Abstract
Intravenous immunoglobulin (IVIg) therapy is increasingly used in the pediatric population, in particular among children with immune-compromising conditions. Pooled immunoglobulin products are routinely tested for hepatitis B surface antigen (HBsAg) and nucleic acid, however screening for hepatitis B core antibody (anti-HBc) is not commonly performed. Thus, the administration of IVIg containing anti-HBc to children with immune-compromising conditions may complicate the interpretation of hepatitis B serologic testing in that a positive anti-HBc test may represent passive transfer of antibody from IVIg or may indicate resolved or chronic hepatitis B infection. Due to the risk of hepatitis B reactivation in immunocompromised patients, a positive anti-HBc test must be carefully considered. As part of a quality improvement initiative, we identified and reviewed the records of all pediatric patients at our institution who tested positive for anti-HBc over an 18-month period. Of 44 total patients with positive anti-HBc tests, we found that 22 (50%) had previously received IVIg in the preceding 4 months. All but one of these, 21/22 (95%), went on to receive immunosuppressive therapy (IS). Among the patients who received IS, 19 (86%) had not undergone hepatitis B serologic testing prior to IVIg administration and 16 (73%) did not have subsequent testing to distinguish between passive acquisition of anti-HBc from IVIg and chronic hepatitis B infection. Our single-center experience reveals that a high proportion of positive anti-HBc tests in children are presumed to be due to passive antibody transfer from IVIg. However, a low proportion of patients undergo confirmatory testing, despite the risk of hepatitis B reactivation during IS. We thus propose a risk-based algorithm for interpretation and monitoring of hepatitis B testing in immunocompromised children.
Keywords: Hepatitis B, virus reactivation, immunocompromised, IVIg
Introduction
The use of intravenous immunoglobulin (IVIg) therapy has become increasingly common in the pediatric population. It is routinely prescribed in a variety of conditions including as replacement therapy for children with primary and acquired immunodeficiency syndromes1,2. Importantly, IVIg is being increasingly utilized in severely immunocompromised patients, including those undergoing hematopoietic stem cell transplantation (HSCT) where it is used as replacement prior to B cell engraftment and occasionally as an adjunctive therapy for graft versus host disease3.
IVIg is a blood product produced from pooled plasma recovered from a range of 15,000–60,000 donors per lot2. IVIg preparations are screened for transmissible infectious agents, including hepatitis B virus (HBV). HBV screening is performed by evaluating for hepatitis B surface antigen (HBsAg) along with nucleic acid testing2. While HBsAg can confirm hepatitis B infection, hepatitis B core antibody (anti-HBc) and hepatitis B surface antibody (anti-HBs) are used to differentiate between acute, chronic, and resolving infections4,5. Universal screening for anti-HBc is not performed on plasma donors or IVIg preparations produced in the United States, as it alone does not confer an infectious risk or distinguish between different stages of infection5. The passive transfer of anti-HBc from IVIg has been described in the adult population6, however, there is a paucity of data in the pediatric population, particularly with respect to immunocompromised patients7.
Failure to fully evaluate an immunocompromised child who has a positive anti-HBc test can have significant consequences. Reactivation of hepatitis B infection is reported in 20–50% of patients receiving immunosuppression (IS) or cancer chemotherapy, leading to hepatitis and sometimes fulminant liver failure5. Anti-viral prophylaxis effectively reduces the risk of hepatitis B reactivation in patients who undergo IS8, thus careful diagnostic evaluation of high-risk patients is essential to prevent hepatitis B-related morbidity and mortality9.
In 2017, we identified a case of HBV reactivation in a patient following allogeneic HSCT who was anti-HBc positive but HBsAg negative pre-transplantation. One year following HSCT, routine repeat testing revealed persistently positive anti-HBc and evidence of acute hepatitis with elevated transaminases (AST 202 U/L, ALT 209 U/L). In addition, she had converted to HBsAg positive status with a hepatitis B viral load of >170,000,000. This case prompted a quality improvement initiative, the goals of which were to (1) define the current practice of evaluating immunocompromised patients with positive anti-HBc tests at our institution, and (2) develop a risk-based algorithm to standardize evaluation and management of these patients. To inform our study, we reviewed the medical records of all patients who tested positive for anti-HBc during an 18-month period. Based on our findings, we propose a standardized approach to the evaluation of pediatric patients that are anti-HBc positive who are immunocompromised or planning to receive IS. This approach is aimed at providing clinicians with a tool for risk stratification, guidance on additional testing, and management decisions regarding hepatitis B infection.
