Abstract
Along with the massive use of implants in orthopaedic surgeries in recent few decades, there has been a tremendous demand for the surface modification of the implants to avoid surgery failure and improve their function. Polydopamine (PDA), being able to adhere to almost all kinds of substrates and possessing copious functional groups for covalently immobilizing biomolecules and anchoring metal ions, has been widely used for surface modification of materials since its discovery in the last decade. PDA and its derivatives can be used for the surface modification of orthopaedic implants to modulate cellular responses, including cell spreading, migration, proliferation, and differentiation, and may thereby enhance the function of existing implants. In addition, the osseointegration and antimicrobial properties of orthopaedic implants may also be improved by PDA-based coatings. The aim of this review is to provide a brief overview of current advances of surface modification technologies for orthopaedic implants using PDA and its derivatives as a medium. Given the versatility of PDA-based adhesion, such PDA-assisted surface modification technologies will certainly benefit the development of new orthopaedic implants.
The translational potential of this article
Surface treatments of orthopaedic implants, which are normally inert materials, are essential for their performance in vivo. This review summarizes recent advances in the surface modification of orthopaedic implants using facile and highly versatile techniques based on the use of polydopamine (PDA) and its derivatives.
Keywords: Implants, Orthopaedic, Polydopamine, Surface modification
Introduction
The past few decades have witnessed a substantial increase in patient population because of ageing and technical advancement of orthopaedic surgeries. Along with these, there has been a tremendous demand for orthopaedic implants, including bone fillers, articular replacements, and internal/external fixation systems for bone fracture and disc fusion cages [1], [2], [3]. With the increasing requirement of orthopaedic surgeries, challenges remain in developing novel bulk biomaterials (such as bioabsorbable bioceramics) and improving the clinical performance of current implants including titanium (Ti) and poly(ether ether ketone) (PEEK) to avoid surgery failure and improve the function of implants [4], [5]. Being able to award the biosubstitutes multiple functions to meet the requirements of tissue regeneration, surface modification of orthopaedic implants has evolved into an essential and promising technology. For example, the osseointegration and antimicrobial properties of orthopaedic implants can be significantly improved on appropriate surface modification [6], [7].
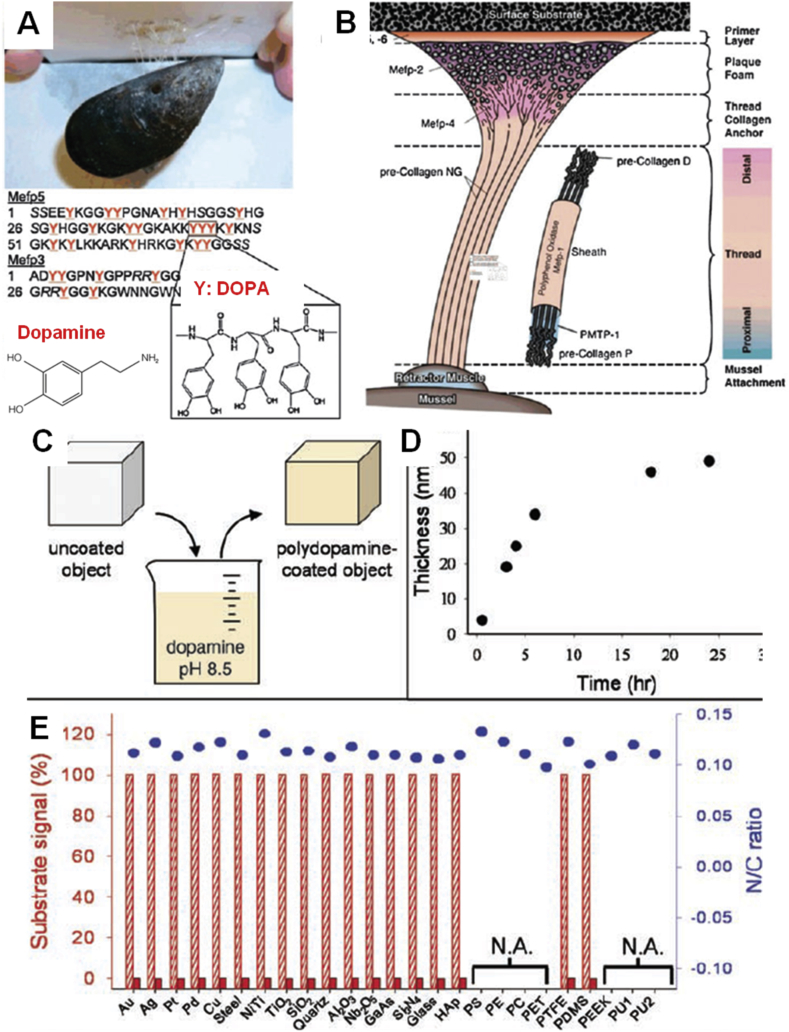
In recent years, mussel adhesive proteins have attracted much attention because they can form strong adhesive interface interaction with various substrates in a wet environment (Fig. 1) [8], [9]. Among them, 3,4-dihydroxy-l-phenylalanine and lysine-enriched proteins near the plaque–substrate interface played important role in the extraordinarily robust adhesion [10], [11]. Therefore, dopamine, with similar molecular structure to 3,4-dihydroxy-l-phenylalanine, became a novel coating material (Fig. 1A). Dopamine is easily oxidized into dopaquinone under aerobic conditions [12], [13]. The oxidation of dopamine can form polydopamine (PDA) coating. PDA coating can be easily deposited on virtually all types of inorganic and organic substrates and show controllable thickness and durable stability. In addition, PDA possesses excellent biocompatibility and unique chemical structure containing many functional groups including catechol, amine, and imine, which can be used to covalently immobilize molecules and adsorb metal ions. Owing to these outstanding properties, PDA has been extensively used for modulating cellular and tissue responses to materials [14], [15], [16]. PDA technique used in surface coating, molecular imprinting, and electrochemistry has shown potential application in biomedical field so far [17], [18].
Figure 1.
(A) Photograph of blue mussel binding to Teflon and molecular structure of DOPA and dopamine [9]. (B) Location of adhesive-related proteins identified in the byssus of Mytilus edulis[9]. (C) A schematic illustration of thin film deposition of PDA by dip coating within an alkaline dopamine solution [8]. (D) Thickness evolution of PDA coating on Si as measured by atomic force microscopy (AFM) of patterned surfaces [8]. (E) X-ray photoelectron spectroscopy (XPS) characterization of 25 different PDA-coated surfaces [8]. Reprinted with permission from ref. [8], 2007, American Association for the Advancement of Science and ref. [9], 2011, Royal Society of Chemistry Publishing Group. HAp = hydroxyapatite; PS = polystyrene; PE = polyethylene; PC = polycarbonate; PET = polyethylene terephthalate; PTFE = polytetrafluoroethylene; PU = polyurethane; DOPA = 3,4-dihydroxy-l-phenylalanine; PDA = polydopamine; PDMS = polydimethylsiloxane; PEEK = poly(ether ether ketone).
The aim of this review is to summarize the recent advances of PDA-modified materials as orthopaedic implants. We will introduce the preparation and polymerization mechanism of PDA. Then, the properties of PDA-modified materials will be briefly elucidated. We will then emphatically discuss the in vitro osteoblast/stem cell adhesion, migration, proliferation, and osteogenic differentiation behaviour on the PDA-modified orthopaedic implants. After that, we will present the in vivo bone repair results of typical PDA-modified orthopaedic implants. We will then summarize other applications of the PDA-modified materials in musculoskeletal tissues. Finally, we will summarize the status and prospect of the PDA-modified materials for musculoskeletal tissue engineering.
Preparation and polymerization mechanism of PDA
Preparation of PDA
Dopamine self-polymerization can form thin PDA coating onto various inorganic and organic materials, including metals, oxides, polymers, semiconductors, and ceramics (Fig. 1) [8], [9]. At present, a variety of PDA polymerization conditions emerge. Solution oxidation method is the most widely used protocol for forming PDA coating. When the dopamine is oxidized and spontaneously self-polymerized under alkaline conditions with oxygen as the oxidant, the thickness of the PDA film can be controlled by changing polymerization condition and time (Table 1). The maximum thickness of a PDA film by a typical reaction (air, pH 8.5) is about 50 nm, but this process may take a few hours up to a few days [8]. To accelerate the deposition rate of PDA coatings, researchers have developed different methods such as UV irradiation, oxidant promotion, and electrochemical actuation (Table 1). Although the deposition rate of PDA can be improved by these methods, some large PDA coatings are found to aggregate together. Zhang et al. [19] reported a new strategy to accelerate the deposition rate of PDA coatings using CuSO4/H2O2 as a trigger. This approach had faster deposition rate and uniform PDA coating. Furthermore, the PDA-coated porous membranes showed excellent antioxidant properties and antibacterial performance. Florian et al. investigated dopamine oxidation under acidic conditions (pH 5.0) with ammonium peroxodisulfate, sodium periodate, and copper sulphate as oxidants [20]. The sodium periodate group showed the thickest and homogeneous PDA coating because of degradation of quinine units to yield carboxyl functions.
