Summary
Background
Phenytoin is the recommended second-line intravenous anticonvulsant for treatment of paediatric convulsive status epilepticus in the UK; however, some evidence suggests that levetiracetam could be an effective and safer alternative. This trial compared the efficacy and safety of phenytoin and levetiracetam for second-line management of paediatric convulsive status epilepticus.
Methods
This open-label, randomised clinical trial was undertaken at 30 UK emergency departments at secondary and tertiary care centres. Participants aged 6 months to under 18 years, with convulsive status epilepticus requiring second-line treatment, were randomly assigned (1:1) using a computer-generated randomisation schedule to receive levetiracetam (40 mg/kg over 5 min) or phenytoin (20 mg/kg over at least 20 min), stratified by centre. The primary outcome was time from randomisation to cessation of convulsive status epilepticus, analysed in the modified intention-to-treat population (excluding those who did not require second-line treatment after randomisation and those who did not provide consent). This trial is registered with ISRCTN, number ISRCTN22567894.
Findings
Between July 17, 2015, and April 7, 2018, 1432 patients were assessed for eligibility. After exclusion of ineligible patients, 404 patients were randomly assigned. After exclusion of those who did not require second-line treatment and those who did not consent, 286 randomised participants were treated and had available data: 152 allocated to levetiracetam, and 134 to phenytoin. Convulsive status epilepticus was terminated in 106 (70%) children in the levetiracetam group and in 86 (64%) in the phenytoin group. Median time from randomisation to cessation of convulsive status epilepticus was 35 min (IQR 20 to not assessable) in the levetiracetam group and 45 min (24 to not assessable) in the phenytoin group (hazard ratio 1·20, 95% CI 0·91–1·60; p=0·20). One participant who received levetiracetam followed by phenytoin died as a result of catastrophic cerebral oedema unrelated to either treatment. One participant who received phenytoin had serious adverse reactions related to study treatment (hypotension considered to be immediately life-threatening [a serious adverse reaction] and increased focal seizures and decreased consciousness considered to be medically significant [a suspected unexpected serious adverse reaction]).
Interpretation
Although levetiracetam was not significantly superior to phenytoin, the results, together with previously reported safety profiles and comparative ease of administration of levetiracetam, suggest it could be an appropriate alternative to phenytoin as the first-choice, second-line anticonvulsant in the treatment of paediatric convulsive status epilepticus.
Funding
National Institute for Health Research Health Technology Assessment programme.
Introduction
Convulsive status epilepticus is the most common paediatric neurological emergency worldwide.1 It has an annual incidence of 20 per 100 000 children, and is the second most common reason for unplanned admissions to paediatric intensive care units (PICUs) in the UK.2 Mortality is low, but morbidity, including neurodisability, learning difficulties, and de-novo and drug-resistant epilepsy, could be as high as 22%.3, 4, 5, 6 The longer the duration of convulsive status epilepticus, the more difficult it is to terminate, and the greater the risk of morbidity.1, 5, 6
Convulsive status epilepticus is treated using an algorithm recommended by Advanced Paediatric Life Support (APLS), which incorporates 10 min intervals between treatments.7 Second-line treatment is given when convulsive status epilepticus persists either after two doses of benzodiazepine or the child's personalised emergency (rescue) treatment. Failure of second-line treatment is followed by anaesthesia via rapid sequence induction (RSI).7 Randomised clinical trial evidence supports the use of benzodiazepines as first-line treatment.8 However, no high-quality randomised clinical trial evidence exists to support any second-line treatment.9
Research in context.
Evidence before this study
We searched Embase and MEDLINE with no language or date restrictions on Jan 1, 2013, using the search strategy of the Cochrane Epilepsy Group publications that described use of intravenous levetiracetam and phenytoin as a first-line or second-line antiepileptic drug or anticonvulsant in the management of status epilepticus, convulsive status epilepticus, and serial seizures in children and adults. There are few randomised clinical trial data for the second-line anticonvulsant treatment of paediatric convulsive status epilepticus. Most published evidence for both phenytoin (the current recommended first-choice, second-line anticonvulsant outside the USA) and levetiracetam is anecdotal, retrospective, or both, and predominantly based on studies in adults. Observational data suggest that levetiracetam is more effective than phenytoin. Two small randomised clinical trials undertaken predominantly in adults and a recent paediatric randomised clinical trial, using a range of methodologies and outcomes, found no significant difference between phenytoin and levetiracetam in the rate of cessation of convulsive status epilepticus or recurrence of convulsive status epilepticus within 24 h.
Added value of this study
This is an adequately powered randomised clinical trial that directly compares anticonvulsants for second-line treatment of paediatric convulsive status epilepticus in an emergency setting. It is also the first randomised clinical trial to compare the efficacy and safety of levetiracetam and phenytoin in this paediatric neurological emergency. We found no significant differences between the two anticonvulsants in any primary or secondary outcomes, including time to seizure cessation and need for additional anticonvulsants. The safety profile was similar between treatments, by contrast with existing observational evidence that phenytoin has a worse safety profile.
Implications of all the available evidence
Our results suggest that levetiracetam could be considered as an alternative treatment to phenytoin for second-line management of paediatric convulsive status epilepticus. Possible benefits of levetiracetam over phenytoin include its ease of preparation and administration, minimal interaction with antiepilepsy and other drugs, and easy conversion to oral maintenance therapy. Further randomised clinical trial and meta-analysis data could help to confirm our results and might lead to levetiracetam being the preferred second-line anticonvulsant in children with benzodiazepine-resistant convulsive status epilepticus.
The currently recommended second-line treatment for convulsive status epilepticus is intravenous phenytoin in the UK and Europe (fosphenytoin in the USA), based on predominantly non-randomised clinical trial data; reported cessation rates vary widely between 50% and 96%.10, 11 Safety concerns are widely reported, particularly cardiovascular disturbance (hypotension and fatal arrhythmias) and Stevens-Johnson syndrome.12, 13, 14 Levetiracetam has been reported to be effective and well tolerated in the management of serial seizures and convulsive status epilepticus, also based on predominantly retrospective and observational trial data, with reported rates of convulsive status epilepticus cessation between 44% and 94%.9, 11, 15, 16, 17 Adverse reactions with levetiracetam seem to be less frequent and less severe than with phenytoin.18 Levetiracetam is administered more rapidly (5–10 min) than phenytoin (a minimum of 20 min), suggesting that more rapid termination of convulsive status epilepticus could be possible with levetiracetam. However, findings of existing studies of second-line treatments cannot be generalised because of methodological issues, including small sample sizes and heterogeneity of primary outcomes. Management of convulsive status epilepticus was therefore identified as one of five priority areas for research by the UK National Institute for Health and Care Excellence in its epilepsy guideline published in 2012.19
The Emergency treatment with Levetiracetam or Phenytoin in convulsive Status Epilepticus in children (EcLiPSE) trial aimed to determine whether intravenous levetiracetam or intravenous phenytoin is the more effective and safer second-line anticonvulsant for emergency management of paediatric convulsive status epilepticus.
Methods
Study design
We did an open-label randomised clinical trial at 30 UK emergency departments, all of which are members of Paediatric Emergency Research in the United Kingdom & Ireland (PERUKI).20 These emergency departments were secondary care (district general hospitals) and tertiary care centres.
Ethical approval was gained from the National Research Ethics Service, Liverpool Central, on March 3, 2016; all participating centres were granted permission from the UK National Health Service before commencing recruitment.
The trial protocol has been published21 and is also available in full online.
Participants
Children of either sex, aged 6 months to under 18 years, presenting with convulsive status epilepticus (generalised tonic-clonic, generalised clonic, or focal clonic seizure) that required second-line treatment were eligible for inclusion. Patients were ineligible if they presented with absence, myoclonic, or non-convulsive status epilepticus, or infantile spasms; were known or suspected to be pregnant; had a contraindication or allergy to levetiracetam or phenytoin; had established renal failure; had received a second-line anticonvulsant during the presenting episode of convulsive status epilepticus, before screening; or were known to have been previously enrolled in the EcLiPSE trial.
