Abstract
Orthopaedic implants are recognised as important therapeutic devices in the successful clinical management of a wide range of orthopaedic conditions. However, implant-related infections remain a challenging and not uncommon issue in patients with implanted instrumentation or medical devices. Bacterial adhesion and formation of biofilm on the surface of the implant represent important processes towards progression of infection. Given the intimate association between infection and the implant surface, adequate treatment of the implant surface may help mitigate the risk of infection. This review summarises the current surface treatment technologies and their role in prevention of implant-related infection from the beginning.
Translational potential of this article
Despite great technological advancements, the prevalence of implant-related infections remains high. Four main challenges can be identified. (i) Insufficient mechanical stability can cause detachment of the implant surface coating, altering the antimicrobial ability of functionalized surfaces. (ii) Regarding drug-loaded coatings, a stable drug release profile is of vital importance for achieving effective bactericidal effect locally; however, burst release of the loaded antibacterial agents remains common. (iii) Although many coatings and modified surfaces provide superior antibacterial action, such functionalisation of surfaces sometimes has a detrimental effect on tissue biocompatibility, impairing the integration of the implants into the surrounding tissue. (iv) Biofilm eradication at the implant surface remains particularly challenging. This review summarised the recent progress made to address the aforementioned problems. By providing a perspective on state-of-the-art surface treatment strategies for medical implants, we hope to support the timely adoption of modern materials and techniques into clinical practice.
Keywords: Implant, Infection, Orthopaedic, Prevention
Introduction
Many types of implants are currently available for helping restore the structure or function of the musculoskeletal system [1]; commonly used implants include bone cements for the filling of large bone defects, joint prostheses for total hip or knee arthroplasty and fracture fixation devices for fracture restoration [2]. However, implant recipients are at high risk for serious complications including infection, aseptic loosening, device wear, displacement and breakage. Indeed, implant-related infection is known as a catastrophic complication, and patients with severe infection often require revision surgery [3]. The infection rate of open fracture is reported at up to 20% but can be as high as 50% in patients with very severe fracture [4]. On average, 2–5% of implants are estimated to be contaminated, and the additional cost associated with the management of each patient with implant-related infection (drug treatment and surgical revision) is estimated to be around 100,000 USD [5]. Some prophylactic measures are used to prevent implant-related infections; such measures often include using strict aseptic techniques and administering systemic antibiotics perioperatively. However, the incidence of implant-related infection remains high. There is an urgent need for developing effective strategies to minimise the risk of implant-related infection and its potentially life-threatening complications. Local strategies for mitigating infection risk have so far shown good prospects, as reflected by the findings of many studies. Local strategies for preventing or managing implant-related infection can be roughly divided into two categories: (i) fabricating implants made of materials with intrinsic antibacterial properties, including Ag, Cu, Zn and polymers such as chitosan [6], [7], [8], [9], [10]; and (ii) applying surface modification technologies to functionalise the surface of the implant, conferring antibacterial properties; such strategies include surface coating, modification of surface chemistry and modification of surface morphology [11].
The aforementioned surface modification strategies represent a topic of high interest because they help render the surface of the implant antiinfective without changing the properties of the constituent material. Thus, coating technology can be widely applied for treating various kinds of implants already used in routine clinical practice, as well as for those still in undergoing testing. Considering the wide range of antibacterial properties achievable by surface coating, as well as the wide application prospects, implant surface modification is expected to represent an excellent strategy for mitigating implant-related infections.
According to the behaviour of independent bacteria or bacterial colonies, bacterial infection is considered to have several stages. Initial exposure to the pathogen and bacterial adhesion are the earliest steps of infection. As soon as bacteria become firmly adhered to a surface, they begin to proliferate [12]. Proliferation of surface bacteria is often accompanied by production of extracellular matrix, leading to biofilm formation. After biofilm maturation, bacteria can diffuse from the edge of the biofilm and invade surrounding tissues [13]. Because most antibacterial agents have poor diffusion into the surrounding tissues, diffuse infection is more difficult to treat. Intracellular invasion and bone degradation in the tissue surrounding the prosthesis are common complications in the late stage of infection and are difficult to treat [14]. Considering the treatment difficulty and serious consequences of implant failure, it is critical to ensure that adequate preventive measures are in place and can act from the early phase of infection. While reading this review, it is important to keep in mind that, in this context, early infection does not refer to infection occurring soon after implantation surgery, which generally be considered within one month [15], but to the beginning of infection, which includes several steps starting from exposure to the pathogen. This definition of the beginning of infection emphasises the specific bacterial behaviour in the early phases after pathogen exposure, without consideration to the time elapsed from surgery.
Several reviews have summarised recently developed surface modification technologies aiming to overcome implant-associated infection. In 2013, Campoccia et al. thoroughly surveyed the numerous kinds of antibacterial biomaterials and the underlying antibacterial strategies involving such materials [16]. Although exhaustive, the information in that previous review is called for update. Arciola et al. recently published an excellent review on implant-associated infection, in which they also mention surface modification strategies but do not provide a systematic review in this direction [17]. We presently focused on the early mechanisms underlying the development of implant-related infection and thus have summarised the state-of-the-art implant surface modification strategies in a systematic manner structured according to the specific pathogen behaviour targeted by each prevention strategy (Table 1).
Table 1.
Bacterial behaviour and treatment strategies in the beginning of implant-related infection.
| Bacterial behaviour | Strategies | Coating technologies |
|---|---|---|
| Bacterial adhesion | Prevention of adhesion | Polymer coatings [21], [22], [23], [24], [25] |
| Morphology [26], [27], [28], [29], [30] | ||
| Wettability [32] | ||
| Proliferation of adhered bacteria | Contact killing | Antibiotics [40], [41], [42], [43] |
| AMPs [38], [46], [47], [48], [49] | ||
| Metal implantation [52], [53], [54], [55], [56] | ||
| Biofilm formation | Antibiofilm | Enzymes [64], [65], [66], [67] |
| QS inhibitors [70], [71] | ||
| Planktonic bacteria (pathogens of initial exposure or bacteria diffused from biofilm edge) | Release killing | Antibiotics [79], [80], [81], [82], [83], [84] AMPs [85], [86], [87], [88] |
| Chitosan and derivatives [93], [94], [95], [96], [97], [98] | ||
| Ag [99], [100]; Cu [103], [104], [105]; Zn [109], [110] |
AMP = antimicrobial peptide; QS = quorum sensing.
Preventing bacterial adhesion to the implant surface
Upon insertion of the implant into the body, native cells and bacteria compete for interaction with the implant surface. Several factors such as long operating time, diabetes and anaemia are known to increase the risk of bacterial adhesion [18], [19]. Therefore, many types of surface functionalisation strategies aim to prevent bacterial adhesion to the implant surface. Interfering with adhesion delays the initial stage of infection, which greatly reduces infection risk [16]. Some surface characteristics including roughness, wettability and microstructure are recognised as important factors in bacterial adhesion. Nowadays, antiadhesion effect is mainly obtained using surface modification technologies that involve coating the implant surface with antiadhesive polymers or modifying surface characteristics such as morphology and wettability.
Polyethylene glycol and other polymer coatings
Applying a layer of physiologically inert polymer on the implant surface is a commonly used antiinfection strategy. Although the coating itself has no bactericidal effect, its inherent antiadhesive nature diminishes bacterial interaction with the surface. Polyethylene glycol (PEG), one of the most widely used polymers for preventing bacterial adhesion, has been the focus of several studies. Polymers can be firmly attached to the surface of medical devices through procedures such as physical adsorption or grafting, creating surfaces resistant to bacterial attachment [20]. The PEG-based coating referred to as poly(DMA-mPEGMA-AA) can effectively inhibit bacterial adhesion under both static and fluidic conditions, providing an eightfold reduction in the adhesion of Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) (compared with the adhesion noted for untreated surfaces). In addition, grafting dextran on titanium surfaces was reported as an effective antiadhesion strategy [21]. However, the antiadhesion properties of polymers grafted on the implant surface effectively prevent the interaction of the implant both with bacterial cells and native cells. Preventing the attachment of mesenchymal stem cells and osteoblasts to the implant may result in inadequate integration of the implant with the native tissue [22]. To avoid this negative effect of polymer coatings, it has been suggested that biologically active proteins such as bone morphogenetic protein-2 (BMP-2) could be grafted on the antiadhesion surface to promote osteoblast spreading, alkaline phosphatase activity and calcium mineral deposition on the modified surface [23]. Interestingly, immobilisation of BMP-2 on the coating did not diminish the antiadhesion properties of the implant surface, which did not bind S. aureus or S. epidermidis. The widely used cell adhesion peptide RGD (Arg-Gly-Asp) can also effectively promote the osteogenesis of mesenchymal stem cells on the implant surface, with a dose-dependent increase in some osteogenesis-related markers such as osteocalcin and alkaline phosphatase [24]. Muszunska et al. designed an antiadhesive polymer brush (Pluronic F-127) functionalised with antimicrobial peptides and RGD. The functionalised polymer brush showed excellent ability to prevent pathogen adhesion while providing better tissue compatibility than that of the original, unfunctionalised brush [25].
