Abstract
Background:
Individuals differ in their sensitivity to alcohol’s physiological effects, including blacking and passing out. Blackouts are periods of impaired memory formation when an individual engages in activities they later cannot recall, while passing out results in loss of consciousness.
Methods:
The sample consisted of 3,292 adult twins from the Australian Twin Registry. Univariate twin analyses were conducted to examine the contributions of genetic and environmental influences to blacking and passing out occurrence and susceptibility (accounting for frequency of intoxication). Evidence for shared etiology of susceptibility to blacking and passing out was examined using bivariate twin analyses.
Results:
Although blacking and passing out were strongly associated (OR = 4.45, 95% CI: [3.85, 5.14]), the genetic epidemiology was quite different. Genetic (43%) and nonshared environmental (57%) influences contributed to liability for blackout occurrence. For passing out occurrence, there was evidence of sex differences. Among men, genetic (32%) and nonshared environmental (68%) influences contributed, whereas among women there were shared (29%) and nonshared environmental (72%) influences. After accounting for frequency of intoxication, genetic influences on blackout susceptibility remained significant; in contrast, only nonshared environmental influences were significant for passing out susceptibility. There was evidence for overlapping genetic and nonshared environmental factors influencing susceptibility to blacking and passing out among men; among women, there were overlapping nonshared environmental influences.
Conclusions:
Blacking and passing out are two common sedative-like effects of heavy drinking, and people differ considerably in their susceptibility to these effects. This study suggests that differences in blackout susceptibility can be explained by genetic factors in both men and women, while differences in susceptibility to pass out after consuming alcohol may be attributable to environmental influences, particularly among women. These environmental factors may include changing social and cultural norms about alcohol use, drinking context, and the type(s) of alcohol consumed.
Keywords: blackout, passing out, alcohol sensitivity, twin study, sex differences
Some individuals experience significant impairment at lower levels of alcohol use, while others are able to use larger quantities of alcohol without seeing many, or even any, effects. This difference in sensitivity to alcohol’s effects has been found to be related to risk of developing an alcohol use disorder (AUD), whereby those with a low level of response to alcohol are at increased risk of an AUD years later (Schuckit et al. 2007). Individuals who have a low level of response to alcohol require more alcohol in order to feel its effects, while those who are highly sensitive feel the effects of alcohol more quickly. Level of response to alcohol has been proposed as a candidate endophenotype for AUD (Salvatore, Gottesman, & Dick, 2015) and shows promise as a Research Domain Criterion (RDoC; Ray, Bujarski, & Roche, 2016). Individuals differ in their sensitivity to a variety of alcohol effects, ranging from more common experiences like unsteadiness or slurred speech, to more extreme effects like experiencing a blackout or passing out.
Blacking and passing out are two consequences associated with alcohol’s sedating effects. Alcohol-induced blackouts refer to periods of time during a drinking episode in which an individual does not lose consciousness and participates in events that they later cannot recall, either partially (fragmentary blackout) or fully (en bloc blackout; Labrie et al., 2011), whereas passing out results in a loss of consciousness due to dangerously high levels of alcohol in the blood. Though originally thought to be a sign of alcoholism (Jellinek, 1946; Jellinek, 1952), over half of drinkers (54.2%) report experiencing a blackout (Barnett et al., 2014). Research shows that passing out may occur at similarly high rates, with one study of college drinkers finding that 13% of males and 16% of females pass out from alcohol use in a typical week (Sugarman et al., 2009). While causes of passing out from alcohol use are not fully understood, the effect of alcohol on lowering blood pressure might be partially responsible, as even low levels of alcohol consumption may inhibit blood vessel constriction (Narkiewicz, Cooley, & Somers, 2000). Blackouts are forms of anterograde amnesia in which memories are not transferred from short-term to long-term memory as a result of a heavy drinking episode (White, 2003) and are thought to result from hippocampal dysfunction.
Blackouts and passing out are significantly correlated with blood alcohol concentration (BAC). For example, the likelihood of a blackout greatly increases at BACs above .24 (Hartzler & Fromme, 2003; Perry et al., 2006), while passing out typically occurs at BACs greater than .30 (National Institute on Alcohol Abuse and Alcoholism, 2015). However, there is considerable variability in the level of alcohol consumption required for an individual to experience these consequences, with some individuals reporting blackouts at BACs as low as .07 (Hartzler & Fromme, 2003). Similarly, passing out may occur at lower or higher BAC levels, depending on a variety of factors.
Some of the factors that account for this variability include environmental, physiological, and psychological factors (see Rose & Grant, 2010 for a review). Low sensitivity to alcohol’s effects is one such physiological factor that has consistently been shown to increase risk of blackout. Those low in sensitivity to alcohol are more likely to adopt patterns of drinking that result in a more rapid increase in BAC (Trela et al., 2016), which is related to liability for experiencing blackouts (White, 2003) and may be related to passing out. Sensitivity to alcohol’s effects has been found to be heritable, with some estimates suggesting that genetic effects account for 60% of the variability (Heath et al., 1999; Martin et al., 1985). However, research on level of response to alcohol among humans has not typically examined the extent to which sensitivity to alcohol’s sedative effects (like blacking and passing out), rather than overall sensitivity, might be influenced by genetic or environmental influences.
Despite how common these alcohol consequences are among drinkers, there has been limited research examining their genetic and environmental underpinnings. Within behavioral genetic models, the influence of genetic and environmental contributions to blackout and passing out experiences can be determined using the inferred genotypic information of twins, as it is known that monozygotic (MZ) twins share all of their genetic material and dizygotic (DZ) twins share just half of their segregating genes. Due to this known relationship, the variance in liability to blacking and passing out may be partitioned into additive genetic influences (known as the A component of variance) and environmental influences, which can be divided into two types: shared and nonshared influences. Factors that are shared between twins and contribute to similarity are referred to as shared environmental influences, or the C component of variance. Nonshared environmental influences, on the other hand, are environmental experiences that cause twins to differ from one another; these factors are referred to as the E component of variance.
