Abstract
In utero alcohol exposure can disrupt the development of the fetal brain and result in a wide-range of neurobehavioral outcomes collectively known as fetal alcohol spectrum disorders (FASD). This paper provides a comprehensive review of the cognitive and behavioral outcomes of prenatal alcohol exposure, including domains of general intelligence, executive functioning, language development, learning and memory, adaptive functioning, academic performance, and concurrent psychopathology. In addition, the current status of the neurobehavioral profile of FASD and its potential as a diagnostic tool will be discussed.
Keywords: fetal alcohol syndrome (FAS), fetal alcohol spectrum disorders (FASD), neurobehavioral profile, brain, cognition, behavior
INTRODUCTION
Since the identification of alcohol as a teratogen in 1973 (Jones and Smith, 1973, Jones et al., 1973), an extensive amount of research focused on the long-term effects of prenatal alcohol exposure indicates that consuming alcoholic beverages during pregnancy can significantly affect fetal development. The physical and neurobehavioral effects of alcohol are collectively known as fetal alcohol spectrum disorders (FASD; Bertrand et al., 2005, Mattson et al., 2011). Diagnoses on the severe end of the spectrum such as fetal alcohol syndrome (FAS) and related disorders (e.g., partial FAS [PFAS]) rely on the presence of distinct facial anomalies and other physical dysmorphology. However, many individuals with histories of prenatal alcohol exposure do not display these characteristic physical features and consequently may go undiagnosed or misdiagnosed despite significantly impairing cognitive and behavioral deficits (Chasnoff et al., 2015). As a result, research has focused on delineating the specific neurobehavioral deficits associated with prenatal alcohol exposure in the absence of physical dysmorphology with the aim of improving identification of affected individuals. This paper will review the literature pertaining to neuropsychological and behavioral features of FASD and discuss the emerging neurobehavioral profile for FASD.
DIAGNOSTIC TERMINOLOGY
Alcohol is a teratogen that can disrupt prenatal development and negatively affect the fetus. FASD refers to the range of effects that can occur due to prenatal alcohol exposure and includes FAS, PFAS, alcohol-related neurodevelopmental disorder (ARND), alcohol-related birth defects (ARBD), and neurobehavioral disorder associated with prenatal alcohol exposure (ND-PAE). Diagnoses on the more severe end of the spectrum (i.e., FAS and PFAS) require presence of characteristic facial anomalies initially identified by Jones and Smith (1973), including short palpebral fissures, thin vermilion border, and smooth philtrum. Additionally, a diagnosis of FAS requires evidence of growth deficiency (height and/or weight ≤ 10th percentile for chronological age), abnormal brain growth (head circumference ≤ 10th percentile, brain structure anomalies, or recurrent nonfebrile seizures), and neurobehavioral impairment, which can include cognitive impairment, behavioral impairment, or both (Hoyme et al., 2016). For those without documented alcohol exposure, a PFAS diagnosis requires the characteristic facial features, presence of either a growth deficiency or abnormal brain growth, and neurobehavioral impairment (Hoyme et al., 2016). Not all alcohol-exposed individuals exhibit physical features to the extent required for diagnosis (Bertrand et al., 2005, Mattson et al., 2011); diagnoses used in such cases may include ARND, ARBD, and ND-PAE. Both ARND and ARBD are used in the absence of physical dysmorphology while ND-PAE can be used with or without a diagnosis of FAS. An ARND diagnosis requires confirmed maternal prenatal alcohol use and evidence of neurobehavioral impairment (Hoyme et al., 2016, Stratton et al., 1996). Individuals diagnosed with ARBD have a documented history of prenatal alcohol exposure and present with one or more physical abnormalities (Hoyme et al., 2016). Lastly, ND-PAE has been included in the Diagnostic and Statistical Manual of Mental Disorders – Fifth Edition (American Psychiatric Association, 2013) under “conditions for further study” and encompasses a range of neurobehavioral effects that are associated with prenatal alcohol exposure. The proposed DSM-5 diagnostic criteria for ND-PAE includes a confirmed history of prenatal alcohol exposure along with deficits in neurocognitive, self-regulation, and adaptive functioning domains. This proposed diagnosis can occur independently of the physical dysmorphology associated with alcohol exposure and both diagnoses (FAS/PFAS and ND-PAE) can be given. Similar diagnostic methods are present internationally; however, several important differences in the criteria listed should be noted, including the number of facial features required to make a diagnosis, the inclusion (or absence) of growth deficits, number of diagnostic categories, and acceptable measures to establish documented prenatal alcohol exposure during pregnancy (American Psychiatric Association, 2013, Coles et al., 2016, Cook et al., 2016, Hoyme et al., 2016). A lack of consistent diagnostic criteria across countries contributes to difficulty in establishing accurate prevalence rates and identifying relevant resources for affected individuals. Developing standard diagnostic criteria and terminology is imperative to be able to accurately evaluate FASD on an international level. In general, deficits occur across the full spectrum of alcohol exposure. For the purposes of this paper, the term FASD will refer to those with histories of prenatal alcohol exposure regardless of dysmorphology. Differences between diagnostic group will be noted where appropriate. Diagnostic criteria are summarized in Table 1.
Table 1.
Summary of diagnoses within the continuum of fetal alcohol spectrum disorders (FASD). Includes updated diagnostic criteria for FAS, PFAS, ARND and ARBD (Hoyme et al., 2016), as well as recommended criteria for ND-PAE (American Psychiatric Association, 2013).
| Diagnostic Categories |
Required Diagnostic Elements |
|---|---|
| FAS | Fetal alcohol syndrome (FAS; with or without documented exposurea)
|
| PFAS | Partial FAS (PFAS; with documented exposurea)
Partial FAS (PFAS; without documented exposure)
|
| ARND | Alcohol-related neurobehavioral disorder (ARND)
|
| ARBD | Alcohol-related birth defects (ARBD)
|
| ND-PAE | Neurobehavioral disorder associated with prenatal alcohol exposure (ND-PAE)
|
Criteria for children three years or older.
- Six or more drinks per week for two or more weeks during pregnancy
- Three or more drinks per occasion on two or more occasions during pregnancy
- Alcohol-related social or legal problems before/during pregnancy
- Evidence of alcohol-use during pregnancy (i.e. blood, breath, urine test)
- Documentation of alcohol-exposure during pregnancy or at birth using established biomarkers
- Increased risk of drinking during pregnancy determined by the use of a validated screening tool
Early diagnosis is important to help determine appropriate early interventions for the child (Bertrand et al., 2005). However, there are many challenges to identification and diagnosis of affected children, including lack of confirmation of prenatal alcohol exposure, lack of clinical expertise, and many possible confounding variables. Several factors, including changes in custody (i.e., children not in the care of their biological mothers), maternal death, or social stigma, make documentation of prenatal alcohol exposure challenging or impossible. In the absence of such documentation, identification of at-risk children is difficult. Even when exposure is known, several factors may affect diagnosis. For example, because the characteristic facial anomalies and growth deficits may become less distinctive after puberty (Streissguth et al., 1991b), older children or adults may not be accurately identified. Similarly, potential ethnic differences in the key facial dysmorphological characteristics (Moore et al., 2007) may limit accuracy of diagnosis. Finally, and perhaps most importantly, the majority of youth affected by prenatal alcohol exposure do not display the outward signs of their exposure. Physical and cognitive deficits can occur for a multitude of reasons and thus without the presence of facial dysmorphology or documented prenatal alcohol exposure, it is difficult to establish an etiologic link between prenatal alcohol exposure and disordered behavior or cognition. Thus, research has focused on developing and refining a neurobehavioral profile for FASD and designing identification tools that do not rely on the presence of physical characteristics solely.
FACTORS IMPACTING EXPOSURE
Important factors that can influence the impact of prenatal alcohol exposure include the dose and developmental timing in which the exposure occurs. In terms of dosage, the amount of alcohol to which the fetus is exposed influences the extent of the damage and severity of developmental deficits (Alvik et al., 2013, Maier and West, 2001). Binge drinking patterns, defined for females as consuming four or more drinks in about two hours (National Institute of Alcohol Abuse and Alcoholism, 2004), exposes the fetus to high alcohol concentrations over relatively short periods of time (Maier and West, 2001). Such drinking patterns may result in more severe behavioral and cognitive deficits than continuous drinking patterns (Flak et al., 2014). Due to the ability to control confounding factors and specific dosage information, animal models have been imperative in elucidating specific dosage and timing questions related to prenatal alcohol exposure. The severity of microcephaly (limited brain growth) in rats is related to the concentration of administered alcohol; rats that receive a highly concentrated alcohol dose (equivalent to a binge drinking pattern) have significant brain growth impairment as compared to rats that receive small, continuous amounts of alcohol (Bonthius and West, 1988). Some observational studies in humans have been conducted and substantiate animal model findings. Binge drinking during pregnancy was predictive of child behavioral problems (Alvik et al., 2013), increased mental health problems (especially in hyperactivity/inattention domains), and lower academic scores (Sayal et al., 2014). Altogether, these studies demonstrate the importance of frequency and concentration of prenatal alcohol consumption and the need for further clinical research to corroborate preclinical findings.
As reviewed by Jones (2011), drinking during any point throughout pregnancy can cause damage to the central nervous system of the fetus. However, the extent of structural damage is affected by the period of development in which exposure occurs (Guerri et al., 2009, Maier and West, 2001). The fetus is especially vulnerable to the damaging effects of prenatal alcohol exposure during critical brain development periods, many of which occur very early in pregnancy (Maier and West, 2001). For example, facial dysmorphology appears to be the outcome of high alcohol concentration occurring during gastrulation, which occurs during week 3 and 4 of human pregnancy (Sulik, 2005). The pattern of alcohol exposure and developmental timing of the exposure can lead to a wide-range of outcomes with mild to severe cognitive and behavioral deficits for the child.
