ABSTRACT
Background
Limited nationally representative data are available on dietary supplement (DS) use and resulting nutrient exposures among infants and toddlers.
Objective
This study evaluated DS use among US infants and toddlers to characterize DS use, estimate nutrient intake from DSs, and assess trends in DS use over time.
Methods
Using nationally representative data from NHANES (2007–2014) and trends over time (1999–2014), we estimated prevalence of DS use and types of products used for US infants and toddlers aged <2 y (n = 2823). We estimated median daily intakes of vitamins and minerals consumed via DSs for all participants aged <2 y, by age groups (0–11.9 mo and 12.0–23.9 mo), and by feeding practices for infants 0–5.9 mo.
Results
Overall, 18.2% (95% CI: 16.2%, 20.3%) of infants and toddlers used ≥1 DS in the past 30 d. Use was lower among infants (0–5.9 mo: 14.6%; 95% CI: 11.5%, 18.1%; 6–11.9 mo: 11.6%; 95% CI: 8.8%, 15.0%) than among toddlers (12–23.9 mo: 23.3%; 95% CI: 20.4%, 26.3%). The most commonly reported DSs were vitamin D and multivitamin infant drops for those <12 mo, and chewable multivitamin products for toddlers (12–23.9 mo). The nutrients most frequently consumed from DSs were vitamins D, A, C, and E for those <2 y; for infants <6 mo, a higher percentage of those fed breast milk than those fed formula consumed these nutrients via DSs. DS use remained steady for infants (6–11.9 mo) and toddlers from 1999–2002 to 2011–2014, but increased from 7% to 20% for infants aged 0–5.9 mo.
Conclusions
One in 5 infants and toddlers aged <2 y use ≥1 DS. Future studies should examine total nutrient intake from foods, beverages, and DSs to evaluate nutrient adequacy overall and by nutrient source.
Keywords: NHANES, dietary supplements, infants, toddlers, vitamins, vitamin D, breast milk, infant formula
Introduction
Optimal nutrition is essential for the healthy growth and development of infants and young children, especially during the critical period of rapid growth from birth to 24 mo. Previous editions of the Dietary Guidelines for Americans (DGA) have not focused on this age group, largely because of a lack of high-quality data (1). However, the 2014 Farm Bill mandates the inclusion of infants and toddlers aged <2 y in the 2020–2025 DGA (2). Information on the prevalence of dietary supplement (DS) use in the United States, particularly among infants, has been identified as a data need to help inform the DGA (1).
Although the DGA are food-based and intended to be met through the consumption of foods and beverages, DSs may be beneficial for certain subgroups of the population to help them obtain adequate amounts of key nutrients. For example, the American Academy of Pediatrics (AAP) recommends a vitamin K supplement immediately after birth for all infants, vitamin D supplementation for all breastfed and partially breastfed infants (3), and iron supplementation beginning around 4 mo for healthy infants born at term who are either fully or partially breastfed (4). Furthermore, it is important to monitor the population to assess the sources of nutrients in diets in order to help inform policy. It is also important to include nutrients from DSs in evaluating the diets of infants and toddlers, because excluding nutrients from DSs when estimating nutrient intake could overestimate nutrient inadequacy and underestimate nutrient excess in the United States (5, 6). The NHANES collects data on dietary intake from foods, beverages, and DSs, providing the opportunity to estimate nutrient intake from all sources for the US population, including infants and toddlers aged 0–23.9 mo.
In this article we characterize the use of DSs and the nutrient contributions from DSs, and examine time trends in DS use among US infants and toddlers (aged <2 y) using the most recently available national data from the NHANES survey. This information would be useful to inform the forthcoming DGA Committee, for the development of nutritional guidance and for informing other clinical and research applications for infants and toddlers aged <2 y.
Methods
Study design
NHANES is a nationally representative cross-sectional survey of the civilian, noninstitutionalized US population, conducted by the CDC's National Center for Health Statistics (NCHS) and is not a clinical trial and therefore does not need to be registered. NHANES monitors the country's health and nutritional status through an in-home interview, followed by a standardized physical examination at a specially designed mobile examination center. Study participants are selected using a complex, multistage probability sampling design. Survey designs are described in detail elsewhere (7, 8). To increase the precision of estimates, NHANES oversampled certain subgroups such as Hispanic individuals, non-Hispanic blacks, non-Hispanic non-black Asians, low-income non-Hispanic whites, and others, in various survey cycles. The NCHS Research Ethics Review board approved the NHANES’ protocol. Written parental consent was obtained for all participants aged <18 y. A parent or proxy provided sociodemographic information for children aged <16 y. All estimates provided for this report were collected via interviewer-administered questionnaires that were answered by a proxy, in most cases the mother. Response rates for infants <1 y and children 1–5 y participating in the home interview were 92% and 88% in 2007–2008; 89% and 90% in 2009–2010; 86% and 82% in 2011–2012; and 82% and 79% in 2013–2014, respectively (9–12).
DS use
Information on DS use was collected as part of the in-home interview by an interviewer who recorded information from DS labels for products used in the past 30 d, combined with the Dietary Supplement Questionnaire that collects information on the consumption frequency, duration, amount taken, and motivations for taking each product. The following gate question is asked to the proxy of the participant: “Has [CHILD] used or taken any vitamins, minerals, herbals or other dietary supplements in the past 30 days? Include prescription and non-prescription supplements.” Although we recognize that the participant did not choose to take a DS, “DS users” throughout this article is defined as those who, via proxy, reported taking or using ≥1 DS in the past 30 d. Types of products were classified according to the categorization scheme provided in Supplemental Table 1. Average daily intake was estimated by multiplying the number of days the DS was taken by the dosage usually consumed, divided by 30 d. Nutrients were converted to units consistent with the DRIs and current US FDA label regulations (13, 14). Please see Supplemental Table 2 for conversion factors.
Motivations for use were based on a categorical question in which participants, via proxy, were shown a hand card with the categories listing various motivations to choose from. If the motivations were not on the hand card, additional motivations could be reported. Participants were also asked if they took the DS for their own reasons or if the DS was recommended by a doctor or another health professional. Frequency of DS use was categorized as 1–14, 15–29, and 30 d. Further details on the NHANES DS component can be found elsewhere (15–18).
Covariates
Demographic variables included participant's age at the time of the screening interview (0–5.9, 6–11.9, and 12–23.9 mo), sex (boys and girls), race, and Hispanic origin (non-Hispanic white, non-Hispanic black, non-Hispanic Asian, and Hispanic). Participants who were proxy-identified as other or multiracial were included in overall estimates but are not reported separately because the findings relate to a very small and heterogeneous group.