We used research without prior consent (also known as deferred consent) because of the time-crucial management of convulsive status epilepticus, in accordance with regulatory requirements, research without prior consent guidance, and pretrial research.22, 23, 24 The process of research without prior consent in EcLiPSE was formally assessed and evaluated in a nested consent study. The primary objective was to assess how well the process of consent was conducted and understood by parents and legal representatives and the medical and nursing staff at each participating site. The nested consent study findings will be published separately.21 Parents, legal representatives or guardians, and patients were approached once the patient's clinical condition was stable (ideally within 24 h of randomisation and before discharge from hospital), at which point written informed consent was sought to continue data collection and use data already collected.21 When consent was not sought before discharge, participants or parents or legal guardians were contacted within 5 working days of randomisation by a member of the research team, and informed of their involvement and trial details. Participants were sent written information, a consent form, and a covering letter stating that they had 4 weeks from the date of the letter to confirm or decline participation. We used an opt-out postal approach; the covering letter explained that the participant would be included in the trial if no response was received.
Randomisation and masking
Participants were randomly assigned (1:1) to receive levetiracetam or phenytoin using random variable block sizes of two and four. A computer-generated randomisation schedule was produced by an independent statistician who had no further involvement in the study, stratified by centre. Sites were provided with randomisation packs, which were sequentially numbered, heavy duty, opaque, A4 cardboard envelopes with tamper-proof closure strips to be opened in ascending order. Each envelope contained the random treatment allocation and relevant case report form. Periodic checks ensured sites had the correct number of envelopes, that they were intact, and that the sequential numbering system was maintained.
Treating clinicians opened randomisation envelopes after confirmation of eligibility. This was undertaken after administration of the final first-line treatment to allow sufficient time to prepare and administer the allocated second-line treatment in accordance with the APLS algorithm.7 If convulsive status epilepticus stopped before administration of the allocated treatment, participants were excluded; however, these participants could be subsequently included if their seizure restarted and required a second-line treatment while in the emergency department. Emergency department team members were aware of the allocated drug, and the treating emergency clinician determined time of cessation of convulsive status epilepticus based on clinical examination.
Participants, parents, legal representatives and guardians, and clinicians were all informed of allocated treatments. The statistician was not masked to treatment allocation because the database containing the trial data included treatments received. Amendments to the analysis plan were considered and implemented by a masked statistician, which included masked collection and analysis of the data before database lock. Inclusion in the analysis sets was determined without reference to treatments received with independent determination by another statistician.
Procedures
Convulsive status epilepticus was managed according to the APLS algorithm, and both study treatments were given intravenously.7, 21 Levetiracetam was administered over 5 min in a dose of 40 mg/kg (maximum dose 2·5 g); phenytoin was administered over a minimum of 20 min in a dose of 20 mg/kg (maximum dose 2 g and with a maximum infusion rate of 1 mg/kg per min). Clinicians treated subsequent ongoing convulsive status epilepticus according to the APLS algorithm.7
Data were recorded on a paper-based case report form by emergency clinicians during the convulsive status epilepticus, including times of randomisation, commencement and completion of infusions, and cessation of convulsive status epilepticus. These key data were collated and highlighted on the first page of the case report form to ensure data accuracy. Additional information included participant demographics, type of convulsive status epilepticus, site of trial treatment administration, need for additional anticonvulsants, RSI, and adverse events. After consent, information was collected on pre-existing epilepsy diagnosis, oral maintenance antiepilepsy drugs, neurological comorbidities, concomitant medications, aetiology of convulsive status epilepticus, patient location on admission and at 24 h, and further seizure activity within 24 h. Final follow-up was undertaken 14 days after enrolment by chart review (recording discharge, readmission, death, and organ failure), and a brief participant postal questionnaire (assessing current participant health, new medical problems, and new antiepilepsy drugs).
At each site initiation visit, training of the site's medical and nursing staff included a simulated demonstration of the processes of screening, randomisation, and assessment of the endpoint to ensure a consistent understanding of the primary outcome measure.
Outcomes
The primary outcome was time from randomisation to cessation of all visible signs of convulsive activity, defined as cessation of all continuous rhythmic clonic activity, as judged by the treating clinician.
Secondary outcomes were need for further anticonvulsants to manage the convulsive status epilepticus after administration of the trial treatment; need for RSI because of ongoing convulsive status epilepticus; need for admission to critical care (either a high-dependency unit or a PICU); and serious adverse reactions (including death, Stevens-Johnson syndrome, rash, airway complications, cardiovascular instability, extravasation injury, and extreme agitation, as well as those listed in the summary product characteristic of each treatment).
All adverse events, including serious adverse events, and adverse reactions and their causes were assessed by the principal investigator at each participating site and within the context of each treatment's summary of product characteristics.
Statistical analysis
The sample size was calculated on the basis of existing reported seizure cessation rates for phenytoin and levetiracetam.10, 18 140 randomised and consented participants per group, with a total of 183 events (of convulsive status epilepticus cessation) were required for a 0·05 level two-sided log-rank test for equality of survival curves to detect an increase in seizure cessation from 60% to 75% (a constant hazard ratio [HR] of 0·661) at 80% power. The sample size was increased to 308 to allow for 10% loss to follow-up. The final sample size was 286 due to low attrition and completeness of primary outcome data.
The primary analysis was based on a modified intention-to-treat (mITT) principle. All randomised and consented participants who received a second-line treatment were included in the analysis according to their allocated treatment. Children who were randomised but whose convulsive status epilepticus stopped without requiring second-line treatment (and did not restart in the emergency department) were excluded. The safety analysis included the same participants, grouped according to actual treatment received. To avoid double-counting, serious adverse events are reported separately from adverse events.
Statistical tests were two-sided at a 5% significance level; results are presented with 95% CIs. The primary outcome was analysed using the log-rank test and is presented as a Kaplan-Meier curve. All participants were followed up to cessation of convulsive status epilepticus, with censoring used in the event of RSI or death. If RSI was administered, time was censored at RSI plus 12 h (720 min); in patients who died before cessation of convulsive status epilepticus, time was censored at time of death plus 48 h (2880 min). Sensitivity analyses were done to test the robustness of results of the analysis approach taken including: Gray's test,25 treating RSI as a competing risk, calculating time to cessation of convulsive status epilepticus from start of infusion instead of randomisation, and censoring participants at the time of an additional second-line treatment after no response to the allocated treatment.
Additional analysis using a Cox proportional hazards model adjusted for baseline characteristics of weight (<12 kg, 12–36 kg, or >36 kg), sex, and whether this was the child's first seizure. Two covariates (site of infusion and additional anticonvulsants given in parallel) specified in the analysis plan were not included because they were measured after randomisation. Additionally, centre could not be included in the Cox model due to lack of convergence. Schoenfeld residual plots were used to check the assumption of proportionality. The binary secondary outcomes of need for further anticonvulsants, RSI, and admission to critical care were analysed using the χ2 test and presented with relative risks. Logistic regression models were fitted as additional analyses to the primary χ2 tests, with adjustments as per the Cox proportional hazards model. No adjustment was made for multiplicity for the secondary outcomes. Baseline categorical data and adverse event data are summarised using numbers and percentages, and continuous data are summarised as medians and IQRs.
A post-hoc analysis was undertaken for the reasons underlying the further management of the presenting episode of convulsive status epilepticus, the assessment of which was done without knowledge of the allocated intervention
A detailed statistical analysis plan is available online. All analyses were done with SAS software, version 9.4. The trial was overseen by an independent data and safety monitoring committee (IDSMC), which made recommendations to a trial steering committee (TSC). The majority of the TSC were independent and remained masked to accumulating data until the end of the trial. The IDSMC and TSC met at least annually and were consulted before the decision to stop recruitment due to low attrition and completeness of data. The Haybittle-Peto approach was used by the IDSMC as a guide to consider stopping the trial within interim reports with 99·9% CIs. This trial is registered with ISRCTN, number ISRCTN22567894.
Role of the funding source
The funder monitored trial progress and approved membership of the oversight committees (IDSMC and TSC). The funder had no role in trial design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the trial and had final responsibility for the decision to submit for publication.