Several maternal factors have been proposed that are linked to an increased risk of FASD (May and Gossage, 2011, May et al., 2014). Maternal risk factors include being of an older age (May et al., 2005, May et al., 2008, Rubio et al., 2008, Chiodo et al., 2010, Skagerstrom et al., 2013, Esper and Furtado, 2014), having a history of alcohol abuse in the family and/or maternal partner (May et al., 2008, Ceccanti et al., 2014, Esper and Furtado, 2014, May et al., 2014), and attending fewer prenatal appointments (May et al., 2014). Additionally, lower maternal body weight, height, and BMI (May et al., 2008, May and Gossage, 2011, May et al., 2016), as well as poor nutrition (Keen et al., 2010, May et al., 2016) are associated with an increased risk of having a child with FASD. Several demographic factors have also been identified, including low maternal education, rural residence, (Viljoen et al., 2002, Rubio et al., 2008, May et al., 2013), and lower socioeconomic status (May et al., 2005, May et al., 2008). Additional factors that are predictive of drinking during pregnancy include late recognition of pregnancy, frequent binge drinking episodes prior to pregnancy (Viljoen et al., 2002, Balachova et al., 2012, Mallard et al., 2013, Parackal et al., 2013, Skagerstrom et al., 2013, Ceccanti et al., 2014, Esper and Furtado, 2014), and drug use, including tobacco (Viljoen et al., 2002, Flynn and Chermack, 2008, Rubio et al., 2008, Mallard et al., 2013, Skagerstrom et al., 2013). Furthermore, mothers who have a child with FASD and continue to drink have an increased risk of subsequent offspring developing more severe forms of FASD (Abel, 1988, Esper and Furtado, 2014). Additional research is needed to identify other possible maternal risk factors and potential targets for prevention of disabilities related to FASD.
PREVALENCE AND COST
The exact prevalence of the full range of FASD is not clear and several different methods have been used to estimate this number. Regarding FAS specifically, one early study found that 0.2 to 1.5 infants for every 1,000 live births were identified as having FAS (Centers for Disease Control and Prevention, 2002) and a study conducted in 2010 found that 0.3 of 1,000 children were diagnosed with FAS (Centers for Disease Control and Prevention, 2015). Estimates across the full range of FASD are higher; a study conducted in a representative Midwestern U.S. community reported that between 24 – 48 per 1,000 first grade students were identified as FASD (May et al., 2014). Other estimates between 2010 and 2016 in four U.S. communities suggested the prevalence of FASD as between 11.3 to 50.0 per 1,000 first-grade children, using a conservative estimating approach, and 31.1 to 98.5 per 1,000 children using a weighted approach (May et al., 2018). These estimates are vastly higher than those previously reported and reflect that FASD continues to be a significant public health concern.
There are numerous societal and economic costs associated with FASD including substance abuse treatment programs, mental health services, foster care, criminal justice system, and long-term care services. The estimated cost for one individual with FAS over his or her lifespan is $2 million, which includes medical care services ($1.6 million) and productivity losses ($0.4 million; Lupton et al., 2004). The estimated overall annual cost associated with FASD in Canada was found to fall between CAD1.3 billion and CAD2.3 billion, with cost of productivity losses mostly due to morbidity and premature mortality (Popova et al., 2016b). It is important to continue to improve upon the methods used to estimate prevalence rates and associated economic costs of FASD in order to appropriately allot adequate health care funding and services.
NEUROLOGICAL ABNORMALITIES
One significant impact of maternal alcohol consumption during pregnancy is the influence it can have on fetal brain development. See brain development specific reviews (Donald et al., 2015, Norman et al., 2009, Moore et al., 2014) for comprehensive examination of the effects of prenatal alcohol exposure on brain structure and function. Briefly, studies involving neuroimaging techniques, such as magnetic resonance imaging (MRI), have found widespread effects of exposure including a decrease in overall brain volume with reduction in grey matter as well as disorganization of the central nervous system (Donald et al., 2016, Roussotte et al., 2012). Some structures, including the parietal lobe (Archibald et al., 2001) and frontal lobe (Sowell et al., 2002), appear to be particularly sensitive to the effects of prenatal alcohol exposure. Abnormalities have also been found in the corpus callosum (Yang et al., 2012), cerebellum, caudate, and hippocampus (Donald et al., 2015, Norman et al., 2009), basal ganglia, diencephalon (Roussotte et al., 2012), thalamus (Roussotte et al., 2012), and amygdala (Nardelli et al., 2011). Furthermore, abnormal network connectivity is present in children with FASD (Wozniak et al., 2013), with atypical activity found in the insula, basal ganglia (Norman et al., 2013, Malisza et al., 2012), cerebellum, and amygdala (Malisza et al., 2012).
The neurologic damage due to prenatal alcohol exposure appears to be widespread, affecting most areas of the brain (Lebel et al., 2011). Importantly, earlier work showing volumetric decreases has been extended to include correlations between brain structural measures and other important variables like behavior and cognition. Decreased volume in several brain structures correlates with deficits in cognitive function. Smaller left hippocampi, which are important in the consolidation and retrieval of memories, correlate with decreased verbal learning skills and spatial memory performance for youth with FASD compared to controls (Willoughby et al., 2008). In particular, within the FASD group, hippocampus size was positively correlated with performance on both short- and long-term delayed verbal recall. Caudate volume correlates with cognitive control, verbal learning, and recall skills, and is the best predictor of neuropsychological performance for children prenatally exposed to alcohol (Fryer et al., 2012). Correlations have also been found between brain structure volumes and facial dysmorphology in alcohol-exposed subjects. Smaller palpebral fissures relate to a decrease in diencephalon volume bilaterally, philtrum size correlates with volume of basal ganglia and diencephalon structures, and decreased IQ scores are associated with smaller basal ganglia structure volumes and increased physical dysmorphology features (Roussotte et al., 2012). Additionally, certain dysmorphological findings (i.e., orbital hypertelorism) indicate that heavy prenatal alcohol exposure during particular points in pregnancy affects brain development and ultimately results in unique facial dysmorphology (Suttie et al., 2017). Functional neuroimaging studies have indicated that children with FASD demonstrate altered brain activation patterns during verbal learning (Sowell et al., 2007), response inhibition (Fryer et al., 2007b), visual attention (Li et al., 2008) and working memory (Malisza et al., 2005, Astley et al., 2009, Spadoni et al., 2009, O’Hare et al., 2005) tasks. Taken together these studies demonstrate the impact that prenatal alcohol exposure can have on the development of the fetal brain and the subsequent effects on cognitive abilities.
COGNITIVE AND BEHAVIORAL FUNCTIONING
In utero exposure to alcohol has been reported to negatively impact a multitude of cognitive domains, including overall general intelligence, motor function, attention and activity levels, language development, executive function, visual perception and construction, learning and memory, and adaptive functioning. In addition, prenatal alcohol exposure has a high comorbidity rate with other learning and behavioral disorders (Jones, 2011, Kingdon et al., 2016, Mattson et al., 1999, Mattson et al., 2011). The remainder of this paper will review findings in these domains; a summary is provided in Table 2.
Table 2.
Summary of neuropsychological deficits seen in individuals with fetal alcohol spectrum disorders (FASD). Results are presented in comparison to typically developing controls and, when available, children with attention-deficit/hyperactivity disorder (ADHD), and non-exposed IQ-matched children.
| Cognitive Domain | Compared to typically developing controls | Compared to ADHD | Compared to IQ-matched |
|---|---|---|---|
| General intelligence |
|
|
|
| Motor skills |
|
|
|
| Attention |
|
|
|
| Executive function | Significant deficits are observed in children with FASD in several executive function domains including:
|
|
|
| Language |
|
|
|
| Learning and Memory |
|
|
|
| Visual spatial abilities |
|
|
|
| Adaptive functioning |
|
|
|
| Academic performance |
|
|
|
| Concurrent psychopathology |
|
|
|
General Intelligence
Although not explicitly a diagnostic requirement, decreased IQ is one of the most commonly reported findings in relation to prenatal alcohol exposure (Mattson et al., 2011). Notably, fetal alcohol spectrum disorders are one of the leading preventable causes of intellectual deficiency, birth defects, and neurodevelopmental disorders in the United States (Williams et al., 2015). General intellectual ability is typically measured with assessments using standard scores that have a mean of 100 and a standard deviation of 15, with an IQ score < 70 signifying an intellectual deficit. Estimates of average IQ scores for individuals with FAS range from about 68 – 79 (Streissguth et al., 1996), while estimates for the full range of FASD fall within a much larger range of 20 – 120, with an average of about 72 (Mattson and Riley, 1998, Streissguth et al., 1996). Among the population of affected children, overall ability levels are lowest in individuals with a diagnosis of FAS, followed by PFAS, and ARND (Ferreira and Cruz, 2017). However, intellectual deficits occur across the spectrum and are not strictly related to the presence of facial dysmorphology; children with and without FAS can exhibit below average IQ scores and the absence of facial dysmorphology does not preclude impaired intellectual functioning (Mattson et al., 1997). Studies involving IQ scores in individuals with FASD have been extensively reviewed in Mattson and Riley (1998).
Motor Skills
Deficits in motor ability have been linked with prenatal alcohol exposure (Connor et al., 2006, Doney et al., 2014). Adults with FASD demonstrate greater deficits in motor function compared to typically developing adults, specifically on tests of balance and fine motor control (Connor et al., 2006) as well as with higher-order cognitive-motor abilities such as hand-eye coordination (Adnams et al., 2001). Deficits have also been found in postural balance for individuals with FASD when compared to controls, especially when somatosensory input was manipulated (Roebuck et al., 1998). These findings suggest that individuals with prenatal alcohol exposure heavily rely upon somatosensory feedback and display greater difficulty in compensating when such feedback is not available.
In general, diagnostic guidelines for disorders under the FASD umbrella recommend the inclusion of both gross and fine motor skills assessments (Bertrand et al., 2005). Deficits in fine motor coordination are frequently present in children with FASD, specifically with complex fine motor skills (e.g., visual-motor integration; Doney et al., 2014). Compared to gross motor skills, fine motor abilities appear to be more severely impacted (Kalberg et al., 2006), however, findings have been inconsistent. A meta-analysis reported that the odds of having gross motor impairment were tripled for those with moderate to heavy prenatal alcohol exposure (Lucas et al., 2014). Taken together, results from these studies indicate that deficits in motor function are common in FASD, yet the pattern of deficits is still unclear. Continued research including proficiency tests of fine and gross motor ability among individuals with FASD is needed.
Attention
Attention ability has been extensively studied within the FASD field as attention deficits are commonly associated with prenatal alcohol exposure (Bertrand et al., 2005, Kodituwakku et al., 1995, Nanson and Hiscock, 1990, Streissguth et al., 1994). Children with heavy prenatal alcohol exposure have overall slower performance and difficulty establishing, organizing, and sustaining attention (Nanson and Hiscock, 1990).
