Abstract
Background:
HIV infection and heavy drinking independently promote microbial translocation and inflammation. However, it is not known how alcohol use may affect these processes in people living with HIV (PLWH). This study tested the hypothesis that alcohol exacerbates innate immune dysfunction in PLWH.
Methods:
Participants were 75 PLWH and 34 uninfected controls. Groups were recruited to have similar proportions of non-drinkers, moderate drinkers, and heavy drinkers. Substance use data and plasma samples were collected at up to three visits over a five-year study period. Recent alcohol use was assessed with the Timeline Followback Interview. Biomarkers of microbial translocation (lipopolysaccharide, LPS) and immune activation (lipopolysaccharide binding protein, LBP; soluble CD14, sCD14; soluble CD163, sCD163) were quantified using ELISA. Analyses tested two hypotheses: 1) that biomarker levels would be significantly higher in PLWH than controls with comparable alcohol use; 2) that current alcohol use would exacerbate biomarker elevations in PLWH. The second analysis included the interaction of alcohol use with hepatitis C virus (HCV) co-infection.
Results:
Groups were matched on alcohol use, smoking, and other drug use. All biomarkers were significantly higher in PLWH relative to controls (LBP: p=.005; LPS: p=.014; sCD14: p<.001; sCD163: p<.001). In PLWH, alcohol use showed a significant, positive association with sCD163, but not with other biomarkers. However, the interaction of alcohol use with HCV co-infection was significant for all biomarkers (LBP: p=.002; LPS: p=.026; sCD14: p=.0004; sCD163: p=.001). In pairwise tests with sequential Bonferroni correction, HIV/HCV co-infected individuals who drank heavily had significantly higher sCD163 compared to co-infected non-drinkers and to HIV mono-infected non-drinkers, moderate drinkers, and heavy drinkers (p’s<.005). Co-infected moderate drinkers had significantly higher sCD163 than each mono-infected group (p’s<.003). In addition, sCD14 was significantly higher in co-infected moderate drinkers than co-infected non-drinkers (p=.027).
Conclusions:
As predicted, PLWH had higher levels of LBP, LPS, sCD14, and sCD163 than uninfected individuals with similar alcohol use. In PLWH, alcohol by itself was significantly associated only with higher sCD163. However, heavy or moderate alcohol use was associated with elevations in macrophage activation (sCD163) and monocyte activation (sCD14) in HIV/HCV co-infected individuals.
Keywords: HIV infection, alcohol, hepatitis C, biomarkers, inflammation
Introduction
Chronic immune activation and inflammation are hallmarks of human immunodeficiency virus (HIV) infection, even with viral suppression on combination antiretroviral therapy (ART) (Appay and Kelleher, 2016). Translocation of gut bacteria into the systemic circulation is a driver of chronic inflammation in people living with HIV infection (PLWH) (Brenchley et al., 2006, Zevin et al., 2016). At the same time, mounting evidence implicates heavy alcohol use as a cause of microbial translocation and immune dysregulation in the general population. Because 47% of PLWH in the United States reported alcohol use and 15% reported heavy drinking in the past 30 days (Centers for Disease Control and Prevention, 2016), there is clear potential for alcohol to exacerbate immune dysfunction in the context of HIV. A recent study showed that even moderate drinking, averaging one drink per day, was associated with significantly increased mortality in men living with HIV (Justice et al., 2016). Some harmful effects of alcohol in PLWH may be mediated by behavioral factors such as decreased adherence to ART (Kalichman et al., 2013). However, the biological bases of alcohol-HIV interactions remain incompletely understood (Hahn and Samet, 2010). The objective of the current study was to investigate associations of alcohol use with systemic markers of microbial translocation and innate immune activation in HIV infection.
A widely used marker of microbial translocation is plasma concentration of lipopolysaccharide (LPS), a component of cell walls of Gram-negative bacteria and a ligand for toll-like receptor 4 (TLR4) (Park and Lee, 2013). Monocyte recognition of LPS by TLR4 requires facilitation by co-receptor cluster of differentiation 14 (CD14) and lipopolysaccharide binding protein (LBP). Binding of LPS to TLR4 induces pro-inflammatory cytokine production and shedding of CD14 from the cellular membrane, leading to elevated plasma levels of soluble CD14 (sCD14). sCD14 is an acute phase protein that modulates monocyte response by either presenting LPS to the TLR4-CD14 receptor complex or diverting it to non-immunoreactive lipoproteins (Landmann et al., 1996, Lu et al., 2008, Kitchens and Thompson, 2005). A related protein, soluble CD163 (sCD163), also is induced by LPS and is a marker of macrophage activation (Moller, 2012).
Numerous clinical studies have reported elevated levels of LPS, LBP, sCD14, and sCD163 in PLWH relative to uninfected individuals, providing strong evidence of microbial translocation and associated immune activation in this population [e.g., (Brenchley et al., 2006, Ancuta et al., 2008, Dinh et al., 2014, Balagopal et al., 2008, Burdo et al., 2011, Royal et al., 2016, Sandler et al., 2011)]. These biomarkers tend not to normalize in ART-treated PLWH, suggesting that viral replication is not the primary driver of chronic inflammation in PLWH (Marchetti et al., 2013). Co-infection with HIV and hepatitis C virus (HCV) leads to greater elevation in sCD163 than HIV mono-infection, consistent with the role of sCD163 in hepatic inflammation (Shmagel et al., 2016, Lidofsky et al., 2018, Mascia et al., 2017). Clinical utility of these biomarkers, particularly sCD14 and sCD163, derives from their ability to predict cognitive impairment, morbidity, and all-cause mortality in PLWH, independent of clinical factors (Ancuta et al., 2008, Burdo et al., 2013, Royal et al., 2016, Knudsen et al., 2016, Sandler et al., 2011, Imp et al., 2016, Hunt et al., 2014, Monnig et al., 2017).
HIV-associated biomarker perturbations described above are paralleled in chronic heavy drinking, presumably due to the shared factor of microbial translocation (Monnig, 2016). Like HIV infection, heavy drinking alters the composition of the intestinal microbiome, depletes gut lymphocytes, and induces intestinal hyperpermeability via disruption of tight junction proteins (Dinh et al., 2015, Mutlu et al., 2014, Vazquez-Castellanos et al., 2015, Nazli et al., 2010, Brenchley et al., 2004, Keshavarzian et al., 2009, Asquith et al., 2014). Individuals in early alcohol withdrawal, with or without liver disease, have elevated plasma LPS, LBP, sCD14, and sCD163 levels (Leclercq et al., 2012, Parlesak et al., 2002, Schafer et al., 2002, Urbaschek et al., 2001, Frank et al., 2004, Sandahl et al., 2014, Liangpunsakul et al., 2017). Experimental evidence in humans is sparse to date but supports a causal role of alcohol: a controlled alcohol administration trial in healthy individuals demonstrated elevations in LPS, LBP, and sCD14 within 30 minutes of consumption (Bala et al., 2014).
