Abstract
Endometriosis is a complex, benign, estrogen-dependent gynecological disorder with an incidence of ~10% women in reproductive age. The implantation and growth of endometrial cells outside the uterus leads to the development of endometriosis. Endometriosis is also associated with comorbid conditions like cardiovascular and autoimmune diseases. The absence of non-invasive diagnostic markers, delayed diagnosis, high risk of recurrence of the disease on surgical removal of the tissue and absence of a definitive cure for endometriosis makes it imperative to gain insights into the complex etiology of endometriosis. A plethora of genes identified from blood and endometrial biopsies, involved in different pathways like steroid metabolism, angiogenesis, inflammation, etc. have been associated with endometriosis. However, the exact mechanism and genetic etiology of endometriosis still remain unclear. The polygenic nature of the disease, incongruent phenotypic manifestations in different ethnic populations and information scattered in literature makes it difficult to delineate the sub-network of genes that will aid in disease diagnosis and effective treatment. Endometriosis Knowledgebase is a manually curated database with information on genes associated with endometriosis. It holds information on 831 genes, their associated polymorphisms, gene ontologys, pathways and diseases. Genes in the database are enriched in pathways important for cell signaling, immune regulation and reproduction. A genetic overlap is seen between endometriosis and cancers, endocrine/reproductive, nervous system, immune and metabolic diseases. Network analysis of genes in the Endometriosis Knowledgebase helped predict 13 new candidate genes for endometriosis. These genes were found to be enriched in biological processes associated with endometriosis. The Endometriosis Knowledgebase and incorporated tools for gene and sequence-based analysis will benefit both researchers and clinicians working in the realm of reproductive biology.
Introduction
Endometriosis is a common, benign, estrogen-dependent, gynecological disorder affecting ~10% of reproductive age women across the globe (1). It is associated with chronic pelvic pain, subfertility, dysmenorrhea, dyspareunia (2) leading to substantial socioeconomic burden and affecting the quality of life (3). A delay of 8–10 years in the diagnosis of endometriosis expected due paucity of reliable non-invasive diagnostic methods (4, 5). An increased risk of developing comorbid conditions like diabetes mellitus, cardiovascular disease, chronic liver disease, pelvic inflammatory diseases and autoimmune disorders such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Sjogren’s syndrome (6), multiple sclerosis (7), inflammatory bowel diseases (such as Crohn’s disease and ulcerative colitis) (8) and celiac disease (9) is observed in women with endometriosis.
The etiology of endometriosis is multifactorial in origin and is often referred to as ‘disease of theories’. Sampson’s theory of retrograde menstruation (RM) is the most commonly accepted theory, which proposes the reflux of viable endometrial cells into the peritoneal cavity leading to the development of endometriotic lesions (10). However, RM is observed in 70–90% of women and endometriosis occurs in only 7–10% of women suggesting the role of other factors in the etiology of endometriosis (11).
Several studies have found a familial aggregation of endometriosis (12–14). The largest twin study among 3096 Australian female twins estimated heritability of ~50% (15). These reports reinforce the role of genes in the pathogenesis of endometriosis.
Both candidate gene-based and high-throughput approaches like Genome-Wide Association Studies (GWAS) (16) have been employed to delineate genes associated with endometriosis. Genes mainly involved in inflammatory processes (e.g. IL1, CCRL2, CCL17) (17–19), steroid-synthesis (e.g. HSD17B7, CYP19A1, HSD17B1) (20, 21), detoxification (e.g. GSTM1, GSTP1, GSTT1) (22), hormone receptors (e.g. PR, AR) (23), estrogen metabolism (e.g. ESR1) (24), growth factors (e.g. GDF15) (25), adhesion molecules (e.g. ICAM) (26), apoptosis (e.g. NFKB1, TNFRSF1A) (27, 28), cell-cycle regulation (e.g. CCNB1) (29) and oncogenes (e.g. EGFR, FOS) (30, 31) were found to be associated with endometriosis. However, these studies were unsuccessful in providing replicable results when evaluated in larger independent cohorts (32).
The absence of a permanent cure for endometriosis, side effects of existing drugs that help to reduce the symptoms of the disease and a reported 35–50% chance of recurrence post-surgery makes developing diagnostic markers and identifying therapeutic targets for endometriosis a necessity (33–36).
