SUMMARY
Endocannabinoids acting on the cannabinoid-1 receptor (CB1R) or ghrelin acting on its receptor (GHS-R1A) both promote alcohol-seeking behavior, but an interaction between the two signaling systems has not been explored. Here we report that the peripheral CB1R inverse agonist JD5037 reduces ethanol drinking in wild-type mice but not in mice lacking CB1R, ghrelin peptide or GHS-R1A. JD5037 treatment of alcohol drinking mice inhibits the formation of biologically active octanoyl-ghrelin without affecting its inactive precursor desacyl-ghrelin. In ghrelin-producing stomach cells, JD5037 reduced the level of the substrate octanoyl-carnitine generated from palmitoyl-carnitine by increasing fatty acid β-oxidation. Blocking gastric vagal afferents abrogated the ability of either CB1R or GHS-R1A blockade to reduce ethanol drinking. We conclude that blocking CB1R in ghrelin-producing cells reduces alcohol drinking by inhibiting the formation of active ghrelin and its signaling via gastric vagal afferents. Thus, peripheral CB1R blockade may have therapeutic potential in the treatment of alcoholism.
In Brief (eTOC blurb)
Endocannabinoids and ghrelin are both implicated in promoting alcohol intake. Godlewski et al. show that cannabinoid-1 receptor (CB1R) signaling in ghrelin-producing stomach cells modulates the formation of biologically active octanoyl-ghrelin and then acts via gastric vagal afferents to promote alcohol consumption. They further show that peripherally-restricted pharmacological inhibition of CB1R reduces ethanol drinking in mice.
Graphical Abstract

INTRODUCTION
C57Bl6/J mice display hedonic traits, as reflected in their preference for eating diets high in fat and carbohydrates or drinking alcohol. Both of these preferences are driven, to a large extent, by endocannabinoids acting via its receptor CB1R, as they are suppressed by pharmacological inhibition (Colombo et al., 2005; Di Marzo et al., 2001) or genetic deletion of CB1R (Hungund et al., 2003; Ravinet Trillou et al., 2004; Wang et al., 2003). Endocannabinoids and CB1R are obligatory components of the hypothalamic and limbic neural circuitries involved in the control of both food and drug seeking behavior (Solinas et al., 2008). Therefore, evidence that CB1R in the CNS may be dispensable for the anorectic effect of CB1R blockade was surprising (Bellocchio et al., 2013; DiPatrizio et al., 2011; Gomez et al., 2002), and it suggested the involvement of a periphery-to-brain axis in this effect, although the nature of the signaling pathway was not identified. Direct evidence for the dominant role of peripheral CB1R in the control of food intake came from studies with the non-brain-penetrant CB1R inverse agonist JD5037 (Chorvat et al., 2012), which was as effective as its brain-penetrant counterpart ibipinabant in reducing food intake in mice with diet-induced obesity (DIO), despite the documented absence of central CB1R occupancy by JD5037 (Tam et al., 2012). Furthermore, the JD5037-induced hypophagia could be attributed to endogenous leptin, as the obesity-related hyperleptinemia thought to be responsible for maintaining leptin resistance (Li et al., 2013) was rapidly normalized by peripheral CB1R blockade that decreased leptin production in adipocytes and increased leptin clearance in the kidney, thus restoring sensitivity to endogenous leptin (Tam et al., 2012). To test if periphery-to-brain signaling may also be involved in the control of alcohol drinking behavior by endocannabinoids, we analyzed the effects of JD5037 treatment on alcohol drinking by male C57Bl6/J mice, using the two-bottle free-choice and the drinking-in-the-dark paradigms.
RESULTS
Peripheral CB1R Mediates High Alcohol Preference and Intake in C57Bl6 Mice
Male C57Bl6/J mice with access to water or a 15% ethanol solution displayed high preference for alcohol, resulting in an average daily intake of 11.5 ± 0.4 mg ethanol/g body weight (n = 84). The high alcohol preference and intake remained unaffected by daily gavage with vehicle, whereas both were sharply and similarly reduced during 5 days of treatment with daily oral doses of 3 mg/kg of the peripherally restricted CB1R inverse agonist JD5037 (Tam et al., 2012) or 10 mg/kg of the brain-penetrant CB1R inverse agonist rimonabant (Figure 1A), the difference in dosing reflecting the higher CB1R binding affinity of JD5037 (Kd: 0.4 nM) compared to rimonabant (Kd: 3.2 nM). Alcohol intake was also significantly reduced by JD5037 treatment in a restricted access/drinking-in-the-dark paradigm that results in inebriating blood levels of ethanol (Figure 1B). JD5037 treatment did not affect total fluid intake, or preference for solutions containing saccharin, sucrose or quinine, suggesting that peripheral CB1R blockade has no effect on the perception of thirst or the taste or caloric value of ethanol (Figure S1A), and also failed to affect the rate of alcohol metabolism (Figure S1B). The role of CB1R in high alcohol preference and its inhibition by JD5037 or rimonabant was further supported by findings in CB1R knockout (Cnr1−/−) mice whose significantly lower ethanol preference compared to wild-type controls was unaffected by CB1R blockade (Figure 1A). This less than 50% preference induced by CB1R blockade or deletion may be interpreted as alcohol ‘avoidance’.
Figure 1. Effect of CB1R Blockade on Alcohol Drinking in Wild-type Mice and in Mice Deficient in CB1R, Ghrelin Peptide or Ghrelin Receptor. (see also Figures S1, S2 and S4 and Table S1).
(A) The effect of CB1R blockade on alcohol preference, intake and total fluid intake in wild-type and Cnr1−/− littermate mice. All 3 groups were treated daily with vehicle from day 2 to 5, and continued with vehicle (open circles), 10 mg/kg/day rimonabant (red) or 3 mg/kg/day JD5037 (blue) by oral gavage from day 6 to 10. Mice had free access to water or a 15% ethanol solution in water, using a two-bottle/free choice paradigm described in Methods. Points and vertical bars represent means ± SEM from the indicated number of experiments. For the bar graph on ethanol consumption, the vehicle groups for rimonabant and JD5037 were combined. Results were analyzed using one or two-way ANOVA, as appropriate. *P < 0.05, **P < 0.01, ***P < 0.001 relative to vehicle group. (B) Alcohol intake and blood ethanol concentrations in mice using a limited access/drinking in the dark paradigm described in Methods. Mean values indicated by horizontal lines; significance of difference between pre and post-JD5037 values, **P < 0.01. (C) Effect of peripheral CB1R blockade on alcohol drinking in wild-type and Ghrl−/− littermate mice. Treatments and symbols as described in panel A. (D) Effect of peripheral CB1R blockade on alcohol drinking in wild-type and Ghsr−/− littermate mice. Treatments and symbols as described in panel (A).
Endocannabinoid Modulation of Alcohol Drinking is Ghrelin-Dependent
To identify a possible hormonal signal that influences alcohol intake in a CB1R-dependent manner, we measured the plasma levels of several appetitive and stress-related hormones in mice drinking either alcohol or water. Plasma levels of leptin, ACTH, corticosterone and neuropeptide Y were unaffected by alcohol drinking. However, total ghrelin plasma levels were significantly higher in alcohol-drinking than in control mice, in agreement with findings in a large, population-based study in human drinkers (Wittekind et al., 2018), and this increase was attenuated by JD5037 treatment (Table S1). Since ghrelin is known for promoting alcohol drinking (Jerlhag et al., 2009; Lee et al., 2018; Leggio et al., 2014; Zallar et al., 2018), we explored its role as a possible link between peripheral endocannabinoids and brain structures involved in the control of alcohol drinking. We analyzed alcohol preference and its modulation by peripheral CB1R blockade in mice lacking ghrelin peptide or its receptor, which is the growth hormone secretagogue receptor-1A (GHS-R1A). Both Ghrl−/− and Ghsr−/− mice had significantly lower alcohol preference and intake than their respective wild-type littermates, and daily treatment with JD5037 significantly reduced ethanol preference and intake in controls but not in the two knockout strains (Figure 1C–D). Food intake remained unaffected by JD5037 treatment in both wild-type and knockout strains (Figure S2).
These findings suggest that endocannabinoids acting via peripheral CB1R promote alcohol drinking by inducing ghrelin production, its post-translational modification and/or its release from ghrelin-producing cells. Any of these mechanisms are predicated on the expression of CB1R by ghrelin-producing cells. We used RNAscope ®, an RNA in situ hybridization technology, to confirm earlier findings of low Cnr1 expression in ghrelin-producing cells of the stomach mucosa (Engelstoft et al., 2013) (Figure S3A). Compared to other tissues, the mouse stomach contains very high levels of endocannabinoids, particularly anandamide, which are further increased in ethanol-drinking animals, whereas no such increase in EC levels was evident in the hypothalamus or plasma (Figure S3B). Endocannabinoids and their degrading enzymes are also present in MGN3–1 mouse gastric ghrelinoma cells (Figure S3C). These cells were selected for our study because they express CB1R (Koyama et al., 2016) similar to primary gastric mucosal cells (Engelstoft et al., 2013). Additionally, they are capable of incorporating and metabolizing long-chain fatty acids to generate octanoic acid for ghrelin acylation, unlike some other ghrelin-producing cell lines that require octanoic acid supplementation to produce octanoyl-ghrelin (Bando et al., 2016).
CB1R Blockade Inhibits the Octanoylation of Ghrelin
To become biologically active, ghrelin is octanoylated by ghrelin O-acyl transferase (GOAT) (Yang et al., 2008). Alcohol-drinking wild-type mice exhibited elevated plasma levels of both octanoyl-ghrelin and its inactive precursor desacyl-ghrelin. JD5037 treatment reversed the increase in octanoyl-ghrelin without affecting desacyl-ghrelin levels (Figure 2A). The selectivity of this effect makes it unlikely that it reflects a change in ghrelin release, in agreement with the lack of effect of CB1R agonism on ghrelin secretion from ghrelin-producing primary stomach cells (Engelstoft et al., 2013). As to the alcohol-induced increase in total plasma ghrelin, alcohol intake is known to increase sympathetic nerve activity (Hering et al., 2011), and sympathetic stimulation increases ghrelin secretion via the β1-adrenergic receptor (Zhao et al., 2010). Accordingly, treatment of alcohol-drinking mice with the β-adrenergic antagonist propranolol reduced alcohol preference and intake, in agreement with earlier observations (Gilpin and Koob, 2010), and decreased the plasma levels of both desacyl- and octanoyl-ghrelin (Figure S4).
Figure 2. Effect of Peripheral CB1R Blockade on Plasma Acyl- and Desacyl-ghrelin in Alcohol Drinking Wild-type and Cnr1−/− Mice and on Alcohol Drinking Behavior in Mboat−/− Mice (see alsoFigure S3).
(A) Plasma hormone levels measured by ELISA in control (no ethanol) and vehicle- or JD5037-treated alcohol-drinking wild-type mice and their similarly treated Cnr1−/− littermates at the end of the 5-day treatment period. Significant difference from corresponding values in ‘no ethanol’ group (*) or between vehicle- and JD5037-treated alcohol group (#), with P values as indicated in the legend for Figure 1 and n values shown in brackets. (B) Mboat−/− mice and their wild-type littermates were subjected to the two-bottle paradigm to monitor ethanol preference and intake in the absence or presence of JD5037 treatment (3 mg/kg/day) as described in the legend of Figure 1.
Peripheral CB1R blockade did not affect octanoyl-ghrelin levels in Cnr1−/− mice, confirming the distinct role of CB1R in this effect (Figure 2A). Given the abundance of endocannabinoids in stomach tissue and their increase in alcohol drinking animals, decreases in octanoyl-ghrelin by JD5037 likely reflect the reversal of a tonic increase in ghrelin acylation by endocannabinoids. Selective targeting of ghrelin acylation by endocannabinoids via CB1R is also supported by findings in mice lacking Mboat4, the gene encoding GOAT. Mboat4−/− mice and their wild-type littermates had similar body weight and food intake. Plasma levels of acyl-ghrelin were 145.3 ±27.6 pg/mL (n = 11) in wild-type mice but undetectable (<10 pg/mL, n = 9) in Mboat4−/− mice whereas desacyl-ghrelin levels were similar in the two strains (1.64 ± 0.43 ng/mL in wt vs 2.56 ± 0.67 ng/mL in ko, n.s.). Mboat4−/− mice had significantly lower ethanol preference and intake than their wild-type littermates, and JD5037 treatment did not reduce alcohol preference and intake in these knockout animals as it did in wild-type counterparts (Figure 2B).