Although few previous studies have examined the possible contribution of alcohol use to microbial translocation in PLWH, early evidence suggests that alcohol may exacerbate this phenomenon. One study in untreated PLWH found that those reporting heavy drinking in the past three months had significantly higher plasma sCD14 than non-drinkers (Carrico et al., 2015). Another study reported that PLWH with alcohol use disorder had higher LPS and LBP compared to PLWH without a substance use disorder (Ancuta et al., 2008). In a longitudinal study of PLWH on ART, greater past-week alcohol consumption was associated with higher LBP and sCD14 (Webel et al., 2017). Recently, our group reported that severity of heavy drinking was associated with higher sCD14 in virally suppressed men living with HIV (Monnig et al., 2016). Moreover, sCD14 levels decreased significantly in individuals who reduced heavy drinking over a 3-month period, but not in those who maintained heavy drinking (Monnig et al., 2016).
This study examined effects of HIV status, alcohol use, and HCV co-infection on biomarkers of microbial translocation and innate immune activation. LPS, LBP, sCD14, and sCD163 were quantified in plasma samples collected in a longitudinal study of PLWH in care and uninfected controls. HIV-infected and uninfected groups were recruited to have similar proportions of non-drinkers, moderate drinkers, and heavy drinkers. We tested two a priori hypotheses: 1) that PLWH would have higher levels of these biomarkers than uninfected controls with comparable alcohol use; 2) that alcohol use would exacerbate biomarker elevations in PLWH.
Materials and Methods
Study Design
This study utilized behavioral data and bioreposited plasma samples from a longitudinal study on biobehavioral interactions of alcohol use and HIV infection conducted by the Alcohol Research Center on HIV (ARCH; P01AA019072, PI: Monti). HIV-infected individuals and uninfected controls were assessed at up to 3 visits over a 5-year period, with an average of 18 months between assessments (range = 8.2–27.8 months). All participants with available plasma samples and behavioral data were included in the current study. A total of 167 plasma samples collected from 109 individuals (75 HIV-infected, 34 uninfected) were available for analysis. The most common reasons for missing samples were participant attrition and sample storage in cryovials not designated endotoxin-free by the manufacturer.
Participants
Recruitment.
Participants were recruited from an immunology clinic specializing in treatment of HIV. Prospective participants underwent chart review, screening, and review by the study physician (Dr. Tashima). Individuals with a full range of drinking behavior (i.e., heavy drinkers, moderate drinkers, and non-drinkers) were approached for participation, based on review of medical records and clinical interviews with the study staff. Other individuals treated at the clinic were recruited to comprise an uninfected control group with similar sociodemographic characteristics. Participants provided informed consent prior to participation. All study procedures were reviewed and approved by the Institutional Review Boards of Brown University and The Miriam Hospital/Lifespan.
Inclusion Criteria:
1) age 21–70 years; 2) HIV serostatus documented by enzyme-linked immunosorbent assay (ELISA) and confirmed by Western blot and plasma HIV RNA.
Exclusion Criteria:
1) history of significant pre-existing neurological disease, including dementia, stroke, seizure disorder, or traumatic brain injury with loss of consciousness >10 minutes; 2) severe psychiatric illness; 3) end-stage disease (life expectancy <12 months); 4) pregnancy; 5) evidence of opportunistic central nervous system infections (e.g., toxoplasmosis) or neoplasm; 6) history of ascites, encephalopathy, esophageal variceal bleeding, or hepatorenal syndrome, or evidence of severe liver disease; 7) current substance use disorder for cocaine, stimulants, heroin, opioids, or any intravenous drug use; 8) any contraindication for magnetic resonance imaging (e.g., ferromagnetic implant, claustrophobia), which was a component of the parent study.
Procedures
Assessment.
Past 90-day alcohol and drug use was assessed with the Timeline Followback Interview (TLFB) (Sobell and Sobell, 1992). The TLFB asked participants to report the number of standard alcoholic drinks (12 oz. of beer, 5 oz. of wine, or 1.5 oz. 80-proof liquor) consumed each day, as well as use of marijuana, opioids, cocaine, hallucinogens, stimulants, and sedatives. Alcohol use disorder (AUD) diagnoses were determined using the Mini-International Neuropsychiatric Interview [MINI (Sheehan et al., 1998)]. Remote AUD was defined as meeting criteria for lifetime but not current AUD. The MINI also was used to assess current drug use disorder as an exclusion criterion (see above). Cigarette smoking was determined by self-report.
Immunoassays.
Plasma was isolated from blood samples collected into BD Vacutainer tubes and stored in endotoxin-free cryovials at −80°C. Behavioral data and blood samples typically were collected on the same day (range=0–17 intervening days). Enzyme-linked immunosorbent assays (ELISA) were used to quantify LPS (MyBioSource, San Diego, CA), LBP (Hycult Biotech, Wayne, PA), sCD14, and sCD163 (R&D Systems, Minneapolis, MN) following manufacturer instructions. ELISA tests were performed in duplicate, and samples with coefficient of variation ≥ 20% were excluded from further analysis. Percentages of samples exceeding this threshold by biomarker were as follows: 0% for LPS; 8% for LBP; 6% for sCD14; and 6% for sCD163. For HIV-infected participants, HCV status was determined at baseline by antibody test followed by HCV RNA testing for confirmation of reactive antibody results. HCV testing was not performed in controls due to resource limitations.
Statistical Analysis
Differences in demographics and substance use between PLWH and controls were assessed using t-tests on continuous variables and Pearson chi-square tests on categorical variables. Differences in baseline characteristics also were tested between participants with available plasma samples versus those without available samples (n=41).
Given the nature of the sample and our research questions, we examined potential for a so-called “sick quitter” effect by testing whether the prevalence of remote AUD (i.e., lifetime but not current AUD diagnosis on the MINI) differed according to current drinking status, HIV status, or HCV coinfection status. Differences in prevalence of remote AUD across the relevant groups were tested using the Pearson chi-square test.