The genetic complexity of endometriosis and its associated comorbid conditions suggest the need for use of an integrated approach to understand the pathophysiology of the disease and delineate its genetic etiology. A knowledgebase with manually curated information on endometriosis-associated genes, their ontologys, pathways and comorbid conditions will help to accelerate the research focused on understanding the etiology and pathogenesis of the disease. The knowledgebase would also be an excellent resource for the design of future research programs on endometriosis. The Endometriosis Knowledgebase is a comprehensive resource developed with an aim to augment research on endometriosis.
Data collection
The aim of Endometriosis Knowledgebase was to develop a resource with all genes associated with endometriosis reported in the literature. The PubMed (37) database was queried for ‘Endometriosis’. The search was limited to human studies only. The genes (37) and single nucleotide polymorphisms (SNPs) (37) linked to the articles were retrieved from the Gene and SNP databases at National Center for Biotechnology Information (NCBI). A search query was built by combining each gene with its alternate names and the ‘endometriosis’ keyword. The query was further used to search the PubMed database. The articles retrieved were manually curated for information on the genes associated with endometriosis. The information mined from the PubMed reference is similar to information present in the CBD database (38). Each relevant PubMed reference abstract was mined for information on study population (population size, ethnicity), polymorphisms/mutations reported and other genes in the reference studied for their role in endometriosis. Genes having a role in endometriosis-associated infertility were annotated accordingly.
SNPs associated with endometriosis were retrieved from the SNP database of NCBI. Protein-related information was obtained from the UniProt database (39) and Gene Ontology (GO) information from the AMIGO2 database was mined using the GOOSE tool (40–43). The pathway information on the genes was retrieved from the KEGG Pathway database (44). The genes were also searched for disease associations in the Genetic Association Database (GAD) (45), KEGG disease database and in the Online Mendelian Inheritance in Man (OMIM) database (46). The information retrieved from the PubMed, Gene, SNP, KEGG, GAD, OMIM, UniProt and GO were compiled together as the Endometriosis Knowledgebase.
Database architecture
Endometriosis Knowledgebase is built on Apache HTTP Server 2.2.11. The database is created using MySQL Server 5.1.33 and the web interfaces are designed using PHP 5.2.9, HTML and JavaScript. These are platform-independent, open source software packages.
Database design
The knowledgebase consists of information on 831 genes and their 302 SNPs, 7032 gene ontologys, 367 pathways and 1390 diseases. The database homepage gives a brief introduction to the database. The user-friendly search and browse options allow efficient data retrieval. Gene and sequence-based tools have been incorporated in the knowledgebase for the benefit of users. These tools can be accessed through the Tools, BLAST and the Analysis link in the database interface. These options are described below:
Tools: This section allows users to identify conserved domains and motifs in their gene of interest using the CDART (47) or Motif Scan (48) link, respectively. The orthologs and SNPs link under the tools section help in retrieving known orthologs and variant effects of SNPs respectively using the g:Profiler program (49). The STRING (50) link allows users to see the known and predicted interactions between the user-selected set of genes.
Blast (37, 51): Users can find homologous protein or nucleotide sequences for the user-selected set of genes using the BLAST link.
Analysis: The analysis tab in the knowledgebase allows users to cluster genes based on diseases, pathways and gene ontologys present in Endometriosis Knowledgebase separately. It also allows parsing user-selected genes to the Panther and g:profiler interface using the Panther (42) and Function (49) links, respectively.
The help page guides the users in the usage of the database. The database statistics can be accessed through the Statistics link. A snapshot of the database results page and the advanced search page are shown in Figures 1 and 2, respectively.
Figure 1.
Search results page of Endometriosis Knowledgebase.
Figure 2.
Advanced search page of Endometriosis Knowledgebase.
Data analysis
The genes present in the knowledgebase were analyzed to identify processes and pathways enriched in endometriosis using the Panther Gene List Analysis tool. The knowledgebase was found to be enriched in cell-signaling molecules and transcription factors indicating a regulatory role for most of the genes. Gonadotropin-releasing hormone receptor pathway, inflammation mediated by chemokine and cytokine signaling, the CCKR signaling pathway, angiogenesis and the integrin signaling pathway were found to be the most represented pathway among genes associated with endometriosis (42). Synchronized regulation of these pathways are important for cell survival, normal secretion of gonadotropins LH and FSH, pubertal development, reproduction, angiogenesis, trafficking and migration of immune cells etc. (42). The dysregulation of the gonadotropin-releasing hormone receptor pathway may result in high secretion of estrogen, a characteristic of patients with endometriosis. Aberrations in genes in the angiogenesis pathways lead to an imbalance in the expression of pro-angiogenic and anti-angiogenic factors that facilitate implantation, growth and/or survival of the endometriotic tissue in patients suffering from endometriosis (52). The dysregulation of the integrin signaling pathway expedites the establishment of endometriotic focii in endometriosis (53). These observations reinstate the complex pathophysiology of endometriosis.
