Abstract
Purpose
Older cancer patients are at increased risk of cancer-related cognitive impairment. The purpose of this study was to assess the alterations in intrinsic brain activity associated with adjuvant chemotherapy in older women with breast cancer.
Methods
Chemotherapy treatment (CT) group included sixteen women aged ≥ 60 years (range 60–82 years) with stage I-III breast cancers, who underwent both resting-state functional magnetic resonance imaging (rs-fMRI) and neuropsychological testing with NIH Toolbox for Cognition before adjuvant chemotherapy, at time point 1 (TP1), and again within 1 month after completing chemotherapy, at time point 2 (TP2). Fourteen age- and sex-matched healthy controls (HC) underwent the same assessments at matched intervals. Three voxel-wise rs-fMRI parameters: amplitude of low-frequency fluctuation (ALFF), fractional ALFF (fALFF), and regional homogeneity (ReHo), were computed at each time point. The changes in rs-fMRI parameters from TP1 to TP2 for each group, the group differences in changes (the CT group vs. the HC group), and the group difference in the baseline rs-fMRI parameters were assessed. In addition, correlative analysis between the rs-fMRI parameters and neuropsychological testing scores was also performed.
Results
In the CT group, one brain region, which included parts of the bilateral subcallosal gyri and right anterior cingulate gyrus, displayed increased ALFF from TP1 to TP2 (cluster p-corrected=0.024); another brain region in the left precuneus displayed decreased fALFF from TP1 to TP2 (cluster level p-corrected=0.025). No significant changes in the rs-fMRI parameters from TP1 to TP2 were observed in the HC group. Although ALFF and fALFF alterations were observed only in the CT group, none of the between-group differences in rs-fMRI parameter changes reached statistical significance.
Conclusions
Our study results of ALFF and fALFF alterations in the chemotherapy-treated women suggest that adjuvant chemotherapy may affect intrinsic brain activity in older women with breast cancer.
Background
Breast cancer is the most commonly diagnosed cancer in women, and almost half of new breast cancers are found in women over 60 years of age [1,2]. Older breast cancer patients are at increased risk of chemotherapy-associated toxicity, such as cancer-related cognitive impairment (CRCI) [3–5]. As the population ages and cancer treatment improves, increasing numbers of older cancer survivors are susceptible to CRCI. Cognitive problems present major challenges to patient care, as minor alterations in cognition can greatly affect independence [6]. However, the underlying mechanism of CRCI is not known, and even less is known about CRCI in older cancer survivors.
Resting-state functional magnetic resonance imaging (rs-fMRI) is commonly used to assess the intrinsic brain activity that is associated with cognitive function [7–9]. Rs-fMRI uses blood oxygenation level-dependent (BOLD) contrast, which has been useful in studying CRCI [7–9]. Also, since rs-fMRI uses a shorter scanning time than task-based fMRI and the patients do not need to perform tasks during scanning, rs-fMRI is more tolerable for patients with cancer undergoing chemotherapy. Rs-fMRI allows assessment of intrinsic brain activity through three parameters: amplitude of low-frequency fluctuation (ALFF), fractional ALFF (fALFF), and regional homogeneity (ReHo). ALFF and fALFF analyze the intensity and power of regional spontaneous resting-state brain activity [10,11]. ReHo is a voxel-based measure of brain activity, which evaluates the synchronization between a given voxel and its neighboring voxels [12,13].
Rs-fMRI analysis for intrinsic brain activity has been used to evaluate and quantify physiological processes, such as cognitive training of young healthy adults [14], for evaluating the impact of pathological conditions, such as psychogenic erectile dysfunction [15], subcortical infarction [16] and brain glioma [17], and for evaluating the impact of cancer treatment [18,19]. One study of people with gastric cancer showed that decreased ALFF in the left frontal gyrus correlated with poor performance in executive function [18]. Another study used rs-fMRI to evaluate 19 women with breast cancer (mean age ± standard deviation [SD]: 43.1 ± 8.8 years) and found a chemotherapy-associated decline in memory performance and significant changes in activity in various brain regions [19]. However, limited data exists regarding intrinsic brain activity changes in older breast cancer female patients receiving adjuvant chemotherapy.
Here, we present the results from a prospective longitudinal study of women aged ≥ 60 years with stage I-III breast cancer receiving adjuvant chemotherapy. The study had the following objectives: 1) to assess the adjuvant chemotherapy-associated alterations in ALFF, fALFF, and ReHo, and 2) to explore the correlations between these rs-fMRI parameters and measures of cognitive performance, which were obtained through neuropsychological (NP) testing with NIH Toolbox for Cognition. We hypothesized that the three rs-fMRI parameters would be altered in one or more brain regions from pre- to post-chemotherapy in older women with breast cancer and that these changes would correlate with changes in cognitive performance.
