Abstract
Previously, vibratory stimulation increased spontaneous swallowing rates in healthy volunteers indicating that sensory stimulation excited the neural control of swallowing. Here we studied patients with severe chronic dysphagia following brain injury or radiation for head and neck cancer to determine if sensory stimulation could excite an impaired swallowing system. We examined: 1) if laryngeal vibratory stimulation increased spontaneous swallowing rates over sham (no stimulation) ; 2) the optimal rate of vibration, device contact pressure, and vibratory mode for increasing swallowing rates; and 3) if vibration altered participants’ urge to swallow, neck comfort, and swallow initiation latency. Vibration was applied to the skin overlying the thyroid lamina bilaterally in thirteen participants to compare vibratory rates 30, 70, 110, 150, or 70+110 Hertz, different device to neck pressures (2, 4, or 6 kilopascals), and pulsed versus continuous vibration. Swallows were confirmed from recordings of laryngeal accelerometry and respiratory apneas and viewing neck movement. Participants’ swallowing rates, urge to swallow, discomfort levels and swallow initiation latencies were measured. Vibration at 70 Hz and at 110 Hz significantly increased swallowing rates over sham. All vibratory frequencies except 70+100 Hz increased participants’ urge to swallow, while no pressures or modes were optimal for increasing urge to swallow. No conditions increased discomfort. Vibration did not reduce measures of swallow initiation latency using accelerometry. In conclusion, as noninvasive neck vibration overlying the larynx increased swallowing rates and the urge to swallow without discomfort in patients with chronic dysphagia, the potential for vibratory stimulation facilitating swallowing during dysphagia rehabilitation should be investigated.
Keywords: Sensory stimulation, swallowing, deglutition, deglutition disorders, stroke, head and neck cancer, rehabilitation
Dysphagia (oropharyngeal swallowing disorders) can be caused by diseases/disorders affecting the central or peripheral nervous systems such as stroke, Parkinson’s disease or following radiation for treatment of head and neck cancer. Dysphagia prevalence ranges from 22%-68% depending on setting (community, acute care, or long-term care) (1, 2). Although swallowing rehabilitation can improve swallowing physiology and lead to functional swallow gains (3-7), most methods have small to moderate effect sizes between 0.3 and 0.6.(8). Thus, new/improved methods are needed to improve rehabilitation benefits for patients with swallowing disorders.
Normally sensory stimulation triggers swallowing via brain stem networks (9, 10) and activates cortical pathways involved in swallowing (11, 12). Electrical stimulation of sensory nerves such as either the glossopharyngeal or the internal branch of the superior laryngeal nerve (iSLN) induces swallowing in anesthetized animals (10, 13, 14). Bilateral sensory input from the iSLN was also shown to be crucial for adequate airway protection during swallowing in healthy volunteers (15).
Studies have shown that intra-oral air pulses presented to the faucial pillars can activate mechanoreceptors innervated by glossopharyngeal afferents, upregulating spontaneous swallowing rates in healthy younger and older adults (16, 17). Further, electrical stimulation of the pharyngeal mucosa can increase cortical activation for swallowing (18) and intra-oral stimulation similarly can increase corticobulbar excitability in healthy adults (19). However, a less invasive sensory stimulation than electrical stimulation of mucosa or intra-oral or intra-pharyngeal devices, may be more practical for aiding patient swallowing.
As vibration continuously alternates direction with each deflection it continues to elicit responses in laryngeal afferents without sensory adaptation (a reduction in responses over time) (20). In contrast, neural responses to tissue pressures, such as occur with air pulses, have shown rapid adaptation in laryngeal afferents (20). Recently healthy infants’ spontaneous swallowing rates were shown to increase when vibration at 100 Hz was applied to the front of the neck overlying the larynx (21). Further, in healthy adults, vibration applied to the neck area overlying the larynx was shown to penetrate to the vocal folds and alter phonation (22). In the same study, vibration at both 70 and 150 Hz increased the rate of spontaneous swallowing, and increased cortical activation during swallowing over sham in healthy adults (22). Such stimulation on the neck overlying the larynx might provide sensory upregulation for swallowing that could be applied during feeding without interfering with oral intake, if appropriately used and monitored in persons with dysphagia.
However, it is unclear whether or not patients with dysphagia will respond to sensory stimulation to enhance swallowing rates when their dysphagia is due to either central nervous system injury or radiation effects on sensory and motor nerves in the head and neck. It is also unknown whether injury to the neural control of swallowing or laryngeal afferents might interfere with the use of laryngeal vibration to upregulate swallowing. As patients with dysphagia, particularly those who rely on enteric feeding, have reduced frequency of spontaneous swallowing (23), it is likely that their neural control for swallowing is impaired. Although sensory stimuli can upregulate cortical activation during swallowing in healthy volunteers when using intra-oral air pressure pulses (11, 12, 19), laryngeal vibration (22), or pharyngeal electrical stimulation (24), the degree to which sensory stimulation can upregulate swallowing in patients with dysphagia is less clear. Two types of sensory stimuli, pharyngeal electrical stimulation (24) and air puff stimulation of the oral mucosa (25), have been used to augment rehabilitation training for dysphagia (26, 27). To date, no studies have examined whether vibratory stimulation to the larynx affects the rate of spontaneous swallowing in patients with oropharyngeal dysphagia. If vibratory stimulation is found to augment spontaneous swallowing rates in patients with severe dysphagia, then laryngeal vibration could be used to upregulate the swallowing system during dysphagia rehabilitation in such patients.
This pilot proof-of-principle study is the first study to examine if laryngeal vibration affects swallowing rate in chronic oropharyngeal dysphagia. It aimed to determine: 1) if vibration overlying the larynx increased the rate of spontaneous swallowing over sham; 2) the optimal vibration rate in Hertz (Hz), device to neck contact pressure in kilopascals (kPa), and vibratory mode (continuous versus pulsed vibration) for increasing spontaneous swallowing rate; and, 3) if vibration reduced swallowing initiation latency over sham in chronic dysphagia. We hypothesized that participants with chronic dysphagia might require higher vibration rates and increased device to neck pressures to increase their swallowing rates in response to vibration. We expected that 4 Hz pulsed epochs might be more effective than continuous vibration as pulsed stimulation might reduce sensory adaptation (20). Further, as laryngeal vibration in healthy volunteers increased the hemodynamic response for swallowing in the motor cortex of the swallowing network (22), we hypothesized that vibration might reduce the initiation latency during volitional saliva swallows in chronic dysphagia.
Methods and Materials
Institutional Review Boards of James Madison University and Sentara Rockingham Memorial Hospital Medical Center (Sentara RMH), Harrisonburg, Virginia both approved the research protocol, consent forms and recruitment plans for the study.
