Abstract
Background.
Mindfulness-based relapse prevention (MBRP) and transcranial direct current stimulation (tDCS) have independently shown benefits for treating alcohol use disorder (AUD). Recent work suggests tDCS may enhance mindfulness. The combination of MBRP with tDCS may provide synergistic benefits and may target both behavioral and neurobiological dysfunction in AUD. The goal of this double-blind sham-controlled randomized trial was to examine the efficacy of a rolling group MBRP treatment combined with tDCS among individuals interested in reducing their drinking.
Methods.
Individuals who were interested in reducing their alcohol use (n=84; 40.5% female; mean age=52.3; 98.9% with current AUD) were randomized to receive active (2.0 milliamps) or sham (0.0 milliamps) anodal tDCS (5cm x 3cm electrode) of the right inferior frontal gyrus with the 5cm x 3cm cathodal electrode applied to the left upper arm, combined with eight weeks of outpatient MBRP rolling group treatment. Assessments were conducted at baseline, post-treatment, and 2-months following treatment. The primary outcome was drinks per drinking day and secondary outcomes were percent heavy drinking days, self-reported craving, alcohol cue reactivity in an alcohol cue task, and response inhibition in a stop signal reaction time task.
Results.
Results indicated significant reductions in drinks per drinking day over time (B(SE)=−0.535 (0.16), p=0.001) and a significant dose effect for number of groups attended (B(SE)=−0.259 (0.11), p=0.01). There were also significant effects of time and dose for number of groups attended on secondary outcomes of percent heavy drinking days and alcohol cue reactivity. There were no effects of active versus sham tDCS on primary or secondary outcomes.
Discussion.
Findings from the current study provide initial support for the effectiveness of rolling group MBRP as an outpatient treatment for drinking reduction. The current study did not find additive effects of this tDCS protocol in enhancing MBRP among individuals with drinking reduction goals.
Keywords: alcohol use disorder, mindfulness-based relapse prevention, drinking reduction, transcranial direct current stimulation, rolling group treatment
Introduction
Alcohol use is the third leading global contributor to the burden of disease (World Health Organization, 2009) and costs of excessive alcohol use in the United States exceeded $220 billion in 2006 (Bouchery et al., 2011). Nearly one-third of Americans meet lifetime criteria for alcohol use disorder (AUD) and 13.9% met criteria for current AUD in 2012–2013 (Grant et al., 2015). Of those with AUD who receive behavioral or pharmacological AUD treatment, most return to drinking within the first year following treatment. As such, AUD has been characterized as a chronic, relapsing disease (McLellan et al., 2000). Behavioral interventions for AUD are the most common form of treatment; however, increased understanding of the neurobiological dysfunction associated with addiction (Koob and Volkow, 2010) has led to greater interest in treatment approaches that target specific neural systems (Koob et al., 2014; Litten et al., 2015). The current study evaluated the efficacy of combining two approaches designed to target behavioral and neurobiological dysfunction in addiction: mindfulness-based relapse prevention (MBRP; Bowen et al., 2011; Witkiewitz et al., 2013, 2005) and transcranial direct current stimulation (tDCS; Coles et al., 2018; Feil and Zangen, 2010; Trojak et al., 2017).
Behavioral and Neurobiological Dysfunction in AUD
Chronic alcohol use is strongly associated with poor behavioral and cognitive control (Goudriaan et al., 2007). Imaging studies have found that AUD is associated with altered functioning in brain regions that support cognitive control such as dorsolateral prefrontal cortex (dlPFC), inferior frontal gyrus (IFG), and anterior cingulate cortex (ACC; Claus et al., 2013) and degree of AUD severity predicts differential brain responses in these regions when performing cognitive control tasks. For example, in a functional magnetic resonance imaging (fMRI) study, Claus and colleagues (2013) found severity of AUD was negatively associated with blood oxygen level dependent (BOLD) signal change in right IFG, pregenual ACC, and inferior parietal lobe (IPL) during correct inhibition trials; and bilateral IFG and dorsal ACC during error trials of a Go/NoGo cognitive inhibition task.
Numerous studies have provided support for the importance of craving (defined as the subjective desire to drink) in the relapse process, including identifying associations between reactivity to alcohol cues, self-reported craving, and drinking outcomes (Claus et al., 2011a). Imaging work has consistently shown a diverse network of regions involved in cue reactivity such as ACC, ventral and dorsal striatum, orbitofrontal cortex (OFC), insula, and brainstem (Claus et al., 2011b; Schacht et al., 2013). In a large sample (n=326) of individuals with AUD who completed a cue reactivity task, Claus and colleagues found that increased AUD severity was associated with greater engagement of ventral and dorsal striatum, ACC, amygdala, and precuneus during cue presentation (Claus et al., 2011b).
Novel Interventions to Target Behavioral and Neurobiological Mechanisms of AUD
Mindfulness-based relapse prevention.
Mindfulness can be defined as the awareness that arises through paying attention to the present moment in a purposeful and non-judgmental way. Previous reviews of neuroimaging studies have posited several putative neural mechanisms of mindfulness (Chiesa et al., 2013; Hölzel et al., 2011), including in addiction treatment (Witkiewitz et al., 2013). In healthy adults, eight weeks of mindfulness was found to be associated with decreased functional connectivity between right insula and ventromedial prefrontal cortex (vmPFC) when mindfully attending to internal states, such as thoughts and emotions (Farb et al., 2007). In a sample of smokers, decreased functional connectivity between the insula and the subgenual ACC was found when mindfully attending to smoking images compared to passively viewing the images, suggesting a decreased association between these reward regions (Westbrook et al., 2011).
MBRP is a manualized intervention combining cognitive behavioral skills with mindfulness practices (Bowen et al., 2011). The evidence base for MBRP, while relatively small and in its early stages, is promising (Wilson et al., 2017). In the largest and most methodologically rigorous randomized controlled trial (RCT) of MBRP to date, MBRP as an aftercare treatment outperformed both standard relapse prevention and treatment-as-usual in reducing rates of heavy drinking and drug use 12-months following treatment (Bowen et al., 2014). Two RCTs have evaluated MBRP as a primary intervention in residential treatment and found that MBRP was significantly more effective than standard relapse prevention in reducing alcohol and drug use following treatment (Davis et al., 2018; Witkiewitz et al., 2014). Of note, there are several manualized interventions similar to MBRP, and recent meta-analytic reviews of the various mindfulness-based interventions for substance use disorders have each shown significant effects of mindfulness-based interventions in reducing substance use (Li et al., 2017).
In regards to mechanisms of change in MBRP, extant research suggests MBRP might be effective in part by reducing craving, decoupling the negative affect-craving association, decoupling the craving-substance use association, and increasing the mindfulness-based processes of awareness, acceptance, and nonjudgment (Wilson et al., 2017). Research on other mindfulness-based interventions for substance use disorder have shown that mindfulness training may reduce alcohol and drug cue reactivity to a greater extent than other treatments (Garland et al., 2014).
One important issue that needs to be addressed in MBRP is suboptimal treatment compliance rates. MBRP treatment compliance rates in outpatient settings range from 50–60% of participants attending the majority of sessions (Bowen et al., 2014, 2009), as compared to compliance of 70% in relapse prevention (Bowen et al., 2014). Clinically, we have observed some participants struggle with adhering to regular mindfulness practice, perhaps due to significant deficits in executive function (Aharonovich et al., 2006) and inhibitory control (Streeter et al., 2008). Those clients who are able to engage with MBRP experience fewer and less severe lapses, and report greater improvements in overall quality of life (Witkiewitz et al., 2014).
Transcranial direct current stimulation (tDCS).