Materials & Methods
Patients aged ≥ 60 years with stage I-III breast cancer with no history of neurological or psychiatric disorders or stroke were recruited prior to receiving adjuvant chemotherapy (CT group). Age- and sex-matched healthy controls without history of cancer or chemotherapy from the community with similar criteria but no cancer diagnosis were enrolled as healthy controls (HC group). The pre-chemotherapy assessment at baseline (time point 1, TP1), which included a brain MRI scan and NP testing with the NIH Toolbox for Cognition, was performed after surgery but before the start of adjuvant chemotherapy. The follow-up assessment (time point 2, TP2) was conducted within one month after the last infusion of chemotherapy. The HC group was tested with the same assessments at matched intervals. This prospective longitudinal study has been reported previously regarding brain volume, gray matter density and subcortical brain iron [20–22] but not the rs-fMRI data reported here. This research protocol was approved by the Institutional Review Board at City of Hope National Medical Center. Written informed consent was obtained from all study participants.
Demographic and disease characteristics:
Demographic information for the study participants, including age, race, and education, was obtained through a self-report questionnaire. We obtained disease stage and treatment information, such as the chemotherapy regimen, through medical record abstraction.
Brain MRI scan acquisition:
All participants underwent brain MRI scans at both time points on the same 3T Verio Siemens scanner (Siemens, Erlangen, Germany) with a 12-channel head coil. The rs-fMRI data were acquired using rapid gradient echo-planar pulse imaging (EPI) with the following parameters: repetition time (TR) = 2000 millisecond (ms), echo time (TE) = 25 ms, field of view (FOV) = 224 × 224 mm2, voxel size = 3.5 × 3.5 × 3.5 mm3 and number of slices = 32 (interleaved). From each participant, 160 volumes were acquired over 5 minutes and 20 seconds. During data acquisition, the participants were instructed to keep their eyes closed without thinking about anything specific or falling into sleep. The anatomical imaging with the sagittal T1-weighted three-dimensional magnetization prepared rapid gradient echo (MPRAGE) sequence was also acquired.
Neuroimaging processing:
The first 10 volumes of each rs-fMRI acquisition were discarded for magnetization stabilization and the remaining 150 volumes were preprocessed using the Conn Toolbox [23] through the following six steps: 1) Slice-timing of the functional images was corrected using the middle slice as the reference. 2) Each volume in the functional image was aligned against the first volume through rigid body transformation. 3) The functional image was co-registered to the anatomical image. 4) The anatomical images were spatially normalized to the Montreal Neurological Institute (MNI) space. 5) The co-registered functional images were spatially normalized and resampled to a voxel size of 2 × 2 × 2 mm3. 6) The time series in the functional images were de-noised through de-trending and linearly regressing out the head motion parameters.
Functions implemented in the “Resting-State fMRI Data Analysis Toolkit” were used to compute ALFF, fALFF and ReHo (normalized and de-noised) [24]. For ALFF and fALFF, the preprocessed images were smoothed (full width at half maximum [FWHM] = 6 mm) and the time series in each voxel was transformed into the frequency domain using fast Fourier transformation to obtain the power spectrum. ALFF was the average of the square root of the power spectrum over the frequency range of 0.01–0.08 Hz [10], while fALFF was the ratio between ALFF and the average square root of the power spectrum over the entire detectable frequency range [11]. ReHo images were obtained by voxel-wise calculation of Kendall’s coefficient of concordance (KCC) for the time series [12].
NP testing:
All study participants were administered the NIH Toolbox Cognition Battery in a quiet area outside the MRI scanner [25]. The NIH Toolbox used a computerized format with national standardization and the Cognition Battery consists of seven measures to target subdomains of executive function, episodic memory, language, processing speed, working memory, and attention. This battery generated 10 scores (3 composite scores and 7 individual scores).
Statistical analysis:
Changes in the three rs-fMRI parameters (ALFF, fALFF and ReHo) between TP1 and TP2 in the HC and CT groups, as well as the group differences in changes, were assessed using a mixed-design repeated-measures two-way analysis of variance (ANOVA) model in Statistical Parametric Mapping version12 (SPM12, Wellcome Trust Centre for Neuroimaging, London, UK). The model included a group factor (the HC group and the CT group), time factor (TP1 and TP2), and a subject factor to account for the subject effect in repeated measurements. The analysis was implemented using the flexible factorial design in SPM12. The design matrix contained columns for the four combinations of the group and time factors (HC*TP1, HC*TP2, CT*TP1, and CT*TP2) implementing the group by time interaction, along with the subject factor columns indicating the corresponding subject for each image. Various statistical hypotheses were tested by constructing appropriate contrasts. The weight vectors for the contrasts were constructed by setting the elements corresponding to the columns of the assessed variables to 1 or −1 and setting all other elements to zero. Uncorrected p < 0.001 was used as the threshold to locate potential statistically significant clusters.
