Abstract
In order to generate complex motor outputs, the nervous system integrates multiple sources of sensory information that ultimately controls motor neurons to generate coordinated movements. The neural circuits that integrate higher order commands from the brain and generate motor outputs are located in the nerve cord of the central nervous system. Recently, genetic access to distinct functional subtypes that make up the Drosophila adult ventral nerve cord has significantly begun to advance our understanding of the structural organization and functions of the neural circuits coordinating motor outputs. Moreover, lineage-tracing and genetic intersection tools have been instrumental in deciphering the developmental mechanisms that generate and assemble the functional units of the adult nerve cord. Together, the Drosophila adult ventral nerve cord is emerging as a powerful system to understand the development and function of neural circuits that are responsible for coordinating complex motor outputs.
Introduction
The central nervous systems of most bilaterian animals can be divided into two components, an anterior brain and a more posterior nerve cord. From among its most primitive forms in annelids, to the complex spinal cord found in vertebrates, the primary function of the nerve cord is to integrate and process information from the brain to produce coordinated locomotor outputs by controlling muscle activities in the periphery. Understanding the development and assembly of circuits in the nerve cord is therefore crucial for understanding how animals respond to their environments by executing motor outputs.
In order to carry out these functions the nerve cord is composed of a large number of neurons that can be classified according to their function and morphology. These include local interneurons that modulate and generate rhythmic motor patterns, ascending and descending neurons that relay information to and from the brain, and motor neurons that synapse onto muscles and are directly responsible for causing muscle contractions and movements. In addition, the nerve cord receives numerous inputs from peripheral sensory neurons. As will be highlighted below, these populations can be further subdivided based on their specific functions and anatomy.
To assemble complex neural circuits, biological systems employ a range of developmental mechanisms that regulate and specify the number, birth order and unique identities of the component neurons. To link the developmental origins of neural circuits with their functional outputs, it is advantageous to use a model where sophisticated tools are available for both types of analyses. The Drosophila model system is well renowned for the range of genetic tools available to dissect the development and functions of individual and small groups of neurons (1–5). Moreover, adult Drosophila execute a large range of complex motor outputs using multi-jointed legs for walking and grooming, and wings and halteres for flying. Additional behaviors that use these appendages are courtship by males and aggression between individuals. Notably, rudimentary forms of some motor programs, including walking, can be executed in the absence of a brain, indicating the autonomy of the circuits in the nerve cord for executing rhythmic motor outputs (6,7). However, intact adult Drosophila simultaneously integrate visual, chemosensory, and proprioceptive inputs while executing these motor behaviors (8). The combination of behavioral complexity, plasticity, and abundance of genetic tools sets this system apart as an exceptionally powerful model to understand the development and function of neural circuits.
In Drosophila, most of the neurons that make up the nerve cord, known as the ventral nerve cord (VNC) due to its ventral position, are born post-embryonically in the larval stages in an immature form (9). Because the larval and adult stages execute significantly different behaviors, the nervous system undergoes a striking transformation during metamorphosis during which adult specific neural circuits develop into their mature forms. In comparison to the adult brain of Drosophila (10–14) much less is known about the functional organization and development of distinct adult VNC circuits. In this review we highlight recent studies that describe the diverse population of cell types and neuropils that make up the Drosophila adult VNC together with the specific tools and techniques that enable a more thorough understanding of their developmental origins and functional contributions to various motor outputs.
Structural and Functional Organization of the Drosophila Adult VNC
