Abstract
Objective
Scoliosis is a common disease characterized by spinal curvature with variable severities. There is no generally accepted theory about the physical origin of the spinal deformation of scoliosis. The aim of this study was to explore a new hypothesis suggesting that the curvatures in scoliosis may be associated with the imbalance growth between thoracic vertebral column and sternum.
Methods
We undertook a comparative computed tomography (CT) based morphology study of thoracic vertebrae and sternum of patients with adolescent idiopathic scoliosis (AIS) and age-gender matched normal subjects. We further measured the ratios between the lengths of the sternum and thoracic vertebra of mice with deficiency of fibroblast growth factor receptor 3 (FGFR3), which exhibit scoliosis. Three-week-old C57BL/6J mice were used to generate bipedal and sternal growth plate injury model. Radiographs and histological images were obtained to observe the presence of sternal and spinal deformity.
Results
There was a significant correlation between the severities of scoliosis and the ratios of the sternum to thoracic vertebral lengths. We also found that FGFR3 deficient mice showed smaller ratio of the sternum to thoracic vertebra lengths than that of the wild-type mice, which were similar with that of the AIS patients. Surgery-induced injuries of sternal growth plates can accelerate and aggravate the scoliosis in bipedal mice and imbalanced development of anterior and posterior thoracic occurred before the appearance of scoliosis.
Conclusions
Our findings suggest that the imbalanced growth between the thoracic vertebral column and the sternum is an important causative factor for the pathogenesis of scoliosis including AIS.
The translational potential of this article
Imbalanced growth between the thoracic vertebral column and the sternum is associated with scoliosis. Surgical or rehabilitation intervention for scoliosis should focus on all components involved in the pathogenesis of curvature to obtain better outcome.
Keywords: Growth plate, Imbalanced growth, Scoliosis, Sternum, Vertebrae
Introduction
Scoliosis, a common spinal disease in humans, is characterized by three-dimensional deformity of spine, which can cause back pain, respiratory and/or cardiac problems [1]. There are multiple types of scoliosis such as congenital scoliosis (CS), idiopathic scoliosis, degenerative scoliosis and neuromuscular scoliosis [2]. Among them, CS and adolescent idiopathic scoliosis (AIS) occur during early or later development stages, i.e., they are maldevelopment-related scoliosis. AIS affects 1–3% of children in the at-risk population of those aged 10–16 years, and CS has an incidence of 0.5–1 per 1000 births [3], [4]. Although the clinical manifestations and symptoms of maldevelopment-related scoliosis are well characterized, the pathogenesis of them is not fully understood. Currently, the major effective therapy for scoliosis is conservative treatment and/or orthopaedic surgery prevention or correction of the primary manifestation of the disorder—i.e., spinal deformity [5]. Better understanding of the aetiology will be helpful for more effective prevention, early diagnosis and intervention of maldevelopment-related scoliosis including AIS.
It has been recognized that genetic factors play important roles in the pathogenesis of maldevelopment-related scoliosis. The skeleton of spine is formed through endochondral ossification (EO). Multiple signalling pathways play significant roles in EO. Disturbance of these pathways may lead to spine deformation such as scoliosis and kyphosis. Beside the well-recognized cause–effect relation between gene mutations and scoliosis, segregation studies support a complex inheritance model in which multiple genetic factors contribute greater than 80% of the overall risk for AIS [6]. Multiple genes such as FGFR3 (fibroblast growth factor receptor 3), Gpr126, Pax1, TBX6 and fibrillin were proved to be associated with maldevelopment-related scoliosis [7], [8], [9], [10]. FGFR3-deficient mice develop a pronounced scoliosis phenotype at 2 months of age [11], [12]. Chondrocyte-specific knockout of tuberous sclerosis 1 (TSC-1) leads to congenital spinal deformity in mice [13]. Chickens will develop scoliosis after removal of pineal body due to lack of melatonin, a crucial molecule for growth plate development [14]. All these facts reveal that the dysregulated EO with either global or regional skeleton maldevelopment plays important roles in the pathogenesis of maldevelopment-related scoliosis.
Previous studies mainly focus on the role of asymmetry/imbalanced development of individual bone such as vertebrae [15]. People with AIS were reported to have more active growth in the anterior portion of vertebrae than in the posterior spinal column [16], [17], [18], [19]. It has been reported that disturbance of the ribcage development can lead to progressive structural scoliosis and a shallow chest, which is associated with the initiation of small thoracic curvatures and thoracic AIS [20]. Crijns et al. [21] using a simplified physical model of the thoracolumbar spine, found that restrained differential growth in the sagittal plane can result in lateral bending and rotation without a preexisting left–right asymmetry, indicating that scoliosis is not just associated with the abnormalities of spine itself, but rather a disturbance of the coordinated development of multiple skeletons and soft tissue including spine, ribcage, ligament and muscles.
AIS mostly occurs in the thoracic spine. The thoracic cavity is coordinately formed ventrally by the sternum and costal cartilages and dorsally by the 12 thoracic vertebrae and the dorsal parts of the 12 ribs. Theoretically, any development imbalance between sternum and vertebrae may lead to disturbed thoracic development and subsequently cause maldevelopment-related scoliosis. We speculated that the mechanism of the distorted spine development might be related to the loss of coupling between the development of sternum and thoracic vertebras.