Three dimensions of socioeconomic status were examined: 1) education level of the head-of-household (less than high school or high school degree/General Equivalency Diploma, and greater than high school education); 2) family poverty-to-income ratio (PIR) (<130%, 130–349%, and ≥350%); and 3) eligibility for, and receipt of food benefits from, the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) (WIC recipient, WIC eligible but not receiving WIC, WIC income ineligible). The PIR is an index calculated from family income divided by a federal poverty guideline specific to family size and location. The PIR is used to assess eligibility for federal nutrition programs like the Supplemental Nutrition Assistance Program (<130%), WIC (<185%), and free and reduced meals in the National School Lunch and Breakfast programs (19). Cutoffs were chosen to harmonize with previous reports that provide estimates of nutrient intake among US infants and toddlers (20, 21).
Feeding practices and breastfeeding status
DS use was also examined by feeding practices and breastfeeding status. It is informative to assess DS use by these covariates since recommendations exist by feeding status [the AAP recommends vitamin D supplementation for all breastfed infants (3)]. Therefore, national estimates are important in order to monitor the prevalence of the population that are following guidelines. Feeding practices were defined based on the reported consumption of breast milk, formula, breast milk and formula, or neither breast milk nor formula from the Diet Behavior and Nutrition Questionnaire. It is important to note that infants and toddlers could have consumed other milks (including cow milk), solids, juice, and water as well. DS use by breastfeeding status was also assessed. This variable included 3 categories: still breastfeeding, ever breastfed, or never breastfed and includes infants that were breastfed or fed breast milk.
Analytic sample
Data from four 2-y cycles of NHANES were combined for most of the analyses: NHANES 2007–2008, 2009–2010, 2011–2012, and 2013–2014. For the analysis of trends in DS use over time, data from previous cycles of NHANES were also used: NHANES 1999–2000, 2001–2002, 2003–2004, and 2005–2006. Please see the flow chart provided as Supplemental Figure 1 for overall exclusions. The analytic sample included boys and girls aged 0–<24 mo whose parents, family members, or caretakers participated in the household interview as their proxies. One child was excluded from the analysis because use of any DS in the past 30 d was unknown. However, respondents whose answers contained missing values for covariates such as PIR (n = 229 missing values), education (n = 686 missing values), WIC eligibility (n = 290 missing values), and feeding practices or breastfeeding status (n = 4 missing values) were not excluded globally in the final analytic sample of 2823. Where applicable, item nonresponse is indicated by a footnote.
Statistical analysis
All analyses were performed with SAS-callable SUDAAN, version 9.4, to account for the complex survey design. Interview survey weights were used to account for oversampling, survey nonresponse, and poststratification. Prevalence estimates were provided for any DS use, types of DSs used, frequency of use in the past 30 d, and the most common motivations for using DSs. The distribution of average daily intakes was highly positively skewed; therefore medians and IQRs (IQR 25th and 75th percentiles) were estimated for daily intake of selected nutrients. Variance estimates were calculated using the Taylor linearization. CIs were constructed using the Clopper–Pearson method, as recommended by the NCHS (22). Differences between nominal groups were tested using a univariate t statistic at the P < 0.05 significance level. Orthogonal polynomials were used to test for linear and quadratic trends by age group, by PIR within each age group, and over time (1999–2014; single survey cycles were tested but 4-y estimates are shown in Figure 1). Adjustments were not made for multiple comparisons. The NCHS Data Presentation Standards for Proportions were used to determine if estimates were reliable (22).
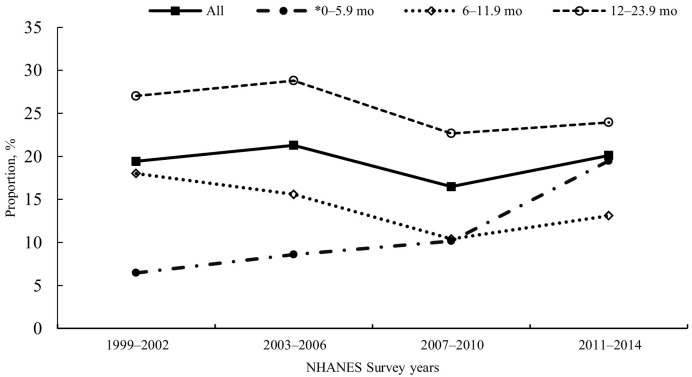
FIGURE 1.
Prevalence of US infants and toddlers aged 0–23.9 mo who reported consuming any dietary supplement in the past 30 d, by age at interview and survey cycle, NHANES 1999–2014. Source: CDC/National Center for Health Statistics, NHANES 1999–2014. *Significant increasing linear trend by survey cycle, P < 0.05.
Results
A total of 2823 infants and toddlers from 0–23.9 mo of age from the NHANES 2007–2014 surveys were included in this analysis. NHANES 2011–2014 captured 99 non-Hispanic Asian infants and toddlers, aged 0–23.9 mo, reflecting the oversampling of Asian individuals that began in NHANES in 2011. Approximately 27% (95% CI: 17.8%, 37.0%) of non-Hispanic Asian infants and toddlers aged <2 y consumed ≥1 DS, but the limited sample size in this group precluded further analysis.
Characteristics associated with DS use
Overall, 18.2% (95% CI: 16.2%, 20.3%) of infants and toddlers aged <2 y used ≥1 DS in the past 30 d (Table 1). Reported use among boys and girls was similar (19.2%; 95% CI: 16.6%, 22.0% compared with 17.1%; 95% CI: 14.7%, 19.8%). Most users reported taking 1 DS (93.5%) (data not shown). For those taking ≥1 DS, frequency of use over the past month varied; 53.0% reported taking the DS every day, 26.5% reported taking the DS 15–29 d out of 30 d, and 20.5% reported taking the DS 1–14 d out of 30 d (data not shown). DS use was higher among toddlers aged 12–23.9 mo (23.3%; 95% CI: 20.4%, 26.3%) than among young infants aged 0–5.9 mo (14.6%; 95% CI: 11.5%, 18.1%) and older infants aged 6–11.9 mo (11.6%; 95% CI: 8.8%, 15.0%). Among infants and toddlers aged 0–23.9 mo, a higher percentage of non-Hispanic whites (20.5%; 95% CI: 17.4%, 23.9%) consumed ≥1 DS than non-Hispanic blacks (13.3%; 95% CI: 10.3%, 16.7%) and Hispanics (15.0%; 95% CI: 12.2%, 18.3%). A similar pattern of difference by race and Hispanic origin group was seen for infants aged 0–5.9 mo and toddlers aged 12–23.9 mo, but the differences were nonsignificant for infants aged 6–11.9 mo.
TABLE 1.