In order to understand how distinct neurons find their place in a functional circuit, it is crucial to characterize the anatomy and organization of the nervous tissue. This involves identifying specific landmarks, such as groups of neuronal cell bodies, neuronal tracts and commissures, anatomically distinct neuropils, and the organization of non-neuronal glial cells, which contribute to the assembly, growth, and homeostasis of nervous tissue (15,16).
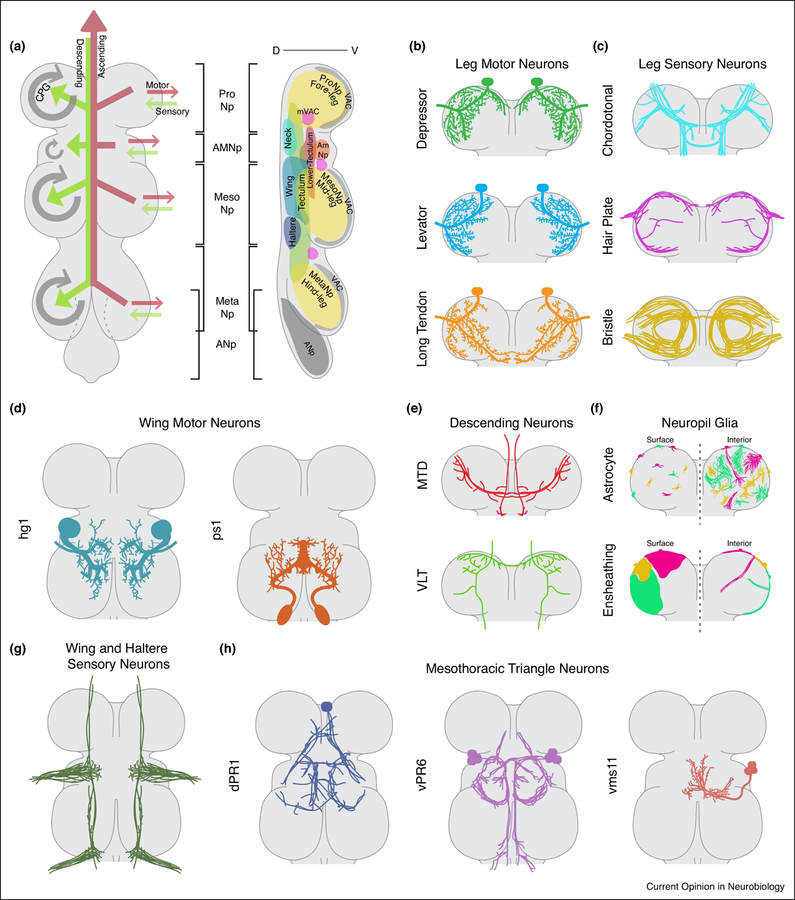
In the insect nerve cord, neuronal cell bodies are located in an outer cortex, while their projections converge into densely packed neuropils that can be recognized by staining for Bruchpilot and N-cadherin, two well-established markers of mature synapses (17,18). Further, tightly fasciculated nerve bundles enter and exit specific neuropils through distinct tracts and commissures, which are clearly identified by anti-Tubulin and anti-Futsch staining. Notably, the organization of the Drosophila adult VNC differs dramatically from its larval counterpart. While the larval VNC is organized as repeating neuropils in each of the thoracic and abdominal segments, the adult VNC is dominated by three major thoracic neuropils, corresponding to the three thoracic segments (prothoracic, mesothoracic and metathoracic), and a fused posterior abdominal ganglia (18,19) (Figure 1A). Shepherd et al., used a developmentally based ‘hemilineage’ approach to define the anatomical framework of the entire adult VNC during the course of metamorphosis (20). Specifically, most adult VNC neurons arise during post-embryonic neurogenesis from 25 distinct neuroblast (NB) progenitors in each thoracic neuromere, and each lineage gives rise to both Notch-ON and Notch-OFF hemilineages, some of which undergo programed cell death (9,21). Of the 33 hemilineages that survive apoptosis, each extends primary neurites into specific tracts with stereotyped points of entry into an immature neuropil. These tracts, readily discerned by Neuroglian staining, remain tightly fasciculated and largely intact throughout metamorphosis, thereby providing a consistent reference for neuropil organization. For example, certain lineage tracts define the boundaries of two smaller neuropils – the accessory mesothoracic neuropil (AmNp), which receives wing sensory afferents, and a dorsal compartment called the tectulum, which consists of intersegmental projecting hemilineages (Figure 1A). Interestingly, this hemilineage-based organization of VNC anatomy is likely to be functionally relevant as distinct hemilineages represent functional ‘modules’ that contribute to specific motor outputs (6) (Table 2).
Figure 1. Structural and Functional Organization of the Drosophila Adult VNC.
(A) Frontal (left) and lateral (right) views of the Drosophila adult VNC depicting five major neuropils – Pro-, Meso- and Metathoracic neuropils corresponding to the three thoracic segments and the accessory mesothoracic neuropil (AMNp), and abdominal neuropil (ANp). For nomenclature refer to (19).
Left – Neuronal subtypes projecting in and out of the Drosophila adult VNC with information flow depicted in the form of arrows. Motor and ascending neurons project axons out of the VNC (red arrows); sensory and descending neurons project into the VNC (green arrows); and CPG neurons are local interneurons contained within the VNC (grey arrows).
Right – Spatial boundaries of the sixteen distinct neuropils that make up the Drosophila adult VNC (adapted from Namiki et al., 2018 (22)). For the complete list of neuropils and associated neuronal hemilineages refer to Table 1.
(B) Dendritic projections of distinct prothoracic leg motor neurons subtypes in the prothoracic VNC segment, including depressor, levator and long tendon muscle targeting motor neurons (56,24,25).
(C) Axonal projections of distinct sensory neuron subtypes projecting into the prothoracic VNC segment from the corresponding legs, including those from the chordotonal organs, hair plates and bristles (27)
(D) Dendritic projections of selected wing motor neuron subtypes, hg1 and ps1 in the wing neuropil compartment of the VNC. Corresponding axon targets are in the fourth axiallary and pleurosternal muscles respectively (23).
(E) Axon projections of leg neuropil targeting descending neurons in the prothoracic VNC segment, belonging to two distinct cervical connective tracts - the median tract of dorsal cervical fasciculus (MTD; also known as the dorsal medial tract (DMT)) and the ventral lateral tract (VLT)(22).
(F) Structural organisation of astrocyte and ensheathing neuropil glia, both on the surface (left of midline) and interior (right of midline), of the prothoracic VNC segment (35).
(G) Axon projections of both wing and haltere sensory neurons in the haltere and accessory mesothoracic neuropil regions (26).
(H) Projections of local intersegmental CPG-like mesothoracic triangle neurons dPR1, vPR6 and vms11 in the VNC that control distinct features of wing extension and pulse song (33,34).
Table 2. Hemilineage-Specific TFs and Corresponding Motor Outputs.