Table 1.
Typical thickness and deposition rate of PDA coatings at different conditions.
| Condition | Time (h) | Thickness (nm) | Thickness/time (nm h−1) | References |
|---|---|---|---|---|
| Pure O2, pH 8.5 | 0.5 | 4.4 | 8.8 | [21] |
| CuSO4/H2O2, pH 8.5 | 0.67 | 30 | 44.8 | [19] |
| Sodium periodate, pH 5.0 | 2 | 100 | 50.0 | [20] |
| UV, pH 8.5/pH 7.0 | 2 | 4.0 | 2.0 | [22] |
| Ammonium persulfate, pH 7.0 | 2 | 70.0 | 35.0 | [23] |
| Air, pH 8.5 | 24 | 50.0 | 2.1 | [8] |
| CuSO4, pH 8.5 | 80 | 70 | 0.9 | [24] |
PDA = polydopamine.
Polymerization mechanism
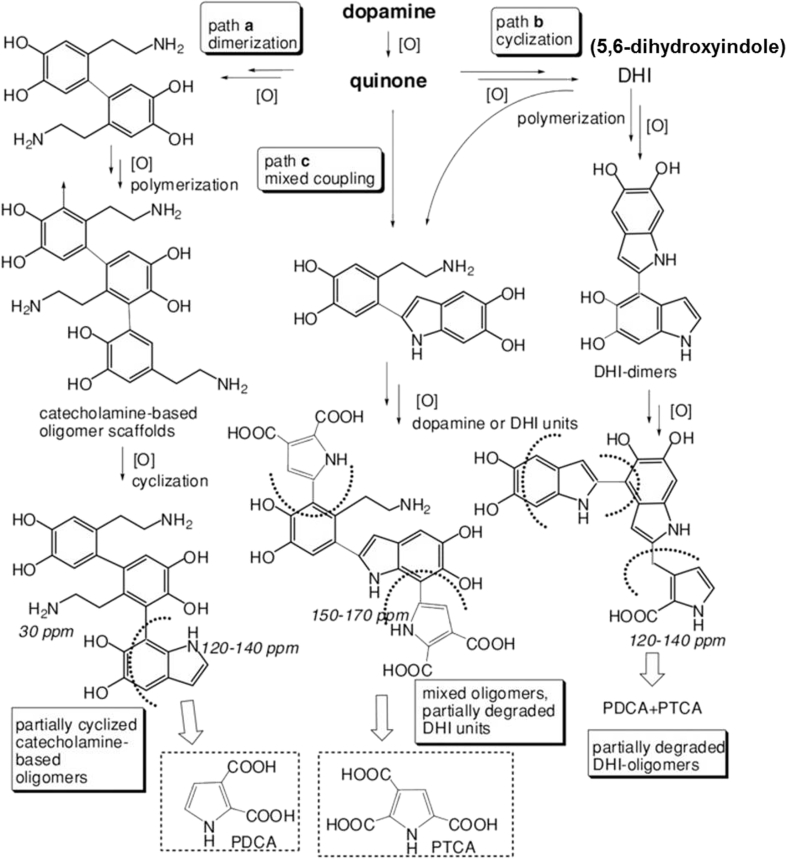
Owing to the complex redox process and a series of intermediates generated during the polymerization, the molecular mechanism behind the formation of PDA has long been the scientific debate. In the early stage, despite little solid experimental evidence, the formation of PDA is believed to follow a mechanism similar to the synthetic mechanism of eumelanin in a living organism. Later, in contrast to the “eumelanin” model, Lee et al. suggested that PDA was formed combining noncovalent self-assembly and covalent polymerization by using high-performance liquid chromatography coupled with mass spectrometry analysis [25]. In 2013, Vecchia et al. [26] expanded the new concepts of the structure of PDA and proposed that the formation of PDA would undergo three competing pathways depending on the preparation conditions, such as the concentration of dopamine and the types of the buffers (Fig. 2). In Path a, dimerization of dopamine would lead to uncyclized, biphenyl-type structures. Uncyclized units would then undergo further oxidation and intramolecular cyclization to generate 5,6-dihydroxyindole (DHI) units unsubstituted at the 2-position. In Path b, DHI-based structures arising along typical eumelanin–forming pathways may grow and suffer partial oxidative cleavage under the autoxidative conditions of the process because of hydrogen peroxide generation. As a result, partial peroxidative fission of o-quinone moieties may occur, leading to pyrrolecarboxylic acid formation. In Path c, the route may operate at different levels following dopamine quinone formation, leading to mixed species, for example, by attack of DHI to various monomer and oligomer quinone species. In 2014, Marco et al. compared the biosynthesis and chemical synthesis mechanism of eumelanin, neuromelanin, and PDA [27]. They thought that natural eumelanin contains a high proportion of 5,6-dihydroxyindole-2-carboxylic acid–derived units, whereas synthetic PDA from dopamine consists mainly of 5,6-dihydroxyindole–related units.
Figure 2.
Overall view of three reaction pathways involved in polydopamine formation. Reprinted with permission from ref. [26], 2013, John Wiley and Sons Publishing Group.
Characteristics of PDA-modified materials
Adhesive property
One of the most important properties of PDA is its strong adhesion to virtually all types of surfaces independent of the substrate's property. It is proved that 3,4-dihydroxyphenylalanine in adhesive proteins, secreted by marine mussels, plays an important role in interfacial binding and intermolecular cross-linking [28]. It was reported that catechol could form strong and reversible bonds with metal oxides reaching 40% of covalent bond strength, the strongest reversible bond involving a biological molecule reported to date [29]. This property of PDA has been widely investigated in biomedical field. For example, Liu et al. [30] developed an injectable dopamine-modified four-armed poly(ethylene glycol) hydrogel containing laponite as the nanocomposite tissue adhesive hydrogel. The incorporation of laponite significantly enhanced the bulk mechanical and adhesive properties of the adhesive. Recently, Gong et al. [31] prepared an injectable hydrogel via simply mixing dopamine-modified poly(α, β-aspartic acid) and FeCl3 solutions. This dopamine-modified poly(α, β-aspartic acid)–Fe3+ hydrogel is composed of covalent and coordination cross-links and suitable to serve as a bioadhesive agent according to the rheological behaviours and the observed significant shear adhesive strength. In addition, catechol is also a reasonable bioinspired candidate for the design of a biopolymer that can function both as an adhesive and cohesive material depending on the external environment. A hyaluronic acid––catechol conjugate can exhibit either adhesiveness, functionalizing the surface of materials, or cohesiveness, building 3D hydrogels [32].
Surface property
Although PDA coating will not change the bulk properties of the materials, it can lead to a big change in the surface properties of the substrate, including surface morphology, wettability, chemical composition, and mechanical properties [8]. The most obvious change is the colour, changing from bulk colour to dark brown. The surface roughness also can be changed after the PDA deposition. Xi et al. [33] reported the membranes of porous polymers became smoother after poly(3,4-dihydroxyphenylalanine) coating. Conversely, a surface transition from smooth to rough in poly(L-Lactide-co-caprolactone) (PLCL) films was observed after treatment with PDA [34]. The PDA coating is hydrophilic; then, the substrates coated by PDA will show a change in hydrophilicity, whether the substrates are hydrophilic or hydrophobic [8], [35].
Chemical reactivity
Most biomacromolecules, including proteins, peptides, DNA, and aptamers, have been reported to successfully anchor on various surfaces via PDA coating. The PDA coating is an extremely versatile platform for further modification of amine- and thiol-containing molecules via Michael addition or Schiff base reactions. In a study, chemically inert materials (Nomex/polytetrafluoroethylene (PTFE) fibres) were coated with PDA to form a bridge to introduce a substantial amino-functionalized silane layer onto the fabric surfaces [36]. The tensile and bonding strength of PDA-modified and subsequently amino-functionalized fabric composite were superior to those of the nontreated and PDA-coated fabric composite only. Liu and Huang [37] formed β-amino carbonyl linkages between PDA and acrylate/acrylamide molecules via aza-Michael addition on various substrates including titanium dioxide, gold, silicon dioxide (SiO2), nitinol alloy, polystyrene, and poly(dimethylsiloxane). Recently, Zhu et al. [38] modified a Ti percutaneous implant by first depositing a PDA coating on Ti6Al4V based on dopamine self-polymerization and then immobilizing collagen chains on PDA. This modification could improve the integration between the implant and soft tissues.
