Abstract
Bone wax, primarily composed of beeswax and softening agent, is a century-old material used to control bleeding of disrupted bone surfaces by acting as a mechanical barrier to seal the wound. The current bone wax products are commonly packed in easy-to-open foil in the form of sterile sticks or plates, with excellent malleability and smooth consistency, enabling cost-effective and easy handling approach for bleeding control. It has also been reported that the inert nature of bone wax causes complications including foreign body reaction, infection promotion and bone healing inhibition. With the advances in biomaterials and the market boost of bone haemostatic materials, the arena of bone wax substitute research has expanded to a wide spectrum of material formulations and forms. However, the development of substitutes of bone wax for translation is a pivotal yet challenging topic because currently a potential candidate is recommended to be just as simple to use, effective and inexpensive to produce as traditional bone wax but also be absorbable and osteogenic. This review provides an overview of bone wax including its history, clinical applications and associated complication. In addition, emerging substitutes of bone wax and outlooks of future directions including the standardised evaluation methods are also discussed as an effort to catalyse the innovation and translation of bone haemostatic agents in the near future.
The translational potential of this article: Occurrence of osseous haemorrhage is common in surgically incised or traumatically fractured bone. It is essential to stop bone bleeding to avoid further pathologic consequences such as tissue necrosis and eventually mortalities due to blood loss. Medical sterile bone wax is a classical material for haemostasis of bone during orthopaedic surgeries, thoracic surgeries, neurological surgeries and so on. Along with its widespread use, complications such as foreign body reaction, bone healing inhibition and infection promotion associated with bone wax are observed. With the growing knowledge in biomaterials and the boost of market of bone haemostatic materials, bone wax substitute research is thriving. An overview of bone and its substitutes together with evolution of their design criteria is carried out in this work, providing information for the innovation and translation of bone haemostatic agents in the near future.
Keywords: Biocompatible materials, Bone regeneration, Bone wax, Haemostatic
Introduction
Bone contains abundant channels for blood and bone marrow. When it is surgically incised or traumatically fractured, osseous haemorrhage can be a difficult problem to control, especially in the highly vascular bones of the spine and sternum. Medical sterile bone wax is an essential material for haemostasis of bone during orthopaedic surgeries, thoracic surgeries and neurological surgeries. This material is commonly defined as a waxy substance used to mechanically control bleeding from bone fractures for previously addressed surgical procedures. Bone wax is strongly hydrophobic and is not metabolised; therefore, it is minimally resorbed from the site of application and causes no effect on pH of the contacted body fluids [1]. Bone wax has no inherent haemostatic quality. In vitro experiments showed that suspension of bone wax in blood can promote platelet aggregation to a modest extent, but its platelet-aggregating effect in vivo is not of significance because of small contact surface [2]. Therefore, bone wax mainly acts as an impenetrable mechanical barrier (tamponade/sealant) at the wound site. In brief, bone haemostasis after local application of bone wax results from the mechanical occlusion of haversian canals in cortical bone and medullary spaces in cancellous bone, blocking blood flow from transected vessels and allowing clotting to occur.
The medical application history of bone wax can be traced back to the eighteenth century [3]. The classical and most widely used formulation was developed by Sir Victor Alexander Haden Horsley in 1885, which composed of seven parts of beeswax, one part of almond oil and one percent of salicylic acid [4]. The first documented evidence of the successful use of bone wax in clinical surgery appeared in 1892, when Rushton Parker used it to stop bleeding from the lateral sinus [5]. Since then, the term “Horsley's wax” was synonymous with bone wax although several formula modifications were developed. For instance, Wharton reported two formulas of bone wax in 1905; one was composed of 2 parts of olive oil, 8 parts of spermaceti, and 1% iodine, and the other was composed of 3 parts of bismuth subnitrate, 0.5 part of white wax and 6 parts of petroleum jelly [6]. Similarly, Simmons [7] proposed a formula of 3 parts of spermaceti, 3 parts of sesame oil and 4 parts of iodoform in 1911. In 1950, Geary and Fhantz [8] introduced the idea of making partially biodegradable bone wax, inventing a formula consisting of 6 parts of Carbowax 1540, 1.5 parts of polyethylene glycol (PEG) and 2.5 parts of oxidised cellulose. Unfortunately, none of the aforementioned alternatives of Horsley's wax made into successful market launching.
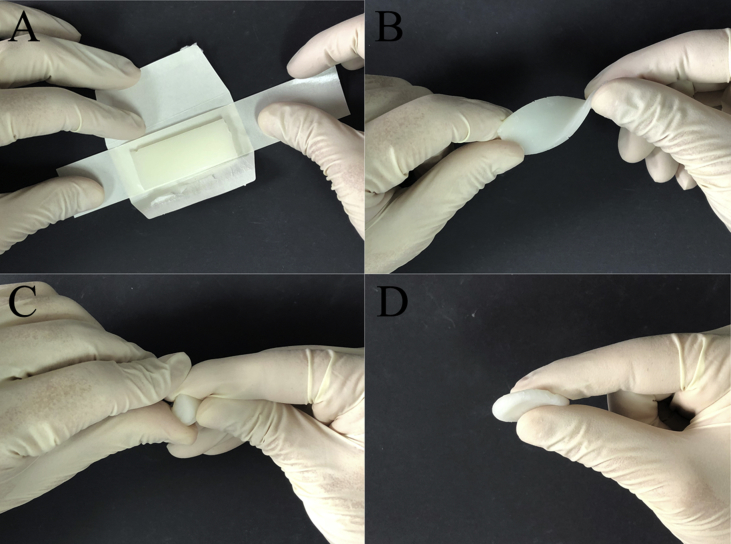
After the evolution of over a century, the current commercial bone wax products still mainly consist of beeswax and softening agent such as vaseline or a mixture of paraffin wax and isopropyl palmitate. Bone wax in the market is categorised as Class 2 medical device by the US Food and Drug Administration (FDA) and is commonly supplied in easy-to-open foil package in the form of sterile sticks or plates. Bone wax is quite cheap. Although prices may differ from country to country, costs for one sterilised ready-to-use 2.5-g strip may not range outside a single-digit dollar frame [3], [9]. It has a shelf life of approximately five years if stored properly. Main bone wax products commercially available at present are provided by several manufacturers such as Aesculap, CP Medical, Covidien, Ethicon, Surgical Specialties and so on. In practice, bone wax should be used immediately after removal from the package; and it should be softened to the desired consistency before applying by moulding with the fingers or by immersing the unopened foil packet in a warm sterile solution [10]. The material displays excellent malleability and smooth consistency and is capable of being smeared across the cut surface to plug the holes in the bone to stop bleeding physically (Fig. 1). The optimum working temperature of bone wax is usually suggested to be 21–23°C.