Results
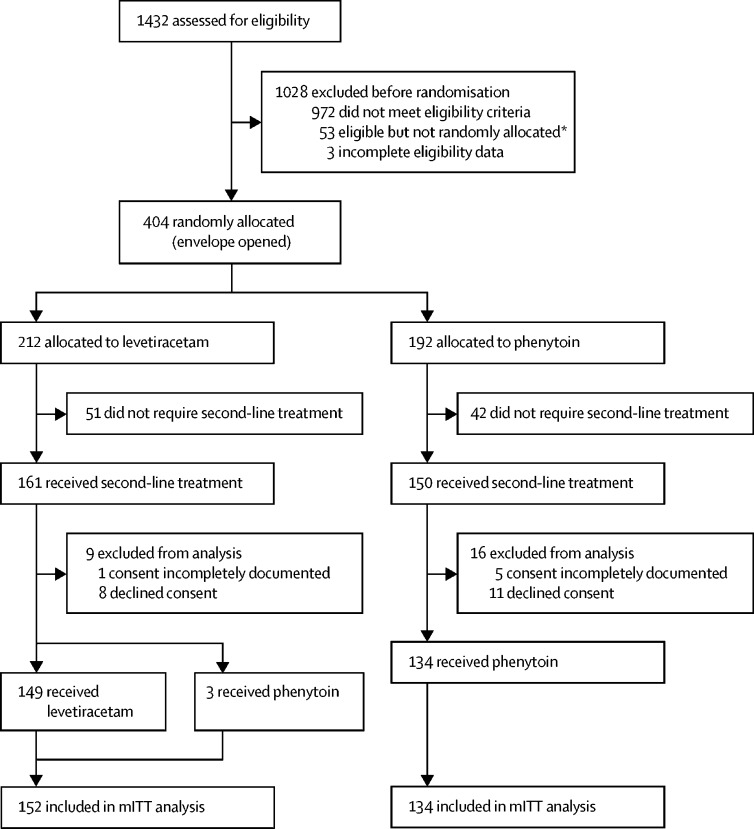
Between July 17, 2015, and April 7, 2018, 1432 children presented to participating emergency departments with convulsive status epilepticus. After exclusion of 1028 ineligible patients, 404 were randomly assigned (212 to levetiracetam and 192 to phenytoin). However, 93 patients did not require second-line treatment and 25 did not provide consent. Therefore, 286 randomised participants were treated, of whom 19 declined consent and six had incompletely documented consent, leaving 152 (53%) allocated to levetiracetam and 134 (47%) allocated to phenytoin in the mITT analysis (figure 1). Written consent was obtained in 250 (87%), and the remaining 36 (13%) were included via the opt-out pathway. Baseline characteristics were similar between groups (table 1).
Figure 1.
Trial profile
mITT=modified intention-to-treat. *Reasons included no trial-trained doctor available, loss of or failure to achieve intravenous access, clinical judgment (eg, child too sick), and treatment given before random assignment.
Table 1.
Baseline demographic and seizure characteristics
| Levetiracetam (n=152) | Phenytoin (n=134) | ||
|---|---|---|---|
| Sex | |||
| Male | 75 (49%) | 72 (54%) | |
| Female | 77 (51%) | 62 (46%) | |
| Age | |||
| 6 months to <2 years | 65 (43%) | 53 (40%) | |
| 2–11 years | 81 (53%) | 74 (55%) | |
| 12–17 years | 6 (4%) | 7 (5%) | |
| Median (IQR), years | 2·7 (1·3–5·9) | 2·7 (1·6–5·6) | |
| Range, years | 0·6–16·1 | 0·6–17·9 | |
| Weight | |||
| <12 kg | 52 (34%) | 42 (31%) | |
| 12–36 kg | 86 (57%) | 80 (60%) | |
| >36 kg | 14 (9%) | 12 (9%) | |
| Median (IQR) | 12·1 (10·0–19·0) | 12·0 (10·0–18·0) | |
| Range | 7·5–70·0 | 6·0–66·0 | |
| Participant's first seizure | 69 (45%) | 49 (37%) | |
| Presenting seizure type | |||
| Generalised tonic-clonic | 107 (70%) | 105 (78%) | |
| Generalised clonic | 12 (8%) | 7 (5%) | |
| Focal clonic | 33 (22%) | 22 (16%) | |
| Seizure cause* | |||
| Febrile convulsion | 63 (41%) | 58 (43%) | |
| Seizure (pre-existing epilepsy) | 46 (30%) | 46 (34%) | |
| First afebrile seizure | 16 (11%) | 12 (9%) | |
| CNS infection | 6 (4%) | 7 (5%) | |
| Intracranial vascular event (bleed or stroke) | 2 (1%) | 2 (1%) | |
| Traumatic brain injury | 0 | 0 | |
| Substance misuse | 1 (<1%) | 0 | |
| Indeterminate | 10 (7%) | 7 (5%) | |
| Other | 27 (18%) | 26 (19%) | |
| Maintenance antiepilepsy drugs at presentation*† | |||
| Levetiracetam | 29 (19%) | 26 (19%) | |
| Sodium valproate | 16 (11%) | 19 (14%) | |
| Carbamazepine | 12 (8%) | 10 (7%) | |
| Clobazam | 9 (6%) | 9 (7%) | |
| Topiramate | 4 (3%) | 8 (6%) | |
| Phenytoin | 0 | 1 (<1%) | |
| Other | 11 (7%) | 18 (13%) | |
Data are n (%), median (IQR), or range.
Categories not mutually exclusive.
Includes participants with an established diagnosis of epilepsy who were reciving antiepilepsy drugs at the time of random allocation to treatment.
Convulsive status epilepticus was terminated by levetiracetam in 106 (70%) participants, and by phenytoin in 86 (64%) participants. Three patients allocated to the levetiracetam group received phenytoin (table 2).
Table 2.
Trial adherence
| Levetiracetam (n=152) | Phenytoin (n=134) | Total (n=286) | |
|---|---|---|---|
| Patient given lower dose of trial treatment | 8 (5%) | 4 (3%) | 12 (4%) |
| Patient given higher dose of trial treatment | 2 (1%) | 1 (<1%) | 3 (1%) |
| Dose administration shorter than expected | 0 | 1 (<1%) | 1 (<1%) |
| Dose administration longer than expected | 27 (18%) | 34 (25%) | 61 (21%) |
| Treatment prematurely discontinued | 0 | 2 (1%) | 2 (<1%) |
| Unauthorised route of administration (intraosseous) | 6 (4%) | 0 | 6 (2%) |
| Received initial second-line treatment other than that allocated | 3 (2%) | 0 | 3 (<1%) |
| Received further second-line treatment* | 22 (14%) | 13 (10%) | 35 (12%) |
Includes those who subsequently received the alternative trial treatment or an additional dose of the allocated treatment, within 24 h.
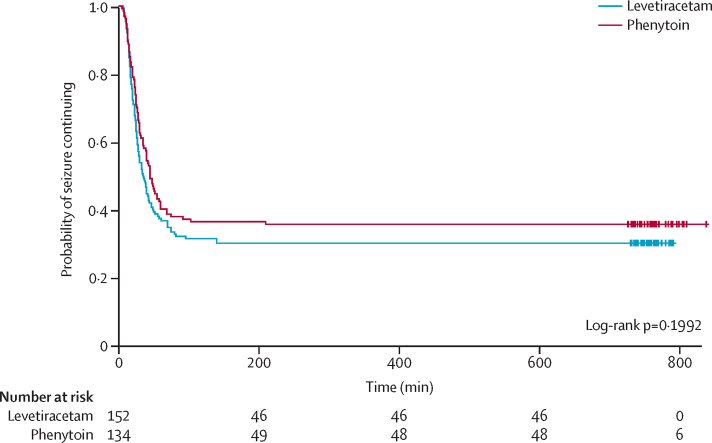
Median time from randomisation to start of infusion was 11 min (IQR 8–15) for levetiracetam and 12 min (8–17) for phenytoin. Infusion duration was longer than expected for 27 (18%) participants allocated to levetiracetam and 34 (25%) allocated to phenytoin. Two participants in the phenytoin group discontinued treatment early because of loss of intravenous access during drug administration (table 2). Median time from randomisation to seizure cessation was 35 min (IQR 20 to not assessable) in the levetiracetam group and 45 min (IQR 24 to not assessable) in the phenytoin group (log-rank test p=0·20; figure 2). Event times were censored for 46 (30%) participants in the levetiracetam group and 48 (36%) in the phenytoin group who received RSI for any reason before seizure cessation. The unadjusted HR was 1·20 (95% CI 0·91–1·60; p=0·20) in favour of levetiracetam. The Schoenfeld residuals for the unadjusted model (p=0·72) indicated the independency of time and the validity of the proportionality assumption. The Schoenfeld residuals for the adjusted model indicated that the assumption of proportionality for weight was not met (p=0·05, p value range from 0·27 to 0·71 for other variables; appendix). The proportionality assumption within each weight subgroup (<12 kg, 12–36 kg, or >36 kg) was supported by the Schoenfeld residuals. Direction of treatment effect was consistent across subgroups, confidence intervals were wide, and results were not significant (appendix). The treatment effect was increased for children in the over 36 kg subgroup; however, numbers within this group are small and the treatment effect was not significant (appendix). Sensitivity analyses undertaken on the primary outcome confirmed the robustness of the results (appendix).