Numerous studies have investigated the patterns of deficits found in various domains of sustained attention. When processing visually presented information children and adolescents with prenatal alcohol exposure are less efficient, make more omission errors (Coles et al., 2002), have lower accuracy rates, and slower reaction times as compared to controls (Mattson et al., 2006). Furthermore, impairments are not uniform. Although a smaller number of studies have been conducted on auditory attention, it appears that deficits are not apparent in the auditory domain or exist to a lesser extent than in the visual domain (Coles et al., 2002, Connor et al., 1999, Mattson et al., 2006, Rasmussen et al., 2013). These results suggest that while significant deficits in attention occur in this population, these deficits are not global. Importantly, attention function measures are able to distinguish children with prenatal alcohol exposure from control children with a high degree of accuracy (Lee et al., 2004). Overall, numerous studies have demonstrated the detrimental effect on attention abilities associated with in utero alcohol exposure and the potential benefit of using such measures to aid in identification of affected individuals. Multiple studies have now compared children with FASD to those with attention-deficit/hyperactivity disorder (ADHD) and these results will be detailed below.
Executive Function
Executive function broadly refers to the higher-order interrelated cognitive processes (e.g., working memory, problem solving, planning, response inhibition) that are involved in goal-directed behavior (Anderson, 2002). Children with FASD have significant deficits across executive function domains as compared to controls. Substantial global executive function impairment occurs in children across the spectrum, suggesting that inclusion of neurocognitive impairment is essential to accurate diagnosis (Mattson et al., 1997, Mattson et al., 1999, Kingdon et al., 2016, Riley and McGee, 2005, Schonfeld et al., 2001).
Verbal fluency.
Verbal fluency tasks assess the ability to produce as many words as possible within a category or starting with a given letter in one minute (Shao et al., 2014). Compared to controls, children with FASD score lower on verbal fluency measures (Schonfeld et al., 2001), display greater difficulty with letter fluency versus category fluency tasks (Kodituwakku et al., 2006, Mattson and Riley, 1999, Vaurio et al., 2008), and produce fewer words in both domains (Kodituwakku et al., 2006, Mattson and Riley, 1999). Letter fluency assessments require strategic search for word subsets while category fluency tasks rely more on lexicosemantic memory retrieval. Thus, although an overlap in required cognitive processes is present, both tasks involve differing cognitive abilities (Kodituwakku et al., 2006) suggesting disruption of several processes in children with FASD.
Inhibition.
Children with prenatal alcohol exposure show impairments in response inhibition, which is the ability to suppress one response in favor of another (Connor et al., 2000, Mattson et al., 1999). Using an auditory Go/NoGo task, which assesses the number of correctly withheld responses, alcohol-exposed children performed as well as controls in their ability to inhibit responses but had significantly slower reaction times in the Go condition (Gerhold et al., 2017). Neuroimaging findings also show differential patterns of regional-activation in areas important for inhibition control among children with FASD (Kodali et al., 2017, Ware et al., 2015). Overall, children with FASD demonstrate deficits in response inhibition, though further research is necessary to clarify patterns of impairment.
Problem solving and planning.
Alcohol-exposed children display lower performance on measures of problem solving and planning abilities as compared to typically developing controls (Kodituwakku et al., 1995). Children with FASD spend less time pre-planning their strategy before solving a problem, use less efficient strategies, show increased rule violations, and require more moves to solve a problem as compared to control groups on planning and strategy-use tasks (Green et al., 2009, Kodituwakku et al., 1995, Mattson et al., 1999). Additionally, this impairment in planning abilities becomes more distinct as difficulty of the task increases (Aragón et al., 2008, Green et al., 2009).
Concept formation and set-shifting.
Difficulties in concept formation and conceptual set shifting have been observed in alcohol-exposed individuals (Kodituwakku et al., 1995, Mattson et al., 2011). Concept formation requires the ability to identify relationships among a given set of stimuli, and heavily relies on the use of prior knowledge as well as other cognitive abilities including selective and shifting attention (Hartman and Stratton-Salib, 2007). Children with prenatal alcohol exposure show deficits in skills necessary to generate and verbalize concepts as well as respond to feedback from the examiner (i.e., cognitive set-shifting; McGee et al., 2008b) and alcohol-exposed children make more errors when learning conceptual rules (Mattson et al., 1998). On measures of concept formation and identification, children with FASD make more errors, display increased perseverations (Coles et al., 1997, Kodituwakku et al., 1995, Olson et al., 1998), and evidence poorer cognitive flexibility (McGee et al., 2008b) as compared to controls. Collectively, these studies demonstrate that children with prenatal alcohol exposure have deficits in detecting and producing concepts as well as poor cognitive flexibility, which is necessary for solving problems in everyday life.
Working memory.
Working memory is a storage system with limited capacity that temporarily holds active information necessary for a variety of tasks including learning, comprehension, and reasoning (Baddeley and Hitch, 1974). Although less research has been done within working memory, a handful of studies have shown a greater impairment in working memory abilities among individuals with prenatal alcohol exposure. As the difficulty of the task increases, alcohol-exposed individuals exhibit deficits in manipulating information in working memory, compared to controls, but these impairments are not generalized to all domains (Kodituwakku et al., 1995). Children with FASD evidence impaired performance on measures of working memory as compared to controls (Aragón et al., 2008) and strong correlations have been shown between prenatal alcohol exposure and tests of working memory (Streissguth et al., 1990). Impairments in spatial working memory are also evident, with deficits becoming more significant as the task increases in complexity (Green et al., 2009). Additionally, neural correlates of working memory impairment have been found in fronto-parietal regions among individuals with prenatal alcohol exposure (Infante et al., 2017).
Language
Although not as well studied as other cognitive domains, language skills are negatively impacted by prenatal alcohol exposure. Alcohol-exposed children have deficits in fundamental language skills such as articulation (Becker et al., 1990), grammatical ability (Thorne, 2017), and expressive and receptive skills (Church et al., 1997). Relative to controls, children with FAS display poorer performance on word ordering, sentence combining, and grammatical comprehension. Furthermore, younger children appear to experience global language deficits whereas older children experience specific difficulties with language syntax (Carney and Chermak, 1991). Children with prenatal alcohol exposure evidence deficits in both receptive and expressive language (Abkarian, 1992, Gentry et al., 1998, Wyper and Rasmussen, 2011), with expressive language more severely impacted (McGee et al., 2009). In addition, children with prenatal alcohol exposure make grammatical errors at higher rates than controls, and this has been shown to be more accurate in predicting the presence of an FASD than measures of productivity and grammatical complexity (Thorne, 2017). These findings suggest that measures of language skills could serve as a significant addition to FASD diagnostic tools (Thorne, 2017).
Learning and Memory
Strong evidence supports deficits in verbal learning and memory following in utero exposure to alcohol (Bertrand et al., 2005, Crocker et al., 2011, Mattson et al., 2011). Compared to non-exposed children, children with heavy prenatal alcohol exposure show slower learning, impaired recall, (Crocker et al., 2011, Mattson and Roebuck, 2002, Lewis et al., 2015, Willoughby et al., 2008), and impaired discrimination (Mattson et al., 1998). Notably, these findings exist even when controlling for IQ suggesting impaired verbal learning and memory is not attributable to overall intellectual ability (Lewis et al., 2015). Retention of learned materials appears to differ based on alcohol-exposure level as heavily exposed individuals retained comparable amounts of information as compared to controls (Crocker et al., 2011, Mattson and Roebuck, 2002, Willford et al., 2004) whereas moderately exposed individuals were impaired on both learning and retention of verbal material (Lewis et al., 2015).
In comparison to verbal learning and memory, less is known about nonverbal learning and memory. Deficits are observed in nonverbal tasks (Olson et al., 1998, Uecker and Nadel, 1996) but results are inconsistent. Children with FAS show intact immediate memory but impaired delayed memory on nonverbal tasks as compared to controls (Uecker and Nadel, 1996). Deficits were also found in spatial recall ability, but not with object recall (Uecker and Nadel, 1998). Neither study examined nonverbal retention specifically. However, other studies suggest that unlike the verbal domain, recall and retention are impaired across learning and memory with nonverbal information (Mattson and Roebuck, 2002, Willoughby et al., 2008). Additionally, impairments in nonverbal learning and recall were present after controlling for IQ (Coles et al., 2010). Most of what is known about visual spatial memory and prenatal alcohol exposure is derived from studies using animal models and suggests deficits in spatial learning and memory (Berman and Hannigan, 2000). Animal models have also shown alterations in hippocampal neurogenesis, an area that is associated with spatial learning and recall and has been shown to be vulnerable to alcohol exposure (Berman and Hannigan, 2000, Klintsova et al., 2007).
Visual Perception and Visual Construction
Although research is limited in this area, impairments in visual-spatial abilities have been reported for children with prenatal alcohol exposure on visual construction tasks (Uecker and Nadel, 1996). Children with histories of prenatal alcohol exposure show increased deficits in the recall of local (smaller) features compared to global (larger) features and have difficulty reproducing the local stimuli (Mattson et al., 1996). Difficulties in visual-spatial abilities are thought to mediate some of the deficits observed in spatial memory, as discussed above, as some have found no differences in spatial memory ability once visual-spatial performance was accounted for (Kaemingk and Halverson, 2000). As such, visual-spatial performance appears to impact visual memory.
Adaptive Functioning
Adaptive functioning encompasses skills necessary for everyday living (Sparrow et al., 2016), including the ability to lead an independent life, keep social relationships, and integrate effectively into society (Fagerlund et al., 2012). Traditional assessment of adaptive behavior includes performance in three domains: communication, socialization, and daily living skills (Sparrow et al., 2016). Deficits in adaptive functioning skills have been reported across the spectrum of FASD and in all three domains (Carr et al., 2010, Crocker et al., 2009, Jirikowic et al., 2008a, Streissguth et al., 2004). In one study of adolescents and adults (mean age 17 years), the average age-equivalent performance was at the 7-year-old level, with the lowest scores in socialization (6-year-old equivalent) and highest scores in daily living skills (9-year-old equivalent; (Streissguth et al., 1991a). Further, adaptive behavior deficits occur across development (Carr et al., 2010, Panczakiewicz et al., 2016) though there is some indication that performance in the communication and socialization domains diminishes with age (Crocker et al., 2009, Thomas et al., 1998, Whaley et al., 2001).
Deficits in adaptive functioning can help account for the daily challenges that children with FASD face in their home, school, and community environments, including high rates of inappropriate sexual behavior, disrupted school experience, alcohol and drug problems, and decreased independent living (Streissguth et al., 2004). Adaptive function deficits may also relate to reported deficits in social problem solving. Social problem solving includes being able to identify a problem, generate and implement the best possible solutions, and examine the effectiveness of solutions (McGee et al., 2008a). Youth with prenatal alcohol exposure demonstrate deficits in this domain and rate themselves as more impaired on their ability to identify problems and execute solutions (McGee et al., 2008a).