Further, clustering the genes in the knowledgebase, based on their disease associations (excluding endometriosis) (Supplementary Table 1), showed a huge percentage of genes associated with other female reproductive disorders majorly polycystic ovary syndrome (PCOS), reproductive cancers and/or infertility (male and female). Endometriosis is associated with ovarian, endometrioid and clear cell cancer (54–56) and this explains the genetic overlap between the two diseases. Endometriosis-associated genes GRB14 and IGF2 play a role in insulin receptor signaling (57). Alterations in the insulin signaling pathway have been observed in endometrial pathologies like endometrial cancers and PCOS (58, 59). In addition to the molecules playing a role in the insulin signaling pathway, the Endometriosis Knowledgebase also consists of several other pleiotropic genes like IL17A, MUC1, etc. that have been reported to be associated with PCOS by independent research groups (60–62). Reports on coexistence of endometriosis and PCOS are rare but are present (63). Another interesting observation from the disease-based clustering is an overlap in genes associated with endometriosis and male infertility (azoospermia, oligospermia, etc.). These genes shared between endometriosis and male infertility were associated with secretion of gonadotropins, pubertal development and reproduction.
Coexistence of endometriosis and autoimmune diseases has been reported. Autoantibodies against syntaxin5, tropomyosin3, stomatin-like protein 2, tropomodulin 3, etc. have been identified in patients suffering from endometriosis (4, 64). Several genes associated with endometriosis are also known to share similarities with other autoimmune diseases (65). Gene polymorphisms like CCL21 (rs2812378) and HLA-DRB1 (rs660895) polymorphisms have been associated with both endometriosis and RA (66). The matrix metalloproteinases are another class of proteins that are associated with the pathogenesis of both arthritis and endometriosis (67). The ESR2 gene polymorphism was found to be associated with Graves’ disease (68, 69). Independent studies have also indicated a higher prevalence of RA, SLE (70) and Sjogren’s syndrome (71) in patients suffering from endometriosis. The genes present in Endometriosis Knowledgebase and associated with RA, SLE, Graves’ disease and Sjogren’s syndrome (6) were analyzed using Panther. The inflammation-mediated chemokine and cytokine signaling pathway and the apoptosis signaling pathway were the two common enriched pathways in genes associated with endometriosis and the above autoimmune diseases.
Other comorbid conditions associated with endometriosis are endocrine/reproductive (e.g. Premature ovarian failure (POF) etc.), nervous system (e.g. Alzheimer’s, Parkinson’s) and metabolic (e.g. obesity) diseases.
The presence of estrogen, progesterone and androgen receptors in the brain is known (72). Estrogen through the estrogen receptor is known to modify the risk of cancer, neurodegenerative diseases, cardiovascular diseases, insulin resistance, etc. (73). The increased exposure to estrogen leads to endometriosis, and its converse leads to neurodegenerative diseases like Alzheimer’s and Parkinson’s disease. While several studies have suggested a decreased risk of neurodegenerative diseases in endometriosis, a case-control study conducted on Danish subjects indicated a moderately increased risk of Parkinson’s disease in women suffering from endometriosis (74). These conflicting observations need to be investigated further. A genome-wide enrichment analysis between endometriosis and obesity-related trait identified the association of GRB14 in both these conditions (75).
The diverse pathways and comorbid disease associations present the complex pathogenesis of endometriosis and also necessitate identification of probable disease targets for therapy and effective disease management. A global approach like gene-disease networks that integrates information on genes their associated gene ontologys, pathways and diseases will aid in gaining a better understanding of the pathophysiology of endometriosis. Endometriosis Knowledgebase would be a useful resource for in-depth study on the genetic etiology of endometriosis.