Both voxel-wise linear regression analysis and region of interest (ROI)-based correlative analysis were performed to assess the relationship between the rs-fMRI parameters and the NP test scores. These analyses were confined within the regions where the CT group displayed statistically significant changes in rs-fMRI parameters over time. For each pair of rs-fMRI parameters and NP score, we assessed the following linear relationships between: 1) two measures, i.e., the rs-fMRI parameters and the NP scores, at TP1; 2) the changes of these two measures from TP1 to TP2; and 3) the rs-fMRI parameters at TP1 and the change in NP score from TP1 to TP2. The total intracranial volume was used as a covariate in all analyses to control for its effects on the rs-fMRI parameters. ROI-based correlative analysis was performed by computing both the pair-wise Spearman (non-parametric) correlation and the associated p-values between the mean rs-fMRI values within the ROI and the ten NP scores. The statistical significance of the correlation was based on the p-values after adjusted for multiple comparison using the Bonferroni methods.
Results
Demographic data:
The detailed demographic characteristics of our study participants have been reported previously [20]. Briefly, 16 women with breast cancer (mean age ± SD: 67.0 ± 5.39 years) and 14 age- and sex-matched healthy controls (mean age ± SD: 67.8 ± 5.24 years) underwent study assessments at two time points. There were no statistically significant differences in age or overall education between the two groups (p > 0.05). The CT group included 11 (68.8%) white and 5 (31.2%) black females, and the HC group included 14 (100%) white females (p = 0.04). The breast cancer staging of the participants was as follows: 5 (31.3%) with stage I, 8 (50.0%) with stage II, and 3 (18.7%) with stage III. In the CT group, 7 (43.8%) received docetaxel and cyclophosphamide (TC regimen) and 9 (56.2%) received a chemotherapy regimen other than TC.
Within-group changes of rs-fMRI parameters from TP1 to TP2:
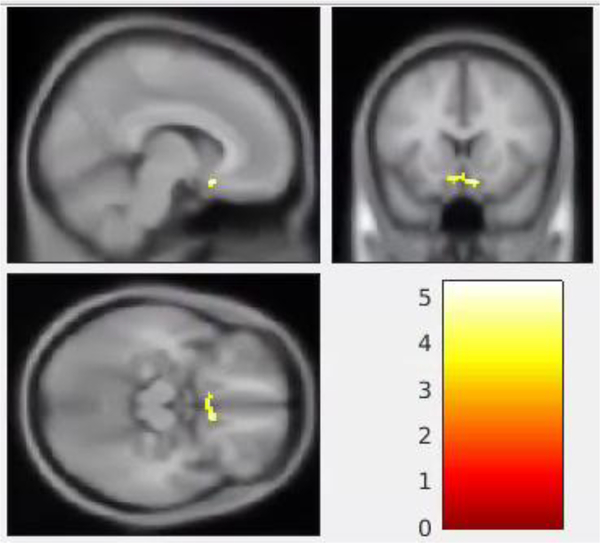
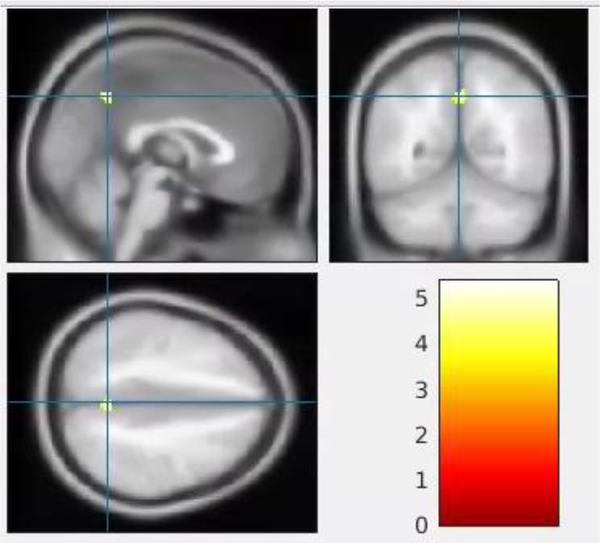
The CT group displayed a statistically significant increase in ALFF from TP1 to TP2 in a single cluster that included parts of the bilateral subcallosal gyri and right anterior cingulate gyrus (BA 32) (cluster p-corrected = 0.024, Table 1 and Figure 1). The CT group also displayed a statistically significant decrease in fALFF from TP1 to TP2 in a single cluster in the left precuneus (cluster p-corrected = 0.025, Table 1 and Figure 2). The spatial distribution of the cluster regarding the fALFF alteration in Figure 2 extended to the right precuneus. However, the cluster was only statistically significant at the left precuneus (cluster p-corrected = 0.025).
Table 1.
