Abstract
Hydrogels, consisting of hydrophilic polymers, can be used as films, scaffolds, nanoparticles and drug carriers. They are one of the hot research topics in material science and tissue engineering and are widely used in the field of biomedical and biological sciences. Researchers are seeking for a type of material that is similar to human tissues and can partially replace human tissues or organs. The hydrogel has brought possibility to solve this problem. It has good biocompatibility and biodegradability. After entering the body, it does not cause immune and toxic reactions. The degradation time can be controlled, and the degradation products are nontoxic and nonimmunogenic; the final metabolites can be excreted outside the body. Owing to the lack of blood vessels and poor migration ability of chondrocytes, the self-healing ability of damaged cartilage is limited. Tissue engineering has brought a new direction for the regeneration of cartilage. Drug carriers and scaffolds made of hydrogels are widely used in cartilage tissue engineering. The present review introduces the natural hydrogels, which are often used for cartilage tissue engineering with respect to synthesis, modification and application methods.
The translational potential of this article
This review introduces the natural hydrogels that are often used in cartilage tissue engineering with respect to synthesis, modification and application methods. Furthermore, the essential concepts and recent discoveries were demonstrated to illustrate the achievable goals and the current limitations. In addition, we propose the putative challenges and directions for the use of natural hydrogels in cartilage regeneration.
Keywords: Alginate, Chitosan, Collagen, Gelatin, Hyaluronan, Natural hydrogel
Introduction
Hydrogels are network systems formed by macromolecule polymers. Their hydrophilic residues combine with water molecules and the hydrophobic residues expand with water to form cross-linked polymers, which can absorb about 1000 folds of their dry weight in water. The network of the physically cross-linked hydrogels is aggregated by molecular entangling, ionic bond, hydrogen bond or hydrophobic interaction force to form a reversible structure, which will gradually degrade in water and is usually not uniform [1]. The network formed by covalent bonds is known as a chemically cross-linked hydrogel, which is stable, homogeneous and difficult to degrade [2]. The source of hydrogels is usually natural or synthetic polymers. Natural polymers have explicit biomedical applications and properties, such as nontoxicity and biodegradability but poor stability; synthetic polymers are exactly opposite. The compressive strength of the hydrogel scaffolds consists of alginate, chitosan, polyvinyl alcohol or hyaluronan that can range from 0.46 to 5.64 MPa [3], [4], [5]. However, the hydrogels made by artificial polymers, such as polylactic-co-glycolic acid (PLGA), polymethyl methacrylate (PMMA), polycaprolactone (PCL) and poly(ethylene glycol) (PEG) can achieve the mechanical strength of ≥20–120 MPa [6], [7], [8]. The natural and synthetic polymers can be prepared using polymeric blending to achieve hydrogels for diverse application in biomedical fields, thereby allowing the adjustment of the physical and chemical properties of both kinds of polymers [9].
To systematically describe the characteristics of a hydrogel, some of the properties are usually used such as mechanical properties, swelling rate, pore size, porosity, substance exchange capacity and biodegradable performance. The mechanical properties of the hydrogels can affect the stability of the material and the behavior of the cells, including cell spreading, migration, and differentiation by affecting the signal transformation process; for example, the mechanical signals are converted into biochemical signals [10]. Thus, hydrogels with different mechanical properties can be synthesized as per unique requirements and the effects of different mechanical strengths on explored cell behavior. Swelling is the phenomenon of volume expansion of hydrogels in water. As the equilibrium of swelling is reached, the ratio of volume before and after the swelling is termed as the swelling ratio. The swelling rate of hydrogels primarily characterizes the hydrophilicity of materials and the internal cross-linking density. Usually, the harder the hydrogels, the smaller the swelling rates. The internal pore size and porosity of the hydrogels can directly affect the exchange capacity, swelling rate, and mechanical properties. Thus, the hydrogels with low swelling ratio, substance exchange capacity, and high Young's modulus exhibit small pore size and porosity. A sufficient biodegradability is essential for the application of hydrogels in tissue engineering. Whether the hydrogels are used as scaffolds or drug delivery devices, their biodegradability plays a vital role and affects the performance of hydrogel products. In comparison with the nondegradable hydrogels, the migration and intercellular interaction of cells are markedly improved in the biodegradable hydrogels.
Owing to the deficiency of blood vessels, nerves and lymphatics, mature cartilage cannot spontaneously heal the defects resulting from osteoarthritis, aging or joint injury [11], [12]. Current strategies for cartilage tissue engineering are not yet capable of fabricating artificial cartilage that is indistinguishable from native cartilage with respect to zonal organization, extracellular matrix (ECM) and mechanical properties [13]. As an ideal strategy for the repair of osteochondral lesions, the structure of cartilage and the subchondral bone must be regenerated layer by layer. Despite the reconstruction of structure, cell seeding, cell differentiation and long-term safety of implants are other difficulties that should be overcome. The use of hydrogels, especially natural hydrogels, could be considered optimal to fulfill such requirements. Since the pioneering work of Wichterle and Lim on the application of cross-linked 2-hydroxyethyl methacrylate hydrogels in biology, the hydrophilicity and biocompatibility of such materials have attracted the attention of researchers in the fields of tissue engineering and biomaterial science [14], [15]. The present review introduces the application of natural hydrogels in cartilage tissue engineering on the aspects of structure, synthesis and utilization forms.
Hyaluronan
General properties
Hyaluronan, also known as hyaluronic acid (HA), is a disaccharide unit composed of N-acetylglucosamine and d-glucuronic acid. It is widely distributed in the human body, including the ECM of the skin, lenses and cartilage [16]. Unlike other mucopolysaccharides, HA does not contain sulphur, and the molecular weight occurs in a wide range in different tissues. HA is a major component of the ECM, and it plays a critical role in cell signal transduction and wound healing [17], [18]. Presently, HA and its derivatives have been widely used in tissue engineering, regenerative medicine and clinical practice [19], [20], [21].
Modification of HA molecules
Esterification
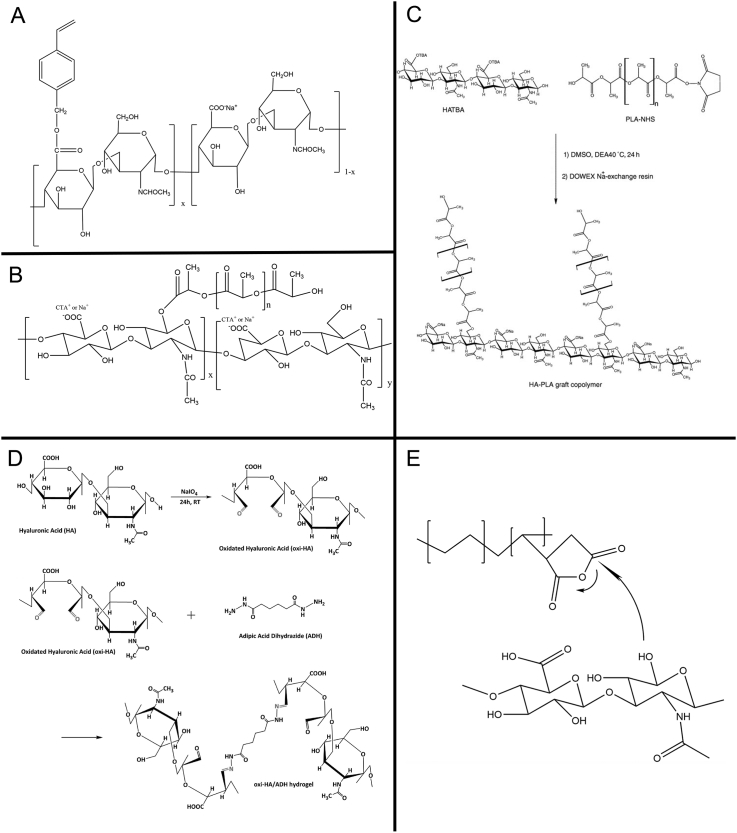
The esterification of the HA includes hydroxyl modification and carboxyl modification, i.e., the hydroxyl groups are esterified with acid or anhydride, or the carboxyl group reacts with alcohols, phenols, epoxides or halogenated hydrocarbons to form esterification derivatives (Figure 1A). Vazquez et al. synthesized an ester compound HA-VB by esterification with dimethyl sulfoxide, chloromethyl styrene and tetrabutylammonium hydroxide [22].
Figure 1.
Modification and preparation of hyaluronic acid. (A) Esterification. (B) Hydrophobic modification. (C) Graft modification. (D) Acylhydrazine cross-linking. (E) Photopolymerization.
PLA-NHS = polylactic acid-N-hydroxysuccinimide; HA-PLA = hyaluronic acid-polylactic acid; DMSO = dimethyl sulfoxide.
Hydrophobic modification
HA is highly hydrophilic and often exists in the form of a sodium salt. It cannot be solubilized in most organic solvents and combined/modified with hydrophobic materials (Figure 1B). According to the report by Pravata et al., cetyl trimethyl ammonium bromide, and polylactic acid (PLA) could be used to modify HA for synthesizing self-assembled hydrogels [23].
Graft modification
The graft modification is grafting small molecules or polymers onto the main chain of hyaluronic acid (HA) (Figure 1C). Oldinski et al. grafted HA on high-density polyethylene to prepare a biomaterial that can be used in cartilage tissue engineering [24]. Palumbo et al. synthesized a copolymer, HA-PLA, using tetrabutylammonium with low molecular weight hyaluronic acid to react with N-hydroxysuccinimide (NHS)–activated polyPLA (PLA-NHS) in dimethyl sulphoxide [25].
Preparation of HA hydrogel
Acylhydrazine cross-linking
The hydrazide compounds can be used as cross-linking agents to decrease the flowability and improve the mechanical strength (Figure 1D). Adipic acid dihydrazide (ADH) is the most frequently used hydrazide derivative, and HA can form a stable HA-ADH compound in the presence of ADH. Some groups used this method to synthesize the injectable hydrogel or film for the regeneration of cartilage [26], [27].
Carbodiimide cross-linking
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) can react with a carboxyl group in an acidic solution to form an N-acyl urea compound. Subsequently, the cross-linked HA derivatives are formed with adequate stability, strong rigidity, large biological density and ability to resist enzyme hydrolysis. Lai et al. explored the EDC–cross-linked HA hydrogel in the anterior chambers of rats. The results showed that compared to glutaraldehyde, the gel film had better biocompatibility and higher tensile strength [28].
Sulphone cross-linking
Divinylsulfone (DVS) can cross-link rapidly with the hydroxyl groups to obtain HA hydrogels with different properties. The cross-linking degree of the hydrogels can be altered by controlling the concentration of HA, the molecular weight, the HA/DVS value and the pH of the reaction medium.
Photopolymerization
Photopolymerization is suitable for the preparation of HA hydrogels owing to satisfactory reproducibility, rapid reaction and nontoxicity (Figure 1E) [29]. Methacrylic anhydride (MA), glycidyl methacrylate (GMA) and other methacrylic acid derivatives are commonly adopted for the esterification with HA. In the presence of photoinitiators, such as 2-hydroxy-4'-(2-hydroxyethoxy)-2-methylpropiophenone (I2959), cross-linking reaction can occur under the exposure to ultraviolet (UV) light at 365 nm [30], [31].
The Click–Huisgen cycloaddition reaction
The Huisgen cycloaddition reaction is a copper-catalyzed azido alkynyl cycloaddition reaction, and the click reaction is a physiological positive cross-linking reaction with adequate biocompatibility. Present studies have demonstrated that hydrogels formed by these reactions exhibit cytocompatibility, especially for chondrocytes [32], [33].
