Abstract
Background/Objectives
Decalcification of bone specimens is necessary for routine paraffin embedding and sectioning. Ethylenediaminetetraacetic acid (EDTA), a chelating agent for decalcification, maintains bone tissue integrity and histological features but requires long decalcification period, especially for cortical bone with dense mineral matrix. We hypothesised that the application of a newly commercially available ultrasound (US) decalcifier would accelerate decalcification of thick cortical bone specimen in EDTA efficiently and that the working temperature at 30–45°C would not affect histological and immunohistochemical analysis. Comparison was made with traditional decalcification method with regards to quality of tissue morphology and antigenicity.
Methods
A fresh human cadaveric femoral shaft was sectioned into 5-mm-thick transverse sections. After fixation, the bone slices were divided into two groups: Ultrasound decalcification group (US DeCal), in which bone sections (n = 3) were placed in a US decalcifier (50 W at a frequency of 40kHz) with EDTA solution, and normal decalcification group (Normal DeCal), in which bone sections (n = 3) were decalcified in EDTA without US. The mineral content of the bone sections was measured with micro-computed tomography and dual-energy X-ray absorptiometry at different time points. Rate of calcium extraction was quantified by measuring the calcium concentration in EDTA solution using inductively coupled plasma optical emission spectrometry. After decalcification, the paraffin sections of the decalcified bone were stained with haematoxylin and eosin or immunohistochemical staining of sclerostin.
Results
Samples in US DeCal contained 2.9 ± 2.8% of the mineral content at Day 6 and were completely decalcified at Day 8. However, sections in Normal DeCal retained 36.3 ± 5.1% and 24.3 ± 4.8% at Day 6 and Day 8, respectively, and took six times longer to complete decalcification. The concentration of calcium in the EDTA solution of the US DeCal group was 70% higher than that of the Normal DeCal group (p < 0.05) in Day 1 and 2. No staining difference was observed in histological sections between the two groups.
Conclusion
The application of US decalcification significantly shortened the decalcification time in EDTA without causing histological artefacts.
The translational potential of this article
This article shows that the application of ultrasound in sample decalcification would shorten the duration that decalcification required. This would accelerate the sample processing for routine bone histology in both basic and clinical research and assessments for diagnostic purposes.
Keywords: Bone histology, Bone histomorphometry, Decalcification, Immunohistochemistry, Ultrasound
Introduction
Bone is a composite material consisting of organic matrix, inorganic minerals, cells and water [1], [2]. In cortical bone, the organic matrix and cells account for 40% of the dry weight of bone tissue, water accounts for 5–8% and inorganic mineral content makes up the remainder and presents in the form of hydroxyapatite (HA) (Ca10(PO4)6(OH)2) crystals [1]. The crystals are aligned along the axis of the matrix collagen fibrils and reinforce the collagen matrix. Decalcification is a process to remove the HA from organic matrix to soften the bone specimen that takes long time and therefore unfavourable for both laboratory studies and clinical diagnosis. The most common chemical process [represented by Equation (1)] for removal of HA is by ionisation in acids or by chelation [2].
| (1) |
After decalcification, the bone samples would be compatible with routine paraffin histologic preparation. However, the traditional decalcification method requires long incubation time for this process to get completed. The duration of the decalcification process is dependent on several factors including the thickness of the sample, type and concentration of decalcifying agent, the temperature at which the decalcification process takes place and the presence or absence of any mechanical agitation such as shaking and stirring [3].
Decalcification is performed usually with two categories of decalcifying agents, namely acids and chelating agents. The use of strong acids, high concentration of acids and high temperature would increase the speed of decalcification, but the tissue might be damaged during the decalcification process. Ethylenediaminetetraacetic acid (EDTA) is a chelating agent that reacts with calcium by binding with the ionised calcium on the outer layer of the apatite crystal. EDTA has no effect on the surrounding tissue or tissue depleted of calcium [2], [4]. This decalcifying agent preserves the tissue integrity and histological features [5]. Therefore, EDTA is used as the decalcifying agent when immunohistochemical staining is anticipated or when the tissue is a composite of both bone and soft tissue. However, the limitation of EDTA is the longer decalcification duration than acids [2], [5], [6].
There are different methods to shorten the decalcification duration, including microwave radiation [7] and ultrasound (US) [8], [9], [10]. The application of US has been reported to reduce the decalcification time in smaller animal bone samples [10], [11]. US is believed to enhance the decalcification by a cavitation mechanism [11], [12] by destroying the boundary layers that can impede the decalcification process [13]. However, US has thermal effect which increases the temperature of decalcification solutions. The working temperature of a newly commercially available decalcifier ranges between 30–45°C while it has been reported that temperature above 37°C would increase the risk of tissue swelling and tissue digestion [2]. The present study investigated the efficacy of US on enhancing decalcification speed of large cortical bone specimens, 5-mm thick, with EDTA, a slow decalcifying agent that preserves the histological features of bone. Accordingly, we hypothesised that the application of the newly commercially available US decalcifier would accelerate the decalcification of thick cortical bone sample in EDTA compared with the conventional decalcification method without damaging bone tissue for histological assessments.