Biocompatibility and biodegradability
The biocompatibility of PDA-modified materials can be improved. PDA as a “eumelanin-like” polymer has excellent biocompatibility. Ku et al. [39] evaluated the cell adhesion and cell cytotoxicity on the PDA-coated nonwetting surfaces. They found that cell viability was not affected by PDA coating. Liu et al. [40] found the rats could remain healthy over a one-month period after intravenous injection of dopamine–melanin colloidal nanospheres for in vivo cancer therapy. Biodegradability is also another vital factor required for a material used in biomedical or clinical applications. Eumelanin would undergo degradation in vitro in the presence of oxidation agents. The PDA nanoparticles also have been observed to degrade with hydrogen peroxide by colour fading. An in vivo study has found that after 8 weeks of implantation, silicone implants showed chronic inflammation response [41]. On the contrary, the melanin implants were almost completely degraded, and the fibrous tissue surrounding the implants mostly subsided.
Antibacterial property
PDA-modified materials have good antibacterial activity. The antibacterial effect of dopamine and PDA was investigated by Iqbal et al. [42]. It was proved that dopamine/PDA exhibits extraordinary antibacterial property. Silver or nanoparticles deposited in situ onto the PDA coating also endow the material with better antibacterial properties [37], [43]. With the help of PDA coating, Gao et al. [6] developed a biocompatible and antibacterial coating for PEEK implants. In other studies, the substrates having both antifouling and antimicrobial properties were fabricated by PDA coating [37], [43]. The active bactericidal surfaces were prepared by in situ formation of Ag nanoparticles in PDA, whereas the antifouling performance was obtained by grafting some antifouling molecules on the surface. Long-term antibacterial activity could also be obtained on the antibiotic-decorated Ti material by PDA coating [44]. The multifunctional coating also could be used in orthopaedics. PDA coating could promote the coating stability of the photocross-linkable gelatin-based hydrogel on Ti implants [45]. After loading cationic antimicrobial peptide and silicate nanoparticles to the hydrogels, this implant showed excellent antibacterial activity and enhanced osteogenesis.
Osteogenesis of orthopaedic implants based on PDA-assisted modification
Mineralization ability of PDA-modified materials
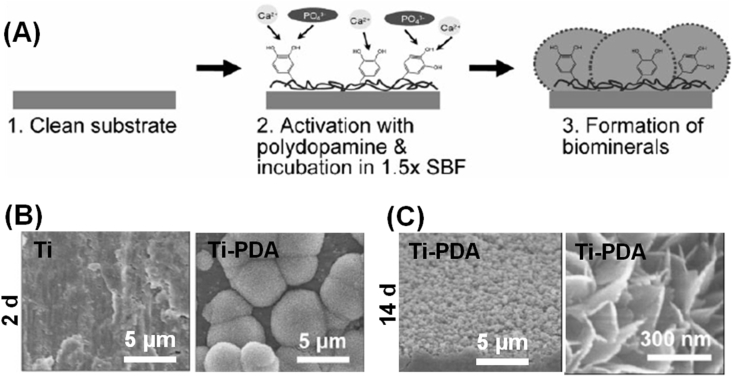
PDA coatings can efficiently assist the biomimetic mineralization of organic, inorganic, or metal materials. In a study, PDA-coated polycaprolactone (PCL) scaffolds immersed in simulated body fluid induced the formation of hydroxyapatite (HA) crystals [46]. PDA modification on the Ti substrate also promotes the formation of calcium phosphate biominerals (Fig. 3) [47]. There is significant difference between pure Ti and Ti-PDA substrates. Recently, electrospun collagen fibres containing catecholamines and Ca2+ were prepared [48]. The presence of Ca2+ could induce simultaneous partial oxidative polymerization of catecholamines and cross-linking of collagen nanofibres and then induce mineralization of the mats by ammonium carbonate. PDA coating also could improve the mineralization ability of porous SiO2 scaffolds [49].
Figure 3.
(A) Scheme for PDA-assisted calcium phosphate crystal formation. (B) The morphology of Ti and PDA-modified Ti substrate (Ti-PDA) after immersing in SBF for two days. (C) The entire surface of Ti-PDA was covered with calcium phosphate minerals and lath-like structures, typically found in natural HA crystals, after incubation for 14 days. Reprinted with permission from ref. [47], 2010, John Wiley and Sons Publishing Group. SBF = simulated body fluid; HA = hydroxyapatite; PDA = polydopamine.
Cell adhesion and proliferation on PDA-modified implants
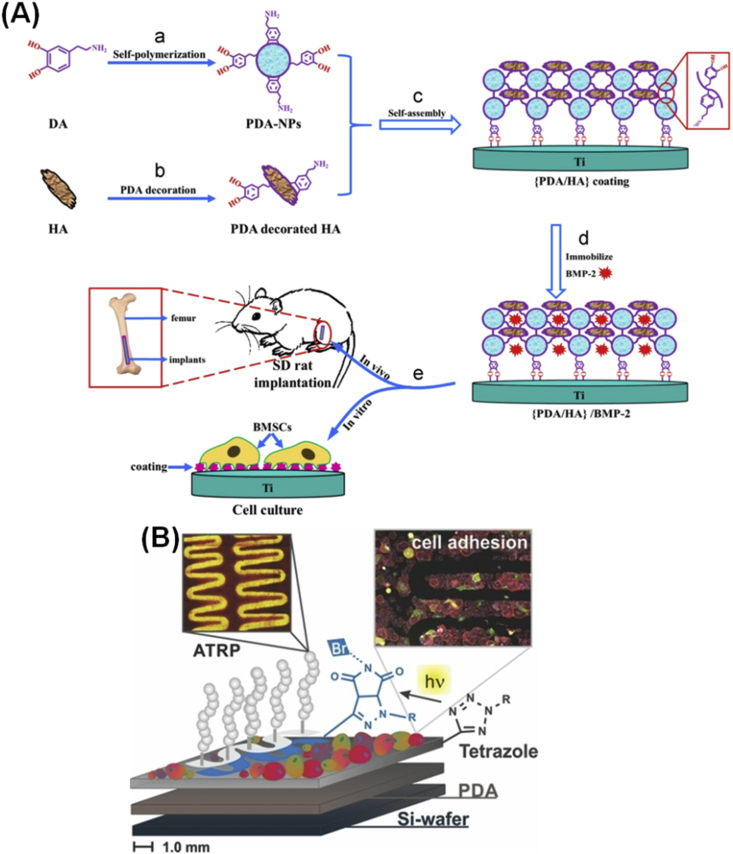
PDA coating could promote cell adhesion and proliferation on the implants. Bone biomaterials play a vital role in bone repair by providing the necessary substrate for cell adhesion and proliferation and by modulating cell activity and function [50]. Cellular interactions with orthopaedic implants may be enhanced by PDA coating alone or subsequent immobilization of adhesive biomolecules. PDA coating could be used for surface modification of bioinert implants and significantly improve cell attachment and proliferation compared with bare substrates [46], [51], [52]. In a study, stereolithography technology and PDA modifications were used to prepare a PDA-coated/HA precipitate [46]. Compared with pure PCL scaffolds, focal adhesion kinase levels, cell attachment, and proliferation were enhanced with increase in PDA content. The stainless steel (SS), a kind of implant, is widely used clinically for orthopaedics. To control cell–SS interactions, SS was coated with PDA, then nonfouling polymer brushes were grown on the surface to prevent protein adsorption and cell adhesion, subsequently functionalized with adhesive peptides to direct cell adhesion and signalling [52]. A facile deposition method by mixing nanostructured HA in an alkaline dopamine solution and then depositing onto Ti was developed for preparation of nanostructured PDA/HA coatings on Ti [53]. The deposition of dopamine/HA greatly enhanced the adhesion, proliferation, and mineralization of osteoblasts. Another study reported that the PDA/HA composite coating on the surface of Ti could adsorb bone morphogenic protein-2 (BMP-2), as shown in Fig. 4a [54]. The presence of PDA and HA coatings and porous and hierarchical micro/nanostructures enhanced the adhesion of bone marrow mesenchymal stem cells (BMSCs).