Application in cartilage regeneration
HA is a component of ECM that can provide cells with three-dimensional microenvironment similar to the natural conditions. Therefore, HA hydrogels can be directly used as a scaffold material for cartilage tissue engineering. Liu et al. synthesized a HA hydrogel to encapsulate the mesenchymal stem cells (MSCs) using hydrosulfonic acid, HA, gelatin and bisacrylamide PEG for repairing the full-thickness cartilage defect of the rabbit femoral trochlea [34]. The sulfated HA exhibits significantly slow degradation, promotes chondrogenesis and suppresses the hypertrophy of encapsulated human mesenchymal stem cells (hMSCs) both in vitro and in vivo. It can also avert the cartilage abrasion and hypertrophy in animal osteoarthritic joints [35]. Moreover, the conjugation of N-cadherin peptides onto HA hydrogels promote both early chondrogenesis of MSCs and cartilage-specific matrix production [36]. The hyaluronan sponges–based scaffolds modified by the lysine methyl ester cross-linking demonstrated adequate morphological, water-uptake, mechanical and stability properties to maintain the lineage identity of the chondrocytes for 3 weeks [37]. The combination of hyaluronan and fibrin has been studied for its potential of promoting cell adhesion, proliferation and differentiation. The chondrogenesis behavior of human MSCs on this mechanically stable hybrid matrix can be observed [38]. A transglutaminase–cross-linked hyaluronan hydrogel could gel rapidly in the presence of factor XIIIa (FXIIIa) and acquire the characteristics of injectability, biocompatibility with encapsulated cells, mitogenicity, chondroinductivity, form-stability and strong adhesion to native cartilage [39]. Photopolymerization is another commonly used method for the fabrication of tissue engineering scaffolds. These kinds of hydrogel generally have good injectability or printability. An injectable MA-modified HA containing a small molecule drug kartogenin has been proved to have good ability to gel in situ and repair the cartilage defects [31]. Another visible green light–activated cross-linking systems are designed using MA-modified HA, eosin Y, triethanolamine and 1-vinyl-2-pyrrolidinone. The mechanical strength and cell compatibility improved significantly as compared with those in UV-activated systems [40]. The drug delivery system constructed using HA hydrogel is widely applied in the field of regenerative medicine. The release duration of bovine serum albumin in HA hydrogel can be sustained from 6 h to several weeks. The difference in the release rate depends on the internal cross-linking degree of the hydrogel and the combination of sustained release microspheres. Using optical coherence tomography, the degradation properties of microspheres and HA hydrogel were characterized [41]. The adamantane-functionalized HA guest polymers and monoacrylate β-cyclodextrins host monomers can self-assemble through the host–guest interaction and achieve the ability of self-healing [42].
Gelatin
General properties
Gelatin, also known as animal gelatin, is produced/synthesized from partial hydrolysis of animal collagen. It is formed by the disintegration of the three-helix structure of collagen and is the main component of the connective tissue, such as the skin, bone, sarcolemma and muscle charm. The thermally reversible change of gelatin gel can occur in the aqueous solution at 30–40 °C, and water was a pore-forming agent during the freeze-drying process to form porous scaffolds. The pore size and porosity could be adjusted by modifying the freezing parameters [43]. The use of gelatin-based composites as scaffolds for tissue engineering and carrier signal molecules is the hotspot topic in biomaterials.
Modification of gelatin molecules
Physical modification
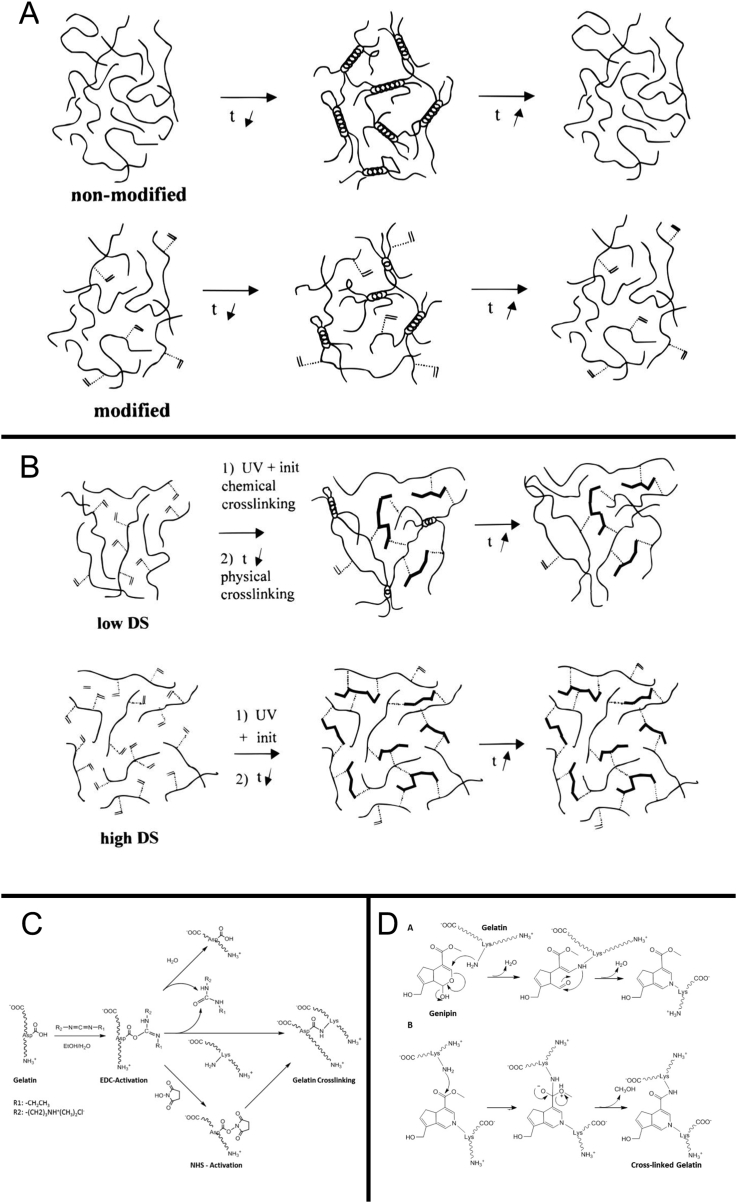
The physical modification is the altering of properties of gelatin by transforming its structure without any additives. In addition, gelatin exists as helical conformation and curled conformation in its products (Figure 2A). The difference in the two conformational ratios greatly influenced the properties of gelatin products. The gelatin solution or film is condensed for a specific time that alters its conformation into that of a high spiral, which is known as “refolding”; it is only a physical modification [44]. This is greatly significant in both theory and practical; however, there are yet several challenges with respect to the modification.
Figure 2.
Modification and preparation of gelatin. (A) Physical modification. (B) Chemical modification. (C) Zero-length cross-linking. (D) Nonzero-length cross-linking.
NHS = N-hydroxysuccinimide.
Chemical modification
The chemical modification of gelatin is the addition of various functional groups on the backbone of the cross-linking (Figure 2B). The methacrylic anhydride (MA) is widely used for the chemical modification of gelatin to produce gelatin methacrylamide (GelMA), a versatile matrix that can be used for engineering tissue analogs, ranging from the vasculature to the cartilage and bone. In brief, the reaction introduces methacryloyl substitution groups on the reactive amine and hydroxyl groups of the amino acid residues [45]. The mechanical properties of GelMA can be adjusted based on the level of methacryloyl substitution, which is related to the ratio and pH of the reaction mixture [46]. Based on the dissolution in aqueous media with catalytic levels of photoinitiator, GelMA can be cross-linked using UV light [47].
Preparation of gelatin hydrogel
Physical cross-linking
Physical cross-linking occurs without any potentially cytotoxic chemicals; however, the constructs are generally weak, and the modulus is low. Plasma, UV radiation and dehydrothermal treatment are the common methods for physical cross-linking; however, the cross-linking is not stringent, and the degree of cross-linking is low [48], [49], [50], [51].
Enzymatic cross-linking
An amide bond between the carboxylic acid groups of glutamic acid and the ε-amino group of lysine can be introduced using calcium-independent microbial transglutaminase [52]. In addition, tyrosinase has also been evaluated to initiate gelatin cross-linking with weak structures [53]. This methodology is appropriate for in situ cross-linking and has been used for the synthesis of injectable gelatin-based hydrogels for bioadhesives and tissue regeneration [54], [55].
Chemical cross-linking
Based on the influence of the cross-linked product on the molecular structure, the chemical cross-linking agents can be differentiated into covalently linked amine residues (nonzero-length) and carboxylic acid and amine residues (zero-length). For zero-length cross-linking, EDC and NHS are widely used carbodiimide linkers, which activate the carboxylic acid residues of aspartic and glutamic acids and convert them into O-acylisourea groups (Figure 2C) [56]. The most representative nonzero-length agents are aldehydes, polyepoxides, isocyanates and natural products such as genipin (Figure 2D) [57], [58], [59], [60], [61], [62]. Aldehydes can react with the α-amino groups of lysine to form a Schiff's base between the polymer chains and the mechanical and biological properties of gelatin can be dramatically altered in this reaction by altering the concentrations of aldehydes [63]. In comparison with the reagents in the nonzero-length cross-linking, the reagents in the zero-length cross-linking will not be released during the degradation of materials, and the cell toxicity is decreased.
Application in cartilage regeneration
Gelatin is ideal for making cartilage tissue engineering scaffolds. The acrylate-grafted gelatin and glucosamine molecules can covalently cross-link under photoradiation to form hydrogels, and the physiochemical properties can be adjusted by altering the proportions of GelMA and N-acryloyl glucosamine [64]. In addition to UV polymerization, visible light polymerization, two-photon polymerization and gamma radiation could be used to form GelMA hydrogels [65], [66], [67], [68], [69], [70]. A gelatin-based hydrogel synthesized by oxidized dextran, amino gelatin and four-arm PEG-acrylate through a two-step process can stimulate cell attachment and proliferation. Furthermore, the mechanical properties, biodegradability and biocompatibility of this hydrogel are suitable for cartilage tissue engineering [71]. The thermosensitive gelatin-based hydrogel with an interconnected, double-thermoresponsive macroporous membrane can promote cell proliferation and penetrate fibroblasts during cell seeding. Different from the abovementioned molecule, poly (N-isopropyl acrylamide) was used as the graft molecule forming the backbone of gelatin [72]. The supramolecular gelatin macromere could be prepared by host–guest complexation between the aromatic residues of gelatin and freely diffusing photocross-linkable acrylate β-cyclodextrin monomers (β-CD). The subsequent weak host–guest interactions between the β-CD and the gelatinous aromatic residues can form highly resilient supramolecular gelatin hydrogels with good biocompatibilities [73]. For improving the compression strength, the microfibrillated cellulose and periodate were introduced to prepare the hydrogel with gelatin. The hybrid hydrogels can reach Young's modulus exceeding 1.6 MPa, which was 41 folds higher than that of pure gelatin. The composite scaffold also demonstrated a good swelling capacity that could successfully maintain its shape in buffer solution [74]. The grafting of ADH to chondroitin sulfate (CS) and gelatin resulted in CS-ADH and gelatin-ADH, respectively. After the subsequent reaction mediated by EDC, the in situ gelatin-CS hydrogel was obtained, which showed excellent biocompatibility, especially the application potential in cartilage tissue engineering [75].