Post-embryonic hemilineages of the Drosophila adult VNC, along with corresponding TF expression patterns described in Lacin and Truman, 2016 (45) and Lacin et al., 2014 (70) and corresponding motor outputs upon hemilineage-specific activation described in Harris et al., 2015 (6).
| Hemilineage | TF Expression | Motor Output | |
|---|---|---|---|
| 0A | En | N/A | |
| 0B | N/A | N/A | |
| 1A | Msh | Walking | |
| 1B | Nmr1 | N/A | |
| 2A | Toy | Wing buzz, takeoff | |
| 2B | - | - | |
| 3A | Nkx6, Nmr1 | N/A | |
| 3B | Dbx | Change in posture | |
| 4A | N/A | N/A | |
| 4B | Nkx6, Lim3, Hb9 | N/A | |
| 5A | - | - | |
| 5B | Vg, Cut, Toy | Change in posture | |
| 6A | ¥Toy | Uncoordinated leg movement | |
| 6B | Vg, Cut, En | Uncoordinated leg movement | |
| 7A | - | - | |
| 7B | Unc4 | Wing buzz, takeoff | |
| 8A | Ey*, Ems | Change in posture | |
| 8B | Lim3*, Acj6 | N/A | |
| 9A | Msh | ¥Ems | Change in posture |
| 9B | Lim3, Isl | N/A | |
| 10A | - | - | |
| 10B | Nkx6, Lim3, Hb9 | Walking | |
| 11A | Nkx6, Unc4 | Wing buzz, takeoff | |
| 11B | Eve* | Wing buzz, takeoff | |
| 12A | Unc4 | ¥Dbx | Wing Buzz, Wing Wave, Walking |
| 12B | Nkx6, Nmr1 | Change in posture | |
| 13A | Dbx | N/A | |
| 13B | D, Vg | Change in posture | |
| 14A | Msh | N/A | |
| 14B | Lim3, Isl | N/A | |
| 15A | - | - | |
| 15B | Nkx6, Isl, Lim3 | N/A | |
| 16A | N/A | ||
| 16B | Lim3, Hb9 | N/A | |
| 17A | Unc4, Isl | N/A | |
| 17B | - | - | |
| 18A | - | - | |
| 18B | Unc4 | Wing buzz, takeoff, walking | |
| 19A | Dbx | Uncoordinated leg movement | |
| 19B | Unc4 | N/A | |
| 20/22A | BarH | Change in posture | |
| 20/22B | |||
| 21A | Msh | ¥Ey | Uncoordinated leg movement |
| 21B | N/A | ||
| 23A | - | - | |
| 23B | Unc4, Acj6 | Change in posture, uncoordinated leg movement | |
| 24A | - | - | |
| 24B | Ems, Toy, Nkx6 | Repetitive leg movements | |
Hemilineage-specific expression is unknown for these TFs.
Personal communication H.Lacin, Truman Lab.
Hemilineages that undergo apoptosis.
Based on the detailed description of the anatomy of the adult VNC and associated nerves, the Drosophila adult VNC is currently viewed as containing sixteen distinct neuropils (22) (Figure 1A, Table 1). The definition of these neuropils and their anatomical boundaries has depended on multiple complementary efforts to characterize the function and development of the diverse populations of cell-types that project into the VNC (Figure 1B–H). These include, but are not restricted to, the motor neurons (MNs) controlling movements of the legs, wings and neck (23–25) (Figure 1B, D); sensory neurons (SNs) such as the proprioceptive chordotonal organ, mechanosensory and chemosensory neurons from the legs, wings and halteres (26–29) (Figure 1C, G); descending neurons (DNs) that bring command-like information from the brain (30,22,31) (Figure 1E); ascending neurons that relay somatosensory information back to the brain (32); the mesothoracic triangle neurons that are CPG-like neurons responsible for generating male-specific courtship songs (33,34) (Figure 1H); and glia that wrap and ensheath cell bodies and projections in the VNC (35,36) (Figure 1F).
Table 1. Neuropils of the Drosophila Adult VNC.
Neuropils of the Drosophila Adult VNC as denoted in Namiki et al., 2018 (22), along with defining projections and contributing hemilineages as denoted in Shepherd et al. 2016 (20) and Harris et al., 2015 (6).
Refer to Figure 1A for spatial boundaries of each neuropil.
| Neuropil | Defining Projections | Contributing Hemilineages | |
|---|---|---|---|
| Leg (Pro, Meso, Meta) | Leg MNs | 15B, 24B, 1B, 3A, 9A, 12B, 13A, 19A, 20A, 21A, 22A, 23B | |
| Neck* | Neck MNs | 2A | |
| Wing* | Wing MNs | 3B, 12A, 9B | |
| Haltere* | Haltere SNs | 8B | |
| Tectulum | Commissural INs | 2A, 6A, 6B, 7B, 8A, 3B, 11A/B, 12A, 18B, 19B | |
| Lower Tectulum* | Peripheral Sensing INs | 10B, 11A/B, 18B | |
| Accessory Mesothoracic | Wing SNs | 12A ♂, 23B | |
| Ventral Association Centers* (3) | SNs | 13B, 14A | |
| Medial Ventral Association Centers (3) | SNs | N/A | |
| Abdominal | N/A | N/A |
Hemilineages contributing to these neuropils were separately inferred from Harris et al., 2015 (6).
Characterizing the morphologies of functionally distinct neurons, especially individual cells within each subtype, has been greatly facilitated by the generation of cell-specific markers that generally consist of transcriptional regulatory elements that drive expression of a downstream reporter in a spatially restricted manner (37–40,5). Combinations of regulatory elements can be genetically intersected generate more limited expression patterns in distinct cell types. For example, we now have genetic access to ~50% of ~100 distinct descending neuron subtypes, 100 distinct subtypes of sex-specific neurons, as well as individual pairs of wing MNs that innervate the flight muscles (23,22,34). Another recent study described different subtypes of proprioceptive neurons that respond to distinct types of mechanical stimulation, such as vibration and joint angles (41). Together, these observations underscore the idea that the stereotyped anatomy of VNC neuropils, a consequence of VNC development, underlies its ability to produce distinct motor behaviors.