Academic Performance
A number of challenges in the school environment are present for children with prenatal alcohol exposure (Millar et al., 2017). Additionally children with FASD have a high rate of being suspended, expelled, or dropping out of school (Streissguth et al., 1996, Streissguth et al., 2004, Popova et al., 2016a). Mathematical abilities appear to be particularly affected (Howell et al., 2006, Jirikowic et al., 2008b, Jacobson et al., 2011, Rasmussen and Bisanz, 2011, Crocker et al., 2015), with deficits persisting even after controlling for global intellectual ability (Jirikowic et al., 2008b, Crocker et al., 2015). In children with FASD, difficulties with arithmetic skills may be related to spatial processing impairments (Crocker et al., 2015), working memory deficits (Rasmussen and Bisanz, 2011), as well as deficits in the ability to process the relative magnitude and distance of numbers (Jacobson et al., 2011). Interventions targeting mathematic skills in children with FASD have been effective in improving some of these academic challenges (Coles et al., 2009). In addition to mathematical impairments, lower performance on spelling and reading is found in association with FASD (Jirikowic et al., 2008, Glass et al., 2015, Glass et al., 2017). These impairments are thought to be related to deficits in working memory (Glass et al., 2015). Continued research on elucidating potential cognitive mechanisms that underlie academic deficits in children with FASD is necessary in order to further improve upon targeted strategies and interventions for affected individuals.
Concurrent Psychopathology Related to FASD
In addition to the broad range of cognitive deficits as described above, children with FASD are at an increased risk for numerous mental health problems, learning disabilities, and specific behavioral disorders (Fryer et al., 2007a, Kingdon et al., 2016, Popova et al., 2016c, Weyrauch et al., 2017). One meta-analysis examined 127 studies and identified 428 comorbidities (i.e., other diagnoses or conditions) that occur in individuals with FASD (Popova et al., 2016c). Among those with FASD, the most prevalent comorbid conditions include ADHD, depression, anxiety disorder, post-traumatic stress disorder, oppositional defiant disorder, conduct disorder, receptive language disorder, and expressive language disorder (O’Connor and Paley, 2009, Pei et al., 2011, Popova et al., 2016c, Weyrauch et al., 2017). Furthermore, alcohol-exposed children have higher rates of psychological disorders in comparison to typically developing children even when matched on age, gender, and socioeconomic status (Fryer et al., 2007a).
Additionally, substantial overlap exists in the behavioral phenotype of FASD and ADHD leading to high rates of ADHD in individuals with FASD (Mattson et al., 2011, Weyrauch et al., 2017) and FASD has been identified as the leading cause of ADHD (Burd, 2016). A systematic review reported that 50% of individuals diagnosed with an FASD also had a diagnosis of ADHD, a rate that is 10 times that of the general population (Weyrauch et al., 2017). Previous studies have corroborated findings of high co-occurrence of FASD and ADHD with rates ranging from 63% (Rasmussen et al., 2010) to 95% (Fryer et al., 2007a). The significant overlap in the symptomatology and occurrence between FASD and other disorders adds to the difficulty of obtaining accurate diagnoses.
NEUROBEHAVIORAL PROFILE
As reviewed above, alcohol has consistently been demonstrated to negatively affect the cognitive and behavioral development of children exposed in utero. This includes diminished general intelligence as well as deficits in motor skills, attention, language, executive functions, and learning and memory (Jones, 2011, Kingdon et al., 2016, Mattson and Riley, 1998, Mattson et al., 2011). While current diagnostic methods are useful in identifying FAS, such methods are insufficient in accurately identifying those affected without the associated dysmorphic characteristics and this comprises the majority of those affected by prenatal alcohol exposure (Bertrand et al., 2005). The wide-range in the severity of impairments, high comorbidity with other clinical populations, and lack of maternal report contribute to the difficulty in obtaining accurate diagnoses (Streissguth et al., 2004). Within a sample of foster and adopted children between 4 to 18 years of age, 80.1% of children with an FASD had never been previously diagnosed and 6.4% were misdiagnosed (Chasnoff et al., 2015). Misdiagnosis can affect the incidence and prevalence rates and consequently the allocated resources, as well as lead to inappropriate interventions and services. Therefore, identifying a specific neurobehavioral profile for FASD is of utmost importance as it can contribute to more accurate and early diagnoses (Mattson and Riley, 2011).
Several studies have been conducted using latent profile analysis to investigate whether a distinct neurobehavioral profile exists for individuals affected by prenatal alcohol exposure. Patterns of neuropsychological performance were used to classify subjects with and without histories of prenatal alcohol exposure. The initial profile was successful in distinguishing between non-exposed controls and those with FAS (92% overall accuracy), and those with heavy prenatal alcohol exposure (88% overall accuracy; Mattson et al., 2010). A second study had slightly reduced classification accuracies with 77% for FAS and 70% for alcohol exposed (no FAS; Mattson et al., 2013). Differences in classification results could be explained by several significant differences between the two studies including the number of sites and the inclusion of a clinical contrast group (ADHD) in the second study. Importantly, use of a neuropsychological profile for group classification in these studies was more accurate than using IQ scores alone. Measures of executive functioning and spatial processing were found to be the most sensitive to identifying prenatal alcohol exposure (Mattson et al., 2013). In a separate study using attention measures, subjects with histories of prenatal alcohol exposure were distinguished from a typically developing control group with 91.7% overall classification accuracy (Lee et al., 2004).
A decision tree composed of four neurobehavioral and physical measures was able to differentiate between children with prenatal alcohol exposure and non-exposed children with accuracy rates ranging from 79.5% – 84.7% (Goh et al., 2016). Accuracy rates remained high (85%) after the removal of children with FAS as these individuals are more reliably identifiable. Furthermore, the highest accuracy came from inclusion of both neurobehavioral and dysmorphology variables rather than dysmorphology variables alone. Importantly, children with prenatal exposure were accurately identified from a clinical-comparison group that included children with other behavioral concerns.
In addition to being sensitive to identifying individuals affected by prenatal alcohol exposure, a neurobehavioral profile must also be specific in that it is able to correctly exclude individuals that have not been affected by prenatal alcohol exposure (Mattson and Riley, 2011). Several studies have investigated the specificity of prenatal alcohol exposure with most studies using IQ-matched controls and non-exposed children with ADHD as comparison groups. IQ-matched controls display both similarities and differences when compared to children with prenatal alcohol exposure. Children with FASD evidence lower adaptive abilities (Fagerlund et al., 2012) and poorer performance on measures of parent-rated behavior problems and verbal learning as compared to IQ-matched children (Vaurio et al., 2011). Similarities between those prenatally exposed to alcohol and IQ-matched controls have been found on measures of visual attention, retention of verbal material, verbally-mediated tasks of executive function, fine motor skill measures (Vaurio et al., 2011), internalizing behaviors (Mattson and Riley, 2000) and measures of expressive and receptive language ability (McGee et al., 2009).
An extensive number of studies have investigated the specificity of neurobehavioral deficits between prenatal alcohol exposure and ADHD. Similar to children with ADHD, children with prenatal alcohol exposure have deficits in organization, increased impulsive behaviors, decreased response inhibition, executive dysfunction, and hyperactivity (Kingdon et al., 2016, Mattson et al., 2011, Rasmussen, 2005). However, greater impairments have been reported for children with FASD compared to those with ADHD (but without histories of prenatal alcohol exposure) on assessments of planning, fluency, set-shifting, working memory (Kingdon et al., 2016), encoding, visual-spatial skills, problem-solving flexibility (Coles et al., 1997), interference control (Graham et al., 2016), IQ (Vaurio et al., 2008), social cognition, and facial emotion processing ability (Greenbaum et al., 2009). Children with FASD have greater deficits in arithmetic, while ADHD children are relatively more impaired on measures of reading (Coles et al., 1997), although both domains are impacted in FASD (Glass et al., 2017). Both clinical groups of children have higher rates of psychiatric disorders compared to controls, with non-exposed children with ADHD displaying higher rates of comorbid generalized anxiety disorder and oppositional defiant disorder compared to children with FASD (Ware et al., 2013). Deficits in adaptive functioning have been reported in both groups; however, children with prenatal alcohol exposure display a pattern that suggests an arrest in the development of adaptive functioning skills while children with ADHD show developmental delay with skills improving with age (Crocker et al., 2009), though these findings are cross-sectional in nature. Deficits on executive functioning measures are predictive of lower adaptive behavior scores for both children with ADHD and those with heavy prenatal alcohol exposure, but the pattern of deficits differs between the two groups (Ware et al., 2012). For those with ADHD, most executive function measures significantly relate to adaptive behavior, whereas nonverbal executive function tasks alone relate to adaptive function among alcohol-exposed youth.
Among other cognitive domains, certain patterns have been found. Deficits in attention have been observed in children with ADHD and FASD, although a unique pattern exists for each group (Coles et al., 1997). Children with FASD have poorer performance in subtests measuring encoding and shifting attention while children with ADHD score lower on subtests that measure the ability to focus and stay on task (Coles et al., 1997, Streissguth et al., 1994). Additional differential deficits exist in the domain of verbal learning and memory. Both children with FASD and those with ADHD display impairments on learning delayed recall trials relative to controls, but only retention of learned materials is impaired for those with ADHD (Crocker et al., 2011). The unique and shared neuropsychological deficits in FASD and ADHD are illustrated in Figure 1 and summarized in Table 2.
Figure 1.