Figure 4.
(A) Schematic illustration of (PDA/HA) composite coating self-assembly and characterization of the biological properties on the Ti surface [54]. (B) Poly(MeOEGMA) modified on the patterned areas and their resistance to cell adhesion [55]. Reprinted with permission from ref. [55], 2013, John Wiley and Sons Publishing Group. HA = hydroxyapatite; NP = nanoparticle; SD = Sprague-Dawley; ATRP = atom transfer radical polymerization; BMP-2 = bone morphogenic protein-2; PDA = polydopamine.
PDA coating also helps to form cell patterns. Because the PDA modification method does not require complex chemical reactions, it enables cell patterning in a simple and versatile manner compared with conventional methods by the immobilization of adhesive proteins. Rodriguez-Emmenegger et al. [55] developed patterned antifouling poly(MeOEGMA) brushes on PDA surfaces to guide rat embryonic fibroblasts to grow in the pattern (Fig. 4b). Ku et al. [56] used a microfluidic approach to pattern PDA on polydimethylsiloxane, and different cell lines (NIH-3T3, MC3T3-E1, and HT1080) were aligned in the direction of striped PDA patterns. Shi et al. [57] also prepared micropatterned PDA-coated parafilms with different groove widths (30 μm, 50 μm, 100 μm, and flat) to induce the osteogenic differentiation of stem cells. The grooved micropatterns on PDA-coated parafilms produced topographic cues for cell regulation. The Wharton's jelly mesenchymal stem cells were directly printed on the surface of 3D-printed PDA-modified chitosan (CS)/PCL scaffolds [58]. The adhesion and proliferation of Wharton's jelly mesenchymal stem cells on the PDA-modified CS/PCL scaffold was faster than that on the traditional CS/PCL scaffold. In another study, PDA coating was used to construct micropatterns (squares/circles) on thermally expandable hydrogels where human nasal turbinate tissue was selectively attached and formed a monolayer [59].
Osteogenic differentiation of cells on PDA-modified implants
PDA coating on the orthopaedic implants can promote the osteogenic activity (Table 2). It was reported PDA coating itself could promote the osteogenic ability of substrates. A study presented that MC3T3-E1 osteoblasts express functional dopamine receptors that enhance proliferation and mineralization [60]. In a comparative study, the PDA-coated PCL scaffolds showed better osteogenic ability than pure PCL scaffolds [46]. Moreover, this effect exhibited concentration dependence. Recently, “self-fitting” PDA-coated materials were prepared by modifying poly(ε-caprolactone) diacrylate with PDA coating [61]. These new materials had enhanced osteoinductivity compared with poly(ε-caprolactone) diacrylate scaffolds without PDA modification. Alginate hydrogels have been used in cell encapsulation for many years, but they are unable to provide enough bioactive properties to interact with cells. Zhang et al. [62] found that alginate modified by PDA could promote osteogenic differentiation of mouse BMSCs. With the help of PDA coating, the formed micropatterns on the surface of substrates also regulated the osteogenic ability [57].
Table 2.
In vitro cell responses on implants modified by PDA-assisted methods.
| Substrates | Surface composition | Cell type | Adhesion | Proliferation | Osteogenic differentiation | References |
|---|---|---|---|---|---|---|
| PCLDA | PDA coating | hMSCs | √ | √ | [61] | |
| Parafilm | PDA coating | ADMSCs | √ | √ | √ | [57] |
| Calcium silicate/PCL | PDA coating | WJMSCs | √ | √ | [58] | |
| Alginate | PDA coating and silver nanoparticles | BMSCs | √ | √ | [62] | |
| PCL | PDA-coated/HA precipitate | hMSCs | √ | √ | √ | [46] |
| op-HA/PLGA | Immobilized peptides by PDA | MC3T3-E1 | √ | √ | √ | [63] |
| Porous SiO2 | PDA layer loading dexamethasone | BMSCs | √ | √ | √ | [49] |
| β-TCP | PDA NPs | BMSCs | √ | √ | [66] | |
| Ti | PDA/HA coatings | MG63 | √ | √ | √ | [53] |
| Ti | PDA/BMP-2 coating | PDLSCs | √ | [64] | ||
| Ti | Microporous PDA architectures adsorbing BMP-2 | BMSCs | √ | √ | √ | [67] |
| Ti | PDA/HA/BMP-2 composite coating | BMSCs | √ | √ | √ | [54] |
BMP-2 = bone morphogenic protein-2; PCLDA = poly(ε-caprolactone) diacrylate; PCL = polycaprolactone; HA = hydroxyapatite; β-TCP = β-tricalcium phosphate; NPs = nanoparticles; BMSCs = bone marrow mesenchymal stem cells; hMSCs = human mesenchymal stem cells; ADMSCs = adipose-derived mesenchymal stem cells; PDA = polydopamine; PDLSC = periodontal ligament stem cell; PLGA = poly(lactic-co-glycolic acid); WJMSC = Wharton's jelly mesenchymal stem cell.
PDA coating also can combine with other osteogenic factors to promote osteogenic ability of substrates. After modifying the collagen mimetic peptide and osteogenic growth peptide onto the surface of op-HA/poly(lactic-co-glycolic acid) (PLGA) substrates by PDA, the cell adhesion and osteogenic differentiation of MC3T3-E1 cells were enhanced [63]. In another study, the osteogenic differentiation of periodontal ligament stem cells on Ti substrates could also be enhanced by immobilizing BMP-2 via PDA [64]. In addition, a multifunctional coating having both osteogenic and antibacterial properties can be easily obtained by PDA-assisted technique. For example, a PDA-assisted hybrid coating composed of HA, Ag nanoparticles, and CS was successfully prepared on the Ti substrate by a layer-by-layer assembly process [65]. This composite PDA/HA/Ag/CS-1 coatings exhibited antibacterial ratios of 63.0% and 51.8% against Escherichia coli and Staphylococcus aureus, respectively. Moreover, high alkaline phosphatase (ALP) activity demonstrated that the composite PDA/HA/Ag/CS-1 coating on the Ti substrate had osteogenic activity.
The formation of the microstructure on the surface of materials assisted by PDA is conducive to the ingrowth of cells and tissues. The generated grooved micropatterns on PDA-coated parafilms could produce topographic cues for cell regulation [57]. In a study, PDA nanoparticle–decorated substrates promoted cell behaviour and tissue ingrowth because of the micro/nanostructures [66]. In another study, Wang et al. [67] prepared porous micro/nanostructured PDA microcapsules/chitosan composite coatings on Ti surfaces using a layer-by-layer self-assembly technique. The bioadhesive microarchitecture and its immobilized BMP-2 synergistically enhanced the activity and osteogenic differentiation of BMSCs (Table 2).