Selective regulation of octanoyl-ghrelin by CB1R may result from altered gene expression or enzyme activity of GOAT, or changes in the availability of the GOAT substrate octanoyl-carnitine. Mboat4 expression was moderately reduced by 16 ± 5% (n = 16) in the stomach of JD5037-versus vehicle-treated, alcohol-drinking mice. Previously, however, a much greater, ~50% reduction in Mboat4 expression was reported to have no effect on plasma octanoyl-ghrelin levels (Kirchner et al., 2009). Therefore, it is unlikely that the modest decrease produced by JD5037 is responsible for the parallel decrease in octanoyl-ghrelin. CB1R blockade is also unlikely to inhibit GOAT enzyme activity directly: JD5037 concentrations resulting in maximal CB1R occupancy significantly reduced octanoyl-ghrelin levels in MGN3–1 cells incubated with 10 μM but not 50 or 250 μM octanoic acid (Figure S5A), whereas a synthetic GOAT inhibitor potently reduced octanoyl-ghrelin, which could not be overcome by increasing the substrate concentration to 250 μM (Figure S5B). Thus, inhibition of ghrelin acylation by JD5037 may be due to the depletion of octanoic acid, and this inhibition can be overcome with excess octanoic acid substrate. This mechanism is plausible as CB1R blockade is known to increase energy expenditure by increasing fatty acid β-oxidation (FAO), as documented in white and brown fat tissue (Jbilo et al., 2005; Perwitz et al., 2010), liver (Tam et al., 2010), kidney (Udi et al., 2017) and sperm cells (Aquila et al., 2010). We sought to test whether a similar mechanism operates in ghrelin-producing stomach cells.
CB1R Blockade Inhibits Ghrelin Acylation by Increasing the Oxidative Degradation of the Substrate Octanoyl-Carnitine
Mammals do not generate medium-chain fatty acids via lipogenesis, so octanoic acid could either be obtained through diet or derived metabolically from long-chain fatty acids from which it is generated by β-oxidation. The standard mouse chow used in our experiments does not contain octanoic acid, which was also undetectable in mouse plasma (not shown), suggesting that octanoic acid must be generated by uptake of long-chain fatty acids and subsequent β-oxidation in ghrelin producing cells. To test this, we incubated MGN3–1 cells with deuterated palmitic acid in the presence of 100 nM JD5037 or vehicle and followed its metabolic fate. The cellular level of [2H31]palmitic acid was similar in the two groups, indicating that fatty acid uptake is unaffected by CB1R blockade (Figure 3A). On the other hand, the conversion of palmitate to palmitoyl-carnitine, catalyzed by carnitine-palmitoyl transferase-1 (CPT1), the rate-limiting enzyme in fatty acid β-oxidation, was significantly increased in the presence of JD5037. The resulting increase in palmitoyl-carnitine:palmitic acid ratio indicates increased CPT-1 activity (Figure 3A). Furthermore, there was a progressive reduction of the ratios of [2H26]myristoyl-carnitine, [2H]lauroyl-carnitine and [222 H14]octanoyl-carnitine in JD5037-treated vs. vehicle-treated cells, resulting in a ~50% decrease in the amount of [2H14]octanoyl-carnitine generated from [2H31]palmitoyl-carnitine in response to JD5037 treatment (Figure 3A). That this reduction was due to increased oxidative degradation rather than decreased production of octanoyl-carnitine was indicated by three lines of evidence.
Figure 3. Effects of CB1R Blockade on Fatty Acid Uptake and Processing by MGN3–1 Stomach Ghrelinoma Cells (see also Figure S5 and Table S2).
(A) MGN3–1 cells seeded in 6-well plates at 2 × 106 cells/plate were allowed to adhere for 2 days before changing the medium to BSA-free, low glucose (1.5 g/L) DMEM. Cells were then incubated with 100 nM JD5037 or its vehicle for 2 h followed by the addition of 50 M of [2H31]palmitic acid for an additional 6 h before measuring the cellular uptake of [2H31]palmitic acid and the generated labeled acyl-carnitine species by LC-MS/MS. (B) Effect of JD5037 on the conversion of octanoyl-carnitine to hexanoyl-carnitine in MGN3–1 cells incubated with 50 μM of either [2H31]palmitic acid or [13C4]octanoic acid and on the generation of acyl-ghrelin.
First, in MGN3–1 cells preincubated with either [2H]-labeled palmitic acid or [13C]-labeled octanoic acid, 100 nM JD5037 caused similar, significant reductions in the amount of labeled octanoyl-carnitine generated, whereas the amount of its immediate degradation product hexanoyl-carnitine was less reduced. The corresponding increase in hexanoyl:octanoyl ratio indicates increased metabolic degradation of octanoic acid, resulting in a decrease in ghrelin acylation (Figure 3B). Second, incubation of MGN3–1 cells with 10–100 nM JD5037 robustly increased the rate of oleic acid oxidation, as measured by the activity of a fluorescent extracellular O2 consumption reagent (Figure 4A), and the effect of JD5037 was prevented in MGN3–1 cells with shRNA-mediated knockdown of Cnr1 (Figure 4B). In parallel experiments, exposure of MGN3–1 cells to JD5037 resulted in significant increases in the gene expression of two enzymes involved in the oxidation of long chain fatty acids, acyl-CoA dehydrogenase (Acadvl) and β-ketoacyl CoA hydrolase (Hadhb), which may have contributed to the increased rate of fatty acid oxidation (Figure S6). Third, treatment of MGN3–1 cells with selective inhibitors of three different enzymes in the fatty acid β-oxidation pathway, including oxfenicine for CPT1, triacsin-C for long chain fatty acylCoA synthase and 4-bromocrotonic acid for β-ketoacyl CoA thiolase, reduced acyl-ghrelin production and occluded the similar effect of JD5037 (Figure 5).
Figure 4. Effect of CB1R Blockade on Fatty Acid β-oxidation and Ghrelin Production in MGN3–1 Cells. (see also Figures S3, S5, S6).
(A) Time course and rate of increase in oleate-driven respiratory activity in MGN3–1 cells. Cells were treated with 10 or 100 nM JD5037, 2.5 M of FCCP (positive control) or vehicle in the presence of the fluorescent extracellular O2 consumption reagent. Oxidative respiration was recorded every 90 sec and the slope of the initial linear increase was calculated. Points and bars are means ± SEM from n = 11–15 experiments, as indicated. *P < 0.05 compared to vehicle. (B) Verification of Cnr1 knockdown by rtPCR in cells used in panel C. Cellular uptake of the constructs was verified by fluorescent microscopy (20x magnification) and the degree of knockdown was determined by rt-PCR, *P < 0.05, n = 4. (C) Oleate-driven oxidative activity in MGN3–1 cells with shRNA-mediated knockdown of Cnr1 expression and their mock-transfected controls, n = 3–8 experiments, as indicated, *P < 0.05.
Figure 5. Effects of Inhibitors of Fatty Acid Oxidation on Acyl- and Desacyl-ghrelin Production and its Inhibition by JD5037. (see also Figure S3).
Oxfenicine (1 mM), or 4-bromo-crotonic acid (50 M) were present in the medium for 2 h whereas triacsin C (5 μM) was present for 24 h before the experiments. The enzyme targets of each inhibitor are illustrated by the FAO scheme on the right (dashed red lines). Cell medium was collected for measurements of acyl- and desacyl-ghrelin. Columns and bars are means ± SEM, number of experiments indicated in brackets. *P < 0.05, **P < 0.01 compared to vehicle (ANOVA followed by Tukey’s multiple comparison test.
Ghrelin Signals via Vagal Afferents to Promote Alcohol Drinking Behavior
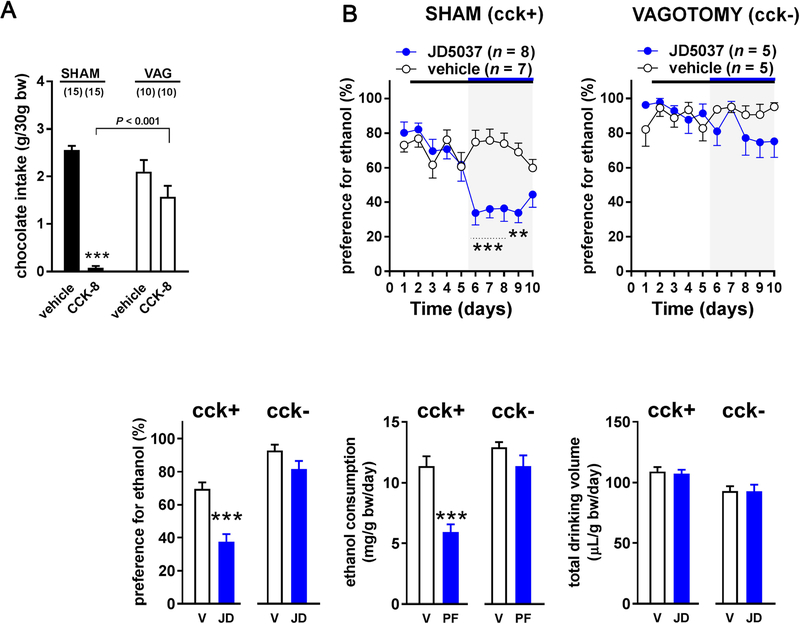
GHS-R1A is expressed in brain regions associated with the control of food intake (Zigman et al., 2006) where it may be activated by blood-borne acyl-ghrelin. However, it is uncertain whether this occurs in mice, which lack a functional blood-to-brain transport mechanism for acyl-ghrelin (Banks et al., 2002). Alternatively, acyl-ghrelin released from ghrelin-producing cells of the stomach may act on GHS-R1A expressed on vagal afferents (Burdyga et al., 2006; Date et al., 2002), which are in close apposition to ghrelin-producing neuroendocrine cells in the stomach mucosa (Gautron et al., 2011), with which they may form true synapses (Kaelberer et al., 2018). We have explored this latter possibility using mice with selective blockade of vagal afferent signaling by neonatal capsaicin treatment. These mice displayed permanent sensory afferent denervation verified by the complete loss of cholecystokinin (CCK)-induced anorexia (Figure 6A). In control (CCK+) mice with intact vagal innervation, treatment with either JD5037 or rimonabant significantly reduced alcohol preference and intake. In contrast, capsaicin-pretreated CCK-unresponsive (CCK-) mice displayed a statistically significant increase in both alcohol preference (pooled data during vehicle-treatment in CCK+: 69.8 ± 2.2%, n = 64, vs CCK-mice: 84.7 ± 1.8%, n = 61, P < 0.0001) and intake (12.4 ± 0.4 vs 16.2 ± 0.4 mg/g/day, P < 0.0001), which was unaffected by either JD5037 or rimonabant treatment (Figure 6B).
Figure 6. Effects of Blockade of Peripheral CB1R or GHS-R1A on Alcohol Intake and Preference in Control and Capsaicin-treated, Cholecystokinin (CCK)-Unresponsive Mice. (see also Figure S2).
(A) Effect of neonatal capsaicin or vehicle treatment on CCK-induced suppression food intake, measured as described in Methods. *P < 0.05, ***P < 0.001 indicate significant difference between pre- and post-CCK levels of intake of chocolate milk. (B) Effect of vehicle or CB1R blockade by JD5037 (3 mg/kg/day) or rimonabant (10 mg/kg/day) treatment on alcohol preference and intake in CCK-responsive control and capsaicin-treated, CCK-unresponsive mice. Symbols and statistics as described in the Legend of Figure 1A. (C) Effect of the GHS-R1A antagonist JMV2959 (JMV, 30 mg/kg/day) or its vehicle on alcohol preference and intake in CCK-responsive control and capsaicin-treated, CCK-unresponsive mice. (D) Effect of the peripherally restricted GHS-R1A antagonist PF-5190457 (3 mg/kg/day) on alcohol preference and intake in control and capsaicin-treated CCK-unresponsive mice.