Generalized estimating equation (GEE) models in SPSS v.24 were used to test the two primary hypotheses. GEE models estimate the population-based effect of regressors of interest while accounting for repeated observations of individuals in longitudinal designs (Zeger et al., 1988). Biomarker distributions did not deviate markedly from normality with the exception of LPS, which was log-transformed for GEE analyses. In the first set of GEE models, we tested the association of HIV status with biomarker concentrations, using age, sex, smoking (number of cigarettes per day), and current alcohol use as covariates. Current alcohol use was a categorical variable coded to reflect drinking behavior on the 90-day TLFB, with groups defined as follows: 1) non-drinkers reported zero drinks; 2) moderate drinkers reported alcohol intake at non-hazardous levels, i.e., ≤7 drinks/week for women, ≤14 drinks/week for men; 3) heavy drinkers exceeded these thresholds. We repeated this analysis with PLWH divided into suppressed and non-suppressed groups, examining pairwise differences using sequential Bonferroni-corrected pairwise tests. This analysis was exploratory given the small number of non-suppressed individuals. In the second set of GEE models, we examined associations of current alcohol use, HCV co-infection, and their interaction with biomarker concentrations in PLWH. Sex, age, or smoking was included as a covariate in the second set of models if the p-value for its association with a specific biomarker in the first set of models was <.10. We followed up on significant interactions with sequential Bonferroni-corrected pairwise tests on the six subgroups (i.e., HIV mono-infected non-drinker, 18% of total samples; HIV mono-infected moderate drinker, 40%; HIV mono-infected heavy drinker, 18%; HIV/HCV co-infected non-drinker, 8.5%; HIV/HCV co-infected moderate drinker, 9.5%; HIV/HCV co-infected heavy drinker, 6%).
Results
Participant Characteristics
Participant characteristics are shown in Table 1. The HIV-infected group was older than the uninfected control group and had a smaller percentage of females. Groups did not differ on education, racial composition, body mass index, smoking status, or number of cigarettes per day. Importantly, alcohol use, whether characterized by group status or quantified by average weekly drinks, percent drinking days, or percent heavy drinking days, did not differ between groups. Frequency of marijuana use also was comparable between groups, and other drug use was minimal (<1% days; data not shown). The majority of PLWH were taking ART and were virally suppressed, and average CD4 count (M±SD = 549.7 ± 277.5) was in the normal range.
Table 1.
Participant baseline demographic and clinical characteristics (N=109)
| HIV-seronegative controls (n=34) | Individuals living with HIV (n=75) | Test of group difference | |
|---|---|---|---|
| Age | 39.4 ± 12.7 | 48.3 ± 9.1 | p < .001 |
| Education (years) | 14.5 ± 2.8 | 13.5 ± 3.0 | p = .083 |
| Sex (% female) | 50% | 27% | p = .017 |
| Racial background | 68% White 29% African-American 3% Latino |
61% White 24% African-American 12% Latino 3% Other |
p = .332 |
| Body mass index | 28.1 ± 6.5 | 26.4 ± 5.6 | p = .183 |
| Current smoker | 44% | 47% | p = .907 |
| Number of cigarettes per day | 4.0 ± 6.2 | 4.6 ± 6.5 | p = .659 |
| Hepatitis C co-infection | --- | 69% uninfected 20% infected 11% missing data |
--- |
| Years since HIV diagnosis | --- | 17.3 ± 8.9 | --- |
| Prescribed ART | --- | 96% | --- |
| HIV RNA viral load | --- | 72% ≤ 75 copies/ml 24% ≥ 75 copies/ml 4% missing data |
--- |
| Current CD4 count | --- | 549.7 ± 277.5 | --- |
| Nadir CD4 count | --- | 222.9 ± 179.6 | --- |
| Current alcohol use | 26.5% non-drinker 47% moderate drinker 26.5% heavy drinker |
24% non-drinker 57% moderate drinker 19% heavy drinker |
p = .552 |
| Average weekly drinks | 11.5 ± 20.8 | 8.1 ± 14.8 | p = .343 |
| Percent drinking days | 25.7 ± 27.6 | 26.1 ± 34.9 | p = .942 |
| Percent heavy drinking days | 13.5 ± 21.3 | 9.5 ± 23.5 | p = .399 |
| Percent days using marijuana | 12.9 ± 30.4 | 15.0 ± 31.1 | p = .745 |
Values are reported as M ± SD except where expressed as percentages. Data were missing for 1–3 participants on smoking status, body mass index, CD4 nadir, and current CD4. For current level of alcohol use, non-drinkers reported zero drinks; moderate drinkers drank at non-hazardous levels (≤7 drinks/week for women, ≤14 drinks/week for men); and heavy drinkers exceeded moderate drinking thresholds.
Compared to participants with available plasma samples, those without samples were significantly younger (p = .017), more likely to be female (p = .049), and more likely to be HIV-seronegative (p = .0003). Participants with and without available samples did not differ on cigarettes per day (p = .477) or current alcohol use (p = .736).
Remote AUD Diagnosis.
As a whole, the sample had a high rate of remote (i.e., lifetime but not current) AUD diagnosis on the MINI, at 47%. Rates of never AUD and current AUD were 33.5% and 19.5%, respectively. Chi-square tests evaluated whether individuals with remote AUD were over-represented among current non-drinkers, PLWH, or PLWH with HCV co-infection, in order to evaluate the likelihood of a “sick quitter” effect. First, rates of remote AUD were comparable in current non-drinkers and moderate drinkers, at 55% and 50%, respectively [χ2(2) = .728, p = .695], indicating that non-drinkers were not more likely to have a history of heavy drinking. (Current heavy drinkers were not included in this comparison because, as would be expected, the majority had current AUD.) Second, rates of remote AUD did not differ in non-drinking controls (55%) versus non-drinking PLWH (55%), suggesting that PLWH were not more likely than controls to have quit heavy drinking [χ2(2) = .872, p = .647]. Third, rates of remote AUD were similar in mono-infected PLWH (44%) and HCV co-infected PLWH (48%), suggesting that co-infected individuals were not more likely to have quit heavy drinking [χ2(2) = 1.997, p = .368]. In summary, prevalence rates of remote AUD in various non-drinker subgroups did not give evidence of a sick quitter bias in this sample.
Biomarker Values by HIV Status
Biomarker concentrations by HIV status are shown in Table 2. GEE model results for group differences by HIV status, adjusting for age, sex, smoking, and alcohol use, are shown in Table 3. As expected, HIV infection was associated with significant elevations on all biomarkers (LBP, p = .005; LPS, p = .014; sCD14, p < .001; sCD163, p < .001). Sex was associated with LBP (p = .001) and sCD14 (p = .003), with women having significantly higher concentrations than men. Women also had higher LPS (p = .072) and sCD163 (p = .067) at the trend level. Age showed a significant inverse association with LPS (p = .002), such that older age was associated with lower LPS. Cigarettes per day showed a positive association with LBP (p = .046). Current level of alcohol use did not exhibit a significant association with any biomarker in the full sample.