Participants
Recruitment procedures included: announcements of the study given to patients referred to the Sentara RMH for dysphagia evaluation, a study announcement placed on the website of the National Foundation of Swallowing Disorders, and letters sent to rehabilitation centers and speech pathologists specializing in adult dysphagia announcing the study. Both a telephone screening and medical records review were used to determine if a potential participant met the inclusion and exclusion criteria. Participants were required to either have a diagnosis of oropharyngeal dysphagia secondary to a cerebrovascular accident (CVA) or dysphagia following radiation treatment for head and neck cancer. Exclusion criteria were that a participant did not have a history of any of the diagnoses listed in Table 1.
Table 1.
Participant characteristics or history that would have precluded participation
| Previous or current conditions: |
|---|
|
Written informed consent was obtained from all participants. Following informed consent, participants were administered the Folstein Mini-Mental State Examination and had to score 23 or better for inclusion (28). They also had to be able to communicate their preferences and score visual analogue scales to participate.
Initial Clinical Examination
Participants were questioned regarding the impact that their swallowing disorder had on their food intake and whether or not they were dependent upon percutaneous endoscopic gastrostomy (PEG) for nutrition and which types of foods/liquids, if any, they were able to intake either for nutrition or pleasure only. This information was used to generate a Functional Oral Intake Scale score (FOIS), which documented the participant’s current dietary level from level 1 (severe nothing by mouth), level 2 (moderate to severe coded as tube dependent with minimal attempts at food or liquid), level 3 (moderate, tube dependent with consistent oral intake), level 4 (total oral diet of a single consistency), up to level 7 (normal with total oral diet with no restrictions) (29). Participants were also interviewed about their swallowing functioning prior to their CVA or head/neck cancer onset and about what therapy they had received for their dysphagia after onset.
If participants were seen in the Voice and Swallowing Service at Sentara RMH, they received a videofluoroscopic modified barium swallow (MBS) study with 5 ml each of thin liquid, nectar thick, honey thick and pudding based on guidelines from the Modified Barium Swallow Impairment Profile (MBSImP) (30) with participant safety considered. With the exception of components 3 and 13, the MBSImP was scored and combined oral and pharyngeal scores were computed. We also examined the rating for component 6 on the timing of initiation of the pharyngeal swallow from 0= bolus head at the posterior angle of the ramus; 1= bolus head in the vallecula; 2= bolus head at the posterior laryngeal surface of the epiglottis, 3= bolus head in the pyriforms; to 4= no visible initiation of a swallow. Dysphagia Outcome and Severity Scale scores (DOSS) were also documented based on penetration, aspiration, and residue on the videofluoroscopy MBS and the subsequent recommended oral intake for each participant ranging from 7 indicating a normal diet to 1 indicating severe dysphagia and the inability to tolerate any per oral consistency safely (31).
Participants were told that the investigators were testing different vibratory motors and would monitor them for safety reasons, but they were not informed about hypothesized differences between conditions to prevent bias. Participants were not given any boluses to swallow nor instructed to swallow their saliva throughout the study except when swallow initiation times were compared between vibration and sham conditions.
Device Characteristics
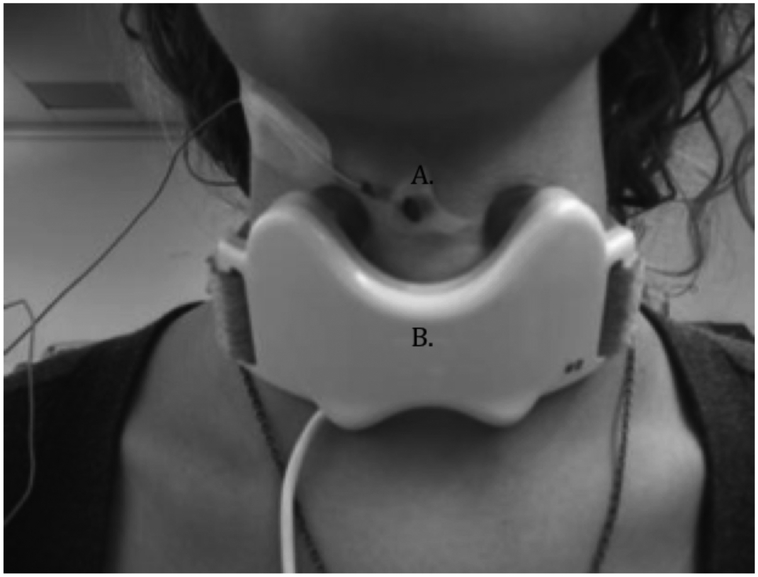
The vibratory device was developed and provided by Passy Muir Inc. (Irvine, CA). It was a molded plastic butterfly shaped neckpiece containing two motors, placed bilaterally on the skin overlying the thyroid lamina on each side (Figure 1). By carefully palpating the thyroid cartilage the motors were placed in the middle of the thyroid lamina. The bilateral motors usually had the same frequency except for one condition when the motors on each side had different frequencies, one side at 70Hz and the other at 110 Hz. Vibration onset and offset was controlled by E-Prime v2.0 pulses programmed for this investigation (Psychology Software Tools, Inc., Sharpsburg, PA 15215-2821, USA.).
Figure 1.
A. Piezoelectric accelerometer to detect laryngeal movement during swallowing. B. Vibratory device with two motors making contact on either side of the thyroid cartilage. The device was attached to the front of the neck with soft adjustable ties and connected to a controller that operated the device. The device was supplied by Passy Muir, Inc.
Vibration Frequency, Pressure & Mode Testing
The first experimental condition compared sham (no vibration with five different vibratory conditions with both motors at 30, 70, 110, and 150 Hz and when the two motors had different frequencies of 70 and 110 Hz. The second experimental condition compared different pressures between motor and the skin of the neck at 2, 4, and 6 kPa. The third experimental condition compared two modes of continuous versus 4 Hz pulsed vibration (125 ms on, 125 ms off) (Table 2). The frequency, pressure and mode conditions were tested on separate days.
Table 2.
Design of Experimental Conditions and Measures
| Independent Factors |
Testing order |
Counterbalanced Conditions |
Constants | Dependent measures |
|---|---|---|---|---|
| Frequency | Day 1 |
|
|
|
| Pressure | Day 2 |
|
|
|
| Mode | Day 3 |
|
|
Refers to specific motor with 70 Hz motor on one side and 110 Hz motor on the other side.