Similar to earlier findings (Gomez et al., 2015), the GHS-R1A antagonist JMV2959 reduced alcohol drinking in control mice; however, it lost its effectiveness in CCK unresponsive mice (Figure 6C). Furthermore, the peripherally restricted GHS-R1A antagonist PF-5190457 (Bhattacharya et al., 2014) also significantly reduced alcohol intake and preference in control but not in capsaicin-treated CCK-unresponsive mice (Figure 6D). Like JD5037, neither of these GHS-R1A antagonists affected food intake (Figure S2).
Because neonatal capsaicin treatment can also affect sensory neurons outside of vagal afferents, we tested another group of mice with subdiaphragmatic surgical vagotomy followed by gastric banding to prevent the post-denervation dilation of the stomach. Although these mice lose both afferent and efferent vagal innervation to the stomach, sensory neurons outside vagal afferents remain intact. These mice also became unresponsive to CCK and, similar to capsaicin-treated mice (Figure 7A) displayed an increase in ethanol preference (sham: 70.0 ± 3.3%, n = 15 vs vagotomy: 91.1 ± 3.1%, n = 10, P = 0.0002) and intake (11.5 ± 0.7 vs 13.4 ± 0.7 mg/g/day, P = 0.08), which was no longer affected by CB1R antagonists (Figure 7B). Together, these findings indicate that intact vagal afferent activity is required for modulation of alcohol drinking behavior by CB1R signaling.
Figure 7. Effects of Subdiaphragmatic Vagotomy on the JD5037-Induced Decrease in Ethanol Intake and Preference in Control and CCK-Unresponsive Vagotomized Mice.
(A) Effect of sham operation and subdiaphragmatic vagotomy with pyloroplasty on CCK-induced suppression food intake, measured as described in Methods. ***P < 0.001 indicates significant difference between pre- and post-cholecystokinin-8 (CCK) levels of intake of chocolate milk. (B) Effect of pharmacological inhibition of CB1R with 3 mg/kg JD5037 (JD) or its vehicle (V) on alcohol consumption in CCK-responsive sham-operated controls and in vagotomized, CCK-unresponsive mice. Symbols and statistics as described in the Legend of Figure 1D.
DISCUSSION
Endocannabinoids acting via CB1R are integral components of the central neural circuits that control the rewarding effects of both food and drugs of abuse, including alcohol. CB1R at such central sites used to be considered the target for both the hypophagic and anhedonic effects of CB1R antagonists, but the brain-penetrance of globally acting CB1R antagonists led to the whole-sale abandonment of this class of compounds as potential therapeutic agents. The present findings represent an important advance on three accounts. First, they demonstrate that addictive behavior such as alcohol preference and drinking can be disrupted via selective blockade of peripheral CB1R in a manner that depends on afferent vagal signaling. Second, they identify ghrelin/GHS-R1A signaling as the downstream target through which CB1R affects drinking behavior. Third, the mechanism of the CB1R/ghrelin interaction in ghrelin-producing stomach cells is unusual: CB1R blockade inhibits the posttranslational activation of ghrelin by its GOAT-catalyzed octanoylation via depleting the substrate octanoyl-carnitine due to its increased oxidative degradation. To our knowledge, this is the first evidence for a GPCR-mediated selective regulation of ghrelin octanoylation.
Although the role of the gut/brain axis in the control of addictive behavior may strike one as surprising, recent evidence implicates the gut-brain axis not only in appetitive functions but also in the control of emotional and motivational states (Breit et al., 2018). In particular, vagal afferents originating in the right nodose ganglion were found to activate a parabrachio-nigral pathway mediating reward-related behaviors, whereas a different subset is likely involved in satiety and aversive behaviors (Han et al., 2018). The present observations are compatible with a scheme whereby an alcohol-induced, CB1R-dependent release of acyl-ghrelin from ghrelin-producing cells in the stomach mucosa activates GHS-R1A on vagal afferent terminals, triggering a signal to the brain to increase the drive to drink alcohol. The loss of the inhibitory effect of peripherally restricted antagonists of either CB1R (JD5037) or GHS-R1A (PF-5190457) on alcohol-drinking in mice with vagal deafferentation strongly supports the above scheme. Nevertheless, an alternative possibility is that vagal deafferentation interrupts a gut-brain-gut pathway that regulates GOAT activity. Studies are in progress to explore the existence of such a regulatory pathway.
Furthermore, a similar loss of the effect of the globally acting antagonists of CB1R (rimonabant) or GHS-R1A (JMV-2959) on alcohol intake in vagal deafferented mice suggests that endocannabinoids acting through ghrelin regulate drinking behavior exclusively via the gut-brain axis; however, they do not negate findings that exogenous ghrelin administered i.c.v. can increase alcohol drinking via GHS-R1A in the CNS (Jerlhag et al., 2009). It was proposed earlier that peripheral ghrelin is not involved in the control of alcohol drinking behavior based on the finding that neutralization of circulating acyl-ghrelin with a high affinity antibody do not affect alcohol drinking in mice (Jerlhag et al., 2014). However, this does not rule out the peripheral mechanism we propose here, as enteroendocrine cells in the GI tract have been shown to form true synapses with sensory afferent terminals (Kaelberer et al., 2018), so released acyl-ghrelin may directly activate GHS-R1A on afferent nerve terminals before gaining access to the circulation.
These findings parallel some earlier observations of a similar dependence of ghrelin-induced hyperphagia on intact vagal afferent innervation in rodents (Date et al., 2002; Date et al., 2005; Senin et al., 2013) and humans (le Roux et al., 2005), although others found gut vagal afferents to be redundant for the orexigenic effect of peripherally administered ghrelin (Arnold et al., 2006), which may occur by activation of GHS-R1A in the medullary dorsal vagal complex (Zigman et al., 2006). Ghrelin activation of GHS-R1A on vagal afferents was shown to reduce the spontaneous firing rate of these neurons (Date et al., 2002), whereas the satiety hormone CCK increases gastric afferent firing (Date et al., 2002; Schwartz et al., 1997). Such findings led to the proposal that the orexigenic effect of ghrelin may involve suppression of the satiety signal of CCK (Burdyga et al., 2006). CCK also suppresses alcohol intake (Geary et al., 2004), which would be compatible with a similar mechanism for the ghrelin-induced increase in alcohol drinking. Such a mechanism is further supported by the present findings that a peripherally restricted GHS-R1A antagonist that had been shown to increase vagal afferent nerve firing (Kong et al., 2016) reduces alcohol intake in mice with intact, but not absent, vagal afferents. The apparent inverse relationship between afferent vagal firing rate and the drive to drink alcohol is also compatible with the significant increase in alcohol preference and intake in mice with vagal deafferentation. Disruption of the vagal innervation of the stomach may explain increased alcohol drinking after Roux-en-Y gastric bypass surgery (Hajnal et al., 2012), which was associated with the loss of regulation of neuronal firing in the ventral tegmental area dopamine neurons by a ghrelin receptor antagonist (Sirohi et al., 2017).
The functional link we have uncovered between endocannabinoids and ghrelin may not be unexpected in view of the fact that both promote alcohol drinking via activation of CB1R (Hungund et al., 2003; Wang et al., 2003) or GHS-R1A (Lee et al., 2018; Leggio et al., 2014; Zallar et al., 2018), respectively, and there are also examples of intriguing similarities and/or interactions between endocannabinoids and ghrelin in the control of food intake (Alen et al., 2013; Kola et al., 2008) and peripheral energy metabolism (Kola et al., 2005; Lim et al., 2013). The mechanism of this interaction, however, is quite unique in ghrelin-producing stomach cells in which endocannabinoids acting via CB1R regulate the availability of the fatty acid substrate for ghrelin acylation. It is well established that CB1R agonists inhibit whereas antagonists promote fatty acid oxidation in various tissues, the latter being the primary mechanism of the peripherally mediated catabolic effect of CB1R blockade. Here we presented several lines of evidence that blockade of CB1R has a similar effect in ghrelin-producing cells where the increased flux through the fatty acid ß-oxidation pathway depletes the cellular level of octanoic acid available for ghrelin acylation. Our conclusion that CB1R selectively targets ghrelin acylation is also supported by the loss of the CB1R-mediated component of ethanol intake in Mboat4−/− mice which produce desacyl- but not acyl-ghrelin. This also indicates that desacyl-ghrelin, which has been implicated in a number of peripheral effects mediated by non-conventional ghrelin receptors (Callaghan and Furness, 2014), is not involved in the control of alcohol drinking behavior in mice.
CB1R at peripheral rather than central sites in the gut-brain axis have also been implicated in the hypophagic effects of the brain-penetrant CB1R antagonist rimonabant (Bellocchio et al., 2013; Gomez del Pulgar et al., 2002), and, in particular, its preferential inhibition of the intake of palatable dietary fats (DiPatrizio et al., 2011). In all these studies, vagal deafferentation abolished the hypophagic effects of rimonabant, but the nature of the vagal afferent signaling affected by CB1R blockade was not identified. In two other studies in rats on regular chow, overnight fasting increased food intake and plasma ghrelin levels and both were inhibited by rimonabant, suggesting a role for ghrelin in the orexigenic effects of endocannabinoids (Cani et al., 2004; Senin et al., 2013). However, a cause-and-effect relationship between the reduction in food intake and plasma ghrelin by rimonabant has not been unequivocally established in either study, and only total ghrelin was measured, which does not allow a distinction between ghrelin release and acylation as the target of CB1R blockade. Despite these parallels between the mechanisms involved in the control of food intake and alcohol drinking, it is noteworthy that in the present experiments peripheral CB1R blockade selectively reduced alcohol intake and preference without having any effect on food intake or the consumption of sucrose or saccharin-containing solutions. The lack of effect of JD5037 on food intake was not surprising, as its hypophagic effect reported in diet-induced obese mice is mediated by endogenous leptin, due to rapid reversal the obesity-induced leptin-resistance (Tam et al., 2012), and this mechanism is not expected to operate in leptin-sensitive lean mice. As for the role of CB1R in promoting the intake of sweets, the suppression of such intake by rimonabant has been shown to be mediated at central sites (DiPatrizio and Simansky, 2008). The possible involvement of CB1R on taste buds, which would be accessible to JD5037, is unlikely in view of a report that a CB1R antagonist did not affect the gustatory nerve response to sucrose in lean mice (Niki et al., 2015). Therefore, the lack of effect of JD5037 on these ingestive behaviors supports the notion that CB1R blockade affects the addictive/rewarding effects of alcohol rather than its caloric or tastant properties, and thus may have therapeutic potential in the pharmacologic management of alcoholism.
Limitations of Study
Numerous studies in rodent models of alcohol drinking indicate that rimonabant and other brain-penetrant CB1R antagonists significantly reduce alcohol preference and intake. In two human studies there was a tendency for rimonabant to reduce alcohol relapse or reduce voluntary drinking by heavy drinkers, but these effects did not reach statistical significance (reviewed in (Maccioni et al., 2010)). The doses of rimonabant used in rodent studies (3–10 mg/kg/day) result in full occupancy of CB1R whereas the dose used in all human studies to date was limited to 20 mg or 0.3 mg/kg/day to minimize the chances of psychiatric side effects, resulting in only 20–30% CB1R occupancy (Hjorth et al., 2016). This limitation can be circumvented by peripherally restricted CB1R antagonists that can be used at doses resulting in full receptor occupancy, which remains to be tested in human patients when peripheral CB1R antagonists, which are in early stages of clinical development, become available for therapeutic use.