Surface morphology
Titanium nanotubes
Titanium nanotube coating is a common surface modification achieved by anodisation of the titanium surface, which results in formation of nanotube arrays. While titanium nanotubes are widely recognised for their potential as effective drug delivery systems, TiO2 nanotubes also inhibit bacterial adhesion and promote bone formation [26]. Specifically, titanium nanotubes increase the adhesion of osteoblasts to the surface, and the effect is inversely proportional to the diameter of the nanotube. At the same time, titanium nanotube arrays have significantly reduced initial adhesion and colonisation of S. epidermidis, with a stronger effect regarding the inhibition of S. epidermidis adhesion noted for arrays with smaller nanotube diameter. In other words, titanium nanotubes improve osteoblast adhesion but reduce bacterial adhesion. This interesting property makes TiO2 nanotubes promising candidates for promoting cell adhesion while concomitantly inhibiting bacterial adhesion, which is of vital importance for implant recipients. One study evaluated the effect of the crystalline phase on the cell adhesion properties of TiO2 nanotubes and found that crystalline titanium nanotube coatings improve platelet adhesion and activation, with nanotubes annealed at 450°C showing the strongest effect [27]. Grafting bioactive molecules such as lectins on the nanotube can further improve the biological properties of the coating [28].
Surface nanoarrays
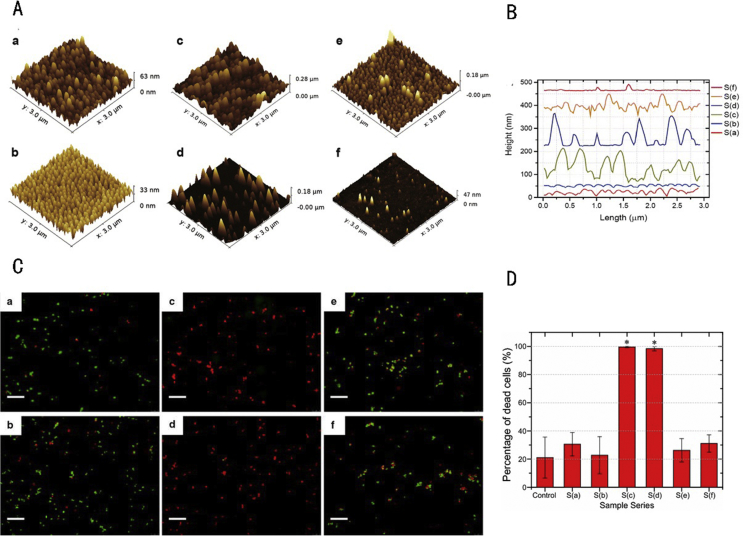
It was recently suggested that some naturally occurring surface morphologies, such as those noted on the wings of insects, have strong antimicrobial activity. The antimicrobial effect of these biological surfaces may be mainly attributed to the mechanical interactions between the adhering bacteria and the nanoscale microstructure and may not be related to the chemical composition of the surface. Such nanoscale microstructures can be modelled in silico (i.e., simulated) and applied to the surface of implants. This strategy can endow the surface with antiadhesion properties without changing the chemical composition of the implant [29]. Recent efforts to explore the influence of surface microstructure (nanopillar arrays of various nanopillar densities and heights) on bacterial adhesion and cell adhesion have revealed that changes in the surface morphology and roughness had an obvious effect on bactericidal activity, resulting in significantly decreased adherence of S. aureus [30] (Figure 1).
Figure 1.
(A) AFM images of nanostructured Ormostamp surfaces (a–f represent structures with different scale); (B) profiles of nanostructured Ormostamp surfaces (a–f extracted from AFM images); (C) characterisation of Staphylococcus aureus viability on various nanostructured surfaces. Images were obtained using SYTO9 and propidium iodide staining followed by fluorescence microscopy. Fluorescent images a–f correspond, respectively, to structures a–f shown in panel (A). Green and red colours indicate live and dead cells, respectively. Scale bar: 20 μm. (D) Bactericidal activities of various nanostructured surfaces. Error bars represent standard errors for at least three images. The statistical significance was determined for each data set using the unpaired, parametric, two-tailed t test. *p < 0.001 versus the control substrate. This figure was adapted from the figures in the study by Wu et al [30]. Copyright (2018) Journal of Nanobiotechnology.
AFM, atomic force microscopy.
Surface wettability
Not only surface roughness but also surface wettability may influence bacterial adhesion to the implant surface [31]. Yue Yuan et al. fabricated several kinds of polystyrene surfaces with different wettability and studied the relationship of surface wettability with bacterial adhesion. They found that both hydrophilic and hydrophobic characteristics were associated with diminished or completely abolished bacterial adhesion. The mechanisms underlying these observations likely involve enhanced repulsive interaction or entrapped air, which reduce the solid area fraction. A surface with moderate hydrophobicity characterised by a water contact angle of about 90° was considered to be most effective for preventing bacterial adhesion [32].
The following challenges remain regarding the application of antiadhesion technologies for preventing implant-related infection. First, the adhesion resistance ability of this type of coatings mainly depends on physical interactions, which are not specific. For this reason, many antiadhesive coatings and surface morphology modification strategies not only are effective in preventing bacterial adhesion but also inhibit the adhesion of osteoblasts. Integration of the implant into the surrounding tissue cannot occur without adhesion and differentiation of native cells on the implant surface [22]. Therefore, additional tethering of bioactive molecules such as RGD or BMP-2 on the modified surface is recommended to improve the interaction between the implant and the native tissue. Second, the stability of the grafted polymers and that of unusual microstructures on the implant surface remains of concern. If the bonding strength between the coating and the implant surface is too low, the coating can be degraded or become separated, and the morphology of the surface can change, which may create a more favourable environment for bacterial adhesion.
Although several challenges remain to be addressed, the application prospects of these antiadhesion strategies are promising. Implant fixation is a classic example of the area in which antiadhesion strategies can bring substantial benefits once certain aspects are addressed. Implants fixed using bone cement are widely used in clinical practice, but uncemented implants with porous surface have been introduced recently. The fixation of the uncemented implant at the surgical site is assisted by enhancing osteogenesis into the implant surface pores. Unfortunately, pore-enriched surfaces have increased surface roughness, which facilitates bacterial adhesion [33]. Thus, such devices are expected to increase cell adhesion at the expense of increased infection potential. Modifying the surface morphology, wettability and chemistry may represent promising solutions, as recently suggested by Mattheys et al and Braem et al [34], [35], [36].
Contact killing of bacteria adhered to the implant surface
Although bacterial adhesion can be greatly diminished by surface coating or microstructuring, it is difficult to completely eliminate adhesion, and some bacteria may still attach to the implant surface. Once bacteria adhere, a biofilm may develop on the implant surface, which is difficult to treat. Therefore, it is necessary to establish a second line of defence to deal with bacteria that overcome the antiadhesion action of the surface treatment. Contact killing is a strategy aiming to completely eradicate adhered pathogens.
This type of antiinfection strategy typically consists of immobilising bactericidal agents on the surface of the implant, thus creating a functional surface with bactericidal capacity. Compared with coatings, the immobilised antimicrobial agent is more stable, which greatly reduces the risk of surface morphology changes caused by coating degradation, thereby reducing the risk of additional infection. Moreover, the immobilised agents remain on the implant surface after binding to and killing bacteria adhered to the surface and thus can regain their antibacterial efficacy on becoming separated from the debris of dead bacteria, thus achieving long-term infection control [37]. Metal ion implantation is another strategy that can endow surfaces with contact-killing ability.
An important concern is whether the loading method affects the efficacy of the loaded antimicrobial agent (drug or metal ion). In addition, considering the long-term risk of infection, it is important to ensure that the functionalised contact-killing surface has long-term stability and long-term, continuous antibacterial effectiveness.
Immobilised antimicrobial agents
Immobilising the antibacterial agent on the surface of the implant can greatly reduce its release. Surface immobilisation is achieved by treating the surface to obtain functional groups that can react with the antibacterial agent, forming covalent bonds that firmly tether the antimicrobial agent to the implant surface [38]. A commonly used approach is to tweak the formation of self-assembled monolayers so that functional reactants are formed on the implant surface, which can act as linkers between the bactericidal agents and the implant surface. On adding the bactericides, a functional surface with effective antibacterial properties is created. The concentration of tethered agents on the surface and the length of the linker are important factors affecting the antibacterial effect of the functional surface. Bagheri et al reported that the length of the linker has a more profound impact on the antibacterial activity of the immobilised surface [39].
Surface immobilisation can achieve high concentration of the drug locally, which reduces the risk of bacterial exposure to sub-bactericidal concentrations of antibiotics and thus reduces the likelihood of drug resistance development. Vancomycin has a broad antimicrobial spectrum and is often chosen for immobilisation on the surface of implants [40], [41]. Furthermore, vancomycin can be easily tethered via its C-terminal carboxylic acid, which is not involved in the antibacterial activity [42]. Antoci et al found high antibacterial efficacy for vancomycin immobilised on titanium surfaces; specifically, the functional surfaces retained their antibacterial activity even after being soaked in phosphate-buffered saline for 1 week, 4 weeks or 11 months, which indicates good stability [43]. Jose et al designed a vancomycin-tethered titanium surface and confirmed its antibacterial activity and long-term stability [41].
Antimicrobial peptides (AMPs) are short-chain compounds with amphoteric or cationic character. The bactericidal effect of AMPs comes mainly from their interaction with the bacterial membrane, in which electrostatic forces play an important role [44]. Specifically, the positive charge on AMPs interacts strongly with the negatively charged lipid components in the bacterial membrane, allowing the AMPs to enter the membrane, which causes a reorganisation of the lipids in the membrane. The subsequent changes in the morphology of the bacterial membrane are accompanied by the formation of pores or even local ruptures in the membrane. Permeabilisation of the bacterial membrane is not the only bactericidal mechanism of AMPs. While inserted into the membrane lipid bilayer, AMPs can enter the cytoplasm and interact with intracellular targets as well, which can have a bactericidal effect if the interaction affects the function of proteins regulating metabolism [45].