The aim of this study is to examine whether the loss of growth coordination between sternum and thoracic vertebras can predispose spine to maldevelopment-related scoliosis, especially AIS. By quantitatively comparing the differences in the growth of the anterior and posterior elements of thorax cage (i.e., sternum and vertebrae) in patients with AIS and normal individuals, as well as two mouse models with maldevelopment-related scoliosis, we demonstrate that the imbalanced growth between the thoracic vertebral column and sternum is one of the causative factors for maldevelopment-related scoliosis including some types of AIS.
Materials and methods
Patients
Fifty AIS patients between the age of 12 and 24 years with Cobb’s angles between 32° and 104°, and 50 age-matched controls were enrolled. All patients and controls underwent a 64-slice three-dimensional reconstructed CT imaging of their thorax. The absolute lengths of the sternum and 1st-10th thoracic vertebrae were subsequently measured and calculated using the Philips EBW software v.4.5 (Philips Healthcare Nederland B.V., Netherlands). The length of the sternum from the midpoint of the upper edge of the presternum to the midpoint between the bilateral 7th rib head was measured. The length of thoracic vertebra was measured from the midpoint of the upper edge of the 1st thoracic vertebrae to the midpoint of the lower edge of the 10th thoracic vertebrae. This project received approval from the Ethical and Protocol Review Committee of Daping Hospital, Chongqing.
Mice
Fgfr3 knockout mice were maintained on C3H/HeJ (C3H) background and the genotyping was conducted as described previously [22]. Female C57BL/6J (C57) mice were purchased from the Nanjing Model Animal Research Center. All experiments were performed in accordance with protocols and approved by the Institutional Animals Care and Use Committee of Daping Hospital (Chongqing, China). All mice were housed in animal room with air conditioning and lighting control system.
Group and surgery
Three-week-old C57 mice were randomly divided into 4 groups: Group 1, 15 quadrupedal mice served as controls; Group 2, 15 mice underwent resection of two forelegs and tail (bipedal mice) [23]; Group 3, 15 quadrupedal mice underwent sternal growth plate injury and Group 4, 15 bipedal mice underwent sternal growth plate injury. Amputation of both bilateral forelimbs at the humeroscapular junction and excision of the tail at the bottom were performed for each mouse in Groups 2 and 4 under anaesthesia with pentobarbital sodium (100 mg/kg), whereas sternum growth plates were destructed using an electrosurgical knife in mice in Groups 3 and 4. Buprenorphine was used for control of postoperative pain.
X-ray
Mice were sacrificed after being subjected to high-resolution X-ray examination using Faxitron MX20 (Faxitron X-ray, Lincolnshire, IL, USA). The acquired images were used to measure the gross anatomical morphology. The length of isolated sternum and thoracic vertebrae was measured based on the X-ray image using ImageJ software (https://imagej.nih.gov/ij/). Their ratio is used as the thoracic anteroposterior length ratio.
Micro-CT
The thorax cages of mice were scanned by micro-CT (VivaCT40; Scanco Medical AG, Switzerland), with the condition of 70 kV and 113 μA. Two-dimensional images were used to generate three-dimensional reconstructions. At each specific time point examined, every measurement used the same filtering and segmentation values to obtain three-dimensional images. The magnitude of the major scoliotic curve (Cobb's angle) was recorded. The curvature of scoliosis greater than 10° was the criterion for scoliosis.
Histology and analysis
A CO2 overdose was used for the euthanasia of all mice. The sternum and thoracic vertebrae were dissected and collected 3 months after surgery, fixed in 4% paraformaldehyde in 0.01 M phosphate buffer saline (PBS) (pH 7.4), decalcified in 15% EDTA (pH 7.4) and embedded in paraffin. Five-micrometre-thick sections were sliced and stained with Safranin-O and Fast Green.
Statistical analysis
All data were statistically analyzed using GraphPad Prism (GraphPad Inc., La Jolla, CA, USA), version 6, and the results were expressed as mean ± standard deviation. Statistics were evaluated using Student t test and two-way analysis of variance test, and P values were considered significant at P < 0.05.