The ability to label and genetically manipulate small groups of neurons in the adult VNC with high resolution is also essential to map or ‘register’ neurons onto a standardized VNC (42,43,17) and can eventually be used alongside a detailed connectome of the VNC derived from electron microscopy (EM) to assemble a more complete picture of the circuits making up the adult VNC. Such an EM connectome is well on its way for the fly brain (10,44) and one for the VNC will hopefully not be far behind.
Developmental Logic of Drosophila Adult VNC Neurons and Glia
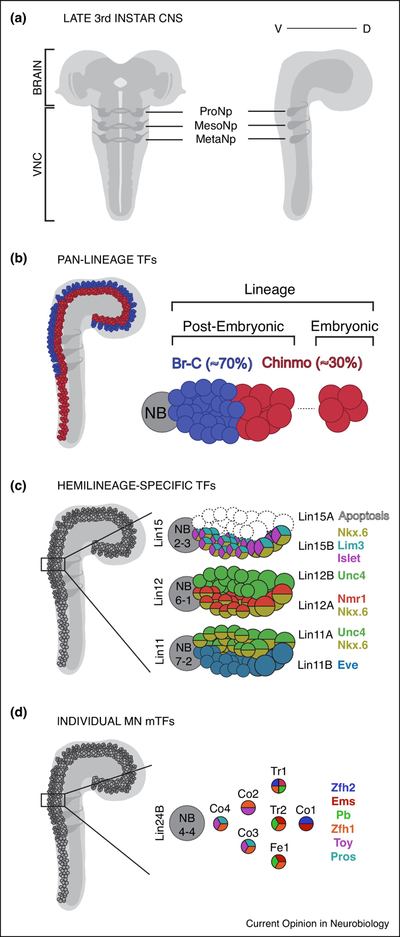
During Drosophila embryogenesis the neuroepithelium gives rise to neural stem cells, neuroblasts (NBs), which first give rise to neurons that are involved in larval function and behaviour (embryonic lineages). Most NBs then enter a quiescent phase, and reinitiate divisions during the early larval stages to give rise to adult-specific neurons (post-embryonic lineages) (45–47). These adult neurons project their neurites into the immature adult neuropils in the larval VNC (Figure 2A). Interestingly, the majority of post-embryonic progeny exhibit a coordinated switch from Chinmo to Broad-Complex (BrC) transcription factor (TF) expression at ~60 hrs after larval hatching such that ~30% of the entire lineage consists of Chinmo expressing cells while the remaining ~70% express genes of the Broad-Complex (48)(Figure 2B). While Chinmo and BrC are known to regulate neuronal cell-fates in the mushroom body of the brain (49), the purpose of this coordinated switch has not been described in the adult VNC. Interestingly, the Chinmo to BrC switch correlates with a 2-fold decrease in cell-size across all thoracic post-embryonic lineages (48), which might be necessary to accommodate the large number of BrC expressing progeny. As mentioned above, in many lineages one of the two hemilineages undergoes Notch-dependent programmed cell death (9,21), suggesting that Notch helps regulate final cell number. The execution of such coordinated events must involve many molecular changes at the progenitor level, and indeed, the Chinmo to Broad transition is controlled by temporal TFs in the early VNC NBs (48) and has been linked to opposing gradients of RNA binding proteins in brain NBs (50). Similarly, Notch expression in progeny neurons is also determined by temporal patterning in the NBs (51). Importantly, extrinsic cues such as ecdysone signaling also play an important role in coordinating temporal transitions in these NB progenitors (52–54).
Figure 2. Transcription Factor Expression in Post-Embryonic VNC Lineages.
(A) Immature adult thoracic VNC neuropils depicted in the late third instar larval CNS. Frontal (left) and lateral (right) views.
(B) Lateral view (left) of the third instar larval CNS depicting cell bodies in the cortex expressing Chinmo (red) and Broad-Complex (Br-C)(blue) TFs. NB lineage (right) comprising of embryonic and post-embryonic progeny; depicting proportions of Chinmo and Broad-Complex (Br-C) TF expression and corresponding to a ~2-fold decrease in cell size (48).
(C) Unique combinations of TF expression identify post-embryonic hemilineages of NB2–3 (Lin15A-B), NB6–1(Lin12A-B) and NB7–2(Lin11A-B). Refer to Table 2. for a complete list of hemilineage-specific TFs (45,71).
(D) Individual leg MNs belonging to hemilineage 24B from NB4–4, express unique combinations of morphological TFs (mTFs) that control their dendritic projections and axon targeting morphologies (56).
Apart from synchronizing the generation and assembly of neurons arising from multiple progenitors, developmental mechanisms must also contribute to the diversity of neuronal cell identities. In the adult fly VNC, additional mechanisms likely define distinct sub-populations of post-mitotic neurons. For instance, although hemilineages are defined by Notch expression across all postembryonic lineages, each hemilineage can also be uniquely identified by a combination of TFs (70) (Figure 2C, Table 2). More recently, multiple lineage-tracing approaches were used to identify the embryonic origins of each post-embryonic lineage (45,1,46,3). The tools generated to uniquely label each adult VNC NB lineage (45), along with powerful clonal analyses like Mosaic Analysis with a Repressible Marker (MARCM) (55), prove to be essential in understanding the developmental logic of distinct neuronal subtypes.