Because the bactericidal mechanism of AMPs involves nonspecific interactions, bacterial resistance is unlikely to develop. Thus, immobilisation of AMPs on the surface of implants may provide adequate bactericidal effect [46]. Humblot et al designed a self-assembled monolayer with a linker based on 11-mercaptoundecanoic acid and 6-mercaptohexanol for tethering magainin I. Immobilisation of magainin I on gold surfaces resulted in a functionalised surface with strong bactericidal effect even after 6 months of storage and four times exposures to bacterial suspensions, indicating satisfactory long-term stability of the antibacterial action [38]. Tethering of gramicidin A on gold surfaces was also associated with good bactericidal effect, as reflected in the significantly lower number of bacteria adhered on the surface (vs. the control group). Effective contact-killing ability was confirmed against gram-positive bacteria, gram-negative bacteria and even fungi, as reflected in the substantial reduction in the number of pathogen cells adhered to the surface (60% decrease for E. coli, 70% for S. aureus and 90% for Candida albicans, respectively) [47]. The urgent need to develop preventive measures against fungal infection has recently become recognised [48]. Braem et al immobilised the antifungal peptide caspofungin through an electrophoretic deposition method and reported that the functionalized surface showed ability to suppress the development of fungal biofilm on the implant [49].
Metal ion implantation
Metal ions or nanoparticles (NPs) loaded on degradable coatings can be released in high concentration around the implant, killing bacteria by multiple mechanisms. On the other hand, metal ion implantation technology does not require the use of degradable coatings and does not cause significant changes to the surface properties. Plasma immersion ion implantation (PIII) is commonly used [50]. Compared to free metal ions, implanted metal ions are characterised by different bactericidal mechanisms, mainly involving electron transport and production of reactive oxygen species (ROS).
The bactericidal effect of AgNPs injected at the implant interface is related to the conductivity of the surface, indicating the importance of electron transport in the antibacterial action of AgNPs. During the process of electron transport, a large number of ROS will be produced on the implant surface, which alters the production of intracellular oxygen and the membrane potential, thus affecting bacterial activity [51]. Cao et al performed PIII of AgNPs into titanium surfaces; scanning electron microscopy revealed NPs ranging from 5 to 8 nm in diameter uniformly distributed on the functionalised surface, which became endowed with both contact bactericidal and osteogenesis promotion properties; this surface treatment reduced bacterial activity by 99% [52].
Jin et al designed a functional surface containing zinc (Zn) and Ag, which showed high bactericidal effect and weak biotoxicity [53]. Furthermore, microarc oxidation in electrolytes was applied to load copper (Cu) NPs on TiO2 coating, achieving significant and dose-dependent bactericidal effect [54].
The previously described strategies showed good results, but certain problems remain to be solved. First, although PIII-treated surfaces typically show no toxicity, metal ion implantation above a certain threshold does cause release of the ions into the surrounding tissue and is thus associated with a toxic effect [55]. Therefore, using an appropriate concentration of injected metal is of vital importance in the application of PIII for the treatment of implant surfaces. Second, although this type of functional surface exerts an excellent effect of contact killing, its effectiveness against planktonic bacteria around the implant is minimal. Therefore, metal implantation technologies should be combined with other antimicrobial strategies. For example, a recent study used PIII to embed AgNPs into TiO2 nanotubes, meanwhile loading vancomycin onto the surface through vacuum extraction and lyophilisation. This multifunctionalised, vancomycin-loaded and Ag-implanted TiO2 nanotubular surface was effective against both planktonic and sessile bacteria, with excellent antimicrobial and antibiofilm effects confirmed in both in vitro and in vivo experiments involving methicillin-resistant S. aureus [56].
Inhibiting biofilm formation
If prevention of bacterial adhesion and killing of adherent bacteria fail, bacterial biofilm will form. The difficulty of treatment is greatly increased once mature biofilm is established, due to two main reasons. First, the extracellular polymeric substance released by the bacteria forms a barrier that restricts the transport of compounds (including antibacterial agents) through the biofilm. Second, bacteria in the biofilm always have severely altered metabolic states, which reduce their sensitivity to treatment with antibiotics [57].
Efforts to prevent biofilm formation and to eradicate the already formed biofilm typically include antibiotics such as vancomycin and daptomycin [58], as well as NPs of Ag [59], Cu [60], Zn and gallium (Ga) [61], [62]. However, the risk of antibiotic resistance development greatly limits the use of antibiotics, whereas toxicity may limit the use of metal ions. As we gained a more comprehensive understanding of biofilm formation and regulation, nonbactericidal strategies have emerged to address biofilm-related infections, mainly through interacting with biofilm-specific components.
Enzyme-based inhibition
Some enzymes can help to degrade the biofilm by targeting the main components of the extracellular polymeric substance matrix. Two such enzymes have been thoroughly studied to date, namely dispersin B (DspB), which degrades polysaccharides, and deoxyribonuclease I (DNase I), which degrades extracellular DNA, ultimately increasing the permeability of the biofilm to antibacterial agents. Such enzymes decrease the biomass of the biofilm and enhance the bactericidal effect of antibiotics [63]. Swartjes et al studied a functional coating containing DNase I; after DNase I treatment, the thickness of the biofilm formed by Pseudomonas aeruginosa and S. aureus reduced to 0.2 and 3 μm, respectively [64]. Pavlukhina et al used a layer-by-layer deposition technique to bind DspB on the surface and achieved an inhibition rate of 98% for the S. epidermidis biofilm, with good biocompatibility [65]. Loading of DspB on carboxymethyl chitosan hydrogel NPs enhanced the thermal stability and reusability of the immobilised enzyme without reducing the biofilm inhibition effect [66].
Certain challenges regarding the use of enzymes as antibiofilm agents remain. After destroying the matrix of biofilm, the bacteria will be released into the surrounding tissue, causing the infection to spread. Thus, although DNase I and DspB can destroy the extracellular matrix of the biofilm effectively, it is recommended to use such enzymes in combination with antimicrobial agents such as antibiotics. Darouiche et al found that DspB and triclosan, which is an antiseptic with broad antimicrobial spectrum, act synergistically to inhibit biofilms [67].
Quorum sensing inhibition
Quorum sensing (QS) is a communication system observed in bacterial populations, especially in those forming biofilms. QS is closely related to several physiological activities and phenotypic conversion of bacteria, such as bacterial expression of virulence, adhesion to the surface and biofilm formation [68]. Small-molecule inhibitors have been developed in an effort to interfere with the signalling processes underlying biofilm formation [69]. The las QS signalling system, in which acetylated homoserine lactone acts as a signal molecule, is an important regulatory system. Zhang et al studied the effect of equisetin on P. aeruginosa biofilm and found that subinhibitory concentrations of equisetin can prevent biofilm formation and inhibit the las system by downregulating the expression of QS-related genes [70]. AgNPs have also been found to affect the biofilm by regulating the QS system [71].
Although some strategies for biofilm prevention have been proposed, it remains very difficult to completely remove the biofilm. In patients with implant-related infections, revision surgery is often the last choice of treatment [72]. The following challenges related to biofilm treatment remain. First, the process underlying biofilm formation is complex and involves a variety of regulatory signals and microenvironment changes. To discover effective targets for biofilm treatment, further understanding of the development and progress of biofilms is needed. Second, the therapeutic targets may show high variability in different bacterial populations, with some target-specific drugs showing reduced or no therapeutic effect in some populations [16]. It is of vital importance to identify targets that can be used to eradicate the biofilms in different bacteria. Third, enzymes that can degrade polysaccharides and extracellular DNA within biofilms may exert their action in other tissues outside the biofilm, increasing the risk for complications. Fourth, when mature biofilm has been established, intracellular invasion and bacterial invasion of the surrounding tissues are commonly found, which will further complicate the treatment of infection [73]. To sum up, complete eradication of mature biofilms is difficult to achieve, and it is of vital importance to prevent biofilm formation.
Controlled release of antimicrobial agents against planktonic bacteria
The strategies discussed previously mainly focus on reducing bacterial adhesion, colonisation and biofilm formation on the implant surface. In high-risk patients, dysfunctional systemic immune response combined with damaged local immunity leads to a weakened ability to eradicate bacteria that invade the circulation system or remain around the implant. For this reason, implants carry an extremely high risk of infection. Reducing the number of local planktonic bacteria is considered a promising strategy for treating implant-related infection. The bacteria that have diffused from the edge of the mature biofilm may also present in planktonic form. To date, several studies have analysed the effectiveness of antimicrobial agents including antibiotics, peptides and metal ions against planktonic bacteria.
Antibiotic-loaded coatings
Antibiotics have long been used in the treatment of infection. However, the inappropriate use of antibiotics has led to the emergence of many types of drug-resistant bacteria, among which methicillin-resistant S. aureus is the most famous. Multidrug-resistant superbugs have also emerged recently, bringing great challenges to the control of clinical infections. Unlike conventional management with systemic antibiotics, local delivery of antibiotics through drug-loaded coatings can achieve effective concentration. The local application of antibiotics has also reduced the risk of drug resistance to a certain extent. Development of coatings with appropriate release profile has been the focus of many researchers.