Collagen
General properties
Collagen is a natural biomaterial that occurs widely in the skin, bone, cartilage, blood vessels, teeth and tendons, and it is commonly used in biology and medicine. Collagen exhibits outstanding biological properties, such as low antigenicity, biodegradability, biocompatibility and cell adaptability. It is a vital component of the ECM that regulates cell function and mimics the tissue characteristics [76] However, the degradation rate and mechanical stability of the natural collagen are insufficient to fulfill the requirements of tissue engineering; whereas modification and cross-linking are essential for utilization of collagen in tissue regeneration.
Modification of collagen molecules
Modification of amino groups
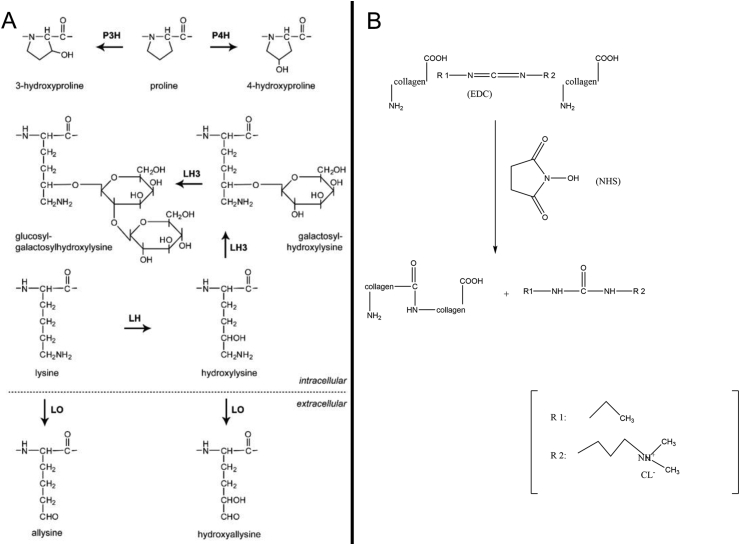
The amino groups on the peptides of collagen, especially Ɛ-amino, may be involved in a series of reactions. The reactions on the amino groups can not only modify the collagen but also protect the amino groups, preventing the subsequent reactions with other groups (Figure 3A). Amidation, phosphorylation and alkylation usually modify the amino groups [77], [78]. The acylation of collagen is the interaction between the nucleophilic group of protein molecules (such as amino) and the electrophilic groups (such as carbonyl) in the acylation reagent. Furthermore, phosphorylation effectuates the esterification between the inorganic phosphate and the specific amino groups on the protein. Under mild alkaline conditions, the amino groups on the protein can be alkylated with aldehydes and ketones to form stable noncross-linked lysine derivatives [79].
Figure 3.
Modification and preparation of collagen. (A) Modification of amino groups. (B) Modification of carboxyl.
NHS = N-hydroxysuccinimide.
Modification of carboxyl
Glutamic acid is a dicarboxylic acid present at a high content in the peptide chain of collagen (Figure 3B). Only one carboxyl group of glutamate is involved in the formation of peptide bonds as the other carboxyl group serves as the side chain of collagen [80], [81]. The carboxyl groups can react with several compounds, and many derivatives can be synthesized using this method. The most commonly used approach is modifying the carboxyl groups by water-soluble carbodiimide, and the products are esters or amides [82], [83], [84].
Preparation of collagen hydrogel
Physical cross-linking
Physical cross-linking primarily includes oxidation, thermal desorption method and UV radiation method. Because the amount of reactant in the physical cross-linking cannot be controlled easily, the physical method is currently used only as an auxiliary method. However, it does not involve the primary structure of the protein, and it is used for solubilization and gelation [85]. The collagen shrinkage temperature, resistance to collagenolytic degradation and durability under load in collagenase are increased during both thermal desorption method and exposure to UV light [86], [87]. The advantage of physical cross-linking was that it could avoid the exogenous toxic chemicals during the cross-linking process, and the drawback was that the degree of collagen cross-linking was low for uniform cross-linking.
Chemical cross-linking
Based on the position of the occurrence of cross-linking, the chemical cross-linking can be divided into two types: in the same spiral and between two different spirals. The former affects the denaturation time and tension of collagen, whereas the latter affects the volume expansion and surface dilatation of collagen [88], [89]. The cross-linking agents can also be divided into two categories: (1) with multiple functional groups and (2) can activate glutamic acid or aspartic acid residues of carboxylic acid [90], [91]. Thus, uniform collagen hydrogel can be obtained via this method; however, the potential toxic effect of residual molecules and/or compounds are observed during in vivo degradation.
Application in cartilage regeneration
Collagen is also a generally used drug delivery material with several advantages, such as good biocompatibility, little toxicity, good biodegradability, high efficiency and prolonged effect. Compared to the hydrophobic polymers, the interaction of the enzyme and other bioactive substances in collagen is weak. An injectable gelatin-transglutaminase and endogenous collagen can create a robust attachment between the hydrogel and tissue ECM, preventing the dissipation of the cells. Kartogenin can be incorporated into the hybrid hydrogel to form a release system and promote high lubricin expression and ECM production of cartilage [92], [93]. The chondrocytes embedded in the hybrid hydrogel containing type I and II collagens maintain good natural morphology and secrete cartilage-specific ECM. The modulus and structure are optimal and can be adjusted by altering the content of collagen type I [94]. The hydrogel made by collagen type II and HA can form in situ scaffolds. The chondrocytes and transforming growth factor β1 encapsulated in the scaffold can maintain chondrocyte viability as well as stimulate cell proliferation, morphology, glycosaminoglycan production and gene expression [95]. The mixed collagen type I and sodium alginate hydrogels were incorporated chondrocytes that can construct cartilage-like tissue in vitro, which could effectively suppress the dedifferentiation of chondrocytes and preserve the phenotype; the mechanical strength and biological functionality were suitable for cartilage regeneration [96]. The self-assembling of collagen and gel cross-linking of genipin forms the hybrid hydrogel composed of three-phase collagen, chondroitin sulfate, and HA, which mimics the function of natural cartilage. The in vivo experiment also demonstrated the successful restoration of cartilage [97]. Riboflavin, a biocompatible vitamin B2, is a generally used non-toxic cross-linking agent in collagen gelling by UV radiation. The mechanical properties are improved, and the enzyme-triggered degradation time is delayed in this hydrogel. The riboflavin-induced photopolymerization collagen hydrogel encapsulated with fibrochondrocytes led to reduced scaffold contraction and enhanced expression levels for the type II collagen and aggrecan genes [98].
Chitosan
General properties
Chitosan, also known as b-(1,4)-2-acetamido-2-deoxy-d-glucose, is commercially obtained by hydrolysis of the aminoacyl group of chitin [99]. The cationic amino groups in the chitosan react with multivalent anions to form hydrogels that are used in biology, medicine, food and cosmetics. Chitosan is a mucopolysaccharide that exists widely in nature. It is the main component of the connective tissues and has properties, such as complexation, bacteriostatic effect, absorbability and antioxidation [100], [101]. Owing to these characteristics, chitosan is considered as the potential material for cartilage repair.
Modification of chitosan molecules
Alkylation modification
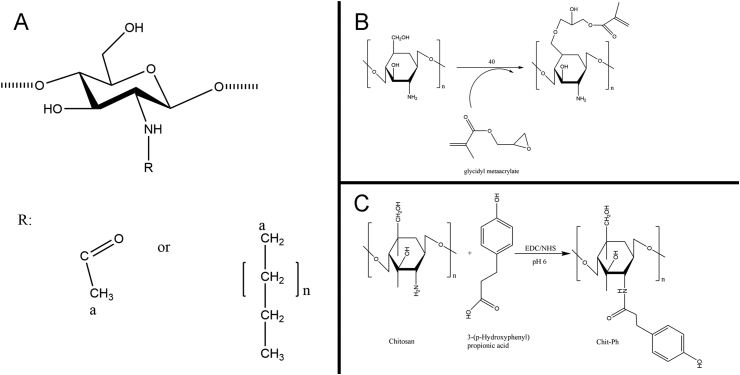
The amino and hydroxyl groups on chitosan can be alkylated to produce the corresponding water-soluble derivatives (Figure 4A). There are 3 principal types of alkylation products: N alkylation, O alkylation and N/O alkylation. These are obtained by reaction between alkylation agents with amino, hydroxyl and amino and hydroxyl, simultaneously [102], [103]. Under the alkaline condition, the reaction is primarily carried out on the hydroxyl groups, and the N-alkylated chitosan is obtained under the acidic condition.
Figure 4.
Modification and preparation of chitosan. (A) Alkyl modification. (B) Photopolymerization. (C) Enzymatic cross-linking.
Quaternary ammonium modification
The quaternary ammonium modification of chitosan is the introduction of quaternary ammonium group on the amino group or the grafting of quaternary ammonium with small molecules onto the amino group. Owing to marked hindrance and strong hydration ability of the quaternary ammonium group, the solubility of chitosan is significantly improved. In addition, the antibacterial, antioxidant, flocculation and moisture retention properties are improved [104].
Other modifications
Except the two commonly used modification methods mentioned previously, etherification, oxidation, carboxylation, acylation and graft copolymerization were introduced to improve the solubility, mechanical strength and biocompatibility of chitosan.
Preparation of chitosan hydrogel
Physical cross-linking
-
(a)
Ionic complexation
Ionic complexation occurs between chitosan and small anionic molecules because of the presence of cationic amino groups in chitosan [105], [106]. The anions and small molecules bind to chitosan via the protonated amino groups to introduce the electrostatic interactions. For metal ions, the coordinate covalent bonds play a vital role in the interaction [107], [108]. Furthermore, other secondary interchain interactions, such as hydrogen bonding between chitosan's hydroxyl groups and ionic molecules or between deacetylated chitosan chains after neutralization of their cationic charge, can synergetically react with ionic complexation [109], [110].
-
(b)
Polyelectrolytic complexation
Unlike ionic complexation, the polyelectrolytic complexation occurs between chitosan and large molecules with a broad range of molecular weight. Although it is an electrostatic interaction, the bonds are stronger than other secondary interactions, such as hydrogen bonds or van der Waals interactions [110]. The charge density, ionic strength, pH, solvent and temperature are the major factors that influence these interactions [111]. The reaction conditions of polyelectrolyte complexation are nonessential for the introduction of the organic precursors, catalysts or reactive agents such that the method can adjust the properties of chitosan safely and effectively.
Chemical cross-linking
-
(a)
Prefunctionalized cross-linking
Many prefunctionalized small molecules and polymers with functional groups are used to cross-link with chitosan. Covalent bonds are formed between chitosan chains and cross-linking agents. Although chitosan hydrogels can be formed in situ, stringent in vivo purifications are essential before application owing to the unclear biocompatibilities of many cross-linking agents. Genipin, HA, glutaraldehyde and diacrylate are the commonly used agents [112], [113], [114], [115]. For a majority of the prefunctionalized cross-linking reactions, the conditions are mild with natural pH, physiological temperature and short reaction time [103].
Photopolymerization
The addition of photosensitive groups to chitosan effectuates the cross-linking of the polymer chains under UV exposure (Figure 4B). Compared to prefunctionalized cross-linking, the abovementioned method is faster, cheaper, easier and safer. After the azide groups are functionalized and activated by UV radiation, the free amino groups of chitosan bind with these reactive nitrene groups [116]. In addition, the acrylate groups and photoreactive azidobenzoic acid are also used for the formation of UV-sensitive chitosan hydrogels [117], [118].
Enzymatic cross-linking
Enzymatic cross-linking is a novel, mild method for in situ gel formation for application in tissue engineering and drug delivery (Figure 4C). Horseradish peroxidase is commonly used for catalyzing the enzymatic cross-linking based on the coupling of phenol or aniline derivatives via the decomposition of hydrogen peroxide [119], [120]. The properties of enzymatic products, especially the stiffness, are usually adjusted by hydrogen peroxide solution, which acts as the oxidant [121]. Another commonly used enzyme catalyst is tyrosinase, an oxidizing enzyme that can introduce an interaction between chitosan and gelatin [53], [122].