The extant behavioral genetic research on blackout has yielded conflicting findings. The earliest examination of the genetic epidemiology of blackout using a large sample of male Vietnam era veterans found there was not a significant genetic contribution to blackout (A = 6%, 95% CI: [0 – 30%]; Slutske et al., 1999). However, more recent research (Nelson et al., 2004) in an Australian sample found that liability for lifetime blackout was significantly heritable, with over half of the variance (53%; 95% CI: [45 – 60%]) in lifetime blackouts being attributed to genetic factors, with no evidence for sex differences. Further analysis showed that a substantial portion of the genetic contribution to blackout was not shared with genetic influences on frequency of intoxication, suggesting that blackout liability is not simply reflecting genetic contributions to frequently drinking to intoxication (Nelson et al. 2004). These findings suggest that genetic propensity for intoxication does not fully explain liability for blackout. Genetic contributions unique to blackout might provide insight into an individual’s sensitivity to alcohol’s sedative effects, which could be an indicator of risk for AUD.
Passing out has been subject to even less behavioral genetic investigation than blackout, with just one prior study examining contributions to frequency of alcohol-induced passing out (Kaprio et al., 1987). The sole study found no evidence for significant genetic influences on the frequency of passing out in a sample of male Finnish twins born before 1958 (Kaprio et al., 1987). However, as the Finnish sample included only men, evidence for sex differences could not be examined. Despite this interesting finding regarding the lack of genetic influences on frequency of passing out, there has been little to no research following up on these results or examining the genetic epidemiology of passing out among women.
There is indirect evidence suggesting that there may be important sex differences in the magnitude or sources of genetic and environmental influences on blackouts and passing out. For example, sensitivity to a number of alcohol’s physiological effects differs for men and women (Nolen-Hoeksema, 2004), with women being more likely to experience blackouts at lower levels of alcohol consumption (Earleywine et al., 2008; Sugarman et al., 2009). This may indicate differences in the relative importance of genes and the environment, or quantitative sex differences, among men and women. The evidence that genetic sources of vulnerability for alcohol abuse and dependence may not be the same for men and women (Prescott, Aggen, & Kendler, 1999) underscores the possibility that similar results might be found for specific alcohol-related consequences, such as blackout and passing out. Such differences in the sources of vulnerability would indicate different factors are important for men and women; this may inform a need for sex-specific intervention targets to reduce consequences of heavy drinking. However, to date, potential sex differences have been explored in just one twin study of blackout (Nelson et al., 2004) and none of passing out.
Current Study
While blackouts and passing out occur commonly among drinkers, there have been just three studies examining their genetic epidemiology (Kaprio et al., 1987; Nelson et al., 2004; Slutske et al., 1999). Additionally, two of these three studies (Kaprio et al., 1987; Slutske et al., 1999) included only male twins and were, therefore, not able to examine evidence for potential sex differences in etiology. The current project aimed to examine the genetic epidemiology of blackout and passing out in a sample of Australian twins and evaluate evidence for sex differences in the quantitative contributions to blackouts and passing out, as well as qualitative sex differences in the sources of liability for these alcohol-related consequences. Contributions to blackout and passing out susceptibility were also examined by controlling for the effects of frequency of intoxication, as individuals who drink to intoxication more frequently would have more chances to blackout or pass out from alcohol use. Finally, evidence for a shared etiology contributing to blacking out and passing out was examined, as well as whether there were blackout-specific or pass out-specific genetic or environmental influences.
Methods
Participants
The sample consisted of members of a cohort of adult twins from the Australian Twin Registry (ATR) born between 1972 and 1979. Sample participants for the current study were initially recruited from the ATR for a cannabis-focused study (Lynskey et al., 2012). The data were collected by computer-assisted telephone interviews that were conducted between 2005 and 2009. The sample for the current study consisted of 3,292 individual twins: 972 monozygotic females (MZF), 479 monozygotic males (MZM), 734 dizygotic females (DZF), 368 dizygotic males (DZM), and 739 DZ opposite sex. Participants were between the ages of 27 and 40 at the time of data collection, with a mean age of 31.84 years (SD = 2.48). Approximately two-thirds (64%) of the sample were female. Participants reported a median household income of $75,000–$99,999/year, which is representative of the population, with the Australian Bureau of Statistics (2017) reporting a mean household income of $89,076/year at the time of data collection. For further details regarding sample recruitment and characteristics, see Lynskey et al. (2012).
Measures
The interview was based on the Australian version of the Semi-Structured Assessment of the Genetics of Alcoholism (SSAGA-OZ), which has been shown to have good reliability and validity for measuring DSM-IV diagnostic criteria for a number of substance use disorders, including AUD (Bucholz et al., 1994). The SSAGA-OZ assessed alcohol abuse and dependence criteria as outlined by the DSM-IV. For the purposes of the current study, responses were scored according to the DSM-5 criteria for AUD, absent the criterion of craving, which was not included in the DSM-IV criteria and, therefore, was not assessed in the interview. Individuals who endorsed having experienced two or more of ten symptoms were considered to have an AUD diagnosis by these approximate DSM-5 criteria. For the current study, lifetime diagnoses were used.
Participants reported on a variety of drinking behaviors and consequences, including blackouts, passing out, intoxication, and the maximum number of drinks ever consumed in a 24-hour period (max drinks). Lifetime blackout was assessed with the following item: “A blackout, which should not be confused with passing out, is when you drank enough so that you could not remember things you had said or done. Have you ever had blackouts, not just passing out from drinking?”. Participants who reported a blackout were then asked if they had experienced three or more in a year, the total number of blackouts experienced over their lifetime, and their age at their first and most recent blackout. Lifetime passing out was assessed with the following item: “Have you ever passed out from drinking, that is, you fell asleep from drinking too much?” Participants who reported passing out were asked the number of times they had passed out, as well as their ages at which the earliest and last occurrences had been.