Results
The thoracic anteroposterior length ratio of patients with AIS is significantly reduced and correlated with the occurrence and severity scores of scoliosis
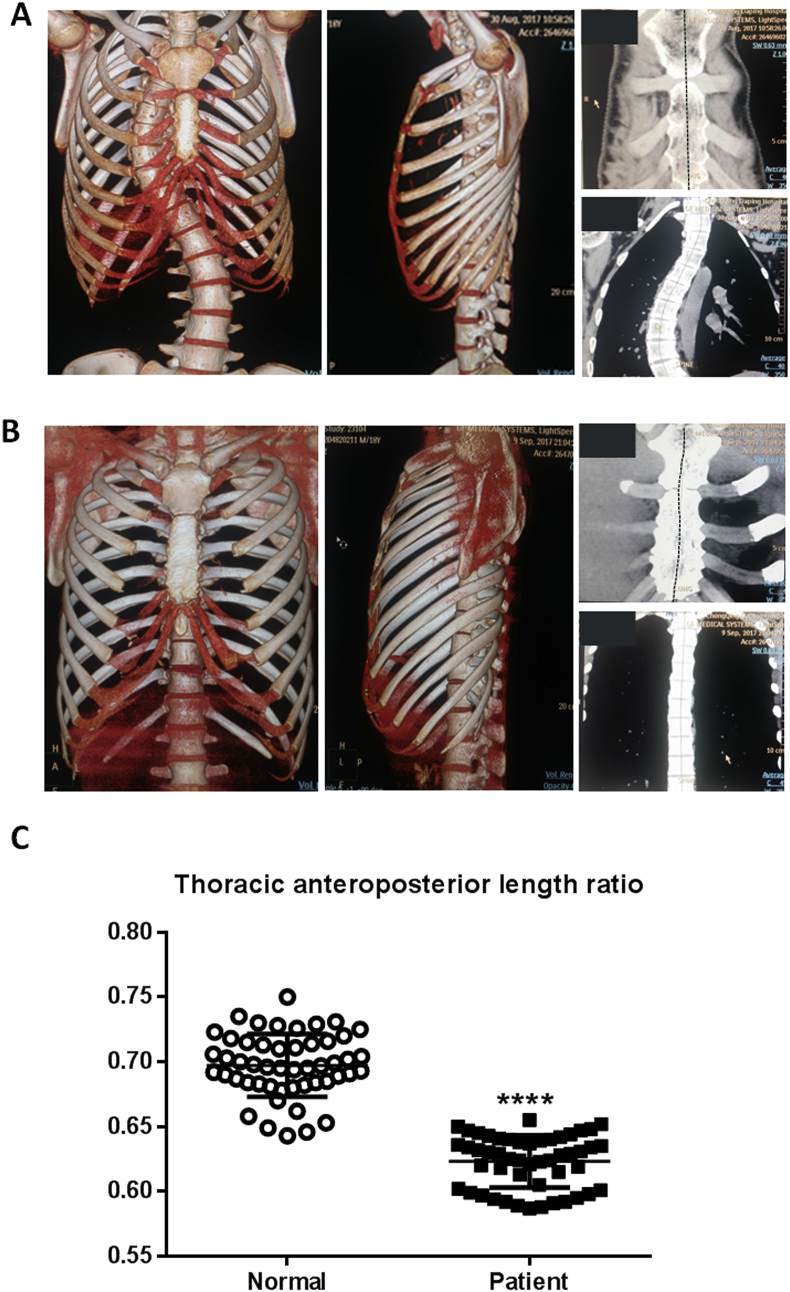
The Cobb's angle is less than 10° in the normal control group without scoliosis phenotype compared with that in the 50 patients with AIS enrolled in this study (Fig. 1A and B). The thoracic anteroposterior length ratio in patients with AIS was 0.623 ± 0.020 (n = 50), whereas that in the control group was 0.695 ± 0.023 (n = 50). Thoracic anteroposterior length ratio of patients with AIS was significantly reduced compared with that of the age-matched control group (Fig. 1C). Based on the Cobb's angle, all patients were divided into three group as follows: 1, 30–45°; 2, 46–60°; 3, 61° and above. The Spearman coefficient analysis of the correlation between ordinal measurements showed a significant negative correlation between the scoliosis severity score and the ratio between sternum and thoracic vertebras (T1–10) (P = 0.008, Rho = −0.370). These data reveal a negative correlation between the extent of disproportionate anteroposterior growth of thorax and the severities of scoliosis, which indicates that the mismatched development between thoracic spine and sternum play an important role in the development of scoliosis.
Figure 1.
The CT images and measurement results obtained from AIS patients and control subject. (A) Representative CT images of thorax in patients with AIS. (B) Representative CT images of thorax of individuals in the normal group. (C) The thoracic anteroposterior length ratio of patients with AIS was significantly decreased. Values are the mean ± SD. ****P < 0.0001 compared with normal group.
AIS = adolescent idiopathic scoliosis; CT = computed tomography; SD = standard deviation.
