Abstract
BACKGROUND:
We previously developed enzyme nanoparticles (ENP) of alcohol metabolism. This study was to evaluate protective effects of facilitated removal of blood alcohol and/or acetaldehyde on anti-HIV drugs and alcohol-induced liver injuries.
METHODS:
ENP were prepared for degrading alcohol completely (ENP1) or partially into acetaldehyde (ENP2), which were applied to mice of acute binge or chronic-binge alcohol feeding in the presence of antivirals (ritonavir and lopinavir). Liver pathologies were examined to assess the protective effects of ENP.
RESULTS:
In the acute model, ENP1 and ENP2 reduced the blood alcohol concentration (BAC) by 41% and 32% respectively within 4 hr whereas in control without ENP, BAC was reduced only by 15%. Blood acetaldehyde concentration (BADC) was increased by 39% in alcohol fed mice treated with ENP2 comparing to control. No significant effects of the anti-HIV drugs on BAC or BADC were observed. Plasma alanine aminotransferase (ALT) and expression of liver TNFα were both significantly increased in the alcohol fed mice, which were normalized by ENP1. In the presence of the antivirals, ALT was partially reduced by ENP1 or ENP2. In the chronic model, inflammation, fatty liver and ALT were increased, which were deteriorated by the antivirals. ENP1 partially reduced BAC, BADC, ALT, and expression of inflammation markers of TNFα, F4/80 and IL-6 and lipogenic factors of ACC, LXRα and SREBP1. ENP2 reduced BAC without significant effects on ALT, inflammation or lipogenesis. Antivirals and alcohol synergistically increased expression of organelle stress markers of CHOP, sXBP-1, ATF6 and GCP60. ENP1 reduced BAC, CHOP and sXbp-1. However, no effects of ENP1 were found on ATF6 or GCP60.
CONCLUSIONS:
Removal of blood alcohol and acetaldehyde by the enzyme nanoparticles protects the liver against alcoholic injuries, and the protection is less effective in chronic alcohol and antiviral feeding due to additional drug-induced organelle stresses.
Keywords: drug/alcohol abuse, enzyme nanoparticles, organelle stress, liver injury, treatment of alcohol intoxication
INTRODUCTION
Alcohol drinking has been playing an important role in human cultures. Excessive alcohol consumption, however, is associated with multiple organ disorders (Gunzerath et al., 2010; Friedmann, 2013; Becker, 2017). It has been estimated that there are about 1.2 million emergency visits per year due to acute alcohol intoxication (https://pubs.niaaa.nih.gov/publications/NEDS&NIS-DRM9/NEDS&NIS-DRM9.pdf). A logical and direct approach to address the alcohol use problems may be to remove alcohol quickly and effectively from the body after alcohol is consumed and enjoyed for cultural moments, which may save lives in the emergency room (Cunradi et al., 2012; Beauchamp and Valento, 2016). This requires an effective delivery of all the alcohol-decomposing enzymes such as alcohol dehydrogenase (ADH), alcohol oxidase, aldehyde dehydrogenase (ALDH) and catalase. In the cell, these enzymes are spatially defined within subcellular organelles catalysing consecutive reactions that break down alcohol (Leiber, 1991; Zakhari, 2006). Close spatial confinement of these enzymes enables enhanced catalytic efficiency and effective elimination of toxic intermediates. Mimicking such enzyme compartmentalization has been highly challenging (Stadler et al., 2009; Schmitt et al., 2016). Previously, we employed a novel nanotechnology enabling effective construction of enzyme nanoparticles with synergic and complementary functions in metabolizing alcohol, and their stability is good enough for intravenous codelivery of the enzymes to the liver, where they degrade alcohol and facilitate reduction of blood alcohol concentrations in animals gavaged with alcohol (Liu et al., 2013; Xu et al, 2018). The enzyme nanoparticles serve as a proof of concept in developing therapeutic strategies aiming to directly eliminate alcohol from the body and protect the body against alcohol-related injuries, which can be tested further in a variety of animal models that reflect situations of alcohol drinking in real life. These enzyme nanoparticles can also be designed to digest alcohol completely or partially, which could broaden their applications in differentiating complex pathophysiological mechanisms by alcohol alone or combined with drug abuse. For instance, either alcohol itself or alcohol metabolites such as acetaldehyde has been recognized to promote development of liver disease (Quertemont, 2004; Zakhari, 2006; Eriksson, 2007; Kwon et al., 2014). The contributory role of acetaldehyde to liver pathology could be verified in vivo through applying the enzyme nanoparticles that digest alcohol partially into acetaldehyde. Combination antiretroviral therapies (cART) have been increasingly used in the HIV-infected patient population (Braithwaite et al., 2007). However, nearly half of them consume or abuse alcohol while under the antiretroviral therapies, which deteriorates certain HIV drug-induced hepatotoxicity leading to greater morbidity and mortality (Kao et al., 2012; Volkow and Normand, 2013; Koob and Mason, 2016). The pathological mechanisms of alcohol and anti-HIV drugs for potential additive/synergistic liver injuries could be differentiated in vivo through applying the enzyme nanoparticles that degrade alcohol. In this study, we compared the effectiveness and mechanisms of protection by the enzyme nanoparticles against liver injuries in animal models of acute versus chronic alcohol feeding and of alcohol alone versus alcohol combined with anti-HIV drugs.
MATERIALS AND METHODS
Enzyme Nanoparticles
All the native enzymes of alcohol metabolism were purchased from Sigma-Aldrich (St. Louis, MO). The enzyme nanoparticles of alcohol oxidase (#9073–63-6) and/or catalase (#C30–1G) were prepared through in situ acrylamide polymerization that encapsulated individual native enzymes, which were described previously (Liu et al., 2013). Aldehyde dehydrogenase (ALDH; #10171832001) nanoparticles were prepared through conjugating acryloyl groups on the surface of native ALDH, followed by in situ polymerization to form a layer of polymer network (Xu et al., 2018). All the enzyme nanoparticles were purified with a size exclusion column (Sepharose 6B; Sigma) to exclude any residual un-encapsulated native enzymes. The enzyme nanoparticles had an average diameter of 32.8±4.0nm under transmission electron microscope and retained greater than 80% of the native enzyme activity after the polymerization and encapsulation. Before being applied to animals, all the enzyme nanoparticles were tested in Hela cells or primary mouse hepatocytes for their thermal and proteolytic stabilities and cellular toxicities at physiological temperature (37C) in the presence of trypsin for 2 to 4 hours. Greater than 70% of the enzyme activities were maintained under these physiological conditions, which were monitored according to the methods described previously (Liu et al., 2013; Xu et al., 2018). Three types of enzyme nanoparticles were formulated for this study. The first was the enzyme nanoparticles for complete alcohol degradation (ENP1), which contained alcohol oxidase, catalase and aldehyde dehydrogenase. The second was the enzyme nanoparticles for partial alcohol degradation into acetaldehyde (ENP2), which contained alcohol oxidase and catalase. The third was the enzyme nanoparticles for acetaldehyde degradation (ENP3), which contained only aldehyde dehydrogenase. The prepared enzyme nanoparticles were stored at −80˚C for later experiments.