Materials and methods
A piece of fresh human femur (Donor ID: L130791) was obtained from Science Care (Arizona, USA). All soft tissue surrounding the femur was removed, and the femur was sawed into proximal, shaft and distal portions. Consecutive sections were prepared and selected for quantitative study to avoid potential variations in shape, cross-sectional area bone mineral density (BMD) [14]. The femoral shaft was sectioned into 5-mm-thick transverse sections because slices thicker than 5mm were not recommended for achieving complete decalcification [2], [14]. Three 5-mm-thick transverse sections were also prepared from a human femoral shaft. The bone slices from the femoral shaft were fixed in 4% phosphate-buffered paraformaldehyde for 24 h. Fixed slices of human femur were randomly divided into two groups: ultrasound decalcification group (US DeCal) and normal decalcification group (Normal DeCal). For US DeCal, the bone sections (n = 3) were place in a US decalcifier (DeCa DX100; Pro-Cure Medical Technology Co. Ltd, Hong Kong, 50 W at a frequency of 40kHz) with 300mL of 0.5 M, pH 7.4, EDTA solution and maintained at 30–45°C. For the Normal DeCal, bone slices (n = 3) were placed in a glass container with 300mL of 0.5 M EDTA and were then placed in a special sample storage room with temperature set at 37°C to avoid temperature-dependent variation in decalcification reaction [6]. All EDTA solution was refreshed daily, and a sample of the EDTA solution would be taken to measure the calcium concentration in the solution. The endpoint of the decalcification was determined radiologically by taking a radiograph of the sample using an X-ray machine for small animals (Faxitron X-ray Corp, Wheeling, Illinois). Human bone sections were place in a US decalcifier (DeCa DX100, Pro-Cure Medical Technology Co. Ltd, 50 W at a frequency of 40kHz) with 300mL of distilled water, maintained at 30–45°C for 6 days.
Quantitative measurements of the mineralised tissue
The volume of mineralised tissue of the bone sections at various time points was measured using a high-resolution micro-computed tomography (microCT) (μCT-40; Scanco Medical, Bruttisellen, Switzerland), whereas the bone mineral content (BMC) and BMD of the bone sections were measured with dual-energy X-ray absorptiometry (DXA) according to an established protocol [11]. The bone sections were placed in a sample holder with their longitudinal axes in the vertical position. Each section was then scanned at an isotropic resolution of 20 μm3. The data were then convoluted using a three-dimensional (3D) Gaussian filter with a width and support equal to 1.2 and 2, respectively. The mineralised tissue was segmented from the marrow and soft tissue for subsequent analysis using a global threshold that was equalled to 165. Values equal to or greater than the threshold represented bone tissue, whereas values below the threshold represented bone marrow and soft tissue [15]. Bone volume (BV), bone volume density (BV density), tissue volume (TV) and the ratio of bone volume to tissue volume (BV/TV) were measured and evaluated. Real BMD and BMC were measured using routine clinical DXA (Norland XR46; Norland, Fort Atkinson, WI, USA) at different time points.
Inductively coupled plasma optical emission spectrometry
At different time points, the decalcification solution of 1mL was collected for measuring the concentration of calcium ions by inductively coupled plasma optical emission spectrometry (Optima 8000; PerkinElmer, USA). For details, the collected decalcification solution was lyophilised to remove all the water content. Then, the remaining particles from the EDTA solution were digested with concentrated HNO3 to remove the EDTA and any organic matter. After thorough digestion, a 5001% HNO3 was added to the remaining particles to make up to a total volume of 5mL for the inductively coupled plasma optical emission spectrometry analysis. The analytical wavelength for calcium ions was set at 396.8nm [16]. The actual concentration of calcium ion was then calculated from the standard curve for statistical analysis.
Histology
After complete decalcification of the bone slices with EDTA, the samples were embedded in paraffin, sectioned at 5μm and stained with haematoxylin and eosin using a standard histological protocol [6], [11]. The stained sections of both the groups were then observed under a light microscope for assessment of cell morphology and to test whether any artefacts or detrimental effects were introduced into the tissues during the decalcification. The ratio of cells with nucleus to total number of cells was measured within a region of interest of mm by mm at a 200× magnification to determine whether the samples had been over decalcified because over decalcification would cause problems with nuclear staining and lead to osteocyte retraction in bone sections [3], [5].