Table 2.
Immune biomarker concentrations
| Control (n=34) |
All PLWH (n=75) |
Virally Suppressed PLWH (n=54) |
Non-Suppressed PLWH (n=18) |
|
|---|---|---|---|---|
| LBP (ng/ml) | 10027 (7131, 14491) | 13998 (7857, 20851) | 14893 (7856, 22003) | 12588 (6451, 17515) |
| LPS (pg/ml) | 79 (60, 135) | 98 (70, 137) | 102 (70, 141) | 86 (69, 126) |
| sCD14 (ng/ml) | 1639 (1471, 1847) | 2081 (1667, 2478) | 2119 (1766, 2552) | 1967 (1639, 2308) |
| sCD163 (ng/ml) | 399 (275, 493) | 620 (390, 851) | 606 (419, 770) | 740 (375, 1267) |
Values are reported as median (interquartile range)
Note: n’s indicate the number of individuals in each group, with each individual contributing 1–3 plasma samples. Details on sample size are as follows: controls, 51 samples from 34 individuals; all PLWH, 116 samples from 75 individuals; suppressed PLWH, 85 samples from 54 individuals; non-suppressed PLWH, 27 samples from 18 individuals. Data on viral load were missing for 3 individuals.
Table 3.
Associations of biomarker concentrations with HIV status, demographics, smoking and alcohol use in the full sample
| B | 95% CI | p-value | |
|---|---|---|---|
| LBP (ng/ml) | |||
| HIV infectiona | 4304.9 | 1293.3, 7316.4 | .005 |
| Sexb | 5574.2 | 2250.6, 8897.8 | .001 |
| Age | 66.8 | −61.7, 195.2 | .308 |
| Cigarettes/day | 209.1 | 3.7, 414.5 | .046 |
| Current alcohol usec | . | ||
| Moderate drinking | −30.3 | −3089.6, 3029.0 | .985 |
| Heavy drinking | −1771.0 | −5960.1, 2418.2 | .407 |
| LPS (pg/ml, log10 transformed) | |||
| HIV infection | .123 | .025, .221 | .014 |
| Sex | .085 | -.008, .179 | .072 |
| Age | −.006 | -.009, −.002 | .002 |
| Cigarettes/day | −.002 | -.007, .003 | .364 |
| Current alcohol use | |||
| Moderate drinking | −.025 | -.134, .083 | .647 |
| Heavy drinking | −.010 | -.137, .116 | .871 |
| sCD14 (ng/ml) | |||
| HIV infection | 538.1 | 349.0, 727.3 | < .001 |
| Sex | 298.9 | 103.6, 494.2 | .003 |
| Age | 2.7 | -7.2, 12.5 | .598 |
| Cigarettes/day | 4.5 | -8.0, 17.0 | .482 |
| Current alcohol use | |||
| Moderate drinking | −26.3 | -238.5, 185.9 | .808 |
| Heavy drinking | −128.1 | -408.2, 152.0 | .370 |
| sCD163 (ng/ml) | |||
| HIV infection | 237.8 | 109.8, 365.8 | < .001 |
| Sex | 118.0 | −8.3, 244.3 | .067 |
| Age | 1.1 | −4.5, 6.8 | .693 |
| Cigarettes/day | −12.4 | −27.4, 2.5 | .103 |
| Current alcohol use | |||
| Moderate drinking | 29.3 | −281.4, 340.0 | .853 |
| Heavy drinking | 103.2 | −166.0, 372.5 | .452 |
Coded to indicate biomarker values in HIV-infected participants relative to uninfected participants.
Coded to indicate biomarker values in women relative to men.
Comparisons are to non-drinking group.
When analyses were repeated with the HIV group divided by viral load status, all biomarkers remained significantly higher in suppressed PLWH relative to controls (p’s < .05). Non-suppressed PLWH did not differ significantly from suppressed PLWH or from controls on LBP (non-suppressed vs. suppressed, p = .996; non-suppressed vs. control, p = .162) or LPS (non-suppressed vs. suppressed, p = .412; non-suppressed vs. control, p = .350). Non-suppressed PLWH had significantly higher sCD14 than controls (p < .001) and did not differ from suppressed PLWH (p = .229). Non-suppressed PLWH showed a trend toward higher sCD163 than suppressed PLWH (p = .080) and had significantly higher sCD163 than controls (p = .002).
Associations of Alcohol Use and HCV Status with Biomarkers in PLWH
Results of GEE models testing associations of current alcohol use group (non-drinker, moderate drinker, heavy drinker), HCV co-infection, and their interaction with each biomarker are presented in Table 4 and Figure 1. Main effects of alcohol use and HCV co-infection are discussed first, followed by interactions of alcohol with HCV.
Table 4.
Associations of biomarkers with alcohol use, HCV co-infection, and their interaction in PLWH
| HCV p-value |
Alcohol p-value |
HCV x Alcohol p-value |
Sex p-value |
Age p-value |
Cig/day p-value |
Pairwise Tests of HCV x Alcohol Interaction with Sequential Bonferroni-Correction |
|
|---|---|---|---|---|---|---|---|
| LBP | .057 | .193 | .002 | .039 | ---- | .003 | HIV/HCV moderate drinker > HIV-only heavy drinker (p = .001) HIV-only non-drinker > HIV-only heavy drinker (p = .040) |
| LPS | .077 | .530 | .026 | .206 | .001 | ---- | HIV/HCV non-drinker > HIV-only non-drinker (p = .004) |
| sCD14 | .350 | .169 | .0004 | .053 | ---- | ---- | HIV/HCV moderate drinker > HIV/HCV non-drinker (p = .027) |
| sCD163 | < .0001 | .011 | .001 | .864 | ---- | ---- | HIV/HCV heavy drinker > HIV/HCV non-drinker (p = .004) HIV/HCV heavy drinker > HIV-only heavy drinker (p < .0001) HIV/HCV heavy drinker > HIV-only moderate drinker (p < .0001) HIV/HCV heavy drinker > HIV-only non-drinker (p < .0001) HIV/HCV moderate drinker > HIV-only heavy drinker (p < .0001) HIV/HCV moderate drinker > HIV-only moderate drinker (p < .0001) HIV/HCV moderate drinker > HIV-only non-drinker (p = .002) |
Figure 1: Interaction of alcohol use and HCV status on biomarker outcomes in PLWH.