Methods
All patients at our institution who had a positive anti-HBc test between April 2016 and October 2017 were eligible for inclusion once during the study period on the date of the index positive test. Patients with positive anti-HBc tests were identified from a list of all patients, including ambulatory and inpatients, who had anti-HBc testing performed using the Abbott ARCHITECT CORE and CORE-M kits (Abbott Diagnostics, Germany) in the Infectious Disease Diagnostics Laboratory. Baseline demographic and clinical characteristics, comorbid medical conditions, history of IVIg exposure, and results of all hepatitis B testing were abstracted from the electronic medical record using a standardized data collection form.
Interpretation of positive anti-HBc tests was categorized based on medical record review. Categories included 1. Resolved infection: defined as patients documented to have history of hepatitis B infection but who are now HBsAg negative, anti-HBc positive, and anti-HBs positive; 2. Chronic infection: defined as persistently positive HBsAg with positive total anti-HBc and negative anti-HBc IgM10; 3. IVIg-related: defined as a positive anti-HBc test in a patient who received IVIg in the preceding 4 months7 and did not meet criteria for resolved or chronic infection; and 4. Unspecificed: defined as a patient who had a positive anti-HBc test without any clear risk factors and in the absence of IVIg administration, and with documentation of follow up negative anti-HBc testing (Fig 1a). Our analysis was limited to descriptive statistics with continuous variables summarized using medians and interquartile ranges and categorical variables summarized as frequencies and percentages. All analyses were performed using Stata, version 14 (StataCorp, College Station, TX).
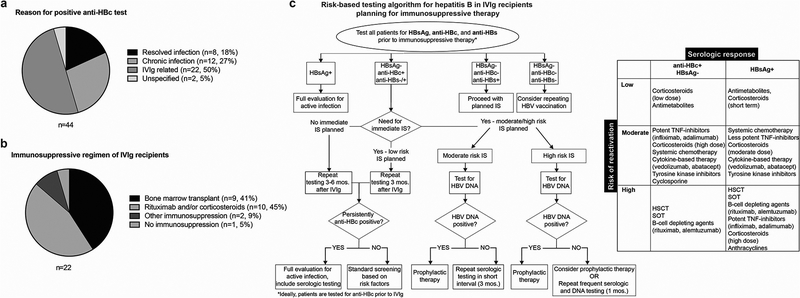
Figure 1. Characteristics of anti-HBc positive patients and risk-based algorithm for diagnostic evaluation prior to immunosuppression.
(a) The reason for positive anti-HBc tests in all patients included in the study. Categorization was determined by review of the medical record of each anti-HBc positive patient. (b) Immunosuppressive regimens administered to patients with positive anti-HBc tests who had received IVIg in the preceding 4 months. (c) The diagnostic algorithm implemented at our institution to risk-stratify patients based on likelihood of hepatitis B reactivation. The algorithm accounts for history of IVIg therapy and risk of hepatitis B reactivation based on planned immunosuppressive therapy. Recommendations are included for full diagnostic evaluations prior to initiation of immunosuppression. Interval re-testing is recommended based timing of IVIg therapy and risk of reactivation during immunosuppression. Table shows immunosuppressive regimens categorized by associated risk of hepatitis B reactivation. Risk stratification (table) reflects that patients with positive HBsAg tests are at highest risk for reactivation.
Results
Over the 18-month study period, a total of 871 patients were tested for total anti-HBc antibody at our institution, and 44 patients were positive (5%). Of these 44 positive patients, 26 (59%) were male and they ranged in age from 2 months to 23 years (median age 15 years, IQR of 5.9 – 17.6 years). IVIg recipients constituted 22 (50%) of the cohort, with the remaining 22 (50%) being documented to have chronic, resolved, or unspecified hepatitis B infection (Fig 1a).
Of the 22 IVIg recipients, the majority were either immunocompromised or were planning for IS (Fig 1b). The highest risk of hepatitis B reactivation occurs in patients receiving HSCT, SOT, or IS containing corticosteroids or rituximab11. Of the 22 IVIg recipients in our cohort, 9 (41%) had received or were planning for HSCT and 21 (95%) subsequently received IS regimens that included corticosteroids and/or rituximab (Fig 1b).