Bone formation ability of orthopaedic implants modified by the PDA-assisted method
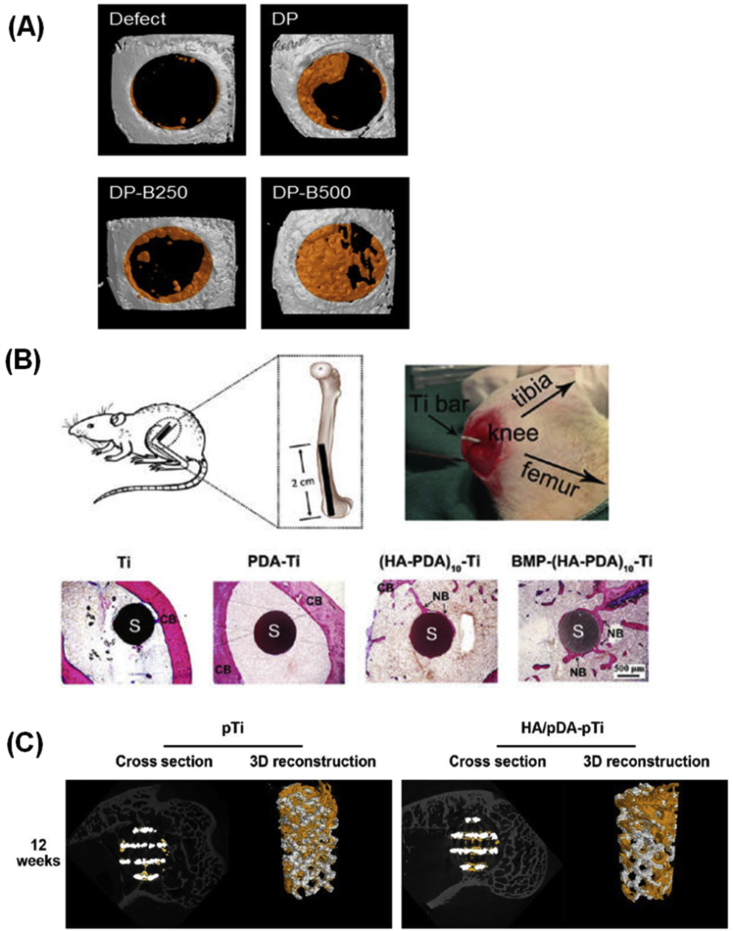
Upon implantation, PDA derivative–modified orthopaedic implants can promote bone healing (Table 3). Lee et al. [68] developed a porous electrospun membrane to act as a barrier to soft tissue and guide bone regeneration. In another work, Lee et al. [69] used a simple method to immobilize bone-forming peptide 1 onto PDA-coated electrospun PLGA fibres. The bone area in a critical-sized calvarial defect of mouse implanted with PDA-coated PLGA and with bone-forming peptide 1–coated PLGA fibres showed significant improvements of 44.27 ± 7.37% and 57.59 ± 15.24%, respectively, whereas the improvement in the defect-only group was small (∼20%). Similarly, Cho et al. [70] developed PDA-coated nanofibres (DP), PDA-coated nanofibres further immobilized with 250 ng/mL BMP-2 (DP-B250) and 500 ng/mL BMP-2 (DP-B500), and found DP-B500 facilitated bone regeneration compared with a control group in a calvarial critical size defect mouse model (Fig. 5A). Moreover, they implanted the fibres with immobilized BMP-2 into the defect and observed better bone repair.
Table 3.
In vivo bone regeneration of implants modified by PDA-assisted method.
| Materials | Surface composition | Animal model | Time | References |
|---|---|---|---|---|
| PLLA electrospun fibres | PDA coating | Mouse calvarial critical size defect | 8 weeks | [68] |
| PLGA electrospun fibres | Immobilize BFP1 onto the surface by PDA coating | Mouse calvarial critical size defect | 8 weeks | [69] |
| PLLA nanofibres | Immobilize BMP-2 onto the surface by PDA coating | Mouse calvarial critical size defect | 8 weeks | [70] |
| Ti | BMP-2–loaded PDA/HA composite coating | Bone marrow cavity of SD rats | 12 weeks | [54] |
| Ti | BMP–HA–PDA multilayer nanofilms | Medullary cavity of SD rats | 12 weeks | [73] |
| Porous Ti6Al4V scaffolds | HA/PDA coating | Rabbit femoral condylar defects | 12 weeks | [7] |
| Ti particles | Ti particle–induced calvarial osteolysis model in mice | 2 weeks | [72] |
PLLA = Poly(L-lactide); SD = Sprague-Dawley; BFP1 = bone-forming peptide 1; BMP-2 = bone morphogenic protein-2; PDA = polydopamine; PLGA = poly(lactic-co-glycolic acid).
Figure 5.
(A) MicroCT analysis of skull bones implanted with nanofibres at two months after surgery [70]. (B) Schematic drawing of the implant and its insertion site and histological sections of the implants stained with methylene blue and basic fuchsin 12 weeks after implantation [73]. (C) MicroCT images of the pTi and HA/PDA-pTi implants at 12 weeks and the yellow colour component was newly formed bone in these scaffolds [7]. Reprinted with permission from ref. [70], 2016, ref. [7] and ref. [72], 2014, ACS Publishing Group. CT = computed tomography; BMP = bone morphogenic protein; CB = cortical bone; NB = new bone; OC = osteocytes; PDA = polydopamine; S = sample.
PDA coating can be used for improving the osseointegration between tissues and implants. With the help of PDA coating, PEEK modified by bioactive lithium doping silica nanospheres remarkably promoted new bone formation [71]. An interesting study demonstrated that local administration of pharmacologic doses of dopamine suppressed wear debris–induced bone resorption through inhibition of osteoclast formation and inflammatory responses via D2-like receptor pathway [72]. Ti scaffolds are widely used in orthopaedics, whereas the osteointegration between Ti and bone tissue is poor. HA–PDA multilayer nanofilms on Ti bars were prepared by a pulse electrochemical–driven layer-by-layer process. While there was nearly no new bone formed around pure Ti after 12 weeks of implantation, Ti modified by BMP-2 and HA was almost completely covered by new bone (Fig. 5B) [73].
The special micro/nanostructure formed, assisted by PDA modification, on the surface of implants can improve the osteogenic activity of implants. In a study, porous Ti6Al4V scaffolds were prepared by the electron beam melting method [7]. HA was further modified on this porous scaffold with the assistance of PDA to obtain HA/PDA-pTi implants, which significantly promoted bone regeneration and enhanced osteointegration after implantation in rabbit femoral condylar defects for 12 weeks (Fig. 5C). PDA nanoparticles and HA nanorods formed a porous and hierarchical micro/nanostructured PDA/HA composite coating on the Ti surface, and BMP-2 was immobilized on the PDA/HA composite coating using the functional groups of PDA [54]. After 12 weeks of implantation in the bone marrow cavity of Sprague-Dawley (SD) rats, there was almost no new bone tissue formation around the bare Ti and PDA coating, whereas new bone tissue formed around the HA and PDA/HA groups. Furthermore, new bone tissue formation increased significantly in the PDA/HA/BMP-2 group. Xu et al. [74] prepared a hierarchical bioceramic scaffold with controlled macropores and nanostructured layers on the strut surface by combining the 3D plotting technique with the PDA/apatite hybrid strategy. Such PDA/apatite nanolayer–modified scaffolds induced more formation of the new bone in and around the scaffolds than unmodified scaffolds after implantation for 12 weeks.
PDA-modified materials for other musculoskeletal tissue repair
PDA-modified materials can also be reported to repair other musculoskeletal tissues. Inspired by the high adhesive strength in a wet environment of PDA, the mussel-inspired tissue adhesives are developed. The developed mussel-inspired easy-to-use double-cross-link tissue adhesive exhibits significantly higher wet tissue adhesion capability than the commercially available fibrin glue when applied on wet porcine skin and cartilage [75]. Tsai et al. [76] showed that the deposition of a poly(dopamine) layer on 3D porous scaffolds is a facile and promising strategy for articular cartilage tissue engineering. They demonstrated that PDA coating could enhance chondrocyte adhesion to a series of biodegradable polymers. PDA-based surface modification has also been applied in ligament and tendon repair [77], [78]. For example, PDA was used to gradually immobilize platelet-derived growth factor-BB on nanofibres to control the differentiation of adipose-derived stem cells into tenocytes in a spatially controlled manner. Furthermore, a symmetrical gradient which had gradient immobilization of platelet-derived growth factor-BB and controlled mineralization on the nanofibre surface was prepared to fabricate a structure-mimicking bone–patellar tendon–bone junction [79].
Summary and perspectives
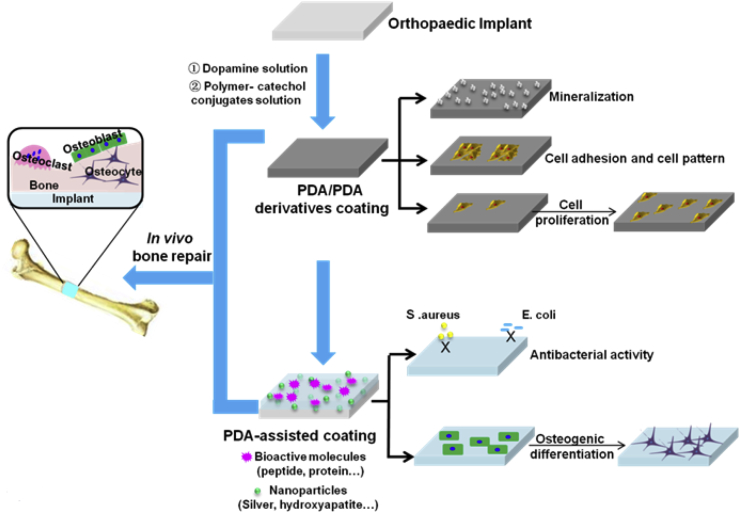
In summary, PDA-assisted surface modification is an easy way to prepare surfaces with excellent antibacterial and osseointegration properties. The surface modification of orthopaedic implants by PDA-assisted techniques is summarized in Fig. 6. Infection and aseptic loosening of the orthopaedic implants are the two major clinical challenges. Improving the cell adhesion, antibacterial property, combination intensity implant–bone interfaces, and osteogenesis are useful methods to resolve the aforementioned problems. PDA-based coatings provide a general surface modification strategy for orthopaedic implants (metallic, inorganic, and organic surfaces), regardless of its chemical and physical nature. The biocompatibility, inflammatory response, cell adhesion, and proliferation of orthopaedic implants could be improved through PDA-assisted coating technology. PDA coating can also endow the osteogenic property of orthopaedic implants by subsequent modification using HA or bioactive molecules which have osteogenic functions. In addition, PDA can also provide antibacterial property for orthopaedic implants by depositing Ag or modifying antibacterial molecules. Further research in this field should aim to achieve precise and microscopic control of material surface properties using PDA-assisted techniques. In-depth understanding of the interactions between cells (e.g., osteoblasts, osteoclasts, stem cells, and immune cells) and PDA-modified orthopaedic implants is also necessary. In addition, the interaction between PDA and extracellular matrix (ECM), which in turn will affect cell behaviours and functions, should also be investigated.