Experiments with Animal Models
Male C57B6 mice were purchased from Jackson Laboratory (Bar Harbor, ME). The acute alcohol binge model was based on the one developed by Carson and Pruett (1996) with a couple of modifications. Briefly, the mice were gavaged with alcohol (30% alcohol in phosphate buffer saline (PBS); 6.5mg/g body weight) in the morning. The enzyme nanoparticles (in 20 μl PBS solutions; 2 μg of each enzyme/g body weight) were intravenously injected into the mice 30 min after the alcohol gavage. Equal amount of PBS or native enzyme mix (EMX) was injected as a background control. A small volume of blood (less than 25 μl) were taken through tail vein sampling for monitoring the blood levels of alcohol and acetaldehyde. In some of the tests, the mice were gavaged with anti-HIV protease inhibitors (Moravek Biochemicals, Brea, CA), ritonavir (M1535) and lopinavir (M1735) (25μg each/g body weight) simultaneously with the alcohol binge. Eight hours after the alcohol gavage, mice were killed for blood and liver tissue samples.
The chronic alcohol diet feeding and alcohol binge (chronic-binge) model was based on the one developed by Ki et al (2010) with modifications. Mice were fed alcohol liquid diet AIN-93G containing 4.5% alcohol (Dyets, Inc) or isocaloric control diet for 10 days. On the 11st day in the morning, mice were gavaged with alcohol (30% alcohol in PBS; 5g/kg body weight) or same volume of isocaloric maltose solution as the control. Mice were intravenously injected with the enzyme nanoparticles (2 mg of each enzyme/kg body weight) within 30 min after the alcohol gavage. At the time of the alcohol binge and thereafter the injection of the enzyme nanoparticles, a small volume of blood (less than 25 μl) were taken through tail vein sampling for monitoring the blood levels of alcohol and acetaldehyde. In some of the experiments, the mice were gavaged with ritonavir and lopinavir (50mg each/kg body weight) every other day during the chronic alcohol diet feeding and simultaneously with the alcohol binge (25mg each/kg body weight). There were three experimental groups of the chronic-binge model for the nanoparticle tests: group 1 (CB) was fed chronic control diet plus alcohol binge; group 2 (EB) was fed chronic alcohol diet plus alcohol binge; group 3 (EPB) was fed chronic alcohol diet and the anti-HIV drugs plus alcohol binge with the anti-HIV drugs. Mice were euthanized for blood and liver tissue samples 8 hours after the alcohol binge. All animals were treated in accordance with the Guide for Care and Use of Laboratory Animals and the study was approved by the local animal care committee.
Measurements of Pathological Parameters and Molecular Markers
Plasma alanine aminotransferase (ALT) and total liver triglyceride were measured as described previously (Han et al., 2013). For hematoxylin and eosin staining (H&E), liver tissues were fixed in 10% formalin overnight at 4 °C and stored in 80% ethanol, which were then embedded in paraffin, sectioned at 5μm and proceeded to H&E. H&E staining kits were purchased from Roche.
Ethanol Assay Kit (K620–100) was purchased from BioVision (Mountain View, CA) or from Analox Instruments (IL, USA). Blood acetaldehyde concentrations (BADC) was measured based on the reaction between acetaldehyde and 3-methyl-2-benzothiazolinone hydrazine (MBTH). In brief, one volume of standard acetaldehyde (MAK139; ACS grade, Sigma) or samples was mixed with one volume of 0.8% MBTH. Meanwhile, another one volume of 0.8% MBTH was dissolved with 1% iron(III) chloride in water. The two mixtures were incubated at room temperature for 15 min and equally mixed together. The blue color MBTH- acetaldehyde complex formed immediately after mixing and was measured by a spectrophotometer at 600nm. A standard curve with different acetaldehyde concentrations (5 to 250 ppm) was prepared as a reference. The concentration of acetaldehyde in the sample was referred to the standard curve.
Extractions of proteins and RNAs from the liver tissues, immunoblotting of proteins, and qPCR of mRNA were described previously (Han et al., 2013). Primary antibodies for SREBP1c (sterol regulatory element-binding protein 1c) (Thermo Fisher Scientific, MA5–11685), and ATF6 (activating transcription factor 6) (Cell Signaling, #65880) and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (Cell Signaling, #8884) were purchased. PCR primers of inflammation markers of TNFα, F4/80 and IL-6 were based on Ki et al. (2010). PCR primers of lipogenic factors of ACC (acetyl-CoA carboxylase), LXRα (liver X receptor α) and SREBP1, ER stress markers of sXbp1 (the alternatively spliced form of X-box binding protein 1) and CHOP (CCAAT-enhancer-binding protein homologous protein), and Golgi stress marker of GCP60 (Golgi complex-associated protein 60) were reported previously (Han et al., 2013; 2017).
Statistics
Data are presented as means ± standard error of mean (SEM) unless otherwise indicated. Statistical analyses were performed with GraphPad Prism® 6 using the one way-ANOVA for comparison of multiple groups and two-way ANOVA for comparison of trends between different treatments. The p values of 0.05 or less are considered significant.
RESULTS
Effects of facilitated removal of blood alcohol and/or acetaldehyde on liver injury in acute alcohol-fed Mice
The primary goal of alcohol detoxification by the enzyme nanoparticles was to achieve faster reduction of blood alcohol concentration (BAC) and protect the liver from alcoholic injury. Three type of enzyme nanoparticles with different enzyme activities were synthesized and formulated for the tests: (1) enzyme nanoparticles (ENP1) that completely degrade alcohol, (2) enzyme nanoparticles (ENP2) that partially break down alcohol into acetaldehyde, and (3) enzyme nanoparticles (ENP3) that only degrade acetaldehyde. A mix of native enzymes (EMX) that have minimal activities in vivo were used as control. The liver of mice gavaged with alcohol at 6.5mg/g and treated with phosphate buffer saline (PBS) showed mild alcoholic foamy and ballooning degenerations, which were accompanied with focal hepatic infiltration of neutrophils indicative of necrotic cell death (Fig. 1A). These histological changes were not seen in the liver of alcohol-fed mice treated with ENP1, whereas the necrotic changes remained in the liver of mice treated with ENP2, ENP3 or EMX.