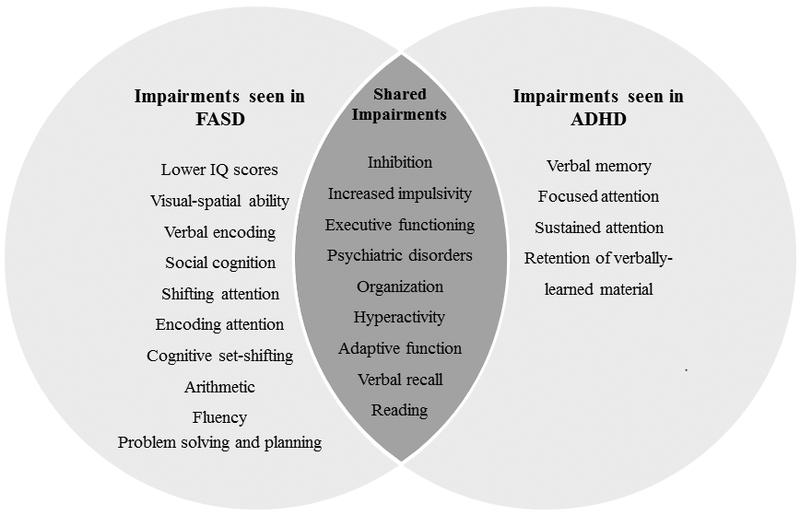
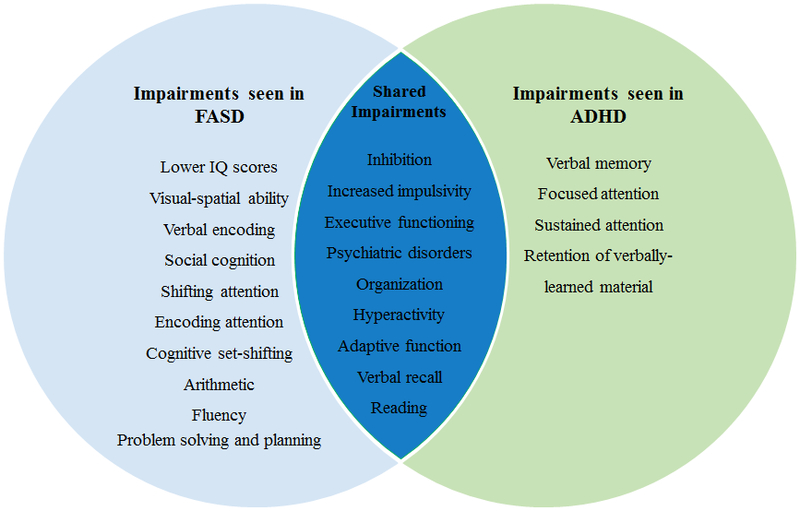
Overlapping and specific neurocognitive impairments between FASD and ADHD. See text for details.
Note: Identified neurobehavioral deficits for children with fetal alcohol spectrum disorders (FASD) and attention-deficit/hyperactivity disorder (ADHD). Impairments listed reflect findings from comparisons between the two clinical groups and not in comparison to typically developing controls. Deficits indicated represent areas where each clinical group display greater impairments compared to the other, not absolute impairment. For details and related references, see text and Table 2.
Overall, differential deficits and patterns of relations amongst domains between those with prenatal alcohol exposure and those with ADHD can assist in differential diagnosis and creation of a specific neurobehavioral profile. Promising classification accuracies using neuropsychological assessments to identify those with prenatal alcohol exposure emphasizes the critical role cognitive measures contribute as a diagnostic tool. Further research is necessary to improve identification of those affected by prenatal alcohol exposure and continue development of a specific neurobehavioral profile.
CONCLUSIONS AND DIRECTIONS FOR FUTURE RESEARCH
In utero alcohol exposure can negatively affect development of the fetus and have long-lasting neurological and behavioral effects on the individual. Deficits exist in numerous areas of functioning including general intelligence, motor function, attention and activity levels, language development, visual perception and construction, learning and memory, adaptive functioning, and executive functioning. Additionally, individuals with FASD also experience other learning and behavioral disorders. The potential benefit of using neuropsychological and physical assessments in the identification of those with FASD was presented, yet continued research is necessary to further refine this profile. Given the high prevalence rates of FASD in the U.S. (May et al., 2018) and frequent missed/misdiagnosis (Chasnoff et al., 2015), a specific neurobehavioral profile is essential to improve early identification, which will ultimately improve intervention and treatment programs.
While an impressive amount of research has been conducted to date, gaps still exist in our current knowledge. Continued investigation into and refinement of diagnostic criteria is needed to improve identification of individuals affected by prenatal alcohol exposure and provide continued monitoring of prevalence rates. In particular, continued study to confirm ND-PAE criteria is necessary. Likewise, identification of biomarkers will aid in accurate identification and diagnosis. Future research should consider using larger datasets in order to discern patterns not apparent in smaller studies. Additionally, research is needed to understand the effects of prenatal alcohol exposure across the lifespan as little is currently known about neurobehavioral impairments into adulthood. Finally, identification of risk and resiliency factors will aid in preventative efforts and development of targeted interventions. Ultimately, enhanced identification, diagnosis, and intervention efforts will lead to improved outcomes for affected individuals, their families, community, and society.
Acknowledgments
Preparation of this paper was supported by NIAAA grant U01 AA014834 (Mattson). Additional support was provided by NIAAA grant F31 AA025256 (Doyle).
Footnotes
The authors thank the families and children who graciously participate in our studies and to the members of the Center for Behavioral Teratology for ongoing assistance and support.
References
- Abel EL (1995) An update on incidence of FAS: FAS is not an equal opportunity birth defect. Neurotoxicol Teratol 17:437–443. [DOI] [PubMed] [Google Scholar]
- Abel EL (1988) Commentary: Fetal alcohol syndrome in families. Neurotoxicol Teratol 10:1–2. [DOI] [PubMed] [Google Scholar]
- Abel EL, Hannigan JH (1995) Maternal risk factors in fetal alcohol syndrome: Provocative and permissive influences. Neurotoxicol Teratol 17:445–462. [DOI] [PubMed] [Google Scholar]
- Abkarian GG (1992) Communication effects of prenatal alcohol exposure. J Commun Disord 25:221–240. [DOI] [PubMed] [Google Scholar]
- Adnams CM, Kodituwakku PW, Hay A, Molteno CD, Viljoen D, May PA (2001) Patterns of cognitive-motor development in children with fetal alcohol syndrome from a community in South Africa. Alcohol Clin Exp Res 25:557–562. [PubMed] [Google Scholar]
- Alvik A, Aalen OO, Lindemann R (2013) Early fetal binge alcohol exposure predicts high behavioral symptom scores in 5.5-year-old children. Alcohol Clin Exp Res 37:1954–1962. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). 5th ed., American Psychiatric Publishing, Inc, Arlington, VA. [Google Scholar]
- Anderson P (2002) Assessment and development of executive function (EF) during childhood. Child Neuropsychol 8:71–82. [DOI] [PubMed] [Google Scholar]
- Aragón AS, Kalberg WO, Buckley D, Barela-Scott LM, Tabachnick BG, May PA (2008) Neuropsychological study of FASD in a sample of American Indian children: Processing simple versus complex information. Alcohol Clin Exp Res 32:2136–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL (2001) Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol 43:148–154. [PubMed] [Google Scholar]
- Astley SJ, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE, Davies J, Dorn S, Gendler B, Jirikowic T, Kraegel P, Maravilla K, Richards T (2009) Functional magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. J Neurodevelop Disord 1:61–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD, Hitch GJ (1974) Working memory, in The Psychology of Learning and Motivation: Advances in Research and Theory, Vol. 8, The Psychology of Learning and Motivation: Advances in Research and Theory (Bower GH ed), pp 47–89, Academic Press, New York. [Google Scholar]
- Balachova T, Bonner B, Chaffin M, Bard D, Isurina G, Tsvetkova L, Volkova E (2012) Women’s alcohol consumption and risk for alcohol-exposed pregnancies in Russia. Addiction 107:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker M, Warr-Leeper GA, Leeper HA Jr. (1990) Fetal alcohol syndrome: A description of oral motor, articulatory, short-term memory, grammatical, and semantic abilities. J Commun Disord 23:97–124. [DOI] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH (2000) Effects of prenatal alcohol exposure on the hippocampus: Spatial behavior, electrophysiology, and neuroanatomy. Hippocampus 10:94–110. [DOI] [PubMed] [Google Scholar]
- Bertrand J, Floyd RL, Weber MK (2005) Guidelines for identifying and referring persons with fetal alcohol syndrome. MMWR Recomm Rep 54:1–10. [PubMed] [Google Scholar]
- Bonthius DJ, West JR (1988) Blood alcohol concentration and microencephaly: A dose-response study in the neonatal rat. Teratology 37:223–231. [DOI] [PubMed] [Google Scholar]
- Burd L (2016) FASD and ADHD: Are they related and How? BMC Psychiatry 16:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney LJ, Chermak GD (1991) Performance of American Indian children with fetal alcohol syndrome on the test of language development. J Commun Disord 24:123–134. [DOI] [PubMed] [Google Scholar]
- Carr JL, Agnihotri S, Keightley M (2010) Sensory processing and adaptive behavior deficits of children across the fetal alcohol spectrum disorder continuum. Alcohol Clin Exp Res 34:1022–1032. [DOI] [PubMed] [Google Scholar]
- Ceccanti M, Fiorentino D, Coriale G, Kalberg WO, Buckley D, Hoyme HE, Gossage JP, Robinson LK, Manning M, Romeo M, Hasken JM, Tabachnick B, Blankenship J, May PA (2014) Maternal risk factors for fetal alcohol spectrum disorders in a province in Italy. Drug Alcohol Depend 145:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2002) Fetal alcohol syndrome - Alaska, Arizona, Colorado, and New York, 1995-1997. MMWR 51:433–435. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2015) Fetal Alcohol Syndome Among Children Aged 7-9 Years - Arizona, Colorado, and New York, 2010. MMWR 64:54–57. [PMC free article] [PubMed] [Google Scholar]
- Chasnoff IJ, Wells AM, King L (2015) Misdiagnosis and missed diagnoses in foster and adopted children with prenatal alcohol exposure. Pediatrics 135:264–270. [DOI] [PubMed] [Google Scholar]
- Chiodo LM, da Costa DE, Hannigan JH, Covington CY, Sokol RJ, Janisse J, Greenwald M, Ager J, Delaney-Black V (2010) The impact of maternal age on the effects of prenatal alcohol exposure on attention. Alcohol Clin Exp Res 34:1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church MW, Eldis F, Blakley BW, Bawle EV (1997) Hearing, language, speech, vestibular, and dentofacial disorders in fetal alcohol syndrome. Alcohol Clin Exp Res 21:227–237. [PubMed] [Google Scholar]
- Coles CD, Gailey AR, Mulle JG, Kable JA, Lynch ME, Jones KL (2016) A comparison among 5 methods for the clinical diagnosis of fetal alcohol spectrum disorders. Alcohol Clin Exp Res 40:1000–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Kable JA, Taddeo E (2009) Math performance and behavior problems in children affected by prenatal alcohol exposure: Intervention and follow-up. J Dev Behav Pediatr 30:7–15. [DOI] [PubMed] [Google Scholar]
- Coles CD, Lynch ME, Kable JA, Johnson KC, Goldstein FC (2010) Verbal and nonverbal memory in adults prenatally exposed to alcohol. Alcohol Clin Exp Res 34:897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Lynch ME, Freides D (2002) Auditory and visual sustained attention in adolescents prenatally exposed to alcohol. Alcohol Clin Exp Res 26:263–271. [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, Smith IE (1997) A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol Clin Exp Res 21:150–161. [PubMed] [Google Scholar]
- Connor PD, Sampson PD, Bookstein FL, Barr HM, Streissguth AP (2000) Direct and indirect effects of prenatal alcohol damage on executive function. Dev Neuropsychol 18:331–354. [DOI] [PubMed] [Google Scholar]
- Connor PD, Sampson PD, Streissguth AP, Bookstein FL, Barr HM (2006) Effects of prenatal alcohol exposure on fine motor coordination and balance: A study of two adult samples. Neuropsychologia 44:744–751. [DOI] [PubMed] [Google Scholar]
- Connor PD, Streissguth AP, Sampson PD, Bookstein FL, Barr HM (1999) Individual differences in auditory and visual attention among fetal alcohol-affected adults. Alcohol Clin Exp Res 23:1395–1402. [PubMed] [Google Scholar]