Prevalence of reported use of any supplement in the past 30 d, by age and characteristics of US infants and toddlers aged 0–23.9 mo, NHANES 2007–20141
| 0–5.9 mo | 6–11.9 mo | 12–23.9 mo | 0–23.9 mo | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | |
| All2 | 863 | 14.6 | (11.5, 18.1) | 845 | 11.6 | (8.8, 15.0) | 1115 | 23.3 | (20.4, 26.3) | 2823 | 18.2 | (16.2, 20.3) |
| Gender | ||||||||||||
| Boys2 | 426 | 13.0 | (9.0, 18.0) | 421 | 13.7 | (9.7, 18.5) | 573 | 25.3 | (21.6, 29.2) | 1420 | 19.2 | (16.6, 22.0) |
| Girls2 | 437 | 16.3 | (11.9, 21.5) | 424 | 9.6 | (6.5, 13.5) | 542 | 21.3 | (17.1, 25.9) | 1403 | 17.1 | (14.7, 19.8) |
| Race and Hispanic origin3 | ||||||||||||
| Non-Hispanic white2 | 289 | 16.9a | (12.4, 22.1) | 281 | 12.7 | (7.9, 19.0) | 320 | 26.6a | (21.6, 32.0) | 890 | 20.5a | (17.4, 23.9) |
| Non-Hispanic black4 | 162 | 9.5b | (4.7, 16.6) | 145 | 8.9 | (4.3, 15.7) | 235 | 17.5b | (12.9, 23.0) | 542 | 13.3b | (10.3, 16.7) |
| Hispanic2 | 336 | 11.0b | (7.0, 16.0) | 348 | 9.8 | (6.8, 13.5) | 443 | 19.4b | (15.7, 23.5) | 1127 | 15.0b | (12.2, 18.3) |
| PIR | ||||||||||||
| <1302 | 391 | 9.26 | (5.8, 13.6) | 390 | 7.26 | (4.5, 10.8) | 528 | 14.96 | (11.2, 19.2) | 1309 | 11.56 | (9.1, 14.3) |
| 130–3492 | 254 | 14.5 | (9.3, 21.1) | 252 | 10.7 | (7.1, 15.2) | 314 | 27.1 | (21.6, 33.1) | 820 | 19.7 | (16.1, 23.8) |
| ≥350 | 146 | 21.4 | (13.4, 31.2) | 129 | 18.4 | (10.3, 29.3) | 190 | 29.9 | (22.5, 38.1) | 465 | 25.1 | (20.6, 30.1) |
| WIC eligibility | ||||||||||||
| WIC recipient2 | 516 | 9.1a | (6.3, 12.5) | 535 | 6.2a | (4.1, 8.9) | 561 | 16.8a | (13.5, 20.5) | 1612 | 11.6a | (9.8, 13.5) |
| WIC eligible, not receiving WIC | 50 | — | — | 31 | — | — | 93 | — | — | 174 | 18.8a,b | (10.2, 30.4) |
| WIC income ineligible4 | 217 | 20.0b | (13.8, 27.5) | 206 | 19.2b | (13.1, 26.6) | 324 | 31.2b | (25.9, 36.9) | 747 | 25.9b | (22.3, 29.7) |
| Head-of-household educational attainment | ||||||||||||
| High school degree or less2 | 327 | 7.4a | (4.4, 11.5) | 358 | 6.3a | (3.4, 10.4) | 460 | 16.5a | (12.7, 20.9) | 1145 | 11.7a | (9.6, 14.1) |
| More than a high school degree2 | 304 | 17.7b | (12.5, 23.9) | 284 | 14.6b | (9.9, 20.5) | 404 | 28.5b | (24.1, 33.1) | 992 | 22.5b | (19.3, 25.9) |
| Feeding practice5 | ||||||||||||
| Breast milk, no formula | 188 | 24.8a | (17.7, 33.0) | 98 | 29.1a | (17.3, 43.5) | 73 | — | — | 359 | 30.3a | (23.2, 38.2) |
| Formula, no breast milk4 | 494 | 6.6b | (4.4, 9.5) | 623 | 8.8b | (5.7, 12.8) | 82 | 16.7 | (7.8, 29.5) | 1199 | 8.7b | (6.5, 11.4) |
| Breast milk and formula | 179 | 23.9a | (15.9, 33.6) | 83 | — | — | 11 | — | — | 273 | 19.3c | (13.4, 26.4) |
| Solids (no breast milk or formula) | 2 | — | — | 41 | — | — | 949 | 22.3 | (19.2, 25.6) | 992 | 21.9c | (18.9, 25.1) |
| Breastfeeding status | ||||||||||||
| Still breastfeeding | 335 | 24.7a | (19.0, 31.5) | 176 | 20.8a | (14.0, 29.0) | 82 | — | — | 593 | 26.7a | (21.8, 32.0) |
| Ever breastfed2 | 310 | 9.9b | (6.5, 14.2) | 459 | 11.4b | (7.7, 16.0) | 711 | 24.2a | (20.1, 28.7) | 1480 | 18.8b | (16.0, 21.9) |
| Never breastfed | 218 | — | — | 209 | — | — | 319 | 15.6b | (10.6, 21.9) | 746 | 9.9c | (7.1, 13.3) |
Source: CDC/NCHS, NHANES 2007–2014. Estimates are percentages and 95% CIs. Labeled percentages, within a column and by covariate, without a common letter are significantly different (P < 0.05). Missing for 0–5 mo: PIR, n = 72; education, n = 232; WIC eligibility, n = 80. Missing for 6–11 mo: breastfeeding status, n = 1; PIR, n = 74; education, n = 203; WIC eligibility, n = 73. Missing for 12–23 mo: breastfeeding status, n = 3; PIR, n = 83; education, n = 251; WIC eligibility, n = 137. Differences were tested using a univariate t statistic, significance was set at P < 0.05. Estimates with — are suppressed and do not meet NCHS data presentation standards (22). NCHS, National Center for Health Statistics; PIR, poverty-to-income ratio; WIC, Special Supplemental Nutrition Program for Women, Infants, and Children.
Significant quadratic trend by age group (P < 0.05).
Other race/Hispanic origin included in totals but not shown separately.
Significant linear trend by age group (P < 0.05).
The variable “Feeding Practice” was derived using 5 questions from the Diet Behavior and Nutrition Questionnaire: “Was [CHILD] ever breastfed or fed breastmilk?,” “How old was [CHILD] when he/she completely stopped breastfeeding or being fed breastmilk?,” “How old was [CHILD] when he/she was first fed formula?,” “How old was [CHILD] when he/she completely stopped drinking formula?,” and “How old was [CHILD] when he/she was first fed anything other than breast milk or formula?” Participants in all 4 categories could have consumed juice; water; other milks, including cow milk; and solids.
Significant linear trend by PIR, within each age group (P < 0.05).
Overall DS use increased with increasing PIR for all infants and toddlers aged 0–23.9 mo, and also within each age group. For example, 11.5% (95% CI: 9.1%, 14.3%), 19.7% (95% CI: 16.1%, 23.8%), and 25.1% (95% CI: 20.6%, 30.1%) of infants and toddlers 0–23.9 mo consumed DSs, by PIR <130, 130–349, and ≥350, respectively. Among infants and toddlers, those from families in the lower household income groups who were WIC recipients had a lower use of DSs than the WIC income-ineligible group. Overall and within each age group, DS use was higher for children from families where the head-of-household had more than high school education as compared with those with high school degree or less.