Application in cartilage regeneration
The hybrid scaffold containing chitosan and poly (vinyl alcohol) can be cross-linked by tetraethoxysilane. The physiochemical properties of the scaffold, especially the tensile strength and fracture strain, can be adjusted by altering the molar ratio of chitosan and poly (vinyl alcohol) that may lead to different influences on the proliferation of chondrocytes [123]. The combination of chitosan, glycerol phosphate and hydroxyethyl cellulose can form a hydrogel in situ to encapsulate MSCs and improve the cell viability, proliferation and differentiation capacity, demonstrating high potential for cartilage repair [124]. The addition of chitosan to PLA, calcium phosphate and hydroxyapatite enhanced the compressive modulus of hydrogel scaffolds [125], [126]. Glutamic acid and 1,5-pentanodiol mixture could be introduced as the cross-linking agents to form chitosan hydrogels. The antioxidant properties of the hydrogels could be enhanced by modification with Tilia platyphyllos extract. The synthesis method was nontoxic and applicable in regenerative medicine and drug delivery [127]. To overcome the limitation of nondissolution in water, a water-soluble N-succinyl chitosan-dialdehyde starch hybrid hydrogels with tighter structure was prepared to repair the cartilage defects [128]. Chitosan and its carboxymethyl derivatives are suitable for drug delivery. The polyelectrolytic complexes, which are nontoxic, well tolerated and biocompatible, play a vital role in designing chitosan drug delivery systems. Alginate, pectin, xanthan gum, collagen and cellulose can form PEC with chitosan to obtain hydrogels for carrying drugs [129], [130], [131]. Furthermore, the chitosan, alginate and collagen can be blended to form a hybrid hydrogel using graphene oxide to enrich the porous structures. After cross-linking by Ca2+, the hydrogel scaffolds showed increased mechanical properties with elongated collagen fibrils and adequate biocompatibility [132].
Alginate
General properties
Alginic acid sodium salt (alginate) is a natural polysaccharide abstracted from brown seaweeds composed of 1,4-linked d-mannuronic acid (M-block) and l-guluronic acid (G-block) residues. It has been widely used for enfolding different cells into hydrogels owing to its desirable biocompatibility, highly hydrated viscoelastic properties and efficient complexation with divalent cations [133], [134]. In addition, it is approved by the US Food and Drug Administration for human use as a food additive and wound dressing material. Compared with other proteins or natural polymers, alginate is cheap, nontoxic to cellular function and convenient for fabricating cell-immobilized beads or 3D porous scaffolds. However, the alginate-only scaffolds showed poor adhesion to anchorage-dependent cells [135], [136].
Modification of alginate molecules
Acetylation of alginate
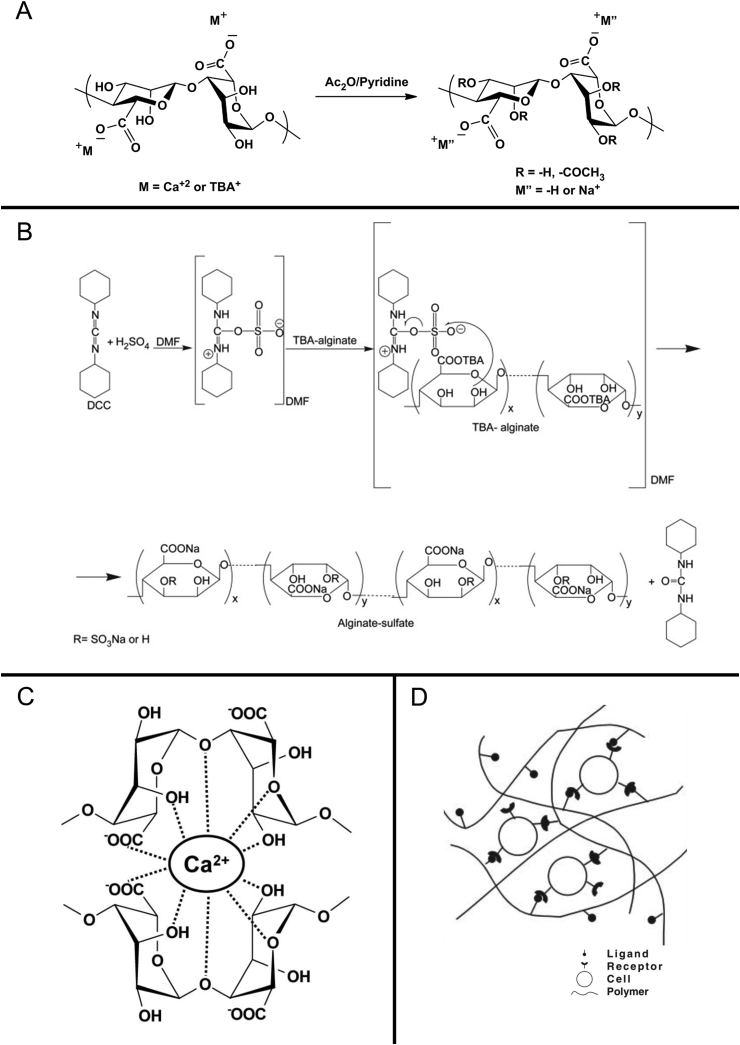
The physiological functions and biosynthesis are mainly affected by acetylation. The partially and fully acetylated alginic acid derivatives can be synthesized using an acid-catalyzed esterification technique by suspending alginic acid in a mixture of acetic acid and acetic anhydride containing perchloric acid (Figure 5A) [137], [138]. Because the H-bonding is robust in the dry state, the hydroxyl groups present in alginic acid yarn could not be reacted with acetic anhydride. However, the presence of water can promote the reaction and acetylation of hydroxyl groups [139]. The addition of acetyl groups to the backbone of alginate causes noticeable chain extension and plays a vital role in defining the polymer conformation [140].
Figure 5.
Modification and preparation of alginate. (A) Acetylation of alginate. (B) Sulfation of alginate. (C) Ionic cross-linking. (D) Cell cross-linking. DCC = dicyclohexylcarbodiimide.
Sulfation of alginate
The alginate sulfates are synthesized by carbodiimide coupling chemistry (Figure 5B). The hydroxyl groups are sulfated using a dicyclohexylcarbodiimide (DCC) sulfuric acid reagent system [141]. The sulfation of alginate using chlorosulfonic acid in formamide enhances the anticoagulation properties, which are greatly influenced by the attachment of quaternary amines and a large number of quaternary ammonium groups [142]. Although a majority of the biological functions in alginates are related to the sulfation pattern and sequence, the control over the placement of sulfate groups along the alginate backbone cannot be achieved easily [143], [144].
Hydrophobic modification
The two hydroxyl groups and the carboxylate ion of the alginate render it hydrophilic and soluble in water at ≥ pH 5.0. To transform the property of the molecule from its predominantly hydrophilic nature to amphipathic or hydrophobicity, the covalent attachment of the hydrophobic moieties to the polymer backbone is the commonly used method [145]. The alginate hydroxyl groups are converted to aldehydes via oxidization and the reactivity increases. Moreover, the carboxylate groups are preserved in the process effectuating the formation of ionic gels from the modified alginate [143].
Preparation of alginate hydrogel
Ionic cross-linking
Combining the aqueous alginate solution with ionic cross-linking agents, such as divalent cations, is considered for the preparation of alginate hydrogel (Figure 5C). Ca2+, Ba2+ and other divalent cations can bind solely to guluronate blocks and form the egg-box model, leading to the gelling process [133], [146]. Calcium chloride is one of the most frequently used agents for ionic cross-linking; the gelation speed is extremely rapid for control. A buffer containing phosphate controls and slows the process as the competition between the phosphate groups in the buffer and the carboxylate groups of alginate react with the calcium ions [147]. The slow gelation introduces high gelation rate, uniform structures, and greater mechanical integrity [148]. However, the ionic cross-linking alginate hydrogel can be solubilized in a short period because of the release of divalent cations and the exchange of monovalent cations.
Covalent cross-linking
Covalent cross-linking has been widely used to improve the mechanical properties of hydrogels. Notably, in the case of alginate hydrogels, the elastic deformation is affected by the inability to dissociate and reform the cross-linking bonds [149] Using different cross-linking molecules and densities, the mechanical properties and swelling ratio of alginate hydrogels can be tightly regulated. The modification with a methacrylate or polyallylamine can cause alginate hydrogels to covalently cross-link to gel by light radiation treatment at different wavelengths in mild reaction conditions in situ [150], [151]. This method can significantly improve the mechanical properties and biological permeability of the hydrogel.
Cell cross-linking
The long-distance and reversible polymer network can be formed because of the occurrence of cells and cell adhesive ligand–modified alginates, such as arginine-glycine-aspartic acid (RGD)-modified alginate (Figure 5D). This system can introduce the cross-link network structure by specific receptor–ligand interactions without any chemical cross-linking agents [147], [152]. It is an appropriate method for gelling within the body because the hybrid solution can transform the solid immediately after reacting with cells in natural tissues.
Application in cartilage regeneration
Alginate hydrogels are synthesized to carry a variety of low molecular weight drugs using primary or secondary bonds. The partially oxidized alginate gels and the amphiphilic alginate gel beads can achieve the control and localized delivery of drugs [153], [154]. The combination of alginate microspheres and HA hydrogel acts as a composite carrier of MSCs and transforming growth factor βeffectively to retain its bioactivity in the scaffold and promote chondrogenesis of MSCs [155]. The alginate grafted by RGD-containing peptides can significantly enhance the adhesive interactions with chondrocytes. Furthermore, the presence of RGD aids in the recovery of hydrogel structure after shear-induced breakdown, thereby controlling the cell phenotype [156]. Except for normal alginate, the oxidized alginate-based hydrogels are commonly used in cartilage repair. Although the elastic modulus is lower than normal alginate-based hydrogel, the oxidized alginate hydrogel demonstrates the better ability to repair the cartilage [157]. Subsequently, chitosan and calcium polyphosphate were added, and the oxidized alginate-based hydrogel exhibited a low degradation rate and high compressive strength. The cell viability and spreading were improved with high calcium polyphosphate content [158]. The gelling time of oxidized alginate-based hydrogel can be shortened by coupling with gelatin and borax. The hydrogel can alleviate the inflammatory effect and generation of reactive oxygen species. The incorporation of dexamethasone, chondroitin sulfate and recombinant mouse platelet–derived growth factor in the hydrogel stimulates the glycosaminoglycan deposition and DNA content [159]. Blending the RGD-modified oxidized alginate, chondrocytes and hyaluronate can form an injectable hydrogel, which expressed the chondrogenesis-related protein and chondrogenic marker gene at 6 weeks post-injection [160]. The gelatin and partially oxidized polysaccharides can gel by self-cross-linking without any cross-linking agents. Based on this phenomenon, the oxidized alginate and oxidized HA can form hydrogels with gelatin by a simple blending operation. Consequently, the swelling degree, cross-linking extent, compressive modulus, viscoelastic behaviors, porous structure and cytocompatibility of the hybrid hydrogels were greatly improved by altering the concentration of the involved components [161].