The thoracic anteroposterior length ratio is significantly reduced in fgfr3 knockout mice
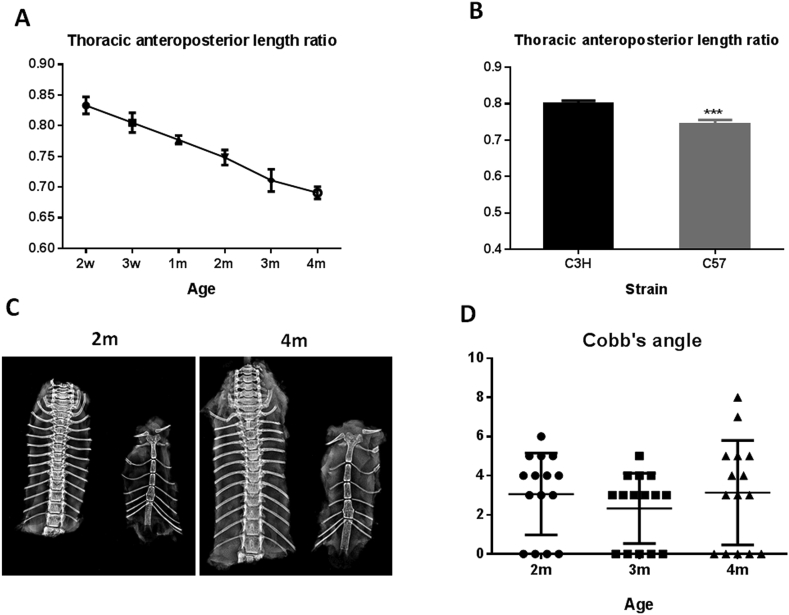
Fgfr3 knockout mice showed overgrowth as previously described [22]. These mice exhibit elongated thoracic vertebrae, which is increasingly apparent (Fig. 2A). All mutant mice show severe scoliosis (greater than 30°) at 2 months of age, whereas none of the age-matched wild-type C3H mice have scoliosis phenotype (Cobb's angle < 10°). The apical vertebrae of scoliosis in fgfr3 knockout mice was mainly located between the 7th and 10th thoracic vertebrae, indicating that the maldevelopment of thorax cage plays an important role in the pathogenesis of scoliosis in fgfr3 knockout mice (Fig. 2B). There was a differential growth rate along the thoracic spinal column between two genotypes. By 2 months, the height of the thoracic vertebrae (T1–T10) in FGFR3-deficient mice (total lengths, 3.708 ± 0.184 cm) was significantly greater than that of the vertebrae in C3H mice (total lengths, 2.802 ± 0.164 cm). The length of sternum in FGFR3-deficient and control mice was 2.425 ± 0.185 cm and 2.243 ± 0.183 cm, respectively (Fig. 2C). The thoracic anteroposterior length ratio of FGFR3-deficient mice was 0.6573 ± 0.016, which was significantly lower than that in the control group (0.8000 ± 0.008) at 2 months (Fig. 2D). Measurement of the lengths of sternum and thoracic vertebrae (T1–10) reveals that fgfr3 knockout mice had relatively more active growth in thoracic vertebrae than in sternum when compared with wild-type mice, leading to reduced thoracic anteroposterior length ratio.
Figure 2.
FGFR3 deficiency results in scoliosis with decreased thoracic anteroposterior length ratio in mice. (A) Representative X-ray images of the sternum and thoracic vertebrae from WT and FGFR3-deficient mice at 2 and 8 weeks. (B) Representative 3D micro-CT images of thoracic vertebrae from WT and FGFR3-deficient mice at 8 weeks. (C) The length of thoracic vertebrae (T1–10) in FGFR3-deficient mice was increased compared with WT mice (n = 8). (D) Thoracic anteroposterior length ratio was significantly reduced in FGFR3-deficient mice (n = 8). (E) The histological section image of the affected thoracic vertebra and intervertebral disc. Red arrows point to apical vertebrae. Values are the mean ± SD. ***P < 0.001; ****P < 0.0001 compared with C3H mice. CT = computed tomography; SD = standard deviation; WT = wild-type.
Reduced thoracic anteroposterior length ratio is sufficient to cause scoliosis
Mice with genetic or pinealectomy-induced melatonin deficiency have scoliosis, which is a commonly used model for idiopathic scoliosis [24], [25]. In C57 mice, arylalkylamine N-acetyltransferase gene is naturally mutated, leading to decreased melatonin synthesis [26]. We checked whether C57 mice showed spontaneous scoliosis. The thoracic anteroposterior length ratio of 2-week-, 3-week-, 1-month-, 2-month-, 3-month- and 4-month-old C57 mice were 0.833 ± 0.014, 0.805 ± 0.016, 0.777 ± 0.006, 0.748 ± 0.011, 0.711 ± 0.018 and 0.691 ± 0.010, respectively. During the rapid development stage (2 weeks–4 months) of C57 mice, the thoracic anteroposterior length ratio was gradually decreased with age (Fig. 3A). Although C57 mice had significantly smaller thoracic anteroposterior length ratios than C3H mice at 2 months, they show no scoliosis phenotype at all age stages (Fig. 3B–D). These data indicate that the decreased thoracic anteroposterior length ratio in C57 mice alone is not enough to cause scoliosis. To obtain scoliosis phenotype, additional scoliosis-promoting factors are needed.
Figure 3.
Analysis of the thoracic anteroposterior length ratio in C57 and C3H mice. (A) The thoracic anteroposterior length ratio in C57 mice was gradually decreased with age in the development stage. (n = 5) (B) C57 mice exhibited significantly lower thoracic anteroposterior length ratios than C3H mice (n = 8) (C) Representative X-ray images of the sternum and thoracic vertebrae from C57 mice at postnatal 2 and 4 months. (D) Scatter plots illustrate that no scoliosis was developed during the development stage in C57 mice (n = 15). Values are the mean ± SD. ***P < 0.001 compared with C3H mice.
SD = standard deviation.