Transcranial direct current stimulation (tDCS) is a safe and non-invasive brain stimulation technique that applies weak electrical current to stimulate brain regions (Clark et al., 2012). Most tDCS studies compare verum/active (1.0 to 2.0 milliamps (mA)) tDCS to a sham/control condition with either reduced current strength (i.e., 0.1 mA) or a ramp-up/ramp-down procedure that introduces an electrical current for a brief amount of time (Clark, 2018). The neurobiological mechanisms of tDCS depend on a variety of factors, including current strength and duration; the number, polarity and specific placement of electrodes; the electrode composition and size; and individual differences in physiology (Li et al., 2015). TDCS is thought to alter neuronal activity indirectly by inducing changes in resting membrane potential, cerebral blood flow, synaptic transmission, oscillatory activity, functional connectivity, and neurotransmitter concentrations (Fonteneau et al., 2018; Kroczek et al., 2016).
Active tDCS versus sham has been shown to enhance the effects of cognitive training in a number of domains, including inhibitory control, memory, attention and other forms of cognition (Coffman, Clark, & Parasuraman, 2012). There have been over 250 clinical trials using tDCS for a variety of disorders and diseases and there are also preliminary data suggesting tDCS has an effect on addictive disorders (Boggio et al., 2008; Coles et al., 2018; Feil and Zangen, 2010; Lapenta et al., 2018; Spagnolo and Goldman, 2017; Trojak et al., 2017). Specifically, tDCS has been shown to impact inhibitory control (Levasseur-Moreau and Fecteau, 2012; Stramaccia et al., 2015), and cue-reactivity and craving (Jansen et al., 2013; Nardone et al., 2012).
Most investigations of tDCS for addictive disorders have focused on intermediate phenotypes, including effects of tDCS on decision making and cue reactivity among individuals with stimulant use disorders (Batista et al., 2015), opioid use disorder (Wang et al., 2016), nicotine use disorders (Boggio et al., 2009), and marijuana use (Boggio et al., 2010). The growing literature of tDCS studies conducted with heavy drinkers and AUD patients have found significant effects of active tDCS on reductions in alcohol craving (Boggio et al., 2008; den Uyl et al., 2015; Jansen et al., 2013; Nardone et al., 2012) and several small RCTs have found significant effects of tDCS in preventing relapse and improving quality of life among patients with AUD (Klauss et al., 2014, 2018a). Yet, there are also negative findings. Recent studies have found no effects of active tDCS targeting the dorsolateral prefrontal cortex (dlPFC) versus sham tDCS on subjective and objective measures of alcohol craving and cue reactivity (den Uyl et al., 2016; Wietschorke et al., 2016), as well as AUD treatment outcomes (den Uyl et al., 2017; Klauss et al., 2018b).
Most prior studies in addiction have focused on stimulation of the right dlPFC, however there is preliminary evidence from our lab that tDCS of the right inferior frontal gyrus (IFG) was associated with reductions in drinking among smokers (Witkiewitz and Clark, 2015). Specifically, we examined the effects of MBRP combined with active tDCS of the right IFG among smokers who were interested in quitting smoking (n=8) in an uncontrolled pre-post design. All participants received 2.0 mA of active tDCS applied to the right IFG during a meditation practice in the first 30 minutes of four weekly 90-minute MBRP group sessions and completed a follow-up assessment at the end of treatment and two months following treatment (87.5% follow-up rate). Results indicated acceptability and feasibility of the tDCS/MBRP intervention, and indicated medium effect size reductions in drinks per drinking day (d=0.64 end of treatment, d=0.43 2-month follow-up) and heavy drinking days (d=0.50 end of treatment, d=0.46 2-month follow-up). Furthermore, prior studies have found stimulation targeting the right IFG to be associated with improvements in inhibitory control (Campanella et al., 2018) and increases in mindfulness in a sham-controlled trial of individuals with chronic pain (McCallion et al., under review).
Current Study
Both MBRP and tDCS have independently shown benefits for treating AUD. Further, there is recent evidence from small pilot studies that tDCS of the frontal cortex can enhance the effects of mindfulness training, potentially by improving working memory (Hunter et al., 2018), increasing positive affective experience (Robinson et al., 2017), or increasing awareness (Badran et al., 2017). The combination of MBRP with tDCS may provide synergistic benefits to reducing symptoms of AUD greater than each can provide alone. To test the hypothesis that tDCS may enhance the effects of MBRP in reducing drinking and targeting craving, cue reactivity, and inhibitory control, the current study was a sham-controlled, double-blind investigation of the efficacy of MBRP in combination with active or sham tDCS as an intervention for AUD. We hypothesized that individuals who received active tDCS in combination with MBRP would report greater reductions in drinks per drinking day (primary outcome) and percent heavy drinking days, self-reported craving and alcohol cue reactivity, as well as improvements in inhibitory control (secondary outcomes), as compared to those in the sham tDCS condition.
Materials and Methods
Participants and Procedures
Participants were recruited for the proposed study by posting flyers in the Albuquerque metro area, advertisements in local papers, and postings on online forums (e.g., Craigslist). Participants were screened and enrolled from November 2016 through March 2018 for the following inclusion criteria: interest in reducing drinking, drinking alcohol in the past 30 days, being able to communicate in English, and right-handedness based on a handedness scale (Oldfield, 1971). Exclusion criteria included inability to tolerate tDCS during an initial baseline session; current symptoms of psychosis or mania; current SUD (including mild SUD) other than nicotine or marijuana assessed via a Structured Clinical Interview for DSM-5 Disorders, Clinical Trials version (SCID-5-CT; First et al., 2015); metal objects in the body that interfere with or could be compromised by tDCS (e.g., metal plates, screws and prosthetics in head, cardiac pacemaker, implantable defibrillator, tattoos and permanent makeup using metal-containing inks, aneurysm clips, neural stimulators, ear implants, insulin pumps, drug-infusion devices, and dental appliances); allergy to latex, rubber, or a conductive medium like saline or electrode gel; pregnancy; history of seizures or a seizure disorder; history of severe alcohol withdrawal; or a score of 8 or greater on the Clinical Institute Withdrawal of Alcohol Assessment Scale – Revised (Sullivan et al., 1989) at any tDCS sessions.
Following phone screen, eligible participants (n=134) were randomized to active (n=68) or sham tDCS (n=66) using urn randomization with stratification by sex, age, and number of drinks on a typical drinking day reported at screening. All eligible participants after phone screen were invited to attend a baseline assessment session where participants completed informed consent, a battery of self-report and behavioral measures, a baseline EEG, and an initial tDCS session to evaluate tolerability of stimulation sensation. Randomization occurred prior to the baseline session so the initial tDCS session (to evaluate tolerance of tDCS) could be based on the assigned treatment condition. Of those initially randomized, only 85 individuals were included after the baseline session due to not attending the baseline assessment (n=29) or being excluded at baseline (n=20). One additional participant was excluded from the study after the baseline assessment because of threats of violence made toward the research team. Thus, the final intent-to-treat sample size available for analyses was 84 individuals (active: n=47; sham: n=37). See Figure 1 for participant flow in the study.
Figure 1.