At present, coatings commonly used for antibiotic loading are made of ceramics and polymers such as hydroxyapatite, poly(lactic-co-glycolic acid) (PLGA) [74], polyacrylic acid and hydrogel [75]. Antibiotics such as penicillin and ceftriaxone attack gram-positive bacteria, whereas antibiotics such as levofloxacin, erythromycin and tetracycline attack gram-negative bacteria and are commonly used to treat implant-related infection. Current challenges include drug release dynamics and bacterial resistance. First, many coatings release a large proportion of the loaded drug in a very short time (burst release). Although a high concentration of antibiotics is needed to prevent infection in high-risk patients, such high doses can induce serious tissue toxicity with severe adverse effects. Moreover, it is difficult to ensure long-term antibacterial protection in situ using such coatings [76]. Second, because implant-associated infection in the adjacent bone tissue is commonly caused by drug-resistant bacteria or by a mixture of several pathogens, traditional antibiotics may not be effective to control infection. While some antibiotics kill only sensitive bacteria, the remaining drug-resistant bacteria may continue to develop. The extracellular DNA released from disrupted cells of antibiotic-sensitive pathogens can promote the expression of virulence-associated genes and formation of biofilm [77]. Finally, it is difficult to achieve controlled release of the antibiotic drug from the coating. Once the coated implants enter the body, they begin to release antibiotic agents immediately. This burst release may miss the bactericidal time window for opportunistic pathogens.
Much research effort has been expended to identify and develop coatings that allow controlled drug release. Hydroxyapatite, which is one of the most commonly used drug-loaded materials, can only maintain effective release for about 48 h [78]. Shukla et al used spray technology to prepare a layer-by-layer, assembled film on gelatin containing vancomycin; this coating showed a more stable drug release curve, extending the release time to approximately 100 h [79]. Noble et al proposed an ultrasound-responsive system made up of hydrogel and a self-assembled multilayer of methylene chains; the coating was initially impermeable while releasing the drug in a controlled manner under ultrasound treatment, with no burst release of antibiotics [80].
Creating a nanotube array on the implant surface and embedding the antibacterial agent into this array is another effective strategy to reinforce the loading efficiency and prolong the release time of antibiotics. We found that drug release time was extended with the increase in tube diameter. The time to reach steady drug release was extended to 9 h in nanotubes with smaller diameter (80 nm and 120 nm) and to 21 h in nanotubes with larger diameter (160 nm and 200 nm). Meanwhile, the total release of the drug increased with tube diameter. The results suggested that the drug-loaded nanotubes could significantly inhibit the formation of bacterial biofilm in vitro [81] and could prevent infection in vivo [82].
Mesoporous SiO2, which was recently proposed as a diffusion barrier, is a promising candidate for achieving long-term controlled release of the antibiotic. De Cremer K et al. applied the drug-loaded mesoporous SiO2 onto a porous titanium surface and found excellent ability to maintain a steady release of the loaded drug (chlorhexidine or toremifene) in vitro, maintaining effective drug concentration for at least 9 days and showing potent antibiofilm effect against Streptococcus mutans and C. albicans; this recently developed drug delivery system may thus be adequate for combating both bacterial and fungal infections [83], [84].
Antimicrobial peptides
As indicated previously, antimicrobial peptides (AMPs) represent a promising alternative to antibiotics in the management of implant-related infection. To reduce the high risk of infection postoperatively, these peptides can be loaded onto degradable coatings. Phosphate coatings are a common type of drug-loading material that can generally maintain effective antimicrobial concentrations for a few hours. GL13K (GKIIKLKASLKLL-CONH2) is a broad-spectrum AMP derived from the human parotid secretory protein. Loading GL13K onto titanium nanotubes on the implant surface provided sustained and slow drug release with great bactericidal effect in vitro [85]. Such coatings cannot entirely avoid the burst release effect, but layer-by-layer, assembled films have provided good results. Ponericin G1 is an AMP known to be highly active against S. aureus. Shukla et al incorporated ponericin G1 into a biodegradable polyelectrolyte multilayer film and found that the coating could release the peptide gradually within 10 days, with effective inhibition of bacterial growth and attachment [86]. Kazemzadeh-Narbat et al used another multilayered coating to deliver HHC-36, a potent broad-spectrum antimicrobial agent, and found a controlled and sustained release profile of HHC-36, showing great efficiency against both S. aureus and P. aeruginosa [87]. OP-145 is an LL-37–derived synthetic peptide effective against S. aureus. Breij et al used a Polymer-Lipid Encapsulation MatriX (PLEX) coating to incorporate OP-145 for controlled release; nonetheless, a burst release of approximately 55% of the peptide by the PLEX-OP-145 coating occurred during the first 48 h, followed by a daily release of about 1% for 30 days, with good bactericidal effects confirmed by both in vitro and in vivo experiments [88].
Although AMPs are known to be effective against bacteria, certain aspects limit their widespread application. First, some AMPs are easily degraded by proteases in vivo. Second, the polycation structure in the peptide is widely considered as the main functional element of AMPs. In addition to effectively killing bacteria, polycations can also cause cell damage, exerting biological toxicity [89]. When used locally for treating implant-related infection, AMP toxicity will affect the integration of the implant within the surrounding tissue.
Several studies aimed to address the two main limitations of AMPs, namely stability and toxicity. For example, using d-amino acids to synthesise antimicrobial peptides can effectively inhibit degradation by proteases in vivo [90]. In addition, using the liposome-encapsulated form may be a promising method to screen the high toxicity of AMPs. The aforementioned PLEX coating is a good example of how cytotoxicity can be reduced by encapsulating the peptides within liposomes [88].
Chitosan and its derivatives
Chitosan is a natural compound with good antibacterial capacity and biocompatibility. However, the poor solubility of chitosan at neutral or even higher pH limits its application as an antibacterial agent in vivo [91]. Reduced purity, which represents the main cause of side effects, represents another limitation of the use of chitosan. To overcome these shortcomings, various chitosan derivatives have been proposed, some of which exhibited both good solubility and antibacterial effects. Quaternized chitosan is a chitosan derivative containing a quaternary ammonium group, which confers increased solubility and stronger antibacterial activity [92].
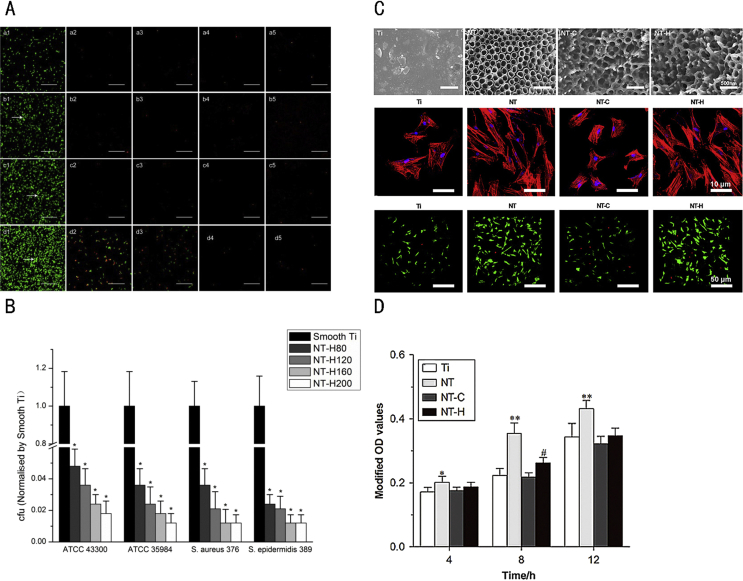
Hydroxypropyltrimethyl ammonium chloride chitosan (HACC) is synthesised through the reaction of chitosan with glycidyl trimethylammonium chloride, producing a chitosan derivative with excellent water solubility and antibacterial effect [93]. On investigating the effect of different substitution degrees at the quaternary ammonium on the antibacterial effect of this derivative, we found that, compared to compounds with 6% and 44% substitution, HACC derivatives with 18% substitution have stronger antibacterial activity and better biocompatibility, which supports its potential use as a coating with controlled drug release [94]. We also found that HACC can effectively reduce the formation of biofilm and inhibit the expression of biofilm-associated gene ica-A [95]. HACC covalently immobilised on titanium surface showed significant inhibition of bacterial adhesion in vitro, while in vivo experiments involving X-ray, bacteriology and histopathology analyses confirmed this effect [96]. Later, we used TiO2 nanotubes with various diameters (80, 120, 160 and 200 nm) and of 200 nm length as drug carriers, loading HACC through a lyophilisation method and vacuum drying. We found significantly lower bacterial adhesion and biofilm formation on HACC-loaded Ti nanotubes (NT-H) than on smooth Ti, with stronger antibacterial activity noted for NT-H with larger diameter (160 or 200 nm), which provided extended HACC release time [97]. In addition to strong antibacterial properties, this HACC-loaded surface also exhibits good biocompatibility compared with simple titanium surfaces (Ti), nanotubes without polymer loading (NT) and nanotubes loaded with chitosan (NT-C) [98] (Figure 2). Based on these findings, HACC can generally be considered an effective antibacterial agent with promising application prospects.
Figure 2.
(A) Confocal laser scanning microscopy analysis of bacterial viability on different surfaces. (1) Smooth Ti; (2) NT-H80; (3) NT-H120; (4) NT-H160; (5) NT-H200 incubated with Staphylococcus aureus (ATCC 43300) for (a) 6 h; (b) 24 h; (c) 48 h and (d) 72 h. The arrow head indicates biofilm. The scale bar is 50 μm. (B) Number of viable bacteria adhered on smooth Ti and NT-H surfaces at 6 h. The number of viable bacteria was counted and normalised to the counts from the smooth Ti control for each bacterial strain. (C) Scanning electron microscopy image of titanium without modification (Ti), titania nanotubes without drug-loading (NT, with diameters of 160 nm), chitosan-loaded titania nanotubes (NT-C) and HACC-loaded titania nanotubes (NT-H). Attachment and spreading of human bone marrow–derived mesenchymal stem cells (hMSCs) on various specimens. (D) Cell attachment assay. Evaluations were conducted using the Cell Counting Kit-8. *p < 0.05 versus Ti; **p < 0.01 versus other groups; #p < 0.05 versus NT-C; ##p < 0.01 versus Ti and NT-C. Panels in this figure were adapted from figures in the studies by Lin et al and Yang et al [97], [98]. Copyright (2016) Materials (Basel) and (2016) Bone Research.