VAS: Visual Analogue Scale; Hz: Hertz; kPa: kilopascals
Each condition contained 28 eight second stimulation epochs alternated with 15 second vibration-free periods for a total of 10.7 minutes per condition. These were programmed using the same E-Prime program to provide the same duration of stimulation and non-stimulation epochs across all participants. This was similar to our previous experience in conducting a similar study in healthy volunteers (22). However, to prevent fatigue in the participants with dysphagia, we shortened the stimulation epochs from 10 seconds down to 8 seconds and the vibration free intervals from a range of 30 to 45 seconds down to 15 seconds. To eliminate the effects of the previous condition, five minute rest periods occurred between each condition, when the participant was encouraged to relax and avoid talking.
Throughout each condition, video-recordings of the participant’s mouth and neck were digitized. Simultaneously a trained observer pressed a button producing a recorded digital pulse whenever they saw laryngeal elevation for a swallow without other head movements or speech. The pulse was recorded in the synchronized real time digitized data on PowerLab 16/30 SP unit with a 16-bit analog-to-digital converter (AD Instruments, model ML880, Colorado Springs, CO, USA).
During each condition, neck to device pressure was set before testing by placing the air-filled bulb of the IOPI pressure sensor (IOPI Medical, model 2.3, Woodinville, WA, USA) between the neck and the strap and adjusting the ties until the target pressure level was read on the IOPI in kPa. The trained observer assured that the strap position was maintained throughout a testing to assure constant pressure.
Within the first condition, the order of frequencies and sham were counter-balanced across participants. In addition, two 10.7 minute non-stimulation conditions were recorded: one when the participant was not wearing the device (no device) and another when the participant was wearing the device but no vibration occurred (sham).
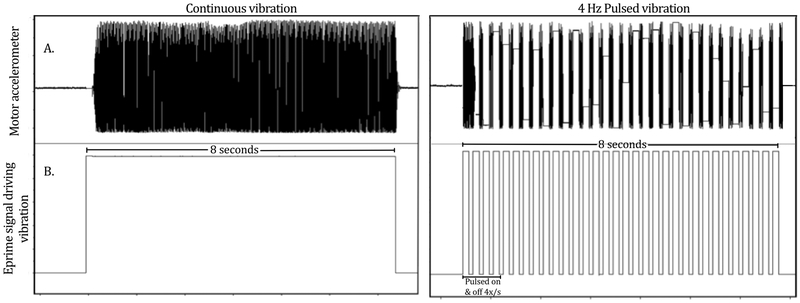
In the second condition examining different pressures, the orders were counter-balanced across participants. During the pulsed versus continuous condition, E-Prime programming controlled the pulsed stimulation condition by providing 8 s of vibration which was pulsed on for 125 ms and off for 125 ms, four times per second (Figure 2).
Figure 2.
Illustration of continuous and pulsed vibration as detected by the motor accelerometer signal (A) and as controlled by E-Prime software (B.) and. For continuous vibration, stimulation was delivered for 8 seconds alternated with 15 seconds of no vibration for 28 cycles. For pulsed vibration, stimulation was delivered at the same frequency and pulsed on and off 4 times/second for 8 seconds alternated with 15 seconds of no vibration for 28 cycles.
Ratings of Urge to Swallow and Discomfort Level
In each of the three experimental testing conditions comparing frequencies, pressure, and pulsed versus continuous stimulation, after each recording the participant was asked to rate their perceptions of their urge to swallow and discomfort level on separate sheets. These were measured using visual analogue scale (VAS) lines of 100 mm labeled “none” on the far left and “greatest ever experienced” on the far right. Scale markings were later converted into 0-100 scores for each condition.
Swallow Initiation Latency Testing
In a separate experimental condition on day 3 of testing, we examined if vibratory stimulation affected the latency of initiation of voluntary swallowing. The optimal vibration settings found during the frequency, pressure, and mode testing to induce the highest rate of swallowing for that participant were used during swallow initiation testing. The participants were instructed to manually push a button to turn on the vibration and then initiate a swallow as quickly as possible. As many of the participants were designated as taking nothing per oral, no substances were administered and participants were only swallowing their own saliva. The examiner randomly selected between equal numbers of trials when the device was turned on and when it was turned off throughout testing so that 50% of a participant’s trials were stimulated regardless of when they fatigued and had to stop. Swallow initiation latencies with stimulation were compared to when the participant pushed the button and swallowed without vibration (sham). Trials were self-paced, participants were allowed as much time between trials as they wanted. The number of trials depended upon the total time available for testing (usually several hours) and participant fatigue.
Identification of Swallows
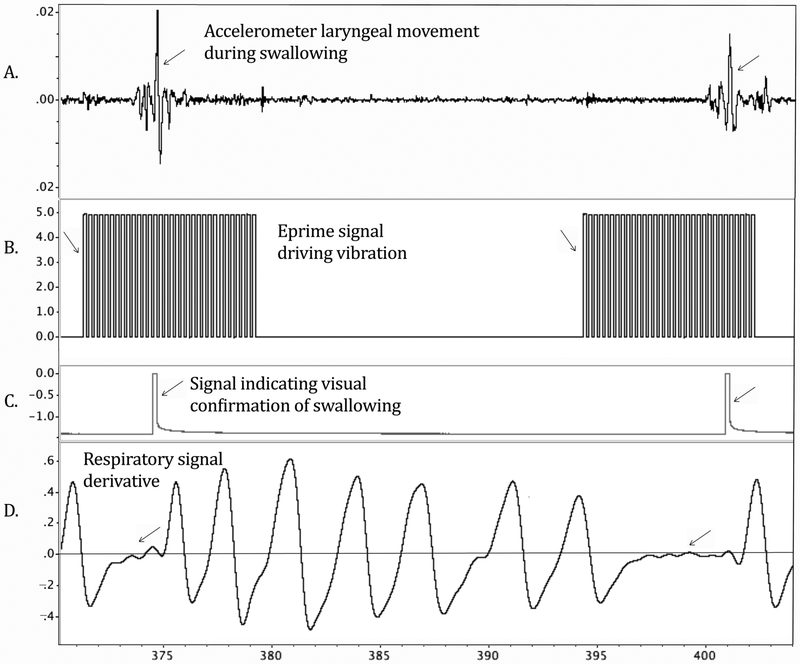
A miniature piezoelectric accelerometer with pre-amplification was placed over the thyroid notch to detect the onset of laryngeal elevation at the beginning of the pharyngeal phase of swallowing (Kistler, model 8778A500, Amherst, New York, USA·). Inductive plethysmography stretching bands were secured around the ribcage and the abdomen, with each channel amplified at 1 and then both summed using Inductotrace model 10.9000, (Ambulatory Monitoring, Inc. Ardsley, N.Y., USA). Realtime digital recordings were made during each 10.7 minute condition while being displayed on the PowerLab 16/30 SP unit. Accelerometry was sampled at 40 kHz and all other signals were sampled at 4 kHz. All signals were low-pass filtered for anti-aliasing prior to digitization. The recordings included the accelerometer reflecting the onset of laryngeal elevation for the pharyngeal phase of swallowing (Figure 3A); another accelerometer provided with the device which was attached to the motor to indicate when the motor was vibrating (Figure 2A); Inductotrace sum signals were down-sampled and the first derivative computed to identify swallowing apnea (Figure 3D), and a pulse produced by the observer button each time they viewed the participant swallowing (Figure 3C). Each digitized recording was coded so that the principal investigator was blind to condition when analyzing swallowing rate offline. When a swallow occurred during vibration the accelerometer signal was low pass filtered at 15 Hz to identify the laryngeal elevation signal after removing the vibration signal. As the participants were on enteric feeding, both their swallowing attempts and completed swallows indicated activation of the swallowing system and were included.