Another potential limitation stems from the finding that peripheral CB1R blockade reduces alcohol preference and intake by limiting the availability of the octanoic acid substrate required for ghrelin acylation in the ghrelin-producing cells, which can be overcome by octanoic acid supplementation (see Figure S5A). Normal human plasma contains very low to negligible levels of octanoic acid (Christinat et al., 2016; Shrestha et al., 2015) as the majority of dietary medium-chain fatty acids is completely metabolized during their first pass through the liver. However, plasma levels of octanoic acid may be high enough to overcome the effect of CB1R blockade in people consuming special diets reinforced by medium-chain fatty acids or containing high levels of coconut and its oils.
STAR ✯ METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, George Kunos (george.kunos@nih.gov). Requests for MGN3–1 cells will be referred to the original provider, Professor Hiroshi Iwakura (hiwaku@wakayama-med.ac.jp) who has agreed to make this material available under a separate agreement to other scientists at non-profit organizations.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee of NIAAA, NIH, and the experiments were carried out in accordance with the guidelines. C57BL/6J mice were purchased from the Jackson Laboratory (USA). Cnr1−/− (Zimmer et al., 1999), Ghrl−/− (Sun et al., 2003) and Ghsr−/− mice (Sun et al., 2004) were generated as described and were propagated by heterozygote breeding, using corresponding wild-type littermates as controls. All 3 knockout strains had been backcrossed at least 8 times to a C57BL/6J background.
Mboat4−/− mice were generated using CRISPR/Cas9-mediated genome engineering by Cyagen US Inc. (USA) on a contract with our laboratory (contract number: NTMCK-1700809-AJW-01), with exon 1 to exon 2 of the mouse Mboat4 gene (GenBank accession number: NM_001126314.2; Ensembl: ENSMUSG00000071113) selected as target sites. Cas9 mRNA and gRNA generated by in vitro transcription were then injected into fertilized C57BL/6J eggs for knockout mouse production. The founders were genotyped by PCR followed by DNA sequencing analysis, confirming a 6334 bp deletion in the knockouts. The positive founders were bred to the next generation (F1) and subsequently genotyped by PCR and DNA sequencing analysis. Three potential off-target sites have been identified by PCR and sequence analysis, and no mutation was detected.
Mice were housed 2–4 animals per cage in air-conditioned racks maintained at 22.0 ± 0.5°C and 50.0 ± 1.0% humidity, under a 12:12 light/dark cycle. They had ad libitum access to food (NIH-31 standard rodent chow) and water. Absence of known pathogenic organisms was verified by regular testing in sentinel mice. Only male mice were used in drinking paradigms to avoid the confounding effect of the oestrus cycle on the orexigenic effect of ghrelin (Clegg et al., 2007; Sakurazawa et al., 2013). Mice used in drinking paradigms were 8 weeks old and weighing 20–25 g at the onset of the study. Since breeding patterns were not synchronous, a small variation of age (± 1 week) was allowed to obtain a cohort of at least 8–10 mutant and wild-type mice per treatment group.
Cell lines
Stomach-derived MGN3–1 ghrelinoma cell line was derived from a male mouse (Iwakura et al., 2010). Cells were maintained in culture in high glucose (4.5 g/L) DMEM, containing 10% heat inactivated fetal bovine serum and 100 U/mL penicillin and 100 g/mL streptomycin sulfate at 37°C under a humidified atmosphere of 5% CO 2 and 5% O2, as described in the original publications. Cells subcultured using Trypsin (0.25%) EDTA underwent no more than 6 passages during the course of the experiments. They exhibited high expression of Mboat4 and Ghrl genes and had the ability to produce acyl-ghrelin and desacyl-ghrelin. In most experiments, cells were subcultured onto 6 well plates at a density of 1.5×106 cells/2 mL, or in 12 well plates at a density of 0.67×106 cells/0.9 mL and allowed to adhere for 2 days. For gene expression and endocannabinoid measurements, cells were seeded onto 100-mm dishes at a density of 4.5×106 cells/10 mL or 107 cells/10 mL, respectively. On day 3, cells were washed 3 times with a sterile phosphate buffered saline and culture medium was replaced with treatment medium: BSA-free, low glucose (1.5 g/L) DMEM, containing 100 U/mL penicillin and 100 μg/mL streptomycin sulfate. Carnitine palmitoyltransferase 1 (CPT1) inhibitor oxfenicin 1 mM, mitochondrial 3-ketoacyl-CoA thiolase and acetoacetyl-CoA thiolase inhibitor 4-bromocrotonic acid 50 μM, CB1 antagonist JD5037 10–100 nM, ghrelin O-acyltransferase (GOAT) inhibitor MRI-2443 1 nM-1 μM or vehicle was added to the medium for 2 hours, followed by albumin solution of [2H31]palmitic acid 50 μM or [13C4]octanoic acid 10 μM, 50 μM or 250 μM or their unlabeled counterparts for another 6 hours. The long-chain fatty-acyl CoA synthase inhibitor triacsin C (5 μM) or its vehicle was present in the cell medium for 24 h before the addition of the labeled fatty acid. For endocannabinoid measurement, cells were incubated with fatty acid amide hydrolase/monoacylglycerol lipase inhibitor JZL195 (1 μM) (Long et al., 2009) or its vehicle for 6 hours.
At the end of treatment, cell culture plates were placed on ice. Media were collected, centrifuged at 953 × g for 3 min at 4°C and acidifi ed with 1N HCl to a final concentration of 0.1 M for measurement of ghrelin. The acidification step was omitted in samples intended for endocannabinoid measurement. Cells were washed 3 times with ice-cold PBS and immediately frozen. Samples were kept at −80°C until assay.
METHOD DETAILS
Two bottle alcohol preference test
The test was performed as described before (Wang et al., 2003) with modifications. Animals were individually housed and acclimated to the paradigm for 5–7 days by having access to two identical water bottles and handled daily to minimize the stress associated with drug testing. Animals were first subjected to a gradual increase in ethanol concentration in drinking bottle (3%, 6%, 9%, 12%, 15%), while the other bottle contained water. The position of bottles was changed every day and alcohol and water bottles will be replaced every 4 days. Once alcohol concentration reached 15%, animals remained on the paradigm for 10 days. Starting on day 2, mice received vehicle by oral gavage for 4 days, followed by a daily treatment with the global or peripheral CB1 antagonist (rimonabant 10 mg/kg or JD5037 3 mg/kg, respectively) or vehicle (control group) for another 5 days, one hour before the dark period. The nonselective beta blocker propranolol (5 mg/kg), the GHS-R1A antagonist JMV2959 (30 mg/kg), the peripheral GHS-R1A antagonist PF-5190457 (3 mg/kg) or vehicle was administered intraperitoneally. All animals were sacrificed for blood and tissue collection 12–16 hours after treatment.
Two bottle taste preference test
Animals were individually housed, acclimated and handled the same way as described in the ‘Two bottle alcohol preference test’ subsection. Following acclimation, mice were given access to 2 bottles, one with water and the other containing 0.3% or 3% sucrose, 0.01% or 0.2% saccharin (w/v), or 2 mg/dL quinine in water, for 10 days. The position of bottles was changed every day and tastant and water bottles will be replaced every 4 days. Starting on day 2, animals were gavaged with vehicle for 4 days, followed by daily treatment with JD5037 3 mg/kg or were continued with vehicle (control group) for another 5 days.
Drinking in the dark
The procedure was performed as described (Rhodes et al., 2005), with modifications. Mice were individually housed and acclimated to the room for 5–7 days before testing and randomly assigned to treatment groups. Starting 3 hours into the dark cycle, water bottles were replaced with 20% ethanol in 25 × 100 mm glass tubes fitted with metal sippers, Access to the ethanol solution was limited to 4 hours every day. One hour before the dark period on day 4, animals were gavaged with JD5037 3 mg/kg or vehicle, and the alcohol session was repeated. Blood samples were collected from mandibular vein immediately after the drinking session. Alcohol concentration was measured in serum using a sample analyzer (Model GM7 Micro-Stat, Analox Instruments, USA).
Neonatal capsaicin treatment
The procedure was originally developed for rats (Jancso et al., 1977) and was subsequently adopted for mice (Shimizu et al., 1984). Neonatal (2-day old) mice were subjected to hypothermia on crushed ice and water to alleviate the neurotoxin-associated pain and distress. Once a lack of motion and/or blanching of the tips of the toes was noted, the pups were transferred to a cooling platform for the drug injection. A single dose 50 mg/kg of capsaicin or its vehicle was injected subcutaneously. Each animal received the same volume (20 μL) based on an average weight of all the pups. The drug was injected into the skin fold on either flank/side of the animal by insulin syringe equipped with the 30-gauge needle under the microscope to ensure the subcutaneous placement of the needle without damaging the abdominal organs. Pups remained on ice for up to 15 minutes to provide post-injection analgesia, then they were transferred to a warm water bath (32–35°C) for 20–30 minutes. Once pups regained consciousness and became active, they were returned to the nest in the cage on the warm water blanket.
Subdiaphragmatic vagal denervation
The procedure was performed under isofluorane anesthesia using sterile instruments as described (Erlanson-Albertsson and Lindqvist, 2008). Briefly, the abdomen was opened to expose the stomach and esophagus. Both vagal trunks were ligated and sectioned immediately below the diaphragm. Pyloroplasty was routinely performed in conjunction with vagal denervation in order to prevent food retention and fatal gastric dilatation. This involved a longitudinal incision across the pyloric sphincter that was closed transversely. After ligation, the intestine was returned to the abdomen, and the surgical site closed using 2 layers of vicryl suture. Sham-operation consisted of a mid-line abdominal incision and gentle manipulation of the stomach without disturbing its innervation. The completeness of vagal denervation was verified by the cholecystokinin test 10–14 days after the surgery.
Cholecystokinin test
In order to check the efficiency of capsaicin-induced deafferentation and subdiaphragmatic denervation, mice were subjected to the cholecystokinin test, which was performed as described elsewhere (Kopin et al., 1999). Briefly, mice were maintained on a 60:40 (vol/vol) solution of chocolate-flavored Ensure (Abbott Nutrition Abbott Laboratories, USA) diluted in water. The liquid diet was presented for 60 min in the morning (08:30–9:30 AM) and in the afternoon (4:30–5:30 PM) using 50-mL plastic centrifuge Falcon tubes (Corning Life Sciences, USA), adapted with leak-proof stainless-steel sippers. Water was freely available before and after the feeding periods. Once baseline chocolate intakes were stable for a period of 4 days, the cholecystokinin test was initiated after overnight fasting. Mice were injected intraperitoneally (i.p.) with 30 μg/kg sulfated cholecystokinin octapeptide (CCK-8) or its vehicle (saline) in a volume of 5 mL/kg body weight. Five min later chocolate-flavored Ensure was presented for 15 min. Injections of CCK-8 or saline into the same animals were repeated in a reversed order 4–5 days apart. Animals were habituated to the procedure with saline injections for 2 days before test.
Ethanol clearance assays
Mice were gavaged with JD5037 3 mg/kg or its vehicle. One hour later, animals were injected i.p. with ethanol (4 g/kg; 15% by volume) in saline. Blood was collected from mandibular vein 1 and 3 hours later into K2EDTA microtainer tubes (Becton, Dickinson and Company, USA) and centrifuged at 3500 x rpm for 10 min. Plasma ethanol levels were measured using the colorimetric ethanol assay kit (Abcam, USA) in accordance with the manufacturers’ instructions.
Blood chemistry
Mouse plasma acylated ghrelin and unacylated ghrelin, leptin, corticosterone, ACTH and neuropeptide Y were determined using commercial sandwich ELISA assays (listed in Key Resources Table) in accordance with the manufacturers’ instructions and measured with the Spectra MAX 190 microplate reader (Molecular Devices, USA).