Predicting novel candidate genes for endometriosis
The interaction network for proteins encoded by genes present in the Endometriosis Knowledgebase was imported from the String database using Cytoscape 3.6 (76) with a confidence cut-off of 0.4. The MCODE plugin (77) in Cytoscape was used to find functional modules in the network. The highest-scoring cluster was analyzed for proteins absent in the Endometriosis Knowledgebase but interacting with endometriosis-associated proteins. The genes corresponding to 20 proteins absent in Endometriosis Knowledgebase were identified from cluster1 (Figure 3). Five of these genes, F2 (78), INS (79), MAPK8 (80), CREB1 (81) and STAT5 (82), have been reported to be differentially regulated in patients with endometriosis. CD34 identified in this study is a marker for endothelial cells that has been reported to be upregulated in endometriosis (83). The JAK2 protein is part of the JAK2/STAT3 signaling pathway, which is important for migration and invasion abilities of endometriotic cells in endometriosis (84). These genes were included in the Endometriosis Knowledgebase. The Panther Tool was used to delineate the enriched pathways in the remaining 13 genes. The most enriched pathways were the angiogenesis pathway and the Ras pathway that are important for cell proliferation, differentiation and survival. Both these pathways are important for the manifestation of the endometriosis phenotype. The probable role of these genes in endometriosis needs to be determined experimentally.
Figure 3.
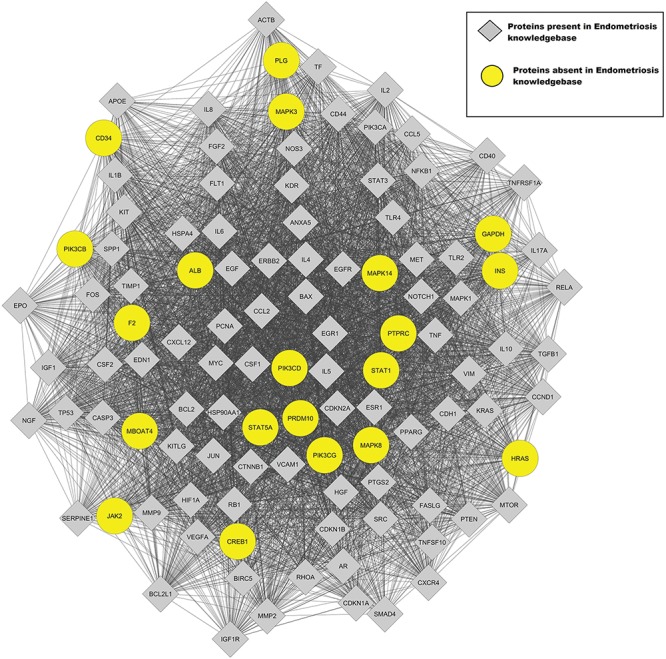
This figure represents the highest-scoring MCODE cluster of the protein–protein interaction network for Endometriosis.
Conclusion
Endometriosis Knowledgebase is a useful resource for genes associated with endometriosis. The knowledgebase currently holds information on 831 genes and 1383 associated diseases. These genes associated with endometriosis have been manually curated and the information on the mutation identified/screened; population size, ethnicity and other pathophenotypes observed in the patients have been included in the database. The database also includes information on gene ontology, KEGG pathways and other associated disorders that could be exploited to build gene-disease networks to identify key causal genes or probable new gene targets for endometriosis. Tools to identify homologs of the genes, identify conserved protein domains and motifs and for SNP analysis have been incorporated in the database. Additionally, the analysis section in the database also allows users to input a user-selected gene list from the database to cluster genes based on common diseases, pathways, gene ontology and protein function. A link has also been provided to PANTHER gene list analysis. Network analysis of genes associated with endometriosis was employed and 13 new candidate genes with a probable role in endometriosis were identified. This resource would be useful to both clinicians and researchers working towards understanding the complex genetic etiology of endometriosis.
Supplementary Material
Acknowledgements
The authors are grateful to Dr Rahul Gajbhiye for critically reviewing the manuscript. We also acknowledge the assistance provided by Mr Ram S. Barai in hosting the knowledgebase.
Database URL: http://www.ek.bicnirrh.res.in
Funding
Indian Council of Medical Research [BIC/12(10)/2013] and National Institute for Research in Reproductive Health (NIRRH) [RA/599/01-2018].
Conflict of interest. None declared.