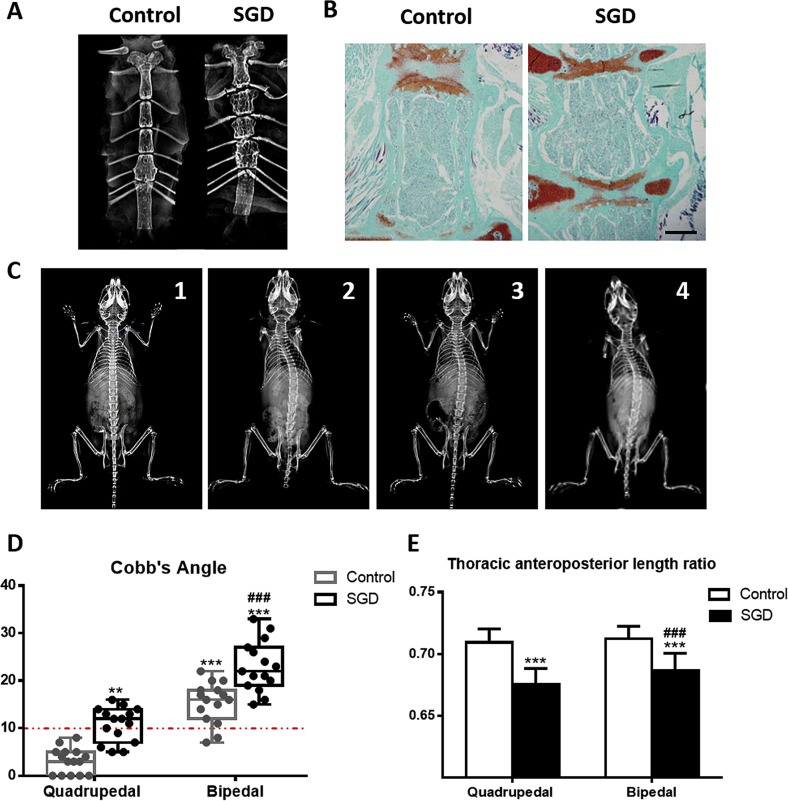
We showed that the normal quadrupedal C57 mice had no scoliosis phenotype at 4 months of age. X-ray and histology study of the sternum found that the growth plates of mice in Groups 3 and 4 were destructed with resultant shortened sternum (Fig. 4A and B). Two months after surgery, although mice in both Groups 3 and 4 showed smaller thoracic anteroposterior length ratio, no significant scoliosis developed (data not shown). Three months after surgery, there was no scoliosis developed in Group 1. There were, however, 13 mice with scoliosis in Group 2 (86.67%), with an average Cobb's angle of 15.4°, 10 mice in Group 3 (66.67%), with an average Cobb's angle of 10.8°, and 15 mice in Group 4 (100%), with an average Cobb's angle of 23.0°. A higher incidence and more severe curvature were observed in Group 4 than in both Groups 2 and 3 (Fig. 4C and D). The thoracic anteroposterior length ratios in Groups 1, 2, 3 and 4 were 0.7092 ± 0.0110, 0.7124 ± 0.0100, 0.6755 ± 0.0127 and 0.6863 ± 0.0143, respectively (Fig. 4E). Our data suggest that sternal growth plate destruction surgery leads to relative overgrowth of the thoracic vertebras, which artificially reduces the thoracic anteroposterior length ratio, leading to scoliosis in quadrupedal mice and increased scoliosis curvature severity in bipedal mice.
Figure 4.
Artificially reducing the thoracic anteroposterior length ratio by surgical destruction of growth plates (SGD) was sufficient to cause scoliosis in quadrupedal C57 mice and increase scoliosis curvature severity in bipedal mice. (A, B) Representative X-ray and histologic images of the sternum with or without SGD at 3 month after surgery. SGD led to shortened sternum. (C) Representative X-ray images of mice in four groups: 1, quadrupedal; 2, bipedal; 3, SGD; 4, bipedal with SGD. (D) SGD led to scoliosis in quadrupedal mice and increased scoliosis curvature severity in bipedal mice. (E) SGD led to reduced thoracic anteroposterior length ratio. Values are the mean ± SD (n = 15 mice per group). **P < 0.01, ***P < 0.001 compared with quadrupedal control mice (Group 1); ###P < 0.001 compared with bipedal mice (Group 3).
SD = standard deviation.
Discussion
Spine curvature is a three-dimensional deformation of the spine which can lead to back pain, skewed body and decreased respiratory function, having severe impact on the life quality and psychological well-being of individuals affected [27]. To date, orthosis and orthopaedic surgery are the most effective therapies for CS. Better understanding of the aetiology of CS and identifying possible risk factors will provide valuable clues for early diagnosis and nonsurgical and surgical management of spine curvature.
Maldevelopment-related spine deformation such as CS and AIS mainly affects the child and young people. The mechanism underlying them is not fully clarified presently, although dysregulated skeleton development is certainly the major reasons. Until now, studies have been mainly focused on the role of asymmetry development of individual vertebrae in spine curvature. Multiple studies demonstrate that spine curvature, especially AIS, is associated with the overgrowth of the anterior column resulting from loss of coupling between the EO and intramembranous ossification during the growth spurt [17], [18], [19].