Immunohistochemistry
Immunohistochemical staining was performed according to an established protocol [11]. Briefly, after removal of paraffin and rehydration, the sections were quenched in 3% hydrogen peroxide for 20 min and then submerged in 10mM citric acid for 10 min at 65°C for antigen retrieval. Blocking solution (UltraVision Protein Block, Thermo Fisher Scientific, Cheshire, UK) was applied for 5 min to block the nonspecific binding sites. The sections were washed with phosphate-buffered saline and were incubated with rabbit anti-Human sclerostin antibody (1:100, ab63097; Abcam) or mouse anti-Human osteocalcin antibody (1:100, ab13420; Abcam) in a humid chamber overnight at 4°C. The sections were then incubated with horseradish peroxidase–conjugated anti-rabbit IgG antibody (1:200, ab6721; Abcam) or anti-mouse IgG antibody (1:200, ab6789; Abcam) at room temperature for 60 min and developed using 3,3′-diaminobenzidine tetrahydrochloride (Thermo Scientific, Fremont, CA, US). These sections were counterstained in Mayer's haematoxylin and were examined under a light microscope (Leica Q500MC; Leica Microsystems Cambridge Ltd, Cambridge, UK). The ratio of the number of sclerostin (Scl)-positive cells to the total number of cells was calculated for US DeCal and Normal DeCal groups. The region of interest was selected to be an area with the dimension of 620μm × 460μm observed under a magnification of 200×.
Statistics
All quantitative measurements were expressed as mean ± standard deviation (SD). All analyses were performed using SPSS (version 16.0 for Windows, SPSS Inc, Chicago, Illinois). Two-way analysis of variance with Bonferroni post hoc test was used to detect temporal changes between the two groups. Correlation of Ca2+ concentration in EDTA solution and mineralised tissue (BMC, BMD, BV and BV/TV) was analysed by Pearson's correlation. Statistical significance was set at p < 0.05.
Results
Quantification of mineralisation in bone sections
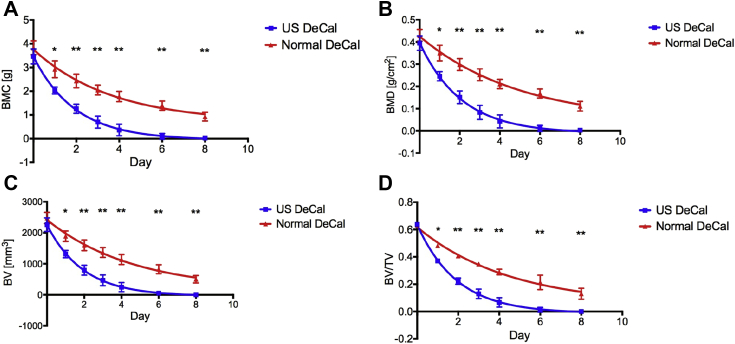
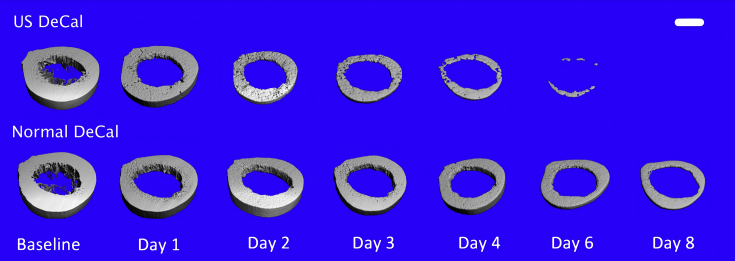
The gross morphology of the bone sections from Normal DeCal and US DeCal groups showed no difference after completion of decalcification (Fig. 1). After 24 h of decalcification, the BMC measured by DXA showed that there remained 58.6 ± 4.3% of the calcium content in the US DeCal bone sample and 76.4 ± 9.3% (Fig. 2) in the Normal DeCal bone sample, whereas 100% in the human bone samples. At Day 6, two of three samples from the US DeCal group completed the decalcification. The last remaining sample contained less than 7% of the mineral content. All the samples of the US DeCal group were completely decalcified at Day 8. For the Normal DeCal group, the three samples retained 36.3 ± 5.1% and 24.3 ± 4.8% at Day 6 and Day 8, respectively. At Day 8, the central core of the bone slice sample remained calcified while the outer layer was decalcified (Fig. 3). As detected by DXA and microCT, Normal DeCal samples took 50 days for achieving complete decalcification.
Figure 1.
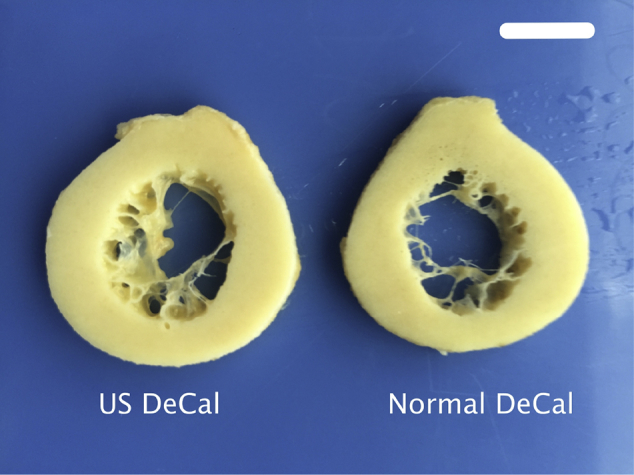
Representative bone slices of Normal DeCal and US DeCal after complete decalcification, without showing significant difference in appearance. Scale bar: 10mm.