Figure 1.
Operating process of bone wax. (A) Bone wax is a sterile and flat slice wrapped in a tiny bag; (B), (C) and (D) softened bone wax is malleable and easy to be shaped.
The applications of bone wax
Both cancellous and cortical bones contain vascular tissues, and when the bone is incised or fractured, damage to its vasculature can cause osseous haemorrhage that is sometimes too severe to be controlled by natural haemostasis. In this case, haemorrhage should be effectively controlled to avoid further pathologic consequences such as tissue necrosis and eventually mortalities due to blood loss [11]. At the present time, some options can be used in clinical settings for bone haemostasis: (a) the use of classical absorbable haemostatic agents such as collagen and oxidised cellulose, (b) the application of electrocautery and (c) use of bone wax. However, the use of oxidised cellulose is limited because of its inappropriate knitted fabric form and lack of adherence within the bone, causing problems in sealing irregular surfaces and pores of defective bone; collagen, on the other hand, in various forms, alone or in combination with fibrin and suspended in various delivery vehicles, has been proposed as a bone haemostatic agent but problems with storage stability, cohesiveness and biocompatibility have prevented practical fruition [12], [13]. The use of electrocautery, which thermally sears oozing blood vessels and closes them, is time-consuming and can easily induce severe thermal damage to tissues, which may further delay osteogenesis and allow soft tissue ingrowth that interferes with normal bone union [13], [14]. Instead, bone wax is highlighted by its ease of operation, satisfactory cohesion to bone, malleability and cost-effectiveness. It is mainly applied for bone haemostasis during orthopaedic surgeries [15], thoracic surgeries [16] and neurological surgeries [3], but can be occasionally extended to dental and jaw surgeries [17], [18], [19]. In addition to directly being applied to the wound site, bone wax can be used to modify surgical tools for blood control purposes. For example, in percutaneous endoscopic cervical discectomy via the anterior transcorporeal approach for cervical intervertebral disc herniation, bone wax was smeared onto the endoscopic burr to control bleeding without obvious interference with bone healing [20]. Similarly, it can also be used to prevent the leakage of blood through the lumen of a cannulated screw after arthroscopic repair of the anterior cruciate ligament [21].
Concerns of bone wax
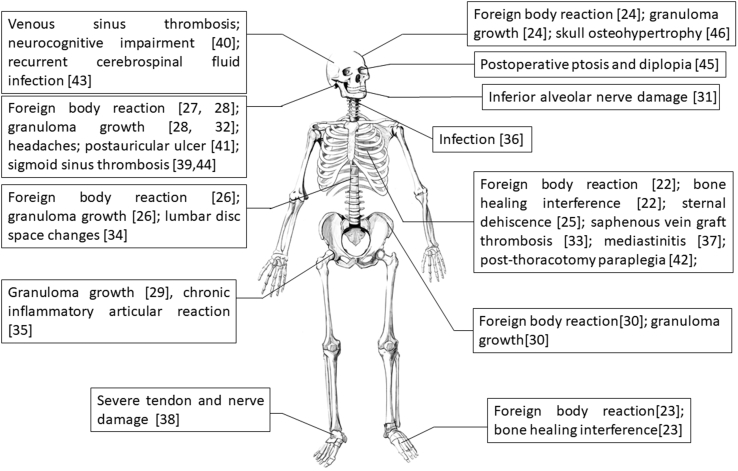
Clinical practice has uncovered numerous complications associated with bone wax since its development. Although bone wax has potential and promise for haemostasis application, the related complications may outweigh its benefits. As shown in Fig. 2, a series of complications in surgeries were reported, such as failed bone healing, foreign body reaction, granuloma growth, thrombosis, infection and nerve compression [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46].
Figure 2.
Summary of reported complications caused by bone wax in the body.
A rat calvarial bone model was used to define the local reactions of bone to bone wax [47]. It was confirmed bone regrowth was markedly impaired by the presence of bone wax. Inert bone wax most typically encompassed the bone margins in a collar-like fashion, and active bone production was observed only beyond this collar and then connected mostly to the external periosteum. Moderate-to-severe inflammation, foreign body reaction and fibrous reaction were observed in the lesion. The observations are in consistent with histologic findings associated with the implantation of bone wax in a rat tibia model, which typically includes foreign body reactions and a lack of bone formation [48]. Sorrenti et al. [49] investigated the response of human tibia to bone wax. At the early stage, a nonspecific inflammatory response was noted (6 months), followed by an increase in fibrous tissue with foreign-body giant cells (9 months). After 13 months, mature fibrous tissue with no inflammatory response was observed. As bone wax dramatically interferes with bone healing, it should be used sparingly and restricted in the sites where fusion is highly desired.
The effect of bone wax on the ability of cancellous bone to clear bacteria was examined using a Staphylococcus aureus and rabbit model, indicating that as a foreign body, bone wax can significantly diminish the ability of bone to clear bacteria [50]. Similarly, in a rat model of chronic S. aureus osteomyelitis, the infection-promoting potential of sterile bone wax was also observed [51]. In a case series of 19 patients presenting with osteomyelitis of the sternum after cardiac surgery, bone wax applied to the oozing sternum halves was postulated to be the possible cause of this problem [52]. Because of the risk of induction of infection, bone wax must never be used in contaminated fields. Besides, cleansing of bone wax in iodine is recommended after manual manipulation during clinical practice.
In addition to these postoperative complications, the efficiency of control of bone bleeding using bone wax is also in dispute. For example, in a prospective randomised study on 400 thoracic surgical patients undergoing isolated coronary bypass surgery, bone wax application after median sternotomy showed no benefits in blood loss control [9]. In contrast, in a report of total knee arthroplasty, the application of bone wax was reported to be safe and effective for reducing total blood loss and maintaining higher haemoglobin levels [15]. Postoperative evaluations revealed that its application on the exposed cancellous bone surface around the femoral and tibia prostheses to seal the nail holes in total knee arthroplasty is safe and is effective for reducing total blood loss and maintaining higher haemoglobin levels. No consensus is achieved yet, and surgeons may readily use bone wax at their own experience and discretion.
So far, the status of bone wax has been overviewed, and a table summarising the advantages and disadvantages of bone wax is thereby presented as a guideline for the development of a new generation of bone haemostatic materials (Table 1).