Frequency of intoxication during the participant’s heaviest period of drinking was assessed by asking, “During that period, how often did you get drunk (that is, your speech would be slurred or you would be unsteady on your feet or you found it was hard to keep your balance)?”. Responses were coded into six categories ranging from less than three times a year to three or more days a week. To assess max drinks, participants were asked to report on the types (beer, wine, spirits, and other), strength, and quantity of alcohol consumed. This was then converted by the interviewer into a number of standard drinks consumed within 24 hours, which was summed across the different types of alcohol to create the max drinks variable. Responses ranged from 0 – 94 drinks, with a mean max drinks of 18.3 (SD = 12.6). Frequency of binge drinking was assessed with the following item: “During that period of at least 12 months when you were drinking the most, how often would you have 5 or more drinks in a single day?”. Response options were coded into ten categories ranging from ‘never’, coded as ‘0’ to ‘every day’, coded as ‘365’.
Data Analysis
Prevalence rates for alcohol use behaviors were calculated using SAS software version 9.4 (SAS Institute, 2015). Additionally, survey analysis procedures in SAS (SAS Institute, 2015) were used to test sex differences in prevalence rates for alcohol use behaviors while taking into account the non-independence of twin pair observations. Correlations between a number of heavy drinking behaviors, including AUD diagnosis, max drinks, and binge drinking frequency, were also calculated within SAS (SAS Institute, 2015).
Twin correlations were calculated in Mplus (Muthén & Muthén, 2017). Univariate twin analyses were conducted in Mplus using full information maximum likelihood robust weighted least squares estimation and fitting models to the raw ordinal data. Analyses included both complete and incomplete twin pairs. The models of the categorical outcomes of blackout and passing out occurrence were based on a liability-threshold model, which assumes a latent liability continuum underlying the categorical outcome (Kendler, 1993; Neale & Cardon, 1992). Structural equation modeling was used to partition the variation in liability into additive genetic (A), shared environmental (C), and nonshared environmental (E) influences. Sex differences in prevalence rates were modeled by allowing thresholds to differ for men and women. To test for quantitative sex differences, the fit of a model in which the estimates of the A and C components of variation were constrained to be equal among men and women was compared to the fit of a model in which they were allowed to differ. Evidence for qualitative sex differences, or different genetic factors contributing to liability in men and women, was tested by comparing the fit of a model in which the genetic correlation was constrained to 0.5 for DZ opposite-sex twin pairs to a model in which it was freely estimated (a genetic correlation of 0.5 is expected when genetic effects are not sex-limited).
Susceptibility to blackout and passing out were derived from regressing the lifetime blackout and passing out occurrence variables on participants’ reported frequency of intoxication. Within SAS (SAS Institute, 2015) the probit regression of the lifetime blackout and passing out variables on frequency of intoxication was modeled, creating a threshold value for each participant that is defined by a probit regression with an overall sex-specific intercept that varies based on individuals’ reported frequency of intoxication. The resulting residuals were then transformed using a Box Cox transformation. After transformation, the blackout susceptibility variable had skewness and kurtosis of −0.20 and −1.54, respectively, while the passing out susceptibility variable had skewness of −0.28 and kurtosis of −1.55. These residual variables were then standardized within Mplus. Following this, genetic and environmental influences on the residual variance, what we have termed blackout and passing out susceptibility, were estimated in Mplus (Muthén & Muthén, 2017). This model takes into account effects of frequency of intoxication on liability for blacking and passing out and is similar to one of the approaches used by Nelson et al (2004) to account for effects of frequency of intoxication on liability for blackout.
Bivariate genetic analyses were conducted within Mplus (Muthén & Muthén, 2017) among the same-sex twin pairs to determine the extent to which sources of variation were common to both lifetime blackout and passing out susceptibility (that is, after taking into account the effects of frequency of intoxication) or were specific to each phenotype. A Cholesky decomposition (Loehlin, 1996) was implemented to partition the genetic and environmental variation into common factors shared by lifetime blackout and passing out, as well as two sets of specific factors that were unique to each phenotype. This information was also used to compute genetic (rA), shared environmental (rC), and nonshared environmental (rE) correlations between blackout and passing out, which indicate the degree of overlap in etiological influences. (Bivariate genetic analyses for lifetime blackout and passing out occurrence were also conducted and can be found in supplemental materials.)
Results
Descriptive Statistics
Almost all participants had consumed alcohol (99%) and gotten drunk (97%) in their lifetime. Similar proportions of participants had experienced a blackout (53%) and passed out (56%) from alcohol. On average, men reported 9.85 lifetime blackouts (SD = 30.17) and 7.43 passing out experiences (SD = 29.19), while women reported 4.51 blackouts (SD = 15.90) and 3.64 passing out experiences (SD = 15.72). Approximately 9% of the sample (12.4% of men and 6.6% of women) reported a past year blackout, and approximately 8% had passed out in the past year (10.8% of men and 6.2% of women), suggesting that these occurrences had continued into adulthood. Men were more likely than women to endorse all drinking behaviors except having ever drank, for which there were no significant differences (see Table 1).
Table 1.
Drinking behaviors of the Australian Twin Registry Cohort 3.