- Cook JL, Green CR, Lilley CM, Anderson SM, Baldwin ME, Chudley AE, Conry JL, LeBlanc N, Loock CA, Lutke J, Mallon BF, McFarlane AA, Temple VK, Rosales T (2016) Fetal alcohol spectrum disorder: A guideline for diagnosis across the lifespan. CMAJ 188:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker N, Riley EP, Mattson SN (2015) Visual-spatial abilities relate to mathematics achievement in children with heavy prenatal alcohol exposure. Neuropsychology 29:108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker N, Vaurio L, Riley EP, Mattson SN (2009) Comparison of adaptive behavior in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Alcohol Clin Exp Res 33:2015–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker N, Vaurio L, Riley EP, Mattson SN (2011) Comparison of verbal learning and memory in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Alcohol Clin Exp Res 35:1114–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald KA, Eastman E, Howells FM, Adnams C, Riley EP, Woods RP, Narr KL, Stein DJ (2015) Neuroimaging effects of prenatal alcohol exposure on the developing human brain: a magnetic resonance imaging review. Acta Neuropsychiatrica 27:251–269. [DOI] [PubMed] [Google Scholar]
- Donald KA, Fouche JP, Roos A, Koen N, Howells FM, Riley EP, Woods RP, Zar HJ, Narr KL, Stein DJ (2016) Alcohol exposure in utero is associated with decreased gray matter volume in neonates. Metab Brain Dis 31:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doney R, Lucas BR, Jones T, Howat P, Sauer K, Elliott EJ (2014) Fine motor skills in children with prenatal alcohol exposure or fetal alcohol spectrum disorder. J Dev Behav Pediatr 35:598–609. [DOI] [PubMed] [Google Scholar]
- Esper LH, Furtado EF (2014) Identifying maternal risk factors associated with fetal alcohol spectrum disorders: A systematic review. Eur Child Adolesc Psychiatry 23:877–889. [DOI] [PubMed] [Google Scholar]
- Fagerlund A, Autti-Ramo I, Kalland M, Santtila P, Hoyme HE, Mattson SN, Korkman M (2012) Adaptive behaviour in children and adolescents with foetal alcohol spectrum disorders: a comparison with specific learning disability and typical development. Eur Child Adolesc Psychiatry 21:221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira VK, Cruz MS (2017) Intelligence and fetal alcohol spectrum disorders: A review. J Popul Ther Clin Pharmacol 24:1–18. [DOI] [PubMed] [Google Scholar]
- Flak AL, Su S, Bertrand J, Denny CH, Kesmodel US, Cogswell ME (2014) The association of mild, moderate, and binge prenatal alcohol exposure and child neuropsychological outcomes: A meta-analysis. Alcohol Clin Exp Res 38:214–226. [DOI] [PubMed] [Google Scholar]
- Flynn HA, Chermack ST (2008) Prenatal alcohol use: The role of lifetime problems with alcohol, drugs, depression, and violence. J Stud Alcohol Drugs 69:500–509. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Mattson SN, Jernigan TL, Archibald SL, Jones KL, Riley EP (2012) Caudate volume predicts neurocognitive performance in youth with heavy prenatal alcohol exposure. Alcohol Clinn Exp Res 36:1932–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer SL, McGee CL, Matt GE, Riley EP, Mattson SN (2007a) Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics 119:733–741. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Tapert SF, Mattson SN, Paulus MP, Spadoni AD, Riley EP (2007b) Prenatal alcohol exposure affects frontal-striatal BOLD response during inhibitory control. Alcoholism Clin Exp Res 31:1415–1424. [DOI] [PubMed] [Google Scholar]
- Gentry B, Griffith L, Dancer J, Davis P, Eaton B, Schulz E (1998) Prenatal alcohol exposure and communication, behavior, and nonverbal intelligence of 3 school-age children. Percept Mot Skills 86:1089–1090. [DOI] [PubMed] [Google Scholar]
- Gerhold MM, Jacobson SW, Jacobson JL, Molteno CD, Meintjes EM, Andrew CM (2017) An ERP study of response inhibition in the auditory domain in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 41:96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass L, Graham DM, Akshoomoff N, Mattson SN (2015) Cognitive factors contributing to spelling performance in children with prenatal alcohol exposure. Neuropsychology 29:817–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass L, Moore EM, Akshoomoff N, Jones KL, Riley EP, Mattson SN (2017) Academic difficulties in children with prenatal alcohol exposure: Presence, profile, and neural correlates. Alcohol Clin Exp Res 41:1024–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh PK, Doyle LR, Glass L, Jones KL, Riley EP, Coles CD, Hoyme HE, Kable JA, May PA, Kalberg WO, Sowell ER, Wozniak JR, Mattson SN (2016) A decision tree to identify children affected by prenatal alcohol exposure. J Pediatr 177:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DM, Glass L, Mattson SN (2016) The influence of extrinsic reinforcement on children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res 40:348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CR, Mihic AM, Nikkel SM, Stade BC, Rasmussen C, Munoz DP, Reynolds JN (2009) Executive function deficits in children with fetal alcohol spectrum disorders (FASD) measured using the Cambridge Neuropsychological Tests Automated Battery (CANTAB). J Child Psy Psychia 50:688–697. [DOI] [PubMed] [Google Scholar]
- Greenbaum RL, Stevens SA, Nash K, Koren G, Rovet J (2009) Social cognitive and emotion processing abilities of children with fetal alcohol spectrum disorders: A comparison with attention deficit hyperactivity disorder. Alcohol Clin Exp Res 33:1656–1670. [DOI] [PubMed] [Google Scholar]
- Guerri C, Bazinet A, Riley EP (2009) Foetal Alcohol Spectrum Disorders and Alterations in Brain and Behaviour. Alcohol Alcohol 44:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman M, Stratton-Salib BC (2007) Age differences in concept formation. J Clin Exp Neuropsychol 29:198–214. [DOI] [PubMed] [Google Scholar]
- Howell KK, Lynch ME, Platzman KA, Smith GH, Coles CD (2006) Prenatal alcohol exposure and ability, academic achievement, and school functioning in adolescence: A longitudinal follow-up. J Pediatr Psychol 31:116–126. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley D, Marais A-S, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Jewett T, Coles CD, Chambers C, Jones KL, Adnams CM, Shah PE, Riley EP, Charness ME, Warren KR, May PA (2016) Updated clinical guidelines for diagnosing fetal alcohol spectrum disorders. Pediatrics 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante MA, Moore EM, Bischoff-Grethe A, Tapert SF, Mattson SN, Riley EP (2017) Altered functional connectivity during spatial working memory in children with heavy prenatal alcohol exposure. Alcohol 64:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Dodge NC, Burden MJ, Klorman R, Jacobson SW (2011) Number processing in adolescents with prenatal alcohol exposure and ADHD: Differences in the neurobehavioral phenotype. Alcohol Clin Exp Res 35:431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Sokol RJ, Ager JW Jr. (1998) Relation of maternal age and pattern of pregnancy drinking to functionally significant cognitive deficit in infancy. Alcohol Clin Exp Res 22:345–351. [DOI] [PubMed] [Google Scholar]
- Jirikowic T, Kartin D, Olson HC (2008b) Children with fetal alcohol spectrum disorders: A descriptive profile of adaptive function. Can J Occup Ther 75:238–248. [DOI] [PubMed] [Google Scholar]
- Jirikowic T, Olson HC, Kartin D (2008a) Sensory processing, school performance, and adaptive behavior of young school-age children with fetal alcohol spectrum disorders. Phys Occup Ther Pediatr 28:117–136. [DOI] [PubMed] [Google Scholar]
- Jones KL (2011) The effects of alcohol on fetal development. Birth Defects Res C Embryo Today 93:3–11. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW (1973) Recognition of the fetal alcohol syndrome in early infancy. Lancet 302. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW, Ulleland CN, Streissguth AP (1973) Pattern of malformation in offspring of chronic alcoholic mothers. Lancet 301:1267–1271. [DOI] [PubMed] [Google Scholar]
- Kaemingk KL, Halverson PT (2000) Spatial memory following prenatal alcohol exposure: More than a material specific memory deficit. Child Neuropsychol 6:115–128. [DOI] [PubMed] [Google Scholar]
- Kalberg WO, Provost B, Tollison SJ, Tabachnick BG, Robinson LK, Hoyme HE, Trujillo PM, Buckley D, Aragón AS, May PA (2006) Comparison of motor delays in young children with fetal alcohol syndrome to those with prenatal alcohol exposure and with no prenatal alcohol exposure. Alcohol Clin Exp Res 30:2037–2045. [DOI] [PubMed] [Google Scholar]
- Keen CL, Uriu-Adams JY, Skalny A, Grabeklis A, Grabeklis S, Green K, Yevtushok L, Wertelecki WW, Chambers CD (2010) The plausibility of maternal nutritional status being a contributing factor to the risk for fetal alcohol spectrum disorders: The potential influence of zinc status as an example. BioFactors 36:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingdon D, Cardoso C, McGrath JJ (2016) Research Review: Executive function deficits in fetal alcohol spectrum disorders and attention-deficit/hyperactivity disorder - a meta-analysis. J Child Psychol Psychiatry 57:116–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintsova AY, Helfer JL, Calizo LH, Dong WK, Goodlett CR, Greenough WT (2007) Persistent impairment of hippocampal neurogenesis in young adult rats following early postnatal alcohol exposure. Alcohol Clin Exp Res 31:2073–2082. [DOI] [PubMed] [Google Scholar]
- Kodali VN, Jacobson JL, Lindinger NM, Dodge NC, Molteno CD, Meintjes EM, Jacobson SW (2017) Differential recruitment of brain regions during response inhibition in children prenatally exposed to alcohol. Alcohol Clin Exp Res 41:334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodituwakku PW, Adnams CM, Hay A, Kitching AE, Burger E, Kalberg WO, Viljoen DL, May PA (2006) Letter and category fluency in children with fetal alcohol syndrome from a community in South Africa. J Stud Alcohol 67:502–509. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, Handmaker NS, Cutler SK, Weathersby EK, Handmaker SD (1995) Specific impairments in self-regulation in children exposed to alcohol prenatally. Alcohol Clin Exp Res 19:1558–1564. [DOI] [PubMed] [Google Scholar]