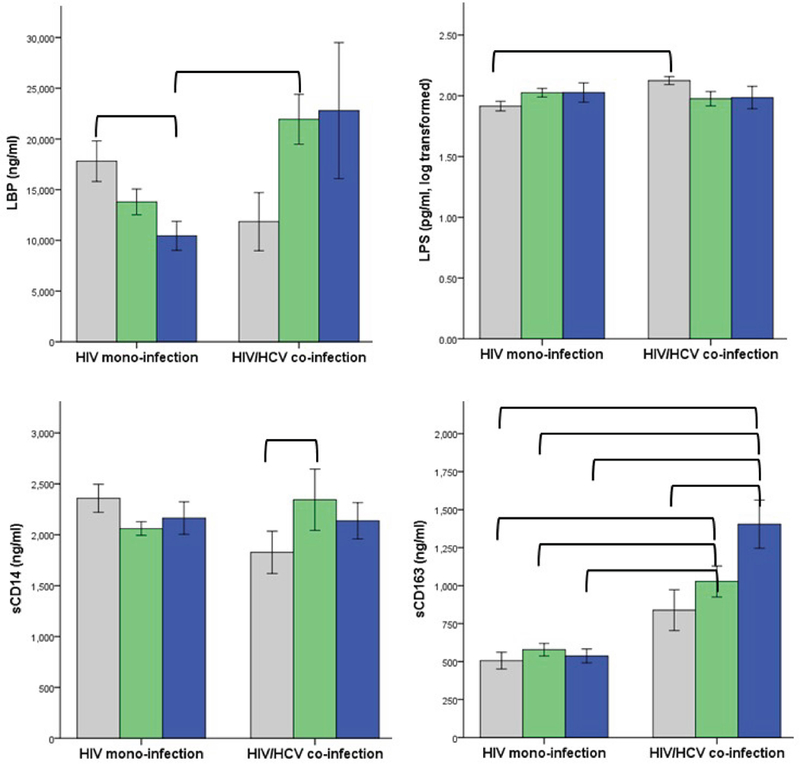
Biomarker concentrations (M ± 1 standard error) are shown in gray for non-drinkers, green for moderate drinkers, and blue for heavy drinkers. The interaction of HCV co-infection with alcohol use was significant for all biomarkers. Brackets indicate that group means differed significantly on follow-up pairwise tests after sequential Bonferroni correction for multiple comparisons (see also Table 4).
Main Effect of Alcohol Use.
The main effect of alcohol was significant for sCD163 (p = .011), with greater alcohol use associated with higher sCD163. The main effect of alcohol was not significant for LBP, LPS, or sCD14.
Main Effect of HCV Co-Infection.
The main effect of HCV co-infection was significant for sCD163 (p < .0001), with HCV associated with higher sCD163. The main effect of HCV was not significant for LBP, LPS, or sCD14.
Interaction of Alcohol Use with HCV Co-Infection.
The interaction of alcohol use with HCV co-infection was significant for each biomarker (LBP, p = .002; LPS, p = .026; sCD14, p = .0004; sCD163, p = .001). Follow-up tests with sequential Bonferroni correction for multiple comparisons examined pairwise differences by alcohol use group and HCV status. For LBP, levels were lowest in HIV mono-infected heavy drinkers. This group had significantly lower LBP than two other groups: HIV/HCV co-infected moderate drinkers (p = .001) and HIV mono-infected non-drinkers (p = .040). For LPS, HIV/HCV co-infected non-drinkers had significantly higher levels than HIV mono-infected non-drinkers (p = .004). For sCD14, levels were significantly higher in HIV/HCV co-infected moderate drinkers compared to co-infected non-drinkers (p = .027).
For sCD163, numerous significant pairwise differences emerged. Within co-infected individuals, heavy drinkers had significantly higher sCD163 than non-drinkers (p = .004). In comparisons to mono-infected groups, HIV/HCV co-infected heavy drinkers had significantly elevated sCD163 relative to each group [i.e., mono-infected heavy drinkers (p < .0001), mono-infected moderate drinkers (p < .0001), and mono-infected non-drinkers (p < .0001)]. HIV/HCV co-infected moderate drinkers did not differ from the other co-infected groups. However, co-infected moderate drinkers showed elevated sCD163 relative to every mono-infected group [i.e., mono-infected heavy drinkers (p < .0001), mono-infected moderate drinkers (p < .0001), and mono-infected non-drinkers (p = .002)]. As is evident from Figure 1, the alcohol main effect for sCD163 was fully accounted for by the co-infected subgroup, and the HCV main effect was driven largely by heavy and moderate drinkers. Therefore, main effects described above for sCD163 should be interpreted in the context of the alcohol-HCV interaction.
Discussion
Consistent with prior research, we found that plasma biomarkers of microbial translocation (LPS) and innate immune activation (LBP, sCD14, sCD163) were significantly higher in PLWH compared to uninfected controls. Few previous studies have matched HIV-infected and uninfected groups on substance use characteristics. In the current study, HIV-infected and uninfected groups were well matched in that each had a full range of alcohol use, high prevalence of cigarette smoking, and minimal use of drugs other than marijuana. In addition, we did not find evidence for a so-called “sick quitter” bias in this sample, as rates of remote AUD were similar across various subgroups formed according to current drinking and health status. As a result, we can conclude that biomarker differences by HIV status were not confounded by substance use.
The study also provides novel evidence for our hypothesis that alcohol use in the context of HIV infection exacerbates inflammation and immune activation. Support for this hypothesis was qualified by the interaction of current drinking with HCV co-infection. Although alcohol use by itself was not significantly associated with most biomarkers in PLWH, the interaction of alcohol use with HCV co-infection was associated with significant elevations on all biomarkers. For example, HIV/HCV co-infected participants who drank heavily had markedly higher sCD163, a marker of monocyte activation, than every other group except co-infected moderate drinkers. Further, co-infected participants who drank moderately had significantly higher sCD163 than all mono-infected groups, regardless of drinking status. Although significant main effects of HCV and alcohol on sCD163 in the current study are consistent with previous research (Shmagel et al., 2016, Lidofsky et al., 2018, Liangpunsakul et al., 2017), these effects should be interpreted in the context of the observed interaction.