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| N/A | ||
| Bacterial and Virus Strains | ||
| mouse CNR1 shRNA silencing Adenovirus (Ad-m-Cnr1-shRNA) | Vector Biolabs | shADV-255795 |
| Scramble shRNA with GFP Adenovirus (Ad-scramble-shRNA) | Vector Biolabs | 1122 |
| Biological Samples | ||
| N/A | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| DMEM high glucose, pyruvate | ThermoFisher Scientific | 11995065 |
| DMEM low glucose, pyruvate | ThermoFisher Scientific | 11885084 |
| DMEM, no glucose, no glutamine, no phenol red | ThermoFisher Scientific | A1443001 |
| Fetal Bovine Serum (FBS), certified, heat inactivated | ThermoFisher Scientific | 10082147 |
| Penicillin-Streptomycin (5,000 U/mL), 100× | ThermoFisher Scientific | 15070063 |
| L-Glutamine(200 mM) | ThermoFisher Scientific | 25030081 |
| Trypsin (0.25%) EDTA | ThermoFisher Scientific | 25200056 |
| PBS (phosphate buffered saline) | ThermoFisher Scientific | 10010 |
| Oxfenicine (4-Hydroxy-D-phenylglycine) | MilliporeSigma | 215333 |
| Triacsin C from Streptomyces sp. | MilliporeSigma | T4540 |
| 4-bromocrotonic acid | Santa Cruz Biotechnology | sc-486695 |
| MRI-2443 | (Martinez-Grau, 2015) | N/A |
| JD5037 | (Chorvat et al., 2012) | N/A |
| Rimonabant (SR141716) | NIDA Drug Supply Proqram | NOCD-082 |
| JMV2959 | MilliporeSigma | 345888 |
| PF-5190457 | MilliporeSigma | PZ0270 |
| (±) Propranolol hydrochloride | MilliporeSigma | P0884 |
| [2H31] Palmitic acid | Cambridge Isotope Laboratories, Inc. | DLM-215–1 |
| 1,2,3,4-[13C4] Octanoic acid | Cambridge Isotope Laboratories, Inc. | CLM-2721–0.25 |
| JZL 195 | Cayman CHEMICAL | 13668 |
| Hydrochloric acid (HCI) | Avantor - Macron Fine Chemicals | 2612–14 |
| Sucrose | MilliporeSigma | S7903 |
| Saccharin | MilliporeSigma | 109185 |
| Quinine hydrochloride dihydrate | MilliporeSigma | Q1125 |
| Capsaicin | MilliporeSigma | M2028 |
| Ensure | Abbott Nutrition Abbott Laboratories | 57231 |
| CCK octapeptide (CCK-8) | Tocris Bioscience | 1166 |
| p-hydroxymercuribenzoic acid (PHMB) | MilliporeSigma | 55540 |
| Aprotinin from bovine lung | MilliporeSigma | A1153 |
| Dimethylsulfoxide (DMSO) | ATCC | 4-X |
| QIAzol Lysis Reagent | QIAGEN | 79306 |
| DNase I DNase I, Amplification Grade | ThermoFisher Scientific | 18068015 |
| RNeasy Lipid Tissue Mini Kit | Qiagen | 74804 |
| iScript™ cDNA synthesiskit | BIO-RAD | 9207996 |
| Fast SYBR™ Green Master Mix | Applied Biosystems | 4385617 |
| Ethanol (ethyl alcohol, U.S.P. 200 proof, anhydrous) | The Warner Graham Company; product obtained through NIH Supply Center | 6505001050000 |
| UltraPureTM Agarose | ThermoFisher Scientific | 16500500 |
| Formalin solution, neutral buffered, 10% | MilliporeSigma | HT501128 |
| RNAscope® LS Multiplex TSA Buffer | Advanced Cell Diagnostics | 322809 |
| GreenGlo Safe DNA Dye | Denville Scientific, Inc. | CA3600 |
| TAE Buffer (Tris-acetate-EDTA) (50X) | ThermoFisher Scientific | B49 |
| DirectPCR Lysis Reagent (Mouse Tail) 100ml | Viagen Biotech | 102-T |
| Proteinase K Powder (≥30 U/mg) | ThermoFisher Scientific | 17916 |
| dNTP Mix(10 mM) | ThermoFisher Scientific | 18427013 |
| Taq DNA Polymerase, recombinant | ThermoFisher Scientific | 10342020 |
| Tween® 80 | MilliporeSigma | P1754–25ML |
| 0.9% Sodium chloride for injection, USP - 50ml | Hospira Inc., obtained through NIH Division of Veterinary Resources | N/A |
| Sodium hydroxide solution | MilliporeSigma | 72068–100ML |
| Bovine Serum Albumin (BSA) solution, 30% in saline, fatty acid free, aseptically filled | MilliporeSigma | A9205–50ML |
| D-(+)-Glucose solution | MilliporeSigma | G8769–100ML |
| Qctanoic acid | MilliporeSigma | W279900-SAMPLE-K |
| Palmitic acid | MilliporeSigma | P0500–25G |
| Palmitoyl-L-carnitine | MilliporeSigma | 61251–10MG |
| Myristoyl-L-carnitine | MilliporeSigma | 61367–10MG |
| Lauroyl-L-carnitine | MilliporeSigma | 39953–10MG |
| Octanoyl-L-Carnitine | MilliporeSigma | 50892–10MG |
| Hexanoyl-L-carnitine | MilliporeSigma | 07439–10MG |
| Anandamide-d4 (N-(2-Hydroxyethyl-1, 1, 2, 2-d4)-5Z, 8Z, 11Z, 14Z-eicosatetraenamide) | Tocris | 3717 |
| Chloroform, stabilized with amylene, Optima™, for HPLC and GC, Fisher Chemical | ThermoFisher Scientific | C297–4 |
| Methanol, Optima™ LC/MS Grade, Fisher Chemical | ThermoFisher Scientific | A456–4 |
| Water, Optima™ LC/MS Grade, Fisher Chemical | ThermoFisher Scientific | W6–4 |
| Boc-Ala-OH (Boc-L-Alanine) | MilliporeSigma | 15380–100G |
| Critical Commercial Assays | ||
| Ethanol assay kit | Abeam | ab65343 |
| Fatty acid oxidation complete assay kit | Abeam | ab222944 |
| Unacylated Ghrelin (mouse, rat) Express EIA Kit | Cayman CHEMICAL | 10008953 |
| Acylated Ghrelin (mouse, rat) Express EIA Kit | Cayman CHEMICAL | 10006307 |
| Mouse Leptin ELISA Kit | As One International, Inc. | KTE71186 |
| Mouse/Rat Corticosterone ELISA | Alpco | 55-CORMS-E01 |
| Neuropeptide Y (NPY) (Human, Rat, Mouse) - EIA Kit | Phoenix Pharmaceuticals, Inc. | EK-049–03 |
| ACTH (Rat, Mouse)-EIA Kit | Phoenix Pharmaceuticals, Inc. | EK-001–21 |
| RNAscope® Multiplex Fluorescent Kit v2 | Advanced Cell Diagnostics | 323100 |
| TSA Cy 3, Cy 5, TMR, Fluorescein Evaluation Kit | Perkin Elmer | NEL760001KT |
| Deposited Data | ||
| Experimental Models: Cell Lines | ||
| MGN3–1 ghrelinomacell line | (Iwakura et al., 2010) | N/A |
| Experimental Models: Organisms/Strains | ||
| C57BL/6J | The Jackson Laboratory | 000664 |
| Cnr1−/− | (Zimmer et al., 1999) | N/A |
| Ghrl−/− | (Sun et al., 2003) | N/A |
| Ghsr−/− | (Sun et al., 2004) | N/A |
| Mboat4−/− | Cyagen US Inc., This paper | N/A |
| Oligonucleotides | ||
| Mm_Acadvl_1_SG QuantiTect Primer Assay (Acadvl) | QIAGEN | QT00253127 |
| Mm_Hadhb_1_SG QuantiTect Primer Assay (Hadhb) | QIAGEN | QT01059968 |
| Mm Mboat4_va.1_SG QuantiTect Primer Assay (Mboat4) | QIAGEN | QT01562232 |
| Mm_MGI:930008_1_SG QuantiTect Primer Assay (Ghrl) | QIAGEN | QT00137536 |
| Mm_Rpl19_2_SG QuantiTect Primer Assay (Rpl19) | QIAGEN | QT01779218 |
| mouse cannabinoid receptor 1 (Cnr1) | GeneCopoeia | MQP091551 |
| RNAscope® Probe-Mm-Cnr1-C3 | Advanced Cell Diagnostics | 420721-C3 |
| RNAscope® Probe-Mm-Mboat4 | Advanced Cell Diagnostics | 415311 |
| RNAscope® Probe-Mm-Ghrl-C2 | Advanced Cell Diagnostics | 41 5301-C2 |
| Mboat4−/− mice wt forward primer ATCCAGAACCTGGTATCCTGTAATA | This paper | N/A |
| Mboat4−/− mice wt reverse primer GTCAATCCCACTGACTCACAACACAG | This paper | N/A |
| Mboat4−/− mice mutant forward primer GAGTTCCTTGTCAAATCTCCAAAGCC | This paper | N/A |
| Mboat4−/− mice mutant reverse primer GTCAATCCCACTGACTCACAACACAG | This paper | N/A |
| Cnr1−/− wild type/mutant forward primer GTACCATCACCACAGACCTCCTC | (Zimmer et al., 1999) | N/A |
| Cnr1−/− wild type reverse primer GGATTCAGAATCATGAAGCACTCCA | (Zimmer et al., 1999) | N/A |
| Cnr1−/− mutant reverse primer AAGAACGAGATCAGCAGCCTCTGTT | (Zimmer et al., 1999) | N/A |
| Ghrl−/− wt forward primer GCTCTGGATGGACATGGCC | (Sun et al., 2003) | N/A |
| Ghrl−/− wt reverse primer CTGCGCATGTCTGCTGCTCC | (Sun et al., 2003) | N/A |
| Ghrl−/− mutant forward primer GAGGCTGAAGTTCAGATGTGCGGCG | (Sun et al., 2003) | N/A |
| Ghrl−/− mutant reverse primer CCTCGAATCAGCAACGGCTTGCCG | (Sun et al., 2003) | N/A |
| Ghsr−/− wt forward primer TCTTCGTGGTGGGCATCTCG | (Sun et al., 2004) | N/A |
| Ghsr−/− wt reverse primer CTTCCTCCCGATGAGACTGT | (Sun et al., 2004) | N/A |
| Ghsr−/− mutant forward primer AGCGCATCGCCTTCTATCGC | (Sun et al., 2004) | N/A |
| Ghsr−/− mutant reverse primer GCTCGACTTTGTCCAGGCC | (Sun et al., 2004) | N/A |
| Recombinant DNA | ||
| mouse CNR1 shRNA silencing Adenovirus (Ad-m-Cnr1-shRNA) | Vector Biolabs | shADV-255795 |
| Scramble shRNA with GFP Adenovirus (Ad-scramble-shRNA) | Vector Biolabs | 1122 |
| Software and Algorithms | ||
| GraphPad Prism 7 | GraphPad Software | N/A |
| MassHunter Workstation Optimizer software | Agilent Technologies | N/A |
| Other | ||
| N/A | ||
For ghrelin measurement, blood was collected from mandibular vein into K2EDTA microtainer tubes containing 1 mM p-hydroxymercuribenzoic acid (PHMB) and aprotinin 0,6 TIU per 1 mL of blood. The total volume of protease inhibitors was 10 μL/1mL of blood. Blood samples were then mixed by inversion, centrifuged at 3,500 rpm for 10 minutes at 4°C and acidified with 1N HCl to a final concentration of 0.1 M. For all other assays, blood was collected without other stabilizers. Plasma samples were assayed immediately or stored at − 80°C and used within 30 days after collection. For endocannabinoid and ethanol measurements, blood was allowed to clot for 30 min followed by centrifugation at 3,500 rpm for 10 minutes at 4°C. Serum samples were stored at −80°C until analysis.
Fatty acid oxidation assay
Oleate was used as substrate to evaluate fatty acid-driven oxygen consumption in MGN3–1 cells according to the manufacturer’s instructions. MGN3–1 cells were seeded in a Costar 96 well assay plates (black wall with clear flat bottom; Corning Incorporated, USA) at a density of 5 × 105 cells/well in 200 μL culture medium and allowed to adhere to the plate for 24 hours. On the day of experiment cells were treated with JD5037 10 nM and 100 nM or its vehicle (DMSO). The mitochondrial oxidative phosphorylation uncoupler carbonylcyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP, 2.5 μM) was used to achieve maximum respiratory activity. The oxygen consumption rate was measured using an Infinite F500 fluorescent microplate reader (Tecan, USA) (excitation 380 nm and emission 650 nm) and was expressed as the initial rate of increase in the fluorescent signal, as recommended by the manufacturer.