- Lebel C, Roussotte F, Sowell ER (2011) Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychol Rev 21:102–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KT, Mattson SN, Riley EP (2004) Classifying children with heavy prenatal alcohol exposure using measures of attention. J Int Neuropsychol Soc 10:271–277. [DOI] [PubMed] [Google Scholar]
- Lewis CE, Thomas KG, Dodge NC, Molteno CD, Meintjes EM, Jacobson JL, Jacobson SW (2015) Verbal learning and memory impairment in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 39:724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Ma X, Peltier S, Hu X, Coles CD, Lynch ME (2008) Occipital-temporal reduction and sustained visual attention deficit in prenatal alcohol exposed adults. Brain Imaging Behav 2:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas BR, Latimer J, Pinto RZ, Ferreiera ML, Doney R, Lau M, Jones T, Dries D, Elliot EJ (2014) Gross motor deficits in children prenatally exposed to alcohol: A meta-analysis. Pediatrics 134:192–209. [DOI] [PubMed] [Google Scholar]
- Lupton C, Burd L, Harwood R (2004) Cost of fetal alcohol spectrum disorders. Am J Med Genet C Semin Med Genet 127C:42–50. [DOI] [PubMed] [Google Scholar]
- Maier SE, West JR (2001) Drinking patterns and alcohol-related birth defects. Alcohol Res Health 25:168–174. [PMC free article] [PubMed] [Google Scholar]
- Malisza KL, Allman AA, Shiloff D, Jakobson L, Longstaffe S, Chudley AE (2005) Evaluation of spatial working memory function in children and adults with fetal alcohol spectrum disorders: A functional magnetic resonance imaging study. Pediatr Res. 58:1150–1157. [DOI] [PubMed] [Google Scholar]
- Malisza KL, Buss JL, Bolster RB, de Gervai PD, Woods-Frohlich L, Summers R, Clancy CA, Chudley AE, Longstaffe S (2012) Comparison of spatial working memory in children with prenatal alcohol exposure and those diagnosed with ADHD; A functional magnetic resonance imaging study. J Neurodevelop Disord 4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard SR, Connor JL, Houghton LA (2013) Maternal factors associated with heavy periconceptional alcohol intake and drinking following pregnancy recognition: A post-partum survey of New Zealand women. Drug Alcohol Rev 32:389–397. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Calarco KE, Lang AR (2006) Focused and shifting attention in children with heavy prenatal alcohol exposure. Neuropsychology 20:361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT (2011) Fetal alcohol spectrum disorders: Neuropsychological and behavioral features. Neuropsychol Rev 21:81–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Goodman AA, Caine C, Delis DC, Riley EP (1999) Executive functioning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res 23:1808–1815. [PubMed] [Google Scholar]
- Mattson SN, Gramling L, Riley EP, Delis DC, Jones KL (1996) Global-local processing in children prenatally exposed to alcohol. Child Neuropsychol 2:165–175. [Google Scholar]
- Mattson SN, Riley EP (1998) A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res 22:279–294. [DOI] [PubMed] [Google Scholar]
- Mattson S, Riley EP (1999) Implicit and explicit memory functioning in children with heavy prenatal alcohol exposure. J Int Neuropsychol Soc 5:462–471. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP (2000) Parent ratings of behavior in children with heavy prenatal alcohol exposure and IQ-matched controls. Alcohol Clin Exp Res 24:226–231. [PubMed] [Google Scholar]
- Mattson SN, Riley EP (2011) The quest for a neurobehavioral profile of heavy prenatal alcohol exposure. Alcohol Res Health 34:51–55. [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL (1997) Heavy prenatal alcohol exposure with or without physical features of fetal alcohol syndrome leads to IQ deficits. J Pediatr 131:718–721. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL (1998) Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology 12:146–153. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Roebuck TM (2002) Acquisition and retention of verbal and nonverbal information in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res 26:875–882. [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Glass L, Deweese BN, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Adnams CM, Jones KL, Riley EP, CIFASD (2013) Further development of a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol Clin Exp Res 37:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Fagerlund A, Autti-Ramo I, Jones KL, May PA, Adnams CM, Konovalova V, Riley EP, CIFASD (2010) Toward a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol Clin Exp Res 34:1640–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Baete A, Russo J, Elliott AJ, Blankenship J, Kalberg WO, Buckley D, Brooks M, Hasken J, Abdul-Rahman O, Adam MP, Robinson LK, Manning M, Hoyme HE (2014) Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics 134:855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, Kopald D, Hasken JM, Xu R, Honerkamp-Smith G, Taras H, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Vaux K, Jewett T, Elliott AJ, Kable JA, Akshoomoff N, Falk D, Arroyo JA, Hereld D, Riley EP, Charness ME, Coles CD, Warren KR, Jones KL, Hoyme HE (2018) Prevalence of fetal alcohol spectrum disorders in 4 US communities. JAMA 319:474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP (2011) Maternal risk factors for fetal alcohol spectrum disorders: Not as simple as it might seem. Alcohol Res Health 34:15–26. [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Brooke LE, Snell CL, Marais A-S, Hendricks LS, Croxford JA, Viljoen DL (2005) Maternal risk factors for fetal alcohol syndrome in the Western Cape Province of South Africa: A population-based study. Am J Public Health 95:1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais A-S, Hendricks LS, Snell CL, Tabachnick BG, Stellavato C, Buckley DG, Brooke LE, Viljoen DL (2008) Maternal risk factors for fetal alcohol syndrome and partial fetal alcohol syndrome in South Africa: A third study. Alcohol Clin Exp Res 32:738–753. [DOI] [PubMed] [Google Scholar]
- May PA, Hamrick KJ, Corbin KD, Hasken JM, Marais AS, Blankenship J, Hoyme HE, Gossage JP (2016) Maternal nutritional status as a contributing factor for the risk of fetal alcohol spectrum disorders. Reprod Toxicol 59:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Tabachnick BG, Gossage JP, Kalberg WO, Marais A-S, Robinson LK, Manning MA, Blankenship J, Buckley D, Hoyme EH, Adnams CM (2013) Maternal factors predicting cognitive and behavioral characteristics of children with fetal alcohol spectrum disorders. J Dev Behav Pediatr 34:314–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee CL, Bjorkquist OA, Riley EP, Mattson SN (2009) Impaired language performance in young children with heavy prenatal alcohol exposure. Neurotoxicol Teratol 31:71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee CL, Fryer SL, Bjorkquist OA, Mattson SN, Riley EP (2008a) Deficits in social problem solving in adolescents with prenatal exposure to alcohol. Am J Drug Alcohol Abuse 34:423–431. [DOI] [PubMed] [Google Scholar]
- McGee CL, Schonfeld AM, Roebuck-Spencer TM, Riley EP, Mattson SN (2008b) Children with heavy prenatal alcohol exposure demonstrate deficits on multiple measures of concept formation. Alcohol Clin Exp Res 32:1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JA, Thompson J, Schwab D, Hanlon-Dearman A, Goodman D, Koren G, Masotti P (2017) Educating students with FASD: Linking policy, research and practice. J Res Special Educ Needs 17:3–17. [Google Scholar]
- Moore EM, Migliorini R, Infante MA, Riley EP (2014) Fetal alcohol spectrum disorders: Recent neuroimaging findings. Curr Dev Disord Rep 1:161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore ES, Ward RE, Wetherill LF, Rogers JL, Autti-Rämö I, Fagerlund A, Jacobson SW, Robinson LK, Hoyme HE, Mattson SN, Foroud T, CIFASD (2007) Unique facial features distinguish fetal alcohol syndrome patients and controls in diverse ethnic populations. Alcohol Clin Exp Res 31:1707–1713. [DOI] [PubMed] [Google Scholar]
- Nanson JL, Hiscock M (1990) Attention deficits in children exposed to alcohol prenatally. Alcohol Clin Exp Res 14:656–661. [DOI] [PubMed] [Google Scholar]
- Nardelli A, Lebel C, Rasmussen C, Andrew G, Beaulieu C (2011) Extensive deep gray matter volume reductions in children and adolescents with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 35:1404–1417. [DOI] [PubMed] [Google Scholar]
- National Institute of Alcohol Abuse and Alcoholism (2004) NIAAA council approves defninition of binge drinking. NIAAA Newsletter 3. [Google Scholar]
- Norman AL, Crocker N, Mattson SN, Riley EP (2009) Neuroimaging and fetal alcohol spectrum disorders. Dev Disabil Res Rev 15:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AL, O’Brien JW, Spadoni AD, Tapert SF, Jones KL, Riley EP, Mattson SN (2013) A functional magnetic resonance imaging study of spatial working memory in children with prenatal alcohol exposure: Contribution of familial history of alcohol use disorders. Alcohol Clin Exp Res 37:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MJ, Paley B (2009) Psychiatric conditions associated with prenatal alcohol exposure. Dev Disabil Res Rev 15:225–234. [DOI] [PubMed] [Google Scholar]
- O’Hare ED, Kan E, Yoshii J, Mattson SN, Riley EP, Thompson PM, Toga AW, Sowell ER (2005) Mapping cerebellar vermal morphology and cognitive correlates in prenatal alcohol exposure. Neuroreport 16:1285–1290. [DOI] [PubMed] [Google Scholar]
- Olson HC, Feldman JJ, Streissguth AP, Sampson PD, Bookstein FL (1998) Neuropsychological deficits in adolescents with fetal alcohol syndrome: Clinical findings. Alcohol Clin Exp Res 22:1998–2012. [PubMed] [Google Scholar]
- Panczakiewicz AL, Glass L, Coles CD, Kable JA, Sowell ER, Wozniak JR, Jones KL, Riley EP, Mattson SN, CIFASD (2016) Neurobehavioral deficits consistent across age and sex in youth with prenatal alcohol exposure. Alcohol Clin Exp Res 40:1971–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parackal SM, Parackal MK, Harraway JA (2013) Prevalence and correlates of drinking in early pregnancy among women who stopped drinking on pregnancy recognition. Maternal Child Health J 17:520–529. [DOI] [PubMed] [Google Scholar]
- Pei J, Denys K, Hughes J, Rasmussen C (2011) Mental health issues in fetal alcohol spectrum disorder. J Ment Health 20:438–448. [DOI] [PubMed] [Google Scholar]