Figure 2.
Kaplan-Meier curve for time to seizure cessation
57 (38%) participants in the levetiracetam group and 50 (37%) in the phenytoin group received additional anticonvulsants (relative risk [RR] 1·01 [95% CI 0·74–1·36]; p=0·97; table 3). Results were similar in a post-hoc analysis restricted to further management for the presenting episode of convulsive status epilepticus. 44 (29%) participants in the levetiracetam group and 47 (35%) in the phenytoin group were given an RSI due to ongoing convulsive status epilepticus (RR 0·83 [95% CI 0·59–1·16]; p=0·27; table 3). 97 (64%) participants in the levetiracetam group and 72 (54%) in the phenytoin group were admitted to critical care (RR 1·19 [95% CI 0·97–1·45]; p=0·08).
Table 3.
Secondary outcomes and 14-day follow-up
| Levetiracetam (n=152) | Phenytoin (n=134) | Relative risk (95% CI) | p value | ||
|---|---|---|---|---|---|
| Secondary outcomes | |||||
| Need for further anticonvulsants* | 57 (37·5%) | 50 (37·3%) | 1·01 (0·74–1·36) | 0·97 | |
| Need for further anticonvulsants for the presenting convulsive status epilepticus† | 24 (15·8%) | 20 (14·9%) | 1·06 (0·61–1·83) | 0·84 | |
| Need for further anticonvulsants for a subsequent seizure (within 24 h)†‡ | 14 (9·2%) | 17 (12·7%) | 0·72 (0·37–1·40) | 0·33 | |
| RSI to terminate an ongoing seizure | 44 (30·0%) | 47 (35·1%) | 0·83 (0·59–1·16) | 0·27 | |
| Admission to critical care | 97 (63·8%) | 72 (53·7%) | 1·19 (0·97–1·45) | 0·08 | |
| Serious adverse reaction | 0 | 2 (1%)§ | .. | .. | |
| 14-day follow-up | |||||
| Discharged from hospital | 145 (95%) | 130 (97%) | .. | .. | |
| Readmitted to hospital | 12 (8%) | 10 (7%) | .. | .. | |
| Patient died | 1 (<1%) | 1 (<1%) | .. | .. | |
| Organ failure | 1 (<1%) | 0 | .. | .. | |
Data are n (%) or relative risk (95% CI). RSI=rapid sequence induction.
Includes all instances of further anticonvulsant being given in following 24 h, including for the presenting seizure, subsequent seizures, or for prophylaxis.
Post-hoc analysis; assessment conducted without knowledge of the allocated intervention.
Excludes nine participants with unavailable data.
Two events in one participant, one of which was a suspected unexpected serious adverse reaction.
132 participants received levetiracetam only and 130 received phenytoin only. The remaining 24 participants received both treatments sequentially; 17 received levetiracetam followed by phenytoin, and seven received phenytoin followed by levetiracetam.
20 adverse events were reported in 16 participants receiving levetiracetam, 23 adverse events were reported in 18 participants receiving phenytoin, and eight adverse events were reported in four participants receiving both treatments. Each individual adverse event had a prevalence of less than 10% (table 4). The most common adverse event was agitation, which occurred in 11 (8%) who received levetiracetam and in four (3%) who received phenytoin (table 4).
Table 4.
Adverse events
|
Levetiracetam (n=132) |
Phenytoin (n=130) |
Both drugs (n=24) |
Total (n=286) |
|||||
|---|---|---|---|---|---|---|---|---|
| Events | Patients | Events | Patients | Events | Patients | Events | Patients | |
| Any adverse event | 20 | 16 (12%) | 23 | 18 (14%) | 8 | 4 (17%) | 51 | 38 (13%) |
| Agitation | 11 | 11 (8%) | 4 | 4 (3%) | 0 | 0 | 15 | 15 (5%) |
| Hypotension | 2 | 2 (2%) | 3 | 3 (2%) | 1 | 1 (4%) | 6 | 6 (2%) |
| Catheter site related | 1 | 1 (<1%) | 1 | 1 (<1%) | 3 | 2 (8%) | 5 | 4 (1%) |
| Extravasation | 0 | 0 | 4 | 4 (3%) | 1 | 1 (4%) | 5 | 5 (2%) |
| Tachycardia | 1 | 1 (<1%) | 3 | 3 (2%) | 1 | 1 (4%) | 5 | 5 (2%) |
| Rash | 2 | 2 (2%) | 1 | 1 (<1%) | 0 | 0 | 3 | 3 (1%) |
| Hypertension | 0 | 0 | 2 | 2 (2%) | 0 | 0 | 2 | 2 (<1%) |
| Reaction to ceftriaxone | 0 | 0 | 0 | 0 | 1 | 1 (4%) | 1 | 1 (<1%) |
| Confusion | 1 | 1 (<1%) | 0 | 0 | 0 | 0 | 1 | 1 (<1%) |
| Decreased consciousness | 0 | 0 | 1 | 1 (<1%) | 0 | 0 | 1 | 1 (<1%) |
| Hallucination | 1 | 1 (<1%) | 0 | 0 | 0 | 0 | 1 | 1 (<1%) |
| Infusion site erythema | 0 | 0 | 1 | 1 (<1%) | 0 | 0 | 1 | 1 (<1%) |
| Mechanical ventilation complication | 0 | 0 | 1 | 1 (<1%) | 0 | 0 | 1 | 1 (<1%) |
| Pallor | 0 | 0 | 1 | 1 (<1%) | 0 | 0 | 1 | 1 (<1%) |
| Stridor | 0 | 0 | 0 | 0 | 1 | 1 (4%) | 1 | 1 (<1%) |
| Vomiting | 0 | 0 | 1 | 1 (<1%) | 0 | 0 | 1 | 1 (<1%) |
| Wheezing | 1 | 1 (<1%) | 0 | 0 | 0 | 0 | 1 | 1 (<1%) |
Only five serious adverse events were observed (three in two participants receiving phenytoin, one in a participant receiving levetiracetam, and one in a participant who received both interventions) Four of these resolved; the remaining serious adverse event occurred in a participant who died as a result of catastrophic cerebral oedema unrelated to either treatment (this participant received levetiracetam followed by phenytoin). Two of the serious adverse events were assessed as being related to treatment. One was a case of hypotension considered to be immediately life-threatening, which was classed as a serious adverse reaction, and the other was a case of increased focal seizures and decreased consciousness considered to be medically significant, which was classed as a suspected unexpected serious adverse reaction. Both occurred in the same participant, who was allocated to, and received phenytoin. The remaining serious adverse event occurred in a levetiracetam-treated participant who had a cardiorespiratory arrest due to an obstructed endotracheal tube, which was considered unrelated to treatment.
Discussion
In this open-label, multicentre trial, we did not detect a significant difference between phenytoin and levetiracetam in the second-line treatment of paediatric convulsive status epilepticus for any outcome, including time to seizure cessation. To our knowledge, this trial is the largest randomised clinical trial to date to compare levetiracetam with phenytoin for treatment of paediatric convulsive status epilepticus that was unresponsive to first-line treatment.
Convulsive status epilepticus cessation rates for levetiracetam (70%) and phenytoin (64%) in our trial were broadly similar to those previously reported in observational and retrospective studies in adult patients.10, 16 Cessation rates as high as 85–95% have been reported, but these studies have significant heterogeneity in design and outcomes.11, 26 One randomised clinical trial undertaken in adults with convulsive status epilepticus compared the efficacy of intravenous phenytoin (20 mg/kg), valproate (30 mg/kg), and levetiracetam (25 mg/kg) in 150 patients unresponsive to intravenous lorazepam.17 Convulsive status epilepticus stopped in 34 (68%) patients treated with phenytoin, 34 (68%) treated with valproate, and 39 (78%) treated with levetiracetam (p=0·44). A recent paediatric randomised clinical trial evaluated 100 children aged 3–12 years receiving levetiracetam (30 mg/kg) or phenytoin (20 mg/kg) if their convulsive status epilepticus continued after one dose of intravenous diazepam.11 Efficacy was high and almost identical in both groups. A lower diastolic blood pressure was recorded in phenytoin-treated patients. However, it is difficult to translate these findings to clinical practice because of the trial's design, including many exclusion criteria and the primary outcome of absence of seizure activity within 24 h, which is not a clinically practical outcome.11 In UK practice, management of paediatric convulsive status epilepticus follows the APLS algorithm, which is applicable to the vast majority of children presenting to emergency departments.7 Our study design therefore used eligibility criteria that were as inclusive as possible, and followed a treatment pathway that reflected clinical practice.