References
- 1. Giudice L.C. (2010) Clinical practice. Endometriosis. N. Engl. J. Med., 362, 2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stratton P. and Berkley K.J. (2011) Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum. Reprod. Update, 17, 327–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simoens S., Dunselman G., Dirksen C. et al. (2012) The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum. Reprod., 27, 1292–1299. [DOI] [PubMed] [Google Scholar]
- 4. Gajbhiye R., Sonawani A., Khan S. et al. (2012) Identification and validation of novel serum markers for early diagnosis of endometriosis. Hum. Reprod., 27, 408–417. [DOI] [PubMed] [Google Scholar]
- 5. Gajbhiye R., McKinnon B., Mortlock S. et al. (2018) Genetic variation at chromosome 2q13 and its potential influence on endometriosis susceptibility through effects on the IL-1 family. Reprod. Sci., 25, 1307–1317. [DOI] [PubMed] [Google Scholar]
- 6. Sinaii N., Cleary S.D., Ballweg M.L. et al. (2002) High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum. Reprod., 17, 2715–2724. [DOI] [PubMed] [Google Scholar]
- 7. Nielsen N.M., Jorgensen K.T., Pedersen B.V. et al. (2011) The co-occurrence of endometriosis with multiple sclerosis, systemic lupus erythematosus and Sjogren syndrome. Hum. Reprod., 26, 1555–1559. [DOI] [PubMed] [Google Scholar]
- 8. Jess T., Frisch M., Jorgensen K.T. et al. (2012) Increased risk of inflammatory bowel disease in women with endometriosis: a nationwide Danish cohort study. Gut, 61, 1279–1283. [DOI] [PubMed] [Google Scholar]
- 9. Aguiar F.M., Melo S.B.C., Galvao L.C. et al. (2009) Serological testing for celiac disease in women with endometriosis. A pilot study. Clin. Exp. Obstet. Gynecol., 36, 23–25. [PubMed] [Google Scholar]
- 10. Sampson J.A. (1927) Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol., 14, 422–469. [Google Scholar]
- 11. D’Hooghe T.M. and Debrock S. (2002) Endometriosis, retrograde menstruation and peritoneal inflammation in women and in baboons. Hum. Reprod. Update, 8, 84–88. [DOI] [PubMed] [Google Scholar]
- 12. Kennedy S., Mardon H. and Barlow D. (1995) Familial endometriosis. J. Assist. Reprod. Genet., 12, 32–34. [DOI] [PubMed] [Google Scholar]
- 13. Painter J.N., Nyholt D.R., Krause L. et al. (2014) Common variants in the CYP2C19 gene are associated with susceptibility to endometriosis. Fertil. Steril., 102, 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Audebert A., Lecointre L., Afors K. et al. (2015) Adolescent endometriosis: report of a series of 55 cases with a focus on clinical presentation and long-term issues. J. Minim. Invasive Gynecol., 22, 834–840. [DOI] [PubMed] [Google Scholar]
- 15. Treloar S.A., O’Connor D.T., O’Connor V.M. et al. (1999) Genetic influences on endometriosis in an Australian twin sample. Fertil. Steril., 71, 701–710. [DOI] [PubMed] [Google Scholar]
- 16. Albertsen H.M. and Ward K. (2017) Genes linked to endometriosis by GWAS are integral to cytoskeleton regulation and suggests that mesothelial barrier homeostasis is a factor in the pathogenesis of endometriosis. Reprod. Sci., 24, 803–811. [DOI] [PubMed] [Google Scholar]
- 17. Sapkota Y., Low S.K., Attia J. et al. (2015) Association between endometriosis and the interleukin 1A (IL1A) locus. Hum. Reprod., 30, 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Agic A., Djalali S., Wolfler M.M. et al. (2008) Combination of CCR1 mRNA, MCP1, and CA125 measurements in peripheral blood as a diagnostic test for endometriosis. Reprod. Sci., 15, 906–911. [DOI] [PubMed] [Google Scholar]
- 19. Bellelis P., Barbeiro D.F., Rizzo L.V. et al. (2013) Transcriptional changes in the expression of chemokines related to natural killer and T-regulatory cells in patients with deep infiltrative endometriosis. Fertil. Steril., 99, 1987–1993. [DOI] [PubMed] [Google Scholar]
- 20. Smuc T., Pucelj M.R., Sinkovec J. et al. (2007) Expression analysis of the genes involved in estradiol and progesterone action in human ovarian endometriosis. Gynecol. Endocrinol., 23, 105–111. [DOI] [PubMed] [Google Scholar]
- 21. Visnovsky J., Galo S., Zubor P. et al. (2008) Semiquantitative analysis of mRNA aromatase expression in eutopic endometrium as a diagnostic marker of endometriosis and estrogen dependent diseases. Ces. Gynekol., 73, 213–217. [PubMed] [Google Scholar]