Figure 6.
Surface modification of orthopaedic implants by PDA-assisted techniques. PDA = polydopamine.
Conflict of interest
The authors have no conflicts of interest to disclose in relation to this article.
Acknowledgements
The authors are grateful to the funding support from the National Natural Science Foundation of China (31872748, 31500779, 81471790, 31530024, 81672213), Jiangsu Provincial Special Program of Medical Science (BL2012004), China Postdoctoral Science Foundation (2016M590500, 2017T100398), Jiangsu Planned Projects for Postdoctoral Research Funds (1601269C), and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2019.04.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ott H.C., Matthiesen T.S., Goh S.-K., Black L.D., Kren S.M., Netoff T.I. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 2.Tian L., Tang N., Ngai T., Wu C., Ruan Y.C., Huang L. Hybrid fracture fixation systems developed for orthopaedic applications: a general review. J Orthop Transl. 2019;16:1–13. doi: 10.1016/j.jot.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yi H., Rehman F.U., Zhao C.Q., Liu B., He N.Y. Recent advances in nano scaffolds for bone repair. Bone Res. 2016;4:16060. doi: 10.1038/boneres.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bekmurzayeva A., Duncanson W.J., Azevedo H.S., Kanayeva D. Surface modification of stainless steel for biomedical applications: revisiting a century-old material. Mat Sci Eng C-Mater. 2018;93:1073–1089. doi: 10.1016/j.msec.2018.08.049. [DOI] [PubMed] [Google Scholar]
- 5.Xu H.H.K., Wang P., Wang L., Bao C.Y., Chen Q.M., Weir M.D. Calcium phosphate cements for bone engineering and their biological properties. Bone Research. 2017;5:17056. doi: 10.1038/boneres.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao C., Wang Y., Han F., Yuan Z., Li Q., Shi C. Antibacterial activity and osseointegration of silver-coated poly(ether ether ketone) prepared using the polydopamine-assisted deposition technique. J Mater Chem B. 2017;5:9326–9336. doi: 10.1039/c7tb02436c. [DOI] [PubMed] [Google Scholar]
- 7.Li Y., Yang W., Li X., Zhang X., Wang C., Meng X. Improving osteointegration and osteogenesis of three-dimensional porous Ti6Al4V scaffolds by polydopamine-assisted biomimetic hydroxyapatite coating. ACS Appl Mater Interfaces. 2015;7:5715–5724. doi: 10.1021/acsami.5b00331. [DOI] [PubMed] [Google Scholar]
- 8.Lee H., Dellatore S.M., Miller W.M., Messersmith P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318:426–430. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye Q., Zhou F., Liu W. Bioinspired catecholic chemistry for surface modification. Chem Soc Rev. 2011;40:4244–4258. doi: 10.1039/c1cs15026j. [DOI] [PubMed] [Google Scholar]
- 10.Harrington M.J., Masic A., Holten-Andersen N., Waite J.H., Fratzl P. Iron-clad fibers: a metal-based biological strategy for hard flexible coatings. Science. 2010;328:216–220. doi: 10.1126/science.1181044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waite J.H., Qin X. Polyphosphoprotein from the adhesive pads of mytilus edulis. Biochemistry. 2001;40:2887–2893. doi: 10.1021/bi002718x. [DOI] [PubMed] [Google Scholar]
- 12.van der Leeden M.C. Are Conformational Changes, Induced by osmotic pressure variations, the underlying mechanism of controlling the adhesive activity of mussel adhesive proteins? Langmuir. 2005;21:11373–11379. doi: 10.1021/la0515468. [DOI] [PubMed] [Google Scholar]
- 13.He C., Feng W., Cao L., Fan L. Crosslinking of poly(L-lactide) nanofibers with triallyl isocyanurate by gamma-irradiation for tissue engineering application. J Biomed Mater Res. 2011;99A:655–665. doi: 10.1002/jbm.a.33235. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y., Ai K., Lu L. Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem Rev. 2014;114:5057–5115. doi: 10.1021/cr400407a. [DOI] [PubMed] [Google Scholar]
- 15.Madhurakkat Perikamana S.K., Lee J., Lee Y.B., Shin Y.M., Lee E.J., Mikos A.G. Materials from mussel-inspired chemistry for cell and tissue engineering applications. Biomacromolecules. 2015;16:2541–2555. doi: 10.1021/acs.biomac.5b00852. [DOI] [PubMed] [Google Scholar]
- 16.Huang S., Liang N., Hu Y., Zhou X., Abidi N. Polydopamine-assisted surface modification for bone biosubstitutes. BioMed Res Int. 2016;2016:2389895. doi: 10.1155/2016/2389895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palladino P., Bettazzi F., Scarano S. Polydopamine: surface coating, molecular imprinting, and electrochemistry-successful applications and future perspectives in (bio) analysis. Anal Bioanal Chem. 2019 doi: 10.1007/s00216-019-01665-w. [DOI] [PubMed] [Google Scholar]
- 18.Ryu J.H., Messersmith P.B., Lee H. Polydopamine surface chemistry: a decade of discovery. ACS Appl Mater Interfaces. 2018;10:7523–7540. doi: 10.1021/acsami.7b19865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang C., Ou Y., Lei W.X., Wan L.S., Ji J., Xu Z.K. CuSO4/H2O2-induced rapid deposition of polydopamine coatings with high uniformity and enhanced stability. Angew Chem Int Ed. 2016;55:3054–3057. doi: 10.1002/anie.201510724. [DOI] [PubMed] [Google Scholar]
- 20.Ponzio F., Barthès J., Bour J., Michel M., Bertani P., Hemmerlé J. Oxidant control of polydopamine surface chemistry in acids: a mechanism-based entry to superhydrophilic-superoleophobic coatings. Chem Mater. 2016;28:4697–4705. [Google Scholar]
- 21.Kim H.W., Mccloskey B.D., Choi T.H., Lee C., Kim M., Freeman B.D. Oxygen concentration control of dopamine-induced high uniformity surface coatingchemistry. ACS Appl Mater Interfaces. 2013;5:233–238. doi: 10.1021/am302439g. [DOI] [PubMed] [Google Scholar]
- 22.Du X., Li L., Li J., Yang C., Frenkel N., Welle A. UV-triggered dopamine polymerization: control of polymerization, surface coating, and photopatterning. Adv Mater. 2014;26:8029–8033. doi: 10.1002/adma.201403709. [DOI] [PubMed] [Google Scholar]
- 23.Wei Q., Zhang F., Li J., Li B., Zhao C. Oxidant-induced dopamine polymerization for multifunctional coatings. Polym Chem. 2010;1:1430–1433. [Google Scholar]
- 24.Ball V.., Gracio J., Vila M., Singh M.K., Metz-Boutigue M.H., Michel M. Comparison of synthetic dopamine-eumelanin formed in the presence of oxygen and Cu2+ cations as oxidants. Langmuir. 2013;29:12754–12761. doi: 10.1021/la4029782. [DOI] [PubMed] [Google Scholar]
- 25.Hong S., Na Y.S., Choi S., Song I.T., Kim W.Y., Lee H. Non-covalent self-assembly and covalent polymerization co-contribute to polydopamine formation. Adv Funct Mater. 2012;22:4711–4717. [Google Scholar]
- 26.Vecchia N.F.D., Avolio R., Alfe M., Errico M.E., Napolitano A., Dischia M. Building-block diversity in polydopamine underpins a multifunctional eumelanin-type platform tunable through a quinone control point. Adv Funct Mater. 2013;23:1331–1340. [Google Scholar]
- 27.d'Ischia M., Napolitano A., Ball V., Chen C.T., Buehler M.J. Polydopamine and eumelanin: from structure-property relationships to a unified tailoring strategy. Accounts Chem Res. 2014;47:3541–3550. doi: 10.1021/ar500273y. [DOI] [PubMed] [Google Scholar]