- Popova S, Lange S, Burd L, Nam S, Rehm J (2016a) Special education of children with fetal alcohol spectrum disorder. Exceptionality 24:165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova S, Lange S, Burd L, Rehm J (2016b) The economic burden of fetal alcohol spectrum disorder in Canada in 2013. Alcohol Alcohol 51:367–375. [DOI] [PubMed] [Google Scholar]
- Popova S, Lange S, Shield K, Mihic A, Chudley AE, Mukherjee RAS, Bekmuradov D, Rehm J (2016c) Comorbidity of fetal alcohol spectrum disorder: a systematic review and meta-analysis. Lancet 387:978–987. [DOI] [PubMed] [Google Scholar]
- Rasmussen C (2005) Executive functioning and working memory in fetal alcohol spectrum disorder. Alcohol Clin Exp Res 29:1359–1367. [DOI] [PubMed] [Google Scholar]
- Rasmussen C, Benz J, Pei J, Andrew G, Schuller G, Abele-Webster L, Alton C, Lord L (2010) The impact of an ADHD co-morbidity on the diagnosis of FASD. Can J Clin Pharmacol 17:165–176. [PubMed] [Google Scholar]
- Rasmussen C, Bisanz J (2011) The relation between mathematics and working memory in young children with fetal alcohol spectrum disorders. J Spec Ed 45:184–191. [Google Scholar]
- Rasmussen C, Tamana S, Baugh L, Andrew G, Tough S, Zwaigenbaum L (2013) Neuropsychological impairments on the NEPSY-II among children with FASD. Child Neuropsychol 19:337–349. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL (2005) Fetal alcohol spectrum disorders: An overview with emphasis on changes in brain and behavior. Exp Biol Med 230:357–365. [DOI] [PubMed] [Google Scholar]
- Roebuck TM, Simmons RW, Mattson SN, Riley EP (1998) Prenatal exposure to alcohol affects the ability to maintain postural balance. Alcohol Clin Exp Res 22:252–258. [PubMed] [Google Scholar]
- Roussotte FF, Sulik KK, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, O’Connor MJ, Narr KL, Sowell ER (2012) Regional brain volume reductions relate to facial dysmorphology and neurocognitive function in fetal alcohol spectrum disorders. Hum Brain Mapp 33:920–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio DM, Kraemer KL, Farrell MH, Day NL (2008) Factors associated with alcohol use, depression, and their co-occurrence during pregnancy. Alcohol Clin Exp Res 32:1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayal K, Heron J, Draper E, Alati R, Lewis SJ, Fraser R, Barrow M, Golding J, Emond A, Davey Smith G, Gray R (2014) Prenatal exposure to binge pattern of alcohol consumption: Mental health and learning outcomes at age 11. Eur Child Adolesc Psychiatry 23:891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld AM, Mattson SN, Lang AR, Delis DC, Riley EP (2001) Verbal and nonverbal fluency in children with heavy prenatal alcohol exposure. J Stud Alcohol 62:239–246. [DOI] [PubMed] [Google Scholar]
- Shao Z, Janse E, Visser K, Meyer AS (2014) What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front Psychol 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skagerstrom J, Alehagen S, Haggstrom-Nordin E, Arestedt K, Nilsen P (2013) Prevalence of alcohol use before and during pregnancy and predictors of drinking during pregnancy: A cross sectional study in Sweden. BMC public health 13:780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Lu LH, O’Hare ED, McCourt ST, Mattson SN, O’Connor MJ, Bookheimer SY (2007) Functional magnetic resonance imaging of verbal learning in children with heavy prenatal alcohol exposure. Neuroreport 18:635–639. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW (2002) Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cereb Cortex 12:856–865. [DOI] [PubMed] [Google Scholar]
- Spadoni AD, Bazinet AD, Fryer SL, Tapert SF, Mattson SN, Riley EP (2009) BOLD response during spatial working memory in youth with heavy prenatal alcohol exposure. Alcohol Clin Exp Res 33:2067–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, Saulnier CA (2016) Vineland Adaptive Behavior Scales, Third Edition (Vineland-3), Pearson, Bloomington, MN. [Google Scholar]
- Stratton K, Howe C, Battaglia F (1996) Fetal alcohol syndrome: Diagnosis, epidemiology, prevention, and treatment, in Series Fetal alcohol syndrome: Diagnosis, epidemiology, prevention, and treatment (Medicine IO ed, pp 213, National Academy Press, Institute of Medicine; Washington, D.C. [Google Scholar]
- Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF (1991a) Fetal alcohol syndrome in adolescents and adults. JAMA 265:1961–1967. [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Kogan J, Bookstein FL (1996) Final report: Understanding the occurrence of secondary disabilities in clients with fetal alcohol syndrome (FAS) and fetal alcohol effects (FAE), University of Washington Publication Services, Seattle, WA. [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD (1990) Moderate prenatal alcohol exposure: Effects on child IQ and learning problems at age 7 ½ years. Alcohol Clin Exp Res 14:662–669. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK (2004) Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr 25:228–238. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Randels SP, Smith DF (1991b) A test-retest study of intelligence in patients with fetal alcohol syndrome: Implications for care. J Am Acad Child Adolesc Psychiatry 30:584–587. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Sampson PD, Olson HC, Bookstein FL, Barr HM, Scott M, Feldman J, Mirsky AF (1994) Maternal drinking during pregnancy: Attention and short-term memory in 14-year-old offspring - A longitudinal prospective study. Alcohol Clin Exp Res 18:202–218. [DOI] [PubMed] [Google Scholar]
- Sulik K (2005) Genesis of alcohol-induced craniofacial dysmorphism. Exp Biol Med 230:366–375. [DOI] [PubMed] [Google Scholar]
- Suttie M, Wetherill L, Jacobson SW, Jacobson JL, Hoyme HE, Sowell ER, Coles C, Wozniak JR, Riley EP, Jones KL, Foroud T, Hammond P, CIFASD (2017) Facial curvature detects and explicates ethnic differences in effects of prenatal alcohol exposure. Alcohol Clin Exp Res 41:1471–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SE, Kelly SJ, Mattson SN, Riley EP (1998) Comparison of social abilities of children with fetal alcohol syndrome to those of children with similar IQ scores and normal controls. Alcohol Clin Exp Res 22:528–533. [PubMed] [Google Scholar]
- Thorne JC (2017) Accentuate the negative: Grammatical errors during narrative production as a clinical marker of central nervous system abnormality in school-aged children with fetal alcohol spectrum disorders. J Speech Lang Hear Res 60:3523–3537. [DOI] [PubMed] [Google Scholar]
- Uecker A, Nadel L (1996) Spatial locations gone awry: Object and spatial memory deficits in children with fetal alcohol syndrome. Neuropsychologia 34:209–223. [DOI] [PubMed] [Google Scholar]
- Uecker A, Nadel L (1998) Spatial but not object memory impairments in children with fetal alcohol syndrome. Amer J Ment Retard 103:12–18. [DOI] [PubMed] [Google Scholar]
- Vaurio L, Riley EP, Mattson SN (2008) Differences in executive functioning in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. J Int Neuropsychol Soc 14:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaurio L, Riley EP, Mattson SN (2011) Neuropsychological comparison of children with heavy prenatal alcohol exposure and an IQ-matched comparison group. J Int Neuropsychol Soc 17:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viljoen D, Croxford J, Gossage JP, Kodituwakku PW, May PA (2002) Characteristics of mothers of children with fetal alcohol syndrome in the Western Cape Province of South Africa: A case control study. J Stud Alcohol 63:6–17. [PubMed] [Google Scholar]
- Ware AL, Crocker N, O’Brien JW, Deweese BN, Roesch SC, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Jones KL, Riley EP, Mattson SN, CIFASD (2012) Executive function predicts adaptive behavior in children with histories of heavy prenatal alcohol exposure and attention-deficit/hyperactivity disorder. Alcohol Clin Exp Res 36:1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware AL, Infante MA, O’Brien JW, Tapert SF, Jones KL, Riley EP, Mattson SN (2015) An fMRI study of behavioral response inhibition in adolescents with and without histories of heavy prenatal alcohol exposure. Behav Brain Res 278:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware AL, O’Brien JW, Crocker N, Deweese BN, Roesch SC, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Jones KL, Riley EP, Mattson SN, CIFASD (2013) The effects of prenatal alcohol exposure and attention-deficit/hyperactivity disorder on psychopathology and behavior. Alcohol Clin Exp Res 37:507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyrauch D, Schwartz M, Hart B, Klug MG, Burd L (2017) Comorbid mental disorders in fetal alcohol spectrum disorders: A systematic review. J Dev Behav Pediatr 38:283–291. [DOI] [PubMed] [Google Scholar]
- Whaley SE, O’Connor MJ, Gunderson B (2001) Comparison of the adaptive functioning of children prenatally exposed to alcohol to a nonexposed clinical sample. Alcohol Clin Exp Res 25:1018–1024. [PubMed] [Google Scholar]
- Willford JA, Richardson GA, Leech SL, Day NL (2004) Verbal and visuospatial learning and memory function in children with moderate prenatal alcohol exposure. Alcohol Clin Exp Res 28:497–507. [DOI] [PubMed] [Google Scholar]
- Williams JF, Smith VC, Committee on Substance Abuse (2015) Fetal Alcohol Spectrum Disorders. Pediatrics 136:e1395–1406. [DOI] [PubMed] [Google Scholar]
- Willoughby KA, Sheard ED, Nash K, Rovet J (2008) Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J Int Neuropsychol Soc 14:1022–1033. [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Mueller BA, Bell CJ, Muetzel RL, Hoecker HL, Boys CJ, Lim KO (2013) Global functional connectivity abnormalities in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 37:748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyper KR, Rasmussen CR (2011) Language impairments in children with fetal alcohol spectrum disorders. J Popul Ther Clin Pharmacol 18:364–376. [PubMed] [Google Scholar]
- Yang Y, Phillips OR, Kan E, Sulik KK, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, O’Connor MJ, Narr KL, Sowell ER (2012) Callosal thickness reductions relate to facial dysmorphology in Fetal Alcohol Spectrum Disorders. Alcohol Clin Exp Res 36:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]