Using MARCM-based approaches, the major lineages that give rise to motor neurons (MNs) that innervate adult leg muscles have been characterized in detail, and reveal a stereotyped topographic organization of leg MN dendrites that correlate with their axon projections to distinct muscles in the adult legs (24,25). Further, the dendritic projections and axon targeting morphologies of individual leg MNs were shown to be controlled by unique combinations of TFs termed morphological TFs (mTFs) (56) (Figure 2D). Interestingly, while individual leg MNs can be identified by their unique morphologies, their dendritic innervation patterns tightly cluster based on axon targeting to one of four segments (coxa, trochanter, femur and tibia). Leg MNs display stereotyped dendritic projection patterns based on the type of muscle being innervated irrespective of leg segment. For instance, prothoracic leg MNs innervating the long tendon muscles (present in both the tibia and femur) project some dendrites across the midline in the corresponding thoracic neuropil, several depressor targeting leg MNs extend projections into the antero-medial region of the neuropil, and levator and reductor targeting leg MNs tend to project their dendrites into the lateral compartments of the thoracic neuropils (Figure 1B). This topological organization suggests that dendritic projections within each leg neuropil are functionally compartmentalized to receive the correct inputs. Consistently, descending neurons that project into the leg neuropils also have highly stereotyped projections and use one of two tracts to project into more lateral or medial compartments (22) (Figure 1E). Interestingly, one such descending neuron specifically triggers backward walking of the adult fly (57,58), while other sets of descending neurons produce distinct behavioral responses when activated (30,59,60). The ability to merge functional studies with neuronal activity measurements, which have recently become possible in the Drosophila VNC, will be essential to further test these hypotheses (61).
Apart from molecular mechanisms that define cell-diversity in post-mitotic progeny, other mechanisms controlling birth-order and modes of divisions also help diversify post-embryonic lineages. For instance one of the major leg MN lineages, Lin15B (also called LinA), exhibits a strong correlation between birth-order and proximal-distal axon targeting in the Femur and Tibia leg segments (24,25). Notably, the Hox TF Antennapedia, which is strongly expressed in the Lin15 distal-targeting MNs is sufficient to increase axon targeting in the distal Femur when ectopically expressed in the entire lineage (62) and Olig, another TF expressed in a subset of Lin15 MNs is important for proper axons targeting in the Femur and Tibia (63). This suggests that the birth order of Lin15 MNs might be important in establishing the post-mitotic mTF code and is likely regulated by the mechanisms described at the progenitor level.
Interestingly, the Lin15 NB also gives rise to leg neuropil glia (Figure 1F) that remain closely associated with the Lin15 leg MNs, suggesting that the shared lineage between MNs and glia may help assemble complex neuropils containing multiple cell types (35). These glia also appear to play a direct role in guiding the growth of MN dendrites through Plexin/Semaphorin signaling (64). Also noteworthy is that even though they come from the same NB lineage, the mode of division of the glial progenitors differs drastically from those of the leg MNs, as the glia greatly increase in number during metamorphosis while the MNs do not. Moreover, unlike the stereotyped morphologies of the leg MNs, the astrocyte-like neuropil glia adopt varied morphologies but strictly ‘tile’ with one another in a non-overlapping fashion. In accordance to these differing developmental strategies, distinct mTF codes have not been identified in the neuropil glia, suggesting that such TF codes may only be required when unique and stereotyped individual cell-type morphologies are being generated.
Future directions
While the above studies have established a solid anatomical framework and some of the developmental mechanisms that operate in the VNC, many questions remain. For example, multiple distinct NB temporal windows, such as those observed in embryonic VNC NBs and the post-embryonic brain (65–67), have yet to be identified in the post-embryonic VNC and likely regulate birth order, number and diversity of NB progeny. Further, since many of the above studies have been conducted in lineages that generate leg MNs and neuropil glia, it is important to map each neuronal subtype to its specific hemilineage. Third, in order to identify and understand the mechanisms controlling neuronal cell diversity in post-embryonic lineages it is essential to compare the entire transcriptome across hemilineages as well as between individual cells belonging to a single lineage, as has been demonstrated in the Drosophila brain and larval VNC (54,68–70).
ACKNOWLEDGEMENTS
This work was funded by NIH grants R01NS070644 and U19NS104655 awarded to R.S.M. We thank Haluk Lacin for sharing unpublished data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Awasaki T, Kao C-F, Lee Y-J, Yang C-P, Huang Y, Pfeiffer BD, et al. Making Drosophila lineage-restricted drivers via patterned recombination in neuroblasts. Nat Neurosci 2014. April;17(4):631–7. [DOI] [PubMed] [Google Scholar]
- 2.Li H-H, Kroll JR, Lennox SM, Ogundeyi O, Jeter J, Depasquale G, et al. A GAL4 Driver Resource for Developmental and Behavioral Studies on the Larval CNS of Drosophila. Cell Rep 2014. August 7;8(3):897–908. [DOI] [PubMed] [Google Scholar]
- 3.Hadjieconomou D, Rotkopf S, Alexandre C, Bell DM, Dickson BJ, Salecker I. Flybow: genetic multicolor cell labeling for neural circuit analysis in Drosophila melanogaster. Nat Methods 2011. March;8(3):260–6. [DOI] [PubMed] [Google Scholar]
- 4.Venken KJT, Schulze KL, Haelterman NA, Pan H, He Y, Evans-Holm M, et al. MiMIC: a highly versatile transposon insertion resource for engineering Drosophila melanogaster genes. Nat Methods 2011. September;8(9):737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfeiffer BD, Jenett A, Hammonds AS, Ngo T-TB, Misra S, Murphy C, et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc Natl Acad Sci 2008. July 15;105(28):9715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.**Harris RM, Pfeiffer BD, Rubin GM, and Truman JW (2015). Neuron hemilineages provide the functional ground plan for the Drosophila ventral nervous system. ELife 4, e04493.Description of the distinct anatomical features and functional contributions of each post-embryonic hemilineage that populates the adult VNC.