Figure 1. Effects of enzyme nanoparticles of alcohol metabolism on liver injury and blood concentrations of alcohol and acetaldehyde in mice with acute alcohol binge.

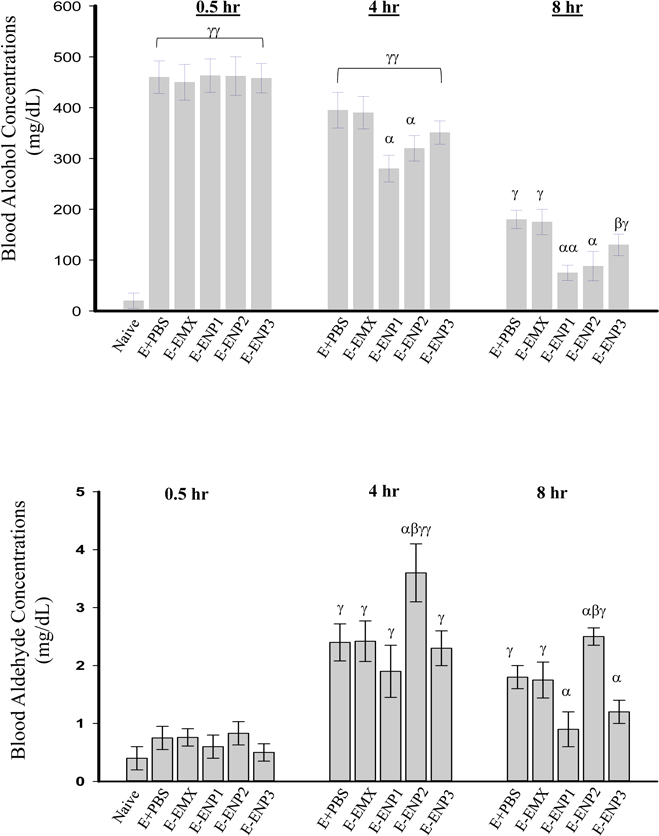

(A) Liver histology revealed by H&E staining of the liver tissues. Original magnification 200x; Arrows identify focal neutrophil infiltrations; (B) Blood alcohol concentrations (BAC); (C) Blood acetaldehyde concentrations (BADC); (D) plasma alanine aminotransferase (ALT); (E) Change of tumor necrosis factor (TNF) α; Naïve, from mice without alcohol binge or enzyme nanoparticles; E+PBS, from mice with alcohol binge and injection of phosphate-buffered saline (PBS); E+EMX, from mice with alcohol binge and injection of a mix of native enzymes of alcohol metabolism; E+ENP1, from mice with alcohol binge and injection of the enzyme nanoparticles of complete alcohol degradation (ENP1); E+ENP2, from mice with alcohol binge and injection of the enzyme nanoparticles of partial alcohol degradation into acetaldehyde (ENP2); E+ENP3, from mice with alcohol binge and injection of the enzyme nanoparticles of acetaldehyde degradation (ENP3); α p<0.05, αα p<0.01 compared to PBS or EMX at the same time point; β p<0.05 compared to ENP1 at the same time point; γ p<0.01 and γγ p<0.005 compared to Naïve; n=5.
At 30 minutes after the alcohol gavage, blood alcohol concentrations (BAC) in the mice were peaked between 430 mg/dL and 490 mg/dL and there were not significant changes among the mice with the different enzyme treatments (Fig. 1B). At 4 hours after the alcohol gavage, BAC was reduced by 15% in mice treated with PBS or EMX, whereas ENP1, ENP2 and ENP3 treatments reduced BAC by 41%, 32% and 22%, respectively. The BAC reductions by ENP1 and ENP2 were significantly different from the reductions by PBS or EMX that represented the mouse endogenous alcohol digestion. At 8 hours after the alcohol gavage, BAC was reduced by about 60% in mice treated with PBS or EMX, by 83%, 80% and 71% in mice treated with ENP1, ENP2 and ENP3, respectively. The BAC differences between PBS/EMX and ENP1/ENP2 were significant. The effect of ENP3 on BAC was not significant at all time points. The blood acetaldehyde concentrations (BADC) in the mice were not significantly different among the treatments at 30 minutes after the alcohol gavage (Fig. 1C). At 4 hours, BADC was increased by 39% in ENP2 compared to that of PBS and there were not significant differences between PBS, EMX, ENP1 and ENP3. At 8 hours, BADC of ENP1 (or ENP3) was lower than in PBS or EMX, and there was significant difference between ENP2 and ENP1 (or ENP3).
The acute alcohol binge significantly increased plasma alanine aminotransferase (ALT) in mice treated with PBS, EMX, or ENP3 compared to that of the naïve mice without treating alcohol or any of the enzyme nanoparticles (Fig. 1D). ALT was normalized by ENP1, partially reduced by ENP2, and not significantly changed by ENP3, suggesting that increasing or removing acetaldehyde only had minimal effects on liver injury in this acute alcohol model. In addition, compared to the naïve control, the alcohol binge increased hepatic tumor necrosis factor α (TNFα) by more than 3-fold, which was reduced by ENP1 (Fig. 1E). Neither ENP2 nor ENP3 reduced the increased TNFα significantly.
In the presence of anti-HIV drugs, the acute alcohol-induced hepatic foamy and ballooning degenerations were much more severe (Fig. 2A). However, ENP1 treatment apparently reduced the histopathological changes. Adding the anti-HIV drugs to alcohol did not have significant effects on BAC, BADC, or ALT in the mice treated with ENP1 or ENP2 (Fig. 2B–D).
Figure 2. Effects of enzyme nanoparticles of alcohol metabolism on liver injury and blood concentrations of alcohol and acetaldehyde in mice fed with acute alcohol binge and anti-HIV drugs.