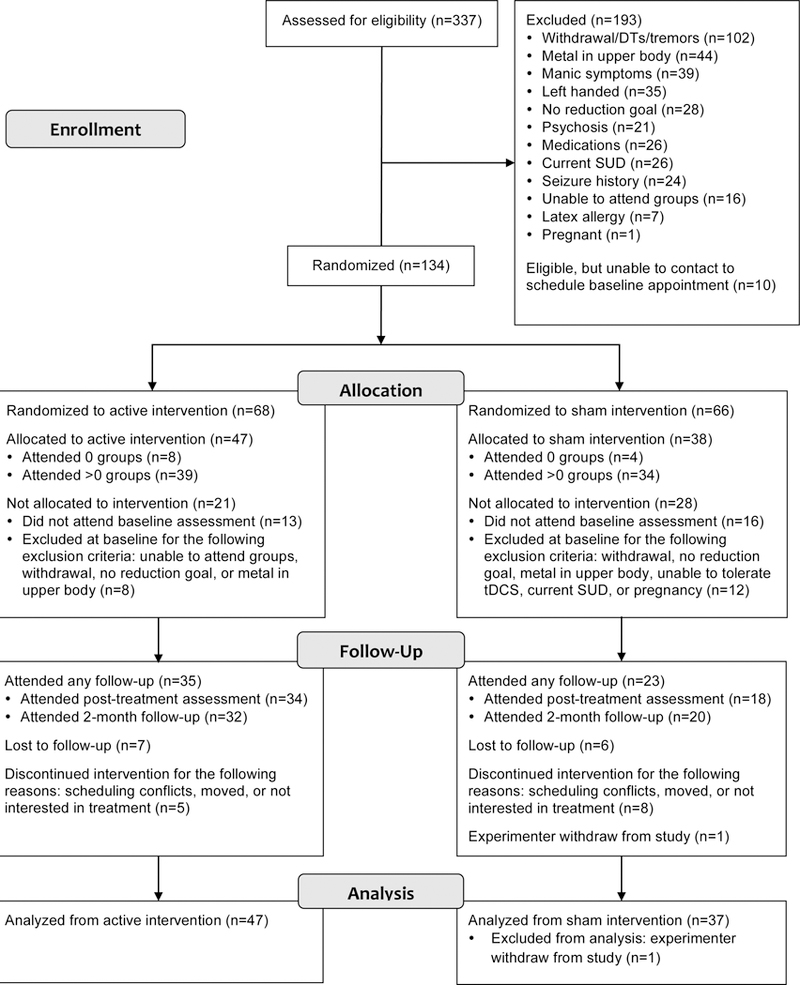
Consort Diagram
After completing the baseline assessment, participants attended the rolling MBRP group treatment once a week for eight weeks. At each session, participants received 30 minutes of tDCS during a guided meditation practice followed by 90 minutes of MBRP content, described below. Follow-up assessments were conducted at post-treatment (approximately 2–3 months after the baseline assessment) and approximately 2-months following treatment (approximately 4–6 months following the baseline assessment) from February 2017 through August 2018. Participants were paid $20/hour for their participation at the following assessment sessions: baseline (up to $100), during 8-sessions of treatment for completing questionnaires ($80), post-treatment (up to $100), and 2-month post-treatment (up to $40). An additional $10 bonus was provided for completing the post-treatment assessment, a $20 bonus was provided for completing the 2-month follow-up assessment, and a $50 bonus was provided for completing all assessment sessions. The maximum compensation was $400. All procedures were approved by the University of New Mexico Main Campus Institutional Review Board and no adverse events were reported. This study was registered with clinicaltrials.gov (NCT02861807).
tDCS Protocol.
All tDCS sessions were double blinded with either 2.0 mA (active) or 0.0 mA (sham) anodal stimulation of the right inferior frontal gyrus (IFG; i.e., F10 according to the modified EEG international 10–20 or 10–10 system) with a left upper arm cathode. Sponge electrodes were 5 cm x 3 cm x 0.56 cm (depth) at saturation and were pre-saturated using saline. The saline electrodes were snapped into stimulation sites (F10 and left upper arm) using a custom headband and armband (SNAPstrap™) designed by Soterix Medical for the purpose of this study. The anode electrode placement was approximately parallel with the zygomatic arch (see Figure 2). Finite element modeling of the electrical fields induced by the F10 versus left arm tDCS montage suggests that the largest effect is located underneath the anodal electrode, in the right IFG, with spread to the lateral and ventral prefrontal and temporal cortices and brainstem (see Scheldrup et al., 2014). In addition to these regions, activity in other regions to which these areas are interconnected may also be altered.
Figure 2.

TDCS placement with anode electrode applied to right inferior frontal gyrus and cathode electrode applied to left upper arm during meditation
Double blinding was achieved using the Soterix Mini-CT device (Soterix Medical, NY) with sham versus active stimulation assigned by codes stored within each device. Research staff entered a code and were not aware of participant stimulation condition. Stimulation was administered in groups during guided meditation as part of the MBRP treatment group sessions. Individuals in the active tDCS condition received a 30 second ramp up to 2.0 mA of current for the remaining 29 minutes, followed by a 30 second ramp down to no current and end of stimulation. The sham condition consisted of a 30 second ramp up to 2.0 mA of current and then a subsequent 30 second ramp down to 0.0 mA of current for 28 minutes. The last minute of sham stimulation included a 30 second ramp up and ramp down to no current at the end of stimulation. Participants rated sensations (heat, tingling, and itching) on a 10 point scale (from 1=no sensation to 10=severe sensation) at 10 minute intervals during each stimulation session. If participants rated a sensation of 7 or greater then tDCS was discontinued. Active and sham participants were treated in the same MBRP groups.
MBRP Protocol.
MBRP groups were co-facilitated by therapists that had at least a Bachelor’s degree in psychology or related field and experience with group treatment of AUD. Therapists were trained and supervised by a licensed clinical psychologist with experience in MBRP therapist training and supervision (KW). Fidelity to MBRP treatment content was monitored in real time by co-facilitation and weekly supervision. All sessions were 2 hours, with the first 30 minutes consisting of tDCS and guided meditation practice. After tDCS, sessions included discussions of mindfulness as a means of coping with craving, cognitions, and emotions; and session-specific mindfulness practices (e.g., urge surfing, SOBER breathing space). MBRP was delivered via a rolling group format so participants could start and end the treatment at varying times, as described by Roos et al (2018). Participants were given audio format files (mp3s or CDs) of guided meditation practices developed specifically for MBRP for practice outside of sessions.
Measures
Measures were assessed at baseline, post-treatment, and a 2-month follow-up assessment.
Demographics.
Demographics, including age, sex, and race/ethnicity, were assessed at baseline using a self-report demographic questionnaire.
Alcohol consumption.
Daily alcohol consumption was measured using a Timeline Follow-Back (TLFB) interview (Sobell and Sobell, 1992), which uses a calendar to assess daily alcohol use. The TLFB was used to derive estimates of the primary outcome: drinks per drinking day, as well as the secondary alcohol consumption outcome: percent heavy drinking days, where a heavy drinking day was defined as 4 or more drinks for women and 5 or more drinks for men (National Institute on Alcohol Abuse and Alcoholism, 2005). The TLFB has demonstrated reliability and validity in the collection of alcohol and drug use data (Sobell et al., 1996). The TLFB assessed daily alcohol consumption over the past 60 days at baseline, post-treatment (approximately 2–3 months following baseline), and a two-month follow-up (approximately 4–6 months following baseline).
Craving and alcohol cue reactivity.
Craving was assessed via the Penn Alcohol Craving Scale (PACS; Flannery et al., 1999), a five item measure of overall craving for alcohol scored with seven response options representing no craving/urges to extreme craving urge (e.g., “How difficult would it have been to resist taking a drink during this period of time if you had known a bottle was in your house?” with response options of “Not at all difficult” to “Would not be able to resist”). Higher scores indicate greater craving. The reliability of the PACS in the current study was excellent at all time points (Cronbach’s α > 0.86).