- 7.Yellman C, Tao H, He B, Hirsh J. Conserved and sexually dimorphic behavioral responses to biogenic amines in decapitated Drosophila. Proc Natl Acad Sci U S A 1997. April 15;94(8):4131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.**Bidaye SS, Bockemühl T, Büschges A Six-legged walking in insects: how CPGs, peripheral feedback, and descending signals generate coordinated and adaptive motor rhythms. J Neurophysiol 2018. 01;119(2):459–75.Initial identification of a small number of descending neurons that, when activated, cause flies to walk in a backwards direction.
- 9.Truman JW, Schuppe H, Shepherd D, Williams DW. Developmental architecture of adult-specific lineages in the ventral CNS of Drosophila. Dev Camb Engl 2004. October;131(20):5167–84. [DOI] [PubMed] [Google Scholar]
- 10.Zheng Z, Lauritzen JS, Perlman E, Robinson CG, Nichols M, Milkie D, et al. A Complete Electron Microscopy Volume of the Brain of Adult Drosophila melanogaster. Cell 2018. July 26;174(3):730–743.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee T Wiring the Drosophila Brain with Individually Tailored Neural Lineages. Curr Biol CB 2017. January 23;27(2):R77–82. [DOI] [PubMed] [Google Scholar]
- 12.Nériec N, Desplan C. From the Eye to the Brain: Development of the Drosophila Visual System. Curr Top Dev Biol 2016;116:247–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito M, Masuda N, Shinomiya K, Endo K, Ito K. Systematic analysis of neural projections reveals clonal composition of the Drosophila brain. Curr Biol CB 2013. April 22;23(8):644–55. [DOI] [PubMed] [Google Scholar]
- 14.Ito K, Shinomiya K, Ito M, Armstrong JD, Boyan G, Hartenstein V, et al. A systematic nomenclature for the insect brain. Neuron 2014. February 19;81(4):755–65. [DOI] [PubMed] [Google Scholar]
- 15.Kremer MC, Jung C, Batelli S, Rubin GM, Gaul U. The glia of the adult Drosophila nervous system. Glia 2017;65(4):606–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamkin ER, Heiman MG. Coordinated morphogenesis of neurons and glia. Curr Opin Neurobiol 2017;47:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boerner J, Duch C. Average shape standard atlas for the adult Drosophila ventral nerve cord. J Comp Neurol 2010. July 1;518(13):2437–55. [DOI] [PubMed] [Google Scholar]
- 18.Power ME. The thoracico-abdominal nervous system of an adult insect, Drosophila melanogaster. J Comp Neurol 1948. June 1;88(3):347–409. [DOI] [PubMed] [Google Scholar]
- 19.Court RC, Armstrong JD, Borner J, Card G, Costa M, Dickinson M, et al. A Systematic Nomenclature for the Drosophila Ventral Nervous System. bioRxiv 2017. January 1;122952.
- 20.Shepherd D, Harris R, Williams DW, Truman JW. Postembryonic lineages of the Drosophila ventral nervous system: Neuroglian expression reveals the adult hemilineage associated fiber tracts in the adult thoracic neuromeres. J Comp Neurol 2016. 01;524(13):2677–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Truman JW, Moats W, Altman J, Marin EC, Williams DW. Role of Notch signaling in establishing the hemilineages of secondary neurons in Drosophila melanogaster. Dev Camb Engl 2010. Jan;137(1):53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.**Namiki S, Dickinson MH, Wong AM, Korff W, and Card GM (2018). The functional organization of descending sensory-motor pathways in Drosophila. ELife 7, e34272.Generation and and anatomical description of intersectional Gal4 tools that provide genetic access to descending neurons.
- 23.**O’Sullivan A, Lindsay T, Prudnikova A, Erdi B, Dickinson M, von Philipsborn AC. Multifunctional Wing Motor Control of Song and Flight. Curr Biol CB 2018. September 10;28(17):2705–2717.e4.Dissection of the functions of wing MNs in both flight and male courship song.
- 24.Brierley DJ, Rathore K, VijayRaghavan K, Williams DW. Developmental origins and architecture of Drosophila leg motoneurons. J Comp Neurol 2012. June 1;520(8):1629–49. [DOI] [PubMed] [Google Scholar]
- 25.Baek M, Mann RS. Lineage and Birth Date Specify Motor Neuron Targeting and Dendritic Architecture in Adult Drosophila. J Neurosci 2009. May 27;29(21):6904–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hampel S, McKellar CE, Simpson JH, and Seeds AM (2017). Simultaneous activation of parallel sensory pathways promotes a grooming sequence in Drosophila. ELife 6, e28804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.**Tuthill JC, Wilson RI. Parallel Transformation of Tactile Signals in Central Circuits of Drosophila. Cell 2016. February 25;164(5):1046–59.Description of three types of VNC interneurons that process incoming sensory information in unique ways.