| Full Sample | Women | Men | F* | p-value | |
|---|---|---|---|---|---|
| % | % | % | |||
| 98.7 | 98.7 | 98.8 | 0.01 | 0.93 | |
| Lifetime intoxication | 96.7 | 95.6 | 98.4 | 17.25 | < .0001 |
| Regular drinker | 92.2 | 90.6 | 95.0 | 19.63 | < .0001 |
| Lifetime blackout | 52.8 | 48.5 | 59.9 | 37.52 | < .0001 |
| Lifetime pass out | 55.6 | 50.3 | 64.4 | 59.32 | < .0001 |
| Past year blackout | 8.7 | 6.6 | 12.4 | 34.31 | < .0001 |
| Past year pass out | 7.9 | 6.2 | 10.8 | 24.01 | < .0001 |
| Blackout 3+ times/year | 17.7 | 14.8 | 24.1 | 46.32 | < .0001 |
| Lifetime AUD diagnosis | 37.7 | 28.6 | 56.3 | 305.11 | < .0001 |
| Mean (SD) | Mean (SD) | Mean (SD) | F* | p-value | |
| # times blackout | 6.5 (22.5) | 4.5 (15.9) | 9.9 (30.2) | 45.79 | < .0001 |
| # times pass out | 5.1 (21.8) | 3.6 (15.7) | 7.4 (29.2) | 24.31 | < .0001 |
| Age of first blackout | 19.8 (3.7) | 19.8 (3.9) | 19.8 (3.5) | 0.21 | 0.64 |
| Age of first pass out | 19.1 (3.5) | 19.3 (3.8) | 18.8 (3.2) | 8.89 | 0.003 |
| Age of most recent blackout | 25.9 (4.9) | 25.2 (5.0) | 26.7 (4.6) | 44.25 | < .0001 |
| Age of most recent pass out | 25.2 (5.0) | 24.5 (5.0) | 26.0 (4.9) | 40.45 | < .0001 |
test of difference between women and men
Blacking and passing out were significantly correlated with other heavy drinking indicators such as the maximum number of drinks consumed in a single day in the past year and binge drinking, and were significantly correlated with each other (r = .56; see Table 2). About three quarters (72.3%) of people who reported experiencing a blackout also reported having passed out from alcohol use. Similarly, of those who reported passing out, 68.7% reported a lifetime blackout. Having passed out from alcohol use was associated with 4.45 times the odds of having a blackout (95% CI: [3.85 – 5.14]). This association was reduced but still significant after accounting for frequency of intoxication (OR = 3.40, 95% CI: [2.92 – 3.96]). Blackouts and passing out were both associated with increased risk of an AUD (blackout: OR = 3.12 [2.70 – 3.60]; passing out: OR = 2.81 [2.44 – 3.25]). This risk was reduced but still significant after taking into account the effects of frequency of intoxication (blackout: OR = 1.95 [1.66 – 2.28]; passing out: OR = 1.98 [1.69 – 2.32]).
Table 2.
Correlations between blackout, passing out, and other drinking behaviors.
| Blackout | Passing Out | Binge Drinking | Max Drinks | Frequency of Intoxication | Lifetime AUD | |
|---|---|---|---|---|---|---|
| Blackout | --- | .50 | .45 | .39 | .46 | .43 |
| Passing Out | .56 | --- | .30 | .29 | .35 | .38 |
| Binge Drinking | .37 | .29 | --- | .61 | .81 | .52 |
| Max Drinks | .38 | .30 | .52 | --- | .54 | .47 |
| Frequency of Intoxication | .42 | .34 | .76 | .47 | --- | .54 |
| Lifetime AUD | .35 | .30 | .47 | .42 | .48 | --- |
Note: Polychoric correlations above the diagonal are for women, correlations below the diagonal are for men. All correlations are significant at p < .0001.
Twin Correlations
Some initial patterns emerged when interpreting the twin correlations, although a number of the confidence intervals for MZ and DZ twins overlapped (Table 3). For example, MZ correlations for lifetime blackout occurrence were larger than the DZ correlations, suggesting a potential genetic contribution to blackout. On the other hand, MZ correlations for lifetime passing out were higher than DZ correlations only among men, suggesting genetic factors might not contribute to passing out among women, for whom DZ correlations were higher than MZ correlations (again, with considerable overlap of confidence intervals). Additionally, twin correlations for passing out occurrence were near zero for opposite sex twins, suggesting potential qualitative sex differences. To explore these patterns further, univariate twin analyses were conducted.
Table 3.
Twin correlations for blackout and passing out.
| Women | Men | OS | |||
|---|---|---|---|---|---|
| MZ | DZ | MZ | DZ | DZ | |
| Lifetime blackout | .36 [.22 2 .51] | .26 [.10 – .43] | .53 [.35 – .71] | .27 [.18 – .36] | .16 [.01 – .31] |
| Lifetime passing out | .14 [−.03 – .30] | .44 [.27 – .61] | .32 [.08 – .57] | .15 [−.15 – .45] | −.02 [−.25 – .21] |
Note: MZ = monozygotic, DZ = dizygotic, and OS = opposite sex twins. Numbers within brackets are 95% confidence intervals. Bold indicates significance.
Univariate Twin Analyses of Blackout
Separate univariate biometric models were fit for lifetime blackout occurrence (not accounting for frequency of intoxication) and lifetime blackout susceptibility1 (accounting for effects of frequency of intoxication). Results for the univariate models are presented in Table 4, and model fit indices are presented in Table 5.
Table 4.
Estimates of variance components from univariate twin models of blackout and passing out.
| Women | Men | Constrained across Sex | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A | C | E | A | C | E | A | C | E | |
| Lifetime blackout | |||||||||
| occurrence | .20 [0 – .56] |
.17 [0 –.48] |
.64
[.50 – .78] |
.53
[.35 – .71] |
0 |
.47
[.29 – .65] |
.43 [.32 – .54] |
0 |
.57 [.45 – .68] |
| susceptibilitya | .16 [0 – .45] |
.02 [0 – .26] |
.83 [.73 – .92] |
.27 [.15 – .40] |
0 |
.73 [.60 – .86] |
.21 [.13 – .28] |
0 |
.79 [.72 – .87] |
| Lifetime passing out | |||||||||
| occurrenceb | 0 |
.29
[.17 – .41] |
.72
[.60 – .83] |
.32
[.09 – .55] |
0 |
.68
[.46 – .91] |
__ | __ | __ |
| susceptibilitya | 0 |
.15 [.08 – .22] |
.85 [.78 – .92] |
.15 [.01 – .29] |
0 |
.85 [.71 – .99] |
.06 [0 – .21] |
.07 [0 – .17] |
.87 [.79 – .95] |
Note: A = additive genetic influences, C = shared environmental influences, E = nonshared environmental influences. Bold indicates significance.
analyses controlling for the effects of frequency of intoxication.
constrained results are not presented because estimates could not be constrained across sex without a significant decrease in model fit.
Table 5.