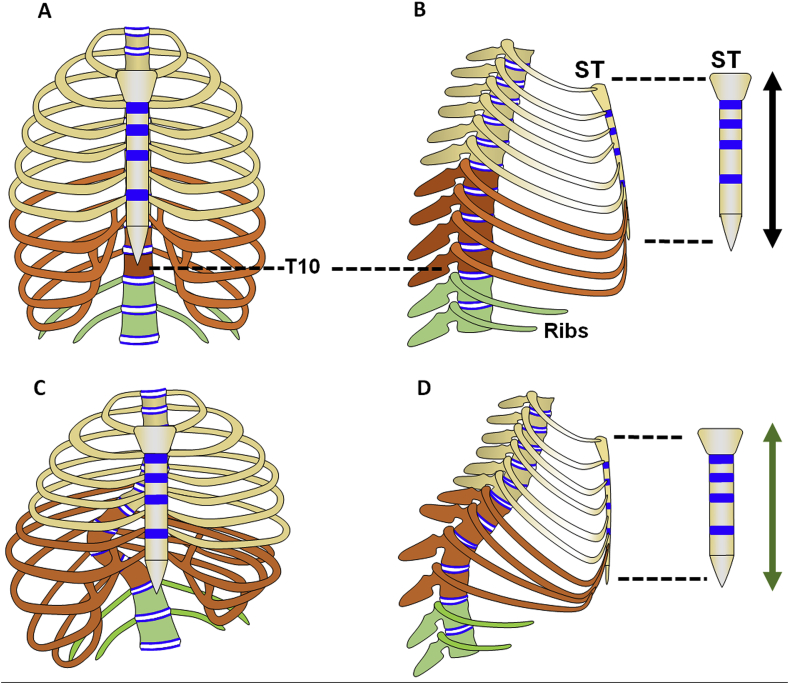
Interestingly, AIS occurs mainly in thoracic vertebrae; the reason for this phenomenon is not clear. The major bony components of thoracic cavity including ribs, sternum and vertebrae are mainly formed through EO (Fig. 5A and B).
Figure 5.
Schematic diagram of the relationship between the sternum and the thoracic vertebrae. (A, B) The schematic diagram of thoracic skeleton structure in normal individuals: (A) anteroposterior; (B) lateral. The diagram of thoracic skeleton structure in thoracic scoliosis patients with bending and rotation in lateral: (C) anteroposterior; (D) lateral. The blue regions represent the growth plates. T, thoracic vertebrae; ST, sternum. The orange regions are the T7–10 and Ribs 7–10, which are the major affected regions of thoracic scoliosis. The green regions are the T11–12 and Rib 11–12 (floating ribs).
Recently, it has been proved that the disturbed ribcage development can cause progressive structural scoliosis. Doi et al [28] found that shallow chest is associated with the initiation of small thoracic curvatures and thoracic AIS. Patients with thoracic scoliosis always showed spinal axial deformities accompanied by flat chest and ribcage rotation [29], [30]. All these data suggest that ribcage maldevelopment is one of the causative factors of AIS.
Indeed, the components of the thoracic cage including sternum, rib and thoracic vertebrae (T1–T12) are structurally and functionally integrated as a unit to meet the basic functional demand of the thoracic spine and organs related, especially the heart and lung. Imbalanced development among these components of thorax resulting from either genetic and/or environmental disturbance may lead to occurrence and/or increased severity of the thoracic spine curvature (Fig. 5C and D). The sternum, connected to the spine via ribs, is one of the major skeleton components composed of the thoracic cavity, indicating its potential essential role in spine development. We here find that patients with scoliosis had a reduced thoracic anteroposterior length ratio resulting from relative longer thoracic vertebra and shorter sternum. We further found that C57BL/6J mice exhibited a decreasing thoracic anteroposterior length ratio with ageing, but there was no scoliosis phenotype even at 12 months (data not shown). When we artificially reduced the thoracic anteroposterior length ratio by surgically destroying the sternal growth plates, scoliosis in quadrupedal mice and increased scoliosis curvature in bipedal mice were found. Consistently, FGFR3 knockout mice exhibited severe scoliosis and had a reduced thoracic anteroposterior length ratio, which further indicate that imbalanced growth between the thoracic vertebral column and the sternum may also participate in the pathogenesis of spine curvature including AIS. We thus hypothesize that the gradually reduced relative length of sternum during development stage also accounts for the spine curvature/scoliosis.
To keep the physiological shape and volume of thoracic cavity, the range of length ratio between the sternum and related thoracic spine must be precisely restricted. The length of the sternum is normally much shorter than that of the thoracic spine connected to the sternum by ribs. It is not clear why the length of sternum is relatively shorter than that of thoracic vertebrae connected to ribs (T1–T10). The development of the spine and sternum is mainly accomplished by their growth plates through EO. There are less numbers of growth plates in the sternum than that of (T1-T10), which may help to explain why the sternum is shorter than the thoracic spine related. Although the growth is accelerated in both the sternum and vertebral bodies of FGFR3-deficient mice, the relatively more active development of vertebrae that is presumptively related to their larger numbers of growth plates still leads to the imbalanced development of the anterior and posterior part of thorax, causing spine curvature. The detailed mechanisms need to be further studied in future.