To measure cue-elicited responses to alcohol, we used a visual cue presentation task, whereby participants viewed pictures of alcohol containing beverages and neutral pictures from the International Affective Pictures Series (Lang et al., 2008) and from the web during electroencephalography (EEG). Alcohol and neutral pictures were matched for color and complexity as well as other potentially important confounds (e.g., presence of people). We examined responses to approximately 100 trials each of alcohol pictures and control pictures in a mixed event design (~15 minutes). Self-reported craving after cue presentation was rated on a scale of 1 to 9. The cue task was administered at baseline and post-treatment.
Inhibitory control.
The Stop Signal Task (Logan et al., 2014) was used to assess inhibitory control during EEG. In the task, participants make left-right judgments of the directionality of an arrow presented on the screen. For each trial, a circle appeared for 500 ms, followed by a left or right-pointing arrow for up to 1 second and between 300 ms and 800 ms jittered inter-trial interval to reduce anticipatory responses. Twenty-five percent of trials were “stop trials”, wherein the inside of the circle containing the arrow turned red to signal participants to inhibit the current response. This timing of the color change was dynamically adjusted to ensure successful inhibition on approximately 50% of trials. There were 400 trials across 10 minutes. Stop signal reaction time was estimated as the measure of inhibitory control. The Stop Signal task was administered at baseline and post-treatment.
Blinding measure.
A single item measure was used to assess whether the tDCS blinding procedures were successful. Specifically, participants were asked “If you had to guess, do you think you were assigned to active treatment (active brain stimulation) or the control treatment (no stimulation)?” The blind question was assessed at post-treatment.
Data Analyses
Preliminary analyses and a priori power analyses.
All assessment data were analyzed for outliers and missing cases to detect any bias that might result from non-normal distributions or differential attrition. Preliminary data checking indicated data were mostly normally distributed and attrition analyses revealed no measures were associated with missing data.
We conducted a priori power analyses using effect sizes from an unblinded pilot study evaluating the effect of active tDCS (2.0 mA) on drinks per drinking day among heavy drinking smokers where we found pre- to post- effect sizes of d=0.64 and d=0.43 on drinks per drinking day at post- and 2-month follow-up, respectively (Witkiewitz and Clark, 2015). Based on these preliminary effect sizes and with desired power>0.80, a two-tailed α<0.05, and three time-points, we needed 70 participants to detect a medium effect of active tDCS + MBRP versus sham tDCS + MBRP on drinks per drinking day at follow-up. Attrition of 15% was anticipated based on our pilot tDCS study and thus we proposed to recruit n=84 participants to allow for attrition.
Primary and secondary outcome models.
The efficacy of active tDCS + MBRP versus sham tDCS + MBRP was examined using a linear mixed effects model with drinks per drinking day as the primary outcome, with fixed effects of treatment and a random effect of time. The secondary outcomes that were assessed at all time points, including percent heavy drinking days, and self-reported craving on the PACS, were also analyzed using a linear mixed model. Binary covariates (e.g., sex and treatment condition) were coded as −0.5 and 0.5 and all continuous covariates (e.g., age, number of treatment groups) were grand mean centered. Time was coded as 0=baseline, 1=post-treatment, and 2=2-month follow-up. Secondary outcomes of cue reactivity and stop signal reaction time were analyzed using regression with baseline measures of the outcome covaried in the analyses.
Variables used in randomization (sex, age, average number of drinks on a typical drinking day reported at screen) were covaried in all analyses. All analyses used an intent to treat approach (Del Re et al., 2013), whereby all participants were analyzed as randomized after screen regardless of attendance at group sessions or receipt of tDCS. Those individuals with no available data (e.g., those who did not attend the baseline session) were excluded from analyses. To account for potential non-independence of observations due to timing of participation in the rolling group (e.g., cohort effects), we included trial month as a clustering variable and analyzed the data using a sandwich estimator to adjust the standard errors for non-independence of observations within cohorts. We used maximum likelihood estimation with robust standard errors for all analyses, which provides the variance-covariance matrix for all available data and is the preferred method for estimation when some data are missing (Hallgren and Witkiewitz, 2013; Schafer and Graham, 2002). All models were estimated in Mplus version 8.2 (Muthén and Muthén, 2017).
Results
Descriptives and Attrition
Participants (n=84; active: n=47; sham: n=37) were predominantly male (n=50; 59.5%), ranged in age from 19 to 77 years old (mean age (SD)=52.27 (13.0); median age=55), and were mostly non-Hispanic white (n=40; 47.6%) or Hispanic (n=32; 38.1%). Other races included non-Hispanic American Indian/Alaska Native (n=4; 4.8%), non-Hispanic Black or African American (n=3; 3.6%), more than one race (n=3; 3.6%), or race was not reported (n=2; 2.4%). Participants were mostly never married (n=26; 31.3%) or divorced (n=25; 30.1%), employed part-time (n=32; 38.6%) or full-time (n=22; 26.5%), and most participants had at least some college (n=25; 30.1%) or a college degree (n=44; 52.4%). Interestingly, most participants (n=50; 61.0%) had never received any form of treatment or mutual help for AUD. Most participants also reported a goal of drinking reduction (n=69; 82.1%) and only a minority of participants had an abstinence goal (n=15; 17.9%). Average drinks per drinking day at baseline was 6.56 drinks (SD=3.57) and participants drank heavily on 42.4% of days (SD=33.66%). Nearly 60% (n=50) met criteria for severe AUD on the clinician administered SCID (average number of symptoms=6.38, SD=2.56), 22.6% met criteria for moderate AUD, and 17.9% met criteria for mild AUD at baseline. Only 1 participant did not meet criteria for current AUD, but this individual did meet criteria for lifetime AUD and was currently drinking. Demographics and drinking measures by tDCS group are shown in Table 1. There were no significant differences between tDCS groups on any measures at baseline, post-treatment, or the 2-month-follow-up.
Table 1.
Demographic Variables, Treatment Attendance, and Primary/Secondary Outcome Measures by tDCS Groups and for the Total Sample (n=84)
| Measure | Total Sample | Active tDCS (n=47) |
Sham tDCS (n=37) |
Cohen’s |
|---|---|---|---|---|
| M (SD) | M (SD) / N (%) | M (SD) / N (%) |
d | |
| Sex N (%) Male | 50 (59.5%) | 28 (59.6%) | 22 (59.5%) | |
| N (%) AUD diagnosis baseline | 83 (98.8%) | 46 (97.9%) | 37 (100%) | |
| N (%) AUD diagnosis 2-months (%, out of 55 with SCID data) |
37 (67.3%) | 25 (73.5%) | 12 (57.1%) | |
| Age | 52.27 (13.00) | 51.43 (14.19) | 53.35 (11.41) | −.15 |
| Number of sessions | 4.08 (3.22) | 4.32 (3.35) | 3.78 (3.06) | .17 |
| DDD baseline | 6.56 (3.57) | 6.32 (3.02) | 6.85 (4.19) | −.15 |
| DDD post-treatment | 5.33 (3.51) | 5.12 (3.14) | 5.64 (4.06) | −.14 |
| DDD 2-months | 5.16 (3.62) | 4.95 (3.18) | 5.48 (4.27) | −.14 |
| PHDD baseline | 42.4% (33.6%) | 41.0% (32.0%) | 44.1% (35.9%) | −.09 |
| PHDD post-treatment | 26.8% (28.9%) | 27.4% (30.5%) | 25.9% (26.8%) | .05 |
| PHDD 2-months | 29.2% (31.6%) | 29.5% (31.1%) | 28.8% (33.2) | .02 |
| Craving baseline | 2.75 (1.25) | 2.82 (1.30) | 2.66 (1.19) | .13 |
| Craving post-treatment | 2.30 (1.28) | 2.42 (1.09) | 2.33 (1.39) | .07 |
| Craving 2-months | 2.30 (1.28) | 2.24 (1.21) | 2.40 (1.42) | −.12 |
| Cue reactivity baseline | 4.26 (2.10) | 4.40 (2.20) | 3.99 (1.95) | .20 |
| Cue reactivity post-treatment | 3.34 (1.73) | 3.49 (1.81) | 3.06 (1.60) | .25 |
| SSRT baseline | 291.22 (75.28) | 287.54 (75.76) | 295.52 (75.58) | −.11 |
| SSRT post-treatment | 278.0 (82.04) | 284.42 (94.85) | 266.06 (51.07) | .24 |
Note. Cohen’s d for differences between active – sham; DDD= drinks per drinking day; PHDD = percent heavy drinking days; SSRT = stop signal reaction time.