- 28.Lee B.P., Messersmith P.B., Israelachvili J.N., Waite J.H. Mussel-inspired adhesives and coatings. Annu Rev Mater Res. 2011;41:99–132. doi: 10.1146/annurev-matsci-062910-100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee H., Scherer N.F., Messersmith P.B. Single-molecule mechanics of mussel adhesion. Proc Natl Acad Sci U S A. 2006;103:12999–13003. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y., Meng H., Konst S., Sarmiento R., Rajachar R., Lee B.P. Injectable dopamine-modified poly(ethylene glycol) nanocomposite hydrogel with enhanced adhesive property and bioactivity. ACS Appl Mater Interfaces. 2014;6:16982–16992. doi: 10.1021/am504566v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong C., Lu C., Li B., Shan M., Wu G. Injectable dopamine-modified poly(alpha, beta-aspartic acid) nanocomposite hydrogel as bioadhesive drug delivery system. J Biomed Mater Res A. 2017;105:1000–1008. doi: 10.1002/jbm.a.35931. [DOI] [PubMed] [Google Scholar]
- 32.Hong S., Yang K., Kang B., Lee C., Song I.T., Byun E. Hyaluronic acid catechol: a biopolymer exhibiting a pH-dependent adhesive or cohesive property for human neural stem cell engineering. Adv Funct Mater. 2013;23:1774–1780. [Google Scholar]
- 33.Xi Z.-Y., Xu Y.-Y., Zhu L.-P., Wang Y., Zhu B.-K. A facile method of surface modification for hydrophobic polymer membranes based on the adhesive behavior of poly(DOPA) and poly(dopamine) J Membr Sci. 2009;327:244–253. [Google Scholar]
- 34.Shin Y.M., Lee Y.B., Shin H. Time-dependent mussel-inspired functionalization of poly(l-lactide-co-ɛ-caprolactone) substrates for tunable cell behaviors. Colloids Surf, B. 2011;87:79–87. doi: 10.1016/j.colsurfb.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Ku S.H., Park C.B. Human endothelial cell growth on mussel-inspired nanofiber scaffold for vascular tissue engineering. Biomaterials. 2010;31:9431–9437. doi: 10.1016/j.biomaterials.2010.08.071. [DOI] [PubMed] [Google Scholar]
- 36.Yuan J., Zhang Z., Yang M., Guo F., Men X., Liu W. Surface modification of hybrid-fabric composites with amino silane and polydopamine for enhanced mechanical and tribological behaviors. Tribol Int. 2017;107:10–17. [Google Scholar]
- 37.Liu C.Y., Huang C.J. Functionalization of polydopamine via the aza-Michael reaction for antimicrobial interfaces. Langmuir. 2016;32:5019–5028. doi: 10.1021/acs.langmuir.6b00990. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Y., Liu D., Wang X., He Y., Luan W., Qi F. Polydopamine-mediated covalent functionalization of collagen on a titanium alloy to promote biocompatibility with soft tissues. J Mater Chem B. 2019;7:2019–2031. doi: 10.1039/c8tb03379j. [DOI] [PubMed] [Google Scholar]
- 39.Ku S.H., Ryu J., Hong S.K., Lee H., Park C.B. General functionalization route for cell adhesion on non-wetting surfaces. Biomaterials. 2010;31:2535–2541. doi: 10.1016/j.biomaterials.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y., Ai K., Liu J., Deng M., He Y., Lu L. Dopamine-melanin colloidal nanospheres: an efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv Mater. 2013;25:1353–1359. doi: 10.1002/adma.201204683. [DOI] [PubMed] [Google Scholar]
- 41.Bettinger C.J., Bruggeman J.P., Misra A., Borenstein J.T., Langer R. Biocompatibility of biodegradable semiconducting melanin films for nerve tissue engineering. Biomaterials. 2009;30:3050–3057. doi: 10.1016/j.biomaterials.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iqbal Z., Lai E.P.C., Avis T.J. Antimicrobial effect of polydopamine coating on Escherichia coli. J Mater Chem. 2012;22:21608–21612. [Google Scholar]
- 43.Wang L., Yang X., Cao W.W., Shi C., Zhou P.H., Li Q. Mussel-inspired deposition of copper on titanium for bacterial inhibition and enhanced osseointegration in a periprosthetic infection model. RSC Adv. 2017;7:51593–51604. [Google Scholar]
- 44.He S., Zhou P., Wang L., Xiong X., Zhang Y., Deng Y. Antibiotic-decorated titanium with enhanced antibacterial activity through adhesive polydopamine for dental/bone implant. J R Soc Interface. 2014;11:20140169. doi: 10.1098/rsif.2014.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng H., Yue K., Kazemzadeh-Narbat M., Liu Y., Khalilpour A., Li B. Mussel-inspired multifunctional hydrogel coating for prevention of infections and enhanced osteogenesis. ACS Appl Mater Interfaces. 2017;9:11428–11439. doi: 10.1021/acsami.6b16779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng Y.-L., Chen Y.-W., Wang K., Shie M.-Y. Enhanced adhesion and differentiation of human mesenchymal stem cell inside apatite-mineralized/poly(dopamine)-coated poly(ɛ-caprolactone) scaffolds by stereolithography. J Mater Chem B. 2016;4:6307–6315. doi: 10.1039/c6tb01377e. [DOI] [PubMed] [Google Scholar]
- 47.Ryu J., Ku S.H., Lee H., Park C.B. Mussel-inspired polydopamine coating as a universal route to hydroxyapatite crystallization. Adv Funct Mater. 2010;20:2132–2139. [Google Scholar]
- 48.Dhand C., Ong S.T., Dwivedi N., Diaz S.M., Venugopal J.R., Navaneethan B. Bio-inspired in situ crosslinking and mineralization of electrospun collagen scaffolds for bone tissue engineering. Biomaterials. 2016;104:323–338. doi: 10.1016/j.biomaterials.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 49.Wu C., Fan W., Chang J., Xiao Y. Mussel-inspired porous SiO2 scaffolds with improved mineralization and cytocompatibility for drug delivery and bone tissue engineering. J Mater Chem. 2011;21:18300–18307. [Google Scholar]
- 50.Gao C.D., Peng S.P., Feng P., Shuai C.J. Bone biomaterials and interactions with stem cells. Bone Research. 2017;5:17059. doi: 10.1038/boneres.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao X., Song J., Ji P., Zhang X., Li X., Xu X. Polydopamine-templated hydroxyapatite reinforced polycaprolactone composite nanofibers with enhanced cytocompatibility and osteogenesis for bone tissue engineering. ACS Appl Mater Interfaces. 2016;8:3499–3515. doi: 10.1021/acsami.5b12413. [DOI] [PubMed] [Google Scholar]
- 52.Alas G.R., Agarwal R., Collard D.M., García A.J. Peptide-functionalized poly[oligo(ethylene glycol) methacrylate] brushes on dopamine-coated stainless steel for controlled cell adhesion. Acta Biomater. 2017;59:108–116. doi: 10.1016/j.actbio.2017.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chien C.-Y., Liu T.-Y., Kuo W.-H., Wang M.-J., Tsai W.-B. Dopamine-assisted immobilization of hydroxyapatite nanoparticles and RGD peptides to improve the osteoconductivity of titanium. J Biomed Mater Res A. 2013;101A:740–747. doi: 10.1002/jbm.a.34376. [DOI] [PubMed] [Google Scholar]
- 54.Wang Z., Li P., Jiang Y., Jia Z., Tang P., Lu X. Mussel-inspired nanostructured coatings assembled using polydopamine nanoparticles and hydroxyapatite nanorods for biomedical applications. Biosurf Biotribol. 2017;3:1–10. [Google Scholar]
- 55.Rodriguez-Emmenegger C., Preuss C.M., Yameen B., Pop-Georgievski O., Bachmann M., Mueller J.O. Controlled cell adhesion on poly(dopamine) interfaces photopatterned with non-fouling brushes. Adv Mater. 2013;25:6123–6127. doi: 10.1002/adma.201302492. [DOI] [PubMed] [Google Scholar]