Table 1.
Advantages and disadvantages of bone wax.
| Advantages | Disadvantages |
|---|---|
| Low cost | Inertness |
| Easy handling | Bone union prevention |
| Malleability | Foreign body reaction induction |
| Inertness | Granuloma growth induction |
| Sealing capacity | Infection promotion |
| Bone adherence | Lack of inherent haemostatic quality |
| Long clinical history | Undesired immigration |
| — | Thrombosis induction |
Substitutes of bone wax
It has been recognised that the major factor causing the aforementioned postoperative complications such as bone union prevention, infection promotion and foreign body reaction is the intrinsic inertness and poor biocompatibility of bone wax. The attempt of developing absorbable bone wax can be dated back to 1950, when Geary and Frantz [8] reported experimental haemostatic bone wax by combining Carbowax, PEG and oxidised cellulose together. Although this formulation failed in dental surgery studies [15], this work guided the direction of development of bone wax substitutes by clarifying that absorbability is the top priority in the design of substitutes.
From 1980 to 2000, numerous bone wax substitute prototypes have been reported in the literature, such as fatty acid salts [53], fibrin/collagen paste [54], [55], gelatin paste [56], glycolic or lactic acid/glycerol oligomers [57], [58], partially deacetylated chitin hydrochloride [59], PEG/microfibrillar collagen paste [60], polydioxanone/natural oils [61] and polyorthoester [54]. Unfortunately, none of these formulations are in widespread use or launched in market, which suggests that it has been difficult to combine the beneficial characteristics of traditional bone wax with the advantages of an absorbable material.
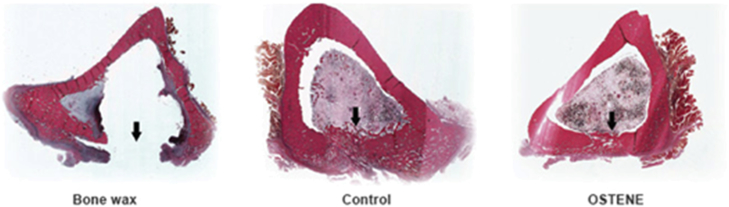
In the 2000s, an ideal bone wax substitute was suggested to be just as simple to use, effective and inexpensive to produce as traditional bone wax but would also be fully absorbable, noninflammatory and biocompatible. According to this criterion, water soluble wax composed solely of alkylene oxide block copolymers (Pluronics) was developed in 2001, which has material, application and haemostatic characteristics that are similar to those of bone wax, but its absorbable property avoids the negative biological effects [62]. Inspired by this pioneering work, a commercially available water-soluble alkylene oxide copolymer–based bone wax substitute (Ostene, “absorbable bone wax”; Ceremed, Inc., Los Angeles, CA, USA) was launched in 2006 [63]. Similar to bone wax, Ostene can be softened by manual manipulation before use and sticks well to bleeding bone as a tamponade. The material dissolves in the implanted site within 24–48 h, allowing the early phases of bone healing to occur. Because of its solubility, Ostene provides the potential to address adverse reactions associated with inert bone wax [64], [65] (Fig. 3). This formula was later revised to develop new products in the literature, such as a putty-like mixture of alkylene oxide copolymers and carboxymethylcellulose sodium salt (Absorbable haemostatic bone putty; Abyrx, Inc., Irvington, NY, USA) [66] or a glue-like miscible blend of PEG–polypropylene glycol–PEG (PEG–PPG–PEG) copolymer and pregelatinized starch [67].
Figure 3.
The rabbit tibia model was inoculated with Staphylococcus aureus, introduced into the intramedullary canal through a defect created at the anteromedial facet of the proximal tibia. After 4 weeks, the cross section of the rabbit tibia shows normal bone development in the cortical window of the Ostene and control samples. The bone wax cross section shows signs of osteomyelitis with no sign of bone healing [64].
Back in 1992, haemostatic agents including microfibrillar collagen flour, absorbable gelatin sponge and oxidised regenerated cellulose powder were applied as alternatives to bone wax in iliac bone procurement, showing no bone regeneration inhibition as planned [68]. Such extended applications of existing haemostatic agents, including hydrated gelatin powder [69], gelatin paste [56], gelatin–thrombin matrix sealant [70], fibrin solution [71], patient-derived fibrin sealant [72], autologous platelet-poor plasma gel [73], fibrin dressing [74] and gel-like mixture of agar and coagulation factors [75], for control of bone bleeding were popular in research. However, whether these agents can act as alternatives to bone wax in bone haemostasis is still questionable because of limited animal studies and lack of clinical trials.