Participant retention was less than expected with only 69% of the sample (n=58) completing either the post-treatment or 2-month follow-up. Fifty participants (60%) completed the post-treatment assessment and 52 completed the 2-month follow-up (62%). There were no differences between those who were lost to follow-up and those who completed assessments on any of the baseline variables. Importantly, retention among those who attended at least one group (n=73) was nearly 80% and there was a significant difference between those who were lost to follow-up and those who completed assessments in treatment attendance (t (82)=5.95, p<0.001). Individuals who were followed attended an average of 5.26 group sessions (SD=3.14) and those who were lost to follow-up attended an average of 1.46 group sessions (SD=1.21).
Treatment Engagement and tDCS Delivery
There were no differences between tDCS groups in the average number of sessions attended (t (82)=−0.76, p=0.45; see Table 1). More participants in the active tDCS condition attended all 8 sessions (n=17; 36.2%) versus the sham condition (n=10; 27.0%), but this difference was not significant (χ2(1)=0.79, p=0.37). A minimal dose of the MBRP rolling group of 2 sessions (as described by Roos et al., 2018) was achieved by 76.4% of participants, with 80% of those in the active tDCS group and 73% of those assigned to sham tDCS receiving the minimal dose of MBRP rolling groups, which was also not significant (χ2(1)=0.45, p=0.50).
The participant blinding of tDCS was somewhat effective. Most participants in the active condition guessed correctly that they received active stimulation (n=26; 81.3% of those assigned to active who answered the blinding question); however, most participants in the sham condition also guessed (incorrectly) that they received active stimulation (n=11; 61.1% of those assigned to sham who answered the blinding question). The difference between groups in guessing active versus sham assignment was not significant (χ2 (1)=2.43, p=0.12). tDCS was discontinued during at least one treatment session throughout the 8 weeks of treatment due to high sensation ratings for 17 participants (33.3% of active participants versus 12.1% of sham participants), which was a significant difference between groups (χ2 (1)=4.46, p=0.04). On average, active tDCS participants reported significantly higher sensation ratings for all three sensations: itching, heat, and tingling (all p <0.05), at all sessions. There were no adverse events of tDCS, however four participants in the active tDCS condition and one participant in the sham condition refused tDCS administration after the first or second session. Reasons for refusing tDCS included wanting to use hearing aids or glasses during the sessions and not tolerating the tDCS sensations.
Primary and Secondary Outcome Models
Results from the mixed models, reported in Table 2, indicated significant effects of time and number of treatment groups attended in predicting drinks per drinking day and percent heavy drinking days. Drinks per drinking day decreased by approximately ½ of a drink, on average, at each time point and by approximately ¼ of a drink, on average, for each additional group attended, at the mean of all covariates. Likewise, percent of heavy drinking days decreased by approximately 5.68% of days, on average, at each time point and by approximately 2.25% of days, on average, for each additional group attended, at the mean of all covariates. For self-reported craving on the PACS, there was a significant effect of time, such that scores on the PACS decreased significantly over time. Typical drinks at screening was the only other significant covariate, with more drinks at screening associated with significantly higher drinks per drinking day, percent heavy drinking days, and craving on the PACS. Active versus sham tDCS was not associated with any of the outcomes in the mixed models.
Table 2.
Mixed Model Results for Primary and Secondary Outcomes (n=84)
| Model Parameters | DDD | PHDD | PACS | |||
|---|---|---|---|---|---|---|
| β | B (SE) | β | B (SE) | β | B (SE) | |
| Time | −.27 | −.52 (.17)** | −.27 | −5.68 (1.88)** | −.34 | −0.26 (.07)*** |
| Sex (male coded +5) | .20 | 1.36 (.71) | −.01 | −0.59 (6.56) | −.12 | −0.27 (.27) |
| Age | −.03 | −0.008 (.02) | .18 | 0.38 (.22) | .15 | 0.01 (.01) |
| Typical drinks at screening | .43 | 0.37 (.11)** | .35 | 2.50 (0.89)** | .22 | 0.06 (.03)* |
| Number of groups attended | −.24 | −0.25 (.11)* | −.26 | −2.25 (.84)** | .17 | 0.06 (.04) |
| Treatment (active tDCS coded +5) | −.11 | −0.72 (.76) | −.03 | −1.81 (8.46) | .04 | 0.09 (.32) |
Note.
p<0.05;
p<0.01;
p<0.001.
β = standardized coefficient; B (SE) = unstandardized regression coefficient (standard error); DDD = Drinks per drinking day; PHDD = Percent heavy drinking days; PACS = Penn Alcohol Craving Scale.
Results from the regression models, reported in Table 3, indicated significant effects of baseline craving and the number of groups attended in predicting self-reported craving after alcohol cue presentation in the cue reactivity task. Individuals who attended more groups reported significantly less craving following the alcohol cue presentation. For the Stop Signal Task, there were no significant effects of covariates on inhibitory control, as measured by the stop signal reaction time. Active versus sham tDCS was not associated with any of the outcomes in the regression models.
Table 3.
Regression Model Results for Secondary Outcomes
| Predictors | Cue reactivity | SSRT | ||
|---|---|---|---|---|
| β | B (SE) | β | B (SE) | |
| Baseline measure of outcome | 0.56 | 0.50 (.10)*** | 0.24 | 0.21 (.15) |
| Sex (male coded +5) | 0.25 | 0.91 (.57) | 0.02 | 2.91 (24.5) |
| Age | 0.02 | 0.002 (.02) | 0.08 | 0.40 (.97) |
| Typical drinks at screening | 0.12 | 0.06 (.09) | 0.10 | 1.80 (2.43) |
| Number of groups attended | −0.32 | −0.20 (.05)*** | −0.10 | −1.95 (2.67) |
| Treatment (active tDCS coded +5) | 0.04 | 0.15 (.46) | 0.07 | 9.28 (16.18) |
Note.
p<0.05;
p<0.01;
p<0.001.
β = standardized regression coefficient; B (SE) = unstandardized regression coefficient (standard error); SSRT = Stop Signal Reaction Time.
Discussion
The current study aimed to examine the synergistic effects of rolling group mindfulness-based relapse prevention (MBRP) and transcranial direct current stimulation (tDCS) applied to the right inferior frontal gyrus (rIFG) as a combination treatment for individuals with alcohol use disorder (AUD) who were interested in reducing their drinking. Results indicated small, statistically significant reductions in drinks per drinking day and percent heavy drinking days over time and a MBRP dose effect, such that attending more MBRP sessions was associated with greater reductions in drinking over time. On average, participants reduced their drinking by just over one drink per drinking day and reduced occasions of heavy drinking by approximately 15% of days. Given that the sample was initially a lower drinking sample compared to most alcohol clinical trials (e.g., average drinks per drinking day at baseline in the COMBINE study was greater than 12 drinks per drinking day, as compared to approximately 7 drinks in the current study; Anton et al., 2006), and that nearly all patients had drinking reduction goals, these findings suggest that small drinking reductions could be clinically meaningful. Indeed, we found nearly one-third of the sample no longer endorsed two or more symptoms of AUD at the two-month follow-up, suggesting the drinking reductions were associated with improvements in functioning. There were also significant reductions in self-reported craving, as measured via a questionnaire (the Penn Alcohol Craving Scale) and an alcohol cue reactivity task. There were no effects of treatment on inhibitory control, measured via a Stop Signal Task.