We did not detect a significant difference between levetiracetam and phenytoin in time to cessation of convulsive status epilepticus. We used a superiority design for three reasons: reported convulsive status epilepticus cessation rates for each drug suggested that levetiracetam would be more effective than phenytoin; no randomised clinical trial data comparing the efficacy of either treatment to placebo were available; and levetiracetam has a shorter infusion time (5 min vs at least 20 min for phenytoin). We selected time from randomisation and instructed sites to undertake randomisation at the latest possible point that would allow reconstitution of the allocated treatment to provide scientific and clinical rigour. Because the median time to commencement of infusion exceeded 10 min in each group, we also undertook a sensitivity analysis using time to cessation from commencement of infusion, which supported the findings of our primary analysis.
Progression to RSI in convulsive status epilepticus can be required for one or a combination of reasons, including continuing convulsive status epilepticus, respiratory depression, clinical deterioration, or to stabilise the patient for transfer or to safely undertake investigations. However, RSI abolishes visible convulsive status epilepticus activity, and might therefore prevent assessment of seizure cessation directly related to trial treatment. Participants were therefore censored if they progressed to RSI, but the censoring time was increased to allow for this to be a negative and potentially informative outcome. Increasing censoring time might have artificially inflated the time to cessation of convulsive status epilepticus. However, sensitivity analyses in which patients were censored at the time of RSI (and in which RSI was defined as a competing risk) did not change our findings.
Observed safety profiles were similar between treatment groups. Because of their relative infrequency in relation to the trial population size, together with good clinical management in participating sites, low rates of serious adverse events or reactions were observed. However, hypotension, cardiac arrhythmias, and severe extravasation injuries are well recognised adverse effects of phenytoin; rarely, the cardiovascular effects might be fatal.12, 13, 14 Levetiracetam was well tolerated when administered over 5 min, a more rapid rate than previously reported.16, 17, 27 Agitation was the most common adverse event in the levetiracetam group, as reported previously.15 There were no new or unexpected serious adverse reactions with levetiracetam. Sedation, somnolence, and dizziness are rare side-effects of levetiracetam in adults, but these rare side-effects might in part be due to use of benzodiazepines or craniotomy in the study population.18, 28 Anxiety has also been reported in adults but was not reported as an adverse event or reaction in this or other paediatric studies. However, anxiety might be similar to the agitation that was seen and reported in EcLiPSE. Because 90% of our study population was younger than 10 years (and 41% aged 2 years or under), anxiety might be difficult, if not impossible, for them or their carers to descibe, instead using the terms agitated or irritable.
The EcLiPSE trial is unique for many reasons. To our knowledge, it is the first adequately powered randomised clinical trial to compare the efficacy and safety of two anticonvulsants for second-line treatment of convulsive status epilepticus. Second, it is an adequately powered randomised clinical trial to evaluate phenytoin as a second-line treatment for convulsive status epilepticus, despite this drug being used as the first-choice second-line treatment for more than 50 years. Third, we incorporated a nested consent study that evaluated the process of research without prior consent in a paediatric emergency medicine trial.22, 23, 24 Finally, this was the first multicentre randomised clinical trial to be supported by, and delivered across, the PERUKI collaborative.20
This trial has several strengths. First, it evaluated a specific step (second-line treatment) in a commonly used UK clinical algorithm for the management of paediatric convulsive status epilepticus.7 A similar trial assessing the first-line, non-intravenous treatment of convulsive status epilepticus in the same algorithm led to a change in national clinical practice.29 Second, this trial showed that research without prior consent is acceptable and successful, with 385 (95%) of 404 randomised participants providing consent; in those who were randomised and treated, 286 (92%) of 311 provided consent. Research without prior consent is essential for the successful delivery of trials in paediatric emergency care. The high consent rate mirrors that found in a previous trial of first-line management of convulsive status epilepticus (consent rate 97%),29 and in a pilot randomised clinical trial that compared fluid boluses in shock (consent rate 100%).30 Third, this was a pragmatic trial, and recorded only key primary and secondary outcomes in the resuscitation room. This approach, supported by focused data-collection materials and simple allocation and enrolment methods, facilitated successful delivery of the study across all sites, as shown by low numbers of missed patients, high protocol adherence, and accurate data capture for key outcomes. Finally, the trial was conducted in emergency departments from secondary and tertiary care centres throughout PERUKI, increasing the generalisability of our findings, and facilitating rapid dissemination and knowledge translation.
This trial has some limitations. First, it was open-label. A double-blind design was considered too complex for most participating sites (partly because of the substantially different infusion rates of the two drugs) and in the context of the life-threatening nature of convulsive status epilepticus. Second, assessment of cessation of all signs of continuous, rhythmic clonic activity (rather using than fixed timepoints to assess cessation of convulsive status epilepticus) was probably subjective. Clearly, these two limitations might collectively increase the risk of bias. However, continual assessment of a child's condition reflects real-life clinical practice in a dynamic situation, in which clinicians constantly evaluate and prepare for the next step in the treatment algorithm. Site training included a simulated demonstration of the endpoint to ensure an understanding of the key outcome measure for the trial. It would not have been feasible or pragmatic for each participant to undergo a video recording or an electroencephalogram (EEG) to determine the time of convulsive status epilepticus cessation more precisely. Without EEGs, it is not possible to state definitively whether any patients developed non-convulsive status epilepticus. However, treatment algorithms for non-convulsive status epilepticus would follow the same flow as for convulsive status epilepticus, and there was no difference between treatment groups in the number of additional anticonvulsants given after trial treatment. Third, the timing of randomisation meant that convulsive status epilepticus terminated before administration of trial treatment in many cases; however, this affected both treatment groups equally, and was essential to maintain high standards of clinical care and to avoid treatment delays. Finally, we included safety measures as key secondary outcomes because of previous reports of harm. However, this trial was not powered to show a difference in the number of serious adverse reactions (a secondary outcome) between treatment groups.
Other treatment-related factors might be relevant to the interpretation of our findings. These include the widespread use of levetiracetam as maintenance therapy for many childhood epilepsies because of its broad-spectrum activity and safety profile. In the EcLiPSE trial participants, levetiracetam was the most commonly used oral antiepilepsy drug at the time of presentation. Anecdotally, clinicians are reluctant to give a loading dose of phenytoin to children in convulsive status epilepticus who are on oral maintenance phenytoin because of potential cardiovascular toxicity. There seemed to be no similar concerns for levetiracetam, and no increase in adverse events was observed when giving 40 mg/kg to children already receiving maintenance levetiracetam. A substantial minority of children who present in convulsive status epilepticus for the first time are subsequently commenced on maintenance therapy. Levetiracetam is more likely than phenytoin to be used as maintenance therapy because of phenytoin's adverse event profile and complex pharmacokinetics. One observational study in adults showed that 8% of patients treated with intravenous fosphenytoin for convulsive status epilepticus were subsequently commenced on oral phenytoin, whereas 78% of those treated with intravenous levetiracetam were subsequently commenced on oral levetiracetam.26 Ease of drug preparation and administration is also a factor in the management of convulsive status epilepticus. Throughout the EcLiPSE trial, levetiracetam was reported by clinical teams in the participating centres to be easier to prepare and administer than phenytoin because of the calculations performed in reconstituting phenytoin, the number of vials required, and procedures needed for its administration; these observations are supported by the scientific literature.14, 18, 28
Strategies for management of convulsive status epilepticus are evolving. This includes the increasing use of two or more second-line treatments before progression to RSI, in preference to the traditional practice of immediate progression to RSI after failure of a single second-line treatment. 24 participants in the EcLiPSE trial received both second-line treatments sequentially. Of whom, 17 were randomly assigned to and received levetiracetam first. This could reflect an acceptability issue of a second second-line treatment being conditional on the amount of time lapsed. Clinicians might consider the risks of RSI to be greater than the risks of administration and assessment of an additional second-line treatment. However, the administration of two second-line treatments might substantially delay the use of RSI, which could be detrimental to the child and contribute to neurological and cognitive impairment. The shorter administration time of levetiracetam (by contrast with 20 min or more for phenytoin) could make it an appealing first-choice second-line treatment. Finally, intravenous levetiracetam has been shown to be as effective (76%) as intravenous lorazepam (the current first-choice first-line treatment for convulsive status epilepticus) in terminating convulsive status epilepticus in adults,27 which might justify further study.