None of the 22 IVIg recipients were documented to be hepatitis B core IgM antibody or HBsAg positive at the time of anti-HBc detection, although 2 patients (9%) had missing HBsAg values. Three (14%) had documented baseline pre-IVIg testing that was negative for anti-HBc, and 19 (86%) did not have pre-IVIg testing for anti-HBc. Despite the majority subsequently receiving high risk IS, 16 patients (73%) did not undergo repeat testing to distinguish between passive transfer of anti-HBc from IVIg and resolved or chronic hepatitis B infection. Six patients (27%) had repeat testing, and of these only one patient (4.5%) had persistently positive anti-HBc tests, with repeat testing performed 2 weeks after the index positive test, which was four weeks after receipt of IVIg. This patient was subsequently lost to follow up. Notably, out of all IVIg recipients only 2/22 (9%) were tested for HBV DNA and 0/2 were positive. Additionally, 2/22 (9%) were tested for anti-HBe and 0/2 were positive. While these low rates of anti-HBe and HBV DNA testing represent missed diagnostic opportunities, the negative results strengthen our hypothesis that for many pediatric patients anti-HBc antibody is transmitted via IVIg and does not represent true infection.
As previously noted, of the remaining 22/44 (50%) patients with a positive anti-HBc test who did not receive IVIg, 20/22 (90%) were documented to have chronic or resolved hepatitis B infection. Twelve (55%) of these patients also had a positive HBsAg test. Eleven (50%) had a previous diagnosis of chronic hepatitis B (Fig 1a). Only one patient with a positive HBsAg test did not have a prior diagnosis of hepatitis B nor did they have prior positive testing for HBsAg. This patient was diagnosed with chronic hepatitis B infection and treated with pre-emptive entecavir due to planned therapy with adalimumab for severe hidradenitis suppurativa. Two patients (9%) with positive anti-HBc tests were suspected to have either false positive results or passive antibody transfer from non-IVIg blood products (Fig 1a). Of note, HBV DNA results were available for 14/22 patients, eight (57%) of which were positive. Additionally, anti-HBe results were available for 15/22 patients, and three (20%) were positive.
Discussion
Our single center review of pediatric patients who were tested for total anti-HBc revealed a 5% positive test rate. A larger cohort review over a 10-year period at a different pediatric institution yielded a similar rate of 3% for either anti-HBc or HBsAg12. The slightly increased proportion at our institution may represent a referral bias given that we are a national liver referral center and also see a large number of immunocompromised patients.
At our institution, half of the positive anti-HBc tests were attributed to passive acquisition of antibodies from IVIg. Ramsay, et al reported detection of anti-HBc in 46% of adult IVIg recipients known to be anti-HBc negative prior to administration6. Another study showed that 10 out of 10 IVIg lots produced in the US tested positive for anti-HBc7. We performed anti-HBc antibody testing on a lot of our formulary IVIg brand (Gamunex) and found total anti-HBc antibody to be reactive and anti-HBc IgM non-reactive. These data suggest that passive antibody transfer is a likely explanation for pediatric patients with positive anti-HBc test results after receiving IVIg. However, the potential for a true anti-Hbc positive result and associated risk of HBV reactivation must be considered in a patient who is or will become immunosuppressed.
Nearly all of the patients we reviewed who tested positive for anti-HBc after receiving IVIg subsequently received IS, but only a small minority had additional testing to evaluate for hepatitis B infection. Hepatitis B infection is relatively common in the US, and testing prior to IS is recommended for patients at the highest risk of reactivation as determined by hepatitis B risk factors and planned IS regimen8,13. The American Gastroenterological Association (AGA) defines a high-risk group as >10% incidence of reactivation8. The IS regimens that portend the greatest risk of HBV reactivation include B cell depletion, high-dose corticosteroids, SOT or HSCT8,13. Risk assessment in pediatrics differs from adults due to the lower likelihood of exposure to hepatitis B virus in children, however the risk of reactivation associated with IS regimens are similar. At our institution, all patients referred for HSCT or SOT undergo pre-transplant serologic evaluation for hepatitis B infection. However, a standard approach to additional testing in patients with a positive anti-HBc has not been adopted, and our retrospective patient review revealed a number of missed opportunities for appropriate additional diagnostic testing.