The significant dose effects of MBRP group attendance on nearly all outcomes suggests that the outpatient rolling group MBRP program may be an effective treatment for individuals with drinking reduction goals. Prior research has also found a greater dose of the MBRP rolling group was associated with significantly greater reduction in craving and negative affect among individuals in residential treatment for substance use disorder (Roos et al., 2018). Lack of a control group for the MBRP condition greatly limits interpretation, given that reductions in alcohol use may reflect motivation to reduce alcohol use or simply regression to the mean. Future work with a comparison group would be necessary to determine whether MBRP is an effective outpatient treatment for individuals with drinking reduction goals.
The lack of findings for tDCS was somewhat surprising given our pilot data that found medium effects of tDCS and mindfulness training on drinks per drinking in an unblinded trial (Witkiewitz and Clark, 2015). However, the finding that tDCS was not significant under rigorous conditions of double blinding is consistent with other recent studies in the field that have also found no effects of active tDCS versus sham tDCS on alcohol craving, cue reactivity (den Uyl et al., 2016; Wietschorke et al., 2016), and AUD treatment outcomes (den Uyl et al., 2017; Klauss et al., 2018b). Increasing blinding, from no blind to single blind to double blind, appears to be associated with corresponding decreases in effect sizes. As such, future work should focus on only double-blinded tDCS trials. Lack of findings for tDCS could also be due to the electrode positions and other aspects of the tDCS protocol used in the current study. The F10/right IFG placement has shown some promise in increasing inhibitory control (Campanella et al., 2018) and mindfulness (McCallion et al., under review), but has received less attention in treatment trials for addiction compared with other protocols. Placing the electrodes over right (anode) and left (cathode) dorsolateral prefrontal cortex, which has been used previously in addiction treatment trials might have yielded different results. Additionaly, the decision to use the left upper arm for the placement of the cathode electrode was based on our prior work (Clark et al., 2012), and we did not consider bihemispheric stimulation of the IFG. Yet, recent work has found that bihemispheric tDCS stimulation of the IFG (with a right IFG anode and left IFG cathode) was not associated with improvements in inhibitory control (Leite et al., 2018) or reductions in craving (den Uyl et al., 2015). Unfortunately, with the various dosing and electrode parameters (current strength, polarity and duration, and electrode number, size and placement), over 4 million tDCS protocols are possible (Clark, 2018). Thus, it is impossible to know if the current study did not find effects of tDCS because tDCS is ineffective, or because we utilized an ineffective combination of tDCS electrode placement and dose parameters.
The current study had several additional limitations. The low follow-up rate is particularly concerning. We did not identify systematic attrition bias and used model-based missing data methods, which are less prone to bias when data are missing at random (Hallgren and Witkiewitz, 2013); however, the interpretation of effects is limited. Our decision to exclude individuals with a history of severe alcohol withdrawal, due to tDCS safety concerns, likely reduced variability in our sample and also resulted in a less severe sample of individuals with AUD. Other tDCS exclusions, including seizure history, left-handedness, and metal in the upper body further limits generalizability. Due to funding limitations we were also unable to assess alcohol biomarkers and relied entirely on self-reported drinking, which may be prone to errors. However, the Timeline Follow-Back method, particularly over short periods, has been shown to produce reliable and valid estimates of alcohol consumption (Sobell et al., 1996). Another potential limitation is the older age and wide age range of this sample (range 19–77, median=55 years old; 75% of the sample older than 45). Prior tDCS studies have found reduced and/or delayed effects of tDCS in older populations (Boggio et al, 2010). Thus, the effects of tDCS may have been reduced or altered in this sample relative to a younger population. Yet, sensitivity analyses testing age by treatment interactions did not indicate differential treatment effects by age. In addition to the broad age range, we also included participants who were tobacco (42.9%) or cannabis users (38.1%), which increased generalizability, but also increased the heterogeneity in the sample. In addition, nicotine use has also been found to alter tDCS effects (Thirugnanasambandam et al. 2011). Future work could explore whether tDCS is effective for individuals with AUD who are not tobacco and/or cannabis users.
Despite these limitations, the current study provides an important test of tDCS as an adjunct to behavioral treatment for AUD, and results from the current study did not find support for our specific implementation of tDCS combined with MBRP. Post-hoc power analyses indicated over 800 subjects per condition would be necessary to detect the small effect size difference between the active and sham tDCS conditions on drinks per drinking day (d=.14). Although tDCS was not effective, the findings provide preliminary support for rolling group MBRP as an outpatient treatment for individuals who are interested in drinking reduction. Future studies with larger samples of individuals with AUD, particularly individuals with more severe AUD, and an active comparison treatment are necessary to further evaluate the efficacy of rolling group MBRP for drinking reduction. Future work with tDCS may consider alternative electrode placements and tDCS dosing parameters, as well as individualized stimulation protocols (Naish et al., 2018).
Acknowledgements.
This research was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (R21AA024926; T32AA018108), and a University of New Mexico Women in STEM Faculty Development Seed Grant.
Footnotes
The authors have no conflicts of interest.
Contributor Information
Katie Witkiewitz, Department of Psychology, University of New Mexico.
Elena R. Stein, Department of Psychology, University of New Mexico
Victoria R. Votaw, Department of Psychology, University of New Mexico
Adam D. Wilson, Department of Psychology, University of New Mexico
Corey R. Roos, Department of Psychology, University of New Mexico
Stevi J. Gallegos, Department of Psychology, University of New Mexico
Vincent P. Clark, Department of Psychology, University of New Mexico
Eric D. Claus, Mind Research Network.