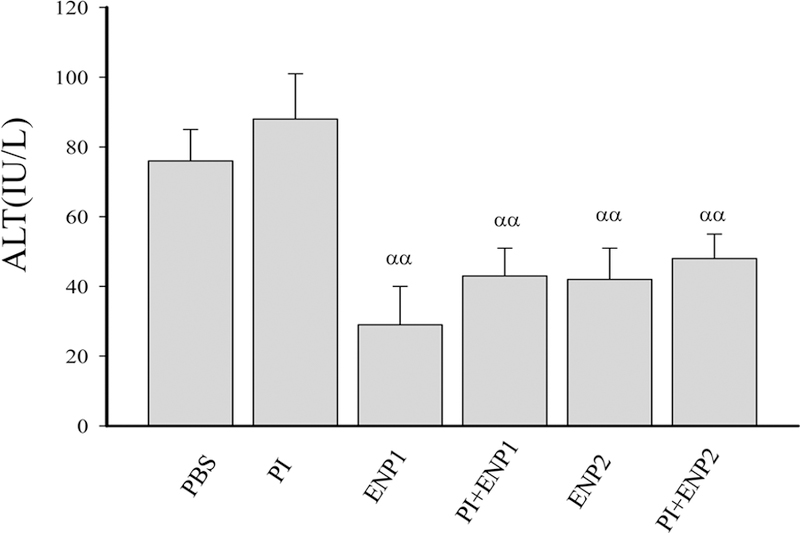
(A) Liver histology revealed by H&E staining. Original magnification 200x; (B) Blood alcohol concentrations (BAC) in mice of acute alcohol binge; (C) Blood acetaldehyde concentrations (BADC); (D) Plasma alanine aminotransferase (ALT); PBS, injected with phosphate-buffered saline after the alcohol binge; E, ethanol; PI, injected with anti-HIV protease inhibitors: ritonavir and lopinavir after the alcohol binge; ENP1, injected with the enzyme nanoparticles of complete alcohol degradation after the alcohol binge; PI+ENP1, injected with PI and ENP1 after the alcohol binge; ENP2, injected with the enzyme nanoparticles of partial alcohol degradation after the alcohol binge; PI+ENP2, injected with PI and ENP2 after the alcohol binge; α p<0.05, αα p<0.01 compared to PBS or PI; β p<0.05 compared to ENP1; n=3–5.
Effects of facilitated removal of blood alcohol and/or acetaldehyde on liver injury in chronic alcohol-binge mice
In control diet feeding and alcohol binge (the CB group), either ENP1 or ENP2 exerted protective effects on BAC, BADC and ALT at all time points, respectively, which were similar to those observed in the acute single alcohol gavage (Fig. 3). In contrast, chronic alcohol diet feeding (the EB group) already increased BAC to 240–250 mg/dL, BDAC to 2 mg/dL, and ALT to 40–60 IU/L at the time of the alcohol binge (Fig. 3A–C). In the presence of anti-HIV drugs (the EPB group), the before-binge background values of BAC, BADC, and ALT were increased further and were significantly higher than those in the CB or EB groups that were without the antivirals. At 4 hours after the alcohol binge, BAC were raised in all groups with the EPB group reached 530–570 mg/dL, which were the highest and nearly doubled the levels of BAC of the CB group. At 8 hours, ENP1 normalized BAC in the CB group, reduced BAC by 79% in the EB group, and only by 45% in the EPB group. The average BCA was above 300 mg/dL in the EPB group at 8 hours. In addition, in the EB group, there was a significant difference of BAC at 8 hours between ENP1 and ENP2 treatments, which was not observed in the EPB group, indicating a possible interference with alcohol degradation by the anti-HIV drugs (Fig. 3A).
Figure 3. Effects of enzyme nanoparticles of alcohol metabolism on blood concentrations of alcohol and acetaldehyde and liver injury in mice fed chronic alcohol diet plus acute alcohol binge and anti-HIV drugs.

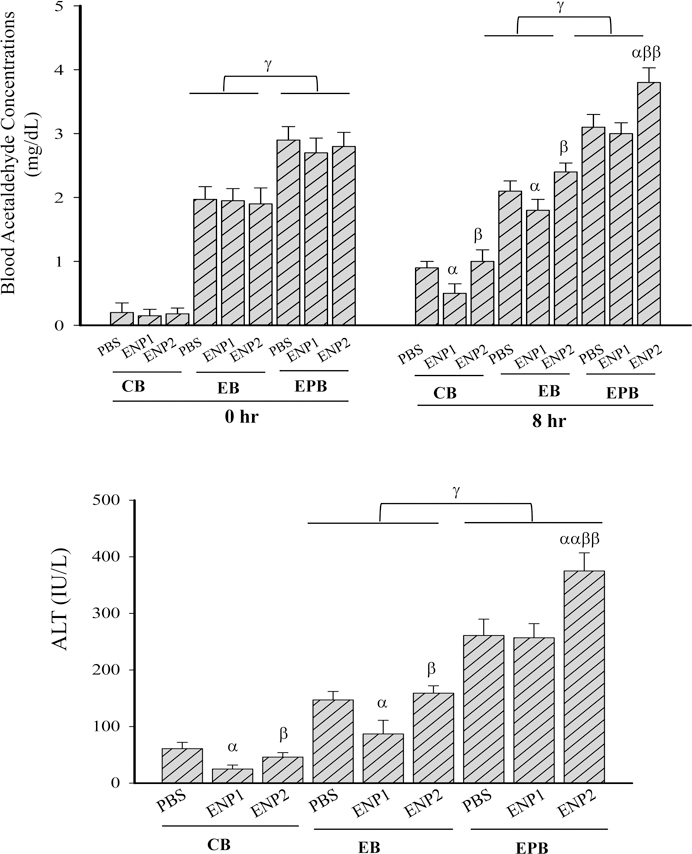
(A) Blood alcohol concentrations (BAC); (B) Blood acetaldehyde concentrations (BADC); (C) Plasma alanine aminotransferase (ALT); CB, from mice fed with isocaloric control diet and acute alcohol binge; EB, from mice fed with chronic alcohol diet and acute alcohol binge; EPB, from mice fed with chronic alcohol diet and ritonavir and lopinavir (PI), and acute alcohol binge plus PI; PBS, injected with phosphate-buffered saline; ENP1, injected with the enzyme nanoparticles of complete alcohol degradation; ENP2, injected with the enzyme nanoparticles of partial alcohol degradation; α p<0.05 and αα p<0.01 compared to PBS in the same group; β p<0.05 compared to ENP1 in the same group; γ p<0.05, γγ p<0.01 and γγγ p<0.005 compared between groups at the same time point; n=5.
At the time of the alcohol binge (0 hour), chronic alcohol diet feeding also increased BADC in the EB and EPB groups, and BADC were significantly higher in the EPB group than in the EB group (Fig. 3B). At 8 hours: in the CB group, ENP1 but not ENP2 reduced BADC to a normal level, which was less than 0.7 mg/dL; in either the EB or EPB groups, ENP1 did not reduce BADC significantly; ENP2 increased BADC by 2.1 fold in the EB group and by 3.2 fold in the EPB group compared to the corresponding BADC in the CB group.