- 28.Tuthill JC, Wilson RI. Mechanosensation and Adaptive Motor Control in Insects. Curr Biol CB 2016. 24;26(20):R1022–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merritt DJ, Murphey RK. Projections of leg proprioceptors within the CNS of the fly Phormia in relation to the generalized insect ganglion. J Comp Neurol 1992. August 1;322(1):16–34. [DOI] [PubMed] [Google Scholar]
- 30.**Cande J, Namiki S, Qiu J, Korff W, Card GM, Shaevitz JW Stern DL, and Berman GJ (2018). Optogenetic dissection of descending behavioral control in Drosophila. ELife 7, e34275.Demonstrated that distinct subtypes of descending neurons produce distinct behaviors when activated.
- 31.Hsu CT, Bhandawat V. Organization of descending neurons in Drosophila melanogaster. Sci Rep 2016. February 3;6:20259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsubouchi A, Yano T, Yokoyama TK, Murtin C, Otsuna H, Ito K. Topological and modality-specific representation of somatosensory information in the fly brain. Science 2017. 03;358(6363):615–23. [DOI] [PubMed] [Google Scholar]
- 33.von Philipsborn AC, Liu T, Yu JY, Masser C, Bidaye SS, Dickson BJ. Neuronal control of Drosophila courtship song. Neuron 2011. February 10;69(3):509–22. [DOI] [PubMed] [Google Scholar]
- 34.Yu JY, Kanai MI, Demir E, Jefferis GSXE, Dickson BJ. Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr Biol CB 2010. September 28;20(18):1602–14. [DOI] [PubMed] [Google Scholar]
- 35.**Enriquez J, Rio LQ, Blazeski R, Bellemin S, Godement P, Mason C, et al. Differing Strategies Despite Shared Lineages of Motor Neurons and Glia to Achieve Robust Development of an Adult Neuropil in Drosophila. Neuron 2018. February 7;97(3):538–554.e5.Demonstrated that the neuropil glia, which surround and innervate the large thoracic neuropil, come from the same lineages that also generate the MNs that innervate the leg.
- 36.Freeman MR. Drosophila Central Nervous System Glia. Cold Spring Harb Perspect Biol [Internet] 2015. November [cited 2019 Jan 9];7(11). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4632667/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tirian L, Dickson B. The VT GAL4, LexA, and split-GAL4 driver line collections for targeted expression in the Drosophila nervous system. bioRxiv 2017. January 1;198648.
- 38.Kvon EZ, Kazmar T, Stampfel G, Yáñez-Cuna JO, Pagani M, Schernhuber K, et al. Genome-scale functional characterization of Drosophila developmental enhancers in vivo. Nature 2014. August;512(7512):91–5. [DOI] [PubMed] [Google Scholar]
- 39.Jenett A, Rubin GM, Ngo T-TB, Shepherd D, Murphy C, Dionne H, et al. A GAL4-Driver Line Resource for Drosophila Neurobiology. Cell Rep 2012. October 25;2(4):991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfeiffer BD, Ngo T-TB, Hibbard KL, Murphy C, Jenett A, Truman JW, et al. Refinement of Tools for Targeted Gene Expression in Drosophila. Genetics 2010. October 1;186(2):735–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.**Mamiya A, Gurung P, Tuthill JC. Neural Coding of Leg Proprioception in Drosophila. Neuron 2018. November 7;100(3):636–650.e6.Initial anatomical and function dissection of subtypes of sensory neurons that come from the femural chordotonal organ of the legs.
- 42.**Otsuna H, Ito M, Kawase T. Color depth MIP mask search: a new tool to expedite Split-GAL4 creation. bioRxiv 2018. January 1;318006.Method for identifying similar neuronal patterns in large databases of confocal images.
- 43.Milyaev N, Osumi-Sutherland D, Reeve S, Burton N, Baldock RA, Armstrong JD. The Virtual Fly Brain browser and query interface. Bioinformatics 2012. February 1;28(3):411–5. [DOI] [PubMed] [Google Scholar]
- 44.Franconville R, Beron C, and Jayaraman V (2018). Building a functional connectome of the Drosophila central complex. ELife 7, e37017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.**Lacin H, Truman JW. Lineage mapping identifies molecular and architectural similarities between the larval and adult Drosophila central nervous system. eLife 2016. March 15;5:e13399.Identified the embryonic origins of adult VNC NB lineages, and mapped transcription factor expression in distinct lineages.
- 46.Birkholz O, Rickert C, Nowak J, Coban IC, Technau GM. Bridging the gap between postembryonic cell lineages and identified embryonic neuroblasts in the ventral nerve cord of Drosophila melanogaster. Biol Open 2015. March 27;4(4):420–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Truman JW, Bate M. Spatial and temporal patterns of neurogenesis in the central nervous system of Drosophila melanogaster. Dev Biol 1988. January;125(1):145–57. [DOI] [PubMed] [Google Scholar]
- 48.Maurange C, Cheng L, Gould AP. Temporal Transcription Factors and Their Targets Schedule the End of Neural Proliferation in Drosophila. Cell 2008. May 30;133(5):891–902. [DOI] [PubMed] [Google Scholar]
- 49.Zhu S, Lin S, Kao C-F, Awasaki T, Chiang A-S, Lee T. Gradients of the Drosophila Chinmo BTB-Zinc Finger Protein Govern Neuronal Temporal Identity. Cell 2006. October 20;127(2):409–22. [DOI] [PubMed] [Google Scholar]
- 50.**Liu Z, Yang C-P, Sugino K, Fu C-C, Liu L-Y, Yao X, et al. Opposing intrinsic temporal gradients guide neural stem cell production of varied neuronal fates. Science 2015. October 16;350(6258):317–20.Demonstrated the role of two opposing gradients of RNA binding proteins in regulating post-mitotic neuronal fates.