- 22. Wu C.H., Guo C.Y., Yang J.G. et al. (2012) Polymorphisms of dioxin receptor complex components and detoxification-related genes jointly confer susceptibility to advanced-stage endometriosis in the Taiwanese Han population. Am. J. Reprod. Immunol., 67, 160–168. [DOI] [PubMed] [Google Scholar]
- 23. Babayev S.N., Park C.W., Keller P.W. et al. (2017) Androgens upregulate endometrial epithelial progesterone receptor expression: potential implications for endometriosis. Reprod. Sci., 24, 1454–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Osinski M., Wirstlein P., Wender-Ozegowska E. et al. (2018) HSD3B2, HSD17B1, HSD17B2, ESR1, ESR2 and AR expression in infertile women with endometriosis. Ginekol. Pol., 89, 125–134. [DOI] [PubMed] [Google Scholar]
- 25. Seo S.K., Nam A., Jeon Y.E. et al. (2010) Expression and possible role of non-steroidal anti-inflammatory drug-activated gene-1 (NAG-1) in the human endometrium and endometriosis. Hum. Reprod., 25, 3043–3049. [DOI] [PubMed] [Google Scholar]
- 26. Prefumo F., Semino C., Melioli G. et al. (2002) A defective expression of ICAM-1 (CD54) on secretory endometrial cells is associated with endometriosis. Immunol. Lett., 80, 49–53. [DOI] [PubMed] [Google Scholar]
- 27. Bianco B., Lerner T.G., Trevisan C.M. et al. (2012) The nuclear factor-kB functional promoter polymorphism is associated with endometriosis and infertility. Hum. Immunol., 73, 1190–1193. [DOI] [PubMed] [Google Scholar]
- 28. Salmeri F.M., Lagana A.S., Sofo V. et al. (2015) Behavior of tumor necrosis factor-alpha and tumor necrosis factor receptor 1/tumor necrosis factor receptor 2 system in mononuclear cells recovered from peritoneal fluid of women with endometriosis at different stages. Reprod. Sci., 22, 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tang L., He R., Zhou C. et al. (2007) Expression of cyclin B1 and cyclin-dependent kanasel in ectopic and eutopic endometrium tissues of patients with endometriosis. Zhonghua Yi Xue Za Zhi, 87, 179–183. [PubMed] [Google Scholar]
- 30. Chatterjee K., Jana S., DasMahapatra P. et al. (2018) EGFR-mediated matrix metalloproteinase-7 up-regulation promotes epithelial-mesenchymal transition via ERK1-AP1 axis during ovarian endometriosis progression. FASEB J., 32, 4560–4572. [DOI] [PubMed] [Google Scholar]
- 31. Pan H., Zhang P., Li J.R. et al. (2016) c-Fos-regulated matrix metalloproteinase-9 expression is involved in 17beta-estradiol-promoted invasion of human endometrial stromal cell. Curr. Mol. Med., 16, 266–275. [DOI] [PubMed] [Google Scholar]
- 32. Rahmioglu N., Montgomery G.W. and Zondervan K.T. (2015) Genetics of endometriosis. Women’s Heal., 11, 577–586. [DOI] [PubMed] [Google Scholar]
- 33. Eisenberg V.H., Weil C., Chodick G. et al. (2017) Epidemiology of endometriosis: a large population-based database study from a healthcare provider with 2 million members. BJOG, 125, 55–62. [DOI] [PubMed] [Google Scholar]
- 34. Grummer R. (2013) Translational animal models to study endometriosis-associated infertility. Semin Reprod Med., 31, 125–132. [DOI] [PubMed] [Google Scholar]
- 35. Bozdag G. (2015) Recurrence of endometriosis: risk factors, mechanisms and biomarkers. Womens Health (Lond)., 11, 693–699. [DOI] [PubMed] [Google Scholar]
- 36. Campbell I.G. and Thomas E.J. (2001) Endometriosis: candidate genes. Hum. Reprod. Update, 7, 15–20. [DOI] [PubMed] [Google Scholar]
- 37. Coordinators N.R. (2015) Database resources of the National Center for Biotechnology Information. Nucleic Acids Res., 43, D6–D17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang X., Sun X.F., Cao Y. et al. (2018) CBD: a biomarker database for colorectal cancer. Database (Oxford), 2018. doi: 10.1093/database/bay046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Consortium U. (2015) UniProt: a hub for protein information. Nucleic Acids Res., 43, D204–D212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ashburner M., Ball C.A., Blake J.A. et al. (2000) Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet., 25, 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Consortium T.G.O. (2017) Expansion of the Gene Ontology knowledgebase and resources. Nucleic Acids Res., 45, D331–D338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mi H., Huang X., Muruganujan A. et al. (2017) PANTHER version 11: expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Res., 45, D183–D189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carbon S., Ireland A., Mungall C.J. et al. (2009) AmiGO: online access to ontology and annotation data. Bioinformatics, 25, 288–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kanehisa M., Furumichi M., Tanabe M. et al. (2017) KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res., 45, D353–D361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Becker K.G., Barnes K.C., Bright T.J. et al. (2004) The genetic association database. Nat. Genet., 36, 431–432. [DOI] [PubMed] [Google Scholar]