Normal DeCal = normal decalcification group; US DeCal = Ultrasound decalcification group.
Figure 2.
Measurements of (A) BMC , (B) BMD , (C) BV (D) and BV/TV Normal decalcification and US decalcification group. * = p < 0.01; ** = p < 0.001.
BMC = bone mineral content; BMD = bone mineral density; BV = bone volume; BV/TV = ratio of bone volume to tissue volume; Normal DeCal = normal decalcification group; US = ultrasound; US DeCal = Ultrasound decalcification group.
Figure 3.
Three-dimensional images of the bone slices over time. US DeCal showed significantly faster decrease in the mineralised tissue than Normal DeCal. The central core of the bone slice in the Normal DeCal group remained calcified at Day 8, whereas the tissue of outer layer had been completely decalcified in the US DeCal group. Scale bar: 10mm.
Normal DeCal = normal decalcification group; US DeCal = Ultrasound decalcification group.
Concentration of calcium ions in EDTA solution
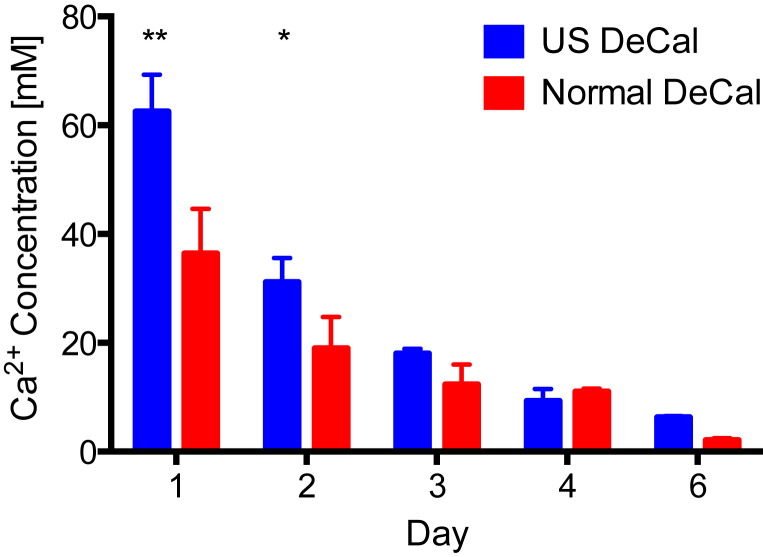
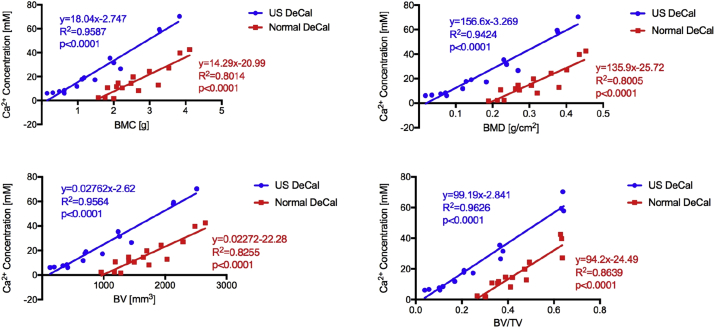
The concentration of calcium ions in the EDTA solution of the US DeCal group was 71.7% higher (p < 0.01) and 66.4% higher (p < 0.05) than that of the Normal DeCal after 24 h and 48 h, respectively (Fig. 4). After 72 h, the US DeCal group had 45.8% higher concentration of calcium ions in the EDTA solution than the Normal DeCal group, but no significant difference was detected between the two groups. Significant linear positive correlations were detected between Ca2+ concentration in the EDTA and BMC and BMD, both measured by DXA, and BV and BV/TV, both measured by microCT (Fig. 5). The slopes of the line of best fit of the US DeCal group and Normal DeCal group were comparable, whereas the lines of the US DeCal group were shifted to the left when compared with those of the Normal DeCal group.
Figure 4.
Concentration of calcium ions in the decalcification solution was measured by ICP-OES. The calcium concentration was significantly higher in the US DeCal group than in the Normal DeCal group by 80% at Day 1 and 2 of decalcification. ** = p < 0.001; * = p < 0.05.
ICP-OES = inductively coupled plasma optical emission spectrometry; Normal DeCal = normal decalcification group; US DeCal = Ultrasound decalcification group.