ALT was reduced to normal levels (less than 30 IU/L) in the CB group treated with ENP1 (Fig. 3C). ENP1 but not ENP2 reduced ALT by 60% compared to EB with PBS in the EB group. However, in the EPB group, no significant protective effects by ENP1 were detected, and ENP2 treatment even deteriorated the ALT significantly.
Interference of anti-HIV drugs with the protective effects of facilitated removal of blood alcohol and/or acetaldehyde on liver injury
Liver histology revealed apparent differences between the CB, EB and EPB groups and between the ENP1 and ENP2 treatments. In the CB group, mild foamy and ballooning degenerations, scattered focal neutrophil infiltration and microsteatosis were observed in the alcohol-binged mice without treatments of the enzyme nanoparticles (Fig. 4A). Both ENP1 and ENP2 treatments eliminated the microsteatosis. In the EB group, moderate fatty liver and inflammation were seen in the alcohol binged mice without the enzyme nanoparticles. ENP1 but not ENP2 treatment apparently reduced the fatty liver which was indicated by the disappearance of macrosteatosis. In the EPB group, the combination of alcohol and anti-HIV drugs induced much severe fatty liver and inflammation, which were still apparent in the mice treated with ENP1 or ENP2.
Figure 4. Effects of enzyme nanoparticles of alcohol metabolism on fatty liver injury and inflammation in mice fed chronic alcohol diet plus acute alcohol binge in the presence of anti-HIV drugs.

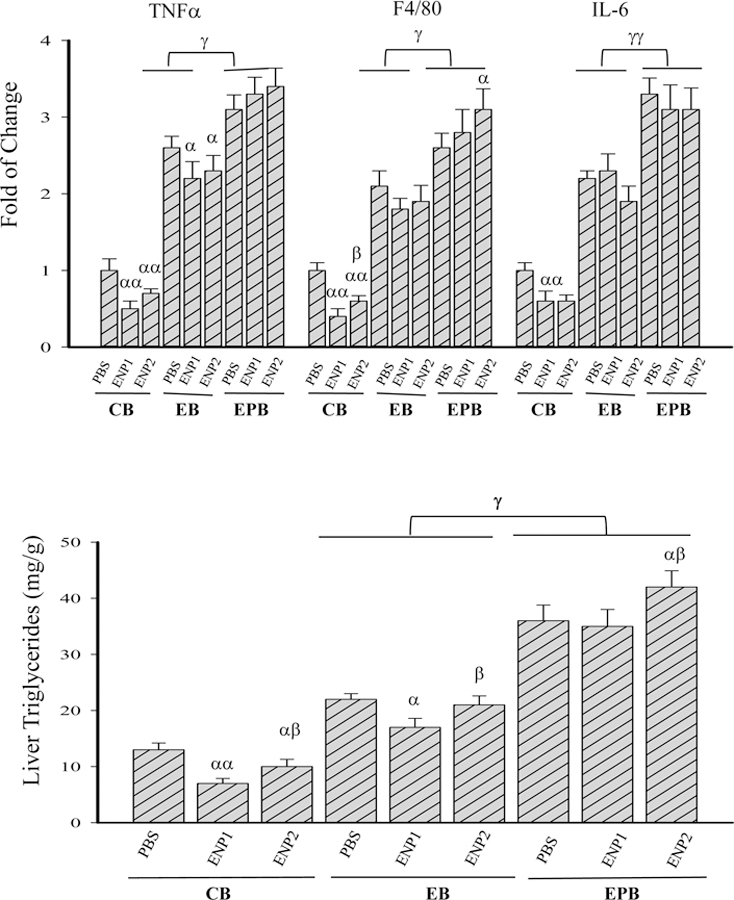
(A) Micro/macrovascular fatty changes revealed by H&E staining of the liver tissues. Original magnification 200x; (B) Expression of inflammation markers; TNFα, tumor necrosis factor α; F4/80, a glycoprotein as a major macrophage marker; IL-6, interleukin 6; (C) Quantitation of the total liver triglycerides (TG); Note, the labels for all the experimental treatments are the same as those described in Figure 3. α p<0.05 and αα p<0.01 compared to PBS in the same group; β p<0.05 compared to ENP1 in the same group; γ p<0.05 and γγ p<0.01 compared between EB and EPB at the same time point; n=4.
Selective molecular markers for inflammation and hepatic lipogenesis were quantified to evaluate further the protective effects by the enzyme nanoparticles. In the CB group, ENP1 treatment reduced the TNFα, F4/80, and interleukin (IL)-6 by 49%, 61% and 40%, respectively; ENP2 treatment reduced the TNFα, F4/80, and IL-6 by 31%, 40% and 38%, respectively (Fig. 4B). In the EB group, the chronic-binge challenge increased TNFα, F4/80, and IL-6 by 2.6, 2.2 and 2.3 fold, respectively, compared to those of the mice without enzyme nanoparticles in the CB group; Neither ENP1 or ENP2 treatment decreased TNFα, F4/80, or IL-6 significantly compared to the PBS control. In the EPB group, the combination of anti-HIV drugs and chronic-binge increased TNFα, F4/80, and IL-6 by 3.1, 2.7 and 3.3 fold, respectively, compared to those of the CB mice without the nanoparticles. ENP1 or ENP2 treatment either increased TNFα and F4/80 or decreased IL-6 slightly, suggesting minimal protective effects by the enzyme nanoparticles on the drug and alcohol-induced inflammation. In addition, the anti-HIV drugs also interfered with the protective effects of the enzyme nanoparticles on alcohol-induced fatty liver. Liver triglycerides (TG) were increased from 13 mg/g in the CB group to 22 mg/g in the EB group and 36 mg/g in the EPB group (Fig. 4C). ENP1 treatment decreased the liver triglycerides by 46% in the CB group and by 22% in the EB group, whereas ENP2 decreased the triglycerides by 23% in the CB group and by 4.6% in the EB group. There was a significant difference of TG between ENP1 and ENP2 treatments in either the CB or the EB group. In contrast in the EPB group, ENP1 treatment did not reduce the liver triglycerides whereas ENP2 even increased the TG further.