DS use also differed by feeding practice and breastfeeding status. Among infants aged 0–5.9 mo, a higher percentage of those consuming only breast milk (24.8%; 95% CI: 17.7%, 33.0%) or breast milk with formula (23.9%; 95% CI: 15.9%, 33.6%) consumed a DS, than of infants only consuming formula (6.6%; 95% CI: 4.4%, 9.5%). The feeding practice of consuming both breast milk and formula was uncommon among infants aged >5.9 mo and therefore no additional comparisons for this feeding practice were made within other age groups. It is important to remember that infants could have also been consuming juice, water, other milks (including cow milk), and solids. Among infants aged 6–11.9 mo, the percentage consuming a DS was higher among those who consumed breast milk (and no formula) (29.1%; 95% CI: 17.3%, 43.5%) than among those who consumed formula (and no breast milk) (8.8%; 95% CI: 5.7%, 12.8%). Overall and by age group, a higher percentage of infants and toddlers who were still breastfeeding consumed a DS than of those who were ever breastfed or never breastfed (where comparisons were possible). For example, among infants and toddlers aged 0–23.9 mo, 26.7% (95% CI: 21.8%, 32.0%) of those still breastfeeding used a DS, compared with 18.8% (95% CI: 16.0%, 21.9%) of those who had ever breastfed and 9.9% (95% CI: 7.1%, 13.3%) of those who had never breastfed (Table 1).
Whereas the prevalence of use of any DS remained steady from 1999 to 2014 for all infants and toddlers aged 0–23.9 mo, use increased significantly for younger infants aged 0–5.9 mo: from 6.5% (95% CI: 4.3%, 9.3%) (NHANES 1999–2002) to 19.5% (95% CI: 14.3%, 25.5%) (NHANES 2011–2014) (Figure 1). This increase in part is due to the increase in vitamin D supplements for infants aged 0–5.9 mo. For the study period 1999–2014, only n = 58 (1.15%; 95% CI: 0.74%, 1.70%) of infants and toddlers aged 0–23.9 mo reported consuming a vitamin D supplement. Of these, virtually all (n = 54) reported use during the 2011–2014 survey cycle.
Types of DSs used
The most commonly reported types of DS products for those aged 0–5.9 mo were vitamin D–only products (drops) (9.8%; 95% CI: 5.5%, 15.8%) and infant multivitamin drops (8.4%; 95% CI: 6.3%, 10.9%), including those containing vitamins A, C, and D (3.4%; 95% CI: 1.9%, 5.6%) and drops containing ≥8 vitamins (4.9%; 95% CI: 3.3%, 7.0%) (Table 2). It is important to note that the estimates for vitamin D–only products were from NHANES 2011–2014 only, because <1% of proxies reported vitamin D–only products during 2007–2010. For all other products, usage remained fairly stable between 2007–2010 and 2011–2014; therefore, estimates for other products were based on NHANES 2007–2014 data in order to provide more reliable estimates. For those aged 6–11.9 mo, 5.6% (95% CI: 2.8%, 9.8%) reported vitamin D–only supplements (drops) and 5.6% (95% CI: 3.6%, 8.3%) reported infant vitamin drops; 2.5% (95% CI: 1.2%, 4.7%) reported infant vitamin drops containing vitamins A, C, and D and 3.2% (95% CI: 2.1%, 4.6%) reported those containing ≥8 vitamins. Single-vitamin products were also reported in this age group, although this was uncommon (1.7%; 95% CI: 0.7%, 3.3%). For toddlers aged 12–23.9 mo, a wider variety of products were taken. Use of infant vitamin drops was still reported in this older age group (6.1%; 95% CI: 4.5%, 8.0%); 2.0% (95% CI: 1.0%, 3.6%) reported products containing vitamins A, C, and D and 4.1% (95% CI: 2.8%, 5.7%) reported infant drops containing ≥8 vitamins. In addition, chewable multivitamin-mineral products were reported by 11.4% (95% CI: 9.3%, 13.8%) of toddlers aged 12–23.9 mo, whereas 3.0% (95% CI: 1.7%, 4.9%) reported single-nutrient products (e.g., iron-only products, fluoride-only products) and 1.7% reported other types of products (e.g., probiotics, n–3 fatty acid products).
TABLE 2.
Prevalence of dietary supplement products reported for US infants and toddlers aged 0–23.9 mo, by age group, NHANES 2007–20141
| 0–5.9 mo | 6–11.9 mo | 12–23.9 mo | 0–23.9 mo | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | |
| Single-nutrient products | ||||||||||||
| Vitamin D only2 (NHANES 2011–2014 only) | 405 | 9.8 | (5.5, 15.8) | 392 | 5.6 | (2.8, 9.8) | 488 | 1.3 | (0.3, 3.9) | 1285 | 4.6 | (2.9, 6.9) |
| Single-nutrient products (excluding vitamin D–only products) | 863 | 0.6 | (0.1, 1.5) | 845 | 1.7 | (0.7, 3.3) | 1115 | 3.0 | (1.7, 4.9) | 2823 | 2.1 | (1.3, 3.1) |
| Multinutrient products | ||||||||||||
| Infant drops | 863 | 8.4 | (6.3, 10.9) | 845 | 5.6 | (3.6, 8.3) | 1115 | 6.1 | (4.5, 8.0) | 2023 | 6.5 | (5.5, 7.8) |
| Products containing vitamins A, C, and D3 | 863 | 3.4 | (1.9, 5.6) | 845 | 2.5 | (1.2, 4.7) | 1115 | 2.0 | (1.0, 3.6) | 2823 | 2.5 | (1.8, 3.5) |
| Products containing ≥8 vitamins3,4 | 863 | 4.9 | (3.3, 7.0) | 845 | 3.2 | (2.1, 4.6) | 1115 | 4.1 | (2.8, 5.7) | 2823 | 4.1 | (3.2, 5.1) |
| Tablet, softgel, chew, capsule | ||||||||||||
| Multivitamin minerals, multivitamins | — | — | — | 845 | 0.4 | (0.1, 1.1) | 1115 | 11.4 | (9.3, 13.8) | 2823 | 5.8 | (4.7, 7.0) |
| Other products5 | 863 | 1.6 | (0.8, 3.0) | 845 | 0.9 | (0.2, 2.8) | 1115 | 1.7 | (0.9, 2.9) | 2823 | 1.5 | (0.9, 2.2) |
Source: CDC/NCHS, NHANES 2007–2014. Estimates are percentages and 95% CIs. Respondents may have taken >1 type of product. Please see Supplemental Table 1 for the categorization scheme. Estimates with — are suppressed and do not meet NCHS data presentation standards (22). NCHS, National Center for Health Statistics.
Estimate based on data from 2011–2014 only, therefore n is smaller; vitamin D use was <1% during 2007–2010. All vitamin D–only products were infant drops.
This estimate includes products that may or may not contain iron and/or fluoride.
Vitamins included: A, C, D, E, may include B-12, B-6, niacin, riboflavin, and thiamin.
Other includes: for infants 0–5 mo: iron-only products, products to soothe digestive issues, and unknown products; for infants 6–11 mo: chewable multivitamin/mineral products, probiotics, fiber, botanical, and unknown products; for toddlers 12–23 mo: probiotics, n–3 fatty acids, fiber, botanical, and unknown products.