References
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV, 2006. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend 81, 313–22. [DOI] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A, 2006. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 295, 2003–17. [DOI] [PubMed] [Google Scholar]
- Badran BW, Austelle CW, Smith NR, Glusman CE, Froeliger B, Garland EL, Borckardt JJ, George MS, Short B, 2017. A double-blind study exploring the use of transcranial direct current stimulation (tDCS) to potentially enhance mindfulness meditation (E-Meditation). Brain Stimul 10, 152–154. [DOI] [PubMed] [Google Scholar]
- Batista EK, Klauss J, Fregni F, Nitsche MA, Nakamura-Palacios EM, 2015. A randomized placebo-controlled trial of targeted prefrontal cortex modulation with bilateral tDCS in patients with crack-cocaine dependence. Int J Neuropsychopharmacol 18, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Campanhã C, Valasek CA, Fecteau S, Pascual-Leone A, Fregni F 2010. Modulation of decision-making in a gambling task in older adults with transcranial direct current stimulation. Eur J Neurosci 31, 593–7. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Liguori P, Sultani N, Rezende L, Fecteau S, Fregni F, 2009. Cumulative priming effects of cortical stimulation on smoking cue-induced craving. Neurosci Lett 463, 82–6. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Sultani N, Fecteau S, Merabet L, Mecca T, Pascual-Leone A, Basaglia A, Fregni F, 2008. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double-blind, sham-controlled study. Drug Alcohol Depend 92, 55–60. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Zaghi S, Villani AB, Fecteau S, Pascual-Leone A, Fregni F, 2010. Modulation of risk-taking in marijuana users by transcranial direct current stimulation (tDCS) of the dorsolateral prefrontal cortex (DLPFC). Drug Alcohol Depend 112, 220–5. [DOI] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD, 2011. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med 41, 516–24. [DOI] [PubMed] [Google Scholar]
- Bowen S, Chawla N, Collins SE, Witkiewitz K, Hsu S, Grow J, Clifasefi S, Garner M, Douglass A, Larimer ME, Marlatt GA, 2009. Mindfulness-based relapse prevention for substance use disorders: a pilot efficacy trial. Subst Abus 30, 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen S, Chawla N, Marlatt GA, 2011. Mindfulness-based relapse prevention for addictive behaviors: A clinician’s guide. Guilford Press, New York, NY. [Google Scholar]
- Bowen S, Witkiewitz K, Clifasefi SL, Grow J, Chawla N, Hsu SH, Carroll HA, Harrop E, Collins SE., Lustyk MK, Larimer ME, 2014. Relative efficacy of mindfulness-based relapse prevention, standard relapse prevention, and treatment as usual for substance use disorders: a randomized clinical trial. JAMA Psychiatry 71, 547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella S, Schroder E, Vanderhasselt M-A, Baeken C, Kornreich C, Verbanck P, Burle B, 2018. Short-term impact of tDCS over the right inferior frontal cortex on impulsive responses in a go/no-go task. Clin EEG Neurosci 49, 398–406. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Serretti A, Christian J, 2013. Mindfulness: Top–down or bottom–up emotion regulation strategy? Clin Psychol Rev 33, 82–96. [DOI] [PubMed] [Google Scholar]
- Clark VP 2018. Coordinated, multimodal neuromodulation and neuroimaging. IEEE Intelligent Informatics Bulletin, 19(2), 1–3. [Google Scholar]
- Clark VP, Coffman BA, Mayer AR, Weisend MP, Lane TDR, Calhoun VD, Raybourn EM, Garcia CM, Wassermann EM, 2012. TDCS guided using fMRI significantly accelerates learning to identify concealed objects. Neuroimage 59, 117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Ewing SWF, Filbey FM, Sabbineni A, Hutchison KE, 2011a. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology 36, 2086–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Ewing SWF, Filbey FM, Sabbineni A, Hutchison KE, 2011b. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology 36, 2086–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Feldstein Ewing SW, Filbey FM, Hutchison KE, 2013. Behavioral control in alcohol use disorders: relationships with severity. J Stud Alcohol Drugs 74, 141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman BA, Clark VP, Parasuraman R (2014). Battery powered thought: A review of methods for cognitive enhancement using transcranial direct current stimulation. NeuroImage 85, 895–908. [DOI] [PubMed] [Google Scholar]
- Coles AS, Kozak K, George TP, 2018. A review of brain stimulation methods to treat substance use disorders. Am J Addict 27, 71–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JP, Berry D, Dumas TM, Ritter E, Smith DC, Menard C, Roberts BW, 2018. Substance use outcomes for mindfulness based relapse prevention are partially mediated by reductions in stress: Results from a randomized trial. J Subst Abuse Treat 91, 37–48. [DOI] [PubMed] [Google Scholar]
- Del Re AC, Maisel NC, Blodgett JC, Finney JW, 2013. Intention-to-treat analyses and missing data approaches in pharmacotherapy trials for alcohol use disorders. BMJ Open 3, e003464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Uyl TE, Gladwin TE, Rinck M, Lindenmeyer J, Wiers RW, 2017. A clinical trial with combined transcranial direct current stimulation and alcohol approach bias retraining. Addict Biol 22, 1632–1640. [DOI] [PubMed] [Google Scholar]
- den Uyl TE, Gladwin TE, Wiers RW, 2015. Transcranial direct current stimulation, implicit alcohol associations and craving. Biol Psychol 105, 37–42. [DOI] [PubMed] [Google Scholar]
- den Uyl TE, Gladwin TE, Wiers RW, 2016. Electrophysiological and Behavioral Effects of Combined Transcranial Direct Current Stimulation and Alcohol Approach Bias Retraining in Hazardous Drinkers. Alcohol Clin Exp Res 40, 2124–2133. [DOI] [PubMed] [Google Scholar]
- Farb NAS, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, Anderson AK, 2007. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Soc Cogn Affect Neurosci 2, 313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil J, Zangen A, 2010. Brain stimulation in the study and treatment of addiction. Neurosci Biobehav Rev 34, 559–74. [DOI] [PubMed] [Google Scholar]
- First MB, Williams JBW, Karg RL, 2015. Structured Clinical Interview for DSM-5 Disorders Clinical Trials Version. American Psychiatric Publishing, Inc., Arlington, VA, US. [Google Scholar]
- Flannery BA, Volpicelli JR, Pettinati HM, 1999. Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res 23, 1289–1295. [PubMed] [Google Scholar]
- Fonteneau C, Redoute J, Haesebaert F, Le Bars D, Costes N, Suaud-Chagny M-F, Brunelin J, 2018. Frontal transcranial direct current stimulation induces dopamine release in the ventral striatum in humans. Cereb Cortex 28, 2636–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Liguori P, Fecteau S, Nitsche MA, Pascual-Leone A, Boggio PS, 2008. Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: a randomized, sham-controlled study. J Clin Psychiatry 69, 32–40. [DOI] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, Howard MO, 2014. Effects of Mindfulness-Oriented Recovery Enhancement on reward responsiveness and opioid cue-reactivity. Psychopharmacol 231, 3229–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan AE, Grekin ER, Sher KJ, 2007. Decision making and binge drinking: a longitudinal study. Alcohol Clin Exp Res 31, 928–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS, 2015. Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 72, 757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren KA, Witkiewitz K, 2013. Missing data in alcohol clinical trials: A comparison of methods. Alcohol Clin Exp Res 37, 2152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago DR, Ott U, 2011. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspect Psychol Sci 6, 537–59. [DOI] [PubMed] [Google Scholar]
- Hunter MA, Lieberman G, Coffman BA, Trumbo MC, Armenta ML, Robinson CSH, Bezdek MA, O’Sickey AJ, Jones AP, Romero V, Elkin-Frankston S, Gaurino S, Eusebi L, Schumacher EH, Witkiewitz K, Clark VP, 2018. Mindfulness-based training with transcranial direct current stimulation modulates neuronal resource allocation in working memory: A randomized pilot study with a nonequivalent control group. Heliyon 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen JM, Daams JG, Koeter MWJ, Veltman DJ, van den Brink W, Goudriaan AE, 2013. Effects of non-invasive neurostimulation on craving: A meta-analysis. Neurosci Biobehav Rev 37, 2472–2480. [DOI] [PubMed] [Google Scholar]