Figure 3.
Illustration of offline analysis of swallow rate in LabChart. A large spike in the accelerometer signal (A) indicated laryngeal elevation at swallowing onset. Eprime software (B) signal indicated vibratory onsets and offsets. The square wave of a pulse generator (C) indicated that the trained observer pushed a button when they visually confirmed a swallow. The first derivative of the sum Inductotrace signal (D) flattened to approximately zero on the y axis, indicating apneic periods during swallowing.
To be counted as a swallow or a swallow attempt, three simultaneous events were required during offline analysis: 1) the peak of the hyolaryngeal accelerometry signal confirming laryngeal elevation (Figure 3A), 2) a simultaneous apneic period on inductive plethysmography (Figure 3D), and 3) an observer button press providing visual confirmation of a swallow or a swallow attempt and no other head movements (Figure 3C). Review of the synchronized video-recording was required to identify a swallow when the accuracy of the 3 events was in question.
Measures of Delay in the Onset of the Pharyngeal Phase from Videofluoroscopy
Two measures were made from the intake videofluoroscopy recording to determine if the participants had delays in the onset of the pharyngeal phase of swallowing. First, scoring on the MBSImP Condition 6 coded when a participant initiated brisk superior-anterior hyoid excursion relative to the position of the bolus at that time was scored as previously described on a 5 point scale. Second, for the 5 ml thin liquid bolus condition pharyngeal initiation time was measured, defined as the time in seconds from when the bolus head first crossed the ramus of the mandible to the time of the first frame of hyoid movement for excursion.
Measurement of Swallow Initiation Latencies with and without Vibration
For offline analysis of swallow initiation latency from PowerLab recordings, the interval in seconds was measured between the onset of the button press signal when the participant turned on the vibration or sham, and the onset of laryngeal elevation in the accelerometer signal for the swallow. Any swallows that began before the button was pushed were excluded from analysis.
Statistical Analysis
Device Effect without Stimulation
To determine if wearing the device on the neck affected spontaneous swallowing, the swallow rates from the no device condition were compared with the sham condition (device on, no vibration) using a two-tailed t test at p=0.05.
Group Comparisons on Spontaneous Swallow Rates During the Sham Conditions
To determine if the baseline swallowing rates differed between the post CVA and post radiation participants, the sham condition swallowing rates were computed as swallows per minute. Healthy volunteer data from the sham condition of ten participants on swallows per minute from a similar previously published study (22) were used for comparison to determine if the participants studied here differed from healthy volunteers in their spontaneous swallowing rates. An ANOVA compared the swallows per minute rates of the three groups: healthy volunteers, participants with dysphagia post CVA and participants post radiation for head and neck cancer.
Vibration Frequency Comparisons with Sham
To determine if swallowing rates increased with vibration during each frequency condition, swallowing rates during vibration were compared with sham (device on, no vibration), on one-tailed Z tests with p=0.025 for unidirectional comparisons with sham for each frequency.
To determine if participants adapted to vibration over time, swallowing rates during vibration on were compared across four 2.6 minute increments within each 10.7 min condition using repeated measures ANOVAs. Only frequency conditions when swallowing rates increased with stimulation compared to sham were examined as these were the only conditions when adaptation could have occurred.
Contact Pressure Comparisons
Repeated measures ANOVAs compared swallowing rates for three pressure conditions (2, 4, and 6kPa) during stimulation at p=0.025.
Vibration Mode Comparisons
Continuous and pulsed conditions were compared using two-tailed paired t tests during stimulation and during non-stimulation intervals at p=0.025 for each.
Swallow Initiation Latency with and without Vibration
First, we examined timing measures of the onset of the pharyngeal phase of swallowing made in each participant on their baseline videofluoroscopy study on a 5 ml thin liquid swallow. We measured time in seconds from when the bolus first crossed the ramus of the mandible to the onset of hyoid movement. We then measured the change in mean swallow onset latency between swallows without vibration and swallows with vibration from the PowerLab recordings. To determine if there was a relationship between participants’ videofluoroscopy measures of pharyngeal onset time and their change scores on swallow initiation time with vibration, we computed a Pearson r and determined if p<0.05. If there was no relationship, then no covariance was needed and the participants’ mean swallow onset latencies for trials with vibration were compared with trials without vibration using a two-tailed paired t test at p=0.05.
Participant Perception Ratings
For each frequency, one-tailed Z tests compared VAS scores after a stimulation condition with those following sham. For pressure effects, repeated measures ANOVAs compared VAS scores across pressures. For mode comparisons, a two-tailed paired t test compared VAS scores between modes. Alpha levels =0.05 were used for each.
Results
Participants
Fourteen participants were consented. One participant did not pass the medical screening post-consent and was excluded from data collection. Thus, the data from 13 participants (2 female, mean age: 60.5, range: 15-80) are described in Table 3. DOSS(31) scores ranged from 1-2. All participants received either partial or total nutrition via PEG tube (Table 4).
Table 3.