Differential effects of facilitated removal of blood alcohol and/or acetaldehyde on drug/alcohol-induced lipogenesis and stress response
Fatty liver is characteristic of alcohol-induced liver injury. It is well established that one of the major pathological mechanisms contributing to anti-HIV drug or alcohol-induced liver steatosis is the cellular organelle stress response (Ji and Kaplowitz, 2003; Ji, 2015). However, the drug-induced organelle stress response appeared different from the alcohol-induced stress response (Han et al., 2017). We wanted to confirm the difference by taking the advantage of the enzyme nanoparticles that facilitate alcohol removal in vivo. Selective lipogenic factors were examined in response to alcohol, the drugs, and the enzyme nanoparticles (Fig. 5A). In the CB group, no significant differences in mRNA of acetyl-CoA carboxylase (ACC), liver X receptor (LXR)α, or protein of sterol regulatory element-binding protein (SREBP)1c were detected between ENP1 and ENP2 treatments. In the EB group, ACC, LXRα and SREBP1c were all significantly increased compared to those of the CB group, but there was no difference between the ENP1 and ENP2 treatments. In the EPB group, either ACC or LXRα was increased slightly compared to the EB group and there was no significant difference between ENP1 and ENP2 treatments. In contrast, SREBP1c was significantly increased compared to the EB group, which was synergistically increased by the enzyme nanoparticle treatments (either ENP1 or ENP2), indicating that the lipogenic factors were differentially affected by the nanoparticles in the presence of the anti-HIV drugs.
Figure 5. Effects of enzyme nanoparticles of alcohol metabolism on hepatic lipogenesis and organelle stresses in mice fed chronic alcohol diet plus acute alcohol binge in the presence of anti-HIV drugs.
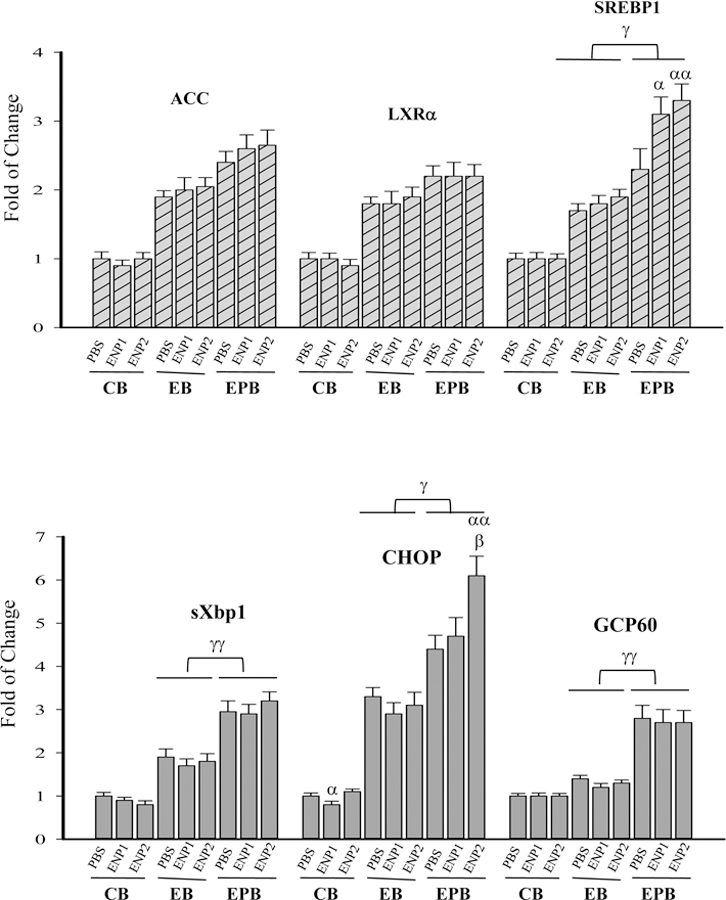

(A) Expression of lipogenic factors; ACC, acetyl-CoA carboxylase; LXRα, liver X receptor α; SREBP1c, sterol regulatory element-binding protein 1c; (B) Expression of selected markers for endoplasmic reticulum (ER) stress and Golgi stress; sXbp1, the alternatively spliced form of X-box binding protein 1; CHOP, CCAAT-enhancer-binding protein homologous protein; GCP60, Golgi complex-associated protein 60; (C) Western blots of ER or Golgi stress related proteins; SREBP1c (p), precursor protein of SREBP1c; SREBP1c (n), nuclear or activated SREBP1c; ATF6, activating transcription factor 6; ATF6 (p), precursor ATF6; ATF6 (n), nuclear or activated ATF6; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; (D) Quantitation of the activated ATF6, which was conducted with ImageJ and normalized with GAPDH; Note, the labels for all the experimental treatments are the same as those described in Figure 3. α p<0.05 and αα p<0.01 compared to PBS in the same group; β p<0.01 compared to ENP1 in the same group; γ p<0.05 and γγ p<0.01 compared between EB and EPB at the same time point; n=4.
Selective organelle stress markers were then examined (Fig. 5B). They included active X-box binding protein (sXbp) 1 for ER stress response, Golgi complex-associated protein (GCP)60 for Golgi stress response, and CCAAT-enhancer-binding protein homologous protein (CHOP) that regulates cell death upon ER and/or Golgi stresses (Ji, 2017). sXbp1 was increased in the EB group compared to the CB group and was synergistically increased in the EPB group compared to the CB group. CHOP expression patterns in the three mouse groups were similar to those of sXbp-1. However, CHOP expression was significantly higher in the EPB mice treated with ENP2 than with ENP1. Interestingly, GCP60 was not responsive to alcohol in the CB or EB groups, whereas the anti-HIV drugs induced significant increase of GCP60 in the EPB group. The increased GCP60 expression was accompanied by increased nuclear SREBP1c and decreased nuclear activating transcription factor (ATF)6 (Fig. 5C&D) in the EPB group, both of SREBP1c and ATF6 are known to be processed in the Golgi apparatus (Bartz and Seemann, 2008).