Statistically significant alterations in rs-fMRI parameters from TP1 to TP2 in the chemotherapy group
| rs-fMRI | comparison | x,y,z (mm) | cluster size | cluster p (corr) | voxel p (FWE) | voxel p (FDR) | voxel T | Region |
|---|---|---|---|---|---|---|---|---|
| ALFF | CT: TP2 > TP1 | 8, 16, −16 | 75 | 0.024 | 0.568 | 0.559 | 5.35 | R Subcallosal Gyrus |
| −8, 14, −14 | 75 | 0.024 | 0.76 | 0.559 | 5.10 | L Subcallosal Gyrus | ||
| 4, 22, −8 | 75 | 0.024 | 1 | 0.559 | 4.26 | R Anterior Cingulate (BA 32) | ||
| fALFF | CT: TP2 < TP1 | 0, −56, 44 | 46 | 0.025 | 0.733 | 0.548 | 5.40 | L Precuneus |
MRI magnetic resonance imaging, rs-fMRI resting-state functional MRI, ALFF amplitude of low-frequency fluctuation, fALFF fractional ALFF, CT chemotherapy group, TP1 time point 1 (prior to chemotherapy), TP2 time point 2 (after chemotherapy), FWE family-wise error rate, FDR false discovery rate, L left, R right, BA Brodmann area, corr correlation.
Fig. 1.
Alterations in the amplitude of low-frequency fluctuation (ALFF) in the chemotherapy group from pre- (time point 1, TP1) to post-chemotherapy (time point 2, TP2). The highlighted area is a single cluster showing a statistically significant increase in ALFF from TP1 to TP2 (p-corrected < 0.05). This cluster includes parts of the right subcallosal gyrus (8, 16, −16), left subcallosal gyrus (−8, 14, −14) and right anterior cingulate gyrus (4, 22, −8; Brodmann 32). The color bar in the right lower panel indicates the scale of the t statistics for the within-group comparison of ALFF between the TP1 and TP2.
Fig. 2.
Alteration of fractional amplitude of low-frequency fluctuation (fALFF) in the chemotherapy group from pre- (time point 1, TP1) to post-chemotherapy (time point 2, TP2). One cluster (indicated in yellow) in the left precuneus (0, −56, 44) shows a statistically significant decrease in fALFF from TP1 to TP2 (p-corrected < 0.05). The blue crosshair indicates the cluster peak (0, −56, 44) in each plane. The color bar in the right lower panel indicates the scale of the t statistics for the within-group comparison of fALFF between TP1 and TP2.
There were no changes from TP1 to TP2 for ReHo in the CT group (p-corrected > 0.05). The HC group did not show any statistically significant changes from TP1 to TP2 for any of the rsfMRI parameters (p-corrected > 0.05).
Between-group comparison of rs-fMRI parameters:
There were no between-group differences (the CT group vs. the HC group) for any of the three rs-fMRI parameters at TP1 or TP2 (p-corrected > 0.05). No between-group differences were noted when comparing the changes from TP1 to TP2 in the CT group to that in the HC group for each of the three rs-fMRI parameters (p-corrected > 0.05).
Correlations between rs-fMRI parameters and NP scores:
No statistically significant between-group differences (the CT group vs. the HC group) for any of the ten NP test scores were noted at TP1 or from TP1 to TP2, which has been reported previously [20]. Here, voxel-wise linear regression analysis did not identify an association of ALFF or fALFF with any of the ten NP scores within the two clusters involving subcallosal gyri and right anterior cingulate gyrus, and left precuneus where the CT group displayed significant changes from TP1 to TP2 (p-corrected > 0.05). ROI-based correlative analysis identified positive correlation between the change in ALFF and the change in the testing scores for Pattern Comparison Processing Speed from TP1 to TP2 in the HC group (r = 0.84, p = 0.0002) (Figure 3), but not in the CT group (r = −0.19, p = 0.47).
Fig. 3.
Spearman correlation and the associated p-values between the changes in ALFF and changes in Pattern Comparison Processing Speed test scores from pre- to post-chemotherapy for the chemotherapy (CT) group (left) and the healthy control (HC) group (right). Linear regression was performed to obtain the fitting curve.
Note: pat_compare_proc: Pattern Comparison Processing Speed
Discussion
To the best of our knowledge, the current study is the first prospective longitudinal study of brain intrinsic activity in older women with breast cancer undergoing adjuvant chemotherapy. In the breast cancer patients treated with chemotherapy, we observed changes from TP1 to TP2 in two intrinsic brain activity parameters: increased ALFF in the bilateral subcallosal gyrus and right anterior cingulate cortex and decreased fALFF in the left precuneus. However, no such changes were observed in the healthy controls.