Participant intake information on entry into the investigation and completion of testing conditions
| Participant | Demographic Data |
Diagnosis | Months Post- Onset |
Functional Oral Intake Scale score* |
Testing completed |
|---|---|---|---|---|---|
| S1 | 80 y; male | Brainstem CVA |
18 | 2, Mod/Sev | All |
| S2 | 56 y; male | Brainstem CVA |
44 | 1, Severe | All |
| S3 | 51 y; male | Brainstem CVA |
12 | 1, Severe | Frequency & Pressure |
| S4 | 62 y; male | Brainstem and cerebellar CVA |
10 | 3, Moderate | All |
| S5 | 70 y; female | Brainstem CVA |
42 | 2, Mod/Sev | Frequency, Pressure, Mode |
| S6 | 15 y; female | Brainstem CVA |
53 | 1, Severe | Frequency |
| S7 | 59 y; male | Brainstem CVA |
36 | 2, Mod/Sev | Frequency, Pressure, Mode |
| S8 | 58 y, male | Brainstem CVA |
31 | 1, Severe | Frequency, Pressure, Mode |
| S9 | 74 y; male | Brainstem CVA |
32 | 2, Mod/Sev | All |
| S10 | 67 y; male | Head/ neck CA |
17 | 1, Severe | All |
| S11 | 76 y; male | Head/ neck CA |
160 | 2, Mod/Sev | All |
| S12 | 50 y; male | Head/ neck CA |
36 | 1, Severe | All |
| S13 | 69 y; male | Head/ neck CA |
131 | 1, Severe | All |
Table 4.
Patient Dietary level and results of Intake Videofluoroscopy Study with scores on the Modified Barium Swallow Impairment Profile (MBSImP) consistencies used on Modified Barium Swallow Study (MBS) and Dysphagia Outcome and Severity Scores (DOSS)
| Participant ID |
Dietary Level |
MBSImP Oral Score* (out of 15) |
MBSImP Pharyngeal Score*(out of 30) |
MBSImP Initiation of the pharyngeal swallow score (out of 4)** |
Consistencies trialed on MBS*** |
DOSS+* |
|---|---|---|---|---|---|---|
| S1 | PEG tube; Puree for pleasure |
Not available | Not available | Not available | Not available | 1, Severe |
| S2 | PEG tube | 7 | 25 | 4 | Thin liquid, honey thick liquid | 1, Severe |
| S3 | PEG tube | Not available | Not available | Not available | Not available | 1, Severe |
| S4 | Partial PEG dependent; Most textures by mouth;avoided dry foods |
12 | 12 | 3 | Thin liquid, nectar thick liquid, honey thick liquid, pudding, solid | 2, Mod/severe |
| S5 | PEG tube; Some liquids and purees for pleasure |
5 | 19 | 4 | Thin liquid, nectar thick liquid, honey thick liquid, pudding | 2, Mod/severe |
| S6 | PEG tube | 5 | 24 | 4 | Thin liquid, nectar thick liquid, honey thick liquid | 1, Severe |
| S7 | PEG tube; some liquids for pleasure |
3 | 17 | 1 | Thin liquid, nectar thick liquid | 1, Severe |
| S8 | PEG tube | 4 | 23 | 3 | Thin liquid, nectar thick liquid | 1, Severe |
| S9 | PEG tube; some liquids and soft solids for pleasure |
6 | 22 | 4 | Thin liquid, nectar thick liquid, honey thick liquid | 1, Severe |
| S10 | PEG tube | 8 | 20 | 4 | Thin liquid, nectar thick liquid, honey thick liquid | 1, Severe |
| S11 | PEG tube; Puree for pleasure |
6 | 16 | 0 | Thin liquid, nectar thick liquid, honey thick liquid, pudding | 1, Severe |
| S12 | PEG tube; water for pleasure |
3 | 24 | 3 | Thin liquid, nectar thick liquid, honey thick liquid | 1, Severe |
| S13 | PEG tube; some liquids for pleasure |
5 | 24 | 3 | Thin liquid, nectar thick liquid, honey thick liquid | 1, Severe |
MBSImP: Modified Barium Swallow Impairment Profile; Both pharyngeal and oral score represent the sum of the worst score each component received. Components 3 and 13 were not included in the oral and pharyngeal sums, respectively. A higher score indicates a worse severity.
Represents the worst score obtained on Component 6 of the MBSImP protocol, which is judged at the first movement of brisk superior-anterior hyoid trajectory. The scoring is as follows: 0=Bolus head at posterior angle of ramus; 1=Bolus head in valleculae; 2=Bolus head at posterior laryngeal surface of epiglottis; 3=Bolus head in pyriforms; 4=No visible initiation at any location.
MBSImP protocol followed with patient safety considered.
DOSS score of 1 = Severe dysphagia requiring complete NPO, unable to tolerate any P.O. safely; DOSS score of 2= Moderately severe dysphagia requiring maximum assistance or use of strategies with partial P.O. only on at least one consistency (31).
None of the participants reported swallowing disorders prior to their CVA or prior to their onset of head and neck cancer. All of the participants had received therapy for their dysphagia after onset and had completed therapy prior to their participation in the study.
Clinical ratings on the MBSImP oral and pharyngeal scores for each of the participants are provided in Table 4. On Condition 6, two participants had ratings of 1 or 0 indicating less delay on initiation of the pharyngeal of a swallow while eight had ratings of 3 or 4 indicating either a significant delay in onset for the pharyngeal phase or no visible swallow initiation. No participant had a rating of 2 on Condition 6 (Table 4).
As different factors (frequency, pressure, mode, and initiation times) were tested on different days; not all participants were able to complete the entire study (Table 3). Thirteen participants completed frequency testing, 12 pressure, 11 mode, and 8 initiation time.
Device Effect without Stimulation on Swallowing Rates
No difference in swallowing rate occurred between not wearing the device and sham (device on, no vibration) (t=−.12, p=0.91). Thus, the sham swallow rate was compared with swallowing for each vibratory frequency on one-way Z tests to determine if the swallowing rate increased.
Group Comparisons on Swallowing Rate Without Vibration.
Similar swallowing rates without vibration (swallows/minute) were found in the three groups on the ANOVA (F=0.761, p= 0.480). The mean swallowing rates were similar: healthy volunteers (mean 1.27, SD=1.22) participants post CVA (mean =0.73, SD =0.75) and participants post radiation for head and neck cancer (Mean= 0.70, SD=1.15).
Effects of Different Frequencies
During stimulation, only vibration frequencies of 70 Hz (Z=3.49, p<.001) and 110 Hz (Z=4.11, p<.001) significantly increased swallowing rate compared to sham, while 30 Hz showed a non-significant trend (Z=1.91, p=.027) (Figure 4). The other vibration frequencies did not differ from sham (p>.025). When the stimulation was off (during interstimulus intervals), no significant differences in swallow rates occurred on any frequencies compared to sham (p>.025) indicating no persistence of effect on swallowing rate after vibration was turned off.
Figure 4.
Box plots comparing swallowing rate (swallows/min) during stimulation and without stimulation for the different vibration frequencies that were tested. 70 Hz and 110 Hz significantly increased swallowing rate during vibration; asterisk indicates p<.001. Sham referred to when the participant had the device on the neck but there was no vibration. Sixteen of the seventeen outliers (empty and filled circles) were from two participants, one with CVA and one with head and neck cancer, and occurred equally across all frequencies and sham conditions.