The EcLiPSE trial did not show that levetiracetam was superior to phenytoin in cessation rate of convulsive status epilepticus, the time taken to terminate convulsive status epilepticus, or adverse reactions and events. However, the results, together with previously reported safety profiles and relative ease of administration of levetiracetam, suggest that it could be an appropriate alternative to phenytoin as the first-choice anticonvulsant for second-line treatment of paediatric convulsive status epilepticus.
Data sharing
Data will be shared upon request to the Clinical Trials Research Centre (CTRC) University of Liverpool (Liverpool, UK). Requests will be checked for compatibility with participant consent and the CTRC data sharing policy will be followed. The CTRC data sharing policy requires investigator assessment and approval of the request and completion of a data sharing agreement. Anonymised data and a copy of the annotated case report forms will be shared. The data will be available following their inclusion in a planned individual participant data meta-analysis.
Acknowledgments
Acknowledgments
This trial was funded by the National Institute for Health Research (NIHR) Health Technology Assessment programme (project grant 12/127/134). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. The EcLiPSE trial was sponsored by the University of Liverpool (Liverpool, UK) and Alder Hey Children's UK National Health Service (NHS) Foundation Trust (Liverpool, UK). We thank all participating families, all NHS sites and staff who participated in screening and enrolment, the independent members of the trial oversight committees, and staff at the Clinical Trials Research Centre at the University of Liverpool, particularly Nadia Al-Najjar, for Trial Management. The EcLiPSE Trial Management Group is grateful to Rachael Kelly, Managing Editor and the Cochrane Epilepsy Group, University of Liverpool, UK, for assistance with the literature searches for this manuscript.
Contributors
REA devised the trial concept, and was the grant holder and chief investigator. REA, CG, MDL, AI, SM, KW, HH, and JN secured the trial grant. REA, CG, MDL, AI, and SM designed the clinical trial and KW designed the consent study. JN, EDL, and SP contributed to the trial design, provided materials, and provided nursing leadership to participating sites. MDL was the PERUKI lead for the trial. CG was the statistical lead and NEAR did the statistical analysis. KW led the consent study and LR conducted the research. HH and AH provided trial management, and PT provided data management. VE provided patient and public involvement. MDL wrote the first draft of the manuscript with input from RA and CG. All authors reviewed and agreed the final manuscript.
Paediatric Emergency Research in the United Kingdom & Ireland (PERUKI) collaborative
Participating sites and investigators: Matthew Pereira, Susie Hardwick (Addenbrookes Hospital, Cambridge University NHS Trust, Cambridge, UK); Shrouk Messahel, Joanne Noblet, Elizabeth D Lee, Rachel Greenwood-Bibby (Alder Hey Children's Hospital NHS Foundation Trust, Liverpool, UK). Mark Buchanan, Sharon Hughes, Lucy Lewis (Arrowe Park Hospital, Wirral University Hospital NHS Trust, Liverpool, UK); Stuart Hartshorn, Louise Rogers, Juliet Hopkins (Birmingham Children's Hospital, UK); Mark D Lyttle, Daphin Fernandez, Sarah Potter, Holly R Lavigne-Smith, Phoebe Moulsdale, Alice Smith, Tracey Bingham (Bristol Royal Hospital for Children, UK); James Ross, Natasha Ramsey, Jo Hacking (Chelsea and Westminster NHS Foundation Trust, London, UK); Niall Mullen, Paul P Corrigan, Sarah Prudhoe (City Hospitals Sunderland NHS Foundation Trust, Sunderland, UK); Hani Faza, Gisela Robinson, Rachel C Sunley, Coral J Smith, Vanessa Unsworth (Derbyshire Children's Hospital, Derby, UK); John Criddle, Martin Laque, Alyce B Sheedy (Evelina London Children's Hospital, London, UK); Mark Anderson, Kirsty Devine, Kathryn Bell (Great North Children's Hospital, Newcastle upon Tyne, UK); Alex Scott, Ramesh Kumar, Sonia Armstrong (James Cook University Hospital, Middlesbrough, UK); Emer Sutherland, Fleur Cantle, Sinead Helyar, Paul Riozzi, Hannah Cotton (King's College Hospital, London, UK); Alice J Downes, Helen Mollard (Leeds General Infirmary, Leeds, UK); Damian Roland, Felix Hay (Leicester Hospitals, Leicester, UK); Chris Gough, Sonya Finucane (Nottingham University Hospitals NHS Trust, Nottingham, UK); Catherine Bevan, Rebecca Ramsay, Emily Walton (Royal Alexandra Children's Hospital, Brighton, UK); Julie-Ann Maney, Elizabeth Dalzell, Muriel Millar (Royal Belfast Hospital for Sick Children, Belfast, UK); Rachel J Howells, Andy Appelboam, Jennie Small, Daisy Mackle (Royal Devon and Exeter NHS Foundation Trust, Exeter, UK); Vince Choudhery, Stewart MacLeod, Ashleigh Neil (Royal Hospital for Children, Glasgow, UK); Jen Browning, Thom O'Neill, Julia Grahamslaw (Royal Hospital for Sick Children, Edinburgh, UK); Ami Parikh, Imogen Skene, Rhys Thomas (Royal London Hospital, London, UK); Katherine Potier de la Morandiere, Jill L Wilson, Donna Danziger (Royal Manchester Children's Hospital, Manchester, UK); Derek Burke, Shammi Ramlakhan, Jayne Evans, Julie Morcombe, Stuart Gormley (Sheffield Children's Hospital NHS Foundation Trust, Sheffield, UK); Jason M Barling, Katrina Cathie, Jane Bayreuther, Ruth Ensom (Southampton Children's Hospital, Southampton, UK); Yasser Iqbal, Sarah Rounding (St George's University Hospitals NHS Foundation Trust, London, UK); Joanne Mulligan, Claire Bell, Shona McLellan, Shona Leighton (University Hospital, Crosshouse, Kilmarnock, UK); Tina Sajjanhar, Maggie Nyirenda, Anastasia Alcock, Laura Crome, Neil Williamson (University Hospital Lewisham, London, UK); Sara Edwards, Jeff Morgan, Colin V E Powell (University Hospital of Wales, Cardiff, UK); Chaniyil A Ramesh, Solomon Kamal-Uddin (Watford GeneralHospital, London, UK); Mike Linney, Katia Vamvakiti, Sharon Floyd, Gill Hobden (Western Sussex Hospitals NHS Foundation Trust, UK).
Declaration of interests
VE was our Patient and Parent Involvement representative and is a parent of a child with a severe epilepsy who had frequently experienced episodes of convulsive status epilepticus. We declare no competing interests.
Contributor Information
Richard E Appleton, Email: richardappleton55@hotmail.co.uk.