Conclusions and future perspectives
The effect of cartilage defect repair is related to the deposition and remodeling of the chondrocyte ECM. If the degradation rate is not appropriate, the ECM cannot be deposited in the defect region, thus affecting the cartilage regeneration. Owing to the controlled degradation rate and optimal biocompatibility, the hydrogels are regarded as appropriate tissue engineering materials in cartilage repair. The hydrogel scaffolds containing drugs or cells for promoting the regeneration of cartilage defects were generally introduced in the past decades. Their structures, components, physicochemical properties, gelling methods and mechanical strength can be customized as per the synthesis techniques and fabrication methodologies. For a majority of the natural hydrogels, the modification and gelling methods are universal, such as acetylation, photopolymerization and covalent cross-linking. Herein, we summarized the properties, synthesis and fabrication options of the most commonly used natural macromolecules in cartilage regeneration. Furthermore, the essential concepts and recent discoveries illustrated the achievable goals and the current limitations.
A critical challenge in cartilage tissue engineering promoted the integration of cartilage and subchondral bone regeneration. The structure and modulus are different in the two types of tissue, which causes difficulties in simulating the structure and function using hydrogel scaffolds. The section related to cartilage repair showed adequate elastic modulus to resist the pressure and friction, to promote ECM and chondrogenesis expression of MSCs or chondrocytes and to inhibit the hypertrophic differentiation and mineralization of chondrocytes. The section related to subchondral bone repair promotes blood vessel network formation in the hydrogel to provide nutrient transport, stimulate osteoblast proliferation and provide support for regenerated cartilage. Furthermore, the incorporation of intelligence or self-guided features, such as self-assembling and functional flexibility for dynamic biological demands, is essential in the hydrogel design for cartilage regeneration [162]. This integration of the surrounding cartilage and the implants can be significantly enhanced. In addition, novel microfabrication techniques, such as 3D printing, should be considered for fabricating the hydrogel scaffolds with complex structure and materials.
Conflicts of interest
All the authors declare that there is no conflict of interest related to the present study.
Acknowledgement
This study was supported by the International Cooperation and Exchanges of the National Natural Science Foundation (81420108021), Key Program of the National Natural Science Foundation (81730067), the National Natural Science Foundation (51575100, 51705259), Jiangsu Provincial Key Medical Center Foundation and Jiangsu Provincial Medical Outstanding Talent Foundation.
Contributor Information
Xingsong Wang, Email: xswang@seu.edu.cn.
Qing Jiang, Email: qingj@nju.edu.cn.
References
- 1.Campoccia D., Doherty P., Radice M., Brun P., Abatangelo G., Williams D.F. Semisynthetic resorbable materials from hyaluronan esterification. Biomaterials. 1998;19:2101. doi: 10.1016/s0142-9612(98)00042-8. [DOI] [PubMed] [Google Scholar]
- 2.Prestwich G.D., Marecak D.M., Marecek J.F., Vercruysse K.P., Ziebell M.R. Controlled chemical modification of hyaluronic acid: synthesis, applications, and biodegradation of hydrazide derivatives. J Contr Release. 1998;53:93. doi: 10.1016/s0168-3659(97)00242-3. [DOI] [PubMed] [Google Scholar]
- 3.Mishra R., Basu B., Kumar A. Physical and cytocompatibility properties of bioactive glass-polyvinyl alcohol-sodium alginate biocomposite foams prepared via sol-gel processing for trabecular bone regeneration. J Mater Sci Mater Med. 2009;20:2493–2500. doi: 10.1007/s10856-009-3814-1. [DOI] [PubMed] [Google Scholar]
- 4.Lin H.R., Yeh Y.J. Porous alginate/hydroxyapatite composite scaffolds for bone tissue engineering: preparation, characterization, and in vitro studies. J Biomed Mater Res B Appl Biomater. 2004;71b:52–65. doi: 10.1002/jbm.b.30065. [DOI] [PubMed] [Google Scholar]
- 5.Qiao P., Wang J., Xie Q., Li F., Dong L., Xu T. Injectable calcium phosphate-alginate-chitosan microencapsulated MC3T3-E1 cell paste for bone tissue engineering in vivo. Mater Sci Eng C Mater Biol Appl. 2013;33:4633. doi: 10.1016/j.msec.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Jiang L., Xiong C., Jiang L., Xu L. Effect of HA with different grain size range on the crystallization behaviors and mechanical property of HA/PLGA composite. Thermochim Acta. 2013;565:52–57. [Google Scholar]
- 7.Pahlevanzadeh F., Bakhsheshi-Rad H.R., Hamzah E. In-vitro biocompatibility, bioactivity, and mechanical strength of PMMA-PCL polymer containing fluorapatite and graphene oxide bone cements. J Mech Behav Biomed Mater. 2018;82:257. doi: 10.1016/j.jmbbm.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Wang L., Liu Y., Bao L., Hu D., Zong Y., Tong G. Preparation of acrylamide-based poly-HIPEs with enhanced mechanical strength using PVDBM-b-PEG-emulsified CO2-in-water emulsions. J Appl Polym Sci. 2018;135(23) [Google Scholar]
- 9.Sionkowska A., Skopinska-Wisniewska J., Planecka A., Kozlowska J. The influence of UV irradiation on the properties of chitosan films containing keratin. Polym Degrad Stabil. 2010;95:2486–2491. [Google Scholar]
- 10.Ingber D.E. Cellular mechanotransduction: putting all the pieces together again. Faseb J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 11.Grabiner, Mark D. Basic orthopaedic biomechanics. J Clin Eng. 1998;16 [Google Scholar]
- 12.Räsänen P., Paavolainen P., Sintonen H., Koivisto A.M., Blom M., Ryynänen O.P. Effectiveness of hip or knee replacement surgery in terms of quality-adjusted life years and costs. Acta Orthop. 2007;78:108–115. doi: 10.1080/17453670610013501. [DOI] [PubMed] [Google Scholar]
- 13.Hunziker E.B. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthr Cartil. 2002;10:432. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 14.Wichterle O., Lím D. Hydrophilic gels for biological use. Nature. 1960;185:117–118. [Google Scholar]
- 15.Hoffman A.S. Hydrogels for biomedical applications. Ann N Y Acad Sci. 2001;944:62. doi: 10.1111/j.1749-6632.2001.tb03823.x. [DOI] [PubMed] [Google Scholar]
- 16.Fraser J.R., Laurent T.C., Laurent U.B. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 17.Toole B.P. Hyaluronan in morphogenesis. Semin Cell Dev Biol. 2001;12:79–87. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- 18.Toole B.P. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Canc. 2004;4:528. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 19.Allison D.D., Grande-Allen K.J. Review. Hyaluronan: a powerful tissue engineering tool. Tissue Eng. 2006;12:2131–2140. doi: 10.1089/ten.2006.12.2131. [DOI] [PubMed] [Google Scholar]
- 20.Prestwich G.D. Engineering a clinically-useful matrix for cell therapy. Organogenesis. 2008;4:42. doi: 10.4161/org.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuo J.W. CRC Press; 2005. Practical aspects of hyaluronan based medical products. [Google Scholar]
- 22.Vázquez C.P., Boudou T., Dulong V., Nicolas C., Picart C., Glinel K. Variation of polyelectrolyte film stiffness by photo-cross-linking: a new way to control cell adhesion. Langmuir. 2009;25:3556. doi: 10.1021/la803577t. [DOI] [PubMed] [Google Scholar]
- 23.Pravata L., Braud C., Boustta M., El G.A., Tømmeraas K., Guillaumie F. New amphiphilic lactic acid oligomer-hyaluronan conjugates: synthesis and physicochemical characterization. Biomacromolecules. 2008;9:340. doi: 10.1021/bm700843m. [DOI] [PubMed] [Google Scholar]
- 24.Oldinski R.A., Ruckh T.T., Staiger M.P., Popat K.C., James S.P. Dynamic mechanical analysis and biomineralization of hyaluronan-polyethylene copolymers for potential use in osteochondral defect repair. Acta Biomater. 2011;7:1184–1191. doi: 10.1016/j.actbio.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Palumbo F.S., Pitarresi G., Mandracchia D., Tripodo G., Giammona G. New graft copolymers of hyaluronic acid and polylactic acid: synthesis and characterization. Carbohydr Polym. 2006;66:379–385. [Google Scholar]
- 26.Su W.Y., Chen Y.C., Lin F.H. Injectable oxidized hyaluronic acid/adipic acid dihydrazide hydrogel for nucleus pulposus regeneration. Acta Biomater. 2010;6:3044–3055. doi: 10.1016/j.actbio.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 27.Ma X., Xu T., Chen W., Wang R., Xu Z., Ye Z. Improvement of toughness for the hyaluronic acid and adipic acid dihydrazide hydrogel by PEG. Fibers Polym. 2017;18:817–824. [Google Scholar]
- 28.Lai J.Y., Ma H.K., Cheng H.Y., Sun C.C., Huang S.J., Li Y.T. Ocular biocompatibility of carbodiimide cross-linked hyaluronic acid hydrogels for cell sheet delivery carriers. J Biomater Sci Polym Ed. 2010;21:359–376. doi: 10.1163/156856209X416980. [DOI] [PubMed] [Google Scholar]
- 29.Zhu M., Qian F., Sun Y., Gang L., Bian L. Effect of cartilaginous matrix components on the chondrogenesis and hypertrophy of mesenchymal stem cells in hyaluronic acid hydrogels. J Biomed Mater Res B Appl Biomater. 2017;105 doi: 10.1002/jbm.b.33760. [DOI] [PubMed] [Google Scholar]
- 30.Oldinski R.A., Cranson C.N., James S.P. Synthesis and characterization of a Hyaluronan-polyethylene copolymer for biomedical applications. J Biomed Mater Res B Appl Biomater. 2010;94B:441–446. doi: 10.1002/jbm.b.31672. [DOI] [PubMed] [Google Scholar]
- 31.Shi D., Xu X., Ye Y., Song K., Cheng Y., Di J. Photo-cross-linked scaffold with kartogenin-encapsulated nanoparticles for cartilage regeneration. Sci Found China. 2016;10 doi: 10.1021/acsnano.5b06663. 19-19. [DOI] [PubMed] [Google Scholar]
- 32.Crescenzi V., Cornelio L., Di M.C., Nardecchia S., Lamanna R. Novel hydrogels via click chemistry: synthesis and potential biomedical applications. Biomacromolecules. 2007;8:1844–1850. doi: 10.1021/bm0700800. [DOI] [PubMed] [Google Scholar]
- 33.Han S.S., Hong Y.Y., Ji Y.Y., Cho M.O., Shim H.E., Jeong J.E. In situ cross-linkable hyaluronic acid hydrogels using copper free click chemistry for cartilage tissue engineering. Polym Chem. 2018;9:20–27. [Google Scholar]
- 34.Liu Y., Shu X.Z., Prestwich G.D. Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue Eng. 2006;12:3405. doi: 10.1089/ten.2006.12.3405. [DOI] [PubMed] [Google Scholar]