HAAC = hydroxypropyltrimethyl ammonium chloride chitosan.
The polycationic structure of HACC is considered an important factor facilitating the interaction with the anions on the surface of the bacterial membrane, further increasing permeability and disrupting the integrity of the membrane. The antibacterial mechanism of HACC has not been fully clarified to date, and it remains unclear whether other bactericidal mechanisms play a role in the bactericidal process.
Metal ions
In addition to killing adhered bacteria, as mentioned previously, metal ions such as Ag, Cu and Zn have traditionally been used as effective agents against planktonic bacteria, exhibiting broad-spectrum bactericidal capacity.
The ability of Ag ions to kill free bacteria has been widely described. Liu et al embedded AgNPs in PLGA coatings and found that PLGA/AgNP exhibits strong bactericidal action against S. aureus and P. aeruginosa. Furthermore, the effect remained unperturbed for 8 weeks, with no bacteria or inflammatory cells around the implant [99]. Agarwal et al studied the antibacterial effect of nano-silver loaded in multilayer polymer coatings. Specifically, they prepared a thin layer of poly(allylamine hydrochloride) and polyacrylic acid polymers using layer-by-layer deposition techniques. The AgNP-loaded coating showed a remarkable antibacterial effect, providing a concentration of 0.4 μg/cm2, which killed 99% of S. epidermidis cells; however, higher doses were associated with strong toxicity [100].
Some studies suggest that the bactericidal mechanism of Cu is based on its interaction with DNA, resulting in rapid fragmentation of DNA [101]. It has also been proposed that Cu exerts a major bactericidal action via destruction of bacterial cell membranes, while DNA damage is not a major cause of bacterial lethality [102]. The application of Cu to the surface of the implant can increase the antibacterial activity of the surface and at the same time improve osteogenic and angiogenic effects, which are beneficial to the implant [103]. The bacteria-killing effect of Cu-loaded polymer coatings was significantly high and was dose dependent [104]. The simultaneous loading of two kinds of metal ions in the same NP has also been described. For example, Cu/AgNP-loaded coatings showed strong antibacterial activity against methicillin-resistant S. aureus [105].
Zn is another widely studied metal bactericide, which can cause dysfunction of transmembrane proton pumps, leading to damage of bacterial proteins [106]. It has also been reported that Zn oxide NPs (ZnNPs) can destroy the integrity of the bacterial membrane. ROS generation is another means by which ZnNPs can interfere with biofilm formation by E. coli and S. aureus. In addition to the superior antibacterial effect, Zn is thought to play an important role in the process of osteoblast mineralisation and bone growth regulation. Upregulation of osteogenic genes including alkaline phosphatase, type I collagen, osteocalcin and osteopontin is commonly involved [107], [108]. Owing to its superior antibacterial effect and osteogenic effect, Zn-based treatment of implant-related infection has gained increasing interest. Huang et al used electrophoretic deposition technology to load Zn into chitosan/gelatin nanocomposite coatings, which not only provided good antibacterial effect against E. coli and S. aureus but also promoted the proliferation and osteogenic activity of bone marrow mesenchymal stem cells in vitro [109]. ZnNPs have excellent antibacterial effect, but their potential toxicity and aggregation tendency have limited their application to some extent. Lin et al improved the biocompatibility of ZnNPs by grafting gelatin on the surface of such particles, which did not affect the bactericidal effect of Zn on E. coli and S. aureus, thus indicating that at least some of the obstacles to the application of ZnNPs have been addressed [110].
Perspective
We have summarised the state-of-the-art strategies for surface modification to reduce the risk of implant-associated infection. Such strategies include surface morphology modification (e.g., nanoarrays) to prevent bacterial adhesion, surface immobilisation of AMPs to combat both bacterial and fungal infections or surface implantation of metal NPs with potent antibacterial activity. However, antibacterial surfaces are yet to be widely adopted for clinical applications. For a surface treatment strategy to become routinely used in the optimisation of orthopaedic implants for clinical applications, the functionalized surface must meet many safety (biocompatibility), efficiency and economic criteria. In particular, the coating should have sufficient mechanical integrity and adhesion to the implant surface to withstand insertion and repeated mechanical loading; local biotoxicity should be minimal; loaded antibiotics should have a broad-spectrum activity and effectiveness against biofilm-forming microbes; the surface treatment procedure should be technically simple and require minimal cost [111]. Substantial progress has been made in these directions. For example, mesoporous silica can release the loaded antibacterial agents in a controlled fashion, endowing the drug-loaded antibacterial surface long-term antibacterial efficacy. PIII seems to be a promising strategy to reduce the biotoxicity of metal ions. Nevertheless, no currently available surface treatment approach fulfils all required criteria. The remaining challenges will continue to be a topic of research, with the ultimate goal of developing surface treatment methods that can confer both potent antibacterial ability and good biocompatibility while fulfilling the safety, practical and economic criteria for clinical translation.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
Acknowledgements
This work was funded by the National Key R&D Programme (2016YFC1102100) and the Shanghai Science and Technology Development Fund (18DZ2291200, 18441902700).
References
- 1.Tang T-t, Qin L. Translational study of orthopaedic biomaterials and devices. J Orthop Transl. 2016;5:69–71. doi: 10.1016/j.jot.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alt V. Antimicrobial coated implants in trauma and orthopaedics-A clinical review and risk-benefit analysis. Injury Int J Care Injured. 2017;48(3) doi: 10.1016/j.injury.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Brown J.M., Mistry J.B., Cherian J.J., Elmallah R.K., Chughtai M., Harwin S.F. Femoral component revision of total hip arthroplasty. Orthopedics. 2016;39(6):1. doi: 10.3928/01477447-20160819-06. [DOI] [PubMed] [Google Scholar]
- 4.Okike K., Bhattacharyya T. Trends in the management of open fractures. A critical analysis. J Bone Jt Surg Am. 2007;88(12):2739–2748. doi: 10.2106/JBJS.F.00146. [DOI] [PubMed] [Google Scholar]
- 5.Darouiche R.O. Treatment of infections associated with surgical implants. N Engl J Med. 2004;172(5):1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 6.Chen M., Yang L., Zhang L., Han Y., Lu Z., Qin G. Effect of nano/micro-Ag compound particles on the bio-corrosion, antibacterial properties and cell biocompatibility of Ti-Ag alloys. Mater Sci Eng C Mater Biol Appl. 2017;75:906–917. doi: 10.1016/j.msec.2017.02.142. [eng] [DOI] [PubMed] [Google Scholar]
- 7.Chen M., Zhang E., Zhang L. Microstructure, mechanical properties, bio-corrosion properties and antibacterial properties of Ti-Ag sintered alloys. Mater Sci Eng C Mater Biol Appl. 2016;62:350–360. doi: 10.1016/j.msec.2016.01.081. [eng] [DOI] [PubMed] [Google Scholar]
- 8.Sun D., Xu D., Yang C., Chen J., Shahzad M.B., Sun Z. Inhibition of Staphylococcus aureus biofilm by a copper-bearing 317L-Cu stainless steel and its corrosion resistance. Mater Sci Eng C Mater Biol Appl. 2016;69:744–750. doi: 10.1016/j.msec.2016.07.050. [eng] [DOI] [PubMed] [Google Scholar]
- 9.Bakhsheshi-Rad H.R., Hamzah E., Low H.T., Kasiri-Asgarani M., Farahany S., Akbari E. Fabrication of biodegradable Zn-Al-Mg alloy: mechanical properties, corrosion behavior, cytotoxicity and antibacterial activities. Mater Sci Eng C Mater Biol Appl. 2017;73:215–219. doi: 10.1016/j.msec.2016.11.138. [eng] [DOI] [PubMed] [Google Scholar]
- 10.Karahaliloglu Z., Kilicay E., Denkbas E.B. Antibacterial chitosan/silk sericin 3D porous scaffolds as a wound dressing material. Artif Cells Nanomed Biotechnol. 2017;45(6):1–14. doi: 10.1080/21691401.2016.1203796. [eng] [DOI] [PubMed] [Google Scholar]