Paediatric Emergency Research in the United Kingdom & Ireland (PERUKI) collaborative:
Matthew Pereira, Susie Hardwick, Shrouk Messahel, Joanne Noblet, Elizabeth D Lee, Rachel Greenwood-Bibby, Mark Buchanan, Lucy Lewis, Sharon Hughes, Stuart Hartshorn, Louise Rogers, Juliet Hopkins, Mark D Lyttle, Daphin Fernandez, Sarah Potter, Holly R Lavigne-Smith, Phoebe Moulsdale, Alice Smith, Tracey Bingham, James Ross, Natasha Ramsey, Jo Hacking, Niall Mullen, Paul P Corrigan, Sarah Prudhoe, Hani Faza, Gisela Robinson, Rachel C Sunley, Coral J Smith, Vanessa Unsworth, John Criddle, Martin Laque, Alyce B Sheedy, Mark Anderson, Kathryn Bell, Kirsty Devine, Alex Scott, Ramesh Kumar, Sonia Armstrong, Emer Sutherland, Fleur Cantle, Sinead Helyer, Paul Riozzi, Hannah Cotton, Alice J Downes, Helen Mollard, Damian Roland, Felix Hay, Christopher Gough, Sonya Finucane, Catherine Bevan, Rebecca Ramsay, Emily Walton, Julie-Ann Maney, Elizabeth Dalzell, Muriel Millar, Rachel J Howells, Andy Appelboam, Daisy Mackle, Jennie Small, Ashleigh Neil, Vince Choudhery, Stewart MacLeod, Jen Browning, Thomas O'Neill, Julia Grahamslaw, Ami Parikh, Imogen Skene, Rhys Thomas, Katherine Potier de la Morandiere, Jill L Wilson, Donna Danziger, Derek Burke, Shammi Ramlakhan, Jayne Evans, Julie Morcombe, Stuart Gormley, Jason M Barling, Katrina Cathie, Jane Bayreuther, Ruth Ensom, Yasser Iqbal, Sarah Rounding, Joanne Mulligan, Claire Bell, Shona McLellan, Shona Leighton, Tina Sajjanhar, Maggie Nyirenda, Laura Crome, Neil Williamson, Anastasia Alcock, Sara Edwards, Jeff Morgan, Colin VE Powell, Chaniyil A Ramesh, Solomon Kamal-Uddin, Mike Linney, Katia Vamvakiti, Sharon Floyd, and Gill Hobden
Supplementary Material
References
- 1.Novorol CL, Chin RFM, Scott RC. Outcome of convulsive status epilepticus: a review. Arch Dis Child. 2007;92:948–951. doi: 10.1136/adc.2006.107516. [DOI] [PMC free article] [PubMed] [Google Scholar]; CL Novorol, RFM Chin, RC Scott. Outcome of convulsive status epilepticus: a review. Arch Dis Child, 92, 2007, 948–951 [DOI] [PMC free article] [PubMed]
- 2.PICANet Annual Report 2010. PICANet. http://www.picanet.org.uk/Documentation; PICANet Annual Report 2010. PICANet, http://www.picanet.org.uk/Documentation
- 3.Metsäranta P, Koivikko M, Peltola J, Eriksson K. Outcome after prolonged convulsive seizures in 186 children: low morbidity, no mortality. Dev Med Child Neurol. 2004;46:4–8. doi: 10.1017/s0012162204000027. [DOI] [PubMed] [Google Scholar]; P Metsäranta, M Koivikko, J Peltola, K Eriksson. Outcome after prolonged convulsive seizures in 186 children: low morbidity, no mortality. Dev Med Child Neurol, 46, 2004, 4–8 [DOI] [PubMed]
- 4.Chin RF, Neville BG, Peckham C, Bedford H, Wade A, Scott RC. Incidence, cause, and short-term outcome of convulsive status epilepticus in childhood: prospective population-based study. Lancet. 2006;368:222–229. doi: 10.1016/S0140-6736(06)69043-0. [DOI] [PubMed] [Google Scholar]; RF Chin, BG Neville, C Peckham, H Bedford, A Wade, RC Scott. Incidence, cause, and short-term outcome of convulsive status epilepticus in childhood: prospective population-based study. Lancet, 368, 2006, 222–229 [DOI] [PubMed]
- 5.Hussain N, Appleton R, Thorburn K. Aetiology, course and outcome of children admitted to paediatric intensive care with convulsive status epilepticus: a retrospective 5-year review. Seizure. 2007;16:305–312. doi: 10.1016/j.seizure.2007.01.007. [DOI] [PubMed] [Google Scholar]; N Hussain, R Appleton, K Thorburn. Aetiology, course and outcome of children admitted to paediatric intensive care with convulsive status epilepticus: a retrospective 5-year review. Seizure, 16, 2007, 305–312 [DOI] [PubMed]
- 6.Eriksson K, Metsäranta P, Huhtala H, Auvinen A, Kuusela A-L, Koivikko M. Treatment delay and the risk of prolonged status epilepticus. Neurology. 2005;65:1316. doi: 10.1212/01.wnl.0000180959.31355.92. [DOI] [PubMed] [Google Scholar]; K Eriksson, P Metsäranta, H Huhtala, A Auvinen, A-L Kuusela, M Koivikko. Treatment delay and the risk of prolonged status epilepticus. Neurology, 65, 2005, 1316 [DOI] [PubMed]
- 7.Advanced Paediatric Life Support: a practical approach to emergencies (APLS) 6th Edition. http://www.alsg.org/uk/Publications; Advanced Paediatric Life Support: a practical approach to emergencies (APLS) 6th Edition, http://www.alsg.org/uk/Publications
- 8.McTague A, Martland T, Appleton R. Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children. Cochrane Database Syst Rev. 2018;1 doi: 10.1002/14651858.CD001905.pub3. CD001905. [DOI] [PMC free article] [PubMed] [Google Scholar]; A McTague, T Martland, R Appleton. Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children. Cochrane Database Syst Rev, 1, 201, 8 CD001905. [DOI] [PMC free article] [PubMed]
- 9.Brigo F, Bragazzi N, Nardone R, Trinka E. Direct and indirect comparison meta-analysis of levetiracetam versus phenytoin or valproate for convulsive status epilepticus. Epilepsy Behav. 2016;64:110–115. doi: 10.1016/j.yebeh.2016.09.030. [DOI] [PubMed] [Google Scholar]; F Brigo, N Bragazzi, R Nardone, E Trinka. Direct and indirect comparison meta-analysis of levetiracetam versus phenytoin or valproate for convulsive status epilepticus. Epilepsy Behav, 64, 2016, 110–115 [DOI] [PubMed]
- 10.Lewena S, Pennington V, Acworth J. Emergency management of pediatric convulsive status epilepticus: a multicenter study of 542 patients. Pediatr Emerg Care. 2009;25:83–87. doi: 10.1097/PEC.0b013e318196ea6e. [DOI] [PubMed] [Google Scholar]; S Lewena, V Pennington, J Acworth. Emergency management of pediatric convulsive status epilepticus: a multicenter study of 542 patients. Pediatr Emerg Care, 25, 2009, 83–87 [DOI] [PubMed]
- 11.Singh K, Aggarwal A, Faridi M, Sharma S. IV levetiracetam versus IV phenytoin in childhood seizures: a randomized controlled trial. J Pediatr Neurosci. 2018;13:158. doi: 10.4103/jpn.JPN_126_17. [DOI] [PMC free article] [PubMed] [Google Scholar]; K Singh, A Aggarwal, M Faridi, S Sharma. IV levetiracetam versus IV phenytoin in childhood seizures: a randomized controlled trial. J Pediatr Neurosci, 13, 2018, 158 [DOI] [PMC free article] [PubMed]
- 12.Craig S. Phenytoin poisoning. Neurocrit Care. 2005;3:161–170. doi: 10.1385/NCC:3:2:161. [DOI] [PubMed] [Google Scholar]; S Craig. Phenytoin poisoning. Neurocrit Care, 3, 2005, 161–170 [DOI] [PubMed]
- 13.Appleton RE, Gill A. Adverse events associated with intravenous phenytoin in children: a prospective study. Seizure. 2003;12:369–372. doi: 10.1016/s1059-1311(02)00338-2. [DOI] [PubMed] [Google Scholar]; RE Appleton, A Gill. Adverse events associated with intravenous phenytoin in children: a prospective study. Seizure, 12, 2003, 369–372 [DOI] [PubMed]
- 14.Risk of death and severe harm from error with injectable phenytoin. NHS Improvement. https://improvement.nhs.uk/news-alerts/risk-death-and-severe-harm-error-injectable-phenytoin; Risk of death and severe harm from error with injectable phenytoin. NHS Improvement, https://improvement.nhs.uk/news-alerts/risk-death-and-severe-harm-error-injectable-phenytoin
- 15.McTague A, Kneen R, Kumar R, Spinty S, Appleton R. Intravenous levetiracetam in acute repetitive seizures and status epilepticus in children: experience from a children's hospital. Seizure. 2012;21:529–534. doi: 10.1016/j.seizure.2012.05.010. [DOI] [PubMed] [Google Scholar]; A McTague, R Kneen, R Kumar, S Spinty, R Appleton. Intravenous levetiracetam in acute repetitive seizures and status epilepticus in children: experience from a children's hospital. Seizure, 21, 2012, 529–534 [DOI] [PubMed]