- 35.Feng Q., Lin S., Zhang K., Dong C., Wu T., Huang H. Sulfated hyaluronic acid hydrogels with retarded degradation and enhanced growth factor retention promote hMSC chondrogenesis and articular cartilage integrity with reduced hypertrophy. Acta Biomater. 2017;53:329–342. doi: 10.1016/j.actbio.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Bian L., Guvendiren M., Mauck R.L., Burdick J.A. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc Natl Acad Sci USA. 2013;110:10117. doi: 10.1073/pnas.1214100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gatta A.L., Ricci G., Stellavato A., Cammarota M., Filosa R., Papa A. Hyaluronan hydrogels with a low degree of modification as scaffolds for cartilage engineering. Int J Biol Macromol. 2017;103:978–989. doi: 10.1016/j.ijbiomac.2017.05.091. [DOI] [PubMed] [Google Scholar]
- 38.Mikael P.E., Kim H.S., Nukavarapu S.P. Hybrid extracellular matrix design for cartilage-mediated bone regeneration. J Biomed Mater Res B Appl Biomater. 2018;106 doi: 10.1002/jbm.b.33842. [DOI] [PubMed] [Google Scholar]
- 39.Broguiere N., Cavalli E., Salzmann G.M., Applegate L.A., Zenobiwong M. Factor XIII cross-linked hyaluronan hydrogels for cartilage tissue engineering. ACS Biomater Sci Eng. 2016;2 doi: 10.1021/acsbiomaterials.6b00378. [DOI] [PubMed] [Google Scholar]
- 40.Fenn S.L., Oldinski R.A. Visible light crosslinking of methacrylated hyaluronan hydrogels for injectable tissue repair. J Biomed Mater Res B Appl Biomater. 2016;104:1229–1236. doi: 10.1002/jbm.b.33476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patterson J., Stayton P.S., Li X. In situ characterization of the degradation of PLGA microspheres in hyaluronic acid hydrogels by optical coherence tomography. IEEE Trans Med Imag. 2009;28:74–81. doi: 10.1109/TMI.2008.927356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei K., Zhu M., Sun Y., Xu J., Feng Q., Lin S. Robust biopolymeric supramolecular “Host−Guest macromer” hydrogels reinforced by in situ formed multivalent nanoclusters for cartilage regeneration. Macromolecules. 2016;49 [Google Scholar]
- 43.Subramanian Anuradha, Vu David, Larsen G., Lin Hsin-Yi. Preparation and evaluation of the electrospun chitosan/PEO fibers for potential applications in cartilage tissue engineering. J Biomater Sci Polym Ed. 2005;16:861–873. doi: 10.1163/1568562054255682. [DOI] [PubMed] [Google Scholar]
- 44.KuciNska-Lipka J., GubaNska I., Janik H. Gelatin-modified polyurethanes for soft tissue scaffold. Sci World J. 2013;2013 doi: 10.1155/2013/450132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.An I.V.D.B., Bogdanov B., Rooze N.D., Schacht E.H., Cornelissen M., Berghmans H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules. 2000;1:31–38. doi: 10.1021/bm990017d. [DOI] [PubMed] [Google Scholar]
- 46.Hoch E., Schuh C., Hirth T., Tovar G.E., Borchers K. Stiff gelatin hydrogels can be photo-chemically synthesized from low viscous gelatin solutions using molecularly functionalized gelatin with a high degree of methacrylation. J Mater Sci Mater Med. 2012;23:2607–2617. doi: 10.1007/s10856-012-4731-2. [DOI] [PubMed] [Google Scholar]
- 47.Bertassoni L.E., Cardoso J.C., Manoharan V., Cristino A.L., Bhise N.S., Araujo W.A. Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication. 2014;6 doi: 10.1088/1758-5082/6/2/024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prasertsung I., Damrongsakkul S., Saito N. Crosslinking of a gelatin solutions induced by pulsed electrical discharges in solutions. Plasma Process Polym. 2014;10:792–797. [Google Scholar]
- 49.Bhat R., Karim A.A. Ultraviolet irradiation improves gel strength of fish gelatin. Food Chem. 2009;113:1160–1164. [Google Scholar]
- 50.Brinkman W.T., Nagapudi K., Thomas B.S., Chaikof E.L. Photo-cross-linking of type I collagen gels in the presence of smooth muscle cells: mechanical properties, cell viability, and function. Biomacromolecules. 2003;4:890–895. doi: 10.1021/bm0257412. [DOI] [PubMed] [Google Scholar]
- 51.Ratanavaraporn J., Rangkupan R., Jeeratawatchai H., Kanokpanont S., Damrongsakkul S. Influences of physical and chemical crosslinking techniques on electrospun type A and B gelatin fiber mats. Int J Biol Macromol. 2010;47:431–438. doi: 10.1016/j.ijbiomac.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 52.Yung C.W., Wu L.Q., Tullman J.A., Payne G.F., Bentley W.E., Barbari T.A. Transglutaminase crosslinked gelatin as a tissue engineering scaffold. J Biomed Mater Res. 2007;83A:1039–1046. doi: 10.1002/jbm.a.31431. [DOI] [PubMed] [Google Scholar]
- 53.Chen T., Embree H.D., Brown E.M., Taylor M.M., Payne G.F. Enzyme-catalyzed gel formation of gelatin and chitosan: potential for in situ applications. Biomaterials. 2003;24:2831. doi: 10.1016/s0142-9612(03)00096-6. [DOI] [PubMed] [Google Scholar]
- 54.Chen T., Janjua R., Mcdermott M.K., Bernstein S.L., Steidl S.M., Payne G.F. Gelatin-based biomimetic tissue adhesive. Potential for retinal reattachment. J Biomed Mater Res B Appl Biomater. 2006;77B:416–422. doi: 10.1002/jbm.b.30439. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto S., Hirata A., Ishikawa S., Ohta K., Nakamura K.I., Okinami S. Feasibility of using gelatin-microbial transglutaminase complex to repair experimental retinal detachment in rabbit eyes. Graefe's Arch Clin Exp Ophthalmol. 2013;251:1109–1114. doi: 10.1007/s00417-012-2245-8. [DOI] [PubMed] [Google Scholar]
- 56.Kuijpers A.J., Engbers G.H., Krijgsveld J., Zaat S.A., Dankert J., Feijen J. Cross-linking and characterisation of gelatin matrices for biomedical applications. J Biomater Sci Polym Ed. 2000;11:225–243. doi: 10.1163/156856200743670. [DOI] [PubMed] [Google Scholar]
- 57.Rose J.B., Pacelli S., Haj A., Dua H.S., Hopkinson A., White L.J. Gelatin-based materials in ocular tissue engineering. Materials. 2014;7:3106. doi: 10.3390/ma7043106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sisson K., Zhang C., Farach-Carson M.C., Chase D.B., Rabolt J.F. Evaluation of cross-linking methods for electrospun gelatin on cell growth and viability. Biomacromolecules. 2009;10:1675–1680. doi: 10.1021/bm900036s. [DOI] [PubMed] [Google Scholar]
- 59.Talebian Kordestani, Rashidi S.S., Dadashian The effect of glutaraldehyde on the properties of gelatin films. Kemija U Industriji. 2007;56:537–541. [Google Scholar]
- 60.Nishi C., Nakajima N., Ikada Y. In vitro evaluation of cytotoxicity of diepoxy compounds used for biomaterial modification. J Biomed Mater Res. 1995;29:829–834. doi: 10.1002/jbm.820290707. [DOI] [PubMed] [Google Scholar]
- 61.Naimark W.A., Pereira C.A., Tsang K., Lee J.M. HMDC crosslinking of bovine pericardial tissue: a potential role of the solvent environment in the design of bioprosthetic materials. J Mater Sci Mater Med. 1995;6:235–241. [Google Scholar]
- 62.Bigi A., Cojazzi G., Panzavolta S., Roveri N., Rubini K. Stabilization of gelatin films by crosslinking with genipin. Biomaterials. 2002;23:4827–4832. doi: 10.1016/s0142-9612(02)00235-1. [DOI] [PubMed] [Google Scholar]
- 63.Bigi A., Cojazzi G., Panzavolta S., Rubini K., Roveri N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials. 2001;22:763–768. doi: 10.1016/s0142-9612(00)00236-2. [DOI] [PubMed] [Google Scholar]
- 64.Suo H., Xu K., Zheng X. Using glucosamine to improve the properties of photocrosslinked gelatin scaffolds. J Biomater Appl. 2015;29:977–987. doi: 10.1177/0885328214551009. [DOI] [PubMed] [Google Scholar]
- 65.Pierce B.F., Tronci G., Rößle M., Neffe A.T., Jung F., Lendlein A. Photocrosslinked Co-networks from glycidylmethacrylated gelatin and poly(ethylene glycol) methacrylates. Macromol Biosci. 2012;12:484–493. doi: 10.1002/mabi.201100232. [DOI] [PubMed] [Google Scholar]
- 66.Loessner D., Meinert C., Kaemmerer E., Martine L.C., Yue K., Levett P.A. Functionalization, preparation and use of cell-laden gelatin methacryloyl-based hydrogels as modular tissue culture platforms. Nat Protoc. 2016;11:727–746. doi: 10.1038/nprot.2016.037. [DOI] [PubMed] [Google Scholar]
- 67.Tsang K.M., Annabi N., Ercole F., Zhou K., Karst D., Li F. Facile one-step micropatterning using photodegradable methacrylated gelatin hydrogels for improved cardiomyocyte organization and alignment. Adv Funct Mater. 2015;25:977–986. doi: 10.1002/adfm.201403124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ovsianikov A., Deiwick A., Vlierberghe S.V., Dubruel P., Möller L., Dräger G. Laser fabrication of three-dimensional CAD scaffolds from photosensitive gelatin for applications in tissue engineering. Biomacromolecules. 2011;12:851–858. doi: 10.1021/bm1015305. [DOI] [PubMed] [Google Scholar]
- 69.Koroleva A., Deiwick A., Nguyen A., Schliewolter S., Narayan R., Timashev P. Osteogenic differentiation of human mesenchymal stem cells in 3-D Zr-Si organic-inorganic scaffolds produced by two-photon polymerization technique. PLoS One. 2015;10 doi: 10.1371/journal.pone.0118164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eid M., Abdel-Ghaffar M.A., Dessouki A.M. Effect of maleic acid content on the thermal stability, swelling behaviour and network structure of gelatin-based hydrogels prepared by gamma irradiation. Nucl Instrum Methods Phys Res. 2009;267:91–98. [Google Scholar]
- 71.Geng X., Mo X., Fan L., Yin A., Fang J. Hierarchically designed injectable hydrogel from oxidized dextran, amino gelatin and 4-arm poly(ethylene glycol)-acrylate for tissue engineering application. J Mater Chem. 2012;22:25130–25139. [Google Scholar]
- 72.Oh B.H., Bismarck A., Chanpark M.B. Injectable, interconnected, high-porosity macroporous biocompatible gelatin scaffolds made by surfactant-free emulsion templating. Macromol Rapid Commun. 2015;36:364–372. doi: 10.1002/marc.201400524. [DOI] [PubMed] [Google Scholar]
- 73.Feng Q., Wei K., Lin S., Xu Z., Sun Y., Shi P. Mechanically resilient, injectable, and bioadhesive supramolecular gelatin hydrogels crosslinked by weak host-guest interactions assist cell infiltration and in situ tissue regeneration. Biomaterials. 2016;101:217–228. doi: 10.1016/j.biomaterials.2016.05.043. [DOI] [PubMed] [Google Scholar]
- 74.Zheng X., Zhang Q., Liu J., Pei Y., Tang K. A unique high mechanical strength dialdehyde microfibrillated cellulose/gelatin composite hydrogel with giant network structure. RSC Adv. 2016;6 [Google Scholar]