- Klauss J, Anders QS, Felippe LV, Ferreira LVB, Cruz MA, Nitsche MA, Nakamura-Palacios EM, 2018a. Lack of additional effects of extended sessions of transcranial Direct Current Stimulation (tDCS) over dorsolateral prefrontal cortex on craving and relapses in crack-cocaine users. Front Pharmacol 9, 1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauss J, Anders QS, Felippe LV, Nitsche MA, Nakamura-Palacios EM, 2018b. Multiple sessions of transcranial direct current stimulation (tDCS) reduced craving and relapses for alcohol use: A randomized placebo-controlled trial in alcohol use disorder. Front Pharmacol 9, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauss J, Penido Pinheiro LC, Silva Merlo B.L., de Almeida Correia Santos G, Fregni F, Nitsche MA, Miyuki Nakamura-Palacios E, 2014. A randomized controlled trial of targeted prefrontal cortex modulation with tDCS in patients with alcohol dependence. Int J Neuropsychopharmacol 17, 1793–803. [DOI] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW, George O, 2014. Addiction as a stress surfeit disorder. Neuropharmacology 76 Pt B, 370–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2010. Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroczek AM, Häußinger FB, Rohe T, Schneider S, Plewnia C, Batra A, Fallgatter AJ, Ehlis AC, 2016. Effects of transcranial direct current stimulation on craving, heart-rate variability and prefrontal hemodynamics during smoking cue exposure. Drug Alcohol Depend 168, 123–127. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (2008). International affective picture System (IAPS): Affective ratings of pictures and instruction manual Technical Report A-8. University of Florida, Gainesville, FL. [Google Scholar]
- Lapenta OM, Marques LM, Rego GG, Comfort WE, Boggio PS, 2018. tDCS in addiction and impulse control disorders. J ECT 00, 1. [DOI] [PubMed] [Google Scholar]
- Leite J, Goncalves OF, Pereira P, Khadka N, Bikson M, Fregni F, Carvalho S 2018. The differential effects of unihemispheric and bihemispheric tDCS over the inferior frontal gyrus on proactive control. Neurosci Res, 130, 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levasseur-Moreau J, Fecteau S, 2012. Translational application of neuromodulation of decision-making. Brain Stimul 5, 77–83. [DOI] [PubMed] [Google Scholar]
- Li LM, Uehara K, Hanakawa T, 2015. The contribution of interindividual factors to variability of response in transcranial direct current stimulation studies. Front Cell Neurosci 9, 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Howard MO, Garland EL, McGovern P, Lazar M, 2017. Mindfulness treatment for substance misuse: A systematic review and meta-analysis. J Subst Abuse Treat 75, 62–96. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, Koob GF, 2015. Heterogeneity of alcohol use disorder: Understanding mechanisms to advance personalized rreatment. Alcohol Clin Exp Res 39, 579–584. [DOI] [PubMed] [Google Scholar]
- Logan GD, Van Zandt T, Verbruggen F, Wagenmakers E-J, 2014. On the ability to inhibit thought and action: general and special theories of an act of control. Psychol Rev 121, 66–95. [DOI] [PubMed] [Google Scholar]
- McCallion E, Robinson CSH, Clark VC, Witkiewitz K under review. Efficacy of a transcranial direct current stimulation (tDCS) enhanced Mindfulness-Based Stress Reduction protocol for chronic pain management: A single-blind randomized sham controlled study. Mindfulness. [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD, 2000. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA 284, 1689–95. [DOI] [PubMed] [Google Scholar]
- Muthén, L.K., Muthén, B.O., 2017. Mplus users guide (Version 8), Los Angeles, CA.
- Naish KR,Vedelago L, MacKillop J, Amlung M 2018. Effects of neuromodulation on cognitive performance in individuals exhibiting addictive behaviors: A systematic review. Drug Alcohol Depend 192, 338–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone R, Bergmann J, Christova M, Lochner P, Tezzon F, Golaszewski S, Trinka E, Brigo F, 2012. Non-invasive brain stimulation in the functional evaluation of alcohol effects and in the treatment of alcohol craving: a review. Neurosci Res 74, 169–76. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism, 2005. Helping patients who drink too much: A clinician’s guide. National Institutes of Health, Bethesda, MD. [Google Scholar]
- Oldfield RC, 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Robinson C, Armenta M, Combs A, Lamphere ML, Garza GJ, Neary J, Wolfe JH, Molina E, Semey DE, McKee CM, Gallegos SJ, Jones AP, Trumbo MC, Al-Azzawi H, Hunter MA, Lieberman G, Coffman BA, Aboseria M, Bikson M, Clark VP, Witkiewitz K, 2017. Modulating affective experience and emotional intelligence with loving kindness meditation and transcranial direct current stimulation: A pilot study. Soc Neurosci doi: 10.1080/17470919.2017.1397054. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H, 2013. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol 18, 121–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL, Graham JW, 2002. Missing data: our view of the state of the art. Psychol Methods 7, 147–77. [PubMed] [Google Scholar]
- Scheldrup M, Greenwood PM, McKendrick R, Strohl J, Bikson M, Alam M, McKinley RA, & Parasuraman R 2014. Transcranial direct current stimulation facilitates cognitive multi-task performance differentially depending on anode location and subtask. Fron Hum Neursci 8, 665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbabaie A, Golesorkhi M, Zamanian B, Ebrahimpoor M, Keshvari F, Nejati V, Fregni F, Ekhtiari H, 2014. State dependent effect of transcranial direct current stimulation (tDCS) on methamphetamine craving. Int J Neuropsychopharmacol 17, 1591–8. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Brown J, Leo GI, Sobell MB, 1996. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend 42, 49–54. [DOI] [PubMed] [Google Scholar]
- Spagnolo PA, Goldman D, 2017. Neuromodulation interventions for addictive disorders: challenges, promise, and roadmap for future research. Brain 140, 1183–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stramaccia DF, Penolazzi B, Sartori G, Braga M, Mondini S, Galfano G, 2015. Assessing the effects of tDCS over a delayed response inhibition task by targeting the right inferior frontal gyrus and right dorsolateral prefrontal cortex. Exp Brain Res 233, 2283–90. [DOI] [PubMed] [Google Scholar]
- Streeter CC, Terhune DB, Whitfield TH, Gruber S, Sarid-Segal O, Silveri MM, Tzilos G, Afshar M, Rouse ED, Tian H, Renshaw PF, Ciraulo DA, Yurgelun-Todd DA, 2008. Performance on the Stroop predicts treatment compliance in cocaine-dependent individuals. Neuropsychopharmacology 33, 827–36. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM, 1989. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict 84, 1353–7. [DOI] [PubMed] [Google Scholar]
- Thirugnanasambandam N, Grundey J, Adam K, Drees A, Skwirba AC, Lang N, Paulus W, Nitsche MA, 2011. Nicotinergic impact on focal and non-focal neuroplasticity induced by non-invasive brain stimulation in non-smoking humans. Neuropsychopharmacol 36, 879–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojak B, Sauvaget A, Fecteau S, Lalanne L, Chauvet-Gelinier J-C, Koch S, Bulteau S, Zullino D, Achab S, 2017. Outcome of non-invasive brain stimulation in substance use disorders: A review of randomized sham-controlled clinical trials. J Neuropsychiatry Clin Neurosci 29, 105–118. [DOI] [PubMed] [Google Scholar]
- Westbrook C, Creswell JD, Tabibnia G, Julson E, Kober H, Tindle HA, 2011. Mindful attention reduces neural and self-reported cue-induced craving in smokers. Soc Cogn Affect Neurosci 8, 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietschorke K, Lippold J, Jacob C, Polak T, Herrmann MJ, 2016. Transcranial direct current stimulation of the prefrontal cortex reduces cue-reactivity in alcohol-dependent patients. J Neural Transm 123, 1173–1178. [DOI] [PubMed] [Google Scholar]
- Wilson AD, Roos CR, Robinson CS, Stein ER, Manuel JA, Enkema MC, Bowen S, Witkiewitz K, 2017. Mindfulness-based interventions for addictive behaviors: Implementation issues on the road ahead. Psychol Addict Behav 31, 888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Clark VP, 2015. Integration of brain stimulation and imaging in clinical research and treatment of addiction. BrainStim 2015. Honolulu, HI. [Google Scholar]
- Witkiewitz K, Lustyk MKB, Bowen S, 2013. Retraining the addicted brain: A review of hypothesized neurobiological mechanisms of mindfulness-based relapse prevention. Psychol Addict Behav 27, 351–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Warner K, Sully B, Barricks A, Stauffer C, Thompson BL, Luoma JB, 2014. Randomized trial comparing mindfulness-based relapse prevention with relapse prevention for women offenders at a residential addiction treatment center. Subst Use Misuse 49, 536–46. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2009. Global health risks: mortality and burden of disease attributable to selected major risks. World Health Organization, Geneva, Switzerland. [Google Scholar]