Adenoviral infection
Adenovirus expressing shRNA for silencing of mouse Cnr1 (Ad-m-Cnr1-shRNA) or containing a scrambled shRNA sequence as a control (Adv-scrambled-shRNA) which, under the control of U6 promoter with the GFP co-expressed under a CMV promoter, were purchased from Vector Biolabs (USA). Mouse MGN3–1 ghrelinoma cells were seeded at a density of 3 × 105 / 200 μL per well in a 96-well plate to achieve full confluence for the measurement of fatty assay oxidation. After overnight cell attachment, the original cell culture medium was replaced with Ad-Cnr1-shRNA or Ad-scramble-shRNA virus-containing medium, which included 2 × 107 plaque-forming units (pfu) per 200 L per well. After 24h incubation of MGN3–1 cells with Ad-Cnr1-shRNA or Ad-scramble-shRNA, the culture media were replaced with glucose-deprivation media for an additional 24h before performing fatty acid oxidation assay as described above. Cellular uptake of the constructs was verified by fluorescent microscopy and the degree of knockdown was determined by rt-PCR.
Quantitative real-time PCR
Total RNA was extracted with QIAzol Lysis Reagent and purified by RNeasy Lipid Tissue Mini Kit, followed by DNase I treatment and evaluation of RNA concentration by NanoDrop 2000c spectrophotometer (Thermo Scientific, USA). A fixed amount of 1 μg RNA was reverse-transcribed to cDNA with iScript cDNA synthesis kit. Quantitative real-time PCR was performed by a StepOne Plus real time PCR system (Applied Biosystems, USA) in a sample containing Fast SYBR™ Green Master Mix, 25 ng of single stranded cDNA transcript and QuantiTect primers against mouse acyl-Coenzyme A dehydrogenase, very long chain (Acadvl), against mouse hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase/enoyl-CoA hydratase (trifunctional protein), beta subunit (Hadhb) or qPCR primer against mouse cannabinoid receptor 1 (Cnr1). Normalization was performed with the primer against mouse ribosomal protein L19 (Rpl19) as a housekeeping gene.
Genotyping
Genotyping was performed from tail or ear clippings using a REDExtract-N-Amp™ tissue PCR kit (MilliporeSigma, USA) according to the manufacturer’s protocol. The following forward (F) and reverse (R) primer sequences were used:
Ghs-r1A:
1F (5’ TCTTCGTGGTGGGCATCTCG 3’) and 1R (5’-CTTCCTCCCGATGAGACTGT-3’) and also F2 (5’ AGCGCATCGCCTTCTATCGC) and R2 (5’-GCTCGACTTTGTCCAGGCC-3’) amplified a 557 bp and 380 bp segments of the wild type and Neo resistance cassette of the mutant mouse, respectively;
Ghrl:
1F (5’-GCTCTGGATGGACATGGCC-3’) and 1R (5’-CTGCGCATGTCTGCTGCTCC-3’) and also 2F (5’-GAGGCTGAAGTTCAGATGTGCGGCG-3’) and 2R (5’-CCTCGAATCAGCAACGGCTTGCCG-3’) amplified a 465 bp and 337 bp segments of the gene in wild type and mutant mouse, respectively;
Cnr1:
F (5’-GTACCATCACCACAGACCTCCTC-3’) and 1R (5’-GGATTCAGAATCATGAAGCACTCCA-3’) and also F (5’ GTACCATCACCACAGACCTCCTC 3’) and 2R (5’-AAGAACGAGATCAGCAGCCTCTGTT-3’) amplified a 350 bp and 150 bp segments of the gene in wild type and mutant mouse, respectively;
Mboat4:
1F (5’-ATCCAGAACCTGGTATCCTGTAATA-3’) and 1R (5’- GTCAATCCCACTGACTCACAACACAG-3’) and also 2F (5’- GAGTTCCTTGTCAAATCTCCAAAGCC-3’) and 2R (5’- GTCAATCCCACTGACTCACAACACAG-3’) amplified a 531 bp and 630 bp segments of the gene in wild type and mutant mouse, respectively.
Products were separated on 3% UltraPure™ Agarose gel in 1xTEA in the presence of GreenGlo Safe DNA Dye and bands were visualized on G:BOX scanner (Syngene, USA).
Fluorescent in situ hybridization.
Pieces of mouse stomach tissues were fixed in 10% neutral buffered formalin (NBF) for 24 hours, embedded in paraffin and sectioned (4 μm) onto glass slides. Tissue sections were processed for fluorescent in situ hybridization (FISH) using the RNAscope® Multiplex Fluorescent Kit v2 according to the manufacturer’s instructions (Wang et al., 2012) (Advanced Cell Diagnostics [ACD], USA). The following mouse RNAscope® FISH probes from ACD were used: Cnr1 in channel 3 (NM_007726.3, region 530 – 1458), Mboat4 in channel 1 (NM_001126314.2, region 435 – 1435), Ghrl in channel 2 (NM_021488.5, region 13 – 550). DAPI was part of the kit and was used to stain for nuclei. For fluorescence detection, sections were analyzed using a Zeiss LSM700 (USA) confocal microscope.
The fluorophores used to visualize the RNAscope® FISH probes were fluorescein, cyanin 3 and cyanin 5 (TSA Cyanin 3 & 5, TMR Fluorescein Evaluation Kit; Perkin Elmer, USA). Fluorophores were diluted in RNAscope® LS Multiplex TSA Buffer (ACD, USA). The fluorophore dilutions for each RNAscope® ISH probe were Cnr1-Fluorescein (1:750), Mboat4-Cyanin 3 (1:750), and Ghrl-Cyanin 5 (1:30000).
Endocannabinoid measurement
Endocannabinoids were extracted and quantified by liquid chromatography–tandem mass spectrometry (LC-MS/MS) as described (Mukhopadhyay et al., 2011). Stomach tissue or MGN- 3 cells were homogenized in 0.5 mL of ice-cold methanol/Tris buffer (50 mM, pH 8.0), 1:1, containing 7 ng of [2H4]arachidonoyl ethanolamide ([2H4]AEA) as internal standard. Homogenates were extracted three times with CHCl3:methanol (2:1, vol/vol), dried under nitrogen and reconstituted with methanol after precipitating proteins with ice-cold acetone. Liquid chromatography tandem mass spectrometry (LC-MS/MS) analyses were conducted on an Agilent 6410 triple quadrupole mass spectrometer (Agilent Technologies) coupled to an Agilent 1200 LC system. Analytes were separated using a Zorbax SB-C18 rapid-resolution HT column. Gradient elution mobile phases consisted of 0.1% formic acid in water (phase A) and 0.1% formic acid in methanol (phase B). Gradient elution (250 μL/min) was initiated and held at 10% B for 0.5 min, followed by a linear increase to 85% B at 1 min and maintained until 12.5 min, then increased linearly to 100% B at 13 min and maintained until 14.5 min. The mass spectrometer was set for electrospray ionization operated in positive ion mode. The source parameters were as follows: capillary voltage, 4,000 V; gas temperature, 350°C; drying gas, 10 L/min; nitrogen was used as the nebulizing gas. Collision-induced dissociation was performed using nitrogen. Level of each compound was analyzed by multiple reactions monitoring. The molecular ion and fragment for each compound were measured as follows: m/z 348.3→62.1 for AEA, m/z 352.3→66.1 for [2H4]AEA and m/z 379.3→91.1 for 2-AG. Analytes were quantified using Mass-Hunter Workstation LC/QQQ Acquisition and MassHunter Workstation Quantitative Analysis software (Agilent Technologies). Levels of AEA and 2-AG in the samples were measured against standard curves. Values are expressed as pmol or nmol/g wet tissue or per number of cultured cells.
Acyl-carnitine measurement
Stable isotope-labelled acyl-carnitines were measured by LC-MS/MS. The sample extraction, chromatography and mass spectrometry conditions were set as described (Cinar et al., 2014). The extraction efficiency and chromatographic validation was performed using unlabeled acyl-carnitine standards obtained from MilliporeSigma (St Louis, MI). The extraction efficiency and chromatographic validation was performed using unlabeled acyl-carnitine standards obtained from MilliporeSigma (St Louis, MI). The labelled acyl-carnitines were generated in vitro in MGN3–1 cells after treatment with either [2H31]palmitic acid or [13C4]octanoic acid as detailed above. The cells were treated with vehicle, unlabeled fatty acids or labelled fatty acids, then cell extracts were injected to the LC-MS/MS under scanning mode to capture the corresponding mass [M+H]+ and chromatography of the generated acyl-carnitines. The labelled acyl-carnitines were analyzed by multiple reactions monitoring (MRM). In MRM mode, detection of each compound is based on fragmentation of the precursor ion [M+H]+ to yield a prominent product ion. Mass spectrometric conditions were optimized with serial injections of [2H31]palmitic acid or [13C4]octanoic acid-treated cell extracts by using MassHunter Workstation Optimizer software (Agilent Technologies, USA). Fragmentation of the molecular ion and mass spectrometric conditions of [2H31]palmitoyl carnitine, [2H26]myristoyl carnitine, [2H21]lauroyl carnitine, [2H14]octanoyl carnitine, [13C4]octanoyl carnitine, [2H10]hexanoyl carnitine and [13C4]hexanoyl carnitine are listed in supplementary Table 2.
Drugs
JD5037 was synthesized as described (Chorvat et al., 2012). Sources of the combined fatty acid amide hydrolase/monoacylglycerol lipase inhibitor JZL195 (Long et al., 2009), JMV2959, cholecystokinin octapeptide (CCK-8), 4-bromocrotonic acid, rimonabant, [2H31]palmitic acid, octanoic acid-1,2,3,4-13C4 are provided in the Key Resources Table. All other chemicals including the peripheral GOAT inhibitor PF-51904577 were from MilliporeSigma (USA). For animal studies, (±)-propranolol hydrochloride was dissolved in saline and sterile filtered for i.p. injections. Other drugs were dissolved in DMSO:Tween 80:saline (5:2:93). Use of this vehicle is critical for keeping JD5037 in clear solution in order to maintain ~25% oral bioavailability. For in vitro studies, drugs were dissolved in DMSO and applied in a volume of 1 μL per 1 mL of cell medium. Protease inhibitors were prepared as 100x concentrated solutions: aprotynin was dissolved in saline, whereas PHMB was dissolved in saline containing 1.2% (v/v) of 10N NaOH and stored aliquoted for one month at −20°C. 10 μL of protease inhibitors was used per 1 mL of blood. Palmitic acid and octanoic acid were prepared as 250 mM stock solutions in 75% ethanol at 70°C for 10 min. To make fatty acid-BSA conjugat es, the fatty acid stock solution was added dropwise into 10% BSA to make a final stock of 10 mM. The solution was continuously mixed at 50°C for 10–60 min, then brought to room temperatur e and sterile filtered. 50 μM octanoate-BSA and palmitate-BSA complexes were used as a final concentration in most cell experiments.
When evaluating the effects of JF5037 and MRI-2443 at 10 μM or 250 μM octanoate-BSA in cell medium, stock solutions of octanoic acid were adjusted correspondingly. Capsaicin was dissolved in a solution of 10% ethanol, 10% Tween-80 in isotonic saline. The capsaicin/vehicle solutions were prepared aseptically. CCK-8 was dissolved aseptically in saline (Hospira Inc., USA).