- 75.Bang S., Jung U.W., Noh I. Synthesis and biocompatibility characterizations of in situ chondroitin sulfate–gelatin hydrogel for tissue engineering. Tissue Eng Regen Med. 2018;15:25–35. doi: 10.1007/s13770-017-0089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miao Z., Lu Z., Wu H., Liu H., Li M., Lei D. Collagen, agarose, alginate and matrigel hydrogels as cell substrates for culture of chondrocytes in vitro: a comparative study. J Cell Biochem. 2018;119:7924–7933. doi: 10.1002/jcb.26411. [DOI] [PubMed] [Google Scholar]
- 77.Diamond A.M., Gorham S.D., Etherington D.J., Robertson J.G., Light N.D. The effect of modification on the susceptibility of collagen to proteolysis: I. Chemical modification of amino acid side chains. Matrix. 1991;11:321. doi: 10.1016/s0934-8832(11)80203-9. [DOI] [PubMed] [Google Scholar]
- 78.Stoichevska V., An B., Peng Y.Y., Yigit S., Vashi A.V., Kaplan D.L. Formation of multimers of bacterial collagens through introduction of specific sites for oxidative crosslinking. J Biomed Mater Res. 2016;104:2369–2376. doi: 10.1002/jbm.a.35772. [DOI] [PubMed] [Google Scholar]
- 79.Hulmes D.J.S. Springer US; 2008. Collagen diversity, synthesis and assembly. [Google Scholar]
- 80.Brzuszkiewicz-Zarnowska H. Glutamic acid participation in the process of collagen biosynthesis. Bull Acad Pol Sci Ser Sci Biol. 1971;19:167. [PubMed] [Google Scholar]
- 81.Li P., Wu G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids. 2017;50:1–10. doi: 10.1007/s00726-017-2490-6. [DOI] [PubMed] [Google Scholar]
- 82.Li J., Ren N., Qiu J., Jiang H., Zhao H., Wang G. Carbodiimide crosslinked collagen from porcine dermal matrix for high-strength tissue engineering scaffold. Int J Biol Macromol. 2013;61:69. doi: 10.1016/j.ijbiomac.2013.06.038. [DOI] [PubMed] [Google Scholar]
- 83.Salehi-Nik N., Amoabediny G., Shokrgozar M.A., Mottaghy K., Klein-Nulend J., Zandieh-Doulabi B. Surface modification of silicone tubes by functional carboxyl and amine, but not peroxide groups followed by collagen immobilization improves endothelial cell stability and functionality. Biomed Mater. 2015;10 doi: 10.1088/1748-6041/10/1/015024. [DOI] [PubMed] [Google Scholar]
- 84.Matsumura K., Hyon S.N., Peng C., Tsutsumi S. Surface modification of poly(ethylene-co-vinyl alcohol) (EVA). Part I. Introduction of carboxyl groups and immobilization of collagen. J Biomed Mater Res A B. 2000;50:512–517. doi: 10.1002/(sici)1097-4636(20000615)50:4<512::aid-jbm6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 85.Friess W. Collagen–biomaterial for drug delivery. Eur J Pharm Biopharm. 1998;45:113. doi: 10.1016/s0939-6411(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 86.Gorham S.D., Light N.D., Diamond A.M., Willins M.J., Bailey A.J., Wess T.J. Effect of chemical modifications on the susceptibility of collagen to proteolysis. II. Dehydrothermal crosslinking. Int J Biol Macromol. 1992;14:129–138. doi: 10.1016/s0141-8130(05)80002-9. [DOI] [PubMed] [Google Scholar]
- 87.Weadock K.S., Miller E.J., Keuffel E.L., Dunn M.G. Effect of physical crosslinking methods on collagen-fiber durability in proteolytic solutions. J Biomed Mater Res. 1996;32:221. doi: 10.1002/(SICI)1097-4636(199610)32:2<221::AID-JBM11>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 88.Gratzer P.F., Pereira C.A., Lee J.M. Solvent environment modulates effects of glutaraldehyde crosslinking on tissue-derived biomaterials. J Biomed Mater Res. 1996;31:533–543. doi: 10.1002/(SICI)1097-4636(199608)31:4<533::AID-JBM14>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 89.Wang L., Stegemann J.P. Glyoxal crosslinking of cell-seeded chitosan/collagen hydrogels for bone regeneration. Acta Biomater. 2011;7:2410–2417. doi: 10.1016/j.actbio.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu Y., Ma L., Gao C. Facile fabrication of the glutaraldehyde cross-linked collagen/chitosan porous scaffold for skin tissue engineering. Mater Sci Eng C. 2012;32:2361–2366. [Google Scholar]
- 91.Sionkowska A., Kozłowska J. Properties and modification of porous 3-D collagen/hydroxyapatite composites. Int J Biol Macromol. 2013;52:250–259. doi: 10.1016/j.ijbiomac.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 92.Liu C., Li T., Yang Z., Liu D., Li Y., Zhou Z. Kartogenin enhanced chondrogenesis in co-cultures of chondrocytes and bone mesenchymal stem cells. Tissue Eng Part A. 2018;24(11–12):990–1000. doi: 10.1089/ten.TEA.2017.0162. [DOI] [PubMed] [Google Scholar]
- 93.Kuwahara K., Yang Z., Slack G.C., Nimni M.E., Han B. Cell delivery using an injectable and adhesive transglutaminase-gelatin gel. Tissue Eng C Meth. 2010;16:609. doi: 10.1089/ten.TEC.2009.0406. [DOI] [PubMed] [Google Scholar]
- 94.Yuan L., Li B., Yang J., Ni Y., Teng Y., Guo L. Effects of composition and mechanical property of injectable collagen I/II composite hydrogels on chondrocytes behaviors. Tissue Eng. 2016;22:899. doi: 10.1089/ten.TEA.2015.0513. [DOI] [PubMed] [Google Scholar]
- 95.Kontturi L.S., Järvinen E., Muhonen V., Collin E.C., Pandit A.S., Kiviranta I. An injectable, in situ forming type II collagen/hyaluronic acid hydrogel vehicle for chondrocyte delivery in cartilage tissue engineering. Drug Deliv Transl Res. 2014;4:149–158. doi: 10.1007/s13346-013-0188-1. [DOI] [PubMed] [Google Scholar]
- 96.Yang X., Lu Z., Wu H., Li W., Zheng L., Zhao J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater Sci Eng C Mater Biol Appl. 2018;83:195. doi: 10.1016/j.msec.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 97.J X., L J., L Q., L Z., Z L., Z J. Therapy for cartilage defects: functional ectopic cartilage constructed by cartilage-simulating collagen, chondroitin sulfate and hyaluronic acid (CCH) hybrid hydrogel with allogeneic chondrocytes. Biomater Sci. 2018;6(6):1616–1626. doi: 10.1039/c8bm00354h. [DOI] [PubMed] [Google Scholar]
- 98.Heo J., Koh R.H., Shim W., Kim H.D., Yim H.G., Hwang N.S. Riboflavin-induced photo-crosslinking of collagen hydrogel and its application in meniscus tissue engineering. Drug Deliv Transl Res. 2016;6:148–158. doi: 10.1007/s13346-015-0224-4. [DOI] [PubMed] [Google Scholar]
- 99.Kato Y., Onishi H., Machida Y. Application of chitin and chitosan derivatives in the pharmaceutical field. Curr Pharmaceut Biotechnol. 2003;4:303–309. doi: 10.2174/1389201033489748. [DOI] [PubMed] [Google Scholar]
- 100.Molinaro G., Leroux J.C., Damas J., Adam A. Biocompatibility of thermosensitive chitosan-based hydrogels: an in vivo experimental approach to injectable biomaterials. Biomaterials. 2002;23:2717–2722. doi: 10.1016/s0142-9612(02)00004-2. [DOI] [PubMed] [Google Scholar]
- 101.Jayakumar R., Prabaharan M., Reis R.L., Mano J.F. Graft copolymerized chitosan--present status and applications. Carbohydr Polym. 2005;62:142–158. [Google Scholar]
- 102.De B.D., de Assis O.B. Synthesis and mechanical properties of quaternary salts of chitosan-based films for food application. Int J Biol Macromol. 2007;41:198. doi: 10.1016/j.ijbiomac.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 103.Bhattarai N., Gunn J., Zhang M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev. 2010;62:83–99. doi: 10.1016/j.addr.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 104.Thanou M.M., Kotzé A.F., Scharringhausen T., Luessen H.L., de Boer A.G., Verhoef J.C. Effect of degree of quaternization of N-trimethyl chitosan chloride for enhanced transport of hydrophilic compounds across intestinal caco-2 cell monolayers. J Contr Release. 2000;64:15–25. doi: 10.1016/s0168-3659(99)00131-5. [DOI] [PubMed] [Google Scholar]
- 105.Shu X.Z., Zhu K.J. Controlled drug release properties of ionically cross-linked chitosan beads: the influence of anion structure. Int J Pharm. 2002;233:217–225. doi: 10.1016/s0378-5173(01)00943-7. [DOI] [PubMed] [Google Scholar]
- 106.Shen E.C., Wang C., Fu E., Chiang C.Y., Chen T.T., Nieh S. Tetracycline release from tripolyphosphate–chitosan cross-linked sponge: a preliminary in vitro study. J Periodontal Res. 2008;43:642. doi: 10.1111/j.1600-0765.2007.01045.x. [DOI] [PubMed] [Google Scholar]
- 107.Brack H.P., Tirmizi S.A., Jr W.M.R. A spectroscopic and viscometric study of the metal ion-induced gelation of the biopolymer chitosan. Polymer. 1997;38:2351–2362. [Google Scholar]
- 108.Dambies L., Vincent T., Domard A., Guibal E. Preparation of chitosan gel beads by ionotropic molybdate gelation. Biomacromolecules. 2001;2:1198–1205. doi: 10.1021/bm010083r. [DOI] [PubMed] [Google Scholar]
- 109.Berger J., Reist M., Mayer J.M., Felt O., Peppas N.A., Gurny R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur J Pharm Biopharm. 2004;57:19. doi: 10.1016/s0939-6411(03)00161-9. [DOI] [PubMed] [Google Scholar]
- 110.Bhattarai N., Gunn J., Zhang M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev. 2010;62:83–99. doi: 10.1016/j.addr.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 111.Tsuchida E., Abe K. Interactions between macromolecules in solution and intermacromolecular complexes. Adv Polym Sci. 1982;45:1–119. [Google Scholar]
- 112.Hoare T.R., Kohane D.S. Hydrogels in drug delivery: progress and challenges. Polymer. 2008;49:1993–2007. [Google Scholar]
- 113.Hennink W.E., Nostrum C.F.V. Novel crosslinking methods to design hydrogels. Adv Drug Deliv Rev. 2012;64:223–236. doi: 10.1016/s0169-409x(01)00240-x. [DOI] [PubMed] [Google Scholar]
- 114.Sung H.W., Liang I.L., Chen C.N., Huang R.N., Liang H.F. Stability of a biological tissue fixed with a naturally occurring crosslinking agent (genipin) J Biomed Mater Res. 2001;55:538–546. doi: 10.1002/1097-4636(20010615)55:4<538::aid-jbm1047>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 115.Tan H., Rubin J.P., Marra K.G. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for adipose tissue regeneration. Organogenesis. 2010;6:173–180. doi: 10.4161/org.6.3.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ono K., Saito Y., Yura H., Ishikawa K., Kurita A., Akaike T. Photocrosslinkable chitosan as a biological adhesive. J Biomed Mater Res A. 2000;49:289–295. doi: 10.1002/(sici)1097-4636(200002)49:2<289::aid-jbm18>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 117.Yeo Y., Geng W., Ito T., Kohane D.S., Burdick J.A., Radisic M. Photocrosslinkable hydrogel for myocyte cell culture and injection. J Biomed Mater Res B Appl Biomater. 2007;81B:312–322. doi: 10.1002/jbm.b.30667. [DOI] [PubMed] [Google Scholar]