- 16.Chakravarthi S, Goyal MK, Modi M, Bhalla A, Singh P. Levetiracetam versus phenytoin in management of status epilepticus. J Clin Neurosci. 2015;22:959–963. doi: 10.1016/j.jocn.2014.12.013. [DOI] [PubMed] [Google Scholar]; S Chakravarthi, MK Goyal, M Modi, A Bhalla, P Singh. Levetiracetam versus phenytoin in management of status epilepticus. J Clin Neurosci, 22, 2015, 959–963 [DOI] [PubMed]
- 17.Mundlamuri RC, Sinha S, Subbakrishna DK. Management of generalised convulsive status epilepticus (SE): a prospective randomised controlled study of combined treatment with intravenous lorazepam with either phenytoin, sodium valproate or levetiracetam—pilot study. Epilepsy Res. 2015;114:52–58. doi: 10.1016/j.eplepsyres.2015.04.013. [DOI] [PubMed] [Google Scholar]; RC Mundlamuri, S Sinha, DK Subbakrishna. Management of generalised convulsive status epilepticus (SE): a prospective randomised controlled study of combined treatment with intravenous lorazepam with either phenytoin, sodium valproate or levetiracetam—pilot study. Epilepsy Res, 114, 2015, 52–58 [DOI] [PubMed]
- 18.Trinka E, Dobesberger J. New treatment options in status epilepticus. Ther Adv Neurol Disord. 2009;2:79–91. doi: 10.1177/1756285608100460. [DOI] [PMC free article] [PubMed] [Google Scholar]; E Trinka, J Dobesberger. New treatment options in status epilepticus. Ther Adv Neurol Disord, 2, 2009, 79–91 [DOI] [PMC free article] [PubMed]
- 19.National Institute for Health and Care Excellence Epilepsies: diagnosis and management. Clinical guideline CG137. April, 2018. https://www.nice.org.uk/guidance/cg137 [PubMed]; National Institute for Health and Care Excellenc, pilepsies: diagnosis and management. Clinical guideline CG137, https://www.nice.org.uk/guidance/cg13, 7 April, 2018 [PubMed]
- 20.Lyttle MD, O'Sullivan R, Hartshorn S. Pediatric Emergency Research in the UK and Ireland (PERUKI): developing a collaborative for multicentre research. Arch Dis Child. 2014;99:602–603. doi: 10.1136/archdischild-2013-304998. [DOI] [PubMed] [Google Scholar]; MD Lyttle, R O'Sullivan, S Hartshorn. Pediatric Emergency Research in the UK and Ireland (PERUKI): developing a collaborative for multicentre research. Arch Dis Child, 99, 2014, 602–603 [DOI] [PubMed]
- 21.Lyttle MD, Gamble C, Messahel S. Emergency treatment with levetiracetam or phenytoin in status epilepticus in children—the EcLiPSE study: study protocol for a randomised controlled trial. Trials. 2017;18:283. doi: 10.1186/s13063-017-2010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; MD Lyttle, C Gamble, S Messahel. Emergency treatment with levetiracetam or phenytoin in status epilepticus in children—the EcLiPSE study: study protocol for a randomised controlled trial. Trials, 18, 2017, 283 [DOI] [PMC free article] [PubMed]
- 22.Woolfall K, Gamble C, Frith L, the CONNECT Advisory Group. Young B. Research Without Prior Consent (deferred consent) in trials investigating the emergency treatment of critically ill children: CONNECT study guidance Version 2.0. 2015. https://www.liverpool.ac.uk/psychology-health-and-society/research/connect; K Woolfall, C Gamble, L Frith, the CONNECT Advisory Group B Young. Research Without Prior Consent (deferred consent) in trials investigating the emergency treatment of critically ill children: CONNECT study guidance Version 2.0, https://www.liverpool.ac.uk/psychology-health-and-society/research/connec, t 2015
- 23.Woolfall K, Young B, Frith L. Doing challenging research studies in a patient-centred way: a qualitative study to inform a randomised controlled trial in the paediatric emergency care setting. BMJ Open. 2014;4:e005045. doi: 10.1136/bmjopen-2014-005045. [DOI] [PMC free article] [PubMed] [Google Scholar]; K Woolfall, B Young, L Frith. Doing challenging research studies in a patient-centred way: a qualitative study to inform a randomised controlled trial in the paediatric emergency care setting. BMJ Open, 4, 2014, e005045 [DOI] [PMC free article] [PubMed]
- 24.Woolfall K, Roper L, Humphreys A. Enhancing practitioner confidence in recruitment and consent in the EcLiPSE trial: a mixed method evaluation of practitioner training—a Paediatric Emergency Research in the United Kingdom & Ireland (PERUKI) study. Trials. 2019;20:181. doi: 10.1186/s13063-019-3273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; K Woolfall, L Roper, A Humphreys. Enhancing practitioner confidence in recruitment and consent in the EcLiPSE trial: a mixed method evaluation of practitioner training—a Paediatric Emergency Research in the United Kingdom & Ireland (PERUKI) study. Trials, 20, 2019, 181 [DOI] [PMC free article] [PubMed]
- 25.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Statist. 1988;16:1141–1154. [Google Scholar]; RJ Gray. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Statist, 16, 1988, 1141–1154
- 26.Nakamura K, Inokuchi R, Daidoji H. Efficacy of levetiracetam versus fosphenytoin for the recurrence of seizures after status epilepticus. Medicine. 2017;96:e7206. doi: 10.1097/MD.0000000000007206. [DOI] [PMC free article] [PubMed] [Google Scholar]; K Nakamura, R Inokuchi, H Daidoji. Efficacy of levetiracetam versus fosphenytoin for the recurrence of seizures after status epilepticus. Medicine, 96, 2017, e7206 [DOI] [PMC free article] [PubMed]
- 27.Misra UK, Kalita J, Maurya PK. Levetiracetam versus lorazepam in status epilepticus: a randomized, open labeled pilot study. J Neurol. 2012;259:645–648. doi: 10.1007/s00415-011-6227-2. [DOI] [PubMed] [Google Scholar]; UK Misra, J Kalita, PK Maurya. Levetiracetam versus lorazepam in status epilepticus: a randomized, open labeled pilot study. J Neurol, 259, 2012, 645–648 [DOI] [PubMed]
- 28.Fuller KL, Wang YY, Cook MJ, Murphy MA, D'Souza WJ. Tolerability, safety, and side effects of levetiracetam versus phenytoin in intravenous and total prophylactic regimen among craniotomy patients: a prospective randomized study. Epilepsia. 2013;54:45–57. doi: 10.1111/j.1528-1167.2012.03563.x. [DOI] [PubMed] [Google Scholar]; KL Fuller, YY Wang, MJ Cook, MA Murphy, WJ D'Souza. Tolerability, safety, and side effects of levetiracetam versus phenytoin in intravenous and total prophylactic regimen among craniotomy patients: a prospective randomized study. Epilepsia, 54, 2013, 45–57 [DOI] [PubMed]
- 29.McIntyre J, Robertson S, Norris E. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: a randomised controlled trial. Lancet. 2005;366:205–210. doi: 10.1016/S0140-6736(05)66909-7. [DOI] [PubMed] [Google Scholar]; J McIntyre, S Robertson, E Norris. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: a randomised controlled trial. Lancet, 366, 2005, 205–210 [DOI] [PubMed]
- 30.Inwald DP, Canter R, Woolfall K. Restricted fluid bolus volume in early septic shock: results of the Fluids in Shock pilot trial. Arch Dis Child. 2018 doi: 10.1136/archdischild-2018-314924. published online Aug 7. [DOI] [PMC free article] [PubMed] [Google Scholar]; DP Inwald, R Canter, K Woolfall. Restricted fluid bolus volume in early septic shock: results of the Fluids in Shock pilot trial. Arch Dis Chil, d 201, 8 published online Aug 7. DOI:10.1136/archdischild-2018-314924 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be shared upon request to the Clinical Trials Research Centre (CTRC) University of Liverpool (Liverpool, UK). Requests will be checked for compatibility with participant consent and the CTRC data sharing policy will be followed. The CTRC data sharing policy requires investigator assessment and approval of the request and completion of a data sharing agreement. Anonymised data and a copy of the annotated case report forms will be shared. The data will be available following their inclusion in a planned individual participant data meta-analysis.