Specific to adaptation, swallowing rates during stimulation over the course of 10 minutes did not change over time during the 70 Hz condition (F(3,36)=.78, p=.51) or during the 110 Hz condition (F(3,36)=.58, p=.63).Thus, there was no effect of time of the study (early, mid, late) that would indicate adaptation to stimulation during the experiment.
Participants reported significantly greater urges to swallow during vibration frequencies of 30 Hz (Z=2.53, p<.01), 70 Hz (Z=1.8, p<.05), 110 Hz (Z=1.85, p<.05), and 150 Hz (Z=1.98, p<.05) compared to the sham condition (Figure 5A). No significant differences in discomfort levels occurred between vibration and sham at any frequency (p>.05) (Figure 5B).
Figure 5.
Box plots comparing urge to swallow and discomfort perceptions across the different tested vibration frequencies and sham. Frequencies 30, 70, 110, and 150 Hz significantly increased participants’ urge to swallow compared to sham. Single asterisk indicates p<.05, double asterisks indicate p<.01.
Effects of Contact Pressures
Device contact pressures did not significantly alter swallowing rates during stimulation (F(2, 10)=0.18, p=.84) or between stimulations (F(2,10)=0.26, p=.77). Also, no differences occurred between contact pressures on urge to swallow ratings (F(2,10)=0.71, p=.51) or discomfort level ratings (F(2,10)=0.86, p=.45). Discomfort levels also did not differ between the CVA and head and neck cancer groups, p=0.22.
Effects of Vibration Mode
No differences occurred in swallowing rates between continuous and pulsed vibration modes, either during vibration (t=0.00, p=1.0) or between vibrations (t=−0.47, p=.65). Changes in mode did not alter ratings of urge to swallow (t=0.96, p=.36) or discomfort levels (t= −0.56, p=.59).
Effects of Vibration on Swallow Initiation Latency
Vibration effects on swallow initiation were measured on an average of 16.8 trials for both vibration on and vibration off conditions across participants (range: 5-36). Swallows occurring with vibration had a mean onset latency of 5.82 seconds from button press to onset of laryngeal elevation while swallows occurring without vibration had a mean onset time of 5.81 seconds between button press and laryngeal elevation. As only one of the eight participants in the study demonstrated a change in swallowing initiation time with and without vibration, but did not have a rating of 3 or 4 on Condition 6 of the MBSImP, we could not relate the MBSImP results to the effects of vibration on swallow initiation time. No relationship was found between the videofluoroscopy measure of pharyngeal initiation time in seconds and the change in swallow initiation time with and without vibration measured in Labchart (r—1.68, p= 0.719). On a paired t-test, vibration did not alter swallowing initiation time compared to sham for the group (t= −0.340, p=0.744).
Discussion
This study demonstrated the feasibility and safety of using vibration to upregulate swallowing rates in participants with dysphagia. The participants tolerated all the procedures well and vibration did not induce discomfort. Vibration increased spontaneous swallowing in participants with chronic dysphagia at vibratory frequencies of 70 and 110 Hz with no adaptation to stimulation over time. Although not all frequencies produced statistically significant increases in swallowing rate, no frequency reduced the participants’ mean swallowing rate (Figure 4). Additionally, the participants reported an increased urge to swallow during all frequencies except the combination of motors 70+110 Hz (Figure 5). Thus, the sensation of vibration at 70 Hz and at 110 Hz increased both the urge to swallow and swallowing rates in the group.
The finding that vibratory frequencies of 70 and 110 Hz increased swallowing rates in participants with dysphagia is similar to the results we previously found in healthy volunteers when swallowing rates increased only in response to 70 and 150 Hz (22). Thus, although we shortened the duration of stimulation epochs from 10 seconds down to 8 seconds and the non-stimulation intervals from between 30 and 45 seconds down to 15 seconds in the participants to prevent fatigue, the effects of vibration on swallowing rates was very similar. This suggests that reducing stimulation and non-stimulation epochs to prevent fatigue did not alter the effects of vibration on swallowing rates in the participants.
An increase in the spontaneous swallowing rate is potentially significant particularly for patients with chronic dysphagia who rely on non-oral feeding and expectorate saliva rather than spontaneously swallow for clearance. In such patients, who are not swallowing spontaneously or ingesting food orally, inactivity of the neural swallowing pathways potentially could result in reduced synaptic connections further impairing function (32). As vibratory stimulation could upregulate neural activity in the swallowing network even when the patient is not directly engaging in rehabilitative activity, it may have potential for enhancing function. The increase in urge to swallow with vibration also suggests that the participants had retained some sensory awareness of their swallowing system.
Other types of sensory stimulation have been shown to upregulate swallowing rates in dysphagia. Theurer et al. presented 2 Hz air-pulse stimulations to the faucial pillars in eight participants with dysphagia post CVA (27) and reported a two standard deviation increase in spontaneous swallowing in 4 of the 8 participants. Their post-hoc analysis indicated that the participants who responded to air-pulse stimulation were also the ones with the highest baseline swallowing rates (27). Post-hoc analysis of our data similarly indicated that the participants with the highest sham rates of spontaneous swallowing were those with the highest swallowing rates during vibration (Figure 6). Therefore, sensory stimulation may be less effective in participants with limited spontaneous swallowing possibly due to greater dysfunction of their swallowing network.
Figure 6.
Scatterplot demonstrating the association between participants’ sham swallowing rate and their swallowing rate during 70 and 110 Hz stimulation. Pearson’s product-moment correlation demonstrated a strong association between the two (r(26)=.89, p<.001). Markers located above the dotted reference line indicated an increase in swallowing with vibration compared to sham for that participant; markers located on or below the reference line, indicated equal or reduced swallowing during vibration compared to sham.
To determine if the effects of vibration on upregulating swallowing rates in participants with dysphagia persisted past the period of vibration, we also examined if swallowing rates increased during the non-stimulation epochs in the stimulation conditions when compared with the sham stimulation condition. The swallowing upregulation during vibration at 70 and 110 Hz were not seen during the epochs without stimulation between stimulation epochs. Thus, once the vibration was turned off the effect of the preceding vibration did not persist to maintain increased swallowing. This also occurred during the pressure and mode conditions. Although persistence of the effects of vibration on swallowing rate were not seen in this short-term study, it needs to be determined if benefits occur when stimulation is used for intensive long-term treatment.