Nutrient content of DSs used
The nutrients commonly consumed from DSs were vitamin D, vitamin A, vitamin C, and vitamin E (Table 3). The prevalence of use of nutrients varied by age group as follows: vitamin D (range: 10.6–18.2%), vitamin C (6.8–18.3%), and vitamin A (7.1–17.5%) (Table 3). The use of iron-containing DSs was very low among infants aged 0–11.9 mo (1.6%). For those consuming vitamin D or a supplement containing vitamin D, the median average daily intake from supplementation was 6.7 μg/d for infants aged 0–11.9 mo and 4.9 μg/d for toddlers aged 12–23.9 mo. For those consuming vitamin A or a supplement containing vitamin A, the median average daily intake from supplements was 293.0 μg retinol activity equivalent/d for infants aged 0–11.9 mo and 299.9 μg retinol activity equivalent/d for toddlers aged 12–23.9 mo.
TABLE 3.
Prevalence of use of selected nutrient-containing DSs and daily intake for users of selected nutrients, US infants and toddlers aged 0–23.9 mo, NHANES 2007–20141
| Total population prevalence2 (0–11.9 mo)% (95% CI) (n = 1708) | Daily intake of selected nutrients among users of DSs3 (0–11.9 mo) | Total population prevalence2 (12–23.9 mo)% (95% CI) (n = 1115) | Daily intake of selected nutrients among users of DSs3 (12–23.9 mo) | Total population prevalence2 (0–23.9 mo)% (95% CI) (n = 2823) | Daily intake of selected nutrients among users of DSs3 (0–23.9 mo) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nutrient | n | Median | IQR | n | Median | IQR | n | Median | IQR | |||
| Vitamin D [ergocalciferol (D2) and cholecalciferol (D3)], μg | 10.6 (8.7, 12.8)4 | 160 | 6.7 | 2.3–9.6 | 18.2 (15.5, 21.1) | 183 | 4.9 | 2.4–8.9 | 14.4 (12.5, 16.4) | 343 | 5.0 | 2.3–9.6 |
| Vitamin A, μg RAE | 7.1 (5.6, 9.0)4 | 113 | 293 | 99–421 | 17.5 (14.8, 20.4) | 177 | 300 | 173–444 | 12.3 (10.6, 14.1) | 290 | 300 | 131–441 |
| Retinol, μg | 7.1 (5.6, 9.0)4 | 113 | 293 | 99–421 | 17.3 (14.6, 20.2) | 175 | 298 | 166–450 | 12.2 (10.5, 14.0) | 288 | 297 | 143–450 |
| Vitamin C, mg | 6.8 (5.3, 8.5)4 | 110 | 22.6 | 7.7–32.3 | 18.3 (15.9, 21.0) | 185 | 17.0 | 8.9–33.5 | 12.5 (11.0, 14.2) | 295 | 18.8 | 8.0–33.0 |
| Vitamin E, mg tocopherol | 4.3 (3.1, 5.7)4 | 79 | 1.8 | 0.6–3.1 | 15.5 (12.9, 18.4) | 156 | 6.7 | 2.7–8.9 | 9.9 (8.3, 11.6) | 235 | 3.5 | 2.0–8.0 |
| Thiamin (vitamin B-1), mg | 3.5 (2.6, 4.7)4 | 78 | 0.3 | 0.1–0.4 | 8.1 (6.3, 10.2) | 94 | 0.4 | 0.2–0.8 | 5.8 (4.7, 7.1) | 172 | 0.4 | 0.1–0.5 |
| Riboflavin (vitamin B-2), mg | 3.5 (2.6, 4.7)4 | 78 | 0.3 | 0.1–0.5 | 8.2 (6.4, 10.3) | 95 | 0.5 | 0.3–1.0 | 5.8 (4.7, 7.1) | 173 | 0.5 | 0.2–0.6 |
| Niacin, mg | 4.2 (3.1, 5.6)4 | 78 | 4.5 | 1.3–7.3 | 8.7 (6.9, 10.8) | 95 | 7.3 | 2.4–9.5 | 6.4 (5.3, 7.8) | 173 | 5.7 | 2.0–7.9 |
| Vitamin B-6, mg | 3.6 (2.6, 4.8)4 | 79 | 0.2 | 0.1–0.4 | 15.1 (12.4, 18.0) | 156 | 0.4 | 0.2–0.9 | 9.3 (7.7, 11.0) | 235 | 0.4 | 0.2–0.8 |
| Vitamin B-12, μg | 3.1 (2.1, 4.4)4 | 60 | 0.9 | 0.3–1.8 | 14.4 (11.9, 17.2) | 143 | 2.0 | 1.0–3.0 | 8.7 (7.3, 10.4) | 203 | 1.8 | 0.9–2.9 |
| Folic acid, μg | 0.1 (0.0, 0.5) | — | — | — | 10.9 (8.9, 13.3) | 104 | 95.1 | 53.7–165.1 | 5.5 (4.4, 6.7) | 108 | 95.1 | 52.7–166.9 |
| Folate (DFE), μg | 0.1 (0.0, 0.5) | — | — | — | 10.9 (8.9, 13.3) | 104 | 161.8 | 91.4–280.6 | 5.5 (4.4, 6.7) | 108 | 161.8 | 89.7–283.6 |
| Total choline, mg | — | — | — | — | 5.8 (4.2, 7.6) | 64 | 0.4 | 0.05–18.3 | 2.9 (2.1, 3.8) | 65 | 0.4 | 0.05–18.3 |
| Calcium, mg | 0.1 (0.0, 0.4) | — | — | — | 4.1 (2.7, 5.9) | 42 | 16.7 | 7.4–71.8 | 2.1 (1.4, 3.0) | 45 | 16.9 | 7.4–73.1 |
| Magnesium, mg | 0.1 (0.0, 0.3) | — | — | — | 5.0 (3.6, 6.8) | 49 | 3.0 | 0.9–10.0 | 2.5 (1.8, 3.4) | 51 | 3.0 | 0.9–10.1 |
| Iodine, μg | 0.1 (0.0, 0.3) | — | — | — | 4.9 (3.3, 6.8) | 47 | 14.1 | 7.8–20.5 | 2.5 (1.7, 3.5) | 48 | 14.0 | 7.6–20.4 |
| Iron, mg | 1.6 (1.0, 2.4)4 | 30 | 8.3 | 2.9–9.6 | 4.8 (3.4, 6.7) | 56 | 7.2 | 2.7–14.1 | 3.2 (2.4, 4.2) | 86 | 7.4 | 2.7–12.2 |
| Zinc, mg | 0.2 (0.0, 0.5) | — | — | — | 10.7 (8.6, 13.1) | 102 | 1.3 | 0.8–2.4 | 5.4 (4.3, 6.7) | 107 | 1.3 | 0.8–2.5 |
Source: CDC/NCHS, NHANES 2007–2014. Estimates with — are suppressed and do not meet NCHS data presentation standards (22). DFE, dietary folate equivalent; DS, dietary supplement; NCHS, National Center for Health Statistics; RAE, retinol activity equivalent.