- 51.Bertet C, Li X, Erclik T, Cavey M, Wells B, Desplan C. Temporal Patterning of Neuroblasts Controls Notch-Mediated Cell Survival through Regulation of Hid or Reaper. Cell 2014. August 28;158(5):1173–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dillard C, Narbonne-Reveau K, Foppolo S, Lanet E, Maurange C. Two distinct mechanisms silence chinmo in Drosophila neuroblasts and neuroepithelial cells to limit their self-renewal. Dev Camb Engl 2018. 25;145(2). [DOI] [PubMed] [Google Scholar]
- 53.Syed MH, Mark B, Doe CQ. Playing Well with Others: Extrinsic Cues Regulate Neural Progenitor Temporal Identity to Generate Neuronal Diversity. Trends Genet TIG 2017;33(12):933–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.**Syed MH, Mark B, and Doe CQ (2017b). Steroid hormone induction of temporal gene expression in Drosophila brain neuroblasts generates neuronal and glial diversity. ELife 6, e26287.Describes a role for ecdysone signaling in specifying temporally distinct fates in brain NBs.
- 55.Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci 2001. May 1;24(5):251–4. [DOI] [PubMed] [Google Scholar]
- 56.Enriquez J, Venkatasubramanian L, Baek M, Peterson M, Aghayeva U, Mann RS. Specification of individual adult motor neuron morphologies by combinatorial transcription factor codes. Neuron 2015. May 20;86(4):955–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sen R, Wu M, Branson K, Robie A, Rubin GM, Dickson BJ. Moonwalker Descending Neurons Mediate Visually Evoked Retreat in Drosophila. Curr Biol CB 2017. March 6;27(5):766–71. [DOI] [PubMed] [Google Scholar]
- 58.Bidaye SS, Machacek C, Wu Y, Dickson BJ. Neuronal Control of Drosophila Walking Direction. Science 2014. April 4;344(6179):97–101. [DOI] [PubMed] [Google Scholar]
- 59.Zacarias R, Namiki S, Card GM, Vasconcelos ML, Moita MA. Speed dependent descending control of freezing behavior in Drosophila melanogaster. Nat Commun 2018. 12;9(1):3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.**Schnell B, Ros IG, Dickinson MH. A Descending Neuron Correlated with the Rapid Steering Maneuvers of Flying Drosophila. Curr Biol CB 2017. April 24;27(8):1200–5.Identification of a descending neuron that is used to initiate rapid changes in flight path.
- 61.Chen C-L, Hermans L, Viswanathan MC, Fortun D, Aymanns F, Unser M, et al. Imaging neural activity in the ventral nerve cord of behaving adult Drosophila. Nat Commun 2018. 22;9(1):4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baek M, Enriquez J, Mann RS. Dual role for Hox genes and Hox co-factors in conferring leg motoneuron survival and identity in Drosophila. Dev Camb Engl 2013. May 1;140(9):2027–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oyallon J, Apitz H, Miguel-Aliaga I, Timofeev K, Ferreira L, Salecker I. Regulation of locomotion and motoneuron trajectory selection and targeting by the Drosophila homolog of Olig family transcription factors. Dev Biol 2012. September 15;369(2):261–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Syed DS, Bm S, Reddy OV, Reichert H, VijayRaghavan K. Glial and neuronal Semaphorin signaling instruct the development of a functional myotopic map for Drosophila walking. eLife 2016. February 29;5:e11572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doe CQ. Temporal Patterning in the Drosophila CNS. Annu Rev Cell Dev Biol 2017. 06;33:219–40. [DOI] [PubMed] [Google Scholar]
- 66.Rossi AM, Fernandes VM, Desplan C. Timing temporal transitions during brain development. Curr Opin Neurobiol 2017;42:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li X, Chen Z, Desplan C. Chapter Three - Temporal Patterning of Neural Progenitors in Drosophila. Current Topics in Developmental Biology 2015. 105; 69–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Konstantinides N, Kapuralin K, Fadil C, Barboza L, Satija R, Desplan C. Phenotypic Convergence: Distinct Transcription Factors Regulate Common Terminal Features. Cell 2018. July 26;174(3):622–635.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li H, Horns F, Wu B, Xie Q, Li J, Li T, et al. Classifying Drosophila Olfactory Projection Neuron Subtypes by Single-Cell RNA Sequencing. Cell 2017. November 16;171(5):1206–1220.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang C-P, Fu C-C, Sugino K, Liu Z, Ren Q, Liu L-Y, et al. Transcriptomes of lineage-specific Drosophila neuroblasts profiled by genetic targeting and robotic sorting. Dev Camb Engl 2016. February 1;143(3):411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lacin H, Zhu Y, Wilson BA, Skeath JB. Transcription factor expression uniquely identifies most postembryonic neuronal lineages in the Drosophila thoracic central nervous system. Development 2014. March 1;141(5):1011–21. [DOI] [PMC free article] [PubMed] [Google Scholar]