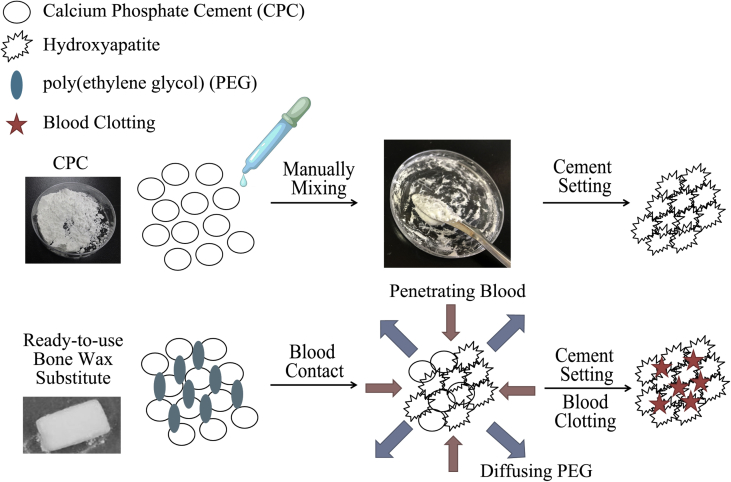
In the past few years, the reciprocal influence between the conceptual strategy of developing absorbable substitutes and the better understanding of haemostasis and bone regeneration has led to the evolution of bone wax substitutes from a sole haemostatic agent to hybrid agent with both haemostatic and bone regeneration capabilities. On the one hand, some fast absorbable haemostatic agents are not free of risk of complications, possibly causing allergic reaction and retarding bone regeneration to some extent, making it a must-addressed issue in designing bone wax substitute [76]. On the other hand, in many surgical scenarios, scaffold-induced bone healing is highly desirable [77], [78]. One typical strategy in the present day is the adoption of bioceramic cement–based paste/putty in bone haemostasis, whose phase and as-formed matrix can help to both stop bleeding and enhance osteogenesis [79], [80], [81]. To further improve blood clotting efficiency and handling properties, supplements such as alginate [82], cellulose [83] and chitosan [84] can be blended. For example, currently Zhang et al. reported a self-curing bone wax substitute by mixing tricalcium silicate (C3S) cement and 58S bioactive glass/chitosan/carboxymethyl cellulose with KH2PO4 setting solution, which enables haemostasis, injection and bone cell proliferation [80]. Calcium Apatite bone tamponade (CAAP; Skeletal Kinetics, LLC., Cupertino, CA, USA) is an FDA-approved product composed of calcium phosphate, sodium silicate solution and a mixing system (mixing bowl, pestle and spatula). Its operation procedure is kind of similar to that of cement: (1) open powder vial, pour powder into the mixing bowl, and gently tap the vial to ensure maximum transfer of powder; (2) slowly pour the liquid vial into the mixing bowl; (3) use the pestle to vigorously mix in circular motion the powder and liquid for approximately 1 min, and make sure to reincorporate the material collected on the pestle into the mixing process to achieve a proper mix and (4) when mixed together, it forms a cement-like paste that can be applied directly to sites of bleeding bone; the resulting hardening scaffold from the paste is composed of hydroxyapatite similar to the mineral phase of native bone tissue, enabling bony ingrowth and bone regeneration [79], [85]. The drawback of this formula is the necessity to manually mix the powder and setting solution before use, increasing operation steps and contamination risk. As a solution to this issue, a ready-to-use paste of calcium phosphate cement, PEG and pregelatinised starch was reported [86] (Fig. 4). After exposure to a humid environment, the PEG phase dissolved and was exchanged by penetrating water that interacted with the calcium phosphate precursor to form highly porous, nanocrystalline hydroxyapatite via a dissolution/precipitation reaction. Simultaneously, pregelatinised starch could gel and supply the mixture with liquid-sealing features. The novel formulation was found to be cohesive and malleable, and after hardening under aqueous conditions, it had a mechanical performance (∼2.5 MPa compressive strength) that is comparable to that of cancellous bone. The concerns of this formula are the sensitivity of calcium phosphate cement to moisture during storage and the lack of clinical evaluations. Nevertheless, the ready-to-use design demonstrates high translational potential as it would simplify the surgery and make the material closer to the handling characteristics of bone wax.
Figure 4.
Traditional calcium phosphate cement (CPC) requires the addition of a liquid curing agent and manual mixing to finally form HA, whereas ready-to-use bone wax substitute can be much simpler and easier to use [86]. After exposure to blood, the PEG phase dissolved and was exchanged by penetrating blood, which stimulates HA matrix formation in situ. HA, hydroxyapatite; PEG, polyethylene glycol.
Owing to the potential risk of infection, antibiotics have been incorporated into bone wax substitutes [87], [88]. Such an attempt paves the possibility of using bone wax substitutes to deliver therapeutic agents to enhance blood clotting or bone regeneration. For example, water-soluble bone wax substitutes were suggested to serve the additional purpose of acting as an absorbable matrix for short-term drug delivery (e.g., bone morphogenic proteins, antibiotics and cytokines) to the damaged bone, providing additional benefits by promoting osteogenesis, improving fusion rates, reducing inflammation and preventing postsurgical infections [62]. The challenges mainly rely on the shelf life of therapeutic agents and their release and cost control. There is increasing amount of evidence over the past decade demonstrating that the delivery of selected bioactive ions can trigger specific biological responses such as microbial inhibition, blood clotting stimulation, angiogenesis and osteogenesis [89], [90], [91]. In reported bone wax substitutes, bioceramics such as bioactive glass, tricalcium silicate and calcium phosphate have been added to promote bone regeneration, which are also potential candidates for bioactive ion delivery. The design of bone wax substitutes as bioactive ion carriers requires the control of different ion release profiles after the material is exposed to blood and analysis of their consequent biological responses in situ in blood clotting and bone regeneration.
Outlook and summary
Since the introduction of bone wax over 125 years, this century-old haemostatic agent is still being used for controlling bone bleeding and sealing. Clinical complications originated from the nature of bone wax spur the continuing research of substitutes, and a potential candidate is recommended to be just as simple to use, effective and inexpensive to produce as traditional bone wax but would also be absorbable and osteogenic.
According to a market report entitled “Bone Wax Market - Global Industry Analysis, Size, Share, Growth, Trends, and Forecast, 2018–2026” by Transparency Market Research, the global market size was estimated to be US$ 68.8 million in 2017 and is projected to expand at a compound annual growth rate of 2% from 2018 to 2026 to reach US$ 84.2 million in 2026 [92]. The US holds the major share of the market owing to the increase in the adoption of emerging new substitute products of bone wax along with high awareness of end-users. In addition, market players in the US are more active in research and development of or introducing new products with improved efficacy to the market. Europe is the second major market for bone wax. The market in these regions is driven by the increase in the number of surgical procedures, innovations in bone wax products, rise of bone diseases and accidental fracture cases. The bone wax market in the Asia–Pacific region is also growing rapidly at a growth rate of 3% during the forecast period, driven primarily by the developing countries such as India and China because of the soaring market needs.
With the growing knowledge and technology advances in biomaterials and the boost of market, the arena of bone wax substitute research has expanded to a wide spectrum of material formulations and forms to meet the evolving design criteria. The innovation and translation of bone wax substitutes is expected to thrive in the near future.
As the outcomes of research move towards translation and commercialisation, it will also be important to elucidate thorough standardised evaluation systems of bone wax and its substitutes, which will lead to improved material design and generation. From an operational point of view, it is highly suggested that bone wax and its substitutes can easily detach from gloves when pressed into cavity but can exhibit strong adhesion to the bleeding bone surfaces or the capability to seal the bleeding defects. In FDA enforcement reports of bone wax, the failure of bone wax is largely attributed to its loss of adhesion to the host site after storage [93]. However, according to the FDA information, such failure reports are largely based on the subjective judgement. Contradictory evaluation results are sometimes achieved between the relevant surgeons and regulatory agency. This controversy definitely calls for the standardised evaluation of the adhesive and sealing capability of bone wax. So far, the haemostatic performance has been generally studied through in vivo animal experiments by drilling holes in the bone and plugging with bone wax. Instead of complicated animal testing, an in vitro model has been proposed by Suwanprateeb et al [94] for sealing capability testing. In brief, acrylic glass tubes with a length of 2.00 m and an inner diameter of 3.00 mm were filled with water up to a height of 1.91 m, which corresponds to systolic blood pressure (18.68 kPa, 140 mmHg), and sealed with cone-shaped samples; the constructs were stored at room temperature and monitored until their failure. However, there are drawbacks for this model design: (1) in body, the blood actually diffuses from the damaged cancellous bone, different from the flowing of water in a tube, and (2) water is far different from blood, unable to reflect blood clotting and other haemodynamic behaviours. Besides, the relevant testing of shelf life and lone-term adhesiveness, preservation of samples is commonly lacking in the research and development of bone wax substitutes. Taken together, reliable and standardised testing methods for bone haemostatic agents with haemostatic and adhesive capabilities are needed in the future.