The anterior cingulate cortex, as part of the frontal lobe, regulates fundamental cognitive process such as motivation, decision-making, and learning [26,27]. Important cognitive functions in the frontal lobe, such as executive function, memory and language, are vulnerable to chemotherapy-associated structural and functional alterations [8,20,28,29]. For instance, a longitudinal study by McDonald and colleagues studying a similar number of chemotherapy patients in a younger age group at around 50 years of age showed an acute reduction in frontal lobe gray matter density one month after completion of chemotherapy [30]. They also showed that decreased frontal gray matter density was accompanied by self-reported difficulties in executive functioning. Additional longitudinal brain structural MRI studies support a similar pattern of frontal gray matter alterations [31,32]. In our current cohort, we previously observed reduced frontal lobe volume in the CT group from TP1 to TP2 [20] and reduced gray matter density mostly in the frontal lobes, including the bilateral inferior frontal gyri, bilateral insula, left anterior cingulate gyrus, left inferior frontal gyrus (BA 47), and right middle frontal gyrus [21]. In this analysis, we observed altered frontal lobe intrinsic brain activity in the frontal lobe in the CT group. Our findings provide additional evidence that the frontal lobe is susceptible to chemotherapy-associated alterations in cancer patients, including older patients.
We also identified a chemotherapy-associated decrease in fALFF in the precuneus brain region in the CT group. The precuneus is in the medial aspect of the parietal lobe; this region is part of the association cortices, which shares connections with other cortical and subcortical regions [33]. In addition, the precuneus is a functional core of the default-mode network (DMN) which supports learning, autobiographical memory, creativity, etc. and is sensitive to chemotherapy toxicity and vulnerable to aging [34–36,8]. The DMN includes the precuneus, posterior cingulate, medial frontal, middle temporal and lateral parietal regions and hippocampus, and it is the one of the most commonly observed resting-state brain networks [35]. Reduced DMN connectivity is a potential neuroimaging biomarker of age-and disease-related cognitive decline [35]. Kesler and colleagues showed that DMN resting-state functional connectivity patterns could be used to discriminate chemotherapy-treated from no-chemotherapy-treated breast cancer survivors or from healthy controls [36]. Another study from the same group showed disrupted organization of the global resting-state functional brain network following chemotherapy in breast cancer survivors [8]. Their study showed the nodal degree and number of hubs were decreased in the orbitofrontal, dorsolateral prefrontal and middle temporal regions, indicating altered functional network topology. Our current study did not directly examine DMN functional connectivity. Instead, we used data-driven methods to evaluate intrinsic brain activation patterns in the whole brain without the need to specify a hypothesis-driven ROI [10–13]. Our finding of decreased fALFF in the precuneus is consistent with the literature regarding the disrupted DMN functional brain connectivity involving the precuneus following chemotherapy [35,36]. Our study results again implicate the precuneus as a critical brain region for chemotherapy-associated brain alterations.
The increased ALFF in the subcallosal gyri and right anterior cingulate gyrus coupled with decreased fALFF in the left precuneus were in general agreement of compensatory efforts described in literature [9]. We speculate that brain activity may increase in one region to compensate for decreased activity in another region. This may help the patients to maintain cognitive function. Such a compensatory mechanism may also explain why we observed no chemotherapy-associated changes in the NP test scores. Neuroplasticity and compensation have been postulated to underlie the relationship between cancer and cognition [9,37]. A study by Cimprich and colleagues showed that cancer patients had increased brain activation by recruiting additional neurocircuitry during a high-memory task load before chemotherapy compared to healthy controls [37]. Their study provided evidence that patients used compensatory efforts to achieve similar cognitive testing scores as the controls. We postulate that the older cancer patients in our study cohort may have also used compensatory efforts; however, further work is needed to test this hypothesis. In addition, we acknowledge that the small sample size of this study may not provide sufficient power to identify subtle changes in cognitive performance.
In order to identify the potential correlation of chemotherapy-associated brain changes between intrinsic brain activity and gray matter density, we co-registered the coordinates of ALFF and fALFF alterations with the coordinates of the clusters showing the gray matter density reduction [21]. We found no overlap of specific brain regions. We speculate that chemotherapy may have affected brain structure and intrinsic brain activity differently, thus resulting in alterations in different brain regions.
We found no significant alterations in the ReHo values after chemotherapy. This is in contrast to prior work showing a chemotherapy-associated decrease in the ReHo values in the frontal lobe such as the right orbitofrontal area and left dorsolateral prefrontal cortex in 19 younger Asian breast cancer patients (mean age ± SD: 43.1 ± 8.8 years) [19]. ReHo is a voxel-based measure of brain activity, which evaluates temporal similarity of a given voxel to its neighbors [12,13] and it has been used to identify alterations in intrinsic brain activity. We speculate that the small sample size of this study, older study participants, potential compensatory efforts in the CT group and short-term follow-up may have limited our ability to detect subtle changes in the ReHo values.