Percentages and 95% CIs of any use of a DS containing the indicated nutrient in the total population (users and nonusers).
Median and IQR (25th and 75th quartile) for average daily intake for users of a DS containing the indicated nutrient.
Statistically significant difference between toddlers aged 12–23 mo, P < 0.05.
Value appears as 0.0 because of rounding; actual value for 25th percentile, 0.001 for 0–23.9 mo and 12–23.9 mo.
For young infants aged 0–5.9 mo, a higher percentage of those who consumed breast milk and no formula took ≥1 DS containing vitamin D (24.3%; 95% CI: 17.3%, 32.4%) than of those that consumed formula and no breast milk (5.6%; 95% CI: 3.4%, 8.5%) (Table 4). This pattern was similar for other nutrients. A higher percentage of those fed breast milk than of formula-fed infants consumed vitamins A, C, and E from supplements (Table 4).
TABLE 4.
Prevalence of the use of selected nutrient-containing dietary supplements by breastfeeding status and age group, US infants aged 0–5.9 mo, NHANES 2007–20141
| Nutrient | 0–5.9 mo | ||
|---|---|---|---|
| Breast milk, no formula (n = 188) | Formula, no breast milk (n = 494) | Breast milk and formula (n = 179) | |
| Vitamin D [ergocalciferol (D2) and cholecalciferol (D3)] | 24.3 (17.3, 32.4)* | 5.6 (3.4, 8.5) | 17.2 (10.4, 26.1) |
| Vitamin A2 | 15.2 (10.0, 21.8)* | 4.2 (2.3, 6.9) | 8.8 (4.5, 15.1) |
| Vitamin C | 15.2 (10.0, 21.8)* | 4.2 (2.3, 6.9) | 8.8 (4.5, 15.1) |
| Vitamin E | 7.9 (4.1, 13.6)* | 2.8 (1.4, 5.0) | — |
| Iron | 2.1 (0.5, 5.5) | 1.5 (0.5, 3.3) | 1.5 (0.3, 4.5) |
Source: CDC/NCHS, NHANES 2007–2014. Estimates are percentages (95% CIs). *Statistically significant difference with formula, no breast milk group, P < 0.05. Estimates with — are suppressed and do not meet NCHS data presentation standards (22). NCHS, National Center for Health Statistics.
All vitamin A in this age group contained retinol.
Reasons for taking DSs
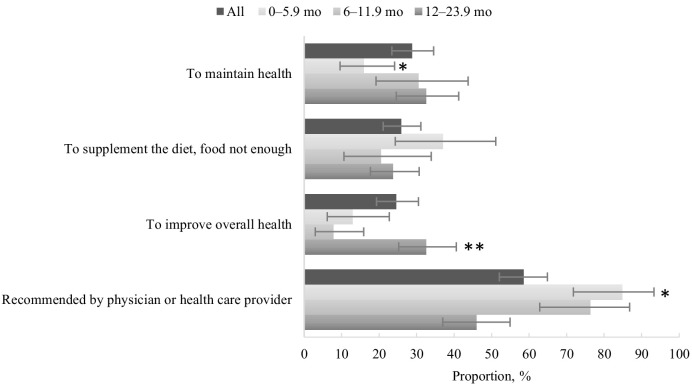
The major reasons reported for taking products, among infants and toddlers that reported using ≥1 DS, were to “maintain health” (28.8%; 95% CI: 23.4%, 34.5%), “supplement the diet, food not enough” (25.9%; 95% CI: 21.1%, 31.3%), and “improve overall health” (24.6%, 95% CI: 19.3%, 30.5%) (Figure 2). Over half (58.6%; 95% CI: 52.1%, 64.9%) of those <2 y reported taking a product recommended by a physician or health care provider. For young infants aged 0–5.9 mo and older infants aged 6–11.9 mo, 84.8% (95% CI: 71.8%, 93.3%) and 76.3% (95% CI: 62.8%, 86.8%), respectively, were given a product recommended by a doctor or another health professional. A significantly lower proportion of toddlers aged 12–23.9 mo (45.9%; 95% CI: 37.0%, 54.9%) were taking a product that was recommended by a doctor or another health professional compared with both younger and older infants.
FIGURE 2.
Prevalence [percentage (95% CI)] of reported motivations for use of dietary supplements among US infants and toddlers aged 0–23.9 mo who reported dietary supplement use in the past 30 d, NHANES 2007–2014. Source: CDC/National Center for Health Statistics, NHANES 2007–2014. Categories are not mutually exclusive. *Significant linear trend by age group, P < 0.05. **Statistically significant difference between the 0–5.9 mo and 6–11.9 mo age groups, P < 0.05.
Discussion
To our knowledge, this is the first report characterizing the use of DSs among infants and toddlers aged <2 y using the most recent available population-based nationally representative data in the United States from NHANES. Noteworthy is our finding that almost 1 in 5 (18%) infants and toddlers was receiving a DS. Characteristics associated with any DS usage were infant and toddler age >12 mo, non-Hispanic white race, higher household income, higher educational status of the head-of-the-household, and consumption of breast milk. The number of micronutrients consumed from DSs increased with age and the t form of DS products tended to shift from drops to chewable forms. Finally, particularly in the youngest age groups, proxies reported that the reason for DS use was primarily due to physician recommendation.
Data on the usage of DSs have been collected in NHANES since the 1970s and these data suggest a decrease in use over time. Among those aged 1–2 y, the prevalence of any DS use decreased from 54.8% in NHANES I (1971–1974) to 30.9% (NHANES 1999–2000) (23). For those <1 y of age such information is very limited; however, data from NHANES 1999–2002 indicate that 11.9% used ≥1 DS (24). Our study suggests that in general, the use of any DS has remained stable from 1999–2002 to 2011–2014 for infants and toddlers aged 0–23.9 mo. However, use of any DS increased significantly among younger infants (aged 0–5.9 mo) from 1999–2002 to 2011–2014. This increase parallels the increase in duration of breastfeeding in the population (25) and recommendations by pediatric professional associations, such as the AAP, that breastfed and partially breastfed infants should be supplemented with vitamin D (3). In terms of the general decline since the early 1970s, this could be partly due to the increase in commercial infant formulas containing all nutrients known to be needed by infants, which were not introduced and widely popular until after the Infant Formula Act of 1980 passed, making supplementation of commercial formula–fed infants unnecessary (24). Furthermore, we observed that the percentage of infants and toddlers aged <2 y using a DS was lower than that reported in the published literature among older children. For example, in a report published by Bailey et al. (26), using data from NHANES 2007–2010, DS use was 45% ± 1.9% (mean ± SE) among children aged 2–5 y and 36% ± 2.0% among children aged 6–11 y.