Conflict of interest
The authors have no conflicts of interest to disclose in relation to this article.
Acknowledgement
This work was supported by the Natural Science Foundation of Jiangsu Province (No. BK20181045), the National Natural Science Foundation of China (No.81622032 and 51672184), the Natural Science Research of Jiangsu Higher Education Institutions (No.17KJA180011) and the Jiangsu Innovation and Entrepreneurship Program.
Contributor Information
Huan Zhou, Email: Huan.Zhou@rockets.utoledo.edu.
Lei Yang, Email: ylei@hebut.edu.cn.
References
- 1.Bjorenson J.E., Grove H.F., Gloria List S.M., Haasch G.C., Peter Austin B. Effects of hemostatic agents on the pH of body fluids. J Endod. 1986;12:289–292. doi: 10.1016/s0099-2399(86)80110-8. [DOI] [PubMed] [Google Scholar]
- 2.Solheim E., Anfinsen O.-G., Holmsen H., Sudmann E. Effect of local hemostatics on platelet aggregation. Eur Surg Res. 1991;23(1):45–50. doi: 10.1159/000129135. [DOI] [PubMed] [Google Scholar]
- 3.Das J.M. Bone wax in neurosurgery: a review. World Neurosurg. 2018;116:72–76. doi: 10.1016/j.wneu.2018.04.222. [DOI] [PubMed] [Google Scholar]
- 4.Ellis H. Horsley's wax. J Perioper Pract. 2007;17:82–83. doi: 10.1177/175045890701700206. [DOI] [PubMed] [Google Scholar]
- 5.Gupta Gaurav, Prestigiacomo Charles J. From sealing wax to bone wax: predecessors to Horsley's development. Neurosurg Focus. 2007;23:1–4. doi: 10.3171/foc.2007.23.1.16. [DOI] [PubMed] [Google Scholar]
- 6.Wharton H. 7th ed. Lea and Febiger; Philadelphia and New York: 1909. Minor surgical procedures in minor and operative surgery including bandaging; p. 176. [Google Scholar]
- 7.Simmons C.C. VI. Bone abscess treated with Moorhof's bone wax: a report of five cases. Ann Surg. 1911;53:67–76. doi: 10.1097/00000658-191101000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geary J.R., Fhantz V.K. New absorbable hemostatic bone wax. Ann Surg. 1950;132:1128–1137. doi: 10.1097/00000658-195012000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jorg P., Martin H., Stock U.A., Kuehnel R.U., Albes J.M. Is bonewax safe and does it help? Ann Thorac Surg. 2008;85:1002–1006. doi: 10.1016/j.athoracsur.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 10.Surgical specialties bone wax. Available at: http://www.hospeq.com/v/vspfiles/photos/pdfs/surgical-specialties-bone-wax.pdf.
- 11.Hickman D.A., Pawlowski C.L., Sekhon U.D.S., Marks J., Gupta A.S. Biomaterials and advanced technologies for hemostatic management of bleeding. Adv Mater. 2018;30:1700859. doi: 10.1002/adma.201700859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Absorbable putty-like implants and methods for their use for mechanical hemostatsis of bone and for the treatment of osseous defects. US Patent 20060002976A1.
- 13.Absorbable implants and methods for their use in hemostasis and in the treatment of osseous defects. US Patent 20060013857A1.
- 14.Saaiq M., Zaib S., Ahmad S. Electrocautery burns: experience with three cases and review of literature. Ann Burns Fire Disaster. 2012;25:203–206. [PMC free article] [PubMed] [Google Scholar]
- 15.Moo I.H., Chen J.Y.Q., Pagkaliwaga E.H., Tan S.W., Poon K.B. Bone wax is effective in reducing blood loss after total knee arthroplasty. J Arthroplasty. 2017;32:1483–1487. doi: 10.1016/j.arth.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Bone wax. Available at: https://enwikipediaorg/wiki/Bone_wax.