Interestingly, the way through which the sternum is connected to the thoracic vertebra may also play an important role in the pathogenesis of the deformed thoracic spine. The costal cartilage of Rib 1–7 directly attaches to the sternum, whereas the costal cartilage of Ribs 8–10 indirectly connects to the sternum by attaching to the cartilage of the next upper rib. These differences may cause lower stability of thoracic 7–10 vertebral bodies than that in thoracic 1–6 vertebral bodies. When the thoracic spine is subjected to lateral curvature and rotation, the less stabilized thoracic 7–10 vertebral bodies are more likely to be distorted. This may also help to explain the phenomenon that the apical vertebrae are mostly located between the thoracic vertebral body 7 and 10 in patients with thoracic AIS and fgfr3 knockout mice.
In summary, using mouse models with maldevelopment-related spine curvature and images from patients with AIS, we revealed that mechanically and genetically induced differential development between sternum and thoracic vertebras can induce severe spine deformation mimicking AIS. Our data support a novel mechanism for the pathogenesis of spine curvature including AIS, suggesting that the prevention and/or treatment of spine curvature should focus on not only spine itself but also surgical or rehabilitation intervention of other components involved in the pathogenesis of spine curvature, which will bring better outcomes for patients.
Conflict of interest
The authors declare that they have no competing interests.
Acknowledgements
This work was supported by Special Funds for Major State Basic Research Program of China (973 program) (No. 2014CB942904), National Natural Science Foundation of China (No. 81830075, 81772306).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2018.12.001.
Contributor Information
Yangli Xie, Email: xieyangli841015@163.com.
Lin Chen, Email: linchen70@163.com.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Altaf F., Gibson A., Dannawi Z., Noordeen H. Adolescent idiopathic scoliosis. BMJ. 2013;346:f2508. doi: 10.1136/bmj.f2508. [DOI] [PubMed] [Google Scholar]
- 2.Burton D.C., Carlson B.B., Place H.M., Fuller J.E., Blanke K., Cho R. Results of the scoliosis Research society morbidity and mortality database 2009–2012: a report from the morbidity and mortality committee. Spine Deformity. 2016;4(5):338–343. doi: 10.1016/j.jspd.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Cho W., Shepard N., Arlet V. The etiology of congenital scoliosis: genetic vs. environmental-a report of three monozygotic twin cases. Eur Spine J. 2018;27(Suppl 3):533–537. doi: 10.1007/s00586-018-5604-2. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein S.L., Dolan L.A., Cheng J.C., Danielsson A., Morcuende J.A. Adolescent idiopathic scoliosis. Lancet. 2008;371(9623):1527–1537. doi: 10.1016/S0140-6736(08)60658-3. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigues L.M.R., Gotfryd A.O. Adolescent idiopathic scoliosis: surgical treatment and quality of life. 2017;25(3):85–89. doi: 10.1590/1413-785220172503157788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grauers A., Einarsdottir E., Gerdhem P. Genetics and pathogenesis of idiopathic scoliosis. Scoliosis Spinal Disord. 2016;11:45. doi: 10.1186/s13013-016-0105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma S., Londono D., Eckalbar W.L., Gao X., Zhang D., Mauldin K. A PAX1 enhancer locus is associated with susceptibility to idiopathic scoliosis in females. Nat Commun. 2015;6:6452. doi: 10.1038/ncomms7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchan J.G., Alvarado D.M., Haller G.E., Cruchaga C., Harms M.B., Zhang T. Rare variants in FBN1 and FBN2 are associated with severe adolescent idiopathic scoliosis. Hum Mol Genet. 2014;23(19):5271–5282. doi: 10.1093/hmg/ddu224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kou I., Takahashi Y., Johnson T.A., Takahashi A., Guo L., Dai J. Genetic variants in GPR126 are associated with adolescent idiopathic scoliosis. Nat Genet. 2013;45(6):676–679. doi: 10.1038/ng.2639. [DOI] [PubMed] [Google Scholar]
- 10.Wu N., Ming X., Xiao J., Wu Z., Chen X., Shinawi M. TBX6 null variants and a common hypomorphic allele in congenital scoliosis. N Engl J Med. 2015;372(4):341–350. doi: 10.1056/NEJMoa1406829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao C., Chen B.P., Sullivan M.B., Hui J., Ouellet J.A., Henderson J.E. Micro CT analysis of spine architecture in a mouse model of scoliosis. Front Endocrinol. 2015;6:38. doi: 10.3389/fendo.2015.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colvin J.S., Bohne B.A., Harding G.W., McEwen D.G., Ornitz D.M. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996;12(4):390–397. doi: 10.1038/ng0496-390. [DOI] [PubMed] [Google Scholar]