We found a correlation between increased ALFF and improved Pattern Recognition Processing Speed scores in the HC group but not in the CT group. Our study findings are divergent from previous reports. Processing speed serves as the foundation for other cognitive process, including working memory, attention, executive function, and memory [38]. Kvale and colleagues reported that chemotherapy exposure in older patients was associated with decreased processing speed [39]. In addition, Lepage and colleagues observed a significant correlation between gray matter reduction in the left insular cortex and processing speed in chemotherapy-treated cancer patients [32]. There are several possible explanations for the discrepancy between our study and the prior work. First, our study participants (ranging from 60 to 82 years) were generally older than the participants in the reported studies, and aging may influence the cognitive response to chemotherapy. Second, the focus of our study was different from the prior studies (31, 38). Our study focused on brain intrinsic activity data obtained from rs-fMRI while prior studies focused on cognitive testing data [39] or brain structural data such gray matter changes in chemotherapy-treated patients [32]. Third, the lack of correlation in our CT group might be partially due to a chemotherapy-associated decrease in the practice effect from repeated administration of cognitive tests, whereas the HC participants may derive more benefit from practice [40].
Our study result showed that the significant correlation between ALFF and Processing Speed scores in the HC group seemingly relied on two control individuals who had worse performance overtime (Figure 3). We speculate that our controls were older adults who might be more susceptible to cognitive changes due to aging and co-morbidities. Nevertheless, there was no statistically significant change in the testing scores at the group level. Our study finding may serve as pilot data for generating hypothesis for a future study of CRCI in older patients.
There are several limitations in this study. The short follow-up duration, small sample size, heterogeneous stages and chemotherapy regimens could contribute to the non-significant correlation between rs-fMRI and NP scores in the chemotherapy group. In addition, we were limited by a lack of breast cancer control group without chemotherapy to evaluate the effect of the cancer on brain activity and cognition. Despite the limitations, there were strengths; this study used data-driven methods to assess intrinsic brain activity patterns in the entire brain and evaluated these patterns as candidate neuroanatomical correlates of cognitive function. Our study focusing on older breast cancer patients undergoing adjuvant chemotherapy contributes much needed information about brain structure and function in the population of older cancer patients, who are potentially more susceptible to cognitive issues from aging.
In summary, we observed a chemotherapy-associated increase in ALFF in bilateral subcallosal gyri and right anterior cingulate gyrus and a decrease in fALFF in left precuneus in older breast cancer patients. We also found a significant correlation between increased ALFF values and cognitive processing speed in older adults with no history of cancer. Our study results support the notion that intrinsic brain activity parameters may serve as neuroimaging biomarkers for cognitive functioning in older patients with cancer undergoing adjuvant chemotherapy. Future studies with a larger cohort and longer follow-up are needed to validate these findings.
Acknowledgements:
This study was funded by National Institutes of Health/National Institute on Aging grants R03 AG045090–02 (BTC) and R01 AG037037-01A1 (Arti Hurria). Nancy Linford, PhD provided editing assistance.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest: JM reports being a consultant/advisory role in the following entities: Puma and Pfizer. YY reports research grants from and being a consultant with an advisory role in the following entities: Puma, Novartis, Genentech, Merck, GTx, Pfizer, and Immunemedics, outside the submitted work. All other authors declare no competing interests.
Ethical approval: All procedures performed in this study involving human participants were in accordance with the ethical standards of the Institutional Review Board of City of Hope and with the 1964 Helsinki Declaration and its later amendments, as well as all local and national laws. This study is registered on ClinicalTrials.gov (NCT01992432).
Informed consent: Informed consent was obtained from all study participants in the study.
Data availability statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable requests.
References
- 1.Hayat MJ, Howlader N, Reichman ME, Edwards BK (2007) Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. The Oncologist 12 (1):20–37. doi:12/1/20 [pii] 10.1634/theoncologist.12-1-20 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A (2017) Cancer Statistics, 2017. CA Cancer J Clin 67 (1):7–30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 3.Hurria A, Somlo G, Ahles T (2007) Renaming “chemobrain”. Cancer Invest 25 (6):373–377. doi: 10.1080/07357900701506672 [DOI] [PubMed] [Google Scholar]