Figure 5.
Correlation between Ca2+ concentration in EDTA solution and mineralised tissue. Positive correlations were observed between Ca2+ concentration and BMC, BMD, BV and BV/TV. Regarding the linear line of best fit, the lines of the US DeCal and Normal DeCal had comparable slopes. Furthermore, the lines of the US DeCal were shifted to the left compared with those of the Normal DeCal.
BMC = bone mineral content; BMD = bone mineral density; BV = bone volume; BV/TV = ratio of bone volume to tissue volume; EDTA = ethylenediaminetetraacetic acid; Normal DeCal = normal decalcification group; US DeCal = Ultrasound decalcification group.
Histology
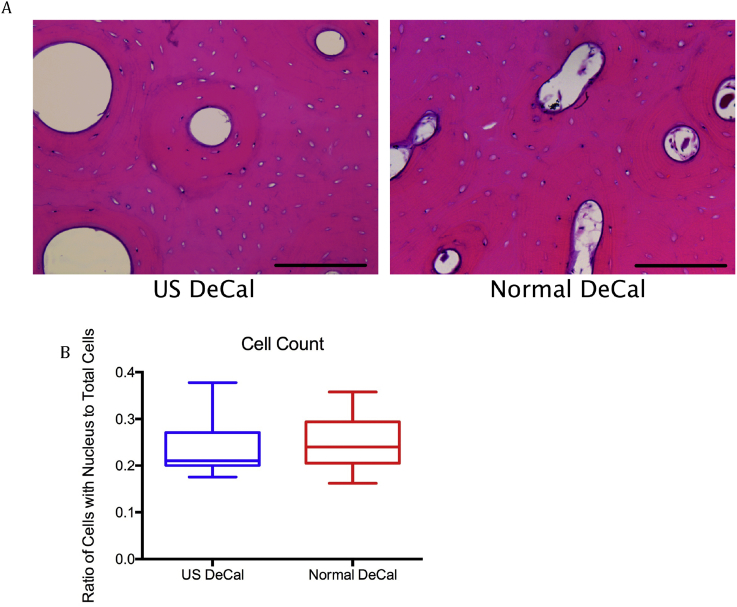
Histological analysis of the haematoxylin and eosin–stained sections showed that there was no significant difference between the Normal DeCal and US DeCal. The transverse sections showed numerous osteons with peripheral cement line and well-stained osteocyte nuclei. There was no architectural alteration or distortion to any of the tissues or cells in morphology in the US DeCal group as compared with those in the Normal DeCal group (Fig. 6A). The ratio of cells with visible nucleus to total number of cells did not show any significant difference between US DeCal and Normal DeCal (Fig. 6B).
Figure 6.
(A) Representative H&E images of samples from US DeCal and Normal DeCal groups. The transverse sections showed numerous osteons with peripheral cement line. Well-stained osteocyte nuclei indicate that the decalcification endpoint was not exceeded. There is no significant difference between the two groups with respect to the histological features and presence of artefacts; (B) The ratio of the osteocytes with identifiable nucleus to the total number of osteocytes did not show any significant difference between US DeCal and Normal DeCal groups. Magnification: 200×. Scale Bar: 200μm.
H&E = haematoxylin and eosin; Normal DeCal = normal decalcification group; US DeCal = Ultrasound decalcification group.
Immunohistochemistry
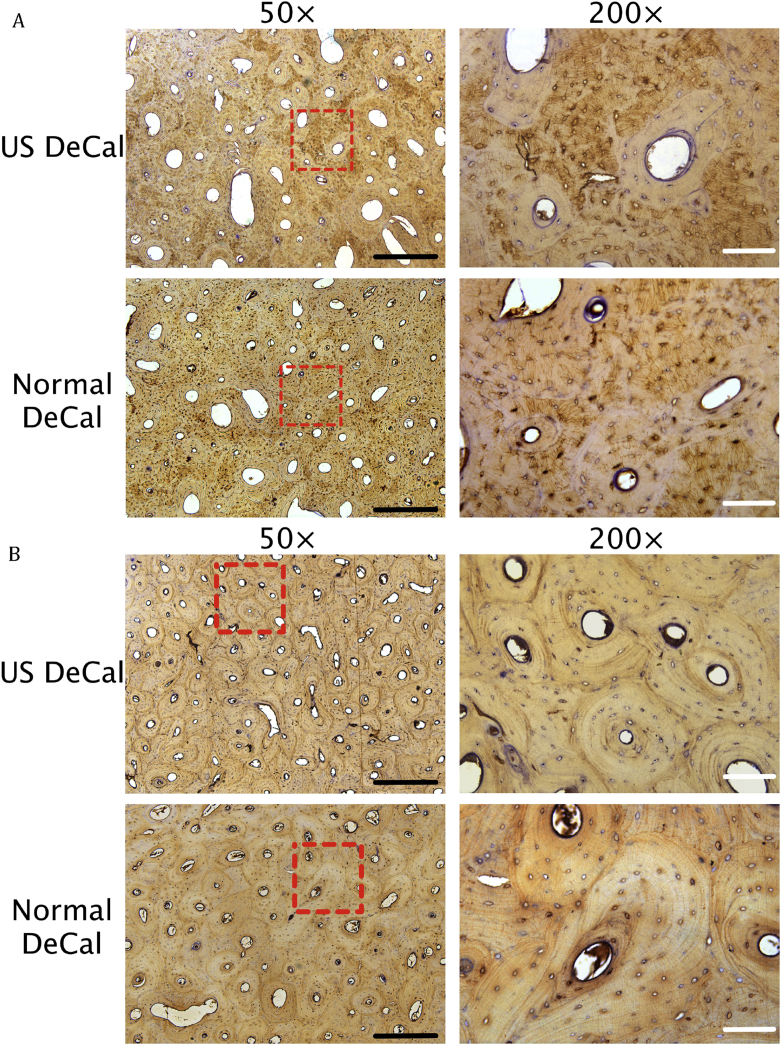
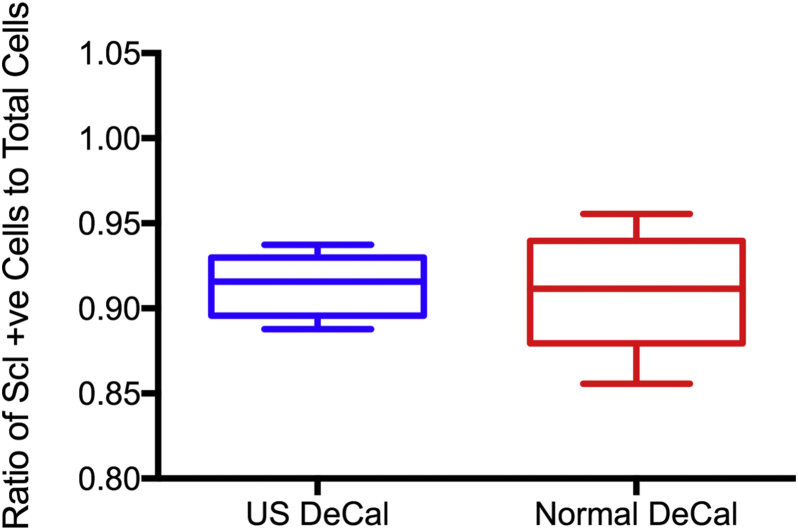
The presence of Scl (Fig. 7A) and osteocalcin (Fig. 7B) in the histological sections was detected in both US DeCal and Normal DeCal groups. The average ratios of the number of Scl-positive cells to the total number of cells for US DeCal and Normal DeCal groups were 0.9134 ± 0.0188 and 0.9100 ± 0.0363, respectively (Fig. 8). Similarly, osteocalcin expression detected was also comparable between US DeCal and Normal DeCal groups. There was no significant difference between the two groups (p < 0.05).
Figure 7.