We therefore propose a risk-based approach to anti-HBc testing and follow up evaluation in immunocompromised pediatric patients (Fig 1c). This approach recommends testing and management based on initial anti-HBc test results, and incorporates the likely passive transfer of antibodies in patients who have recently received IVIg. While an evaluation of T-cell response to hepatitis B core antigen could distinguish passive acquisition of antibody from true infection, this is not currently a clinically available test, thus longitudinal testing of anti-HBc antibody is indicated following IVIg. The algorithm adapts risk stratification, as defined in the AGA guidelines8, of patients according to IS regimen. Children at highest risk for hepatitis B reactivation are those undergoing HSCT, SOT, or B-cell depleting therapy, and thus should have the most comprehensive evaluation and frequent follow-up testing for hepatitis B reactivation, even if they have recently received IVIg (Fig 1c). Follow-up testing intervals for patients who have received IVIg must be balanced with the timing of planned IS. The AGA recommendations include antiviral prophylaxis with entecavir or lamivudine for patients at high risk of hepatitis B reactivation8, thus our algorithm recommends prophylactic antiviral treatment for pediatric patients with evidence of acute or chronic hepatitis B infection who are undergoing high-risk IS regimens. However, in the more common situation of positive anti-HBc testing and negative HBV DNA testing preceding high risk IS, the benefit of antiviral prophylaxis over interval monitoring for hepatitis B reactivation in high-risk patients receiving IVIg has yet to be defined and should be considered on a case-by-case basis. In line with AGA recommendations, we advise consideration of a serial testing interval of 1–3 months, depending on IS duration and potential risk (Fig 1c). If a patient remains persistently positive for anti-HBc in the absence of IVIg, and/or has HBV nucleic acid detected, immediate antiviral therapy and close clinical and laboratory monitoring are recommended. The ideal duration of prophylaxis remains unclear however we recommend a minimum of 6 months after discontinuation of immunosuppressive therapy, and at least 12 months for B-cell depleting agents8. This algorithm has been instituted at the Children’s Hospital of Philadelphia and will be evaluated prospectively to determine impact on clinical outcomes.
Conclusion
Our single-center retrospective review identified 44 pediatric patients with positive total anti-HBc tests during an 18-month period. Positive anti-HBc tests were attributed to passive transfer of antibodies from IVIg in 50% of cases. Subsequent evaluation for hepatitis B infection was infrequent despite administration of immunosuppressive therapy to the majority (95%) of these patients. While our study is limited by a small sample size and a lack of long-term follow up to determine the true rate of hepatitis B reactivation, our findings confirm and highlight the difficult and inconsistent interpretation of hepatitis B serologic testing in immunocompromised children who have received IVIg. Our algorithm provides a starting point to risk-stratify patients based on likelihood of hepatitis B reactivation determined by IS regimen, which will aid in the long-term management of medically complex pediatric patients.
Acknowledgements
This work was supported by NIH grant K08 CA212299 awarded to A. M. Green. The authors thank members of the Division of Infectious Diseases at the Children’s Hospital of Philadelphia, in particular Dr. Kathleen Chiotos and Dr. Brian T. Fisher, for their thoughtful evaluation of this manuscript.
References
- 1.Krivan G et al. New insights in the use of immunoglobulins for the management of immune deficiency (PID) patients. Am J Clin Exp Immunol 6, 76–83, (2017). [PMC free article] [PubMed] [Google Scholar]
- 2.Perez EE et al. Update on the use of immunoglobulin in human disease: A review of evidence. J Allergy Clin Immunol 139, S1–S46 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Jolles S, Sewell WA & Misbah SA Clinical uses of intravenous immunoglobulin. Clin Exp Immunol 142, 1–11, (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinbaum CM et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep 57, 1–20 (2008). [PubMed] [Google Scholar]
- 5.Lok AS & McMahon BJ Chronic hepatitis B: update 2009. Hepatology 50, 661–662 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Ramsay I et al. Transmission of Hepatitis B Core Antibody and Galactomannan Enzyme Immunoassay Positivity via Immunoglobulin Products: A Comprehensive Analysis. Clin Infect Dis 63, 57–63 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Thibault V, Pinte L, Vergez J, Leger JM & Liou A Too Often Forgotten: Passive Transfer of Antibodies. Clin Infect Dis 63, 709–710 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Reddy KR et al. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 148, 215–219; quiz e216–217 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Doo EC, Hoofnagle JH & Rodgers GP NIH consensus development conference: management of Hepatitis B. Introduction. Hepatology 49, S1–32009). [DOI] [PubMed] [Google Scholar]
- 10.Krajden M, McNabb G & Petric M The laboratory diagnosis of hepatitis B virus. Can J Infect Dis Med Microbiol 16, 65–72, (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bessone F & Dirchwolf M Management of hepatitis B reactivation in immunosuppressed patients: An update on current recommendations. World J Hepatol 8, 385–394, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung DH et al. Unrecognized hepatitis B in pre-screened children with hematologic and oncologic conditions. Pediatr Blood Cancer 61, 865–868 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Loomba R & Liang TJ Hepatitis B Reactivation Associated With Immune Suppressive and Biological Modifier Therapies: Current Concepts, Management Strategies, and Future Directions. Gastroenterology 152, 1297–1309, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]