepsy: Surgical and cognitive outcomes with and without mesial temporal sclerosis. Epilepsia 2018; 59: 1421–1432. [DOI] [PubMed] [Google Scholar]
- 12.Le S, Ho AL, Fisher RS, et al. Laser interstitial thermal therapy (LITT): Seizure outcomes for refractory mesial temporal lobe epilepsy. Epilepsy Behav 2018; 89: 37–41. [DOI] [PubMed] [Google Scholar]
- 13.Wu C, Boorman DW, Gorniak RJ, Farrell CJ, Evans JJ, Sharan AD. The Effects of Anatomic Variations on Stereotactic Laser Amygdalohippocampectomy and a Proposed Protocol for Trajectory Planning. Neurosurgery 2015; 11: 345–357. [DOI] [PubMed] [Google Scholar]
- 14.D’Haese P-F, Konrad PE, Pallavaram S, et al. CranialCloud: a cloud-based architecture to support trans-institutional collaborative efforts in neurodegenerative disorders. Int J Comput Assist Radiol Surg 2015; 10: 815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakravorti S, Jermakowicz WJ, Wu C, et al. Evaluation of Nonrigid Registration Around the Hippocampus for the Construction of Statistical Maps in a Multicenter Dataset of Epilepsy Laser Ablation Patients. Proc. SPIE Med Imaging 2019. Image-Guided Procedures, Robotic Interventions, and Modeling, 109511J (8 March 2019). [Google Scholar]
- 16.Ivanovic J, Larsson PG, Østby Y, et al. Seizure outcomes of temporal lobe epilepsy surgery in patients with normal MRI and without specific histopathology. Acta Neurochir (Wien) 2017; 159: 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Youngerman BE, Oh JY, Anbarasan D, et al. Laser ablation is effective for temporal lobe epilepsy with and without mesial temporal sclerosis if hippocampal seizure onsets are localized by stereoelectroencephalography. Epilepsia 2018; 59: 595–606. [DOI] [PubMed] [Google Scholar]
- 18.Kahane P, Bartolomei F. Temporal lobe epilepsy and hippocampal sclerosis: lessons from depth EEG recordings. Epilepsia 2010; 51: 59–62. [DOI] [PubMed] [Google Scholar]
- 19.Jobst BC, Cascino GD. Resective Epilepsy Surgery for Drug-Resistant Focal Epilepsy: A Review. JAMA 2015; 313: 285–293. [DOI] [PubMed] [Google Scholar]
- 20.Maillard L, Vignal JP, Gavaret M, Guye M, Biraben A, McGonigal A, Chauvel P, Bartolomei F. Semiologic and electrophysiologic correlations in temporal lobe seizure subtypes. Epilepsia 2004; 45: 1590–1599. [DOI] [PubMed] [Google Scholar]
- 21.Sun Z, Zuo H, Yuan D, et al. Predictors of prognosis in patients with temporal lobe epilepsy after anterior temporal lobectomy. Exp Ther Med 2015; 10: 1896–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radhakrishnan A, Menon R, Thomas SV, et al. “Time is Brain”-How early should surgery be done in drug-resistant TLE? Acta Neurol Scand. 2018; 138: 531–540. [DOI] [PubMed] [Google Scholar]
- 23.Joo EY, Han HJ, Lee EK, et al. Resection extent versus postoperative outcomes of seizure and memory in mesial temporal lobe epilepsy. Seizure 2005; 14: 541–551. [DOI] [PubMed] [Google Scholar]
- 24.Wyler AR, Hermann BP, Somes G. Extent of medial temporal resection on outcome from anterior temporal lobectomy: a randomized prospective study. Neurosurgery 1995; 37: 982–990. [DOI] [PubMed] [Google Scholar]
- 25.Wendling A-S, Hirsch E, Wisniewski I, et al. Selective amygdalohippocampectomy versus standard temporal lobectomy in patients with mesial temporal lobe epilepsy and unilateral hippocampal sclerosis. Epilepsy Res 2013; 104: 94–104. [DOI] [PubMed] [Google Scholar]
- 26.Schramm J Temporal lobe epilepsy surgery and the quest for optimal extent of resection: A review. Epilepsia 2008; 49: 1296–1307. [DOI] [PubMed] [Google Scholar]
- 27.Schramm J, Lehmann TN, Zentner J, et al. Randomized controlled trial of 2.5-cm versus 3.5-cm mesial temporal resection in temporal lobe epilepsy–Part 1: intent-to-treat analysis. Acta Neurochir (Wien) 2010; 153: 209–219. [DOI] [PubMed] [Google Scholar]
- 28.Haegelen C, Perucca P, Châtillon C-E, et al. High-frequency oscillations, extent of surgical resection, and surgical outcome in drug-resistant focal epilepsy. Epilepsia 2013; 54:848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayer S, Maier A, Ostermeier M, Fahrig R. Intraoperative Imaging Modalities and Compensation for Brain Shift in Tumor Resection Surgery. Int J Biomed Imaging 2017. Article ID 6028645, 18 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vismer MS, Forcelli PA, Skopin MD, Gale K, Koubeissi MZ. The piriform, perirhinal, and entorhinal cortex in seizure generation. Front Neural Circuits 2015; 9: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin D, Thompson JA, Drees C, et al. Optic Radiation Tractography and Visual Field Deficits in Laser Interstitial Thermal Therapy for Amygdalohippocampectomy in Patients with Mesial Temporal Lobe Epilepsy. Stereotact Funct Neurosurg 2017; 95: 107–113. [DOI] [PubMed] [Google Scholar]
- 32.Mathon B, Navarro V, Bielle F, et al. Complications After Surgery for Mesial Temporal Lobe Epilepsy Associated with Hippocampal Sclerosis. World Neurosurg 2017; 102: 639–650. [DOI] [PubMed] [Google Scholar]
- 33.Pruitt R, Gamble A, Black K, Schulder M, Mehta AD. Complication avoidance in laser interstitial thermal therapy: lessons learned. J Neurosurg 2017; 126: 1238–1245. [DOI] [PubMed] [Google Scholar]
- 34.Zrinzo L, Foltynie T, Limousin P, Hariz MI. Reducing hemorrhagic complications in functional neurosurgery: a large case series and systematic literature review. J Neurosurg 2012; 116: 84–94. [DOI] [PubMed] [Google Scholar]
- 35.Wrench J, Wilson SJ, Bladin PF. Mood Disturbance before and after Seizure Surgery: A Comparison of Temporal and Extratemporal Resections. Epilepsia 2004; 45: 534–543. [DOI] [PubMed] [Google Scholar]
- 36.Schmeiser B, Daniel M, Kogias E, et al. Visual field defects following different resective procedures for mesiotemporal lobe epilepsy. Epilepsy Behav 2017; 76: 39–45. [DOI] [PubMed] [Google Scholar]
- 37.Cohen-Gadol AA, Leavitt JA, Lynch JJ, Marsh WR, Cascino GD. Prospective analysis of diplopia after anterior temporal lobectomy for mesial temporal lobe sclerosis. J Neurosurg 2003; 99: 496–499. [DOI] [PubMed] [Google Scholar]
- 38.Mehta S, Inoue K, Rudrauf D, Damasio H, Tranel D, Grabowski T. Segregation of anterior temporal regions critical for retrieving names of unique and non-unique entities reflects underlying long-range connectivity. Cortex 2016; 75: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