DISCUSSION
We have been developing strategies to directly remove blood alcohol and acetaldehyde in persons who consume excessive alcohol and are bound to cause multiple organ pathologies. As a proof of concept, we previously created hepatocyte-mimicking antidotes through synthesizing and formulating enzyme nanoparticles that co-deliver alcohol oxidase, catalase and aldehyde dehydrogenase (ALDH) to the liver, where these enzyme nanoparticles lowed blood levels of alcohol and acetaldehyde in alcohol-intoxicated mice (Liu et al., 2013; Xu et al., 2018). In this study, we applied these enzyme nanoparticles of alcohol metabolism to animals fed both anti-HIV drugs (e.g. ritonavir and lopinavir) and alcohol, which resemble a significant portion of HIV-infected/AIDS patients (Braithwaite, 2007). We compared the protective effects of the enzyme nanoparticles on blood alcohol (BAC) and blood acetaldehyde (BADC) concentrations and liver injuries in two animal models of acute single alcohol binge (acute) and chronic alcohol plus alcohol binge (chronic-binge), which closely mimic the drinking patterns of real life. Our results revealed several interesting outcomes. In the acute model, BAC was reduced faster and more effective by the enzyme nanoparticles of complete alcohol metabolism (ENP1) than by endogenous enzymes of alcohol metabolism as, at 4 hours after the alcohol binge, ENP1 reduced BAC by ~40% and the endogenous enzymes reduced by 15%; and at 8 hours, ENP1 reduced BAC by more than 80% and the endogenous enzymes reduced BAC by 60%. Similar effects by ENP1 occurred to BADC. The usage of anti-HIV drugs did not have any significant effects on the speed and effectiveness on the BAC reduction by ENP1. However, in the chronic-binge model, the anti-HIV drugs not only increased BAC from 410 mg/dL in drug-free mice to 540 mg/dL at 4 hours after the alcohol binge, but also dramatically decreased the BAC reduction by ENP1 at 8 hours after the binge. These results suggest that, for the direct alcohol removal strategy, the application of the enzyme nanoparticles could not be effective in the situation of chronic alcohol consumption plus anti-HIV drugs. Combination of the enzyme nanoparticles with protective agents against off-target effects of certain antiviral drugs would be required in dealing with the liver injuries from chronic alcohol consumption and anti-HIV regimen.
Through comparisons, this study with the enzyme nanoparticles of different enzyme activities also revealed in vivo some clues regarding the contributory roles of alcohol, alcohol metabolite acetaldehyde, and the anti-HIV drugs to liver injuries. In the acute model, alcohol induced mild hepatic ballooning degenerations, inflammation and increase of ALT. These pathological changes seemed to be attributed solely by alcohol itself not by acetaldehyde or the anti-HIV drugs. This is supported by the observations that there was no difference in the ALT reduction by ENP1 that completely degrade alcohol and by the ENP2 that partially degrade alcohol causing an accumulation of acetaldehyde, and that the ALT was significantly higher in the mice treated with ENP3 that degrade only acetaldehyde without reducing BAC than the ALT in the mice treated with ENP2 that reduced BAC but increased BADC. In addition, the levels of ALT and TNFα in the acute model appeared to be relevant to BAC even in the presence of anti-HIV drugs. However, in the chronic-binge model, the background values of BAC and BADC at zero time before the alcohol binges were already increased significantly due to the chronic alcohol diet feeding. The alcohol-induced liver pathologies indicated by ALT, inflammation markers and total triglycerides were all remarkable, which were not contributed only by alcohol/BAC. For instance, despite the significant differences in BAC and BADC between the ENP1 and ENP2 treated mice, the alcohol-induced inflammation as indicated by TNFα, F4/80 and IL-6 in the ENP1 treatment was not different from that of ENP2 treatment. The BADC appeared correlated well to the levels of ALT or the total liver triglycerides. In the presence of the anti-HIV drugs, the functions of the enzyme nanoparticles in degrading alcohol and acetaldehyde were diminished. The possible reason for that could be that under chronic conditions, the antivirals and alcohol induce downstream pathophysiological changes that interfere with the functions of the enzyme nanoparticles, which could be worse in the already “sick” hepatocytes. One culprit factor can be the protein/enzyme adduct-forming acetaldehyde (Ishii, 1994; Kwon et al., 2014; Lopez-Islas et al., 2016), which was already increased before the alcohol binge. Thus, alcohol, acetaldehyde, anti-HIV drugs, and other downstream pathological factors such as induction of Cyp2E1, oxidative stress, inflammation and dysbiosis of gut microbiota, all may collectively contribute to the liver injury, which might explain why the application of the enzyme nanoparticles did not protect the liver in the chronic-binge model as effectively as in the acute model.
The mechanisms by which chronic alcohol and anti-HIV drugs interfere with the protective effects of the facilitated in vivo removals of alcohol and acetaldehyde can be complex. We focused on the cellular organelle stress mechanism as it is one of the major pathological mechanisms promoting liver disease, and it is well established that either excessive alcohol or certain anti-HIV drugs such as ritonavir and lopinavir induce ER stress response in the hepatocytes and prolonged ER stress leads to fatty liver disease (Kao et al, 2012; Ji, 2015). The ER stress mediated hepatic lipogenesis involves lipogenic factors such as LXRα, ACC, and SREBP1c, which were all increased in response to alcohol and the anti-HIV drugs in the chronic-binge model. The major ER stress markers, sXbp1 and CHOP were also increased in the chronic model, suggesting that the ER stress mechanism did play a role in promoting the observed liver pathologies. In the presence of anti-HIV drugs, CHOP that regulates ER stress-related cell death was much more increased in ENP1 of complete alcohol digestion than in ENP2 of partial alcohol digestion, suggesting possible synergistic effects by the antivirals and the toxic acetaldehyde. However, it is of note that there were no differential protective effects on the three lipogenic factors by the two enzyme nanoparticles. The use of anti-HIV drugs increased further SREBP1c but not LXRα or ACC. The results are interesting as LXRα is known to be upstream of ACC and SREBP1c (Repa et al., 2000) and the alcohol and antivirals-induced ER stress should have equal effects on all the three lipogenic factors being examined. There must be some additional effects by the antivirals on SREBP1c that were independent of LXRα. Based on our previous observation that anti-HIV drugs induced Golgi stress which subsequently contributed to the ER stress response (Han et al, 2017; Ji, 2017), we assumed that additional organelle stresses by the antivirals would enhance SREBP1c expression. Indeed, the Golgi stress response indicated by GCP60 was significantly increased only in the chronic model fed both alcohol and the antivirals. We further confirmed that the antiviral-induced Golgi stress blocked the inter-organelle (ER to Golgi) proteolytic processing and nuclear translocation of ATF6 that resulted in enhanced nuclear accumulation of SREBP1c and severe fat accumulation. Therefore, the toxic effects of acetaldehyde and additional organelle stresses by the antivirals might interfere with the protective effects by the enzyme nanoparticles, and the nanoparticles with defined enzyme activities could be utilized to differentiate pathological mechanisms by factors other than alcohol/acetaldehyde.
In summary, the bio-mimetic enzyme nanoparticles of alcohol degradation facilitate removal of blood alcohol and acetaldehyde and protects the liver against alcoholic injuries in animal models, and the protection is more effective in acute than chronic alcohol feeding. Antiviral-induced additional organelle stresses may undermine the protective effects by the enzyme nanoparticles in the chronic alcohol feeding.
ACKNOWLEDGEMENT
This work is supported by the U.S. National Institute of Health (NIH) grant DA042632. The authors thank the California NanoSystems Institute of UCLA for providing alcohol-metabolizing enzyme nanoparticles.