- 4.Dutta V (2011) Chemotherapy, neurotoxicity, and cognitive changes in breast cancer. J Cancer Res Ther 7 (3):264–269. doi: 10.4103/0973-1482.87008 [DOI] [PubMed] [Google Scholar]
- 5.Wefel JS, Schagen SB (2012) Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep 12 (3):267–275. doi: 10.1007/s11910-012-0264-9 [DOI] [PubMed] [Google Scholar]
- 6.Fried TR, Bradley EH, Towle VR, Allore H (2002) Understanding the treatment preferences of seriously ill patients. N Engl J Med 346 (14):1061–1066. doi: 10.1056/NEJMsa012528 [DOI] [PubMed] [Google Scholar]
- 7.Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34 (4):537–541 [DOI] [PubMed] [Google Scholar]
- 8.Bruno J, Hosseini SM, Kesler S (2012) Altered resting state functional brain network topology in chemotherapy-treated breast cancer survivors. Neurobiol Dis 48 (3):329–338. doi: 10.1016/j.nbd.2012.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Ruiter MB, Schagen SB (2013) Functional MRI studies in non-CNS cancers. Brain Imaging Behav 7 (4):388–408. doi: 10.1007/s11682-013-9249-9 [DOI] [PubMed] [Google Scholar]
- 10.Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8 (9):700–711. doi: 10.1038/nrn2201 [DOI] [PubMed] [Google Scholar]
- 11.Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, Wang YF, Zang YF (2008) An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods 172 (1):137–141. doi: 10.1016/j.jneumeth.2008.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zang Y, Jiang T, Lu Y, He Y, Tian L (2004) Regional homogeneity approach to fMRI data analysis. Neuroimage 22 (1):394–400. doi: 10.1016/j.neuroimage.2003.12.030 [DOI] [PubMed] [Google Scholar]
- 13.Zuo XN, Xu T, Jiang L, Yang Z, Cao XY, He Y, Zang YF, Castellanos FX, Milham MP (2013) Toward reliable characterization of functional homogeneity in the human brain: preprocessing, scan duration, imaging resolution and computational space. Neuroimage 65:374–386. doi: 10.1016/j.neuroimage.2012.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeuchi H, Taki Y, Nouchi R, Sekiguchi A, Kotozaki Y, Nakagawa S, Makoto Miyauchi C, Sassa Y, Kawashima R (2017) Neural plasticity in amplitude of low frequency fluctuation, cortical hub construction, regional homogeneity resulting from working memory training. Sci Rep 7 (1):1470. doi: 10.1038/s41598-017-01460-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin C, Guan M, Dong M, Wu J, He Z, Chen X, Shi D, Ren J, Shi G, Zhang X (2017) Aberrant baseline brain activity in psychogenic erectile dysfunction patients: a resting state fMRI study. Brain Imaging Behav. doi: 10.1007/s11682-017-9805-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu G, Dang C, Peng K, Xie C, Chen H, Xing S, Chen X, Zeng J (2015) Increased spontaneous neuronal activity in structurally damaged cortex is correlated with early motor recovery in patients with subcortical infarction. Eur J Neurol 22 (12):1540–1547. doi: 10.1111/ene.12780 [DOI] [PubMed] [Google Scholar]
- 17.Wu J, Qian Z, Tao L, Yin J, Ding S, Zhang Y, Yu Z (2015) Resting state fMRI feature-based cerebral glioma grading by support vector machine. Int J Comput Assist Radiol Surg 10 (7):1167–1174. doi: 10.1007/s11548-014-1111-z [DOI] [PubMed] [Google Scholar]
- 18.Kim HG, Shin NY, Bak Y, Kim KR, Jung YC, Han K, Lee SK, Lim SM (2017) Altered intrinsic brain activity after chemotherapy in patients with gastric cancer: A preliminary study. Eur Radiol 27 (7):2679–2688. doi: 10.1007/s00330-016-4578-x [DOI] [PubMed] [Google Scholar]
- 19.Mo C, Lin H, Fu F, Lin L, Zhang J, Huang M, Wang C, Xue Y, Duan Q, Lin W, Chen X (2017) Chemotherapy-induced changes of cerebral activity in resting-state functional magnetic resonance imaging and cerebral white matter in diffusion tensor imaging. Oncotarget 8 (46):81273–81284. doi: 10.18632/oncotarget.18111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen BT, Sethi SK, Jin T, Patel SK, Ye N, Sun CL, Rockne RC, Haacke EM, Root JC, Saykin AJ, Ahles TA, Holodny AI, Prakash N, Mortimer J, Waisman J, Yuan Y, Somlo G, Li D, Yang R, Tan H, Katheria V, Morrison R, Hurria A (2018) Assessing brain volume changes in older women with breast cancer receiving adjuvant chemotherapy: a brain magnetic resonance imaging pilot study. Breast Cancer Res 20 (1):38. doi: 10.1186/s13058-018-0965-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen BT, Jin T, Patel SK, Ye N, Sun CL, Ma H, Rockne RC, Root JC, Saykin AJ, Ahles TA, Holodny AI, Prakash N, Mortimer J, Waisman J, Yuan Y, Li D, Somlo G, Vazquez J, Levi A, Tan H, Yang R, Katheria V, Hurria A (2018) Gray matter density reduction associated with adjuvant chemotherapy in older women with breast cancer. Breast Cancer Res Treat 172 (2):363–370. doi: 10.1007/s10549-018-4911-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen BT, Ghassaban K, Jin T, Patel SK, Ye N, Sun CL, Kim H, Rockne RC, Mark Haacke E, Root JC, Saykin AJ, Ahles TA, Holodny AI, Prakash N, Mortimer J, Waisman J, Yuan Y, Somlo G, Li D, Yang R, Tan H, Katheria V, Morrison R, Hurria A (2018) Subcortical brain iron deposition and cognitive performance in older women with breast cancer receiving adjuvant chemotherapy: A pilot MRI study. Magn Reson Imaging 54:218–224. doi: 10.1016/j.mri.2018.