Findings for LPS and LBP also reflect multifactorial influences. First, the HCV-associated elevation in LPS was significant only within non-drinkers. This finding is consistent with exacerbated microbial translocation due to the “double hit” of HIV and HCV but is inconclusive with respect to hypothesized alcohol effects. For LBP, co-infected drinkers had the highest levels, as expected, yet mono-infected heavy drinkers unexpectedly had the lowest LBP. LBP modulates host response to LPS by transferring LPS either to lipoproteins or to the TLR4 complex (Kitchens and Thompson, 2005) and plays a mechanistic role in development of alcoholic liver damage through the pro-inflammatory LPS-TLR4 pathway (Uesugi et al., 2002). Because chronic heavy drinking is associated with elevated LBP in uninfected individuals (e.g., Schafer et al., 2002), low levels of this acute phase protein in mono-infected heavy drinkers is a novel finding that could represent a shift in its complex immunomodulatory activity and/or impaired host defense. At present, this premise is speculative and will require further investigation. Interesting to note, number of cigarettes per day was positively associated with LBP in the overall sample and in PLWH, giving broad evidence of substance-related inflammation.
Although this study did not examine associations of biomarker elevations with clinical outcomes, sCD163 and sCD14 have proven to be robust predictors of morbidity and mortality in PLWH in previous research (Hunt et al., 2014, Knudsen et al., 2016). Our findings of alcohol-related elevations in these biomarkers are consistent with a recent report linking moderate drinking to increased risk of mortality in PLWH (Justice et al., 2016). Results for sCD163 also are consistent with a previous finding that any alcohol use by HIV/HCV co-infected individuals was associated with a dramatically increased prevalence of advanced liver fibrosis (Lim et al., 2014). Plasma sCD163 has been identified as a candidate marker of active liver fibrogenesis in HIV/HCV co-infection (Lidofsky et al., 2018) and predicts mortality in alcoholic liver disease (Sandahl et al., 2014). Given associations of sCD163 and sCD14 with all-cause mortality in PLWH, our findings of alcohol-related elevations in these biomarkers support strong initiatives to reduce alcohol use in PLWH, particularly those with HCV co-infection.
Pending further research, a similar precaution about alcohol use may pertain to PLWH with non-suppressed viral load, as we observed a trend toward higher sCD163 in this group compared to suppressed individuals. Suppressed and non-suppressed groups did not differ significantly on LPS, LBP, or sCD14 levels. It is likely that the small number of non-suppressed PLWH limited our ability to detect biomarker differences by viral load status. Important to note, virally suppressed PLWH had significantly higher levels of all biomarkers than controls, demonstrating that the main effect of HIV was not driven by the small group of non-suppressed individuals. These results are consistent with previous research showing that biomarker levels typically decrease but do not fully normalize with viral suppression (Vassallo et al., 2012, Wada et al., 2015, Hileman and Funderburg, 2017).
Significant limitations include the lack of HCV testing in uninfected controls and absence of objective data (i.e., lab tests) on liver function in all participants. Prevalence of HCV co-infection in this sample (20%) was slightly lower than national rates (~25%), and so the number of samples from HIV/HCV co-infected participants was small. Future studies may benefit from oversampling co-infected individuals. Strengths include a representative sample of PLWH in care, the majority of whom were virally suppressed on ART; careful assessment of alcohol and drug use; and absence of confounds related to smoking status or drug use. In future studies, it would be helpful to use continuous measures of alcohol use in order to examine linear relationships with immune biomarkers. In sum, this study offers preliminary evidence that alcohol may interact with HCV co-infection to drive inflammation in PLWH, highlighting the need to better understand mechanisms and to reduce alcohol-related health harms in PLWH.
Funding Sources:
This research was supported by National Institute of Alcohol Abuse and Alcoholism (NIAAA) grants P01AA019072 (PI: Monti), K05AA019681 (PI: Monti), U01AA020797 (Multiple PI: Cohen), and K23AA024704 (PI: Monnig); and National Institute of Child Health and Human Development grant K24HD080539 (PI: Ramratnam). Research reported in this publication also was supported by the Proteomics Core of the COBRE Center for Cancer Research Development, funded by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM103421, previously P20RR017695. This work was facilitated by the Providence/Boston Center for AIDS Research (P30AI042853). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- ANCUTA P, KAMAT A, KUNSTMAN KJ, KIM EY, AUTISSIER P, WURCEL A, ZAMAN T, STONE D, MEFFORD M, MORGELLO S, SINGER EJ, WOLINSKY SM & GABUZDA D 2008. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One, 3, e2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APPAY V & KELLEHER AD 2016. Immune activation and immune aging in HIV infection. Curr Opin HIV AIDS, 11, 242–9. [DOI] [PubMed] [Google Scholar]
- ASQUITH M, PASALA S, ENGELMANN F, HABERTHUR K, MEYER C, PARK B, GRANT KA & MESSAOUDI I 2014. Chronic ethanol consumption modulates growth factor release, mucosal cytokine production, and microRNA expression in nonhuman primates. Alcohol Clin Exp Res, 38, 980–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALA S, MARCOS M, GATTU A, CATALANO D & SZABO G 2014. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS One, 9, e96864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALAGOPAL A, PHILP FH, ASTEMBORSKI J, BLOCK TM, MEHTA A, LONG R, KIRK GD, MEHTA SH, COX AL, THOMAS DL & RAY SC 2008. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology, 135, 226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRENCHLEY JM, PRICE DA, SCHACKER TW, ASHER TE, SILVESTRI G, RAO S, KAZZAZ Z, BORNSTEIN E, LAMBOTTE O, ALTMANN D, BLAZAR BR, RODRIGUEZ B, TEIXEIRA-JOHNSON L, LANDAY A, MARTIN JN, HECHT FM, PICKER LJ, LEDERMAN MM, DEEKS SG & DOUEK DC 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med, 12, 1365–71. [DOI] [PubMed] [Google Scholar]
- BRENCHLEY JM, SCHACKER TW, RUFF LE, PRICE DA, TAYLOR JH, BEILMAN GJ, NGUYEN PL, KHORUTS A, LARSON M, HAASE AT & DOUEK DC 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med, 200, 749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURDO TH, LENTZ MR, AUTISSIER P, KRISHNAN A, HALPERN E, LETENDRE S, ROSENBERG ES, ELLIS RJ & WILLIAMS KC 2011. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis, 204, 154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURDO TH, WEIFFENBACH A, WOODS SP, LETENDRE S, ELLIS RJ & WILLIAMS KC 2013. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. Aids, 27, 1387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARRICO AW, HUNT PW, EMENYONU NI, MUYINDIKE W, NGABIRANO C, CHENG DM, WINTER MR, SAMET JH & HAHN JA 2015. Unhealthy Alcohol Use is Associated with Monocyte Activation Prior to Starting Antiretroviral Therapy. Alcohol Clin Exp Res [DOI] [PMC free article] [PubMed]
- CENTERS FOR DISEASE CONTROL AND PREVENTION. Behavioral and clinical characteristics of persons receiving medical care for HIV infection--Medical Monitoring Project, United States, 2013 Cycle (June 2013-May 2014) 2016.