This work is supported by the U.S. National Institute of Health (NIH) grant R01DA042632.
REFERENCES
- Bartz R, Seemann J (2008). Mitotic regulation of SREBP and ATF6 by separation of the Golgi and ER. Cell Cycle, 7(14):2100–5. [DOI] [PubMed] [Google Scholar]
- Becker HC 2017) Influence of stress associated with chronic alcohol exposure on drinking. Neuropharmacology, 122:115–126. doi: 10.1016/j.neuropharm.2017.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp GA, Valento M (2016). Toxic Alcohol Ingestion: Prompt Recognition and Management in The Emergency Department. Emerg Med Pract, 18 (9), 1–20. [PubMed] [Google Scholar]
- Braithwaite RS, Conigliaro J, Roberts MS, Shechter S, Schaefer A, McGinnis K, Rodriguez MC, Rabeneck L, Bryant K, Justice AC (2007). Estimating the impact of alcohol consumption on survival for HIV+ individuals. AIDS Care, 19(4):459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson EJ; Pruett SB (1996). Development and characterization of a binge drinking model in mice for evaluation of the immunological effects of ethanol. Alcohol. Clin. Exp. Res, 20, 132–138. [DOI] [PubMed] [Google Scholar]
- Cunradi CB, Mair C, Ponicki W, & Remer L (2012). Alcohol Outlet Density and Intimate Partner Violence-Related Emergency Department Visits. Alcohol Clin Exp Res, 36 (5), 847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson CJ (2007). Measurement of acetaldehyde: what levels occur naturally and in response to alcohol? Novartis Found Symp, 285, 247–260. [DOI] [PubMed] [Google Scholar]
- Friedmann PD (2013). Clinical practice. Alcohol use in adults. N Engl. J Med, 368 (4), 65–73. [DOI] [PubMed] [Google Scholar]
- Gunzerath L, Hewitt BG, Li T, & Warren KR (2010). Alcohol research: past, present, and future. Annals of the New York Academy of Sciences, 1216 (1), 1–23. [DOI] [PubMed] [Google Scholar]
- Han H, Hu J, Lau MY, Feng M, Petrovic LM, Ji C (2013). Altered methylation and expression of ER-associated degradation factors in long-term alcohol and constitutive ER stress-induced murine hepatic tumors. Front Genet, October 31, 4, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H, He Y, Hu J, Lau R, Lee H, Ji C (2017). Disrupted ER-to-Golgi Trafficking Underlies Anti-HIV Drugs and Alcohol-Induced Cellular Stress and Hepatic Injury. Hepatol Commun, 1(2):122–139. doi: 10.1002/hep4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii H (1994). Formation of acetaldehyde adducts and their significance in the pathogenesis of alcoholic liver disease. Hepatology, 19 (4), 177. [Google Scholar]
- Ji C (2015). Advances and New Concepts in Alcohol-Induced Organelle Stress, Unfolded Protein Responses and Organ Damage. Biomolecules, 52, 1099–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C (2017). Dissecting the Role of Disturbed ER-Golgi Trafficking in Antivirals and Alcohol Abuse-Induced Pathogenesis of Liver Disorders. J Drug Abuse, 3(3). pii: 14. doi: 10.21767/2471-853X.100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao E, Shinohara M, Feng M, Lau MY, Ji C (2012). Human immunodeficiency virus protease inhibitors modulate Ca2+ homeostasis and potentiate alcoholic stress and injury in mice and primary mouse and human hepatocytes. Hepatology, 56(2):594–604. doi: 10.1002/hep.25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ki SH; Park O; Zheng M; Morales-Ibanez O; Kolls JK; Bataller R; Gao B (2010). Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: Role of signal transducer and activator of transcription 3. Hepatology, 52, 1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Mason BJ (2016). Existing and Future Drugs for the Treatment of the Dark Side of Addiction. Annu Rev Pharmacol Toxicol, 56, 299–322. [DOI] [PubMed] [Google Scholar]
- Kwon HJ, Won YS, Park O, Chang B, Duryee MJ, Thiele GE, Matsumoto A, Singh S, Abdelmegeed MA, Song BJ, Kawamoto T, Vasiliou V, Thiele GM, Gao B (2014). Aldehyde dehydrogenase 2 deficiency ameliorates alcoholic fatty liver but worsens liver inflammation and fibrosis in mice. Hepatology, 60(1), 146–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber C (1991). Metabolism of ethanol and associated hepatotoxicity. Drug and Alcohol Review, 10, 175–202. [DOI] [PubMed] [Google Scholar]
- Liu Y, Du J, Yan M, et al. (2013). Biomimetic enzyme nanocomplexes and their use as antidotes and preventive measures for alcohol intoxication. Nature nanotechnology, 8(3), 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Islas A, Chagoya-Hazas V, Pérez-Aguilar B, Palestino-Domínguez M, Souza V, Miranda RU, Bucio L, Gómez-Quiroz LE, Gutiérrez-Ruiz MC (2016). Cholesterol Enhances the Toxic Effect of Ethanol and Acetaldehyde in Primary Mouse Hepatocytes. Oxidative Medicine and Cellular Longevity, 2016, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quertemont E (2004). Genetic polymorphism in ethanol metabolism: acetaldehyde contribution to alcohol abuse and alcoholism. Mol Psychiatry, 9(6):570–81. [DOI] [PubMed] [Google Scholar]
- Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ (2000). Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev, 14(22):2819–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt C, Lippert AH, Bonakdar N, Sandoghdar V, Voll LM (2016). Compartmentalization and Transport in Synthetic Vesicles. Frontiers in Bioengineering and Biotechnology, 2016, 4, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Städler B, Chandrawati R, Price AD, Chong SF, Breheney K, Postma A, Connal LA, Zelikin AN, Caruso F (2009). A microreactor with thousands of subcompartments: enzyme-loaded liposomes within polymer capsules. Anl Rew Chem Int Ed Engl, 48 (24), 4359–62. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Normand J (2013). The international HIV/AIDS pandemic has been closely inter-twined with drug abuse and addiction from the time it began. Preface. Drug Alcohol Depend, 132 Suppl 1:S1. doi: 10.1016/j.drugalcdep.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Xu D, Han H, He Y, Lee H, Wu D, Liu F, Liu X, Liu Y, Lu Y, Ji C (2018). A Hepatocyte-Mimicking Antidote for Alcohol Intoxication. Adv Mater, 30(22):e1707443. doi: 10.1002/adma.201707443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhari S (2006). Overview: how is alcohol metabolized by the body? Alcohol Res. Health, 29 (4), 245–54. [PMC free article] [PubMed] [Google Scholar]