Expression and presence of (A) sclerostin and (B) osteocalcin in US DeCal and Normal DeCal samples at different magnifications (50× and 200×). No significant difference was observed between the two groups. Red dotted box: Zoom-in area of 200× image. Black scale bar: 500μm; white scale bar: 100μm.
Normal DeCal = normal decalcification group; US DeCal = Ultrasound decalcification group.
Figure 8.
The ratio of sclerostin (Scl)-positive cells to total number of cells between US DeCal and Normal DeCal. There was no significant difference between the two groups.
Normal DeCal = normal decalcification group; US DeCal = Ultrasound decalcification group.
Discussion
Paraffin processing of tissue is the most widely used methodology in histologic slide preparation. Decalcification is a necessary step for preparing bone samples compatible with the setup used in routine histological sectioning. However, decalcification usually takes a long time that may delay the study progress and clinical diagnosis. Different methods, such as modifying the decalcification conditions including higher concentration of acid and increasing the temperature of the decalcifying agent, have been demonstrated to accelerate and shorten decalcification. However, these methods may damage the tissue integrity and introduce artefacts to the samples, such as osteocyte retraction [3].
For unknown reasons, there are only random reports on US-accelerated decalcification since the first report by Thorpe et al in 1963 [17]. All previous publications reported US-accelerated decalcification without mentioning the working temperature during decalcification [17], [18]. The present study demonstrated that US with a working temperature between 30 and 45°C accelerated the decalcification of human cortical bone sample in EDTA by six times comparing with the normal decalcification. This result is also supported by other studies with a 4–12 times decrease in decalcification duration depending on the thickness and size of the sample and the type of tissues [9], [17]. The application of US enhanced the decalcification of the central core of the thick bone slice. From the microCT images, the central core of the bone slice in the Normal DeCal group took the longest for the decalcification to complete. This phenomenon can be observed at the plateauing of the measurements of the mineralised tissue using microCT and DXA. However, the decalcification with application of US showed diminishing rate, but no plateauing of the measurements was observed. The application of US would still overcome this difficulty in decalcifying the core of the sample and effectively accelerate the decalcification [3].