- DINH DM, VOLPE GE, DUFFALO C, BHALCHANDRA S, TAI AK, KANE AV, WANKE CA & WARD HD 2014. Intestinal Microbiota, Microbial Translocation, and Systemic Inflammation in Chronic HIV Infection. J Infect Dis [DOI] [PMC free article] [PubMed]
- DINH DM, VOLPE GE, DUFFALO C, BHALCHANDRA S, TAI AK, KANE AV, WANKE CA & WARD HD 2015. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J Infect Dis, 211, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANK J, WITTE K, SCHRODL W & SCHUTT C 2004. Chronic alcoholism causes deleterious conditioning of innate immunity. Alcohol Alcohol, 39, 386–92. [DOI] [PubMed] [Google Scholar]
- HAHN JA & SAMET JH 2010. Alcohol and HIV disease progression: weighing the evidence. Curr HIV/AIDS Rep, 7, 226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILEMAN CO & FUNDERBURG NT 2017. Inflammation, Immune Activation, and Antiretroviral Therapy in HIV. Curr HIV/AIDS Rep, 14, 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT PW, SINCLAIR E, RODRIGUEZ B, SHIVE C, CLAGETT B, FUNDERBURG N, ROBINSON J, HUANG Y, EPLING L, MARTIN JN, DEEKS SG, MEINERT CL, VAN NATTA ML, JABS DA & LEDERMAN MM 2014. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis, 210, 1228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IMP BM, RUBIN LH, TIEN PC, PLANKEY MW, GOLUB ET, FRENCH AL & VALCOUR VG 2016. Monocyte Activation is Associated with Worse Cognitive Performance in Virologically Suppressed HIV-Infected Women. J Infect Dis [DOI] [PMC free article] [PubMed]
- JUSTICE AC, MCGINNIS KA, TATE JP, BRAITHWAITE RS, BRYANT KJ, COOK RL, EDELMAN EJ, FIELLIN LE, FREIBERG MS, GORDON AJ, KRAEMER KL, MARSHALL BD, WILLIAMS EC & FIELLIN DA 2016. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend [DOI] [PMC free article] [PubMed]
- KALICHMAN SC, GREBLER T, AMARAL CM, MCNEREY M, WHITE D, KALICHMAN MO, CHERRY C & EATON L 2013. Intentional non-adherence to medications among HIV positive alcohol drinkers: prospective study of interactive toxicity beliefs. J Gen Intern Med, 28, 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESHAVARZIAN A, FARHADI A, FORSYTH CB, RANGAN J, JAKATE S, SHAIKH M, BANAN A & FIELDS JZ 2009. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol, 50, 538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KITCHENS RL & THOMPSON PA 2005. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J Endotoxin Res, 11, 225–9. [DOI] [PubMed] [Google Scholar]
- KNUDSEN TB, ERTNER G, PETERSEN J, MOLLER HJ, MOESTRUP SK, EUGEN-OLSEN J, KRONBORG G & BENFIELD T 2016. Plasma Soluble CD163 Level Independently Predicts All-Cause Mortality in HIV-1-Infected Individuals. J Infect Dis, 214, 1198–204. [DOI] [PubMed] [Google Scholar]
- LANDMANN R, KNOPF HP, LINK S, SANSANO S, SCHUMANN R & ZIMMERLI W 1996. Human monocyte CD14 is upregulated by lipopolysaccharide. Infect Immun, 64, 1762–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LECLERCQ S, CANI PD, NEYRINCK AM, STARKEL P, JAMAR F, MIKOLAJCZAK M, DELZENNE NM & DE TIMARY P 2012. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav Immun, 26, 911–8. [DOI] [PubMed] [Google Scholar]
- LIANGPUNSAKUL S, TOH E, ROSS RA, HEATHERS LE, CHANDLER K, OSHODI A, MCGEE B, MODLIK E, LINTON T, MANGIACARNE D, JIMENEZ C, DONG XC, WANG L, TU W & NELSON DE 2017. Quantity of alcohol drinking positively correlates with serum levels of endotoxin and markers of monocyte activation. Sci Rep, 7, 4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIDOFSKY A, HOLMES JA, FEENEY ER, KRUGER AJ, SALLOUM S, ZHENG H, SEGUIN IS, ALTINBAS A, MASIA R, COREY KE, GUSTAFSON JL, SCHAEFER EA, HUNT PW, DEEKS S, SOMSOUK M, CHEW KW, CHUNG RT & ALATRAKCHI N 2018. Macrophage Activation Marker Soluble CD163 is a Dynamic Marker of Liver Fibrogenesis in HIV/HCV Coinfection. J Infect Dis [DOI] [PMC free article] [PubMed]
- LIM JK, TATE JP, FULTZ SL, GOULET JL, CONIGLIARO J, BRYANT KJ, GORDON AJ, GIBERT C, RIMLAND D, GOETZ MB, KLEIN MB, FIELLIN DA, JUSTICE AC & LO RE V 3RD 2014. Relationship between alcohol use categories and noninvasive markers of advanced hepatic fibrosis in HIV-infected, chronic hepatitis C virus-infected, and uninfected patients. Clin Infect Dis, 58, 1449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LU YC, YEH WC & OHASHI PS 2008. LPS/TLR4 signal transduction pathway. Cytokine, 42, 145–51. [DOI] [PubMed] [Google Scholar]
- MARCHETTI G, TINCATI C & SILVESTRI G 2013. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev, 26, 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASCIA C, LICHTNER M, ZUCCALA P, VITA S, TIEGHI T, MAROCCO R, SAVINELLI S, ROSSI R, IANNETTA M, CAMPAGNA M, SCHIAVONE F, MENGONI F, RUSSO G, MASTROIANNI CM & VULLO V 2017. Active HCV infection is associated with increased circulating levels of interferon-gamma (IFN-gamma)-inducible protein-10 (IP-10), soluble CD163 and inflammatory monocytes regardless of liver fibrosis and HIV coinfection. Clin Res Hepatol Gastroenterol, 41, 644–655. [DOI] [PubMed] [Google Scholar]
- MOLLER HJ 2012. Soluble CD163. Scand J Clin Lab Invest, 72, 1–13. [DOI] [PubMed] [Google Scholar]
- MONNIG MA 2016. Immune activation and neuroinflammation in alcohol use and HIV infection: evidence for shared mechanisms. Am J Drug Alcohol Abuse, 1–17. [DOI] [PMC free article] [PubMed]