Model fit indices and tests of quantitative and qualitative sex differences from univariate twin modelling of blackout and passing out.
| χ2 | df | p-value | Δχ2 | df | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blackout Occurrence | ||||||||||||
| Base Model | 2.83 | 8 | 0.94 | -- | -- | -- | ||||||
| mACE=fACE | 5.52 | 10 | 0.85 | 2.69 | 2 | 0.26 | ||||||
| OS rg = 0.5 | 3.41 | 9 | 0.95 | 0.58 | 1 | 0.45 | ||||||
| Blackout Susceptibility | ||||||||||||
| Base Model | 3.36 | 8 | 0.91 | -- | -- | -- | ||||||
| mACE = fACE | 8.29 | 10 | 0.60 | 4.93 | 2 | 0.09 | ||||||
| OS rg = 0.5 | 3.79 | 9 | 0.92 | 0.43 | 1 | 0.51 | ||||||
| Passing Out Occurrence | ||||||||||||
| Base Model | 10.20 | 8 | 0.25 | -- | -- | -- | ||||||
| mACE = fACE | 17.84 | 10 | 0.06 | 7.64 | 2 | 0.02 | ||||||
| Passing Out Susceptibility | ||||||||||||
| Base Model | 8.37 | 8 | 0.40 | -- | -- | -- | ||||||
| mACE = fACE | 14.13 | 10 | 0.17 | 5.76 | 2 | 0.06 | ||||||
| OS rg = 0.5 | 8.37 | 9 | 0.50 | -- | -- | -- | ||||||
Note: m = men, f = women, ACE = genetic (A), shared environmental (C), and nonshared environmental (E) influences, OS = opposite sex.
mACE = fACE: test of quantitative sex differences (equating parameter estimates for men and women); OS rg = 0.5: test of qualitative sex differences (constraining OS genetic correlation to 0.5, or the genetic correlation for same-sex dizygotic twins).
The univariate model examining contributions to liability for lifetime blackout occurrence provided a heritability estimate of 53.3% for men and 19.7% for women. Shared environmental influences accounted for 16.5% of variance among women, but did not contribute among men. Nonshared environmental influences accounted for the remainder of variance in blackout occurrence. Despite the apparent differences in heritability for men and women, constraining ACE estimates across sex did not result in a significant decrease in fit. Additionally, there was no evidence of qualitative sex differences. When combining men and women, heritability of lifetime blackout occurrence was estimated to be 42.9%, with the remainder of the variance accounted for by nonshared environmental factors.
A univariate twin model examining contributions to blackout susceptibility fit the data well, with results showing that 21.2% of the variance in blackout susceptibility was due to genetic factors after constraining estimates equal for men and women. The remainder of the variance in susceptibility to blackout was accounted for by nonshared environmental factors.
Univariate Twin Analyses of Passing Out
Separate univariate twin analyses examined influences on liability for lifetime passing out occurrence and lifetime passing out susceptibility2. Results for the univariate models are presented in Table 4, with model fit indices presented in Table 5.
Constraining ACE estimates to be equal for men and women led to a significant decrease in model fit, suggesting sex differences in the magnitude of genetic and environmental contributions to liability for lifetime passing out occurrence. Among men, about a third (31.8%) of the variance in liability for passing out was due to genetic factors. The remainder of the variance was accounted for by nonshared environmental factors (68.2%). Among women, genetic factors did not contribute to liability for lifetime passing out occurrence; instead, shared (28.5%) and nonshared (71.5%) environmental factors accounted for variance. The presence of qualitative sex differences was not tested because there was no evidence for shared environmental influences among men or genetic influences among women.
After accounting for frequency of intoxication, among women, 15% of the variance in susceptibility to passing out was due to shared environmental factors. The remainder of the variance was accounted for by nonshared environmental factors. Among men, 15.1% of the variance in susceptibility to passing out could be attributed to genetic factors, with nonshared environmental influences accounting for the remaining variance. Constraining estimates for men and women did not result in a significant decrease in model fit, providing an overall nonsignificant heritability estimate of 6% for lifetime passing out susceptibility. Shared environmental influences were also nonsignificant, accounting for an estimated 7% of the variance. Nonshared environmental influences accounted for the remainder of the variance in susceptibility.
Bivariate Twin Analyses of Lifetime Blackout and Passing Out Susceptibility
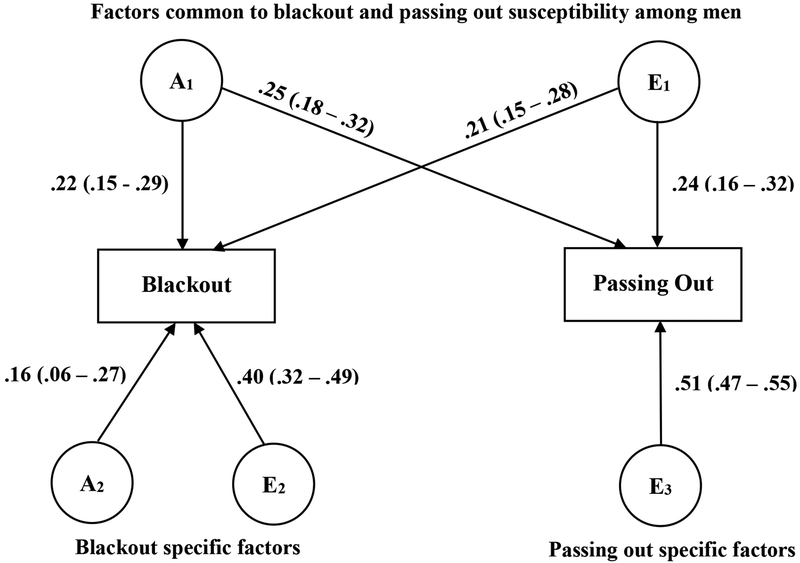
Bivariate models were fit to the data after accounting for the effects of frequency of intoxication on liability to blackout and passing out. Models were fit separately for men and women, as the influences on passing out appeared to differ considerably across sex. The bivariate twin model examining susceptibility to blackout and passing out fit the data well (men: χ2 = 9.80, df = 20, p = .97; women: χ2 = 19.13, df = 18, p = .38). Among men (Figure 1), there was evidence for common genetic (rA = .91; 95% CI: .32 – 1) and nonshared environmental (rE = .20; 95% CI: .08 – 0.33) factors influencing susceptibility to both blackouts and passing out. Additionally, genetic (16.4%) and nonshared environmental (40.3%) factors specific to blackout susceptibility were significant. There was no evidence for genetic factors specific to passing out susceptibility, as all of the genetic influences on passing out (24.5%) were shared with blackout.