- 46. Amberger J.S., Bocchini C.A., Schiettecatte F. et al. (2015) OMIM.org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res., 43, D789–D798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Geer L.Y., Domrachev M., Lipman D.J. et al. (2002) CDART: protein homology by domain architecture. Genome Res., 12, 1619–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pagni M., Ioannidis V., Cerutti L. et al. (2007) MyHits: improvements to an interactive resource for analyzing protein sequences. Nucleic Acids Res., 35, W433–W437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reimand J., Arak T., Adler P. et al. (2016) g:Profiler-a web server for functional interpretation of gene lists (2016 update). Nucleic Acids Res., 44, W83–W89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Szklarczyk D., Franceschini A., Wyder S. et al. (2015) STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res., 43, D447–D452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Altschul S.F., Gish W., Miller W. et al. (1990) Basic local alignment search tool. J. Mol. Biol., 215, 403–410. [DOI] [PubMed] [Google Scholar]
- 52. Djokovic D. and Calhaz-jorge C. (2014) Angiogenesis as a Therapeutic Target in Endometriosis. Acta Med Port., 27, 489–497. [DOI] [PubMed] [Google Scholar]
- 53. Ahn S.H., Monsanto S.P., Miller C. et al. (2015) Pathophysiology and immune dysfunction in endometriosis. Biomed Res Int., 2015, 795976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Heidemann L.N., Hartwell D., Heidemann C.H. et al. (2014) The relation between endometriosis and ovarian cancer—a review. Acta Obstet. Gynecol. Scand., 93, 20–31. [DOI] [PubMed] [Google Scholar]
- 55. Pavlidou A. and Vlahos N.F. (2014) Endometriosis and ovarian cancer: clinical and molecular aspects. Minerva Endocrinol., 39, 155–165. [PubMed] [Google Scholar]
- 56. Nezhat F.R., Pejovic T., Reis F.M. et al. (2014) The link between endometriosis and ovarian cancer: clinical implications. Int. J. Gynecol. Cancer, 24, 623–628. [DOI] [PubMed] [Google Scholar]
- 57. Bereziat V., Kasus-Jacobi A., Perdereau D. et al. (2002) Inhibition of insulin receptor catalytic activity by the molecular adapter Grb14. J. Biol. Chem., 277, 4845–4852. [DOI] [PubMed] [Google Scholar]
- 58. Wang C.F., Zhang G., Zhao L.J. et al. (2013) Overexpression of the insulin receptor isoform A promotes endometrial carcinoma cell growth. PLoS One, 8, e69001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59. Orostica L., Rosas C., Plaza-Parrochia F. et al. (2016) Altered steroid metabolism and insulin signaling in PCOS endometria: impact in tissue function. Curr. Pharm. Des., 22, 5614–5624. [DOI] [PubMed] [Google Scholar]
- 60. Sabbaghi M., Aram R., Roustaei H. et al. (2014) IL-17A concentration of seminal plasma and follicular fluid in infertile men and women with various clinical diagnoses. Immunol. Invest., 43, 617–626. [DOI] [PubMed] [Google Scholar]
- 61. Margarit L., Taylor A., Roberts M.H. et al. (2010) MUC1 as a discriminator between endometrium from fertile and infertile patients with PCOS and endometriosis. J. Clin. Endocrinol. Metab., 95, 5320–5329. [DOI] [PubMed] [Google Scholar]
- 62. Whitehead S.A., Peattie A.B., Shakil T. et al. (1996) Endometriosis and polycystic ovary syndrome: enhanced stimulatory effect of peritoneal fluid on progesterone release from human granulosa-lutein cells. Fertil. Steril., 66, 487–489. [PubMed] [Google Scholar]
- 63. Kichukova D. (1996) Polycystic ovaries in association with pelvic endometriosis in infertile women diagnosed by laparoscopy. Folia Med. (Plovdiv)., 38, 71–73. [PubMed] [Google Scholar]
- 64. Nabeta M., Abe Y., Takaoka Y. et al. (2011) Identification of anti-syntaxin 5 autoantibody as a novel serum marker of endometriosis. J. Reprod. Immunol., 91, 48–55. [DOI] [PubMed] [Google Scholar]