- 13.Yang C., Chen Y., Li Z., Cao H., Chen K., Lai P. Chondrocyte-specific knockout of TSC-1 leads to congenital spinal deformity in mice. BioMed Res Int. 2017;2017:8215805. doi: 10.1155/2017/8215805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Latalski M., Danielewicz-Bromberek A., Fatyga M., Latalska M., Kröber M., Zwolak P. Current insights into the aetiology of adolescent idiopathic scoliosis. Arch Orthop Trauma Surg. 2017;137(10):1327–1333. doi: 10.1007/s00402-017-2756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fortin C., Grunstein E., Labelle H., Parent S., Ehrmann Feldman D. Trunk imbalance in adolescent idiopathic scoliosis. Spine J : official journal of the North American Spine Society. 2016;16(6):687–693. doi: 10.1016/j.spinee.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 16.Zhu F., Qiu Y., Yeung H.Y., Lee K.M., Cheng J.C. Histomorphometric study of the spinal growth plates in idiopathic scoliosis and congenital scoliosis. Pediatr Int. 2006;48(6):591–598. doi: 10.1111/j.1442-200X.2006.02277.x. [DOI] [PubMed] [Google Scholar]
- 17.Guo X., Chau W.W., Chan Y.L., Cheng J.C. Relative anterior spinal overgrowth in adolescent idiopathic scoliosis. Results of disproportionate endochondral-membranous bone growth. J Bone Joint Surg Br Vol. 2003;85(7):1026–1031. doi: 10.1302/0301-620x.85b7.14046. [DOI] [PubMed] [Google Scholar]
- 18.Guo X., Chau W.W., Chan Y.L., Cheng J.C., Burwell R.G., Dangerfield P.H. Relative anterior spinal overgrowth in adolescent idiopathic scoliosis--result of disproportionate endochondral-membranous bone growth? Summary of an electronic focus group debate of the IBSE. Eur Spine J. 2005;14(9):862–873. doi: 10.1007/s00586-005-1002-7. [DOI] [PubMed] [Google Scholar]
- 19.Guo X., Chau W.W., Chan Y.L., Cheng J.C.Y. Relative anterior spinal overgrowth in adolescent idiopathic scoliosis. J Bone Joint Surg. 2003;85(7):1026–1031. doi: 10.1302/0301-620x.85b7.14046. [DOI] [PubMed] [Google Scholar]
- 20.Yagi M., Takemitsu M., Machida M. Chest cage angle difference and rotation of main thoracic curve are independent risk factors of postoperative shoulder imbalance in surgically treated patients with adolescent idiopathic scoliosis. Spine. 2013;38(19):E1209–E1215. doi: 10.1097/BRS.0b013e31829e0309. [DOI] [PubMed] [Google Scholar]
- 21.Crijns T.J., Stadhouder A., Smit T.H. Restrained differential growth: the initiating event of adolescent idiopathic scoliosis? Spine. 2017;42(12):E726–E732. doi: 10.1097/BRS.0000000000001946. [DOI] [PubMed] [Google Scholar]
- 22.Deng C., Wynshaw-Boris A., Zhou F., Kuo A., Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996;84(6):911–921. doi: 10.1016/s0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- 23.Wu T., Sun X., Zhu Z., Yan H., Guo J., Cheng J.C. Role of enhanced central leptin activity in a scoliosis model created in bipedal amputated mice. Spine. 2015;40(19):E1041–E1045. doi: 10.1097/BRS.0000000000001060. [DOI] [PubMed] [Google Scholar]
- 24.Wai M.G.C., Jun W.W.W., Yee Y.A.P., Ho W.J., Bun N.T., Ping L.T. A review of pinealectomy-induced melatonin-deficient animal models for the study of etiopathogenesis of adolescent idiopathic scoliosis. Int J Mol Sci. 2014;15(9):16484–16499. doi: 10.3390/ijms150916484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zamecnik J., Krskova L., Hacek J., Stetkarova I., Krbec M. Etiopathogenesis of adolescent idiopathic scoliosis: expression of melatonin receptors 1A/1B, calmodulin and estrogen receptor 2 in deep paravertebral muscles revisited. Mol Med Rep. 2016;14(6):5719–5724. doi: 10.3892/mmr.2016.5927. [DOI] [PubMed] [Google Scholar]
- 26.Roseboom P.H., Namboodiri M.A., Zimonjic D.B., Popescu N.C., Rodriguez I.R., Gastel J.A. Natural melatonin 'knockdown' in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase. Brain Res Mol Brain Res. 1998;63(1):189–197. doi: 10.1016/s0169-328x(98)00273-3. [DOI] [PubMed] [Google Scholar]
- 27.Kim H.S. Response to: evidence-based of nonoperative treatment in adolescent idiopathic scoliosis. Asian Spine J. 2015;9(2):315. doi: 10.4184/asj.2015.9.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doi T., Matsumoto Y., Tono O., Tarukado K., Harimaya K., Okada S. A shallow chest correlates with the aortic position in the normal spine: features resembling those observed in structural scoliosis. Scoliosis. 2014;9:14. doi: 10.1186/1748-7161-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doi T., Harimaya K., Matsumoto Y., Iwamoto Y. Aortic location and flat chest in scoliosis: a prospective study. Fukuoka Igaku Zasshi. 2011;102(1):14–19. [PubMed] [Google Scholar]
- 30.Hong J.Y., Suh S.W., Park H.J., Kim Y.H., Park J.H., Park S.Y. Correlations of adolescent idiopathic scoliosis and pectus excavatum. J Pediatr Orthoped. 2011;31(8):870–874. doi: 10.1097/BPO.0b013e31822da7d5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.