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitfield-Gabrieli S, Nieto-Castanon A (2012) Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2 (3):125–141. doi: 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- 24.Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF (2011) REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One 6 (9):e25031. doi: 10.1371/journal.pone.0025031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, Carlozzi NE, Slotkin J, Blitz D, Wallner-Allen K, Fox NA, Beaumont JL, Mungas D, Nowinski CJ, Richler J, Deocampo JA, Anderson JE, Manly JJ, Borosh B, Havlik R, Conway K, Edwards E, Freund L, King JW, Moy C, Witt E, Gershon RC (2013) Cognition assessment using the NIH Toolbox. Neurology 80 (11 Suppl 3):S54–64. doi: 10.1212/WNL.0b013e3182872ded [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Apps MA, Rushworth MF, Chang SW (2016) The Anterior Cingulate Gyrus and Social Cognition: Tracking the Motivation of Others. Neuron 90 (4):692–707. doi: 10.1016/j.neuron.2016.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS (2012) Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci 12 (2):241–268. doi: 10.3758/s13415-011-0083-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDonald BC, Conroy SK, Smith DJ, West JD, Saykin AJ (2012) Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: A replication and extension study. Brain Behav Immun. doi: 10.1016/j.bbi.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simo M, Rifa-Ros X, Rodriguez-Fornells A, Bruna J (2013) Chemobrain: a systematic review of structural and functional neuroimaging studies. Neurosci Biobehav Rev 37 (8):1311–1321. doi: 10.1016/j.neubiorev.2013.04.015 [DOI] [PubMed] [Google Scholar]
- 30.McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ (2010) Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat 123 (3):819–828. doi: 10.1007/s10549-010-1088-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald BC, Conroy SK, Smith DJ, West JD, Saykin AJ (2013) Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: a replication and extension study. Brain Behav Immun 30 Suppl:S117–125. doi: 10.1016/j.bbi.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lepage C, Smith AM, Moreau J, Barlow-Krelina E, Wallis N, Collins B, MacKenzie J, Scherling C (2014) A prospective study of grey matter and cognitive function alterations in chemotherapy-treated breast cancer patients. Springerplus 3:444. doi: 10.1186/2193-1801-3-444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cavanna AE, Trimble MR (2006) The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129 (Pt 3):564–583. doi: 10.1093/brain/awl004 [DOI] [PubMed] [Google Scholar]
- 34.Utevsky AV, Smith DV, Huettel SA (2014) Precuneus is a functional core of the default-mode network. J Neurosci 34 (3):932–940. doi: 10.1523/JNEUROSCI.4227-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kesler SR (2014) Default mode network as a potential biomarker of chemotherapy-related brain injury. Neurobiol Aging 35 Suppl 2:S11–19. doi: 10.1016/j.neurobiolaging.2014.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kesler SR, Wefel JS, Hosseini SM, Cheung M, Watson CL, Hoeft F (2013) Default mode network connectivity distinguishes chemotherapy-treated breast cancer survivors from controls. Proc Natl Acad Sci U S A 110 (28):11600–11605. doi: 10.1073/pnas.1214551110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cimprich B, Reuter-Lorenz P, Nelson J, Clark PM, Therrien B, Normolle D, Berman MG, Hayes DF, Noll DC, Peltier S, Welsh RC (2010) Prechemotherapy alterations in brain function in women with breast cancer. J Clin Exp Neuropsychol 32 (3):324–331. doi: 10.1080/13803390903032537 [DOI] [PubMed] [Google Scholar]
- 38.Carlozzi NE, Tulsky DS, Chiaravalloti ND, Beaumont JL, Weintraub S, Conway K, Gershon RC (2014) NIH Toolbox Cognitive Battery (NIHTB-CB): the NIHTB Pattern Comparison Processing Speed Test. J Int Neuropsychol Soc 20 (6):630–641. doi: 10.1017/S1355617714000319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kvale EA, Clay OJ, Ross-Meadows LA, McGee JS, Edwards JD, Unverzagt FW, Ritchie CS, Ball KK (2010) Cognitive speed of processing and functional declines in older cancer survivors: an analysis of data from the ACTIVE trial. Eur J Cancer Care (Engl) 19 (1):110–117. doi: 10.1111/j.1365-2354.2008.01018.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cerulla N, Arcusa A, Navarro JB, de la Osa N, Garolera M, Enero C, Chico G, Fernandez-Morales L (2018) Cognitive impairment following chemotherapy for breast cancer: The impact of practice effect on results. J Clin Exp Neuropsychol:1–10. doi: 10.1080/13803395.2018.1546381 [DOI] [PubMed] [Google Scholar]