The AAP recommends that exclusively breastfed infants be given iron supplements around the time that their innate iron stores start to deplete, starting around 4 mo (4). This is in contrast to infants who exclusively consume commercial infant formulas that typically contain iron. Our study suggests that 2.1% of breastfed infants aged 0–5.9 mo and 1.5% of infants formula fed are taking a DS containing iron, which was not statistically different. These data may indicate that a very small percentage of US infants fed only breast milk take a DS containing iron and therefore it may be possible that this subgroup of the US population may not obtain enough iron to meet their needs as they approach later infancy and fetal hemoglobin stores are depleted (27). However, more data are needed with an adequate sample size to provide more detailed analysis of specific ages (e.g., only those aged 4–6 mo) and complementary feeding practices.
We found that ∼10% of infants aged 0–5.9 mo consumed a vitamin D–only product and ∼8% consumed vitamin D in a combination or multinutrient product. However, only about a quarter (24%) of breast milk–fed (no formula) infants (<6 mo) were using a vitamin D–containing DS despite the vitamin D supplement recommendations of the AAP (3). Vitamin D supplementation was lowest among infants consuming only formula, with mixed-feeding usage rates in between exclusive breastfeeding and formula, respectively.
For toddlers aged 12–23.9 mo, DSs may contribute significant amounts of certain nutrients, such as vitamins A and E, with the result that the distribution of intakes is shifted to the right and hence the percentage of the population with inadequate intakes may be decreased or the percentage of the population at risk of excess increased, or both. Vitamin E is a nutrient contained in most multivitamin formulations and use of a DS is likely to significantly contribute to intake of this nutrient, especially considering the finding from our current study that 23% of toddlers aged 12–23.9 mo take a DS. In a report of usual nutrient intakes using data from the Feeding Infants and Toddlers Study (FITS) conducted in 2008, DSs were included in nutrient estimations (20) and 24.8% of toddlers aged 12–23 mo reported supplement use. In comparison, Ahluwalia et al. (28) described usual intakes based on NHANES 2009–2012 data on similar age groups, but did not include intakes from DSs. The proportion of toddlers with inadequate intakes of vitamin E was 63% in FITS (including DSs), compared with 82% in NHANES (not including DSs) (20, 28). Similarly, 16% of toddlers in the latter report, based on NHANES data, met or exceeded the Tolerable Upper Intake Level for vitamin A based on dietary sources only, whereas the estimate in FITS based on total intakes including diet and DS sources was 31% (20, 28). Although direct comparisons are difficult between studies because of differences in study periods and in study design as well as differences in the sources of nutrients included in usual intake distributions that were calculated, results highlight that certain nutrients commonly consumed through supplementation may affect the percentage not meeting or exceeding DRIs. Despite these differences, data from both FITS and NHANES underscore the need to examine total nutrient intakes in future studies.
The data reported here should be interpreted with the following caveats in mind. First, very little is known about the reporting errors that exist for DSs, especially among this age group. However, because the use of DSs was assessed during the home interview, products’ containers or bottles were seen by trained interviewers and recorded while on site. These data were further reviewed by NHANES nutritionists to ensure completeness and accuracy. However, reporting errors can arise since DS use (frequency and dosage) data may be reported by nonparent proxies.
It is also possible that product formulations changed over the data collection period; however, NHANES makes every effort to ensure that DS formulations on the market at the time of data collection are matched to participant data. Nutrient estimates presented in this article rely on label declarations made by the product manufacturer. Although the label declarations on adult and older children nutrient products tend to be higher when chemically analyzed than what is presented on the label (29), little is known about how label declarations compare among infant and toddler products. Finally, although this analysis uses the most recently available NHANES DS-use data, it should be noted that the data are from 2007–2014 and may not reflect DS use in more recent years. However, a recent article by Bailey et al. (27) using data from the FITS study conducted in 2016 does suggest that DS use among infants aged 6–11.9 mo and toddlers aged 12–23.9 mo has remained stable, whereas usage among infants aged 0–5.9 mo may have increased between 2007–2014 and 2016 (14.6%; 95% CI: 11.5%, 18.1%; compared with 23% ± 2.3%).
This analysis has many strengths. Population-based national data like NHANES are critical to provide information on the sources of nutrient intakes, to assess adherence to dietary recommendations, and to monitor changes over time (30, 31). NHANES provides detailed high-quality data on the population's use of DSs from birth onwards that can be used to characterize the prevalence of DS use; to assess nutrient intake from DSs as well as total nutrient intake from foods, beverages, and DSs; and to examine associations between DSs and other demographic, lifestyle, and health factors (32).
In conclusion, the present analysis provides estimates of DS use based on the most recent data available from NHANES. Our findings indicate that DS use tends to be higher among those aged 12–23.9 mo than among infants and that the types of supplements used by infants were chiefly a few vitamins, rather than multivitamin, multimineral DSs used by toddlers and older children. We found that 24.3% of infants aged 0–5.9 mo that were fed breast milk (no formula) were taking a DS containing vitamin D, which is an increase over time but is still low considering the AAP recommendation of vitamin D supplementation for infants exclusively fed breast milk. Continued monitoring of DS use among infants and toddlers is warranted. In addition, future studies should examine total nutrient intake from food, beverages, and DSs among infants and toddlers and evaluate the nutrient adequacy of US infants and toddlers aged <2 y based on total intakes.
Supplementary Material
Acknowledgments
The authors’ contributions were as follows—JJG and KAH: analyzed the data and had primary responsibility for final content; and all authors: designed the research (project conception, development of the overall research plan, and study oversight), reviewed the manuscript, and read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: JJG, KAH, NP, RLB, and NA, no conflicts of interest. JTD has served as a consultant for Gerber/Nestlé and holds stock in several food and drug companies.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the NIH/Office of Dietary Supplements, the CDC, or any other entity of the US Government.
Supplemental Tables 1 and 2 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
JJG and KAH contributed equally to this work.
Abbreviations used: AAP, American Academy of Pediatrics; DGA, Dietary Guidelines for Americans; DS, dietary supplement; FITS, Feeding Infants and Toddlers Study; NCHS, National Center for Health Statistics; PIR, family poverty-to-income ratio; WIC, Special Supplemental Nutrition Program for Women, Infants, and Children.