Figure 1.
Bivariate biometric models of liability for blackout and passing out susceptibility (that is, after taking into account frequency of intoxication) among men. A = additive genetic influences, C = shared environmental influences, and E = nonshared environmental influences.
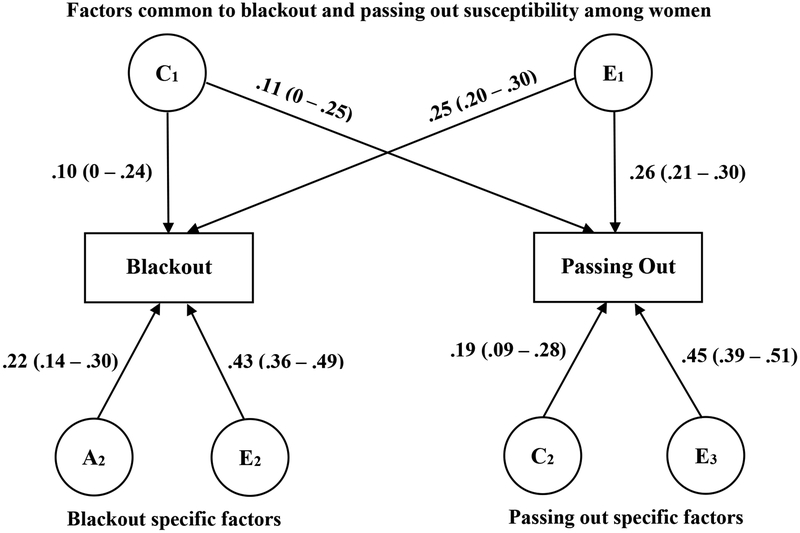
Among women (Figure 2), there was evidence for significant nonshared environmental factors common to susceptibility for both blacking and passing out (rE = .26; 95% CI: .19 - .33); common shared environmental influences on susceptibility to blackout and passing out (rC = .84; 95% CI: 0 – 1) were nonsignificant. Genetic influences specific to blackout susceptibility (22.4%) and shared environmental influences specific to passing out susceptibility (18.8%) were significant. Nonshared environmental influences specific to blackout (43%) and those specific to passing out (45.3%) were also significant.
Figure 2.
Bivariate biometric models of liability for blackout and passing out susceptibility (that is, after taking into account frequency of intoxication) among women. A = additive genetic influences, C = shared environmental influences, and E = nonshared environmental influences.
Results for bivariate models of lifetime blackout and passing out occurrence produced results similar to those for susceptibility (see Supplemental Materials).
Discussion
Genetic Epidemiology of Blackout Experiences
The current study provided additional evidence confirming the contribution of genetic factors to liability for experiencing blackout based on a cohort of Australian twins born in 1972–79, similar to those previously obtained in an earlier cohort of Australian twins born in 1964–71 (Nelson et al, 2004). However, because individual differences in drinking habits might explain these findings, it is important to disentangle the genetic susceptibility to blacking out from the genetic contributions to excessive drinking. As an alternative to experimentally controlling for level of alcohol consumption, we statistically controlled for it in multivariate analyses. Analyses controlling for the effects of frequency of intoxication on blackout also confirmed the existence of a genetic susceptibility to blackout. Although individuals vary in their genetic propensity to frequently drink to intoxication, this cannot fully explain susceptibility to experiencing a blackout.
Genetic Epidemiology of Passing Out Experiences
Little is known about the etiology of another indicator of sensitivity to alcohol’s sedative effects, that is, alcohol-related passing out. To our knowledge, just one prior study has examined genetic and environmental contributions to passing out. Previous research conducted with a sample of male Finnish twins found no genetic contributions to frequency of passing out (Kaprio et al., 1987). The current findings differed by providing evidence for significant, albeit low, genetic influences on passing out among men, with no significant genetic influences among women. Instead, shared environmental influences contributed significantly among women, but not men.
The finding of a modest estimate of genetic contributions to passing out experiences among men and no evidence for genetic influences among women suggests that passing out may be influenced mostly by the environment. For women, this may be social and cultural factors such as changing norms that have increased the social acceptability of heavy drinking. Additionally, some qualitative research suggests that women who engage in excessive drinking may do so because of increased sexual attention from men (Young et al., 2005). These motivations among women likely differ across drinking contexts and age groups. For men, similar social norms may be at play; male participation in drinking games, a context associated with heavy, rapid drinking that often leads to negative consequences, may be driven by sexual motivations (Hone, Carter, & McCullough, 2013). Other environmental influences, such as the type of alcohol being consumed (liquor vs. beer) in a given drinking episode, may also be important for influencing susceptibility to the physiological effects of alcohol, with evidence showing that drinking shots of liquor significantly increased the risk of blacking out in a sample of college students (Labrie et al., 2011). Together, these and other environmental influences seem to account for a large degree of the variability in one’s susceptibility to pass out from alcohol.
There is also evidence that genetic pathways to alcohol sensitivity may be more important for men than women, with findings suggesting that the familial association between parental AUD and offspring’s alcohol sensitivity is greater among men than women (Quinn & Fromme, 2011). This is consistent with findings for passing out in the current study. These potential sex differences will be important to consider in future research examining contributions to liability for physiological alcohol-related consequences. Additionally, these findings may inform sex-specific intervention strategies for reducing negative consequences of heavy drinking. Particularly among women, targeting risky environmental contexts and increasing use of protective behavioral strategies (i.e., reducing drinking game participation, not mixing types of alcohol, not taking shots) may reduce the likelihood of experiencing a blackout or passing out.
Despite sex differences in the etiology of passing out occurrence, estimates of genetic and environmental contributions were not significantly different for men and women after taking into account the effects of frequency of intoxication. This finding suggests that sex differences in the etiology of passing out may be due to contributions to frequency of intoxication, rather than contributions specific to passing out. Models that assumed similar estimates of genetic and environmental contributions for men and women suggested the importance of environmental influences for susceptibility to passing out. These environmental influences may include the type of alcohol being consumed (liquor vs. beer), the context in which drinking occurs (with friends vs. with family), and social and cultural norms regarding gender and alcohol use.