- 17.Douglas B.L. Clinical observations on the use of absorbable hemostatic bone wax in dental and oral surgery. Oral Surg Oral Med Oral Pathol. 1953;6:1195–1198. doi: 10.1016/0030-4220(53)90007-3. [DOI] [PubMed] [Google Scholar]
- 18.Selden H.S. Bone wax as an effective hemostat in periapical surgery. Oral Surg Oral Med Oral Pathol. 1970;29:262–264. doi: 10.1016/0030-4220(70)90095-2. [DOI] [PubMed] [Google Scholar]
- 19.Marta K., Kornel K., Piotr F. Safety and efficacy of bone wax in patients on oral anticoagulant therapy. Acta Pol Pharm. 2014;71:683–686. [PubMed] [Google Scholar]
- 20.Chu L., Yang J.-S., Yu K.-X., Chen C.-M., Hao D.-J., Deng Z.-L. Usage of bone wax to facilitate percutaneous endoscopic cervical discectomy via anterior transcorporeal approach for cervical intervertebral disc herniation. World Neurosurg. 2018;118:102–108. doi: 10.1016/j.wneu.2018.07.070. [DOI] [PubMed] [Google Scholar]
- 21.Bohy B., Feyen J., Smits P., Nuyts R. Bone wax as a way to prevent hematoma after arthroscopic anterior cruciate ligament reconstruction. Arthroscopy. 2002;18:1–2. doi: 10.1053/jars.2002.36463. [DOI] [PubMed] [Google Scholar]
- 22.Ozerdem G., Hidiroglu M., Kucuker A., Kunt A., Cetin L. Bone wax as a cause of a foreign body granuloma in a resternotomy: a case report. J Cardiothorac Surg. 2013;8:P121. [Google Scholar]
- 23.Hill J., Little J., Ford T. Bone wax: a foreign body/giant cell reaction in the foot. Foot Ankle Spec. 2013;6:236–238. doi: 10.1177/1938640013484797. [DOI] [PubMed] [Google Scholar]
- 24.Wolvius E.B., van der Wal K.G.H. Bone wax as a cause of a foreign body granuloma in a cranial defect: a case report. Int J Oral Maxillofac Surg. 2003;32:656–658. doi: 10.1054/ijom.2002.0394. [DOI] [PubMed] [Google Scholar]
- 25.Alhan C., Arıtürk C., Senay S., Okten M., Güllü A.U., Kilic L. Use of bone wax is related to increased postoperative sternal dehiscence. Kardiochir Torakochi. 2014;11:385–390. doi: 10.5114/kitp.2014.47337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eser O., Cosar M., Aslan A., Sahin O. Bone wax as a cause of foreign body reaction after lumbar disc surgery: a case report. Adv Ther. 2007;24:594–597. doi: 10.1007/BF02848783. [DOI] [PubMed] [Google Scholar]
- 27.Ateş Ö., Çayli S.R., Gürses İ. Bone wax can cause foreign body granuloma in the medulla oblongata. Br J Neurosurg. 2004;18:538–540. doi: 10.1080/02688690400012566. [DOI] [PubMed] [Google Scholar]
- 28.Patel R.B., Kwartler J.A., Hodosh R.M. Bone wax as a cause of foreign body granuloma in the cerebellopontine angle. J Neurosurg. 2000;92:362. doi: 10.3171/jns.2000.92.2.0362. [DOI] [PubMed] [Google Scholar]
- 29.Lavigne M., Boddu Siva Rama K.R., Doyon J., Vendittoli P.-A. Bone-wax granuloma after femoral neck osteoplasty. Can J Surg. 2008;51:E58–E60. [PMC free article] [PubMed] [Google Scholar]
- 30.Qayum A., Koka A.H. Foreign body reaction to bone wax an unusual cause of persistent serous discharge from iliac crest graft donor site and the possible means to avoid such complication – a case report. Case J. 2009;2:9097. doi: 10.1186/1757-1626-2-9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katre C., Triantafyllou A., Shaw R.J., Brown J.S. Inferior alveolar nerve damage caused by bone wax in third molar surgery. Int J Oral Maxillofac Surg. 2010;39:511–513. doi: 10.1016/j.ijom.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 32.Low W.K., Sim C.S. Bone wax foreign body granuloma in the Mastoid. ORL. 2002;64:38–40. doi: 10.1159/000049267. [DOI] [PubMed] [Google Scholar]
- 33.Chun P.K.C., Virmani R., Mason T.E., Johnson F. Bone wax granuloma causing saphenous vein graft thrombosis. Am Heart J. 1988;115:1310–1313. doi: 10.1016/0002-8703(88)90029-4. [DOI] [PubMed] [Google Scholar]
- 34.Ozdemir N., Gelal M., Minoglu M., Celik L. Reactive changes of disc space and foreign body granuloma due to bone wax in lumbar spine. Neurol India. 2009;57:493–496. doi: 10.4103/0028-3886.55606. [DOI] [PubMed] [Google Scholar]
- 35.Solomon L.B., Guevara C., Büchler L., Howie D.W., Byard R.W., Beck M. Does bone wax induce a chronic inflammatory articular reaction? Clin Orthop Relat Res. 2012;470:3207–3212. doi: 10.1007/s11999-012-2457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi B.-K., Yang E.-J. Delayed infection after using bone wax in maxillofacial surgery: a rare complication after reduction mandibuloplasty. Wound Med. 2017;17:18–23. [Google Scholar]
- 37.Bhatti F., Dunning J. Does liberal use of bone wax increase the risk of mediastinitis? Interact Cardiovasc Thorac Surg. 2003;2:410–412. doi: 10.1016/S1569-9293(03)00180-4. [DOI] [PubMed] [Google Scholar]
- 38.Allen-Wilson N., Beatty R., Sharpe J. Severe bone wax foreign-body reaction causing peroneal tendon destruction. J Am Podiat Med Assn. 2015;105:74–79. doi: 10.7547/8750-7315-105.1.74. [DOI] [PubMed] [Google Scholar]
- 39.Crocker M., Nesbitt A., Rich P., Bell B. Symptomatic venous sinus thrombosis following bone wax application to emissary veins. Br J Neurosurg. 2008;22:798–800. doi: 10.1080/02688690802256399. [DOI] [PubMed] [Google Scholar]
- 40.Fahradyan A., Ohanisian L., Tsuha M., Park M.J., Hammoudeh J.A. An unusual complication of bone wax utilization. J Craniofac Surg. 2018;29:976–979. doi: 10.1097/SCS.0000000000004321. [DOI] [PubMed] [Google Scholar]
- 41.Baird S.M., Teh B.M., Lim K.K.M., Campbell M.C. Bone wax extrusion through postauricular wounds: a case series. Laryngoscope. 2018;128:369–372. doi: 10.1002/lary.26697. [DOI] [PubMed] [Google Scholar]
- 42.Kumar A., Kale S.S., Dutta R., Kumar A. Post-thoracotomy paraplegia due to epidural migration of bone wax. Eur J Cardio Thorac. 2009;35:734–736. doi: 10.1016/j.ejcts.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 43.Spennato P., Escamilla-Rodrìguez I.E., Di Martino G., Imperato A., Mirone G., Cinalli G. Intraventricular bone wax as cause of recurrent cerebrospinal fluid infection: a neuroradiologic pitfall. World Neurosurg. 2016;88 doi: 10.1016/j.wneu.2015.11.030. 690.e7–.e9. [DOI] [PubMed] [Google Scholar]
- 44.Byrns K., Khasgiwala A., Patel S. Migration of bone wax into the sigmoid sinus after posterior fossa surgery. Am J Neuroradiol. 2016;37:2129–2133. doi: 10.3174/ajnr.A4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maki Y., Ishibashi R., Yamada D., Morita T., Chin M., Yamagata S. Postoperative ptosis and diplopia induced by the intraoperative application of bone wax. World Neurosurg. 2017;103 doi: 10.1016/j.wneu.2017.03.102. 951.e1–.e3. [DOI] [PubMed] [Google Scholar]
- 46.Kamide T., Nakada M., Hirota Y., Hayashi Y., Hayashi Y., Uchiyama N. Skull osteohypertrophy as a complication of bone wax. J Clin Neurosci. 2009;16:1658–1660. doi: 10.1016/j.jocn.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 47.Alberius P., Klinge B., Sjögren S. Effects of bone wax on rabbit cranial bone lesions. J Cranio Maxillofac Surg. 1987;15:63–67. doi: 10.1016/s1010-5182(87)80020-3. [DOI] [PubMed] [Google Scholar]
- 48.Howard T.C., Kelley R.R. The effect of bone wax on the healing of experimental rat tibial lesions. Clin Orthop Relat Res. 1969;63:226. [PubMed] [Google Scholar]
- 49.Sorrenti S.J., Cumming W.J., Miller D. Reaction of the human tibia to bone wax. Clin Orthop Relat Res. 1984;182:293–296. [PubMed] [Google Scholar]
- 50.Johnson P.,., Fromm D. Effects of bone wax on bacterial clearance. Surgery. 1981;89:206–209. [PubMed] [Google Scholar]
- 51.Nelson D.R., Buxton T.B., Luu Q.N., Rissing J.P. The promotional effect of bone wax on experimental Staphylococcus aureus osteomyelitis. J Thorac Cardiovasc Surg. 1990;99:977–980. [PubMed] [Google Scholar]
- 52.Robicsek F.,., Daugherty H.K., Cook J.W., Selle J.G., Masters T.N., O'Bar P.R. Mycobacterium fortuitum epidemics after open-heart surgery. J Thorac Cardiovasc Surg. 1978;75:91–96. [PubMed] [Google Scholar]
- 53.Synthetic absorbable hemostatic composition. US Patent 4440789.