- 65. Nothnick W.B. (2001) Treating endometriosis as an autoimmune disease. Fertil. Steril., 76, 223–231. [DOI] [PubMed] [Google Scholar]
- 66. Sundqvist J., Falconer H., Seddighzadeh M. et al. (2011) Endometriosis and autoimmune disease: association of susceptibility to moderate/severe endometriosis with CCL21 and HLA-DRB1. Fertil. Steril., 95, 437–440. [DOI] [PubMed] [Google Scholar]
- 67. Amalinei C., Caruntu I.D., Giusca S.E. et al. (2010) Matrix metalloproteinases involvement in pathologic conditions. Rom. J. Morphol. Embryol., 51, 215–228. [PubMed] [Google Scholar]
- 68. Baranov V.S., Ivaschenko T.E., Liehr T. et al. (2015) Systems genetics view of endometriosis: a common complex disorder. Eur. J. Obstet. Gynecol. Reprod. Biol., 185, 59–65. [DOI] [PubMed] [Google Scholar]
- 69. Yuk J.S., Park E.J., Seo Y.S. et al. (2016) Graves disease is associated with endometriosis: a 3-year population-based cross-sectional study. Medicine (Baltimore), 95, e2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Harris H.R., Costenbader K.H., Mu F. et al. (2016) Endometriosis and the risks of systemic lupus erythematosus and rheumatoid arthritis in the Nurses’ Health Study II. Ann. Rheum. Dis., 75, 1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Haga H.J., Gjesdal C.G., Irgens L.M. et al. (2005) Reproduction and gynaecological manifestations in women with primary Sjogren’s syndrome: a case-control study. Scand. J. Rheumatol., 34, 45–48. [DOI] [PubMed] [Google Scholar]
- 72. Fauser B.C.J.M., Laven J.S.E., Tarlatzis B.C. et al. (2011) Sex steroid hormones and reproductive disorders: impact on women’s health. Reprod. Sci., 18, 702–712. [DOI] [PubMed] [Google Scholar]
- 73. Deroo B.J. and Korach K.S. (2006) Estrogen receptors and human disease. J. Clin. Invest., 116, 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Latourelle J.C., Dybdahl M., Destefano A.L. et al. (2010) Estrogen-related and other disease diagnoses preceding Parkinson’s disease. Clin. Epidemiol., 2, 153–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rahmioglu N., Macgregor S., Drong A.W. et al. (2015) Genome-wide enrichment analysis between endometriosis and obesity-related traits reveals novel susceptibility loci. Hum. Mol. Genet., 24, 1185–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shannon P., Markiel A., Ozier O. et al. (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res., 13, 2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bader G.D. and Hogue C.W.V. (2003) An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinformatics, 4, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hirota Y., Osuga Y., Hirata T. et al. (2005) Possible involvement of thrombin/protease-activated receptor 1 system in the pathogenesis of endometriosis. J. Clin. Endocrinol. Metab., 90, 3673–3679. [DOI] [PubMed] [Google Scholar]
- 79. Marianna S., Alessia P., Susan C. et al. (2017) Metabolomic profiling and biochemical evaluation of the follicular fluid of endometriosis patients. Mol. Biosyst., 13, 1213–1222. [DOI] [PubMed] [Google Scholar]
- 80. Young V.J., Brown J.K., Saunders P.T.K. et al. (2014) The peritoneum is both a source and target of TGF-β in women with endometriosis. PLoS One, 9, e106773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lv X., Wang D., Ma Y. et al. (2018) Analysis of the oncogene BRAF mutation and the correlation of the expression of wild-type BRAF and CREB1 in endometriosis. Int. J. Mol. Med., 41, 1349–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Guo Y., Chen Y., Liu L.B. et al. (2013) IL-22 in the endometriotic milieu promotes the proliferation of endometrial stromal cells via stimulating the secretion of CCL2 and IL-8. Int. J. Clin. Exp. Pathol., 6, 2011–2020. [PMC free article] [PubMed] [Google Scholar]
- 83. Wingfield M., Macpherson A., Healy D.L. et al. (1995) Cell proliferation is increased in the endometrium of women with endometriosis. Fertil. Steril., 64, 340–346. [DOI] [PubMed] [Google Scholar]
- 84. Ahn J.H., Choi Y.S. and Choi J.H. (2015) Leptin promotes human endometriotic cell migration and invasion by up-regulating MMP-2 through the JAK2/STAT3 signaling pathway. Mol. Hum. Reprod., 21, 792–802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