The application of US with EDTA at a working temperature of 30–45°C for the decalcification of cortical bone slices did not impair the tissue morphology or create any artefacts such as osteocyte retraction in over decalcified bone samples. Histological analysis did not show differences between Normal DeCal and US DeCal groups. Our previous histological investigations on the rat bone samples that were decalcified with US did not show any architectural alterations or distortion to the growth plate, cortical bone, trabecular bone, chondrocytes and osteoblasts in morphology as compared with the tissue process in conventional decalcification [11]. The antigenicity of decalcified bone samples was also preserved according to the result of immunohistochemistry of Scl and osteocalcin. Reineke et al also reported that decalcification did not affect the morphology and antigenicity of their samples of bone marrow by using a commercially available decalcifier with temperature controller, but they have not mentioned the working temperature of the machine [18]. For the application of US in other bone samples, Thorpe et al showed that using an US cleaner sped the rate of decalcification without significant tissue damage in bone samples with variety of pathological conditions. The working temperature of the US cleaner was not reported [17]. During the application of US, heat would be produced by the machine. This rise in temperature would increase the decalcification rate [14], [19]. On the other hand, there is a concern that a rise in temperature would increase risks of damaging the tissue integrity, resulting in tissue swelling and tissue digestion when temperature was above 37°C [2], [13]. The result of this study demonstrated the US decalcification at temperature between 30 and 45°C for a duration as long as 8 days did not introduce any histological or immunohistological artefacts to the bone samples. Sangeetha et al used microwave to heat the EDTA solution to 41–43°C and reported that tissue preservation and staining efficacy were optimal [20].
It was considered that US-accelerated decalcification was mainly through cavitation effect destroying the boundary layers between the HA and collagen in bone [11], [12], [13]. US could only accelerate decalcification in combination with decalcification agents. The result suggested that cavitation and vibration produced by ultrasound might contribute only to removing the chemically detached HA particles from the surface and cavity of bone samples. Under normal condition without US, bone sections up to 3-mm thick are decalcified evenly and rapidly, whereas specimens with a thickness between 3 mm and 6 mm would decalcify at a slower rate [2]. It takes much time to decalcify dense cortical bone with thickness greater than 6 mm [2]. The reason might be the detached HA obstructing the openings of microcavities in bone; thus, the decalcifying agents could not reach the deep bone tissues efficiently. US helped with removing the obstructions and enabled the contact between the decalcifying agent and deeper tissue. In this way, US did not impair both microstructure and antigenicity of the bone tissue.
The decalcification frequency of the machine was 40kHz. The frequency of US can also affect the efficiency of the decalcification. US is defined as sound frequency above 20kHz. However, as frequency increases, attenuation would also increase, which leads to loss in energy and decrease in penetration depth [20]. This increase in frequency would proportionally increase the time required for decalcification. At higher frequencies, agitation and cavitation are less evident, so it is believed that less effective work is accomplished [17].
Decalcification protocols used in this experiment aimed to create sections with good quality while minimising the time required for sample processing without affecting its quality for sectioning and staining. A problem that is often encountered in large samples with dense cortical bone is having centralised area of undecalcified tissue even the outer tissue has been completely decalcified [2]. However, a thickness of 4–5mm for dense samples with a large cutting face would be optimal because this prevents the sample from coming out of the paraffin block by physical forces generated during sectioning [8]. Therefore, thicker specimens (4- to 5-mm thick) are more appropriate than thinner specimen (<2-mm thick) to ensure the quality of the histological sections [2].
The disadvantage of the US decalcifier is that it creates persistent noise during operation. This is because the US waves are converted to cavitations as they impact the samples and the water tank [21].
Conclusion
The present study demonstrated that US described in this study significantly reduced the decalcification time for large cortical bone samples by six times without impairing histological quality of the bone sections embedded in paraffin. The application of our technology would accelerate the routine sample decalcification process for bone histology in both basic and clinical research and diagnosis.
Declaration of interest
None declared.
Funding/Acknowledgements
This project was supported by the Public Sector Trial Scheme in the Innovation and Technology Commission of the Hong Kong SAR (Ref. ITT/003/13GP), the SMART Program, Lui Che Woo Institute of Innovative Medicine, Faculty of Medicine, the Chinese University of Hong Kong, and Li Ka Shing Institute of Health Sciences (LiHS), the Chinese University of Hong Kong.