- 54.Pinholt E.M., Solheim E., Bang G., Sudmann E. Bone induction by composites of bioresorbable carriers and demineralized bone in rats: a comparative study of fibrin-collagen paste, fibrin sealant, and polyorthoester with gentamicin. J Oral Maxillofac Surg. 1992;50:1300–1304. doi: 10.1016/0278-2391(92)90231-n. [DOI] [PubMed] [Google Scholar]
- 55.Harris P., Capperauld I. Clinical experience in neurosurgery with absele: a new absorbable haemostatic bone sealant. Surg Neurol. 1980;13:231–235. [PubMed] [Google Scholar]
- 56.Wilkinson Harold A., Baker Steve, Rosenfeld Steven. Gelfoam paste in experimental laminectomy and cranial trephination. J Neurosurg. 1981;54:664–667. doi: 10.3171/jns.1981.54.5.0664. [DOI] [PubMed] [Google Scholar]
- 57.Resorbable bone wax. US patent 5308623.
- 58.Resorbable bone wax. US Patent 5482717.
- 59.Sugamori T.,., Iwase H.,., Maeda M.,., Inoue Y.,., Kurosawa H. Local hemostatic effects of microcrystalline partially deacetylated chitin hydrochloride. J Biomed Mater Res. 2000;49:225–232. doi: 10.1002/(sici)1097-4636(200002)49:2<225::aid-jbm10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 60.Orgill D.P., Ehret F.W., Regan J.F., Glowacki J., Mulliken J.B. Polyethylene glycol/microfibrillar collagen composite as a new resorbable hemostatic bone wax. J Biomed Mater Res. 1998;39:358–363. doi: 10.1002/(sici)1097-4636(19980305)39:3<358::aid-jbm3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 61.Absorbable hemostatic composition. US Patent 4439420.
- 62.Wang M.Y., Armstrong J.K., Fisher T.C., Meiselman H.J., McComb G.J., Levy M.L. A new, pluronic-based, bone hemostatic agent that does not impair osteogenesis. Neurosurgery. 2001;49:962–967. doi: 10.1097/00006123-200110000-00031. [DOI] [PubMed] [Google Scholar]
- 63.Wellisz T., Armstrong J.K., Cambridge J., Fisher T.C. Ostene, a new water-soluble bone hemostasis agent. J Craniofac Surg. 2006;17:420–425. doi: 10.1097/00001665-200605000-00006. [DOI] [PubMed] [Google Scholar]
- 64.Wellisz T., An Y X., Kang Q., Hill C., Armstrong J. Infection rates and healing using bone wax and a soluble polymer material. Clin Orthop Relat Res. 2008;466:481–486. doi: 10.1007/s11999-007-0067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tham T., Costantino P., Roberts K., Burban J., Shanahan J. Analysis of bone healing with a novel bone wax substitute compared to bone wax in a porcine bone defect model. bioRxiv. 2017 doi: 10.4155/fsoa-2018-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.HEMASORB resorbable hemostatic bone putty. Available at: http://www.abyrx.com/products.hemasorb.asp.
- 67.Suwanprateeb J., Kiertkrittikhoon S., Kintarak J., Suvannapruk W., Thammarakcharoen F., Rukskul P. In vivo assessment of new resorbable PEG-PPG-PEG copolymer/starch bone wax in bone healing and tissue reaction of bone defect in rabbit model. J Mater Sci Mater Med. 2014;25:2131–2139. doi: 10.1007/s10856-014-5249-6. [DOI] [PubMed] [Google Scholar]
- 68.Finn M D., Schow S., Schneiderman E. Osseous regeneration in the presence of four common hemostatic agents. J Oral Maxillofac Surg. 1992;06:608–612. doi: 10.1016/0278-2391(92)90443-4. [DOI] [PubMed] [Google Scholar]
- 69.Magyar C.E., Aghaloo T., Atti E., Tetradis S. Gelatine based “bone wax”. Neurosurgery. 2010;67 E521-E. [Google Scholar]
- 70.Gurcan O., Gurcay A.G., Kazanci A., Onder E., Senturk S., Bavbek M. Is the use of hemostatic matrix (Floseal) and alkylene oxide copolymer (Ostene) safe in spinal laminectomies? Peridural fibrosis assessment. Acta Orthop Traumatol. 2017;51:165–168. doi: 10.1016/j.aott.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu L., Gu T., Song L., Shi E., Fang Q., Wang C. Fibrin sealant provides superior hemostasis for sternotomy compared with bone wax. Ann Thorac Surg. 2012;93:641–644. doi: 10.1016/j.athoracsur.2011.08.087. [DOI] [PubMed] [Google Scholar]
- 72.Kjaergard H.K., Trumbull H.R. Bleeding from the sternal marrow can be stopped using vivostat patient-derived fibrin sealant. Ann Thorac Surg. 2000;69:1173–1175. doi: 10.1016/s0003-4975(99)01560-x. [DOI] [PubMed] [Google Scholar]
- 73.Cillo J.E., Marx R.E., Stevens M.R. Evaluation of autologous platelet-poor plasma gel as a hemostatic adjunct after posterior iliac crest bone harvest. J Oral Maxillofac Surg. 2007;65:1734–1738. doi: 10.1016/j.joms.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 74.Floyd C.T., Padua R.A., Olson C.E. Hemostasis and safety of a novel fibrin dressing versus standard gauze in bleeding cancellous bone in a caprine spine surgery model. Spine Deform. 2017;5:310–313. doi: 10.1016/j.jspd.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 75.Stehrer R., Hunger S., Schotten K.-J., Parsaei B., Malek M., Jacob M. Reduction of transfusion requirements in pediatric craniosynostosis surgery by a new local hemostatic agent. J Cranio Maxillofac Surg. 2016;44:1246–1251. doi: 10.1016/j.jcms.