References
- 1. Raiten DJ, Raghavan R, Porter A, Obbagy JE, Spahn JM. Executive summary: evaluating the evidence base to support the inclusion of infants and children from birth to 24 mo of age in the Dietary Guidelines for Americans–“the B-24 Project”. Am J Clin Nutr. 2014;99(3):663S–91S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. U.S. Congress. Pub. L. 113–79. The 2014 Farm Bill. Washington, DC: 113th Congress; 2014. [Google Scholar]
- 3. Wagner CL, Greer FR, American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–52. [DOI] [PubMed] [Google Scholar]
- 4. Baker RD, Greer FR, Committee on Nutrition American Academy of Pediatrics. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age). Pediatrics. 2010;126(5):1040–50. [DOI] [PubMed] [Google Scholar]
- 5. Bailey RL, Fulgoni VL, Keast DR, Lentino CV, Dwyer JT. Do dietary supplements improve micronutrient sufficiency in children and adolescents?. J Pediatr. 2012;161(5):837–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Briefel R, Hanson C, Fox MK, Novak T, Ziegler P. Feeding Infants and Toddlers Study: do vitamin and mineral supplements contribute to nutrient adequacy or excess among US infants and toddlers?. J Am Diet Assoc. 2006;106(1 Suppl 1):S52–65. [DOI] [PubMed] [Google Scholar]
- 7. Curtin LR, Mohadjer LK, Dohrmann SM, Kruszon-Moran D, Mirel LB, Carroll MD, Hirsch R, Burt VL, Johnson CL. National Health and Nutrition Examination Survey: sample design, 2007–2010. Vital Health Stat 2. 2013;160:1–23. [PubMed] [Google Scholar]
- 8. Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National Health and Nutrition Examination Survey: sample design, 2011–2014. Vital Health Stat 2. 2014;162:1–33. [PubMed] [Google Scholar]
- 9. National Center for Health Statistics. Unweighted Response Rates for NHANES 2007–2008 by Age and Gender. Hyattsville, MD: National Center for Health Statistics; 2009. [Google Scholar]
- 10. National Center for Health Statistics. Unweighted Response Rates for NHANES 2009–2010 by Age and Gender. Hyattsville, MD: National Center for Health Statistics; 2011. [Google Scholar]
- 11. National Center for Health Statistics. Unweighted Response Rates for NHANES 2011–2012 by Age and Gender. Hyattsville, MD: National Center for Health Statistics; 2013. [Google Scholar]
- 12. National Center for Health Statistics. Unweighted Response Rates for NHANES 2013–2014 by Age and Gender. Hyattsville, MD: National Center for Health Statistics; 2015. [Google Scholar]
- 13. Otten JJ, Hellwig JP, Meyers LD. DRI, Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 14. USDA Agriculture Research Service. Dietary Supplement Ingredient Database: Unit Conversions. [Internet]. USDA; 14 Aug, 2017. [cited 31 October 2017]. Available from: https://dietarysupplementdatabase.usda.nih.gov/Conversions.php. [Google Scholar]
- 15. National Center for Health Statistics. National Health and Nutrition Examination Survey 2007–2008: data documentation, codebook, and frequencies: dietary supplement use 30-day. Department of Health and Human Services, editor. Hyattsville, MD: National Center for Health Statistics; 2010. [Google Scholar]
- 16. National Center for Health Statistics. National Health and Nutrition Examination Survey 2009–2010: data documentation, codebook, and frequencies: dietary supplement use 30-day. Department of Health and Human Services, editor. Hyattsville, MD: National Center for Health Statistics; 2012. [Google Scholar]
- 17. National Center for Health Statistics. National Health and Nutrition Examination Survey 2011–2012: data documentation, codebook, and frequencies: dietary supplement use 30-day. Department of Health and Human Services, editor. Hyattsville, MD: National Center for Health Statistics; 2014. [Google Scholar]
- 18. National Center for Health Statistics. National Health and Nutrition Examination Survey 2013–2014: data documentation, codebook, and frequencies: dietary supplement use 30-day. Department of Health and Human Services, editor. Hyattsville, MD: National Center for Health Statistics; 2016. [Google Scholar]
- 19. USDA Food and Nutrition Service. Supplemental Nutrition Assistance Program (SNAP). Am I eligible for SNAP?. [Internet]. USDA; 27 Jun, 2018; [cited 5 Jul, 2018]. Available from: http://www.fns.usda.gov/snap/eligibility. [Google Scholar]
- 20. Butte NF, Fox MK, Briefel RR, Siega-Riz AM, Dwyer JT, Deming DM, Reidy KC. Nutrient intakes of US infants, toddlers, and preschoolers meet or exceed dietary reference intakes. J Am Diet Assoc. 2010;110(12 Suppl):S27–37. [DOI] [PubMed] [Google Scholar]
- 21. Devaney B, Ziegler P, Pac S, Karwe V, Barr SI. Nutrient intakes of infants and toddlers. J Am Diet Assoc. 2004;104(1 Suppl 1):s14–21. [DOI] [PubMed] [Google Scholar]
- 22. Parker JD, Talih M, Malec DJ, Beresovsky V, Carroll M, Gonzalez JF, Hamilton BE, Ingram DD, Kochanek K, McCarty F et al.. National Center for Health Statistics data presentation standards for proportions. Vital Health Stat 2. 2018;2(175):1–22. [PubMed] [Google Scholar]
- 23. Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Annu Rev Nutr. 2004;24:401–31. [DOI] [PubMed] [Google Scholar]
- 24. Picciano M, Dwyer JT, Radimer KL, Wilson DH, Fisher KD, Thomas PR, Yetley EA, Moshfegh AJ, Levy PS, Nielsen SJ et al.. Dietary supplement use among infants, children, and adolescents in the United States, 1999–2002. Arch Pediatr Adolesc Med. 2007;161(10):978–85. [DOI] [PubMed] [Google Scholar]
- 25. Herrick KA, Rossen LM, Kit BK, Wang CY, Ogden CL. Trends in breastfeeding initiation and duration by birth weight among US children, 1999–2012. JAMA Pediatr. 2016;170(8):805–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bailey RL, Gahche JJ, Thomas PR, Dwyer JT. Why US children use dietary supplements. Pediatr Res. 2013;74(6):737–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bailey RL, Catellier D, Jun S, Dwyer JT, Jacquier EF, Anater AF, Eldridge AL. Total usual nutrient intakes of US children (under 48 months): findings from the Feeding Infants and Toddlers Study (FITS) 2016. J Nutr. 2018;148(9S):1557S–66S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ahluwalia N, Herrick KA, Rossen LM, Rhodes D, Kit B, Moshfegh A, Dodd KW. Usual nutrient intakes of US infants and toddlers generally meet or exceed Dietary Reference Intakes: findings from NHANES 2009–2012. Am J Clin Nutr. 2016;104(4):1167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. USDA Agricultural Research Service. USDA Dietary Supplement Ingredient Database Release 4.0 DSID-4 Adult Multivitamin/mineral (AMVM-2017) dietary supplement study research summary. Beltsville Human Nutrition Research Center Nutrient Data Laboratory, editor. Beltsville, MD: USDA Agricultural Research Service; 2017. [Google Scholar]
- 30. Ahluwalia N, Dwyer J, Terry A, Moshfegh A, Johnson C. Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr. 2016;7(1):121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ahluwalia N, Herrick K, Paulose-Ram R, Johnson C. Data needs for B-24 and beyond: NHANES data relevant for nutrition surveillance of infants and young children. Am J Clin Nutr. 2014;99(3):747S–54S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gahche JJ, Bailey RL, Potischman N, Ershow AG, Herrick KA, Ahluwalia N, Dwyer JT. Federal monitoring of dietary supplement use in the resident, civilian, noninstitutionalized US population, National Health and Nutrition Examination Survey. J Nutr. 2018;148(8S):1436S–44S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.