- 11.Raphel J., Holodniy M., Goodman S.B., Heilshorn S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials. 2016;84:301. doi: 10.1016/j.biomaterials.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.An Y.H., Friedman R.J. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J Biomed Mater Res B Appl Biomater. 1998;43(3):338–348. doi: 10.1002/(sici)1097-4636(199823)43:3<338::aid-jbm16>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 13.Moormeier D.E., Bayles K.W. Staphylococcus aureus biofilm: a complex developmental organism. Mol Microbiol. 2017;104(3):365–376. doi: 10.1111/mmi.13634. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dapunt U., Giese T., Stegmaier S., Moghaddam A., Hansch G.M. The osteoblast as an inflammatory cell: production of cytokines in response to bacteria and components of bacterial biofilms. BMC Muscoskel Disord. 2016;17:243. doi: 10.1186/s12891-016-1091-y. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmerli W. Clinical presentation and treatment of orthopaedic implant-associated infection. J Int Med. 2014;276(2):111–119. doi: 10.1111/joim.12233. [eng] [DOI] [PubMed] [Google Scholar]
- 16.Campoccia D., Montanaro L., Arciola C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials. 2013;34(34):8533–8554. doi: 10.1016/j.biomaterials.2013.07.089. [DOI] [PubMed] [Google Scholar]
- 17.Arciola C.R., Campoccia D., Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nat Rev Microbiol. 2018;16(7):397–409. doi: 10.1038/s41579-018-0019-y. [DOI] [PubMed] [Google Scholar]
- 18.ter Boo G.J., Grijpma D.W., Moriarty T.F., Richards R.G., Eglin D. Antimicrobial delivery systems for local infection prophylaxis in orthopedic- and trauma surgery. Biomaterials. 2015;52:113–125. doi: 10.1016/j.biomaterials.2015.02.020. [eng] [DOI] [PubMed] [Google Scholar]
- 19.Greenky M., Gandhi K., Pulido L., Restrepo C., Parvizi J. Preoperative anemia in total joint arthroplasty: is it associated with periprosthetic joint infection? Clin Orthop Relat Res. 2012;470(10):2695–2701. doi: 10.1007/s11999-012-2435-z. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caro A., Humblot V., Méthivier C., Minier M., Salmain M., Pradier C.M. Grafting of lysozyme and/or poly(ethylene glycol) to prevent biofilm growth on stainless steel surfaces. J Phys Chem B. 2009;113(7):2101–2109. doi: 10.1021/jp805284s. [DOI] [PubMed] [Google Scholar]
- 21.Park J.Y., Kim J.S., Nam Y.S. Mussel-inspired modification of dextran for protein-resistant coatings of titanium oxide. Carbohydr Polym. 2013;97(2):753–757. doi: 10.1016/j.carbpol.2013.05.064. [DOI] [PubMed] [Google Scholar]
- 22.Harris L.G., Tosatti S., Wieland M., Textor M., Richards R.G. Staphylococcus aureus adhesion to titanium oxide surfaces coated with non-functionalized and peptide-functionalized poly(L-lysine)-grafted-poly(ethylene glycol) copolymers. Biomaterials. 2004;25(18):4135–4148. doi: 10.1016/j.biomaterials.2003.11.033. [eng] [DOI] [PubMed] [Google Scholar]
- 23.Shi Z., Neoh K.G., Kang E.T., Poh C., Wang W. Titanium with surface-grafted dextran and immobilized bone morphogenetic protein-2 for inhibition of bacterial adhesion and enhancement of osteoblast functions. Tissue Eng Part A. 2009;15(2):417–426. doi: 10.1089/ten.tea.2007.0415. [DOI] [PubMed] [Google Scholar]
- 24.Yang F., Williams C.G., Wang D.A., Lee H., Manson P.N., Elisseeff J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials. 2005;26(30):5991. doi: 10.1016/j.biomaterials.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 25.Muszanska A.K., Rochford E.T., Gruszka A., Bastian A.A., Busscher H.J., Norde W. Antiadhesive polymer brush coating functionalized with antimicrobial and RGD peptides to reduce biofilm formation and enhance tissue integration. Biomacromolecules. 2014;15(6):2019–2026. doi: 10.1021/bm500168s. [DOI] [PubMed] [Google Scholar]
- 26.Peng Z., Ni J., Zheng K., Shen Y., Wang X., He G. Dual effects and mechanism of TiO2 nanotube arrays in reducing bacterial colonization and enhancing C3H10T1/2 cell adhesion. Int J Nanomed. 2013;8(default):3093. doi: 10.2147/IJN.S48084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L., Liao X., Fok A., Ning C., Ng P., Wang Y. Effect of crystalline phase changes in titania (TiO2) nanotube coatings on platelet adhesion and activation. Mater Sci Eng C. 2017;82:91–101. doi: 10.1016/j.msec.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 28.Oliveira W.F., Irs A., Gmm S., Machado G., Lcbb C., Mts C. Functionalization of titanium dioxide nanotubes with biomolecules for biomedical applications. Mater Sci Eng C Mater Biol Appl. 2017;81:597–606. doi: 10.1016/j.msec.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 29.Elbourne A., Crawford R.J., Ivanova E.P. Nano-structured antimicrobial surfaces: from nature to synthetic analogues. J Colloid Interface Sci. 2017;508:603–616. doi: 10.1016/j.jcis.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 30.Wu S., Zuber F., Maniuraweber K., Brugger J., Ren Q. Nanostructured surface topographies have an effect on bactericidal activity. J Nanobiotechnol. 2018;16(1):20. doi: 10.1186/s12951-018-0347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dou X.Q., Zhang D., Feng C., Jiang L. Bioinspired hierarchical surface structures with tunable wettability for regulating bacteria adhesion. ACS Nano. 2015;9(11):10664–10672. doi: 10.1021/acsnano.5b04231. [eng] [DOI] [PubMed] [Google Scholar]
- 32.Yuan Y., Hays M.P., Hardwidge P.R., Kim J. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 2017;7(23):14254–14261. [Google Scholar]
- 33.Harris L.G., Richards R.G. Staphylococci and implant surfaces: a review. Injury. 2006;37(Suppl. 2):S3–S14. doi: 10.1016/j.injury.2006.04.003. [eng] [DOI] [PubMed] [Google Scholar]
- 34.Mattheys T., Braem A., Neirinck B., Biest O.V.D., Vleugels J. Ti coatings with macropores for improved implant fixation obtained by electrophoretic deposition of TiH2 stabilized emulsions. Adv Eng Mater. 2012;14(6):371–376. [Google Scholar]
- 35.Braem A., Mattheys T., Neirinck B., Schrooten J., Biest O.V.D., Vleugels J. Porous titanium coatings through electrophoretic deposition of TiH2 suspensions. Adv Eng Mater. 2011;13(6):509–515. [Google Scholar]
- 36.Braem A., Van Mellaert L., Mattheys T., Hofmans D., De Waelheyns E., Geris L. Staphylococcal biofilm growth on smooth and porous titanium coatings for biomedical applications. J Biomed Mater Res A. 2014;102(1):215–224. doi: 10.1002/jbm.a.34688. [DOI] [PubMed] [Google Scholar]
- 37.Costa F., Carvalho I.F., Montelaro R.C., Gomes P., Martins M.C. Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater. 2011;7(4):1431–1440. doi: 10.1016/j.actbio.2010.11.005. [eng] [DOI] [PubMed] [Google Scholar]
- 38.Humblot V., Yala J.F., Thebault P., Boukerma K., Héquet A., Berjeaud J.M. The antibacterial activity of Magainin I immobilized onto mixed thiols self-assembled monolayers. Biomaterials. 2009;30(21):3503. doi: 10.1016/j.biomaterials.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 39.Bagheri M., Beyermann M., Dathe M. Immobilization reduces the activity of surface-bound cationic antimicrobial peptides with no influence upon the activity spectrum. Antimicrob Agents Chemother. 2009;53(3):1132–1141. doi: 10.1128/AAC.01254-08. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawson M.K.C., Hoth K.C., Deforest C.A., Bowman C.N., Anseth K.S. Inhibition of Staphylococcus epidermidis biofilms using polymerizable vancomycin derivatives. Clin Orthop Relat Res. 2010;468(8):2081–2091. doi: 10.1007/s11999-010-1266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jose B., Antoci V., Jr., Zeiger A.R., Wickstrom E., Hickok N.J. Vancomycin covalently bonded to titanium beads kills Staphylococcus aureus. Chem Biol. 2005;12(9):1041–1048. doi: 10.1016/j.chembiol.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 42.Jose B., Antoci V., Jr., Zeiger A.R., Wickstrom E., Hickok N.J. Vancomycin covalently bonded to titanium beads kills Staphylococcus aureus. Chem Biol. 2005;12(9):1041–1048. doi: 10.1016/j.chembiol.2005.06.013. [eng] [DOI] [PubMed] [Google Scholar]
- 43.Antoci V.J., Adams C.J., Ducheyne P., Shapiro I., Hickok N. Covalently attached vancomycin provides a nanoscale antibacterial surface. Clin Orthop Relat Res. 2007;461(461):81. doi: 10.1097/BLO.0b013e3181123a50. [DOI] [PubMed] [Google Scholar]
- 44.Fjell C.D., Hiss J.A., Hancock R.E., Schneider G. Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov. 2012;11(1):37–51. doi: 10.1038/nrd3591. [DOI] [PubMed] [Google Scholar]
- 45.Yeung A.T.Y., Gellatly S.L., Hancock R.E.W. Multifunctional cationic host defence peptides and their clinical applications. Cell Mol Life Sci. 2011;68(13):2161–2176. doi: 10.1007/s00018-011-0710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeaman M.R., Yount N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev. 2003;55(1):27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- 47.Yala J.F., Thebault P., Héquet A., Humblot V., Pradier C.M., Berjeaud J.M. Elaboration of antibiofilm materials by chemical grafting of an antimicrobial peptide. Appl Microbiol Biotechnol. 2011;89(3):623. doi: 10.1007/s00253-010-2930-7. [DOI] [PubMed] [Google Scholar]