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 76.Schonauer C., Tessitore E., Moraci A., Barbagallo G., Albanese V. Springer Berlin Heidelberg; Berlin, Heidelberg: 2005. The use of local agents: bone wax, gelatin, collagen, oxidized cellulose; pp. 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murakami Y., Honda Y., Anada T., Shimauchi H., Suzuki O. Comparative study on bone regeneration by synthetic octacalcium phosphate with various granule sizes. Acta Biomater. 2010;6:1542–1548. doi: 10.1016/j.actbio.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 78.Xu H.H., Takagi S., Quinn J.B., Chow L.C. Fast-setting calcium phosphate scaffolds with tailored macropore formation rates for bone regeneration. J Biomed Mater Res A. 2010;68A:725–734. doi: 10.1002/jbm.a.20093. [DOI] [PubMed] [Google Scholar]
- 79.Muehrcke D.D., Barberi P., Shimp W.M. Calcium phosphate cements to control bleeding in osteoporotic sternums. Ann Thorac Surg. 2007;84:259–261. doi: 10.1016/j.athoracsur.2007.02.086. [DOI] [PubMed] [Google Scholar]
- 80.Weijing C., Yangyang P., Yin Z., Fan Q., Mingming L., Jun T. Novel bone wax based on tricalcium silicate cement and BGs mixtures. Biomed Mater. 2018;13:065001. doi: 10.1088/1748-605X/aad73c. [DOI] [PubMed] [Google Scholar]
- 81.Momota Y., Miyamoto Y., Ishikawa K., Takechi M., Yuasa T., Tatehara S. Evaluation of feasibility of hydroxyapatite putty as a local hemostatic agent for bone. J Biomed Mater Res. 2002;63:542–547. doi: 10.1002/jbm.10332. [DOI] [PubMed] [Google Scholar]
- 82.Huan Z., Mengmeng Y., Xinye N., Lei Y., Kutty M.G. Using calcium sulfate cement-hydroxypropyl methyl cellulose/sodium alginate composites as substitutes of bone wax. Int J Appl Ceram Technol. 2018;15:903–909. [Google Scholar]
- 83.Hoffmann B., Volkmer E., Kokott A., Weber M., Hamisch S., Schieker M. A new biodegradable bone wax substitute with the potential to be used as a bone filling material. J Mater Chem. 2007;17:4028–4033. [Google Scholar]
- 84.Chen C., Li H., Pan J., Yan Z., Yao Z., Fan W. Biodegradable composite scaffolds of bioactive glass/chitosan/carboxymethyl cellulose for hemostatic and bone regeneration. Biotechnol Lett. 2015;37:457–465. doi: 10.1007/s10529-014-1697-9. [DOI] [PubMed] [Google Scholar]
- 85.Muehrcke D.D., Shimp W.M., Rafael A.L. Calcium phosphate cements improve bone density when used in osteoporotic sternums. Ann Thorac Surg. 2009;88:1658–1661. doi: 10.1016/j.athoracsur.2009.05.058. [DOI] [PubMed] [Google Scholar]
- 86.Brückner T., Schamel M., Kübler A.C., Groll J., Gbureck U. Novel bone wax based on poly(ethylene glycol)–calcium phosphate cement mixtures. Acta Biomater. 2016;33:252–263. doi: 10.1016/j.actbio.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 87.Madsboell T.K., Vestergaard R.F., Andelius T.C., Hauge E.M., Hasenkam J.M. Gentamicin-enriched, water-soluble polymer wax reduces the burden of infection after sternotomy in pigs. Eur J Cardio Thorac. 2014;45:476–480. doi: 10.1093/ejcts/ezt410. [DOI] [PubMed] [Google Scholar]
- 88.Ragusa R., Faggian G., Rungatscher A., Cugola D., Marcon A., Mazzucco A. Use of gelatin powder added to rifamycin versus bone wax in sternal wound hemostasis after cardiac surgery. Interact Cardiovasc Thorac Surg. 2007;6:52–55. doi: 10.1510/icvts.2005.126250. [DOI] [PubMed] [Google Scholar]
- 89.Bose S., Fielding G., Tarafder S., Bandyopadhyay A. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 2013;31:594–605. doi: 10.1016/j.tibtech.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Henderson S., Xia J., Wu H., Stafford A.R., Leslie B.A., Fredenburgh J.C. Zinc promotes clot stability by accelerating clot formation and modifying fibrin structure. Thromb Haemostasis. 2016;115:533–542. doi: 10.1160/TH15-06-0462. [DOI] [PubMed] [Google Scholar]
- 91.Eltohamy M., Kundu B., Moon J., Lee H.Y., Kim H.W. Anti-bacterial zinc-doped calcium silicate cements: bone filler. Ceram Int. 2018;44:13031–13038. [Google Scholar]
- 92.Research TM. Global Bone Wax Market to Reach US$ 84.2 Mn in 2026; Innovations in Bone Wax Products and Usage as a Drug Carrier for Medications to Fuel Market Growth. Available at: https://www.prnewswirecom/news-releases/global-bone-wax-market-to-reach-us-842-mn-in-2026-innovations-in-bone-wax-products-and-usage-as-a-drug-carrier-for-medications-to-fuel-market-growth-682010421html. Accessed 2018.
- 93.Available at: https://wwwaccessdatafdagov/cdrh_docs/pdf11/K111538pdf.
- 94.Suwanprateeb J., Suvannapruk W., Thammarakcharoen F., Chokevivat W., Rukskul P. Preparation and characterization of PEG–PPG–PEG copolymer/pregelatinized starch blends for use as resorbable bone hemostatic wax. J Mater Sci Mater Med. 2013;24:2881–2888. doi: 10.1007/s10856-013-5027-x. [DOI] [PubMed] [Google Scholar]