- 118.Yoo H.S. Photo-cross-linkable and thermo-responsive hydrogels containing chitosan and pluronic for sustained release of human growth hormone (hGH) J Biomater Sci Polym Ed. 2007;18:1429. doi: 10.1163/156856207782246803. [DOI] [PubMed] [Google Scholar]
- 119.Lukaszczyk J., Rmiga M., Jaszcz K., Adler H.J., Jähne E., Kaczmarek M. Evaluation of oligo(ethylene glycol) dimethacrylates effects on the properties of new biodegradable bone cement compositions. Macromol Biosci. 2005;5:64. doi: 10.1002/mabi.200400135. [DOI] [PubMed] [Google Scholar]
- 120.Sakai S., Yamada Y., Zenke T., Kawakami K. Novel chitosan derivative soluble at neutral pH and in-situ gellable via peroxidase-catalyzed enzymatic reaction. J Mater Chem. 2008;19:230–235. [Google Scholar]
- 121.Wang L.S., Chung J.E., Chan P.P., Kurisawa M. Injectable biodegradable hydrogels with tunable mechanical properties for the stimulation of neurogenesic differentiation of human mesenchymal stem cells in 3D culture. Biomaterials. 2010;31:1148–1157. doi: 10.1016/j.biomaterials.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 122.Chen T., Embree H.D., Wu L.Q., Payne G.F. In vitro protein-polysaccharide conjugation: tyrosinase-catalyzed conjugation of gelatin and chitosan. Biopolymers. 2002;64:292–302. doi: 10.1002/bip.10196. [DOI] [PubMed] [Google Scholar]
- 123.Islam A., Yasin T., Gull N., Khan S.M., Munawar M.A., Shafiq M. Evaluation of selected properties of biocompatible chitosan/poly(vinyl alcohol) blends. Int J Biol Macromol. 2016;82:551–556. doi: 10.1016/j.ijbiomac.2015.09.073. [DOI] [PubMed] [Google Scholar]
- 124.Naderimeshkin H., Andreas K., Matin M.M., Sittinger M., Bidkhori H.R., Ahmadiankia N. Chitosan-based injectable hydrogel as a promising in situ forming scaffold for cartilage tissue engineering. Cell Biol Int. 2014;38:72–84. doi: 10.1002/cbin.10181. [DOI] [PubMed] [Google Scholar]
- 125.Lou T., Wang X., Yan X., Miao Y., Long Y.Z., Yin H.L. Fabrication and biocompatibility of poly(l-lactic acid) and chitosan composite scaffolds with hierarchical microstructures. Mater Sci Eng C Mater Biol Appl. 2016;64:341–345. doi: 10.1016/j.msec.2016.03.107. [DOI] [PubMed] [Google Scholar]
- 126.Tsiourvas D., Tsetsekou A., Papavasiliou A., Arkas M., Boukos N. A novel hybrid sol–gel method for the synthesis of highly porous silica employing hyperbranched poly(ethyleneimine) as a reactive template. Microporous Mesoporous Mater. 2013;175:59–66. [Google Scholar]
- 127.Piątkowski M., Janus Ł., Radwanpragłowska J., Bogdał D., Matysek D. Biodegradable, pH-sensitive chitosan beads obtained under microwave radiation for advanced cell culture. Colloids Surf B Biointerfaces. 2018;164:324–331. doi: 10.1016/j.colsurfb.2018.01.061. [DOI] [PubMed] [Google Scholar]
- 128.Kamoun E.A. N-succinyl chitosan–dialdehyde starch hybrid hydrogels for biomedical applications. J Adv Res. 2016;7:69–77. doi: 10.1016/j.jare.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Il'Ina A.V., Varlamov V.P. Chitosan-based polyelectrolyte complexes: a review. Appl Biochem Microbiol. 2005;41:9. [PubMed] [Google Scholar]
- 130.Berger J., Reist M., Mayer J.M., Felt O., Gurny R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur J Pharm Biopharm. 2004;57:35–52. doi: 10.1016/s0939-6411(03)00160-7. [DOI] [PubMed] [Google Scholar]
- 131.Hamman J.H. Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems. Mar Drugs. 2010;8:1305–1322. doi: 10.3390/md8041305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kolanthai E., As P., Khajuria D.K., Veerla S.C., Kuppuswamy D., Catalani L.H. Graphene oxide-A tool for preparation of chemically crosslinking free alginate-chitosan-collagen scaffold for bone tissue engineering. Acs Appl Mater Interfaces. 2018;10(15):12441–12452. doi: 10.1021/acsami.8b00699. [DOI] [PubMed] [Google Scholar]
- 133.Sang H.C., Mooklim S., Dong K.H., Hongyuk S., Ilim G., Jin H.L. Time-dependent alginate/polyvinyl alcohol hydrogels as injectable cell carriers. J Biomater Sci Polym Ed. 2009;20:863–876. doi: 10.1163/156856209X444312. [DOI] [PubMed] [Google Scholar]
- 134.Cho S.H., Oh S.H., Lee J.H. Fabrication and characterization of porous alginate/polyvinyl alcohol hybrid scaffolds for 3D cell culture. J Biomater Sci Polym Ed. 2005;16:933–947. doi: 10.1163/1568562054414658. [DOI] [PubMed] [Google Scholar]
- 135.El K.D., Goff H.D., Berengut S., Kubant R., Anderson G.H. Effect of sodium alginate addition to chocolate milk on glycemia, insulin, appetite and food intake in healthy adult men. Eur J Clin Nutr. 2014;68:613. doi: 10.1038/ejcn.2014.53. [DOI] [PubMed] [Google Scholar]
- 136.Zehnder T., Sarker B., Boccaccini A.R., Detsch R. Evaluation of an alginate-gelatine crosslinked hydrogel for bioplotting. Biofabrication. 2015;7 doi: 10.1088/1758-5090/7/2/025001. [DOI] [PubMed] [Google Scholar]
- 137.Schweiger R.G. Acetylation of alginic acid. I. Preparation and viscosities of algin acetates. J Org Chem. 1962;27 [Google Scholar]
- 138.Schweiger R.G. Acetylation of alginic acid. II. Reaction of algin acetates with calcium and other divalent ions. J Org Chem. 1962;27 [Google Scholar]
- 139.Speakman J.B. Alginic acid diacetate. Nature. 1946;158 553-553. [Google Scholar]
- 140.Skjåk-Bræk G., Zanetti F., Paoletti S. Effect of acetylation on some solution and gelling properties of alginates. Carbohydr Res. 1989;185:131–138. [Google Scholar]
- 141.Freeman I., Kedem A., Cohen S. The effect of sulfation of alginate hydrogels on the specific binding and controlled release of heparin-binding proteins. Biomaterials. 2008;29:3260–3268. doi: 10.1016/j.biomaterials.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 142.Huang R., Du Y., Yang J. Preparation and in vitro anticoagulant activities of alginate sulfate and its quaterized derivatives. Carbohydr Polym. 2003;52:19–24. [Google Scholar]
- 143.Pawar S.N., Edgar K.J. Alginate derivatization: a review of chemistry, properties and applications. Biomaterials. 2012;33:3279–3305. doi: 10.1016/j.biomaterials.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 144.Rogers C.J., Clark P.M., Tully S.E., Abrol R., Garcia K.C., Hsieh-Wilson L.C. Elucidating glycosaminoglycan-protein-protein interactions using carbohydrate microarray and computational approaches. Proc Natl Acad Sci U S A. 2011;108:9747–9752. doi: 10.1073/pnas.1102962108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Carré M.C., Delestre C., Hubert P., Dellacherie E. Covalent coupling of a short polyether on sodium alginate: synthesis and characterization of the resulting amphiphilic derivative. Carbohydr Polym. 1991;16:367–379. [Google Scholar]
- 146.Grant G.T., Morris E.R., Rees D.A., Smith P.J.C., Thom D. Biological interactions between polysaccharides and divalent cations: the egg-box model. FEBS Lett. 1973;32:195–198. [Google Scholar]
- 147.Lee K.Y., Mooney D.J. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37:106. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kuo C., Ma P. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part 1. Structure, gelation rate and mechanical properties. Biomaterials. 2001;22:511–521. doi: 10.1016/s0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 149.Zhao X., Huebsch N., Mooney D.J., Suo Z. Stress-relaxation behavior in gels with ionic and covalent crosslinks. J Appl Phys. 2010;107:1869. doi: 10.1063/1.3343265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Smeds K.A., Pfister-Serres A., Miki D., Dastgheib K., Inoue M., Hatchell D.L. Photocrosslinkable polysaccharides for in situ hydrogel formation. J Biomed Mater Res B Appl Biomater. 2001;54:115–121. doi: 10.1002/1097-4636(200101)54:1<115::aid-jbm14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 151.Lu M.Z., Lan H.L., Wang F.F., Chang S.J., Wang Y.J. Cell encapsulation with alginate and alpha-phenoxycinnamylidene-acetylated poly(allylamine) Biotechnol Bioeng. 2000;70:479–483. doi: 10.1002/1097-0290(20001205)70:5<479::aid-bit1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 152.Lee K.Y., Kong H.J., Larson R.G., Mooney D.J. Hydrogel formation via cell crosslinking. Adv Mater. 2003;15:1828–1832. [Google Scholar]
- 153.Bouhadir K.H., Alsberg E., Mooney D.J. Hydrogels for combination delivery of antineoplastic agents. Biomaterials. 2001;22:2625–2633. doi: 10.1016/s0142-9612(01)00003-5. [DOI] [PubMed] [Google Scholar]
- 154.Colinet I., Dulong V., Mocanu G., Picton L., Le C.D. New amphiphilic and pH-sensitive hydrogel for controlled release of a model poorly water-soluble drug. Eur J Pharm Biopharm. 2009;73:345–350. doi: 10.1016/j.ejpb.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 155.Bian L., Zhai D.Y., Tous E., Rai R., Mauck R.L., Burdick J.A. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials. 2011;32:6425–6434. doi: 10.1016/j.biomaterials.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Park H., Kang S.W., Kim B.S., Mooney D.J., Lee K.Y. Shear-reversibly crosslinked alginate hydrogels for tissue engineering. Macromol Biosci. 2009;9:895–901. doi: 10.1002/mabi.200800376. [DOI] [PubMed] [Google Scholar]
- 157.Bouhadir K.H., Lee K.Y., Alsberg E., Damm K.L., Anderson K.W., Mooney D.J. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol Prog. 2001;17:945–950. doi: 10.1021/bp010070p. [DOI] [PubMed] [Google Scholar]
- 158.Wang J., Fu W., Zhang D., Yu X., Li J., Wan C. Evaluation of novel alginate dialdehyde cross-linked chitosan/calcium polyphosphate composite scaffolds for meniscus tissue engineering. Carbohydr Polym. 2010;79:705–710. [Google Scholar]
- 159.Balakrishnan B., Joshi N., Jayakrishnan A., Banerjee R. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater. 2014;10:3650–3663. doi: 10.1016/j.actbio.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 160.Park H., Lee K.Y. Cartilage regeneration using biodegradable oxidized alginate/hyaluronate hydrogels. J Biomed Mater Res. 2014;102:4519. doi: 10.1002/jbm.a.35126. [DOI] [PubMed] [Google Scholar]
- 161.Khorshidi S., Karkhaneh A. A self-crosslinking tri-component hydrogel based on functionalized polysaccharides and gelatin for tissue engineering applications. Mater Lett. 2016;164:468–471. [Google Scholar]
- 162.Li R., Xu J., Wong D., Li J., Zhao P., Bian L. Self-assembled N-cadherin mimetic peptide hydrogels promote the chondrogenesis of mesenchymal stem cells through inhibition of canonical Wnt/β-catenin signaling. Biomaterials. 2017;145:33–43. doi: 10.1016/j.biomaterials.2017.08.031. [DOI] [PubMed] [Google Scholar]