GOAT inhibitor MRI-2443: (S)-N-(1-(4-(2-(4-amino-6-chloro-2-methylpyrimidin-5-yl)ethyl)piperidin-1-yl)-1-oxopropan-2-yl)cyclopropanecarboxamide (Martinez-Grau, 2015),
 was synthesized using commercially available starting materials and (2S)-2-(tert-butoxycarbonylamino)propanoic acid (Boc-L-Alanine) using the procedures outlined in PCT/2014/064202. The structure and purity were verified by NMR spectroscopy and LC-MS. 1H-NMR (400 MHz, CDCl3): δ 6.84 (d, J = 7.1 Hz, 1H), 5.05 (s, 2H), 4.90 (dd, J = 11.2, 6.6 Hz, 1H), 4.60–4.55 (m, 1H), 3.91–3.86 (m, 1H), 3.02 (q, J = 10.0 Hz, 1H), 2.60–2.53 (m, 2H), 2.43 (s, 3H), 1.90–1.80 (m, 3H), 1.64–1.56 (m, 1H), 1.47 (t, J = 7.6 Hz, 2H), 1.39 (dd, J = 7.6, 3.9 Hz, 1H), 1.28 (q, J = 8.0 Hz, 3H), 1.25–1.12 (m, 2H), 0.92 (d, J = 3.7 Hz, 2H), 0.71 (d, J = 7.0 Hz, 2H). LC-MS detection was carried out on Agilent 1200 using Luna C18 3 um (3 × 75 mm). The mobile phase was 4% to 100% acetonitrile (0.05% TFA) standard gradient. LC-MS m/z for C19H28ClN5O2 (MH+) 394.1.
was synthesized using commercially available starting materials and (2S)-2-(tert-butoxycarbonylamino)propanoic acid (Boc-L-Alanine) using the procedures outlined in PCT/2014/064202. The structure and purity were verified by NMR spectroscopy and LC-MS. 1H-NMR (400 MHz, CDCl3): δ 6.84 (d, J = 7.1 Hz, 1H), 5.05 (s, 2H), 4.90 (dd, J = 11.2, 6.6 Hz, 1H), 4.60–4.55 (m, 1H), 3.91–3.86 (m, 1H), 3.02 (q, J = 10.0 Hz, 1H), 2.60–2.53 (m, 2H), 2.43 (s, 3H), 1.90–1.80 (m, 3H), 1.64–1.56 (m, 1H), 1.47 (t, J = 7.6 Hz, 2H), 1.39 (dd, J = 7.6, 3.9 Hz, 1H), 1.28 (q, J = 8.0 Hz, 3H), 1.25–1.12 (m, 2H), 0.92 (d, J = 3.7 Hz, 2H), 0.71 (d, J = 7.0 Hz, 2H). LC-MS detection was carried out on Agilent 1200 using Luna C18 3 um (3 × 75 mm). The mobile phase was 4% to 100% acetonitrile (0.05% TFA) standard gradient. LC-MS m/z for C19H28ClN5O2 (MH+) 394.1.
QUANTIFICATION AND STATISTICAL ANALYSIS
Values are presented as mean ± SEM, with the number of replicates and the level of significance reported in figures, figure legends and supplementary tables. Statistical data analysis was performed using GraphPad Prism 7 for Windows (GraphPad Software, USA). A two-tailed Student’s t-test for paired or unpaired data was used for comparison of values between two groups. Data were assumed to have normal distribution as indicated by the D’Agostino & Pearson normality test. For multiple groups, ordinary one-way ANOVA followed by the Dunnett’s post-hoc test was applied. Time-dependent variables were analyzed by two-way ANOVA with Bonferroni’s correction when one group was compared to the control or Tukey’s multiple comparisons test. Differences were considered significant when P < 0.05.
Sample sizes for in vivo experiments were based on prior experience that 8 to 10 mice per treatment group provided reproducible values and differences. A much larger group of mice was used in the initial phase of the project (n = 17–24 mice per group, see Figure 1A) to obtain biological samples for screening purposes before ghrelin was selected as the focus of the study. Sample size was also dependent on the availability of animals through in-house breeding, which aimed to generate age- and weight-matched male littermates of 8–10 mice per treatment group. Randomization of animals into different treatment groups was done after the initial control drinking period to assure that mean values of alcohol intake and preference were similar in the vehicle versus drug treatment groups. The number of animals that underwent subdiaphragmatic vagotomy with pyloroplasty was limited by the Institutional Animal Care and Use Committee. The investigator monitoring the drinking pattern of these mice was blinded to their sham or vagotomized status. In vitro experiments were performed in duplicates. Cells from two dishes (acyl carnitine measurement) or three dishes (endocannabinoid measurement) were combined as a single biological replicate for more accurate detection. The initial slope of the fluorescence intensity in the Fatty Acid Oxidation assay was assessed from the same linear portion of the slope between 3 and 9 minutes, using GraphPad Prism. No data were excluded from in vitro studies, whereas a total of 10 mice were excluded from the drinking studies because of excessive weight loss caused by malocclusion (5 mice), seizures (2 mice) or complications of surgery (3 mice).
Supplementary Material
Highlights.
CB1R and ghrelin work together to promote alcohol intake via a gut-brain axis
Alcohol intake is inhibited by CB1R blockade, but only when ghrelin signaling is intact
Inhibition of peripheral CB1R reduces plasma levels of biologically active ghrelin
CB1R blockade promotes the degradation of the substrate needed to activate ghrelin
Context and Significance.
Endocannabinoids and the stomach-derived protein ghrelin both act as signaling molecules, and both are implicated in promoting appetite, including that for alcohol. Godlewski et al. show that these two molecules are functionally linked as they find that endocannabinoids promote the formation of biologically active ghrelin in the stomach, where the latter then promotes a gut-brain signaling pathway that leads to increased alcohol intake. They further show that peripherally-restricted pharmacological inhibition of this pathway reduces voluntary alcohol drinking in mice. As direct pharmacological targeting of the brain is undesired given the potential for adverse neuropsychological effects, the peripherally-centered approach described here may be a safer approach for the treatment of alcoholism.
Acknowledgement.
We thank Dr. Jeffrey M. Zigman for valuable discussions and advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in Its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary information includes 6 figures and 2 tables and can be found with this article online at https://doi.org/10.2016/j.cmet.2019
Declaration of Interests.The authors confirm that there are no competing interests.
REFERENCES
- Alen F, Crespo I, Ramirez-Lopez MT, Jagerovic N, Goya P, de Fonseca FR, de Heras RG, and Orio L (2013). Ghrelin-induced orexigenic effect in rats depends on the metabolic status and is counteracted by peripheral CB1 receptor antagonism. PLoS One 8, e60918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquila S, Guido C, Santoro A, Gazzerro P, Laezza C, Baffa MF, Ando S, and Bifulco M (2010). Rimonabant (SR141716) induces metabolism and acquisition of fertilizing ability in human sperm. Br J Pharmacol 159, 831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold M, Mura A, Langhans W, and Geary N (2006). Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci 26, 11052–11060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bando M, Iwakura H, Koyama H, Hosoda H, Shigematsu Y, Ariyasu H, Akamizu T, Kangawa K, and Nakao K (2016). High incorporation of long-chain fatty acids contributes to the efficient production of acylated ghrelin in ghrelin-producing cells. FEBS Lett 590, 992–1001. [DOI] [PubMed] [Google Scholar]
- Banks WA, Tschop M, Robinson SM, and Heiman ML (2002). Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther 302, 822–827. [DOI] [PubMed] [Google Scholar]
- Bellocchio L, Soria-Gomez E, Quarta C, Metna-Laurent M, Cardinal P, Binder E, Cannich A, Delamarre A, Haring M, Martin-Fontecha M, et al. (2013). Activation of the sympathetic nervous system mediates hypophagic and anxiety-like effects of CB(1) receptor blockade. Proc Natl Acad Sci U S A 110, 4786–4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya SK, Andrews K, Beveridge R, Cameron KO, Chen C, Dunn M, Fernando D, Gao H, Hepworth D, Jackson VM, et al. (2014). Discovery of PF-5190457, a Potent, Selective, and Orally Bioavailable Ghrelin Receptor Inverse Agonist Clinical Candidate. ACS Med Chem Lett 5, 474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breit S, Kupferberg A, Rogler G, and Hasler G (2018). Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front Psychiatry 9, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G, Varro A, Dimaline R, Thompson DG, and Dockray GJ (2006). Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am J Physiol Gastrointest Liver Physiol 290, G1289–1297. [DOI] [PubMed] [Google Scholar]
- Callaghan B, and Furness JB (2014). Novel and conventional receptors for ghrelin, desacyl-ghrelin, and pharmacologically related compounds. Pharmacological reviews 66, 984–1001. [DOI] [PubMed] [Google Scholar]
- Cani PD, Montoya ML, Neyrinck AM, Delzenne NM, and Lambert DM (2004). Potential modulation of plasma ghrelin and glucagon-like peptide-1 by anorexigenic cannabinoid compounds, SR141716A (rimonabant) and oleoylethanolamide. Br J Nutr 92, 757–761. [DOI] [PubMed] [Google Scholar]
- Chorvat RJ, Berbaum J, Seriacki K, and McElroy JF (2012). JD-5006 and JD-5037: peripherally restricted (PR) cannabinoid-1 receptor blockers related to SLV-319 (Ibipinabant) as metabolic disorder therapeutics devoid of CNS liabilities. Bioorg Med Chem Lett 22, 6173–6180. [DOI] [PubMed] [Google Scholar]
- Christinat N, Morin-Rivron D, and Masoodi M (2016). High-Throughput Quantitative Lipidomics Analysis of Nonesterified Fatty Acids in Human Plasma. J Proteome Res 15, 2228–2235. [DOI] [PubMed] [Google Scholar]
- Cinar R, Godlewski G, Liu J, Tam J, Jourdan T, Mukhopadhyay B, Harvey-White J, and Kunos G (2014). Hepatic cannabinoid-1 receptors mediate diet-induced insulin resistance by increasing de novo synthesis of long-chain ceramides. Hepatology 59, 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, and Geary N (2007). Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes 56, 1051–1058. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Vacca G, Carai MA, and Gessa GL (2005). Endocannabinoid system and alcohol addiction: pharmacological studies. Pharmacol Biochem Behav 81, 369–380. [DOI] [PubMed] [Google Scholar]
- Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, and Nakazato M (2002). The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 123, 1120–1128. [DOI] [PubMed] [Google Scholar]
- Date Y, Toshinai K, Koda S, Miyazato M, Shimbara T, Tsuruta T, Niijima A, Kangawa K, and Nakazato M (2005). Peripheral interaction of ghrelin with cholecystokinin on feeding regulation. Endocrinology 146, 3518–3525. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, et al. (2001). Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 410, 822–825. [DOI] [PubMed] [Google Scholar]
- DiPatrizio NV, Astarita G, Schwartz G, Li X, and Piomelli D (2011). Endocannabinoid signal in the gut controls dietary fat intake. Proc Natl Acad Sci U S A 108, 12904–12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPatrizio NV, and Simansky KJ (2008). Activating parabrachial cannabinoid CB1 receptors selectively stimulates feeding of palatable foods in rats. J Neurosci 28, 9702–9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelstoft MS, Park WM, Sakata I, Kristensen LV, Husted AS, Osborne-Lawrence S, Piper PK, Walker AK, Pedersen MH, Nohr MK, et al. (2013). Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Molecular metabolism 2, 376–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlanson-Albertsson C, and Lindqvist A (2008). Vagotomy and accompanying pyloroplasty down-regulates ghrelin mRNA but does not affect ghrelin secretion. Regulatory peptides 151, 14–18. [DOI] [PubMed] [Google Scholar]
- Gautron L, Sakata I, Udit S, Zigman JM, Wood JN, and Elmquist JK (2011). Genetic tracing of Nav1.8-expressing vagal afferents in the mouse. J Comp Neurol 519, 3085–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary N, Wolfe A, Polidori C, Policani F, and Massi M (2004). Exogeneous and endogenous CCK inhibit ethanol ingestion in Sardinian alcohol-preferring rats. Peptides 25, 1185–1194. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, and Koob GF (2010). Effects of beta-adrenoceptor antagonists on alcohol drinking by alcohol-dependent rats. Psychopharmacology 212, 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez del Pulgar T, Velasco G, Sanchez C, Haro A, and Guzman M (2002). De novo-synthesized ceramide is involved in cannabinoid-induced apoptosis. Biochem J 363, 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JL, Cunningham CL, Finn DA, Young EA, Helpenstell LK, Schuette LM, Fidler TL, Kosten TA, and Ryabinin AE (2015). Differential effects of ghrelin antagonists on alcohol drinking and reinforcement in mouse and rat models of alcohol dependence. Neuropharmacology 97, 182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, Cippitelli A, Nava F, Piomelli D, and Rodriguez de Fonseca F (2002). A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci 22, 9612–9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnal A, Zharikov A, Polston JE, Fields MR, Tomasko J, Rogers AM, Volkow ND, and Thanos PK (2012). Alcohol reward is increased after Roux-en-Y gastric bypass in dietary obese rats with differential effects following ghrelin antagonism. PLoS One 7, e49121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Tellez LA, Perkins MH, Perez IO, Qu T, Ferreira J, Ferreira TL, Quinn D, Liu ZW, Gao XB, et al. (2018). A Neural Circuit for Gut-Induced Reward. Cell 175, 887–888. [DOI] [PubMed] [Google Scholar]