Increases in device to neck pressure greater than 2 kPa did not alter the rate of spontaneous swallowing or the participant’s perceived urge to swallow. Although no association occurred between contact pressure and discomfort, some patients might experience difficulty tolerating a tight-fitting device on their neck. The results here indicate that a light pressure was just as effective as medium to high pressure and future applications need not require a tightness level beyond a patient’s comfort level.
It was hypothesized that vibration would improve swallow initiation compared to swallows without vibration. Previous literature demonstrated faster swallowing initiation times with a sour tasting bolus, after thermal tactile stimulation, and with carbonation (33-35). However, the vibration stimulus did not alter initiation times of swallows compared to sham. The effects of vibration on the timing of physiological events when a bolus is used should be studied using videofluoroscopy, to determine if ongoing vibration alters motions during swallowing. On the other hand, vibration did not slow initiation times, which is important from a safety standpoint when considering the feasibility of using laryngeal vibration as a stimulus during dysphagia therapy.
We hypothesized that vibration of the laryngeal tissues most likely served as a sensory stimulus to upregulate brainstem and cortical swallowing networks. In healthy volunteers, laryngeal vibration enhanced the hemodynamic response in the motor cortex during swallowing likely due to increased afferent input (22). Vibration plays a wide role in rehabilitation science during motor tasks and has been explored in the physical therapy and orthopedic literature (36-40). Vibratory stimulation can increase muscular contraction via the tonic vibration reflex involving stimulation of muscle spindles providing feedback to brain stem motor neurons that can potentiate muscle fiber recruitment (36). However, the laryngeal muscles may not have muscle spindles (41) and stretch of either the thyroarytenoid or cricothyroid muscles does not induce stretch reflexes in laryngeal muscles in humans although stretch reflexes were found in the sternohyoid muscles (42). Thus vibration overlying the larynx may both activate mechanoreceptors in the mucosa in the larynx and potentiate recruitment of suprahyoid and infrahyoid muscles, which have spindles and are involved in swallowing. Vibration of the neck tissues overlying the thyroid cartilage, as performed here, most likely also affected the thyrohyoid muscle, which contracts to bring the thyroid cartilage and hyoid bone closer together for laryngeal vestibule closure during swallowing. In exercise physiology, vibration applied during isometric limb exercises increased muscle power output and whole-body vibration in elderly participants improved muscle strength, balance and mobility (36-40). Therefore, although vibration in this study was only aimed at increasing spontaneous swallowing through sensory upregulation of the swallowing centers, vibration during swallowing rehabilitation may have additional effects on swallowing musculature. Further studies are needed to determine if vibratory stimulation during dysphagia rehabilitation could benefit muscle physiology to facilitate recovery from dysphagia and if so, which pathologies causing dysphagia might benefit from this stimulation.
Limitations
This study only included participants with chronic moderate to severe dysphagia as a convenience sample. Further research in acute and subacute populations is needed. The sample size was small, and results may not be generalizable to a larger sample or dysphagic patients with other etiologies. Salivary flow rates were not controlled as this was a within participant study. As vibration was previously shown to increase oral salivary flow (43), this could be another possible mechanism for increased swallowing rates found with laryngeal vibration.
As the experiment required three days of testing days with two to four hours of recording time each day, neither endoscopy nor fluoroscopy could be used to visualize swallowing throughout the study. Therefore, the immediate effect of the vibratory stimulation on swallow physiology is unknown. Physiological instrumentation was used to simultaneously record laryngeal elevation and respiratory apnea, with simultaneous visual confirmation that the participants were attempting a swallow. However, whether a swallow was fully completed is unknown, particularly given the dysphagia severity of the participants studied. Further research is required to understand the short and long-term effects of vibratory stimulation on swallowing physiology and whether these effects differ with different types of dysphagia pathophysiology. A videofluoroscopic study with kinematic analysis of oral and pharyngeal movement during swallowing with and without vibration is needed to determine the immediate effects of vibration on swallowing physiology in dysphagia.
Conclusions
Vibratory stimulation of the laryngeal tissues can be safely and comfortably applied in patients with dysphagia. Optimal vibratory characteristics for increasing spontaneous swallowing rate in patients were vibration at 70 and at 110 Hz with a comfortable skin to device contact pressure of 2 kPa. The same frequencies of vibration also increased the participants urge to swallow. Although vibration did not improve swallow initiation times, it did not interfere with swallowing initiation or induce discomfort. It needs to be determined if vibratory stimulation can augment traditional dysphagia rehabilitation techniques by increasing spontaneous swallowing and the urge to swallow in patients with dysphagia.
References
- 1.Lindgren S, Janzon L: Prevalence of swallowing complaints and clinical findings among 50-79-year-old men and women in an urban population. Dysphagia 6: 187–192, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Steele CM, Greenwood C, Ens I, Robertson C, Seidman-Carlson R: Mealtime difficulties in a home for the aged: not just dysphagia. Dysphagia 12: 43–50; discussion 51, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Carnaby-Mann G, Crary MA, Schmalfuss I, Amdur R: "Pharyngocise": Randomized controlled trial of preventative exercises to maintain muscle structure and swallowing function during head-and-neck chemoradiotherapy. International Journal of Radiation Oncology Biology Physics 83: 210–219, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Kang JH, Park RY, Lee SJ, Kim JY, Yoon SR, Jung KI: The effect of bedside exercise program on stroke patients with dysphagia. Annals of Rehabilitation Medicine 36: 512–520, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCullough GH, Kamarunas E, Mann GC, Schmidley JW, Robbins JA, Crary MA: Effects of Mendelsohn maneuver on measures of swallowing duration post stroke. Top Stroke Rehabil 19: 234–243, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang EJ, Baek SR, Shin J, Lim JY, Jang HJ, Kim YK, Paik NJ: Effects of transcranial direct current stimulation (tDCS) on post-stroke dysphagia. Restorative Neurology and Neuroscience 30: 303–311, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Zhen Y, Wang JG, Tao D, Wang HJ, Chen WL: Efficacy survey of swallowing function and quality of life in response to therapeutic intervention following rehabilitation treatment in dysphagic tongue cancer patients. European Journal of Oncology Nursing 16: 54–58, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Drulia TC, Ludlow CL: Relative Efficacy of Swallowing versus Non-swallowing Tasks in Dysphagia Rehabilitation: Current Evidence and Future Directions. Current physical medicine and rehabilitation reports 1: 242–256, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chi-Fishman G, Capra NF, McCall GN: Thermomechanical facilitation of swallowing evoked by electrical nerve stimulation in cats. Dysphagia 9: 149–155, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Kitagawa J, Nakagawa K, Hasegawa M, Iwakami T, Shingai T, Yamada Y, Iwata K: Facilitation of reflex swallowing from the pharynx and larynx. J Oral Sci 51: 167–171, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Lowell SY, Poletto CJ, Knorr-Chung BR, Reynolds RC, Simonyan K, Ludlow CL: Sensory stimulation activates both motor and sensory components of the swallowing system. NeuroImage 42: 285–295, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soros P, Lalone E, Smith R, Stevens T, Theurer J, Menon RS, Martin RE: Functional MRI of oropharyngeal air-pulse stimulation. Neuroscience 153: 1300–1308, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Doty RW: Influence of stimulus pattern on reflex deglutition. Am J Physiol 166: 142–158, 1951. [DOI] [PubMed] [Google Scholar]