- 48.Giles C., Lamont-Friedrich S.J., Michl T.D., Griesser H.J., Coad B.R. The importance of fungal pathogens and antifungal coatings in medical device infections. Biotechnol Adv. 2018;36(1):264–280. doi: 10.1016/j.biotechadv.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 49.Braem A., De Brucker K., Delattin N., Killian M.S., Roeffaers M.B., Yoshioka T. Alternating current electrophoretic deposition for the immobilization of antimicrobial agents on titanium implant surfaces. ACS Appl Mater Interfaces. 2017;9(10):8533–8546. doi: 10.1021/acsami.6b16433. [DOI] [PubMed] [Google Scholar]
- 50.Qiao S., Cao H., Zhao X., Lo H., Zhuang L., Gu Y. Ag-plasma modification enhances bone apposition around titanium dental implants: an animal study in Labrador dogs. Int J Nanomed. 2015;10(default):653–664. doi: 10.2147/IJN.S73467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang G., Jin W., Qasim A.M., Gao A., Peng X., Li W. Antibacterial effects of titanium embedded with silver nanoparticles based on electron-transfer-induced reactive oxygen species. Biomaterials. 2017;124:25–34. doi: 10.1016/j.biomaterials.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 52.Cao H., Zhang W., Meng F., Guo J., Wang D., Qian S. Osteogenesis catalyzed by titanium-supported silver nanoparticles. ACS Appl Mater Interfaces. 2017;9(6):5149–5157. doi: 10.1021/acsami.6b15448. [DOI] [PubMed] [Google Scholar]
- 53.Jin G., Qin H., Cao H., Qiao Y., Zhao Y., Peng X. Zn/Ag micro-galvanic couples formed on titanium and osseointegration effects in the presence of S. aureus. Biomaterials. 2015;65:22–31. doi: 10.1016/j.biomaterials.2015.06.040. [DOI] [PubMed] [Google Scholar]
- 54.Zhang X., Li J., Wang X., Wang Y., Hang R., Huang X. Effects of copper nanoparticles in porous TiO2 coatings on bacterial resistance and cytocompatibility of osteoblasts and endothelial cells. Mater Sci Eng C Mater Biol Appl. 2018:110–120. doi: 10.1016/j.msec.2017.08.061. [DOI] [PubMed] [Google Scholar]
- 55.Schrock K., Schneider H., Lutz J., Hacker M.C., Mandl S., Kamprad M. Cytocompatibility of nitrogen plasma ion immersed medical cobalt-chromium alloys. J Biomed Mater Res A. 2014;102(6):1744–1754. doi: 10.1002/jbm.a.34842. [eng] [DOI] [PubMed] [Google Scholar]
- 56.Wang J., Li J., Qian S., Guo G., Wang Q., Tang J. Antibacterial surface design of titanium-based biomaterials for enhanced bacteria-killing and cell-assisting functions against periprosthetic joint infection. Appl Mater Interfaces. 2016;8(17):11162–11178. doi: 10.1021/acsami.6b02803. [DOI] [PubMed] [Google Scholar]
- 57.Costerton J.W., Stewart P.S., Greenberg E.P. Bacterial biofilms: a common cause of persistent infections. Science (New York, NY) 1999;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. [eng] [DOI] [PubMed] [Google Scholar]
- 58.Hickok N.J., Shapiro I.M. Immobilized antibiotics to prevent orthopedic implant infections. Adv Drug Deliv Rev. 2012;64(12):1165–1176. doi: 10.1016/j.addr.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim M.H. Nanoparticle-based therapies for wound biofilm infection: opportunities and challenges. IEEE Trans Nanobiosci. 2016;15(3):294–304. doi: 10.1109/TNB.2016.2527600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lewisoscar F., Mubarakali D., Nithya C., Priyanka R., Gopinath V., Alharbi N.S. One pot synthesis and anti-biofilm potential of copper nanoparticles (CuNPs) against clinical strains of Pseudomonas aeruginosa. Biofouling. 2015;31(4):379–391. doi: 10.1080/08927014.2015.1048686. [DOI] [PubMed] [Google Scholar]
- 61.Ma H., Darmawan E.T., Zhang M., Lei Z., Bryers J.D. Development of a poly (ether urethane) system for the controlled release of two novel anti-biofilm agents based on gallium or zinc and its efficacy to prevent bacterial biofilm formation. J Contr Release Off J Contr Release Soc. 2013;172(3):1035–1044. doi: 10.1016/j.jconrel.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bullen J.J., Rogers HJSpalding P.B., Ward C.G. Iron and infection: the heart of the matter. Pathog Dis. 2013;43(3):325–330. doi: 10.1016/j.femsim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 63.Tetz G.V., Artemenko N.K., Tetz V.V. Effect of DNase and antibiotics on biofilm characteristics. Antimicrob Agents Chemother. 2009;53(3):1204–1209. doi: 10.1128/AAC.00471-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Swartjes J.J.T.M., Das T., Sharifi S., Subbiahdoss G., Sharma P.K., Krom B.P. A functional DNase I coating to prevent adhesion of bacteria and the formation of biofilm. Adv Funct Mater. 2013;23(22):2843–2849. [Google Scholar]
- 65.Pavlukhina S.V., Kaplan J.B., Li X., Wei C., Yu X., Madhyastha S. Noneluting enzymatic antibiofilm coatings. Appl Mater Interfaces. 2011;4(9):4708–4716. doi: 10.1021/am3010847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan Y., Ma S., Liu C., Yu W., Han F. Enhancing the stability and antibiofilm activity of DspB by immobilization on carboxymethyl chitosan nanoparticles. Microbiol Res. 2015;178:35–41. doi: 10.1016/j.micres.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 67.Darouiche R.O., Mansouri M.D., Gawande P.V., Madhyastha S. Antimicrobial and antibiofilm efficacy of triclosan and DispersinB combination. J Antimicrob Chemother. 2009;64(1):88. doi: 10.1093/jac/dkp158. [DOI] [PubMed] [Google Scholar]
- 68.Ma L., Feng S., de la Fuente-Nunez C., Hancock R.E.W., Lu X. Development of molecularly imprinted polymers to block quorum sensing and inhibit bacterial biofilm formation. ACS Appl Mater Interfaces. 2018;10(22):18450–18457. doi: 10.1021/acsami.8b01584. [DOI] [PubMed] [Google Scholar]
- 69.Galloway W.R., Hodgkinson J.T., Bowden S., Welch M., Spring D.R. Applications of small molecule activators and inhibitors of quorum sensing in Gram-negative bacteria. Trends Microbiol. 2012;20(9):449–458. doi: 10.1016/j.tim.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 70.Zhang M., Wang M., Zhu X., Yu W., Gong Q. Equisetin as potential quorum sensing inhibitor of Pseudomonas aeruginosa. Biotechnol Lett. 2018:1–6. doi: 10.1007/s10529-018-2527-2. [DOI] [PubMed] [Google Scholar]
- 71.Ravindran D., Ramanathan S., Arunachalam K., Jeyaraj G.P., Shunmugiah K.P., Arumugam V.R. Phytosynthesized silver nanoparticles as anti-quorum sensing and antibiofilm agent against the nosocomial pathogen Serratia marcescens: an in vitro study. J Appl Microbiol. 2018:1425–1440. doi: 10.1111/jam.13728. [DOI] [PubMed] [Google Scholar]
- 72.Osmon D.R., Berbari E.F., Berendt A.R., Lew D., Zimmerli W., Steckelberg J.M. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1–e25. doi: 10.1093/cid/cis803. [eng] [DOI] [PubMed] [Google Scholar]
- 73.Tuchscherr L., Heitmann V., Hussain M., Viemann D., Roth J., von Eiff C. Staphylococcus aureus small-colony variants are adapted phenotypes for intracellular persistence. J Infect Dis. 2010;202(7):1031–1040. doi: 10.1086/656047. [eng] [DOI] [PubMed] [Google Scholar]
- 74.Neut D., Dijkstra R.J., Thompson J.I., Kavanagh C., van der Mei H.C., Busscher H.J. A biodegradable gentamicin-hydroxyapatite-coating for infection prophylaxis in cementless hip prostheses. Eur Cell Mater. 2015;29:42–55. doi: 10.22203/ecm.v029a04. discussion 55–6. [eng] [DOI] [PubMed] [Google Scholar]
- 75.Drago L., Boot W., Dimas K., Malizos K., Hansch G.M., Stuyck J. Does implant coating with antibacterial-loaded hydrogel reduce bacterial colonization and biofilm formation in vitro? Clin Orthop Relat Res. 2014;472(11):3311–3323. doi: 10.1007/s11999-014-3558-1. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahmed A.R., Elkharraz K., Irfan M., Bodmeier R. Reduction in burst release after coating poly(D,L-lactide-co-glycolide) (PLGA) microparticles with a drug-free PLGA layer. Pharmaceut Dev Technol. 2012;17(1):66–72. doi: 10.3109/10837450.2010.513989. [eng] [DOI] [PubMed] [Google Scholar]
- 77.Vorkapic D., Pressler K., Schild S. Multifaceted roles of extracellular DNA in bacterial physiology. Curr Genet. 2016;62(1):71–79. doi: 10.1007/s00294-015-0514-x. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Belcarz A., Zima A., Ginalska G. Biphasic mode of antibacterial action of aminoglycoside antibiotics-loaded elastic hydroxyapatite-glucan composite. Int J Pharmaceut. 2013;454(1):285–295. doi: 10.1016/j.ijpharm.2013.06.076. [DOI] [PubMed] [Google Scholar]
- 79.Shukla A., Fang J.C., Puranam S., Hammond P.T. Release of vancomycin from multilayer coated absorbent gelatin sponges. J Contr Release. 2012;157(1):64–71. doi: 10.1016/j.jconrel.2011.09.062. [DOI] [PubMed] [Google Scholar]