Shared Etiology of Blackout and Passing Out
The experience of blacking and passing out from alcohol use were highly correlated, with most people who reported experiencing one effect also endorsing experiencing the other. There was evidence for significant shared etiological factors contributing to this co-occurrence. However, bivariate genetic analyses showed that the causes of this co-occurrence were quite different for men and women. Models accounting for frequency of intoxication, which likely contributes to jointly experiencing blacking and passing out by increasing the number of opportunities for these consequences to co-occur, showed that common genetic and nonshared environmental factors contributed to the shared etiology among men. In fact, the possibility that the same set of genes contributed to blackout and passing-out susceptibility among men could not be ruled out. Among women, common nonshared environmental influences accounted for the covariation in susceptibility to blackouts and passing out, with no evidence for significant familial causes of overlap, that is, either common genetic or shared environmental factors.
In addition to evidence for the shared etiology of susceptibility to blackout and passing out, there was also support for etiological factors specific to each phenotype. For example, there were significant genetic and nonshared environmental factors specific to blackout susceptibility, but no evidence for genetic factors specific to passing out susceptibility among men or women. Among women, shared environmental and nonshared environmental influences specific to passing out susceptibility were significant. Although blackout and passing out experiences are phenotypically similar commonly co-occurring sedative effects of excessive alcohol consumption, they also appear to have distinct underlying etiological influences.
Limitations
Several limitations must be considered when interpreting these findings. The sample size may have been inadequate to detect qualitative sex differences (Verhulst, 2017); it is possible that qualitative sex differences for blackout might be revealed in more adequately powered studies. Additionally, retrospective recall for lifetime alcohol use behaviors is subject to a number of biases and errors. Blackout, in particular, has been notoriously difficult to assess (Wetherill & Fromme, 2016), as knowledge of a blackout often requires some significant event to occur during the blackout or for others to cue the individual to events that he/she does not recall. Furthermore, polydrug use, particularly co-occurring cannabis use, might have contributed to some reports of blackout or passing out.
It is also important to consider the potential impact of the different wording that has been used in studies of blackout. In the Nelson et al. (2004) study, participants were asked to report experiencing a blackout when they did not pass out from drinking, while in the present study, individuals could have endorsed experiencing both a blackout and passing out from alcohol arising from the same drinking occasion. This could have led to the higher rates of lifetime blackout seen in the current study, as rates increased from 49% and 34% for men and women in the previous study to 60% and 48% for men and women in the present study. It is possible that these higher estimates better reflect reality, as people can and likely often do experience both a blackout and passing out from alcohol in a single drinking episode. To our knowledge, there is no previous research that has ascertained whether blackouts and passing out co-occurred within a drinking episode. This is an important direction for future research.
Finally, the current study was unable to distinguish between the two forms of blackout: fragmentary (partial blackouts) and en bloc (complete blackouts). These two types of blackouts have different characteristics and occur at different BAC ranges (Hartzler & Fromme, 2003). As en bloc blackouts indicate complete memory loss for a period of time in a drinking episode, instances of this form of blackout may be more difficult to detect via self-report and may have been underrepresented in the current study. Future studies should assess the type of blackout experienced and investigate whether differences exist in the genetic epidemiology of fragmentary versus en bloc blackouts.
Conclusions
Blackouts and passing out occur frequently among regular drinkers, but little is known about the genetic epidemiology of these experiences, with only three previous studies (Kaprio, 1987; Nelson et al, 2004; Slutske et al, 1999). This was the first study to provide evidence for a shared etiology for blackout and passing out, as well as for specific etiologic factors that are unique to each. Additionally, there was evidence for distinct etiological mechanisms for passing out among men and women that seemed to be explained by the frequency of intoxication. Continued research and improved assessment of blackouts and passing out will be critical to better understand these common and often consequential physiological effects of alcohol.
Supplementary Material
Acknowledgements:
We thank Dixie Statham, Richard Parker, Soad Hancock, Judith Moir, Sally Rodda, Pieta-Maree Shertock, Heather Park, Jill Wood, Pam Barton, Fran Husband, and Adele Somerville, who worked on this project and the twins and their siblings for participating.
Funding: This work was supported by National Institutes of Health Grants DA18267 (MTL) and T32AA013526 (CND).
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
Univariate biometric models were also fit for number of times blacking out and experiencing 3+ blackouts in a year. Results for number of times blacking out were very similar to those for lifetime blackout occurrence, with significant genetic (47%) and nonshared environmental (50%) influences. For experiencing 3+ blackouts in a year, only nonshared environmental influences (43%) were significant, with genetic influences accounting for 28% of the remaining variance.
Univariate biometric models were also fit for number of times passing out. Results were similar to those for lifetime passing out occurrence, with significant shared (35%) and nonshared environmental (65%) influences among women. Among men, genetic (30%) and nonshared environmental (69%) influences contributed significantly to variance.