- 14.Jean A: Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev 81: 929–969., 2001. [DOI] [PubMed] [Google Scholar]
- 15.Jafari S, Prince RA, Kim DY, Paydarfar D: Sensory regulation of swallowing and airway protection: a role for the internal superior laryngeal nerve in humans. J Physiol 550: 287–304, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theurer JA, Bihari F, Barr AM, Martin RE: Oropharyngeal stimulation with air-pulse trains increases swallowing frequency in healthy adults. Dysphagia 20: 254–260, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Theurer JA, Czachorowski KA, Martin LP, Martin RE: Effects of oropharyngeal air-pulse stimulation on swallowing in healthy older adults. Dysphagia 24: 302–313, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Fraser C, Rothwell J, Power M, Hobson A, Thompson D, Hamdy S: Differential changes in human pharyngoesophageal motor excitability induced by swallowing, pharyngeal stimulation, and anesthesia. Am J Physiol Gastrointest Liver Physiol 285: G137–144, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Power M, Fraser C, Hobson A, Rothwell JC, Mistry S, Nicholson DA, Thompson DG, Hamdy S: Changes in pharyngeal corticobulbar excitability and swallowing behavior after oral stimulation. Am J Physiol Gastrointest Liver Physiol 286: G45–50, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Davis PJ, Nail BS: Quantitative analysis of laryngeal mechanosensitivity in the cat and rabbit. J Physiol (Lond) 388: 467–485, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szynkiewicz SH, Mulheren RW, Palmore KW, O'Donoghue CR, Ludlow CL: Using devices to upregulate nonnutritive swallowing in typically developing infants. Journal of applied physiology: jap 00797 02015, 2016. [DOI] [PubMed] [Google Scholar]
- 22.Mulheren RW, Ludlow CL: Vibration over the larynx increases swallowing and cortical activation for swallowing. J Neurophysiol 118: 1698–1708., 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crary MA, Carnaby GD, Sia I, Khanna A, Waters MF: Spontaneous swallowing frequency has potential to identify dysphagia in acute stroke. Stroke 44: 3452–3457, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamdy S, Rothwell JC, Aziz Q, Singh KD, Thompson DG: Long-term reorganization of human motor cortex driven by short-term sensory stimulation. Nat Neurosci 1: 64–68, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Power ML, Fraser CH, Hobson A, Singh S, Tyrrell P, Nicholson DA, Turnbull I, Thompson DG, Hamdy S: Evaluating oral stimulation as a treatment for dysphagia after stroke. Dysphagia 21: 49–55, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Jayasekeran V, Singh S, Tyrrell P, Michou E, Jefferson S, Mistry S, Gamble E, Rothwell J, Thompson D, Hamdy S: Adjunctive functional pharyngeal electrical stimulation reverses swallowing disability after brain lesions. Gastroenterology 138: 1737–1746, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Theurer JA, Johnston JL, Fisher J, Darling S, Stevens RC, Taves D, Teasell R, Hachinski V, Martin RE: Proof-of-principle pilot study of oropharyngeal air-pulse application in individuals with dysphagia after hemispheric stroke. Archives of Physical Medicine and Rehabilitation 94: 1088–1094, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR: "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198, 1975. [DOI] [PubMed] [Google Scholar]
- 29.Crary MA, Mann GD, Groher ME: Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil 86: 1516–1520, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Martin-Harris B, Brodsky MB, Michel Y, Castell DO, Schleicher M, Sandidge J, Maxwell R, Blair J: MBS measurement tool for swallow impairment--MBSImp: establishing a standard. Dysphagia 23: 392–405, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Neil KH, Purdy M, Falk J, Gallo L: The Dysphagia Outcome and Severity Scale. Dysphagia 14: 139–145, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Kleim JA, Jones TA: Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res 51: S225–239, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Leow LP, Huckabee ML, Sharma S, Tooley TP: The influence of taste on swallowing apnea, oral preparation time, and duration and amplitude of submental muscle contraction. Chem Senses 32: 119–128, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Logemann JA, Pauloski BR, Colangelo L, Lazarus C, Fujiu M, Kahrilas PJ: Effects of a sour bolus on oropharyngeal swallowing measures in patients with neurogenic dysphagia. J Speech Hear Res 38: 556–563, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Regan J, Walshe M, Tobin WO: Immediate effects of thermal-tactile stimulation on timing of swallow in idiopathic Parkinson's disease. Dysphagia 25: 207–215, 2010. [DOI] [PubMed] [Google Scholar]
- 36.Cerciello S, Rossi S, Visona E, Corona K, Oliva F: Clinical applications of vibration therapy in orthopaedic practice. Muscles Ligaments Tendons J 6: 147–156, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rittweger J: Vibration as an exercise modality: how it may work, and what its potential might be. Eur J Appl Physiol 108: 877–904, 2010. [DOI] [PubMed] [Google Scholar]
- 38.Bautmans I, Van Hees E, Lemper JC, Mets T: The feasibility of Whole Body Vibration in institutionalised elderly persons and its influence on muscle performance, balance and mobility: a randomised controlled trial [ISRCTN62535013]. BMC Geriatr 5: 17, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rees SS, Murphy AJ, Watsford ML: Effects of whole-body vibration exercise on lower-extremity muscle strength and power in an older population: a randomized clinical trial. Phys Ther 88: 462–470, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Verschueren SM, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, Boonen S: Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Miner Res 19: 352–359, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Brandon CA, Rosen C, Georgelis G, Horton MJ, Mooney MP, Sciote JJ: Staining of human thyroarytenoid muscle with myosin antibodies reveals some unique extrafusal fibers, but no muscle spindles. J Voice 17: 245–254, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loucks TM, Poletto CJ, Saxon KG, Ludlow CL: Laryngeal muscle responses to mechanical displacement of the thyroid cartilage in humans. J Appl Physiol 99: 922–930, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hiraba H, Yamaoka M, Fukano M, Fujiwara T, Ueda K: Increased secretion of salivary glands produced by facial vibrotactile stimulation. Somatosens Mot Res 25: 222–229, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]