- 80.Noble M.L., Mourad P.D., Ratner B.D. Digital drug delivery: on-off ultrasound controlled antibiotic release from coated matrices with negligible background leaching. Biomater Sci. 2014;2(6):893–902. doi: 10.1039/C3BM60203F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin W., Tan H., Duan Z., Yue B., Ma R., He G. Inhibited bacterial biofilm formation and improved osteogenic activity on gentamicin-loaded titania nanotubes with various diameters. Int J Nanomed. 2014;2014(1):1215. doi: 10.2147/IJN.S57875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang Y., Ao H.Y., Yang S.B., Wang Y.G., Lin W.T., Yu Z.F. In vivo evaluation of the anti-infection potential of gentamicin-loaded nanotubes on titania implants. Int J Nanomed. 2016;11:2223–2234. doi: 10.2147/IJN.S102752. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Cremer K., Braem A., Gerits E., De Brucker K., Vandamme K., Martens J.A. Controlled release of chlorhexidine from a mesoporous silica-containing macroporous titanium dental implant prevents microbial biofilm formation. Eur Cell Mater. 2017;33:13–27. doi: 10.22203/eCM.v033a02. [DOI] [PubMed] [Google Scholar]
- 84.Braem A., De Cremer K., Delattin N., De Brucker K., Neirinck B., Vandamme K. Novel anti-infective implant substrates: controlled release of antibiofilm compounds from mesoporous silica-containing macroporous titanium. Colloids Surf B Biointerfaces. 2015;126:481–488. doi: 10.1016/j.colsurfb.2014.12.054. [DOI] [PubMed] [Google Scholar]
- 85.Li T., Wang N., Chen S., Lu R., Li H., Zhang Z. Antibacterial activity and cytocompatibility of an implant coating consisting of TiO2 nanotubes combined with a GL13K antimicrobial peptide. Int J Nanomed. 2017;12:2995–3007. doi: 10.2147/IJN.S128775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shukla A., Fleming K.E., Chuang H.F., Chau T.M., Loose C.R., Stephanopoulos G.N. Controlling the release of peptide antimicrobial agents from surfaces. Biomaterials. 2010;31(8):2348–2357. doi: 10.1016/j.biomaterials.2009.11.082. [DOI] [PubMed] [Google Scholar]
- 87.Kazemzadeh-Narbat M., Lai B.F., Ding C., Kizhakkedathu J.N., Hancock R.E., Wang R. Multilayered coating on titanium for controlled release of antimicrobial peptides for the prevention of implant-associated infections. Biomaterials. 2013;34(24):5969–5977. doi: 10.1016/j.biomaterials.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 88.de Breij A., Riool M., Kwakman P.H., de Boer L., Cordfunke R.A., Drijfhout J.W. Prevention of Staphylococcus aureus biomaterial-associated infections using a polymer-lipid coating containing the antimicrobial peptide OP-145. J Contr Release. 2016;222:1–8. doi: 10.1016/j.jconrel.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 89.Ramesh S., Govender T., Kruger H.G., de la Torre B.G., Albericio F. Short AntiMicrobial Peptides (SAMPs) as a class of extraordinary promising therapeutic agents. J Pept Sci. 2016;22(7):438–451. doi: 10.1002/psc.2894. [eng] [DOI] [PubMed] [Google Scholar]
- 90.Carmona G., Rodriguez A., Juarez D., Corzo G., Villegas E. Improved protease stability of the antimicrobial peptide Pin2 substituted with D-amino acids. Protein J. 2013;32(6):456–466. doi: 10.1007/s10930-013-9505-2. [eng] [DOI] [PubMed] [Google Scholar]
- 91.Sahariah P., Masson M. Antimicrobial chitosan and chitosan derivatives: a review of the structure-activity relationship. Biomacromolecules. 2017;18(11):3846–3868. doi: 10.1021/acs.biomac.7b01058. [eng] [DOI] [PubMed] [Google Scholar]
- 92.Tan H., Ma R., Lin C., Liu Z., Tang T. Quaternized chitosan as an antimicrobial agent: antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int J Mol Sci. 2013;14(1):1854–1869. doi: 10.3390/ijms14011854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang Y., Yang S.-B., Wang Y.-G., Zhang S.-H., Yu Z.-F., Tang T.-T. Bacterial inhibition potential of quaternised chitosan-coated VICRYL absorbable suture: an in vitro and in vivo study. J Orthop Transl. 2017;8:49–61. doi: 10.1016/j.jot.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peng Z.-X., Wang L., Du L., Guo S.-R., Wang X.-Q., Tang T.-T. Adjustment of the antibacterial activity and biocompatibility of hydroxypropyltrimethyl ammonium chloride chitosan by varying the degree of substitution of quaternary ammonium. Carbohydr Polym. 2010;81(2):275–283. [Google Scholar]
- 95.Peng Z.X., Tu B., Shen Y., Du L., Wang L., Guo S.R. Quaternized chitosan inhibits icaA transcription and biofilm formation by Staphylococcus on a titanium surface. Antimicrob Agents Chemother. 2011;55(2):860–866. doi: 10.1128/AAC.01005-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peng Z., Ao H., Wang L., Guo S., Tang T. Quaternised chitosan coating on titanium provides a self-protective surface that prevents bacterial colonisation and implant-associated infections. RSC Adv. 2015;5(67):54304–54311. [Google Scholar]
- 97.Lin W.T., Zhang Y.Y., Tan H.L., Ao H.Y., Duan Z.L., He G. Inhibited bacterial adhesion and biofilm formation on quaternized chitosan-loaded titania nanotubes with various diameters. Materials. 2016;9(3) doi: 10.3390/ma9030155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang Y., Ao H., Wang Y., Lin W., Yang S., Zhang S. Cytocompatibility with osteogenic cells and enhanced in vivo anti-infection potential of quaternized chitosan-loaded titania nanotubes. Bone Res. 2016;4:16027. doi: 10.1038/boneres.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu Y., Zheng Z., Zara J.N., Hsu C., Soofer D.E., Lee K.S. The antimicrobial and osteoinductive properties of silver nanoparticle/poly (DL-lactic-co-glycolic acid)-coated stainless steel. Biomaterials. 2012;33(34):8745–8756. doi: 10.1016/j.biomaterials.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 100.Agarwal A., Weis T.L., Schurr M.J., Faith N.G., Czuprynski C.J., Mcanulty J.F. Surfaces modified with nanometer-thick silver-impregnated polymeric films that kill bacteria but support growth of mammalian cells. Biomaterials. 2010;31(4):680–690. doi: 10.1016/j.biomaterials.2009.09.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ibrahim M., Wang F., Lou M.M., Xie G.L., Li B., Bo Z. Copper as an antibacterial agent for human pathogenic multidrug resistant Burkholderia cepacia complex bacteria. J Biosci Bioeng. 2011;112(6):570–576. doi: 10.1016/j.jbiosc.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 102.Hong R., Kang T.Y., Michels C.A., Gadura N. Membrane lipid peroxidation in copper alloy-mediated contact killing of Escherichia coli. Appl Environ Microbiol. 2012;78(6):1776–1784. doi: 10.1128/AEM.07068-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang D., Ma K., Cai X., Yang X., Hu Y., Huang P. Evaluation of antibacterial, angiogenic, and osteogenic activities of green synthesized gap-bridging copper-doped nanocomposite coatings. Int J Nanomed. 2017;12:7483–7500. doi: 10.2147/IJN.S141272. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wei X., Yang Z., Wang Y., Tay S.L., Gao W. Polymer antimicrobial coatings with embedded fine Cu and Cu salt particles. Appl Microbiol Biotechnol. 2014;98(14):6265–6274. doi: 10.1007/s00253-014-5670-2. [DOI] [PubMed] [Google Scholar]
- 105.Ballo M.K.S., Rtimi S., Pulgarin C., Hopf N., Berthet A., Kiwi J. In vitro and in vivo effectiveness of an innovative silver-copper nanoparticle coating of catheters to prevent methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2016;60(9):5349–5356. doi: 10.1128/AAC.00959-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lemire J.A., Harrison J.J., Turner R.J. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol. 2013;11(6):371. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 107.Wang T., Zhang J.C., Chen Y., Xiao P.G., Yang M.S. Effect of zinc ion on the osteogenic and adipogenic differentiation of mouse primary bone marrow stromal cells and the adipocytic trans-differentiation of mouse primary osteoblasts. J Trace Elem Med Biol Organ Soc Miner Trace Elem. 2007;21(2):84–91. doi: 10.1016/j.jtemb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 108.Hadley K.B., Newman S.M., Hunt J.R. Dietary zinc reduces osteoclast resorption activities and increases markers of osteoblast differentiation, matrix maturation, and mineralization in the long bones of growing rats. J Nutr Biochem. 2010;21(4):297–303. doi: 10.1016/j.jnutbio.2009.01.002. [eng] [DOI] [PubMed] [Google Scholar]
- 109.Huang P., Ma K., Cai X., Huang D., Yang X., Ran J. Enhanced antibacterial activity and biocompatibility of zinc-incorporated organic-inorganic nanocomposite coatings via electrophoretic deposition. Colloids Surf B Biointerfaces. 2017;160:628–638. doi: 10.1016/j.colsurfb.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 110.Lin J., Ding J., Dai Y., Wang X., Wei J., Chen Y. Antibacterial zinc oxide hybrid with gelatin coating. Mater Sci Eng C Mater Biol Appl. 2017;81(2):321. doi: 10.1016/j.msec.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 111.Tobin E.J. Recent coating developments for combination devices in orthopedic and dental applications: a literature review. Adv Drug Deliv Rev. 2017;112:88–100. doi: 10.1016/j.addr.2017.01.007. [DOI] [PubMed] [Google Scholar]