References
- Australian Bureau of Statistics (2017). ABS Survey of Income and Housing. Available at: http://abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/6523.0~2015-16~Main%20Features~Key%20Findings~1
- Barnett NP, Clerkin EM, Wood M, Monti PM, O’Leary Tevyaw T, Corriveau D, Fingeret A, Kahler CW (2014) Description and predictors of positive and negative alcohol-related consequences in the first year of college. J Stud Alcohol Drugs 75:103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI Jr, Reich T, Schmidt I, Schuckit MA (1994) A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol 55:149–158. [DOI] [PubMed] [Google Scholar]
- Earleywine M, LaBrie JW, Pedersen ER (2008) A brief Rutgers Alcohol Problem Index with less potential for bias. Addict Behav 33:1249–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler B, Fromme K (2003) Fragmentary and en bloc blackouts: similarity and distinction among episodes of alcohol-induced memory loss. J Stud Alcohol 64:547–550. [DOI] [PubMed] [Google Scholar]
- Heath AC, Madden PAF, Bucholz KK, Dinwiddie SH, Slutske WS, Bierut LJ, Rohrbaugh JW, Statham DJ, Dunne MP, Whitfield JB, Martin NG (1999) Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychol Med 29:1069–1081. [DOI] [PubMed] [Google Scholar]
- Hone LS, Carter EC, McCullough ME (2013) Drinking games as a venue for sexual competition. Evol Psychol 11:889–906. [PubMed] [Google Scholar]
- Jellinek EM (1946) Phases in the drinking history of alcoholics. Analysis of a survey conducted by the official organ of Alcoholics Anonymous (Memoirs of the Section of Studies on Alcohol). Quarterly Journal of Studies on Alcohol 7:1–88. [PubMed] [Google Scholar]
- Jellinek EM (1952) Phases of alcohol addiction. Quarterly Journal of Studies on Alcohol 13:673–684. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Koskenvuo M, Langinvainio H, Romanov K, Sarna S, Rose RJ (1987) Genetic influences on use and abuse of alcohol: a study of 5638 adult Finnish twin brothers. Alcohol Clin Exp Res 11:349–356. [DOI] [PubMed] [Google Scholar]
- Kendler KS (1993) Twin studies of psychiatric illness: current status and future directions. Arch Gen Psychiatry 50:905–915. [DOI] [PubMed] [Google Scholar]
- LaBrie JW, Hummer J, Kenney S, Lac A, Pedersen E (2011) Identifying factors that increase the likelihood for alcohol-induced blackouts in the prepartying context. Subst Use Misuse 46:992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loehlin JC (1996) The Cholesky approach: A cautionary note. Behav Genet 26:65–69. [Google Scholar]
- Lynskey MT, Agrawal A, Henders A, Nelson EC, Madden PA, Martin NG (2012) An Australian twin study of cannabis and other illicit drug use and misuse, and other psychopathology. Twin Res Hum Genet 15:631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin NG, Oakeshott JG, Gibson JB, Starmer GA, Perl J, Wilks AV (1985) A twin study of psychomotor and physiological responses to an acute dose of alcohol. Behav Genet 15:305–347. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO (2017) Mplus User’s Guide. Eighth Edition. Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Narkiewicz K, Cooley RL, Somers VK (2000) Alcohol potentiates orthostatic hypotension: implications for alcohol-related syncope. Circulation 101:398–402. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (2015) Alcohol overdose: The dangers of drinking too much. Available at: https://pubs.niaaa.nih.gov/publications/alcoholoverdosefactsheet/overdoseFact.pdf.
- Neale MC, Cardon LR (1992) Methodology for genetic studies of twins and families. Kluwer Academic Publishers, Netherlands. [Google Scholar]
- Nelson EC, Heath AC, Bucholz KK, Madden PA, Fu Q, Knopik V, Lynskey MT, Whitfield JB, Statham DJ, Martin NG (2004) Genetic epidemiology of alcohol-induced blackouts. Arch Gen Psychiatry 61:257–263. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S (2004) Gender differences in risk factors and consequences for alcohol use and problems. Clin Psychol Rev 24:981–1010. [DOI] [PubMed] [Google Scholar]
- Perry PJ, Argo TR, Barnett MJ, Liesveld JL, Liskow B, Hernan JM, & Brabson MA (2006) The association of alcohol‐induced blackouts and grayouts to blood alcohol concentrations. J Forensic Sci 51:896–899. [DOI] [PubMed] [Google Scholar]
- Polderman TJ, Benyamin B, De Leeuw CA, Sullivan PF, Van Bochoven A, Visscher PM, Posthuma D (2015) Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat Genet 47:702–709. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Aggen SH, Kendler KS (1999) Sex differences in the sources of genetic liability to alcohol abuse and dependence in a population‐based sample of US twins. Alcohol Clin Exp Res 23:1136–1144. [DOI] [PubMed] [Google Scholar]
- Quinn PD, Fromme K (2011) Subjective response to alcohol challenge: a quantitative review. Alcohol Clin Exp Res 35:1759–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Roche DJ (2016) Subjective response to alcohol as a research domain criterion. Alcohol Clin Exp Res 40:6–17. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC (2003) Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Arch Gen Psychiatry 60:1256–1264. [DOI] [PubMed] [Google Scholar]
- Rose ME, Grant JE (2010) Alcohol-induced blackout: phenomenology, biological basis, and gender differences. J Addict Med 42:61–73. [DOI] [PubMed] [Google Scholar]
- Salvatore JE, Gottesman II, Dick DM (2015) Endophenotypes for alcohol use disorder: an update on the field. Curr Addict Rep 2:76–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute (2015) Base SAS 9.4 Procedures Guide. SAS Institute. [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Pierson J, Hesselbrock V, Bucholz KK, Kramer J, Kuperman S, Dietiker C, Brandon R, Chan G (2007) The ability of the Self-Rating of the Effects of Alcohol (SRE) Scale to predict alcohol-related outcomes five years later. J Stud Alcohol Drugs 68:371–378. [DOI] [PubMed] [Google Scholar]
- Slutske WS, True WR, Scherrer JF, Heath AC, Bucholz KK, Eisen SA, Goldberg J, Lyons MJ, Tsuang MT (1999) The heritability of alcoholism symptoms:”indicators of genetic and environmental influence in alcohol‐dependent individuals” revisited. Alcohol Clin Exp Res 23:759–769. [DOI] [PubMed] [Google Scholar]
- Sugarman DE, DeMartini KS, Carey KB (2009) Are women at greater risk? An examination of alcohol-related consequences and gender. Am J Addict 18:194–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trela CJ, Piasecki TM, Bartholow BD, Heath AC, Sher KJ (2016) The natural expression of individual differences in self-reported level of response to alcohol during ecologically assessed drinking episodes. Psychopharmacology 233:2185–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst B (2017) A power calculator for the classical twin design. Behav Genet 47:255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Fromme K (2016) Alcohol‐induced blackouts: A review of recent clinical research with practical implications and recommendations for future studies. Alcohol Clin Exp Res 40:922–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM (2003) What happened? Alcohol, memory blackouts, and the brain. Alcohol Res Health 27:186–196. [PMC free article] [PubMed] [Google Scholar]
- Young AM, Morales M, McCabe SE, Boyd CJ, D’arcy H (2005) Drinking like a guy: Frequent binge drinking among undergraduate women. Subst Use Misuse 40:241–267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.