- Hering D, Kucharska W, Kara T, Somers VK, and Narkiewicz K (2011). Potentiated sympathetic and hemodynamic responses to alcohol in hypertensive vs. normotensive individuals. J Hypertens 29, 537–541. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Karlsson C, Jucaite A, Varnas K, Wahlby Hamren U, Johnstrom P, Gulyas B, Donohue SR, Pike VW, Halldin C, et al. (2016). A PET study comparing receptor occupancy by five selective cannabinoid 1 receptor antagonists in non-human primates. Neuropharmacology 101, 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungund BL, Szakall I, Adam A, Basavarajappa BS, and Vadasz C (2003). Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. Journal of neurochemistry 84, 698–704. [DOI] [PubMed] [Google Scholar]
- Iwakura H, Li Y, Ariyasu H, Hosoda H, Kanamoto N, Bando M, Yamada G, Hosoda K, Nakao K, Kangawa K, et al. (2010). Establishment of a novel ghrelin-producing cell line. Endocrinology 151, 2940–2945. [DOI] [PubMed] [Google Scholar]
- Jancso G, Kiraly E, and Jancso-Gabor A (1977). Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature 270, 741–743. [DOI] [PubMed] [Google Scholar]
- Jbilo O, Ravinet-Trillou C, Arnone M, Buisson I, Bribes E, Peleraux A, Penarier G, Soubrie P, Le Fur G, Galiegue S, et al. (2005). The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 19, 1567–1569. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, Datta R, Perrissoud D, Dickson SL, and Engel JA (2009). Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci U S A 106, 11318–11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E, Ivanoff L, Vater A, and Engel JA (2014). Peripherally circulating ghrelin does not mediate alcohol-induced reward and alcohol intake in rodents. Alcoholism, clinical and experimental research 38, 959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, Shen X, and Bohorquez DV (2018). A gut-brain neural circuit for nutrient sensory transduction. Science 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner H, Gutierrez JA, Solenberg PJ, Pfluger PT, Czyzyk TA, Willency JA, Schurmann A, Joost HG, Jandacek RJ, Hale JE, et al. (2009). GOAT links dietary lipids with the endocrine control of energy balance. Nat Med 15, 741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kola B, Farkas I, Christ-Crain M, Wittmann G, Lolli F, Amin F, Harvey-White J, Liposits Z, Kunos G, Grossman AB, et al. (2008). The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS One 3, e1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kola B, Hubina E, Tucci SA, Kirkham TC, Garcia EA, Mitchell SE, Williams LM, Hawley SA, Hardie DG, Grossman AB, et al. (2005). Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. J Biol Chem 280, 25196–25201. [DOI] [PubMed] [Google Scholar]
- Kong J, Chuddy J, Stock IA, Loria PM, Straub SV, Vage C, Cameron KO, Bhattacharya SK, Lapham K, McClure KF, et al. (2016). Pharmacological characterization of the first in class clinical candidate PF-05190457: a selective ghrelin receptor competitive antagonist with inverse agonism that increases vagal afferent firing and glucose-dependent insulin secretion ex vivo. Br J Pharmacol 173, 1452–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopin AS, Mathes WF, McBride EW, Nguyen M, Al-Haider W, Schmitz F, Bonner-Weir S, Kanarek R, and Beinborn M (1999). The cholecystokinin-A receptor mediates inhibition of food intake yet is not essential for the maintenance of body weight. J Clin Invest 103, 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama H, Iwakura H, Dote K, Bando M, Hosoda H, Ariyasu H, Kusakabe T, Son C, Hosoda K, Akamizu T, et al. (2016). Comprehensive Profiling of GPCR Expression in Ghrelin-Producing Cells. Endocrinology 157, 692–704. [DOI] [PubMed] [Google Scholar]
- le Roux CW, Neary NM, Halsey TJ, Small CJ, Martinez-Isla AM, Ghatei MA, Theodorou NA, and Bloom SR (2005). Ghrelin does not stimulate food intake in patients with surgical procedures involving vagotomy. The Journal of clinical endocrinology and metabolism 90, 4521–4524. [DOI] [PubMed] [Google Scholar]
- Lee MR, Tapocik JD, Ghareeb M, Schwandt ML, Dias AA, Le AN, Cobbina E, Farinelli LA, Bouhlal S, Farokhnia M, et al. (2018). The novel ghrelin receptor inverse agonist PF-5190457 administered with alcohol: preclinical safety experiments and a phase 1b human laboratory study. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Zywiak WH, Fricchione SR, Edwards SM, de la Monte SM, Swift RM, and Kenna GA (2014). Intravenous ghrelin administration increases alcohol craving in alcohol-dependent heavy drinkers: a preliminary investigation. Biological psychiatry 76, 734–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Schmidt SF, and Friedman JM (2013). Developmental role for endocannabinoid signaling in regulating glucose metabolism and growth. Diabetes 62, 2359–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CT, Kola B, Feltrin D, Perez-Tilve D, Tschop MH, Grossman AB, and Korbonits M (2013). Ghrelin and cannabinoids require the ghrelin receptor to affect cellular energy metabolism. Mol Cell Endocrinol 365, 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, Jin X, Burston JJ, Sim-Selley LJ, Lichtman AH, Wiley JL, et al. (2009). Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci U S A 106, 20270–20275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccioni P, Colombo G, and Carai MA (2010). Blockade of the cannabinoid CB1 receptor and alcohol dependence: preclinical evidence and preliminary clinical data. CNS Neurol Disord Drug Targets 9, 55–59. [DOI] [PubMed] [Google Scholar]
- Martinez-Grau MA (2015). Substituted piperidyl-ethyl-pyrimidineas ghrelin-o-acyl transferase inhibitor. WIPO/PCT, ed. [Google Scholar]
- Mukhopadhyay B, Cinar R, Yin S, Liu J, Tam J, Godlewski G, Harvey-White J, Mordi I, Cravatt BF, Lotersztajn S, et al. (2011). Hyperactivation of anandamide synthesis and regulation of cell-cycle progression via cannabinoid type 1 (CB1) receptors in the regenerating liver. Proc Natl Acad Sci U S A 108, 6323–6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki M, Jyotaki M, Yoshida R, Yasumatsu K, Shigemura N, DiPatrizio NV, Piomelli D, and Ninomiya Y (2015). Modulation of sweet taste sensitivities by endogenous leptin and endocannabinoids in mice. The Journal of physiology 593, 2527–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perwitz N, Wenzel J, Wagner I, Buning J, Drenckhan M, Zarse K, Ristow M, Lilienthal W, Lehnert H, and Klein J (2010). Cannabinoid type 1 receptor blockade induces transdifferentiation towards a brown fat phenotype in white adipocytes. Diabetes Obes Metab 12, 158–166. [DOI] [PubMed] [Google Scholar]
- Ravinet Trillou C, Delgorge C, Menet C, Arnone M, and Soubrie P (2004). CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity.Int J Obes Relat Metab Disord 28, 640–648. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, and Crabbe JC (2005). Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav 84, 53–63. [DOI] [PubMed] [Google Scholar]
- Sakurazawa N, Mano-Otagiri A, Nemoto T, and Shibasaki T (2013). Effects of intracerebroventricular ghrelin on food intake and Fos expression in the arcuate nucleus of the hypothalamus in female rats vary with estrous cycle phase. Neurosci Lett 541, 204–208. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Moran TH, White WO, and Ladenheim EE (1997). Relationships between gastric motility and gastric vagal afferent responses to CCK and GRP in rats differ. Am J Physiol 272, R1726–1733. [DOI] [PubMed] [Google Scholar]
- Senin LL, Al-Massadi O, Folgueira C, Castelao C, Pardo M, Barja-Fernandez S, Roca-Rivada A, Amil M, Crujeiras AB, Garcia-Caballero T, et al. (2013). The gastric CB1 receptor modulates ghrelin production through the mTOR pathway to regulate food intake. PLoS One 8, e80339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Fujita S, Izumi K, Koja T, Ohba N, and Fukuda T (1984). Corneal lesions induced by the systemic administration of capsaicin in neonatal mice and rats. Naunyn Schmiedebergs Arch Pharmacol 326, 347–351. [DOI] [PubMed] [Google Scholar]
- Shrestha R, Hui SP, Imai H, Hashimoto S, Uemura N, Takeda S, Fuda H, Suzuki A, Yamaguchi S, Hirano K, et al. (2015). Plasma capric acid concentrations in healthy subjects determined by high-performance liquid chromatography. Ann Clin Biochem 52, 588–596. [DOI] [PubMed] [Google Scholar]
- Sirohi S, Richardson BD, Lugo JM, Rossi DJ, and Davis JF (2017). Impact of Roux-en-Y gastric bypass surgery on appetite, alcohol intake behaviors, and midbrain ghrelin signaling in the rat. Obesity (Silver Spring) 25, 1228–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR, and Piomelli D (2008). The endocannabinoid system in brain reward processes. Br J Pharmacol 154, 369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Ahmed S, and Smith RG (2003). Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol 23, 7973–7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Wang P, Zheng H, and Smith RG (2004). Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci U S A 101, 4679–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J, Cinar R, Liu J, Godlewski G, Wesley D, Jourdan T, Szanda G, Mukhopadhyay B, Chedester L, Liow JS, et al. (2012). Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab 16, 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J, Vemuri VK, Liu J, Batkai S, Mukhopadhyay B, Godlewski G, Osei-Hyiaman D, Ohnuma S, Ambudkar SV, Pickel J, et al. (2010). Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest 120, 2953–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udi S, Hinden L, Earley B, Drori A, Reuveni N, Hadar R, Cinar R, Nemirovski A, and Tam J (2017). Proximal Tubular Cannabinoid-1 Receptor Regulates Obesity-Induced CKD. J Am Soc Nephrol 28, 3518–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, Wu X, Vo HT, Ma XJ, and Luo Y (2012). RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn 14, 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu J, Harvey-White J, Zimmer A, and Kunos G (2003). Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci U S A 100, 1393–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittekind DA, Kratzsch J, Mergl R, Enzenbach C, Witte AV, Villringer A, and Kluge M (2018). Alcohol consumption is positively associated with fasting serum ghrelin in non-dependent adults: Results from the population-based LIFE-Adult-Study. Psychoneuroendocrinology 97, 143–148. [DOI] [PubMed] [Google Scholar]
- Yang J, Brown MS, Liang G, Grishin NV, and Goldstein JL (2008). Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 132, 387–396. [DOI] [PubMed] [Google Scholar]
- Zallar LJ, Beurmann S, Tunstall BJ, Fraser CM, Koob GF, Vendruscolo LF, and Leggio L (2018). Ghrelin receptor deletion reduces binge-like alcohol drinking in rats. J Neuroendocrinol, e12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao TJ, Sakata I, Li RL, Liang G, Richardson JA, Brown MS, Goldstein JL, and Zigman JM (2010). Ghrelin secretion stimulated by {beta}1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proc Natl Acad Sci U S A 107, 15868–15873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, and Elmquist JK (2006). Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 494, 528–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, and Bonner TI (1999). Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci U S A 96, 5780–5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.