- 56.Ku S.H., Lee J.S., Park C.B. Spatial control of cell adhesion and patterning through mussel-inspired surface modification by polydopamine. Langmuir. 2010;26:15104–15108. doi: 10.1021/la102825p. [DOI] [PubMed] [Google Scholar]
- 57.Shi X., Li L., Ostrovidov S., Shu Y., Khademhosseini A., Wu H. Stretchable and micropatterned membrane for osteogenic differentiation of stem cells. ACS Appl Mater Interfaces. 2014;6:11915–11923. doi: 10.1021/am5029236. [DOI] [PubMed] [Google Scholar]
- 58.Chen Y.-W., Shen Y.-F., Ho C.-C., Yu J., Wu Y.-H.A., Wang K. Osteogenic and angiogenic potentials of the cell-laden hydrogel/mussel-inspired calcium silicate complex hierarchical porous scaffold fabricated by 3D bioprinting. Mater Sci Eng C. 2018;91:679–687. doi: 10.1016/j.msec.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 59.Lee Y.B., Kim E.M., Byun H., Chang H-k, Jeong K., Aman Z.M. Engineering spheroids potentiating cell-cell and cell-ECM interactions by self-assembly of stem cell microlayer. Biomaterials. 2018;165:105–120. doi: 10.1016/j.biomaterials.2018.02.049. [DOI] [PubMed] [Google Scholar]
- 60.Lee D.J., Tseng H.C., Wong S.W., Wang Z.Y., Deng M., Ko C.C. Dopaminergic effects on in vitro osteogenesis. Bone Research. 2015;3:15020. doi: 10.1038/boneres.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Erndt-Marino J.D., Munoz-Pinto D.J., Samavedi S., Jimenez-Vergara A.C., Diaz-Rodriguez P., Woodard L. Evaluation of the osteoinductive capacity of polydopamine-coated poly(ε-caprolactone) diacrylate shape memory foams. ACS Biomater Sci Eng. 2015;1:1220–1230. doi: 10.1021/acsbiomaterials.5b00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang S., Xu K., Darabi M.A., Yuan Q., Xing M. Mussel-inspired alginate gel promoting the osteogenic differentiation of mesenchymal stem cells and anti-infection. Mater Sci Eng C. 2016;69:496–504. doi: 10.1016/j.msec.2016.06.044. [DOI] [PubMed] [Google Scholar]
- 63.Wang Z., Chen L., Wang Y., Chen X., Zhang P. Improved cell adhesion and osteogenesis of op-HA/PLGA composite by poly(dopamine)-assisted immobilization of collagen mimetic peptide and osteogenic growth peptide. ACS Appl Mater Interfaces. 2016;8:26559–26569. doi: 10.1021/acsami.6b08733. [DOI] [PubMed] [Google Scholar]
- 64.Lee J.S., Lee J.-C., Heo J.S. Polydopamine-assisted BMP-2 immobilization on titanium surface enhances the osteogenic potential of periodontal ligament stem cells via integrin-mediated cell-matrix adhesion. J Cell Commun Signal. 2018:1–12. doi: 10.1007/s12079-018-0468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li M., Liu X., Xu Z., Yeung K.W.K., Wu S. Dopamine modified organic-inorganic hybrid coating for antimicrobial and osteogenesis. ACS Appl Mater Interfaces. 2016;8:33972–33981. doi: 10.1021/acsami.6b09457. [DOI] [PubMed] [Google Scholar]
- 66.Wang Z., Wang K., Zhang Y., Jiang Y., Lu X., Fang L. Protein-affinitive polydopamine nanoparticles as an efficient surface modification strategy for versatile porous scaffolds enhancing tissue regeneration. Part Part Syst Char. 2016;33:89–100. [Google Scholar]
- 67.Wang Z., Li C., Xu J., Wang K., Lu X., Zhang H. Bioadhesive microporous architectures by self assembling polydopamine microcapsules for biomedical applications. Chem Mater. 2015;27:848–856. [Google Scholar]
- 68.Lee J.H., Lee Y.J., Cho H.J., Shin H. Guidance of in vitro migration of human mesenchymal stem cells and in vivo guided bone regeneration using aligned electrospun fibers. Tissue Eng. 2014;20:2031–2042. doi: 10.1089/ten.tea.2013.0282. [DOI] [PubMed] [Google Scholar]
- 69.Lee Y.J., Lee J.H., Cho H.J., Kim H.K., Yoon T.R., Shin H. Electrospun fibers immobilized with bone forming peptide-1 derived from BMP7 for guided bone regeneration. Biomaterials. 2013;34:5059–5069. doi: 10.1016/j.biomaterials.2013.03.051. [DOI] [PubMed] [Google Scholar]
- 70.Cho H.J., Perikamana S.K., Lee J.H., Lee J., Lee K.M., Shin C.S. Effective immobilization of BMP-2 mediated by polydopamine coating on biodegradable nanofibers for enhanced in vivo bone formation. ACS Appl Mater Interfaces. 2014;6:11225–11235. doi: 10.1021/am501391z. [DOI] [PubMed] [Google Scholar]
- 71.Zhang J., Cai L., Wang T., Tang S., Li Q., Tang T. Lithium doped silica nanospheres/poly(dopamine) composite coating on polyetheretherketone to stimulate cell responses, improve bone formation and osseointegration. Nanomed Nanotechnol Biol Med. 2018;14:965–976. doi: 10.1016/j.nano.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 72.Yang H., Xu Y., Zhu M., Gu Y., Zhang W., Shao H. Inhibition of titanium-particle-induced inflammatory osteolysis after local administration of dopamine and suppression of osteoclastogenesis via D2-like receptor signaling pathway. Biomaterials. 2016;80:1–10. doi: 10.1016/j.biomaterials.2015.11.046. [DOI] [PubMed] [Google Scholar]
- 73.Xie C., Lu X., Wang K., Yuan H., Fang L., Zheng X. Pulse electrochemical driven rapid layer-by-layer assembly of polydopamine and hydroxyapatite nanofilms via alternative redox in situ synthesis for bone regeneration. ACS Biomater Sci Eng. 2016;2:920–928. doi: 10.1021/acsbiomaterials.6b00015. [DOI] [PubMed] [Google Scholar]
- 74.Xu M., Zhai D., Xia L., Li H., Chen S., Fang B. Hierarchical bioceramic scaffolds with 3D-plotted macropores and mussel-inspired surface nanolayers for stimulating osteogenesis. Nanoscale. 2016;8:13790–13803. doi: 10.1039/c6nr01952h. [DOI] [PubMed] [Google Scholar]
- 75.Fan C., Fu J., Zhu W., Wang D.-A. A mussel-inspired double-crosslinked tissue adhesive intended for internal medical use. Acta Biomater. 2016;33:51–63. doi: 10.1016/j.actbio.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 76.Tsai W.B., Chen W.T., Chien H.W., Kuo W.H., Wang M.J. Poly(dopamine) coating of scaffolds for articular cartilage tissue engineering. Acta Biomater. 2011;7:4187–4194. doi: 10.1016/j.actbio.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 77.Chen W.-C., Chen C.-H., Tseng H.-W., Liu Y.-W., Chen Y.-P., Lee C.-H. Surface functionalized electrospun fibrous poly(3-hydroxybutyrate) membranes and sleeves: a novel approach for fixation in anterior cruciate ligament reconstruction. J Mater Chem B. 2017;5:553–564. doi: 10.1039/c6tb02671k. [DOI] [PubMed] [Google Scholar]
- 78.Li Q., Sun L., Zhang L., Xu Z., Kang Y., Xue P. Polydopamine-collagen complex to enhance the biocompatibility of polydimethylsiloxane substrates for sustaining long-term culture of L929 fibroblasts and tendon stem cells. J Biomed Mater Res A. 2017;106:408–418. doi: 10.1002/jbm.a.36254. [DOI] [PubMed] [Google Scholar]
- 79.Madhurakkat Perikamana S.K., Lee J., Ahmad T., Kim E.M., Byun H., Lee S. Harnessing biochemical and structural cues for tenogenic differentiation of adipose derived stem cells (ADSCs) and development of an in vitro tissue interface mimicking tendon-bone insertion graft. Biomaterials. 2018;165:79–93. doi: 10.1016/j.biomaterials.2018.02.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.