References
- 1.Einhorn T.A., Simon S.R. 2nd ed. American Academy of Orthopaedic Surgeons; Rosemont, Ill: 2000. American Academy of Orthopaedic Surgeons. Orthopaedic basic science : biology and biomechanics of the musculoskeletal system. [Google Scholar]
- 2.An Y.H., Martin K.L. Humana Press; Totowa, NJ: 2003. Handbook of histology methods for bone and cartilage. [Google Scholar]
- 3.Kapila S.N., Natarajan S., Boaz K., Pandya J.A., Yinti S.R. Driving the mineral out faster: simple modifications of the decalcification technique. J Clin Diagn Res. 2015;9(9):ZC93–ZC97. doi: 10.7860/JCDR/2015/14641.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirita T. Springer Science+Business Media; New York, NY: 2015. Oral cancer. [Google Scholar]
- 5.Prasad P., Donoghue M. A comparative study of various decalcification techniques. Indian J Dent Res. 2013;24(3):302–308. doi: 10.4103/0970-9290.117991. [DOI] [PubMed] [Google Scholar]
- 6.Suvarna S.K., Layton C., Bancroft J.D. Bancroft's theory and practice of histological techniques. 7th ed. Churchill Livingstone Elsevier; Oxford: 2013. [Google Scholar]
- 7.Imaizumi K., Taniguchi K., Ogawa Y. An evaluation of the effect of microwave irradiation on bone decalcification aimed to DNA extraction. Leg Med (Tokyo) 2013;15(5):272–277. doi: 10.1016/j.legalmed.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Poston F. Bone decalcification expedited by ultrasonic sound. Am J Med Technol. 1967;33(4):263–268. [PubMed] [Google Scholar]
- 9.Milan L., Trachtenberg M.C. Ultrasonic decalcification of bone. Am J Surg Pathol. 1981;5(6):573–579. doi: 10.1097/00000478-198109000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Hatta H., Tsuneyama K., Nomoto K., Hayashi S., Miwa S., Nakajima T. A simple and rapid decalcification procedure of skeletal tissues for pathology using an ultrasonic cleaner with D-mannitol and formic acid. Acta Histochem. 2014;116(5):753–757. doi: 10.1016/j.acthis.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Guo X., Lam W.L. Acceleration of bone decalcification by ultrasound. In: Leung K.S., Qin L., editors. A practical manual for musculoskeletal research. World Scientific; Singapore: 2008. pp. 201–218. [Google Scholar]
- 12.Carstensen E.L., Gracewski S., Dalecki D. The search for cavitation in vivo. Ultrasound Med Biol. 2000;26(9):1377–1385. doi: 10.1016/s0301-5629(00)00271-4. [DOI] [PubMed] [Google Scholar]
- 13.Lillie R.D. Blakiston Division; New York: 1965. Histopathologic technic and practical histochemistry. [Google Scholar]
- 14.Verdenius H.H., Alma L. A quantitative study of decalcification methods in histology. J Clin Pathol. 1958;11(3):229–236. doi: 10.1136/jcp.11.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin L., Wang L., Wong M.W.N., Wen C., Wang G., Zhang G. Osteogenesis induced by extracorporeal shockwave in treatment of delayed osteotendinous junction healing. J Orthop Res. 2010;28(1):70–76. doi: 10.1002/jor.20948. [DOI] [PubMed] [Google Scholar]
- 16.Harrington J.M., Young D.J., Essader A.S., Sumner S.J., Levine K.E. Analysis of human serum and whole blood for mineral content by ICP-MS and ICP-OES: development of a mineralomics method. Biol Trace Elem Res. 2014;160(1):132–142. doi: 10.1007/s12011-014-0033-5. [English] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thorpe E.J., Bellomy B.B., Sellers R.F. Ultrasonic decalcification of bone. An experimental and clinical study. J Bone Joint Surg Am. 1963;45(6):1257–1259. [PubMed] [Google Scholar]
- 18.Reineke T., Jenni B., Abdou M.T., Frigerio S., Zubler P., Moch H. Ultrasonic decalcification offers new perspectives for rapid FISH, DNA, and RT-PCR analysis in bone marrow trephines. Am J Surg Pathol. 2006;30(7):892–896. doi: 10.1097/01.pas.0000213282.20166.13. [DOI] [PubMed] [Google Scholar]
- 19.Sangeetha R., Uma K., Chandavarkar V. Comparison of routine decalcification methods with microwave decalcification of bone and teeth. J Oral Maxillofac Pathol. 2013;17(3):386–391. doi: 10.4103/0973-029X.125204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oláh L. Ultrasound principles. In: Baracchini C., Csiba L., editors. Manual of neurosonology. Cambridge University Press; Cambridge: 2016. pp. 1–14. [Google Scholar]
- 21.Apfel R.E. Sonic effervescence: a tutorial on acoustic cavitation (reprinted from J. Accoust. Soc. Am vol 101, pg 1227, 1997) Nato Adv Sci I C Mat. 1999;524:1–24. [English] [Google Scholar]