- MONNIG MA, KAHLER CW, CIOE PA, MONTI PM, MAYER KH, PANTALONE DW, COHEN RA & RAMRATNAM B 2017. Markers of Microbial Translocation and Immune Activation Predict Cognitive Processing Speed in Heavy-Drinking Men Living with HIV. Microorganisms, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONNIG MA, KAHLER CW, CIOE PA, TUCKER L, MONTI PM, MAYER KH & RAMRATNAM B 2016. Alcohol use predicts elevation in inflammatory marker soluble CD14 in men living with HIV. AIDS Care, 28, 1434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUTLU EA, KESHAVARZIAN A, LOSURDO J, SWANSON G, SIEWE B, FORSYTH C, FRENCH A, DEMARAIS P, SUN Y, KOENIG L, COX S, ENGEN P, CHAKRADEO P, ABBASI R, GORENZ A, BURNS C & LANDAY A 2014. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog, 10, e1003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAZLI A, CHAN O, DOBSON-BELAIRE WN, OUELLET M, TREMBLAY MJ, GRAY-OWEN SD, ARSENAULT AL & KAUSHIC C 2010. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog, 6, e1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK BS & LEE JO 2013. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med, 45, e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARLESAK A, SCHAFER C & BODE C 2002. IgA against gut-derived endotoxins: does it contribute to suppression of hepatic inflammation in alcohol-induced liver disease? Dig Dis Sci, 47, 760–6. [DOI] [PubMed] [Google Scholar]
- ROYAL W 3RD, CHERNER M, BURDO TH, UMLAUF A, LETENDRE SL, JUMARE J, ABIMIKU A, ALABI P, ALKALI N, BWALA S, OKWUASABA K, EYZAGUIRRE LM, AKOLO C, GUO M, WILLIAMS KC & BLATTNER WA 2016. Associations between Cognition, Gender and Monocyte Activation among HIV Infected Individuals in Nigeria. PLoS One, 11, e0147182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANDAHL TD, GRONBAEK H, MOLLER HJ, STOY S, THOMSEN KL, DIGE AK, AGNHOLT J, HAMILTON-DUTOIT S, THIEL S & VILSTRUP H 2014. Hepatic macrophage activation and the LPS pathway in patients with alcoholic hepatitis: a prospective cohort study. Am J Gastroenterol, 109, 1749–56. [DOI] [PubMed] [Google Scholar]
- SANDLER NG, WAND H, ROQUE A, LAW M, NASON MC, NIXON DE, PEDERSEN C, RUXRUNGTHAM K, LEWIN SR, EMERY S, NEATON JD, BRENCHLEY JM, DEEKS SG, SERETI I, DOUEK DC & GROUP, I. S. S. 2011. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis, 203, 780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAFER C, PARLESAK A, SCHUTT C, BODE JC & BODE C 2002. Concentrations of lipopolysaccharide-binding protein, bactericidal/permeability-increasing protein, soluble CD14 and plasma lipids in relation to endotoxaemia in patients with alcoholic liver disease. Alcohol Alcohol, 37, 81–6. [DOI] [PubMed] [Google Scholar]
- SHEEHAN DV, LECRUBIER Y, SHEEHAN KH, AMORIM P, JANAVS J, WEILLER E, HERGUETA T, BAKER R & DUNBAR GC 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry, 59 Suppl 20, 22–33;quiz 34–57. [PubMed] [Google Scholar]
- SHMAGEL KV, SAIDAKOVA EV, SHMAGEL NG, KOROLEVSKAYA LB, CHERESHNEV VA, ROBINSON J, GRIVEL JC, DOUEK DC, MARGOLIS L, ANTHONY DD & LEDERMAN MM 2016. Systemic inflammation and liver damage in HIV/hepatitis C virus coinfection. HIV Med, 17, 581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOBELL LC & SOBELL MB 1992. Timeline Followback: A technique for assessing self-reported alcohol consumption. In: ALLEN JP & COLUMBUS M (eds.) Assessing Alcohol Problems: A Guide for Clinicians and Researchers Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism. [Google Scholar]
- UESUGI T, FROH M, ARTEEL GE, BRADFORD BU, WHEELER MD, GABELE E, ISAYAMA F & THURMAN RG 2002. Role of lipopolysaccharide-binding protein in early alcohol-induced liver injury in mice. J Immunol, 168, 2963–9. [DOI] [PubMed] [Google Scholar]
- URBASCHEK R, MCCUSKEY RS, RUDI V, BECKER KP, STICKEL F, URBASCHEK B & SEITZ HK 2001. Endotoxin, endotoxin-neutralizing-capacity, sCD14, sICAM-1, and cytokines in patients with various degrees of alcoholic liver disease. Alcohol Clin Exp Res, 25, 261–8. [PubMed] [Google Scholar]
- VASSALLO M, MERCIE P, COTTALORDA J, TICCHIONI M & DELLAMONICA P 2012. The role of lipopolysaccharide as a marker of immune activation in HIV-1 infected patients: a systematic literature review. Virol J, 9, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAZQUEZ-CASTELLANOS JF, SERRANO-VILLAR S, LATORRE A, ARTACHO A, FERRUS ML, MADRID N, VALLEJO A, SAINZ T, MARTINEZ-BOTAS J, FERRANDO-MARTINEZ S, VERA M, DRONDA F, LEAL M, DEL ROMERO J, MORENO S, ESTRADA V, GOSALBES MJ & MOYA A 2015. Altered metabolism of gut microbiota contributes to chronic immune activation in HIV-infected individuals. Mucosal Immunol, 8, 760–72. [DOI] [PubMed] [Google Scholar]
- WADA NI, JACOBSON LP, MARGOLICK JB, BREEN EC, MACATANGAY B, PENUGONDA S, MARTINEZ-MAZA O & BREAM JH 2015. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. Aids, 29, 463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBEL AR, SATTAR A, FUNDERBURG NT, KINLEY B, LONGENECKER CT, LABBATO D, ALAM SK & MCCOMSEY GA 2017. Alcohol and dietary factors associate with gut integrity and inflammation in HIV-infected adults. HIV Med, 18, 402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZEGER SL, LIANG KY & ALBERT PS 1988. Models for longitudinal data: a generalized estimating equation approach. Biometrics, 44, 1049–60. [PubMed] [Google Scholar]
- ZEVIN AS, MCKINNON L, BURGENER A & KLATT NR 2016. Microbial translocation and microbiome dysbiosis in HIV-associated immune activation. Curr Opin HIV AIDS, 11, 182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]


